Abstract
Inflammatory diseases such as arthritis are chronic conditions that fail to resolve spontaneously. While the cytokine and cellular pathways triggering arthritis are well defined, those responsible for the resolution of inflammation are incompletely characterized. Here we identified IL-9-producing type 2 innate lymphoid cells (ILC2s) as a molecular and cellular pathway that orchestrates the resolution of chronic inflammation. In mice, the absence of IL-9 impaired ILC2 proliferation, activation of regulatory T cells (Treg) and resulted in chronic arthritis with excessive cartilage destruction and bone loss. In contrast, treatment with IL-9 promoted ILC2-dependent Treg activation and effectively induced resolution of inflammation and protection of bone. Rheumatoid arthritis patients in remission demonstrated high numbers of IL-9+ ILC2s in the joints and in the circulation. Hence, fostering IL-9-mediated ILC2 activation may offer a novel therapeutic approach inducing resolution of inflammation rather than suppression of inflammatory responses.
Keywords: Resolution of inflammation, chronic inflammation, IL-9, innate lymphoid cells, arthritis, bone loss
Introduction
Resolution of inflammation is still incompletely understood. In chronic inflammatory diseases the physiological process of resolution of inflammation is impaired 1,2. Diseases such as rheumatoid arthritis (RA) often start in young adulthood, but fail to wane requiring life-long immunosuppressive treatment3. Furthermore, the body’s failure to effectively terminate inflammation leads to pronounced tissue damage such as bone loss4,5. Current treatment strategies for chronic inflammatory diseases like RA target pro-inflammatory cytokines and hence the activation process of inflammation rather than promoting its resolution6,7. While lipid mediators such as resolvins have been implicated in resolution of inflammation8, cytokine pathways for governing this process are largely undefined to date. Identification of such pathways, however, would allow rebalancing of inflammatory responses rather than generally suppressing inflammation and may substantially add to the development of new treatment possibilities. We were therefore interested in identifying novel pathways governing the resolution of arthritis. We identified interleukin (IL)-9 as a master regulator for resolution of arthritis and found that type 2 innate lymphoid cells (ILC2s), which produce IL-9 are essential for the initiation of this resolution process.
Results
Antigen-induced arthritis (AIA) is a standard model of arthritis with spontaneous resolution (Supplementary Fig. 1a)9,10. In contrast, we observed that the course of AIA in Il9−/− mice was highly chronic (Fig. 1a). While joint swelling resolved spontaneously within 12–16 days in WT mice, it persisted beyond 42 days without signs of resolution in Il9−/− mice. The chronic inflammatory phenotype of Il9−/− mice was rescued by overexpression of IL-9 via hydrodynamic gene transfer (HDGT), using mini-circle (MC) vectors encoding IL-9 (Il9 MC) (Fig. 1b). Histological analysis of the affected knee joints of Il9−/− mice at d42 confirmed persistent synovitis and demonstrated excessive degradation of cartilage and bone and higher numbers of osteoclasts in Il9−/− compared to WT mice (Fig. 1c–e). Microcomputed tomography showed a pronounced loss of the trabecular network and bone volume as signs of inflammation-induced osteopenia in Il9−/− mice (Fig. 1f). In contrast to AIA, absence of IL-9 did not affect the course of acute inflammatory arthritis induced by monosodium urate crystal, which exclusively relies on neutrophil activation (Supplementary Fig. 1b).
Figure 1. Chronic arthritis in Il9-deficient mice.
(A, B) Antigen-induced arthritis (AIA) in littermates of (A) wild-type (WT) (n=9) and Il9−/− mice (n=11) of 3 independent experiments; Y-axis shows knee swelling and the area under the curve (AUC) of knee swelling. (B) Overexpression of IL-9 by hydrodynamic gene therapy (HDGT) with mini-circles (MC) encoding for IL-9 in WT (n=7) and Il9−/− (n=8) mice at day 22 of AIA. Control mini-circles (ctrl MC) were also injected into WT (n=6) and Il9−/− (n=8) mice. (C, D) Sections of knee joints from control (ctrl) and AIA-induced mice stained with (C) hematoxylin and eosin or (D) Safranin-O at day 42 of AIA. Histomorphometric analysis (n>6) of the area of inflammation, proteoglycan loss, chondrocyte number and cartilage thickness. A representative image of each group is included. (E) Histomorphometric analysis of osteoclast numbers in the tibia of WT and Il9−/− mice (n>6) at day 42 of AIA. A representative image of each group is included. N.Oc/B.Pm, number of osteoclasts per bone parameter; Oc.S/BS, osteoclast surface per bone surface. (F) Micro-computed tomography scans and assessment of microarchitectural parameters of tibial bone in ctrl and AIA WT and Il9−/− mice (n=6 each) at 14–16 weeks of age. BV/TV, bone volume per total volume. Data are shown as the mean ± SEM. Scale bars correspond to 100 μm. *p<0.05, **p<0.01, ***p<0.001 determined by Student’s t test (a, c–f) or one-way analysis of variance (ANOVA) with Tukey’s post hoc test (a, b) for experiments including more than 2 groups in one experiment.
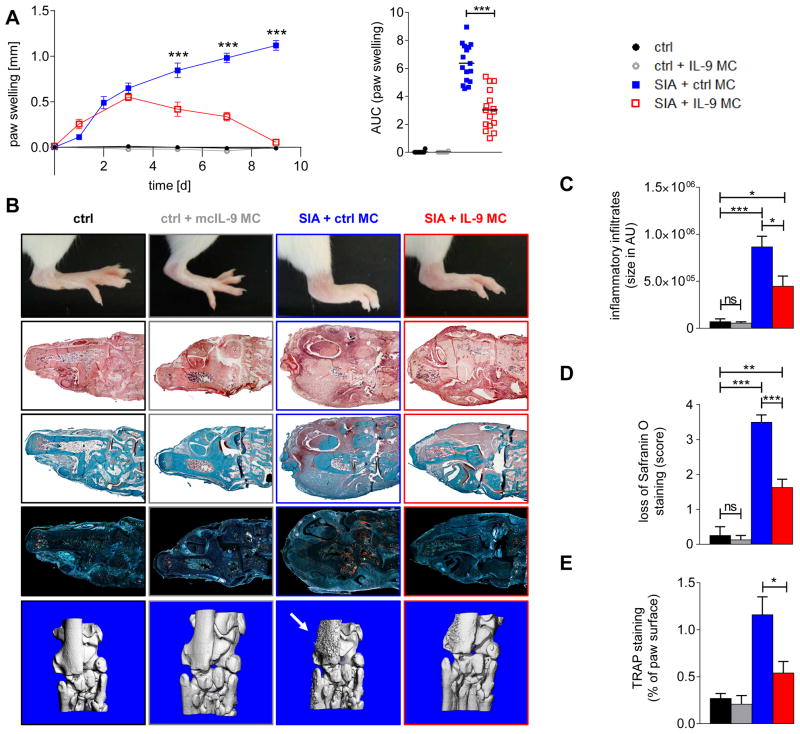
Mice expressing the transgenic T cell receptor (TCR) KRN and the MHC class II allele Ag7 (K/BxN mice) develop autoantibodies against glucose-6-phosphate isomerase11. Passive transfer of serum from those mice is commonly used to initiate chronic arthritis (SIA)12 with inflammation persisting over several weeks. To assess the therapeutic potential of IL-9 to promote its resolution, we overexpressed IL-9 using HDGT during the effector phase of SIA (3 days after induction of SIA). Consistent with our previous findings in AIA, overexpression of IL-9 did not have major effects on the initiation phase of SIA or on the maximal intensity of arthritis, but strongly accelerated its resolution (Fig. 2a). Joint swelling completely resolved within 9 days in mice with forced expression of IL-9, whereas arthritis in mice injected with the control vector was still worsening at day 9 (Fig. 2a, b). Histological analysis at day 9 showed substantially less synovitis in the paws of mice injected with Il9 MC (Fig. 2b, c). Accelerated resolution of arthritis by IL-9 translated into reduced tissue damage with preservation of cartilage integrity, reduced osteoclast counts and decreased bone erosions (Fig. 2b–e).
Figure 2. IL-9 accelerates the resolution of arthritis.
(A) K/BxN serum induced arthritis (SIA) and non-arthritic mice (ctrl) receiving hydrodynamic gene therapy (HDGT) with mini-circles over-expressing IL-9 (Il9 MC) (SIA: n=16; ctrl: n=10) or control vector (SIA: n=10; ctrl: n=10). Y-axis shows paw swelling and the area under the curve (AUC) of paw swelling. (B) Representative images of paw swelling (line 1), hematoxylin and eosin (H&E) staining of joint tissue (line 2), safranin O staining (line 3), tartrate-resistant acid phosphatase (TRAP) staining (line 4) and micro-computed tomographies (line 5) of the respective groups. (C) Quantification of inflammation on H&E stains (D) cartilage damage on safranin-O stains and (E) bone erosions on TRAP stains. All data are shown as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test.
To further characterize the mechanism by which IL-9 fosters the resolution of arthritis, the kinetics of pro- and anti-inflammatory mediators were analyzed in the serum and joints of arthritic Il9−/− and WT mice (Fig. 3a, Supplementary. Fig. 2a, b). Time kinetics and concentrations of most key cytokine mediators such as tumor necrosis factor α (TNF-α), IL-6, interferon (IFN)-γ, IL-2 and IL-4 did not significantly differ between WT and Il9−/− mice. However, pronounced differences in IL-17 levels were observed which remained persistently high in Il9−/− AIA mice, but returned to baseline levels in WT AIA mice. The selective upregulation of IL-17 was associated with enhanced Th17 polarization particularly in inflamed joints of Il9−/− mice, whereas naïve, memory and effector T cell counts were comparable in WT and Il9−/− mice (Fig. 3b, c). These data demonstrate a shift to a persistent Th17-cell-driven immune response in Il9−/− mice. To address the possibility that IL-9 serves as an intrinsic regulator of Th17 differentiation, CD4 T cells from WT and Il9−/− mice were stimulated under Th17-inducing conditions. Differentiation into conventional as well as into inflammatory Th17 cells was comparable in WT and Il9−/− mice (Fig. 3d) providing no evidence for an intrinsic defect in Th17 development in Il9−/− mice but arguing for a central role of other cell types for the IL-9-induced inhibition of Th17 polarization.
Figure 3. Altered Th17 and Treg responses in arthritic Il9−/− mice.
(A) Heat map of serum levels of indicated cytokines from AIA WT (n=9) and Il9−/− (n=11) littermates before immunization (d0), after inoculation (d22) and after resolution of inflammation in WT mice (d42). (B) CD4pos cell compartment in control (ctrl) and draining lymph nodes (LN) of AIA WT (n=6) and Il9−/− (n=6) mice analyzed for memory (CD44high), effector (CD44low) and naïve (CD62Lpos) T cells at day 42 of AIA. Representative dot plot is included. (C) Th17 cells in ctrl/draining LN, and ctrl/affected knee joint of AIA WT (n=6) and Il9−/− (n=6) mice assessed by flow cytometry at day 24, 27 and 42 of AIA. (D) Differentiation of CD4+ T cells from WT (n=4) and Il9−/− (n=4) mice into conventional (c)Th17 and inflammatory (i)Th17 cells. Th0-stimulating condition served as control. (E) Quantification of CD4posCD25posFoxP3pos Tregs in ctrl and draining LN of AIA WT (n=6) and Il9−/− (n=6) mice at day 42 of AIA. (F) Treg suppression assay: suppressive capacity of CD4posCD25posFoxP3pos Tregs from WT (n=7) and Il9−/− (n=7) mice. Cell proliferation of CD4posFoxP3neg responder cells (Teff) was assessed by the dilution of the fluorescent dye CFSE to dividing daughter cells. Representative histograms are included. Suppression was calculated using the division index. (G) mRNA expression levels of FoxP3 and co-stimulatory receptors GITR and ICOS on sorted CD4posCD25highFoxP3pos Tregs (n=8 each) stimulated with anti-CD3/28 in the presence and absence of recombinant IL-9 for 48h. (H) Treg suppression assay: susceptibility of WT and Il9−/− (n=6 each) Teff to become suppressed by WT Tregs. (I) WT Treg-induced suppression of cytokines released from WT and Il9−/− Teffs (n=6 each). All data are shown as the mean ± SEM of 3–6 independent experiments of each group. *p<0.05, **p<0.01, ***p<0.001 determined by Student’s t test (b, e) or ordinary one-way ANOVA with Tukey’s post hoc test (c, d, f–i).
To identify these target cells of IL-9, we next analyzed regulatory T cells (Tregs) in WT and Il9−/− mice at day 42 after immunization, when inflammation was resolved in WT mice, but still persisted in Il9−/− mice. Total numbers of CD4posCD25posFoxp3pos Tregs were comparable in Il9−/− compared to WT mice (Fig. 3e). However, the suppressive capacity of Tregs from Il9−/− mice was significantly decreased as shown by co-culture of CFSE-labeled CD25negFoxp3neg effector T cells (Teffs) with Foxp3pos Tregs and subsequent analyses of proliferation of Teffs (Fig. 3f). The functional defect of Il9−/− Tregs was associated with significantly decreased expression levels of functionally important effector molecules13,14 such as GITR and ICOS in Il9−/− Tregs (Fig. 3g). To exclude the possibility that effector T cells from Il9−/− mice are resistant to the effects of Tregs, the susceptibility of Il9−/− and WT effector T cells to the suppressive effects of WT Tregs was assessed. Both, proliferation and cytokine production did not differ between Il9−/− and WT responder T cells (Fig. 3h, i).
In line with previous reports, exogenous IL-9 modestly enhanced Treg function in vitro (data not shown)15. However, prestimulation of Tregs from Il9−/− mice with IL-9 and adoptive transfer of these cells into Il9−/− mice did not prevent chronification of AIA (Supplementary Fig. 3a). We therefore further aimed to define cellular intermediates that might be involved in IL-9-induced resolution of inflammation. To explore the source of IL-9 in arthritis, AIA was induced in Il9citrine reporter mice16. The majority of IL-9-producing cells did not express lineage (Lin) specific markers that define Linpos lymphocytic and myeloid populations including potential IL-9-producing cell types such as Th9 cells17–20 and mast cells21 (Fig. 4a). More than 80% of the Linneg citrinepos cells expressed ST2, ICOS (Fig. 4a), CD25, CD90, and Sca-1 (Supplementary Fig. 3b) suggesting that innate lymphoid cells type 2 (ILC2) are the predominant source of IL-9 during the resolution phase of arthritis, as defined as the segment of time between the peak of inflammation and 50% regression22 (Fig. 4a). These data were supported by quantitative analysis of synovial tissue sections from AIA WT mice stained with immunofluorescence labeled antibodies. ILC2s were identified as the major source of IL-9 during resolution of AIA (Supplementary Fig. 3c). Furthermore, expression levels of mast cell-specific genes in inflamed joints did not differ between WT and Il9−/− mice (Supplementary Fig. 3d, e). ILC2 numbers were profoundly decreased in the synovium of Il9−/− mice (Fig. 4b). Impaired proliferation may account for the reduced numbers of ILC2s, as the number of proliferating Ki67 positive ILC2s was significantly decreased in arthritic knee joints of Il9−/− mice (Fig. 4b). In line with previous reports that IL-9 production by ILC2 acts in an autocrine loop to promote ILC proliferation23,24, induction of ILC2s by overexpression of IL-25 and IL-33 using HDGT confirmed an intrinsic defect of ILC2 proliferation in Il9−/− mice (Supplementary Fig. 4a). Addition of IL-9 led to complete reconstitution of ILC2s. To confirm the relevance of this finding for the pathogenesis of chronic arthritis, multicolor immunofluorescence microscopy (IF) revealed that IL-9-producing ILC2s are located in close proximity to CD3posFoxp3pos Tregs in the inflamed synovium (Fig. 4c). The co-localization of ILC2s and Tregs in the inflamed tissue supports cellular interactions that might be of functional relevance for Treg suppression. Indeed, stimulation of ILC2s with IL-9 induced upregulation of the Treg-receptor-associated ligands GITRL and ICOSL (Fig. 4d).
Figure 4. ILC2s sustain suppressive capacity of Tregs by co-stimulation via ICOS-L/ICOS and GITR-L/GITR.
(A) Flow cytometric analysis of AIA in Il9citrine reporter mice (n=7). Cells isolated from joints at day 27 were stratified due to viability, IL-9, CD45, expression of lineage markers (Lin; CD3ε, CD11b, CD11c, CD45R, CD49b, FcER1a, Gr-1, TER-119), ICOS, and ST2. Representative plots are shown. (B) Quantification of ILC2s in ctrl and arthritic joints from AIA WT and Il9−/− mice at day 27 (n=5 each) by flow cytometry. Ki67 was co-stained to assess proliferation of ILC2s. (C) IF microscopy of inflamed joints of WT mice (n=5) with AIA at day 27 stained for Foxp3, IL-9, ICOS and CD3ε. Randomly chosen Tregs from 5 tissue sections per mouse were analyzed for surrounding cells stratified by IL-9 production and surface markers. Data are shown as mean ± SEM. Representative images are shown. (D) mRNA expression levels of GITRL and ICOSL in sorted ILC2s cultured in the presence and absence of recombinant IL-9 for 72h (n=5 each). (E–G) Treg suppression assay; suppressive capacity of CD4posFoxP3pos Tregs from WT and Il9−/− mice: (E) co-cultures w/o ILC2s in the presence/absence of blocking antibodies (Ab) against GITRL or ICOSL (n>5 each). (F) Pre-stimulation of Il9−/− Tregs with recombinant ICOSL and agonistic anti-GITR before co-culture with responder cells (Teff; n>5 each); isotype control (iso ctrl) antibody was used as control. Representative histograms are included. Suppression was calculated using the division index. Data are shown as the mean ± SEM of 3 independent experiments of each group. (G) Il9−/− Tregs were pre-stimulated ex vivo with recombinant ICOSL and agonistic anti-GITR before adoptive transfer into Il9−/− mice induced for AIA (n=5 each). (H) ILC2s were adoptively transferred into Il9−/− mice induced for AIA (n=5 each). Y-axis shows knee swelling and the area under the curve (AUC) of knee swelling. All data are shown as the mean ± SEM of 3 independent experiments of each group. *p<0.05, **p<0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test (a–c, e–h) or Student’s t test (b, d).
To determine the functional impact of interactions between ILC2s and Tregs, we performed Treg suppression assays in the presence and absence of ILC2s. These assays demonstrated that ILC2s stimulated the suppressive capacity of Tregs. Whereas Il9−/− Tregs alone did not suppress Teffs proliferation, addition of ILC2s completely rescued this impaired suppressive capacity of Il9−/− Tregs (Fig. 4e). Transwell experiments revealed that these ILC2-mediated effects on Il9−/− Tregs required direct cell contacts (Supplementary Fig. 4b). Screening for potential mediators of this contact dependent effect revealed that ILC2s express high levels of GITRL and ICOSL, which are known to promote the suppressive capacity of Tregs13,14. Blockade of GITR/GITRL and ICOS/ICOSL reversed the ILC2 mediated effects on the suppressive capacity of Il9−/− Tregs (Fig. 4e). Consistent with this model, ligand binding to GITR and ICOS in Il9−/− Tregs also restored the suppressive capacity of Il9−/− Tregs in the absence of ICOSL/GITRL-bearing ILC2s (Fig. 4f). In vivo, adoptive transfer of Il9−/− Tregs, which had been pre-activated via GITR and ICOS ex vivo, led to a regain of their suppressive activity in the AIA model (Fig. 4g), highlighting that receptor/ligand specific interactions between ILC2s and Tregs activate the suppressive capacity of Tregs. In order to confirm the functional impact of ILC2s on the resolution of inflammation, ILC2s were adoptively transferred into Il9−/− mice with AIA. Adoptive transfer of ILC2s inhibited activation of Th17 cells and promoted resolution of inflammation in Il9−/− mice (Fig. 4h, Supplementary Fig. 4c).
To investigate the role of IL-9 for the resolution of inflammation in humans, synovial tissues of patients with RA as prototypical chronic inflammatory joint disease were analyzed. In line with previous reports25,26, the dominant part of IL-9 expression in the synovial membranes of active RA patients came from Linpos cells (Fig. 5a, b). Only low numbers of IL-9pos ILC2s were found in active RA despite extensive synovial inflammation. In contrast, RA patients in clinical remission exhibited high numbers of Linneg IL-9pos ILC2s and a significant decline of Linpos IL-9pos cells (Fig. 5a, b). Patients with acute joint inflammation after trauma demonstrated only a tendency towards increased numbers of Linpos IL-9pos and Linneg IL-9pos cells compared to healthy individuals. Longitudinal analysis of infiltrates in synovial tissue of RA patients before and 6 months after start of anti-inflammatory treatment showed a shift in the cellular source of IL-9 from Linpos IL-9pos cells during active disease to Linneg IL-9pos ILC2s 6 months later when anti-rheumatic drugs had led to remission of arthritis (Fig. 5c). Stratification of RA patients according to disease activity revealed that ILC2 numbers in the blood were particularly low in RA patients with persistent inflammatory activity, but significantly higher in RA patients that were in disease remission (Fig. 5d, e). ILC2 numbers significantly correlated with disease activity in RA as measured by standardized disease activity score 28 (DAS28) (Fig. 5e). Longitudinal observations of patients revealed a reciprocal link between ILC2s and disease activity (Fig. 5f). These data, taken in combination with the results from the mouse models, indicate a pivotal role of ILC2s in the resolution of chronic inflammation and prevention of immunochronicity.
Figure 5. IL-9 during resolution of human arthritis.
(A–C) IF microscopy of human synovial biopsies stained for lineage markers (Lin; CD3, CD11b, CD16, mast cell tryptase), ICOS, IL-9 and DAPI. (A) Histomorphometric analysis was performed by quantification of Linpos, Linpos/IL-9pos and Linneg/ICOSpos/IL-9pos (ILC2) cells per 0.3 mm2 of synovial tissue by five independent and blinded researchers. Data are shown as the mean ± SEM. Included are normal controls (n=11), patients with acute trauma (n=8), rheumatoid arthritis (RA) patients with active disease (n=19; DAS28 score >3.2) and RA patients in remission (n=19; DAS28 score <2.6). (B) Representative IF images; (C) Quantification of Linpos, Linpos/IL-9pos and Linneg/ICOSpos/IL-9pos (ILC2) cells in RA patients with high disease activity (DAS28: 5.1 ± 1.2; n=10) before and after 6 months of treatment with anti-rheumatic drugs inducing disease remission. (D–F) Absolute counts of ILC2s in the blood of RA patients (n=111); (D) ILC2 counts in patients with active disease (DAS28≥3.2; n=61) and inactive disease (DAS28<2.6; n=50); data are shown as the mean ± SEM. (E) Correlation between ILC2 counts and disease activity score (DAS28); the correlation analysis used was nonparametric (Spearman’s correlation). (F) Longitudinal observation of ILC2 counts at baseline and 6–12 month follow-up in RA patients (n=63) stratified into 4 groups according to baseline and follow-up disease activity (inactive vs. active). *p<0.05, **p<0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test (a) or Student’s t test (c, d).
Discussion
Our data demonstrate that IL-9 fosters resolution of inflammation and prevents the chronification of arthritis. IL-9 virtually exclusively affected the resolution phase of the disease, while only minor effects were observed in the induction phase. In some experiments a tendency to higher peak inflammation was observed in IL-9 deficient mice although these effects were only mild and not consistent among the experiments. These observations may be explained by the regulatory action of IL-9 starting already before the peak of inflammation has been reached. Nonetheless induction of resolution of arthritis clearly came up as the primary action of IL-9 in arthritis contrasting the effects of other known cytokines involved in arhritis such as TNFα, IL-6 and IL-17, which are primarily involved in the induction phase and which are successfully targeted by modern cytokine inhibitors. Our data show that resolution of arthritis is mechanistically based on the induction of ILC2s by IL-9, which in turn elicits GITR/GITRL and ICOS/ICOSL dependent activation of Tregs. This IL-9-mediated forced resolution of arthritis translates into reduced tissue damage such as bone and cartilage loss, which usually result from chronic inflammation in the context of arthritis. Our data provide evidence that IL-9 has a dichotomous function in different phases of inflammation. Previous studies have shown that under certain circumstances IL-9 can promote inflammation in acute models15,16,27–28. In chronic arthritis however, IL-9 acts as a cytokine governing the resolution phase of the disease. These findings are remarkable as resolution of inflammation is preferentially attributed to lipid mediators such as resolvins to date and little was known about innate/adaptive immune system interactions in orchestrating the resolution process2. IL-9 and the function of ILC2s in this process provide strong support for the existence of immune pathways, which primarily foster the resolution of inflammation and restore immune homeostasis in chronic inflammatory diseases. From the therapeutic perspective such approaches are highly attractive as they provide an anchor for allowing re-balancing of the pathologic inflammatory response in the near future rather than exposing patients to a broad suppression of inflammatory responses.
Supplementary Material
(A) Schematic protocol of AIA model. (B) Monosodium urate (MSU) crystals induced acute arthritis in littermates of WT (n=9) and Il9−/− mice (n=9); Y-axis shows paw swelling and the area under the curve (AUC) of paw swelling. Data are shown as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test.
(A) Serum levels of indicated cytokines at day 0, 22 and 42 in AIA WT (n=9) and Il9−/− mice (n=9) assessed by bead-based ELISA. (B) Levels of indicated cytokines in knee joints of AIA WT (n=5) and Il9−/− mice (n=5) at day 24, 27 and 42. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test.
(A) AIA in WT (n=6) and Il9−/− mice (n=6); Y-axis shows knee swelling relative to control and the area under the curve (AUC) of knee swelling. As indicated, sorted Tregs from Il-9−/− mice were pre-stimulated (ps) with recombinant IL-9 for 72h before adoptive transfer into Il9−/− mice (n=6). Transfer of WT Tregs into Il9−/− mice (n=6) served as control. (B) Cytometric co-staining of citrine positive cells isolated from inflamed joints of Il9citrine reporter mice (n=8) at day 27. Representative histograms of fluorescence-minus controls (FMO; light grey) and the respective markers CD25, CD90, Sca-1 and FcERIa (dark grey) are shown; respective geometric mean fluorescence intensity (gMFI) of 8 independent experiments. (C) Quantitative analysis of IL-9 positive cells in the affected knee joints from WT mice upon induction of AIA (n=6) with immunofluorescence staining for CD3ε, DAPI, ICOS, IL-9. CD3+/IL-9+ (T cells), ICOS+/IL-9+/CD3− (ILC2s) and IL-9+/CD3−/ICOS− (others) cells were counted per 0.3 mm2 of synovial tissue. (D, E) mRNA expression levels of mast cell associated genes in inflamed joints of (D) AIA and (E) SIA of 4–6 mice of each group; expression levels were normalized to B2m. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ** p≤0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test (a, c, e) or Student’s t test (d).
(A) Induction of ILC2s by hydrodynamic gene therapy with IL-25 and IL-33 in WT and Il9−/− mice with and without supplementation of IL-9. Data are shown as the mean ± SEM of 3–6 independent experiments of each group. (B) Suppressive capacity of CD4posFoxP3pos Tregs from WT and Il9−/− mice co-cultured w/o ILC2s in a transwell system. Cell proliferation of CD4posFoxP3neg responder cells (Teff) was assessed by the dilution of the fluorescent dye CFSE to dividing daughter cells. Suppression was calculated using the division index. (C) Serum levels of IL-17 at day 0, 22 and 44 in AIA WT and Il9−/− mice w/o adoptive transfer of ILC2s. Data are shown as the mean ± SEM of at least 6 mice of each group. (D) Cytometric gating strategy to identify human ILC2s. Representative dot plots are shown. (E) Survival and persistence of ILC2s within the knee joint of WT and Il9−/− mice with AIA upon intraarticular injection (CD45.2; n=4 respectively). ILC2s were sorted from CD45.1 mice and injected intra-articularly together with mBSA at day 21. Cytometric single-cell analysis of digested synovial tissue was performed at day 27 of AIA. Representative dot plots are included. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ** p≤0.01 determined by one-way ANOVA with Tukey’s post hoc test.
Acknowledgments
The authors thank to Mónica Pascual, Katja Dreissigacker, Regina Kleinlein, Maria Comazzi, Daniela Weidner and Barbara Happich for excellent technical assistance. We thank Uwe Appelt and Markus Mroz of the Core Unit Cell Sorting and Immunomonitoring Erlangen for cell sorting. This work was supported by the Deutsche Forschungsgemeinschaft (RA 2506/3-1, RA 2506/4-1 to A.R.; DI 1537/5-1, DI 1537/7-1, DI 1537/8-1, DI 1537/9-1, DI 1537/11-1 to J.H.W.D.; SCHE 1583/7-1 to G.S.; SPP1468-IMMUNOBONE and CRC1181 to G.S. and J.H.W.D.), the Bundesministerium für Bildung und Forschung (METHARTHROS to G.S. and J.H.W.D.), the Marie Curie project OSTEOIMMUNE (to G.S. and J.H.W.D.), the TEAM project of the European Union and the IMI funded project RTCure (to G.S.), Else Kröner-Fresenius-Stiftung 2014_A184 (to A.R.), the Interdisciplinary Centre for Clinical Research, Erlangen (A64 to J.H.W.D., J40 to A.R.), the ELAN Fonds of the Universitätsklinikum Erlangen (16-10-05-1 to A.R.), the Career Support Award of Medicine of the Ernst Jung Foundation (to J.H.W.D.), SNF Sinergia CRSII3_154490 (to O.D.), UK-MRC (UI015178805; to A.N.J.M.), the Wellcome Trust (100963/Z/13/Z; to A.N.J.M.) as well as NIH grant AI057459 (to M.H.K.).
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Author Contributions
Design of the study: S.R., G.S., J.H.W.D., A.R.
Acquisition of data: S.R., M.L., S.We., L.M., A.S., T.W., N.-Y.L., K.D., M.G., A.R.
Interpretation of data: S.R., A.B., M.H., A.N.J.M., B.W., M.M.Z., U.F., D.J.V., J.D.C., O.D., F.R., C.P., S.W., M.F.N., G.S., J.H.W.D., A.R.
Support of material: A.B., M.H., M.H.K., B.W., U.F., D.J.V., J.D.C., O.D., F.R., C.P., M.F.N., A.N.J.M., S.W.
Manuscript preparation: S.R., G.S., J.H.W.D., A.R.
References
- 1.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 4.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nature reviews Rheumatology. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman L, Merrill JT, McInnes IB, Peakman M. Optimization of current and future therapy for autoimmune diseases. Nature medicine. 2012;18:59–65. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- 7.Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nature medicine. 2013;19:822–824. doi: 10.1038/nm.3260. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kehoe O, Cartwright A, Askari A, El Haj AJ, Middleton J. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. Journal of translational medicine. 2014;12:157. doi: 10.1186/1479-5876-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brackertz D, Mitchell GF, Mackay IR. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977;20:841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- 11.Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Current protocols in immunology/edited by John E. Coligan … [et al.] 2008;Chapter 15(Unit 15):22. doi: 10.1002/0471142735.im1522s81. [DOI] [PubMed] [Google Scholar]
- 12.Kollias G, et al. Animal models for arthritis: innovative tools for prevention and treatment. Ann Rheum Dis. 2011;70:1357–1362. doi: 10.1136/ard.2010.148551. [DOI] [PubMed] [Google Scholar]
- 13.Ji HB, et al. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 14.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 15.Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlach K, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–686. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhoen M, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 20.Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A. Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood. 2012;119:4665–4674. doi: 10.1182/blood-2011-11-392589. [DOI] [PubMed] [Google Scholar]
- 21.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 22.Bannenberg GL, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JE, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciccia F, et al. Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology. 2015;54:2264–72. doi: 10.1093/rheumatology/kev252. [DOI] [PubMed] [Google Scholar]
- 26.Kundu-Raychaudhuri S, et al. IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine. 2016;79:45–51. doi: 10.1016/j.cyto.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Chang HC, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic protocol of AIA model. (B) Monosodium urate (MSU) crystals induced acute arthritis in littermates of WT (n=9) and Il9−/− mice (n=9); Y-axis shows paw swelling and the area under the curve (AUC) of paw swelling. Data are shown as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test.
(A) Serum levels of indicated cytokines at day 0, 22 and 42 in AIA WT (n=9) and Il9−/− mice (n=9) assessed by bead-based ELISA. (B) Levels of indicated cytokines in knee joints of AIA WT (n=5) and Il9−/− mice (n=5) at day 24, 27 and 42. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test.
(A) AIA in WT (n=6) and Il9−/− mice (n=6); Y-axis shows knee swelling relative to control and the area under the curve (AUC) of knee swelling. As indicated, sorted Tregs from Il-9−/− mice were pre-stimulated (ps) with recombinant IL-9 for 72h before adoptive transfer into Il9−/− mice (n=6). Transfer of WT Tregs into Il9−/− mice (n=6) served as control. (B) Cytometric co-staining of citrine positive cells isolated from inflamed joints of Il9citrine reporter mice (n=8) at day 27. Representative histograms of fluorescence-minus controls (FMO; light grey) and the respective markers CD25, CD90, Sca-1 and FcERIa (dark grey) are shown; respective geometric mean fluorescence intensity (gMFI) of 8 independent experiments. (C) Quantitative analysis of IL-9 positive cells in the affected knee joints from WT mice upon induction of AIA (n=6) with immunofluorescence staining for CD3ε, DAPI, ICOS, IL-9. CD3+/IL-9+ (T cells), ICOS+/IL-9+/CD3− (ILC2s) and IL-9+/CD3−/ICOS− (others) cells were counted per 0.3 mm2 of synovial tissue. (D, E) mRNA expression levels of mast cell associated genes in inflamed joints of (D) AIA and (E) SIA of 4–6 mice of each group; expression levels were normalized to B2m. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ** p≤0.01, ***p<0.001 determined by one-way ANOVA with Tukey’s post hoc test (a, c, e) or Student’s t test (d).
(A) Induction of ILC2s by hydrodynamic gene therapy with IL-25 and IL-33 in WT and Il9−/− mice with and without supplementation of IL-9. Data are shown as the mean ± SEM of 3–6 independent experiments of each group. (B) Suppressive capacity of CD4posFoxP3pos Tregs from WT and Il9−/− mice co-cultured w/o ILC2s in a transwell system. Cell proliferation of CD4posFoxP3neg responder cells (Teff) was assessed by the dilution of the fluorescent dye CFSE to dividing daughter cells. Suppression was calculated using the division index. (C) Serum levels of IL-17 at day 0, 22 and 44 in AIA WT and Il9−/− mice w/o adoptive transfer of ILC2s. Data are shown as the mean ± SEM of at least 6 mice of each group. (D) Cytometric gating strategy to identify human ILC2s. Representative dot plots are shown. (E) Survival and persistence of ILC2s within the knee joint of WT and Il9−/− mice with AIA upon intraarticular injection (CD45.2; n=4 respectively). ILC2s were sorted from CD45.1 mice and injected intra-articularly together with mBSA at day 21. Cytometric single-cell analysis of digested synovial tissue was performed at day 27 of AIA. Representative dot plots are included. All data are shown as the mean ± SEM. Asterisks symbol p-value levels: ns p>0.5, * p≤0.05, ** p≤0.01 determined by one-way ANOVA with Tukey’s post hoc test.