Abstract
Purpose
This multi-disciplinary, evidence-based guideline for clinically non-metastatic muscle-invasive bladder cancer focuses on the evaluation, treatment, and surveillance of muscle-invasive bladder cancer guided toward curative intent.
Materials and Methods
A systematic review utilizing research from the Agency for Healthcare Research and Quality (AHRQ) as well as additional supplementation by the authors and consultant methodologists was used to develop the guideline. Evidence-based statements were based on body of evidence strengths Grade A, B, or C and were designated as Strong, Moderate, and Conditional Recommendations with additional statements presented in the form of Clinical Principles or Expert Opinions. (Table 1)
Results
For the first time, for any type of malignancy, the American Urological Association (AUA), the American Society of Clinical Oncology (ASCO), the American Society for Radiation Oncology (ASTRO), and the Society of Urologic Oncology (SUO) have formulated an evidence-based guideline based on a risk-stratified clinical framework for the management of muscle-invasive urothelial bladder cancer. This document is designed to be used in conjunction with the associated treatment algorithm.
Conclusions
The intensity and scope of care for muscle-invasive bladder cancer should focus on the patient, disease, and treatment response characteristics. This guideline attempts to improve a clinician's ability to evaluate and treat each patient, but higher quality evidence in future trials will be essential to improve level of care for these patients.
MeSH: Urinary bladder, neoplasm, radiotherapy, cystectomy, drug therapy
Introduction
Epidemiology
There are 79,030 new cases of bladder cancer and 16,870 bladder cancer deaths predicted for 2017 in the United States.1 Approximately 25% of newly diagnosed patients have muscle-invasive disease,2,3 a rate that has not changed over the last 10 years based on data from the Surveillance, Epidemiology, and End Results (SEER) registry.4
Etiology
Smoking tobacco remains the most important and common risk factor for bladder cancer and is estimated to contribute to the development of 50% of bladder tumors.5,6 Other well-documented risk factors include occupational exposure to carcinogens (e.g., aromatic amines, polycyclic aromatic hydrocarbons, chlorinated hydrocarbons), pelvic radiation for other malignancies, exposure to S. haematobium infection and genetic predisposition.7,8
Guideline Statements
Initial Patient Evaluation and Counseling
Prior to treatment consideration, a full history and physical exam should be performed, including an exam under anesthesia at the time of transurethral resection of bladder tumor (TURBT) for a suspected invasive cancer. (Clinical Principle)
Prior to muscle-invasive bladder cancer (MIBC) management, clinicians should perform a complete staging evaluation, including imaging of the chest and cross sectional imaging of the abdomen and pelvis with intravenous contrast if not contraindicated. Laboratory evaluation should include a comprehensive metabolic panel (complete blood count, liver function tests, alkaline phosphatase and renal function). (Clinical Principle)
An experienced genitourinary pathologist should review the pathology of a patient when variant histology is suspected or if muscle invasion is equivocal (e.g., micropapillary, nested, plasmacytoid, neuroendocrine, sarcomatoid, extensive squamous or glandular differentiation). (Clinical Principle)
For patients with newly diagnosed MIBC, curative treatment options should be discussed before determining a plan of therapy that is based on both patient comorbidity and tumor characteristics. Patient evaluation should be completed using a multidisciplinary approach. (Clinical Principle)
Prior to treatment, clinicians should counsel patients regarding complications and the implications of treatment on quality of life (e.g., impact on continence, sexual function, fertility, bowel dysfunction, metabolic problems). (Clinical Principle)
Following the pretreatment evaluation, the patient should be engaged in a shared decision making process when determining a treatment plan that involves a multi-disciplinary discussion of the role and impact of surgery, chemotherapy, and radiotherapy.
A thorough history and physical exam is important in evaluating not only bladder cancer risk but also the overall health of the patient and his or her comorbidities. This examination in conjunction with appropriate imaging will help to determine optimal management and may impact both the readiness for surgery and the type of procedure or urinary diversion that is best suited for the patient.9,10 This information contributes to the overall determination of clinical stage and assessment of potential benefit of neoadjuvant chemotherapy (NAC). 11,12
Treatment
Neoadjuvant/Adjuvant Chemotherapy
6. Utilizing a multidisciplinary approach, clinicians should offer cisplatin-based NAC to eligible radical cystectomy patients prior to cystectomy. (Strong Recommendation; Evidence Level: Grade B)
7. Clinicians should not prescribe carboplatin-based NAC for clinically resectable stage cT2-T4aN0 bladder cancer. Patients ineligible for cisplatin-based NAC should proceed to definitive locoregional therapy. (Expert Opinion)
8. Clinicians should perform radical cystectomy as soon as possible following a patient's completion of and recovery from NAC. (Expert Opinion)
9. Eligible patients who have not received cisplatin-based NAC and have non-organ confined (pT3/T4and/or N+) disease at cystectomy should be offered adjuvant cisplatin- based chemotherapy. (Moderate Recommendation; Evidence Level: Grade C)
Cisplatin eligibility is a major determinant of candidacy for NAC. Toxicities of cisplatin, including nephrotoxicity, diminished cardiac function, neurotoxicity, and hearing loss, preclude 30-50% of MIBC patients from safe receipt of cisplatin-based chemotherapy.13 In choosing to pursue treatment with cisplatin-based NAC, clinicians should note the following:
There are no validated predictive factors or clinical characteristics associated with an increased or decreased probability of response and benefit using cisplatin-based NAC.
The best regimen and duration for cisplatin-based NAC remains undefined.
The decision regarding eligibility for cisplatin-based NAC should be based on comorbidities and performance status.
There is insufficient data to recommend non-cisplatin-based regimens as either NAC or adjuvant chemotherapy (AC) for MIBC. Although some suggestive cohort and clinical trial data exist,14 there is no high level evidence that carboplatin-based regimens lead to increased survival in this setting for MIBC.
The Panel advocates that cisplatin-eligible patients with high-risk pathologic features who do not receive NAC be offered AC following radical cystectomy on the basis of a multi-disciplinary consultation with a thorough informed consent. No single randomized clinical trial has demonstrated a significant improvement in overall survival with AC; however, meta-analyses have suggested a possible benefit, albeit based on data of variable quality.15,16 In patients who are non-cisplatin-eligible, consideration of referral to a clinical trial is reasonable.
Radical Cystectomy
10. Clinicians should offer radical cystectomy with bilateral pelvic lymphadenectomy for surgically eligible patients with resectable non-metastatic (M0) MIBC. (Strong Recommendation; Evidence Level: Grade B).
11. When performing a standard radical cystectomy, clinicians should remove the bladder, prostate, and seminal vesicles in males and should remove the bladder, uterus, fallopian tubes, ovaries, and anterior vaginal wall in females. (Clinical Principle)
12. Clinicians should discuss and consider sexual function preserving procedures for patients with organ-confined disease and absence of bladder neck, urethra, and prostate (male) involvement. (Moderate Recommendation; Evidence Level: Grade C)
For non-metastatic MIBC, radical cystectomy combined with NAC is the standard of treatment.17 Preservation of sexual function may be feasible in patients undergoing radical cystectomy. In all patients who desire sexual function preservation, a nerve-sparing procedure should be discussed and offered as long as it will not compromise oncologic control.18
Patients considering a radical cystectomy should be counseled regarding the high rate of complications, both acute and chronic.19,20 This is particularly critical given that patients undergoing cystectomy are usually older and have multiple comorbid conditions.
Urinary Diversion
13. In patients undergoing radical cystectomy, ileal conduit, continent cutaneous, and orthotopic neobladder urinary diversions should all be discussed. (Clinical Principle)
14. In patients receiving an orthotopic urinary diversion, clinicians must verify a negative urethral margin. (Clinical Principle)
The choice of urinary diversion has a significant impact on long-term quality of life for patients who undergo radical cystectomy, and each type of diversion is associated with its own unique potential complications. Discussing the pros and cons of each approach is an important component of preoperative education. The Panel emphasized that clinicians should first determine whether or not a patient is a candidate for each of the diversion options, and patients should be counseled regarding all three categories of urinary diversion, if not contraindicated.
Perioperative Surgical Management
15. Clinicians should attempt to optimize patient performance status in the perioperative setting. (Expert Opinion)
16. Perioperative pharmacologic thromboembolic prophylaxis should be given to patients undergoing radical cystectomy. (Strong Recommendation; Evidence Level: Grade B)
17. In patients undergoing radical cystectomy μ -opioid antagonist therapy should be used to accelerate gastrointestinal recovery, unless contraindicated. (Strong Recommendation; Evidence Level: Grade B)
18. Patients should receive detailed teaching regarding care of urinary diversion prior to discharge from the hospital (Clinical Principle).
Given the significant risk of morbidity and significant recovery time associated with radical cystectomy, the Panel recommends perioperative patient optimization.21 While a specific enhanced recovery after surgery (ERAS) protocol was not recommended, there are a number of important components that should be considered for any patient undergoing radical cystectomy. Overall, utilization of clinical pathways is associated with decreased narcotic usage, lower incidence of postoperative ileus, and shorter hospital length of stay.22
Pelvic Lymphadenectomy
19. Clinicians must perform a bilateral pelvic lymphadenectomy at the time of any surgery with curative intent. (Strong Recommendation; Evidence Level: Grade B)
20. When performing bilateral pelvic lymphadenectomy, clinicians should remove, at a minimum, the external and internal iliac and obturator lymph nodes (standard lymphadenectomy). (Clinical Principle)
Mapping studies from patients with invasive bladder cancer have documented the pathways of progression of invasive bladder cancer.23,24 Sequential dissemination from the lower pelvic to the more proximal lymph nodes in the pelvis and retroperitoneum is the general pattern of spread, and the risk of regional lymph node metastases is associated with the depth of invasion of the primary tumor. Data from a variety of studies has shown that a pelvic lymphadenectomy can improve disease specific survival and pelvic recurrence risk compared to no pelvic lymphadenectomy at the time of radical cystectomy.25-28
Bladder Preserving Approaches
Patient Selection
21. For patients with newly diagnosed non-metastatic MIBC who desire to retain their bladder, and for those with significant comorbidities for whom radical cystectomy is not a treatment option, clinicians should offer bladder preserving therapy when clinically appropriate. (Clinical Principle)
22. In patients under consideration for bladder preserving therapy, maximal debulking TURBT and assessment of multifocal disease/carcinoma in situ should be performed. (Strong Recommendation; Evidence Level: Grade C)
A multi-modal bladder preserving approach with its merits and disadvantages should be discussed in each individual case. The studies that evaluate curative bladder preserving strategies, as a general rule, have highly select patient populations. The Panel found no strong evidence to determine whether or not immediate cystectomy improved survival when compared to initial bladder sparing protocols that employ salvage cystectomy as therapy for persistent bladder cancer.
Maximal Turbt and Partial Cystectomy
23. Patients with MIBC who are medically fit and consent to radical cystectomy should not undergo partial cystectomy or maximal TURBT as primary curative therapy. (Moderate Recommendation; Evidence Level: Grade C)
Although to date there are no randomized, head-to-head trials, radical cystectomy offers a significant therapeutic benefit for the vast majority of patients compared to partial cystectomy or maximal TURBT.29 With the exception of multi-modal bladder preserving regimens that include maximal TURBT, chemotherapy and radiation therapy, therapies other than radical cystectomy (e.g., partial cystectomy, TURBT alone, chemotherapy alone, or radiation alone) are associated with increased risk of all-cause mortality in unadjusted analyses.29-31
Primary Radiation Therapy
24. For patients with MIBC, clinicians should not offer radiation therapy alone as a curative treatment. (Strong Recommendation; Evidence Level: Grade C)
Radiation therapy alone has been associated with high rates of pelvic failure; five-year local control rates of approximately 30-50% have been reported, but these may be under-estimates as those who develop metastatic disease within this interval are less likely to undergo continued bladder surveillance.32-37
Multi-Modal Bladder Preserving Therapy
25. For patients with MIBC who have elected multi-modal bladder preserving therapy, clinicians should offer maximal TURBT, chemotherapy combined with external beam radiation therapy, and planned cystoscopic re-evaluation. (Strong Recommendation; Evidence Level: Grade B)
26. Radiation sensitizing chemotherapy regimens should include cisplatin or 5-fluorouracil and mitomycin C. (Strong Recommendation; Evidence Level: Grade B.)
27. Following completion of bladder preserving therapy, the clinician should perform regular surveillance with CT scans, cystoscopy and urine cytology. (Strong Recommendation; Evidence Level: Grade C)
The Panel believes that multi-modal bladder preserving therapy is the preferred treatment in those patients who desire bladder preservation and understand the unique risks associated with this approach and/or those who are medically unfit for surgery.
The rationale for combining TURBT, concurrent chemotherapy, and external beam radiation therapy is two-fold. Certain cytotoxic agents may sensitize tumor cells to radiation, thus increasing cell kill in a synergistic fashion. In addition, up to 50% of those with MIBC may harbor occult metastases. The addition of systemic chemotherapy has the potential to improve loco-regional control, and incorporating cisplatin-based multi-agent regimens in the neoadjuvant setting may provide additional benefit for control of occult metastatic disease at an early stage.
For patients receiving staged multi-modal therapy who are otherwise surgical candidates, clinicians should offer a mid-course evaluation to allow for the early selection of non-responders before consolidation radiotherapy is given. For patients who are medically unfit for surgery, this mid-course evaluation may be omitted, and these patients can be treated uninterrupted with a definitive dose of radiation along with concurrent chemotherapy.
Those who are biopsy-proven complete responders to bladder preserving protocols remain at risk for both invasive and non-invasive recurrences as well as new tumors in the upper tracts. Recurrences may be successfully managed by prompt salvage therapy, (e.g. radical cystectomy). Although there is no direct evidence to determine optimal frequency of surveillance, most bladder preserving protocols encourage careful follow up.
Bladder Preserving Treatment Failure
28. In patients who are medically fit and have residual or recurrent muscle-invasive disease following bladder preserving therapy, clinicians should offer radical cystectomy with bilateral pelvic lymphadenectomy (Strong Recommendation; Evidence Level: Grade C).
29. In patients who have a non-muscle invasive bladder cancer (NMIBC) recurrence after bladder preserving therapy, clinicians may offer either local measures, such as TURBT with intravesical therapy, or radical cystectomy with bilateral pelvic lymphadenectomy. (Moderate Recommendation; Evidence Level: Grade C)
Approximately 30% of those selected for treatment by multi-modal bladder preserving therapy will have an invasive bladder recurrence.38 For patients who remain surgical candidates, cystectomy should be offered as a salvage procedure. While there is no direct evidence demonstrating the value of salvage cystectomy, the relatively high survival rates achieved in bladder preserving series are likely, in part, due to the use of close surveillance and the use of early salvage cystectomy for patients with invasive disease. The presence of an NMIBC relapse predicts an increased likelihood for further future relapses, including both NMIBC and MIBC recurrences.39
Patient Surveillance and Follow Up
Imaging
30. Clinicians should obtain chest imaging and cross sectional imaging of the abdomen and pelvis with CT or MRI at 6-12 month intervals for 2-3 years and then may continue annually. (Expert Opinion)
The Panel recommends chest imaging and cross sectional imaging preferably with intravenous contrast and delayed images to evaluate the upper tracts and also other sites for disease recurrence. Radiographic evaluation of the abdomen and pelvis is important for 1) detection of upper tract cancer; 2) disease detection in the most common sites of recurrence, progression, and metastasis, including the pelvis and retroperitoneum, liver, lungs and bones; and 3) urinary diversion concerns like hydronephrosis.
Laboratory Values and Urine Markers
31. Following therapy for MIBC, patients should undergo laboratory assessment at three to six month intervals for two to three years and then annually thereafter. (Expert Opinion)
32. Following radical cystectomy in patients with a retained urethra, clinicians should monitor the urethral remnant for recurrence. (Expert Opinion)
Following cystectomy and urinary diversion, all patients should undergo assessment of electrolytes and renal function.40-43 In follow up, vitamin B 12 levels should be assessed as there is an increased risk of deficiency and consequent neurological damage in patients with resection of > 60 cm of ileum and in those patients in whom the terminal ileum is utilized.44,45 Routine frequent complete blood count and liver function testing for cancer surveillance has not been validated.
In addition, patients should undergo physical examination of the urethra and discussion of any urethral symptoms such as urethral discharge or spotting.
Patient Survivorship
33. Clinicians should discuss with patients how they are coping with their bladder cancer diagnosis and treatment and should recommend that patients consider participating in a cancer support group or consider receiving individual counseling. (Expert Opinion).
34. Clinicians should encourage bladder cancer patients to adopt healthy lifestyle habits, including smoking cessation, exercise, and a healthy diet to improve long-term health and quality of life. (Expert Opinion).
Over the last 25 years, there has been extensive research on the positive effects of support groups as a method of coping with cancer and improving quality of life. In addition to providing emotional support, clinicians should encourage patients to follow an overall healthy lifestyle. Cancer survivors have special health needs, in part because of the risks of the late effects of cancer recurrence.
Variant Histology
35. In patients diagnosed with variant histology, clinicians should consider unique clinical characteristics that may require divergence from standard evaluation and management for urothelial carcinoma. (Expert Opinion)
As variant histologies become recognized, the most appropriate care and evaluation may also become better understood as well as increasingly defined. Importantly, treatment recommendations previously outlined may NOT apply to these patients who represent a small but significant number.
Future Research
Several key areas of future research need emphasis to improve clinical care and provide a path to better patient outcomes with invasive bladder cancer with a particular focus on detection and markers, genetic evaluation and characterization, improved systemic therapy, and appropriate, and accurate surveillance.
Conclusions
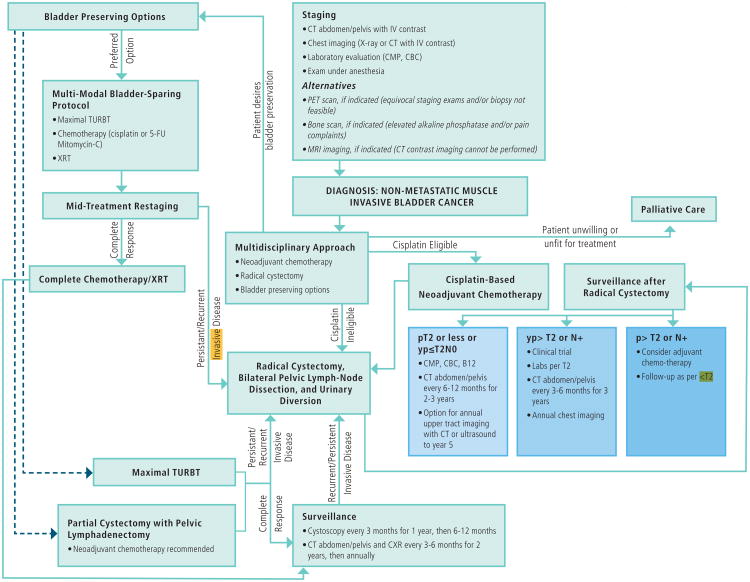
These guidelines serve as the first multidisciplinary constructed guidelines for a genitourinary malignancy and represent the best available evidence for the management of MIBC. In addition, the guidelines present a framework for counseling patients regarding the management of MIBC. A comprehensive treatment algorithm (Figure 1) summarizes the principles discussed in this document.
Figure 1. Non-Metastatic Muscle-Invasive Bladder Cancer: Treatment Algorithm.
CBC=complete blood count; CMP=comprehensive metabolic panel; CXR=chest X-ray; p=pathologic stage; TURBT=trans-urethral resection of bladder tumor; XRT=external beam radiation therapy; Yp=pathologic stage after neoadjuvant chemotherapy
Table 1. AUA Nomenclature Linking Statement Type to Level of Certainty, Magnitude of Benefit or Risk/Burden, and Body of Evidence Strength.
| Evidence Strength A (High Certainty) | Evidence Strength B (Moderate Certainty) | Evidence Strength C (Low Certainty) | |
|---|---|---|---|
|
Strong Recommendation (Net benefit or harm substantial) |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) is substantial Applies to most patients in most circumstances and future research is unlikely to change confidence |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) is substantial Applies to most patients in most circumstances but better evidence could change confidence |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) appears substantial Applies to most patients in most circumstances but better evidence is likely to change confidence (rarely used to support a Strong Recommendation) |
|
Moderate Recommendation (Net benefit or harm moderate) |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) is moderate Applies to most patients in most circumstances and future research is unlikely to change confidence |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) is moderate Applies to most patients in most circumstances but better evidence could change confidence |
Benefits > Risks/Burdens (or vice versa) Net benefit (or net harm) appears moderate Applies to most patients in most circumstances but better evidence is likely to change confidence |
|
Conditional Recommendation (No apparent net benefit or harm) |
Benefits = Risks/Burdens Best action depends on individual patient circumstances Future research unlikely to change confidence |
Benefits = Risks/Burdens Best action appears to depend on individual patient circumstances Better evidence could change confidence |
Balance between Benefits & Risks/Burdens unclear Alternative strategies may be equally reasonable Better evidence likely to change confidence |
| Clinical Principle | A statement about a component of clinical care that is widely agreed upon by urologists or other clinicians for which there may or may not be evidence in the medical literature | ||
| Expert Opinion | A statement, achieved by consensus of the Panel, that is based on members' clinical training, experience, knowledge, and judgment for which there is no evidence | ||
Acknowledgments
Funding of the Panel was provided by the AUA. Panel members received no remuneration for their work. Each member of the Panel provides an ongoing conflict of interest disclosure to the AUA.
Abbreviations
- AC
Adjuvant chemotherapy
- AHRQ
Agency for Healthcare Research and Quality
- ASCO
American Society of Clinical Oncology
- ASTRO
American Society for Radiation Oncology
- AUA
American Urological Association
- MIBC
Muscle-invasive bladder cancer
- NAC
Neoadjuvant chemotherapy
- NMIBC
Non-muscle invasive bladder cancer
- SUO
Society of Urologic Oncology
- TURBT
Transurethral resection of bladder tumor
Footnotes
Disclaimer: This document was written by the Muscle Invasive Bladder Cancer Guideline Panel of the American Urological Association Education and Research, Inc., which was created in 2015. The Practice Guidelines Committee (PGC) of the AUA selected the committee chair. Panel members were selected by the chair. Membership of the Panel included specialists in urology/medical oncology/radiation oncology with specific expertise on this disorder. The mission of the Panel was to develop recommendations that are analysis-based or consensus-based, depending on Panel processes and available data, for optimal clinical practices in the treatment of muscle-invasive bladder cancer.
While these guidelines do not necessarily establish the standard of care, AUA seeks to recommend and to encourage compliance by practitioners with current best practices related to the condition being treated. As medical knowledge expands and technology advances, the guidelines will change. Today these evidence-based guidelines statements represent not absolute mandates but provisional proposals for treatment under the specific conditions described in each document. For all these reasons, the guidelines do not pre-empt physician judgment in individual cases.
Treating physicians must take into account variations in resources, and patient tolerances, needs, and preferences. Conformance with any clinical guideline does not guarantee a successful outcome. The guideline text may include information or recommendations about certain drug uses (‘off label‘) that are not approved by the Food and Drug Administration (FDA), or about medications or substances not subject to the FDA approval process. AUA urges strict compliance with all government regulations and protocols for prescription and use of these substances. The physician is encouraged to carefully follow all available prescribing information about indications, contraindications, precautions and warnings. These guidelines and best practice statements are not in-tended to provide legal advice about use and misuse of these substances.
Although guidelines are intended to encourage best practices and potentially encompass available technologies with sufficient data as of close of the literature review, they are necessarily time-limited. Guidelines cannot include evaluation of all data on emerging technologies or management, including those that are FDA-approved, which may immediately come to represent accepted clinical practices.
For this reason, the AUA does not regard technologies or management which are too new to be addressed by this guideline as necessarily experimental or investigational.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2016;67:7. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Smith AB, Deal AM, Woods ME, et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int. 2014;114:719. doi: 10.1111/bju.12601. [DOI] [PubMed] [Google Scholar]
- 3.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Charlton ME, Adamo MP, Sun L, et al. Bladder cancer collaborative stage variables and their data quality, usage, and clinical implications: a review of SEER data, 2004-2010. Cancer. 2014;120:3815. doi: 10.1002/cncr.29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samanic C, Kogevinas M, Dosemeci M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15:1348. doi: 10.1158/1055-9965.EPI-06-0021. [DOI] [PubMed] [Google Scholar]
- 7.Abern MR, Dude AM, Tsivian M, et al. The characteristics of bladder cancer after radiotherapy for prostate cancer. Urol Oncol. 2013;31:1628. doi: 10.1016/j.urolonc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Rothman N, Garcia-Closas M, Chatterjee N, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. 2010;42:978. doi: 10.1038/ng.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384. doi: 10.1002/cncr.23462. [DOI] [PubMed] [Google Scholar]
- 10.Lotan Y, Amiel G, Boorjian SA, et al. Comprehensive handbook for developing a bladder cancer cystectomy database. Bladder Cancer Think Tank; Bladder Cancer Advocacy Network. doi: 10.1016/j.urolonc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Marshall, VF The relation of the preoperative estimate to the pathologic demonstration of the extent of vesicle neoplasms. J Urol. 1952;68:714. doi: 10.1016/S0022-5347(17)68271-5. [DOI] [PubMed] [Google Scholar]
- 12.Culp SH, Dickstein RJ, Grossman HB, et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J Urol. 2014;191:40. doi: 10.1016/j.juro.2013.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12:211. doi: 10.1016/S1470-2045(10)70275-8. [DOI] [PubMed] [Google Scholar]
- 14.Koie T, Ohyama C, Yamamoto H, et al. Neoadjuvant gemcitabine and carboplatin followed by immediate cystectomy may be associated with a survival benefit in patients with clinical T2 bladder cancer. Med Oncol. 2014;31:949. doi: 10.1007/s12032-014-0949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Adjuvant chemotherapy for invasive bladder cancer (individual patient data) Cochrane Database Syst Rev. 2006;19:CD06018. doi: 10.1002/14651858.CD006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenzl A, Witjes JA. Comperat E: Guidelines on bladder cancer muscle-invasive and metastatic. EAU. 2012 [Google Scholar]
- 18.Schoenberg MP, Walsh PC, Breazeale DR, et al. Local recurrence and survival following nerve sparing radical cystoprostatectomy for bladder cancer: 10-year follow-up. J Urol. 1996;155:490. [PubMed] [Google Scholar]
- 19.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Lowrance WT, Rumohr JA, Chang SS, et al. Contemporary open radical cystectomy: analysis of perioperative outcomes. J Urol. 2008;179:1313. doi: 10.1016/j.juro.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 21.Collins JW, Patel H, Adding C, et al. Enhanced recovery after robot-assisted radical cystectomy: EAU Robotic Urology Section Scientific Working Group consensus view. Eur Urol. 2016;70:649. doi: 10.1016/j.eururo.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Xu W, Daneshmand S, Bazargani ST, et al. Postoperative pain management after radical cystectomy: comparing traditional versus enhanced recovery protocol pathway. J Urol. 2015;194:1209. doi: 10.1016/j.juro.2015.05.083. [DOI] [PubMed] [Google Scholar]
- 23.Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004;171:139. doi: 10.1097/01.ju.0000102302.26806.fb. [DOI] [PubMed] [Google Scholar]
- 24.Vazina A, Dugi D, Shariat SF, et al. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol. 2004;171:1830. doi: 10.1097/01.ju.0000121604.58067.95. [DOI] [PubMed] [Google Scholar]
- 25.Abdollah F, Sun M, Schmitges J, et al. Stage-specific impact of pelvic lymph node dissection on survival in patients with non-metastatic bladder cancer treated with radical cystectomy. BJU Int. 109:1147. doi: 10.1111/j.1464-410X.2011.10482.x. 212. [DOI] [PubMed] [Google Scholar]
- 26.Herr HW, Bochner BH, Dalbagni G, et al. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 167:1295. 202. [PubMed] [Google Scholar]
- 27.Konety BR, Joslyn SA, O'Donnell MA. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: analysis of data from the Surveillance, Epidemiology and End Results Program data base. J Urol. 2003;169:946. doi: 10.1097/01.ju.0000052721.61645.a3. [DOI] [PubMed] [Google Scholar]
- 28.Shirotake S, Kikuchi E, Matsumoto K, et al. Role of pelvic lymph node dissection in lymph node-negative patients with invasive bladder cancer. Jpn J Clin Oncol. 2010;40:247. doi: 10.1093/jjco/hyp147. [DOI] [PubMed] [Google Scholar]
- 29.Herr HW. Outcome of patients who refuse cystectomy after receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2008;54:126. doi: 10.1016/j.eururo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcome. J Clin Oncol. 1998;16:1298. doi: 10.1200/JCO.1998.16.4.1298. [DOI] [PubMed] [Google Scholar]
- 31.Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004;172:878. doi: 10.1097/01.ju.0000135530.59860.7d. [DOI] [PubMed] [Google Scholar]
- 32.Duncan W, Quilty PM. The results of a series of 963 patients with transitional cell carcinoma of the urinary bladder primarily treated by radical megavoltage x-ray therapy. Radiother Oncol. 1986;7:299. doi: 10.1016/s0167-8140(86)80059-7. [DOI] [PubMed] [Google Scholar]
- 33.Blandy JP, Jenkins BJ, Fowler CG, et al. Radical radiotherapy and salvage cystectomy for T2/3 cancer of the bladder. Progress in Clinical and Biological Research. 1988;260:447. [PubMed] [Google Scholar]
- 34.Jenkins BJ, Caulfield MJ, Fowler CG, et al. Reappraisal of the role of radical radiotherapy and salvage cystectomy in the treatment of invasive (T2/T3) bladder cancer. Br J Urol. 1988;62:342. doi: 10.1111/j.1464-410x.1988.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 35.Gospodarowicz MK, Rider WD, Keen CW, et al. Bladder cancer: long term follow-up results of patients treated with radical radiation. Clin Oncol. 1991;3:155. doi: 10.1016/s0936-6555(05)80838-6. [DOI] [PubMed] [Google Scholar]
- 36.Jahnson S, Pedersen J, Westman G. Bladder carcinoma - a 20-year review of radical irradiation therapy. Radiother Oncol. 1991;22:111. doi: 10.1016/0167-8140(91)90006-3. [DOI] [PubMed] [Google Scholar]
- 37.Fossa SD, Waehre H, Aass N, et al. Bladder cancer definitive radiation therapy of muscle-invasive bladder cancer. A retrospective analysis of 317 patients. Cancer. 1993;72:3036. doi: 10.1002/1097-0142(19931115)72:10<3036::aid-cncr2820721028>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Coppin C, Gospodarowicz M, James K, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14:2901. doi: 10.1200/JCO.1996.14.11.2901. [DOI] [PubMed] [Google Scholar]
- 39.Zietman AL, Grocela J, Zehr E, et al. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: management and consequences of Ta, T1, and Tis recurrence within the retained bladder. Urology. 2001;58:380. doi: 10.1016/s0090-4295(01)01219-5. [DOI] [PubMed] [Google Scholar]
- 40.Hall MC, Koch MO, McDougal WS. Metabolic consequences of urinary diversion through intestinal segments. Urol Clin North Am. 1991;18:725. [PubMed] [Google Scholar]
- 41.Harraz AM, Mosbah A, El-Assmy A, et al. Renal function evaluation in patients undergoing orthotopic bladder substitution: a systematic review of literature. BJU Int. 2014;114:484. doi: 10.1111/bju.12632. [DOI] [PubMed] [Google Scholar]
- 42.Amini E, Djaladat H. Long-term complications of urinary diversion. Curr Opin Urol. 2015;25:570. doi: 10.1097/MOU.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 43.Krajewski W, Piszczek R, Krajewska M, et al. Urinary diversion metabolic complications - underestimated problem. Adv Clin Exp Med. 2014;23:633. doi: 10.17219/acem/28251. [DOI] [PubMed] [Google Scholar]
- 44.Tan WS, Lamb BW, Kelley D. Complications of radical cystectomy and orthotopic reconstruction. Adv Urol. 2015;2015:323157. doi: 10.1155/2015/323157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van der Aa F, Joniau S, Van Den Branden VD, et al. Metabolic changes after urinary diversion. Adv Urol. 2011;2011 doi: 10.1155/2011/764325. [DOI] [PMC free article] [PubMed] [Google Scholar]