Abstract
Pulmonary artery pseudoaneurysms (PAPs) are uncommon but potentially lethal. They may be incidentally discovered on imaging, or following massive haemoptysis if they rupture, with high risk of mortality. The most frequent causes of PAP are trauma and infectious disease. Vasculitis, in particular Behçet's disease, neoplasm, congenital disease and pulmonary hypertension are rarer causes of PAP. A PAP can be suspected from chest X-ray and contrast CT, but requires confirmation by CT angiography. Arteriography is no longer performed for diagnostic purposes, but can be useful in preparing endovascular occlusion of the PAP. In rare cases, surgery is necessary. The aim of this pictorial review was to illustrate the most common causes of acquired PAPs.
INTRODUCTION
Pulmonary artery pseudoaneurysms (PAPs) are defined as the focal dilatation of a segment of a pulmonary artery. Histologically, a pseudoaneurysm involves only the external layers of the arterial wall (the media and adventitia), while a true aneurysm involves all three layers. PAPs are thus associated with a higher risk of rupture than true aneurysms owing to the relatively low resistance of the surrounding tissue.1
PAPs are classified as proximal or peripheral.2 Proximal PAPs are situated on the pulmonary trunk, defined by a pulmonary trunk diameter >29 mm or right interlobar artery diameter >17 mm. They are often associated with pulmonary hypertension.3 Peripheral PAPs are situated on an intrapulmonary artery, and they are life threatening.
The clinical presentation of PAPs varies, ranging from no symptoms to massive, life-threatening haemoptysis. If the trachea is compressed, patients may present with dyspnoea and cough, or fever may be present in the case of infection-related PAP. Although rare, it is important to detect PAPs as early as possible in order to improve the prognosis.4
PAPs can be detected on standard chest X-ray as round, well-circumscribed nodules or hilar enlargements. Pulmonary angiography is the gold standard for diagnosing PAPs; however, it is no longer used for diagnostic purposes because it is invasive and provides only a luminal image with little characterization of the surrounding anatomy. CT angiography is currently the technique of choice, since it can detect certain PAPs that would not have been identified on a classic angiogram because of their peripheral position, the presence of a flap of vascular tissue acting as a valve, the presence of a thrombus or because of the slow blood flow inside the pseudoaneurysm.5 It is used to detect, describe and precisely locate PAPs on the arterial tree. Moreover, maximal intensity projection and three-dimensional reconstructions are essential to guide endovascular treatment or to prepare for surgery. CT angiography is also useful for determining the aetiology of the PAP as well as to monitor it. CT angiography can reveal enlargement of the pulmonary arteries, with possible mural thrombus in the case of a proximal PAP. The mural thrombus may be calcified. A peripheral PAP usually presents as a saccular distal aneurysm on the angiogram. The bronchial or intercostal arteries may also be dilated, and this should be treated during interventional angiography. If there is associated pulmonary hypertension, the right ventricle and pulmonary trunk will often be enlarged and mosaic perfusion will be seen. A localized area of consolidation or ground-glass opacities may indicate pulmonary haemorrhage from rupturing of the PAP or vasculitis. Angio-MRI can also be useful for determining the characteristics of the PAP, but is not used routinely.
PAPs can be congenital or acquired. Causes of congenital PAPs include deficiency of the vessel wall, valvular and post-valvular stenosis and increased flow due to left-to-right shunts.6 These shunts can be related to ductus arteriosus, ventricular or atrial septal defects or congenital myocardiopathies such as tetralogy of Fallot. Common causes of acquired PAPs are trauma (often iatrogenic), infection, vasculitis (especially Behçet's disease) and neoplasm.7,8 Acquired PAPs can also develop secondary to pulmonary arterial hypertension, commonly owing to chronic pulmonary embolism or owing to any other pre-capillary, capillary or post-capillary cause.
The aim of this article was to review the common causes of acquired PAPs and to provide pictorial examples from our practice in a university hospital.
TRAUMA RELATED
Two types of traumatic PAP are described in the literature: extravascular and intravascular.2 The main cause of intravascular PAP is iatrogenic injury resulting from a malpositioned Swan–Ganz catheter.9 This rare complication occurs in 0.001–0.47% of catheterizations. It is a serious condition, associated with a mortality rate of around 50%4 from massive haemoptysis. A PAP may form subsequent to distal insertion of the tip of the catheter that erodes the pulmonary artery wall, causing weakening and dilatation (Figures 1–3). PAPs may also form following distal migration of the catheter during balloon deflation, retraction of an inflated balloon or too high-pressure inflation of the balloon.4 Risk factors include: age >60 years, female gender, pulmonary hypertension, systemic anticoagulation therapy, long-term corticosteroid use, surgically induced hypothermia and cardiac manipulation during surgery. Other less frequent causes of intravascular PAPs include chest tube insertion, pulmonary angiography and surgical resection or biopsy (Figure 4).
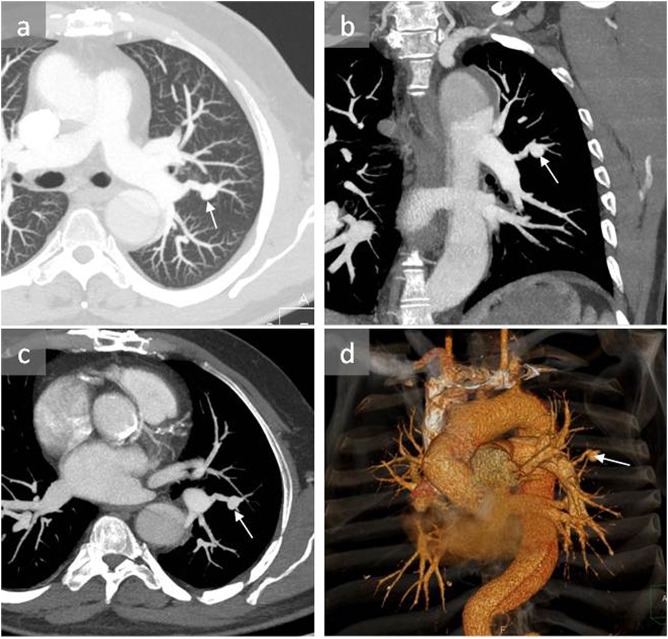
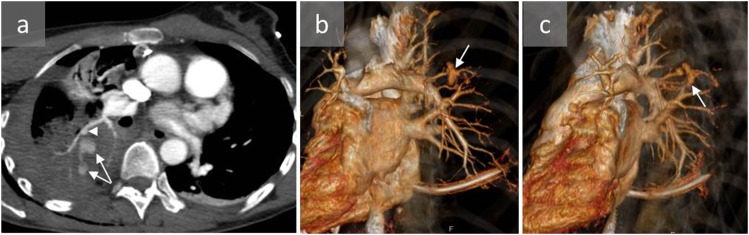
Figure 1.
A 53-year-old male with pulmonary artery pseudoaneurysm (PAP) induced by Swan–Ganz catheterization: axial CT angiography (a, c), coronal reconstruction (b) and three-dimensional reconstruction (d) show a PAP in the apicoposterior segment of the left upper lobe (arrows). The patient was managed conservatively and remained stable.
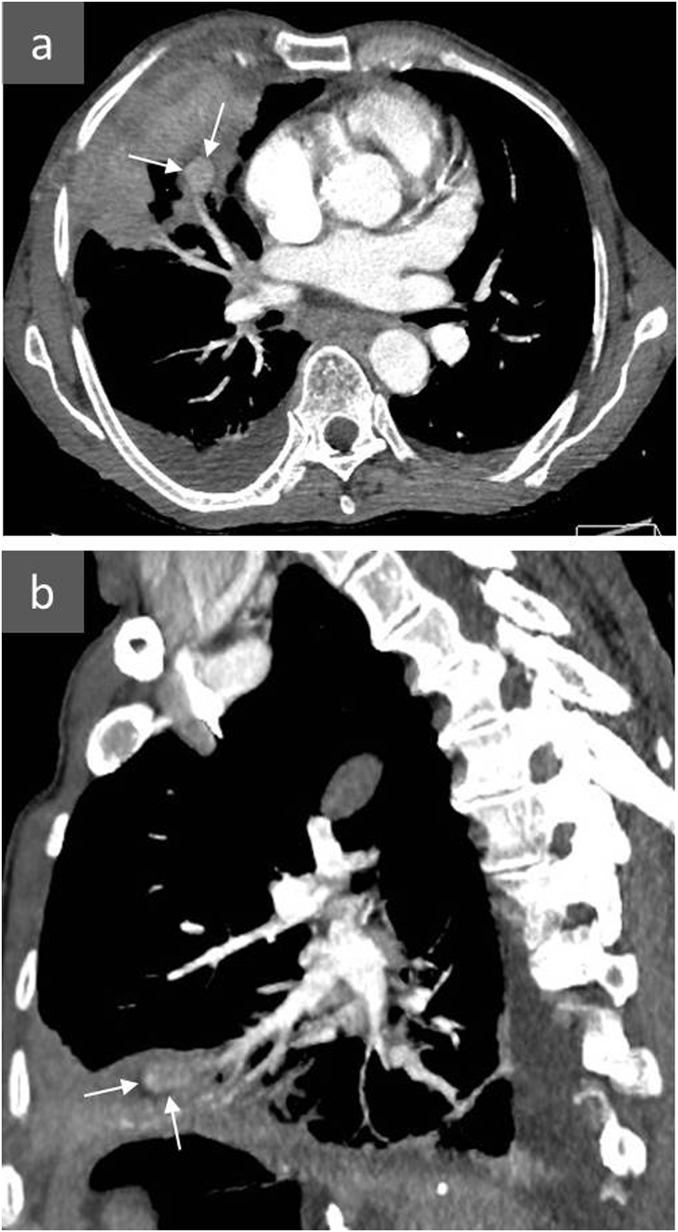
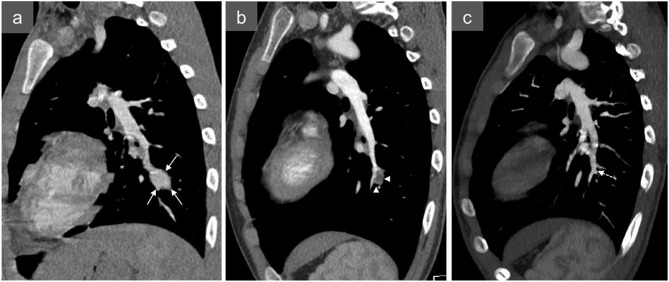
Figure 3.
An 80-year-old female presenting with massive pulmonary haemorrhage after Swan–Ganz catheterization: axial (a) and sagittal (b) CT angiography and three-dimensional reconstruction (c) show a distal pulmonary artery pseudoaneurysm following Swan–Ganz catheterization (arrows). A fragment of the Swan–Ganz catheter remained in the pulmonary artery (arrowheads) next to the pseudoaneurysm. The patient died before any treatment could be carried out.
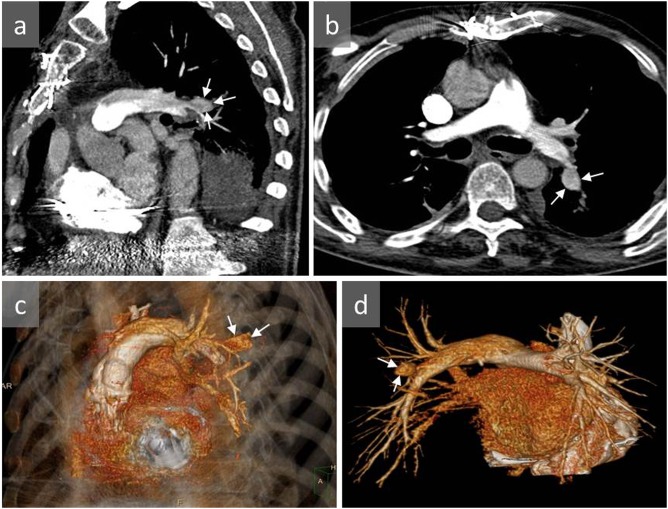
Figure 4.
A 67-year-old male with a pulmonary artery pseudoaneurysm (PAP) identified 2 days after surgical removal of a large hepatic hydatid cyst located within the hepatic dome: axial (a) and sagittal (b) contrast-enhanced CT show a PAP in the right middle lobe (arrows). The pseudoaneurysm was successfully treated with endovascular coil embolization.
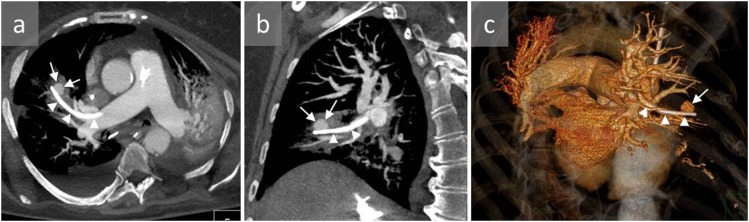
Figure 2.
A 65-year-old male with a history of right heart failure and acute haemoptysis 10 days after right heart catheterization who developed pulmonary artery pseudoaneurysm (PAP): sagittal (a) and axial (b) CT angiography and three-dimensional reconstructions (c, d) show a distal PAP in the apicoposterior segment of the left upper lobe (arrows). The patient died 1 day later of massive haemoptysis and haemothorax.
Extravascular PAPs may be caused by penetrating trauma such as stabbing or gunshot wounds (Figures 5–7).
Figure 5.
A 47-year-old female who was hospitalized after falling from a horse: axial CT angiography (a) and three-dimensional reconstructions (b, c) show a peripheral pulmonary artery pseudoaneurysm (arrowhead) and active bleeding (arrows) within a large pulmonary consolidation within the apical segment of the right lower lobe. The pseudoaneurysm was successfully treated by pulmonary artery embolization with coils.
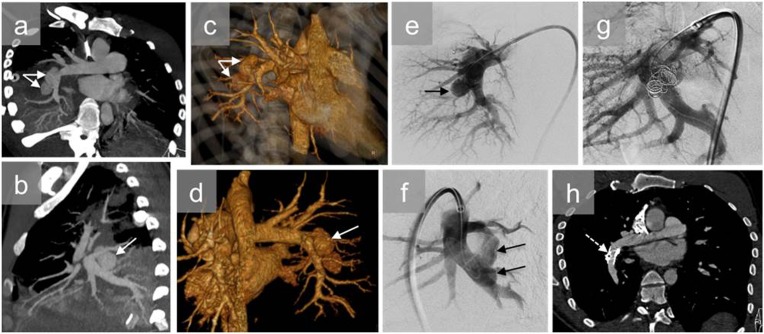
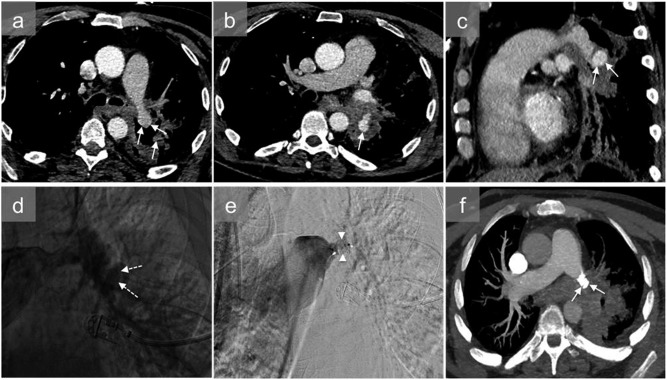
Figure 7.
A young male with a single right thoracic gunshot wound who presented with haemoptysis: axial (a) and sagittal (b) CT angiography and three-dimensional reconstructions (c, d) show bilobulated pulmonary pseudoaneurysms (white arrows) at the origin of the right interlobar pulmonary artery and right lower lobe pulmonary consolidation. Pulmonary angiography confirmed two pseudoaneurysms (black arrows) (e, f) and was used to plan endovascular treatment consisting of coil embolization of the pseudoaneurysms and reinforcement of the pulmonary artery wall by insertion of an endovascular stent (g). The contrast-enhanced CT scan (h) 30 days after the endovascular treatment shows elimination of the pseudoaneurysms (dashed arrow).
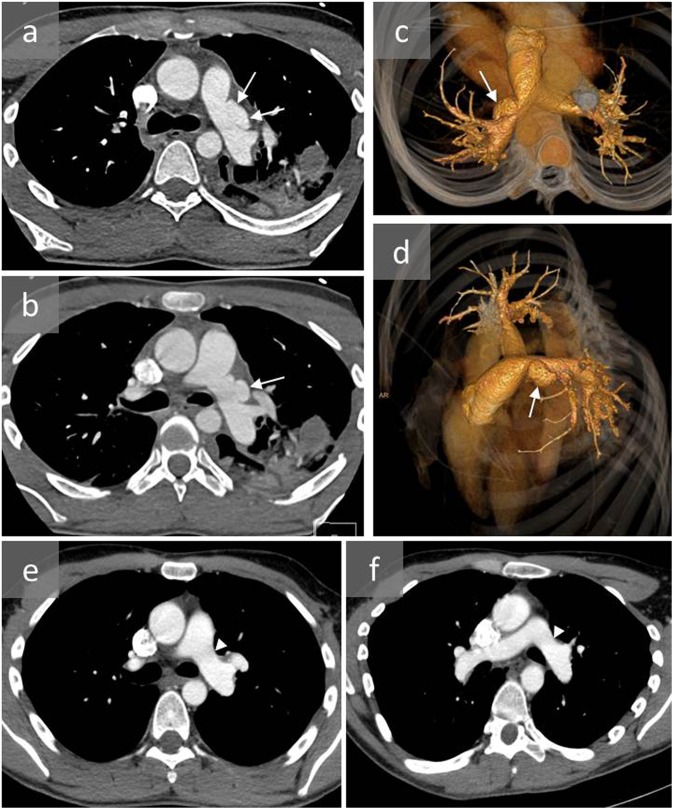
Figure 6.
A young male involved in a severe car accident presenting with dyspnoea and desaturation: axial CT angiography (a, b) and three-dimensional reconstructions (c, d) show a proximal left pulmonary artery pseudoaneurysm (arrows), mild haematoma of the mediastinum and left pulmonary contusion. CT angiography (e, f) after surgical repair of the pseudoaneurysm confirmed successful treatment (arrow head).
INFECTION—MYCOTIC PSEUDOANEURYSM
PAPs can be caused by infectious diseases such as tuberculosis, infection by pyogenic bacteria, necrotizing pneumonia, syphilis or fungi.6,10 A Rasmussen aneurysm is a PAP that results from weakening of the pulmonary artery wall because of a contiguous tuberculous cavern. These are commonly found in the upper lobes, on a peripheral pulmonary artery (Figure 8). These PAPs develop following the destruction of elastic fibres within the media and their replacement by granulation tissues, resulting in segmental artery dilatation. This occurs during the healing of the disease, which explains the delayed onset of the PAPs. In the case of active pulmonary tuberculosis, bleeding originates from the bronchial arteries more often than from PAPs. Nonetheless, if haemoptysis occurs, the pulmonary arterial circulation should be evaluated, particularly in the case of an unremarkable bronchial angiogram.11 A PAP should be suspected if a round mass is seen on chest X-ray or on CT angiogram. Pyogenic and mycotic PAPs can occur in i.v. drug users and/or associated with bacterial endocarditis or septicaemia.6 Risk factors for mycotic PAPs include: age >60 years, pulmonary hypertension, anticoagulation therapy, coagulopathy, long-term steroid therapy and cardiopulmonary surgery.
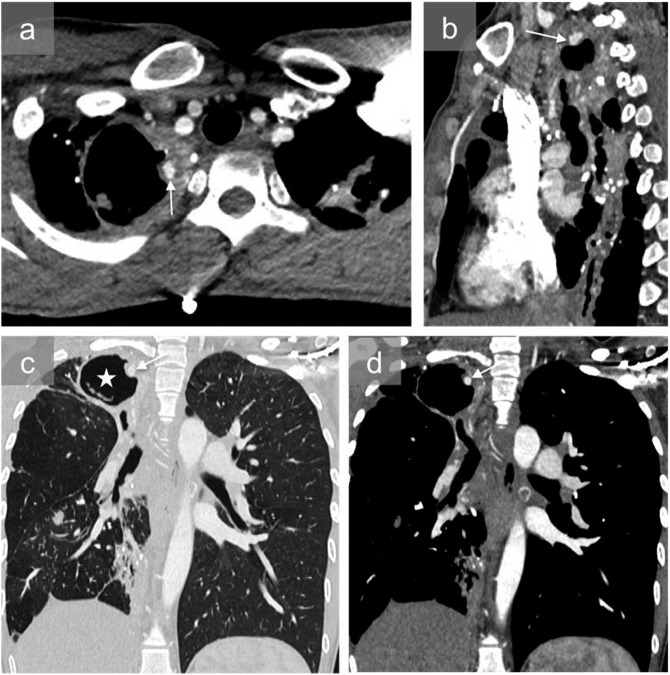
Figure 8.
A 45-year-old male with a history of pulmonary tuberculosis and recurrent tuberculosis under antibiotics who was admitted to the hospital for acute haemoptysis: axial (a), sagittal (b) and coronal mediastinal and pulmonary images at the same level (c, d) show a large right apical cavity (white star) and pseudoaneurysm in the wall of the cavity (arrows). The patient was successfully treated with coil embolization.
VASCULITIS—BEHÇET'S DISEASE
Behçet's disease is a rare chronic systemic disorder of unknown origin that affects the collagen and vascular tissue. It is characterized by recurrent oral and genital ulcers and relapsing uveitis.12,13 This disease commonly affects young males (sex ratio: 10 : 1) particularly from Turkey, the Middle East and Southeast Asia, along the ancient silk road. Signs of Behçet's disease occur in the thoracic region in 1–18% of cases, and particularly in the late stages of the disease. These symptoms include venous thrombosis, pulmonary infarction and haemorrhage, recurrent pneumonia, organizing pneumonia, eosinophilic pneumonia, pleural effusion, aneurysms and PAPs. CT angiogram is usually insufficient for differentiating true aneurysms from PAPs. True aneurysms are caused by destruction of the media following loss of the elastic tissue and massive collagen deposition, along with degenerative and necrotic endothelial cells and perivascular inflammatory cells. PAPs can occur following minor trauma such as a previous surgical intervention, for example on an anastomosis line, making management of the disease even more difficult.14 PAPs are typically situated on the arteries of the right lower lobe and are frequently associated with thrombosis and surrounding inflammation due to vasculitis and transmural necrosis. In most patients, they are multiple, bilateral, saccular and partially or completely thrombosed. Medical treatment with cytostatic agents and corticosteroids may induce regression; however, recurrent haemoptysis or progression in size are common. The presence of a PAP suggests a poor prognosis (Figure 9).
Figure 9.
An 18-year-old male with Behçet's disease who presented with severe haemoptysis: sagittal reconstruction of the contrast-enhanced CT scan (a) shows a pseudoaneurysm (arrows) of the left lower pulmonary artery. The patient underwent careful endovascular embolization with particles, followed by chemotherapy. Contrast-enhanced CT scans 1 month (b) and 3 months (c) later showed thrombosis (white arrowhead) and complete disappearance of the pulmonary artery pseudoaneurysm (dashed arrow).
Radiological signs of Behçet's disease include PAP and thrombosis of the pulmonary artery and systemic veins. PAPs appear as round perihilar opacities, measuring from 1 cm to 3 cm in diameter. CT scan shows the thickness of the pulmonary artery wall. They are usually multiple, ranging from 1 cm to 7 cm in diameter. PAPs typically involve the right interlobar artery, followed by the lobar and segmental arteries. CT scan may also reveal venous thrombosis of the superior vena cava associated with the development of collateral circulation.
NEOPLASM
Primary lung tumours (such as necrotic cavitating lung carcinoma) and lung metastases can cause PAPs secondary to erosion into the pulmonary arteries (Figure 10); however, this is rare. Focal dilatation can also be caused by primary tumours in the pulmonary arteries, such as leiomyosarcomas or angiosarcomas.
Figure 10.
A 50-year-old patient with cancer of the left lower pulmonary lobe who presented with haemoptysis: axial (a, b) and sagittal (c) contrast-enhanced CT scans show a proximal left lower pulmonary artery pseudoaneurysm (arrows). Owing to his poor haemodynamic status, diagnostic angiography was performed, confirming pulmonary pseudoaneurysm (dashed arrows) (d). It was treated by an endovascular approach, with the insertion of a 12-mm Amplatzer™ vascular plug II (St. Jude Medical Inc.; St. Paul, MN) (arrowheads) (e) within the left lower lobar artery. The haemoptysis stopped, the patient status improved and CT angiography (f) 1 week after embolization showed complete occlusion of the pseudoaneurysm (arrows).
TREATMENT
The current treatment for PAPs involves endovascular occlusion of the feeding vessel using coils, plugs or stents.15 CT angiography is useful for determining the vessels that require occlusion. Both the vessels proximal and distal to the PAP should be occluded, although this is associated with a higher risk of iatrogenic rupture.15 Surgery is rarely indicated.
CONCLUSION
PAPs are rare but dangerous entities, associated with a high rate of mortality if left untreated. The most common causes are trauma and iatrogenic causes, related to Swan–Ganz catheterization. CT angiography is the gold standard for diagnosis and facilitates planning of endovascular or surgical procedures for preventing rupture.
Acknowledgments
ACKNOWLEDGMENTS
The authors are grateful to Johanna Robertson, PhD, for assistance in preparing the final version of the article.
Contributor Information
Benedicte Guillaume, Email: bguillaume@chu-grenoble.fr.
Anne Vendrell, Email: avendrell@chu-grenoble.fr.
Xavier Stefanovic, Email: xavier-stefano@hotmail.fr.
Frederic Thony, Email: fthony@chu-grenoble.fr.
Gilbert R Ferretti, Email: gferretti@chu-grenoble.fr.
REFERENCES
- 1.Shuaib W, Tiwana MH, Vijayasarathi A, Sadiq MF, Anderson S, Amin N, et al. Imaging of vascular pseudoaneurysms in the thorax and abdomen. Clin Imaging 2015; 39: 352–62. doi: https://doi.org/10.1016/j.clinimag.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 2.Theodoropoulos P, Ziganshin BA, Tranquilli M, Elefteriades JA. Pulmonary artery aneurysms: four case reports and literature review. Int J Angiol 2013; 22: 143–8. doi: https://doi.org/10.1055/s-0033-1347907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duijnhouwer AL, Navarese EP, Van Dijk AP, Loeys B, Roos-Hesselink JW, De Boer MJ. Aneurysm of the pulmonary artery, a systematic review and critical analysis of current literature. Congenit Heart Dis 2016; 11: 102–9. doi: https://doi.org/10.1111/chd.12316 [DOI] [PubMed] [Google Scholar]
- 4.Pelage JP, El Hajjam M, Lagrange C, Chinet T, Vieillard-Baron A, Chagnon S, et al. Pulmonary artery interventions: an overview. RadioGraphics 2005; 25: 1653–67. doi: https://doi.org/10.1148/rg.256055516 [DOI] [PubMed] [Google Scholar]
- 5.Shin TB, Yoon SK, Lee KN, Choi JS, Kim YH, Sung CG, et al. The role of pulmonary CT angiography and selective pulmonary angiography in endovascular management of pulmonary artery pseudoaneurysms associated with infectious lung diseases. J Vasc Interv Radiol 2007; 18: 882–7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ET, Silva CI, Seely JM, Chong S, Lee KS, Müller NL. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007; 188: W126–34. [DOI] [PubMed] [Google Scholar]
- 7.Lafita V, Borge MA, Demos TC. Pulmonary artery pseudoaneurysm: etiology, presentation, diagnosis, and treatment. Semin Intervent Radiol 2007; 24: 119–23. doi: https://doi.org/10.1055/s-2007-971202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castañer E, Gallardo X, Rimola J, Pallardó Y, Mata JM, Perendreu J, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. RadioGraphics 2006; 26: 349–71. [DOI] [PubMed] [Google Scholar]
- 9.Ferretti GR, Thony F, Link KM, Durand M, Wollschläger K, Blin D, et al. False aneurysm of the pulmonary artery induced by a Swan–Ganz catheter: clinical presentation and radiologic management. AJR Am J Roentgenol 1996; 167: 941–5. [DOI] [PubMed] [Google Scholar]
- 10.Sbano H, Mitchell AW, Ind PW, Jackson JE. Peripheral pulmonary artery pseudoaneurysms and massive hemoptysis. Am J Roentgenol 2005; 184: 1253–9. [DOI] [PubMed] [Google Scholar]
- 11.Santelli ED, Katz DS, Goldschmidt AM, Thomas HA. Embolization of multiple Rasmussen aneurysms as a treatment of hemoptysis. Radiology 1994; 193: 396–8. doi: https://doi.org/10.1148/radiology.193.2.7972750 [DOI] [PubMed] [Google Scholar]
- 12.Erkan F, Gül A, Tasali E. Pulmonary manifestations of Behçet's disease. Thorax 2001; 56: 572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durieux P, Bletry O, Huchon G, Wechsler B, Chretien J, Godeau P. Multiple pulmonary arterial aneurysms in Behcet's disease and Hughes–Stovin syndrome. Am J Med 1981; 71: 736–41. [DOI] [PubMed] [Google Scholar]
- 14.Alpagut U, Ugurlucan M, Dayioglu E. Major arterial involvement and review of Behcet's disease. Ann Vasc Surg 2007; 21: 232–9. [DOI] [PubMed] [Google Scholar]
- 15.Khalil A, Fedida B, Parrot A, Haddad S, Fartoukh M, Carette MF. Severe hemoptysis: from diagnosis to embolization. Diagn Interv Imaging 2015; 96: 775–88. doi: https://doi.org/10.1016/j.diii.2015.06.007 [DOI] [PubMed] [Google Scholar]