Abstract
Background
Options for patients with non-squamous non-small cell lung cancer (NSCLC) whose disease progresses after first-line chemotherapy are limited. This randomized, open-label, international phase 3 study evaluated efficacy and safety of nivolumab versus docetaxel in this patient population after failure of platinum doublet chemotherapy.
Methods
Patients were randomized to nivolumab 3 mg per kilogram every 2 weeks or docetaxel 75 mg per square meter every 3 weeks. The primary endpoint was overall survival.
Results
Nivolumab improved overall survival versus docetaxel. Median overall survival was 12.2 months (95% CI, 9.7 to 15.0) for nivolumab (n=292) and 9.4 months (95% CI, 8.1 to 10.7) for docetaxel (n=290) (hazard ratio, 0.73; 96% CI, 0.59 to 0.89; P=0.002). One-year overall survival rates were 51% (95% CI, 45 to 56) for nivolumab and 39% (95% CI, 33 to 45) for docetaxel. Updated efficacy results with additional follow up are available for overall survival only: 18-month overall survival rates were 39% (95% CI, 34 to 45) for nivolumab and 23% (95% CI, 19 to 28) for docetaxel. Response rates were 19% for nivolumab and 12% for docetaxel (P=0.02). Although progression-free survival did not favor nivolumab (2.3 months for nivolumab versus 4.2 months for docetaxel), 1-year progression-free survival was higher for nivolumab (19%) than docetaxel (8%). Nivolumab further improved efficacy across all endpoints at predefined ≥1%, ≥5%, and ≥10% programmed death-1 ligand 1 (PD-L1) tumor membrane expression levels. Grade 3–5 treatment-related adverse events were reported in 10% of nivolumab and 54% of docetaxel-treated patients.
Conclusions
Compared to docetaxel, nivolumab demonstrated superior overall survival, with PD-L1 expression conferring enhanced efficacy in patients with advanced non-squamous NSCLC after failure of platinum-based chemotherapy. The safety profile of nivolumab was favorable versus docetaxel.
Introduction
Effective options for patients with non-squamous non-small cell lung cancer (NSCLC) whose disease progresses after first-line chemotherapy are limited. Docetaxel was approved as second-line treatment for advanced NSCLC based on improvement in survival versus best supportive care.1–3 More tolerable newer agents, such as pemetrexed and erlotinib, were either shown to be non-inferior or have failed to show superiority in overall survival compared to docetaxel in this setting.4,5
The programmed death-1 (PD-1) receptor expressed on activated T cells is engaged by tumor-expressed ligands PD-L1 and PD-L2 to downregulate T-cell activation and promote tumor immune escape.6 Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, disrupts PD-1-mediated signaling and may restore antitumor immunity.7–9 In phase 1 studies, nivolumab monotherapy demonstrated durable anti-tumor activity and encouraging survival in all NSCLC subtypes.7,9,10 In heavily pretreated patients with advanced non-squamous NSCLC, nivolumab demonstrated a response rate of 17.6%, 1-, 2-, and 3-year overall survival rates of 42%, 23%, and 16%, respectively, and a 1-year progression-free survival rate of 18%.10 Nivolumab is approved in the United States for treatment of patients with metastatic squamous NSCLC and progression on or after platinum-based chemotherapy11 and in the European Union for locally advanced or metastatic squamous NSCLC after prior chemotherapy.12 We report results of a phase 3 study (CheckMate 057; NCT01673867) comparing nivolumab to docetaxel in previously treated advanced non-squamous NSCLC.
Methods
Patients
Eligible patients had documented stage IIIB/IV or recurrent non-squamous NSCLC following radiation therapy or surgical resection, and disease recurrence or progression during or after one prior platinum-based regimen. An additional line of tyrosine kinase inhibitor therapy in patients with known EGFR mutation or ALK translocation and continuation or switch maintenance therapy with pemetrexed, bevacizumab or erlotinib was allowed. Patients 18 years of age or older, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (a 5-point scale in which higher numbers indicate greater tumor-related disability), adequate hematologic, hepatic, and renal function, and treated stable central nervous system (CNS) metastases were eligible. Pretreatment tumor tissue for biomarker analyses was required but not used for patient selection. Exclusion criteria included autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression, prior treatment with immune-stimulatory antitumor agents including checkpoint-targeted agents, or docetaxel. Complete eligibility details are provided in the study protocol available at NEJM.org.
Study design and treatments
From November, 2012 to December, 2013, 792 patients were enrolled and 582 randomized to either nivolumab 3 mg per kilogram every 2 weeks (n = 292), or docetaxel 75 mg per square meter every 3 weeks (n = 290), both intravenously (Fig. S1A). Patients were treated until disease progression or discontinuation due to toxicity or other reasons (Fig. S1B).
Randomization was stratified by prior maintenance treatment and line of therapy (second- vs third-line). Nivolumab patients could continue treatment beyond initial progression if the investigator assessed that the patient was having clinical benefit and tolerating study drug. Criteria for treatment delay or discontinuation for treatment-related adverse events, and docetaxel dose reductions for toxicities, per product label, were defined. Nivolumab dose reductions were not permitted.
Endpoints and assessments
The primary endpoint was overall survival, which was assessed while on treatment, and every 3 months after treatment discontinuation. All randomized patients were followed for survival, unless they had withdrawn consent from survival follow up. Survival information was obtained through a search of publicly available sources for patients who withdrew consent for or were lost to follow up. Secondary efficacy endpoints included investigator-assessed confirmed objective response rate and progression-free survival, efficacy by tumor PD-L1 expression, and patient-reported outcomes. All randomized patients were followed for disease progression, except for those who had withdrawn consent from the study or who were lost to follow up. Tumor response was assessed per Response Evaluation Criteria In Solid Tumors, version 1.1 (RECIST v1.1)13 at week 9 and every 6 weeks thereafter until disease progression. Safety was assessed by evaluating the incidence of adverse events and laboratory parameters, graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Select adverse events (those with potential immunologic etiologies) were grouped according to predefined categories. Analyses of patient-reported outcome measures are ongoing.
PD-L1 biomarker analysis
Tumor PD-L1 protein expression was retrospectively assessed in prospectively collected, pretreatment (archival or recent) tumor biopsies using a validated, automated immunohistochemical assay (Dako North America, Carpinteria, CA) using a rabbit anti-human PD-L1 antibody (clone 28–8; Epitomics Inc, Burlingame, CA). Predefined expression levels were defined by tumor cell membrane staining (any intensity) in a section containing at least 100 evaluable tumor cells.
Study oversight
The study was designed by the authors in collaboration with the sponsor, which worked jointly with investigators to collect and analyze data. The study protocol was approved by an Institutional Review Board or ethics committee at each participating center, and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice; the protocol and statistical analysis plan are available with the full text of the manuscript at www.nejm.org. Written informed consent was collected from all patients before enrollment.
An independent data monitoring committee provided oversight of safety and efficacy. On April 16, 2015, the committee declared superiority in overall survival for nivolumab versus docetaxel. We report the interim analysis results here, including overall survival, objective response rate, progression free survival and safety, based on a March 18, 2015 database lock. Updated efficacy results with additional follow up are reported for overall survival only, based on a July 2, 2015 database lock.
All authors attest that the study was conducted in accordance with the protocol and vouch for data accuracy. The first draft of this manuscript was written by first and last authors; all authors contributed to subsequent drafts, agreed to submit the manuscript for publication, and signed a confidentiality agreement with the sponsor. Medical-writing support, funded by the sponsor, was provided by StemScientific.
Statistical analyses
Overall survival and progression-free survival were analyzed using a two-sided log-rank test stratified by prior maintenance treatment and line of therapy. Hazard ratios and confidence intervals (CIs) were estimated using a stratified, Cox proportional hazards model. Survival curves and rates were estimated using the Kaplan-Meier method. Objective response rates were compared using a stratified, two-sided Cochran-Mantel Haenszel test. Non-conventional benefit (i.e., a reduction in the size or number [or both] of target lesions with simultaneous appearance of new lesions or initial progression followed by either tumor reduction or no further progression for at least two tumor assessments) in patients treated beyond initial progression was not included for response-based analyses (objective response rate or progression-free survival). Prespecified subgroup analyses were performed on overall survival, objective response rate and progression-free survival to assess the consistency of treatment effects among patient subpopulations. All prespecified subgroup analyses of survival are reported in the supplementary appendix using unstratified hazard ratios and 95% CIs; hazard ratios were not computed for subgroups with fewer than 10 patients per treatment group. Additional prespecified analyses were performed to evaluate the prognostic and predictive roles of PD-L1 expression, with an interaction P value of <0.2 considered a signal of predictive association.
Demographic and efficacy analyses included all randomized patients. Safety analyses included all treated patients (received at least one dose of study drug). At the time of interim analysis, 413 patients had expired (93% of the 442 deaths required for the final analysis). The boundary for declaring superiority for overall survival at the interim analysis was P<0.0408, based on the O’Brien-Fleming alpha spending function. If superiority for overall survival was demonstrated, then response rate and progression-free survival were tested hierarchically at the 5% alpha level. The formal primary endpoint testing was based on the interim analysis. For full description of the additional follow-up data, an updated p-value is provided based on July 2, 2015 database lock.
Results
Patients and treatment
Of randomized patients, 287 were treated with nivolumab and 268 were treated with docetaxel. Median age was 62 years. Most patients were ECOG performance status 1, stage IV, and current/former smokers (Table 1 and S1). Baseline characteristics were balanced between treatment groups, with slight imbalances for male sex and age 65 or younger.
Table 1.
Baseline Characteristics, Stratification Factors and Prior Therapy of All Randomized Patients.
| Nivolumab n = 292 |
Docetaxel n = 290 |
Total N = 582 |
|
|---|---|---|---|
|
| |||
| Median age, years (range) | 61 (37, 84) | 64 (21,85) | 62 (21, 85) |
|
| |||
| Age categorization, n (%) | |||
| ≥75 | 20 (7) | 23 (8) | 43 (7) |
|
| |||
| Gender, n (%) | |||
| Male | 151 (52) | 168 (58) | 319 (55) |
|
| |||
| Race, n (%) | |||
| White | 267 (91) | 266 (92) | 533 (92) |
| Asian | 9 (3) | 8 (3) | 17 (3) |
| Black/African American | 7 (2) | 9 (3) | 16 (3) |
| Other* | 9 (3) | 7 (2) | 16 (3) |
|
| |||
| ECOG performance status, n (%) | |||
| 0 | 84 (29) | 95 (33) | 179 (31) |
| 1 | 208 (71) | 193 (67) | 401 (69) |
|
| |||
| Disease stage, n (%) | |||
| Stage IIIB | 20 (7) | 24 (8) | 44 (8) |
| Stage IV | 272 (93) | 266 (92) | 538 (92) |
|
| |||
| Smoking status, n (%) | |||
| Current/former | 231 (79) | 227 (78) | 458 (79) |
| Never | 58 (20) | 60 (21) | 118 (20) |
|
| |||
| EGFR mutation status, † n (%) | |||
| Positive | 44 (15) | 38 (13) | 82 (14) |
|
| |||
| ALK translocation status, † n (%) | |||
| Positive | 13 (4) | 8 (3) | 21 (4) |
|
| |||
| KRAS mutation status, † n (%) | |||
| Positive | 28 (10) | 34 (12) | 62 (11) |
|
| |||
| Prior maintenance, n (%) | 122 (42) | 111 (38) | 233 (40) |
|
| |||
| Number of prior systemic regimens, ‡ n (%) | |||
| 1 | 256 (88) | 259 (89) | 515 (89) |
| 2 | 35 (12) | 31 (11) | 66 (11) |
|
| |||
| Type of prior systemic therapy,§ n (%) | |||
| Prior platinum-based therapy | 292 (100) | 290 (100) | 582 (100) |
| ALK inhibitors | 1 (<1) | 2 (1) | 3 (1) |
| EGFR-TKI | 29 (10) | 24 (8) | 53 (9) |
|
| |||
| Best response to most recent prior systemic regimen (per investigator), n (%) | |||
| Complete or partial response | 73 (25) | 68 (23) | 141 (24) |
| Stable disease | 103 (35) | 96 (33) | 199 (34) |
| Progressive disease | 111 (38) | 116 (40) | 227 (39) |
| Unknown/not reported | 5 (2) | 10 (3) | 15 (3) |
Includes other plus 1 patient American Indian/Alaska Native for nivolumab and 1 patient Native Hawaiian or other Pacific Islander for docetaxel.
Mutation status (EGFR or KRAS) or ALK translocation status was not determined through centralized testing; it was not mandatory per the protocol, but was reported by the investigator and collected from case report forms.
Derived as number of lines of prior therapy received for advanced, metastatic or recurrent disease received. One patient in the nivolumab group had one prior regimen in the neo-adjuvant setting.
Some patients may have been treated with more than 1 type of therapy.
ALK = anaplastic lymphoma kinase; ECOG = Eastern Cooperative Oncology Group; EGFR = epidermal growth factor receptor; KRAS = Kirsten rat sarcoma viral oncogene homolog; TKI = tyrosine kinase inhibitor.
A median of 6 doses (range, 1–52) of nivolumab and 4 doses (range, 1–23) of docetaxel were administered. In the nivolumab and docetaxel groups, respectively, 83% and 66% of patients received at least 90% of the planned dose intensity. At least one dose delay occurred in 39% of nivolumab and 37% of docetaxel patients. Most nivolumab (53%, 117/219 cycles) and docetaxel (67%, 99/147 cycles) delays lasted ≤7 days; 26% of patients required a docetaxel dose reduction. Forty-five percent of nivolumab and 46% of docetaxel delays were due to adverse events.
At the time of interim analysis, 15% of nivolumab and no docetaxel patients were continuing treatment (Table S2). Subsequent systemic therapy was received by 42% of nivolumab and 50% of docetaxel patients. Of nivolumab patients, 23% received subsequent docetaxel; 2% of docetaxel patients received subsequent immunotherapy (Table S3).
Efficacy
Nivolumab significantly improved overall survival versus docetaxel (Fig. 1A). At the time of interim analysis (minimum follow-up for overall survival, 13.2 months), median overall survival was 12.2 months (95% CI, 9.7 to 15.0) for nivolumab and 9.4 months (95% CI, 8.1 to 10.7) for docetaxel, a 27% reduction in risk of death (hazard ratio, 0.73; 96% CI, 0.59 to 0.89; P=0.002). One-year overall survival rates were 51% (95% CI, 45 to 56) and 39% (95% CI, 33 to 45) for nivolumab and docetaxel, respectively. Overall survival hazard ratios favored nivolumab across most predefined patient subgroups, except for third-line therapy (n = 66), rest of world region (n = 98), CNS metastases (n = 68), never smokers (n = 118) and EGFR mutation-positive status (n = 82) (Fig. 1B and S2). With additional follow-up (minimum, 17.2 months), median overall survival was 12.2 months (95% CI, 9.7 to 15.1) for nivolumab and 9.4 months (95% CI, 8.1 to 10.7) for docetaxel, a 28% reduction in risk of death (hazard ratio, 0.72; 95% CI, 0.60 to 0.88; P=0.0009) (Fig. S3). Eighteen-month overall survival rates were 39% (95% CI, 34 to 45) and 23% (95% CI, 19 to 28) for nivolumab and docetaxel, respectively.
Fig. 1. Efficacy of nivolumab versus docetaxel in patients with advanced non-squamous NSCLC.
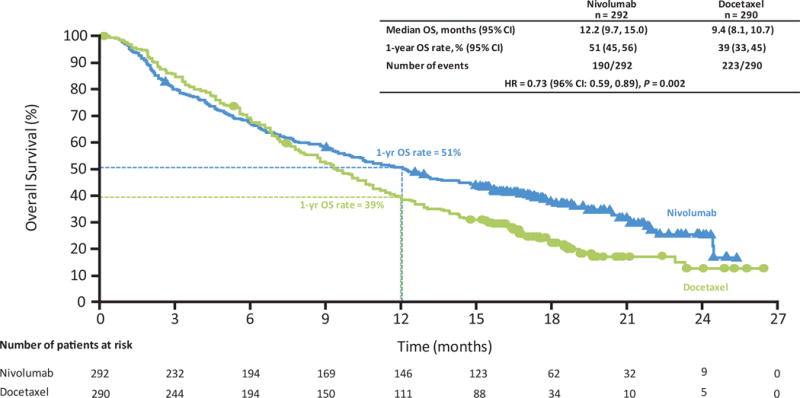



Data are based on a March 18, 2015 database lock. (A) Kaplan-Meier plot of overall survival. All randomized patients (nivolumab, n = 292; docetaxel, n = 290). Symbols represent censored observations. (B) Treatment effect on overall survival in pre-defined subsets. All randomized patients (nivolumab, n = 292; docetaxel, n = 290). HR was not computed for other subsets with less than 10 patients per treatment group. (C) Characteristics of response and progression as assessed per investigator. Ongoing response at last tumor assessment before censoring. Bar indicates progression-free survival. (D) Kaplan-Meier plot of progression-free survival. Progression-free survival was defined as the time from randomization to date of first documented tumor progression, death or last evaluable tumor assessment (censoring date). All randomized patients (nivolumab, n = 292; docetaxel, n = 290). Symbols represent censored observations. CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR = epidermal growth factor receptor; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria In Solid Tumors.
Confirmed objective response rate was significantly higher for nivolumab versus docetaxel (Table 2 and Fig. S4); 19% (95% CI, 15 to 24) versus 12% (95% CI, 9 to 17) (P=0.02). Median time to response was 2.1 months (range, 1.2–8.6) for nivolumab and 2.6 months (range, 1.4–6.3) for docetaxel (Table 2 and Fig. 1C). Median duration of response was 17.2 months (range, 1.8–22.6+) for nivolumab and 5.6 months (range, 1.2+ to 15.2+) for docetaxel.
Table 2.
Tumor Response with Nivolumab versus Docetaxel in Patients with Advanced Non-squamous NSCLC.
| Nivolumab n = 292 |
Docetaxel n = 290 |
|
|---|---|---|
|
| ||
| Objective response rate,* n (%) [95% CI] | 56 (19) [15, 24] | 36 (12) [9, 17] |
|
| ||
| Estimated odds ratio†,‡ (95% CI) | 1.7 (1.1, 2.6) | |
| P value§ | 0.02 | |
|
| ||
| Best overall response, n (%) | ||
| Complete response | 4 (1) | 1 (<1) |
| Partial response | 52 (18) | 35 (12) |
| Stable disease | 74 (25) | 122 (42) |
| Progressive disease | 129 (44) | 85 (29) |
| Unable to determine | 33 (11) | 47 (16) |
|
| ||
| Median time to response,¶,ǁ months (range) | 2.1 (1.2, 8.6) | 2.6 (1.4, 6.3) |
|
| ||
| Median duration of response,¶,** months (range) | 17.2 (1.8, 22.6+) | 5.6 (1.2+, 15.2+) |
Data are based on a March 18, 2015 database lock.
Confirmed complete and partial responses per RECIST v1.1 criteria, as assessed by the investigator. CI based on the Clopper-Pearson method.
Stratified by prior maintenance therapy and prior line of therapy (second- versus third-line).
Strata-adjusted odds ratio (nivolumab over docetaxel) using Cochran-Mantel-Haenszel method.
Two-sided P value from stratified Cochran-Mantel-Haenszel method test.
Values are for all responders (nivolumab, n = 56; docetaxel, n = 36).
Time to response was defined as the time from randomization to the date of first documented complete or partial response.
Calculated using the Kaplan-Meier method. Duration of response was defined as the time between date of first response to date of first documented progression, death, or last evaluable tumor assessment.
CI = confidence interval; Symbol + indicates a censored value.
Median progression-free survival and 1-year progression-free survival rates were 2.3 months (95% CI, 2.2 to 3.3) and 19% (95% CI, 14 to 23), respectively, for nivolumab and 4.2 months (95% CI, 3.5 to 4.9) and 8% (95% CI, 5 to 12), respectively, for docetaxel (Fig. 1D). The hazard ratio for progression-free survival was 0.92 (95% CI, 0.77 to 1.1; P=0.39) (Fig. 1D). Progression-free survival hazard ratios numerically favored nivolumab across most predefined patient subgroups, except for third-line therapy, rest of world region, never smokers, KRAS mutation not detected (n = 123) and EGFR mutation-positive status (Fig. S5).
In total, 71 (25%) nivolumab patients continued treatment beyond initial progression, 16 (23%) of whom demonstrated a non-conventional pattern of benefit. Characteristics of patients treated beyond progression, including change in tumor burden over time, are provided (Fig. S6 and Table S4).
Of randomized patients, 78% (455/582) had quantifiable PD-L1 expression. Rates of PD-L1 expression were balanced between groups (Table S5). At the time of interim analysis, test for interaction suggested a strong predictive association between PD-L1 and clinical outcome at all expression levels for all efficacy endpoints (Table S6). Nivolumab demonstrated improved overall survival, progression-free survival (Fig. S7) and objective response rates (Table S5) at prespecified ≥1%, ≥5% and ≥10% PD-L1 expression levels. Progression-free survival across all prespecified PD-L1 subgroups based on the interim analysis database lock is provided in Fig. S8A. Overall survival by PD-L1 expression level based on the July 2015 database lock is shown in Fig. S8B; the difference in overall survival between nivolumab and docetaxel among patients whose tumors express PD-L1 was still evident with additional follow-up. Median duration of response was longer with nivolumab versus docetaxel across all PD-L1 expression levels (Table S5).
Safety
The frequencies of all-causality, any-grade adverse events were similar between arms, but fewer grade 3– 4 adverse events were reported with nivolumab than docetaxel (Table S7). Treatment-related adverse events were low in severity with nivolumab and less frequent (any-grade: 69%; grade 3–4: 10%) than with docetaxel (any-grade: 88%; grade 3–4: 54%) (Table 3 and S8). The most frequently reported any-grade treatment-related adverse events for nivolumab were fatigue (16%), nausea (12%), decreased appetite (10%), and asthenia (10%). The most frequently reported any-grade treatment-related adverse events for docetaxel were neutropenia (31%), fatigue (29%), nausea (26%), and alopecia (25%). Treatment-related serious adverse events were less frequent with nivolumab (any-grade: 7%; grade 3–4: 5%) than docetaxel (any-grade: 20%; grade 3–4: 18%) (Table S9).
Table 3.
Treatment-related Adverse Events reported in ≥10% of Patients Treated with Nivolumab or Docetaxel.
| Total patients with an event | Nivolumab* n = 287 |
Docetaxel n = 268 |
||
|---|---|---|---|---|
| Any Grade n (%) |
Grade 3–4† n (%) |
Any Grade n (%) |
Grade 3–4† n (%) |
|
| 199 (69) | 30 (10) | 236 (88) | 144 (54) | |
| Fatigue | 46 (16) | 3 (1) | 78 (29) | 13 (5) |
| Nausea | 34 (12) | 2 (1) | 70 (26) | 2 (1) |
| Decreased appetite | 30 (10) | 0 | 42 (16) | 3 (1) |
| Asthenia | 29 (10) | 1 (<1) | 47 (18) | 6 (2) |
| Diarrhea | 22 (8) | 2 (1) | 62 (23) | 3 (1) |
| Peripheral edema | 8 (3) | 0 | 28 (10) | 1 (<1) |
| Myalgia | 7 (2) | 1 (<1) | 30 (11) | 0 |
| Anemia | 6 (2) | 1 (<1) | 53 (20) | 7 (3) |
| Alopecia | 1 (<1) | 0 | 67 (25) | 0 |
| Neutropenia | 1 (<1) | 0 | 83 (31) | 73 (27) |
| Febrile neutropenia | 0 | 0 | 27 (10) | 26 (10)‡ |
| Leukopenia | 0 | 0 | 27 (10) | 22 (8) |
Data are based on a March 18, 2015 database lock.
One death was attributed to nivolumab (encephalitis); association to nivolumab was changed after database lock.
No grade 5 events were reported at database lock; 1 grade 5 event was reported for nivolumab post-database lock.
Docetaxel-related death was due to grade 4 febrile neutropenia.
The most frequently-reported (≥2.5% of patients) any-grade treatment-related select adverse events (nivolumab versus docetaxel) were rash (9% versus 3%), pruritus (8% versus 1%), erythema (1% versus 4%), diarrhea (8% versus 23%), hypothyroidism (7% versus 0%), increased alanine aminotransferase (3% versus 1%), increased aspartate aminotransferase (3% versus 1%), infusion related reaction (3% versus 3%), and pneumonitis (3% versus <1%) (Table S10). Across categories, median times to onset of any-grade treatment-related select adverse events for nivolumab ranged from 0.9 to 31.1 weeks (Table S11). Of those who experienced treatment-related select adverse events across categories (Table S11), 11% to 70% were treated with immune-modulating agents (generally glucocorticoids), per protocol guidelines. Across categories, 44% to 100% of treatment-related select adverse events resolved, with median times to resolution ranging from 0.1 to 12.1 weeks (Table S11). Median time to resolution of treatment-related select endocrinopathies was not reached, as a proportion of these events are not expected to resolve. The frequencies of treatment-related adverse events, serious adverse events and adverse events leading to discontinuation were similar between patients with ≥1% PD-L1and <1% PD-L1 expression (Table S12).
Discontinuation due to treatment–related adverse events was less frequent with nivolumab (5%) than docetaxel (15%) (Tables S13 and S14). The most common treatment-related adverse event leading to discontinuation was pneumonitis (1%) for nivolumab, and fatigue (3%) for docetaxel. One death each in the nivolumab (encephalitis, reported before but causality changed after the database lock) and docetaxel (febrile neutropenia) groups were assessed as treatment-related.
Discussion
Despite the increased number of treatment options for NSCLC, minimal improvement in overall survival has been noted, except in patients with EGFR mutations or ALK translocations. Docetaxel is regarded as the standard of care for previously treated advanced NSCLC and is an appropriate comparator for this trial. In our phase 3 study in advanced non-squamous NSCLC, nivolumab was associated with a significant survival benefit versus docetaxel (27% reduction in risk of death at 13.2 months minimum follow up) that persisted with extended follow up (28% reduction in risk of death at 17.2 months minimum follow up).
The observed overall survival curve for nivolumab in this population is consistent with a prior nivolumab study.10 Furthermore, a delay in the separation of nivolumab and docetaxel overall survival curves is consistent with results of other immune system-modifying agents in advanced melanoma.14
In a recently published phase 3 study, docetaxel with ramucirumab, a vascular endothelial growth factor 2 inhibitor, showed a 1.4 month improvement in survival over docetaxel alone (11.1 versus 9.7 months; hazard ratio, 0.83) in patients with non-squamous NSCLC.15 In our study, the overall survival improvement is 2.8 months (12.2 versus 9.4 months; hazard ratio, 0.73) with nivolumab monotherapy which persisted with extended follow-up. A percentage of patients who were randomized to receive docetaxel were never treated (8%, 22/290); however, those patients were followed for overall survival, and the impact on the overall results is minimal. Benefit of nivolumab was further reflected by a significantly higher objective response rate (19% versus 12%) and markedly better response durability (17.2 versus 5.6 months) as compared to other treatment options for patients in this setting.4,16,17
Nivolumab showed significant improvement in overall survival and response rate but not in progression-free survival. A crossing of progression-free survival curves was noted with a delay in benefit of nivolumab supported by a 1-year progression-free survival rate of 19% versus 8% for docetaxel, which may be typical for immunotherapy. The numerically lower median progression-free survival observed for nivolumab is not due to underperformance, as median progression-free survival reported here is consistent with another nivolumab study,10 but may be explained in part by the higher median progression-free survival of docetaxel in this study (4.2 months) versus previously reported data in non-squamous NSCLC (2.8 to 3.7 months).15,18 It is also possible that the observed progression-free survival results may be driven by patient subgroups, as suggested by the subgroup analyses for smoking status and EGFR mutation. Outcomes observed for patients with EGFR mutation-positive tumors may be attributed to better outcomes on the docetaxel arm. However, interpretation of these results is limited by the wide CIs for calculated hazard ratios in a small subset of patients, and possibly by incomplete collection of mutation data. A biological rationale for different outcomes in never smokers and EGFR mutation-positive patients may be related to low levels of mutational heterogeneity, as preliminary data suggest that sensitivity to immune-checkpoint inhibitors may be higher in tumors bearing high levels of somatic mutations.19,20 However, this study is not designed to test this hypothesis, and additional studies are warranted to address this question.
The current study, which included patients regardless of tumor PD-L1 expression level and comparison to a control arm, is the first to demonstrate a predictive association between PD-L1 expression and benefit from anti-PD-1 treatment. Analyzed tumor samples included archived tissue, suggesting applicability in a real world setting where fresh tissue may not be available or repeat biopsy not feasible. For each of the predefined expression levels examined, the descriptive treatment-biomarker interaction P value met the predefined threshold, suggesting a predictive association with clinical benefit. Although benefit of nivolumab was observed in the overall population, the magnitude of benefit across all efficacy endpoints was greater among those whose tumors express PD-L1 (Figs. S7 and S8 and Table S5). Consistent with a recently reported phase 1 study of pembrolizumab in NSCLC,21 there was a trend toward greater response rate as PD-L1 expression level increased; however, a meaningful separation of the overall survival curves was observed across all predefined expression levels. Among patients whose tumors express PD-L1 (≥1%, ≥5% and ≥10% expression levels), nivolumab nearly doubled median overall survival versus docetaxel. No meaningful differences in overall survival were noted between nivolumab and docetaxel among patients whose tumors have no PD-L1 expression. These data are in contrast to results with squamous NSCLC, where PD-L1 expression did not impact nivolumab clinical activity.22,23 This may imply inherent differences in the immune milieu of squamous and non-squamous histologies, suggesting two distinct diseases.
Although there was no difference in overall survival between nivolumab and docetaxel among patients whose tumors have no PD-L1 expression, the improved safety profile and durability of responses to nivolumab suggests it might be a reasonable option for patients regardless of PD-L1 expression. Additional work is warranted to characterize sub-populations whose disease progresses early and who may benefit from combination therapies.
The safety profile of nivolumab observed in this study (Table 3) is consistent with prior studies and was favorable versus docetaxel, with most patients experiencing adverse events of low severity. Only a small percentage of nivolumab patients reported immune-related adverse events, including pneumonitis, which were managed using protocol guidelines.
In summary, nivolumab led to a statistically superior survival benefit versus docetaxel in unselected patients with advanced previously treated non-squamous NSCLC.
Supplementary Material
Acknowledgments
We thank the patients and their families, as well as the participating study teams for making this study possible. We also thank Dako for collaborative development of the automated PD-L1 immunohistochemistry assay and Anna Labrosciano, MPH for her role as protocol manager. This study was funded by Bristol-Myers Squibb, which worked jointly with investigators to collect and analyze the study results. All drafts of this manuscript were prepared with medical writing and editorial assistance provided by Elyse Smith, PhD and Lisa Sullivan, MA at StemScientific, funded by Bristol-Myers Squibb.
Funded by Bristol-Myers Squibb. CheckMate057; Clinical trial number: NCT01673867.
References
- 1.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 3.Taxotere (docetaxel) US Prescribing Information. 2010 May; Accessed March 23, 2015, at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf.
- 4.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 5.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–88. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–56. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-PD-1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.3708. (in press). Epub ahead of print: http://jco.ascopubs.org/content/early/2015/04/15/JCO.2014.58.3708.long. [DOI] [PMC free article] [PubMed]
- 11.FDA News Release. FDA expands approved use of Opdivo to treat lung cancer. 2015 Mar 4; Accessed April 8, 2015, at http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm436534.htm.
- 12.BMS press release. European commission approves nivolumab BMS, the first PD-1 immune checkpoint inhibitor in Europe proven to extend survival for patients with previously-treated advanced squamous non-small cell lung cancer. 2015 Jul 20; Accessed August 26, 2015, at http://news.bms.com/press-release/european-commission-approves-nivolumab-bms-first-pd-1-immune-checkpoint-inhibitor-euro.
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomized phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 16.Khozin S, Blumenthal GM, Zhang L, et al. FDA Approval: Certinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-3157. (in press.) Epub ahead of print: http://clincancerres.aacrjournals.org/content/early/2015/05/07/1078-0432.CCR-14-3157.abstract. [DOI] [PubMed]
- 17.Shepherd FA, Pereira JR, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 18.Reck MR, Kaiser R, Mellemgarad A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blined, randomised controlled trial. Lancet Oncol. 2014;15:143–55. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 19.Champiat S, Ferté C, Lebel-Binay S, et al. Exomics and immunogenics bridging mutational load and immune checkpoints efficacy. Oncoimmunology. 2014;3:e27817-1–e27817-5. doi: 10.4161/onci.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Cancer Immunology. 2015;348:124–28. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small cell lung cancer. New Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer JR, Reckamp K, Baas P, et al. Nivolumab versus docetaxel in advanced squamous non-small cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


