Abstract
We sought to classify endocervical adenocarcinomas (ECAs) based on morphological features linked to etiology (i.e. HPV infection), unlike the WHO 2014 classification. The International Endocervical Adenocarcinoma Criteria and Classification (IECC criteria), described herein, distinguishes between HPV-associated adenocarcinoma (HPVA), recognized by the presence of luminal mitoses and apoptosis seen at scanning magnification, and no or limited HPVA features (NHPVA). HPVAs were then subcategorized based on cytoplasmic features (mostly to provide continuity with pre-existing classification schemes), while NHPVAs were subclassified based on established criteria (i.e. gastric-type, clear cell, etc.). Complete slide sets from 409 cases were collected from 7 institutions worldwide. Tissue microarrays representing 297 cases were constructed; immunohistochemistry (p16, p53, Vimentin, PR) and chromogenic in situ hybridization (CISH) using an RNA-based probe set that recognizes 18 varieties of high-risk HPV were performed to validate IECC diagnoses. The 5 most common IECC diagnoses were usual-type (HPVA) (73% of cohort), gastric-type (NHPVA) (10%), mucinous adenocarcinoma of HPVA type, including intestinal, mucinous NOS, signet-ring and invasive stratified mucin-producing carcinoma categories (9%), clear cell carcinoma (NHPVA) (3%) and adenocarcinoma, not otherwise specified (2%). Only 3 endometrioid carcinomas were recognized and all were NHPVA. When excluding cases thought to have suboptimal tissue processing, 90% and 95% of usual-type IECC cases overexpressed p16 and were HPV-positive, while 37% and 3% of NHPVAs were p16-positive and HPV-positive, respectively. The one HPV-positive gastric-type carcinoma was found to have hybrid HPVA/NHPVA features on secondary review. NHPVA tumors were larger and occurred in significantly older patients, compared to HPVA tumors (p<0.001). The high-risk HPV CISH probe set had superior sensitivity, specificity, and positive and negative predictive values (0.955, 0.968, 0.992, 0.833, respectively) compared to p16 immunohistochemistry (0.872, 0.632, 0.907, 0.545, respectively) to identify HPV-related usual- and mucinous carcinomas. IECC reliably segregates ECAs into HPVA and NHPVA types using morphology alone. This study confirms that usual-type ECAs are the most common type worldwide and that mucinous carcinomas comprise a mixture of HPVA and NHPVA, with gastric-type carcinoma being the major NHPVA type. Endometrioid and serous carcinomas of the endocervix are extraordinarily rare. Should clinical outcomes and genomic studies continue to support these findings, we recommend replacement of the WHO 2014 criteria with the IECC 2017.
Keywords: Cervix, Endocervical, Adenocarcinoma, HPV, Classification
Introduction
The relative and real incidence of endocervical adenocarcinomas (ECAs) has been increasing worldwide, with up to 20–25% of all invasive cervical tumors now being assigned to this category.1,2 According to the latest World Health Organization (WHO) Classification of Tumours of Female Reproductive Organs, the most frequent type of ECA is usual-type, which is HPV-related.3–5 In the past decade, there has been growing awareness of HPV-unassociated endocervical adenocarcinomas.6,7 The gastric-type adenocarcinoma, for example, was recognized in the latest WHO classification (2014) as the second most frequent subtype of ECA. It is HPV-unrelated, despite being categorized as a subtype of “mucinous ECA,” which is itself a combination of HPV-positive and HPV–negative adenocarcinomas.4 Comparison of usual-type HPV-associated ECA with gastric-type HPV-unassociated ECA reveals important differences in tumor behavior and patient survival,8 with significantly worse clinical outcomes for patients with gastric-type adenocarcinoma,6,9 even when matched for stage.9 Gastric-type adenocarcinomas more frequently metastasize to distant sites, including viscera and peritoneum.9 This, as well as data from studies of the vulva10,11 and oropharynx,12,13 suggest that a classification based on pathogenesis is most likely more clinically informative and reproducible than the current WHO scheme.
Invasive ECAs are classified based on descriptive morphological characteristics, particularly cytoplasmic features,4 as assessed on hematoxylin-eosin (H&E) stained slides. Categorizing ECAs using the WHO 2014 classification has important limitations, as subjective definitions are derived empirically, rather than being linked to clinical or biological features.4 For example, WHO 2014 defines usual-type ECA as a tumor composed of mucin-poor glands, but criteria to distinguish it from mucinous ECAs with more intracytoplasmic mucin, and the so-called “endometrioid” carcinomas with less intracytoplasmic mucin4 are obscure. In addition to being mucin-depleted, the so-called “endometrioid” carcinomas are defined as having “endometrioid morphologic features.” Similarly vague definitions used in the past led to misclassification of many endometrial and ovarian carcinomas.14–19 Since WHO 2014 maintains heterogeneous categories of ECAs that are currently not used in clinical management,20 we convened an international panel of pathologists to establish a morphological classification of endocervical adenocarcinomas that is linked to etiology, as is commonplace in other organs, including the vulva and oropharynx.
Materials and Methods
Selection criteria
An international panel of experienced pathologists studied ECAs and adenosquamous carcinomas from seven pathology laboratories (USA: Memorial Sloan Kettering Cancer Center (MSK), New York, and Massachusetts General Hospital, Boston; Romania: University of Medicine and Pharmacy of Targu Mures and Regional Institute of Oncology, Iasi; Japan: Jikei University School of Medicine, Tokyo; Mexico: Hospital de Oncología Mexico City, Mexico City; Israel: Sheba Medical Center, Tel-Hashomer, Ramat Gan; Italy: Ospedale Sacro Cuore Don Calabria, Negrar).
Slides from 462 ECAs and adenosquamous carcinomas from each of these sites were initially collected. Only invasive tumors with at least 5-year follow-up were included; in situ carcinomas, squamous carcinomas, tumors with a neuroendocrine component, carcinosarcomas, and any tumor demonstrating clinical, macroscopic or microscopic features suggesting a lower uterine segment, uterine corpus, or adnexal primary were excluded. Tumors represented by only biopsies and curettings as well as excisions lacking lymph node assessment were also excluded. Patients treated with neoadjuvant chemotherapy and/or radiotherapy were also excluded. Seventy LEEPs, 8 trachelectomies, 41 conizations, 338 hysterectomies, and 5 exenterations were collected. From this group, 53 cases were excluded due to failure to meet entry criteria, missing blocks, or concern that the available slides were not representative of the lesion. As a result, 409 cases remained for study.
Morphological assessment
All microscopic subtypes of ECA were included in the study. Slides from endocervical adenosquamous carcinomas were reviewed in anticipation that some would be reclassified as ECA after histological re-examination. We required examination of all H&E slides with tumor present (average of 12 slides per case). A consensus diagnosis was reached in every case, with at least 2 and as many as 4 study pathologists reviewing slides at a multi-head microscope. Two diagnoses were assigned to each case: one based on WHO 2014, and a second based on our proposal for a new classification of ECAs: the International Endocervical Adenocarcinoma Criteria and Classification (IECC) 2017. The presence and type of any pre-invasive lesion was also recorded.
IECC criteria follow:
HPV-associated adenocarcinoma (HPVA)
Apical mitotic figures and apoptotic bodies appreciable at scanning magnification. If those features were not seen at scanning magnification, a cursory exam at 200x was performed to detect additional cases. Those with easily-identified apical mitotic figures and apoptotic bodies were considered HPVA.
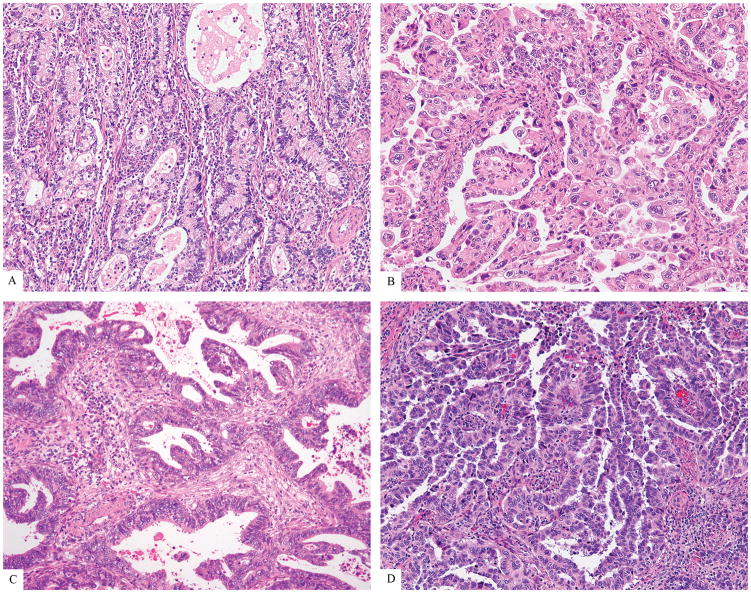
HPVA ECAs (Figure 1) were further subcategorized, mostly based on cytoplasmic features, to provide continuity with pre-existing classification schemes, as follows:
Figure 1.
HPV-associated adenocarcinomas (HPVA): Usual-type (A), mucinous (B), invasive adenocarcinoma with features reminiscent of stratified mucin-producing intraepithelial lesion (iSMILE) (C), villoglandular (D)
Usual-type: 0–50% of cells with appreciable intracytoplasmic mucin, with or without benign-appearing squamous differentiation
Villoglandular: usual-type cytomorphology with exophytic long slender papillae
Mucinous, not otherwise specified (NOS): >50% of cells with intracytoplasmic mucin in a background of usual-type
Mucinous, intestinal type: ≥50% of cells with goblet morphology in a background of usual adenocarcinoma
Mucinous, signet ring cell type: ≥50% of tumor cells with signet-ring morphology present in a background of usual adenocarcinoma
Invasive stratified mucin-producing carcinoma (iSMILE): invasive nests of stratified columnar cells with peripheral palisading and variable amounts of intracytoplasmic mucin,21 resembling its in-situ counterpart (SMILE)22
Thirty-eight tumors remained in the adenosquamous carcinoma category (unequivocal malignant glandular and squamous differentiation) and were excluded; cases retained for study were morphological mimickers of adenosquamous carcinoma, principally represented by iSMILE and usual-type adenocarcinoma with metaplastic-appearing bland squamous differentiation.
Non-HPV-associated adenocarcinoma (NHPVA)
No easily identifiable apical mitotic activity and apoptotic bodies at scanning magnification. If focal or equivocal HPVA features were appreciable at 200x, tumors were considered to show “limited HPVA” features and tentatively classified as NHPVA adenocarcinoma.
NHPVA ECAs (Figure 2) were subclassified based on established published criteria as follows:
Figure 2.
HPV-unassociated adenocarcinomas (NHPVA): Gastric-type (A), clear cell (B), endometrioid (C), serous (D)
Endometrioid adenocarcinoma: endometrioid morphology with “confirmatory endometrioid features” (at least focally identified low-grade endometrioid glands lined by columnar cells, with pseudostratified nuclei demonstrating no more than moderate atypia, with or without squamous differentiation and/or endometriosis present) and lacking HPVA features.17
Gastric-type adenocarcinoma: tumor contains cells with abundant clear, foamy or pale eosinophilic cytoplasm, distinct cytoplasmic borders, generally low nuclear-cytoplasmic ratios and irregular basally-located nuclei, with no or limited HPVA-like features. Minimal deviation adenocarcinoma of mucinous type was considered part of the spectrum of gastric-type adenocarcinoma.6,7 Intestinal differentiation in the form of goblet cells and neuroendocrine-like eosinophilic granular cytoplasm was permitted.
Serous adenocarcinoma: papillary and/or micropapillary architecture with cells showing diffusely distributed, highly atypical nuclei in stratified and pseudostratified cells with relative lack of intercellular adhesion. Mimics, such as drop metastases as well as other carcinomas with papillary architecture including usual-type, clear cell and mesonephric carcinoma were excluded
Clear cell adenocarcinoma: solid, papillary and/or tubulocystic architecture with polygonal cells and highly atypical, but uniform, nuclei.
Mesonephric carcinoma: tumors showing an admixture of growth patterns (ductal, tubular, papillary, cord-like and others), as well as intraluminal eosinophilic colloid-like material resembling mesonephric remnants.
Invasive adenocarcinoma NOS: any tumor that could not be classified by WHO or IECC criteria.
Tissue microarray construction and immunohistochemical study
Tissue microarrays (TMA) were constructed using previously described methods,23,24 and included 297 cases from New York, Boston, Mexico, Japan and Romania, to perform p16, p53, progesterone receptor (PR) and vimentin (Table 1). Briefly, each tumor was represented by three 0.6 mm cores. p16 was interpreted as positive if diffuse, block-like staining was found in all cores; whereas no staining, or patchy staining, was interpreted as negative. p53 was scored as positive if ≥75% of tumor cell nuclei were strongly positive or if no staining was present in a background of an intact internal control. PR was interpreted as positive if ≥1% of tumor cell nuclei were positive. Vimentin was scored as positive if ≥50% of tumor cells showed membranous/cytoplasmic staining.
Table 1.
Immunohistochemical antibodies
| Antibody | CLONE | VENDOR | Instrument |
|---|---|---|---|
| Vimentin | V9 | Roche | Roche Benchmark Ultra |
| p53 | D07 | Roche | Roche Benchmark Ultra |
| p16 | E6H4 | Roche | Roche Benchmark Ultra |
| PR | 16 | Leica Biosystems (Novocastra) | Leica Bond III |
HPV detection
HPV detection for high-risk HPV subtypes (Figure 3) was performed on all ECAs in the TMA that had sufficient tissue to score (n=268). HPV in-situ hybridization with a chromogen was performed using the Advanced Cell Diagnostics (ACD) (Hayward, CA) RNAscope® system (catalogue no.312598). The RNAscope® Probe “HPV HR18” contains probes targeting E6 and E7 mRNA for the following high-risk subtypes: HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82. The methodology is found in Table 2. Tumors known to contain HR-HPV were used as positive controls, while those known to be HR-HPV negative were used as negative controls during assay optimization. Subsequently, a negative control slide lacking application of the probes was prepared and examined, particularly when adjudicating cases with rare or equivocal signals. A full range of cytoplasmic and nuclear signals were encountered, as described by Evans, et al.25
Figure 3.
HPV-positivity in a usual type adenocarcinoma using the ACD RNAscope® Probe HPV HR18. This recognizes 18 types of high-risk HPV
Table 2.
RNAscope® HPV HR18 methodology
| Processing Instrument | LEICA BOND | ||
|---|---|---|---|
| ANTIGEN RETRIEVAL | Leica Epitope Retrieval 2* 20 minutes | ||
| ANTIGEN RETRIEVAL | ACD RNAscope® Protease 15 minutes | ||
| Probe CONCENTRATION | HPV HR18 Ready To Use | ||
| Probe INCUBATION (Hybridization) TIME | Hybridization 2 hours | ||
| Probe INCUB. (Hyb) TEMP | Ambient | ||
| DETECTION KIT | BOND RNAscope® Detection Reagents Brown | VENDOR: Leica | CATALOG #: DS9790 |
(Catalog No: AR9640 Bond Epitope Retrieval Solution 2 contains an EDTA based buffer and surfactant pH 8.9–9.1).
RNA-based in-situ hybridization probes recognize not only nuclear HPV, but also that which is present in cytoplasm. The presence of only rare signals (dots) confined to tumor cells is sufficient to score a case as HPV positive, as long as the corresponding negative control shows no dots.
Statistical analysis
We used standard statistical methods, including analysis of variance, using the statistical package program STATA 13 (StataCorp, College Station, TX, USA).
Tumors from one of the sites were excluded from calculations of sensitivity, specificity, and positive and negative predictive values, comparing the performance of p16 immunohistochemistry (IHC) and HPV ISH since only approximately 50% of IECC usual ECAs were found to be p16 or HPV+ in samples from this site.
Results
Clinical and demographic information based on the IECC classification is shown in Table 3. The median age of patients with HPVAs was 42 years, compared to 55 years for patients with NHPVAs (p<0.001). There were no statistically significant differences in the age of patients with different subtypes of HPVA ECAs. The median size of HPVAs was 21 mm, compared to 38 mm in NHPVAs (p<0.001). Among HPVA tumors, usual-type ECAs tended to be larger than mucinous adenocarcinoma inclusive of all subcategories (23 mm versus 9 mm; p<0.001). Fifty-one percent of gastric-type ECAs presented at FIGO stage II or higher, unlike other tumor types.
Table 3.
Clinical and demographic information of IECC-classified cases
NHPVA tumors were larger and occurred in significantly older patients than HPVA tumors (p<0.001 for both measures).
Abbreviation: IQR, interquartile range (25th – 75th percentile).
The most common IECC diagnoses, exclusive of adenosquamous carcinoma, were usual-type (73% of cohort), gastric-type (10%), mucinous adenocarcinoma of HPVA type, including intestinal, mucinous NOS, signet-ring and invasive stratified mucin-producing carcinoma categories (9%), clear cell carcinoma (3%) and adenocarcinoma, NOS (2%) (Table 4). A summary of the frequencies of the different ECA subtypes based on the WHO classification systems can be found in Supplemental Table 1.
Table 4.
ECA tumor types, IECC classifications*
| IECC (%)** N=371 | |
|---|---|
|
| |
| USUAL | 74 |
| USUAL-TYPE | 73 |
| VILLOGLANDULAR | 0.8 |
|
| |
| GASTRIC(MUCINOUS NHPVA) | 10 |
|
| |
| MUCINOUS HPVA | 8.6 |
| INTESTINAL | 3.0 |
| MUCINOUS NOS | 3.0 |
| iSMILE | 2.4 |
| SIGNET RING | 0.3 |
|
| |
| CLEAR CELL | 3.0 |
|
| |
| ADENOCARCINOMA, NOS | 2.4 |
|
| |
| ENDOMETRIOID | 1.1 |
|
| |
| SEROUS | 0.5 |
|
| |
| MESONEPHRIC | 0.3 |
Entities are listed in descending order of prevalence according to the IECC classification scheme.
Adenosquamous carcinomas are not tabulated, as they are not ECAs, but they represented 9.3% (N=38) of carcinomas reviewed
Supplemental Table 2 summarizes the distribution and types of precursor lesions, stratified by IECC tumor type. Seventy-two percent of HPVA IECC adenocarcinomas contained an HPV-associated precursor lesion, such as adenocarcinoma in situ, stratified mucin-producing intraepithelial lesion, high-grade squamous intraepithelial lesion (HSIL) or combinations thereof. In contrast, only 7 NHPVA adenocarcinomas (14%), including 6 gastric-types and one endometrioid carcinoma contained an HPV-associated precursor lesion (HSIL). Six further IECC gastric-type carcinomas (19%) exhibited gastric-type adenocarcinoma in-situ,26 one of which arose in association with lobular endocervical glandular hyperplasia. Gastric-type adenocarcinoma in-situ was not associated with other tumor types studied.
Only 3 endometrioid carcinomas were identified by IECC, and all were HPV-negative. Comparison of endometrioid carcinomas reclassified as usual-type by IECC, and all usual-type ECAs classified by IECC revealed similar results for p16 overexpression and HPV positivity: 77.9 and 83.3% of cases were p16-positive, respectively; 82.1 and 86.7% were HPV-positive, respectively.
Table 5 presents p16, p53, PR, vimentin, and HPV data for all IECC-classified ECAs, inclusive of cases known to have been processed suboptimally. Seventy-six percent of carcinomas classified as usual-type by the IECC were p16 and HPV-positive (Figure 4), 4.7% were p16-positive/HPV-negative (Figure 5), 11.5% were p16-negative/HPV-positive (Figure 5), and 7.4% were negative for both.
Table 5.
Immunophenotype status of IECC-classified cases*
The number of evaluable TMA cores and cases varied from slide to slide. Cases from one center were not tested for HPV (n=26).
“Selected cohort” lacks data from one center whose IECC usual-type ECAs were only approximately 50% positive for p16 and HPV (n=36).
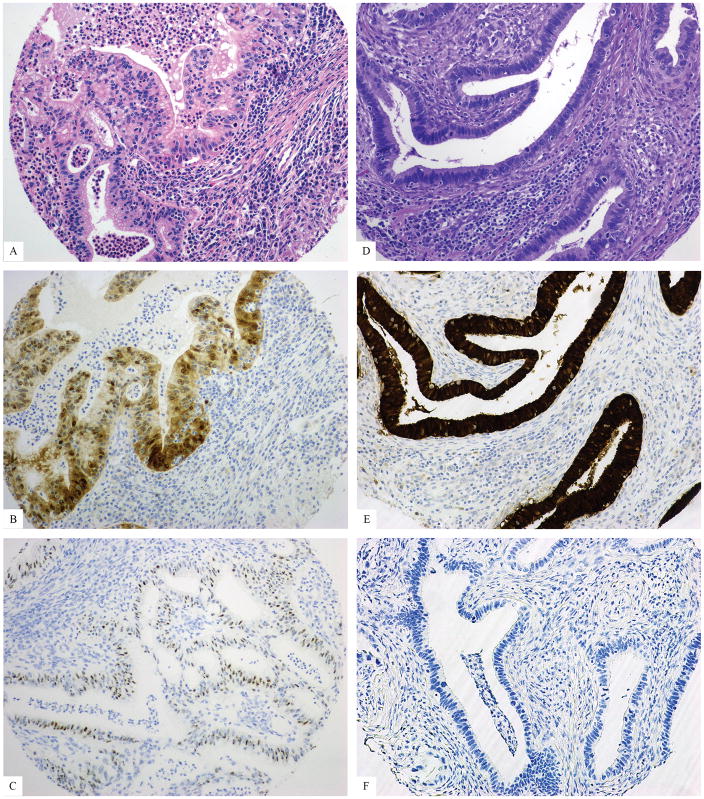
Figure 4.
Usual-type adenocarcinoma with both p16 and HPV positivity: H&E (A), p16 (B), HPV (C)
Figure 5.
Usual-type adenocarcinomas with either p16 or HPV positivity: H&E (A), patchy p16 (B), positive HPV (C), H&E (D), block-like p16 (E), negative HPV (F)
Mucinous ECAs comprise a spectrum of HPVA (NOS, intestinal and signet-ring) and non-HPVA (gastric) tumor subtypes. All the IECC mucinous NOS and intestinal adenocarcinomas were HPV-positive, with 100% of NOS cases and 3 of 4 intestinal cases being p16-positive. The one signet ring cell-type mucinous adenocarcinoma was not included in the TMA. In contrast, only 33% of gastric-type carcinomas were p16-positive; only 1 of 23 was HPV-positive on initial review (Figure 6). This case, a presumptive gastric-type with “limited HPVA features,” as defined in the methods, was both p16- and HPV-positive, and was subsequently re-classified as mucinous adenocarcinoma, HPVA type.
Figure 6.
Unusual gastric-type adenocarcinomas: H&E (A), block-like p16; note presence of goblet cells (B); negative HPV (C); H&E (D); block-like p16 (E); positive HPV (F). The latter case, represented in figures D–F, was reclassified as usual-type adenocarcinoma with a gastric-like appearance on H&E
The ACD RNAscope® HPV HR18 probe set had superior sensitivity, specificity, and positive and negative predictive values (0.955, 0.968, 0.992, 0.833, respectively) compared to p16 immunohistochemistry (0.872, 0.632, 0.907, 0.545, respectively) to identify HPVA usual-type and mucinous carcinomas when excluding cases from one institution that reported suboptimal tissue processing. The latter were excluded from these calculations since only approximately 50% of IECC usual type carcinomas were found to be p16 or HPV+ in samples from this site. Some tissues from this site had been subjected to intense heat in order to mitigate a problem with paraffin quality during a specific time period, a problem that was only identified when comparing differences in results across all tumors. However, even when including such cases, calculations still favored the use of the ACD RNAscope® HPV HR18 probe set over p16. Sensitivity, specificity, and positive and negative predictive values using ISH were 0.879, 0.970, 0.993 and 0.615, respectively, compared to p16 immunohistochemistry (0.817, 0.625, 0.910, 0.424, respectively).
Discussion
This is one of the largest series reporting clinical and demographic information for pathologically well-annotated invasive ECAs and is the largest series reporting p16 expression and HR-HPV ISH using a new RNA ISH assay that recognizes 18 high-risk HPV types. The most important results were as follows: 1) IECC segregates ECAs into HPVA and NHPVA using morphology alone - both categories can be further stratified using existing morphologic criteria; 2) the validity of the HPVA and NHPVA categories is supported by p16 immunophenotype and HPV status; 3) the high-risk HPV probe set is more sensitive and specific than p16 in recognizing usual-type ECA; 4) optimal tissue processing is essential for performance of this assay and p16; 5) HPVA and NHPVA categories differ significantly with respect to age, stage and tumor size; 6) usual-type ECAs are the most common type of ECA worldwide; 7) mucinous carcinomas comprise a mixture of HPVA and NHPVA types, with gastric-type carcinoma being the major NHPVA type; 8) gastric-type carcinoma is the second most common type of ECA, comprising 10% of cases in this international cohort; 9) primary endometrioid and serous carcinomas of the endocervix are extraordinarily rare and, in the case of serous carcinoma, possibly non-existent.
Ninety-three percent of IECC usual-type ECAs collected from all centers before excluding suboptimally fixed cases overexpressed p16 or were HPV-positive by ISH, and the vast majority (76%) showed both p16 overexpression and HPV positivity, validating the IECC criteria. A recent large and comprehensive clinico-pathological study of ECAs27 reported similar results by detecting HPV on whole-tissue sections by laser-capture microdissection and L1-based broad-spectrum SPF10-DEIA-LiPA25 PCR,27 which cannot be easily translated for diagnostic use. Applying IECC criteria with or without HPV ISH is more feasible for clinical practice.
The ACD RNAscope® HPV HR18 probe set performs more robustly than p16 IHC for the identification of HPVAs, with ISH distinguishing between HPV-positive/p16-positive HPVAs and HPV-negative/p16-positive NHPVAs, while recognizing HPV-positive/p16-negative HPVAs as well. Despite this, occasional p16 and/or HPV-negative HPVAs were identified. When outlying cases were excluded from statistical analysis, as summarized in the results, 20 of 156 HPVAs and 6 of 134 HPVAs were p16- and HPV-negative, respectively. All HPV-negative usual-type ECAs in this study were confirmed as usual-type on re-review. While rare p16-negative/HPV-negative usual-type ECAs may represent unusual morphological variants of gastric-type carcinoma,28 all 10 p16-negative/HPV-negative usual-type ECAs were negative for HIK1083 (data not shown), making it improbable that these tumors were, in fact, of gastric-type. Another theoretical consideration is that rare HPV types not included in the RNA ISH probe set could be responsible for negative HPV results. There are precedents in the literature for p16-negative, HPV-associated neoplasia. p16-negative invasive squamous carcinomas of the cervix have been reported,29 as has methylation-induced inactivation of the p16 gene30,31 and allelic loss of p16.31 There are also data reporting that the HPV genome can be differentially expressed in primary tumors, compared to metastases, and within different disease sites, suggesting that some of our “HPV-negative” usual-type adenocarcinomas might have had detectable HPV had multiple sections from the primary carcinoma and any metastastic site been tested.27,32–34
Specific diagnostic criteria for primary endometrioid ECA are lacking in the literature, which has led to discrepancies in its frequency,4 ranging from ~7% to more than 50% among invasive endocervical adenocarcinomas.5, 35–37 Furthermore, rare endocervical endometrioid adenocarcinomas have been reported to arise in association with cervical endometriosis.38,39 Using our interpretation of the WHO criteria, a plurality of tumors in this series would have been diagnosed as endometrioid ECA (41%) (see Supplemental methodology and data); however, by applying IECC criteria and identifying HPVA features in almost every WHO endometrioid adenocarcinoma in this series, only 3 remained in this category. Two of these cases were p16-positive—owing, at least theoretically, to their high-grade histology—but all were HPV-negative (Figure 7). Therefore, endometrioid adenocarcinoma of the cervix, a rare NHPVA type, should not be reflexively diagnosed when evaluating a mucin-poor ECA. In order to accurately diagnose endometrioid ECA, one must be certain that confirmatory endometrioid features are present, as specified in the Methods section. Lastly, origin in the uterine corpus and lower uterine segment must be rigorously excluded. Given the differential diagnosis of usual-type HPVA and endometrioid adenocarcinoma, a positive HPV assay would confirm the former diagnosis in the appropriate context. The differential diagnosis of endometrioid adenocarcinoma also includes mesonephric carcinoma, another HPV-negative tumor, which is characterized by an admixture of growth patterns (ductal, tubular, papillary, cord-like, spindled and others), as well as intraluminal eosinophilic colloid-like material resembling mesonephric remnants.40 Although careful morphological study will usually distinguish between these entities, GATA-3 and TTF-1 immunohistochemistry can be diagnostically helpful, since they are frequently expressed in mesonephric carcinomas and only rarely in endometrioid carcinomas.41
Figure 7.
Endometrioid adenocarcinoma. This rare case (A) was negative for HPV (B), as expected.
Mucinous ECAs represent a spectrum of tumors, the majority being NHPVA/gastric-type. Despite this, 33% of our gastric-type cases were p16-positive in contrast to only 4/18 gastric-type ECAs in a prior study.42 Different methodologies may account for this discordance. However, all unequivocal examples of gastric -type ECAs in this study were HPV-negative, providing further evidence that these tumors are, indeed, HPV-unassociated carcinomas.7,43–46 Herein, we also report one case that resembled gastric-type ECA on scanning magnification, but which focally exhibited apparent mitotic activity and scattered apoptotic bodies on high-power examination. This case, showing “limited HPVA features,” was both p16- and HPV-positive. The most likely explanation is that this was a mucinous HPVA with cytoplasmic features that suggested gastric-type differentiation (a recently reported phenomenon),28 or part of a mixed carcinoma, with one component being HPV-positive.47 An alternative explanation is based on the theoretical coincidence of GNAS mutation with HPV infection, with GNAS underpinning the development of some gastric-type tumors in both the endocervix and extra-gynecologic sites.48 It is of interest that 19% of IECC gastric-type carcinomas studied here harbored HSIL despite the absence of identifiable HPV in the invasive adenocarcinoma. An additional 19% contained gastric-type adenocarcinoma in situ,26 some of which demonstrated intestinal differentiation.49 It should be emphasized that intestinal differentiation, predominantly but not exclusively in the form of goblet cells, can be observed in in-situ and invasive adenocarcinomas of both HPVA and NHPVA types, and is therefore not diagnostically informative.
In contrast to gastric-type ECA, all mucinous NOS and intestinal-type HPVAs were HPV-positive. This concurs with most publications reporting that mucinous NOS, signet-ring and intestinal-type ECAs are usually HPV-related.50 In the current study, a review of cases originally thought to represent adenosquamous carcinomas identified 21 with a partial (n=12) or complete (n=9) “invasive SMILE” (iSMILE) appearance consisting of invasive nests of stratified columnar cells with peripheral palisading and variable amounts of intracytoplasmic mucin,21 resembling stratified mucin-producing intraepithelial lesion (SMILE).22 All tested iSMILE carcinomas in our study were HPV-positive, which is consistent with published results.51 With the possible exception of smaller tumor sizes, mucinous HPVAs and usual-type HPVAs are similar from a demographic and immunophenotypic viewpoint. We would consider mucinous HPVAs to be merely morphologic variants of usual-type ECA if a more detailed clinical and genomic analysis confirmed similarity. This would be comparable with the increasing tendency to diagnose mucinous carcinomas of the endometrium as endometrioid carcinomas with extensive mucinous differentiation.
Non-mucinous, non-endometrioid NHPVAs are rare (Table 5). All 6 clear cell carcinomas were HPV-negative, 17% overexpressed p16, and 14% had an aberrant p53 staining pattern, similar to published reports.7,43,45,46,52 One p16-positive mesonephric carcinoma was found in this cohort, but insufficient material was available for HPV analysis. All such cases reported to date have been HPV-negative.7,45,53 Two tumors morphologically resembled serous carcinoma, but both were p53 wild-type and the one case evaluated for HPV was negative. The literature presents conflicting data with respect to the existence of true serous carcinomas of the endocervix, their immunophenotype, and the association with HPV infection.27,43,45,54–59 It has been suggested that the bimodal age distribution of cases historically diagnosed as endocervical serous carcinoma may be due to HPVA in young patients and drop metastasis from bona fide serous carcinomas in older patients.54,55,60 Neither of the cases we diagnosed as serous carcinoma was, in fact, a serous carcinoma from an immunohistochemical perspective. Review of our data does not confirm the existence of primary endocervical serous carcinoma. One must remain aware of drop metastasis and direct extension of serous carcinoma from the adnexa or corpus and the occasional serous-like appearance of microsatellite-unstable endometrioid carcinoma extending from lower uterine segment to cervix, clear cell carcinoma and mesonephric carcinoma.
IECC was not able to confidently classify only 2.4% of adenocarcinomas (i.e. adenocarcinomas NOS). Immunophenotype and HPV status indicate that this provisional category contains HPVAs and NHPVAs. RNA HPV-ISH with or without p16 can therefore be used to distinguish the two in practice.
There are both practical and theoretical reasons to establish a classification based on the presence of HPV infection. From a practical standpoint, the RNA HPV assay can be used to rule in HPV-associated ECAs and rule out NHPVAs and HPV-unassociated carcinomas of the lower uterine segment, uterine corpus, adnexa and non-gynecologic sites of origin, regardless of p16 status. It is well known that a variety of high-grade endometrial carcinomas, endocervical clear cell carcinomas and rare gastric-type carcinomas may show p16 overexpression without HPV infection.27,43,61,42 Theoretically, a classification system based on linking H&E features to HPV status would have therapeutic, predictive and prognostic implications. Although treatment algorithms incorporating HPV status do not yet exist for ECAs, partly because of the mistaken impression that all ECAs are HPV-related, there is a precedent for this in other organ systems, most notably the head and neck.12,13,62 A comparison of clinical outcomes between HPVA and gastric-type ECA indicates that this type of NHPVA is significantly more aggressive.6,8,9
Conclusions
The IECC segregates ECAs into different pathogenetic categories, all of which can be recognized on H&E slides, as summarized in Figure 8. Immunophenotype and HPV status are concordant with morphology. Further clinical outcomes and genomic studies should be performed to validate the present findings, but based on these results, we recommend replacing the WHO 2014 criteria with IECC 2017.
Figure 8.
IECC Classification 2017
1: HPV associated adenocarcinomas encompass those with and without intracytoplasmic or stromal mucin. Examples with mucinous differentiation may contain glands, goblet cells, signet ring cells or solid nests of tumor cells with intracytoplasmic mucin (i.e. iSMILE). Further work is needed to determine whether these histological variants are biologically and clinically distinctive. The designation “usual-type” HPV-associated endocervical adenocarcinoma can be used for mucin-poor tumors, whereas the designation “mucinous” HPV-associated endocervical adenocarcinoma can be used for cases with obvious intracytoplasmic mucin.
2: Gastric-type adenocarcinoma may contain goblet cells
3: It is uncertain whether true serous carcinomas arise in the endocervix. Together with the HPV-unassociated NOS category (0.8–1.6%), miscellaneous tumors represent at most 2% of all endocervical adenocarcinomas.
Supplementary Material
Acknowledgments
Robert J. Monroe, MD, PhD, Advanced Cell Diagnostics; Adam Kuten, MD, University of Washington Medical Center; Luis Chiriboga, MD, PhD, Department of Pathology, New York University; Carlos Parra-Herran, MD, Sunnybrook Health Sciences Centre, Toronto; Sarah Chiang, MD, Gulisa Turashvili, MD, and Rene Serrette, Department of Pathology, Memorial Sloan Kettering Cancer Center; Jenifer Levin, Editor, GYN Academic Office, Memorial Sloan Kettering Cancer Center
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748 (Dr. Soslow, Dr. Park and Dr. Pike)
Footnotes
Conflicts of Interest: None declared
References
- 1.Smith HO, Tiffany MF, Qualls CR, et al. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 2.Vesterinen E, Forss M, Nieminen U. Increase of cervical adenocarcinoma: a report of 520 cases of cervical carcinoma including 112 tumors with glandular elements. Gynecol Oncol. 1989;33:49–53. doi: 10.1016/0090-8258(89)90602-1. [DOI] [PubMed] [Google Scholar]
- 3.An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18:528–534. doi: 10.1038/modpathol.3800316. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of tumors of female reproductive organs. 4. IARC. WHO Press; 2014. [Google Scholar]
- 5.Young RH, Clement PB. Endocervical adenocarcinoma and its variants: their morphology and differential diagnosis. Histopathology. 2002;41:185–207. doi: 10.1046/j.1365-2559.2002.01462.x. [DOI] [PubMed] [Google Scholar]
- 6.Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 7.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocar- cinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Carunchio L, Soveral I, Steenbergen RD, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG. 2015;122:119–127. doi: 10.1111/1471-0528.13071. [DOI] [PubMed] [Google Scholar]
- 9.Karamurzin YS, Kiyoaka T, Parkash V, et al. Gastric-type endocervical adenocarcinoma. An aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol. 2015;39:1449–1457. doi: 10.1097/PAS.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong F, Kojiro S, Borger DR, et al. Squamous Cell Carcinoma of the Vulva: A Subclassification of 97 Cases by Clinicopathologic, Immunohistochemical, and Molecular Features (p16, p53, and EGFR) Am J Surg Pathol. 2015;39:1045–1053. doi: 10.1097/PAS.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 11.McAlpine JN, Leung S, Cheng A, et al. Human papillomavirus (HPV)-independent vulvar squamous cell carcinoma has a worse prognosis than HPV-associated disease: a retrospective cohort study. Histopathology. 2017;71:238–246. doi: 10.1111/his.13205. [DOI] [PubMed] [Google Scholar]
- 12.Ajila V, Shetty H, Babu S, et al. Human Papilloma Virus Associated Squamous Cell Carcinoma of the Head and Neck. J Sex Transm Dis. 2015;2015:791024. doi: 10.1155/2015/791024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedghizadeh PP, Billington WD, Paxton D, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol. 2016;54:15–27. doi: 10.1016/j.oraloncology.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Gilks CB, Ionescu DN, Kalloger SE, et al. Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39:1239–1251. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Howitt BE, Hanamornroongruang S, Lin DI, et al. Evidence for a dualistic model of high-grade serous carcinoma: BRCA mutation status, histology, and tubal intraepithelial carcinoma. Am J Surg Pathol. 2015;39:287–293. doi: 10.1097/PAS.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 16.Hussein YR, Ducie JA, Arnold AG, et al. Invasion patterns of metastatic extrauterine high-grade serous carcinoma with BRCA germline mutation and correlation with clinical outcomes. Am J Surg Pathol. 2016;40:404–409. doi: 10.1097/PAS.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim D, Murali R, Murray MP, et al. Morphological and Immunohistochemical Reevaluation of Tumors Initially Diagnosed as Ovarian Endometrioid Carcinoma With Emphasis on High-grade Tumors. Am J Surg Pathol. 2016;40:302–312. doi: 10.1097/PAS.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes MC, Arnold AG, Kauff ND, et al. Invasion patterns of metastatic high-grade serous carcinoma of ovary or fallopian tube associated with BRCA deficiency. Mod Pathol. 2014;27:1405–1411. doi: 10.1038/modpathol.2013.237. [DOI] [PubMed] [Google Scholar]
- 19.Soslow RA, Han G, Park KJ, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Gilks CB. The changing landscape of gynaecological cancer diagnosis: implications for histopathological practice in the 21st century. Histopathology. 2017;70:56–69. doi: 10.1111/his.13080. [DOI] [PubMed] [Google Scholar]
- 21.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol. 2016;40:262–269. doi: 10.1097/PAS.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 22.Park JJ, Sun D, Quade BJ, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000;24:1414–1419. doi: 10.1097/00000478-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hedvat CV, Hegde A, Chaganti RS, et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol. 2002;33:958–974. doi: 10.1053/hupa.2002.127438. [DOI] [PubMed] [Google Scholar]
- 24.Kocken M, Baalbergen A, Snijders PJ, et al. High-risk human papillomavirus seems not involved in DES-related and of limited importance in nonDES related clear-cell carcinoma of the cervix. Gynecol Oncol. 2011;122:297–302. doi: 10.1016/j.ygyno.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Evans MF, Peng Z, Clark KM, et al. HPV E6/E7 RNA in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PLoS One. 2014;9:e91142. doi: 10.1371/journal.pone.0091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talia KL, Stewart CJ, Howitt BE, et al. HPV-negative Gastric Type Adenocarcinoma In Situ of the Cervix: A Spectrum of Rare Lesions Exhibiting Gastric and Intestinal Differentiation. Am J Surg Pathol. 2017;41:1023–1033. doi: 10.1097/PAS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 27.Molijn A, Jenkins D, Chen W, et al. Chinese HPV Typing Group. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer. 2016;138:409–416. doi: 10.1002/ijc.29722. [DOI] [PubMed] [Google Scholar]
- 28.Wada T, Ohishi Y, Kaku T, et al. Endocervical Adenocarcinoma With Morphologic Features of Both Usual and Gastric Types: Clinicopathologic and Immunohistochemical Analyses and High-risk HPV Detection by In Situ Hybridization. Am J Surg Pathol. 2017;41:696–705. doi: 10.1097/PAS.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 29.Masoudi H, Van Niekerk DJ, Gilks CB, et al. Loss of p16 INK4 expression in invasive squamous cell carcinoma of the uterine cervix is an adverse prognostic marker. Histopathology. 2006;49:542–545. doi: 10.1111/j.1365-2559.2006.02510.x. [DOI] [PubMed] [Google Scholar]
- 30.Nuovo GJ, Plaia T, Belinsky SA, et al. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci U S A. 1999;96:1254–1259. doi: 10.1073/pnas.96.22.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poetsch M, Hemmerich M, Kakies C, et al. Alterations in the tumor suppressor gene p16(INK4A) are associated with aggressive behavior of penile carcinomas. Virchows Arch. 2011;458:221–229. doi: 10.1007/s00428-010-1007-4. [DOI] [PubMed] [Google Scholar]
- 32.Beyer-Finkler E, Girardi F, Sillem M, et al. Human papillomavirus DNA in genital cancers, metastases, and lymph nodes. Intervirology. 1995;38:173–180. doi: 10.1159/000150429. [DOI] [PubMed] [Google Scholar]
- 33.Ikenberg H, Teufel G, Schmitt B, et al. Human papillomavirus DNA in distant metastases of cervical cancer. Gynecol Oncol. 1993;48:56–60. doi: 10.1006/gyno.1993.1009. [DOI] [PubMed] [Google Scholar]
- 34.Liang WS, Aldrich J, Nasser S, et al. Simultaneous characterization of somatic events and HPV-18 integration in a metastatic cervical carcinoma patient using DNA and RNA sequencing. Int J Gynecol Cancer. 2014;24:329–338. doi: 10.1097/IGC.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfsen GC, Thoresen SO, Kristensen GB, et al. Histopathologic subtyping of cervical adenocarcinoma reveals increasing incidence rates of endometrioid tumors in all age groups: a population based study with review of all nonsquamous cervical carcinomas in Norway from 1966 to 1970, 1976 to 1980, and 1986 to 1990. Cancer. 2000;89:1291–1299. [PubMed] [Google Scholar]
- 36.Zaino RJ. The fruits of our labors: Distinguishing endometrial from endocervical adenocarcinomas. Int J Gynecol Pathol. 2002;21:1–3. doi: 10.1097/00004347-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Schorge JO, Lee KR, Lee SJ, et al. Early cervical adenocarcinoma: selection criteria for radical surgery. Obstet Gynecol. 1999;94:386–390. doi: 10.1016/s0029-7844(99)00312-9. [DOI] [PubMed] [Google Scholar]
- 38.Chang SH, Maddox WA. Adenocarcinoma arising within cervical endometriosis and invading the adjacent vagina. Am J Obstet Gynecol. 1971;110:1015–1017. doi: 10.1016/0002-9378(71)90559-x. [DOI] [PubMed] [Google Scholar]
- 39.Hirschowitz L, Sen C, Murdoch J. Primary endometrioid adenocarcinoma of the cervix with widespread squamous metaplasia--a potential diagnostic pitfall. Diagn Pathol. 2007;2:40. doi: 10.1186/1746-1596-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferry JA, Scully RE. Mesonephric remnants, hyperplasia, and neoplasia in the uterine cervix. A study of 49 cases. Am J Surg Pathol. 1990;14:1100–1111. doi: 10.1097/00000478-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Roma AA, Goyal A, Yang B. Differential Expression Patterns of GATA3 in Uterine Mesonephric and Nonmesonephric Lesions. Int J Gynecol Pathol. 2015;34:480–486. doi: 10.1097/PGP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 42.Carleton C, Hoang L, Sah S, et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 2016;40:636–644. doi: 10.1097/PAS.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houghton O, Jamosin J, Wilson R, et al. p16 Immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342–350. doi: 10.1111/j.1365-2559.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 44.Kusanagi Y, Kojima A, Mikami Y, et al. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. doi: 10.2353/ajpath.2010.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirog EC, Kleter B, Olgac S, et al. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcino. Am J Pathol. 2000;157:1055–1062. doi: 10.1016/S0002-9440(10)64619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pirog EC, Lloveras B, Molijn A, et al. RIS HPV TT study group. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–1567. doi: 10.1038/modpathol.2014.55. [DOI] [PubMed] [Google Scholar]
- 47.Stewart CJ, Frost F, Leake R, et al. Foamy gland changes in gastric-type endocervical neoplasia. Pathology. 2015;47:653–658. doi: 10.1097/PAT.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 48.Matsubara A, Sekine S, Ogawa R, et al. Lobular endocervical glandular hyperplasia is a neoplastic entity with frequent activating GNAS mutations. Am J Surg Pathol. 2014;38:370–376. doi: 10.1097/PAS.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 49.Howitt BE, Herfs M, Brister K, et al. Intestinal-type endocervical adenocarcinoma in situ: an immunophenotypically distinct subset of AIS affecting older women. Am J Surg Pathol. 2013;37:625–633. doi: 10.1097/PAS.0b013e318285be00. [DOI] [PubMed] [Google Scholar]
- 50.Balci S, Saglam A, Usubutun A. Primary signet-ring cell carcinoma of the cervix: case report and review of the literature. Int J Gynecol Pathol. 2010;29:181–184. doi: 10.1097/PGP.0b013e3181b70176. [DOI] [PubMed] [Google Scholar]
- 51.Onishi J, Sato Y, Sawaguchi A, et al. Stratified mucin-producing intraepithelial lesion with invasive carcinoma: 12 cases with immunohistochemical and ultrastructural findings. Hum Pathol. 2016;55:174–181. doi: 10.1016/j.humpath.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 52.McCluggage WG. Recent Developments in Non-HPV-related Adenocarcinomas of the Lower Female Genital Tract and Their Precursors. Adv Anat Pathol. 2016;23:58–69. doi: 10.1097/PAP.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 53.Kenny SL, McBride HA, Jamison J, et al. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- 54.Domfeh AB, Kuhn E, Park K, et al. Abstract 1131: Papillary serous carcinoma of the cervix - two diseases with distinct clinico-pathologic profiles? Mod Pathol. 2013;26:272A. [Google Scholar]
- 55.Hong X, Gilks B, Crum CP, et al. Papillary serous carcioma of the uterine cervix is HPV and P16 positive. Mod Pathol. 2007;20:201A. [Google Scholar]
- 56.Nofech-Mozes S, Khalifa M, Ismiil N, et al. Detection of HPV-DNA by a PCR-based method in ormalin-fixed paraffin-embeded tissue from are endocervical carcinoma types. Appl Immunohistochem Mol Morphol. 2010;18:80–85. doi: 10.1097/PAI.0b013e3181ae7240. [DOI] [PubMed] [Google Scholar]
- 57.Togami S, Sasajima Y, Kasamatsu T, et al. Immunophenotype and human papillomavirusstatus of serous adenocarcinoma of the uterine cervix. Pathol Oncol Res. 2015;21:487–494. doi: 10.1007/s12253-014-9854-y. [DOI] [PubMed] [Google Scholar]
- 58.Watrowski R, Striepecke E, Jäger C, et al. Papillary-serous adenocarcinoma of the uterine cervix during tamoxifen therapy after bilateral breast cancer. Anticancer Res. 2012;32:5075–5078. [PubMed] [Google Scholar]
- 59.Zhou C, Gilks CB, Hayes M, et al. Papillary serous carcinoma of the uterine cervix: a clinicopathologic study of 17 cases. Am J Surg Pathol. 1998;22:113–120. doi: 10.1097/00000478-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 60.McCluggage WG, Hurrell DP, Kennedy K. Metastatic carcinomas in the cervix mimicking primary cervical adenocarcinoma and adenocarcinoma in situ: report of a series of cases. Am J Surg Pathol. 2010;34:735–741. doi: 10.1097/PAS.0b013e3181d6b8fd. [DOI] [PubMed] [Google Scholar]
- 61.Reid-Nicholson M, Iyengar P, Hummer AJ, et al. Immunophenotypic diversity of endometrial adenocarcinomas: implications for differential diagnosis. Mod Pathol. 2006;19:1091–1100. doi: 10.1038/modpathol.3800620. [DOI] [PubMed] [Google Scholar]
- 62.Nelson HH, Pawlita M, Michaud DS, et al. Immune Response to HPV16 E6 and E7 Proteins and Patient Outcomes in Head and Neck Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.4500. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.