Summary
Background
For many years, first-line treatment for locally advanced or metastatic soft-tissue sarcoma has been doxorubicin. This study compared gemcitabine and docetaxel versus doxorubicin as first-line treatment for advanced or metastatic soft-tissue sarcoma.
Methods
The GeDDiS trial was a randomised controlled phase 3 trial done in 24 UK hospitals and one Swiss Group for Clinical Cancer Research (SAKK) hospital. Eligible patients had histologically confirmed locally advanced or metastatic soft-tissue sarcoma of Trojani grade 2 or 3, disease progression before enrolment, and no previous chemotherapy for sarcoma or previous doxorubicin for any cancer. Patients were randomly assigned 1:1 to receive six cycles of intravenous doxorubicin 75 mg/m2 on day 1 every 3 weeks, or intravenous gemcitabine 675 mg/m2 on days 1 and 8 and intravenous docetaxel 75 mg/m2 on day 8 every 3 weeks. Treatment was assigned using a minimisation algorithm incorporating a random element. Randomisation was stratified by age (≤18 years vs >18 years) and histological subtype. The primary endpoint was the proportion of patients alive and progression free at 24 weeks in the intention-to-treat population. Adherence to treatment and toxicity were analysed in the safety population, consisting of all patients who received at least one dose of their randomised treatment. The trial was registered with the European Clinical Trials (EudraCT) database (no 2009–014907–29) and with the International Standard Randomised Controlled Trial registry (ISRCTN07742377), and is now closed to patient entry.
Findings
Between Dec 3, 2010, and Jan 20, 2014, 257 patients were enrolled and randomly assigned to the two treatment groups (129 to doxorubicin and 128 to gemcitabine and docetaxel). Median follow-up was 22 months (IQR 15·7–29·3). The proportion of patients alive and progression free at 24 weeks did not differ between those who received doxorubicin versus those who received gemcitabine and docetaxel (46·3% [95% CI 37·5–54·6] vs 46·4% [37·5–54·8]); median progression-free survival (23·3 weeks [95% CI 19·6–30·4] vs 23·7 weeks [18·1–20·0]; hazard ratio [HR] for progression-free survival 1·28, 95% CI 0·99–1·65, p=0·06). The most common grade 3 and 4 adverse events were neutropenia (32 [25%] of 128 patients who received doxorubicin and 25 [20%] of 126 patients who received gemcitabine and docetaxel), febrile neutropenia (26 [20%] and 15 [12%]), fatigue (eight [6%] and 17 [14%]), oral mucositis (18 [14%] and two [2%]), and pain (ten [8%] and 13 [10%]). The three most common serious adverse events, representing 111 (39%) of all 285 serious adverse events recorded, were febrile neutropenia (27 [17%] of 155 serious adverse events in patients who received doxorubicin and 15 [12%] of 130 serious adverse events in patients who received gemcitabine and docetaxel, fever (18 [12%] and 19 [15%]), and neutropenia (22 [14%] and ten [8%]). 154 (60%) of 257 patients died in the intention-to-treat population: 74 (57%) of 129 patients in the doxorubicin group and 80 (63%) of 128 in the gemcitabine and docetaxel group. No deaths were related to the treatment, but two deaths were due to a combination of disease progression and treatment.
Interpretation
Doxorubicin should remain the standard first-line treatment for most patients with advanced soft-tissue sarcoma. These results provide evidence for clinicians to consider with their patients when selecting first-line treatment for locally advanced or metastatic soft-tissue sarcoma.
Funding
Cancer Research UK, Sarcoma UK, and Clinical Trial Unit Kantonsspital St Gallen.
Research in context.
Evidence before this study
In 2008, when this study was designed, we searched PubMed for randomised controlled trials that compared single-agent doxorubicin with other schedules used in first-line treatment of advanced soft-tissue sarcoma, published in English, with no start date restriction, until Dec 15, 2008. We used the following search terms: “soft tissue”, “sarcoma”, “chemotherapy”, “doxorubicin”, “gemcitabine”, “docetaxel”, “randomised”, and “trial”. We identified six published randomised controlled trials, none of which showed a survival advantage for any schedule over single-agent doxorubicin. We identified a further randomised controlled study comparing single-agent doxorubicin versus doxorubicin and ifosfamide chemotherapy, which was recruiting at that time, and which has since been published in 2014, showing an advantage for the combination for progression-free survival but not overall survival. We also searched for randomised controlled trials comparing doxorubicin versus gemcitabine and docetaxel. Although we identified three phase 2 studies (one of which was a randomised phase 2 study comparing gemcitabine versus gemcitabine and docetaxel), we were unable to find any phase 3 trials comparing this combination with doxorubicin.
Added value of this study
To our knowledge, this is the first randomised controlled trial that compares two commonly used treatments—doxorubicin versus gemcitabine and docetaxel—as first-line treatment in advanced soft-tissue sarcoma. We have shown that gemcitabine and docetaxel was not superior to doxorubicin for either progression-free survival or overall survival. Furthermore, planned subgroup analyses have not identified any subgroup for which gemcitabine and docetaxel was superior, and in particular we did not observe superiority for either leiomyosarcoma or uterine leiomyosarcoma, for which gemcitabine and docetaxel has previously been believed to be particularly active. We found worse treatment adherence with gemcitabine and docetaxel compared with doxorubicin, with more dose delays, lower dose intensity, and more patients stopping treatment early due to toxicity, and lower quality-of-life scores.
Implications of all the available evidence
These results provide evidence for clinicians to consider with patients when selecting first-line treatment for advanced soft-tissue sarcoma. Although the observed similar progression-free survival and overall survival of gemcitabine and docetaxel and doxorubicin might support a conclusion that either schedule can be used according to patient or clinician preference, our results indicate the need for caution with such an approach given the greater difficulty in delivery and toxicity of gemcitabine and docetaxel, and indeed the higher cost of this combination regimen. The data support the conclusion that doxorubicin should remain standard of care as first-line treatment for most patients with advanced soft-tissue sarcoma, and that there is no subgroup of patients for whom gemcitabine and docetaxel should be routinely recommended.
Introduction
Soft-tissue sarcoma comprises a number of rare malignancies, with 3298 patients newly diagnosed with the disease in the UK in 2010.1 Surgery with radiotherapy is the most common treatment for localised disease, with associated 5-year overall survival of 55% in 2006–10.1 Survival outcomes for locally advanced or metastatic soft-tissue sarcoma are poor, with a median overall survival of 12·8–14·3 months after diagnosis.2, 3 Doxorubicin has been used as first-line treatment for locally advanced or metastatic soft-tissue sarcoma for more than 40 years. A randomised controlled phase 3 trial2 comparing combination doxorubicin and ifosfamide versus doxorubicin alone showed a significant increase in progression-free survival in the combination treatment group, but with no increase in overall survival. Toxicity was predictably higher in the combination group, and the authors concluded their results “do not support the use of intensified doxorubicin and ifosfamide for palliation of advanced soft-tissue sarcoma unless the specific goal is tumour shrinkage”. Subsequently, two first-line phase 3 trials4, 5 have combined doxorubicin with novel agents (doxorubicin and palifosfamide compared with doxorubicin and placebo,4 and doxorubicin and evofosfamide compared with doxorubicin alone5), and neither study was able to show improved progression-free survival or overall survival for the combination treatments. Thus, no regimen has proved to be unequivocally superior to doxorubicin as first-line treatment for locally advanced or metastatic soft-tissue sarcoma.
Gemcitabine and docetaxel was first reported as a treatment for locally advanced or metastatic soft-tissue sarcoma in 2002. Hensley and colleagues6 did a phase 2 study in patients with leiomyosarcoma and reported that 53% of patients achieved an objective response. This study also compared plasma levels of gemcitabine achieved with a 90-min infusion versus a 30-min infusion, and showed that the 90-min infusion was associated with a longer duration of plasma gemcitabine concentrations above 10 μmol/L, which is the threshold for saturation of intracellular accumulation of the active form of the drug, gemcitabine triphosphate. A further retrospective review of gemcitabine and docetaxel in patients with locally advanced or metastatic disease included an in-vitro study to investigate the dosing sequence of gemcitabine and docetaxel, finding that gemcitabine followed by docetaxel was synergistic, whereas docetaxel followed by gemcitabine was antagonistic.7 Hence, the currently used schedule of gemcitabine and docetaxel in locally advanced or metastatic soft-tissue sarcoma was established. Subsequently, several retrospective studies and phase 2 studies, in both leiomyosarcoma8, 9, 10, 11 and unselected soft-tissue sarcoma,7, 12, 13 have all showed activity of the combination. The observed responsiveness of leiomyosarcoma in particular has led to some clinicians adopting gemcitabine and docetaxel as a first-line treatment option for locally advanced or metastatic leiomyosarcoma, in the absence of evidence from phase 3 trials. With increasing use of the combination in both leiomyosarcoma and locally advanced or metastatic soft-tissue sarcoma, robust evidence is needed to establish the roles of gemcitabine and docetaxel and doxorubicin as first-line treatments for this disease.
The GeDDiS trial aimed to compare the efficacy of gemcitabine and docetaxel versus doxorubicin in the first-line setting for locally advanced or metastatic soft-tissue sarcoma. We also compared toxicity and quality of life for the two regimens to aid clinical decision-making in treatment selection for individual patients. A pharmacogenomics study was also done to investigate the influence of single-nucleotide polymorphisms (SNPs) on treatment efficacy and toxicity.
Methods
Study design and participants
GeDDiS was a multicentre, randomised, phase 3 trial, which recruited patients from 24 UK hospitals, and one Swiss Group for Clinical Cancer Research (SAKK) hospital in Switzerland (appendix p 1). Eligible patients were at least 13 years old (with the aim to encourage participation of the teenage and young adult population), with histological confirmation of high-grade advanced soft-tissue sarcoma (defined as Trojani grade 2 or 3), measurable disease according to the Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST 1.1),14 evidence of disease progression in the previous 6 months (defined as radiological progression when comparing current imaging to a previous disease assessment done within the previous 6 months; clinical progression was accepted in patients for whom there were concerns regarding treatment delays incurred by awaiting radiological disease progression, on discussion with the chief investigator), no previous chemotherapy for sarcoma, no previous doxorubicin for any previously treated cancer, WHO performance status 0–2, and a life expectancy of at least 3 months. Patients were required to have adequate organ function (absolute neutrophil count ≥1·0 × 109 per L; platelet count ≥100 × 109 per L; bilirubin ≤1·5 × upper limit of normal [ULN]; aspartate transaminase, alanine transaminase, or both ≤3·0 × ULN; alkaline phosphatase ≤3·0 × ULN [patients were eligible with a higher alkaline phosphatase concentration if this was shown to be due to bone isoenzyme]; measured or calculated creatinine clearance ≥30 mL/min; and cardiac ejection fraction within local normal limits). Tumour tissue was required to be available for central review.
Patients were excluded from the trial if they had alveolar soft part sarcoma, gastrointestinal stromal tumour, Ewing's sarcoma, alveolar or embryonal rhabdomyosarcoma, desmoplastic small round cell tumour, extraskeletal myxoid chondrosarcoma, dermatofibrosarcoma protuberans, malignant mixed mesodermal tumour or carcinosarcoma of the uterus, smooth muscle tumours of uncertain malignant potential of uterus, known active or uncontrolled brain metastases, active uncontrolled infection, or grade 3 or 4 peripheral neuropathy. Pregnant or lactating women were excluded, as were patients with a history of malignancy other than sarcoma (exceptions included basal or squamous cell carcinoma of the skin and carcinoma in situ of the cervix, breast, or prostate) within 3 years before enrolment.
Samples of formalin-fixed, paraffin-embedded tumour tissue and haematoxylin and eosin-stained slides were submitted for central review for confirmation of sarcoma diagnosis and histological subtype, although patients were enrolled on the basis of the local pathology report. All samples were reviewed by a single histopathologist (RT).
The trial was approved by the National Research Ethics Service Committee: London, Bloomsbury (10/H0713/54), the Medicines and Healthcare Products Regulatory Agency (Clinical Trials Authorisation number 20363/0285/001–0001), and by the Research and Development department of each participating NHS Trust.
The trial was done according to the principles of the International Conference on Harmonisation on Good Clinical Practice. All patients gave written informed consent before enrolment and undergoing any trial-specific procedures. The trial protocol is available online.
Randomisation and masking
Patients were randomly allocated in a 1:1 ratio to receive either gemcitabine and docetaxel or doxorubicin. Treatment was assigned centrally by computer at the Cancer Research UK and University College London Cancer Trials Centre (UCL CTC; London, UK) using a minimisation algorithm incorporating a random element. Patients were stratified by age (≤18 years vs >18 years) and histological subtype (uterine leiomyosarcoma vs synovial sarcoma vs pleomorphic sarcoma vs other eligible sarcomas). We chose these specific histological strata on the basis of available evidence at the time of trial design suggesting potential differential disease response to chemotherapy in the different strata (uterine leiomyosarcoma and pleomorphic sarcomas might be more sensitive to gemcitabine and docetaxel, and synovial sarcomas are recognised as being generally more sensitive to chemotherapy than other subtypes). Treatment allocation was communicated electronically to the site randomising the patient. Treatment allocation was not masked.
Procedures
Patients received either intravenous doxorubicin 75 mg/m2 on day 1 every 3 weeks, or intravenous gemcitabine 675 mg/m2 on day 1 and intravenous gemcitabine 675 mg/m2 followed by intravenous docetaxel 75 mg/m2 on day 8 every 3 weeks. Gemcitabine was administered over 90 min and docetaxel was administered over 60 min. Dose capping according to sites' local policy and dose banding to within plus or minus 5% of the calculated dose were permitted. Pre-treatment and post-treatment anti-emetics were given for all trial treatments, as per local anti-emetics policy. In both groups, patients completed up to six cycles of treatment in the absence of disease progression, intolerable side-effects, or withdrawal of consent.
Laboratory monitoring (blood count and biochemistry) was done within 72 h of day 1 of each cycle, with additional monitoring on day 8 (blood count only) for gemcitabine and docetaxel treatment. To proceed with treatment on day 1 of each cycle (both groups) the following were required: absolute neutrophil count of at least 1·0 × 109 per L, platelet count of at least 100 × 109 per L, bilirubin of up to 1·5 × ULN, and aspartate transaminase, alanine transaminase, or both of up to 3·0 × ULN. For administration of treatment on day 8 (in the gemcitabine and docetaxel group) the following were required: absolute neutrophil count of at least 1·0 × 109 per L and platelets of at least 75 × 109 per L. Dose modifications for adverse events were made according to prespecified criteria: dose reductions of 20% (first occurrence) ND then 33% (second occurrence) were permitted for doxorubicin (in the case of grade 3 or worse febrile neutropenia) and gemcitabine and docetaxel (for grade 3 or worse febrile neutropenia, grade 2 neuropathy, or any grade pulmonary toxicity); a third occurrence required the patient to be withdrawn from treatment. Granulocyte colony-stimulating factor was permitted after an episode of febrile neutropenia, at the discretion of the treating investigator and according to local policy. Dose delays of up to 2 weeks for haematological toxicity and up to 3 weeks for non-haematological toxicity were allowed; delays longer than this required the patient to be withdrawn from treatment. For doxorubicin, patients were advised to be withdrawn from treatment if left ventricular ejection fraction was less than 45% or reduced by 20% from baseline (cardiotoxicity monitoring was done according to local institutional policies). For gemcitabine and docetaxel, patients were withdrawn for the following events: grade 3 pulmonary fibrosis, any grade 4 pulmonary toxicity, grade 3 or 4 hypersensitivity reactions (docetaxel only), or grade 3 weight gain.
Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, from informed consent until 30 days after last trial treatment administration. Serious adverse events were reported from informed consent until 30 days after last trial treatment administration, or later if the investigator felt that an event was related to trial treatment.
Disease status was assessed by CT scan, MRI scan, or both at baseline, 12 and 24 weeks after randomisation, and then every 12 weeks until disease progression (including patients who discontinued treatment for any reason other than disease progression). Response was assessed by local investigators according to RECIST 1.1. Additionally, scans for 15% of recruited patients were centrally reviewed at University College Hospital. At least one patient from every site had a central scan review (10% of patients for sites recruiting ten patients or more). All scan images (baseline and subsequent disease assessments) available for these patients were reviewed.
Quality of life was assessed at baseline and at 12, 18, and 24 weeks after randomisation, using the EORTC QLQ-C30, and fatigue-specific FA-13 questionnaires. Health economic assessment was also done at the same timepoint using the EuroQoL EQ-5D questionnaire, and by prospectively collecting information about health-care resource use.
For the pharmacogenomics analyses, DNA was extracted from 4 mL whole blood using a QIAamp DNA Blood Maxi kit (Qiagen, Manchester, UK), according to the manufacturer's instructions. All samples were diluted to 10 ng/μL DNA in AE buffer, before being genotyped by Taqman PCR on a 7900HT PCR machine. Individual candidate SNPs in genes associated with the pharmacology of the three drugs were identified from relevant published literature,15, 16, 17, 18, 19, 20, 21 and Taqman Assay on Demand probes (Applied Biosystems, Paisley, UK) were used to genotype the samples.
Outcomes
The primary endpoint was the proportion of patients alive and progression free at 24 weeks after the date of randomisation. Secondary endpoints were the proportion of patients alive and progression free at 12 weeks after the date of randomisation, progression-free survival (time from randomisation to date of progression or death from any cause, whichever occurred first), and overall survival (time from randomisation to date of death from any cause), the proportion of patients achieving an objective response by RECIST 1.1, the proportion of patients achieving an objective response by Choi criteria (retrospective analysis), assessment of adverse events, quality of life, and health economics evaluation. Time to progression, proportion of patients achieving an objective response as assessed by Choi criteria, the health economics assessment, and the planned sensitivity analyses will all be published separately at a later date. We also did a pharmacogenomics analysis to assess the influence of SNPs on response and toxicity in patients assigned to each treatment group.
Statistical analysis
Under the assumption of a hazard ratio (HR) of 0·63, 250 patients (and 148 progressions or deaths) were required to achieve 80% power with a two-sided α of 5%. We assumed a median progression-free survival of 3·5 months for doxorubicin22 (corresponding with 30% of patients achieving 24-week progression-free survival) and a median progression-free survival of 5 months (corresponding with 47% of patients achieving 24-week progression-free survival) for gemcitabine and docetaxel.6, 13
We did the efficacy analysis in the intention-to-treat population of all randomised patients. We analysed adverse events in the safety population consisting of all patients who received at least one dose of their randomly assigned treatment. We calculated median follow-up using the Kaplan-Meier method, censoring patients at date last seen. We plotted Kaplan-Meier curves for progression-free survival and overall survival; treatment effect HRs (with 95% CIs and p values) were obtained from Cox proportional hazards regression models, adjusted for randomisation stratification factors. A HR of less than 1 favoured the gemcitabine and docetaxel group. We tested the proportionality assumption of the Cox model with Schoenfeld residuals.
Although the trial was not powered for subgroup analyses, we prospectively planned subgroup analyses for histological subtypes. These analyses were exploratory by nature, and were performed using tests for interaction.
We presented adverse events as the worst grade per patient per event, and comparison between groups was done using a test of proportions.
We assessed quality of life at the 12-week post-randomisation visit. We used ANCOVA and fitted a linear regression model adjusting for baseline score, stratification factors, and actual time between baseline and 12-week assessments (to allow for variation in the actual timings of assessments). We prospectively planned in our statistical analysis plan to use 99% CIs to account for multiple comparisons within the different scales of quality of life, which is a robust approach when the risk of a type 1 error is inflated by making multiple comparisons.
To assess the effect of SNPs on efficacy and toxicity within treatment groups, we used Cox proportional hazards regressions on overall survival and progression-free survival and logistic regressions on any grade 3 or 4 adverse events.
We calculated dose intensity relative to the total planned dose using a formula that incorporated delays and dose reductions.23
We used STATA version 14.2 for all statistical analyses.
An external independent data monitoring committee oversaw the trial and assessed the safety and efficacy approximately annually. This study is registered with the European Clinical Trials (EudraCT) database (no 2009–014907–29) and as an International Standard Randomised Controlled Trial, number ISRCTN07742377.
Role of the funding source
The trial was sponsored by University College London (UCL) and coordinated centrally by Cancer Research UK and UCL CTC. Funding from Cancer Research UK (C2921/A11561) was used to support the central coordination of the trial at UCL CTC. Analysis of the pharmacogenomics samples was funded by Sarcoma UK (SUK16.2015). The Clinical Trial Unit Kantonsspital St Gallen funded the trial in Switzerland. UCL CTC, on behalf of the sponsor, was responsible for the study design and conduct, including the collection, management, statistical analysis and interpretation of the data, and the writing of the report, in conjunction with the chief investigator (BS). BS, H-MD, SN, SF, and SB had access to all the raw data. GJV, DJ, and KK had access to the raw data associated with the pharmacogenomics analyses. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Results
Between Dec 3, 2010, and Jan 20, 2014, 257 patients from 24 UK hospitals and one Swiss hospital (appendix p 1) were enrolled and randomly assigned to receive doxorubicin (129 patients) or gemcitabine and docetaxel (128 patients; figure 1). Our intention-to-treat population consisted of these 257 patients. Two randomised patients (one in each group) were later found to be ineligible (figure 1); however, both are included in the intention-to-treat analysis and received treatment. Our safety population included 254 of the 257 randomised patients, because one patient who was assigned to doxorubicin and two patients who were assigned to gemcitabine and docetaxel did not receive at least one dose of their allocated treatment (figure 1).
Figure 1.
Trial profile
*Other reasons were: 43 multidisciplinary team decision not to approach patient, five deaths, three referred to or treated at other hospital, one patient did not fully understand trial, one trial closed before patient finished radiotherapy. †Clinical decision made not to treat (doxorubicin group), withdrawn consent (gemcitabine and docetaxel group), disease progression (gemcitabine and docetaxel group). ‡Histology review reclassified as ineligible histological subtypes (one gastrointestinal tumour in the doxorubicin group and one extra-skeletal myxoid chondrosarcoma in the gemcitabine and docetaxel group). §Two moved away, one being treated locally. ¶One moved away. ||See appendix p 6 for a breakdown of reasons for treatment discontinuation.
Patient characteristics were similar in the two treatment groups at baseline (table 1). Because there was only one patient aged 18 years or younger, we excluded this stratification factor from all analyses. The analysis is based on the dataset in its version of Sept 8, 2015; at this point, the estimated median follow-up for all patients was 22 months (IQR 15·7–29·3).
Table 1.
Baseline characteristics
| Doxorubicin (n=129) | Gemcitabine and docetaxel (n=128) | |||
|---|---|---|---|---|
| Sex | ||||
| Male | 50 (39%) | 51 (40%) | ||
| Female | 79 (61%) | 77 (60%) | ||
| Age | 56 (49·4–64·0) | 55 (45·6–64·0) | ||
| ≤18 years | 1 (1%) | 0 | ||
| Weight (kg) | 77·0 (65·7–89·4) | 77·7 (63·3–90·9) | ||
| WHO performance status | ||||
| 0 | 55 (43%) | 52 (41%) | ||
| 1 | 63 (49%) | 67 (52%) | ||
| 2 | 11 (9%) | 9 (7%) | ||
| Trojani grade | ||||
| 2 | 29 (22%) | 34 (27%) | ||
| 3 | 85 (66%) | 85 (66%) | ||
| Not known | 15 (12%) | 9 (7%) | ||
| Histology | ||||
| Uterine leiomyosarcoma | 36 (28%) | 35 (27%) | ||
| Synovial sarcoma | 5 (4%) | 6 (5%) | ||
| Pleomorphic sarcoma | 16 (12%) | 16 (13%) | ||
| Other eligible sarcomas | 72 (56%) | 71 (55%) | ||
| Non-uterine leiomyosarcoma | 24 (19%) | 23 (18%) | ||
Data are n (%) or median (IQR).
Of the 257 randomised patients, 244 (95%) had central histopathology review done. 51 (21%) of 244 reports differed between the local histology report and the central review report, with 36 (14%) major discrepancies resulting in reclassification of histology, and 15 (6%) minor discrepancies (eg, high-grade spindle cell sarcoma being reclassified as pleomorphic sarcoma). A full list of major and minor discrepancies is in the appendix (pp 3–4). Of the 13 (5%) of 257 patients whose histology was not reviewed, six samples were never received, one patient had no tissue available, and for six patients there was no tumour tissue in the submitted blocks.
More patients in the doxorubicin group (71 [56%] of 128 patients) received the full six cycles of chemotherapy than those in the gemcitabine and docetaxel group (49 [39%] of 126 patients). In the gemcitabine and docetaxel group, some patients received day 1 gemcitabine but not day 8 gemcitabine and docetaxel (gemcitabine: cycle 1, eight [6%] of 126 patients; cycle 2, seven [6%] of 110 patients; cycle 3, five [6%] of 89 patients; cycle 4, six [8%] of 75 patients; cycle 5, eight [14%] of 59 patients; and cycle 6, three [6%] of 52 patients; appendix p 5). Mean dose intensity23 (incorporating dose delays and reductions) was 93·7% (SD 0·09) in the doxorubicin group and 83·4% (SD 0·20) in the gemcitabine and docetaxel group. More patients in the gemcitabine and docetaxel group experienced dose delays (71 [56%] of 126 patients) than in the doxorubicin group (59 [46%] of 128 patients). The main reasons for dose delays in both groups were febrile neutropenia (seven [12%] of 59 reasons in the doxorubicin group vs six [4%] of 155 reasons in the gemcitabine and docetaxel group), other haematological toxicities (16 [27%] vs 44 [28%]), non-haematological toxicities (five [8%] vs seven [5%]), other adverse events (14 [24%] vs 29 [18%]), and other practical or social reasons (11 [19%] vs 32 [21%]). More patients had dose reductions in the doxorubicin group (34 [27%] of 128 patients) than in the gemcitabine and docetaxel group (23 [18%] of 126; appendix p 17); the main reasons for dose reductions were febrile neutropenia (eight [20%] of 41 reasons in the doxorubicin group vs one [1%] of 89 reasons in the gemcitabine and docetaxel group) and other haematological toxicities (seven [17%] of 41 vs 26 [29%] of 89). One (1%) of 128 patients in the doxorubicin group and 13 (10%) of 126 in the gemcitabine and docetaxel group stopped treatment early because of toxicity (appendix p 6).
At the time of data analysis, 223 (87%) of 257 patients in the intention-to-treat population had experienced disease progression; 106 (82%) of 129 patients in the doxorubicin group and 117 (91%) of 128 patients in the gemcitabine and docetaxel group. An additional ten (8%) patients in the doxorubicin group and five (4%) in the gemcitabine and docetaxel group died without confirmation of progression. 154 (60%) of 257 patients died in the intention-to-treat population; 74 (57%) of 129 patients in the doxorubicin group and 80 (63%) of 128 patients in the gemcitabine and docetaxel group. No deaths were related to the treatment, but two deaths were due to a combination of disease progression and treatment (see appendix p 10 for the breakdown of causes of death by treatment group). As planned, CT or MRI scan images were centrally reviewed for 38 (15%) of 257 patients. No discrepancies were noted between the hospitals and the central review for any patients.
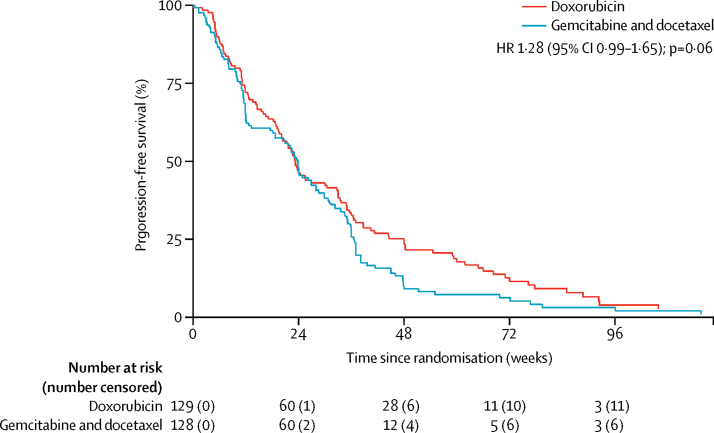
Progression-free survival at 24 weeks did not differ between the treatment groups (46·3% [95% CI 37·5–54·6] in the doxorubicin group vs 46·4% [37·5–54·8] in the gemcitabine and docetaxel group; figure 2). Progression-free survival at 12 weeks was 72·1% (95% CI 63·5–79·0) in the doxorubicin group vs 63·8% (54·8–71·5) in the gemcitabine and docetaxel group. Median progression-free survival was 23·3 weeks (95% CI 19·6–30·4) in the doxorubicin group and 23·7 weeks (18·1–20·0) in the gemcitabine and docetaxel group. The unadjusted HR was 1·28 (95% CI 0·99–1·65; p=0·06); after adjusting for histological subtype, the HR was 1·26 (0·97–1·63; p=0·08) in favour of doxorubicin. Although the Kaplan-Meier curves did not violate the proportional hazards assumption (p=0·46 for the adjusted model), they initially overlapped, and then separated after 24 weeks.
Figure 2.
Progression-free survival
HR=hazard ratio.
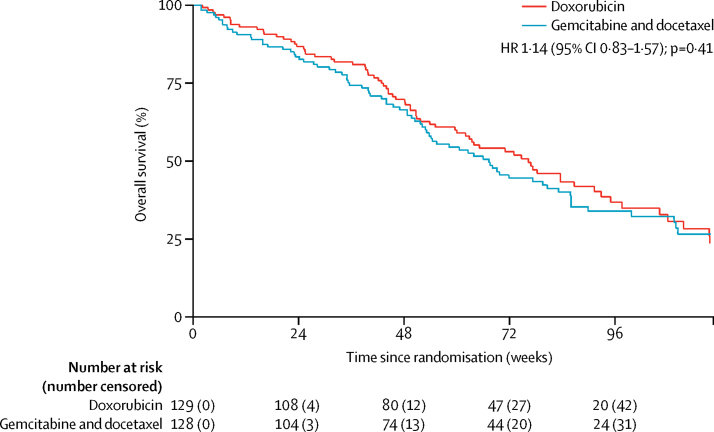
Overall survival did not significantly differ between the two groups. Overall survival at 24 weeks was 86·8% (95% CI 79·6–91·6) in the doxorubicin group and 82·6% (74·8–88·2) in the gemcitabine and docetaxel group (figure 3). Median overall survival was 76·3 weeks (95% CI 60·0–91·3) in the doxorubicin group and 67·3 weeks (53·1–83·1) in the gemcitabine and docetaxel group (unadjusted HR 1·14, 95% CI 0·83–1·57; p=0·41).
Figure 3.
Overall survival
HR=hazard ratio.
The proportion of patients achieving an objective response by RECIST 1.1 (complete or partial response) was similar in the two groups: 25 (19%) of 129 patients in the doxorubicin group and 25 (20%) of 128 in the gemcitabine and docetaxel group (table 2).
Table 2.
Objective responses
| Doxorubicin (n=129) | Gemcitabine and docetaxel (n=128) | |
|---|---|---|
| Complete response | 2 (2%) | 0 |
| Partial response | 23 (18%) | 25 (20%) |
| Stable disease | 60 (47%) | 50 (39%) |
| Progressive disease | 25 (19%) | 27 (21%) |
| Not evaluable | 19 (15%) | 26 (20%) |
Data are n (%).
In our prospectively planned exploratory subgroup analysis, no evidence of a differential treatment effect by histological subtype was recorded (p=0·24; appendix p 11). Further subgroup analyses were done comparing leiomyosarcoma versus other sarcomas (p=0·14), and uterine leiomyosarcoma versus other sarcomas (p=0·38), but again no differential effect was evident between the two treatment groups.
The most common low-grade non-haematological adverse event was grade 1 and 2 alopecia, which occurred in 110 (86%) of 128 patients in the doxorubicin group and 95 (75%) of 126 patients in the gemcitabine and docetaxel group (table 3). The most common low-grade haematological adverse event was anaemia (grade 1–2 in 91 [71%] patients in the doxorubicin group vs 104 [83%] in the gemcitabine and docetaxel group). The most common grade 3 and 4 adverse events were neutropenia (32 [25%] of 128 patients for doxorubicin vs 25 [20%] of 126 patients for gemcitabine and docetaxel), febrile neutropenia (26 [20%] vs 15 [12%]), fatigue (eight [6%] vs 17 [14%]), oral mucositis (18 [14%] vs two [2%]), and pain (ten [8%] vs 13 [10%]; table 3). The three most common serious adverse events, accounting for 111 (39%) of all 285 serious adverse events, were febrile neutropenia (27 [17%] of 155 serious adverse events in the doxorubicin group vs 15 [12%] of 130 in the gemcitabine and docetaxel group), fever (18 [12%] vs 19 [15%]), and neutropenia (22 [14%] vs ten [8%]; appendix pp 7–9).
Table 3.
Adverse events
|
Doxorubicin (n=128) |
Gemcitabine and docetaxel (n=126) |
|||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Haematological | ||||||
| Anaemia | 91 (71%) | 10 (8%) | 0 | 104 (83%) | 8 (6%) | 0 |
| White blood cell decreased | 33 (26%) | 4 (3%) | 6 (5%) | 54 (43%) | 8 (6%) | 1 (1%) |
| Neutropenia | 26 (20%) | 15 (12%) | 17 (13%) | 46 (37%) | 15 (12%) | 10 (8%) |
| Platelet count decreased | 13 (10%) | 1 (1%) | 0 | 31 (25%) | 0 | 0 |
| Febrile neutropenia | 0 | 25 (20%) | 1 (1%) | 0 | 13 (10%) | 2 (2%) |
| Non-haematological | ||||||
| Alopecia | 110 (86%) | 0 | 0 | 95 (75%) | 0 | 0 |
| Fatigue | 108 (84%) | 8 (6%) | 0 | 91 (72%) | 17 (14%) | 0 |
| Nausea | 82 (64%) | 5 (4%) | 0 | 69 (55%) | 3 (2%) | 0 |
| Pain | 70 (55%) | 10 (8%) | 0 | 66 (52%) | 11 (9%) | 2 (2%) |
| Oral mucositis | 64 (50%) | 18 (14%) | 0 | 59 (47%) | 2 (2%) | 0 |
| Anorexia | 58 (45%) | 5 (4%) | 0 | 53 (42%) | 2 (2%) | 1 (1%) |
| Constipation | 54 (42%) | 1 (1%) | 0 | 49 (39%) | 2 (2%) | 0 |
| Diarrhoea | 47 (37%) | 3 (2%) | 0 | 42 (33%) | 9 (7%) | 1 (1%) |
| Vomiting | 46 (36%) | 3 (2%) | 0 | 32 (25%) | 0 | 0 |
| Alkaline phosphatase increased | 41 (32%) | 2 (2%) | 0 | 46 (37%) | 2 (2%) | 0 |
| Alanine aminotransferase increased | 34 (27%) | 1 (1%) | 0 | 36 (29%) | 1 (1%) | 0 |
| Dyspepsia | 27 (21%) | 1 (1%) | 0 | 18 (14%) | 0 | 0 |
| Hypoalbuminaemia | 24 (19%) | 4 (3%) | 0 | 40 (32%) | 0 | 0 |
| Fever | 24 (19%) | 1 (1%) | 0 | 27 (21%) | 1 (1%) | 0 |
| Dysgeusia | 24 (19%) | 0 | 0 | 25 (20%) | 0 | 0 |
| Cough | 23 (18%) | 1 (1%) | 0 | 26 (21%) | 0 | 0 |
| Limb oedema | 22 (17%) | 2 (2%) | 0 | 55 (44%) | 0 | 0 |
| Hyponatraemia | 17 (13%) | 3 (2%) | 0 | 16 (13%) | 7 (6%) | 0 |
| Dyspnoea | 16 (13%) | 3 (2%) | 0 | 24 (19%) | 4 (3%) | 1 (1%) |
| Abdominal pain | 16 (13%) | 4 (3%) | 0 | 14 (11%) | 2 (2%) | 0 |
| Peripheral sensory neuropathy | 14 (11%) | 0 | 0 | 31 (25%) | 0 | 0 |
| Aspartate aminotransferase increased | 14 (11%) | 0 | 0 | 12 (10%) | 1 (1%) | 0 |
| Dry mouth | 12 (10%) | 0 | 0 | 5 (4%) | 0 | 0 |
| Urea increased | 11 (9%) | 0 | 0 | 12 (10%) | 0 | 0 |
| Back pain | 11 (9%) | 1 (1%) | 0 | 11 (9%) | 1 (1%) | 0 |
| Hyperkalaemia | 10 (8%) | 1 (1%) | 0 | 18 (14%) | 0 | 0 |
| Insomnia | 10 (8%) | 0 | 0 | 9 (7%) | 1 (1%) | 0 |
| Urinary tract infection | 9 (7%) | 0 | 0 | 7 (6%) | 1 (1%) | 0 |
| Dizziness | 8 (6%) | 2 (2%) | 0 | 4 (3%) | 0 | 0 |
| Hypokalaemia | 7 (6%) | 2 (2%) | 0 | 7 (6%) | 1 (1%) | 0 |
| Myalgia | 5 (4%) | 0 | 0 | 12 (10%) | 0 | 0 |
| Pneumonitis | 4 (3%) | 0 | 0 | 7 (6%) | 1 (1%) | 0 |
| Lower respiratory tract infection | 4 (3%) | 0 | 0 | 4 (3%) | 2 (2%) | 0 |
| Rash | 3 (2%) | 0 | 0 | 16 (13%) | 0 | 0 |
| Epistaxis | 2 (2%) | 0 | 0 | 13 (10%) | 0 | 0 |
| Herpes zoster virus infection | 2 (2%) | 0 | 1 (1%) | 0 | 0 | 0 |
| Hiccups | 2 (2%) | 0 | 0 | 2 (2%) | 1 (1%) | 0 |
| Muscle weakness lower limb | 2 (2%) | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
| Nail infection | 2 (2%) | 0 | 0 | 0 | 1 (1%) | 0 |
| Tumour pain | 1 (1%) | 1 (1%) | 0 | 3 (2%) | 0 | 0 |
| Hypertension | 1 (1%) | 1 (1%) | 0 | 1 (1%) | 0 | 0 |
| Hyperglycaemia | 1 (1%) | 0 | 0 | 1 (1%) | 1 (1%) | 1 (1%) |
| Allergic reaction | 1 (1%) | 0 | 0 | 3 (2%) | 1 (1%) | 0 |
| Pleural effusion | 1 (1%) | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
| Chest pain—cardiac | 1 (1%) | 0 | 0 | 0 | 2 (2%) | 0 |
| Urticaria | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 |
| Thromboembolic event | 0 | 6 (5%) | 2 (2%) | 0 | 2 (2%) | 2 (2%) |
| Lung infection | 0 | 5 (4%) | 0 | 0 | 3 (2%) | 1 (1%) |
| Syncope | 0 | 3 (2%) | 0 | 0 | 0 | 0 |
| Upper respiratory infection | 0 | 2 (2%) | 0 | 0 | 2 (2%) | 0 |
| Vascular access complication | 0 | 2 (2%) | 0 | 0 | 1 (1%) | 0 |
| Rectal haemorrhage | 0 | 1 (1%) | 0 | 3 (2%) | 0 | 0 |
| Infection not otherwise specified | 0 | 1 (1%) | 0 | 0 | 3 (2%) | 0 |
| Skin infection | 0 | 1 (1%) | 0 | 0 | 2 (2%) | 0 |
| Non-cardiac chest pain | 0 | 1 (1%) | 0 | 0 | 2 (2%) | 0 |
| Wound infection | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 |
| Acute kidney injury | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Anal abscess | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Bone pain | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Breast pain | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Bronchopulmonary haemorrhage | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Colonic ulcer | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Creatinine decreased | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| GGT increased | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Heart failure | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Left ventricular systolic dysfunction | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Lichen sclerosus | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Lower gastrointestinal haemorrhage | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Oral candidiasis | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Pneumothorax | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Premature menopause | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Renal vein thrombosis | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders: Hickman line | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Tremor | 0 | 1 (1%) | 0 | 0 | 0 | 0 |
| Abdominal distension | 0 | 0 | 0 | 4 (3%) | 1 (1%) | 0 |
| Infusion-related reaction | 0 | 0 | 0 | 4 (3%) | 1 (1%) | 0 |
| Skin ulceration | 0 | 0 | 0 | 2 (2%) | 1 (1%) | 0 |
| Cellulitis | 0 | 0 | 0 | 1 (1%) | 3 (2%) | 0 |
| Bronchial Infection | 0 | 0 | 0 | 1 (1%) | 2 (2%) | 0 |
| Night sweats | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
| Anaphylaxis | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Ascites | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Catheter-related infection | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Diverticulitis | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Escherichia coli infection at tumour site | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Electrolyte imbalance | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Fluid retention | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Fracture | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Radiation recall reaction (dermatological) | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Rectal tenesmus | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Small intestinal obstruction | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Stoma site abscess | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
| Hypercalcaemia | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Intestinal perforation | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Sepsis* | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Myocardial infarction† | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
| Sudden death not otherwise specified‡ | 0 | 0 | 0 | 0 | 0 | 0 |
Data are n (%).
Sepsis (grade 5) recorded in one patient who received doxorubicin and one patient who received gemcitabine and docetaxel.
Myocardial infarction (grade 5) recorded in one patient who received doxorubicin.
Sudden death not otherwise specified (grade 5) recorded in one patient who received gemcitabine and docetaxel. GGT=γ glutamyltransferase.
We compared quality of life between the groups at 12 weeks postrandomisation. Insufficient questionnaires were returned to be able to assess quality of life at 18 weeks and 24 weeks (83 [32%] of 257 questionnaires were returned at both 18 weeks and 24 weeks, compared with 132 [51%] of 257 at 12 weeks). Quality-of-life measures did not differ between the treatment groups at 12 weeks postrandomisation (table 4). However, the mean Global Health Status score at 12 weeks was 63·8 (SD 22·5) in the doxorubicin group (based on 64 [50%] of 129 patients) and 59·1 (SD 21·8) in the gemcitabine and docetaxel group (based on 63 [49%] of 128 patients). After adjusting for baseline score and histological subtype, the mean treatment difference was 5·1 (99% CI −4·7 to 15·0; p=0·17; figure 4).
Table 4.
Difference in quality-of-life outcomes at 12 weeks after randomisation
| Treatment effect of gemcitabine and docetaxel versus doxorubicin (99% CI)* | ||
|---|---|---|
| C30 scales† | ||
| Global Health Status (n=120)† | −5·1 (−15·0 to 4·7) | |
| Functional scales† | ||
| Physical functioning (n=122) | −0·5 (−9·8 to 8·8) | |
| Role functioning (n=122) | −7·8 (−21·6 to 6·1) | |
| Emotional functioning (n=121) | −5·6 (−14·6 to 3·4) | |
| Cognitive functioning (n=121) | −3·1 (−12·2 to 6·1) | |
| Social functioning (n=121) | 1·5 (−9·8 to 12·9) | |
| Symptom scales‡ | ||
| Fatigue (n=122) | −0·4 (−12·7 to 12·0) | |
| Nausea and vomiting (n=122) | −4·6 (−13·5 to 4·2) | |
| Pain (n=122) | 5·5 (−7·4 to 18·4) | |
| Dyspnoea (n=122) | 1·5 (−11·6 to 14·5) | |
| Insomnia (n=122) | 2·2 (−11·6 to 16·0) | |
| Appetite loss (n=122) | −11·0 (−25·6 to 3·5) | |
| Constipation (n=121) | −2·2 (−14·0 to 9·6) | |
| Diarrhoea (n=119) | 2·3 (−6·6 to 11·2) | |
| Financial difficulties (n=121) | −0·8 (−11·8 to 10·1) | |
| FA13 scores‡ | ||
| Physical fatigue (n=112) | 8·3 (−3·9 to 20·6) | |
| Emotional fatigue (n=112) | 8·5 (−3·4 to 20·4) | |
| Cognitive fatigue (n=112) | 3·9 (−4·6 to 12·4) | |
| Interference with daily life (n=111) | 5·5 (−8·3 to 19·4) | |
| Social sequelae (n=111) | 2·4 (−8·5 to 13·2) | |
From a linear regression model, adjusting for baseline score, histological subtype, and time since baseline. Treatment effect is calculated as: (gemcitabine and docetaxel change from baseline) – (doxorubicin change from baseline).
Higher scores are associated with better quality of life; a positive number indicates better functioning and quality of life on gemcitabine and docetaxel than on doxorubicin.
Lower scores are associated with better quality of life; a positive number indicates worse symptoms on gemcitabine and docetaxel than on doxorubicin. C30=EORTC core quality-of-life questionnaire. FA13=fatigue questionnaire.
Figure 4.
Quality-of-life outcomes at 12 weeks post-randomisation
The plotted points represent the mean treatment effect between the groups (a positive number for the treatment effect indicates a better quality of life on gemcitabine and docetaxel than doxorubicin). For symptom scales and FA13 scores, lower scores are associated with better quality of life. Consequently, values are the opposite between table 4 and figure 4); horizontal lines are 99% CIs. FA13=fatigue questionnaire.
For the pharmacogenomics analysis in our translational study, total DNA was successfully extracted from 240 patients: 119 in the doxorubicin group and 121 in the gemcitabine and docetaxel group. Frequency and distribution of the SNPs investigated were well balanced between the treatment groups (appendix p 12). Several associations were observed between the SNPs studied, and survival and toxicity (appendix pp 12–15). Within the doxorubicin group, three of the four SNPs in the SLC22A16 gene, all in linkage disequilibrium with each other, were associated with a worse progression-free survival (appendix pp 12, 15), as was the minor allele of the SLC29A1 SNP (rs9394992, in both heterozygotes and homozygotes; appendix p 14). By contrast, the PRDX4 SNP (rs518329) minor allele was associated with improved overall survival in the doxorubicin group in both heterozygotes and homozygotes (appendix p 13). Analysis of the gemcitabine and docetaxel treatment group indicated a possible association of the ABCB1 rs1045642 minor allele with worse progression-free survival in both heterozygotes and homozygotes (appendix p 12); the CDA rs2072671 SNP was associated with worse overall survival (appendix p 14), and the CMPK1 rs4492666 SNP was associated with improved overall survival in both heterozygotes and homozygotes (appendix p 14). The SLC22A16 rs723685 minor allele was associated with a reduced frequency of grade 3–4 adverse events compared with wild type (ten [48%] of 21 patients vs 69 [71%] of 97 patients) in the doxorubicin treatment group but not in the gemcitabine and docetaxel group. No other SNPs were associated with grade 3–4 adverse events (appendix pp 18–19).
After completion of treatment within the trial, 58 (23%) of 254 patients in the safety population (28 [22%] of 128 patients receiving doxorubicin and 30 (24%) of 126 receiving gemcitabine and docetaxel) did not receive any additional treatment. The remaining 196 (77%) patients received at least one additional treatment: chemotherapy for 139 (71%) of 196 patients (62 [62%] of 100 patients in the doxorubicin group and 77 [80%] of 96 patients in the gemcitabine and docetaxel group), and local therapies for 57 (29%) of 196 patients (38 [38%] and 19 [20%]). Local therapies consisted of radiotherapy for 44 (22%) of 196 patients (28 [28%] and 16 [17%]) and surgery for 13 (7%) of 196 patients (ten [10%] and three [3%]). The most frequently used second-line chemotherapy regimens were doxorubicin (61 [42%] of 145 prescriptions; appendix p 16), gemcitabine and docetaxel (19 [13%]), ifosfamide (17 [12%]), trabectedin (13 [9%]), pazopanib (nine [6%]), and gemcitabine (seven [5%]; appendix p 16). Information about the outcomes of these additional treatments (local therapies and chemotherapy) was not collected.
Discussion
In this randomised phase 3 trial of gemcitabine and docetaxel compared with doxorubicin as first-line therapy for locally advanced or metastatic soft-tissue sarcoma, we found no significant difference between the two treatment groups for the primary endpoint of the proportion of patients alive and progression free at 24 weeks. Similarly, we found no significant difference in overall survival between the two treatment groups. Soft-tissue sarcoma is recognised to be a very heterogeneous disease with a large number of histological subtypes, and indeed our trial included 22 different subtypes, with very small numbers of patients with many of these subtypes. This heterogeneity is an inevitable feature of most large soft-tissue sarcoma trials, and as such we believe that the GeDDiS trial population is representative of the general population with advanced soft-tissue sarcoma.
Gemcitabine and docetaxel has been used in locally advanced or metastatic soft-tissue sarcoma since 2002, and has been investigated in several small studies, which have shown activity of the combination therapy.6, 7, 8, 9, 11, 12 A randomised phase 2 study compared gemcitabine and docetaxel with gemcitabine alone in 122 patients with advanced soft-tissue sarcoma who had received between zero and three previous chemotherapy regimens, and reported superior median progression-free survival for gemcitabine and docetaxel compared with gemcitabine alone (6·2 months vs 3·0 months) and overall survival (17·9 months vs 11·5 months).13 A subsequent randomised phase 2 study compared gemcitabine and docetaxel versus gemcitabine alone as second-line treatment in 90 patients with leiomyosarcoma, reporting that both schedules were effective second-line therapies, with similar proportions of patients achieving a response.10 Gemcitabine and docetaxel has therefore become an accepted treatment option in metastatic soft-tissue sarcoma after first-line therapy.24 Subsequent phase 1b/2 studies have combined gemcitabine and docetaxel with bevacizumab showing feasibility and activity,25, 26 and also with pazopanib,27 although that trial closed early because of slow accrual and substantial toxicity. A placebo-controlled phase 3 trial28 of gemcitabine and docetaxel in combination with bevacizumab in metastatic uterine leiomyosarcoma for first-line treatment did not show a benefit for progression-free survival or overall survival.
Since the first published study6 of gemcitabine and docetaxel, which was confined to patients with leiomyosarcoma, some clinicians have assumed that the combination is more active in leiomyosarcoma and uterine leiomyosarcoma than in other histological subtypes. Indeed, several studies8, 9, 10, 11, 28 have restricted the use of gemcitabine and docetaxel to leiomyosarcoma and uterine leiomyosarcoma. However, the results of the GeDDiS trial refute these claims of superior activity in particular histological subtypes of locally advanced or metastatic soft-tissue sarcoma versus others, since our results show no evidence that treatment effect was influenced by histological subtype. We did planned subgroup analyses to investigate whether patients with either leiomyosarcoma or uterine leiomyosarcoma responded better to gemcitabine and docetaxel than other soft-tissue sarcoma histological subtypes, but found no indication of a superior response to gemcitabine and docetaxel in either of these subgroups.
We chose 13 years as the minimum age for the trial, specifically to try to increase participation of the teenage and young adult population, because clinical trial recruitment of this age group is recognised to be poor.29 However, only one patient younger than 18 years of age was recruited, which we believe reflects the differing approaches to treatment of advanced soft-tissue sarcoma by UK paediatric and adult oncologists—with paediatric oncologists using more intensive chemotherapy regimens than those used in this trial. Additionally, competing paediatric trials in this population were running in the UK at the time of recruitment to GeDDiS, such that we believe that younger patients were preferentially recruited to those paediatric trials.
Why did gemcitabine and docetaxel fail to show superiority to doxorubicin? This outcome might have been partly due to the choice of lower dose and fewer cycles of treatment; the 2002 study by Hensley and colleagues6 delivered more cycles (eight vs six), at higher doses (gemcitabine 900 mg/m2 and docetaxel 100 mg/m2 vs gemcitabine 675 mg/m2 and docetaxel 75 mg/m2) than in GeDDiS. Our regimen was chosen on the basis of the randomised phase 2 study of Maki and colleagues,13 which had used the higher dose schedule, and had reported high frequency (46%) of dose reductions for gemcitabine and docetaxel, lower dose intensity than gemcitabine, and more than 40% of patients on gemcitabine and docetaxel stopping within 6 months of starting therapy, for non-haematological toxicities such as fatigue and myalgias, despite dose reductions. The authors concluded that “this dose and schedule…are too high for long-term use”. Additionally, a previous phase 2 study11 that the GeDDiS chief investigator had led using this schedule had found that quite substantial toxicity was experienced in the UK population, such that eight (18%) of 45 patients stopped treatment early due to toxicity, and only ten (20%) patients received the full eight cycles. We therefore made a pragmatic decision to dose modify the schedule to make it more suitable for patients receiving palliative chemotherapy. The lower doses used are reflected by the absence of grade 3–4 thrombocytopenia recorded (no patients on gemcitabine and docetaxel experienced this toxicity) compared with a 40% frequency in the previous randomised phase 2 study.13 It might be suggested that the gemcitabine and docetaxel doses we selected were too low. However, despite modifying the gemcitabine and docetaxel schedule for the GeDDiS trial, our results were similar for gemcitabine and docetaxel to those of Maki and colleagues' study,13 with median progression-free survival of 5·5 months versus 6·2 months, and median overall survival of 15·5 months versus 17·9 months, respectively.
An additional factor in the lack of superiority of gemcitabine and docetaxel over doxorubicin at least for overall survival might be the lower overall exposure of patients to gemcitabine and docetaxel, as first-line and second-line treatments combined. Of 96 patients in the gemcitabine and docetaxel group receiving a subsequent treatment, 60 received doxorubicin, whereas of 100 patients in the doxorubicin group receiving a subsequent treatment, only 18 received gemcitabine and docetaxel. This difference is because for many UK hospitals at the time of the GeDDiS trial, the combination of gemcitabine and docetaxel was not funded and thus unavailable to clinicians.
Despite the use of a modified gemcitabine and docetaxel schedule in our study, treatment adherence to gemcitabine and docetaxel was inferior to that for doxorubicin. Patients in the gemcitabine and docetaxel group experienced more dose delays and lower dose intensity than those in the doxorubicin group, with fewer patients in the gemcitabine and docetaxel group receiving all six cycles of chemotherapy and more patients stopping treatment early due to toxicity. These findings might have a number of explanations. A proportion of patients (4% to 10%) at each cycle did not receive day 8 docetaxel, thus affecting dose intensity. Docetaxel was omitted at these points according to protocol criteria for low neutrophils or platelets, or toxicity leading to a clinical decision to omit docetaxel (notably, the most common non-haematological grade 3–4 toxicity in the gemcitabine and docetaxel group was fatigue). The excess of patients stopping gemcitabine and docetaxel early might also have reflected a bias in clinicians to stop treatment earlier in the experimental group, in the knowledge that patients could go on to receive standard doxorubicin-based treatment. It could be argued that the gemcitabine and docetaxel doses that were delayed or missed represent undertreatment in this group, which could explain the separation of the progression-free survival curves in figure 2.
Although no significant difference was seen in any of the individual quality-of-life parameters between the treatment groups, the global health status was numerically lower for gemcitabine and docetaxel than in the doxorubicin group. Potential contributing factors are that gemcitabine and docetaxel requires an additional visit to hospital in each 3-week cycle, and gemcitabine and docetaxel takes longer to deliver than doxorubicin, prolonging each hospital visit and resulting in an added burden on patients with incurable disease receiving palliative chemotherapy. The implication of these results is that gemcitabine and docetaxel was more difficult to deliver (lower dose intensity, more treatment delays, and more patients stopping early due to toxicity), and was associated with a worse global health status than doxorubicin. Thus, there does seem to be a disadvantage to patients in receiving gemcitabine and docetaxel as first-line treatment. Furthermore, there are economic, as well as personal, disadvantages to gemcitabine and docetaxel because it is a more expensive treatment regimen than doxorubicin because of higher drug costs, more frequent and longer hospital visits are needed for treatment, and increased requirements for supportive medications (data not shown).
Importantly, the results of the current study are consistent with two other first-line studies in locally advanced or metastatic soft-tissue sarcoma that included doxorubicin as the control group, with the median progression-free survival of 23·3 weeks in the doxorubicin group in the current study similar to the 22·5 weeks4 and 26 weeks5 reported in the other studies. This is also true of median overall survival, which was 76·3 weeks in the doxorubicin group in the current study, compared with 73·2 weeks4 and 82·3 weeks5 with doxorubicin in the other studies. Interestingly, all three studies showed an improvement in progression-free survival and overall survival compared with the preceding published trial of Judson and colleagues2 (progression-free survival of 19·9 weeks and overall survival of 55·4 weeks), which had recruited patients several years earlier, suggesting that outcomes have improved for patients with locally advanced or metastatic soft-tissue sarcoma in recent years. The explanation for this is likely to be multifactorial. Clinicians might be better at selecting patients most likely to benefit from palliative chemotherapy for advanced soft-tissue sarcoma, and are treating patients more aggressively in terms of minimising dose reductions and treatment delays, with greater use of supportive medications such as growth factors. A further factor might be increasing use of local therapies such as radiotherapy and surgery for patients with metastatic disease, which has been associated with longer overall survival than for patients not receiving such therapies.30 Indeed, 57 patients on the GeDDiS trial went on to receive surgery or radiotherapy after completing study treatment.
The pharmacogenomics data obtained in the current study suggest that SNPs in the organic cation transporter SLC22A16 are associated with reduced efficacy and decreased toxicity following doxorubicin treatment. These findings are in keeping with previously published data from a breast cancer patient cohort and are consistent with a loss of function in the transporter that results in reduced intracellular influx of doxorubicin.20 Validation of these effects in an independent cohort of sarcoma patients would be valuable to ascertain the potential for this SNP to influence clinical decision-making. Two other SNPs, SLC29A1 rs9394992 and PRDX4 rs518329, were also associated with outcomes in the doxorubicin group; however, these genes are not known to be involved in the pharmacology of doxorubicin, so further investigation is required to understand these effects. There were indications that three SNPs predicted to affect gemcitabine pharmacokinetics were associated with an effect on overall survival, most notably the CDA rs2072671 SNP, which was associated with reduced overall survival in the gemcitabine and docetaxel group, with worse survival in patients homozygous (rather than heterozygous) for the minor allele (this is referred to as a gene-dose effect). However, the same SNP was not associated with a difference in progression-free survival, and insufficient evidence is currently available to advocate routine testing of these SNPs to influence clinical decision-making.
Limitations of the current study include the fact that we used a gemcitabine and docetaxel schedule that used fewer cycles and lower doses than in the originally published schedule,6 which is widely used in other countries. Although we had a clear rationale for this decision, nevertheless we acknowledge that some might conclude that this limits the applicability of the trial results to the wider population of patients with advanced soft-tissue sarcoma beyond our trial. Additionally, more patients stopped treatment early on gemcitabine and docetaxel than doxorubicin, which might have reflected the fact that clinicians knew that patients could go on to receive doxorubicin, whereas the reverse was not true due to the limited availability of gemcitabine and docetaxel in the UK at that time outside of clinical trials.
How should these results inform our discussions with patients with advanced soft-tissue sarcoma? The data do not support superiority for gemcitabine and docetaxel over doxorubicin on survival outcomes. Subgroup analyses have not identified any subgroup for which gemcitabine and docetaxel was superior, and in particular we did not observe superiority for either leiomyosarcoma or uterine leiomyosarcoma. Although the similar progression-free survival and overall survival of gemcitabine and docetaxel and doxorubicin might support a conclusion that either schedule can be used according to patient or clinician preference, our results indicate the need for caution with such an approach, in that gemcitabine and docetaxel is more difficult to deliver than doxorubicin, and patients find it more toxic than doxorubicin, with some effects on quality of life. Furthermore, economic factors should be considered with the higher cost of the gemcitabine and docetaxel schedule. Thus, the overall clinical conclusion should be that doxorubicin remains standard of care as first-line treatment for locally advanced or metastatic soft-tissue sarcoma, and that gemcitabine and docetaxel is not recommended as routine first-line treatment. There might of course be occasions when different choices are made depending on patient factors and preferences, such as using combination doxorubicin and ifosfamide for selected patients who need to optimise chances of tumour shrinkage, or using gemcitabine and docetaxel in patients with cardiac dysfunction that contraindicates use of doxorubicin. However, for most patients, these results will hopefully provide evidence for clinicians to consider with their patients when selecting first-line treatment for advanced soft-tissue sarcoma. The study also highlights the importance of doing randomised trials in rare cancers to rigorously compare new treatments with established standard treatments, rather than extrapolating promising results from smaller non-comparative trials.
Acknowledgments
Acknowledgments
We thank all patients participating in GeDDiS and their families. The GeDDiS trial was funded by Cancer Research UK (C2921/A11561), with separate funding obtained from Sarcoma UK (SUK16.2015) to support the pharmacogenomics studies described. Funding from Cancer Research UK supported the central coordination of the trial. We thank Ian Judson (Royal Marsden Hospital, London, UK; retired) for his review of the manuscript and Shonit Punwani (University College London, London, UK) for his input into the central CT scan review. BS, SJS, and JW were supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. CB was supported by the National Institute for Health Research Royal Marsden and Institute of Cancer Research Biomedical Research Centre. CR's work on the trial was supported at the Kantonsspital St Gallen by a grant of the Clinical Trials Unit commission, CTU 12/14.
Contributors
GeDDiS was developed through and supported by the UK National Cancer Research Institute Sarcoma Clinical Studies Group and was led by the trial management group (composed of BS, SJS, JW, ML, PJW, FC, CR, ZW, SF, and SB). The trial was designed by BS, with input from the trial management group. H-MD and SN were responsible for the statistical analysis. BS had full access to all the data in the trial and had final responsibility for the decision to submit for publication. GJV, DJ, KK, and H-MD were responsible for the pharmacogenomics analysis. Central histopathological review was done by RT. The translational aspects of the study were led by SJS. SF and SB were responsible for the conduct of the trial, ensuring all required approvals were in place, and for collection and verification of the integrity of the data. Study results were interpreted by the trial management group, who also drafted the manuscript and collated responses from all co-authors. CB, NA, and MM were involved in patient recruitment. All authors gave final approval of the version to be published.
Declaration of interests
BS has received honoraria and travel grants from Novartis, Pharmamar, Ariad, Clinigen, Daiichi, and Lilly. SJS has received honoraria and travel grants from Lilly Oncology, Pharmamar, and Pfizer. PJW has received honoraria from Amgen, Bristol-Myers Squibb, Lilly, and Theradex, and research grants from AstraZeneca, Pfizer, and Virtuu. CR has received honoraria from Pfizer, GlaxoSmithKline, Novartis, and Astellas and a research grant from Astellas. MM has received honoraria from Pharmamar and Pierre Fabre, and sponsorship for conferences from Roche and Bristol-Myers Squibb. NA has received sponsorship and funding for conferences from Pharmamar and Roche. SB has received grants from AstraZeneca and professional fees from Biocompatibles. JW, ML, FC, ZW, CB, GJV, DJ, KK, RT, SF, SN, and H-MD declare no competing interests.
Supplementary Material
References
- 1.Francis MDN, Charman J, Lawrence G, Grimer R. Bone and soft tissue sarcomas UK Incidence and Survival: 1996 to 2010. National Cancer Intelligence Network. 2013. http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/sarcomas/ (accessed Aug 25, 2017).
- 2.Judson I, Verweij J, Gelderblom H. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 3.Karavasilis V, Seddon BM, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591. doi: 10.1002/cncr.23332. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CW, Merimsky O, Agulnik M. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34:3898–3905. doi: 10.1200/JCO.2016.67.6684. [DOI] [PubMed] [Google Scholar]
- 5.Tap WD, Papai Z, van Tine BA. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1089–1103. doi: 10.1016/S1470-2045(17)30381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hensley ML, Maki R, Venkatraman E. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Leu KM, Ostruszka LJ, Shewach D. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma. J Clin Oncol. 2004;22:1706–1712. doi: 10.1200/JCO.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pautier P, Floquet A, Penel N. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study) Oncologist. 2012;17:1213–1220. doi: 10.1634/theoncologist.2011-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon B, Scurr M, Jones RL. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clin Sarcoma Res. 2015;5:13. doi: 10.1186/s13569-015-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bay JO, Ray-Coquard I, Fayette J. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer. 2006;119:706–711. doi: 10.1002/ijc.21867. [DOI] [PubMed] [Google Scholar]
- 13.Maki RG, Wathen JK, Patel SR. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Alvarellos ML, Lamba J, Sangkuhl K. PharmGKB summary: gemcitabine pathway. Pharmacogenet Genomics. 2014;24:564–574. doi: 10.1097/FPC.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew SC, Singh O, Chen X. The effects of CYP3A4, CYP3A5, ABCB1, ABCC2, ABCG2 and SLCO1B3 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of docetaxel in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol. 2011;67:1471–1478. doi: 10.1007/s00280-011-1625-9. [DOI] [PubMed] [Google Scholar]
- 17.Edvardsen H, Brunsvig PF, Solvang H. SNPs in genes coding for ROS metabolism and signalling in association with docetaxel clearance. Pharmacogenomics J. 2010;10:513–523. doi: 10.1038/tpj.2010.6. [DOI] [PubMed] [Google Scholar]
- 18.Bray J, Sludden J, Griffin MJ. Influence of pharmacogenetics on response and toxicity in breast cancer patients treated with doxorubicin and cyclophosphamide. Br J Cancer. 2010;102:1003–1009. doi: 10.1038/sj.bjc.6605587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sissung TM, Danesi R, Price DK. Association of the CYP1B1*3 allele with survival in patients with prostate cancer receiving docetaxel. Mol Cancer Ther. 2008;7:19–26. doi: 10.1158/1535-7163.MCT-07-0557. [DOI] [PubMed] [Google Scholar]
- 20.Baker SD, Verweij J, Cusatis GA. Pharmacogenetic pathway analysis of docetaxel elimination. Clin Pharmacol Ther. 2009;85:155–163. doi: 10.1038/clpt.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamieson D, Cresti N, Bray J. Two minor NQO1 and NQO2 alleles predict poor response of breast cancer patients to adjuvant doxorubicin and cyclophosphamide therapy. Pharmacogenet Genomics. 2011;21:808–819. doi: 10.1097/FPC.0b013e32834b6918. [DOI] [PubMed] [Google Scholar]
- 22.Lorigan P, Verweij J, Papai Z. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25:3144–3150. doi: 10.1200/JCO.2006.09.7717. [DOI] [PubMed] [Google Scholar]
- 23.Wampler GL, Fryer JG. Calculation of received dose intensity for combinations of drugs using small-cell lung carcinoma treatment regimens as examples. Cancer Chemother Pharmacol. 1992;30:199–206. doi: 10.1007/BF00686312. [DOI] [PubMed] [Google Scholar]
- 24.Group EESNW. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii92–vii99. doi: 10.1093/annonc/mds253. [DOI] [PubMed] [Google Scholar]
- 25.Verschraegen CF, Arias-Pulido H, Lee SJ. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen. Ann Oncol. 2012;23:785–790. doi: 10.1093/annonc/mdr299. [DOI] [PubMed] [Google Scholar]
- 26.Dickson MA, D'Adamo DR, Keohan ML. Phase II trial of gemcitabine and docetaxel with bevacizumab in soft tissue sarcoma. Sarcoma. 2015;2015:532478. doi: 10.1155/2015/532478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munhoz RR, D'Angelo SP, Gounder MM. A phase Ib/II study of gemcitabine and docetaxel in combination with pazopanib for the neoadjuvant treatment of soft tissue sarcomas. Oncologist. 2015;20:1245–1246. doi: 10.1634/theoncologist.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hensley ML, Miller A, O'Malley DM. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33:1180–1185. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fern LA, Lewandowski JA, Coxon KM. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 2014;15:e341–e350. doi: 10.1016/S1470-2045(14)70113-5. [DOI] [PubMed] [Google Scholar]
- 30.Falk AT, Moureau-Zabotto L, Ouali M. Effect on survival of local ablative treatment of metastases from sarcomas: a study of the French sarcoma group. Clinical Oncol. 2015;27:48–55. doi: 10.1016/j.clon.2014.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.