Abstract
Triple-negative breast cancers (TNBCs) have poor prognosis and lack targeted therapies. Interrogation of genomic datasets identified increased PIM1 copy number-driven expression in TNBC. RNA interference in breast cancer and non-malignant mammary epithelial cell models revealed a PIM1 dependency in TNBC cells for proliferation and apoptotic protection, absent in non-malignant cells. PIM1 knockdown reduced BCL2 expression, and dynamic BH3 profiling analysis revealed that PIM1 prevents mitochondrial-mediated apoptosis in TNBC cell lines. PIM1 expression associates with several MYC-transcriptional signatures and promotes cell population growth through regulation of c-MYC and transcription of MYC-targets, including MCL1. The pan-PIM kinase inhibitor AZD1208 inhibited growth and sensitized TNBC cell lines, xenografts and patient-derived xenografts to standard of care chemotherapy. We identify PIM1 as a malignant cell-selective target in TNBC, illustrating relationships with MYC activation, regulation of anti-apoptotic BCL2 and MCL1, established TNBC oncogenic proteins SHP2 and EPHA2 and cell cycle inhibitor p27. Finally we identify a potential use of PIM1 inhibitors to abrogate TNBC's high threshold to TNBC standard of care chemotherapy induced apoptotic cell death.
Keywords: PIM1, Triple-Negative Breast Cancer (TNBC), c-MYC, AZD1208, MCL1, BCL2, Histone-H3, Cell Proliferation, Apoptosis, Chemotherapy
Triple-Negative Breast Cancer (TNBC) is defined by the lack of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). No targeted therapy is currently available and the mainstay of therapy remains chemotherapy to which many patients are highly resistant. Identification of novel drug targets, especially those that might impact chemotherapy resistance, is an important unmet medical need1. Chemotherapy-resistant TNBCs are associated with very poor prognosis2,3 and often display co-amplification and overexpression of the c-MYC oncogene4 and its transcriptional target5 the anti-apoptotic gene MCL1.
Previous studies identified a recurrent amplification in the genomic region of 6p21-p25 in TNBC6-8, which includes the proto-oncogene PIM1. PIM1 belongs to the PIM kinase family and has been implicated in the control of cancer cell proliferation, migration and apoptosis9, particularly in prostate cancer and leukemia10,11. Since Pim1-null mice develop normally and are fertile12, PIM1 is not essential for physiologic tissue homeostasis, suggesting manageable side effects if targeted specifically for therapy13. Indeed, a number of specific and potent PIM inhibitors have been developed13,14. Yet, to optimize drug positioning it is critical to understand the tumor types and specific molecular frameworks where PIM1's function is essential.
This study was undertaken to test the hypothesis that PIM1 provides a proliferation advantage and raises the threshold for cell death, resisting selective pressures exerted on the tumor including those chemotherapies commonly used in TNBC.
Herein, we show PIM1 is a novel target in TNBC. We demonstrate that PIM1 gene expression is often gene copy number-dependent and elevated in primary TNBCs, and that TNBC models are addicted to PIM1 for protection from spontaneous and chemotherapy-induced apoptosis. We identify cellular and molecular mechanisms that underpin TNBC's cellular addiction to PIM1, including a functional link between PIM1 and c-MYC, control of anti-apoptotic MCL1 and BCL2 expression as well as of known regulators of important malignant phenotypes in TNBC such as SHP2/PTPN1115 and EPHA216,17. Finally, we demonstrate that the pan-PIM kinase inhibitor AZD120811 selectively impaired growth, reduced apoptotic threshold and sensitized malignant TNBC cell lines, xenografts and patient-derived xenotransplants (PDXs) to standard of care TNBC chemotherapy.
Results
Copy-number dependent PIM1 gene expression in TNBC
PIM1 is located on chromosome 6p21-p25, a recurrent amplicon in TNBC6-8. We investigated whether PIM1's copy-number status and expression levels are increased in TNBC by interrogating three independent published datasets: the Guy's Hospital TNBC-enriched18,19, the TCGA Breast20 and the METABRIC21 cohorts. PIM1 mRNA levels were significantly higher in TNBC compared to non-TNBC (Fig. 1a). PAM50 classification22 of these datasets demonstrated increased PIM1 expression levels in the basal-like molecular subtype (Suppl. Fig. 1a). PIM1 gene expression was significantly correlated with its copy-number across TNBCs in the Guy's Hospital and TCGA cohorts (Fig. 1b) and basal-like tumors in the TCGA and METABRIC datasets (Sup. Fig. 1b). A considerable amount of gene expression variability was observed across the datasets. Nevertheless, 75-85% of basal-like breast cancers consistently show PIM1 expression levels that are significantly higher than the top quartile for PIM1 expression levels in breast cancers of the non-TNBC HER2-enriched, luminal A and luminal B molecular subtypes (Sup. Fig. 1c). Notably, such upregulation is underpinned by copy-number gains and amplifications in a significant proportion of TNBCs. Limitations with antibody performance in IHC precluded analysis of protein in large tumor series, yet both PIM1 mRNA (Fig. 1c) and protein levels (Fig. 1d-e) are increased and correlated in cellular models of TNBC when compared to non-TNBC.
Figure 1. PIM1 gene expression is upregulated in TNBC and associated with its gene copy-number levels.
(a) PIM1 gene expression was determined in the Guy's Hospital TNBC-enriched cohort18,19, the TCGA Breast20 and METABRIC21 datasets. The cohorts were divided into TNBCs (red) and non-TNBCs (blue) according to their IHC-defined receptor status. Gene expression is reported as median-centred expression log2 values. The number of patients (n) per group is shown. p-values were determined using a Wilcoxon rank-sum test. (b) PIM1 gene expression values (y-axis) were plotted against absolute gene copy-number (CN) across the TNBCs from the Guy's Hospital, the TCGA and the METABRIC datasets. Tumors were segregated according to their PIM1 gene CN status in those with high amplification (CN > 4), moderate gain (CN = 3-4), neutral copy-number (CN = 2) or deletion (CN < 2). The number of samples (n) per group is shown. p-values were determined using Kruskal-Wallis analysis of variance. (c) PIM1 gene expression was assessed in the Neve et al. cancer cell line expression dataset23. Cell lines were divided into TNBCs (red) and non-TNBCs (blue) according to their receptor status. p-values were determined using a Welch's t-test (Satterthwaite's approximation). (d) PIM1 protein expression was assessed by Western Blotting in a panel of breast cancer and non-malignant cell lines. (e) Relative PIM1 protein expression in TNBC versus non-TNBC cell lines, as quantified by densitometry using ImageJ from three independent experiments and shown as fold change over control (-). β-ACTIN was used a loading control for normalization. p-value was determined using a two-tailed unpaired t-test.
Selective PIM1 kinase addiction in malignant cellular models of breast cancer
To investigate PIM1's specific role in malignant breast cancer cells, we used a TNBC-enriched cell line panel23,24 and three non-malignant cellular models. We assessed the effect of PIM1 knockdown on cell population growth using a Cell Titer-Blue assay and multiple siRNA and shRNAs. The sh/siRNA specificity for PIM1 over PIM2 and PIM3 was validated by standard qRT-PCR (Sup. Fig. 2).
Using two distinct shRNAs targeting PIM1 and a non-targeting (NT) control, 4 out of 5 TNBC cell lines were sensitive to PIM1 knockdown, in contrast to luminal BT474 and non-malignant HMEC cells (Fig. 2a). Comparable results were obtained when Hoechst-33342 was used to measure cell growth (Sup. Fig. 3), demonstrating that the reduction in metabolic activity reflected a reduction in total cell number.
Figure 2. PIM1 supports cell population growth and clonogenic survival of TNBC cells.
(a) Cell population growth over time of HCC38, SUM149, MDA-MB-231, CAL51, SUM159, BT474, and HMEC cells upon treatment with two distinct PIM1 shRNAs (PIM1#1 and PIM1#2) or non-targeting (NT) control shRNA. PIM1 protein expression was assessed by WB and percentage of knockdown (KD) quantified by densitometry using ImageJ. β-ACTIN protein levels were used as internal control. (b) Rescue-of-function experiment of cell population growth over time in SUM149 cells treated with doxycycline-inducible PIM1 shRNA#1 or NT shRNA control and infected with lentivirus stably overexpressing a shRNA#1-resistant and V5-tagged PIM1 or control V5-tagged LacZ. Cells were grown in the absence (-) or presence of doxycycline (+PIM1 KD). Expression of V5-tagged PIM1 and LacZ proteins and KD of endogenous PIM1 were confirmed by WB. GAPDH was used as an internal control. (c) Representation and quantification of the clonogenic survival of HCC38, SUM149, MDA-MB-231 and SUM159 cells upon treatment with PIM1 or NT shRNAs. The data in (a), (b) and (c) represent the mean ± standard error of the mean values of three independent experiments. The last time point (day 3 in (a) and day 6 in (b)) were analysed using a two-tailed unpaired t-test (* = p-value < 0.05, ** = p-value < 0.01, *** = p-value < 0.001).
Next, a broader range of breast cancer and non-malignant models was tested using transient siRNA transfection targeting PIM1 (Sup. Fig. 4a). Silencing PIM1 impaired growth of 6 out of 7 TNBC cell lines, the exception being SUM159, a low PIM1-expressing cell line. 2 out of 4 non-TNBC models showed a degree of PIM1 dependency under these conditions. Of note, none of the non-malignant models were sensitive to PIM1 knockdown (Sup. Fig. 4a). Our experiments showed a significant difference in response to PIM1 knockdown between malignant and non-malignant cells (Sup. Fig. 4b), compatible with the lack of phenotype seen in normal tissues of Pim1 knockout mice12.
We performed rescue-of-function experiments by expressing an sh/siRNA-resistant V5-tagged PIM1 in SUM149 cells and transduced an shRNA against endogenous PIM1 to exclude off target effects. PIM1 knockdown reduced cell population growth in LacZ-expressing control cells, but was ineffective in the sh/siRNA-resistant V5-tagged PIM1-expressing cells (Fig. 2b). Moreover, we generated cell lines stably expressing a doxycycline-inducible sh/siRNA-resistant HA-tagged PIM1 (PIM1WT) or PIM1 kinase-dead mutant (PIM1K67M). PIM1WT rescued the effect of PIM1 knockdown in contrast to the PIM1 kinase-dead mutant (PIM1K67M) (Suppl. Fig. 4c) indicating the phenotypes were specific to PIM1 and required the activity of PIM1's kinase-domain.
We then investigated the effects of silencing PIM1 on TNBC clonogenic survival capacity. PIM1 knockdown reduced the colony formation of TNBC cell lines, with the exception of the low PIM1-expressing SUM159 cells (Fig. 2c), indicating requirement for PIM1 for perpetual cell survival and/or proliferation across multiple cell divisions.
To investigate a potential role for the other members of the PIM kinase family, we silenced PIM2 or PIM3. We did not observe an effect on the cell population growth in the TNBC cell lines MDA-MB-231, SUM149 or SUM159 upon knockdown of PIM2 or PIM3 (Sup. Fig. 5).
PIM1 prevents activation of mitochondrial-mediated apoptosis in TNBC cells
Time-course experiments in MDA-MB-231 cells showed that, following siRNA-mediated PIM1 knockdown, an increase in caspase 3/7 activity (a marker for early apoptosis induction) (Fig. 3a) preceded the impairment of cell population growth (Fig. 3b). This indicated that PIM1's effect on TNBC growth is at least in part due to protection from apoptosis. PIM1 knockdown using multiple RNA interference (RNAi) methods consistently led to increased caspase 3/7 activity only in the TNBC cell lines that had previously shown a population growth defect (Sup. Fig. 6a-b).
Figure 3. PIM1 inhibits activation of mitochondrial-mediated apoptosis in TNBC cells.
MDA-MB-231 cells were transfected with 20nM PIM1 siRNA or control NT siRNA prior to measure changes in (a) Caspase 3/7 activation by Caspase-Glo3/7 assay and (b) cell population growth by Cell-Titer blue assay in a time-dependent manner. The time points represent the mean ± standard deviation of three independent biological replicates (** = p-value < 0.01 (by two-tailed unpaired t-test)). (c) Representative WB analysis of lysates from MDA-MB-231, SUM149, SUM159 and BT474 cells exposed for 4 days to PIM1 or NT siRNA. PIM1, BCL2 and GAPDH expression were assessed. (d) MDA-MB-231 cells overexpressing BCL2 or RFP control were transfected with PIM1 siRNA or NT siRNA control. Caspase 3/7 activation was assessed 72h post-transfection. BCL2 expression was assessed by WB and β-ACTIN was used as internal loading control. The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using an ANOVA with Tukey procedure (*** = p-value < 0.001). (e) Representation of BIM-induced mitochondrial membrane permeabilization (ΔΨn priming determined by fluorescence RFU 590nm) achieved with 0.3 μM of BIM peptide in MDA-MB-231, SUM149, SUM159, BCL2-overexpressing MDA-MB-231 cells treated with NT and PIM1 siRNAs. The data represent the mean ± standard error of the mean values of three independent experiments. (f) Cell population growth of MDA-MB-231 cells overexpressing BCL2 or RFP control treated with PIM1 or NT siRNA was measured every two days, starting at 2-days post-transfection (Day 0 = 48h post-transfection, Day 2 = 96h post-transfection, Day 4 = 144h post-transfection). The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using a Welch's ANOVA (*= p-value < 0.05).
We then examined the effect of PIM1 knockdown on the expression of the anti-apoptotic protein BCL2 in TNBC as it is known to be under the control of PIM1 in other contexts25,26. PIM1 knockdown reduced the expression of BCL2 in MDA-MB-231 and SUM149 cells, while it did not affect the non-responsive SUM159 and BT474 cell lines (Fig. 3c and Sup. Fig. 7), implicating the regulation of this pathway as a potential mechanism for addiction to PIM1. To determine if PIM1's effect on caspase activation was mediated through BCL2, we silenced PIM1 in exogenous BCL2-overexpressing MDA-MB-231 cells and demonstrated suppression of the caspase 3/7 activation phenotype (Fig. 3d).
BCL2 is an anti-apoptotic molecule acting through the mitochondrial apoptotic pathway27. We performed dynamic BH3 profiling (DBP), a novel methodology to measure changes in the pro-apoptotic signalling at the mitochondria28, to test the effect of PIM1 on the threshold at which increasing concentrations of BIM peptide promotes apoptosis (Sup. Fig. 8). At 0.3μM of BIM peptide, PIM1 knockdown led to a reduction of this threshold in MDA-MB-231 and SUM149 but not in SUM159. While knockdown of PIM1 results in depletion of BCL2, enhanced mitochondrial priming and elevated activation of caspases, ectopic expression of BCL2 in MDA-MB-231 fully rescued priming of the mitochondrial permeabilization by PIM1 knockdown (Fig. 3e) suggesting that PIM1 prevents mitochondrial-mediated apoptosis in TNBC at least in part by modulation of BCL2.
Despite fully rescuing the caspase activation phenotype, BCL2 overexpression only partially rescued cell growth upon PIM1 knockdown (Fig. 3f), showing protection from the mitochondrial-apoptotic pathway is not the only mechanism by which PIM1 promotes tumorigenesis.
PIM1 acts through the MYC-activation pathway
PIM1 has been found to synergize with the c-MYC oncogene to drive the progression of prostate cancer and leukemia10,29. MYC-transcriptional activation relies on PIM1-dependent phosphorylation of Histone-H3 at Ser10 in HEK293 cells and transformed rat fibroblasts30, and PIM1-dependent phosphorylation of c-MYC at Ser62 caused oncogenic transformation in prostate cancer10. As c-MYC is known to be upregulated in aggressive chemotherapy-resistant TNBC4 we asked how PIM1 regulated Histone-H3 and c-MYC in relation to the PIM1-addiction status of TNBC models. PIM1 knockdown led to decreased phosphorylation of Histone-H3 at Ser10 and c-MYC at Ser62, and a reduction of total protein levels of c-MYC and its transcriptional target5 MCL1, in MDA-MB-231 and SUM149 (PIM1 knockdown-sensitive cells) but not in SUM159 and BT474 (PIM1 knockdown-insensitive cells) (Fig. 4a and Sup. Fig 9a). We also show that SUM159 cells are sensitive to MYC knockdown (Sup. Fig 9b), indicating that the MYC-activation pathway is likely to be controlled by other mechanisms31,32 in this cellular model.
Figure 4. PIM1 acts through the MYC-activation pathway.
(a) Representation of PIM1 expression, c-MYC phosphorylation at Ser62, total c-MYC expression, Histone H3 phosphorylation at Ser10, total Histone H3 levels and MCL1 expression, as determined by WB in MDA-MB-231, SUM149, SUM159 and BT474 cells upon PIM1 siRNA-mediated knockdown. (b) Histograms representing mRNA levels of PIM1, MCL1 and c-MYC in MDA-MB-231 cells 72h post-transfection with PIM1 siRNA assessed by qRT-PCR. GAPDH was used as housekeeping gene for normalization. The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using a two-tailed unpaired t-test (* = p-value < 0.05, *** = p-value < 0.001). (c) Cell population growth over time in Myc-overexpressing SUM149 cells or empty-vector (EV) control. Cells were grown in the presence of doxycycline and transfected with PIM1 siRNA. Expression of MYC, MCL1 and PIM1 were detected by WB. GAPDH was used as an internal control. The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using an ANOVA with Tukey procedure (*** = p-value < 0.001). (d) The Chandriani et al. MYC-transcriptional signature is plotted over PIM1 gene expression for Guy's Hospital, the TCGA and the METABRIC datasets. Tumor classification by PAM50 molecular intrinsic subtypes is shown. Correlation values and p-values were determined using Pearson's correlation. (e) Volcano plot illustrating changes of mRNA expression (log2 values) of 730 genes in MDA-MB-231 and SUM149 upon PIM1 knockdown as determined by nCounter PanCancer Pathways gene expression analysis. Top 20 gene expression changes are colored with regards to their associated signalling pathways as described by nCounter PanCancer Pathways codeset. (f) Representation of PIM1, p27, SHP2 and EPHA2 expression levels, as determined by WB in SUM149 and MDA-MB-231 cells upon PIM1 siRNA-mediated knockdown.
MCL1 mRNA was also reduced upon PIM1 knockdown (Fig. 4b), suggesting a mechanism involving PIM1 regulation of MYC-driven MCL1 transcription. MYC mRNA levels however were not affected upon PIM1 knockdown, indicating that PIM1 regulates MYC at the protein level (Fig. 4b). To further understand whether PIM1 acts through MYC, we engineered TNBC cell lines expressing a doxycycline-inducible Myc vector33. Exogenous Myc expression, abolished the effects of PIM1 knockdown on the cell population growth phenotype (Fig. 4c) indicating PIM1 is acting directly through regulation of MYC and its downstream effectors. Importantly, we further demonstrated that Myc overexpression rescued MCL1 expression (Fig. 4c).
Taken together, these data indicate direct effects of PIM1 on MYC-driven transcription led us to investigate whether PIM1 and c-MYC gene expression correlated across the Guy's Hospital18,19, the TCGA Breast20 and METABRIC21 cohorts and found moderate positive correlation (Sup. Fig. 9c). We therefore sought evidence of any functional consequence on MYC-driven transcription. 34 MYC-transcriptional signatures have been reported (Sup. Table 1), among the first was that of Chandriani et al34, derived by overexpressing MYC in primary human fibroblasts and found associated with basal-like breast cancer34,35. Consistently, we found this signature upregulated in TNBC tumors and cellular models (Sup. Fig. 9d). We determined the activation score of all 34 gene signatures across the three cohorts and the breast cancer cell line dataset23, using a weighted average mean36. Their activation was then correlated to PIM1 expression and a modest but significant correlation for 8 of the MYC-transcriptional signatures was observed in all 4 datasets (Sup. Table 1). The correlation of Chandriani's MYC activation signature and PIM1 across all datasets is depicted in Fig. 4d.
We then asked if PIM1 had a direct role in driving the upregulation of genes in the Chandriani MYC gene signature we had found associated with PIM1 upregulation in basal-like breast cancer in our correlative analysis (Fig. 4d). We performed NanoString nCounter PanCancer Pathways codeset gene expression analysis following PIM1 knockdown using multiple RNAi methods. Changes on PIM1 mRNA levels upon PIM1 silencing compared to control were confirmed for each cell line (Sup. Fig. 9e). 24 MYC-driven genes present in the Chandriani signature could be interrogated in the codeset. 11 of these were downregulated by PIM1 knockdown in the sensitive cell lines (Sup. Fig. 9f and Sup. Table 2). In the insensitive SUM159 cells, in which there was no evidence of PIM1's regulation of MYC protein level, its phosphorylation at Ser62 or phosphorylation of Histone H3 at Ser10 (Fig 4a), only 5 were downregulated. We believe that PIM1 regulation of MYC-dependent transcription will only partially overlap with that seen in a fibroblast model system, which may explain why we only found 11 out of the 24 MYC-target genes downregulated upon PIM1 knockdown.
Secondly, we investigated the effect of PIM1 knockdown on wider transcriptional measures of pathway activity in the PIM1-dependent MDA-MB-231 and SUM149 cell lines, using the whole nCounter PanCancer Pathways gene expression codeset (Fig. 4e). We sought log2 fold changes > 0.5 or < –0.5 with p-values ≤0.05 showing significant effects on the JAK/STAT pathway, and apoptotic and cell cycle codeset genes, consistent with known functions of PIM19 in other systems (Fig. 4e and Sup. Table 3). By example we observed increased expression of CDKN1B, encoding cell cycle inhibitor p27, shown to be regulated by PIM137. We also showed for the first time that PIM1 regulates PTPN11, encoding SHP2, known to be important for maintenance of tumor initiating cells and clonogenic survival in TNBC15 and its regulatory kinase EPHA238. These results were independently validated at the protein level (Fig. 4f) and suggest wider additional mechanisms by which PIM1 regulates malignant cell phenotypes in TNBC.
The pan-PIM kinase inhibitor AZD1208 impairs in vitro and in vivo growth of TNBC models and enhances chemosensitivity through apoptosis induction
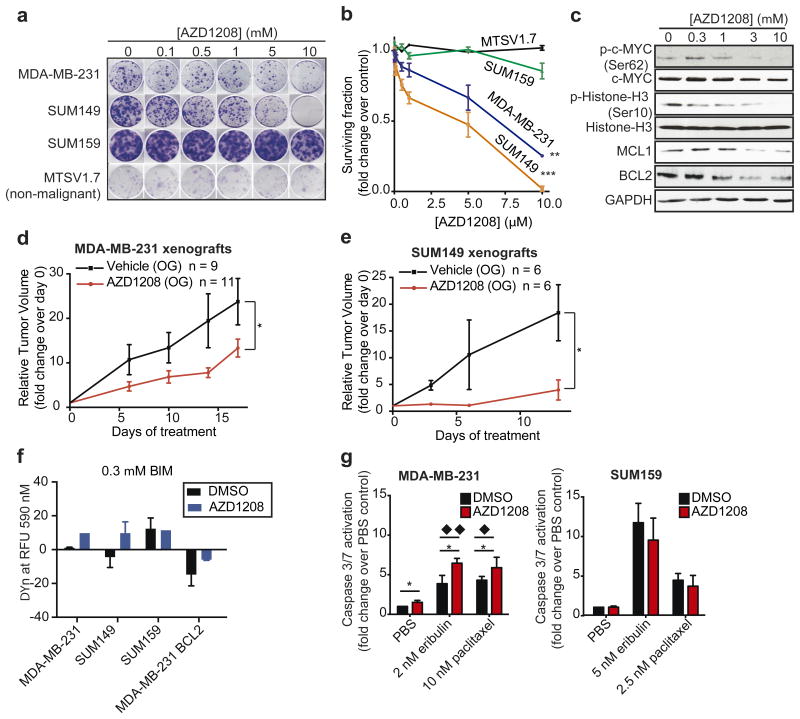
The pan-PIM kinase inhibitor AZD1208 is a highly selective inhibitor of all three PIM kinases11. To examine the potential of PIM inhibitors for targeting PIM1 dependent TNBC, we exposed TNBC cell lines and the non-malignant MTSV1.7 cells to AZD1208 in colony formation assays. In concordance with orthogonal RNAi results, MDA-MB-231 and SUM149 were sensitive to PIM kinase inhibition, in contrast to SUM159 and MTSV1.7 cells (Fig. 5a-b). AZD1208 also significantly reduced anchorage-independent growth of TNBC cells (Sup. Fig. 10). AZD1208 treatment also reduced MCL1 and BCL2 protein levels and decreased phosphorylation of Ser10 Histone-H3 and Ser62 c-MYC (Fig. 5c) consistent with the effects seen with PIM1 RNAi.
Figure 5. The pan-PIM kinase inhibitor AZD1208 impairs clonogenic survival and reduces tumor growth in in vivo xenograft models of TNBC.
(a) Representation and (b) quantification of the clonogenic survival/growth of SUM159, MDA-MB-231, SUM149 and MTSV1.7 cells upon treatment with increasing concentrations of AZD1208. The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using a linear regression of log10 [AZD1208] dose response and % of survival fraction (** = p-value < 0.01 and *** = p-value < 0.001). (c) c-MYC phosphorylation at Ser62, c-MYC expression, Histone H3 phosphorylation at Ser10, Histone H3 expression, MCL1, BCL2 and GAPDH expression (housekeeping protein) were analyzed by WB in SUM149 cells following 48h of incubation with increasing concentrations of AZD1208. (d-e) Relative tumor volume (mm3) over time (days) was assessed in (d) MDA-MB-231 and (e) SUM149-tumor bearing mice following AZD1208 or vehicle control oral gavage (OG) therapy. Relative tumor volume was calculated by normalizing to tumour volume at the start of the treatment. The data represent the mean ± standard error of the mean. The number of treated mice (n) per group is shown. The last time points were analysed using a two-tailed unpaired t-test (* = p-value < 0.05). (f) Representation of BIM-induced mitochondrial membrane permeabilization (ΔΨn priming determined by fluorescence RFU 590nm) achieved with 0.3 μM of BIM peptide in MDA-MB-231, SUM149, SUM159, BCL2-overexpressing MDA-MB-231 cells treated with AZD1208 or DMSO control. The data represent the mean ± standard error of the mean values of three independent experiments. (g) Caspase 3/7 activation levels were measured in MDA-MB-231 and SUM159 cells following 72h of incubation with eribulin or paclitaxel in the presence or absence of 3μM AZD1208. The data represent the mean ± standard error of the mean values of three independent experiments, and were analysed using a two-tailed unpaired t-test (* = p-value < 0.05) and an ANOVA with Tukey procedure (◆ ◆= p-value < 0.01, ◆ = p-value < 0.05)
We treated MDA-MB-231 and SUM149 cell line xenografts with AZD1208, substantially impairing tumor growth (Fig. 5d-e) and inducing an increase in p27-positive cells in treated xenografts (Sup. Fig. 11). This is consistent with the gene expression analysis results and PIM1's known cell cycle progression role through regulation of p2737.
Consistent with the effects of PIM1 RNAi and failure of a kinase-dead PIM1 to rescue knockdown phenotypes, DBP demonstrated that PIM kinase inhibition led to mitochondrial membrane permeabilization at 0.3μM of BIM peptide in both MDA-MB-231 and SUM149, while no differences were seen in SUM159. Notably, BCL2 exogenous overexpression rescued this phenotype in AZD1208 treated cells (Fig. 5f and Sup. Fig. 12).
Apoptosis induction is a major mechanism of action of chemotherapy, which remains the mainstay of TNBC treatment despite limited efficacy in metastatic disease39. The standard advanced TNBC chemotherapy agents eribulin and paclitaxel were given in combinations with AZD1208 to TNBC cells. Inhibition of the PIM1 by AZD1208 increased chemotherapy-induced caspase 3/7 activity in PIM1-dependent MDA-MB-231 but not PIM1-independent SUM159 (Fig. 5g). We further demonstrate that exogenous BCL2 expression prevented mitochondrial-membrane permeabilization induced by combinations of chemotherapy and AZD1208 (Sup. Fig. 13).
We investigated the therapeutic potential of combining PIM1 inhibition with chemotherapy in TNBC cell lines and xenografts. AZD1208 sensitized MDA-MB-231 and SUM149 but not SUM159 or MTSV1.7 to paclitaxel (Fig. 6a). MDA-MB-231 and SUM149 but not SUM159 were also sensitized to eribulin (Fig. 6b) (N.B. MTSV1.7 showed exquisite sensitivity to eribulin precluding combination testing). Reduction of long-term colony formation was associated with increased caspase 3/7 activity suggesting the effect was mediated through induction of apoptosis.
Figure 6. The pan-PIM kinase inhibitor AZD1208 enhances response to chemotherapy in TNBC cells and xenografts.
(a and b) Representation and quantification of MDA-MB-231, SUM149, SUM159 and MTSV1.7 colony formation during dose-response curves to (a) paclitaxel and (b) eribulin in the presence of AZD1208 (3μM in MDA-MB-231, SUM159 and MTSV1.7 cells and 1μM in SUM149 cells) or DMSO vehicle control (-). The data represent the mean ± standard error of the mean values of the surviving fraction for each chemotherapy dose point with DMSO control or AZD1208 across at least three independent experiments, and were analysed using a paired t-test. (c-d) Relative tumor volume (mm3) over time (days) was assessed in (c) MDA-MB-231 and (d) SUM149 xenografts following treatment with AZD1208 by oral gavage (OG), eribulin by intravenous tail-vein injection (IV) combinations of eribulin and AZD1208. (e-f) Relative tumor volume (mm3) over time (days) was assessed in the PDXs (e) PDX93 and (f) PDX156 following treatment with AZD1208 by oral gavage, eribulin by intraperitoneal injection (IP) or combinations of eribulin and AZD1208. Vehicle-treated tumors were used as control. The number of treated mice (n) per group is shown. Data represent the mean ± standard error of the mean and were analysed using a Welch's ANOVA (* = p-value < 0.05, ** = p-value < 0.01, *** = p-value < 0.001). (g-j) Representative images of hematoxylin and eosin staining of (g) MDA-MB-231 xenografts, (h) SUM149 xenografts, (i) PDX93 and (j) PDX156 tumors and (k-n) quantification of cell density (number of tumor cells/area) of (k) MDA-MB-231 xenografts, (l) SUM149 xenografts,(m) PDX93 and (n) PDX156 tumors at the end of the treatment with vehicle control, AZD1208, eribulin or combinations of AZD1208 and eribulin. Data in (k-n) were analysed using a one-way ANOVA Tukey multiple comparisons test (* = p-value < 0.05, ** = p-value < 0.01, *** = p-value < 0.001).
Next, we treated two TNBC cell line xenografts (Fig. 6c-d) and two TNBC PDXs (Fig. 6e-f) which display high levels of PIM1 (Sup. Fig. 14a) with combinations of AZD1208 and eribulin. These combinations led to conversion of the stable disease, achieved with chemotherapy alone, to tumor objective response (Fig. 6c-f and Sup. Fig. 14b-g) and showed marked reduction in viable tumor cell density and increased fibrosis (Fig. 6g-n) demonstrating the potential efficacy of the combination of PIM kinase inhibition and eribulin. There was no evidence that the PIM kinase inhibitor, despite known activity against murine Pim140, lead to loss of animal weight (Sup. Fig. 14h-k), distress or lethality, given alone or in combination with chemotherapy, supporting the notion that PIM inhibition could be well-tolerated in patients.
Overall, our data support the hypothesis that targeting PIM1 can downregulate anti-apoptotic BCL2-family proteins, c-MYC-driven transcripts, cell signalling and cell cycle proteins used by TNBC cells to drive cell proliferation and to enable malignant cells to resist apoptosis despite treatment with approved TNBC chemotherapy.
Discussion
Our data reveal the PIM1 kinase as a potential target for therapy in TNBC. We demonstrate elevated expression of PIM1 and frequent gene copy-number aberrations (CNA) in a high proportion of basal-like TNBC. Using complementary but orthogonal RNAi methods and a small-molecule PIM kinase inhibitor in cell lines and in vivo models, we demonstrate that PIM1 kinase activity is required for the survival of TNBC cells, especially when subjected to the apoptotic stimulus of chemotherapy.
TNBC includes of a number of biologically distinct entities17. While genome profiling studies in TNBC indicate significant diversity of driver mutations with most being of low frequency. Some mutations and gene CNAs are, however, recurrent. For example, oncogenic c-MYC is frequently amplified/overexpressed, and drives oncogenic pathway activation and tumorigenicity17,35. c-MYC is also associated with poor prognosis41 and chemotherapy resistance in TNBC4 but is notoriously hard to target42. Herein, not only do we demonstrate that most TNBC cellular models are addicted to PIM1 for survival and proliferation, but also that PIM1 acts in part through its effects on c-MYC. Overexpression of exogenous Myc abrogated the PIM1 knockdown cell growth phenotype and MYC-transcriptional signatures were associated with PIM1 expression in TNBCs. PIM1 knockdown downregulated transcription of a subset of MYC-target genes including MCL1 through mechanisms such as phosphorylation of Histone-H3 at Ser10 and phosphorylation of c-MYC at Ser62, known to be required for c-MYC-driven transcriptional activation and oncogenic transformation10,30. Altogether, our data support PIM1 as a potential target in basal-like TNBCs, including the MYC-driven group, and are supported by the independent study by Horiuchi et al.43 (personal communication), which demonstrates that c-MYC overexpression generates PIM1 essentiality in mammary epithelial cells and further proposes that PIM1 function is needed to support c-MYC-dependent malignancy in TNBC cells43.
Balko et al. showed that c-MYC and MCL1 are co-amplified in residual TNBC after neoadjuvant chemotherapy proposing that MCL1 amplification can lead to its overexpression and circumvent oncogene and chemotherapy-induced apoptosis4. Petrocca et al. demonstrated that most basal-like TNBCs shared high dependency on MCL144. c-MYC transcriptionally controls MCL1 expression in gastric cancer cells5. Herein we show that MCL1 expression is increased upon exogenous expression of Myc in TNBC, and that PIM1 regulates c-MYC transcriptional activity specifically, including MCL1 expression levels. PIM1 was shown to phosphorylate the BCL2-partner BAD in leukemia11 and kidney COS-7 cells25 and this is now been demonstrated in TNBC by Horiuchi et al.43. Herein, we specifically elucidated a role of PIM1 in raising the TNBC cell's threshold for mitochondrial-mediated apoptosis, and that this can be mediated by BCL2-family members, providing insight to the mechanisms by which PIM1 might overcome oncogene-induced and chemotherapy-induced apoptosis.
BCL2 and MCL1 are, like c-MYC, hard-to-target proteins27. Inhibitors of BCL2-family members have frequently shown off-target effects, triggering cell death independently from on-target mitochondria-mediated apoptosis27. A number of specific and potent PIM1 inhibitors are in late stages of development14 suggesting PIM1 may be a more feasible drug target that would impact therapeutic resistance mediated by these mechanisms. In this study we show that the kinase activity of PIM1 is specifically required for cell population growth and apoptosis protection. Moreover, the pan-PIM kinase inhibitor AZD1208 could not only impair clonogenicity of TNBC cells and tumor growth in TNBC xenografts, but also increased sensitivity to licensed chemotherapeutics used in early and metastatic TNBC by promoting apoptosis initiation. Current development focus for PIM inhibitors is in hematological malignancy45 but our data suggest additional repurposing as a potential combination strategy with chemotherapy to address the major unmet clinical need of chemotherapy-resistance in TNBC.
Beside the cooperation between PIM1 and c-MYC, the role of PIM1 in TNBC's survival and proliferation is excerted by multiple mechanisms. Indeed, gene expression analysis revealed PIM1 regulation of JAK/STAT, apoptotic and cell cycle genes, consistent with previously described PIM1 functions9. We found that PIM1 knockdown upregulated CDKN1B/p27, suggesting a mechanism by which PIM1 promotes cell cycle progression in TNBC. Our findings concord with the findings from Horiuchi et al.43 (personal communication) and indeed, others have shown that PIM1 downregulates p27 at transcriptional and post-transcriptional levels37 in other contexts. We show for the first time that PIM1 knockdown decreased the expression of PTPN11 and EPHA2, genes of significant relevance in breast cancer15,16: PTPN11, encodes phosphatase SHP2, involved in breast cancer progression and maintainance of tumor-initiating cells15. EPHA2 is both associated with regulation of SHP238, and together with PIM1, belongs to a cluster of kinases associated with poor prognosis in ER-negative breast cancers16,17.
We found PIM1 expression was essential in most malignant cell line models of breast cancer, including some of non-TNBC origin but was expendable in non-malignant models including mammary epithelial cells. Indeed, although the evidence of upregulation of PIM1, particularly as driven by copy-number aberration is associated with basal-like breast cancer, PIM kinase function may be relevant to a broader range of higher grade breast tumors including ER positive forms of the disease, as recently proposed by Malinen et al.46. Importantly, PIM1 is not essential for physiologic tissue homeostasis, as the Pim1-null mice develop normally and are fertile12. This is consistent with early evidence of good tolerability47,48 of PIM1 inhibitors in early phase clinical trials.
Altogether, our study provides insights into the mechanisms by which PIM1 drives malignant phenotypes in TNBC and suggests investigation of PIM1 inhibition combined with chemotherapy as a therapeutic strategy in basal-like TNBC cancers with evidence of PIM1 gene expression. Moreover, it may provide a much needed means of targeting tumors that amplify and express c-MYC and its co-amplified gene and transcriptional target MCL15 as well as other anti-apoptotic BCL2-family members that underpin resistance to licensed breast cancer chemotherapy.
Online Methods
Cell lines
The breast cancer cell lines BT20, BT474, CAL51 HCC1143, HCC1428, HCC1954, HCC38, MCF7, MDA-MB-231, MDA-MB-436, SKBR3 and the non-malignant MCF10A cells were from The American Type Culture Collection (ATCC, Teddington, UK). The breast cancer cell lines SUM149 and SUM159 were from Asterand (Royston, UK). The non-malignant HMEC and MTSV-1.7 cells were from Invitrogen (Life Technologies, Paisley, UK). HEK293T cells were also from ATCC. MDA-MB-231 expressing pTIPZ BCL2 or RFP control were kindly given by Prof. Pascal Meier's lab (The Institute of Cancer Research, London, UK). All cell lines were maintained as recommended by the suppliers. The cell lines were authenticated by short tandem repeat (STR) analysis and matched to the German Collection of Microorganisms and Cell Cultures (DSMZ)-database and used no more than 25 passages from STR-typed samples. Mycoplasma tests were routinely performed using MycoAlert Mycoplasma Detection Kit (Lonza, Basel, Switzerland). Although MCF7 and BT20 are included in the database of commonly misidentified cell lines, they were authenticated by STR and included in our work as part of a comprehensive validation. Nevertheless, we focussed on other cell lines for main part of this study.
Gene expression and DNA copy number analysis
Gene expression data (PIM1, c-MYC and genes from 34 MYC-transcriptional signatures1-13) were obtained from the Guy's Hospital TNBC enriched cohort14,15 (177 primary breast tumors), the TCGA dataset16 (526 primary breast tumors), the METABRIC dataset17 (1552 primary breast tumors) and a publicly available cell lines dataset18 (51 cell lines). Breast cancer specimens were classified into TNBC or non-TNBC based on immunohistochemistry (IHC) as per guidelines of the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP)19, or into PAM50 molecular subtypes20. To identify subtypes based on the molecular PAM50 classification, a nearest centroid classification was performed as described previously20 for the METABRIC and TCGA cohort. For the Guy's Hospital TNBC enriched cohort we adapted the PAM50 classification by performing random sampling15. Assignment of tumors to the molecular subtypes was based on their highest Spearman's rank correlation. All statistical analyses were performed in the R environment, using several CRAN packages (http://cran.r-project.org/). Absolute DNA copy number was obtained using Affymetrix SNP6.0 genome profiles and human genome build hg18 mapping for 196 cancers in our Guy's Hospital cohort and for 940 primary breast carcinomas from the TCGA dataset. Raw copy number information was processed for 1501 breast cancers from the METABRIC dataset. To account for normal tissue contamination and ploidy, allele-specific copy number profiling was performed with Allele-Specific Copy number Analysis of Tumors (ASCAT)21. SNP 6.0 copy number data were also extracted from our internal breast cancer cell lines dataset22 and analysed using Tumor Aberration Prediction Suite (TAPS)23.
Western blotting (WB)
The cells were lysed directly in 1X Laemmli buffer (50 mmol/L Tris-HCl pH 6.8, pH 6.8, 2% SDS, 10% glycerol, 2.5% β-mercaptoethanol and 0.1% bromophenol blue). Alternatively, the cells were lysed by scraping them in a pH 7.4 lysis buffer containing 1% NP-40 (Sigma-Aldrich, Gillingham, UK), 50 mmol/L Tris, 10% glycerol, 0.02% NaN3, 150 mmol/L NaCl, and a cocktail of phosphatase and protease inhibitors (Sigma-Aldrich, Gillingham, UK). Cell lysates were passed 5 times through a 30-gauge needle before centrifugation and further processing of the samples. Snap-frozen tumor tissues were suspended in a pH 7.4 lysis buffer containing 50 mmol/L Tris-Base, 150 mmol/L NaCl, 2% TritonX-100, 1% SDS, 10 mmol/L EDTA and a cocktail of phosphatase and protease inhibitors (Sigma-Aldrich, Gillingham, UK). Tissue destruction was done using the bullet blender homogenizer (Next Advance, New York, USA). 20-100 μg of proteins were separated in reducing conditions (2.5% β-mercaptoethanol) by SDS-PAGE (SDS-Polyacrylamide Gel Electrophoresis) and transferred to nitrocellulose membrane (BioRad, Hemel Hempstead, UK) for further processing, following standard WB procedures.
Primary antibodies used in this study were: the anti-PIM1 rabbit monoclonal antibody (ab75776), the anti-c-MYC rabbit monoclonal antibody (ab32072) and the anti-phosphorylated (p-) -c-MYC (Ser62) rabbit polyclonal antibody (ab51156) from Abcam (Cambridge, UK), the anti-PIM1 rabbit antibody PIM-1:NOV22-39-5 from Novartis (Basel, Switzerland). The anti-PIM2 (D1D2) (#4730), anti-PIM3 (D17C9) (#4165), anti-MCL1 (#4572), anti-BCL2 (50E3) (#2870), anti-Histone H3 (D1H2) XP (#4499), anti-p-Histone H3 (Ser10) (D2C8) (#3377) and anti-GAPDH (14C10) (#2118) rabbit antibodies from Cell Signalling Technologies (Massachusetts, USA). The anti-β-Actin mouse antibody (A5441) from Sigma-Aldrich (Gillingham, UK). The anti-V5 tag rabbit antibody from Life Technologies (Paisley, UK). The anti-HA tag rabbit antibody (GTX115044) from GeneTex (Irvine, USA). The secondary peroxidase-conjugated anti-mouse and anti-rabbit antibodies as well as the ECL (Enhanced Chemiluminescence) substrate were obtained from GE Healthcare (Amersham, UK). Image J was used to quantify western blotting results by densitomentry.
Short hairpin RNA (shRNA) and Small interfering RNA (siRNA)- mediated gene knockdown
Two pLKO.1-puro lentiviral MISSION® shRNA constructs targeting PIM1 (TRCN0000010115 (#1) and TRCN0000010118 (#2)) and a non-targeting (NT) control shRNA (TRC1/1.5) were from Sigma-Aldrich (Gillingham, UK). The shRNA#1 sequence targeting PIM1 and the NT shRNA were also subcloned into the inducible Tet-pLKO-puro lentiviral vector (Addgene, Massachusetts, USA). Lentiviral particles were produced in HEK293T cells and titrated on breast cancer cells in medium containing puromycin (1.5 μg/ml) in order to achieve optimal knockdown (KD) of the target protein with minimal viral load. Transduced cells were treated with doxycycline (0.5 μg/mL) to achieve KD, where appropriate.
siGENOME siRNA targeting PIM1 (D-003923-01),, MYC (L-003282-02-0005) and non-targeting siRNA pool#2 (D-001206-14) control were purchased from Dharmacon (Thermo Scientific, Hemel Hempstead, UK), Silencer Select siRNA targeting PIM2 (s21751) was purchased from Ambion (Thermo Fisher Scientific, Massachusetts, USA). FlexiTube PIM3 siRNA#1 (S100684915), PIM3 siRNA#5 (S103084543) and non-targeting siRNA (S103650325) were purchased from Qiagen Ltd. (Manchester, UK). Cells were transfected with 20 nmol/L siRNA using Lipofectamine®RNAiMax transfection reagent (Invitrogen, Life Technologies, Paisley, UK), following manufacturer's instruction.
Overexpression vector
The full PIM1 cDNA sequence (NCBI Reference Sequence: NM_001243186.1) subcloned into the pDONR221 entry vector was obtained from Geneart (Life Technologies, Paisley, UK). This cDNA sequence incorporated silent mutations in the third codon positions of the sequence targeted by PIM1 shRNA#1, and the stop codon (TAG) was replaced with a Tyrosine (TAC) to generate a rescue construct insensitive to PIM1 shRNA. PIM1 cDNA from the entry vector was Gateway recombined into the pLenti6.2/C-Lumio/V5-DEST vector by using the Virapower C-Lumio Lentiviral Expression kit (Life Technologies, Paisley, UK). The kinase-dead PIM1 (PIM1K67M) construct was generated by introducing a site-specific mutation in the full PIM1 cDNA sequence (pDONR221 entry vector) corresponding to Lysine 67 to mutate it into a methionine residue, using the PCR-based site-directed mutagenesis kit (Stratagene, CA, USA). Primers for mutation were designed following Stratagene guidelines. The Myc overexpression vector was obtained from Addgene (Massachusetts, USA)24. The PIM1WT, PIM1K67M and the Myc sequences were Gateway recombined into the HA-tagged pInducer20 vector25. All the vectors were sequenced by SourceBioscience (Cambridge, UK). Lentiviral particles were produced in HEK293T cells.
Quantitative (q) RT-PCR
RNA was extracted from cells using the QIAZOL reagent (Qiagen Ltd., Manchester, UK), following manufacturer instruction. cDNA was prepared from 2μg total RNA in the presence of random examers and using an Applied Biosystems kit, according to manufacturer instruction. PCR was performed using a SYBR green-based detection system and employing a 7900HT Fast RT-PCR thermocycler (Applied Biosystems, Massachusetts, USA) at the Guy's genomics laboratory. We used the following primers: 5′-CGAGCATGACGAAGAGATCAT-3′ and 5′-TCGAAGGTTGGCCTATCTGA-3′ for PIM1; 5-GAGTCAACGGATTTGGTCGT-3′ and 5′-TTGATTTTGGAGGGATCTCG-3′ for GAPDH; 5-GGGCAGGATTGTGACTCTCATT-3′ and 5-GATGCAGCTTTCTTGGTTTATGG-3′ for MCL1. Primers for MYC (QT00035406), PIM2 (QT00086884) and PIM3 (QT00092197) were obtained from Qiagen Ltd. (Manchester, UK). Each set of primers was validated following standard PCR procedures: generation of one product only at the expected molecular weight. GAPDH was used as housekeeping target and the data were normalized using a standard comparative Ct method.
Cell population growth assay
Cells were plated in triplicates at 4,000 cells/well in 96-well plates. Cell population viability was determined over time using Cell Titer-Blue® (Promega, Southampton, UK), following manufacturer instruction, or by labelling DNA with Hoechst 33342 (Invitrogen, Life Technologies, Paisley, UK). Fluorescence was read at the FLUOstar Omega plate reader (BMG LabTech, Aylesbury, UK).
Caspase 3/7 activation assay
Cells were plated in triplicates at 4,000 cells/well in 96-well plates. Caspase activation was determined using the Caspase-Glo3/7® Assay (Promega, Southampton, UK), following manufacturer's instruction.
2D colony-formation assay
Cells were plated in triplicates at low density in 6-well plates. After 15-21 days, the cells were fixed with cold methanol and stained with 0.5% crystal violet solution. Colonies were counted with an e-count pen (Heathrow Scientific, Nottingham, UK)26. For drug treatments, paclitaxel and eribulin were provided as remnants by the Guy's Hospital Oncology Pharmacy. AZD1208 was provided by AstraZeneca (Wilmington, Delaware, USA). The culture medium was replaced every other day. For the drug combination treatments, AZD1208 was given 24 hours before chemotherapy, together with chemotherapy and every other day until the end of the experiment.
3D anchorage-independent growth assay (soft agar assay)
A total of 1000 single-suspended MDA-MB-231 cells were mixed in culture medium (37°C) containing 0.3% low melting point agar (Fermentas, Vilnius, Lithuania) and then seeded in 12-well plate dishes on top of a 0.6% agar (Life Technologies, Paisley, UK) layer. The AZD1208 drug (AstraZeneca) was given in a top/feeding culture medium, which was replaced every 4 days. After 4 weeks in culture, the colonies (composed of at least ∼20 cells) were counted by optical microscopy at ×200 magnification.
Dynamic BH3 profiling (DBP)
15 μl of BIM BH3 peptide at four different concentration of 0.2, 0.6, 2, and 6 μM was prepared in DTEB (300 mM Trehalose, 10 mM HEPES-KOH [pH 7.7], 80 mM KCl, 1 mM EGTA, 1 mM EDTA, 0.1% BSA, 5 mM succinate). 15 μl of each peptide concentration was added in triplicates per well of a black 384-well plate (NUNC). The plate was left at room temperature during the preparation of the single cell suspension. Cells, previously plated at 150,000 cells/well in 6-well plates, were trypsinized and collected 72h after NT or PIM1 siRNA transfection, or following AZD1208 or DMSO treatment. Single-cell suspensions were pelleted at 500g and resuspended and washed in DTEB before being resuspended in DTEB at a density of 2.67×106 (4× density). One volume of the cell suspension was added to one volume of the 4× dye solution previously prepared (4 μM JC-1, 40 μg/ml oligomycin, 0.02% digitonin, 20 mM 2-mercaptoethanol in DTEB). The 2× cell/dye solution was left at RT for 10 min to allow permeabilization and dye equilibration. 15 μl of the 2× cell/dye mix was then added to each treatment well of the plate. This led to a 0.1, 0.3, 1 and 3 μM final concentration of the BIM peptide in each well. The plate was then placed into the Infinite M200 pro TECAN plate reader. The plate was shaken for 15 s and individual wells were read at 590 nm over 3 hours time course every 5 min at 30°C. The obtained values were plotted as Δpriming. The difference in priming was determined by calculating the area under the DMSO control and the FCCP control minus the area under the individual peptide concentrations and the FCCP. These differences where then plotted as ΔΨn at RFU 590 nM.
Nanostring nCounter PanCancer Pathways gene expression analysis
RNA was extracted from cells using the QIAZOL reagent (Qiagen Ltd., Manchester, UK), following manufacturer instructions. Samples were quantitated using Nanodrop (Thermo Scientific, Hemel Hempstead, UK) and Qubit Fluorimetric Quantitation (Thermo Scientific, Hemel Hempstead, UK). The NanoString nCounter System (NanoString Technologies, Seattle, WA, USA) was used to measure gene expression of 12 samples. 100 ng total RNA was assessed using the nCounter PanCancer Pathways Panel targeting 730 genes representing all major cancer pathways including key driver genes. Expression data was normalized using nSolver analysis module and custom scripts in R 2.13.1. Background correction was done by subtracting the geometric mean of the 8 negative control probes. Expression values were normalized with most stable 31 housekeeping genes selected based on the geNorm algorithm. Expression values were then log2 transformed and standardized within each sample. All 730 genes in the present study were detected. Significant differential genes were identified by two-class comparison (control vs. experimental condition); volcano plot was used to display the fold change and p-value (using package ggplot and calibrate).
Animal studies
All animal experiments with cell line xenografts were approved by the King's College London Institutional Committees on Animal Welfare, and in compliance with the United Kingdom Home Office Animals Scientific Procedures Act, 1986. Female CD-1 Nu/Nu mice were obtained from Charles River UK Ltd. Procedures were carried out after 28-35 days of age. One million MDA-MB-231 or SUM149 cells mixed 1:1 in PBS/Matrigel™ were injected into inguinal fat pad of mice following standard procedures. When tumors reached 4 mm in diameter (assessed by palpation and calliper measurement) mice were randomized into groups (vehicle and AZD1208-treated, eribulin or combinations of eribulin and AZD1208). Animals were then dosed daily by oral gavage at 30 mg/kg (MDA-MB-231 xenografts) or 15 mg/kg (SUM149 xenografts) of AZD1208 or vehicle control (0.1% Tween 80, 0.5% methyl cellulose). Eribulin was administered by intravenous tail-vein injection at 0.1 mg/kg. Saline solution 0.9% was used as a vehicle. Tumor growth was monitored over time. When vehicle-treated tumors had reached 10mm in diameter the experiment was ended and tumors were excised and processed for Western blotting analysis (snap-frozen) and fixation in formalin for histological examination (FFPE).
Patient-derived xenotransplants (PDX) studies were performed at the Experimental Therapeutics Group (Vall d'Hebron Institute of Oncology, Barcelona, Spain), approved by the Ethical Committee on Animal's Healthcare and under the Patient's Informed Consent of the Vall d'Hebron's University Hospital. Foxn1-/- Nude mice were obtained from Janvier Labs (Le Genest-Saint-Isle, France). Procedures were carried out after 42-56 days of age. One 2 mm tumor piece was implanted into both inguinal fat pads of mice per model following standard procedures. When tumors reached 6 mm in diameter (assessed by palpation and caliper measurement) mice were randomized into groups (vehicle and AZD1208-treated, eribulin or combinations of eribulin and AZD1208). Animals were then dosed daily by oral gavage at 30 mg/kg AZD1208 or vehicle (0.1% Tween 80, 0.5% methyl cellulose). Eribulin was administered by intraperitoneal injection at 0.1 mg/kg. Saline solution 0.9% was used as a vehicle. When vehicle-treated tumors had reached 14mm in diameter the experiment was ended and tumors were excized and processed for Western blotting analysis (snap-frozen) and fixation in formalin for histological examination (FFPE).
For both studies, tumor volume was calculated using the formula: V= (π × length × width2 / 6), where length is the largest tumor diameter and width is the perpendicular diameter. For data analysis tumor volume was normalized to the volume at which treatment was started and expressed as a fold change relative to this. Statistical analysis was performed using Prism.
Histological analysis
Histological analysis was preformed on 3 μm thick tissue sections stained with routine hematoxylin (Hematoxylin Solution, Gill No.III, Sigma) and eosin (H&E) staining protocol. All the histological slides were digitalized at magnification ×20 (0.46 μm/pixel) using Hamamatsu Nanozoomer 2.0 HT (Hamamatsu Photonics, Hamamatsu, Japan). Digital images in ndp format were submitted for quantitative image analysis using HistoQuest 4.2 (Tissugnostic, Vienna, Austria) software. HistoQuest nucleus detection algorithm on the hematoxylin shade was used as a master channel to identify all the tumor cell nuclei and enabled cells quantification. The surface area of the tumor was also measured in the system and the value for tumor cell density calculated as number of cells/mm2.
In order to assess p27 staining HistoQuest software was optimized to detect the individual tumor cells and assess intensity of DAB staining in the cell nucleus. The software automatically detects tissue that is subjected to color separation module to differentiate between the blue and the brown shade. Nucleus detection algorithm on the hematoxylin shade was used to detect tumor cell nuclei and was defined as master channel. A nuclear mask was used to analyse the staining intensity of non-master chromogen (DAB). The staining intensity was measured as mean intensity of all pixels of a cell and the range of values is from 0 to 255.
Statistical analysis
Gene expression and copy-number statistical analyses were performed in the R environment, using several CRAN packages (http://cran.r-project.org/) as described above. Unpaired two-sided t-tests and one-way ANOVA Tukey multiple comparisons tests were performed using GraphPad Prism software for the analysis of the data obtained in all in vitro experiments and in vivo animal studies. For in vitro studies, no samples were processed and then excluded, all completed experiments are reported. For in vivo studies, we estimated that we need at least 6 samples per treatment to see a difference in effect, having a power of 80% with the probability of Type I Error (α = 0.05). Experiments were repeated to confirm treatment response. The sample size for all in vitro experiments was not chosen with consideration of adequate power to detect a pre-specified effect size. For in vivo studies, the total number of mice processed is indicated. Animals were excluded from the study if body weight reduced during treatment from more than 15% of weight at start of treatment. For the animal studies, investigators were blinded to the group allocation during the experiment and drug treatment. Investigators were also blinded when assessing the outcome by immunohistochemistry. For the animal studies, mice were randomized to treatment groups when tumors reached a predetermined diameter on a per experiment basis, as described above. For each data set, the data meet the assumptions of the statistical test used, as determined by distribution and variance.
Supplementary Material
Acknowledgments
This research was supported by the Breast Cancer Now funding at King's College London and the Institute of Cancer Research London and by the National Institute for Health Research Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. Patient samples and data were provided by King's Health Partners Cancer Biobank, London, UK, which is supported by the Experimental Cancer Medicine Centre at King's College London and the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award. We thank Sora Utting and Melanie Ferrao for technical and administrative assistance. We thank AstraZeneca for providing the AZD1208 compound and thank Kristen McEachern (AstraZeneca) and Dennis Huszar (AstraZeneca) for information provided on use of AZD1208. We thank Hasan Mirza (King's College London) for his support with bioinformatics analysis and Jacob Hurst (The Institute of Cancer Research London) for helping in the analysis of NanoString nCounter PanCancer Pathway analysis. We thank Mitch Dowsett (The Institute of Cancer Research London) for providing staff support for the NanoString work. We thank Tencho Tenev (The Institute of Cancer Research) for providing MDA-MB-231 with BCL2 pTIPZ overexpression vector. We thank the Guy's Hospital Pharmacy for providing the chemotherapeutic drugs. We also thank the NIH grant P30 CA008748 and The Breast Cancer Research Foundation.
Abbreviations
- [ ]
concentration
- ∼
around
- Ab
Antibody
- CN
Copy-Number
- BAD
BCL2-associated death promoter
- BCL2
B-cell lymphoma 2
- c-MYC
Cellular Myelocytomatosis Oncogene
- CNA
Copy-Number Aberration
- DBP
Dynamic BH3 Profiling
- KD
Knockdown
- MCL1
Induced myeloid leukemia cell differentiation protein
- Phosphorylated
(p-)
- n
number of samples
- NT
Non-Targeting
- PDX
patient-derived xenotransplant
- PIM1
proviral integration site for Moloney murine leukemia virus 1
- quantitative
q
- RNAi
RNA interference Ser, Serine
- SNP
single nucleotide polymorphism
- TNBC
Triple-negative breast cancer
- WB
Western Blotting
Footnotes
Breast Cancer Now Unit, Division of Cancer Studies, Faculty of Life Sciences and Medicine, King's College London, Guy's Hospital, London, SE1 9RT (UK)
Author Contributions: ANT, AG, FBM, SF and PM conceived and designed the research. FBM and SF performed the in vitro experiments with crucial help from SC, EN, SM, MS and APR. JW and JQ performed the bioinformatics analyses. RM, EFD, DP and LZ performed the cell line xenograft experiments. VS and AGO performed the PDX experiments. PG, EFD, LZ and FN performed IHC stainings. GL performed dynamic BH3 profiling analysis. RB performed the NanoString nCounter experiments and MC performed the statistical analysis of the gene expression analysis. PC and MS performed Western Blot analysis with the PIM-1:NOV22-39-5 antibody FBM analysed most of the results. FBM wrote the manuscript. ANT coordinated this work. All authors helped with data interpretation and manuscript editing.
Competing Financial Interests: The authors declare no competing financial interests
References
- 1.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 2.Symmans WF, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 3.Masuda H, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balko JM, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer discovery. 2014;4:232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labisso WL, et al. MYC directs transcription of MCL1 and eIF4E genes to control sensitivity of gastric cancer cells toward HDAC inhibitors. Cell cycle. 2012;11:1593–1602. doi: 10.4161/cc.20008. [DOI] [PubMed] [Google Scholar]
- 6.Andre F, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:441–451. doi: 10.1158/1078-0432.CCR-08-1791. [DOI] [PubMed] [Google Scholar]
- 7.Bergamaschi A, et al. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes, chromosomes & cancer. 2006;45:1033–1040. doi: 10.1002/gcc.20366. [DOI] [PubMed] [Google Scholar]
- 8.Turner N, et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29:2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nature reviews Cancer. 2011;11:23–34. doi: 10.1038/nrc2986. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene. 2010;29:2477–2487. doi: 10.1038/onc.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keeton EK, et al. AZD1208, a potent and selective pan-Pim kinase inhibitor, demonstrates efficacy in preclinical models of acute myeloid leukemia. Blood. 2014;123:905–913. doi: 10.1182/blood-2013-04-495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird PW, et al. In vivo analysis of Pim-1 deficiency. Nucleic acids research. 1993;21:4750–4755. doi: 10.1093/nar/21.20.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnuson NS, Wang Z, Ding G, Reeves R. Why target PIM1 for cancer diagnosis and treatment? Future oncology. 2010;6:1461–1478. doi: 10.2217/fon.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narlik-Grassow M, Blanco-Aparicio C, Carnero A. The PIM family of serine/threonine kinases in cancer. Medicinal research reviews. 2014;34:136–159. doi: 10.1002/med.21284. [DOI] [PubMed] [Google Scholar]
- 15.Aceto N, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nature medicine. 2012;18:529–537. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- 16.Speers C, et al. Identification of novel kinase targets for the treatment of estrogen receptor-negative breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6327–6340. doi: 10.1158/1078-0432.CCR-09-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Rinaldis E, et al. Integrated genomic analysis of triple-negative breast cancers reveals novel microRNAs associated with clinical and molecular phenotypes and sheds light on the pathways they control. BMC genomics. 2013;14:643. doi: 10.1186/1471-2164-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazinska P, et al. Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:955–966. doi: 10.1038/modpathol.2012.244. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canbay E. Erb-B2 homodimerization inhibits MUC1 transcription in cultured human mammary epithelial cells. Cell biology international. 2003;27:477–481. doi: 10.1016/s1065-6995(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 25.Aho TL, et al. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS letters. 2004;571:43–49. doi: 10.1016/j.febslet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 26.Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18:4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- 27.Juin P, Geneste O, Gautier F, Depil S, Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nature reviews Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 28.Montero J, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;160:977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbeek S, et al. Mice bearing the E mu-myc and E mu-pim-1 transgenes develop pre-B-cell leukemia prenatally. Molecular and cellular biology. 1991;11:1176–1179. doi: 10.1128/mcb.11.2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zippo A, De Robertis A, Serafini R, Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nature cell biology. 2007;9:932–944. doi: 10.1038/ncb1618. [DOI] [PubMed] [Google Scholar]
- 31.Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes & development. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguchi K, et al. Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. The Journal of biological chemistry. 1999;274:32580–32587. doi: 10.1074/jbc.274.46.32580. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, et al. Phosphorylation regulates c-Myc's oncogenic activity in the mammary gland. Cancer research. 2011;71:925–936. doi: 10.1158/0008-5472.CAN-10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandriani S, et al. A core MYC gene expression signature is prominent in basal-like breast cancer but only partially overlaps the core serum response. PloS one. 2009;4:e6693. doi: 10.1371/journal.pone.0006693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horiuchi D, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. The Journal of experimental medicine. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigoriadis A, et al. Molecular characterisation of cell line models for triple-negative breast cancers. BMC genomics. 2012;13:619. doi: 10.1186/1471-2164-13-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer research. 2008;68:5076–5085. doi: 10.1158/0008-5472.CAN-08-0634. [DOI] [PubMed] [Google Scholar]
- 38.Miura K, et al. Involvement of EphA2-mediated tyrosine phosphorylation of Shp2 in Shp2-regulated activation of extracellular signal-regulated kinase. Oncogene. 2013;32:5292–5301. doi: 10.1038/onc.2012.571. [DOI] [PubMed] [Google Scholar]
- 39.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. The oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 40.Kirschner AN, et al. PIM kinase inhibitor AZD1208 for treatment of MYC-driven prostate cancer. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/dju407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner NC, Reis-Filho JS. Tackling the diversity of triple-negative breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6380–6388. doi: 10.1158/1078-0432.CCR-13-0915. [DOI] [PubMed] [Google Scholar]
- 42.Morton JP, Sansom OJ. MYC-y mice: from tumour initiation to therapeutic targeting of endogenous MYC. Molecular oncology. 2013;7:248–258. doi: 10.1016/j.molonc.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horiuchi D, et al. PIM kinase inhibition presents a novel targeted therapy against triple-negative breast tumors with elevated MYC expression. doi: 10.1038/nm.4213. (Under review - cosubmitted with this manuscript) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrocca F, et al. A genome-wide siRNA screen identifies proteasome addiction as a vulnerability of basal-like triple-negative breast cancer cells. Cancer cell. 2013;24:182–196. doi: 10.1016/j.ccr.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia PD, et al. Pan-PIM kinase inhibition provides a novel therapy for treating hematologic cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:1834–1845. doi: 10.1158/1078-0432.CCR-13-2062. [DOI] [PubMed] [Google Scholar]
- 46.Malinen M, et al. Proto-oncogene PIM-1 is a novel estrogen receptor target associating with high grade breast tumors. Molecular and cellular endocrinology. 2013;365:270–276. doi: 10.1016/j.mce.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Raab MS, et al. 55th ASH Annual meeting and exposition. New Orleans, LA: 2013. Phase 1 Study Of The Novel Pan-Pim Kinase Inhibitor LGH447 In Patients With Relapsed/ Refractory Multiple Myeloma. [Google Scholar]
- 48.McEachern KA, et al. 56th ASH Annual meeting and exposition. San Francisco, CA: 2014. Preclinical and Clinical Pharmacodynamics of Pan-Pim Inhibition By AZD1208 in Acute Myeloid Leukemia: Assessment of Pim Isoform Dependency for Bad and 4EBP1 Phosphorylation. [Google Scholar]
- 1.Chandriani S, et al. A core MYC gene expression signature is prominent in basal-like breast cancer but only partially overlaps the core serum response. PloS one. 2009;4:e6693. doi: 10.1371/journal.pone.0006693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori S, et al. Utilization of pathway signatures to reveal distinct types of B lymphoma in the Emicro-myc model and human diffuse large B-cell lymphoma. Cancer research. 2008;68:8525–8534. doi: 10.1158/0008-5472.CAN-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bild AH, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 4.Zeller KI, Jegga AG, Aronow BJ, O'Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome biology. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donnell KA, et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Molecular and cellular biology. 2006;26:2373–2386. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez PC, et al. Genomic targets of the human c-Myc protein. Genes & development. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YH, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene. 2006;25:130–138. doi: 10.1038/sj.onc.1208997. [DOI] [PubMed] [Google Scholar]
- 8.Lastowska M, et al. Comprehensive genetic and histopathologic study reveals three types of neuroblastoma tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3080–3090. doi: 10.1200/JCO.2001.19.12.3080. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nature genetics. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 10.Menssen A, Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6274–6279. doi: 10.1073/pnas.082005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlosser I, et al. Dissection of transcriptional programmes in response to serum and c-Myc in a human B-cell line. Oncogene. 2005;24:520–524. doi: 10.1038/sj.onc.1208198. [DOI] [PubMed] [Google Scholar]
- 12.Schuhmacher M, et al. The transcriptional program of a human B cell line in response to Myc. Nucleic acids research. 2001;29:397–406. doi: 10.1093/nar/29.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu D, Cozma D, Park A, Thomas-Tikhonenko A. Functional validation of genes implicated in lymphomagenesis: an in vivo selection assay using a Myc-induced B-cell tumor. Annals of the New York Academy of Sciences. 2005;1059:145–159. doi: 10.1196/annals.1339.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rinaldis E, et al. Integrated genomic analysis of triple-negative breast cancers reveals novel microRNAs associated with clinical and molecular phenotypes and sheds light on the pathways they control. BMC genomics. 2013;14:643. doi: 10.1186/1471-2164-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazinska P, et al. Comparison of basal-like triple-negative breast cancer defined by morphology, immunohistochemistry and transcriptional profiles. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:955–966. doi: 10.1038/modpathol.2012.244. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoriadis A, et al. Molecular characterisation of cell line models for triple-negative breast cancers. BMC genomics. 2012;13:619. doi: 10.1186/1471-2164-13-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen M, et al. Allele-specific copy number analysis of tumor samples with aneuploidy and tumor heterogeneity. Genome biology. 2011;12:R108. doi: 10.1186/gb-2011-12-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, et al. Phosphorylation regulates c-Myc's oncogenic activity in the mammary gland. Cancer research. 2011;71:925–936. doi: 10.1158/0008-5472.CAN-10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meerbrey KL, et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3665–3670. doi: 10.1073/pnas.1019736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nature protocols. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.