Abstract
Aicardi syndrome (AS) is an X-linked inherited disorder characterized by infantile spasms, chorioretinal lacunae, and agenesis or hypogenesis of the corpus callosum. The syndrome is more frequently seen in females but is observed in XXY male patients. Central nervous system, ocular, and costovertebral malformations may also seen in AS. Eye findings are of a considerable diagnostic importance; the chorioretinal lacunae are pathognomonic for AS and are generally bilateral. The outcome of the disease is generally severe, with a high mortality rate and poor developmental outcome. It is not clear which characteristics of the syndrome are related to a good prognosis in terms of psychomotor development, epileptic seizures, and survival. The purpose of this report was to demonstrate the spectrum of the clinical findings and the course of AS in two Turkish patients with different ocular and cranial MRI findings.
Aicardi syndrome (AS) was first described by Jean Aicardi in 1965 and is characterized by agenesis or hypogenesis of the corpus callosum, chorioretinal lacunae, and early infantile spasms. Most of AS patients develop normally until 3 months of age, and then, epileptic seizures and developmental delay start. It is an X-linked dominant genetic disorder and lethal in males and, therefore, almost exclusively observed in females. Almost all patients are females; however, XXY male patients have been reported.1 Central nervous system, ocular and costovertebral malformations may also be associated with AS.2,3 Eye findings are of considerable diagnostic importance. The choroidoretinal lacunae are pathognomonic for AS and are generally bilateral. A relationship between ocular findings and severity of the agenesis or hypogenesis of the corpus callosum and prognosis of AS has been claimed, but a few data are available about the clinical features that can predict clinical outcome. A unilateral involvement of the optic nerve coloboma and small chorioretinal lacunae are also related to a better prognosis.4,5 The purpose of this report was to demonstrate the spectrum of the clinical findings and the course of AS in two Turkish patients.
CASE 1
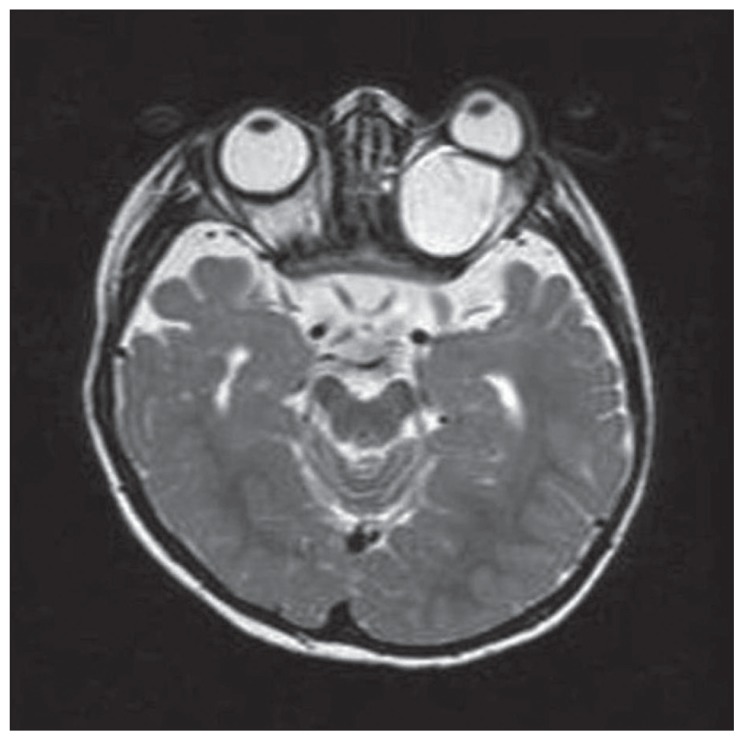
A 2-year-old female patient presented with intractable seizures, microphtalmia, and proptosis of the left eye. At 3 months of age, the patient developed flexure spasms and abnormal eye movements. Antiepileptic agents were ineffective to control seizures. After adrenocorticotropic hormone (ACTH) treatment, at 4 months of age the patient was seizure-free for a 3-month period. On the physical examination, the head circumference was 44.5 cm (−2,39 standard deviation score), and horizontal nystagmus and proptosis on the left eye were detected. The patient exhibited severe psychomotor retardation and hyperactive deep tendon reflexes, and the left eye exhibited microphthalmia. The eye fundus examination revealed bilateral peripapillary pigmentation areas of peripapillary underpigmented chorioretinal lacunae and retinal detachment in the left eye. MRI scans of the brain showed cortical dysplasia involving bilateral frontal and parietal lobes, right occipital lobe, and dysgenesis—developmental atrophy of the corpus callosum. Microphtalmia of the left side, bilateral optic nerve atrophy, and a large retro-orbital coloboma cyst on the left side were observed on orbital MRI scans (Figure 1). The abdominal ultrasound examination and costovertebral x-ray images were normal.
Figure 1.
T2 weighted transverse orbital MR image shows bilateral optic nerve atrophy(black arrows) and retro-orbital big coloboma cyst on the left( arrowheads).
The electroencepahlogram (EEG) showed welllocalized seizure discharges on the right parieto-occipital region of the brain. Clinical and radiological findings of the patient are summarized in Table 1.
Table 1.
Clinical characteristics of the patients.
| Characteristics | Case 1 | Case 2 | ||
|---|---|---|---|---|
|
| ||||
| Eye Fundus | Right | Left | Right | Left |
| Chorioretinal lacunae | + | + | + | + |
| Optic disc coloboma | + | |||
| Retinal detachment | + | |||
| Propitosis | + | |||
| Microphtalmia | + | |||
|
| ||||
| Cranial MRI | ||||
| Cortical dysplasia | + | + | ||
| Total/partial agenesis of corpus callosum | + | + | ||
| Periventricular heterotopia | − | + | ||
| Quadrigeminal cistern cyst | − | + | ||
| Optic nerve atrophy | + | + | ||
| Psychomotor development | Severe retardation | Normal | ||
| Epileptic seizures | + | + | ||
CASE 2
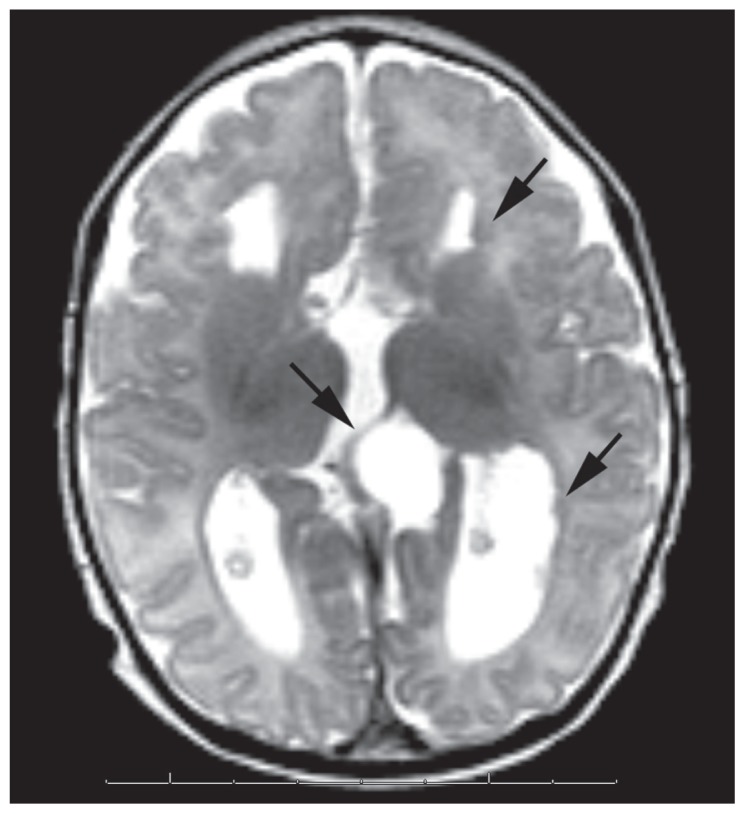
A 3-month-old girl presented with flexure spasms. The patient had a prenatal diagnosis of agenesis of the corpus callosum and a cystic formation of the quadrigeminal cistern by ultrasound and MRI during the 24th week of gestational age. The patient was born by normal delivery, and the physical examination was normal at birth. The patient was asymptomatic during the first 3 months of life. At 3 months of age the patient developed epileptic seizures and the EEG recordings showed an asymmetrical suppression-burst pattern. On the physical examination, the head circumference was 40 cm (0.44 SDS), and cognitive studies showed a normal psychomotor development. The left eye fundus examination revealed a bilateral peripapillary pigment and areas of peripapillary underpigmented chorioretinal lacunae, and the right eye fundus exhibited a colobomatous optic disc. The brain MRI at 3 months of age revealed agenesis of the corpus callosum, periventricular nodular heterotopias, bilateral frontoparietal cortical dysplasia, and a cystic formation of the quadrigeminal cistern (Figure 2). Bilateral optic nerve atrophy was found on orbital MRI. The abdominal ultrasound examination and costovertebral x-ray were normal. After ACTH treatment at 3 months of age, the patient was seizure-free for a 4-month period, and psychomotor development was normal at 7 months of age. Clinical and radiological findings of the patient are summarized in Table 1.
Figure 2.
Transverse T2 weighted MR image shows parallel oriented lateral ventricles due to agenesis of corpus callosum, periventricular nodular heterotopias(arrows on the left brain side), cystic formation of the quadrigeminal cistern(arrow at the midline).
DISCUSSION
AS is an X-linked dominant genetic disorder, and the diagnosis of the syndrome is based on clinical features including the characteristic triad.6 AS is lethal in males, but no gene or candidate region on the X-chromosome has been definitively identified.7
Cortical dysplasia, periventricular heterotopias, cystic formations, vermian anomalies, and choroid plexus papillomas can be detected in this syndrome.8–10 The prognosis varies with the severity of underlying brain abnormalities and symptoms.3 An improved prognosis is reported if the agenesis of the corpus callosum was only partial and the presence or absence of cortical heterotopias has not been related to prognosis.5,11 Costovertebral malformations such as hemivertebrae, kyphoscoliosis, fusion of vertebrae, and absent ribs can be seen in AS.3
The chorioretinal lacunae are pathognomonic for AS. Pigment deposits are frequently present and may increase with age; however, the size of the lacunae do not change over the years.12,13 Colobomas of the optic disc are present in about half of the cases, which are commonly unilateral. Aziz et al reported a patient with right optic nerve aplasia and bilateral iris colobomas.5 Microphthalmia is always unilateral or predominant on one side.14 Our case 2 patient’s eye fundus examination revealed bilateral chorioretinal lacunae, and coloboma of the optic disc on the right eye. Guerriero et al reported an AS patient with choroidoretinal lacunae, seizures, and cerebral cyst but a normal corpus callosum. 12 An eye fundus examination can have not only diagnostic value for the AS, but also prognostic significance. A unilateral involvement of the optic nerve coloboma and small chorioretinal lacunae are also related to an improved prognosis, and the outlook is better for those with fewer ocular abnormalities.4,5 Proptosis, retinal detachment, and a large retro-orbital coloboma cyst on the left eye were found in Case 1, and she had severe psychomotor retardation and epilepsy with poor clinical control. The left eye of the first patient exhibited a distinct proptosis and microphtalmia, but most AS patients have normal faces, except for the possibility of microphthalmia and no skin or other peripheral abnormalities.16
The treatment is symptomatic for AS and generally involves management of the epileptic seizures. Patients with AS usually require multiple antiepileptic drugs for adequate seizure control.7 Infantile spasms are the most common type of seizure in AS. In series of infantile spasms, AS may account for less than 1% to up to 4% of cases.15 Antiepileptic agents were ineffective or partially effective to control epileptic seizures.17 In our study, both patients developed flexure spasms at 3 months of age and responded to ACTH treatment.
In conclusion, we emphasize that a careful ophthalmologic examination is necessary for patients with flexure spasms and corpus callosum abnormalities. We believe that a large series of cases are required for detecting the prognostic significance of the clinical findings.
REFERENCES
- 1.Aicardi J, Lefebvre J, Lerique-Koechlin A. A new syndrome: spasm in flexion, callosal agenesis, ocular abnormalities. Electroencephalogr Clin Neurophysiol. 1965;19:609–610. [Google Scholar]
- 2.Aicardi J, Chevrie JJ, Rousselie F. Spasm-inflexion syndrome, callosal agenesis, chorioretinal abnormalities. Arch Fr Pediatr. 1969;26(10):1103–20. [PubMed] [Google Scholar]
- 3.Aicardi J. Aicardi syndrome: old and new findings. Int Pediatr. 1999;14:5–9. [Google Scholar]
- 4.Aicardi J. Aicardi syndrome. Brain Dev. 2005;27(3):164–71. doi: 10.1016/j.braindev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Aziz HA, Sisk RA, Berrocal AM, Murray TG. Optic nerve aplasia in aicardi syndrome. J Pediatr Ophthalmol Strabismus. 2010;47:e1–4. doi: 10.3928/01913913-20090818-01. [DOI] [PubMed] [Google Scholar]
- 6.Ballabio A, Andria G. Deletions and translocations involving the distal short arm of the human X chromosome:review and hypotheses. Hum Mol Genet. 1992;1(4):221–7. doi: 10.1093/hmg/1.4.221. [DOI] [PubMed] [Google Scholar]
- 7.Billette de Villemeur T, Chiron C, Robain O. Unlayered polymicrogyria and agenesis of the corpus callosum: a relevant association? Acta Neuropathol. 1992;83(3):265–70. doi: 10.1007/BF00296788. [DOI] [PubMed] [Google Scholar]
- 8.Chevrie JJ, Aicardi J. The Aicardi syndrome. In: Pedley TA, Meldrum BS, editors. Recent advances in epilepsy. Vol. 3. New York: Churchill-Livingstone; 1986. pp. 189–210. [Google Scholar]
- 9.Cruz-Velarde JA, Garzo C, Garcia-Munoz S, Gil R, Munoz L. Clinical and prognostic heterogeneity in Aicardi’s syndrome: a description of two cases. Rev Neurol. 1999;28(8):16–30. 784–5. [PubMed] [Google Scholar]
- 10.Del Pero RA, Mets MB, Tripathi RC, Torczynski E. Anomalies of retinal architecture in Aicardi syndrome. Arch Ophthalmol. 1986;104(11):1659–64. doi: 10.1001/archopht.1986.01050230097041. [DOI] [PubMed] [Google Scholar]
- 11.Galdos M, Martínez R, Prats JM. Clinical Outcome of Dıstınct Aicardi Syndrome Phenotypes. Arch Soc Esp Oftalmol. 2008;83(1):29–36. doi: 10.4321/s0365-66912008000100007. [DOI] [PubMed] [Google Scholar]
- 12.Guerriero S, Sciruicchio V, De Blasi R, Furino C, Smaldone G, Ciraci L, et al. Chorioretinal lacunae: pathognomonic findings for aicardi syndrome. J Pediatr Ophthalmol Strabismus. 2010;47:e1–3. doi: 10.3928/01913913-20100324-03. [DOI] [PubMed] [Google Scholar]
- 13.Hamano S, Yagishita S, Kawakami M, Ito F, Maekawa K. Aicardi syndrome: postmortem findings. Pediatr Neurol. 1989;5(4):259–61. doi: 10.1016/0887-8994(89)90088-x. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G. Neuroimaging aspects of Aicardi syndrome. Am J Med Genet A. 2008;146A(22):2871–8. doi: 10.1002/ajmg.a.32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes AV, MacGregor DL, Buncic JR. Aicardi syndrome: natural history and possible predictors of severity. Pediatr Neurol. 1994;11(4):313–8. doi: 10.1016/0887-8994(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 16.Menezes AV, Lewis TL, Buncic JR. Role of ocular involvement in the prediction of visual development and clinical prognosis in Aicardi syndrome. Br J Ophthalmol. 1996;80(9):805–11. doi: 10.1136/bjo.80.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park BL, Chung HJ, Coe CJ. A case of Aicardi’s syndrome. J Korean Pediatr Soc. 1982;12:1285–1292. [Google Scholar]