Abstract
Flagella and motile cilia share a 9 + 2 microtubule-doublet axoneme structure, and asthenozoospermia (reduced spermatozoa motility) is found in 76% of men with primary ciliary dyskinesia (PCD). Nevertheless, causal genetic variants in a conserved axonemal component have been found in cases of isolated asthenozoospermia: 30% of men with multiple morphological anomalies of sperm flagella (MMAF) carry bi-allelic mutations in DNAH1, encoding one of the seven inner-arm dynein heavy chains of the 9 + 2 axoneme. To further understand the basis for isolated asthenozoospermia, we used whole-exome and Sanger sequencing to study two brothers and two independent men with MMAF. In three men, we found bi-allelic loss-of-function mutations in WDR66, encoding cilia- and flagella-associated protein 251 (CFAP251): the two brothers were homozygous for the frameshift chr12: g.122359334delA (p.Asp42Metfs∗4), and the third individual was compound heterozygous for chr12: g.122359542G>T (p.Glu111∗) and chr12: g.122395032_122395033delCT (p.Leu530Valfs∗4). We show that CFAP251 is normally located along the flagellum but is absent in men carrying WDR66 mutations and reveal a spermatozoa-specific isoform probably generated during spermatozoon maturation. CFAP251 is a component of the calmodulin- and radial-spoke- associated complex, located adjacent to DNAH1, on the inner surface of the peripheral microtubule doublets of the axoneme. In Tetrahymena, the CFAP251 ortholog is necessary for efficient coordinated ciliary beating. Using immunofluorescent and transmission electron microscopy, we provide evidence that loss of CFAP251 affects the formation of the mitochondrial sheath. We propose that CFAP251 plays a structural role during biogenesis of the spermatozoon flagellum in vertebrates.
Keywords: male infertility, asthenozoospermia, spermatogenesis, flagellum, mitochondrial sheath, human, genetic disorder
Main Text
It is estimated that 10%–15% of couples trying to have children fail to conceive without medical intervention after 1 year.1 In approximately 60% of infertile couples there is evidence of male infertility: reduced spermatozoa numbers (oligozoospermia or azoospermia) or an increased incidence of spermatozoa with abnormal morphology (teratozoospermia) or diminished motility (asthenozoospermia). Because artificial reproductive technology now allows many of these men to father their own children, there is a vital need to understand the causes of these male infertilities and their consequences for gamete quality. This will enable the improvement and extension of infertility treatment, as well as open the way to informed risk assessment tailored to each couple. It will also provide a wealth of information about the biology of gametogenesis and the proteins that are essential for human fertility.
There are close links between asthenozoospermia and primary ciliary dyskinesia (PCD [MIM: 244400]) given that spermatozoa flagella and motile cilia share the same highly conserved 9 + 2 microtubule-doublet axoneme structure.2 PCD is associated with mutations in genes required for the biogenesis of a fully functional axoneme and is characterized by inefficient or no ciliary beating in the respiratory tract, as well as situs inversus in 50% of affected individuals.3 Fertility is often also affected through compromised motility of cilia in the fallopian tubes (61% of affected females) or immotile spermatozoa flagella (76% of affected males).4 Nevertheless, some men with asthenozoospermia show multiple anomalies of the flagella but no clinical signs of PCD. This phenotype was originally characterized as a dysplasia of the fibrous sheath, the structure that normally forms around, and strengthens, the axoneme of the flagellum.5 Because the spermatozoa in each of these men manifest a heterogeneous set of flagellar malformations (short, absent, bent, coiled, and/or irregular width), this syndrome has recently been renamed as multiple morphological anomalies of the flagella, or MMAF (MIM: 617576).6
The genetic basis of MMAF is beginning to emerge. Bi-allelic genetic variants that cause MMAF were first identified in dynein axonemal heavy chain 1, DNAH1 (MIM: 603332), encoding a component of IDA3, one of the three inner dynein arms that repeat along the length of the peripheral microtubule doublets. This established that isolated asthenozoospermia can result from defects in axoneme components common to sperm flagella and motile cilia.6 Bi-allelic loss-of-function (LoF) mutations in DNAH1 have been found in 30%–50% of MMAF-affected men of North African or Chinese origin.6, 7, 8, 9 In other MMAF-affected individuals, mainly from China or North Africa, bi-allelic LoF mutations have recently been discovered in two genes, CFAP43 (MIM: 617558; 12 individuals [7%–10%]) and CFAP44 (MIM: 617559; nine individuals [5%–8%]), encoding the cilia- and flagella-associated proteins CFAP43 and CFAP44, respectively.10, 11, 12 These proteins are orthologs of FAP43 and FAP44, respectively, isolated from purified flagella in Chlamydomonas reinhardtii.13 CFAP43 and CFAP44 are tryptophan-aspartate (WD)-repeat-containing proteins and are detected along the human and mouse spermatozoon flagellum.10, 12 In Trypanosoma brucei, they are more precisely localized between the outer surface of the axoneme and the paraflagellar rod,10 a deformable lattice structure necessary for motility.14 On this basis, it was proposed that in mammals, CFAP43 and CFAP44 might link the axoneme to auxiliary structures such as the outer dense fibers and the fibrous sheath (FS) in the spermatozoon flagellum.10
In order to better understand the pathophysiology of isolated MMAF, we studied a group of four infertile men presenting with an asthenozoospermia (0%–4% motile spermatozoa) associated with MMAF. In particular, there was an abnormally high frequency of short flagella (20%–41%) and absent flagellum (10%–36%) (Table 1). The group of infertile men is composed of two brothers from a consanguineous family recruited in Lebanon and two unrelated men recruited in Marseilles. Our use of human material to investigate the genetic causes of male infertility was approved by ethics committees in France and Lebanon.
Table 1.
Selected Semen Parameters of the Four Men with Asthenozoospermia and MMAF
| Individual with MMAF | WDR66 LoF Alleles as CFAP251 Change | gnomAD Allele Frequency | Sperm Concentration (Million/mL) | Volume (mL) | Motile Sperm (%) | Normal Forms (%)a |
Flagellar Abnormalities (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Short | Absent | Coiled | Bent | Variable Width | |||||||
| 18668 | homozygous p.Asp42Metfs∗4 | 1/15,375 SA | 12 | 4.5 | 0 | 1 | 20 | 36 | 16 | 36 | 0 |
| 18671 | homozygous p.Asp42Metfs∗4 | 1/15,375 SA | 30 | 3.5 | 0 | ND | ND | ND | ND | ND | ND |
| 21141 | p.Glu111∗ | 1/545 Eu | 1.1 | 5 | 0 | 2 | 41 | 11 | 24 | 18 | 18 |
| p.Leu530Valfs∗4 | 0 | ||||||||||
| 21226 | none | – | 13 | 3.5 | 4 | 10 | 28 | 10 | 19 | 3 | 8 |
Morphology was based on the modified David classification. Abbreviations are as follows: ND, not determined; LoF, loss of function; SA, South Asian; and Eu, European (non-Finnish).
Modified David classification.15
We initially studied the consanguineous Lebanese family, in which six of ten brothers show complete asthenozoospermia (Figure 1). We performed whole-exome sequencing analysis on two infertile brothers (18668 and 18671) and one fertile brother (18669). We used the VarAFT 2.12 software16 to annotate and filter the variants discovered on the basis of autosomal-recessive or X-linked inheritance; allele frequency in gnomAD17 was <1:100 (autosomal) or <1:10,000 (X). We also filtered out variants scored as polymorphic or probably polymorphic by UMD-Predictor or MutationTaster.18, 19 In this way we identified no candidate X-linked variants and four candidate autosomal variants in ALDH2 (MIM: 100650; c.283C>T [p.Arg95Cys] [GenBank: NM_000690.3; gnomAD: 4/275,858]), MCM7 (MIM: 600592; c.1570C>T [p.Arg524Trp] [GenBank: NM_005916.4; gnomAD: absent; dbSNP: ss2137543930]), PPOX (MIM: 600923; c.82C>T [p.Pro28Ser] [GenBank: NM_000309.4; gnomAD: absent; dbSNP: ss2137543929), and WDR66 (MIM: 612573; c.123delA [p.Asp42Metfs∗4] [GenBank: NM_144668.5; gnomAD: 2/245,760]). Screening for these variants in the second fertile brother (18670) by Sanger sequencing allowed us to exclude the missense variants in PPOX and MCM7 because they were found to be homozygous (data not shown). Neither fertile brother was homozygous for ALDH2 c.283C>T (data not shown) or WDR66 c.123delA (Figure 1). We excluded ALDH2 3 c.283C>T on the basis of the fact that a dominant allele resulting in loss of ALDH2 catalytic function has risen to a frequency of 30% in Asian populations and thus loss of ALDH2 function has no major negative effect on male fertility.20 In gnomAD, WDR66 c.123delA (p.Asp42Metfs∗4) is present only in the South Asian group at a low incidence of 2/30,748 alleles. We concluded that WDR66 c.123delA, a base-pair deletion in the first coding exon of WDR66, is the only candidate causal variant revealed by our exome analysis. WDR66 encodes CFAP251, a protein containing nine WD repeats bounded by a 30 kDa N-terminal Glu-rich acidic domain and a C-terminal calcium-binding EF-hand domain. CFAP251 is the only human ortholog of a Tetrahymena thermophila protein, FAP251, required for wild-type cilia function.21
Figure 1.
Bi-allelic LoF WDR66 Mutations in Two Independent Cases of Asthenozoospermia Associated with MMAF
(A–C) Pedigree of family LIBM (A), Sanger sequencing of variant sites in asthenozoospermic brothers 18668 and 18671 and fertile brothers 18669 and 18670 (B), and representative morphologies observed on histological analysis of spermatozoa from individual 18668 (C).
(D–F) Pedigree of family FNBM (D), Sanger sequencing of variant sites in asthenozoospermic man 21141 and his parents (E), and representative morphologies observed on histological analysis of spermatozoa from individual 21141 (F).
In (B) and (E), the reference sequence at the variant site is underlined in homozygotes, the presence of the variant is indicated by inverted black triangles, and the position of CFAP251 codons is indicated by the thin line above the base codes. The reference sequence for genomic nomenclature is RefSeq: NC000012.11.
To seek further genetic evidence that loss of WDR66 function causes asthenozoospermia, we next sequenced the 21 coding exons of WDR66 in the two other unrelated men with MMAF (Table 1). In one of these men (21141), we identified two rare LoF variants, stop gain c.331G>T (p.Glu111∗) (GenBank: NM_144668.5; gnomAD: 1/986 and 1/545 in the non-Finnish European group) in exon 2 and frameshift c.1588_1589delCT (p.Leu530Valfs∗4) (gnomAD: absent; ClinVar: SCV000778846) in exon 11 (Figure 1 and Figure S1). Sanger sequencing of individual 21141’s parents revealed that each is heterozygous for one of the variants, showing that the variants are on separate chromosomes in individual 21141. Thus, individual 21141 represents a second independent case of asthenozoospermia in a man with bi-allelic LoF mutations in WDR66.
To determine the localization of CFAP251 in normal spermatozoa, we performed immunofluorescence (IF) analysis with an anti-CFAP251 antibody (Figures 2A and 2B). A signal was detected along the length of the flagella in spermatozoa from the control individual. No signal was detected along the flagellum of spermatozoa from MMAF individual 21141, confirming both the specificity of the flagellar signal detected with this antibody and the loss of CFAP251 from individual 21141. We also performed western blot analysis on sperm lysates from individual 21141 and one of the brothers from the familial individual, 18668 (Figure 2C). We did not detect the 140 kDa band corresponding to CFAP251 in either case. Of note, in our western blot analysis with control spermatozoa, we observed a strong 100 kDa band that was not visible in whole-testis lysates. This band is almost certainly a signal specific for CFAP251 because it was not detected in the spermatozoa of either asthenozoospermic man. Given that the antibody used detects a C-terminal part of CFAP251 and the 100 kDa band was not detected in whole testis, we propose that CFAP251 could be produced as a precursor protein from which the Glu-rich N terminus is removed during spermatozoa maturation. In support of this hypothesis, we determined the expected migration size of CFAP251 with and without the Glu-rich N-terminal domain to be 140 and 100 kDa respectively, by transfection into HeLa cells (Figure S2). We conclude that, as predicted from the genetic data, CFAP251 is absent from the spermatozoa produced by asthenozoospermic men 21141 and 18668.
Figure 2.
CFAP251 Localizes along the Spermatozoon Flagellum and Is Absent from Spermatozoa in Two Independent MMAF Individuals Carrying Distinct Bi-allelic LoF Mutations in WDR66
(A) Immunofluorescence analysis of spermatozoa from a control individual. The second antibody alone gives no labeling. With the anti-CFAP251 antibody, control spermatozoa are labeled (red) along the full length of the flagellum.
(B) No CFAP251 signal was detected on spermatozoa from individual 21141.
(C) Lysates of purified spermatozoa from affected individuals 18668 and 21141 were migrated with control samples from purified spermatozoa and testis and incubated with an anti-CFAP251 antibody (Sigma, HPA040005). No bands detected by the anti-CFAP251 antibody in control samples were detected in affected individual 18668 or 21141, indicating that they are specific to CFAP251. The predicted molecular weight of the full-length CFAP251 is 130 kDa, and its expected mobility is at 140 kDa (Figure S2). Of note is the strong spermatozoa-specific fragment at approximately 100 kDa. Because the antibody is against a C-terminal domain, this could correspond to an isoform from which the Glu-rich N-terminal domain has been removed during spermatozoa maturation. Lysate from 5 × 104 spermatozoa was loaded except for lanes marked with an asterisk, where lysate from 2 × 106 spermatozoa was loaded. Loading was controlled with an anti-α-tubulin antibody (Abcam, ab15246).
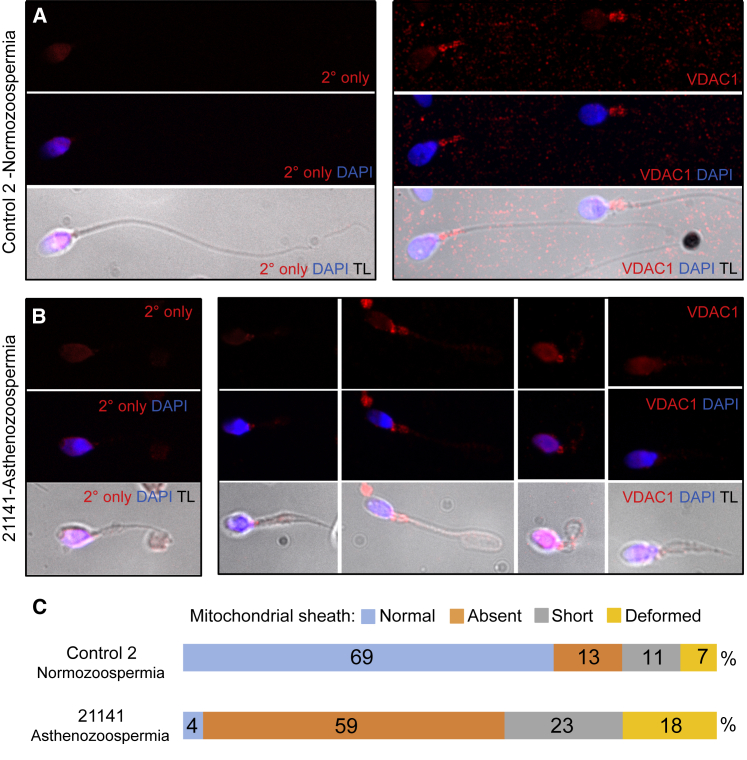
It has been reported that mitochondria can be absent from the spermatozoa in some cases of asthenozoospermia with short flagella or dysplasia of the fibrous sheath.22 However, this has not previously been observed in cases with a defined genetic basis. We therefore investigated whether the mitochondrial sheath (MS) formed normally when the axoneme lacked CFAP251 by IF analysis of spermatozoa from individual 21141 with an antibody against the mitochondrial marker VDAC1 (Figure 3 and Figure S3). We found that whereas the antibody labeled the midpiece of most control spermatozoa, labeling of spermatozoa from individual 21141 was weak or absent. In addition, in the ejaculate of individual 21141, but not of the control individual, many isolated bodies were labeled for VDAC1, suggesting that recruitment of mitochondria had occurred but that their attachment was deficient, leading to detachment of the MS after spermiation (Figure S3).
Figure 3.
The Mitochondrial Sheath Is Abnormal on the Spermatozoa Flagella of Affected Individual 21141
(A and B) IF analysis of spermatozoa from a normospermic man (A) and individual 21141 (B) with an antibody against the mitochondrial outer-membrane protein VDAC1 (red). No labeling was seen on the flagella in the absence of the anti-VDAC1 antibody (secondary [2°] antibody only). DNA was labeled with DAPI (blue). The fluorescent merge is presented alone and overlayed with the transmitted light (TL) image to show the extent of the flagella.
(C) Comparison of the mitochondrial labeling observed in individual 21141 and the control individual. The percentage of different types of labeling is represented (n = 100 for the control individual and individual 21141). In individual 21141, normal labeling of the proximal flagellum by anti-VDAC1 (4%) was much lower than in the control individual (69%). Additional images showing isolated anti-VDAC1-labeled structures, specific to individual 21141, that could be detached MS fragments are shown in Figure S3.
Transmission electron microscopy (TEM) confirmed the presence of MS dysplasia in individual 21141; on flagella with a discernible MS and a FS (n = 4), we observed a short MS with very low mitochondrial density (Figure 4 and Figure S4). Indeed, we found only a single MS axoneme transverse section (Figure S5). Principal-piece transverse axonemal sections were observed to have a normal 9 + 2 structure or lack the central pair but were too few for us to conclude that CFAP251 loss has an effect on axoneme structure (Figure S5). Sections of the end piece were also found in individual 21141 but showed varied structures, as in the control individual (data not shown). In individual 21141, mitochondria were recruited to the flagella and the annulus was detected at the MS-FS junction, but the MS was short and barely extended distal to the segmented columns of the connecting piece (Figure 4 and Figure S4). The MS is normally 1–1.5 times longer than the sperm head but was 2–3 times shorter in individual 21141. The different flagellar forms observed in individual 21141 were confirmed to contain an axoneme by IF with an anti-α-tubulin antibody (Figure S6). We conclude that CFAP251 might be required for the extension of the MS along the midpiece of the spermatozoon flagellum, although this must now be confirmed in additional cases of bi-allelic mutations in WDR66.
Figure 4.
TEM Analysis of Spermatozoa from Affected Individual 21141 Reveals a Short Midpiece with Low Mitochondrial Density
Representative images of spermatozoa from a normozoospermic man (A and B) and spermatozoa from individual 21141 (C and D). The arrows point to the transition between the mitochondrial sheath (MS) and the fibrous sheath (FS). In contrast to the MS of the control individual (B), the MS from individual 21141 does not extend far beyond the segmented column (SC) of the connecting piece. Normally the MS of the spermatozoon is 1–1.5 times the length of the head. The annulus (AN) is seen in individual 21141 (C) correctly positioned at the junction between the short MS and the FS, as in the control individual (B). Further images are shown in Figure S4 and together represent all observed sections with a contiguous head, midpiece, and proximal principal piece. No midpiece of normal length was observed.
Our results demonstrate that loss of CFAP251 causes asthenozoospermia and MMAF in humans. We have shown that CFAP251 is absent in two independent cases of MMAF with complete asthenozoospermia. The absence of CFAP251 in individual 21141 is associated with a failure of normal MS assembly. We conclude that CFAP251 is critical for flagellar biogenesis in mammals.
Human CFAP251 is the ortholog of flagellar-associated protein 251 (FAP251), which was identified in C. reinhardtii by proteomic analysis of isolated flagella.13 In C. reinhardtii, it has been shown that FAP251, along with FAP61 and FAP91, is part of a calmodulin- and radial-spoke-associated complex (CSC) that is necessary for efficient flagellar beating and wild-type motility.23, 24 A large N-terminal Glu-rich domain in human CFAP251 is most pronounced in eutherian mammals, but shorter Glu-rich domains are evident at the N terminus of CFAP251 orthologs in other mammals, birds, reptiles, and fish, suggesting that it could be a feature of vertebrate CFAP251 proteins.
In the 9 + 2 motile axoneme, the nine peripheral microtubule doublets are organized around a central pair of microtubules to which they are connected by radial spokes. Each peripheral microtubule doublet of the axoneme is organized length-wise in repeating 96 nm subunits. Along each subunit there are four outer dynein arms (ODAs); three radial spokes (RS1, RS2, and RS3), associated with three inner dynein arms (IDA1, IDA2, and IDA3, respectively) containing seven distinct dyneins (a–g); the CSC; and the nexin-dynein regulatory complex (N-DRC), which links adjacent microtubule doublets. The CSC is located on the inner surface of the peripheral microtubule doublet at the base of RS3, from where it extends to contact the base of the adjacent RS2 and the N-DRC.25 In support of a physical link between CFAP251, IDA3, and RS3, T. thermophila FAP251 immununoprecipitates with the dynein heavy chain g of IDA3, and knockout of FAP251 led to the loss of an arch-like structure at the base of RS3 and the loss of RS3 from 16% of 96 nm subunits.21 The absence of FAP251 in T. thermophila did not affect the number, length, or rate of assembly of cilia but did diminish the efficiency and coordination of ciliary beating. Thus, currently the evidence supports roles for CFAP251 in stabilizing the base of RS3 and in controlling ciliary and flagellar beating.
Murine DNAH1 has been localized close to the base of RS3 in IDA3.26 Its C. reinhardtii ortholog, the heavy chain of dynein d, is also part of IDA3.27 In MMAF men and mice carrying bi-allelic LoF mutations in DNAH1, the length of the MS is normal,8 but less extreme anomalies of the MS have been identified.6, 8, 15, 28 Thus, loss of either DNAH1 or CFAP251 function potentially affects IDA3 and RS3 and compromises the organization of the MS. Furthermore, CFAP43 and CFAP44, also found mutated in cases of MMAF, have been located between the outer surface of the axoneme and the paraflagellar rod in T. brunei,10 implying that they could be involved in the recruitment or maintenance of auxiliary structures around the axoneme. Thus, genetic variants that cause asthenozoospermia without PCD might predominantly affect proteins involved in the assembly of spermatozoa-specific auxiliary structures: the outer dense fibers, the MS, or the FS.
Nevertheless, because WDR66 is the only human gene that encodes a FAP251 ortholog, it is almost certain that its absence will compromise not only the beating of flagella but also that of motile cilia. The fact that PCD symptoms were not observed in the individuals studied here implies that the disruption of RS3 and the CSC in humans remains compatible with at least some ciliary activity. In keeping with this, reduced efficiency and coordination of beating was observed for cilia in T. thermophila lacking FAP25121 and for flagella in C. reinhardtii when the CSC components CFAP61 and CFAP91 were knocked down.23 Reduced beat frequency of tracheal cilia was also observed in DNAH1-null mice that otherwise manifested no signs of PCD.15
Our findings indicate that in humans, CFAP251 is probably required for the formation of the MS around the axoneme during flagellogenesis. Indeed, given that the MS is short in individual 21141, CFAP251 could be involved in positioning the MS-FS junction by enabling the specific intraflagellar transport that normally moves the annulus caudally away from the nucleus immediately before mitochondrial recruitment. Our observations suggest that in individual 21141, mitochondria are recruited to the short midpiece, where, unable to spread along the axoneme, they form an unstable structure that remains attached to the flagellum during spermiation but subsequently detaches from the flagellum during maturation or ejaculation. Even though it is inside the axoneme, on the peripheral microtubule doublets, CFAP251 could conceivably influence the intraflagellar transport required to define the caudal limit of the MS through a chain of interactions between the axoneme and the auxiliary sheath, through a mechanical effect on the rigidity of the axoneme, or by directing specific proteins to the outer surface of the axoneme during its assembly. In mice and humans, it has been shown that the annulus itself is dispensable for the recruitment of mitochondria to the midpiece or the positioning of the MS-FS junction.29, 30 The study of CFAP251 could therefore provide insights into how the MS is delimited along the flagellum.
Independent of any effect on MS formation, the Glu-rich N terminus of CFAP251 might be part of an intraflagellar stabilizing link that is subsequently cleaved during maturation in the epididymis, when the rigidity of the flagellum is reduced to optimize spermatozoa motility.31 In MMAF-affected individuals, therefore, the absence of CFAP251 could weaken the developing flagellum or compromise its assembly and thus increase the production of spermatozoa with short, deformed, or no flagella.
The nonsense variant WDR66 c.331G>T (p.Glu111∗) is rare but has an allelic frequency of 1/545 in European populations (gnomAD). This will increase the chance of finding bi-allelic mutations in WDR66 in infertile, MMAF-affected men of European origin. This is the case for DNAH1 in Chinese populations, where the frameshift mutation c.11726_11727delCT (p.Pro3909Argfs∗33), which has an allelic frequency of 1/700 in the East Asian group in gnomAD,17 has been found in 8 of 27 independent cases of MMAF in Han Chinese men.8, 9 Bi-allelic mutations in WDR66 could therefore be a major cause of MMAF in men of European origin. This possibility can now be explored by multicenter studies.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We are extremely grateful to the affected individuals and the members of their families who gave their informed consent for the use of their samples in this research study. We thank Alexandre Altié from the Electronic Microscopy core facility in the La Timone Medical Faculty at Aix-Marseille University for ultrastructural analyses of sperm samples. We also thank gnomAD and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about. This work was supported by core funding from INSERM to M.J.M. Y.A. was supported by a PhD fellowship from the French Guiana region (Le Conseil Régional de la Guyane).
Published: August 16, 2018
Footnotes
Supplemental Data include Supplemental Material and Methods, six figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.07.013.
Accession Numbers
The accession numbers for the genetic variants reported in this paper are dbSNP: ss2137543929, dbSNP: ss2137543930, and ClinVar: SCV000778846.
Web Resources
Supplemental Data
References
- 1.Thonneau P., Marchand S., Tallec A., Ferial M.L., Ducot B., Lansac J., Lopes P., Tabaste J.M., Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum. Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Inaba K. Sperm flagella: Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 2011;17:524–538. doi: 10.1093/molehr/gar034. [DOI] [PubMed] [Google Scholar]
- 3.Knowles M.R., Zariwala M., Leigh M. Primary ciliary dyskinesia. Clin. Chest Med. 2016;37:449–461. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanaken G.J., Bassinet L., Boon M., Mani R., Honoré I., Papon J.F., Cuppens H., Jaspers M., Lorent N., Coste A. Infertility in an adult cohort with primary ciliary dyskinesia: Phenotype-gene association. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.00314-2017. [DOI] [PubMed] [Google Scholar]
- 5.Chemes H.E., Brugo S., Zanchetti F., Carrere C., Lavieri J.C. Dysplasia of the fibrous sheath: An ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil. Steril. 1987;48:664–669. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 6.Ben Khelifa M., Coutton C., Zouari R., Karaouzène T., Rendu J., Bidart M., Yassine S., Pierre V., Delaroche J., Hennebicq S. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amiri-Yekta A., Coutton C., Kherraf Z.E., Karaouzène T., Le Tanno P., Sanati M.H., Sabbaghian M., Almadani N., Sadighi Gilani M.A., Hosseini S.H. Whole-exome sequencing of familial cases of multiple morphological abnormalities of the sperm flagella (MMAF) reveals new DNAH1 mutations. Hum. Reprod. 2016;31:2872–2880. doi: 10.1093/humrep/dew262. [DOI] [PubMed] [Google Scholar]
- 8.Sha Y., Yang X., Mei L., Ji Z., Wang X., Ding L., Li P., Yang S. DNAH1 gene mutations and their potential association with dysplasia of the sperm fibrous sheath and infertility in the Han Chinese population. Fertil. Steril. 2017;107:1312–1318.e2. doi: 10.1016/j.fertnstert.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Jin H., Han F., Cui Y., Chen J., Yang C., Zhu P., Wang W., Jiao G., Wang W. Homozygous DNAH1 frameshift mutation causes multiple morphological anomalies of the sperm flagella in Chinese. Clin. Genet. 2017;91:313–321. doi: 10.1111/cge.12857. [DOI] [PubMed] [Google Scholar]
- 10.Coutton C., Vargas A.S., Amiri-Yekta A., Kherraf Z.E., Ben Mustapha S.F., Le Tanno P., Wambergue-Legrand C., Karaouzène T., Martinez G., Crouzy S. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018;9:686. doi: 10.1038/s41467-017-02792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sha Y.W., Wang X., Xu X., Su Z.Y., Cui Y., Mei L.B., Huang X.J., Chen J., He X.M., Ji Z.Y. Novel mutations in CFAP44 and CFAP43 cause multiple morphological abnormalities of the sperm flagella (MMAF) Reprod. Sci. 2017 doi: 10.1177/1933719117749756. [DOI] [PubMed] [Google Scholar]
- 12.Tang S., Wang X., Li W., Yang X., Li Z., Liu W., Li C., Zhu Z., Wang L., Wang J. Biallelic mutations in CFAP43 and CFAP44 cause male infertility with multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2017;100:854–864. doi: 10.1016/j.ajhg.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazour G.J., Agrin N., Leszyk J., Witman G.B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastin P., Sherwin T., Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- 15.Neesen J., Kirschner R., Ochs M., Schmiedl A., Habermann B., Mueller C., Holstein A.F., Nuesslein T., Adham I., Engel W. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 2001;10:1117–1128. doi: 10.1093/hmg/10.11.1117. [DOI] [PubMed] [Google Scholar]
- 16.Desvignes J.P., Bartoli M., Delague V., Krahn M., Miltgen M., Béroud C., Salgado D. VarAFT: A variant annotation and filtration system for human next generation sequencing data. Nucleic Acids Res. 2018;46(W1):W545–W553. doi: 10.1093/nar/gky471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado D., Desvignes J.P., Rai G., Blanchard A., Miltgen M., Pinard A., Lévy N., Collod-Béroud G., Béroud C. UMD-Predictor: A high-throughput sequencing compliant system for pathogenicity prediction of any human cDNA substitution. Hum. Mutat. 2016;37:439–446. doi: 10.1002/humu.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz J.M., Rödelsperger C., Schuelke M., Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 20.Luo H.R., Wu G.S., Pakstis A.J., Tong L., Oota H., Kidd K.K., Zhang Y.P. Origin and dispersal of atypical aldehyde dehydrogenase ALDH2487Lys. Gene. 2009;435:96–103. doi: 10.1016/j.gene.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Urbanska P., Song K., Joachimiak E., Krzemien-Ojak L., Koprowski P., Hennessey T., Jerka-Dziadosz M., Fabczak H., Gaertig J., Nicastro D., Wloga D. The CSC proteins FAP61 and FAP251 build the basal substructures of radial spoke 3 in cilia. Mol. Biol. Cell. 2015;26:1463–1475. doi: 10.1091/mbc.E14-11-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemes H.E., Olmedo S.B., Carrere C., Oses R., Carizza C., Leisner M., Blaquier J. Ultrastructural pathology of the sperm flagellum: association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum. Reprod. 1998;13:2521–2526. doi: 10.1093/humrep/13.9.2521. [DOI] [PubMed] [Google Scholar]
- 23.Dymek E.E., Heuser T., Nicastro D., Smith E.F. The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol. Biol. Cell. 2011;22:2520–2531. doi: 10.1091/mbc.E11-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dymek E.E., Smith E.F. A conserved CaM- and radial spoke associated complex mediates regulation of flagellar dynein activity. J. Cell Biol. 2007;179:515–526. doi: 10.1083/jcb.200703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heuser T., Dymek E.E., Lin J., Smith E.F., Nicastro D. The CSC connects three major axonemal complexes involved in dynein regulation. Mol. Biol. Cell. 2012;23:3143–3155. doi: 10.1091/mbc.E12-05-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vernon G.G., Neesen J., Woolley D.M. Further studies on knockout mice lacking a functional dynein heavy chain (MDHC7). 1. Evidence for a structural deficit in the axoneme. Cell Motil. Cytoskeleton. 2005;61:65–73. doi: 10.1002/cm.20066. [DOI] [PubMed] [Google Scholar]
- 27.Bui K.H., Yagi T., Yamamoto R., Kamiya R., Ishikawa T. Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme. J. Cell Biol. 2012;198:913–925. doi: 10.1083/jcb.201201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolley D.M., Neesen J., Vernon G.G. Further studies on knockout mice lacking a functional dynein heavy chain (MDHC7). 2. A developmental explanation for the asthenozoospermia. Cell Motil. Cytoskeleton. 2005;61:74–82. doi: 10.1002/cm.20067. [DOI] [PubMed] [Google Scholar]
- 29.Kissel H., Georgescu M.M., Larisch S., Manova K., Hunnicutt G.R., Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell. 2005;8:353–364. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Lhuillier P., Rode B., Escalier D., Lorès P., Dirami T., Bienvenu T., Gacon G., Dulioust E., Touré A. Absence of annulus in human asthenozoospermia: Case report. Hum. Reprod. 2009;24:1296–1303. doi: 10.1093/humrep/dep020. [DOI] [PubMed] [Google Scholar]
- 31.Dacheux J.L., Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2013;147:R27–R42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.