Abstract
Investigations in the last 10 years have revealed a new category of neurological diseases mediated by antibodies against cell surface and synaptic proteins. There are currently 16 such diseases all characterized by autoantibodies against neuronal proteins involved in synaptic signaling and plasticity. In clinical practice these findings have changed the diagnostic and treatment approach to potentially lethal, but now treatable, neurological and psychiatric syndromes previously considered idiopathic or not even suspected to be immune-mediated. Studies show that patients' antibodies can impair the surface dynamics of the target receptors eliminating them from synapses (e.g., NMDA receptor), block the function of the antigens without changing their synaptic density (e.g., GABAb receptor), interfere with synaptic protein-protein interactions (LGI1, Caspr2), alter synapse formation (e.g., neurexin-3α), or by unclear mechanisms associate to a new form of tauopathy (IgLON5). Here we first trace the process of discovery of these diseases, describing the triggers and symptoms related to each autoantigen, and then review in detail the structural and functional alterations caused by the autoantibodies with special emphasis in those (NMDA receptor, amphiphysin) that have been modeled in animals.
I. INTRODUCTION
Memory, behavior, and cognition are dependent on the normal function of neurotransmitter receptors, ion channels, and other regulatory cell surface proteins involved in synaptic transmission and plasticity (189, 288). Recent studies have identified a group of human disorders in which these synaptic receptors and proteins are directly targeted by autoantibodies (184). Most of these disorders manifest as a rapidly progressive encephalitis (115) but can also occur as a cerebellar syndrome (293) or a chronic encephalopathy resembling a degenerative process (273). The discovery of these disorders stemmed from clinical observations of patients with unusual syndromes that improved after immunotherapy, or whose cerebrospinal fluid (CSF) and brain MRI findings were consistent with an inflammatory disorder leading to investigations that revealed the presence of autoantibodies against neuronal cell surface proteins (7, 328). In this review we use the simplified term autoimmune encephalopathies (AE) to refer to this group of diseases. There are currently 16 AE in which the autoantibodies are directed against excitatory or inhibitory neuronal receptors or proteins involved in somatodendritic signal integration, clustering and modulation of receptors, synaptic vesicle reuptake, or synaptogenesis (Table 1). For most of these disorders, the accessibility of the cell surface antigens to circulating antibodies and the reversibility of patients' symptoms after removing the antibodies or antibody-producing cells suggested a direct pathogenic role of the antibodies, a hypothesis that has been confirmed in in vitro and in vivo models (144, 251, 258, 293, 335). In this review we first trace the background for the clinical and immunological studies that led to the identification of the autoantibodies, and then provide details of the neuronal targets, main syndrome associations, and underlying mechanisms that delineate the pathophysiology of these disorders.
Table 1.
Antibodies to neuronal cell surface proteins and synaptic receptors
| Antibody | IgG Class | Epitope(s) | In Vitro Effects | In Vivo Effects |
|---|---|---|---|---|
| NMDA receptor (60) | IgG1 | N368/G369 region of GluN1 | Internalization of NMDA receptors in neurons | Memory and behavioral deficits associated with a decrease of total cell surface and synaptic NMDA receptors (258) |
| AMPA receptor (181) | IgG1 | GluA1 or GluA2 | Internalization of AMPA receptors in neurons | Unknown |
| GABAb receptor (186) | IgG1 | B1 subunit | Antagonist of baclofen effects in neurons | Unknown |
| LGI1 (151, 182) | IgG4/IgG1 | Epitempin and leucine-rich repeat domain | Inhibition of interaction with ADAMs; decrease postsynaptic AMPA receptor; presynaptic effects? | Unknown |
| CASPR2 (151, 185) | IgG4/IgG1 | Discoidin-like and lamininG1 domains | Alter gephyrin clusters in inhibitory synapses in cultured neurons | Unknown |
| GABAa receptor (252) | IgG1 | Extracellular epitope of the α1, β3 or γ2 subunits | Selective reduction of GABAa receptor at synapses | Unknown |
| DPPX (33) | IgG4/IgG1 | Unknown | Hyperexcitability of enteric neurons; decrease of expression of DPPX and Kv4.2 in hippocampal neurons | Unknown |
| Dopamine-2 receptor (55) | Unknown | N terminus | Internalization of receptors (studies on HEK cells) | Unknown |
| mGluR5 (187) | IgG1 | Unknown | Unknown | Unknown |
| Neurexin-3α (119) | IgG1 | Unknown | Decrease expression of neurexin-3α on synapsis; decrease synapse formation | Unknown |
| IgLON5 (273) | IgG4/IgG1 | Ig-like domain 2 | Decrease expression of IgLON5 on neurons | Unknown |
| DNER (Tr) (66) | IgG1 | Extracellular domain between 2nd and 3rd EDG-like domain | Unknown | Unknown |
| P/Q-type VGCC (219) | Unknown | P/Q type VGCC | Unknown | Transient ataxia(213) |
| mGluR1 (293) | Unknown | Unknown | Reduction of basal activity of Purkinje cells | Transient ataxia (293) |
| Glycine receptor (147) | IgG1 | Unknown | Internalization of receptors (studies on HEK cells) | Unknown |
| Amphiphysin (65) | IgG1 | N-BAR domain protein enriched in the presynaptic nerve ending | Disrupt vesicle endocytosis in cultures of neurons | Motor hyperactivity, stiffness, and muscle spasms (297) |
NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABAb, gamma-aminobutyric acid type B; LGI1, leucine-rich glioma inactivated 1; CASPR2, contactin-associated protein-like 2; GABAa, gamma-aminobutyric acid type A; DPPX, dipeptidyl-peptidase-like protein-6; mGluR, metabotropic glutamate receptor; DNER, delta/notch-like epidermal growth factor-related receptor; VGCC, voltage-gated calcium channel; Gly, glycine; ADAM, a disintegrin and metalloprotease. Reference numbers are given in parentheses.
II. IDENTIFICATION OF CNS DISEASES MEDIATED BY AUTOANTIBODIES TO NEURONAL CELL SURFACE PROTEINS
The concept that some CNS diseases can be mediated by neuronal autoantibodies has evolved over the years influenced by the study of two groups of disorders, the paraneoplastic syndromes of the CNS, which contributed to their discovery (62), and the myasthenic syndromes, which helped to understand how autoantibodies can alter synaptic function (74, 75, 238, 332).
Paraneoplastic syndromes of the CNS are remote effects of cancer that in many instances are mediated by the immunological system (see Refs. 59, 62 for reviews on these disorders). They include many different syndromes that may involve any part of the nervous system and are associated with immune responses against intraneuronal proteins that are also expressed by the associated cancer (onconeuronal antigens). It is believed that the ectopic expression of these neuronal proteins by the tumor triggers an immune response that is misdirected against the nervous system. In addition to antibodies against onconeuronal antigens, these patients develop prominent cytotoxic T-cell responses against the nervous system. In studies using cultures of live neurons, the antibodies cannot reach the intracellular antigen and do not have pathogenic effects (304). However, autopsy studies of patients show extensive infiltrates of cytotoxic T-cells surrounding neurons and causing degeneration via perforin and granzyme B mechanisms (Figure 1) (26, 30, 31). Consequently, patients with these syndromes rarely respond to immunotherapies focused on removing the antibodies or antibody-producing cells (116, 167, 168, 268).
FIGURE 1.
Autoantibodies in classic paraneoplastic syndromes of the CNS and in novel autoimmune encephalopathies. A: in classic paraneoplastic syndromes of the CNS, the autoantibodies are directed against intracellular neuronal proteins that are also expressed by an underlying systemic tumor (onconeuronal proteins). These antibodies are useful diagnostic biomarkers, but there is no evidence they are pathogenic. Biopsy and autopsy studies of these patients show prominent inflammatory infiltrates of cytotoxic T-cells surrounding and indenting neurons, and causing neuronal degeneration (e.g., perforin or granzyme B cytotoxic mechanisms). In studies using live cultured neurons, the antibodies do not show binding to the target intracellular antigens. Patients with these syndromes rarely respond to treatments aimed to remove the antibodies or antibody-producing cells. Asterisks in A represent the intracellular location of the antigens. B: in the new category of autoantibodies against neuronal cell surface proteins or synaptic proteins [collectively called in this review autoimmune encephalopathies (AE)], the antibodies target epitopes exposed on the neuronal cell surface. Many patients with AE do not have an underlying tumor, and the autoantibodies have a direct pathogenic effect on the target neuronal proteins. Patients with these syndromes often respond to treatments aimed to remove the autoantibodies or antibody-producing cells.
In contrast to these syndromes, the antibodies associated with myasthenia gravis and the Lambert-Eaton syndrome (LEMS) can access their cell surface targets [acetylcholine receptor and the voltage-gated calcium channels (VGCC)] and alter their structure and function. In these disorders, the antibodies produce cross-linking and internalization of the receptors, functional blocking of the AChR, or complement-mediated changes in the neuromuscular junction (74, 75, 238, 332). The characterization of these antibody-induced alterations led to effective treatment approaches beyond immunotherapy (e.g., anticholinesterase drugs that increase the levels of acetylcholine, or 3,4-diaminopyridine that enhances the presynaptic release of acetylcholine) that compensate or antagonize the antibody effects (73, 339).
In recent years the observation of patients with syndromes similar to the paraneoplastic CNS disorders, but without cancer or antibodies against intracellular proteins, and who frequently responded to immunotherapy, suggested they could harbor pathogenic antibodies as those occurring in the myasthenic syndromes (7). One of the initial studies that elucidated one of these novel antibodies described two patients who developed symptoms similar to paraneoplastic limbic encephalitis (a disorder that causes memory loss and seizures) and had antibodies against cell surface proteins interacting with the voltage-gated potassium channels (VGKC). None of the two patients had cancer (one had a benign thymoma) and both improved after immunotherapy (39, 328). Another study identified four young women with prominent neuropsychiatric symptoms and neuronal antibodies that produced a characteristic pattern of immunostaining of the neuropil of rat brain and intense immunolabeling of the cell surface of live neurons (329) (Figure 2). All four patients had an underlying ovarian teratoma, and three recovered with immunotherapy; the target antigen was eventually characterized as the GluN1 subunit of the NMDA receptor, and the disorder is now known as anti-NMDA receptor encephalitis (57, 60).
FIGURE 2.
Comparison of brain and neuronal reactivity of antibodies against a cell surface and an intracellular antigen. Coronal section of rat hippocampus immunolabeled with an autoantibody against a synaptic receptor (NMDA) from a patient with anti-NMDA receptor encephalitis (A), compared with the autoantibody against an intracellular neuronal protein (HuD) from a patient with small cell lung cancer and paraneoplastic encephalitis (B). Magnification of the reactivity is shown in C and D, respectively. Compared with the NMDA receptor autoantibody that shows intense reactivity with the neuropil of hippocampus, the HuD autoantibody does not react with the neuropil, and only shows intracellular staining after tissue permeabilization. In cultures of dissociated rat hippocampal neurons, only the NMDA receptor autoantibody reacts with the autoantigen in live nonpermeabilized neurons (E). The HuD autoantibody does not reach the target intracellular antigen in live neurons (F). Scale bars: A, B = 500 μm; C, D = 20 μm; E, F = 10 μm.
The approach used for the discovery of these two syndromes and antibody associations was adapted and used to identify a large number of neuronal targets in several forms of encephalitis suspected to be immune mediated (Table 1). This approach associates groups of patients with similar symptoms or comorbidities with the demonstration of specific neuronal antibodies, each with a distinct pattern of brain immunostaining (Figure 3) along with neuronal cell surface immunolabeling (similar to that shown in Figure 2E) (33, 60, 181, 186). The antibodies were then used to precipitate the target antigens that were characterized by mass spectrometry. In some instances, the candidate antigen was suggested by the pattern of brain reactivity of the antibodies (e.g., NMDA receptor) (60), whereas in others, it was suggested by the clinical phenotype (e.g., rigidity, hyperekplexia, and the glycine receptor) (147). Once the antigen was identified, the development of cell-based assays (CBA) in which recombinant HEK cells express the antigen of interest led to useful diagnostic tests. Because the epitope targets of many of these disorders are conformational, most techniques using denatured or recombinant proteins are inadequate for antibody detection.
FIGURE 3.
Rat brain immunostaining with autoantibodies of patients targeting neuronal cell surface and synaptic proteins. Sagittal and coronal sections of rat brain immunostained with 13 autoantibodies against neuronal cell surface and synaptic proteins. For DNER and mGluR1, which predominantly react with cerebellum, the coronal section has been replaced by a sagittal section of cerebellum. Autoantibodies against VGCC, dopamine 2R, and GlyR with patterns of immunostaining poorly visible with this technique have been excluded. Technique of immunostaining was reported in Ref. 7. All tissue sections have been mildly counterstained with hematoxylin. Scale bars: all panels = 2 mm. [From Dalmau et al. (56).]
These pioneering studies set in motion the field of antibody-mediated disorders of the CNS leading to a better definition of the syndromes and investigations on the antibody pathogenicity at the cellular, synaptic, and circuitry levels.
III. TRIGGERS OF AUTOIMMUNITY: TUMORS AND VIRUSES
A. Tumors
Different from the classical paraneoplastic syndromes of the CNS that almost always associate with cancer (111), AE may occur with or without tumor association, with a frequency and type of tumor that vary according to the type of disease and autoantibody (Table 2). In this respect the AE are similar to the myasthenic syndromes in which the frequency of thymoma is ∼10% in patients with myasthenia, whereas the frequency of small-cell lung cancer (SCLC) is at least 50% in patients with LEMS (310). There are AE that rarely associate with tumors, for example, LGI1 encephalitis (151, 182), whereas others show a robust and specific cancer association, such as the encephalitis associated with GABAb receptor antibodies and SCLC (138, 158).
Table 2.
Main clinical features associated with antibodies to neuronal cell surface proteins and synaptic receptors
| Antibody | Main Presenting Symptoms | Main Syndrome | MRI FLAIR/T2 Sequences | PET | Frequency of Cancer | Types of Cancer |
|---|---|---|---|---|---|---|
| NMDA receptor | Psychiatric (adults); seizures, dyskinesias (children) | NMDA receptor encephalitis (57, 89, 309) | Normal or transient non-region specific changes | Increased frontal and temporal FDG uptake; decreased occipital FDG update | Overall 40%; 58% in women 18–45 yr | Teratoma* |
| AMPA receptor | Memory loss | Limbic encephalitis (137, 181) | Hyperintense signal highly restricted to medial temporal lobes | FDG uptake in temporal lobes | 65% | Thymoma, SCLC, other |
| GABAb receptor | Memory loss, seizures | Limbic encephalitis with early and prominent seizures (138, 158, 186) | Hyperintense signal highly restricted to medial temporal lobes | FDG uptake in temporal lobes | 50% | SCLC |
| LGI1 | Memory loss, FBD seizures | Limbic encephalitis (11, 323) | Hyperintense signal highly restricted to medial temporal lobes | Basal ganglia and temporal FDG uptake | 5–10% | Thymoma |
| CASPR2 | Sleep disorder, neuromyotonia | Morvan; limbic encephalitis (154, 159, 321) | Normal or hyperintense signal in medial temporal lobes | Unknown | Overall 20%. In Morvan syndrome (20–50%) | Thymoma† |
| GABAa receptor | Seizures | Encephalitis with refractory seizures, status epilepticus (252, 298) | Hyperintense signal in multiple cortical and subcortical areas | Unknown | 25% | Thymoma, other |
| DPPX | Confusion, diarrhea, hyperplexia | Encephalitis, hyperekplexia (21, 33, 311) | Normal or non-region specific changes | Unknown | <10% | Lymphoma |
| Dopamine-2 receptor | Lethargy, psychiatric symptoms, abnormal movements, gait disturbance | Basal ganglia encephalitis (55) | Hyperintense signal in basal ganglia | Unknown | 0% | n/a |
| mGluR5 | Memory loss | Encephalitis (187) | Normal or hyperintense signal in various brain regions | Unknown | A few cases described | Hodgkin disease |
| Neurexin-3α | Confusion, seizures | Encephalitis (119) | Normal | Unknown | 0% | n/a |
| IgLON5 | Sleep disorder | NREM and REM sleep disorder, and brain stem dysfunction (104, 273) | Normal | Unknown | 0% | n/a |
| DNER (Tr) | Gait instability | Cerebellar ataxia (27, 66) | Normal or cerebellar atrophy | Unknown | >90% | Hodgkin disease |
| P/Q-type VGCC | Gait instability | Cerebellar ataxia (114, 219) | Normal or cerebellar atrophy | Unknown | >90%‡ | SCLC |
| mGluR1 | Gait instability | Cerebellar ataxia (211, 293) | Normal or cerebellar atrophy | Unknown | A few cases described | Hodgkin disease |
| Glycine receptor | Muscle rigidity, spasms | PERM, stiff-person syndrome (42, 214) | Normal or non-region specific changes | Unknown | <5% | Thymoma, lung, Hodgkin |
| Amphiphysin | Rigidity, spasms | Stiff-person, encephalomyelitis (256) | Normal or non-region specific changes | Unknown | >90% | Breast cancer, SCLC |
The association with teratoma is sex and age dependent. While young adult females frequently have an ovarian teratoma, the presence of a tumor is uncommon in children or young adult males (89, 309). †Patients with Caspr2 antibodies and Morvan syndrome are more likely to have thymoma than those without Morvan syndrome. (154, 185). ‡This number refers to patients with P/Q-type VGCC antibodies and paraneoplastic cerebellar degeneration. In patients with Lambert-Eaton myasthenic syndrome, the frequency of SCLC is 50–60% (307). NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABAb, gamma-aminobutyric acid type B; LGI1, leucine-rich glioma inactivated 1; CASPR2, contactin-associated protein-like 2; GABAa, gamma-aminobutyric acid type A; DPPX, dipeptidyl-peptidase-like protein-6; mGluR, metabotropic glutamate receptor; DNER, delta/notch-like epidermal growth factor-related receptor; VGCC, voltage-gated calcium channel; Gly, glycine; REM, rapid-eye-movement sleep; NREM, non-rapid-eye-movement sleep; PERM, progressive encephalomyelitis with rigidity and myoclonus; MRI FLAIR, magnetic resonance imaging fluid-attenuated inversion recovery; PET, positron emission tomography; FDG, fluorodeoxyglucose; SCLC, small-cell lung cancer; n/a, not applicable. Reference numbers are given in parentheses.
For AE with a frequent cancer association (Table 2), the cancer cells express the neuronal cell surface or synaptic protein against which the antibody is directed. Therefore, it is thought that the ectopic expression of neuronal proteins by the tumor breaks immune tolerance for these proteins contributing to the development of the immune response (69). The mechanism by which this occurs is unknown. In classical paraneoplastic syndromes mediated by cytotoxic T-cell mechanisms, where the associated antibodies are directed against intracellular proteins, there is evidence that antigens released by apoptotic tumor cells are taken up and processed by antigen-presenting cells at the regional lymph nodes and appropriately presented to the immunological system eliciting an anti-tumor immune response (6). This becomes an autoimmune response against the same protein expressed in the nervous system leading to extensive infiltrates of cytotoxic T cells and neuronal degeneration via perforin or granzyme-related mechanisms (Figure 1A) (30, 31). For some AE [e.g., GABAb receptor and SCLC, or AMPA receptor and lung or other cancers (181)], the trigger may be similar but predominantly priming B-cell immune responses leading to the production of autoantibodies with functional and reversible, rather than structural and irreversible, neuronal alteration.
In the case of ovarian teratoma (which frequently associates with anti-NMDA receptor encephalitis), the tumor itself contains mature or immature neural tissue, sometimes difficult to differentiate microscopically from normal brain tissue (60, 280). As in brain, these neurons contain NMDA receptors that likely contribute in triggering the autoimmune response. In line with this hypothesis, the tumors of these patients contain larger amounts of inflammatory infiltrates compared with the teratomas from patients who do not develop anti-NMDA receptor encephalitis (318).
Tumors of the thymus, including thymomas and carcinomas of the thymus, frequently associate with systemic and neurologic autoimmunity including paraneoplastic syndromes of the CNS, myasthenia gravis, and AE (Table 2) (303, 325). All theories used to explain this predisposition to autoimmunity converge in a loss of self-tolerance produced by alterations in the mechanisms of positive and negative T-cell selection that normally occur in the thymus (52, 223, 287). These alterations lead to production of autoreactive T-cells, which may also affect B-cell function. A clear molecular link between thymoma and autoimmunity was demonstrated in a patient whose tumor tissue lacked expression of the autoimmune regulator gene AIRE, and who developed a clinical picture corresponding to the autoimmune polyglandular syndrome type 1 (APS1), a monogenic syndrome that arises from defects in the AIRE gene (52). This gene mediates the expression of tissue specific self-antigens by medullary thymic epithelial cells, and therefore the altered mechanism of negative selection promoted autoimmunity. There are no similar studies in thymoma tissue of patients with AE, but these patients often develop multiple autoantibodies and overlapping syndromes, e.g., GABAb receptor and GAD antibodies, suggesting the presence of altered mechanisms of immune self-tolerance (13, 186).
An exception to these models is the association between Hodgkin lymphoma and several AE with antibodies against DNER (or Tr), mGluR1, or mGluR5 (Table 2) (27, 187, 293). In these disorders the target antigens are not expressed by the tumor, and the mechanisms underlying the predominant association with Hodgkin lymphoma are unclear.
B. Herpes Simplex and Other Viruses as Triggers of AE
The discovery that ∼20% of patients with herpes simplex virus encephalitis (HSE) develop antibodies against cell surface neuronal proteins (mainly NMDA receptor) demonstrates a link between a CNS viral infection and brain autoimmunity (16, 18). This finding provided a biological explanation for a previously well-known complication of HSE, described as “relapsing neurologic symptoms post-HSE” or “choreoathetosis post-HSE,” that usually occurs a few weeks after successful treatment of the viral infection (67). In children the clinical picture associates with predominant choreoathetoid movements and in adults with psychiatric and cognitive alterations (18, 125, 229, 308). When the CSF and serum of these patients are compared with samples obtained at the time of the viral infection, most cases show new synthesis of antibodies against the NMDA receptor and less frequently against other receptors or neuronal cell surface proteins (GABAa, dopamine, uncharacterized cell surface proteins) (15, 16, 229). Treatment with immunotherapy rather than viral therapy associates with neurological improvement, although patients may have sequelae from the viral encephalitis and the outcome is not as good as that of patients with anti-NMDA receptor encephalitis unrelated to HSE. A possible link between other infections and anti-NMDA receptor encephalitis has been suggested (varicella zoster virus, mycoplasma), but the number of cases is too small to confirm this association (97, 282).
These findings suggest a mechanism whereby a viral-induced inflammatory disorder within the brain triggers autoantibodies against cell surface and synaptic receptors released by the neuronal destructive infection. An alternative mechanism of molecular mimicry seems less likely given that most patients appear to develop autoantibodies against multiple neuronal proteins (15). It is unclear why this autoimmunity predominantly occurs with HSE, but this highly destructive neurotropic virus (337) results in the release of neuronal proteins into a milieu of extensive inflammatory infiltrates that may lead to activation of the immune system. This activation most likely occurs in the deep cervical lymph nodes which receive soluble and cell transported antigens from the CNS (265). The role of deep cervical lymph nodes as site of processing and presentation of CNS-derived antigens to the immunological system is well known for T-cell mediated immune responses, but it is less clear for antibody-mediated disorders (204). Interestingly, the NMDA receptor antibody seroconversion of these patients is first noted in CSF than in serum (15, 16). A proposed model of how anti-NMDA receptor encephalitis can be triggered by a tumor (teratoma) or HSE is shown (Figure 4). According to this model, the initial presentation of the antigen to the immunological system takes place outside the CNS, but the antibodies are eventually synthesized on both sides of the blood-brain barrier (BBB). Two recent studies (107, 173) in which recombinant NMDA receptor antibodies were generated from B cells and expanded plasma cells present in the CSF of patients with anti-NMDA receptor encephalitis, along with investigations showing intrathecal synthesis of antibodies (57), provide robust support of the intra-CNS production of antibodies.
FIGURE 4.
Triggers of anti-NMDA receptor encephalitis and a proposed model of B-cell activation. The figure shows two identified triggers of the disorder: a tumor (usually ovarian teratoma) and much less frequently herpes simplex encephalitis. In ovarian teratoma, the nervous tissue present in the tumor contains neurons and NMDA receptors that are likely released by tumor-related necrotic changes, reaching the local, pelvic-abdominal lymph nodes (318). In cases of herpes simplex encephalitis, the prominent viral-induced inflammation, tissue necrosis, and neuronal degeneration may release the antigen which is transported to the local brain-draining deep cervical lymph nodes (an alternative route is through the venous sinuses). In the lymph nodes (pelvic-abdominal or deep cervical), the antigen is presented to naive B cells by antigen-presenting cells in cooperation with CD4+ T cells leading to generation of memory B cells and antibody-producing plasma cells. Activated memory B cells reach the brain through the bloodstream, crossing the choroidal plexus; in the brain the activated B cells undergo restimulation, antigen-driven affinity maturation, and differentiation into plasma cells. A smaller contribution would be the crossing of a leaky or disrupted BBB by autoantibodies. [From Dalmau et al. (56).]
In a substantial number of patients with AE, the trigger of the autoimmune response is unknown; in these cases screening studies for an occult tumor or recent viral infections are negative. The young age and lack of tumor risk factors in some patients, as well as long clinical follow-up during which patients remain tumor free, support the existence of other unknown immunological triggers. The fact that some patients, even the very young, have other autoantibodies [antinuclear antibodies (ANA), thyroid antibodies] with a frequency higher than expected in the normal population suggest an underlying predisposition to autoimmunity (89, 181, 186, 317). Genetic susceptibility to develop AE has only been investigated in a few of these disorders. Recent studies show specific HLA associations in patients with LGI1 and IgLON5 antibodies (104, 324). Future studies on peptide binding prediction and in silico docking may provide the disease-specific epitopes and a better knowledge of the underlying pathogenic mechanisms.
IV. NEURONAL CELL SURFACE SYNAPTIC PROTEINS, AUTOANTIBODIES, AND SYNDROMES
There is a wide spectrum of symptoms resulting from AE. Some autoantibodies result in predictable and highly characteristic syndromes (and have been used to name the new disease), whereas others associate with less recognizable syndromes that may show substantial clinical overlap with each other (Table 2). Guidelines to the clinical recognition and diagnostic criteria of AE have recently been published (115). In this section, we discuss these disorders according to either the target synaptic protein that defines a disease, or in cases with substantial overlap of symptoms according to the main resulting syndrome.
A. NMDA Receptor: Autoantigen of Anti-NMDA Receptor Encephalitis and Other Disorders
The NMDA receptors are ionotropic glutamate receptors comprised of two GluN1 and two GluN2 or GluN3 subunits (Figure 5) (49, 71). There are eight alternatively spliced GluN1 isoforms, four GluN2 subunits (A-D), and two GluN3 subunits (A-B). GluN1 (which are obligatory subunits) and GluN3 bind glycine, while GluN2 subunits bind glutamate. The GluN subunits have three large extracellular domains, the amino terminal domain (ATD) which is further subdivided into two lobes (163), and the S1 and S2 domains which contain the agonist binding site; three membrane-spanning domains (TM1, 3, 4); a membrane loop (TM2); and an intracellular carboxy-terminal domain that connects the receptor to scaffolding proteins and messenger systems. With maturity many GluN1/GluN2B receptors become largely extrasynaptic in hippocampal neurons, and GluN1/GluN2A/GluN2B become the major synaptic receptors in hippocampus and forebrain. GluN1 knockout mice die within hours after birth (90). Hippocampal CA1 region-specific GluN1 knockouts show impaired spatial and temporal learning with severe impairment of formation of long-term potentiation (LTP) in the Schaffer collateral-CA1 synapse, demonstrating the role of the NMDA receptor in establishing synaptic plasticity and memory formation (143, 315).
FIGURE 5.
Schematic representation of GluN1 and GluN2 subunits of the NMDA receptor and the main antibody-binding site. Antibodies from patients with anti-NMDA receptor encephalitis predominantly bind to an epitope region in the amino-terminal domain (ATD) of GluN1 that includes amino acids N368/G369 (106). This region is between a top and bottom lobe that confer a clamshell-like structure to the ATD. Point mutations at this region (N368/G369) abolish the reactivity of most patients' autoantibodies (106, 120). LBD, ligand binding domain; TMD, transmembrane domain; CTD, COOH-terminal domain.
Autoantibodies to the NMDA receptor have been described in multiple different neurological disorders. The clinical and pathological significance of these antibodies depend on the IgG subclass, target subunit of the receptor, and presence of antibodies in the CSF (described below).
1. Anti-NMDA receptor encephalitis
Patients with this disease develop antibodies detectable with techniques that preserve the native conformation of the antigen, such as brain immunohistochemistry, live cultured neurons, or heterologous cells (e.g., human embryonic kidney 293 or HEK293) expressing the GluN1 subunit of the NMDA receptor, the latter now used as a diagnostic CBA (57, 60). Deletion mutants of GluN1 expressed in a CBA system demonstrated that the N368/G369 region of the ATD was essential for creation of immunoreactivity; in addition, a top lobe deletion mutant showed a wide range of reactivity with patients' antibodies that was increased in some and reduced in others (106, 120, 173). These antibodies cause internalization of the NMDA receptors and disrupt the interaction of the receptor with EphB2 by mechanisms described in detail in section V.
This disease predominantly affects young women (median age 22 yr; 80% women), and ∼40% of all patients are younger than 18 yr (309). Potential triggers of the disease are tumors, mostly teratomas of the ovary, and much less frequently other tumors and HSE, as previously discussed (see sect. III). The presence of a tumor is extremely rare in children younger than 12 yr or men. In a single institution study, children with anti-NMDA receptor encephalitis were more likely to have their symptoms during warm months, clustered between April and September; the reason for this summer predominance was unclear, but the authors argued that it was against a role of vaccinations, which are given throughout the year, or winter pathogens such as influenza (4). Overall, in ∼55-60% of the patients, no trigger is identified (18, 89, 309).
The clinical syndrome suggests an immune-mediated decrease of receptor function, resembling in many aspects the models of noncompetitive antagonists of the NMDA receptors (e.g., phencyclidine, ketamine) (43, 126, 175, 334) or genetic reduction of NMDA receptors (230). Indeed, patients with IgG antibodies to the GluN1 subunit usually present with a rapid progression of neuropsychiatric manifestations that may lead to coma in a few days or weeks. After prodromal headache or fever, which occur in 70% of the patients, there is a sequential progression of psychiatric manifestations that may include anxiety, insomnia, delusional thinking (e.g., grandiose delusions, hyper-religiosity), hallucinations, paranoid thoughts, pressured speech, mood disorder (predominantly manic), or aggressive behavior, with alternating episodes of extreme agitation and catatonia (58, 309). At this stage many patients are suspected to be using drugs or of having an acute psychotic break and are frequently admitted to psychiatric centers (50, 133, 158, 209). However, most patients progress to develop seizures, reduced verbal output, decreased level of consciousness, highly characteristic orofacial and limb dyskinesias, choreoathetosis, dystonic postures, rigidity, and autonomic dysfunction (19, 152, 309, 326). This may include tachycardia (less frequently bradycardia with cardiac pauses), high blood pressure, hyperthermia, profuse salivation, and hypoventilation (280). Eventually, many patients become comatose, in a state resembling akinetic mutism or catatonia alternating with periods of agitation. Dissociative responses such as resisting eye opening but a lack of response to painful stimuli (similar to those obtained with dissociative anesthetics such as ketamine) are frequently noted during the examination. In young children, the first symptoms of the disease are more frequently seizures, abnormal movements, and change of behavior (irritability, insomnia, and disintegration of language) (Figure 6) (18, 309).
FIGURE 6.
Symptom development and time course of anti-NMDA receptor encephalitis. The graph shows the typical course of symptoms in a young adult with full-blown anti-NMDA receptor encephalitis. In children and young males, the symptom onset can be abnormal movements, seizures, or psychiatric symptoms. Otherwise, the progression of symptoms is remarkably similar in most patients. Milder forms of the disorder, without symptoms requiring intensive support care, are becoming more frequent as the disease is better known and diagnosed and treated earlier. [Modified from Kayser and Dalmau (164).]
This clinical picture may be accompanied by high serum levels of creatine kinase and rhabdomyolysis, which in combination with a decreased level of consciousness and rigidity may suggest the diagnosis of neuroleptic malignant syndrome; however, similar symptoms can occur in patients who do not receive neuroleptics (280). There are reports suggesting a high susceptibility of these patients to develop neuroleptic-related complications (164, 191).
The CSF shows IgG antibodies against the GluN1 subunit of the NMDA receptor often accompanied by inflammatory changes including pleocytosis or elevated IgG index, and normal or mild increase of the total concentration of proteins (120, 326). The EEG shows general slow activity in the theta or delta range, often with superimposed epileptic activity. A pattern of EEG activity named “extreme delta brush,” where bursts of rhythmic 20- to 30-Hz beta frequency activity ride on rhythmic delta (1–3 Hz) waves is highly suggestive of this disorder but occurs only in a small subgroup of patients (285). Conventional clinical MRI is normal in 60% of the patients, and the other 40% show mild or transient cortical or subcortical brain, cerebellar, or brain stem abnormalities (309).
The paradox of the occurrence of severe neurological symptoms with normal or mildly abnormal MRI was recently investigated using advanced MRI techniques. This showed a frequently reduced functional connectivity of the hippocampi with the anterior default mode network that correlated with memory performance, along with white matter changes, more prominent in the cingulum, which correlated with disease severity (86). In contrast, T1/T2 weighted structural imaging and grey matter morphology were similar to those of controls. Interestingly, the effects of ketamine also manifest with severe disruption of default mode network connectivity that correlates with working memory impairment and ketamine-induced psychiatric symptoms (8). Schizophrenic patients also show reduced functional connectivity of the hippocampus with default mode network nodes, and fractional anisotropy changes in the cingulum that correlate with cognitive performance (331, 350).
There are no serological biomarkers that help in clinical decision making during the course of the disease. The demonstration of antibodies is critical to initially confirm the diagnosis, but the correlation between antibody titers and symptoms during the disease is imperfect and correlates better with CSF than serum titers (120). A study showed a correlation between the intrathecal synthesis of NMDA receptor antibodies and the CSF concentration of C-X-C motif chemokine 13 (CXCL13), a B-cell attracting chemokine that associates with the presence of plasma cells in the CNS. Prolonged or secondary elevation of CXCL13 was associated with limited response to treatment and relapses (195). One theoretical advantage of CXCL13 over NMDA receptor antibodies as a biomarker of disease course is that the change of levels of CXCL13 appears to occur faster than that of the antibodies.
About 80% of the patients recover or substantially improve with immunotherapy directed to remove the antibodies and antibody-producing plasma cells (corticosteroids, intravenous immunoglobulins, plasma exchange, rituximab, or cyclophosphamide), tumor resection (when needed), symptomatic care (e.g., seizures, dyskinesias, autonomic dysfunction, mood and psychotic symptoms), and physical therapy (309, 326). The process of recovery is slow (over months), with initial improvement of autonomic dysfunction, seizures, and abnormal movements and gradual improvement (over months) of deficits of memory, attention, behavior, and other executive functions that interfere with the social reintegration of the patient (120). We postulate that this slow process of recovery is due in part to the prolonged presence of antibodies synthesized by long-lived mature plasma cells within the CNS, and the resulting antibody-mediated alterations at the cellular, synaptic, and circuitry levels (discussed in sect. V). This is supported by the consistent detection of intrathecal synthesis of antibodies (57) and autopsy studies showing infiltrates of plasma cells (Figure 7), deposits of IgG, and decreased brain expression of NMDA receptors (144, 215).
FIGURE 7.
Intrathecal synthesis of antibodies and brain infiltrates of plasma cells. A: comparison of antibody titers in CSF and serum of 53 patients with anti-NMDA receptor encephalitis; the total IgG was normalized between CSF and serum. The intensity of reactivity was measured by ELISA of HEK cell membranes expressing NMDA receptors. Rfu = relative fluorescence units. B and C: infiltrates of CD138+ cells (plasma cells and plasmablasts) in the brain biopsy of a patient with anti-NMDA receptor encephalitis. Note that CD+138 cells (brown cells) are present in perivascular (B), Virchow-Robin (V-R) (B), and interstitial spaces (C). In Virchow-Robin spaces, the CD+ 138 cells are adjacent to the tissue surface that delineates the spaces containing CSF and small vessels (v). Scale bars: 20 μm. [A from Dalmau et al. (57), with permission from Elsevier. B and C from Martinez-Hernandez et al. (215).]
2. Other NMDA receptor antibodies in other CNS disorders
The prospect of finding a reversible disorder among patients with psychoses has prompted a number of investigators to search for autoantibodies in patients with schizophrenia, major depression, and other psychiatric disorders. To date, all these studies have been done using serum, not CSF, and in most cases the method of testing was a CBA that can lead to false-positive results (53, 98), requiring confirmatory studies that were not done (e.g., tissue or live neuronal staining) (120). The initial excitement caused by the identification of NMDA receptor antibodies (mostly IgA and IgM) in ∼10% of patients with schizophrenia (300) was soon tempered by reports identifying these types of antibodies in a similar proportion of patients with other diseases, such as Parkinson's, stroke, dementia, or healthy controls (40, 53, 72, 347). It has been postulated that a transient opening of the BBB allows entry of serum antibodies to the CNS, worsening the symptoms of the associated disorders (127). However, most studies lack CSF investigations that when performed did not detect CSF antibodies (44, 45, 300). Additionally, biological evidence of BBB disruption and the presence of antibodies in human brain tissue have not been demonstrated. Additional tasks for the future are to determine the epitope targets and demonstrate in animal models whether the transfer of these antibodies causes symptoms related to specific antibody binding to brain and alteration of the structure and function of synaptic networks (262).
Other studies have identified IgG NMDA receptor antibodies in a small proportion of patients with a first episode of psychosis or schizophrenia, but not in healthy control groups (165, 192, 250, 345). The disease specificity and clinical relevance of the antibodies in some of the studies are unclear given that the same antibody testing resulted in detection of NMDA receptor antibodies in 23% of patients with a wide range of diseases including some that are not immune mediated (346), leading to inaccurate diagnosis and unnecessary immunotherapy (17). Experience by us and others with large series of patients with anti-NMDA receptor encephalitis show that none of these patients developed schizophrenia during the subsequent years of follow-up even though after recovery some patients had circulating low titers of NMDA receptor antibodies (120, 128, 309, 326).
The most extensively studied antibodies against the GluN2 subunit of the NMDA receptor are DNA antibodies that cross-react with the peptide sequence D/EWD/EYS/G contained in the GluN2A and GluN2B subunits (68). The presence of these antibodies has been ascribed to patients with systemic lupus erythematosus (SLE) and neuropsychiatric symptoms (188), but they have also been identified in patients without neuropsychiatric manifestations and in healthy family members (301). A correlation between the detection of these antibodies in CSF (suggesting spontaneous or inflammatory-induced leakage of the BBB) and neurological symptoms has been suggested (14, 91). In peptide immunized mice, elevated antibody titers cause neuronal excitotoxicity that preferentially occurs in one brain region over the other according to the mechanism used to open the BBB. For example, opening of the BBB with epinephrine resulted in antibody-mediated neuronal toxicity in the amygdala, whereas disruption of the BBB with lipopolysaccharide caused antibody-mediated toxicity in the hippocampus (142). In humans, the specificity of these antibodies for a well-defined set of symptoms has not been established (130, 146).
Other less-defined antibodies to GluN2 have been reported in Rasmussen's encephalitis, epilepsia partialis continua, viral encephalitis, and neurodegenerative diseases among others (109). The clinical and pathogenic significance of these antibodies has not been demonstrated, and the most important reason for knowing about them is to avoid confusion with the pathogenic GluN1 antibodies of patients with anti-NMDA receptor encephalitis.
B. AMPA Receptor, GABAb Receptor, and LGI1: Autoantigens of Treatment-Responsive Limbic Encephalitis
The term limbic encephalitis refers to an inflammatory process involving the limbic system, including the medial temporal lobes, hippocampus, amygdala, and frontobasal and cingulate cortex (115). This disorder was first reported in 1968 as a remote inflammatory or paraneoplastic manifestation of SCLC (48). Other causes of limbic encephalitis predominantly include AE and viral infections (190, 316). The clinical picture of paraneoplastic or AE limbic encephalitis includes the rapid (usually days or a few weeks) development of mood changes, depression, anxiety, and dramatic loss of the ability to form new memories (clinically referred as “short term memory”). In addition, there is a variable component of retrograde amnesia predominantly encompassing a few weeks or months before disease onset. These symptoms are usually accompanied by temporal lobe seizures and EEG or MRI findings demonstrating involvement of the temporal lobes (Figure 8A) (316). The hallmark of the syndrome is the anterograde memory deficit for people, places, objects, facts, and events as a failure of declarative or explicit memory mechanisms, which is remarkably similar to that of the famous patient Henry Molaison (“Henry M”), who in 1953 underwent bilateral medial temporal lobectomy (anterior 2/3 of the hippocampi) in an attempt to control the seizures (Figure 8B) (281). The difference, however, is that Henry M's deficits remained until his death at the age of 82, whereas patients with antibody-mediated limbic encephalitis usually recover. It is unclear whether these patients are able to learn motor skills (procedural or implicit memory) as Henry M did.
FIGURE 8.
MRI findings in limbic encephalitis compared with the surgical resection of the hippocampi of “Henry M.” MRI from a patient with autoimmune limbic encephalitis (A); the arrows point to the hippocampi which show increased signal intensity in fluid-attenuated inversion recovery (FLAIR) sequences, representing areas of inflammation and edema. The drawing in B shows in green the area of both hippocampi that was removed from patient “Henry M,” who was extensively studied over many years by Dr. Brenda Milner and whose findings were fundamental to understand the role of hippocampus in memory formation (281).
Among all autoantibodies associated with AE, there are three that preferentially affect the limbic system, sometimes with a highly restricted hippocampal involvement. These antibodies target the AMPA receptor, GABAb receptor, or LGI1 (discussed below). Contactin-associated protein-like 2 (Caspr2) is another autoantigen of limbic encephalitis, but patients with these antibodies often have additional symptoms (e.g., neuromyotonia, Morvan syndrome), and will be discussed separately. It is unclear why an antibody attack against these proteins, which are widely expressed in brain, results in selective involvement of the limbic system, or why different pathophysiological mechanisms resulting from different synaptic proteins converge into a similar syndrome.
1. AMPA receptor
The AMPA receptor is a ionotropic glutamate receptor that mediates most of the fast excitatory transmission in the brain (288). The majority of AMPA receptors are tetramers composed of GluA1, 2, 3, or 4 subunits that combine in a brain region-dependent manner (248). The highest levels of GluA1/2 and GluA2/3 receptors are found in the synaptic CA3-CA1 region of the hippocampus, subiculum, cerebellum, caudate-putamen, and cerebral cortex (299).
The AMPA receptor autoantibodies of patients with AE are directed against extracellular epitopes of the GluA1 or GluA2 subunits of the receptor (181). Most patients (median age 62 yr, range: 23–81; 64% female) develop a typical syndrome of limbic encephalitis, 40% of them showing additional symptoms beyond the limbic system, and only a few patients presenting with a different syndrome, such as rapidly progressive dementia or psychosis (137, 181). The clinical picture related to GluA1 antibodies is similar to that of patients with GluA2 or both antibodies. About 70% of the patients have an underlying tumor including SCLC, thymoma and less frequently ovarian or breast cancer, or teratoma. Approximately 70% of the patients respond to immunotherapy or treatment of the tumor, most showing a partial neurological response. Patients who do not receive aggressive immunotherapy, such as rituximab or cyclophosphamide, are more likely to have clinical relapses. The presence of concurrent paraneoplastic antibodies, mainly directed against intracellular antigens (suggesting cytotoxic T-cell mechanisms), were found associated with additional symptoms and a poor prognosis (137).
Initial experimental studies showed intense binding of patients' GluA1 and GluA2 antibodies to the neuropil of rat hippocampus, cerebellum, cerebral cortex, and putamen (Figure 3) (181). The primary epitope region of patients' antibodies appears to be the bottom lobe of the extracellular ATD of either subunit (105). However, the binding epitope is not restricted to a small defined region as reported for antibodies targeting the GluN1 subunit of the NMDA receptor in anti-NMDA receptor encephalitis (106). Preincubation of cultured rodent neurons with patients' IgG to either GluA1 or GluA2 led to a decrease of synaptic clusters of AMPA receptor subunits and to a reduction of fluorescence intensity of the remaining AMPA receptor clusters without affecting other pre- and postsynaptic marker proteins or other ionotropic receptors (181, 251). Western blot analysis revealed a decrease of surface expression but not of the amount of intracellular located receptor subunits. These changes affected both subunits regardless of the subunit specificity of the applied patient IgG and suggest a global reduction of synaptic AMPA receptors by internalization and degradation (251). Consequently, frequency and peak amplitude of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSC) were reduced in primary neurons following incubation with antibodies to the GluA1 and GluA2 receptor subunit (181, 251). In parallel to reduction of AMPA-receptor mediated mEPSC, the frequency of GABA-A receptor inhibitory synaptic transmission and the intrinsic excitability of neurons were also altered. This has been interpreted as a homeostatic compensatory regulation following AMPA-receptor internalization (251). These preliminary findings point toward a direct pathogenic effect of patients' AMPA receptor autoantibodies on synaptic function. Still, evidence is sparse, and important questions remain to be answered. First, the current findings are restricted to evaluation of quantal currents in primary neurons with methodological limitations that need to be considered in interpretation of the results (e.g., the predominant effect of antibodies directed to postsynaptic receptors on mEPSC frequency compared with peak amplitude). Second, the presence of the GluA2 subunit is critical for the properties of the AMPA receptor regarding its calcium permeability, channel conductivity, de- and resensitization kinetics, and inward rectification (36, 156). Therefore, selective targeting of the GluA2 subunit by patients' antibodies may influence essential aspects of AMPA receptor signaling. Moreover, the GluA1 and the GluA2 subunits are distributed differently in the CNS (299). Third, morphological analysis of changes in AMPA receptor expression or localization in small synaptic spots is limited due to the diffraction limit of conventional and confocal light microscopy. Recent developments of superresolution light microscopy, e.g., STED and STORM/PALM microscopy (171, 320), can overcome these limitations as recently shown by anti-amphiphysin antibody-induced changes of vesicle-associated proteins in presynaptic nerve terminals (335).
Autoantibodies against the GluA3 subunit of the AMPA receptor were suggested to be related to Rasmussen's encephalitis in several studies published in the 1990s (267). The observation that a few rabbits immunized with GluA3 developed symptoms resembling Rasmussen's encephalitis, and the subsequent detection of GluR3 antibodies in a few patients who improved with plasma exchange suggested a pathogenic role of the antibodies. Yet, over subsequent years extensive studies carried out by other investigators were unable to reproduce these findings (333). We investigated 20 patients with Rasmussen's encephalitis for antibodies against neuronal cell surface antigens, using cultured live neurons and HEK cells expressing GluA3, and none of the cases showed reactivity with any of the assays (data not shown). Moreover, the poor prognosis of patients with Rasmussen's encephalitis who are usually refractory to all types of antibody-depleting treatments, and neuropathological studies showing predominant T-cell infiltrates do not support an antibody-mediated disorder (29).
Taken together, antibodies against extracellular epitopes of GluA1 and GluA2 have disease relevance in causing AE with prominent limbic dysfunction, while antibodies against GluA3 do not appear to be disease relevant and are not detectable in most patients with Rasmussen's encephalitis or other syndromes.
2. GABAb receptor
Antibodies against the GABAb receptor associate with limbic encephalitis accompanied by prominent seizures or status epilepticus, with ∼50% of patients having an underlying SCLC (138, 158, 186). Most patients have complete or substantial neurological improvement after immunotherapy and tumor therapy when needed. The neurological outcome is similar in patients with or without tumor, but the long-term prognosis is dictated by the presence of SCLC and cancer recurrences; the causes of death are cancer recurrence or progression and, less frequently, refractory status epilepticus.
This disorder emphasizes the clinical importance of differentiating the autoantibodies against cell surface or intracellular onconeuronal antigens. For example, patients with SCLC and limbic encephalitis but who develop classical paraneoplastic antibodies against intraneuronal proteins, such as HuD, or CRMP5 (and therefore the immune mechanism is T-cell driven) rarely respond to treatment (5).
The GABAb receptor is a G protein-coupled receptor for the inhibitory neurotransmitter GABA. The receptors are heterodimers comprised of two subunits, GABA-B1 and GABA-B2, which are both necessary for receptor function (28). GABA-B1 binds GABA with its large extracellular domain, and GABA-B2 activates G proteins intracellularly. GABA-B1 has two isoforms: the GABA-B1a isoform is expressed in presynaptic receptors while GABA-B1b is expressed in postsynaptic receptors (327). Presynaptic GABAb receptors can robustly suppress neurotransmitter release by decreasing presynaptic calcium influx. Postsynaptic GABAb receptors cause hyperpolarization through activation of G protein-activated inward-rectified potassium (GIRK) channels. GABAb receptors activate adenylate cyclase and modulate ion channels and cell signaling pathways.
GABAb receptor autoantibodies bind the extracellular domain of the GABA-B1 subunit (186). The potential pathogenic effects of patients' autoantibodies were recently examined using cultured dissociated rat hippocampal neurons which express GABAb receptor. In culture, neurons develop numerous synapses and spontaneously produce synaptic currents and actions potentials. This electrical activity is powerfully attenuated by the application of the GABAb agonist baclofen. Application of patients' autoantibodies did not modify the levels of cell surface or synaptic receptors, but abrogated the effects of baclofen on culture excitability, suggesting that GABAb receptor antibodies may directly block the function of the receptor (Figure 9) (157). Although the exact mechanism whereby antibodies block GABAbR function is unknown, the findings provide a plausible explanation for the extremely common seizures and life-threatening status epilepticus seen in patients with limbic encephalitis related to these autoantibodies. The existence of allosteric agonists on the GABA-B2 subunit (46), bypassing the GABA-B1 subunit targeted by patients' antibodies, provides a potential treatment strategy for patients with this disorder (157). In this case, in addition to immunotherapy, the activation of the GABA-B2 subunit by an allosteric agonist could circumvent the pathogenic effects of the antibodies.
FIGURE 9.

Schematic representation of the GABAb receptor, binding site, and effects of patients' autoantibodies. The GABAb receptor (A) is a heterodimer that comprises the B1 subunit, which has an extracellular domain that binds GABA or baclofen, and the B2 subunit that activates G proteins (Gi and Go) intracellularly. Both subunits are necessary for receptor function. The B1 subunit has two isoforms, B1a and B1b, that are present in presynaptic and postsynaptic GABAb receptors, respectively. The autoantibodies in GABAb receptor encephalitis predominantly target the NH2-terminal extracellular region of B1. Cultures of dissociated rat hippocampal neurons robustly express GABAb receptors. These neurons have numerous synapses with each other and spontaneously produce synaptic currents and action potentials. This electrical activity is powerfully attenuated by the application of baclofen. Treatment of the neurons with patient but not control CSF abrogates the effects of baclofen on neuronal excitability, suggesting that GABAb receptor antibodies may directly block GABAb receptor function (B).
3. Leucine-rich glioma-inactivated 1 (LGI1)
Autoantibodies attributed to the VGKC were first described in 1995 in some patients with neuromyotonia using immunoprecipitation of 125I-α-dendrotoxin-labeled VGKCs from brain (289). Subsequent studies demonstrated that these antibodies did not recognize the Kv1.1 or Kv1.2 subunits of the Shaker family of VGKC, suggesting that the antigens were other proteins that precipitated with the dendrotoxin-labeled VGKCs. Immunoprecipitation and cell-based assays with proteins interacting with the VGKC led to the identification of LGI1 and Caspr2 as the target antigens (151, 182). Patients with either of these antibodies are often referred to as having diseases related to “VGKC-complex antibodies,” although this term is currently being replaced by the more specific concept of diseases related to LGI1 or Caspr2 antibodies (112, 322). These proteins are localized on the cell surface of neurons (LGI1, Caspr2) (182, 255) and the juxtaparanodal region of myelinated axons (Caspr2) (185) and associate with various neurological syndromes.
The form of limbic encephalitis that occurs in association with LGI1 autoantibodies preferentially affects older patients (median age 60 yr) with a slight male predominance, and 60% of the patients have hyponatremia at symptom presentation (151, 182). The memory deficit and other symptoms of limbic dysfunction can be preceded by episodes of facio-brachial or crural seizures that last a few seconds and may repeat many times during the day; these episodes have been described as facio-brachial dystonic seizures (153, 155). MRI studies show basal ganglia hyperintensity in 42% of patients with this type of seizure, suggesting a basal ganglia localization (87); in addition, comprehensive electrophysiological recordings show that most patients with LGI1 antibodies also develop epileptic activity in frontal cortical and hippocampal regions (239). About 70% of the patients show substantial neurological improvement after immunotherapy, but only 35% are able to return to their baseline cognitive function. Clinical relapses occur in 24–35% of the patients (11, 323). Different from other forms of paraneoplastic or autoimmune limbic encephalitis, most patients with LGI1 antibodies do not have cancer (<5% have thymoma). In a Dutch cohort, a significant association of LGI1-antibody associated encephalitis with HLA-DR7 and HLA-DRB4 was identified. Interestingly, this haplotype association did not appear to apply to patients who developed the disorder in the context of a systemic tumor, suggesting that the absence of those haplotypes could raise suspicion for an underlying tumor or paraneoplastic mechanism (324).
Approximately 13% of patients with LGI1 antibodies have a slowly progressive encephalopathy without evidence of CSF inflammation (11). This atypical presentation is important to recognize because different from many untreatable rapidly progressive dementias, patients with LGI1 antibodies are often responsive to immunotherapy.
In contrast to most AE in which the antibody subclass is predominantly IgG1, LGI1 antibodies (11) as well as Caspr2 antibodies (321) (discussed below) are predominantly IgG4, which do not fix complement and due to their unique feature of being hetero-bispecific (continuously undergoing half-antibody exchange) are less effective than IgG1 in crosslinking and internalizing the target antigen (as for example the IgG1 antibodies in anti-NMDA receptor encephalitis) (145). A proposed mechanism for most IgG4-mediated autoimmune disorders is that the antibodies interfere with protein-protein interactions disrupting the normal function of the target (e.g., cell adhesion in case of desmoglein 1 antibodies in pemphigus, or clustering of AChR by MuSK antibodies in a subtype of myasthenia gravis) (145).
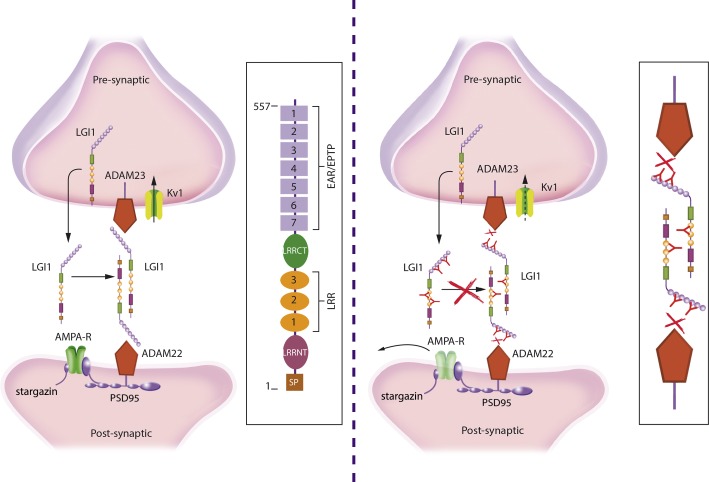
LGI1 is a developmentally regulated secreted neuronal glycoprotein that interacts with presynaptic ADAM23 and postsynaptic ADAM22, organizing a trans-synaptic protein complex which includes presynaptic Kv1.1 potassium channels and postsynaptic AMPA receptors (92–94). In a LGI1 knockout mouse model that reexpresses LGI1 exclusively in the dentate granule cells, LGI1 protein was found in the dentate molecular layer (which corresponds to the dendritic region of granule cells) and the CA3 substratum lucidum (which corresponds to the granule cell mossy fiber-CA3 synapse region), suggesting that LGI1 is secreted from both axonal presynapses and dendritic postsynapses (205). Mutations of LGI1 cause autosomal dominant partial epilepsy with auditory seizures, also known as autosomal dominant lateral temporal lobe epilepsy (ADLTE) (160, 233). Several truncating and missense mutations of LGI1 seem to prevent the secretion of this protein resulting in similar human phenotypes (243). LGI1-null mice develop a lethal epileptic phenotype, predominantly characterized by myoclonic seizures (344). Similar epileptic phenotypes and premature death are obtained with targeted disruption of ADAM22, ADAM23, and Kv channels, suggesting a functional relationship with LGI1 (247, 275, 295). A transgenic mouse model expressing a mutant form of LGI1 similar to that found in ADLTE shows an increase of spine density along with inhibition of dendritic pruning resulting in marked increase of excitatory synaptic transmission compared with wild-type mice (351). These findings together with electrophysiological experiments on LGI1 function in presynaptic Kv1.1-associated protein complexes suggest that LGI1 decreases presynaptic release probability by preventing inactivation of the Kvβ1 subunit of Kv1 channel complexes (286).
The reasons why the syndrome of patients with LGI1 antibody-associated encephalitis is different from that of patients with ADLTE are unclear. Some mutations alter the postnatal maturation of presynaptic and postsynaptic functions, including glutamatergic circuits in an animal model (351). In contrast, LGI1 antibody-associated encephalitis is an acquired disorder that occurs in patients with normal glutamatergic circuits. There is preliminary data suggesting that LGI1 autoantibodies may have pathogenic effects. Similar to what is described in the LGI1 knockout model, preincubation of primary neurons with patients' LGI1 antibodies induces downregulation of synaptic AMPA receptors (244). It has been suggested that LGI1 antibodies interfere with LGI binding to ADAM22 and ADAM23 (244). LGI1 contains a leucine-rich repeat (LRR) and epitempin (EPTP) repeat domain. Binding of patients' antibodies is not restricted to a specific domain, suggesting a polyclonal antibody repertoire with different epitopes (Figure 10) (244). It is not yet clear why downregulation of synaptic AMPA receptors either due to genetic deficiency of LGI1or by LGI1 antibody-induced mechanisms increases neuronal excitability and leads to an epileptic phenotype. Since ADAM22 and ADAM23 are also expressed in inhibitory interneurons, it has been proposed that reduced AMPAR function in these interneurons may contribute to network disinhibition (93, 94, 244). Alternatively, other authors have proposed that LGI1 mutant mice exhibit enhanced AMPAR-mediated synaptic transmission due to the enhanced release of glutamate (344). To date, there is no direct functional evidence of how LGI1 antibodies affect neuronal excitability and synaptic transmission. Since antibodies bind to several LGI1 domains, a selective effect on LGI1/postsynaptic ADAM 22 interaction seems unlikely. Moreover, disruption of the interaction of LGI1 with the presynaptic Kvβ1 subunit of Kv1.1 channel complexes may result in ineffective inactivation of presynaptic Kv1 channels leading to action potential broadening and increase of transmitter release (100, 286). One study using IgG preparations of patients with limbic encephalitis and antibodies to the VGKC showed an increasing fire rate of CA3 pyramidal cells in acute hippocampal slices from rats upon application of patient's IgG (183). Since this study was done before identification of LGI1 as the cognate antigen, one may assume that the effects were mediated by antibodies to LGI1, but the findings await validation with antibodies specific for LGI1.
FIGURE 10.
LGI1 interactions at the synapse and proposed mechanism of dysfunction by LGI1 autoantibodies. LGI1 is a secreted neuronal glycoprotein that interacts with presynaptic ADAM23 and postsynaptic ADAM22 organizing a trans-synaptic protein complex that includes presynaptic Kv1.1 potassium channels and postsynaptic AMPA receptor (left panel; structure and main domains of LGI1 shown in the frame). Autoantibodies to LGI1 react with several different epitopes of the protein (right panel). It has been postulated that the antibodies interfere with the normal interactions of LGI1 probably decreasing the levels of the postsynaptic AMPA receptors and altering the function of the presynaptic Kv1 channels, leading to an increase of neuronal excitability (antibody-mediated disruption of interactions shown in the frame).
In addition to experiments evaluating the pathogenic effect of LGI antibodies on synaptic function and levels of synaptic proteins (AMPA receptor, Kv1 channels), further studies in intact hippocampal slices and in animal models are needed to understand the mechanisms underlying LGI1-antibody mediated hyperexcitability in neuronal networks.
C. Contactin-Associated Protein-like 2 (Caspr2): Autoantigen of Encephalitis and Morvan Syndrome
Caspr2 is a transmembrane axonal protein of the Neurexin IV superfamily that organizes and concentrates VGKCs at the juxtaparanodes of myelinated axons. Its extracellular domain has 8 distinct subdomains and 12 potential N-linked glycosylation sites, and interacts with TAG-1, a cell adhesion protein expressed on both the axon and myelinating cells (51). Caspr2, the cytoskeleton interacting Protein 4.1b, and TAG-1 are each necessary to concentrate Kv1.1/Kv1.2 potassium channels at the juxtaparanodal region (124, 313). This clustering of potassium channels is important for the proper electrical function of axons (349). The mechanisms by which Caspr2 associates with Kv1 channels are not completely understood (140).
Autoantibodies to Caspr2 react with the juxtaparanodal region of myelinated peripheral nerves and also with the cell surface of neurons in the CNS (Figure 3) (185). A study of 38 patients (median age 66 yr, range: 25–77; 34 male) with Caspr2 antibodies showed that 77% had three or more of the following symptoms: encephalopathy, cerebellar symptoms, peripheral nervous system hyperexcitability (also called neuromyotonia), autonomic dysregulation, insomnia, neuropathic pain, or weight loss (321). In some patients, the central nervous system dysfunction resembles limbic encephalitis, and in others Morvan syndrome (151, 154, 159, 185). Morvan syndrome, which may also occur without Caspr2 antibodies, is characterized by the combination of neuromyotonia, neuropathic pain, encephalopathy with hallucinations, and a characteristic sleep disorder described as agrypnia excitata (200, 261). This term (agrypnia = loss of sleep; excitata = increased motor activity and restlessness) was developed to describe the sleep disturbances that occur in several pathogenically unrelated diseases such as fatal familial insomnia (a prion disease) or delirium tremens (232, 260, 261). Patients with agrypnia excitata have severe insomnia, dreamlike stupor (hallucinations and enacted dreams), sympathetic hyperactivity (hyperthermia, perspiration, tachypnea, tachycardia, and hypertension), and motor agitation. Key neurophysiological features include the loss of slow-wave sleep, which represents the transitional process of falling sleep, and the presence of abnormal REM sleep without atonia in the antigravity muscles (232).
Approximately 20% of patients with Caspr2-antibody associated symptoms have an underlying thymoma (321). For reasons that are unknown, this tumor association is higher (20–50%) in patients who develop Morvan syndrome (1, 154).
The Caspr2 antibody subclass is predominantly IgG4, although ∼60% of the patients also have IgG1 Caspr2 antibodies (321). Immunotherapy and treatment of the tumor (when appropriate) resulted in improvement in 93% of the patients, and 25% had relapses (321).
With the use of deletion mutants representing the multiple extracellular subdomains of Caspr2, patients' antibodies were found to react with a major epitope within its discoidin-like domain, with other epitopes concentrated within the amino-terminal half of the protein (246). A plausible explanation for the multiple epitopes identified is that some antibodies disrupt the interaction between Caspr2 and TAG1 in cis (expressed on the axon), whereas others disrupt the interaction between Caspr2 in trans (expressed on myelinated cells opposed to the axon) (246).
Similar conclusions were reached by a study in which the authors focused on the effects of Caspr2 antibodies on the CNS using IgG from patients with symptoms limited to the limbic system (255). In this study, Caspr2 autoantibodies were also found to be directed against the amino-terminal discoidin and lamininG1 modules. With the use of cultures of dissociated rat hippocampal neurons, the antibodies predominantly reacted with inhibitory interneurons. High levels of Caspr2 were found on GAD65-positive axons and at the VGAT-positive inhibitory presynaptic contacts. A Caspr2-Fc chimera revealed that Caspr2 receptors were localized at the somatodendritic compartment and postsynapse, and the binding was strongly increased on TAG-1 transfected neurons (255). Taken together, these findings suggest that within the CNS Caspr2 operates as a cell recognition molecule in inhibitory networks, and therefore, the Caspr2 autoantibodies may potentially impair inhibitory interneuron activity.
D. GABAa Receptor: Autoantigen of Encephalitis With Frequent Seizures and Multifocal Brain MRI Abnormalities
Autoantibodies to the GABAa receptor were recently reported in some patients with AE characterized by encephalitis, prominent seizures or status epilepticus, and multifocal MRI abnormalities (244, 252). The GABAa receptor is a ligand-gated ion channel that mediates the vast majority of fast inhibitory transmission in the brain (208). GABAa receptors are involved in the pathophysiology of epileptic and psychiatric disorders, cognitive deficits, substance abuse, and many other CNS diseases. These receptors are also the main drug target of anticonvulsive, anxiolytic, and sedative medications (270). GABAa receptors are heteropentamers consisting of five homologous subunits forming the channel pore. To date, 19 different GABAa receptor subunits have been identified, subclassified as α (n = 6 different isoforms), β (n = 3), γ (n = 3), δ (n = 1), ε (n = 3), θ (n = 1), π (n = 1), and ρ (n = 1) subunits (292). Most of the GABAa receptors contain two α subunits, two β subunits, and one γ or δ subunit. In patients with autoantibodies against the GABAa receptor, the predominant targets are α1 and β3, and less frequently the γ subunit (244, 252, 298). The clinical significance of antibodies against the γ subunit is unclear (253); in our experience, antibodies to the γ subunit always occur in association with antibodies against the other dominant subunit targets (298). The syndrome of children and adults harboring high-titer serum and CSF antibodies against any of the indicated subunits is mainly characterized by treatment-refractory seizures, epilepsia partialis continua, and status epilepticus (252, 298). Together with epileptic activity, patients show signs of encephalopathy with changes in behavior, cognition, or consciousness including coma. Some patients also develop focal neurological signs, such as hemiparesis, dyskinesias, aphasia, or oculomotor disturbances. CSF findings are variable and abnormal in most cases and include pleocytosis, elevated protein concentration, or oligoconal bands (252). Coexistence of other autoantibodies occurs in some patients including thyroid peroxidase, GAD65, and GABAb receptor antibodies (244, 252). Approximately 75% of the patients develop multifocal, extensive FLAIR and T2 hyperintense MRI abnormalities involving various cortical and subcortical brain regions (298) (Figure 11). This finding is highly unusual in other AE and provides a clue towards the identity of the disorder. Approximately 30% of the patients have an underlying tumor, mainly a thymoma; older patients are more likely to have a tumor than younger patients (298). In rare instances, GABAa receptor autoantibodies are triggered by HSE; these patients develop relapsing neurological symptoms a few weeks after recovering from the viral infection (298).
FIGURE 11.
MRI of a patient with encephalitis and GABAa receptor antibodies. This disorder usually associates with cortical and subcortical FLAIR MRI abnormalities (A, B) that resolve along with the associated symptoms after immunotherapy (C, D).
Treatment with anticonvulsants is frequently ineffective, and pharmacologically induced coma (barbiturates) can be necessary to control seizure activity. In a recent review of all reported cases, 86% of the patients improved with immunotherapy and the other 14% died of status epilepticus or secondary medical complications (298).
The reactivity of the antibodies with live cultured neurons and the type of subunit targets suggests a specific interaction of the antibodies with synaptic GABAa receptors (244, 252). However, interaction with other subunits of the GABAa receptor cannot be excluded, since this has not been investigated in detail. Given the rarity of the syndrome, availability of patient material (especially CSF) is a critical issue that limits experimental approaches to investigate potential effects of the antibodies in a passive-transfer animal model. In cultured primary neurons, application of patients' antibodies led to a reduction of synaptic and extrasynaptic density of GABAa receptors (244, 252). Preliminary functional experiments investigating GABAergic miniature IPSC (mIPSC) in primary cultures of neurons revealed severe reduction of the frequency of GABAergic mIPSCs and to a lesser extent also reduction of the mIPSC peak amplitude (244).
In animal models with experimental status epilepticus, the continuous epileptic activity leads to a downregulation of synaptic GABAa receptors (240). This is induced by decreased phosphorylation of the GABAa receptor β3 subunit and subsequent better association with the adaptor protein 2 (AP2) leading to increased endocytosis of the receptors (305). Additional autoantibody-induced reduction of synaptic GABAa receptors or specific interaction of autoantibodies with the β3 subunit may directly cause or amplify this process and may be the pathophysiological correlate of intractable seizures in these patients. More detailed investigations of how antibodies interact with the different subunits of the GABAa receptor, the consequences of antibody binding on the interaction with anchoring molecules and synaptic delocalization of the receptor, and the effects on GABAergic signaling in intact neuronal networks are exciting topics for future research.
E. Dipeptidyl-peptidase-like protein-6: Autoantigen of Encephalitis With Frequent Gastrointestinal Manifestations
Dipeptidyl-peptidase-like protein-6 (DPPX) is a membrane glycoprotein that has an important role in tuning up the voltage-gated A-type Kv4.2 channels by remodeling channel gating (237). These channels operate in the subthreshold range of membrane potentials and are critical to ensemble voltage-gated ion currents that determine somatodendritic signal integration (169). The transient subthreshold A-type K+ currents in dendrites attenuates the back-propagation of action potentials.
Autoantibodies to DPPX associate with cognitive dysfunction, memory deficits, hyperekplexia, myoclonus, tremor, and seizures.(33, 311). Prodromal weight loss, diarrhea, or other gastrointestinal symptoms occur in ∼66% of the patients, and some patients may develop cardiac dysrhythmia. Overall, the clinical picture of most patients is compatible with a nonspecific syndrome with a variety of manifestations indicating CNS hyperexcitability. Different from other AE, the symptom presentation of these patients can be insidious causing a diagnostic delay of many months. Infrequently, some patients may develop a clinical picture resembling a variant of stiff-person syndrome named progressive encephalomyelitis with rigidity and myoclonus (PERM, discussed later) (21). Most patients show partial or complete neurological response to immunotherapy, accompanied also by resolution of gastrointestinal symptoms (33, 311).
Patients' antibodies are IgG1 and IgG4 and immunoreact with the brain of wild-type but not DPPX-null mice (Figure 3) (33). The antibodies also show intense reactivity with neurons of the myenteric plexus, likely explaining the frequent and severe gastrointestinal problems (33). In preparations of guinea pig myenteric and human submucous plexus, patients' antibodies caused an increase of the excitability and action potential frequency (254). In addition, incubation of hippocampal neurons with patients' antibodies caused a decrease of DPPX and Kv4.2 in neuronal membranes (254). The findings support a pathogenic role of the antibodies, although an animal model of the disorder has not yet been developed.
F. Dopamine-2 Receptor: Autoantigen of Encephalitis of the Basal Ganglia and Movement Disorders
Dopamine signaling is mediated through dopamine receptors, which are a family of G protein-coupled seven transmembrane domains receptors. To date, five receptors have been identified in humans which are divided in two subgroups, D1 (D1R and D5R) and D2 class (D2R, D3R, and D4R) (166). Both classes of receptors are highly expressed in the striatum (caudate-putamen) and also in cortex, hippocampus, and substantia nigra (24). Mutations of DRD2, the gene that codes for D2R, associates with a form of myoclonus dystonia (172). D2R is a therapeutic target of Parkinson's disease, Tourette's syndrome, and schizophrenia (24).
Antibodies to D2R have been demonstrated in patients, mostly children, with basal ganglia encephalitis manifesting with abnormal movements (e.g., dystonia, chorea, Parkinsonism, or oculogyric crisis) along with behavioral change and psychosis. The disorder is rare; for example, in a study including 17 patients with basal ganglia encephalitis identified during 11 yr in a referral institution, 12 had these antibodies. Similar antibodies were identified in control groups including Sydenham's chorea (30%) and Tourette's syndrome (9%), but were absent in patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) (55). These findings differ from other studies showing the presence of D2R antibodies in patients with PANDAS (37). A potential explanation for this discrepancy is the location and type of epitopes, which appear to be extracellular and conformational in cases of basal ganglia encephalitis (55), whereas in cases of PANDAS, the location of the epitope is unknown and the antibody reactivity does not depend on the conformation of the antigen (37). Given the extracellular location of the D2R epitopes in cases of basal ganglia encephalitis, it is likely that the antibodies have pathogenic effects, but studies are not yet available. On the other hand, the pathogenicity of antibodies to D2R in patients with PANDAS and some cases with Sydenham chorea is uncertain. In patients with PANDAS, additional antibodies have been described reacting with group A streptococci and inducing calcium/calmodulin-dependent kinase (CaMK) II signaling and dopamine release in a neuroblastoma cell line (37, 170). However, the exact targets of these antibodies are unknown.
Autoantibodies to cell surface epitopes of the D2R have also been described in some patients who develop autoimmune encephalitis after HSE, usually accompanied by NMDA receptor antibodies (Figure 4) (229), and in a subgroup of children who develop isolated symptoms of psychosis (250). It is unclear whether these antibodies also occur in the CSF or their presence in serum represents a secondary phenomenon of a neurological disease without pathogenic implications. Studies comparing serum and CSF antibody titers and investigations with cultured neurons and in vivo models are needed to establish the role of D2R antibodies in human disease.
G. Metabotropic Glutamate Receptor 5 (mGluR5) and Ophelia Syndrome (Encephalitis Associated With Hodgkin Lymphoma)
Among eight mammalian mGluR receptors, the two of group 1 (mGluR1 and mGluR5) are targets of autoantibodies that associate with human autoimmune CNS disorders, mGluR1 with cerebellar ataxia and motor incoordination (293) (see sect. IVJ) and mGluR5 with a form of encephalitis named “Ophelia syndrome” (41, 187). None of the other receptors from group 2 (mGluR2, mGluR3) or group 3 (mGluR4, mGluR6, mGluR7 and mGluR8) associates with known AE. Interestingly, the two disorders associated with antibodies against mGluR1 and mGluR5 occur with frequent association with Hodgkin's lymphoma (the reason for the association with this specific tumor is unknown) (187, 220, 293). The two receptors have a similar structure consisting of homodimers with a large extracellular domain and seven transmembrane domains (242). Even though mGluR1 and mGluR5 share 85% amino acid sequence homology, the mGluR1 autoantibodies of patients with cerebellar ataxia do not crossreact with the mGluR5 antibodies of patients with Ophelia syndrome (187). Both receptors act via Gq protein and phospholipase C to influence calcium/IP3 signaling (242).
The mGluR5 is particularly important for long-term depression (LTD) and regulates rapid synaptic transmission in the hippocampus (81). Partly because of their functional interaction with NMDA receptors, mGluR5 are involved in the regulation of LTP (180). In addition to these direct roles in LTD and LTP at CA1 of the hippocampus, mGluR5 play a crucial role in some forms of metaplasticity (plasticity of synaptic plasticity) (2); the mechanisms by which activation of mGluR5 results in metaplasticity have been postulated to involve CaMKII and protein kinase C (34, 35). In several human disorders, such as Huntington's disease (266), Alzheimer's disease (179), depression (249), and Fragile X syndrome (283), mGluR5 is being considered as a potential therapeutic target.
The term Ophelia syndrome was used in the description of the first patient reported with AE associated with Hodgkin's lymphoma (likely representing a case of mGluR5 antibody-mediated symptoms) (41). Since then, several patients have been reported, all with a clinical picture that included confusion, agitation, memory loss, delusions, paranoid ideation, hallucinations, psychosis, or seizures (187, 220). Patients' antibodies show intense reactivity with brain (predominantly hippocampus, striatum, and cortex) of wild-type mice but no reactivity with mGluR5-null mice brain. The importance of recognizing this disorder is that it is remarkably responsive to immunotherapy (187, 220).
There are no studies investigating the effects of mGluR5 antibodies on the receptor. The accessibility of the epitopes to circulating antibodies and the rapid response of the disease to immunotherapy suggest pathogenicity of the antibodies. In line with the symptoms described by patients with mGluR antibodies, genetic disruption of mGluR5 impairs behavioral learning, particularly those involving extinction of non-reinforced behaviors.
H. Neurexin-3α: Autoantigen of Encephalitis With Antibodies Altering Synapse Formation
Neurexin-3α belongs to a family of synaptic cell-adhesion molecules involved in synapse formation and maturation that comprises three genes: NRX1, 2 and 3, each of them providing two alternative splice products, a long α and a short β form (302). Neurexins act as a link between pre- and postsynaptic compartments, with the intracellular domain interacting with the presynaptic machinery for neurotransmitter release, and the extracellular domain binding to postsynaptic cell adhesion molecules such as neuroligins, cerebellins, and leucine-rich repeat transmembrane neuronal proteins (LRRTMs) (10, 174, 291, 302). Mutations in the neurexin genes have been associated with schizophrenia (99, 149, 272) and autism (83, 99, 202, 319). Ablation of neurexin-3 demonstrates different brain region-specific pre- and postsynaptic functions; in the hippocampus, extracellular sequences of presynaptic neurexin-3 mediate trans-synaptic regulation of postsynaptic AMPA receptors, whereas in the olfactory bulb, intracellular sequences of neurexin-3 are required for GABA release (9).
Autoantibodies to neurexin-3α were recently identified in five patients (median age 44 yr, range 23–50; 4 female) who after prodromal fever, headache, or gastrointestinal symptoms developed confusion, seizures, and decreased level of consciousness (119). In addition, two patients developed facial dyskinesias, and three needed ventilatory support. Three partially recovered with immunotherapy, one had a fulminant progression to death due to seizures, and the other died of sepsis after partial response to immunotherapy. The most interesting finding was that all five patients had serum and CSF antibodies reacting against extracellular epitopes of neurexin-3α but not with its ligand LRRTM2 (119).
In cultures of dissociated rat embryonic neurons, studies performed during development of functional synaptic networks (days in vitro 1 to 14) showed that patients' antibodies caused a specific reduction of the levels of neurexin-3α and a decrease of the total number of synapses (119). The findings are in accord with studies showing that disruption of endogenous neurexin-neuroligin interaction by recombinant neurexin reduces the number of presynaptic terminals and inhibitory and excitatory synapses (194, 284). Although no animal models are available, these preliminary studies support the pathogenicity of the antibodies.
I. Anti-IgLON5 Disease
The IgLON5 gene was identified during the sequencing of human chromosome 19 and is predominantly expressed in the nervous system (121). IgLON5 is the most recently identified member of a family of proteins that belongs to the immunoglobulin superfamily of cell adhesion molecules (162). IgLON proteins are highly glycosylated, contain three immunoglobulin-like domains, and are attached to the plasma membrane through a glycosylphosphatidylinositol (GPI) anchor (162). The family of IgLON proteins has important functions in neuronal pathfinding and synaptic formation during brain development (131). The exact function of IgLON5 protein is unknown; preliminary studies indicate that it is expressed in the neuronal membrane including the synapse and is widely distributed throughout the brain (273).
Patients with IgLON5 autoantibodies develop an intriguing disorder that reinforces the links between autoimmunity and neurodegeneration. The syndrome was originally identified in a Sleep Disease Center in three patients who showed a disrupted pattern of sleep with some features resembling agrypnia excitata along with gait instability and dysautonomia (273). Investigations for Caspr2 autoantibodies (which often occur with agrypnia excitata) were negative, but the studies revealed a novel autoantibody that reacted with brain (Figure 3) and precipitated IgLON5.
Most patients develop sleep apneas and abnormal movements that predominate in the first part of the night. These symptoms do not improve with continuous positive airway pressure (CPAP) therapy, and sleep studies with video-polysomnograpy show a unique temporal sequence of sleep stages and behaviors, transitioning from very abnormal at the beginning of the night to close to normal by the end. The initiation and reentering of sleep after awakening are characterized by undifferentiated non-rapid-eye-movement (NREM) sleep and poorly structured N2 phase with frequent vocalizations, stereotyped upper limb movements, and finalistic behaviors (parasomnias). Rapid-eye-movement (REM) sleep is present but only in the form of REM sleep behavior disorder. In addition, most patients have a sleep breathing disorder characterized by stridor and obstructive sleep apnea (139, 273, 294).
In addition to the sleep disorder, other symptoms may include gait instability, frequent falls, supranuclear gaze palsy, dysphagia, dysautonomia, chorea, and cognitive decline. These symptoms can evolve over several years, sometimes preceding the sleep disorder and leading to consideration of other diagnoses such as progressive supranuclear palsy or multiple system atrophy. Life-threatening complications include acute respiratory distress requiring tracheostomy and intensive care support, and dysautonomia that likely was responsible from the sudden death (while awake) of some patients (273).
Two immunological features suggest the disorder is immune mediated. First, the human leukocyte antigens (HLA) available in seven patients were HLA-DQB1*0501 and HLA-DRB1*1001 (20, 273). The frequency of these alleles among the normal population is 1.6 and 14.4%, indicating that this association represents a genetic susceptibility for this disorder. Second, all patients have serum or CSF antibodies against IgLON5. IgLON5 antibodies are predominantly IgG4, but lower levels of IgG1 antibodies are also detected. Studies with cultures of dissociated rat hippocampal neurons show that patients' antibodies cause a decrease of clusters of cell surface IgLON5 which is long-lasting or only partially reversible (274); the pathogenic implication of this finding is currently unknown. Autopsy studies showed a novel neuronal tauopathy without evidence of inflammatory infiltrates or complement. Deposits of phosphorylated tau including 3R and 4R tau isoforms were found highly restricted to neurons (Figure 12), predominantly involving the hypothalamus, brain stem tegmentum, and upper spinal cord (104). In retrospect, patients with this disorder may have previously been described as atypical cases of progressive supranuclear palsy (161, 199, 259). A pathologically based set of diagnostic criteria have been recently proposed (Table 3) (104).
FIGURE 12.
Neuropathology of the IgLON5 syndrome. Autopsy studies of the brain of a patient with the IgLON5 syndrome demonstrating absence of inflammatory infiltrates (A and C), a neurofibrillary tangle (B), and neuronal deposits of hyperphosphorylated tau (D). Scale bar: A, C, D = 50 μm; B = 20 μm.
Table 3.
Pathological criteria of anti-IgLON5 disease
| Criteria of Anti-IgLON5 Disease |
|---|
| Possible |
| All of the following requirements: |
| 1. Neurodegenerative features with neuronal loss and gliosis in brain areas showing hyperphosphorylated (hp)Tau pathology without the presence of inflammatory infiltrates. |
| 2. Selective neuronal involvement by deposition of hp Tau in the form of neurofibrillary tangles, pretangles, and neuropil threads with both 3R-tau and 4R-tau isoforms contributing to the inclusions. |
| 3. The hpTau pathology predominantly affects subcortical structures, including the hypothalamus, brain stem tegmentum, and upper spinal cord. |
| Probable |
| Criteria of “possible” AND at least one of the following: |
| 1. Clinical history suggestive of a sleep disorder (NREM and REM parasomnia with sleep apnea), or brain stem, mainly bulbar dysfunction. |
| 2. Presence of HLA-DRB1*1001 and HLA-DQB1*0501 alleles. |
| Definite |
| Criteria of “possible” AND presence of IgLON5 antibodies in CSF or serum. |
See Gelpi et al. (104) for details.
At present, it is unclear if the anti-IgLON5 syndrome is a primary tauopathy that results in a secondary immune response against IgLON5 or a primary autoimmune disorder that through unknown mechanisms leads to abnormal tau phosphorylation and deposition in neurons. In favor of the first is the chronic symptom progression and poor response to immunotherapy. However, this hypothesis does not explain the specific presence of IgLON5 autoantibodies and the association to an uncommon HLA haplotype.
J. Delta/notch-like Epidermal Growth Factor-related Receptor, the P/Q-type Voltage-gated Calcium Channels, and the Metabotropic Glutamate Receptor 1: Autoantigens of Cerebellar Ataxia
The cerebellum, and in particular the Purkinje cells, are frequent targets of autoimmunity. Autoimmune cerebellar ataxia may occur without autoantibodies such as post-varicella cerebellitis (3) or in association with antibodies against intracellular or cell surface neuronal antigens (59). Most intracellular or onconeuronal antigens are expressed by Purkinje cells and other neurons and can be used as biomarkers of paraneoplastic (e.g., Yo antibodies) (59) or idiopathic autoimmunity (e.g., GAD antibodies) (12). There are three antibodies against neuronal cell surface proteins that predominantly associate with cerebellar ataxia: Tr [delta/notch-like epidermal growth factor-related receptor (DNER)] (66), the P/Q-type voltage-gated calcium channels (VGCC) (219), and the metabotropic glutamate receptor 1 (mGluR1) (293).
Regardless of the type of antibody, patients with autoimmune cerebellar dysfunction usually develop rapid progression, over weeks or less commonly months, of truncal (wide-based gait) and limb ataxia associated with dysarthria, nystagmus, vertigo, and diplopia. Symptoms often stabilize within 6 mo of disease onset, leaving the patient physically dependent in most cases. Brain MRI studies are initially normal and later demonstrate cerebellar atrophy (Figure 13A). In rare instances, there is increased signal of the cerebellar white matter on T2-weighted images and transient contrast enhancement of cerebellar folia that may suggest leptomeningeal inflammation or tumor infiltration (59). The CSF shows inflammatory changes or oligoclonal bands in ∼60% of the patients. A common feature of the cerebellar syndromes associated with antibodies against cell surface antigens is that symptoms are less responsive to immunotherapy than those related to limbic and other types of encephalitis (66, 293).
FIGURE 13.
Cerebellar degeneration associated with DNER (Tr) antibodies. MRI of a patient with cerebellar ataxia associated with DNER antibodies; the arrow points to the cerebellum that shows prominent atrophy (A). The CSF of the patient showed robust reactivity with rodent cerebellum (B), including the Purkinje cells (inset) and molecular layer in a characteristic fine dotlike pattern representing the labeling of Purkinje cell dendrites.
The pathological hallmark of autoimmune cerebellar ataxia is a widespread loss of Purkinje cells (47, 95). Other findings may include thinning of the molecular and granular layers of the cerebellar cortex without marked cell loss, and proliferation of Bergmann astrocytes. The deep cerebellar nuclei are usually well preserved, inflammatory infiltrates are rarely present, and there is microglial proliferation in the white matter (59).
1. DNER
The first antibody identified in association with paraneoplastic cerebellar ataxia was named “Tr” after the name of the investigator that first reported the antibody reactivity with Purkinje cells of the cerebellum (110, 314). Patients with this antibody usually present with rapidly progressive cerebellar ataxia and have an underlying Hodgkin's lymphoma. The target antigen of Tr antibodies was identified as DNER, a transmembrane protein containing 10 epidermal growth factor-like repeats (66, 117). This protein is strongly expressed by Purkinje cells of the cerebellum (Figure 13B) and is essential for the normal development of these neurons and Bergmann glia, which express one or more Notch receptors (276, 312). DNER is located in the plasma membrane and endosomes of the dendrites but not in the axons.
Tr antibodies recognize glycosylated epitopes located in the extracellular domain of DNER between the second and third epidermal growth factor (EGF)-like repeats (66). Interestingly, the first two EGF domains of DNER are sufficient and necessary to bind Notch (78); this region does not contain the immunodominant epitope or the glycosylation sites (66). Bergmann glial cells are intimately associated with Purkinje cells throughout development (342), so an immune disruption of the signaling between Bergmann glia and Purkinje neurons by patients' antibodies may have profound effects on cerebellar function. Unlike other antigens in paraneoplastic cerebellar ataxia, DNER has not been found expressed in the tumor tissue of the patients, suggesting that the immune response is not triggered by the tumor expression of the antigen, but likely results from an immune dysregulation caused by the tumor (27, 66).
2. P/Q-type VGCC
Autoantibodies against these calcium channels typically associate with the Lambert-Eaton myasthenic syndrome (LEMS) in patients with or without SCLC (235). However, these antibodies also occur in some patients with SCLC and paraneoplastic cerebellar ataxia without clinical or electrophysiological evidence of LEMS (114, 219). Given that Purkinje cells express P/Q-type VGCC in the dendritic plasma membrane, the occurrence of these antibodies in some patients suggested they could be pathogenic (95). To test this hypothesis, one study showed that passive transfer of IgG from a patient with cerebellar ataxia and LEMS into the cerebellar subarachnoid space of mice caused reversible ataxia, whereas this effect was not observed when the IgG came from a patient with LEMS without ataxia, or from a normal subject (213). A possible explanation for these findings is that the target VGCC epitopes are different in LEMS patients with or without ataxia. In line with this hypothesis, antibodies raised against the extracellular part of the S5–S6 linker segments in domain III of the mouse α1A subunit of P/Q-type VGCC altered synaptic transmission in cerebellar slices and caused cerebellar ataxia in mice (197).
3. mGluR1
This receptor and the mGluR5 (see sect. IVG) belong to group 1 of metabotropic glutamate receptors (242). The mGluR1 is highly expressed by cerebellar Purkinje neurons and modulates rapid calcium signaling in the dendritic spines of these neurons (135). Mice with genetic deletion of mGluR1 have ataxia, abnormal innervation of Purkinje neurons, and impaired LTD at parallel fiber synapses of Purkinje neurons (148, 245).
The cerebellar ataxia with autoantibodies against mGluR1 was identified in 2000 in two patients with cerebellar ataxia and serum and CSF antibodies that showed a similar pattern of reactivity with mice brain and cerebellum (Figure 3) (293). This pattern of immunostaining suggested the target antigen could be mGluR1α, which was confirmed in a cell-based assay using Chinese hamster ovary (CHO) cells expressing mGluR1. Both patients had past history of Hodgkin's lymphoma (2 yr and 9 yr before neurological symptom onset), but no tumor recurrence was identified. One of the patients was diagnosed and treated early, and improved with immunotherapy; the other patient was treated 1 yr after symptom onset and did not improve (293).
Patients' antibodies reacted with the amino-terminal extracellular domain of the receptor and blocked in a dose-dependent manner the glutamate-stimulated formation of inositol phosphates in mGluR1α-expressing CHO cells. Injection of the antibodies in the subarachnoid space of mice, near the cerebellum, resulted in progressive ataxia with wide gait and severe difficulty in walking. The cerebellar dysfunction occurred within 30 min and peaked at ∼2–4 h after injection of the IgG and subsided in 24 h (293). In another study, the application of patients' antibodies to cerebellar slices of mice decreased the basal activity of Purkinje cells, whereas in vivo application to the flocculus of mice evoked acute disturbances in the performance of compensatory eye movements (47). In cultured mouse Purkinje cells, the same antibodies blocked the induction of LTD. Consistent with the prominent antibody-induced dysfunction of Purkinje cells, the autopsy of a patient who for 5 yr had mGluR1 antibodies showed substantial loss of Purkinje cells (47).
Since the above cases were reported, we and others have identified 18 additional patients (187, 203) (4 not reported), all with cerebellar ataxia, but most of them without tumors.
K. Glutamic Acid Decarboxylase, Glycine Receptor, and Amphiphysin: Autoantigens of Stiff-Person Spectrum Disorders
Stiff-person syndrome is characterized by muscle stiffness, rigidity, and painful spams predominantly involving the paraspinal, abdominal, and lower extremity muscles. The arms, neck, and face are less commonly involved. The spasms can be spontaneous or triggered by movement or sensory (tactile, auditory) and emotional stimuli. Electrophysiological studies show sustained motor unit activity leading to cocontraction of agonist and antagonist muscles. Symptoms improve during sleep and with diazepam and other GABAergic drugs (22). The resulting clinical syndrome has been known since 1956 and is easy to recognize on clinical grounds (227). Patients with stiff-person syndrome have a normal brain and spinal MRI, and the CSF is usually normal except for the presence of oligoclonal bands in 35% of the patients (277). There are several variants of the disorder including stiff-limb syndrome (predominant or isolated involvement of one extremity), and PERM (38).
PERM has a more aggressive clinical course compared with stiff-person syndrome. In addition to encephalomyelitis with rigidity and myoclonus, other symptoms of PERM include sensory problems (pruritus and neuropathic pain), brain stem dysfunction (nystagmus, ophthalmoparesis, dysphagia, trismus), and dysautonomia (profuse sweating, dry mouth, bladder dysfunction). Brain and spinal MRI are usually normal, but the CSF frequently shows pleocytosis. Postmortem examination demonstrates degeneration of long tracts and widespread neuronal loss and inflammatory infiltrates in the brain stem and spinal cord. The pathological findings are particularly severe in the central grey matter of the spinal cord that contains inhibitory interneurons accounting for the muscle spasms and rigidity. These findings contrast with those of classical stiff-person syndrome which usually shows no pathological abnormalities. The antibodies more frequently encountered in patients with PERM are directed against the alpha subunit of the GlyR (42).
Depending on the studies, 60–80% of the patients with stiff-person and related syndromes have antibodies against one or more proteins of the GABAergic or glycinergic synapses, including 1) glutamic acid decarboxylase 65 (GAD65, an enzyme that catalyzes the conversion of glutamate to GABA) (296), which is the target of 75% of antibody positive cases; these patients usually have diabetes; 2) glycine receptor (GlyR, a postsynaptic receptor mainly concentrated in the brain stem and spinal cord, involved in inhibitory synaptic transmission and fine regulation of motor neuron excitability) (42), which is the target of 20% of antibody positive cases; and 3) amphiphysin (a N-BAR domain protein involved in the process of clathrin-mediated endocytosis and retrieval of presynaptic vesicles) (65), which is the target of 5% of antibody-positive cases (Figure 14).
FIGURE 14.
Autoantibodies in stiff-person spectrum disorders. Schematic representation of an inhibitory synapse including the three main targets of autoantibodies in patients with stiff-person spectrum disorders: the presynaptic proteins GAD and amphiphysin and the postsynaptic glycine receptor (GlyR). Although autoantibodies to any of these proteins can result in a similar syndrome [stiff-person, stiff-limb, or progressive encephalomyelitis with rigidity and myoclonus (PERM)], GAD autoantibodies predominantly associate with nonparaneoplastic stiff-person syndrome, GlyR antibodies occur more frequently with nonparaneoplastic PERM, and amphiphysin antibodies usually associate with cancer-related stiff-person syndrome or PERM. GABAT, GABA transporter; GLYT, glycine transporter; VIAAT, vesicular inhibitory amino acid transporter.
A series of 121 patients with stiff-person syndrome and related disorders showed that the outcome was more dependent on the severity of the symptoms and type of autoimmunity than on the syndrome (214). In cases of stiff-person syndrome or PERM where the antibodies are directed against cell surface antigens (e.g., GlyR), the symptoms are responsive to immunotherapy (42), but in those in which the antibodies are against intracellular antigens (GAD) or there is evidence of associated cytotoxic T-cell mechanisms (amphiphysin), the symptoms are less responsive to immunotherapy (214).
There is controversy on whether GAD65 antibodies have a direct pathogenic effect. Investigations similar to those used for antibodies against cell surface proteins showed no reactivity of patients GAD65 antibodies with cultured live neurons and no antibody internalization (118). In passive-transfer experiments, intrathecal application of patients IgG fractions containing GAD65 antibodies induced disease signs that resembled those of the donor patients, but results were inconsistent between different IgG preparations (102, 129). Accordingly, depletion of GAD65 antibodies from total IgG of patients with stiff-person syndrome did not alter the effect of the IgG on presynaptic neuronal dysfunction, and did not abolish presynaptic IgG binding suggesting the co-existence of other, potentially pathogenic antibodies (335).
1. Glycine receptor
The glycine receptor (GlyR) is a chloride channel receptor that mediates inhibitory neurotransmission in the spinal cord and brain stem (207). In these locations, the receptor is clustered at inhibitory synapses by the tubulin-binding protein gephyrin (82). The GlyR is also expressed in GABAergic synapses in a subset of hippocampal interneurons and pyramidal neurons (193). The adult form of the GlyR is a heteropentameric α1β receptor that has a stoichiometry of three or four α1 and two or one β subunits (176, 177). The receptor is selectively blocked by the high-affinity competitive antagonist strychnine (263); strychnine poisoning results in severe muscle spasms, opisthotonic posturing, and convulsions which are triggered by minimal stimuli. Mutations of the α1 subunit of the receptor cause hyperekplexia, a disorder characterized by prominent startle responses to tactile or acoustic stimuli along with hypertonia (306).
Interestingly, antibodies to the α1 subunit of the GlyR were initially identified in a 54-yr-old man who presented with violent jerks, spontaneous or triggered by sensory and auditory stimuli; his upper limbs would abduct and flex and the trunk and legs extended. In addition, he had symptoms of brain stem dysfunction and hyperekplexia, and improved with immunotherapy (147). Subsequent studies have shown that these antibodies preferentially associate with PERM (42) but may also occur with stiff-person syndrome (214, 222), seizures, dysautonomia, opisthotonus, hypersomnia, limbic encephalitis, brain stem encephalitis, and pruritus caused by local involvement of the spinal cord (42, 217).
In a series of 52 patients with GlyR antibodies, most responded to immunotherapy (although 4 patients died in the acute stage of the disease), and after a median follow-up of 3 yr, 5 patients had relapses(42). The study also confirmed a low frequency of tumors (3 of 45 patients had benign thymomas, 1 B-cell marginal zone lymphoma, and 1 metastatic breast cancer), and the coexistence in some patients of GlyR and GAD antibodies (42). Although the literature emphasizes the association of GlyR antibodies with the above spectrum of symptoms, these antibodies also occur in 5–10% of patients with unrelated disorders such as optic neuritis or multiple sclerosis (216). In these patients, the CSF is negative for antibodies, whereas in patients with PERM, the antibodies are usually encountered in CSF, suggesting intrathecal synthesis or crossing of the BBB by the antibodies.
A pathogenic effect of the antibodies has been suggested by in vitro studies with HEK cells expressing GlyR. In this setting, patients' antibodies (which are of the IgG class) cause internalization of the receptors and show complement fixation (42). However, it is unclear whether these effects also occur in neurons or in the human disease.
2. Amphiphysin
Antibodies to amphiphysin occur in the paraneoplastic variant of stiff person-syndrome, and less frequently with other syndromes such as PERM, encephalitis, or sensory neuronopathy (64, 65). The tumors more frequently involved are breast cancer and SCLC, and the neurological syndrome usually precedes the detection of the cancer (256, 278). Compared with patients with GAD antibodies, those with amphiphysin antibodies are older, do not develop diabetes, and the pattern of stiffness is different with predominant involvement of neck and arm muscles.
Amphiphysin, first described in 1992 (198), exists as two splice variants (amphiphysin 1 and 2) (264) and is an abundant N-BAR domain protein enriched in the presynaptic nerve ending. In resting conditions amphiphysin is found associated with synaptic vesicles, and during high synaptic activity it localizes at the sites of endocytosis (79). Together with binding partners, amphiphysin is an important regulatory endocytic protein involved in several steps of clathrin-mediated endocytosis. With its clathrin binding domain (CLAP), amphiphysin directly interacts with clathrin and AP2 which is important for presynaptic membrane invagination and clathrin coat assembly (63). The carboxy-terminal src homology 3 (SH3) domain of amphiphysin binds to the prolin-rich region of the GTPase dynamin that mediates clathrin-coated vesicle fission from the membrane (63, 108), and to synaptojanin which in turn is a strong interacting partner of endophilin, both important for endocytic steps from membrane invagination to vesicle uncoating (23, 224, 226). The functional aspects of how autoantibodies to amphiphysin interfere with vesicle endocytosis and synaptic transmission and finally lead to characteristic disease symptoms in animal models are discussed in the next section.
V. FUNCTIONAL INTERACTIONS OF AUTOANTIBODIES WITH SYNAPTIC PROTEINS: TWO MODELS OF ANTIBODY-MEDIATED DISEASES OF THE SYNAPSE
For those disorders in which the functional effects of the antibodies have been examined in cultured neurons, including antibodies to NMDA (144), AMPA (251), GABAb (157), and GABAa receptors (252), or antibodies to LGI1 (244), Caspr2 (255), DPPX (254), neurexin-3 alpha (119), and amphiphysin (335) or in animals models [mGluR1 (293), NMDA receptor (258), and amphiphysin (297)], there is evidence that the antibodies specifically alter the structure or function of the target receptor or synaptic protein. These antibody-mediated effects have only recently started to be elucidated, and findings were briefly described above. However, two autoantibodies have been more extensively studied, including the antibodies against amphiphysin (a presynaptic protein) and those against the NMDA receptor (a postsynaptic receptor). In this section we focus in these two models of antibody-mediated disorders of the synapse.
A. Functional Effects of Amphiphysin Autoantibodies
As noted above, amphiphysin is an important regulatory endocytic protein involved in several steps of clathrin-mediated endocytosis. Even though amphiphysin is not essential for vesicle recycling, amphiphysin knockout mice develop severe dysfunction in vesicle recycling and show reduced learning abilities and increased mortality due to irreversible seizures (70). At the cellular level, neurons from these mice exhibit stimulus-dependent slower recycling time of synaptic vesicles and a reduced pool size of vesicles (70).
In comparison to the genetic deletion of amphiphysin, acute interference with amphiphysin function by specific antibodies or blocking peptides specifically targeting the SH3 domain in the lamprey giant synapse led to a more severe phenotype in endocytic dysfunction (70, 290). In these in vitro studies, it has been demonstrated that the SH3 domain but not the central CLAP domain is accessible to antibodies. The amphiphysin SH3 domain is functionally important because it is the binding part for the interaction with dynamin which mediates the formation of clathrin-coated intermediates during vesicle endocytosis (79, 108). Interestingly, in patients with stiff-person syndrome, the autoantibodies also specifically target the SH3 domain (64). Passive-transfer experiments with polyclonal IgG fractions and with affinity-purified antibodies from patients with stiff person-syndrome induced some of the characteristic symptoms of the disease in experimental animals (103), fulfilling the modified Koch-Witebsky postulates for antibody-mediated disease (269). Motor hyperactivity, stiffness, and muscle spasms were observed after systemic administration of high titer amphiphysin antibodies after opening the BBB using a model of mild experimental autoimmune encephalomyelitis induced by adoptive transfer of encephalitogenic T-cells (32, 297). More specifically, symptoms of muscle hyperactivity along with increased anxiety-related behavior were also obtained in an animal model of repetitive intrathecal application of specific, affinity-purified IgG amphiphysin antibodies via implanted catheters (101, 103). Super-resolution STED microscopy demonstrated human IgG in subcompartments of spinal presynaptic terminals at the site of vesicle endocytosis and in the vicinity of the active zone of vesicle release.
One of the most potent inhibitory mechanisms in the spinal cord is presynaptic inhibition caused by local GABAergic interneurons (last-order GABAergic interneurons). These interneurons form axo-axonal synapses on the presynaptic endings of Ia afferent fibers that project to spinal motor neurons (271). Activation of the GABAa receptors leads to opening of chloride channels and depolarization of the membrane, thereby blocking the invasion of action potentials into the nerve terminals (or reducing its amplitude), resulting in a decrease of calcium influx and finally in a reduction of excitatory transmitter release from the Ia afferent terminal onto the motor neuron (76, 271). This depolarization can be measured by recording voltage changes in a dorsal root (dorsal root potentials) or indirectly by measuring changes in the modification of the monoynaptic Hoffmann (H)-reflex (Figure 15). In symptomatic animals after intrathecal passive transfer of pathogenic amphiphysin antibodies, in vivo H-reflex and dorsal root potential recording revealed markedly decreased presynaptic inhibition as a consequence of severely affected primary afferent depolarization due to disturbed GABAergic inhibition (77, 103, 122) (Figure 15). Interestingly, the function of spinal GABAergic interneurons and presynaptic inhibition has also been shown to be affected in patients with stiff-person syndrome examined with H-reflex recordings and reciprocal and vibration-induced modulation of the monosynaptic reflex arc (88). In addition to reduced spinal GABAergic inhibition, there is evidence of decreased supraspinal inhibition as shown by neurophysiological studies including transcranial magnetic stimulation and blink reflex recordings (231, 279). In vitro experiments with patch-clamp recordings and FM-dye imaging showed deficits in endocytic function in synapses of primary neurons after preincubation with purified human amphiphysin antibodies (Figure 16). These deficits were stimulus dependent and more pronounced in GABAergic compared with glutamatergic synapses (103). This is compatible with a hypothesized interaction of autoantibodies with the amphiphysin SH3 domain and interference with amphiphysin binding to partners in the endocytic process that bind to the SH3 domain, such as dynamin (221). In experiments using dynamin deficient neurons, synaptic function is severely impaired upon stimulation leading to an accumulation of plasma membrane invaginations and reduction of the presynaptic vesicle pool (84). Comparing excitatory and inhibitory synapses by electron tomography, dynamin deficiency unmasks a striking heterogeneity between the synaptic subclasses where inhibitory synapses are primarily affected (132). This indicates that inhibitory synapses may be exceptionally vulnerable to deficits in endocytic protein function which is likely due to their higher tonic activity and unmet need for continuous vesicle recycling to build up vesicle pools for proper exocytosis and neurotransmission (80, 132). Recently, it has been shown that presynaptic vesicle pools and clathrin-coated intermediates are markedly reduced in experimental rats after intrathecal passive-transfer of affinity-purified amphiphysin antibodies (Figure 17) (335). In analogy to the above-mentioned findings, neurons with genetic deficiency of endocytic proteins showed prominent defects in presynaptic vesicle endocytosis after sustained stimulation. In contrast to the depletion induced during sustained stimulation, vesicle pools were even enlarged compared with control animals at resting conditions (335). This is suggestive of a compensatory mechanism to maintain a basal pool of releasable vesicles in vivo that is then exhausted under sustained stimulation and continued synaptic activity. Supporting this hypothesis, the molecular composition of vesicular proteins is changed in primary GABAergic neurons following incubation with human amphiphyin antibodies (335). Super-resolution direct stochastic optical reconstruction microscopy (dSTORM) revealed different composition of presynaptic vesicles with v-SNARE proteins, indicating changes in dynamic properties of vesicle pools with reduction of the resting pool vesicles revealed by the presence of synaptobrevin 7 (141). Moreover, long-term application of amphiphysin antibodies to primary neurons over 24 h induced a switch of endocytic pathways from adaptor-protein (AP) 2-dependent clathrin-mediated endocytosis to the AP3-dependent pathway of vesicle formation from bulk endosomes. This change in endocytic pathways could be reversed by the use of brefeldin A, a fungal inhibitor that blocks the AP3 dependent pathway (330, 335).
FIGURE 15.
Effects of anti-amphiphysin antibodies on spinal inhibitory pathways. A: spinal reflex pathways of antagonistic muscle groups: afferent fibers project to Ia interneurons (gray) mediating disynaptic reciprocal inhibition (recurrent inhibition by glycinergic transmission) onto motor neurons (MN) supplying antagonistic muscle groups. In addition, afferents also project to local interneurons via polysynaptic transmission. Last-order interneurons (black) mediate primary afferent depolarization (PAD) of afferent fibers by activation of GABAa receptors (axo-axonal synapses; presynaptic inhibition). B: examples of Hofmann (H)-reflex recording at higher frequency (10 Hz) after stimulation of a peripheral (tibial) nerve in rats. The first deflection is the anterograde muscle response (M), the second response is the H-reflex after monosynaptic transmission in the spinal cord. In normal conditions, the H-reflex is fully suppressed at high-frequency simulation (green trace). Note that upon long-term intrathecal application of human anti-amphiphysin antibodies, the suppression of the H-reflex is insufficient (red trace). C: H-reflex recordings after tibial nerve stimulation together with stimulation of a nerve supplying antagonistic muscles (peroneal nerve). Top trace shows simultaneous stimulation, and bottom trace represents recordings with a 50 ms preceding volley of peroneal nerve stimulation resulting in reduction of H-reflex amplitude (green traces). In rats with intrathecal application of anti-amphiphysin antibodies (red trace), GABAergic presynaptic inhibition is reduced as shown by absent H-reflex depression after heteronymous stimulation. D: time course of H-reflex inhibition after heteronymous stimulation. Depression of the H-reflex is most pronounced at interstimulus intervals (delay) of 25–100 ms demonstrating polysynaptic mechanisms of presynaptic inhibition. In rats with application of anti-amphiphysin antibodies, H-reflex inhibition is absent. E and F: determination of presynaptic inhibition by in vivo recording of dorsal root potentials (dorsal root L5 is cut on one side and afferent volleys are delivered by tibial nerve stimulation). Dorsal root potentials (long-lasting upward deflection as shown in F) are mediated by presynaptic GABAergic inhibition due to primary afferent depolarization. Dorsal root potential amplitude is severely reduced in rats after intrathecal treatment with anti-amphiphysin antibodies. [From Geis et al. (103), by permission of Oxford University Press.]
FIGURE 16.
Anti-amphiphysin antibodies reduce vesicle release in GABAergic presynaptic terminals. A: vesicles in presynaptic terminals of primary neurons were loaded with FM-dyes (white). Subsequent stimulation results in reduction of fluorescence intensity indicating exocytosis of presynaptic vesicles. Presynaptic terminals were assigned to GABA or glutamatergic boutons by identification of vesicular GABA or glutamate transporter (VGAT and VGLUT, respectively) immunoreactivity (not shown). B: in control conditions, GABA and glutamatergic terminals of neurons preincubated with control IgG show regular patterns of vesicle release. In neurons pretreated with anti-amphiphysin antibodies, initial fluorescence intensity as a measure for vesicle content is reduced in GABAergic terminals and vesicle release is slowed. Schematic depicts the situation of reduced vesicle loading in GABAergic synapses that result in reduced synaptic transmission upon stimulation. [From Geis et al. (103), by permission of Oxford University Press.]
FIGURE 17.
Proposed model of activity-induced synaptic dysfunction due to amphiphysin antibodies. Antibodies to amphiphysin interact with their target antigen at the step of vesicle recycling (A). Binding of antibodies to the amphiphysin SH3 domain blocks the interaction with dynamin and other endocytic proteins (right panel in A). This leads to defective endocytosis with a reduction of clathrin-coated intermediates and subsequently to a reduced number of presynaptic vesicles filled with neurotransmitter and available for exocytosis. Blocking of endocytic function induces an accumulation of adaptor proteins (AP2, AP180) at the cell membrane. B: electron microscopy images of spinal presynaptic boutons (light blue) of rats after intrathecal passive-transfer of IgG from a healthy subject (normal) or anti-amphiphysin antibodies and continuous stimulation of Ia afferent fibers. Vesicle pool and GABA immunoreactivity (postembedding immunogold stain, black dots) are depleted in the synapse of an animal treated with pathogenic amphiphysin antibodies. Scale bar: 200 nm.
Together, the evidence of pathogenic interference of amphiphysin autoantibodies with endocytic function, the unique vulnerability of tonic GABAergic synapses to deficits in clathrin-mediated endocytosis, the transfer of symptoms of stiff-person syndrome upon application of patients' antibodies to experimental animals, and the clinical improvement in patients after antibody removal and immunotherapy (236, 336) makes this rare enigmatic disease an example of AE likely mediated by autoantibodies against a presynaptic protein (Figure 17). Yet, there are several caveats that need to be taken into account when considering amphiphysin antibodies a potential cause of stiff-person syndrome. First, it is not yet clear how systemic or intrathecal antibodies interact with a presumably intracellular antigen (338). Second, although stiff-person syndrome manifests with distinct clinical features, the clinical spectrum of the disorder is heterogeneous, and patients often harbor IgG antibodies to other neuronal antigens such as P/Q-type VGCC and nuclear antigens (256). It is conceivable that coexisting neuronal antibodies (except for anti-nuclear antibodies) contribute to and determine clinical differences among patients. Third, there is evidence in autopsy studies that cytotoxic T-cell infiltrates, mainly CD8+ T cells, are prominent and may play a role in the pathogenesis of the disease (256) (336). Fourth, an active immunization model with production of autoantibodies and induction of disease has not yet been developed.
The first evidence that intrasynaptically located amphiphysin can be accessed by autoantibodies comes from the observation that intrathecally administered affinity-purified antibodies can be detected in spinal synaptic nerve endings by super-resolution microscopy. These pathogenic IgG antibodies also appear to be internalized in primary neurons in an epitope-specific process as revealed by competition and preabsorption experiments (103). One possible explanation for the ability of autoantibodies to access amphiphysin may be the transient exposure of amphiphysin to the extracellular compartment during the process of vesicle recycling. This might be a unique property of synaptic antigens that are located in nerve terminals and closely associated with synaptic vesicles, recently called “transient moonlighting” (150). Supporting this hypothesis is the demonstration that the carboxy-terminal ending of the vesicular GABA transporter (VGAT) is facing the luminal side of synaptic vesicles and can be exposed to the extracellular space during vesicle recycling (212). This short episode could be sufficient for specific antibodies to bind and subsequently be internalized in GABAergic nerve endings. Moreover, this uptake is regulated by synaptic vesicle recycling, thus providing an epitope- and activity-dependent mechanism of specific antibody internalization in neurons. Alternatively, IgG and other macromolecules can also be taken up in a nonspecific manner (e.g., Fc receptor binding or after binding to gangliosides) (136, 228, 343). One can also argue that these mechanisms of antibody uptake may be nonpathogenically relevant given that it is unclear how antibodies would avoid degradation in endosomes and lysosomes and reach their intracellular cognate antigen.
B. Functional Effects of IgG NMDA Receptor (GluN1) Autoantibodies
When the encephalitis associated with antibodies against NMDA receptor was first described in 2005 (329) [GluN1 as the target was not recognized until 2007 (60)], the prevailing concept about most neuronal autoantibodies related to human disease was that they were good biomarkers of the disease but had limited pathogenic relevance. This idea was supported by multiple failed attempts to demonstrate the pathogenicity of some of these antibodies (e.g., anti-Hu, anti-Yo) in cultured neurons or in vivo models (113, 304). Thus the identification of autoantibodies reacting with the cell surface of live neurons (e.g., Figure 2E, shows a patient GluN1 antibodies), the similarity of the syndrome with pharmacological or genetic models in which the function of NMDA receptors is altered, and the clinical response to immunotherapy prompted studies to determine if the antibodies were pathogenic. Initial studies using dissociated rat hippocampal neurons incubated for 3–7 days with patients' antibodies showed a selective and reversible decrease of NMDA receptor surface density and synaptic localization that correlated with the titers of antibodies present in patients' serum or CSF samples (57, 144) (a reproduction of these findings is shown in Figure 18). These effects were not observed using Fab fragments derived from the autoantibodies, but subsequent crosslinking of the Fab fragments with Fab antibodies recapitulated the antibody-induced reduction of cell surface and synaptic NMDA receptors. As a result, whole cell patch-clamp recordings of mEPSCs in cultured neurons showed that patients' antibodies specifically decreased synaptic NMDA receptor-mediated currents, without altering the AMPA receptor-mediated currents (144). In contrast to the marked effects on the levels of NMDA receptors, patients' antibodies did not alter other synaptic proteins (e.g., GluA2/3, GABAa), number of synapses, dendritic spines, dendritic complexity, or neuronal cell survival (144). To determine if the autoantibodies had effects in vivo also, CSF from patients with high-titer GluN1 antibodies or control CSF from individuals without these antibodies was infused directly into the hippocampus of adult rats for 2 wk, followed by analysis of tissue-bound human IgG and levels of NMDA receptors. Rats infused with patients' but not control CSF showed deposits of human IgG in the hippocampus in a pattern that was dependent on NMDA receptor density (e.g., high density in proximal dendrites of dentate gyrus) (144). This pattern was similar to that identified in the hippocampus of two patients who had died of anti-NMDA receptor encephalitis. Moreover, no neuronal death or deposits of complement were observed, which is also similar to the autopsy findings of the patients even though the autoantibodies are predominantly IgG1 and potentially able to fix complement (144). Overall, results from these studies provided a compelling link between the clinical syndrome and the presence of these antibodies, strongly suggesting they have a pathogenic role in the disease.
FIGURE 18.
Patient's NMDA receptor autoantibodies cause a specific reduction of the density of cell surface and synaptic NMDA receptor clusters. Cultures of dissociated rat hippocampal neurons immunolabeled to demonstrate the cell surface NMDA receptors (green clusters) after being exposed for 24 h to control CSF (without NMDA receptor antibodies) or CSF from a patient with high titers NMDA receptor antibodies (A). The framed dendrites are shown at higher magnification in B, which demonstrate the density of cell surface NMDA receptor clusters (green), PSD95 (red), and synaptic NMDA receptor clusters defined by the colocalization of NMDA receptors with PSD95 (yellow). Neurons exposed to patients' CSF showed a significant reduction of cell surface NMDA receptors (not shown) and synaptic NMDA receptors compared with neurons exposed to control CSF or not treated; no effects were noted on PSD95 (C and D). ***P < 0.001. For additional information, see Refs. 144, 234. Each circle indicates the number of clusters per 20 μm length in a different dendrite. Scale bars: A = 20 μm; B = 10 μm.
These findings led to the formulation of several interesting questions that were addressed by Moscato et al. (234). For example, the potential different effects of patients' antibodies on inhibitory (GAD positive) and excitatory (GAD negative) neurons was investigated in cultured neurons showing that the autoantibodies caused a similar decrease of NMDA receptor density in both types of neurons (234). However, these in vitro data did not rule out that at the circuitry level (e.g., in vivo) the antibody effects could be different; for example, networks of neurons and inhibitory interneurons might be required to observe an antibody-induced imbalance of inhibitory and excitatory systems leading to hyperexcitability. This hypothesis was investigated in vivo using microdialysis and infusion of patients' antibodies in the CA1 region of hippocampus and premotor cortex of rats. Analysis of microdialysates showed that in the presence of patients' but not control IgG, very high concentrations of glutamate were found in the extracellular space during NMDA infusion (210). This was consistent with dysfunction of the NMDA-related glutamatergic turnover or impaired turnover of receptors, resulting in NMDA-related excitotoxicity. Concomitant administration of NMDA and AMPA induced a rise in extracellular concentrations of glutamate up to toxic levels, suggesting a potential mechanism of why patients' with persistent, very high levels of CSF antibodies (refractory to immunotherapy) may develop irreversible deficits or have a fatal outcome (234). Data from these microdialysis studies support the concept that patients' antibodies decrease NMDA receptors (and function) not only at the postsynaptic level of the glutamatergic synapse but also at the level of inhibitory interneurons, contributing to the hyperglutamatergic state.
The dynamics of antibody-induced internalization of receptors were investigated in cultured neurons exposed for 15 min to 48 h to patients IgG (234). Although a progressive decrease of NMDA receptor density started to be noted 2 h after exposure to the patients' antibodies, the most significant effects were noted at 12 h with no further reduction of NMDA receptors with longer treatment. In parallel, the internalized NMDA receptor clusters followed the same time course. Similar studies using Fab fragments did not cause significant effects (234). Given that a potential agonistic effect of patient's antibodies could induce internalization by mechanisms independent of crosslinking, similar experiments were conducted in the presence or absence of DL-2-amino-5-phosphonovaleric acid (APV), an NMDA receptor blocker. The presence of the blocker did not attenuate the effects of the antibodies, suggesting that the main mechanism of NMDA receptor internalization was due to the effects of receptor crosslinking (234). Further studies using colabeling of internalized antibody-bound NMDA receptors with recycling endosomal and lysosomal biomarkers (Rab11, Lamp1) showed that a greater percentage of internalized receptors colocalized with recycling endosomes than lysosomes, similar to the trafficking observed in other conditions (e.g., exposing neurons to picrotoxin, NMDA/glycine) (234). Therefore, the postendocytic trafficking did not seem to be affected by antibody binding to the receptor. Another study confirming the antibody-mediated internalization of receptors showed a decrease of total GluN1, suggesting that the internalized receptors were more prone to degradation (225).
The hypothesis that patients' antibodies could potentially modulate receptor function independently of their ability to internalize NMDA receptors led to investigations using whole cell patch-clamp recordings of mEPSCs from primary neurons after short exposure (30 min) to patients and control CSF. These studies showed no significant differences between patients' and control CSF, suggesting that the reduction of mEPSCs after prolonged (24 h) exposure to patients' antibodies is predominantly due to a decrease of synaptic NMDA receptor density rather than other potential modulatory changes of the receptor by patients' antibodies. This concept is also supported by the lack of an effect on mEPSCs after 24-h exposure to patients' Fab fragments (which do not reduce NMDA receptor levels) (234). In contrast to these findings, single-channel recordings on HEK293 cells cotransfected with GluN1 mutants and GluN2B showed that patients' antibodies bound more robustly to GluN1 ATD forms that are prone to more frequent channel opening (106). The data led to studies to explore whether patients' antibodies had an effect on receptor function. Single-channel recordings on outside-out patches from GluN1/GluN2B expressing HEK293 cells showed that application of agonist (glutamate/glycine) with patients' CSF resulted in prolonged open duration of the receptor compared with conditions in which agonist alone or agonist with control CSF were applied. No consistent effects on receptor closed time were observed, suggesting that patients' antibodies bind to a receptor that is prone to opening and probably stabilizes the open state (106). Overall, these data suggest that although the main pathogenic effect of patients' antibodies is receptor internalization, these antibodies may also directly affect receptor function.
The question of whether patients' NMDA receptor antibodies caused homeostatic plasticity (or synaptic scaling) was addressed in cultures of primary neurons determining first a possible upregulation of NMDA receptor transcription after antibody-induced internalization. Quantitative polymerase chain reaction in neurons treated for 24 h to 7 days with patients' CSF antibodies showed no changes in the levels of mRNA of GluN1, GluN2A, GluN2B, or any of the GluN1 carboxy-terminal splice variants. Homeostatic plasticity was also assayed in inhibitory synapses in response to NMDA receptor hypofunction caused by patients' antibodies. However, the amplitude and inter-event interval of miniature inhibitory postsynaptic currents (mIPSCs) were unchanged in cultured neurons treated for 24 h with patients' antibodies, and the gene expression of GAD1 and GAD2 (enzymes involved in the synthesis of GABA by inhibitory neurons) was also unchanged (234). In contrast, patients' antibodies caused a significant decrease in inhibitory synapse density onto excitatory neurons [demonstrated by a decrease of vesicular GABA transporter (VGAT) stained presynaptic terminals] (234). The apparent difference between electrophysiological findings and immunolabeling experiments was attributed to the increased power of the latter (∼30 neurons per condition versus electrophysiology experiments measuring mIPSCs in ∼6 neurons per condition) (234). Overall, results from these studies suggested a mechanism of synaptic scaling whereby neurons were able to partially adjust their inhibitory tone in a compensatory direction. Therefore, although a loss of glutamatergic drive to inhibitory GABAergic neurons has been postulated to lead to disinhibition in models of hypofunction of NMDA receptor [e.g., schizophrenia, anti-NMDA receptor encephalitis (218)], the homeostatic downregulation of inhibitory synapses onto excitatory neurons could potentially enhance excitability.
Other studies conducted by Mikasova et al. (225) showed that in neurons transfected with either the GluN2A or GluN2B subunit tagged with SEP [a pH-sensitive variant of enhanced green fluorescent protein (GFP) that exhibits bright fluorescence when exposed to the exterior of the cell], the exposure to patients' NMDA receptor antibodies caused a decrease of the surface GluN2A- and GluN2B-containing receptors. These changes were not caused by loss of synaptic contacts, since the overall number of glutamatergic synapses was not affected, consistent with the findings of Hughes et al. (144). In addition, neurons exposed to patients' CSF antibodies, but not control CSF for 20 h, failed to increase the synaptic content of GluA1-AMPA receptor after undergoing chemically induced LTP (206), suggesting that patients' antibodies reduced potentiation of glutamatergic synapses. Additional studies using nanoparticle/molecule tracking showed that in neurons treated with patients' IgG the lateral diffusion of the GluN2-NMDA receptor was dramatically increased compared with GluA1-AMPAR (that was mildly increased) and the α2-GABAaR and potassium channel Kv1.3 which were unaffected (225). These findings suggested that patients' antibodies had a rather specific action on NMDA receptor trafficking.
Considering the altered surface dynamics caused by patients' antibodies, further studies focused on the effects of the antibodies on direct partners of the NMDA receptor at the extracellular level, such as the Ephrin B2 receptor (EphB2) and how activation of this receptor would modify or prevent the antibody effects. EphB2 is a member of a family of receptor tyrosine kinases that modulate LTP probably through their interaction with NMDA receptors that results in stabilization and clustering of these receptors in the postsynaptic membrane (61, 134, 178, 201). Ephrin signaling, such as ephrin-B2 ligand binding to the EphB2, is important to establish LTP in CA3-CA1 synapses for which downstream kinase signaling upon EphB2 activation is not critical as intracellular truncated forms of EphB2 do not interfere with LTP (123, 134). In neurons exposed to patients' NMDA receptor antibodies, the tracking of EphB2 using quantum dots showed that the antibodies caused a marked increase of EphB2 diffusion at the synapse. These findings coupled to a reduction of coimmunoprecipitated EphB2/NMDA receptor when neurons were exposed to patients' but not control IgG suggest that the antibodies prevent the surface interaction between EphB2 and NMDA receptors leading to a lateral diffusion of both EphB2 and synaptic NMDA receptors. Notably, activation of EphB2 by ephrin-B2 ligand prevented the increased surface diffusion and lateral synaptic escape of NMDA receptors induced by patients' antibodies (225). Similar protective effects of ephrin-B2 ligand were suggested in vivo after injection of patients' antibodies in the dorsal hippocampus in rats. In this setting, the decrease of NMDA receptor immunostaining (attributed to a decrease of density of receptors) was abrogated when patient antibodies were coinjected with ephrin-B2 ligand (225). These data provided evidence that patients' antibodies alter surface dynamics and density of NMDA receptors which can be potentially prevented by EphB2 stimulation.
Combined data from these reports demonstrated that anti-NMDA receptor encephalitis fulfilled most of the Koch-Witebsky criteria to be an antibody-mediated disease (269) with only the transfer of symptoms to animals pending. To develop such a model in C57BL6/J mice, Planaguma et al. (258) used bilateral ventricular catheters connected to subcutaneous osmotic pumps to deliver a continuous infusion of patients' or control CSF for 14 days. During and after the infusion period, tasks for memory (novel object recognition), anhedonic behaviors (sucrose preference test), depressive-like behaviors (tail suspension, force swimming test), anxiety (black and white, elevated plus maze tests), aggressiveness (resident-intruder test), and locomotor activity were investigated, and the potential association with brain antibody binding and levels of NMDA receptors were examined. The infusion of patients' but not control CSF caused progressive memory deficits, along with anhedonic and depressive-like behaviors, without affecting locomotor activity (258). The most dramatic effects occurred in the novel object recognition task that was maximally impaired on day 18 (4 days after the ventricular infusion stopped) and recovered over 1 wk. Brain tissue studies confirmed the progressive presence of brain-bound human IgG (maximal on day 18, mainly in hippocampus). Extraction and characterization of this IgG confirmed it to be NMDA receptor antibodies. Additionally, analysis of NMDA receptor clusters in the hippocampus showed a progressive decrease of the density of cell surface and synaptic NMDA receptors (maximum on day 18) without affecting the density of AMPA receptors or PSD95. These effects developed in parallel with memory and other behavioral alterations and gradually improved after day 18, with reversibility of symptoms, progressive reduction of brain-bound antibodies, and reestablishment of the normal levels of cell surface and synaptic NMDA receptors (Figure 19). Pathological studies did not reveal inflammatory infiltrates or deposits of complement (258). These findings provided robust evidence that antibodies from patients with anti-NMDA receptor encephalitis alter memory and behavior through reduction of cell surface and synaptic NMDA receptors. The model is in line with the concept that this disorder is predominantly antibody mediated, as supported by detection of high levels of B-cell attracting chemokines (e.g., CXCL13) in the CSF (195), and autopsy or biopsy studies showing absent or very rare neuronophagic T-cell infiltrates, but abundant plasma cells or deposits of IgG (30, 215).
FIGURE 19.
Cerebroventricular infusion of patients' NMDA receptor antibodies causes memory and behavioral deficits in mice. Mice underwent placement of bilateral ventricular catheters connected to subcutaneous osmotic pumps that during 14 days continuously infused CSF from patients with high-titer NMDA receptor antibodies or control CSF (without antibodies). During and after the infusion, cohorts of animals underwent multiple behavioral studies or were killed to determine the effects of the antibodies. Animals infused with patients' but not control CSF showed a progressive accumulation of brain-bound IgG that was maximal on day 18 (A and B) in a pattern identical to that seen by direct brain immunostaining with patients' NMDA receptor antibodies (compare A with panel NMDA receptor of Figure 3). Extraction and characterization of the brain-bound IgG confirmed it to be NMDA receptor antibodies (not shown). Quantitative analysis of the NMDA receptor clusters in the hippocampus (representative squares in B) showed a significant reduction of cell surface and synaptic NMDA receptors, but not PSD95, in animals infused with patients' antibodies (C–E; gray columns: patients' CSF, white columns: control CSF). These effects were maximal on day 18. The reduction of NMDA receptors in hippocampus of mice infused with patients' antibodies was accompanied by severe memory impairment (novel object recognition test) and anhedonic behaviors (indifference to sugar-containing water) (gray circles in F and G) that did not occur in animals infused with control CSF (white circles in F and G). All antibody effects, from reduction of NMDA receptors to memory and behavioral deficits, recovered 10 days after stopping the antibody infusion. *P < 0.05; $$P < 0.01; *** $$$P < 0.001; for experimental details, see Ref. 258. Scale bars: A = 2 mm; B = 200 μm. [From Planaguma et al. (258), by permission of Oxford University Press.]
Considering previous studies that showed that stimulation of EphB2 by ephrin-B2 ligand antagonized the effects of the antibodies, Planaguma et al. (257) used the passive transfer model to investigate the effects on synaptic plasticity (e.g., induction of LTP by stimulation of the Schaffer collateral pathway and recording in CA1 in acute brain sections from infused mice) and to determine whether the antibody-induced memory and behavioral deficits could be prevented by administration of soluble ephrin-B2 ligand. Mice were infused with patients' or control CSF with or without ephrin-B2 ligand added to the osmotic pumps. Animals that received patients' antibodies without ephrin-B2 ligand developed a phenotype identical to that described above, along with severe impairment of long-term hippocampal synaptic plasticity and memory formation (Figure 20). These findings resembled those obtained in hippocampal CA1 region-specific GluN1 knockouts which also show deficits of memory and learning accompanied by severe impairment of LTP in the Schaffer collateral-CA1 synapse, demonstrating the role of NMDA receptors in establishing synaptic plasticity and memory formation (143, 315). Moreover, in contrast to the dramatic effects observed in mice receiving patients' antibodies without ephrin-B2 ligand, the coadministration of ephrin-B2 ligand antagonized the pathogenic effects of the antibodies at all levels, including memory, depressive-like behavior, density of cell-surface and synaptic NMDA receptors, and significantly restored the long-term synaptic plasticity (257). Although the passive transfer of patients' antibodies to mice has been important to confirm their pathogenicity, the phenotype of this model has several limitations and does not reproduce the entire spectrum of the disease. First, mice did not develop seizures or stereotypic or repetitive movements suggesting dyskinesias. This could be due to the strain of mice used (C57BL6/J are optimal for memory and behavioral studies but are resistant to seizures) (85), and the dynamics of CSF antibody distribution when it is infused in the ventricles, favoring the binding to the closer hippocampi over other brain regions. An extended infusion or higher antibody concentration may lead to the involvement of other areas such as cortex or basal ganglia, resulting in seizures or dyskinesias. Second, different from the mouse model in which the infusion of antibodies is automatically discontinued after 14 days (explaining their rapid recovery), the exposure of patient's brain to CNS antibodies is prolonged and decreases slowly over months, likely contributing to the protracted recovery (120, 195). A main source of autoantibodies appears to be the plasma cells that have been demonstrated in the CNS of patients' (from autopsy or biopsy studies) (30, 215). Two recent studies have shown the presence of NMDA receptor-specific B cells (173) and expanded clones of plasma cells showing antigen-driven expansion and ongoing somatic hypermutations in the CSF of patients with anti-NMDA receptor encephalitis (Goebels et al., unpublished data). With the use of single-cell cloning of full-length immunoglobulin heavy and light chain genes, recombinant GluN1 antibodies were generated, which in preliminary studies showed similar pathogenic effects to those demonstrated by patients' CSF in cultured neurons (173) and in the model of chronic cerebroventricular transfer of antibodies to mice (258)(Goebels et al., unpublished data). Therefore, the prolonged presence of antibodies in human brain may disrupt synaptic networks as suggested by the decrease of synaptic NMDA receptor density and alteration of synaptic plasticity in the mouse model (257, 258). Finally, compared with this model in which there are no inflammatory infiltrates, patients have brain inflammatory infiltrates and often develop complications (e.g., autonomic instability, intensive care unit infections) that contribute to the course and outcome of the disorder (58). A model of active immunization with the GluN1 subunit of the NMDA receptor may overcome some of these limitations, but the fact that the epitopes targeted by the antibodies are conformational (e.g., are lost in most manipulations of the protein) has complicated to date the development of such a model.
FIGURE 20.
Patients' NMDA receptor antibodies cause severe impairment of long-term synaptic plasticity in the hippocampus of mice that is partially prevented by ephrin-B2. Experiments conducted with acute sections of mice chronically treated with ventricular infusion of CSF of patients with anti-NMDA receptor encephalitis, or control CSF, with or without soluble ephrin-B2 added to the CSF. The Schaffer collateral pathway was stimulated, and field potentials were recorded in the CA1 region of the hippocampus; long-term potentiation (LTP) was induced by theta-burst stimulation (TBS). A: example traces of individual recordings showing average traces of baseline recording before LTP induction (black traces) and after LTP (red traces). Slope and peak amplitude of fEPSPs are increased following TBS in mice infused with control CSF (Ct CSF) and Ct CSF + ephrin-B2, whereas manifestation of LTP is strongly impaired in the animals infused with patients' CSF antibodies (Pt CSF). In mice infused with Pt CSF + ephrin-B2, there is an increase of slope compared with mice infused with Pt CSF without ephrin-B2. Note that initial peak amplitude of fEPSP may vary within individual recordings. B: time course of fEPSP recordings demonstrating robust changes in fEPSP slope in the Ct CSF (n = 7 recordings, blue open circles) and Ct CSF + ephrin-B2 group (n = 7, cyan open squares) which is stable throughout the recording period after TBS (arrow). In animals chronically infused with Pt CSF (n = 7, red solid circles) the induction of synaptic LTP is markedly impaired. Recordings from the Pt CSF + ephrin-B2 group (n = 5, gray solid squares) show partially resolved effects on synaptic plasticity following LTP induction. C: quantitative analysis of LTP-induced changes in fEPSPs in the plateau interval after TBS depicted compared with each individual baseline value (slope increase as median values ± SE in the consolidation phase during the last 50 min of each recording, starting 15 min after TBS). Chronic application of Pt CSF results in marked reduction of LTP (13.3 ± 4.1% slope increase vs. 73.6 ± 19.3% and 68.3 ± 14.7% in Ct CSF and Ct CSF + ephrin-B2, respectively). Coadministration of soluble ephrin-B2 improved fEPSP potentiation to levels of 33.7 ± 5.5%. Significance of treatment effect was assessed by two-way ANOVA (P < 0.0001 for treatment group) and by post hoc analysis with Bonferroni correction (***P < 0.001). [From Planaguma et al. (257), with permission from John Wiley and Sons, Inc.]
Other studies using a passive transfer model of antibody-mediated symptoms included the assessment of spatial memory (water maze test) in mice and rats (196, 341), and the susceptibility to develop seizures in mice (340). Results of these studies also support the pathogenicity of the antibodies even though some investigations were not included, such as the evaluation for the presence of brain-bound human NMDA receptor specific antibodies (340, 341), the demonstration that the antibodies caused a specific effect on the density of NMDA receptors (196, 340, 341), or the assessment of recovery (340). In one of these studies, bilateral injection of patients' CSF into the dentate gyrus of rats associated with impairment of spatial memory along with a reduction of NMDA receptor-evoked excitatory postsynaptic potentials and LTP (341). The effects were attributed to the antibodies, but the alterations did not reverse, and similar effects were obtained after the injection of a commercial GluN1 antibody against an intracellular epitope of the receptor (the binding of which requires permeabilization of the cell), suggesting that antibody-independent mechanisms may have contributed to the findings. The other study aimed to develop a model of NMDA receptor antibody-induced seizures after a single cerebroventricular injection of patients' or control IgG to mice (340). No spontaneous seizures were observed, although animals injected with patients' IgG showed a diminished threshold for pentylenetetrazol-induced seizures. The alteration of synaptic NMDA receptors (e.g., density of clusters or synaptic currents) by patients' antibodies was not investigated leaving it unclear whether the seizure propensity was specifically related to the dysfunction of the NMDA receptors.
Overall, taking into account the clinical experience with this disorder and the extensive data generated from modeling the effects of antibodies in vitro and in vivo, there is compelling evidence that anti-NMDA receptor encephalitis is an antibody-mediated disease. A summary of the antibody effects is shown (Figure 21). Moreover, the identification that ephrin-B2 ligand antagonizes the pathogenic effects of the antibodies provides a potential strategy beyond immunotherapy on how to treat this disease (e.g., small molecule ephrin-B2 ligand-like agonists able to cross the BBB). Such an approach may shorten the duration of symptoms by antagonizing the antibody effects while immunotherapy would eliminate the antibodies or antibody-producing plasma cells. Additional tasks for the future include obtaining a better understanding of the triggers of the immune response, genetic susceptibility, the relative contribution of systemic versus CNS synthesized antibodies to patients' symptoms, and the participation of T-cell mechanisms and other mediators of inflammation (cytokines) in the disease.
FIGURE 21.
Proposed model of antibody-mediated disruption of NMDA receptors leading to neuropsychiatric disease. Specific binding of patients' antibodies to the GluN1 subunit of the NMDA receptor alters the normal interaction between NMDA receptor and EphB2 displacing them from synaptic to extrasynaptic sites before the NMDA receptors are internalized (A). Inset in A shows the normal cross-talk between EphB2 and the NMDA receptor at the extracellular and intracellular levels (the latter via kinase signaling; phosphorylation sites in both receptors shown with green circles). The internalization of NMDA receptors caused by patients' antibodies leads to reduced NMDA receptor-mediated synaptic currents (144, 234), impaired long-term potentiation (257, 348), and the syndrome characterized by encephalopathy (usually with EEG alterations) (285), memory deficits, and other neuropsychiatric manifestations (58).
VI. IMPLICATIONS, CONCLUDING REMARKS, AND FUTURE DIRECTIONS
The discovery that autoantibodies highly specific for synaptic receptors and other cell surface proteins impair memory, behavior, and cognition and may result in psychosis, seizures, and other neurological deficits that are reversible has changed the landscape and diagnostic approach to many neuropsychiatric disorders. New syndromes and others that were previously defined with descriptive terms, and not suspected to be autoimmune, have been revealed as AE. Examples include subgroups of patients with “encephalitis lethargica” (54, 97), “choreo-athetosis post-HSE” (308), rapidly progressive dementia (11), or “post-partum psychosis” (25) that are now classified as AE. In other instances, the detection of a novel autoantibody, such as anti-IgLON5, has defined a disease in which the underlying mechanisms are at the cross-roads of autoimmunity and neurodegeneration, leading to a new form of tauopathy (273).
The autoantibodies associated with AE are unique in many respects; they show unambiguous and intense reactivity with brain tissue and the neuronal cell surface, which makes them easy to detect with appropriate techniques. To date, the pathogenic effects of most antibodies have been demonstrated in cultured neurons, and for three (mGluR1, amphiphysin, and the GluN1 subunit of the NMDA receptor) (103, 258, 293, 297) have been shown in animal models. Combining available data, these three antibodies and their associated syndromes fulfil the Koch-Witebsky postulates of antibody-mediated disease (269). Therefore, these diseases are naturally occurring human models of immune-mediated dysfunction of neuronal cell surface proteins or synaptic receptors with syndromes that often resemble the clinical phenotypes of pharmacological or genetic alterations of the same proteins.
Concepts applicable to antigen accessibility by patients' antibodies in systemic disorders such as myasthenia gravis are inadequate for most types of AE, where the antibodies are also synthesized in the CNS. Although these disorders are eminently treatable (except for IgLON5-related tauopathy), the response to antibody-removing strategies such as plasma exchange or IVIg is not as fast and predictable as in the systemic disorders. Most treatments currently used as first line therapies (e.g., plasma exchange or IVIg) do not eliminate the antibodies produced in the CNS (96). On the other hand, second-line immunotherapies are also suboptimal; for example, rituximab targets CD20 expressed on B cells and therefore eliminates these cells and prevents their development into plasma cells. However, the already present mature plasma cells in the CNS of the patients do not express CD20, making them resistant to rituximab (30, 215). These cells are further protected by the BBB which limits the penetration of other second-line therapies such as cyclophosphamide.
These considerations and the young stage of the field of AE leave many tasks for the future. From the clinical side, better syndromic characterization of the known disorders and of others as they are discovered, and a systematic investigation of the triggers and genetic predisposition of AE are needed. Multicenter clinical trials will help to determine the best therapies for each disorder and whether chronic prophylactic immunotherapy to prevent relapses is needed.
From the immunologic side, the contribution of the T-cell arm of immunity, currently unexplored for all disorders, needs to be investigated. The exact mechanisms by which synaptic proteins and receptors largely confined to the CNS become targets of autoimmunity and how the autoimmune response reaches the CNS are major challenges to better understand AE and other autoimmune disorders such aquaporin or myelin-associated glycoprotein (MOG) antibody associated demyelinating syndromes (not considered in this review).
Finally, one envisions for AE that a better knowledge of the physiopathological underpinnings will lead to development of treatments that antagonize the antibody effects. The paradigm is similar to that of the antibody-mediated disorders of the neuromuscular junction, such as myasthenia gravis or the LEMS, in which the discovery of the underlying mechanisms led to the development of drugs (e.g., anticholinesterases, 3,4-diaminopyridine) (241) that ameliorate symptoms while patients are also treated with immunotherapy.
GRANTS
This study was supported in part by National Institutes of Health Grant RO1NS077851, Instituto Carlos III Fondo de Investigaciones Sanitarias/FEDER FIS 14/00203 and CIBERER, and Fundació CELLEX (to J. Dalmau); German Research Council, DFG, GE 2519/3-1 and CRC-TR 166/1 B2 (to C. Geis); and Instituto Carlos III Fondo de Investigaciones Sanitarias/FEDER FIS 15/00377 and Fundació la Marató TV3 2014-1830 (to F. Graus).
DISCLOSURES
J. Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test; royalties from Euroimmun for the use of NMDA receptor and GABAB receptor as autoantibody tests; a licensing fee from Euroimmun for the use of DPPX, GABAA receptor, and IgLON5 antibodies as diagnostic tests; and has received an unrestricted research grant from Euroimmun. F. Graus received a licensing fee from Euroimmun for the use of IgLON5 as a diagnostic test. C. Geis has no disclosures, financial or otherwise.
acknowledgments
We are grateful for the critical review provided by Dr. Myrna R. Rosenfeld and for the assistance of Dr. Jesus Planagumà and Esther Aguilar in developing some of the figures.
C. Geis and F. Graus contributed equally.
Address for reprint requests and other correspondence: J. Dalmau, IDIBAPS-Hospital Clínic, Universitat de Barcelona, Department of Neurology, c/Villarroel 170, 08036 Barcelona, Spain (e-mail: jdalmau@clinic.cat).
REFERENCES
- 1.Abou-Zeid E, Boursoulian LJ, Metzer WS, Gundogdu B. Morvan syndrome: a case report and review of the literature. J Clin Neuromuscul Dis 13: 214–227, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9: 387, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Adams C, Diadori P, Schoenroth L, Fritzler M. Autoantibodies in childhood post-varicella acute cerebellar ataxia. Can J Neurol Sci 27: 316–320, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Adang LA, Lynch DR, Panzer JA. Pediatric anti-NMDA receptor encephalitis is seasonal. Ann Clin Transl Neurol 1: 921–925, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alamowitch S, Graus F, Uchuya M, Rene R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain 120: 923–928, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med 11: 1321–1324, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, Galetta SL, Dichter M, Alavi A, Rosenfeld MR, Dalmau J. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 128: 1764–1777, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT, Xu K, Wang F, Krystal JH. N-methyl-d-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77: 569–580, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Aoto J, Foldy C, Ilcus SM, Tabuchi K, Sudhof TC. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci 18: 997–1007, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 154: 75–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arino H, Armangue T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, Lancaster E, Saiz A, Dalmau J, Graus F. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology 87: 759–765, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arino H, Gresa-Arribas N, Blanco Y, Martinez-Hernandez E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau JO, Saiz A, Graus F. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol 71: 1009–1016, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arino H, Hoftberger R, Gresa-Arribas N, Martinez-Hernandez E, Armangue T, Kruer MC, Arpa J, Domingo J, Rojc B, Bataller L, Saiz A, Dalmau J, Graus F. Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol 72: 874–881, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 58: 1130–1135, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Armangue T, Leypoldt F, Malaga I, Raspall-Chaure M, Marti I, Nichter C, Pugh J, Vicente-Rasoamalala M, Lafuente-Hidalgo M, Macaya A, Ke M, Titulaer MJ, Hoftberger R, Sheriff H, Glaser C, Dalmau J. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 75: 317–323, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armangue T, Moris G, Cantarin-Extremera V, Conde CE, Rostasy K, Erro ME, Portilla-Cuenca JC, Turon-Vinas E, Malaga I, Munoz-Cabello B, Torres-Torres C, Llufriu S, Gonzalez-Gutierrez-Solana L, Gonzalez G, Casado-Naranjo I, Rosenfeld M, Graus F, Dalmau J. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 85: 1736–1743, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armangue T, Santamaria J, Dalmau J. When a serum test overrides the clinical assessment. Neurology 84: 1379–1381, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armangue T, Titulaer MJ, Malaga I, Bataller L, Gabilondo I, Graus F, Dalmau J. Pediatric anti-N-methyl-d-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 162: 850–856, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baizabal-Carvallo JF, Stocco A, Muscal E, Jankovic J. The spectrum of movement disorders in children with anti-NMDA receptor encephalitis. Mov Disord 28: 543–547, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Balas A, Garcia-Sanchez F, Vicario JL. Allelic and haplotypic HLA frequency distribution in Spanish hematopoietic patients. Implications for unrelated donor searching. Tissue Antigens 77: 45–53, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blocker IM, Bahtz R, Komorowski L, Stocker W, Kastrup A, Kuthe M, Meinck HM. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 82: 1521–1528, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Barker RA, Revesz T, Thom M, Marsden CD, Brown P. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 65: 633–640, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauerfeind R, Takei K, De Camili P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem 272: 30984–30992, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Bergink V, Armangue T, Titulaer MJ, Markx S, Dalmau J, Kushner SA. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry 172: 901–908, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal F, Graus F, Pifarre A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol 103: 509–515, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Bernal F, Shams'ili S, Rojas I, Sanchez-Valle R, Saiz A, Dalmau J, Honnorat J, Sillevis SP, Graus F. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin's disease. Neurology 60: 230–234, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84: 835–867, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, Kurthen M, Lassmann H, Mantegazza R, Villemure JG, Spreafico R, Elger CE. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128: 454–471, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Bien CG, Vincent A, Barnett MH, Becker AJ, Blumcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J, Sutton I, Lassmann H, Bauer J. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135: 1622–1638, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal DT, Salzman KL, Digre KB, Jensen RL, Dunson WA, Dalmau J. Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis. Neurology 67: 146–149, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Boettger MK, Weishaupt A, Geis C, Toyka KV, Sommer C. Mild experimental autoimmune encephalitis as a tool to induce blood-brain barrier dysfunction. J Neural Transm 117: 165–169, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Boronat A, Gelfand JM, Gresa-Arribas N, Jeong HY, Walsh M, Roberts K, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R, Graus F, Rudy B, Dalmau J. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 73: 120–128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bortolotto ZA, Collingridge GL. Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. Neuropharmacology 37: 535–544, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Bortolotto ZA, Collingridge GL. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. Eur J Neurosci 12: 4055–4062, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15: 453–462, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D. Behavioral, pharmacological, and immunological abnormalities after Streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology 37: 2076–2087, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown P, Marsden CD. The stiff man and stiff man plus syndromes. J Neurol 246: 648–652, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Buckley C, Oger J, Clover L, Tuzun E, Carpenter K, Jackson M, Vincent A. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 50: 73–78, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Busse S, Busse M, Brix B, Probst C, Genz A, Bogerts B, Stoecker W, Steiner J. Seroprevalence of N-methyl-d-aspartate glutamate receptor (NMDA-R) autoantibodies in aging subjects without neuropsychiatric disorders and in dementia patients. Eur Arch Psychiatry Clin Neurosci 264: 545–550, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Carr I. The Ophelia syndrome: memory loss in Hodgkin's disease. Lancet 1: 844–845, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Carvajal-Gonzalez A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 137: 2178–2192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellani S, Giannini AJ, Adams PM. Physostigmine and haloperidol treatment of acute phencyclidine intoxication. Am J Psychiatry 139: 508–510, 1982. [DOI] [PubMed] [Google Scholar]
- 44.Castillo-Gomez E, Kastner A, Steiner J, Schneider A, Hettling B, Poggi G, Ostehr K, Uhr M, Asif AR, Matzke M, Schmidt U, Pfander V, Hammer C, Schulz TF, Binder L, Stocker W, Weber F, Ehrenreich H. The brain as immunoprecipitator of serum autoantibodies against N-methyl-d-aspartate receptor subunit NR1. Ann Neurol 79: 144–151, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Castillo-Gomez E, Oliveira B, Tapken D, Bertrand S, Klein-Schmidt C, Pan H, Zafeiriou P, Steiner J, Jurek B, Trippe R, Pruss H, Zimmermann WH, Bertrand D, Ehrenreich H, Hollmann M. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry 2016, doi: 10.1038/mp.2016.125. [DOI] [PubMed] [Google Scholar]
- 46.Cedillo LN, Miranda F. Effects of co-administration of the GABAB receptor agonist baclofen and a positive allosteric modulator of the GABAB receptor, CGP7930, on the development and expression of amphetamine-induced locomotor sensitization in rats. Pharmacol Rep 65: 1132–1143, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, van Alphen AM, Luo C, van der Geest JN, Kros JM, Gaillard CA, Frens MA, de Zeeuw CI. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol 53: 325–336, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Corsellis JA, Goldberg GJ, Norton AR. “Limbic encephalitis” and its association with carcinoma. Brain 91: 481–496, 1968. [DOI] [PubMed] [Google Scholar]
- 49.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004: re16, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Chapman MR, Vause HE. Anti-NMDA receptor encephalitis: diagnosis, psychiatric presentation, and treatment. Am J Psychiatry 168: 245–251, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, Goureau O, Watanabe K, Goutebroze L, Gaspar P, Zalc B, Karagogeos D, Thomas JL. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Neurosci 28: 7624–7636, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng MH, Fan U, Grewal N, Barnes M, Mehta A, Taylor S, Husebye ES, Murphy EJ, Anderson MS. Acquired autoimmune polyglandular syndrome, thymoma, and an AIRE defect. N Engl J Med 362: 764–766, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S, Hammer C, Borowski K, Begemann M, Lemke S, Rentzsch K, Probst C, Martens H, Wienands J, Spalletta G, Weissenborn K, Stocker W, Ehrenreich H. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol 76: 82–94, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Dale RC, Irani SR, Brilot F, Pillai S, Webster R, Gill D, Lang B, Vincent A. N-methyl-d-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Ann Neurol 66: 704–709, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain 135: 3453–3468, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Dalmau J. NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture. Neurology 87: 2471–2482, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7: 1091–1098, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10: 63–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 7: 327–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61: 25–36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103: 945–956, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 349: 1543–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 63.David C, McPherson PS, Mundigl O, De Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA 93: 331–335, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David C, Solimena M, De Camilli P. Autoimmunity in Stiff-Man syndrome with breast cancer is targeted to the C-terminal region of human amphiphysin, a protein similar to the yeast proteins, Rvs167 and Rvs161. FEBS Lett 351: 73–79, 1994. [DOI] [PubMed] [Google Scholar]
- 65.De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, Meinck HM, Austoni M, Fassetta G, Bottazzo G, Bates D, Cartlidge N, Solimena M, Kilimann MW. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med 178: 2219–2223, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Graaff E, Maat P, Hulsenboom E, van den Berg R, van den Bent M, Demmers J, Lugtenburg PJ, Hoogenraad CC, Sillevis SP. Identification of delta/notch-like epidermal growth factor-related receptor as the Tr antigen in paraneoplastic cerebellar degeneration. Ann Neurol 71: 815–824, 2012. [DOI] [PubMed] [Google Scholar]
- 67.De Tiege X, Rozenberg F, Des Portes V, Lobut JB, Lebon P, Ponsot G, Heron B. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology 61: 241–243, 2003. [DOI] [PubMed] [Google Scholar]
- 68.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med 7: 1189–1193, 2001. [DOI] [PubMed] [Google Scholar]
- 69.DeLuca I, Blachere NE, Santomasso B, Darnell RB. Tolerance to the neuron-specific paraneoplastic HuD antigen. PLoS One 4: e5739, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Paolo G, Sankaranarayanan S, Wenk MR, Daniell L, Perucco E, Caldarone BJ, Flavell R, Picciotto MR, Ryan TA, Cremona O, De Camili P. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron 33: 789–804, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999. [PubMed] [Google Scholar]
- 72.Doss S, Wandinger KP, Hyman BT, Panzer JA, Synofzik M, Dickerson B, Mollenhauer B, Scherzer CR, Ivinson AJ, Finke C, Schols L, Muller Vom HJ, Trenkwalder C, Jahn H, Holtje M, Biswal BB, Harms L, Ruprecht K, Buchert R, Hoglinger GU, Oertel WH, Unger MM, Kortvelyessy P, Bittner D, Priller J, Spruth EJ, Paul F, Meisel A, Lynch DR, Dirnagl U, Endres M, Teegen B, Probst C, Komorowski L, Stocker W, Dalmau J, Pruss H. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann Clin Transl Neurol 1: 822–832, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drachman DB. Myasthenia gravis. N Engl J Med 330: 1797–1810, 1994. [DOI] [PubMed] [Google Scholar]
- 74.Drachman DB, Adams RN, Josifek LF, Self SG. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med 307: 769–775, 1982. [DOI] [PubMed] [Google Scholar]
- 75.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298: 1116–1122, 1978. [DOI] [PubMed] [Google Scholar]
- 76.Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol 159: 147–166, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eccles JC, Schmidt RF, Willis WD. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol 161: 282–297, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci 8: 873–880, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Evergren E, Marcucci M, Tomilin N, Low P, Slepnev V, Andersson F, Gad H, Brodin L, PDC, Shupliakov O. Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse. Traffic 5: 514–528, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Evergren E, Zotova E, Brodin L, Shupliakov O. Differential efficiency of the endocytic machinery in tonic and phasic synapses. Neuroscience 141: 123–131, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Faas GC, Adwanikar H, Gereau RW, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: a differential role in acute depression of synaptic transmission and long-term depression. J Neurosci 22: 6885–6890, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282: 1321–1324, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH Jr, Skinner C, Schwartz CE, Sommer SS. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neurosci Lett 409: 10–13, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O'Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camili P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science 316: 570–574, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Ferraro TN, Golden GT, Smith GG, DeMuth D, Buono RJ, Berrettini WH. Mouse strain variation in maximal electroshock seizure threshold. Brain Res 936: 82–86, 2002. [DOI] [PubMed] [Google Scholar]
- 86.Finke C, Kopp UA, Scheel M, Pech LM, Soemmer C, Schlichting J, Leypoldt F, Brandt AU, Wuerfel J, Probst C, Ploner CJ, Pruss H, Paul F. Functional and structural brain changes in anti-N-methyl-d-aspartate receptor encephalitis. Ann Neurol 74: 284–296, 2013. [DOI] [PubMed] [Google Scholar]
- 87.Flanagan EP, Kotsenas AL, Britton JW, McKeon A, Watson RE, Klein CJ, Boeve BF, Lowe V, Ahlskog JE, Shin C, Boes CJ, Crum BA, Laughlin RS, Pittock SJ. Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol Neuroimmunol Neuroinflamm 2: e161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Floeter MK, Valls-Sole J, Toro C, Jacobowitz D, Hallett M. Physiologic studies of spinal inhibitory circuits in patients with stiff-person syndrome. Neurology 51: 85–93, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 66: 11–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 13: 325–338, 1994. [DOI] [PubMed] [Google Scholar]
- 91.Fragoso-Loyo H, Cabiedes J, Orozco-Narvaez A, Davila-Maldonado L, Atisha-Fregoso Y, Diamond B, Llorente L, Sanchez-Guerrero J. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS ONE 3: e3347, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukata Y, Adesnik H, Iwanaga T, Bredt DS, Nicoll RA, Fukata M. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science 313: 1792–1795, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, Shigemoto R, Nicoll RA, Fukata M. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA 107: 3799–3804, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukata Y, Yokoi N, Miyazaki Y, Fukata M. The LGI1-ADAM22 protein complex in synaptic transmission and synaptic disorders. Neurosci Res pii: S0168-0102(16)30173-0, 2016, doi: 10.1016/j.neures.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 95.Fukuda T, Motomura M, Nakao Y, Shiraishi H, Yoshimura T, Iwanaga K, Tsujihata M, Eguchi K. Reduction of P/Q-type calcium channels in the postmortem cerebellum of paraneoplastic cerebellar degeneration with Lambert-Eaton myasthenic syndrome. Ann Neurol 53: 21–28, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Furneaux HF, Reich L, Posner JB. Autoantibody synthesis in the central nervous system of patients with paraneoplastic syndromes. Neurology 40: 1085–1091, 1990. [DOI] [PubMed] [Google Scholar]
- 97.Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, Collins A, Dengel A, Dalmau J, Glaser CA. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis 28: 1421–1429, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gastaldi M, Thouin A, Franciotta D, Vincent A. Pitfalls in the detection of N-methyl-d-aspartate-receptor (NMDA-R) antibodies. Clin Biochem pii: S0009-9120(16)30589-6, 2016. doi: 10.1016/j.clinbiochem.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 99.Gauthier J, Siddiqui TJ, Huashan P, Yokomaku D, Hamdan FF, Champagne N, Lapointe M, Spiegelman D, Noreau A, Lafreniere RG, Fathalli F, Joober R, Krebs MO, DeLisi LE, Mottron L, Fombonne E, Michaud JL, Drapeau P, Carbonetto S, Craig AM, Rouleau GA. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum Genet 130: 563–573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28: 927–939, 2000. [DOI] [PubMed] [Google Scholar]
- 101.Geis C, Grunewald B, Weishaupt A, Wultsch T, Toyka KV, Reif A, Sommer C. Human IgG directed against amphiphysin induces anxiety behavior in a rat model after intrathecal passive transfer. J Neural Transm 119: 981–985, 2012. [DOI] [PubMed] [Google Scholar]
- 102.Geis C, Weishaupt A, Grunewald B, Wultsch T, Reif A, Gerlach M, Dirkx R, Solimena M, Perani D, Heckmann M, Toyka KV, Folli F, Sommer C. Human stiff-person syndrome IgG induces anxious behavior in rats. PLoS One 6: e16775, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geis C, Weishaupt A, Hallermann S, Grunewald B, Wessig C, Wultsch T, Reif A, Byts N, Beck M, Jablonka S, Boettger MK, Uceyler N, Fouquet W, Gerlach M, Meinck HM, Siren AL, Sigrist SJ, Toyka KV, Heckmann M, Sommer C. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain 133: 3166–3180, 2010. [DOI] [PubMed] [Google Scholar]
- 104.Gelpi E, Hoftberger R, Graus F, Ling H, Holton JL, Dawson T, Popovic M, Pretnar-Oblak J, Hogl B, Schmutzhard E, Poewe W, Ricken G, Santamaria J, Dalmau J, Budka H, Revesz T, Kovacs GG. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol 132: 531–543, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gleichman AJ, Panzer JA, Baumann BH, Dalmau J, Lynch DR. Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis. Ann Clin Transl Neurol 1: 180–189, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci 32: 11082–11094, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goebels NMM, Barman S, Melzer N, Elger C, Wiendl H, Hartung HP. Identification of autoantibody producing plasma cells in the CSF of autoimmune encephalitis (AIE) patients. Neurology 78: P6–132., 2016. [Google Scholar]
- 108.Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camili P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem 272: 13419–13425, 1997. [DOI] [PubMed] [Google Scholar]
- 109.Graus F, Dalmau J. Neuronal antibodies in Creutzfeldt-Jakob disease–reply. JAMA Neurol 71: 514–515, 2014. [DOI] [PubMed] [Google Scholar]
- 110.Graus F, Dalmau J, Valldeoriola F, Ferrer I, Rene R, Marin C, Vecht CJ, Arbizu T, Targa C, Moll JW. Immunological characterization of a neuronal antibody (anti-Tr) associated with paraneoplastic cerebellar degeneration and Hodgkin's disease. J Neuroimmunol 74: 55–61, 1997. [DOI] [PubMed] [Google Scholar]
- 111.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler C, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75: 1135–1140, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Graus F, Gorman MP. Voltage-gated potassium channel antibodies: game over. Neurology 86: 1657–1658, 2016. [DOI] [PubMed] [Google Scholar]
- 113.Graus F, Illa I, Agusti M, Ribalta T, Cruz-Sanchez F, CJ. Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J Neurol Sci 106: 82–87, 1991. [DOI] [PubMed] [Google Scholar]
- 114.Graus F, Lang B, Pozo-Rosich P, Saiz A, Casamitjana R, Vincent A. P/Q type calcium-channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology 59: 764–766, 2002. [DOI] [PubMed] [Google Scholar]
- 115.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Hoftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Pruss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostasy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15: 391–404, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Graus F, Vega F, Delattre JY, Bonaventura I, Rene R, Arbaiza D, Tolosa E. Plasmapheresis and antineoplastic treatment in CNS paraneoplastic syndromes with antineuronal autoantibodies. Neurology 42: 536–540, 1992. [DOI] [PubMed] [Google Scholar]
- 117.Greene M, Lai Y, Baella N, Dalmau J, Lancaster E. Antibodies to Delta/notch-like epidermal growth factor-related receptor in patients with anti-Tr, paraneoplastic cerebellar degeneration, and Hodgkin lymphoma. JAMA Neurol 71: 1003–1008, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gresa-Arribas N, Arino H, Martinez-Hernandez E, Petit-Pedrol M, Sabater L, Saiz A, Dalmau J, Graus F. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One 10: e0121364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gresa-Arribas N, Planaguma J, Petit-Pedrol M, Kawachi I, katada S, Glaser CA, Simabukuro MM, Armangue T, Martinez-Hernandez E, Graus F, Dalmau J. Human neurexin-3alpha antibodies associate with encephalitis and alter synapse development. Neurology 86: 2235–2242, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 13: 167–177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grimwood J, Gordon LA, Olsen A, Terry A, Schmutz J, Lamerdin J, Hellsten U, Goodstein D, Couronne O, Tran-Gyamfi M, Aerts A, Altherr M, Ashworth L, Bajorek E, Black S, Branscomb E, Caenepeel S, Carrano A, Caoile C, Chan YM, Christensen M, Cleland CA, Copeland A, Dalin E, Dehal P, Denys M, Detter JC, Escobar J, Flowers D, Fotopulos D, Garcia C, Georgescu AM, Glavina T, Gomez M, Gonzales E, Groza M, Hammon N, Hawkins T, Haydu L, Ho I, Huang W, Israni S, Jett J, Kadner K, Kimball H, Kobayashi A, Larionov V, Leem SH, Lopez F, Lou Y, Lowry S, Malfatti S, Martinez D, McCready P, Medina C, Morgan J, Nelson K, Nolan M, Ovcharenko I, Pitluck S, Pollard M, Popkie AP, Predki P, Quan G, Ramirez L, Rash S, Retterer J, Rodriguez A, Rogers S, Salamov A, Salazar A, She X, Smith D, Slezak T, Solovyev V, Thayer N, Tice H, Tsai M, Ustaszewska A, Vo N, Wagner M, Wheeler J, Wu K, Xie G, Yang J, Dubchak I, Furey TS, DeJong P, Dickson M, Gordon D, Eichler EE, Pennacchio LA, Richardson P, Stubbs L, Rokhsar DS, Myers RM, Rubin EM, Lucas SM. The DNA sequence and biology of human chromosome 19. Nature 428: 529–535, 2004. [DOI] [PubMed] [Google Scholar]
- 122.Grunewald B, Geis C. Measuring spinal presynaptic inhibition in mice by dorsal root potential recording in vivo. J Vis Exp 85: 51473, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP, Bonhoeffer T, Klein R. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 32: 1027–1040, 2001. [DOI] [PubMed] [Google Scholar]
- 124.Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem 286: 25835–25847, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, Menson E, Lin JP, Tardieu M, Vincent A, Lim MJ. N-methyl-d-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord 29: 90–96, 2014. [DOI] [PubMed] [Google Scholar]
- 126.Haggerty GC, Forney RB, Johnson JM. The effect of a single administration of phencyclidine on behavior in the rat over a 21-day period. Toxicol Appl Pharmacol 75: 444–453, 1984. [DOI] [PubMed] [Google Scholar]
- 127.Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Siren AL, Pardo LA, Sperling S, Mohd JS, Gurvich A, Jensen N, Ostmeier K, Luhder F, Probst C, Martens H, Gillis M, Saher G, Assogna F, Spalletta G, Stocker W, Schulz TF, Nave KA, Ehrenreich H. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry 19: 1143–1149, 2014. [DOI] [PubMed] [Google Scholar]
- 128.Hansen HC, Klingbeil C, Dalmau J, Li W, Weissbrich B, Wandinger KP. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 70: 117–119, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hansen N, Grunewald B, Weishaupt A, Colaco MN, Toyka KV, Sommer C, Geis C. Human Stiff person syndrome IgG-containing high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp Neurol 239: 202–209, 2013. [DOI] [PubMed] [Google Scholar]
- 130.Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum 54: 2515–2522, 2006. [DOI] [PubMed] [Google Scholar]
- 131.Hashimoto T, Yamada M, Maekawa S, Nakashima T, Miyata S. IgLON cell adhesion molecule Kilon is a crucial modulator for synapse number in hippocampal neurons. Brain Res 1224: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- 132.Hayashi M, Raimondi A, O'Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camili P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci USA 105: 2175–2180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heekin RD, Catalano MC, Frontera AT, Catalano G. Anti-NMDA receptor encephalitis in a patient with previous psychosis and neurological abnormalities: a diagnostic challenge. Case Rep Psychiatry 2015: 253891, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Henderson JT, Georgiou J, Jia Z, Robertson J, Elowe S, Roder JC, Pawson T. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron 32: 1041–1056, 2001. [DOI] [PubMed] [Google Scholar]
- 135.Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, Schneider T, Hescheler J, Kano M, Sakimura K, Watanabe M, Dieudonne S, Snutch TP. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci 29: 9668–9682, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hill KE, Clawson SA, Rose JW, Carlson NG, Greenlee JE. Cerebellar Purkinje cells incorporate immunoglobulins and immunotoxins in vitro: implications for human neurological disease and immunotherapeutics. J Neuroinflammation 6: 31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoftberger R, vS A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, Paredes M, Sabater L, Saiz A, Titulaer MJ, Graus F, Dalmau J. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology 84: 2403–2412, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, dF A, Saiz A, Dalmau J, Graus F. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 81: 1500–1506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hogl B, Heidbreder A, Santamaria J, Graus F, Poewe W. IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet 385: 1590, 2015. [DOI] [PubMed] [Google Scholar]
- 140.Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E. Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci 28: 14213–14222, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, Edwards RH. v-SNARE composition distinguishes synaptic vesicle pools. Neuron 71: 474–487, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA 103: 678–683, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron 25: 473–480, 2000. [DOI] [PubMed] [Google Scholar]
- 144.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 30: 5866–5875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huijbers MG, Querol LA, Niks EH, Plomp JJ, van der Maarel SM, Graus F, Dalmau J, Illa I, Verschuuren JJ. The expanding field of IgG4-mediated neurological autoimmune disorders. Eur J Neurol 22: 1151–1161, 2015. [DOI] [PubMed] [Google Scholar]
- 146.Husebye ES, Sthoeger ZM, Dayan M, Zinger H, Elbirt D, Levite M, Mozes E. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis 64: 1210–1213, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, Hager H, Yu P, Becker CM, Vincent A. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 71: 1291–1292, 2008. [DOI] [PubMed] [Google Scholar]
- 148.Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288: 1832–1835, 2000. [DOI] [PubMed] [Google Scholar]
- 149.Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I, Ito Y, Nakamura Y, Kishi T, Okumura T, Fukuo Y, Williams HJ, Hamshere ML, Ivanov D, Inada T, Suzuki M, Hashimoto R, Ujike H, Takeda M, Craddock N, Kaibuchi K, Owen MJ, Ozaki N, O'Donovan MC, Iwata N. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry 69: 472–478, 2011. [DOI] [PubMed] [Google Scholar]
- 150.Irani SR. “Moonlighting” surface antigens: a paradigm for autoantibody pathogenicity in neurology? Brain 139: 304–306, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 133: 2734–2748, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 133: 1655–1667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJ, Zagami S, Bleasel A, Somerville ER, Smith SM, Vincent A. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 69: 892–900, 2011. [DOI] [PubMed] [Google Scholar]
- 154.Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C, Vincent A. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol 72: 241–255, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A, Voets NL, Cardoso MJ, Cash DM, Manning EN, Lang B, Smith SJ, Vincent A, Johnson MR. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 136: 3151–3162, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Jain A, Lancaster E, Dalmau J, Balice-Gordon RJ. Autoantibodies in the CSF of anti-GABA receptor encephalitis patients block activation of GABA receptors in vitro. Ann Neurol 78: S77, 2015. [Google Scholar]
- 158.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology 81: 882–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Joubert B, Saint-Martin M, Noraz N, Picard G, Rogemond V, Ducray F, Desestret V, Psimaras D, Delattre JY, Antoine JC, Honnorat J. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 73: 1115–1124, 2016. [DOI] [PubMed] [Google Scholar]
- 160.Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli BF, Choi C, Morozov P, Das K, Teplitskaya E, Yu A, Cayanis E, Penchaszadeh G, Kottmann AH, Pedley TA, Hauser WA, Ottman R, Gilliam TC. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet 30: 335–341, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaphan E, Pellissier JF, Rey M, Robert D, Auphan M, Ali CA. Esophageal achalasia, sleep disorders and chorea in a tauopathy without ophthalmoplegia, parkinsonian syndrome, nor dementia (progressive supranuclear palsy?): clinicopathological study. Rev Neurol 164: 377–383, 2008. [DOI] [PubMed] [Google Scholar]
- 162.Karagogeos D. Neural GPI-anchored cell adhesion molecules. Front Biosci 8: s1304–s1320, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J 28: 3910–3920, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev 7: 189–193, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 70: 1133–1139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature 277: 93–96, 1979. [DOI] [PubMed] [Google Scholar]
- 167.Keime-Guibert F, Graus F, Broet P, Rene R, Molinuevo JL, Ascaso C, Delattre JY. Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology 53: 1719–1723, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Keime-Guibert F, Graus F, Fleury A, Rene R, Honnorat J, Broet P, Delattre JY. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (Anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry 68: 479–482, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol 100: 1835–1847, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 9: 914–920, 2003. [DOI] [PubMed] [Google Scholar]
- 171.Klar TA, Hell SW. Subdiffraction resolution in far-field fluorescence microscopy. Opt Lett 24: 954–956, 1999. [DOI] [PubMed] [Google Scholar]
- 172.Klein C, Brin MF, Kramer P, Sena-Esteves M, de Leon D, Doheny D, Bressman S, Fahn S, Breakefield XO, Ozelius LJ. Association of a missense change in the D2 dopamine receptor with myoclonus dystonia. Proc Natl Acad Sci USA 96: 5173–5176, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kreye J, Wenke NK, Chayka M, Leubner J, Murugan R, Maier N, Jurek B, Ly LT, Brandl D, Rost BR, Stumpf A, Schulz P, Radbruch H, Hauser AE, Pache F, Meisel A, Harms L, Paul F, Dirnagl U, Garner C, Schmitz D, Wardemann H, Pruss H. Human cerebrospinal fluid monoclonal N-methyl-d-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain 139: 2641–2652, 2016. [DOI] [PubMed] [Google Scholar]
- 174.Krueger DD, Tuffy LP, Papadopoulos T, Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr Opin Neurobiol 22: 412–422, 2012. [DOI] [PubMed] [Google Scholar]
- 175.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214, 1994. [DOI] [PubMed] [Google Scholar]
- 176.Kuhse J, Betz H, Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol 5: 318–323, 1995. [DOI] [PubMed] [Google Scholar]
- 177.Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron 11: 1049–1056, 1993. [DOI] [PubMed] [Google Scholar]
- 178.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol 3: 475–486, 2002. [DOI] [PubMed] [Google Scholar]
- 179.Kumar A, Dhull DK, Mishra PS. Therapeutic potential of mGluR5 targeting in Alzheimer's disease. Front Neurosci 9: 215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kwag J, Paulsen O. Gating of NMDA receptor-mediated hippocampal spike timing-dependent potentiation by mGluR5. Neuropharmacology 63: 701–709, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Mata S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 65: 424–434, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 9: 776–785, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lalic T, Pettingill P, Vincent A, Capogna M. Human limbic encephalitis serum enhances hippocampal mossy fiber-CA3 pyramidal cell synaptic transmission. Epilepsia 52: 121–131, 2011. [DOI] [PubMed] [Google Scholar]
- 184.Lancaster E, Dalmau J. Neuronal autoantigens-pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 8: 380–390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 69: 303–311, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 9: 67–76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, Liebers E, Kornblum C, Bien CG, Honnorat J, Wong S, Xu J, Contractor A, Balice-Gordon R, Dalmau J. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 77: 1698–1701, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck-Spencer T, Weickert T, Bleiberg J, Rosenstein D, Pao M, Patronas N, Steele S, Manzano M, van der Veen JW, Lipsky PE, Marenco S, Wesley R, Volpe B, Diamond B, Illei GG. Anti-N-methyl-d-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum 54: 2505–2514, 2006. [DOI] [PubMed] [Google Scholar]
- 189.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8: 413–426, 2007. [DOI] [PubMed] [Google Scholar]
- 190.Lawn ND, Westmoreland BF, Kiely MJ, Lennon VA, Vernino S. Clinical, magnetic resonance imaging, and electroencephalographic findings in paraneoplastic limbic encephalitis. Mayo Clin Proc 78: 1363–1368, 2003. [DOI] [PubMed] [Google Scholar]
- 191.Lejuste F, Thomas L, Picard G, Desestret V, Ducray F, Rogemond V, Psimaras D, Antoine JC, Delattre JY, Groc L, Leboyer M, Honnorat J. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm 3: e280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lennox BR, Palmer-Cooper EC, Pollak T, Hainsworth J, Marks J, Jacobson L, Lang B, Fox H, Ferry B, Scoriels L, Crowley H, Jones PB, Harrison PJ, Vincent A, PPiP Study Team. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry 4: 42–48, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci 24: 207–217, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem 280: 17312–17319, 2005. [DOI] [PubMed] [Google Scholar]
- 195.Leypoldt F, Hoftberger R, Titulaer MJ, Armangue T, Gresa-Arribas N, Jahn H, Rostasy K, Schlumberger W, Meyer T, Wandinger KP, Rosenfeld MR, Graus F, Dalmau J. Investigations on CXCL13 in anti-N-methyl-d-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol 72: 180–186, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Y, Tanaka K, Wang L, Ishigaki Y, Kato N. Induction of memory deficit in mice with chronic exposure to cerebrospinal fluid from patients with anti-N-methyl-d-aspartate receptor encephalitis. Tohoku J Exp Med 237: 329–338, 2015. [DOI] [PubMed] [Google Scholar]
- 197.Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc Natl Acad Sci USA 105: 2705–2710, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J 11: 2521–2530, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lidov HG, Duchen LW, Thomas PK, Thrush DC. Progressive medullary failure associated with neurofibrillary degeneration. J Neurol Neurosurg Psychiatry 52: 643–647, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, Baruzzi A, Carey T, Gambetti P, Lugaresi E, Montagna P. Morvan's syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 124: 2417–2426, 2001. [DOI] [PubMed] [Google Scholar]
- 201.Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol 5: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu Y, Hu Z, Xun G, Peng Y, Lu L, Xu X, Xiong Z, Xia L, Liu D, Li W, Zhao J, Xia K. Mutation analysis of the NRXN1 gene in a Chinese autism cohort. J Psychiatr Res 46: 630–634, 2012. [DOI] [PubMed] [Google Scholar]
- 203.Lopez-Chiriboga AS, Komorowski L, Kumpfel T, Probst C, Hinson SR, Pittock SJ, McKeon A. Metabotropic glutamate receptor type 1 autoimmunity: Clinical features and treatment outcomes. Neurology 86: 1009–1013, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lovero KL, Fukata Y, Granger AJ, Fukata M, Nicoll RA. The LGI1-ADAM22 protein complex directs synapse maturation through regulation of PSD-95 function. Proc Natl Acad Sci USA 112: E4129–4137, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254, 2001. [DOI] [PubMed] [Google Scholar]
- 207.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84: 1051–1095, 2004. [DOI] [PubMed] [Google Scholar]
- 208.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci 17: 569–602, 1994. [DOI] [PubMed] [Google Scholar]
- 209.Mangalwedhe SB, Pandurangi AA, Pandurangi AK, Dugani RI. Anti-N-methyl-d-aspartate receptor encephalitis presenting with psychiatric symptoms. J Neuropsychiatry Clin Neurosci 27: e152–e153, 2015. [DOI] [PubMed] [Google Scholar]
- 210.Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J Rare Dis 5: 31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Marignier R, Chenevier F, Rogemond V, Sillevis SP, Renoux C, Cavillon G, Androdias G, Vukusic S, Graus F, Honnorat J, Confavreux C. Metabotropic glutamate receptor type 1 autoantibody-associated cerebellitis: a primary autoimmune disease? Arch Neurol 67: 627–630, 2010. [DOI] [PubMed] [Google Scholar]
- 212.Martens H, Weston MC, Boulland JL, Gronborg M, Grosche J, Kacza J, Hoffmann A, Matteoli M, Takamori S, Harkany T, Chaudhry FA, Rosenmund C, Erck C, Jahn R, Hartig W. Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J Neurosci 28: 13125–13131, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Martin-Garcia E, Mannara F, Gutierrez-Cuesta J, Sabater L, Dalmau J, Maldonado R, Graus F. Intrathecal injection of P/Q type voltage-gated calcium channel antibodies from paraneoplastic cerebellar degeneration cause ataxia in mice. J Neuroimmunol 261: 53–59, 2013. [DOI] [PubMed] [Google Scholar]
- 214.Martinez-Hernandez E, Arino H, McKeon A, Iizuka T, Titulaer MJ, Simabukuro MM, Lancaster E, Petit-Pedrol M, Planaguma J, Blanco Y, Harvey RJ, Saiz A, Graus F, Dalmau J. Clinical and immunological investigations in patients with Stiff-person spectrum disorder. JAMA Neurol 73: 714–20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology 77: 589–593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Martinez-Hernandez E, Sepulveda M, Rostasy K, Hoftberger R, Graus F, Harvey RJ, Saiz A, Dalmau J. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor alpha1 subunit in patients with isolated optic neuritis. JAMA Neurol 72: 187–193, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mas N, Saiz A, Leite MI, Waters P, Baron M, Castano D, Sabater L, Vincent A, Graus F. Antiglycine-receptor encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 82: 1399–1401, 2011. [DOI] [PubMed] [Google Scholar]
- 218.Masdeu JC, Dalmau J, Berman KF. NMDA receptor internalization by autoantibodies: a reversible mechanism underlying psychosis? Trends Neurosci 2016, doi: 10.1016/j.tins.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mason WP, Graus F, Lang B, Honnorat J, Delattre JY, Valldeoriola F, Antoine JC, Rosenblum MK, Rosenfeld MR, Newsom-Davis J, Posner JB, Dalmau J. Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert-Eaton myasthenic syndrome. Brain 120: 1279–1300, 1997. [DOI] [PubMed] [Google Scholar]
- 220.Mat A, Adler H, Merwick A, Chadwick G, Gullo G, Dalmau JO, Tubridy N. Ophelia syndrome with metabotropic glutamate receptor 5 antibodies in CSF. Neurology 80: 1349–1350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.McClure SJ, Robinson PJ. Dynamin, endocytosis and intracellular signalling. Mol Membr Biol 13: 189–215, 1996. [DOI] [PubMed] [Google Scholar]
- 222.McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto JY, Harvey RJ, McEvoy KM, Pittock SJ, Lennon VA, Dalmau J. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol 70: 44–50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Meager A, Peterson P, Willcox N. Hypothetical review: thymic aberrations and type-I interferons; attempts to deduce autoimmunizing mechanisms from unexpected clues in monogenic and paraneoplastic syndromes. Clin Exp Immunol 154: 141–151, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J Biol Chem 272: 27239–27245, 1997. [DOI] [PubMed] [Google Scholar]
- 225.Mikasova L, PDR, Bouchet D, Georges F, Rogemond V, Didelot A, Meissirel C, Honnorat J, Groc L. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 135: 1606–1621, 2012. [DOI] [PubMed] [Google Scholar]
- 226.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camili P. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72: 587–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Moersch F, Woltman H. Progressive fluctuating muscular rigidity and spasm (“stiffman syndrome”): report of a case and some observations in 13 other cases. Mayo Clin Proc 31: 421–427, 1956. [PubMed] [Google Scholar]
- 228.Mohamed HA, Mosier DR, Zou LL, Siklos L, Alexianu ME, Engelhardt JI, Beers DR, Le WD, Appel SH. Immunoglobulin Fc gamma receptor promotes immunoglobulin uptake, immunoglobulin-mediated calcium increase, and neurotransmitter release in motor neurons. J Neurosci Res 69: 110–116, 2002. [DOI] [PubMed] [Google Scholar]
- 229.Mohammad SS, Sinclair K, Pillai S, Merheb V, Aumann TD, Gill D, Dale RC, Brilot F. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-d-aspartate receptor or dopamine-2 receptor. Mov Disord 29: 117–122, 2014. [DOI] [PubMed] [Google Scholar]
- 230.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98: 427–436, 1999. [DOI] [PubMed] [Google Scholar]
- 231.Molloy FM, Dalakas MC, Floeter MK. Increased brainstem excitability in stiff-person syndrome. Neurology 59: 449–451, 2002. [DOI] [PubMed] [Google Scholar]
- 232.Montagna P, Lugaresi E. Agrypnia Excitata: a generalized overactivity syndrome and a useful concept in the neurophysiopathology of sleep. Clin Neurophysiol 113: 552–560, 2002. [DOI] [PubMed] [Google Scholar]
- 233.Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, Saenz A, Poza JJ, Galan J, Gesk S, Sarafidou T, Mautner VF, Binelli S, Staub E, Hinzmann B, French L, Prud'homme JF, Passarelli D, Scannapieco P, Tassinari CA, Avanzini G, Marti-Masso JF, Kluwe L, Deloukas P, Moschonas NK, Michelucci R, Siebert R, Nobile C, Perez-Tur J, Lopez de Munain A. Mutations in the LGI1/Epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet 11: 1119–1128, 2002. [DOI] [PubMed] [Google Scholar]
- 234.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-d-aspartate receptor encephalitis. Ann Neurol 76: 108–119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Motomura M, Lang B, Johnston I, Palace J, Vincent A, Newsom-Davis J. Incidence of serum anti-P/O-type and anti-N-type calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. J Neurol Sci 147: 35–42, 1997. [DOI] [PubMed] [Google Scholar]
- 236.Murinson BB, Guarnaccia JB. Stiff-person syndrome with amphiphysin antibodies: distinctive features of a rare disease. Neurology 71: 1955–1958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nadal MS, Ozaita A, Amarillo Y, EVSd M, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37: 449–461, 2003. [DOI] [PubMed] [Google Scholar]
- 238.Nagel A, Engel AG, Lang B, Newsom-Davis J, Fukuoka T. Lambert-Eaton myasthenic syndrome IgG depletes presynaptic membrane active zone particles by antigenic modulation. Ann Neurol 24: 552–558, 1988. [DOI] [PubMed] [Google Scholar]
- 239.Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, Psimaras D, Habert MO, Baulac M, Delattre JY, Honnorat J, collaborators. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain 139: 1079–1093, 2016. [DOI] [PubMed] [Google Scholar]
- 240.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 25: 7724–7733, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Newsom-Davis J, Buckley C, Clover L, Hart I, Maddison P, Tuzum E, Vincent A. Autoimmune disorders of neuronal potassium channels. Ann NY Acad Sci 998: 202–210, 2003. [DOI] [PubMed] [Google Scholar]
- 242.Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60: 1017–1041, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nobile C, Michelucci R, Andreazza S, Pasini E, Tosatto SC, Striano P. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat 30: 530–536, 2009. [DOI] [PubMed] [Google Scholar]
- 244.Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 33: 18161–18174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ohtani Y, Miyata M, Hashimoto K, Tabata T, Kishimoto Y, Fukaya M, Kase D, Kassai H, Nakao K, Hirata T, Watanabe M, Kano M, Aiba A. The synaptic targeting of mGluR1 by its carboxyl-terminal domain is crucial for cerebellar function. J Neurosci 34: 2702–2712, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Olsen AL, Lai Y, Dalmau J, Scherer SS, Lancaster E. Caspr2 autoantibodies target multiple epitopes. Neurol Neuroimmunol Neuroinflamm 2: e127, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Owuor K, Harel NY, Englot DJ, Hisama F, Blumenfeld H, Strittmatter SM. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci 42: 448–457, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev 57: 253–277, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Palucha A, Branski P, Szewczyk B, Wieronska JM, Klak K, Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 81: 901–906, 2005. [DOI] [PubMed] [Google Scholar]
- 250.Pathmanandavel K, Starling J, Merheb V, Ramanathan S, Sinmaz N, Dale RC, Brilot F. Antibodies to surface dopamine-2 receptor and N-methyl-d-aspartate receptor in the first episode of acute psychosis in children. Biol Psychiatry 77: 537–547, 2015. [DOI] [PubMed] [Google Scholar]
- 251.Peng X, Hughes EG, Moscato EH, Parsons TD, Dalmau J, Balice-Gordon RJ. Cellular plasticity induced by anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor encephalitis antibodies. Ann Neurol 77: 381–398, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcala C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 13: 276–286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pettingill P, Kramer HB, Coebergh JA, Pettingill R, Maxwell S, Nibber A, Malaspina A, Jacob A, Irani SR, Buckley C, Beeson D, Lang B, Waters P, Vincent A. Antibodies to GABAA receptor alpha1 and gamma2 subunits: clinical and serologic characterization. Neurology 84: 1233–1241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Piepgras J, Holtje M, Michel K, Li Q, Otto C, Drenckhahn C, Probst C, Schemann M, Jarius S, Stocker W, Balint B, Meinck HM, Buchert R, Dalmau J, Ahnert-Hilger G, Ruprecht K. Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology 85: 890–897, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pinatel D, Hivert B, Boucraut J, Saint-Martin M, Rogemond V, Zoupi L, Karagogeos D, Honnorat J, Faivre-Sarrailh C. Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Front Cell Neurosci 9: 265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, Kim KK, Kilimann MW, Lennon VA. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol 58: 96–107, 2005. [DOI] [PubMed] [Google Scholar]
- 257.Planaguma J, Haselmann H, Mannara F, Petit-Pedrol M, Grunewald B, Aguilar E, Ropke L, Martin-Garcia E, Titulaer MJ, Jercog P, Graus F, Maldonado R, Geis C, Dalmau J. Ephrin-B2 prevents N-methyl-d-aspartate receptor antibody effects on memory and neuroplasticity. Ann Neurol 80: 388–400, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Planaguma J, Leypoldt F, Mannara F, Gutierrez-Cuesta J, Martin-Garcia E, Aguilar E, Titulaer MJ, Petit-Pedrol M, Jain A, Balice-Gordon R, Lakadamyali M, Graus F, Maldonado R, Dalmau J. Human N-methyl d-aspartate receptor antibodies alter memory and behaviour in mice. Brain 138: 94–109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pretnar-Oblak J, Zaletel M, Hajnsek TM, Meglic B, Hocevar-Boltezar I, Popovic M. Isolated bulbar paralysis in a patient with medullar tau pathology: a case report. J Neurol Neurosurg Psychiatry 81: 847–849, 2010. [DOI] [PubMed] [Google Scholar]
- 260.Provini F, Cortelli P, Montagna P, Gambetti P, Lugaresi E. Fatal insomnia and agrypnia excitata: sleep and the limbic system. Rev Neurol 164: 692–700, 2008. [DOI] [PubMed] [Google Scholar]
- 261.Provini F, Marconi S, Amadori M, Guaraldi P, Pierangeli G, Cortelli P, Lugaresi E, Montagna P, Tinuper P. Morvan chorea and agrypnia excitata: when video-polysomnographic recording guides the diagnosis. Sleep Med 12: 1041–1043, 2011. [DOI] [PubMed] [Google Scholar]
- 262.Pruss H, Holtje M, Maier N, Gomez A, Buchert R, Harms L, Ahnert-Hilger G, Schmitz D, Terborg C, Kopp U, Klingbeil C, Probst C, Kohler S, Schwab JM, Stoecker W, Dalmau J, Wandinger KP. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology 78: 1743–1753, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rajendra S, Lynch JW, Schofield PR. The glycine receptor. Pharmacol Ther 73: 121–146, 1997. [DOI] [PubMed] [Google Scholar]
- 264.Ramjaun AR, Micheva KD, Bouchelet I, McPherson PS. Identification and characterization of a nerve terminal-enriched amphiphysin isoform. J Biol Chem 272: 16700–16706, 1997. [DOI] [PubMed] [Google Scholar]
- 265.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12: 623–635, 2012. [DOI] [PubMed] [Google Scholar]
- 266.Reilmann R, Rouzade-Dominguez ML, Saft C, Sussmuth SD, Priller J, Rosser A, Rickards H, Schols L, Pezous N, Gasparini F, Johns D, Landwehrmeyer GB, Gomez-Mancilla B. A randomized, placebo-controlled trial of AFQ056 for the treatment of chorea in Huntington's disease. Mov Disord 30: 427–431, 2015. [DOI] [PubMed] [Google Scholar]
- 267.Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, Crain B, Hughes TE, Heinemann SF, McNamara JO. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science 265: 648–651, 1994. [DOI] [PubMed] [Google Scholar]
- 268.Rojas I, Graus F, Keime-Guibert F, Rene R, Delattre JY, Ramon JM, Dalmau J, Posner JB. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology 55: 713–715, 2000. [DOI] [PubMed] [Google Scholar]
- 269.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today 14: 426–430, 1993. [DOI] [PubMed] [Google Scholar]
- 270.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10: 685–697, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. [DOI] [PubMed] [Google Scholar]
- 272.Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI, Hardarsson G, Jonsdottir GA, Gustafsson O, Fossdal R, Giegling I, Moller HJ, Hartmann AM, Hoffmann P, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Djurovic S, Melle I, Andreassen OA, Hansen T, Werge T, Kiemeney LA, Franke B, Veltman J, Buizer-Voskamp JE, Sabatti C, Ophoff RA, Rietschel M, Nothen MM, Stefansson K, Peltonen L, St Clair D, Stefansson H, Collier DA. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet 18: 988–996, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, Contreras A, Giometto B, Compta Y, Embid C, Vilaseca I, Iranzo A, Santamaria J, Dalmau J, Graus F. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 13: 575–586, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sabater L, Planaguma J, Dalmau J, Graus F. Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome. J Neuroinflammation 13: 226, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sagane K, Hayakawa K, Kai J, Hirohashi T, Takahashi E, Miyamoto N, Ino M, Oki T, Yamazaki K, Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci 6: 33, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Saito SY, Takeshima H. DNER as key molecule for cerebellar maturation. Cerebellum 5: 227–231, 2006. [DOI] [PubMed] [Google Scholar]
- 277.Saiz A, Blanco Y, Sabater L, Gonzalez F, Bataller L, Casamitjana R, Ramio-Torrenta L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 131: 2553–2563, 2008. [DOI] [PubMed] [Google Scholar]
- 278.Saiz A, Dalmau J, Butler MH, Chen Q, Delattre JY, De Camilli P, Graus F. Anti-amphiphysin I antibodies in patients with paraneoplastic neurological disorders associated with small cell lung carcinoma. J Neurol Neurosurg Psychiatry 66: 214–217, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sandbrink F, Syed NA, Fujii MD, Dalakas MC, Floeter MK. Motor cortex excitability in stiff-person syndrome. Brain 123: 2231–2239, 2000. [DOI] [PubMed] [Google Scholar]
- 280.Sansing LH, Tuzun E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol 3: 291–296, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neuropsychiatry Clin Neurosci 12: 103–113, 2000. [DOI] [PubMed] [Google Scholar]
- 282.Schabitz WR, Rogalewski A, Hagemeister C, Bien CG. VZV brainstem encephalitis triggers NMDA receptor immunoreaction. Neurology 83: 2309–2311, 2014. [DOI] [PubMed] [Google Scholar]
- 283.Scharf SH, Jaeschke G, Wettstein JG, Lindemann L. Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr Opin Pharmacol 20: 124–134, 2015. [DOI] [PubMed] [Google Scholar]
- 284.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657–669, 2000. [DOI] [PubMed] [Google Scholar]
- 285.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 79: 1094–1100, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schulte U, Thumfart JO, Klocker N, Sailer CA, Bildl W, Biniossek M, Dehn D, Deller T, Eble S, Abbass K, Wangler T, Knaus HG, Fakler B. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron 49: 697–706, 2006. [DOI] [PubMed] [Google Scholar]
- 287.Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol 8: 199–202, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23: 613–643, 2007. [DOI] [PubMed] [Google Scholar]
- 289.Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ, Plomp JJ, van Kempen GT, Chauplannaz G, Wintzen AR, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol 38: 714–722, 1995. [DOI] [PubMed] [Google Scholar]
- 290.Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, PDC, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276: 259–263, 1997. [DOI] [PubMed] [Google Scholar]
- 291.Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci 30: 7495–7506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem 287: 40224–40231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sillevis SP, Kinoshita A, De LB, Moll W, Coesmans M, Jaarsma D, Henzen-Logmans S, Vecht C, De ZC, Sekiyama N, Nakanishi S, Shigemoto R. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med 342: 21–27, 2000. [DOI] [PubMed] [Google Scholar]
- 294.Simabukuro MM, Petit-Pedrol M, Castro LH, Nitrini R, Lucato L, Zambon AA, Silva LG, Fortes GC, Soares Neto HR, Dalmau JO. GABAA receptor and LGI1 antibody encephalitis in a patient with thymoma. Neurol Neuroimmunol Neuroinflamm 2: e73, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron 20: 809–819, 1998. [DOI] [PubMed] [Google Scholar]
- 296.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med 322: 1555–1560, 1990. [DOI] [PubMed] [Google Scholar]
- 297.Sommer C, Weishaupt A, Brinkhoff J, Biko L, Wessig C, Gold R, Toyka KV. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet 365: 1406–1411, 2005. [DOI] [PubMed] [Google Scholar]
- 298.Spatola M, Petit-Pedrol M, Simabukuro MM, Armangué T, Castro FJ, Barcelo-Artigues MI, Julià Benique MR, Benson L, Gorman M, Felipe A, Caparó-Oblitas R, Rosenfeld MR, Graus F, Dalmau J. Investigations in GABAa receptor antibody-associated encephalitis. Neurology 88: 1–9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sprengel R. Role of AMPA receptors in synaptic plasticity. Cell Tissue Res 326: 447–455, 2006. [DOI] [PubMed] [Google Scholar]
- 300.Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, Kastner A, Skalej M, Jordan W, Schiltz K, Klingbeil C, Wandinger KP, Bogerts B, Stoecker W. Increased prevalence of diverse N -methyl-d-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-d-aspartate glutamate receptor encephalitis. JAMA Psychiatry 1–8, 2013. [DOI] [PubMed] [Google Scholar]
- 301.Steup-Beekman G, Steens S, vB M, Huizinga T. Anti-NMDA receptor autoantibodies in patients with systemic lupus erythematosus and their first-degree relatives. Lupus 16: 329–334, 2007. [DOI] [PubMed] [Google Scholar]
- 302.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455: 903–911, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sun L, Li H, Luo H, Zhao Y. Thymic epithelial cell development and its dysfunction in human diseases. Biomed Res Int 2014: 206929, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Tanaka M, Tanaka K, Idezuka J, Tsuji S. Failure to detect cytotoxic T cell activity against recombinant Yo protein using autologous dendritic cells as the target in a patient with paraneoplastic cerebellar degeneration and anti-Yo antibody. Exp Neurol 150: 337–338, 1998. [DOI] [PubMed] [Google Scholar]
- 305.Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 28: 376–384, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tijssen MA, Shiang R, van DJ, Boerman RH, Wasmuth JJ, Sandkuijl LA, Frants RR, Padberg GW. Molecular genetic reevaluation of the Dutch hyperekplexia family. Arch Neurol 52: 578–582, 1995. [DOI] [PubMed] [Google Scholar]
- 307.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol 10: 1098–1107, 2011. [DOI] [PubMed] [Google Scholar]
- 308.Titulaer MJ, Leypoldt F, Dalmau J. Antibodies to N-methyl-d-aspartate and other synaptic receptors in choreoathetosis and relapsing symptoms post-herpes virus encephalitis. Mov Disord 29: 3–6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12: 157–165, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Titulaer MJ, Soffietti R, Dalmau J, Gilhus NE, Giometto B, Graus F, Grisold W, Honnorat J, Sillevis Smitt PA, Tanasescu R, Vedeler CA, Voltz R, Verschuuren JJ. Screening for tumours in paraneoplastic syndromes: report of an EFNS Task Force. Eur J Neurol 18: 19–e13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Aksamit AJ, Appendino JP, Lucchinetti CF, Matsumoto JY, Pittock SJ, Sandroni P, Tippmann-Peikert M, Wirrell EC, McKeon A. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 83: 1797–1803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tohgo A, Eiraku M, Miyazaki T, Miura E, Kawaguchi SY, Nishi M, Watanabe M, Hirano T, Kengaku M, Takeshima H. Impaired cerebellar functions in mutant mice lacking DNER. Mol Cell Neurosci 31: 326–333, 2006. [DOI] [PubMed] [Google Scholar]
- 313.Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol 162: 1161–1172, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Trotter JL, Hendin BA, Osterland K. Cerebellar degeneration with Hodgkin's disease. An immunological study. Arch Neurol 33: 660–661, 1976. [DOI] [PubMed] [Google Scholar]
- 315.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87: 1327–1338, 1996. [DOI] [PubMed] [Google Scholar]
- 316.Tuzun E, Dalmau J. Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist 13: 261–271, 2007. [DOI] [PubMed] [Google Scholar]
- 317.Tuzun E, Erdag E, Durmus H, Brenner T, Turkoglu R, Kurtuncu M, Lang B, Akman-Demir G, Eraksoy M, Vincent A. Autoantibodies to neuronal surface antigens in thyroid antibody-positive and -negative limbic encephalitis. Neurol India 59: 47–50, 2011. [DOI] [PubMed] [Google Scholar]
- 318.Tuzun E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol 118: 737–743, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Vaags AK, Lionel AC, Sato D, Goodenberger M, Stein QP, Curran S, Ogilvie C, Ahn JW, Drmic I, Senman L, Chrysler C, Thompson A, Russell C, Prasad A, Walker S, Pinto D, Marshall CR, Stavropoulos DJ, Zwaigenbaum L, Fernandez BA, Fombonne E, Bolton PF, Collier DA, Hodge JC, Roberts W, Szatmari P, Scherer SW. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet 90: 133–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Van de Linde S, Loschberger A, Klein T, Heidbreder M, Wolter S, Heilemann M, Sauer M. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat Protoc 6: 991–1009, 2011. [DOI] [PubMed] [Google Scholar]
- 321.Van Sonderen A, Arino H, Petit-Pedrol M, Leypoldt F, Kortvelyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Graus F, Dalmau J, Titulaer MJ. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87: 521–528, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Van Sonderen A, Schreurs MW, de Bruijn MA, Boukhrissi S, Nagtzaam MM, Hulsenboom ES, Enting RH, Thijs RD, Wirtz PW, Sillevis Smitt PA, Titulaer MJ. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 86: 1692–1699, 2016. [DOI] [PubMed] [Google Scholar]
- 323.Van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MA, van Coevorden-Hameete MH, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Titulaer MJ. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology 87: 1449–1456, 2016. [DOI] [PubMed] [Google Scholar]
- 324.Van Sonderen ARD, Stoop JA, Verdijk RM, Haasnoot GW, Thijs RD, Wirtz PW, Schreurs MWJ, Claas FHJ, Sillevis Smitt PAE, Titulaer MJ. Anti-LGI1 encephalitis is strongly associated with HLA-DR7, and HLA-DRB4. Ann Neurol 81: 193–198, 2017. [DOI] [PubMed] [Google Scholar]
- 325.Vernino S, Lennon VA. Autoantibody profiles and neurological correlations of thymoma. Clin Cancer Res 10: 7270–7275, 2004. [DOI] [PubMed] [Google Scholar]
- 326.Viaccoz A, Desestret V, Ducray F, Picard G, Cavillon G, Rogemond V, Antoine JC, Delattre JY, Honnorat J. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology 82: 556–563, 2014. [DOI] [PubMed] [Google Scholar]
- 327.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50: 589–601, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, Clover L, Parkinson A, Bien CG, Omer S, Lang B, Rossor MN, Palace J. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 127: 701–712, 2004. [DOI] [PubMed] [Google Scholar]
- 329.Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol 58: 594–604, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51: 71–84, 2006. [DOI] [PubMed] [Google Scholar]
- 331.Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 133: 1494–1504, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Waterman SA, Lang B, Newsom-Davis J. Effect of Lambert-Eaton myasthenic syndrome antibodies on autonomic neurons in the mouse. Ann Neurol 42: 147–156, 1997. [DOI] [PubMed] [Google Scholar]
- 333.Watson R, Jiang Y, Bermudez I, Houlihan L, Clover L, McKnight K, Cross JH, Hart IK, Roubertie A, Valmier J, Hart Y, Palace J, Beeson D, Vincent A, Lang B. Absence of antibodies to glutamate receptor type 3 (GluR3) in Rasmussen encephalitis. Neurology 63: 43–50, 2004. [DOI] [PubMed] [Google Scholar]
- 334.Weiner AL, Vieira L, McKay CA, Bayer MJ. Ketamine abusers presenting to the emergency department: a case series. J Emerg Med 18: 447–451, 2000. [DOI] [PubMed] [Google Scholar]
- 335.Werner C, Pauli M, Doose S, Weishaupt A, Haselmann H, Grunewald B, Sauer M, Heckmann M, Toyka KV, Asan E, Sommer C, Geis C. Human autoantibodies to amphiphysin induce defective presynaptic vesicle dynamics and composition. Brain 139: 365–379, 2016. [DOI] [PubMed] [Google Scholar]
- 336.Wessig C, Klein R, Schneider MF, Toyka KV, Naumann M, Sommer C. Neuropathology and binding studies in anti-amphiphysin-associated stiff-person syndrome. Neurology 61: 195–198, 2003. [DOI] [PubMed] [Google Scholar]
- 337.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res 71: 141–148, 2006. [DOI] [PubMed] [Google Scholar]
- 338.Wigge P, McMahon HT. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci 21: 339–344, 1998. [DOI] [PubMed] [Google Scholar]
- 339.Wirtz PW, Titulaer MJ, Gerven JM, Verschuuren JJ. 3,4-Diaminopyridine for the treatment of Lambert-Eaton myasthenic syndrome. Expert Rev Clin Immunol 6: 867–874, 2010. [DOI] [PubMed] [Google Scholar]
- 340.Wright S, Hashemi K, Stasiak L, Bartram J, Lang B, Vincent A, Upton AL. Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain 138: 3159–3167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wurdemann T, Kersten M, Tokay T, Guli X, Kober M, Rohde M, Porath K, Sellmann T, Bien CG, Kohling R, Kirschstein T. Stereotactic injection of cerebrospinal fluid from anti-NMDA receptor encephalitis into rat dentate gyrus impairs NMDA receptor function. Brain Res 1633: 10–18, 2015. [DOI] [PubMed] [Google Scholar]
- 342.Yamada K, Watanabe M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int 77: 94–108, 2002. [DOI] [PubMed] [Google Scholar]
- 343.Yoshimi K, Woo M, Son Y, Baudry M, Thompson RF. IgG-immunostaining in the intact rabbit brain: variable but significant staining of hippocampal and cerebellar neurons with anti-IgG. Brain Res 956: 53–66, 2002. [DOI] [PubMed] [Google Scholar]
- 344.Yu YE, Wen L, Silva J, Li Z, Head K, Sossey-Alaoui K, Pao A, Mei L, Cowell JK. Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum Mol Genet 19: 1702–1711, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P, Coles AJ, Vincent A, Lennox BR. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol 258: 686–688, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zandi MS, Paterson RW, Ellul MA, Jacobson L, Al-Diwani A, Jones JL, Cox AL, Lennox B, Stamelou M, Bhatia KP, Schott JM, Coles AJ, Kullmann DM, Vincent A. Clinical relevance of serum antibodies to extracellular N-methyl-d-aspartate receptor epitopes. J Neurol Neurosurg Psychiatry 86: 708–713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zerche M, Weissenborn K, Ott C, Dere E, Asif AR, Worthmann H, Hassouna I, Rentzsch K, Tryc AB, Dahm L, Steiner J, Binder L, Wiltfang J, Siren AL, Stocker W, Ehrenreich H. Preexisting serum autoantibodies against the NMDAR subunit NR1 modulate evolution of lesion size in acute ischemic stroke. Stroke 46: 1180–1186, 2015. [DOI] [PubMed] [Google Scholar]
- 348.Zhang Q, Tanaka K, Sun P, Nakata M, Yamamoto R, Sakimura K, Matsui M, Kato N. Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiol Dis 45: 610–615, 2012. [DOI] [PubMed] [Google Scholar]
- 349.Zhou L, Messing A, Chiu SY. Determinants of excitability at transition zones in Kv1.1-deficient myelinated nerves. J Neurosci 19: 5768–5781, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res 100: 120–132, 2008. [DOI] [PubMed] [Google Scholar]
- 351.Zhou YD, Lee S, Jin Z, Wright M, Smith SE, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med 15: 1208–1214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]