Abstract
Well into the 21st century, we still triage acute myocardial infarction based on the presence or absence of ST segment elevation, a century-old technology. Meanwhile, we have learned a great deal regarding the pathophysiology and mechanisms of acute coronary syndromes (ACS) at clinical, pathological, cellular, and molecular levels. Contemporary imaging studies have shed new light into the mechanisms of ACS. This review discusses these advances and their implications for clinical management of the ACS for the future. Plaque rupture has dominated our thinking about ACS pathophysiology for decades. Yet, current evidence suggests that a sole focus on plaque rupture vastly oversimplifies this complex collection of diseases, and obscures other mechanisms that may mandate different management strategies. We propose to segment coronary artery thrombosis due to plaque rupture into cases with or without signs of concomitant inflammation. This distinction may have substantial therapeutic implications as direct anti-inflammatory interventions for atherosclerosis emerge. Coronary artery thrombosis due to plaque erosion may be on the rise in an era of intense lipid lowering. Identification of patients with of ACS due to erosion may permit a less invasive approach to management than the current standard of care. We also now recognize ACS that occur without apparent epicardial coronary artery thrombus or stenosis. Such events may arise from spasm, microvascular disease, or other pathways. Emerging management strategies may likewise apply selectively to this category of ACS. We advocate this more mechanistic approach to the categorization of ACS to provide a framework for future tailoring, triage, and therapy for patients in a more personalized and precise manner.
Keywords: myocardial infarction, thrombosis, superficial erosion, coronary microvasculature, plaque rupture
Introduction
Willem Einthoven introduced electrocardiography at the dawn of the 20th century. Well into the 21st century, we still triage acute myocardial infarction based on this venerable technology: ST segments up or not. Although this approach remains the appropriate evidence-based strategy today, aiming toward the future goal of more individualized therapy, can we apply a more pathophysiogically-based categorization of the acute coronary syndromes (ACS). Indeed, we have learned regarding the mechanisms of ACS since we last reviewed this topic in these pages.1,2 Moreover, the human disease of atherosclerosis has evolved considerably in the interim.3–5 This review aims to reach toward a categorization of the ACS that reaches beyond equating all ACS with plaque rupture or fissue, guided by the latest insights in the underlying mechanisms of ACS, and points a way forward toward the eventual clinical application of these considerations.
Post-mortem studies carried out in the 1980s proposed that plaque rupture (also sometimes referred to as fissure) caused most fatal myocardial infarctions6. These findings led to the notion of the “vulnerable” or “high-risk” plaque characterized by a large central lipid core, abundance of inflammatory cells, a paucity of smooth muscle cells, and a thin fibrous cap7. These observations spawned the widely accepted concept that instability of coronary atheromata resulted from fissuring of a “thin-capped fibroatheroma” or “TCFA” due to weakening of its collagen structure wrought by inflammatory mechanisms8.
This concept stimulated manifold attempts to develop methods to detect the “vulnerable” plaque, a quest predicated on the postulate that local interventions could preclude plaque thrombosis and possibly prevent ACS. Yet, attempts to identify vulnerable plaques proved disappointing because of the low predictive value9. Pathologic studies of human coronary arteries show that plaque rupture occurs commonly in individuals without symptomatic ACS10. Moreover, fewer than 5% of plaques with the features of TCFA as determined by intravascular ultrasound interrogation actually cause clinical events during 3it’s refyears of follow-up11. Thus, most TCFA are clinically quite stable, a realization that renders “vulnerable plaque” a misnomer. Indeed, plaques with TCFA morphology as assessed by radiofrequency backscatter data from intravascular ultrasound (“virtual histology”) commonly convert to an apparently more “stable” character during a one year follow-up period. 12 Such evolution in plaques may reflect mutability during the “natural history” of human atherosclerosis, but could also reflect adherence of many of these patients to a contemporary “secondary prevention” regimen including statins and agent that target the renin-angiotensin axis.
Moreover, while we have witnessed wide acceptance of the formerly controversial concept that inflammation contributes to atherogenesis 13, inflammation may not drive all transitions from stable atherosclerosis to acute thrombotic events. In one large study, about half of ACS occurred in the presence of normal levels of C-reactive protein (CRP), a marker of inflammation14. Another recent study using optical coherence tomography (OCT) demonstrated that among patients with ACS and plaque rupture, two-thirds of patients had imaging evidence of inflammatory cell infiltration in the region of the ruptured fibrous cap but one-third did not and the latter had lower levels of CRP15. In addition, plaque erosion causes up to a third of ACS in the current era16,17. Finally, about one-fifth of ACS occurs in the apparent absence of coronary thrombosis, suggesting that functional alterations beyond thrombus formation can contribute to ACS pathogenesis18, 19.
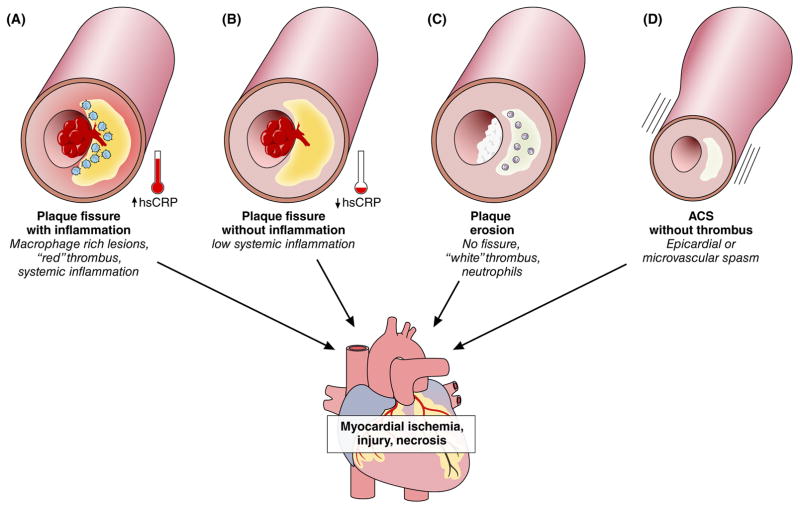
The ensemble of post-mortem studies and in vivo studies using intravascular imaging point to four pathologic pathways to ACS. While these mechanisms may overlap and coexist in some patients, we present them separately for heuristic simplicity: 1) plaque rupture with systemic inflammation; 2) plaque rupture without systemic inflammation; 3) plaque erosion; or 4) plaque without thrombus (Figure 1).20
Figure 1. Four diverse mechanisms cause acute coronary syndromes (ACS).
(A) Plaque rupture, also referred to as fissure, traditionally considered the dominant substrate for ACS, usually associates with inflammation both local (as depicted by the blue monocytes) and systemic, as indicated by the gauge showing an increased in blood C-reactive protein (measured with a high-sensitivity assay, hsCRP.) (B) In some cases, plaque rupture complicates atheromata that do not harbor large collections of intimal macrophages, as identified by optical coherence tomography (OCT) criteria, and do not associate with elevations in circulating CRP. Plaque rupture usually provokes the formation of fibrin-rich “red” thrombi. (C) Plaque erosion appears to account for a growing portion of ACS, often provoking non-ST segment elevation myocardial infarction. The thrombi overlying patches of intimal erosion generally exhibit characteristics of “white” platelet-rich structures. (D) Vasospasm can also cause ACS, long recognized as phenomenon in the epicardial arteries, but also affecting coronary microcirculation.
This review considers the causes of these four predominant pathways to ACS. To strive for a more precision approach, we will explore the therapeutic implications of these distinct mechanisms that provoke ACS. Moreover, we advocate the future goal to move beyond triage of therapy based merely on electrocardiographic (ECG) current of injury by harnessing the recent advances in arterial biology, and human studies that shed new mechanistic light on ACS pathogenesis. Further improvements in the appropriate deployment of technologies and in outcomes should evolve from a more mechanistically-based classification of ACS.
Plaque rupture with systemic inflammation
Mechanisms
Several studies have implicated systemic inflammation in ACS, as assessed by the biomarker C-reactive protein (Figure 1A)21. Laboratory studies, as well as observations on human plaques, point to inflammatory mechanisms as key regulators of the fragility of the fibrous cap, as well as of the thrombogenic potential of the lipid core (Figure 2). Macrophages likely pave the way for the rupture of the plaque’s fibrous cap: when activated, these cells elaborate enzymes that degrade all components of the arterial extracellular matrix. These enzymes include matrix metalloproteinases (MMPs) and certain cathepsins. Multiple mechanisms regulate these matrix-degrading proteinases: transcription, translation, activation of zymogen precursors, and balance with endogenous inhibitors such as the tissue inhibitors of MMPs (TIMPS) or the cystatins. Thus, increased amounts of activated proteinases or reduced levels of their corresponding inhibitors can enhance breakdown of the plaque’s extracellular matrix22.
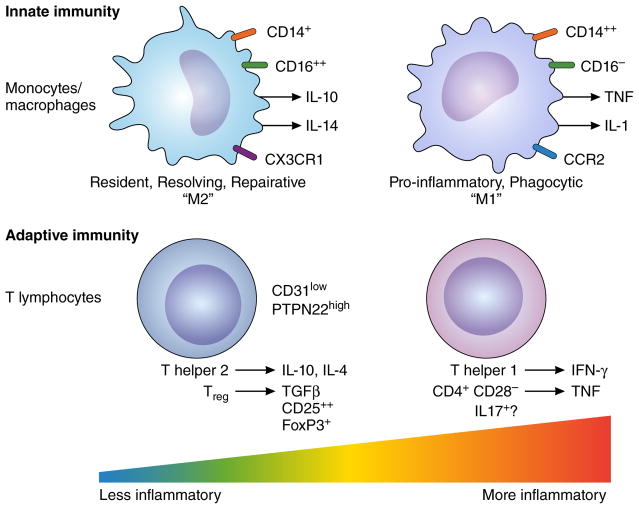
Figure 2. Imbalance in adaptive immune pathways can modulate atherosclerotic plaque activity.
Subsets of T lymphocytes, major participants in adaptive immunity, can either promote local plaque inflammation (effector T cells), or, in the case of regulatory T cells (Treg) suppress inflammation. While many pathways regulate T cell functions, the markers and mechanisms depicted here illustrate the principle that imbalances in T cell activities can prevail in plaques. Low levels of expression of resurface marker CD 31 and high activity of PTPN22 (protein tyrosine phosphatase N22, also known as Lyp) characterize effector T cells. High levels of activation of CREB (cAMP-responsive element binding protein) characterize Treg that can dampen local adaptive immune responses in the plaque.
Adaptive immunity also appears altered in coronary plaque instability23. Patients with ACS have an increased population of particularly pro-inflammatory CD4+ T-cells characterized by low cell surface expression of CD28, a co-stimulatory molecule critically involved in determining the outcome of antigen recognition by T-cells24. ACS also profoundly perturb the numbers of two other circulating T-cell subsets: type 17 helper T-cells (Th17) and CD4+CD25+ regulatory T-cells (Treg). The net role of IL-17 in atherosclerosis remains controversial, however; some experimental studies in mice support a predominantly proatherogenic function for IL-17, yet Th17 cells activated in plaques promote the formation of thick collagen fibers which might increase plaque stability25, 26. Treg normally help to maintain homeostasis of cells involved in adaptive immunity, including antigen-presenting cells and effector T-cells. Treg mediate these modulatory effects by contact-dependent suppression or by releasing anti-inflammatory cytokines, such IL-10 or TGF-β127. Patients with ACS have reduced number and suppressive function of circulating Treg compared to patients with stable angina and healthy controls28. The molecular mechanisms responsible for the T cell dysregulation in ACS remain largely unknown. Recent work highlights the importance of the strength of the stimulation of the T cell receptor for antigen (TCR) in the differentiation of helper T-cell subsets. The altered activity observed in ACS of proteins involved in the regulation of upstream TCR signaling, such as CD31 and PTPN22, may modulate T cell functions29, 30.
Therapeutic implications
Several anti-inflammatory treatments might provide benefit in this setting. Colchicine, a time-honored anti-inflammatory drug, reduced major cardiovascular events in a medium-size, unblinded randomized study in patients with stable ischemic heart disease31. Two trials underway are evaluating colchicine treatment in patients with coronary artery disease (CAD) – although not in the acute phase.32 A phase 3 trial is testing the potential beneficial effects of low-dose methotrexate in patients post myocardial infarction or with coronary artery disease and diabetes or elements of the metabolic syndrome33.
Targeting proinflammatory cytokines offers another approach. Because of the potentially detrimental effects on cardiovascular risk profile and/or lack of compelling evidence of benefit in experimental atherosclerosis, monoclonal antibodies that neutralize TNF-alpha, IFN-gamma, or IL-17 may not prove appropriate for treating atherosclerosis. In a small (n=182) phase II study, the IL-1 receptor antagonist anakinra reduced the area under the curve of high sensitivity C-reactive protein (hsCRP) in patients in the 7 days following presentation with ACS. 34 A large (n>10,000) phase 3 trial with clinical end-points that tested a fully humanized anti-IL-1 β monoclonal antibody, canakinumab, in patients with previous MI and CRP levels > 2 mg/L, has reportedly met its primary endpoint.35
T-cell activation signaling offers possible targets. A synthetic CD31-derived peptide can suppresses immunity in vivo by restoring an inhibitory pathway to a CD31 negative T cell subset 36. Moreover, the development of a novel series of inhibitors of the phosphatase PTPN22 might contribute to a better understanding of its role in immune dysregulation in ACS patients37. Therapeutic strategies that target Treg include administration of low doses of IL-2 and infusion of autologous Tregs that have undergone differentiation and expansion in vitro.38 Various vaccination strategies can limit experimental atherogenesis as well, and may act by augmenting the activity of Treg.39
Plaque rupture without systemic inflammation
Mechanisms
When plaque rupture occurs in the absence of systemic inflammatory activation other mechanisms may contribute to pathogenesis, including extreme emotional disturbance – ranging from external events of short duration, such as earthquakes and a beloved team’s loss of a football (soccer) match, to acute manifestations of more chronic emotional disturbances (Figure 1B). Intense physical exertion, as well as local mechanical stress at the level of the artery wall – either increased circumferential stress or reduced shear stress – may also predispose to plaque rupture40. In addition, subclinical inflammation in the microenvironment of the culprit stenosis might compound the chain of events leading to coronary instability, although the triggers for and effectors of such local inflammation may differ from those that operate in patients with systemic inflammation41. While many studies have clarified the molecular mechanisms leading to coronary instability in individuals with systemic inflammation (gauged for example by CRP elevations), patients without systemic inflammation have undergone less extensive investigation. The precise causes of instability remain poorly understood, providing a strong stimulus for further research.
The possible relationship between psychological stress and plaque rupture may relate to sympathetic nervous system activation and catecholamine release associated with increase in heart rate, blood pressure, and coronary vasoconstriction favoring plaque disruption and platelet activation, hypercoagulability, and intense coronary microvascular constriction.42 Morover, beta3 adrenergic stimulation can stimulate release from the bone marrow of pro-inflammatory monocytes that can home to experimental atheromata and amplify local inflammation43. Although physical or emotional stress per se may not suffice to cause coronary thrombosis, it might trigger instability in plaques already predisposed to provoke events44.
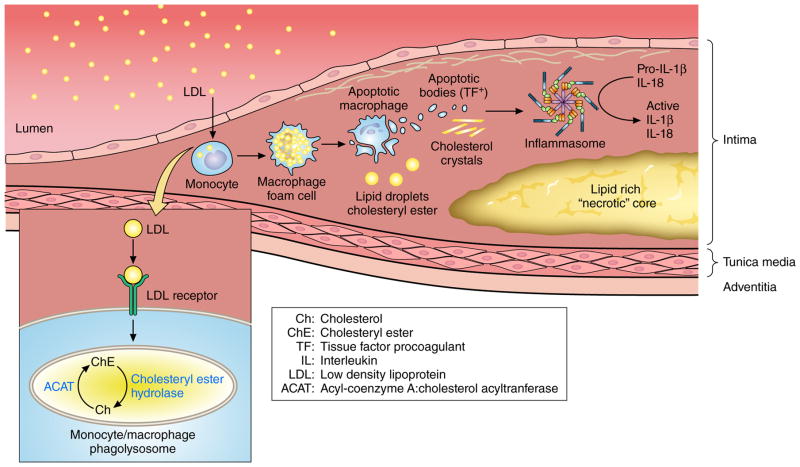
Local changes in the equilibrium between esterified and free cholesterol might promote plaque rupture45 (Figure 3). Cholesterol crystal formation in the lipid core could heighten risk of plaque rupture and thrombosis, and can also co-activate the inflammasome, an intracellular multimeric complex that generates active IL-1 beta and IL-1846.
Figure 3. Cholesterol crystals activate local innate immune pathways in the atherosclerotic plaque.
Plasma low-density lipoprotein (LDL) can enter the arterial wall and accumulate in mononuclear phagocytes via scavenger receptors. Lipid-laden macrophage foam cells can die, contributing to the accumulation of extracellular cholesteryl ester and cholesterol monohydrate crystals in the plaque’s lipid rich “necrotic” core. The dying macrophages can also release apoptotic bodies and microparticles that contain the potent procoagulant tissue factor (TF +). The cholesterol crystals can co-activate the inflammasome, an intracellular supramolecular structure, that generates the biologically active forms of the pro-inflammatory cytokines interleukin (IL)-1 beta and IL-18. Large crystals might also cause mechanical disruption of the fibrous cap.
Optical coherence tomography (OCT) has colocalized cholesterol crystals in human coronary arteries with thin-capped plaques47. The development of micro-OCT with a 2μm resolution may shed new light on this potential mechanism of instability by assessing its occurrence in vivo48. Cholesterol crystals might also theoretically resist plaque rupture by increasing the stiffness of the lipid pool, although this mechanism probably contributes little to plaque stability49. The role of calcium mineral as causal factors in ACS remains controversial. Large collections of calcified tissue do not augment biomechanical instability of plaques or associate with lesions that provoke ACS.50,51 Small foci of calcification within the atherosclerotic intima, some causing “spotty calcification” visible on CT, can also introduce biomechanical inhomogeneities that can favor plaque destabilization.3, 52, 53
Therapeutic implications
As it is difficult to limit environmental, physical, or emotional triggers, the altered plaque characteristics produced by intensive lipid lowering treatment might protect from this form of plaque destabilization54. In particular, statins and ezetimibe may interfere with cholesterol crystal formation55, 56. Cyclodextrin can solubilize cholesterol, and limits experimental atherogenesis, and might thus provide a pharmacologic approach to combatting cholesterol crystal accumulation in plaques. 57
Enhancement of cholesterol efflux might promote plaque stabilization, but strategies to enhance this process by manipulation of HDL concentrations or by administering apolipoprotein A-1 have generally failed to confer clinical benefit58 A large outcome trial with anacetrapib has reportedly met its primary endpoint, but that this agent not only raises HDL, but lowers LDL, renders it difficult to attribute event reduction to raising HDL. In addition, inhibition of cholesteryl ester hydrolase, an enzyme that converts cholesteryl esters to free cholesterol might prevent cholesterol crystallization. Inhibition of ACAT-1, which in contrast promotes cholesterol crystallization, increased atheroma volume and major cardiovascular events59.
Plaque erosion
Mechanisms
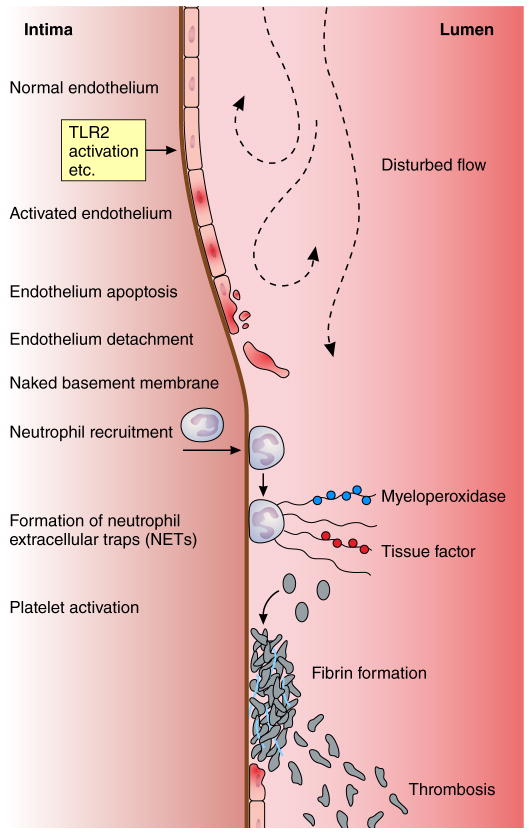
Current evidence suggests that mechanisms of plaque disruption distinct from macrophage-driven rupture of the plaque’s fibrous cap can commonly cause acute coronary syndromes. 60 The mechanism of plaque thrombosis described by pathologists as “superficial erosion” appears not to involve inflammation mediated by macrophages, as in the case of fibrous cap fracture (Figure 1C). Superficial erosion complicates lesions with a distinct epidemiology and morphology, and involves pathophysiologic mechanisms that differ from rupture of the fibrous cap. Indeed, neutrophil activation seems to play a pivotal role in plaque erosion4. Patients who presented with ACS associated with plaque erosion defined by OCT had higher systemic myeloperoxidase (MPO) levels compared to concentrations in patients with plaque rupture. Moreover, in post-mortem coronary specimens, luminal thrombi superimposed on eroded plaques contain many more MPO-positive cells than thrombi associated with ruptured plaques61 (Figure 4).
Figure 4. Pathways implicated in arterial thrombosis due to superficial erosion.
Stimuli such as disturbed flow or engagement of innate immune receptors such as toll-like receptor two (TLR2) can activate the endothelial cells that line the arterial intima. These cells can undergo cell death for example by apoptosis, depicted by the cell with the interrupted membrane an pyknotic nucleus. Injured or dying endothelial cells can desquamate uncovering the basement membrane. Neutrophils attracted by chemokines produced by activated endothelial cells can congregate on the denuded intima and can in turn gegranulate and die releasing neutrophil extracellular traps (NETs). These strands of extruded DNA can bind contents of the neutrophil granules and other proteins, for example myeloperoxidase or tissue factor. The NETs can constitute a solid-state reactor that generates oxidants such as hypochorus acid and stimulate coagulation locally. Platelets interacting with the basement membrane can activate, release their granular contents including chemokines that can recruit further leukocytes, and formed the nidus of a “white”thrombus.
Autopsy studies suggest that fatal plaque erosion associates more commonly with hypertriglyceridemia, diabetes mellitus, female sex, and elderly62. The morphology of lesions that produce thrombosis due to superficial erosion differs radically from the features associated with fibrous cap rupture. Superficially eroded lesions contain few macrophages or T lymphocytes, inflammatory cells considered pathogenic in plaque rupture. Rather than depleted collagen, the lesions associated with superficial erosion contain abundant proteoglycan and glycosaminoglycans. Instead of a paucity of smooth muscle cells, eroded lesions can contain abundant arterial smooth muscle cells. Plaques complicated by fibrous cap fracture typically harbour large necrotic, lipid-rich cores. In contrast, the more fibrous lesions associated with superficial erosion generally lack prominent central lipid cores63. A loss of intimal endothelial cells defines lesions that provoke thrombosis by the mechanism denoted superficial erosion.
Recent work has implicated engagement of the innate immune receptor Toll-like receptor 2 (TLR2) in promoting the susceptibility of endothelial cells to apoptotic stimuli. Hyaluronic acid, a prominent constituent of the extracellular matrix of eroded lesions, could serve as an endogenous ligand of TLR2, implicated in promoting endothelial cell apoptosis.64 Experiments that have evaluated gain and loss of TLR2 function in mice support these observations.65 Indeed, hyaluronan and its receptor, CD44, co-localize along the plaque/thrombus interface in eroded plaque but not in ruptured or stable plaque66. Accumulation of hyaluronan and expression of CD44 along the plaque/thrombus interface of eroded plaques may promote de-endothelization, resulting in CD44-dependent platelet adhesion and subsequent thrombus formation, in part mediated by a direct action of hyaluronan on fibrin polymerization67, 68.
We have hypothesized that thrombosis due to superficial erosion occurs in two phases. The first “hit,” mediated for example by TLR2, would jeopardize endothelial viability or adherence, leading to a breach in the integrity of the endothelial monolayer overlying the atherosclerotic plaque. The denuded patch on the intimal surface would then attract platelets that could undergo activation by contact with collagen and other components of the arterial extracellular matrix usually protected from the blood compartment by the endothelial lining of the intima. The second “hit,” mediated by the release of granular contents from activated platelets and the local production of chemoattractants for polymorphonuclear leukocytes (PMN), would beckon these granulocytes to join the platelets at local sites of endothelial denudation. In turn, the activation of PMN could generate structures known as neutrophil extracellular traps (NETs), comprised of strands of unwound DNA released by the dying granulocytes.69 This uncoiled DNA becomes decorated with proteases, tissue factor, and pro-oxidant enzymes that could propagate local amplification of an innate immune response, thrombin activation, fibrin generation, and entrap further platelets and fibrin strands in an evolving thrombus49.
This hypothetical scenario posits a mechanism of thrombosis independent of the chronic inflammatory cells typically implicated in fibrous cap rupture: macrophages and T lymphocytes. Rather, this schema implicates different arms of innate immunity in thrombus initiation, propagation, and stabilization. These mechanistic differences with fibrous cap fracture have potential therapeutic implications.
Therapeutic implications
As statins alter the lipid profile primarily by lowering LDL rather than triglycerides, therapies that address triglyceride-rich lipoproteins may address the residual risk of events due to erosion that persists in the statin era, more effectively than more intense LDL lowering therapies70. Moreover, therapies that destabilize neutrophil extracellular traps such as deoxyribonuclease (DNAase) administration might limit the propagation of thrombi resulting from superficial erosion71. Receptors such as CD44 expressed by neutrophils may bind hyaluronan and glycosaminoglycan enriched in plaques complicated by erosion, and furnish a potential therapeutic target72.
Jia et al. recently reported a series of patients with ACS with OCT-defined erosion and treated with anti-coagulant/anti-platelet therapy rather that mechanical revascularization. About 80% of the patients showed a > 50% reduction of thrombus volume after 1 month. More than a third of patients lacked detectable thrombus after 1 month. This study suggests that pharmacologic rather than interventional treatment can effectively manage ACS due to superficial erosion, a proposition that merits and requires rigorous testing in outcome trials73, 74.
Plaque without thrombus
Mechanisms
In patients with ACS without plaque thrombus, a functional alteration of coronary circulation likely causes the acute ischemia involving large epicardial coronary arteries or the coronary microcirculation (Figure 1D). Epicardial coronary vasospasm may occur in patients in whom coronary angiography does not demonstrate an obstructive atherosclerotic plaque75. In the CASPAR study, coronary angiography failed to show “culprit lesions” in about 30% of patients with suspected ACS76. Intracoronary acetylcholine (ACh) administration elicited coronary spasm in nearly 50% of these patients. Similar findings pertained to a Japanese population77. Coronary artery spasm may cause coronary instability also in patients with obstructive atherosclerosis. Indeed, in a classical study Bertrand et al. found that ergonovine induced spasm in 20% of patients with recent myocardial infarction and in 40% of patients with unstable angina78. In a more recent study ACh induced spasm in 20% of Caucasian patients and in 60% of patients with MI within the previous 14 days79. Japanese people have a higher prevalence of coronary spasm than Caucasians for unknown reasons.
Microvascular spasm also can cause myocardial ischemia80. This mechanism likely operates in patients with Takotsubo cardiomyopathy81, which frequently occurs in the absence of obstructive atherosclerosis, although about 15% of these patients exhibit concomitant obstructive atheroslerosis82. Microvascular spasm can also contribute to ischemia in the face of angiographically normal appearing epicardial coronary arteries. Microvascular ischemia often manifests as angina pectoris, but can also cause non-ST segment elevation ACS as defined by EKG alterations and biomarker evidence of myocyte injury83. Coronary spasm can also contribute to plaque instability by causing endothelial damage as shown in an infarction-prone strain of the Watanabe heritable hyperlipidemic rabbits.42
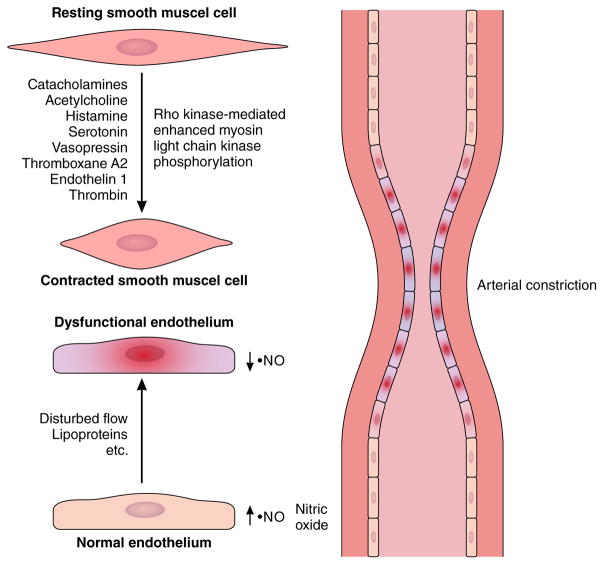
Either macro- or microvascular spasm can result from impaired vasodilatation and/or from vasoconstrictor stimuli acting on hyperreactive vascular SMC (Figure 5)84, 85. Shimokawa et al. demonstrated SMC hyperreactivity in a coronary segment in pigs following adventitial exposure of a coronary segment to an inflammatory stimulus (IL-1β)86. Subsequent experimental and clinical data suggested that an increase in Rho-kinase activity comprised a major mechanisms of SMC hyperreactivity87–89. Perivascular fibrosis in the coronary microvasculature can contribute to myocardial ischemia. 90 This observation highlights the potential contribution of fixed structural changes in coronary arterioles beyond impaired endothelial vasodilator function as a contributor to myocardial ischemia in the absence of obstructive disease of the epicardial coronary arteries.
Figure 5. Cellular mechanisms of arterial epicardial spasm relevant to acute coronary syndrome pathogenesis.
Smooth muscle cells in the media layer of coronary arterial vessels can contract in response to stimuli from the autonomic nervous system (e.g. acetylcholine), local responses to autacoids (e.g. histamine), and to pharmacologic stimuli. Local hyper-reactivity of smooth muscle cells mainly mediated by enhanced Rho kinase activity results in spasm in response to constrictor stimuli. Normal endothelial cells produce endogenous vasodilator substances including nitric oxide. A large body of literature supports the contribution of dysfunctional endothelium to inappropriate constriction of coronary and other arteries.
Therapeutic implications
Several vasoconstrictor substances implicated as triggers of epicardial coronary spasm may cause ischemia in the same patient under different conditions91. Accordingly, the vasoactive substances themselves may not respond to therapeutic targeting. The current treatment of epicardial spasm uses non-specific vasodilators such as long-acting nitrates and calcium-channel blockers92. Nitrates and calcium channel antagonists may alleviate ischemia due to vasospasm. Yet, we need to identify the molecular alterations responsible for SMC hyperreactivity, as many patients do not respond satisfactorily to standard doses of vasodilators93. Knowledge of the post-receptor mechanisms responsible for SMC hyperreactivity and epicardial coronary spasm might enable the development of new therapeutic options for patients who present poor clinical response to nonspecific vasodilator therapy. Fasudil, a rho kinase inhibitor, reduces the rate of coronary spasm episodes in patients with vasospastic angina94. Similarly, further efforts are warranted to unravel the molecular mechanisms responsible for coronary microvascular dysfunction in Takotsubo cardiomyopathy and in ischemia with normal coronary arteries.20 The observation that Takotsubo cardiomyopathy may have worse outcomes than previously considered, and the challenges of managing patients with episodic ischemia due to microvascular dysfunction render this quest urgent82. Preventing or reversing perivascular fibrosis in the coronary arterial microvasculature represents another novel target beyond traditional anti-atherosclerotic therapies.
Conclusions and perspectives
A dichotomous approach based on the EKG has traditionally dominated our clinical management of ACS: STEMI vs. NSTEMI. Our thinking regarding the pathophysiologic bases of the ACS has also evolved separately and in parallel, dominated by the concept of the “vulnerable plaque” as a substrate for fibrous cap rupture, or of a “minority” mechanism: superficial erosion. We and others have championed and explored the role of inflammation as a pathway to the ACS.
Yet, we now have a greater appreciation that not all cases of ACS fit this mold. To move forward we need to remain vigilant not to cling to our cherished notions and remain open to shifts in the nature of the diseases we treat, and to new vistas opened by scientific advances. As discussed here, we need to consider more deeply in the current era arterial spasm, rupture without inflammation, and distinct aspects of innate immunity or dyslipidemia as an instigators of plaque erosion. The complexity of clinical ACS surpasses our traditional categorization of this condition we should evolve beyond the current constrained concepts by expanding our understanding.
We have achieved considerable mastery of plaque rupture with inflammation from a mechanistic and therapeutic viewpoint. Control of traditional risk factors such as hypercholesterolemia, hypertension, and smoking address the pathophysiology of this mechanism of ACS. The other categories that we discuss here may account for an increasing proportion of ACS, now that we are addressing more effectively the traditional risk factors.5 We should remain open to the possibility – even likelihood – that tailored therapeutic approaches may apply to the prevention and treatment of the less well recognized pathways to acute myocardial ischemia discussed here.
We must aim to link the clinical presentation of ACS more tightly to pathophysiologic mechanisms. More than a century after the introduction of EKG we still triage our ACS patients on the basis of this mature technology. To move forward in management of ACS, we should strive for a more personalized approach to therapy based on criteria more tightly linked to the diverse pathophysiologic mechanisms than EKG repolarization abnormalities. We need to develop and validate soluble and imaging biomarkers that reflect the underlying mechanism that yields acute ischemia. Point-of-care assessment of such biomarkers would help render their use clinically practical in the triage of patients who present with ACS, with the goal of sparing some the need for urgent invasive diagnostic or therapeutic measures64, 95. We then need rigorous clinical evaluation of targeted therapies guided by a more precise pathophysiologic classification of ACS that reach beyond our current dichotomous approach based on the EKG. If these ventures succeed, we may one day look back on today’s treatment of ACS as oversimplified and rather primitive. We should strive as a community not to be satisfied with our current approach to ACS management but reach to realize the promise of a more personalized precision deployment of treatment strategies including new ones based on greater mechanistic understanding of the diverse underlying causes of acute myocardial ischemia.
Footnotes
Disclosures
Dr. Libby reports receiving grants from Novartis (no personal compensation), and reports holding scientific advisory board positions for Olatec (uncompensated) and Interleukin Genetics (company no longer operating).
References
- 1.Libby P. The molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;369:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. Eur Heart J. 2015;36:2984–2987. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 5.Pasterkamp G, den Ruijter HM, Libby P. Temporal shifts in clinical presentation and underlying mechanisms of atherosclerotic disease. Nat Rev Cardiol. 2017;14:21–29. doi: 10.1038/nrcardio.2016.166. [DOI] [PubMed] [Google Scholar]
- 6.Davies MJ, Thomas A. Thrombosis and Acute Coronary-Artery Lesions in Sudden Cardiac Ischemic Death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 7.Falk E, Shah P, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 8.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol. 2015;66:337–346. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 10.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease role of healed plaque disruption. Heart. 1999;82:265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 12.Kubo T, Maehara A, Mintz GS, Doi H, Tsujita K, Choi S-Y, Katoh O, Nasu K, Koenig A, Pieper M, Rogers JH, Wijns W, Böse D, Margolis MP, Moses JW, Stone GW, Leon MB. The Dynamic Nature of Coronary Artery Lesion Morphology Assessed by Serial Virtual Histology Intravascular Ultrasound Tissue Characterization. J Am Coll Cardiol. 2010;55:1590–1597. doi: 10.1016/j.jacc.2009.07.078. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 14.Cristell N, Cianflone D, Durante A, Ammirati E, Vanuzzo D, Banfi M, Calori G, Latib A, Crea F, Marenzi G, De Metrio M, Moretti L, Li H, Uren NG, Hu D, Maseri A. High-sensitivity C-reactive protein is within normal levels at the very onset of first ST-segment elevation acute myocardial infarction in 41% of cases: a multiethnic case-control study. J Am Coll Cardiol. 2011;58:2654–2661. doi: 10.1016/j.jacc.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 15.Scalone G, Niccoli G, Refaat H, Vergallo R, Porto I, Leone AM, Burzotta F, D’Amario D, Liuzzo G, Fracassi F, Trani C, Crea F. Not all plaque ruptures are born equal: an optical coherence tomography study. Eur Heart J Cardiovasc Imaging. 2016 doi: 10.1093/ehjci/jew208. Epub ahead of print, December 26, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82:269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 18.Niccoli G, Montone RA, Cataneo L, Cosentino N, Gramegna M, Refaat H, Porto I, Burzotta F, Trani C, Leone AM, Severino A, Crea F. Morphological-biohumoral correlations in acute coronary syndromes: pathogenetic implications. Int J Cardiol. 2014;171:463–466. doi: 10.1016/j.ijcard.2013.12.238. [DOI] [PubMed] [Google Scholar]
- 19.Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 22.Schonbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, Libby P. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res. 1997;81:448–454. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 24.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Crother TR, Arditi M. Emerging role of IL-17 in atherosclerosis. J Innate Immun. 2010;2:325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gistera A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 27.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 28.Liuzzo G, Montone RA, Gabriele M, Pedicino D, Giglio AF, Trotta F, Galiffa VA, Previtero M, Severino A, Biasucci LM, Crea F. Identification of unique adaptive immune system signature in acute coronary syndromes. Int J Cardiol. 2013;168:564–567. doi: 10.1016/j.ijcard.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Flego D, Severino A, Trotta F, Previtero M, Ucci S, Zara C, Pedicino D, Massaro G, Biasucci LM, Liuzzo G, Crea F. Altered CD31 expression and activity in helper T cells of acute coronary syndrome patients. Basic Res Cardiol. 2014;109:448. doi: 10.1007/s00395-014-0448-3. [DOI] [PubMed] [Google Scholar]
- 30.Flego D, Severino A, Trotta F, Previtero M, Ucci S, Zara C, Massaro G, Pedicino D, Biasucci LM, Liuzzo G, Crea F. Increased PTPN22 expression and defective CREB activation impair regulatory T-cell differentiation in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. 2015;65:1175–1186. doi: 10.1016/j.jacc.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Tardif J-C, L’Allier P. Colchicine Cardiovascular Outcomes Trial (COLCOT) 2017 https://clinicaltrials.gov/ct2/show/NCT02551094.
- 33.Everett BM, Pradhan AD, Solomon DH, Paynter N, MacFadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AAK, Glynn RJ, Ridker PM. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–384. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Fornasa G, Clement M, Groyer E, Gaston AT, Khallou-Laschet J, Morvan M, Guedj K, Kaveri SV, Tedgui A, Michel JB, Nicoletti A, Caligiuri G. A CD31-derived peptide prevents angiotensin II-induced atherosclerosis progression and aneurysm formation. Cardiovasc Res. 2012;94:30–37. doi: 10.1093/cvr/cvs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanford SM, Krishnamurthy D, Falk MD, Messina R, Debnath B, Li S, Liu T, Kazemi R, Dahl R, He Y, Yu X, Chan AC, Zhang ZY, Barrios AM, Woods VL, Jr, Neamati N, Bottini N. Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells. J Med Chem. 2011;54:1640–1654. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah PK, Chyu KY, Dimayuga PC, Nilsson J. Vaccine for atherosclerosis. J Am Coll Cardiol. 2014;64:2779–2791. doi: 10.1016/j.jacc.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Koskinas KC, Sukhova GK, Baker AB, Papafaklis MI, Chatzizisis YS, Coskun AU, Quillard T, Jonas M, Maynard C, Antoniadis AP, Shi GP, Libby P, Edelman ER, Feldman CL, Stone PH. Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler Thromb Vasc Biol. 2013;33:1494–1504. doi: 10.1161/ATVBAHA.112.300827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatzizisis YS, Baker AB, Sukhova GK, Koskinas KC, Beigel R, Jonas M, Coskun AU, Stone BV, Maynard C, Shi GP, Libby P, Feldman CL, Edelman ER, Stone PH. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress co-localize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011;123:621–630. doi: 10.1161/CIRCULATIONAHA.110.970038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiomi M, Ishida T, Kobayashi T, Nitta N, Sonoda A, Yamada S, Koike T, Kuniyoshi N, Murata K, Hirata K, Ito T, Libby P. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction-prone strain of the Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2013;33:2518–2523. doi: 10.1161/ATVBAHA.113.301303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libby P, Nahrendorf M, Swirski FK. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease. J Am Coll Cardiol. 2016;67:1091–1103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA. 1999;281:921–926. doi: 10.1001/jama.281.10.921. [DOI] [PubMed] [Google Scholar]
- 45.Janoudi A, Shamoun FE, Kalavakunta JK, Abela GS. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur Heart J. 2016;37:1959–1967. doi: 10.1093/eurheartj/ehv653. [DOI] [PubMed] [Google Scholar]
- 46.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura S, Inami S, Murai K, Takano M, Takano H, Asai K, Yasutake M, Shimizu W, Mizuno K. Relationship between cholesterol crystals and culprit lesion characteristics in patients with stable coronary artery disease: an optical coherence tomography study. Clin Res Cardiol. 2014;103:1015–1021. doi: 10.1007/s00392-014-0748-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, Tearney GJ. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011;17:1010–1014. doi: 10.1038/nm.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loree HM, Tobias BJ, Gibson LJ, Kamm RD, Small DM, Lee RT. Mechanical properties of model atherosclerotic lesion lipid pools. Arterioscler Thromb. 1994;14:230–234. doi: 10.1161/01.atv.14.2.230. [DOI] [PubMed] [Google Scholar]
- 50.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 51.Virmani R, Joner M, Sakakura K. Recent highlights of ATVB: calcification. Arterioscler Thromb Vasc Biol. 2014;34:1329–1332. doi: 10.1161/ATVBAHA.114.304000. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz JL, Weinbaum S, Aikawa E, Hutcheson JD. Zooming in on the genesis of atherosclerotic plaque microcalcifications. J Physiol. 2016;594:2915–2927. doi: 10.1113/JP271339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camici PG, Rimoldi OE, Gaemperli O, Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33:1309–1317. doi: 10.1093/eurheartj/ehs067. [DOI] [PubMed] [Google Scholar]
- 54.Libby P. How does lipid lowering prevent coronary events? New insights from human imaging trials. Eur Heart J. 2015;36:472–474. doi: 10.1093/eurheartj/ehu510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am J Cardiol. 2011;107:1710–1717. doi: 10.1016/j.amjcard.2011.02.336. [DOI] [PubMed] [Google Scholar]
- 56.Patel R, Janoudi A, Vedre A, Aziz K, Tamhane U, Rubinstein J, Abela OG, Berger K, Abela GS. Plaque rupture and thrombosis are reduced by lowering cholesterol levels and crystallization with ezetimibe and are correlated with fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011;31:2007–2014. doi: 10.1161/ATVBAHA.111.226167. [DOI] [PubMed] [Google Scholar]
- 57.Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T, Skjelland M, De Nardo D, Labzin LI, Kerksiek A, Hempel C, Heneka MT, Hawxhurst V, Fitzgerald ML, Trebicka J, Bjorkhem I, Gustafsson JA, Westerterp M, Tall AR, Wright SD, Espevik T, Schultze JL, Nickenig G, Lutjohann D, Latz E. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med. 2016;8:333ra350. doi: 10.1126/scitranslmed.aad6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammadpour AH, Akhlaghi F. Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective. Clin Pharmacokinet. 2013;52:615–626. doi: 10.1007/s40262-013-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meuwese MC, de Groot E, Duivenvoorden R, Trip MD, Ose L, Maritz FJ, Basart DC, Kastelein JJ, Habib R, Davidson MH, Zwinderman AH, Schwocho LR, Stein EA, Investigators C. ACAT inhibition and progression of carotid atherosclerosis in patients with familial hypercholesterolemia: the CAPTIVATE randomized trial. JAMA. 2009;301:1131–1139. doi: 10.1001/jama.301.11.1131. [DOI] [PubMed] [Google Scholar]
- 60.Luscher TF. Substrates of acute coronary syndromes: new insights into plaque rupture and erosion. Eur Heart J. 2015;36:1347–1349. doi: 10.1093/eurheartj/ehv149. [DOI] [PubMed] [Google Scholar]
- 61.Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122:2505–2513. doi: 10.1161/CIRCULATIONAHA.110.955302. [DOI] [PubMed] [Google Scholar]
- 62.Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice - Implications for Superficial Erosion. Circ Res. 2017;121:31. doi: 10.1161/CIRCRESAHA.117.310694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolodgie FD, Burke AP, Wight TN, Virmani R. The accumulation of specific types of proteoglycans in eroded plaques: a role in coronary thrombosis in the absence of rupture. Curr Opin Lipidol. 2004;15:575–582. doi: 10.1097/00041433-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Day AJ. The structure and regulation of hyaluronan-binding proteins. Biochem Soc Trans. 1999;27:115–121. doi: 10.1042/bst0270115. [DOI] [PubMed] [Google Scholar]
- 68.Butler LM, Rainger GE, Nash GB. A role for the endothelial glycosaminoglycan hyaluronan in neutrophil recruitment by endothelial cells cultured for prolonged periods. Exp Cell Res. 2009;315:3433–3441. doi: 10.1016/j.yexcr.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doring Y, Soehnlein O, Weber C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ Res. 2017;120:736–743. doi: 10.1161/CIRCRESAHA.116.309692. [DOI] [PubMed] [Google Scholar]
- 70.Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36:774–776. doi: 10.1093/eurheartj/ehu500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter MP, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM. Coronary Neutrophil Extracellular Trap Burden and Deoxyribonuclease Activity in ST-Elevation Acute Coronary Syndrome Are Predictors of ST-Segment Resolution and Infarct Size. Circ Res. 2015;116:1182–1192. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 72.Aguirre-Alvarado C, Segura-Cabrera A, Velazquez-Quesada I, Hernandez-Esquivel MA, Garcia-Perez CA, Guerrero-Rodriguez SL, Ruiz-Moreno AJ, Rodriguez-Moreno A, Perez-Tapia SM, Velasco-Velazquez MA. Virtual screening-driven repositioning of etoposide as CD44 antagonist in breast cancer cells. doi: 10.18632/oncotarget.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, Xu M, Yao Y, Hu S, Yamamoto E, Lee H, Zhang S, Yu B, Jang IK. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study) Eur Heart J. 2017;38:792–800. doi: 10.1093/eurheartj/ehw381. [DOI] [PubMed] [Google Scholar]
- 74.Libby P. Superficial erosion and the precision management of acute coronary syndromes: not one-size-fits-all. Eur Heart J. 2017;38:801–803. doi: 10.1093/eurheartj/ehw599. [DOI] [PubMed] [Google Scholar]
- 75.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN. The Who, What, Why, When, How and Where of Vasospastic Angina. Circ J. 2016;80:289–298. doi: 10.1253/circj.CJ-15-1202. [DOI] [PubMed] [Google Scholar]
- 76.Ong P, Athanasiadis A, Borgulya G, Voehringer M, Sechtem U. 3-year follow-up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow-up. J Am Coll Cardiol. 2011;57:147–152. doi: 10.1016/j.jacc.2010.08.626. [DOI] [PubMed] [Google Scholar]
- 77.Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2:e000227. doi: 10.1161/JAHA.113.000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FA, Delforge MR, Carre AG, Asseman P, Berzin B, Libersa C, Laurent JM. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299–1306. doi: 10.1161/01.cir.65.7.1299. [DOI] [PubMed] [Google Scholar]
- 79.Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, Cianflone D, Sanna T, Sasayama S, Maseri A. Major Racial Differences in Coronary Constrictor Response Between Japanese and Caucasians With Recent Myocardial Infarction. Circulation. 2000;101:1102. doi: 10.1161/01.cir.101.10.1102. [DOI] [PubMed] [Google Scholar]
- 80.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 81.Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 82.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 83.Arrebola-Moreno AL, Arrebola JP, Moral-Ruiz A, Ramirez-Hernandez JA, Melgares-Moreno R, Kaski JC. Coronary microvascular spasm triggers transient ischemic left ventricular diastolic abnormalities in patients with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2014;236:207–214. doi: 10.1016/j.atherosclerosis.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Kaski JC, Maseri A, Vejar M, Crea F, Hackett D. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989;14:1456–1463. doi: 10.1016/0735-1097(89)90382-3. [DOI] [PubMed] [Google Scholar]
- 85.Lanza GA, Pedrotti P, Pasceri V, Lucente M, Crea F, Maseri A. Autonomic changes associated with spontaneous coronary spasm in patients with variant angina. J Am Coll Cardiol. 1996;28:1249–1256. doi: 10.1016/S0735-1097(96)00309-9. [DOI] [PubMed] [Google Scholar]
- 86.Shimokawa H, Tomoike H, Nabeyama S, Yamamoto H, Araki H, Nakamura M, Ishii Y, Tanaka K. Coronary artery spasm induced in atherosclerotic miniature swine. Science. 1983;221:560–562. doi: 10.1126/science.6408736. [DOI] [PubMed] [Google Scholar]
- 87.Ito A, Shimokawa H, Nakaike R, Fukai T, Sakata M, Takayanagi T, Egashira K, Takeshita A. Role of protein kinase C-mediated pathway in the pathogenesis of coronary artery spasm in a swine model. Circulation. 1994;90:2425–2431. doi: 10.1161/01.cir.90.5.2425. [DOI] [PubMed] [Google Scholar]
- 88.Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, Kuwata K, Kandabashi T, Egashira K, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 89.Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J. 2014;35:3180–3193. doi: 10.1093/eurheartj/ehu427. [DOI] [PubMed] [Google Scholar]
- 90.Dai Z, Aoki T, Fukumoto Y, Shimokawa H. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol. 2012;60:416–421. doi: 10.1016/j.jjcc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, Davies G, Maseri A. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. 1986;74:1255. doi: 10.1161/01.cir.74.6.1255. [DOI] [PubMed] [Google Scholar]
- 92.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 93.Abbate A, Bussani R, Liuzzo G, Biondi-Zoccai GG, Barresi E, Mellone P, Sinagra G, Dobrina A, De Giorgio F, Sharma R, Bassan F, Severino A, Baldi F, Biasucci LM, Pandolfi F, Silvestri F, Vetrovec GW, Baldi A, Crea F. Sudden coronary death, fatal acute myocardial infarction and widespread coronary and myocardial inflammation. Heart. 2008;94:737–742. doi: 10.1136/hrt.2007.115329. [DOI] [PubMed] [Google Scholar]
- 94.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 95.King KR, Grazette LP, Paltoo DN, McDevitt JT, Sia SK, Barrett PM, Apple FS, Gurbel PA, Weissleder R, Leeds H, Iturriaga EJ, Rao AK, Adhikari B, Desvigne-Nickens P, Galis ZS, Libby P. Point-of-Care Technologies for Precision Cardiovascular Care and Clinical Research. JACC: Basic to Translational Science. 2016;1:73–86. doi: 10.1016/j.jacbts.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]