Abstract
Belatacept, a T cell costimulation-blocker demonstrated superior renal function, lower cardiovascular risk, and improved graft/patient survival in renal transplant recipients. Despite the potential benefits, adoption of belatacept has been limited in part due to concerns regarding higher rates and grades of acute rejection in clinical trials. Since July 2011 we have utilized belatacept-based immunosuppression regimens in clinical practice. In this retrospective analysis of 745 patients undergoing renal transplantation at our center, we compared patients treated with belatacept (n=535) to a historical cohort receiving a tacrolimus-based protocol (n=205). Patient and graft survival were equivalent between all groups. An increased rate of acute rejection was observed in an initial cohort treated with a protocol similar to the low-intensity regimen from the BENEFIT trial vs the historical tacrolimus group (50.5% vs 20.5%). The addition of a transient course tacrolimus reduced rejection rates to acceptable levels (16%). Treatment with belatacept was associated with superior eGFR (belatacept 63.8ml/min vs tacrolimus 46.2ml/min at 4 years, p<0.0001). There were no differences in serious infections including rates of CMV or BK viremia. We describe the development of a costimulatory blockade-based strategy that ultimately allows renal transplant recipients to achieve CNI-free immunosuppression.
Introduction
Kidney transplantation improves the length and quality of life in patients with end-stage renal disease.(1, 2) Increasingly effective immunosuppressive regimens have resulted in significant improvements in short-term results over the last 30 years. Unfortunately late outcomes remain less than optimal with the majority of kidneys eventually failing and many patients returning to dialysis within 10 years.(3–5) Several mechanisms significantly contribute to late allograft loss including medication non-adherence, the development of donor specific antibodies, chronic injury due to calcineurin inhibitor toxicity, and recurrent disease.(6, 7) In addition to nephrotoxicity, current immunosuppressants are known to predispose patients to higher rates of diabetes, hypertension, and hyperlipidemia, contributing to accelerated rates cardiovascular disease and higher overall mortality compared to the general population.(8, 9)
Belatacept, a high affinity derivative of the receptor fusion protein CTLA-4-Ig, blocks signaling through CD28 by binding to its ligands CD80 and CD86, thereby inhibiting T cell activation and effector function without many of the detrimental side effects seen with conventional immunosuppression.(10) A large, multi-center, randomized phase III trial, the Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppresion Trial (BENEFIT), evaluated the efficacy and safety of belatacept as compared to cyclosporine in renal transplant recipients.(11) Belatacept treatment was associated with significantly better renal function as well as an improved metabolic profile presumably translating into decreased cardiovascular risk. (12) Outcomes at seven years confirmed a sustained improvement in renal function in belatacept treated patients as well as a 43% risk reduction for the combined endpoint of graft loss and death. (13) Interestingly, belatacept treatment was associated with higher rates and grades of early rejection. Despite the observed benefits of superior renal function, reduced cardiovascular risk, and increased survival, a combination of logistical considerations and concerns about increased rates of rejection may limit widespread adoption of belatacept as a primary therapy.
As with most clinical trials, the BENEFIT trial was limited by exclusion criteria that remove certain patient populations from consideration, some of whom significantly contribute to the general recipient pool. Those with higher immunologic risk such as re-transplants or high panel reactive antibody levels as well as those from a more ethnically diverse population were underrepresented in the trial. In addition the immunosuppressive regimen utilized in the trial over 10 years ago may not reflect currently employed immunosuppressive regimens, i.e. cyclosporine instead of tacrolimus in the control group as well as non-prescribed steroid treatment which resulted in increased dosage and duration of the steroid taper compared to currently utilized regimens where minimization is routine. This seems to be supported by the results of the BENEFIT trial where the cyclosporine control arm had a notably low rejection rate in the first year (~7%). (11) Thus it is important to evaluate and report the outcomes of belatacept-based regimens in clinical practice.
The purpose of this report was to detail our experience in the iterative development of a belatacept-based standard of care immunosuppressive regimen among a large, diverse cohort of renal transplant recipients and describe the effectiveness of belatacept- vs. tacrolimus-based regimens on renal function, incidence of acute rejection, infection, donor specific antibody development, graft failure, and mortality.
Materials and Methods
Patient Population
We retrospectively reviewed the outcomes of 745 adult renal transplants performed at our institution between May 1, 2010 and February 28, 2015. All patients undergoing renal transplant, either living or deceased donor, were evaluated for belatacept treatment. Those who were Epstein barr virus (EBV) seronegative, HIV+, had a history of post-transplant lymphoproliferative disease (PTLD), lymphoma or other hematologic malignancy, were undergoing treatment for latent TB or were the recipient of a simultaneous extra-renal organ were excluded.
For the first part of the review period, May 1, 2010 to July 2011, a tacrolimus-based immunosuppression regimen was the standard of care at our center. 229 patients underwent renal transplant during this period of which 205 received the standard tacrolimus regimen. These 205 patients were used as the tacrolimus control/comparator group for this retrospective study (Tac, Figure 1). The remaining 24 patients were enrolled in clinical trials.
Figure 1. Treatment Groups.

Description of the various belatacept-based immunosuppression regimens. Included are the reasons for discontinuation of belatacept therapy as well as assessment of follow-up. The final boxes in each column represent the number of patients who remained on treatment and were available for analysis at the latest time point for each group. These numbers include those who experienced graft loss or death but whose data was included in the analysis with imputation.
Following FDA approval of belatacept in June 2011 we transitioned our standard of care immunosuppression regimen from a tacrolimus-based regimen to a belatacept-based regimen. From July 2011 to February 28, 2015 an additional 728 patients received a kidney transplant alone at our center. 540 (74%) of these patients received a belatacept-based immunosuppression regimen. There were various reasons why the other 188 patients did not receive belatacept treatment during this time. These include- 118/188 were enrolled in a clinical trial using a different immunosuppression regimen, 25/188 were EBV seronegative precluding belatacept use per label, 14/188 patients were HIV+, and 31/188 patients were in the other category which included history of hematologic malignancy, language barriers, transportation/distance issues that would potentially include difficulties in returning to the transplant center for infusions long-term. This retrospective review was approved by the Institutional Review Board at Emory University (#00059283).
Immunosuppression and Treatment Regimens
The standard tacrolimus-based regimen (Tac) also included anti-IL-2R induction (basiliximab 20mg iv on days 0 and 3 or 4), mycophenolate mofetil (MMF, 1g twice daily) and a short steroid taper (methylprednisolone 500mg iv intra-operatively, 250 mg iv d1, 125mg iv d2, and prednisone 5mg d3 and daily thereafter). The initial belatacept regimen (Bela, Figure 1) followed the package insert including intravenous infusion of belatacept (10mg/kg) during surgery and on post-transplant days 4, 14, 28, 56, and 84 with subsequent doses (5mg/kg) given every 4 weeks thereafter. Patients also received basiliximab induction, MMF and steroid as described above in the standard tacrolimus control regimen (except for BelaB 20mg d3–28, 15mg d29–35, 10mg d36–42, 5mg daily thereafter). Details of the combined belatacept/tacrolimus regimens are contained in figures 1 and 2 and are similar to a regimen that has been previously reported.(14) In short patients receiving Bela/TacShort regimen were treated with a modified belatacept regimen which eliminated doses at days 4 and 14. They also received tacrolimus twice daily to achieve trough doses 8-12ng/ml from the day of transplant through the first three months and then tapered over the next two months to end by 5 months post-transplant. Patients receiving Bela/TacLong regimens were treated with the same modified belatacept dosing regimen described above and received tacrolimus twice daily for the first 9 months post transplant and then tapered to end dosing after month 11 post-transplant. Bela/TacLong(A) trough level targets months 0–3 (8–12ng/ml), months 4–6 (5–8ng/ml), months 7–9 (3–5ng/ml), then tapered months 10–11. Bela/TacLong(B) months 0–1 (8–12ng/ml), months 2–6 (5-8ng/ml), months 7–9 (3–5ng/ml), then tapered months 10–11.
Figure 2. Immunosuppression Protocols.

Belatacept dosing and tacrolimus trough targets as well as timing of tacrolimus discontinuation in the various immunosuppression protocols.
All patients received PCP prophylaxis with daily bactrim or equivalent. CMV prophylaxis was determined based on the serologic status of the donor and recipient. All patients who were CMV IgG+ prior to transplant were treated with valganciclovir daily for 3 months with dosing adjusted for renal function (eGFR>60, 450mg daily). Patients who were CMV IgG− and received a kidney from a CMV IgG+ donor were considered high risk for CMV infection and were treated with valganciclovir daily for 6 months with dosing adjusted for renal function (eGFR>60, 900mg daily). All patients who were CMV IgG+ prior to transplant were tested monthly for evidence of CMV viremia using standard PCR lab test until month 12. In addition all patients had BK PCR tested monthly through the first year post-transplant.
Treatment for biopsy proven rejection was according to Banff grade. Borderline and grade 1A were treated with corticosteroid pulse. All rejections grade IB or higher were treated with thymoglobulin (1.5mg/kg iv daily – IB and IIA-7 days, IIB-10 days, III-14 days) and a 6 week steroid taper.
Outcomes and Statistical Analyses
Kaplan-Meier curves were generated for freedom from rejection, development of donor specific antibody as well as patient and graft survival. A log rank test was used to assess statistical significance. Graft loss was defined as return to dialysis for greater than 2 weeks. For freedom from graft loss calculations both graft loss and death were considered as an event, whichever came first. Rejection was defined as biopsy proven rejection, grade IA or greater as determined by a staff pathologist using standard Banff criteria.(15) For freedom from rejection calculations patients who died were censored at the time of their death. A chi-squared analysis was used to determine significance between the treatment groups for histologic grade of rejection, incidence of viremia, and incidence as well as cause for conversion. For freedom from development of donor specific antibody the data was censored at either patient death or conversion from belatacept treatment. Estimated glomerular filtration rates (eGFR) were calculated using the Modification of Diet in Renal Disease (MDRD) equation at defined time points post-transplant. Mean eGFR values at these time points were compared between treatment regimens and statistical significance was determined by T-test.
To examine the effect of treatment on eGFR values longitudinally over time a repeated measures model was constructed. Initial models treated time as a categorical variable and an unstructured covariance matrix was used. When the model did not converge either a compound symmetry or toeplitz matrix were employed. In these analyses eGFR values from patients who experienced graft loss or death were imputed as zeros after the event. These models were labeled as “intent to treat, imputed”. In the “on treatment” analyses patients who transitioned off of belatacept were censored from the analysis at the time they stopped belatacept therapy. Patients who experienced death or graft loss prior to cessation of belatacept therapy were included in the analyses and subsequent time points after death or graft loss were imputed as zeros. A repeated measures model where patients with death and graft loss were censored at the time of the event without imputation of the missing values was also generated. Lastly, a slope-based model with imputation of missing values was also used to determine whether there was a difference between the slope for the belatacept- and tacrolimus-based treatment regimens, where linearity of the eGFR values between months 1 and 48 was assumed. Time was regarded as a continuous variable, treatment as a fixed effect, and the intercept and time as random effects; no adjustment was made for other potentially confounding variables. All analyses were conducted using SAS 9.4 (Cary, NC).
Results
Calcineurin inhibitor-free, belatacept–based immunosuppression is associated with high rates of rejection early after renal transplantation
Following FDA approval of belatacept in June 2011 we implemented a costimulation blockade-based standard of care immunosuppressive regimen using belatacept at our center. All patients undergoing renal transplant, either living or deceased donor, were evaluated for belatacept treatment. The initial belatacept-based standard of care regimen included anti-IL-2R induction, mycophenolate mofetil, a short steroid taper, and belatacept consistent with the original package insert. 97 patients received this belatacept treatment regimen and were followed to assess post-transplant outcomes. Outcomes from all belatacept treatment groups were compared in a retrospective fashion to a historical cohort of renal transplant recipients that received a similar treatment regimen except that tacrolimus was used as the primary immunosuppressant instead of belatacept. These patients (n=205) underwent transplantation at our center in the 15 months preceding belatacept approval. The details of the belatacept dosing, tacrolimus trough target levels and concomitant immunosuppression for all treatment regimens are detailed in figures 1 and 2. Baseline transplant and demographic characteristics were similar between all groups. (Table 1)
Table 1.
Transplant Recipient Characteristics for Belatacept and Tacrolimus Treatment Regimens.
| Tac (N=205) |
Bela (N=97) |
Bela/TacShort (N=87) |
Bela/TacExtended (N=356) |
Total (N=745) |
|
|---|---|---|---|---|---|
| Mean age, years | 51 | 51 | 55 | 51 | 51 |
| Male, % | 60 | 57 | 64 | 56 | 58 |
| Donor Type, % | |||||
| Living Donor | 30 | 28 | 31 | 33 | 31 |
| Deceased Donor | 70 | 72 | 69 | 67 | 69 |
| SCD | 80 | 80 | 73 | 84 | 81 |
| ECD | 11 | 13 | 17 | 10 | 11 |
| DCD | 3 | 4 | 7 | 5 | 5 |
| PD En Bloc | 6 | 3 | 3 | 1 | 3 |
| Race, % | |||||
| Black/African-American | 53 | 47 | 54 | 53 | 52 |
| White | 40 | 46 | 38 | 38 | 40 |
| Asian | 1 | 2 | 6 | 1 | 2 |
| Hispanic | 2 | 2 | 1 | 5 | 3 |
| Other | 4 | 2 | 1 | 2 | 2 |
| Cause of ESRD, % | |||||
| Hypertensive Nephrosclerosis | 29 | 35 | 34 | 30 | 31 |
| Diabetes | 25 | 17 | 24 | 26 | 24 |
| Glomerulonephritis | 12 | 0 | 3 | 5 | 6 |
| FSGS | 8 | 7 | 11 | 11 | 10 |
| PCKD | 11 | 14 | 10 | 8 | 10 |
| Other | 15 | 25 | 17 | 20 | 19 |
| Previous transplant, % | 3 | 5 | 15 | 8 | 7 |
| Highest Historical PRA, % | |||||
| 0% | 37 | 36 | 47 | 33 | 36 |
| 0-10% | 10 | 5 | 7 | 10 | 9 |
| 11-20% | 15 | 16 | 11 | 17 | 16 |
| 21-80% | 26 | 27 | 22 | 23 | 24 |
| >80% | 13 | 15 | 13 | 17 | 15 |
| HLA Mismatch, % | |||||
| 0 | 9 | 6 | 8 | 6 | 7 |
| 1-2 | 5 | 13 | 5 | 6 | 7 |
| 3-4 | 44 | 44 | 41 | 38 | 41 |
| 5-6 | 42 | 35 | 46 | 45 | 43 |
Compared to tacrolimus-treated patients there was a significantly increased rate of biopsy proven acute cellular rejection in patients treated with the belatacept regimen. (1 yr rejection, Bela vs Tac, 50.5% vs 20.5% Figure 3A) This increased acute rejection rate did not improve with an increase in the steroid taper from 5mg on day 3 to 20mg for the first month and then tapered to 5mg by 6 weeks. (BelaB data not shown) In addition the grades of rejection were more severe in the belatacept treated patients. (Figure 3B) Despite the higher rates and grades of rejection the overall rates of patient and graft survival at 4 years were not different. (Tac vs Bela, 4yr patient 90.2% vs 91.8%, p=0.73, 4yr graft 85.4% vs 89.7%, p=0.46, Figures 4A&B)
Figure 3. Time to Rejection and Pathologic Grades.

Kaplan-Meier curves for freedom from rejection and summary of histologic grades of rejection for the belatacept-based regimens compared to historic control tacrolimus-based immunosuppression. A-B Bela vs Tac, C-D Bela/Tacshort vs Tac, and E-F Bela/Tacextended vs Tac. Shaded bar represents timing of tacrolimus wean/discontinuation. * indicates p<0.05
Figure 4. Patient and Graft Survival.

Kaplan-Meier curves for patient and graft survival, belatacept-based regimens compared to historic control tacrolimus-based immunosuppression. A-B Bela vs Tac, C-D Bela/Tacshort vs Tac, E-F Bela/Tacextended vs Tac and G-H all Bela vs Tac. A, C, E, & G Patient survival, B, D, F, & H Graft survival.
Use of short course tacrolimus prevents early rejection seen with belatacept-based immunosuppression
Given the high rates of rejection associated with our initial belatacept regimen we altered the regimen to include the use of a short course of tacrolimus in the early post-transplant period in combination with a modified belatacept dosing regimen. (Bela/Tacshort, Figures 1 and 2) Doses of belatacept initially given on days 4 and 14 were eliminated while tacrolimus target trough levels were kept similar to our standard tacrolimus regimen for the first 3 months after discharge (8–12ng/ml). Similar to depleting antibody-based induction therapies that have immunomodulatory effects for several months after administration, all patients received tacrolimus induction for 3 months at therapeutic target trough levels after which it was tapered over a two month period with the goal of discontinuation by the end of the 5th month after transplant.
Initial rejection rates were similar to the historical tacrolimus treated cohort and superior to the initial belatacept treated group. (3month rejection rates, Tac 17.1% vs Bela/Tacshort 14.9%, Bela/Tacshort 14.9% vs Bela 38.2% at 3 months, Figure 3C) However after tapering of the tacrolimus therapy there was an additional cohort of patients that experienced rejection. (12mo rejection rates, Tac 20.5% vs Bela/Tacshort 33.3%, Bela/Tacshort 33.3% vs Bela 50.5%, Figure 3C) As in the initial protocol these rejections tended to be of a higher histologic grade. (Figure 3D) Again despite the increased rates and grades of rejection the overall patient and graft survival at 3 years remained comparable between the treatment groups. (Tac vs Bela/Tacshort, 3yr patient 93.7% vs 92%, p=0.99, 3yr graft 87.8% vs 90.8%, Figures 4C&D)
It has long been recognized that the risk of acute rejection diminishes with time after transplant. Given the rates of rejection were still unacceptable when tacrolimus was tapered off between 3 and 5 months, we reasoned that a longer duration of tacrolimus therapy might avoid the higher rate of rejection observed with belatacept therapy. This hypothesis was supported by the phase II conversion study where patients transitioned from CNI therapy to belatacept with low rates of rejection as early as 6 months post-transplant.(16) Accordingly we modified the protocol to provide extended tacrolimus exposure. (Bela/TacextendedA, see Figures 1&2) Tacrolimus trough level targets were 8–12ng/ml for the first 3 months, 5–8ng/ml for months 4–6, and 3–5ng/ml for months 7–9. Patients were then tapered off of tacrolimus over the next two months with a goal of discontinuation by the end of month 11.
Rates of acute rejection in patients receiving extended tacrolimus induction were similar to our tacrolimus treated cohort and significantly better than either the belatacept regimen (Bela) or the belatacept regimen with the short tacrolimus induction (Bela/Tacshort). Histologic grades of rejection were similar between the Tac and Bela/TacextendedA groups. Given that the goal was to limit CNI exposure and possibly reduce DSA formation in order to improve long-term allograft function we reasoned that the length of tacrolimus induction was more important than achieving higher trough levels to avoid early rejection. We next modified the extended tacrolimus induction regimen to include a more rapid reduction in goal trough levels of 8–12ng/ml for only the first month, levels of 5–8ng/ml for months 2–6, and 3–5ng/ml for months 7–9, with planned discontinuation by month 11. (Bela/TacextendedB, Figure 2) For analysis purposes the two Bela/Tacextended treatment groups were combined. Patients treated with the Bela/Tacextended regimens experienced similar rates and grades of acute rejection when compared to the Tac cohort and significantly less rejection than either the Bela or Bela/Tacshort cohorts. (12 month rejection rates Bela/Tacextended 16% vs Tac 20.5%, Bela/Tacextended 16% vs Bela/Tacshort 33.3%, Bela/Tacextended 16% vs Bela 50.5% Figure 3)
Improved renal function with belatacept-based therapy
One of the primary benefits identified in the clinical trials of belatacept was an improvement in renal function. Recent reporting of the 7-year follow-up confirmed a stable to increased GFR in belatacept-treated patients versus worsening renal function over time in the group treated with cyclosporine (~70 vs 45ml/min @ 7yrs). (13) All patients were followed for renal function and other factors per our standard of care protocol. Estimated glomerular filtration rates (eGFR) were calculated using the Modification of Diet in Renal Disease (MDRD) equation at defined time points post-transplant in a retrospective fashion. Those patients who experienced death or graft loss during the follow-up period continued to be included in an intent to treat analysis. For this analysis eGFR values were imputed as zero from the time of the event. (Figure 5A–D). In addition a repeated measures model was generated to compare mean eGFR values between treatment groups. Time was held as a categorical variable and results were generated and analyzed with and without imputation of values of patients who experienced death and graft loss. (Supplementary Figures 1A–D & 2A–D)
Figure 5. Renal Function.

Mean estimated glomerular filtration rates displayed over time for the various treatment groups. A&E Bela vs Tac, B&F Bela/Tacshort vs Tac, C&G Bela/Tacextended vs Tac and D&H all Bela vs Tac. A-D Intent to treat analysis includes all patients within the cohort, E-H On treatment analysis censors those patients who discontinued belatacept therapy. Missing eGFR values from patients who experienced death or graft loss on belatacept therapy remained in the analysis and were imputed as zero after the event. Error bars represent standard error of the mean. Time points with p value p<0.05 indicated with *.
Similar to the published clinical trial experience, patients treated with any of the belatatcept-based regimens had significantly higher eGFR values than those patients treated with tacrolimus. (Figure 5 and Supplementary Figures 1 & 2) This improvement in renal function was accentuated when patients who stopped belatacept therapy for various reasons were censored from the analysis at the time of their discontinuation, labeled “on-treatment” analysis. (Figure 5E–H, Supplementary Figures 1E–H & 2E–H) When all belatacept-treated patients who remained on therapy were compared to those who received only tacrolimus-based immunosuppression there was a significant difference in renal function at 4 years post transplant. (Figure 5H, All Bela 63.8ml/min vs Tac 46.2ml/min, p<0.0001) Consistent with the phase III study results (17), when the slope of the combined cohort was analyzed using a repeated measures model with time held as a continuous variable there was a slight uptrend in eGFR over time in the belatacept treated patients in contrast to the negative slope, indicating loss of renal function over time, in those treated with tacrolimus alone (All Bela +2.62 ml/min/yr vs Tac −1.60 ml/min/yr, p<0.0001).
Rates of CMV and BK viremia were similar between groups
With the development of a new immunosuppressant there is always the desire to strike the right balance between providing sufficient immunosuppressive activity to prevent rejection while avoiding over-immunosuppression, which could result in problems with protective immunity. As a strategy to assess the overall level of immunosuppression in our belatacept-based immunosuppressive regimen we evaluated the rates and severity of both CMV and BK viremia during the first year post-transplant. In an attempt to quantify the significance of viremia for both CMV and BK we stratified the results based on a cut off of 10,000 IU-copies/ml.
Overall there were no significant differences in the rates of either CMV or BK viremia when we compared belatacept- and tacrolimus-based treatments. (Figure 6 A&B) When we examined the belatacept treatment groups individually we did find that there was a significantly higher rate of detectable CMV viremia in the initial belatacept treatment regimen (Bela), which did not include any adjunct tacrolimus. (Figure 6B, Bela 27.8% vs Tac 14.2% p=0.02) This is likely due to the significantly higher rate of rejection in that group and the subsequent administration of thymoglobulin and steroid used to treat those rejection episodes rather than the result of the initial immunosuppression regimen itself, which included the least immunosuppression of all the belatacept treatment groups. BK nephropathy was a rare event with no significant differences between treatment groups. In addition there were no differences in serious infections requiring hospital admission (data not shown).
Figure 6. CMV and BK Viremia.

Percentage of cohort that developed detectable CMV (A) or BK (B) viremia. Positive patients were stratified by highest level of viremia recorded within the first year post-transplant-low positive, <10,000 copies/ml, or >10,000 copies/ml. Those patients who were not tested were presumed to be negative.
The majority of patients achieved CNI-free immunosuppression using a belatacept-based regimen
Not all patients who were initially treated with a belatacept-containing regimen remained on therapy. By protocol any patient who developed a grade III rejection or had recurrent FSGS was transitioned off of belatacept therapy and maintained with tacrolimus as the principal immunosuppressant. Most patients continued to receive belatacept as their primary immunosuppressant. (Figure 7) In the Bela and Bela/Tacshort regimens there were patients who were transitioned back to tacrolimus following episodes of rejection (10.3% and 6.9% respectively, Table 2). In the Bela/Tacextended cohort the rate of acute rejection was significantly less (Figure 3 E&F) as was the conversion rate for rejection (Table 2, Bela 10.3% vs Belaextended 1.4% p<0.0001, Belashort 6.9% vs Belaextended 1.4% p= 0.003). Additional reasons for conversion off of belatacept included issues related to intravenous infusion- poor iv access, patient preference, distance from infusion center, infection-persistent viremia, or recurrent FSGS. The overall rate of conversion appeared to decrease with protocol optimization. (Figure 7, Bela 23%, Bela/Tacshort 21%, Bela/Tacextended 17%)
Figure 7. Conversion.
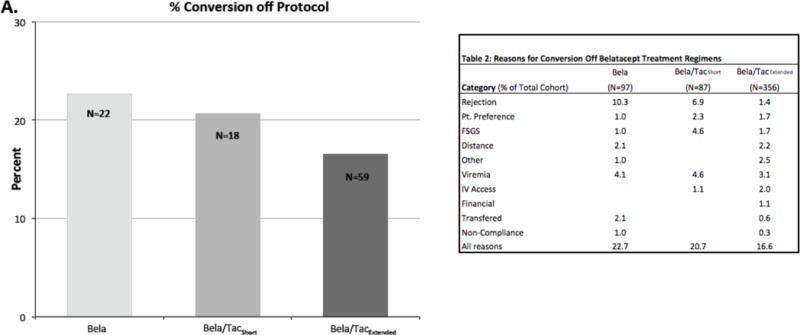
Percentage of cohort who converted off of belatacept.
Table 2.
Reasons for Conversion Off Belatacept Treatment Regimens
| Category (% of Total Cohort) | Bela (N = 97) | Bela/TacShort (N = 87) | Bela/TacExtended (N = 356) |
|---|---|---|---|
| Rejection | 10.3 | 6.9 | 1.4 |
| Pt. Preference | 1.0 | 2.3 | 1.7 |
| FSGS | 1.0 | 4.6 | 1.7 |
| Distance | 2.1 | 2.2 | |
| Other | 1.0 | 2.5 | |
| Viremia | 4.1 | 4.6 | 3.1 |
| IV Access | 1.1 | 2.0 | |
| Financial | 1.1 | ||
| Transferred | 2.1 | 0.6 | |
| Non-Compliance | 1.0 | 0.3 | |
| All Reasons | 22.7 | 20.7 | 16.6 |
Belatacept-based regimens are associated with lower rates of donor specific antibody formation and improved long-term outcomes
In addition to the significant improvement in renal allograft function reported in the 7 year data from the BENEFIT trial, patients treated with belatacept had a significantly lower risk of donor specific antibody (DSA) formation, ~18% in the cyclosporine treated patients vs ~2-4% in those receiving belatacept. (13) Given the association between the development of DSA and inferior long-term allograft function we evaluated the rates of DSA formation in our patients. Despite the significantly higher rates and grades of rejection seen in the Bela and Bela/Tacshort cohorts, the rate of DSA formation in those patients treated with belatacept was significantly lower compared to those receiving a standard tacrolimus-based regimen (Figure 8, Tac 8.82% vs All Bela 4.14% at 1 year, p=0.04)
Figure 8. Donor Specific Antibody Formation.

Kaplan-Meier curves for freedom from the development of donor specific antibody.
The goal of renal transplantation is the long-term survival of both the patient and the transplanted kidney. In order to evaluate the impact of belatacept treatment on long-term patient and graft function we assessed the number of patients who were alive with a functioning graft and an eGFR>30. Those patients who experienced death or graft loss had eGFR values imputed as zero after the event. In an intent to treat analysis, patients treated with belatacept-based immunosuppression were less likely to have a sustained eGFR<30 when compared to the cohort treated with tacrolimus although this did not reach statistical significance (4yr Tac 19.5% vs all Bela 13.3%, p=0.40, Figure 9A). There was however a statistically significant difference when those patients who had stopped belatacept therapy were censored from the analysis reflecting the benefit of avoiding CNI treatment. (4yr, Tac 19.5% vs Bela 7.6%, p=0.0003, Figure 9B) Similar to the phase III clinical trial results and suggesting a potential impact on long-term graft function/survival.(17)
Figure 9. Probability of Survival with eGFR >30.

Kaplan-Meier curves for plot of time to eGFR<30, death, or graft loss. Missing values due to death or graft loss were imputed as zero. A- includes all patients treated with belatacept as an intent to treat analysis. B- Patients who discontinued belatacept therapy were censored from the analysis at the time of discontinuation, on-treatment analysis.
Discussion
In 2011 after completion of a multi-year international clinical development program belatacept was approved as an alternative to CNI in renal transplantation. Based on the high utilization of tacrolimus in the 2015 OPTN annual report there is evidence that use of belatacept in clinical practice is comparatively low. There have been few reports of outcomes observed with belatacept in diverse recipient populations as opposed to selected groups that are eligible for and choose to participate in clinical trials. In this retrospective analysis we have reported on our experience with 540 recipients treated with belatacept-based regimens as standard of care as well as the outcomes of a historical comparator group that received a tacrolimus-based regimen. There were a number of key observations. First, rates of short-term patient and graft survival were comparable for all groups. Second there was a significantly higher rate of rejection with belatacept-regimens that modeled the regimens in the BENEFIT trial (BelaA and BelaB). When a 3-month course of tacrolimus was combined with belatacept (Bela/Tacshort) the rejection rate was significantly reduced although not to the same level previously seen with standard tacrolimus-based therapy. Only when the tacrolimus taper was extended to 9 months (Bela/Tacextended) was the rejection controlled to a level comparable to that seen with standard tacrolimus dosing. The majority of belatacept-treated patients were able to transition to a CNI-free regimen by one year post-transplant. Additionally we found that the rates and levels of BK and CMV viremia were similar across regimens. Maybe most importantly we found that the rates of de-novo DSA formation were significantly lower in the groups treated with belatacept, a finding consistent with the published clinical trials, and that eGFR was superior in belatacept treated subjects compared to those on the tacrolimus-based maintenance regimen (15ml/min at 4 years).
One important finding from our experience was the significantly increased rate of acute rejection seen when we employed belatacept in a regimen similar to that reported in the BENEFIT trial. Based on the results from the clinical trial higher rates and grades of rejection with belatacept treatment were not unexpected, however the frequency and severity of rejection in our initial cohort was significant. There are several factors that may have contributed to the observed increase in rejection. First, the BENEFIT trial population was considerably different from our patient population. The percent of patients who had received a previous transplant, those with higher PRAs, the degree of HLA mismatches and the percentage of African American recipients were all significantly higher in our cohort. In addition the steroid regimen utilized in the BENEFIT trial was not explicitly prescribed. A post-hoc analysis of steroid dosing in the trial showed that patients received significantly higher doses of steroid for a longer period of time post-transplant in contrast to our regimen where the steroid dosing more closely approximated contemporary practice (mean dose of prednisone at discharge, 1 and 3 months after transplant, BENEFIT 41mg, 22mg, 18mg vs BelaA 5mg, 5mg, 5mg vs BelaB 20mg, 15mg, 5mg). (BENEFIT data-personal communication Mary Beth Harler, Bristol-Myers Squibb) This combination of recipients with higher immunologic risk and lower mean steroid dosing in the first few months after transplant may have contributed to the significantly higher rate of rejection seen with our experience. In addition there are likely other yet undefined immunologic risk factors, including the level of immune memory or costimulation resistant T cell subsets that may contribute to the risk of rejection with belatacept-based treatment.
When faced with the higher rates and grades of rejection in the initial belatacept treatment regimen there were several options for optimization including the use of T cell depletion for induction. Previous reports utilizing belatacept in steroid avoidance regimens have had mixed results. An early trial assessing the combination of belatacept, thymoglobulin induction and either MMF or sirolimus in primary renal transplant recipients suggested a higher rate of rejection in patients treated with belatacept/MMF compared to belatacept/sirolimus or a tacrolimus-based control regimen although this was not statistically significant given the small sample size.(18) The combination of alemtuzumab, belatacept and sirolimus has been successfully used in a select cohort to achieve low rejection rates and excellent graft function with many patients eventually weaning to belatacept monotherapy.(19) A more recent study supported by the NIH’s clinical trials in organ transplantation (CTOT-16) evaluating the combination of thymoglobulin, belatacept and MMF in a steroid avoidance regimen was halted due to lack of efficacy, specifically unacceptably high rejection rates.(20)
Our choice to use an adjunct extended period of tacrolimus induction to control the early post-transplant rejection seen with belatacept allows for the ability to titrate the level of immunosuppression if necessary by modifying tacrolimus target levels depending on the individual circumstance. This strategy stands in contrast to traditional depletional therapy where once given one is unable to alter its immunosuppressive effects for the next several months. The current clinical trial evaluating the use of thymoglobulin, belatacept and everolimus will provide additional insight into the efficacy of the combination of T cell depletion and belatacept in a larger study population. (Clinicaltrials.gov #NCT02137239)
The observation that later weaning of tacrolimus facilitates a significantly lower rate of rejection when patients transition to a CNI-free regimen defines an early period of immunologic instability in which a subset of patients will experience costimulation-blockade independent rejection. Current research efforts by our group and others seek to define potential immunologic parameters that might identify those patients at risk of rejection when treated with belatacept.(21, 22) Accurate identification of those patients who are at low risk of rejection may permit us to stratify those to avoid adjunct tacrolimus treatment as well as determine the optimal timing of the transition off of tacrolimus therapy for others.
Perhaps the most meaningful data included in this report is the inclusion of our tacrolimus treated cohort with long-term evaluation of both renal function and rates of donor specific antibody (DSA). While this analysis is retrospective and carries with it all of the inherent caveats associated with that type of analysis it is nonetheless informative. In a diverse group of renal transplant recipients that mirrors the patient population served by many transplant centers we report that there is a steady decline in renal function over time with tacrolimus treatment. These data are consistent with the observed association of long-term tacrolimus exposure on renal function. In contrast, treatment with belatacept-containing regimens is associated with maintenance or even improvement in renal function following transplantation. Our data confirm the recently published report detailing the 7 year results of the BENEFIT trial including sustained improvements in renal function over time. Despite transient tacrolimus exposure early post transplant, our patients also experienced a significant benefit in renal function compared to patients treated with tacrolimus. The 7 year data also demonstrated a benefit in the combined endpoint of patient and graft survival. When we examined the probability of patient survival with an eGFR>30 there was a significant benefit for those patients treated with and maintained on belatacept therapy. This is congruent with the 7 year data from both the BENEFIT trial showing a 43% risk reduction in patient/graft survival as well as the BENEFIT-EXT analysis showing a significant improvement for a similar combined endpoint.(13) Our most recent protocol modification includes lower tacrolimus target trough levels from the time of transplant (5–8ng/ml months 0–6, 3–5ng/ml months 7–9 then tapered off over months 9–11) with rates of acute rejection comparable to our previous regimen (Bela/TacextendedC, data not shown). Ideally less exposure to tacrolimus earlier should minimize any negative impact on renal function.
The development of donor specific antibody is an increasingly recognized risk factor for late allograft failure.(23–25) We report here that treatment with belatacept therapy significantly reduces donor specific antibody formation when compared to tacrolimus-based immunosuppression. Even with the high rates of acute cellular rejection observed in the first belatacept cohort (Bela) DSA formation was low. This confirms the finding seen in the recent long-term follow-up report of the BENEFIT trial where ~18% in the cyclosporine treated patients developed DSA compared to 2-4% of the patients receiving belatacept. This is consistent with the substantial experimental evidence that supports the efficacy of belatacept preventing DSA formation.(10, 26) There was a significant reduction in DSA formation within the first year post-transplant supporting the initiation of belatacept at the time of transplant instead of waiting to convert later. In addition to the potential biologic benefit in DSA reduction with initial belatacept therapy there is also the practical consideration of the potential difficulty of convincing patients to change to an intravenous infusion at a later time point after they are already accustomed to only oral medications versus starting from the time of transplant on a regimen that includes monthly infusions.
The goal of kidney transplantation is to extend and improve the lives of patients with end-stage renal disease. While there are many strategies to improve long-term outcomes one approach with increasing support is the use of immunosuppressive agents, such as belatacept, to avoid the deleterious effects of current immunosuppressants that negatively influence long-term patient and graft survival. We have developed a non-depletional, costimulation blockade-based immunosuppression regimen that achieves equivalent patient and graft survival to a tacrolimus-based regimen. This regimen achieved acceptable rates of acute rejection and promoted improved renal function providing a viable strategy for long-term CNI-free immunosuppression in contemporary clinical practice. The long-term benefits of improved renal function and presumably lower cardiovascular risk should translate into increases in patient and graft survival.
Supplementary Material
Figure S1: eGFR without imputation. The estimated glomerular filtration rate (eGFR) was determined by repeated measures modeling, with time as a categorical variable and does not include imputation for missing values for patients who experienced death or graft loss. Error bars represent 95% CI, * indicates a significant difference between treatment groups, p<0.05. A–D all patients were included in an intent to treat analysis, E–H patients who stopped belatacept therapy were censored at the time of discontinuation, on-treatment analysis.
Figure S2: eGFR with imputation. The estimated glomerular filtration rate (eGFR) was determined by repeated measures modeling, with time as a categorical variable and includes imputation of zeros for values missing in patients after death or graft loss. Error bars represent 95% CI, * indicates a significant difference between treatment groups, p<0.05. A–D all patients were included in an intent to treat analysis, E–H patients who stopped belatacept therapy were censored at the time of discontinuation, on-treatment analysis.
Figure S3: Tacrolimus trough levels. Bela/Tacshort (dashed line) and Bela/Tacextended (solid line) regimens.
Acknowledgments
The authors wish to thank Cameron Evans, Bennett Evans, Hunter Roarke and Jahan Saedi for their assistance with the manuscript as well as Ulf Meier-Kriesche for his critical reading of the manuscript.
Abbreviations
- CMV
cytomegalovirus
- CNI
calcineurin inhibitor
- DSA
donor specific antibody
- EBV
epstein barr virus
- eGFR
estimated glomerular filtration rate
- FSGS
focal segmental glomerulosclerosis
- HIV
human immunodeficiency virus
- MMF
mycophenolate mofetil
- MDRD
Modification of Diet in Renal Disease
- PRA
panel reactive antibody
- PTLD
post-transplant lymphoproliferative disorder
- Tac
tacrolimus
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. ABA and REP have received research support from Bristol-Myers Squibb. ABA and TCP have received consulting fees from Bristol-Myers Squibb. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 3.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:1289–1295. doi: 10.1111/j.1600-6143.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 5.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Kasiske BL, Israni AK. Kidney. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(Suppl 2):11–46. doi: 10.1111/ajt.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. The New England journal of medicine. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 7.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. The New England journal of medicine. 2002;346:580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. Journal of the American Society of Nephrology: JASN. 2000;11:1735–1743. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler DC, Steiger J. Evolution and etiology of cardiovascular diseases in renal transplant recipients. Transplantation. 2000;70:SS41–45. [PubMed] [Google Scholar]
- 10.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 11.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, Massari P, Mondragon-Ramirez GA, Agarwal M, Di Russo G, Lin CS, Garg P, Larsen CP. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 12.Vanrenterghem Y, Bresnahan B, Campistol J, Durrbach A, Grinyo J, Neumayer HH, Lang P, Larsen CP, Mancilla-Urrea E, Pestana JM, Block A, Duan T, Glicklich A, Gujrathi S, Vincenti F. Belatacept-based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT-EXT studies) Transplantation. 2011;91:976–983. doi: 10.1097/TP.0b013e31820c10eb. [DOI] [PubMed] [Google Scholar]
- 13.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP. Belatacept and Long-Term Outcomes in Kidney Transplantation. The New England journal of medicine. 2016;374:333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 14.Wen X, Casey MJ, Santos AH, Hartzema A, Womer KL. Comparison of Utilization and Clinical Outcomes for Belatacept- and Tacrolimus-Based Immunosuppression in Renal Transplant Recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13853. [DOI] [PubMed] [Google Scholar]
- 15.Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, Nankivell BJ, Colvin RB, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell L, Drachenberg C, Dragun D, de Kort H, Gibson IW, Kraus ES, Lefaucheur C, Legendre C, Liapis H, Muthukumar T, Nickeleit V, Orandi B, Park W, Rabant M, Randhawa P, Reed EF, Roufosse C, Seshan SV, Sis B, Singh HK, Schinstock C, Tambur A, Zeevi A, Mengel M. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17:28–41. doi: 10.1111/ajt.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostaing L, Massari P, Garcia VD, Mancilla-Urrea E, Nainan G, del Carmen Rial M, Steinberg S, Vincenti F, Shi R, Di Russo G, Thomas D, Grinyo J. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clinical journal of the American Society of Nephrology: CJASN. 2011;6:430–439. doi: 10.2215/CJN.05840710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, Reyes-Acevedo R, Rice K, Rostaing L, Steinberg S, Xing J, Agarwal M, Harler MB, Charpentier B. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson R, Grinyo J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, Marks WH, Agarwal M, Wu D, Dong Y, Garg P. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirk AD, Guasch A, Xu H, Cheeseman J, Mead SI, Ghali A, Mehta AK, Wu D, Gebel H, Bray R, Horan J, Kean LS, Larsen CP, Pearson TC. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:1142–1151. doi: 10.1111/ajt.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannon R, Stock P, Mehta A, Ikle D, Watson N, Bridges N, Robien M, Newell K. Avoidance of CNI and Steroids Using Belatacept-A Preliminary Report of CTOT-16. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of TransplantSurgeons. 2016;16 http://www.atcmeetingabstracts.com/abstract/avoidance-of-cni-and-steroids-using-belatacept-a-preliminary-report-of-ctot-16/. Accessed May 14, 2016. [Google Scholar]
- 21.Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB, 3rd, George R, Cheeseman J, Stempora L, Townsend R, Durrbach A, Kirk AD. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16:1102–1112. doi: 10.1111/ajt.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krummey SM, Cheeseman JA, Conger JA, Jang PS, Mehta AK, Kirk AD, Larsen CP, Ford ML. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:607–614. doi: 10.1111/ajt.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 24.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 25.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:1367–1373. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB, 3rd, Iwakoshi NN, Villinger F, Kirk AD, Knechtle SJ. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14:59–69. doi: 10.1111/ajt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: eGFR without imputation. The estimated glomerular filtration rate (eGFR) was determined by repeated measures modeling, with time as a categorical variable and does not include imputation for missing values for patients who experienced death or graft loss. Error bars represent 95% CI, * indicates a significant difference between treatment groups, p<0.05. A–D all patients were included in an intent to treat analysis, E–H patients who stopped belatacept therapy were censored at the time of discontinuation, on-treatment analysis.
Figure S2: eGFR with imputation. The estimated glomerular filtration rate (eGFR) was determined by repeated measures modeling, with time as a categorical variable and includes imputation of zeros for values missing in patients after death or graft loss. Error bars represent 95% CI, * indicates a significant difference between treatment groups, p<0.05. A–D all patients were included in an intent to treat analysis, E–H patients who stopped belatacept therapy were censored at the time of discontinuation, on-treatment analysis.
Figure S3: Tacrolimus trough levels. Bela/Tacshort (dashed line) and Bela/Tacextended (solid line) regimens.


