Abstract
Early recognition of pathogens by the innate immune system is crucial for bacterial clearance. Many pattern recognition receptors (PRRs) such as Toll-like (TLRs) and (NOD)-like (NLRs) receptors have been implicated in initial sensing of bacterial components. The intracellular signaling cascades triggered by these receptors result in transcriptional upregulation of inflammatory pathways. Although this step is crucial for bacterial elimination, it is also associated with the potential for substantial immunopathology, which underscores the need for tight control of inflammatory responses. The leading human bacterial pathogen Staphylococcus aureus expresses over 100 virulence factors that exert numerous effects upon host cells. In this manner, the pathogen seeks to avoid host recognition or perturb PRR-induced innate immune responses to allow optimal survival in the host. These immune system interactions may result in enhanced bacterial proliferation but also provoke systemic cytokine responses associated with sepsis. This review summarizes recent findings on the various mechanisms applied by S. aureus to modulate or interfere with inflammatory responses through PRRs. Detailed understanding of these complex interactions can provide new insights toward future immune-stimulatory therapeutics against infection or immunomodulatory therapeutics to suppress or correct dysregulated inflammation.
Keywords: Staphylococcus aureus, pattern recognition receptors, inflammatory responses, evasion, virulence, biotherapeutic
Microbial pathogens have a perpetual co-evolutionary interaction with the innate immune system of their respective hosts. This review outlines the multitude of mechanisms utilized by the leading human bacterial pathogen Staphylococcus aureus to modulate and evade proinflammatory responses induced by Toll-like (TLR), (NOD)-like (NLR) and C-type lectin (CLR) receptors.
INTRODUCTION
Classical inflammation can be triggered by a variety of stimuli such as microbial pathogens or tissue damage, which increase energy expenditure and robustly activate various proinflammatory signaling and effector pathways (Han and Ulevitch 2005). The inflammatory response is rapid and characterized by enhancement of vascular flow and permeability, allowing leakage of serum components into the tissue microenvironment. Extravasation of immune cells to the affected tissue normally promotes healing and regeneration. However, overreaction or failure to appropriately shut down the acute inflammatory response may lead to an uncontrolled ‘cytokine storm’, which can be detrimental to the host. This can be precipitated by a wide range of infectious (e.g. septic shock) as well as noninfectious diseases (e.g. asthma or inflammatory bowel disease) (Collins 1996; Tisoncik et al.2012). Thus, the immune system must carefully fine-tune the level of inflammation to clear the pathogenic threat while avoiding additional tissue damage (Weiss et al.2008).
Innate immune responses are composed of multiple dynamic defense systems, which together play the sentinel role in early recognition of invading pathogens, deployment of generalized antimicrobial factors, induction of proinflammatory responses, recruitment of phagocytic cells and activation of acquired immunity (Medzhitov and Janeway 2000). Acquired immunity subsequently aids in clearance of pathogens with a higher level specificity and the generation of immunological memory (Iwasaki and Medzhitov 2004). The innate immune response is mediated through several factors, including recognition of conserved microbial structures termed ‘pathogen-associated molecular patterns’ (PAMPs) via a broad array of germline-encoded pattern recognition receptors (PRRs). In addition, PRRs can recognize endogenous molecules produced by damaged cells or tissues, called damage-associated molecular patterns (Mogensen 2009). PRRs comprise Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs), cytosolic DNA sensors and retinoic acid-inducible (RIG)-I-like receptors (RLRs) (Xia et al.2016; Ori, Murase and Kawai 2017). RLRs comprise retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), and probable ATP-dependent RNA helicase DHX58 (LGP2), which coordinate antiviral responses (Loo and Gale 2011) and thus will not be further discussed in this review.
A distinctive pattern of subcellular distribution for each of the different PRR classes provides functional redundancy and synergy towards recognition of an extensive range of PAMPs derived from a diverse array of potential pathogens including bacteria, fungi, protozoa and viruses. PRR-based pathogen detection results in activation of several signaling pathways that converge on key transcription factors such as nuclear factor-kappa B (NF-κB), activator protein 1 (AP1) and interferon (IFN) regulatory factors. The cumulative effect of PRR signaling is the differential expression of key proinflammatory as well as antiinflammatory genes (Takeuchi and Akira 2010). Activation of PRRs-mediated inflammatory responses must be tightly controlled: too weak of a response heightens host susceptibility to infection; on the other hand, an overactive response may promote lethal systemic inflammation, autoimmunity, or the development of acute or chronic inflammatory diseases (O’Neill, Bryant and Doyle 2009).
Staphylococcus aureus is among the most medically important bacterial pathogens that persistently colonizes the anterior nares of 30% of healthy individuals. In addition, this bacterium can cause a wide range of serious human infections such as bacteremia, endocarditis, pneumonia, skin and wound infections and deep tissue abscesses (Weidenmaier, Goerke and Wolz 2012; Krismer et al.2017). Genome-wide analysis on S. aureus suggests downregulation of virulence genes during colonization and upregulation of them in the course of infection (Novick 2003). The treatment of S. aureus infections is complicated by the increasing number of antibiotic-resistant strains (DeLeo et al.2010; Tong et al.2015) and the induction of hemodynamic changes that may prove resistant to therapeutic modalities (Polat et al.2017). Moreover, when pyogenic infection arises, a robust host inflammatory response is induced both locally and systemically, often termed sepsis syndrome. The peak cytokine response in Gram-positive pyogenic infections like S. aureus occurs 50–75 h after the challenge (Opal and Cohen 1999). Despite significant advancement in treatment modalities, sepsis-induced mortality rate is 20%–30% in Gram-positive bacterial sepsis and can be as high as 70%–90% when shock and multiorgan failure are present (Polat et al.2017).
In the struggle to avoid immune clearance, the pathogen deploys several mechanisms to subvert the fast-acting innate immune responses through interference with PRR recognition, restraining complement deposition or activation and delaying neutrophil recruitment (Foster 2005; Rooijakkers, van Kessel and van Strijp 2005; Kim et al.2012; Foster et al.2014). The understanding of staphylococcal immune evasion of both innate and adaptive immunity has been an area of intense research. In this light, initial recognition of S. aureus by PRRs is pivotal in setting the course of the host-pathogen interaction and orchestrating the accompanying immune responses. Our aim in this review is to highlight and discuss recent findings on staphylococcal strategies to modulate host innate immune and inflammatory responses via PRRs (Table 1), a multifaceted interaction that brings into play a large array of the pathogen's evasion factors and virulence determinants.
Table 1.
TLRs, NLRs and CLRs involved in S. aureus recognition.
| Receptor-family | Specific receptor | S. aureus—derived ligand | Reference |
|---|---|---|---|
| TLR | TLR2 | LTA, lipoprotein | Schwandner et al. (1999), Takeuchi et al. (1999), Iwaki et al. (2002), Sabroe et al. (2003) |
| TLR2-TLR1/TLR6 | Lipopeptide, lipoprotein | Ozinsky et al. (2000), Morr et al. (2002), Nishiya and DeFranco (2004), Travassos et al. (2004) | |
| TLR2-CD14 | Peptidoglycan, LTA | Schroder et al. (2003) | |
| TLR2-CD36 | Diacylglycerides, LTA | Hoebe et al. (2005) | |
| TLR2- Asialo-GM1 | Unknown (probably a surface molecule regulated by Agr) | Ratner et al. (2001), Soong et al. (2004) | |
| TLR8 | S. aureus RNA | Bergstrom et al. (2015) | |
| TLR9 | S. aureus unmethylated CPG DNA | Parker and Prince (2012) | |
| NLR | NOD2 | Peptidoglycan (MDP) | Volz et al. (2010) |
| CLRs | MBL | LTA | Polotsky et al. (1996) |
| L-ficolin | LTA | Lynch et al. (2004) | |
| SP-A | LTA, CWG | Lawson and Reid (2000) |
LTA: lipoteichoic acid; CPG: cytosine-phosphate-guanosine; MDP: muramyl dipeptide; MBL: mannose binding lectin; CWG: cell wall glycopolymer; SP: surfactant protein.
Toll-like receptor signaling
Human TLRs (TLR1-TLR10) are transmembrane glycoproteins composed of extracellular leucine-rich repeats (LRRs), a transmembrane domain and a cytoplasmic tail containing a Toll/IL-1 receptor (TIR) domain. Each TLR can recognize a distinct set of PAMPs (Takeda and Akira 2004; Akira, Uematsu and Takeuchi 2006) either through direct interaction, e.g. TLR1/TLR2, TLR3 and TLR9 (Jin et al.2007; Latz et al.2007; Liu et al.2008), or indirectly via an accessory PAMP-binding molecule, e.g. interaction between LPS and the MD2-TLR4 complex (Jin et al.2007). TLRs are differentially expressed and distributed in different cell types. Some TLRs are displayed on the cell surface and specialized in recognition of PAMPs and endogenous misplaced proteins, whereas other TLRs are mainly localized in intracellular compartments and recognize nucleic acids or other intracellular PAMPs (Iwasaki and Medzhitov 2004; Mogensen 2009; Kawai and Akira 2010) (Fig. 1). TLR expression can be inducible, e.g. in keratinocytes, or constitutive, e.g. in antigen-presenting cells (APCs) (Mogensen 2009). Binding of the cognate ligand (PAMP or DAMP) to the extracellular leucine-rich repeats (LRRs) induces TLR homo- or heterodimerization. TLR2, for example, is functionally active as a heterodimer in cooperation with TLR1 or TLR6 to achieve specificity and discriminate subtle differences in the repertoire of bacterial lipoproteins (reviewed in Farhat et al. (2008)). Dimerization subsequently results in recruitment of TIR-containing adaptor protein(s) to the intracellular TIR domain of the receptor (Takeda and Akira 2004; O’Neill and Bowie 2007; Song and Lee 2012). Five different TIR-containing adaptors are known to be recruited depending on the stimuli and TLRs involved (Akira, Takeda and Kaisho 2001; Takeda and Akira 2004): Myeloid differentiation factor 88 (MyD88), TIR-associated protein (TIRAP, also known as MyD88 adaptor-like protein (Mal)), TIR-domain-containing adaptor protein including interferon-β (TRIF), TRIF-related adaptor molecule (TRAM), or sterile adaptor α- and armadillo-motif-containing protein (SARM).
Figure 1.
Toll-like receptor (TLR), NOD-like receptor (NLR) and C-type lectin receptor (CLR) localization and example of ligand. TLRs can be located at plasma membrane and at endosomes, while NLRs are localized in cytoplasm. CLRs include soluble molecules such as MBL, and plasma membrane located receptors. See text for details. LTA: Lipoteichoic acid; LPS: Lipopolysaccharide; ssRNA: Single-stranded ribonucleic acid; iE-DAP: D-γ-glutamyl-meso-DAP dipeptide; MDP: Muramyl dipeptide; MBL: mannose binding lectin; ITIM: immunoreceptor tyrosine-based inhibition motifs; ITAM: immune-receptor tyrosine-based activation motif.
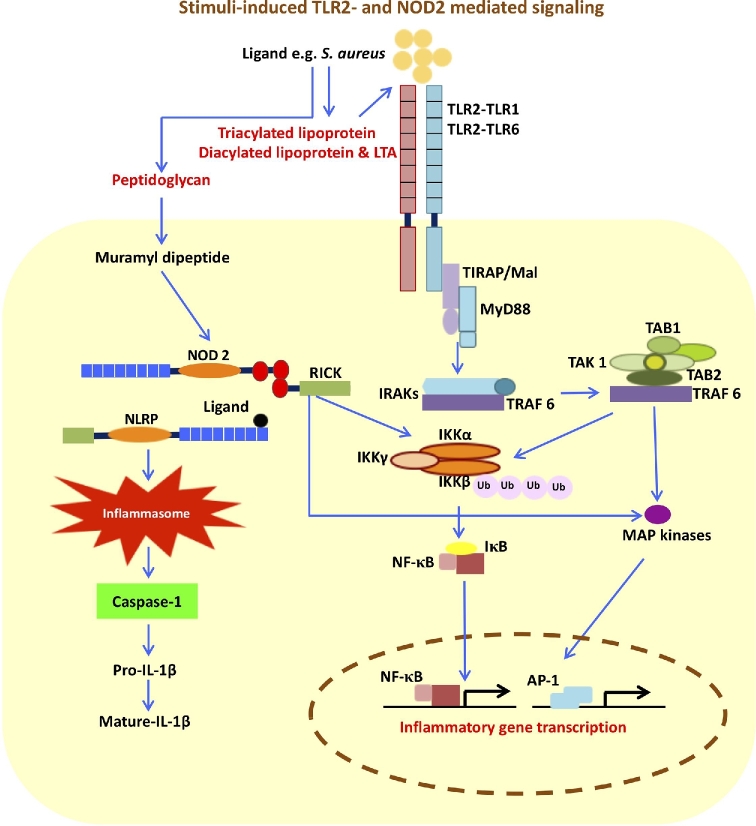
TIRAP has been distinctly involved in bridging MyD88 to TLR2 and TLR4 (Akira, Takeda and Kaisho 2001; Takeda and Akira 2004). MyD88 has a modular structure composed of an N-terminal death domain (DD), an intermediate domain (ID) and a C-terminal TIR domain (TIR) (Takeda and Akira 2004). The three domains of MyD88 are associated with interleukin-1 receptor-associated kinase 4 (IRAK4), IRAK1 and the TIR domain of TLRs, respectively. Binding of IRAK4 mediates the phosphorylation of IRAK1 (Janssens et al.2003; Takeda and Akira 2004) and recruitment of tumor necrosis factor receptor-associated factor 6 (TRAF6) to the receptor complex. Phosphorylated IRAK1 and TRAF6 dissociate from the receptor complex and associate with another complex composed of transforming growth factor-β-activated kinase-1 (TAK1), TAK1 binding protein 1 (TAB 1) and TAB2. This assembly, in turn, triggers activation of two distinct signaling pathways via the inhibitor of nuclear factor-κB (IKK) complex and the mitogen-activated protein kinases (MAPKs) (Akira, Uematsu and Takeuchi 2006; Kawai and Akira 2007; Kawai and Akira 2010). IKK complex formation, composed of IKKα, IKKβ and IKKγ/NEMO, is the bottleneck for multiple pathways ultimately leading to NF-κB activation, and thus important in regulating immune and inflammatory signaling pathways (Hacker and Karin 2006; Mogensen 2009; Kawai and Akira 2010) (Fig. 2). The TAK1 complex functions as a MAPK kinase kinase kinase (MAP3K), initiating a cascade involving phosphorylation of MAP kinase kinase (MAP2K) followed by activation of the MAP kinase (MAPK) subfamilies p38 and Jun-N-terminal kinase (JNK). The MAPK extracellular signal-regulated kinase (ERK) is also activated by TAK1 via the IKK complex. Stimulation of these pathways results in activation factor-1 (AP-1) induction (Ninomiya-Tsuji et al.1999; Kawai and Akira 2011). The TLR induced-activation of NF-κB and AP-1 play a critical role in the induction of inflammatory and innate immune responses, by regulating the expression of a vast number of cytokines, chemokines and antimicrobial effectors (Li and Verma 2002; Lawrence 2009; Xia et al.2016).
Figure 2.
Stimuli-induced TLR2- and NOD2- mediated signaling. The TLR- (e.g. TLR2) pathway is mainly mediated through MyD88. TIRAP is a sorting adaptor used by TLR2 and TLR4. MyD88 recruits IRAKs and TRAF6 and ultimately induces pro-inflammatory responses through activation of NF-κB and AP-1. NOD- (e.g. NOD2) induced NF-κB activation is mediated through RICK by triggering the K63-type polyubiquitination of IKKβ. The cooperation between NODs and NLRPs induces inflammasome formation resulting in production of mature IL-1β. MyD88: myeloid differentiation factor-88, TIRAP: TIR associated protein, IRAK: IL-1 receptor associated kinase, TRAF: tumor necrosis factor receptor-associated factor, TAK1: growth factor-β-activated kinase-1, TAB: TAK1 binding protein 1, IKK: IkappaB kinase, MAPK: mitogen activated protein kinase, NF-κB: nuclear factor kappa B, AP-1: activator protein 1, RICK: CARD-containing serine/threonine kinase.
The importance of TLR-mediated signaling in bacterial clearance
The primary staphylococcal PRR located on the plasma membrane is TLR2, which recognizes bacteria-derived PAMPs such as lipoteichoic acid (LTA) and lipoproteins (Schwandner et al.1999; Iwaki et al.2002; Schroder et al.2003; Dziarski and Gupta 2005). TLR2 is expressed by different cells involved in the inflammatory response such as neutrophils, monocytes/macrophages, dendritic cells, mast cells and astrocytes (reviewed in Fournier and Philpott (2005)). The dual ligand specificity of TLR2 is determined by its dimerization partner. The formation of TLR2-TLR6 heterodimers is initiated by diacyl lipopeptides from Gram-positive bacteria, including S. aureus, (Takeuchi et al.2001), while triacyl lipopeptides from Gram-negative bacteria induce the formation of heterodimers with TLR1 (Takeuchi et al.2002) (Fig. 1).
TLR2 plays an important role in protection against sepsis caused by S. aureus strains that produce phenol-soluble modulin (PSM) (Hanzelmann et al.2016). In addition, a critical role for TLR2 signaling in innate immunity is corroborated by the impact of naturally occurring mutations or polymorphisms in the receptor or downstream components involved in TLR2 signaling, increasing the risk of developing pyogenic bacterial infections. For instance, TLR2- (Takeuchi, Hoshino and Akira 2000; Gonzalez-Zorn et al.2005; Hoebe et al.2005; Strunk et al.2010) and MyD88-deficient (Takeuchi, Hoshino and Akira 2000) mice are predisposed to S. aureus infection and MRSA nasal colonization. Also children carrying mutations in genes encoding TLR- signaling components, such as MyD88, or IRAK4 (Picard et al.2010; von Bernuth et al.2012) or children with the TLR2 polymorphisms Arg753Gln, are at high risk of developing infections with S. aureus and other Gram-positive bacterial pathogens (Carpenter and O’Neill 2007).
Downregulation of TLR2-mediated NF-κB activation by S. aureus
Staphylococcus aureus is detected by the host through its multiple PAMPs, which leads to the release of proinflammatory mediators and antimicrobial peptides (AMPs) through PRR signaling and inflammasome activation (reviewed in Fournier and Philpott (2005)). TLR2 activates the immune cells in response to its agonists, bacterial lipoproteins (Takeuchi, Hoshino and Akira 2000; Hashimoto et al.2006). Staphylococcus aureus PSMs could influence the activation of TLR2 since the protein supports mobilizing and release of lipoprotein (Hanzelmann et al.2016). Notably, S. aureus deficient in lipoprotein synthesis lose their capability to activate this receptor (Stoll et al.2005). In addition, the pathogen dampens appropriate TLR2 activation by at least three distinct mechanisms: inhibition of heterodimer formation, structural mimicry of the TIR domain and activation of inhibitory receptor pathways. These three mechanisms are described below.
Evasion of initial recognition and heterodimer formation
The role of staphylococcal superantigen-like proteins (SSLs) in pathogenesis has been proven in a large number of studies (Baker et al.2007; Bestebroer et al.2007; de Haas et al.2009; Walenkamp et al.2009; Itoh et al.2010a,b; Patel et al.2010; Walenkamp et al.2010; Koymans et al.2017). The SSL family consist of 14 different members (SSL1-SSL14), out of which SSL1-SSL11 and SSL12-SSL14 proteins are encoded by genes located on staphylococcal pathogenicity island 2 (SaPI2) and immune evasion cluster 2 (IEC2), respectively (Fitzgerald et al.2003; Jongerius et al.2007). Among these, SSL3, and to a lesser extent SSL4, negatively interfere with TLR2 recognition, with a net outcome of suppressing chemokine (e.g. IL-8) production by HEK cells explicitly expressing TLR1/2 and TLR2/6 dimers (Bardoel et al.2012). SSL3 has also been shown to block TLR2-mediated secretion of tumor necrosis factor (TNF) in murine macrophages (Yokoyama et al.2012). In the latter case, SSL3 blocked binding of bacterial lipopeptides to the extracellular domain of TLR2 and in the case of ligand binding, prevented the formation of TLR2–TLR1 and TLR2–TLR6 heterodimers (Koymans et al.2015).
Evasion through structural mimicry
Structural mimicry of host proteins is an important mechanism by which pathogens manipulate host immune responses (Stebbins and Galan 2001), including TLR-mediated signaling through PAMP recognition (reviewed in Johannessen et al. (2014)). Bacterial TIR-homologous proteins have been identified in a wide range of Gram-negative bacteria, and more recently, in Gram-positive species. Members of the bacterial TIR-homologous protein family are typically 230-310 amino acids in size, wherein the conserved TIR domain, 150-200 amino acids in length, is localized at the C-terminus or N-terminus, while the remaining protein sequence shows a high degree of variation (reviewed in Rana et al. (2013)). The majority of investigations on bacterial TIR proteins have demonstrated that these proteins negatively interfere with TLR signaling pathways and consequently inhibit NF-κB activation (Newman et al.2006; Low et al.2007; Cirl et al.2008; Radhakrishnan et al.2009; Rana et al.2011; Askarian et al.2014; Kraemer et al.2014; Zou et al.2014). However, on one occasion, the bacterial TIR protein resulted in activation of same pathway (Patterson et al.2014). Recently a TIR-containing protein (TirS) was identified in the genome of S. aureus strain MSSA476 and found to be localized at the mobile genetic element ‘staphylococcal chromosomal cassettes’ (SCC)476. TirS exerted a specific inhibitory effect against stimulus-induced TLR2-mediated NF-κB activation, JNK phosphorylation, and production of proinflammatory cytokines. Furthermore, a comparison of pathogenicity of the MSSA476 wild-type strain vs. its isogenic ΔtirS mutant in an intravenous mouse infection model revealed that presence of TirS increased the bacterial load in multiple organs (Askarian et al.2014). Later, TirS was reported to be present in 12% of S. aureus (both MSSA and MRSA) strains, and found to downregulate NF-κB pathway through inhibition of not only TLR2, but also TLR4, TLR5 and TLR9 (Patot et al.2017). TirS in these S. aureus strains was localized within SCC together with genes encoding fusidic acid resistance and, in some cases, genes providing resistance to methicillin. The characterization of these SSC elements links two critical clinco-pathological features of contemporary staphylococcal infection, immune evasion and antibiotic resistance, since exposure to fusidic acid increased TirS expression (Patot et al.2017). TirS has also been described in an animal isolate of S. aureus (Patterson et al.2014). The only other Gram-positive bacterial TIR protein known to date was identified in Entereococcus faecalis, where it is localized in a genomic region of phage origin, which might suggest horizontal gene transfer (Kraemer et al.2014; Zou et al.2014).
Manipulation of TLRs by activating inhibitory receptors
Inhibitory immune receptors negatively interfere with signal transduction induced by activating receptors. Many inhibitory receptors have specific intracellular sequence motifs, like immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Activation of ITIM-bearing receptors results in phosphorylation of ITIMs and subsequent recruitment of mediators, which causes them to negatively regulate inflammatory signals from activating receptors such as TLRs (van Avondt, van Sorge and Meyaard 2015). Murine paired Ig-like receptor (PIR)-B is identified as a receptor for S. aureus LTA. PIR-B has ITIMs in the cytoplasmic domain and negatively regulates inflammatory cytokine production in response to heat-killed S. aureus in vitro. Mice that are deficient in PIR-B (Pirb-/-) have reduced bacterial load in blood, increased clearance of S. aureus and survive longer than wildtype mice after infection, showing the adverse effect of PIR-B activation for S. aureus clearance (Nakayama et al.2007, 2012).
Potential biotherapeutic implications of interference with TLR2 signaling
Insights into the negative regulation of TLR signaling by microbes such as S. aureus may open new avenues to develop treatments of uncontrolled inflammation.
Therapeutic interference with anomalous immune responses may in theory be possible. The therapeutic potential of microbial TIR domain proteins may be utilized in a highly specific way as they come in a wide variety and have fine differences in their action on TLR signaling (Rana et al.2013). Derived from a vaccinia virus A46R protein, VIPER was generated as a potent inhibitor of TLR4-mediated responses (Lysakova-Devine et al.2010). Cell-penetrating inhibitory peptides are targeting TLR adapter-receptor interactions were based on the BB loop region of the TIR domain and the intermediate domain within MyD88 (reviewed in Fekonja, Avbelj and Jerala (2012)). Interestingly their action could be enhanced by applying concepts discovered in microbial TIR domain proteins. Since coiled–coiled mediated TIR domain dimerization is a method exerted by bacterial TIR domain proteins (Newman et al.2006), this strategy can be utilized to improve wide-spectrum TLR inhibitors (reviewed in Fekonja, Avbelj and Jerala (2012)). Thus research on eukaryotic and bacterial TIR domains or modified variants of them together is a valuable source of templates and strategies for drug design to downregulate an overactive innate immune response. Taken together, these drug development strategies suggest that antiinflammatory bacterial proteins targeting TLRs could be co-opted prevent, dampen or treat inflammatory diseases.
TLR2 also has a unique role in cancer progression and metastasis (Huang et al.2008), and the potential for anti-TLR2 antibody or TLR2 antagonist therapy in cancer immunotherapy has been suggested (Yang et al.2009, 2012). Negative regulators of TLRs have been identified and characterized (Kondo, Kawai and Akira 2012) and interference with TLR2 could also be of therapeutic interest for certain infections. For example, the addition of anti-TLR2 antibody to monocytes infected with Mycobacterium avium subsp. paratuberculosis enhances phagosome maturation and intracellular bacterial clearance (Weiss et al.2008). Moreover, immune-neutralization of TLR2 function with a monoclonal antibody against the extracellular domain of TLR2 was reported to induce a modulatory response to S. aureus during acute infection in vivo (Meng et al.2004). In contrast, pretreatment of animals with corticosteroids did not reduce mortality during S. aureus infection (Opal and Cohen 1999) and anticytokine therapies had a detrimental effect in Gram-positive sepsis (Opal and Cohen 1999; Tracey and Abraham 1999). In all cases, the collaborative activity among different PRRs must be considered in the development of therapeutic strategies against inflammation, cancer and/or infection.
NOD like receptor signaling
The nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) are a family of intracellular/cytosolic PRRs, consisting of more than 20 members in humans. NLRs recognize PAMPs and endogenous molecules introduced into host cell cytosol (Moreira and Zamboni 2012). These receptors are classified to five different subfamilies based on N-terminal structural characteristics and include NLRA (A for acidic transactivation domain), NLRB (B for baculovirus inhibitor of apoptosis protein repeat), NLRC (C for caspase recruitment domain CARD), NLRP (P for pyrin domain) and NLRX (X for no significant homology). The activation of NLRs can result in transcriptional activation (e.g. NLRA), autophagy (e.g. NLRCs and NLRX), inflammasome formation (e.g. NLRPs and NLRB) and/or signal transduction (e.g. NLRCs (Motta et al.2015)). In the following, NLRPs and NLRCs are described in more detail.
Activation of NLRPs induces the assembly of a multiprotein platform called the inflammasome, triggers activation of caspase-1 to promote maturation and secretion of caspase 1-dependent inflammatory cytokines such as IL-1β and IL-18, and ultimately initiates programmed cellular pyroptosis (Munoz-Planillo et al.2009; Schroder and Tschopp 2010), an extremely rapid form of programmed host cell death. Pyroptosis leads to the sudden release of numerous host molecules that further amplify inflammation (Aachoui et al.2013). In general, NLRP3 can be activated upon sensing a wide range of stress signals, e.g. lysosomal damage, reactive oxygen species (ROS), the efflux of potassium, and pathogen-derived molecules (Willingham et al.2007; Schroder and Tschopp 2010). The genetic background, at least in mice, can influence the level of macrophage pyroptosis, which in turn can affect the progression of S. aureus infection (Accarias et al.2015).
The NLRCs (NOD1 and NOD2) play key roles in both bacterial and non-bacterial infections. Their structures consist of a C-terminal LRR domain, which mediates protein–protein interaction, a central nucleotide-binding domain, and either a single or two N-terminal CARD domain(s). Activation of NOD1 and NOD2 is triggered by two different peptidoglycan motifs: D-glutamyl-meso-diaminopimelic acid (iE-DAP) of Gram-negative bacteria or muramyl dipeptide (MDP), which is the minimal bioactive structure of peptidoglycan in Gram-positive bacteria (Strober et al.2006; Moreira and Zamboni 2012; Oviedo-Boyso, Bravo-Patino and Baizabal-Aguirre 2014) (Fig. 1). Recombinant NOD2 binds MDP with high affinity, confirming a direct interaction and that NOD2 functions as a cytosolic PRR (Grimes et al.2012). Monomeric peptidoglycan fragments of S. aureus induce NOD2 activation (Volz et al.2010). Furthermore, the S. aureus α-hemolysin, a pore-forming cytotoxin, facilitates NOD2-dependent recognition of S. aureus during cutaneous infection (Hruz et al.2009).
Activation of NF-κB signaling pathways through NOD1 and NOD2 is associated with recruitment of a CARD-containing serine/threonine kinase called receptor-interacting serine/threonine protein kinase 2 (RICK, also called RIP2 and RIPK2), which interacts with the CARD domain(s) of NOD1 and NOD2. Homotopic CARD–CARD interaction is achieved through recruitment of E3 ubiquitin ligase TRAF6 to RICK, which results in a series of ubiquitination-dependent events and ultimately activation of the TAK1 complex. Activated TAK1 triggers K63-type polyubiquitination of IKKβ and subsequently NF-κB activation (Ogura et al.2001; Strober et al.2006; Hasegawa et al.2008; Moreira and Zamboni 2012) (Fig. 2). Furthermore, activation of other signaling pathways, including the p38, ERK and JNK through NODs-RICK interaction, has been reported. NODs can also interact with NLRPs (e.g. NLRP1, NLRP3 and NLRP12) and result in caspase-1 activation and maturation of L-1β and IL-18 (Strober et al.2006; Moreira and Zamboni 2012 and references therein) (Fig. 2).
Consequence of S. aureus activation of NLRs
The importance of NOD2 in induction of various cytokines, e.g. IL-1β and IL-6, and/or antimicrobial peptides, e.g. β-defensin, in host defense against S. aureus infection, has been demonstrated (Deshmukh et al.2009; Hruz et al.2009). For instance, NOD2-deficient mice show increased susceptibility and mortality upon intraperitoneal S. aureus infection. This susceptibility phenotype was associated with failure of neutrophil phagocytosis, high bacterial load in several organs and magnified production of Th-1 derived cytokines (Deshmukh et al.2009). Subcutaneous infection of NOD2-deficient mice with S. aureus resulted in large skin ulcerations, impaired bacterial clearance and delayed proinflammatory responses (Hruz et al.2009). Other data demonstrate that while NOD2 is not essential for bacterial clearance in experimental S. aureus pneumonia, it contributes significantly to the regulation of the corresponding lung inflammation during infection. Notably, pulmonary lesions and inflammation, which are a critical factor during the recovery of host from pneumonia, are less severe in NOD2-deficient mice (Kapetanovic et al.2010). Involvement of NOD2 in the initiation of host inflammatory responses in resident brain cells infected with S. aureus has also been reported (Liu, Chauhan and Marriott 2010). In addition, mutations in the NOD2 gene are associated with the development of clinically important chronic inflammatory diseases, such as an injured intestinal epithelial barrier in Crohn's disease (Abreu et al.2002; Cuthbert et al.2002).
Although S. aureus is commonly regarded as an extracellular bacterium, it can internalize and invade several types of non-professional phagocytes, including human keratinocytes (Kintarak et al.2004). NOD2 is functional in keratinocytes (Voss et al.2006) and its expression is elevated in the presence of several PAMPs (Kobayashi et al.2009) and during S. aureus infection (Roth et al.2014). Staphylococcus aureus-induced NOD2 expression is also found in mammary glands during bacterial mastitis (Wang et al.2015). Hence, these data indicate a likely role of NOD2 in intracellular recognition of S. aureus. Recently, a contribution of NOD2 in keratinocytes in mediating S. aureus-induced IL-17C expression was identified (Roth et al.2014). IL-17C is a member of IL-17 cytokine family, which plays a prominent role in the regulation of epithelial immune responses (Xie et al.2012). Patients suffering from atopic dermatitis (AD), a chronically relapsing inflammatory skin disease, are often colonized with S. aureus (Ong 2006). Notably, an association between NOD2 polymorphism and AD has been identified (Kabesch et al.2003; Macaluso et al.2007), suggesting impairment of S. aureus recognition among these patients. Additionally, a role of NOD2 in the induction of type I IFN signaling and secretion of IFN-β in response to S. aureus has been described (Parker et al.2014).
NLRP3 inflammasome activation can be triggered by phagocytosis of S. aureus and the following lysosome-mediated degradation of its peptidoglycan. However, S. aureus O-acetyl transferase A modifies the peptidoglycan, rendering it more resistant to lysosome-mediated degradation. In this manner, induction of inflammasome is thereby blunted, and the critical IL-1β-mediated immune response is subverted (Shimada et al.2010). NLRP3 can be triggered by S. aureus α-hemolysin in cultured cells in vitro and in murine models of pneumonia and skin infection (Craven et al.2009; Munoz-Planillo et al.2009; Cho et al.2012; Kebaier et al.2012). Notably, S. aureus α-hemolysin is expressed by the majority of S. aureus isolates (Menzies and Kourteva 2000; Haslinger et al.2003). In addition, S. aureus β- and γ- hemolysins (Munoz-Planillo et al.2009), Panton–Valentine leukocidin (PVL) (Holzinger et al.2012) and leukocidin A/B (LukAB, also known as LukGH) (Melehani et al.2015) activate the NLRP3 inflammasome in monocytes/macrophages resulting in maturation and secretion of IL-1β and IL-18 and induction of necrotic cell death. However, the role of this NLRP3-driven response in immunity against S. aureus in vivo remains controversial. The NLRP3-deficient host-signaling pathway is not required for bacterial clearance in a murine pneumonia infection model though inducing necrotic pulmonary injury (Kebaier et al.2012). However, in a murine S. aureus skin infection model, induction of IL-1β promotes abscess formation and bacterial clearance (Cho et al.2012). An antitoxin platform has been contemplated for a potential antiinfective/antiinflammatory therapy targeting the NLRP3 inflammasome. Immunization of mice with inactivated α-hemolysin or pharmaceutical inhibition of α-hemolysin attenuates the severity of S. aureus pneumonia (Ragle and Bubeck Wardenburg 2009; Ragle, Karginov and Bubeck Wardenburg 2010; Hua et al.2014). Recently, nanosponges have been suggested as a detoxification treatment for a variety of injuries and disease caused by pore-forming toxins such as α-hemolysin. These particles mimic red blood cell membrane and absorb different pore-forming toxins regardless of their molecular structures, and blunt inflammatory activation and pathology (Hu et al.2013; Copp et al.2014; Pang et al.2015; Escajadillo et al.2017).
Another member of this family is NLRP7, which can form an inflammasome after recognizing bacterial acylated lipopeptides, once again resulting in caspase-1-dependent IL-1β and IL-18 maturation. In one study, activation of NLRP7 by S. aureus was shown to diminish bacterial replication inside human macrophages (Khare et al.2012).
C-type lectin receptors and recognition of S. aureus
Conserved microbial carbohydrate structures are recognized by another class of PRRs called C-type lectin receptors (CLRs) and trigger innate and acquired immune responses (reviewed in Sancho and Reis e Sousa (2012); Hoving, Wilson and Brown (2014)). CLRs are widely expressed by different cell types including APCs. The term ‘C-type’ refers to their ability to recognition carbohydrates in calcium (Ca2+)-dependent manner. However, CLRs are able to recognize many non-carbohydrate ligands, such as lipids and proteins, through mechanisms that are not yet fully understood (Drickamer and Fadden 2002; Zelensky and Gready 2005). The lectin activity of these receptors is mediated by conserved carbohydrate-recognition domains (CRDs). Type I and type II CLRs typically carry multiple or a single CRD(s) domain, respectively. In addition, soluble/circulating CLRs exist and include mannose-binding lectin (MBL), which is an oligomeric protein that binds an array of carbohydrate patterns on pathogen surfaces (Zizzari et al.2015) (Fig. 1). The outcome of CLR-microbe engagement depends on the signaling motif. Generally, phosphorylation of cytoplasmic ITIMs leads to downregulation of cellular processes and the recruitment of phosphatases (e.g. SHP-1 and SHP-2), whereas phosphorylation of immune-receptor tyrosine-based activation motifs (ITAMs/hemITAM), results in an induction of kinase cascades (e.g. spleen tyrosine kinase (Syk)) and cellular activation. However, still another group of CLRs does not possess intracellular signaling motifs and signaling cascades proceed independently of kinases and/or phosphatases. Activation of CLRs ultimately triggers phagocytosis and antimicrobial responses (Sancho and Reis e Sousa 2012; Lepenies, Lee and Sonkaria 2013; Hoving, Wilson and Brown 2014).
Staphylococcal evasion factors for CLR recognition have not been described so far. However, S. aureus uses diverse strategies to interfere with later stages of the complement cascades that are induced by CLRs among others (Lambris, Ricklin and Geisbrecht 2008; Zipfel and Skerka 2014). The CLRs Clec-60 and Clec-87 play a role during host immune responses against staphylococcal infection in the invertebrate C. elegans model system (JebaMercy and Balamurugan 2012). In humans, low MBL levels and/or MBL2 polymorphism are associated with a high susceptibility to S. aureus bacteremia (Chong et al.2014). Even though MBL binds LTA of Gram-positive bacteria, a comparative study found that the LTA of S. aureus, which lacks terminal sugars, binds only weakly to MBL (Polotsky et al.1996). L-ficolin, whose structure closely resembles MBL, is another soluble LTA-binding lectin that has been proposed as the major staphylococcal complement activating receptor (Lynch et al.2004). Additionally, S. aureus LTA and cell wall glycopolymer (CWG) can be recognized by the surfactant proteins A and D, which are collectins, a subgroup of mammalian lectins (Lawson and Reid 2000; van de Wetering et al.2001). MBL is involved in the activation of the lectin pathway (LP) of the complement system (Matsushita et al.2012) and the number of inhibitors triggered by this pathway by S. aureus is noteworthy. For instance, S. aureus extracellular adherence protein (Eap) blocks activation of the LP and classical pathway (CP) of complement (Woehl et al.2014, 2017). Unique mechanisms of action employed by staphylococcal complement inhibitors have been reviewed in several publications (Lambris, Ricklin and Geisbrecht 2008; Laarman et al.2010; Zecconi and Scali 2013).
Type I IFN signaling and recognition of S. aureus
Type I IFN signaling was initially identified during viral infections, but the pathway was later demonstrated to be activated by bacteria as well through multiple intracellular and cytosolic receptors (Kovarik et al.2016). More specifically, ligand binding to PRRs, such as TLRs, NLRs, RLRs or DNA sensors, results in activation of interferon regulatory factor (IRF) family of transcription factors, which results in expression of IFN-α/β. The interferons stimulate cells in an autocrine and paracrine manner (Boxx and Cheng 2016). Mechanistically, IFN-α/β binds to its cognate receptor, interferon α/β receptor (IFNAR), which leads to activation of hundreds of interferon-stimulated genes (ISGs) through phosphorylation of the homodimeric signal transducer and activation of transcription (STAT)-1 and the heterotrimeric STAT1–STAT2–IRF9 (i.e. interferon-stimulated gene factor (ISGF3) transcription factors) (Decker, Muller and Stockinger 2005; Monroe, McWhirter and Vance 2010) (Fig. 3). The outcome of bacterial infection as a consequence of type I interferon signaling is influenced by many factors including the route and site of infection, bacterial replication and strain-specific virulence factors (Boxx and Cheng 2016).
Figure 3.
PRR-mediated Type I IFN signaling in response to S. aureus. Staphylococcal molecules such as protein A, DNA and RNA are recognized by TLRs, e.g. TLR8 and TLR9, or other types of cytosolic receptors, e.g. NOD2 and STING, resulting in Type I IFN production. NOD2 recognizes the structurally unique MDP, while STING is receptor for e.g. cyclic di-AMP. In either case, an activation of type I IFN signaling occur in a TBK1-IRF3 mediated manner. The TLR-, NOD-, or STING mediated activation of IRF(s) family of transcription factors, results in expression of IFN-α/β. The type I IFNs, e.g. IFN-α and IFN-β, bind to their specific receptors IFNRs resulting in phosphorylation and activation JAK1 and TYK2. This leads to phosphorylation of STAT proteins, and consequently, rapid assembly of ISGF-3. The ISGF-3 complex then translocates to the nucleus, where it binds the promoter sequence ISRE. MDP: Muramyl Dipeptides, TBK1: Serine/threonine-protein kinase TBK1, IRF: interferon regulatory factor, JAK1: Janus kinase 1, TYK2: tyrosine kinase 2, STAT: signal transducer and activator of transcription, ISGF-3: IFN-stimulated gene factor 3, ISRE: IFN-stimulated response elements.
Staphylococcus aureus PAMPs induces type I IFN signaling by various PRRs, depending on the host cell types. Degradation of S. aureus in phagosomes can lead to exposure of bacterial deoxycytidylyl-deoxyguanosine dinucleotide (CpG) motifs that trigger a TLR9-mediated enhancement of classical inflammatory cytokines production along with induction of type I interferons (IFNs) (Wolf et al.2011). TLR9 requires an adaptor protein such as MyD88 and enhances mitogen-activated protein kinase and NF-κB activation (Wagner 2004). Injection of staphylococcal DNA in a mouse model of cutaneous inflammation causes a robust inflammatory response and arthritis (Deng et al.1999; Molne, Collins and Tarkowski 2003). TLR9 is the pivotal receptor mediating the induction of type I IFN signaling in dendritic cells in response to S. aureus. Interestingly, S. aureus load was enhanced in the lungs of TLR9−/− mice, which has no defect in recruitment of neutrophils and diminished TNF production (Parker and Prince 2012). Another example is TLR8, which recognizes degradation products of U-rich RNA in the form of uridine and short U-containing oligomers RNA (Heil et al.2004). TLR8 triggers a type I IFN response via an interferon regulatory factor (IRF)-5 signaling axis in primary human monocytes and macrophages. The TLR8-IRF5-type I IFN response in phagocytes is antagonized by TLR2 when the receptor is simultaneously activated by S. aureus. Indeed, S. aureus lipoprotein activates TLR2 to attenuate TLR8-induced secretion of IFNβ and IL12 by bacteria degraded within the phagosome (Bergstrom et al.2015). Novel approaches to avoid dysregulation of TLR2/TLR8 signaling may thus improve clinical outcomes in S. aureus sepsis.
Type I IFN signaling can also be induced by bacterial second messengers such as cyclic-di-AMP (c-di-AMP) or cyclic-di-GMP (c-di-GMP) (Parvatiyar et al.2012). These second messengers are sensed by the cytosolic DNA sensors DDX4 and IFI16, followed by activation of the transmembrane adaptor at the endoplasmatic reticulum, stimulator of IFN genes (STING) (Boxx and Cheng 2016). Recently, extracellular c-di-AMP was shown to promote S. aureus intracellular survival in human monocyte-derived macrophages (Gries et al.2016). However, STING signaling appears to be independent of other DNA sensing pathways such as the TLR9 pathway, although both pathways predominantly use TBK1 (Serine/threonine-protein kinase TBK1)-IRF3 and NF-κB to control gene expression (Kumar, Kawai and Akira 2011). Induction of the STING pathway in response to intracellular pathogens such as Listeria monocytogenes (Archer, Durack and Portnoy 2014) and M. tuberculosis (Manzanillo et al.2012) reduce host defense to infection. In a cutaneous S. aureus infection model, induction of STING antagonized innate immunity and allowed infection spread by impairing neutrophil recruitment and IL-1β secretion (Scumpia et al.2017).
Bacterial infection-mediated activation of type I IFNs, which influences the regulation of immune and tissue homeostasis, may have detrimental or beneficial consequences for the host (Kovarik et al.2016). Staphylococcus aureus invades both phagocytic and non-phagocytic cells (Kintarak et al.2004), and induces type I IFN signaling in various cell types (Kasahara et al.1982; Smith, Johnson and Blalock 1983; Svensson et al.1996; Liljeroos et al.2008; Parcina et al.2008; Martin et al.2009; Kaplan et al.2012; Parker and Prince 2012). Depending on context and site of infection, S. aureus-induced type I IFN signaling has been found to be either adverse (Martin et al.2009) or protective (Roquilly et al.2010; Kaplan et al.2012; Lizak and Yarovinsky 2012) to the experimentally infected host. For instance, in models of primary respiratory infection with S. aureus using Ifnar−/− mice, type I IFN signaling appears to play a negative role in the outcome of infection (Martin et al.2009). In contrast, in a subcutaneous model of infection, the induction of Ifnb expression is suggested to be important in controlling the infection. Indeed, supplementation of IFN-β to mice in a skin model of infection resulted in enhanced clearance of S. aureus and reduced lesion sizes (Kaplan et al.2012). Pathogenicity of S. aureus strains has been mainly correlated to the expression level of type I IFNs induced during infection and resulting exaggeration of inflammatory responses (Parker et al.2014). However, the full role of type I IFNs on the outcome and pathogenesis of staphylococcal infection and involvement of PRRs remains poorly understood. Future studies may open novel avenues in immunomodulatory therapy.
Collaboration between PRRs during S. aureus infections
PRRs as mediators of innate immunity orchestrate together to ensure host protection from pathogen invasion by fortifying epithelial barriers and cellular defenses. Collaboration and interplay broaden the spectrum of activity of PRRs, enabling integration of various pathways. However, the molecules that are associated with the synergistic activation of the several pathways merits further investigation.
MBL, as described above, functions as an opsonin and is involved in activation of the lectin pathway of the complement system through recognition of polysaccharide (i.e. glycan) (reviewed in van Kooyk and Rabinovich (2008); Ip et al. (2009)). Accumulating studies suggest a collaboration between MBL and TLRs (Wang et al.2011; Liu et al.2014), which is demonstrated to modulate cytokine responses during bacterial and viral infections (Jack et al.2001; Ip et al.2008; Ling et al.2012). For example, MBL cooperation with TLR2/TLR6 is initiated upon engulfment of S. aureus into the phagosome of macrophages. For this purpose, MBL binds to the LTA and complexes with TLR2 to enhance ligand delivery, resulting in enhancement of NF-κB activation (Ip et al.2008).
The synergistic interplay between TLR2 and NOD2 (Kobayashi et al.2005; Chen et al.2008; Volz et al.2010; Schaffler et al.2014) contributes to both induction or inhibition of specific immune responses (Netea et al.2004). Dendritic cells (DCs) are one of most important innate immune cells. The interplay between NOD2 and TLR2 results in cellular activation observed in murine DCs stimulated with S. aureus-derived PAMPs (Volz et al.2010). TLR2 can boost the NOD2 response to S. aureus, and NOD2-TLR2 co-stimulation leads to increased activation and maturation of DCs (Schaffler et al.2014). Notably, DCs lacking TLR2 were unresponsive to S. aureus-derived peptidoglycan (Volz et al.2010).
In addition, the cumulative effect of NOD2 and TLR2 activation in murine keratinocytes from oral epithelium resulted in induction of IL-6 and IL-1 β secretion. Importantly, the production of these cytokines was reduced by 50% in the absence of either NOD2 or TLR2 (Muller-Anstett et al.2010). However, NOD2 is shown to downregulate TLR1/2 mediated IL-1 β gene expression in macrophages (Dahiya, Pandey and Sodhi 2011). In line with that, NOD2 stimulation, but not NOD2-TLR2 costimulation, promoted intestinal homeostasis through downregulation of inflammatory pathways. This is supported by the finding that activation of NOD2 protected mice from experimental staphylococcal colitis (Watanabe et al.2008). Furthermore, a loss of function due to mutations in NOD2 increases the inflammatory pathogenesis of Crohn's disease (Schaffler et al.2014).
A selective form of autophagy, xenophagy, is involved in the degradation of long-lived cellular proteins, organelles or microbes within eukaryotic cells. In this process, targets are recognized, whereupon entrapment and sequestration of infectious agents into autophagic vesicles is promoted. Thereafter, lysosomes fuse with these autophagic vesicles to degrade their contents. Xenophagy is important for the direct and indirect killing of intracellular and extracellular pathogens, for the generation of bactericidal peptides, as well as for antigen presentation (Sorbara and Girardin 2015). Autophagy can also serve to limit the availability of inflammasome components and decrease proinflammatory signaling (Levine, Mizushima and Virgin 2011; Shi et al.2012). In host cells, both TLR- and NLR-stimulation is associated with induction of xenophagy as a method of defense against bacterial infection (Netea-Maier et al.2016). While xenophagy enhances host cell tolerance to S. aureus toxins (Maurer, Torres and Cadwell 2015), defective xenophagy is associated with cystic fibrosis and leads to overproduction of inflammatory cytokines as well as failure of S. aureus clearance (Jarry and Cheung 2006). Impaired xenophagy is also associated with Crohn's disease development in patients with NOD2 mutation, since NOD2 is needed to promote autophagosome formation (Travassos et al.2010; Caruso et al.2014). Thus, restoration of xenophagy may be considered as a promising area for research on novel therapeutic strategies targeting the intracellular stages of S. aureus infection.
Both TLR2 and NOD2 are known to participate in autophagy and phagocytosis activation via JNK signaling upon S. aureus infection (Fang et al.2014). However, the exact type of autophagy triggered by the bacteria has not been described so far. To evade killing by nonprofessional phagocytes, S. aureus can subvert xenophagy by inhibiting autophagosome maturation. By this, staphylococci replicate within the autophagosome, and subsequently escape to the cytoplasm whereupon they can induce host cell death (Schnaith et al.2007; O’Keeffe et al.2015; Soong et al.2015). Furthermore, S. aureus can evade keratinocyte-mediated clearance in skin infection. Differentiating keratinocytes, which generally recognize S. aureus via NOD2, are actively undergoing autophagy. The bacterium activates caspase-1-mediated bacterial clearance through pyroptosis (Aymard et al.2011). If keratinocytes fail to induce clearance, intracellular staphylococci stimulate autophagy, which in turn promotes degradation of inflammasome components, ultimately aiding the survival of S. aureus (Soong et al.2015).
All these findings reflect the synergy between various PRRs, their signaling cascades and induced defense mechanisms against S. aureus, and also how the pathogen uses multiple vantage points to circumvent these defenses. Moreover, this collaborative activity could potentially be exploited to modulate or improve the host response against pathogenic bacteria that induce an exacerbated inflammatory response.
CONCLUSION REMARKS AND FUTURE PERSPECTIVE
A fundamental role of the innate immunity is to evoke an appropriate response against invading pathogens or to tissue injury. However, genetic polymorphisms and consequently the expression levels of receptors for bacterial virulence factors can determine the mode and manner of host immune response (Peacock et al.2003; Johannessen, Sollid and Hanssen 2012; Weidenmaier, Goerke and Wolz 2012). Staphylococcus aureus, like many other pathogens, evolved various ways to induce tolerance or evade eradication by the host immune system. A wide range of bacterial proteins has been identified that interfere with the induction of proinflammatory responses (Gordon and Lowy 2008; Miller and Diep 2008; Krishna and Miller 2012). Importantly, these antiinflammatory virulence factors targeting NF-κB and MAKP pathways can be served as templates in the development of therapeutic agents to prevent, dampen, or treat chronic inflammatory conditions caused by dysregulation of the very same signaling pathways. Thus unraveling of the underlying mechanism of staphylococcal survival in host, besides a basic understanding of infection process, promotes two important fields of drug development: discovery of novel target points for antimicrobial therapy and new immune-modifying applications to fight against inflammatory diseases.
FUNDING
This work was supported by the Northern Norway Regional Health Authority [Helse Nord RHF SFP1231-15] and U.S. National Institutes of Health [U01 AI124316 & R01 HL125352].
Conflicts of interest. None declared.
REFERENCES
- Aachoui Y, Sagulenko V, Miao EA et al. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol 2013;16:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT, Taylor KD, Lin YC et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology 2002;123:679–88. [DOI] [PubMed] [Google Scholar]
- Accarias S, Lugo-Villarino G, Foucras G et al. Pyroptosis of resident macrophages differentially orchestrates inflammatory responses to Staphylococcus aureus in resistant and susceptible mice. Eur J Immunol 2015;45:794–806. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001;2:675–80. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog 2014;10:e1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarian F, van Sorge NM, Sangvik M et al. A Staphylococcus aureus TIR domain protein virulence factor blocks TLR2-mediated NF-kappaB signaling. J Innate Immun 2014;6:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard E, Barruche V, Naves T et al. Autophagy in human keratinocytes: an early step of the differentiation? Exp Dermatol 2011;20:263–8. [DOI] [PubMed] [Google Scholar]
- Baker HM, Basu I, Chung MC et al. Crystal structures of the staphylococcal toxin SSL5 in complex with sialyl Lewis X reveal a conserved binding site that shares common features with viral and bacterial sialic acid binding proteins. J Mol Biol 2007;374:1298–308. [DOI] [PubMed] [Google Scholar]
- Bardoel BW, Vos R, Bouman T et al. Evasion of Toll-like receptor 2 activation by staphylococcal superantigen-like protein 3. J Mol Med 2012;90:1109–20. [DOI] [PubMed] [Google Scholar]
- Bergstrom B, Aune MH, Awuh JA et al. TLR8 senses Staphylococcus aureus RNA in human primary monocytes and macrophages and induces IFN-beta production via a TAK1-IKKbeta-IRF5 signaling pathway. J Immunol 2015;195:1100–11. [DOI] [PubMed] [Google Scholar]
- Bestebroer J, Poppelier MJ, Ulfman LH et al. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 2007;109:2936–43. [DOI] [PubMed] [Google Scholar]
- Boxx GM, Cheng G. The roles of Type I interferon in bacterial infection. Cell Host Microbe 2016;19:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S, O’Neill LAJ. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol 2007;9:1891–901. [DOI] [PubMed] [Google Scholar]
- Caruso R, Warner N, Inohara N et al. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 2014;41:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Zhang L, Huang J et al. Cooperation between NOD2 and Toll-like receptor 2 ligands in the up-regulation of mouse mFPR2, a G-protein-coupled Abeta42 peptide receptor, in microglial cells. J Leukoc Biol 2008;83:1467–75. [DOI] [PubMed] [Google Scholar]
- Cho JS, Guo Y, Ramos RI et al. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog 2012;8:e1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong YP, Park KH, Kim ES et al. Association of mannose-binding lectin 2 gene polymorphisms with persistent Staphylococcus aureus bacteremia. PLoS One 2014;9:e89139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirl C, Wieser A, Yadav M et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med 2008;14:399–406. [DOI] [PubMed] [Google Scholar]
- Collins SM. Similarities and dissimilarities between asthma and inflammatory bowel diseases. Aliment Pharmacol Ther 1996;10:25–31. [DOI] [PubMed] [Google Scholar]
- Copp JA, Fang RH, Luk BT et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci USA 2014;111:13481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RR, Gao X, Allen IC et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 2009;4:e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AP, Fisher SA, Mirza MM et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 2002;122:867–74. [DOI] [PubMed] [Google Scholar]
- Dahiya Y, Pandey RK, Sodhi A. Nod2 downregulates TLR2/1 mediated IL1beta gene expression in mouse peritoneal macrophages. PLoS One 2011;6:e27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas CJ, Weeterings C, Vughs MM et al. Staphylococcal superantigen-like 5 activates platelets and supports platelet adhesion under flow conditions, which involves glycoprotein Ibalpha and alpha IIb beta 3. J Thromb Haemost 2009;7:1867–74. [DOI] [PubMed] [Google Scholar]
- Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol 2005;5:675–87. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Otto M, Kreiswirth BN et al. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010;375:1557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng GM, Nilsson IM, Verdrengh M et al. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat Med 1999;5:702–5. [DOI] [PubMed] [Google Scholar]
- Deshmukh HS, Hamburger JB, Ahn SH et al. Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun 2009;77:1376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K, Fadden AJ. Genomic analysis of C-type lectins. Biochem Soc Symp 2002;69:59–72. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Gupta D. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infect Immun 2005;73:5212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escajadillo T, Olson J, Luk BT et al. A red blood cell membrane-camouflaged nanoparticle counteracts Streptolysin O-mediated virulence phenotypes of invasive Group A Streptococcus. Front Pharmacol 2017;8:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Wu HM, Ding PS et al. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell Signal 2014;26:806–14. [DOI] [PubMed] [Google Scholar]
- Farhat K, Riekenberg S, Heine H et al. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol 2008;83:692–701. [DOI] [PubMed] [Google Scholar]
- Fekonja O, Avbelj M, Jerala R. Suppression of TLR signaling by targeting TIR domain-containing proteins. Curr Protein Pept Sci 2012;13:776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JR, Reid SD, Ruotsalainen E et al. Genome diversification in Staphylococcus aureus: molecular evolution of a highly variable chromosomal region encoding the Staphylococcal exotoxin-like family of proteins. Infect Immun 2003;71:2827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ. Immune evasion by Staphylococci. Nat Rev Micro 2005;3:948–58. [DOI] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK et al. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 2014;12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 2005;18:521–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Zorn B, Senna JP, Fiette L et al. Bacterial and host factors implicated in nasal carriage of methicillin-resistant Staphylococcus aureus in mice. Infect Immun 2005;73:1847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46:S350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gries CM, Bruger EL, Moormeier DE et al. Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage Type I interferon response. Infect Immun 2016;84:3564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CL, Ariyananda LD, Melnyk JE et al. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 2012;134:13535–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE 2006(357):re13. [DOI] [PubMed] [Google Scholar]
- Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol 2005;6:1198–205. [DOI] [PubMed] [Google Scholar]
- Hanzelmann D, Joo HS, Franz-Wachtel M et al. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat Commun 2016;7:12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Fujimoto Y, Lucas PC et al. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J 2008;27:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Tawaratsumida K, Kariya H et al. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol 2006;177:3162–9. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Strangfeld K, Peters G et al. Staphylococcus aureus alpha-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-alpha and the mitochondrial death pathway. Cell Microbiol 2003;5:729–41. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004;303:1526–9. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S et al. CD36 is a sensor of diacylglycerides. Nature 2005;433:523–7. [DOI] [PubMed] [Google Scholar]
- Holzinger D, Gieldon L, Mysore V et al. Staphylococcus aureus Panton–Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol 2012;92:1069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoving JC, Wilson GJ, Brown GD. Signalling C-type lectin receptors, microbial recognition and immunity. Cell Microbiol 2014;16:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz P, Zinkernagel AS, Jenikova G et al. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci USA 2009;106:12873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Fang RH, Copp J et al. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol 2013;8:336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L, Hilliard JJ, Shi Y et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 2014;58:1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Zhao J, Unkeless JC et al. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 2008;27:218–24. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Ezekowitz RA et al. Mannose-binding lectin and innate immunity. Immunol Rev 2009;230:9–21. [DOI] [PubMed] [Google Scholar]
- Ip WK, Takahashi K, Moore KJ et al. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med 2008;205:169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Hamada E, Kamoshida G et al. Staphylococcal superantigen-like protein 5 inhibits matrix metalloproteinase 9 from human neutrophils. Infect Immun 2010a;78:3298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S, Hamada E, Kamoshida G et al. Staphylococcal superantigen-like protein 10 (SSL10) binds to human immunoglobulin G (IgG) and inhibits complement activation via the classical pathway. Mol Immunol 2010b;47:932–8. [DOI] [PubMed] [Google Scholar]
- Iwaki D, Mitsuzawa H, Murakami S et al. The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem 2002;277:24315–20. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987–95. [DOI] [PubMed] [Google Scholar]
- Jack DL, Read RC, Tenner AJ et al. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis 2001;184:1152–62. [DOI] [PubMed] [Google Scholar]
- Janssens S, Burns K, Vercammen E et al. MyD88S, a splice variant of MyD88, differentially modulates NF-kappaB- and AP-1-dependent gene expression. FEBS Lett 2003;548:103–7. [DOI] [PubMed] [Google Scholar]
- Jarry TM, Cheung AL. Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun 2006;74:2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JebaMercy G, Balamurugan K. Effects of sequential infections of Caenorhabditis elegans with Staphylococcus aureus and Proteus mirabilis. Microbiol Immunol 2012;56:825–35. [DOI] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007;130:1071–82. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Sollid JE, Hanssen AM. Host- and microbe determinants that may influence the success of S. aureus colonization. Front Cell Inf Microbiol 2012;2:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Askarian F, Sangvik M et al. Bacterial interference with canonical NF kappa B signalling. Microbiology 2013;159:2001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongerius I, Kohl J, Pandey MK et al. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med 2007;204:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M, Peters W, Carr D et al. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol 2003;111:813–7. [DOI] [PubMed] [Google Scholar]
- Kapetanovic R, Jouvion G, Fitting C et al. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect 2010;12:759–67. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Ma J, Kyme P et al. Failure to induce IFN-beta production during Staphylococcus aureus infection contributes to pathogenicity. J Immunol 2012;189:4537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T, Harada H, Shioiri-Nakano K et al. Potentiation of natural killer cell activity of human lymphocytes in vitro: the participation of interferon in stimulation with Staphylococcus aureus Cowan I bacteria but not with protein A. Immunology 1982;45:687–95. [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappa B by Toll-like receptors. Trends Mol Med 2007;13:460–9. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–84. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011;34:637–50. [DOI] [PubMed] [Google Scholar]
- Kebaier C, Chamberland RR, Allen IC et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 2012;205:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S, Dorfleutner A, Bryan NB et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 2012;36:464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Thammavongsa V, Schneewind O et al. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012;15:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintarak S, Whawell SA, Speight PM et al. Internalization of Staphylococcus aureus by human keratinocytes. Infect Immun 2004;72:5668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005;307:731–4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yoshiki R, Sakabe J et al. Expression of toll-like receptor 2, NOD2 and dectin-1 and stimulatory effects of their ligands and histamine in normal human keratinocytes. Brit J Dermatol 2009;160:297–304. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 2012;33:449–58. [DOI] [PubMed] [Google Scholar]
- Kovarik P, Castiglia V, Ivin M et al. Type I interferons in bacterial infections: a balancing act. Front Immunol 2016;7:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koymans KJ, Feitsma LJ, Brondijk TH et al. Structural basis for inhibition of TLR2 by staphylococcal superantigen-like protein 3 (SSL3). Proc Natl Acad Sci USA 2015;112:11018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koymans KJ, Goldmann O, Karlsson CAQ et al. The TLR2 antagonist staphylococcal superantigen-like protein 3 acts as a virulence factor to promote bacterial pathogenicity in vivo. J Innate Immun. 2017;9:561–73. [DOI] [PubMed] [Google Scholar]
- Kraemer TD, Quintanar Haro OD, Domann E et al. The TIR domain containing locus of Enterococcus faecalis is predominant among urinary tract infection isolates and downregulates host inflammatory response. Int Microbiol 2014;2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Miller LS. Host–pathogen interactions between the skin and Staphylococcus aureus. Curr Opin Microbiol 2012;15:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krismer B, Weidenmaier C, Zipperer A et al. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat Rev Microbiol 2017;15:675–87. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 2011;30:16–34. [DOI] [PubMed] [Google Scholar]
- Laarman A, Milder F, van Strijp J et al. Complement inhibition by gram-positive pathogens: molecular mechanisms and therapeutic implications. J Mol Med 2010;88:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol 2008;6:132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A et al. Erratum: Corrigendum: Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol 2007;8:1266. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev 2000;173:66–78. [DOI] [PubMed] [Google Scholar]
- Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev 2013;65:1271–81. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011;469:323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QT, Verma IM. NF-kappa B regulation in the immune system. Nat Rev Immunol 2002;2:725–34. [DOI] [PubMed] [Google Scholar]
- Liljeroos M, Vuolteenaho R, Rounioja S et al. Bacterial ligand of TLR2 signals Stat activation via induction of IRF1/2 and interferon-alpha production. Cell Signal 2008;20:1873–81. [DOI] [PubMed] [Google Scholar]
- Ling MT, Tu W, Han Y et al. Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. J Infect Dis 2012;205:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhou J, Ma D et al. Mannan binding lectin attenuates double-stranded RNA-mediated TLR3 activation and innate immunity. FEBS Lett 2014;588:866–72. [DOI] [PubMed] [Google Scholar]
- Liu L, Botos I, Wang Y et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 2008;320:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Chauhan VS, Marriott I. NOD2 contributes to the inflammatory responses of primary murine microglia and astrocytes to Staphylococcus aureus. Neurosci Lett 2010;474:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizak M, Yarovinsky TO. Phospholipid scramblase 1 mediates type I interferon-induced protection against staphylococcal alpha-toxin. Cell Host Microbe 2012;11:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M Jr. Immune signaling by RIG-I-like receptors. Immunity 2011;34:680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LY, Mukasa T, Reed JC et al. Characterization of a TIR-like protein from Paracoccus denitrificans. Biochem Biophys Res Commun 2007;356:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch NJ, Roscher S, Hartung T et al. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol 2004;172:1198–202. [DOI] [PubMed] [Google Scholar]
- Lysakova-Devine T, Keogh B, Harrington B et al. Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J Immunol 2010;185:4261–71. [DOI] [PubMed] [Google Scholar]
- Macaluso F, Nothnagel M, Parwez Q et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol 2007;16:692–8. [DOI] [PubMed] [Google Scholar]
- Manzanillo PS, Shiloh MU, Portnoy DA et al. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 2012;11:469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FJ, Gomez MI, Wetzel DM et al. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest 2009;119:1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Kilpatrick DC, Lee BL et al. Soluble host-defense lectins. J Biomed Biotechnol 2012;2012:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer K, Torres VJ, Cadwell K. Autophagy is a key tolerance mechanism during Staphylococcus aureus infection. Autophagy 2015;11:1184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C Jr.. Innate immunity. N Engl J Med 2000;343:338–44. [DOI] [PubMed] [Google Scholar]
- Melehani JH, James DB, DuMont AL et al. Staphylococcus aureus Leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog 2015;11:e1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng GX, Rutz M, Schiemann M et al. Antagonistic antibody prevents toll-like receptor 2–driven lethal shock-like syndromes. J Clin Invest 2004;113:1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies BE, Kourteva I. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol Med Microbiol 2000;29:39–45. [DOI] [PubMed] [Google Scholar]
- Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008;46:752–60. [DOI] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009;22:240–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molne L, Collins LV, Tarkowski A. Inflammatogenic properties of bacterial DNA following cutaneous exposure. J Invest Dermatol 2003;121:294–9. [DOI] [PubMed] [Google Scholar]
- Monroe KM, McWhirter SM, Vance RE. Induction of type I interferons by bacteria. Cell Microbiol 2010;12:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LO, Zamboni DS. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol 2012;3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morr M, Takeuchi O, Akira S et al. Differential recognition of structural details of bacterial lipopeptides by toll-like receptors. Eur J Immunol 2002;32:3337–47. [DOI] [PubMed] [Google Scholar]
- Motta V, Soares F, Sun T et al. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev 2015;95:149–78. [DOI] [PubMed] [Google Scholar]
- Muller-Anstett MA, Muller P, Albrecht T et al. Staphylococcal peptidoglycan co-localizes with NOD2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One 2010;5:e13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Planillo R, Franchi L, Miller LS et al. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol 2009;183:3942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Underhill DM, Petersen TW et al. Paired Ig-like receptors bind to bacteria and shape TLR-mediated cytokine production. J Immunol 2007;178:4250–9. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Kurokawa K, Nakamura K et al. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J Immunol 2012;189:5903–11. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, de Jong DJ et al. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur J Immunol 2004;34:2052–9. [DOI] [PubMed] [Google Scholar]
- Netea-Maier RT, Plantinga TS, van de Veerdonk FL et al. Modulation of inflammation by autophagy: consequences for human disease. Autophagy 2016;12:245–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RM, Salunkhe P, Godzik A et al. Identification and characterization of a novel bacterial virulence factor that shares homology with mammalian Toll/interleukin-1 receptor family proteins. Infect Immun 2006;74:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 1999;398:252–6. [DOI] [PubMed] [Google Scholar]
- Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem 2004;279:19008–17. [DOI] [PubMed] [Google Scholar]
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 2003;48:1429–49. [DOI] [PubMed] [Google Scholar]
- O’Keeffe KM, Wilk MM, Leech JM et al. Manipulation of autophagy in phagocytes facilitates Staphylococcus aureus bloodstream infection. Infect Immun 2015;83:3445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007;7:353–64. [DOI] [PubMed] [Google Scholar]
- O’Neill LAJ, Bryant CE, Doyle SL. Therapeutic targeting of toll-Like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev 2009;61:177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Inohara N, Benito A et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappa B. J Biol Chem 2001;276:4812–8. [DOI] [PubMed] [Google Scholar]
- Ong PY. Is/are pattern recognition receptor(s) for Staphylococcus aureus defective in atopic dermatitis? Dermatology 2006;212:19–22. [DOI] [PubMed] [Google Scholar]
- Opal SM, Cohen J. Clinical Gram-positive sepsis? Crit Care Med 1999;27:1608–16. [DOI] [PubMed] [Google Scholar]
- Ori D, Murase M, Kawai T. Cytosolic nucleic acid sensors and innate immune regulation. Int Rev Immunol 2017;36:74–88. [DOI] [PubMed] [Google Scholar]
- Oviedo-Boyso J, Bravo-Patino A, Baizabal-Aguirre VM. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators Inflamm 2014;2014:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA 2000;97:13766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Hu CM, Fang RH et al. Detoxification of organophosphate poisoning using nanoparticle bioscavengers. ACS Nano 2015;9:6450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcina M, Wendt C, Goetz F et al. Staphylococcus aureus-induced plasmacytoid dendritic cell activation is based on an IgG-mediated memory response. J Immunol 2008;181:3823–33. [DOI] [PubMed] [Google Scholar]
- Parker D, Prince A. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol 2012;189:4040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Planet PJ, Soong G et al. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog 2014;10:e1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvatiyar K, Zhang Z, Teles RM et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol 2012;13:1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Wines BD, Langley RJ et al. Specificity of staphylococcal superantigen-like protein 10 toward the human IgG1 Fc domain. J Immunol 2010;184:6283–92. [DOI] [PubMed] [Google Scholar]
- Patot S, Imbert PR, Baude J et al. Correction: The TIR homologue lies near resistance genes in staphylococcus aureus, coupling modulation of virulence and antimicrobial susceptibility. PLoS Pathog 2017;13:e1006291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson NJ, Gunther J, Gibson AJ et al. Two TIR-like domain containing proteins in a newly emerging zoonotic Staphylococcus aureus strain sequence type 398 are potential virulence factors by impacting on the host innate immune response. Front Microbiol 2014;5:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock SJ, Justice A, Griffiths D et al. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol 2003;41:5718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, von Bernuth H, Ghandil P et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 2010;89:403–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat G, Ugan RA, Cadirci E et al. Sepsis and septic shock: current treatment strategies and new approaches. Eurasian J Med 2017;49:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotsky VY, Fischer W, Ezekowitz RA et al. Interactions of human mannose-binding protein with lipoteichoic acids. Infect Immun 1996;64:380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan GK, Yu Q, Harms JS et al. Brucella TIR domain-containing protein mimics properties of the Toll-like receptor adaptor protein TIRAP. J Biol Chem 2009;284:9892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 2009;77:2712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragle BE, Karginov VA, Bubeck Wardenburg J. Prevention and treatment of Staphylococcus aureus pneumonia with a beta-cyclodextrin derivative. Antimicrob Agents Ch 2010;54:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana RR, Zhang MH, Spear AM et al. Bacterial TIR-containing proteins and host innate immune system evasion. Med Microbiol Immunol 2013;202:1–10. [DOI] [PubMed] [Google Scholar]
- Rana RR, Simpson P, Zhang MH et al. Yersinia pestis TIR-domain protein forms dimers that interact with the human adaptor protein MyD88. Microb Pathogenesis 2011;51:89–95. [DOI] [PubMed] [Google Scholar]
- Ratner AJ, Bryan R, Weber A et al. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem 2001;276:19267–75. [DOI] [PubMed] [Google Scholar]
- Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol 2005;13:596–601. [DOI] [PubMed] [Google Scholar]
- Roquilly A, Gautreau L, Segain JP et al. CpG-ODN and MPLA prevent mortality in a murine model of post-hemorrhage-Staphyloccocus aureus pneumonia. PLoS One 2010;5:e13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SA, Simanski M, Rademacher F et al. The Pattern recognition receptor NOD2 mediates Staphylococcus aureus –Induced IL-17C expression in keratinocytes. J Invest Dermatol 2014;134:374–80. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Prince LR, Jones EC et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 2003;170:5268–75. [DOI] [PubMed] [Google Scholar]
- Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 2012;30:491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler H, Demircioglu DD, Kuhner D et al. NOD2 stimulation by Staphylococcus aureus-derived peptidoglycan is boosted by Toll-Like receptor 2 costimulation with lipoproteins in dendritic cells. Infect Immun 2014;82:4681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaith A, Kashkar H, Leggio SA et al. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem 2007;282:2695–706. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821–32. [DOI] [PubMed] [Google Scholar]
- Schroder NW, Morath S, Alexander C et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 2003;278:15587–94. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Dziarski R, Wesche H et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 1999;274:17406–9. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, Botten GA, Norman SN et al. Opposing roles of Toll-like receptor and cytosolic DNA-STING signaling pathways for Staphylococcus aureus cutaneous host defense. PLoS Pathog 2017;13:e1006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Shenderov K, Huang NN et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 2012;13:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ et al. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 2010;7:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Johnson HM, Blalock JE. Staphylococcus aureus protein A induces the production of interferon-alpha in human lymphocytes and interferon-alpha/beta in mouse spleen cells. J Immunol 1983;130:773–6. [PubMed] [Google Scholar]
- Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev 2012;250:216–29. [DOI] [PubMed] [Google Scholar]
- Soong G, Reddy B, Sokol S et al. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest 2004;113:1482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong G, Paulino F, Wachtel S et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio 2015;6:e00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara MT, Girardin SE. Emerging themes in bacterial autophagy. Curr Opin Microbiol 2015;23:163–70. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Galan JE. Structural mimicry in bacterial virulence. Nature 2001;412:701–5. [DOI] [PubMed] [Google Scholar]
- Stoll H, Dengjel J, Nerz C et al. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun 2005;73:2411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A et al. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol 2006;6:9–20. [DOI] [PubMed] [Google Scholar]
- Strunk T, Power Coombs MR, Currie AJ et al. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One 2010;5:e10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson H, Cederblad B, Lindahl M et al. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus. J Interferon Cytokine Res 1996;16:7–16. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004;16:3–9. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000;165:5392–6. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999;11:443–51. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 2001;13:933–40. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002;169:10–14. [DOI] [PubMed] [Google Scholar]
- Tisoncik JR, Korth MJ, Simmons CP et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012;76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong SY, Davis JS, Eichenberger E et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Abraham E. From mouse to man: or what have we learned about cytokine-based anti-inflammatory therapies? Shock 1999;11:224–5. [PubMed] [Google Scholar]
- Travassos LH, Girardin SE, Philpott DJ et al. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep 2004;5:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos LH, Carneiro LA, Ramjeet M et al. NOD1 and NOD2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol 2010;11:55–62. [DOI] [PubMed] [Google Scholar]
- van Avondt K, van Sorge NM, Meyaard L. Bacterial immune evasion through manipulation of host inhibitory immune signaling. PLoS Pathog 2015;11:e1004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering JK, van Eijk M, van Golde LM et al. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J Infect Dis 2001;184:1143–51. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol 2008;9:593–601. [DOI] [PubMed] [Google Scholar]
- Volz T, Nega M, Buschmann J et al. Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals. FASEB J 2010;24:4089–102. [DOI] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Puel A et al. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur J Immunol 2012;42:3126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss E, Wehkamp J, Wehkamp K et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem 2006;281:2005–11. [DOI] [PubMed] [Google Scholar]
- Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol 2004;25:381–6. [DOI] [PubMed] [Google Scholar]
- Walenkamp AM, Bestebroer J, Boer IG et al. Staphylococcal SSL5 binding to human leukemia cells inhibits cell adhesion to endothelial cells and platelets. Cell Oncol 2010;32:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenkamp AM, Boer IG, Bestebroer J et al. Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia 2009;11:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu GT, Yu H et al. Characterization of TLR2, NOD2, and related cytokines in mammary glands infected by Staphylococcus aureus in a rat model. Acta Vet Scand 2015;57:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen Y, Zhang Y et al. Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol 2011;8:265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Asano N, Murray PJ et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest 2008;118:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C, Goerke C, Wolz C. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol 2012;20:243–50. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Souza CD, Evanson OA et al. Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. J Leukoc Biol 2008;83:48–55. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Bergstralh DT, O’Connor W et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2007;2:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehl JL, Ramyar KX, Katz BB et al. The structural basis for inhibition of the classical and lectin complement pathways by S. aureus extracellular adherence protein. Protein Sci 2017;26:1595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehl JL, Stapels DAC, Garcia BL et al. The extracellular adherence protein from Staphylococcus aureus inhibits the classical and lectin pathways of complement by blocking formation of the C3 proconvertase. J Immunol 2014;193:6161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Arruda A, Reyes CN et al. Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J Immunol 2011;187:6002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Wang S, Gao P et al. DNA sensor cGAS-mediated immune recognition. Protein Cell 2016;7:777–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XH, Wang LL, Gong FY et al. Intracellular Staphylococcus aureus-induced NF-kappa B activation and proinflammatory responses of P815 cells are mediated by NOD2. J Huazhong Univ Sci Technolog Med Sci 2012;32:317–23. [DOI] [PubMed] [Google Scholar]
- Yan J, Hua F, Liu HZ et al. Simultaneous TLR2 inhibition and TLR9 activation synergistically suppress tumor metastasis in mice. Acta Pharmacol Sin 2012;33:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HZ, Cui B, Liu HZ et al. Blocking TLR2 activity attenuates pulmonary metastases of tumor. PLoS One 2009;4:e6520. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yokoyama R, Itoh S, Kamoshida G et al. Staphylococcal superantigen-like protein 3 binds to the Toll-Like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infect Immun 2012;80:2816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecconi A, Scali F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol Lett 2013;150:12–22. [DOI] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J 2005;272:6179–217. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Staphylococcus aureus: the multi headed hydra resists and controls human complement response in multiple ways. Int J Med Microbiol 2014;304:188–94. [DOI] [PubMed] [Google Scholar]
- Zizzari IG, Napoletano C, Battisti F et al. MGL receptor and immunity: when the ligand can make the difference. J Immunol Res 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Baghdayan AS, Payne SJ et al. A TIR domain protein from E. faecalis attenuates MyD88-mediated signaling and NF-kappaB activation. PLoS One 2014;9:e112010. [DOI] [PMC free article] [PubMed] [Google Scholar]