Abstract
Inner ear spiral ganglion neurons were cultured from day 4 postnatal mice and loaded with a fluorescent Ca2+ indicator (fluo-4, -5F, or -5N). Pulses of infrared radiation (IR; 1,863 nm, 200 µs, 200–250 Hz for 2–5 s, delivered via an optical fiber) produced a rapid, transient temperature increase of 6–12°C (above a baseline of 24–30°C). These IR pulse trains evoked transient increases in both nuclear and cytosolic Ca2+ concentration ([Ca2+]) of 0.20–1.4 µM, with a simultaneous reduction of [Ca2+] in regions containing endoplasmic reticulum (ER). IR-induced increases in cytosolic [Ca2+] continued in medium containing no added Ca2+ (±Ca2+ buffers) and low [Na+], indicating that the [Ca2+] increase was mediated by release from intracellular stores. Consistent with this hypothesis, the IR-induced [Ca2+] response was prolonged and eventually blocked by inhibition of ER Ca2+-ATPase with cyclopiazonic acid, and was also inhibited by a high concentration of ryanodine and by inhibitors of inositol (1,4,5)-trisphosphate (IP3)-mediated Ca2+ release (xestospongin C and 2-aminoethoxydiphenyl borate). The thermal sensitivity of the response suggested involvement of warmth-sensitive transient receptor potential (TRP) channels. The IR-induced [Ca2+] increase was inhibited by TRPV4 inhibitors (HC-067047 and GSK-2193874), and immunostaining of spiral ganglion cultures demonstrated the presence of TRPV4 and TRPM2 that colocalized with ER marker GRP78. These results suggest that the temperature sensitivity of IR-induced [Ca2+] elevations is conferred by TRP channels on ER membranes, which facilitate Ca2+ efflux into the cytosol and thereby contribute to Ca2+-induced Ca2+-release via IP3 and ryanodine receptors.
NEW & NOTEWORTHY Infrared radiation-induced photothermal effects release Ca2+ from the endoplasmic reticulum of primary spiral ganglion neurons. This Ca2+ release is mediated by activation of transient receptor potential (TRPV4) channels and involves amplification by Ca2+-induced Ca2+-release. The neurons immunostained for warmth-sensitive channels, TRPV4 and TRPM2, which colocalize with endoplasmic reticulum. Pulsed infrared radiation provides a novel experimental tool for releasing intracellular Ca2+, studying Ca2+ regulatory mechanisms, and influencing neuronal excitability.
Keywords: Ca2+ photocontrol, endoplasmic reticulum, infrared stimulation, optical stimulation, spiral ganglion neurons, TRP channels
INTRODUCTION
Pulses of infrared radiation (IR) have been investigated as a noninvasive technique for altering activity of excitable cells such as nerve and muscle (Cayce et al. 2014b, 2015; Dittami et al. 2011; Jenkins et al. 2010; Rajguru et al. 2010, 2011). Effects of IR stimulation reported to date have been mixed: for example, IR stimulation is reported to evoke action potentials in cochlear neurons (Tian et al. 2017) and vestibular axons (Rabbitt et al. 2016), but to decrease action potential activity in some hippocampal neurons (Walsh et al. 2016). Some effects of IR stimulation might be mediated or modulated by changes in cytosolic (and/or nuclear) Ca2+ concentration ([Ca2+]). IR stimulation has been shown to increase intracellular [Ca2+] in cardiomyocytes (Dittami et al. 2011), in cortical neurons and glia (Cayce et al. 2014a), and in neuronal and glial cell lines (Moreau et al. 2018). However, Lumbreras et al. (2014) reported that IR stimulation decreased intracellular [Ca2+] in cultured spiral ganglion neurons. The present study reinvestigated IR-induced [Ca2+] changes in cultured spiral ganglion neurons, and we present evidence supporting a different conclusion: namely, that for initial temperatures of ≥24°C, IR stimulation transiently increases cytosolic (and nuclear) [Ca2+]. This [Ca2+] increase was driven by IR photothermal stimulation and was due mainly to release of Ca2+ from endoplasmic reticulum (ER).
Major established pathways for Ca2+ release from ER are ryanodine receptors and inositol (1,4,5)-trisphosphate (IP3) receptors, both of which can participate in Ca2+-induced Ca2+ release (reviewed by Verkhratsky 2005; Wang et al. 2018). We present pharmacological evidence that both of these receptors contribute to the IR-induced increase in intracellular [Ca2+] in spiral ganglion neurons. However, neither of these pathways displays the temperature dependence required to mediate the effects of IR stimulation. Using patch clamp, immunostaining, and pharmacological techniques Albert et al. (2012) presented evidence that TRPV4, a warmth-sensitive vanilloid transient receptor potential (TRP) channel, is present in mouse retinal and rat vestibular ganglion neurons and mediates IR-induced depolarizing responses that can initiate action potentials. This activation of TRPV4 was mediated by the temperature increase produced by IR stimulation. TRPV4 channels are also present in the spiral ganglion neurons studied in the present investigation (Ishibashi et al. 2008) and can be present in ER as well as plasma membranes (e.g., García-Elias et al. 2008, 2015). We present evidence that TRPV4 channels are present in ER of spiral ganglion neurons and that activation of these channels contributes to the IR-induced increase in cytosolic [Ca2+] produced by release of intracellular Ca2+ stores.
METHODS
Cell culture.
All procedures were approved by the University of Miami Institutional Animal Care and Use Committee. Spiral ganglia were dissected from C57BJ mice at postnatal day 4. Mice were euthanized by cervical dislocation, cochlea were removed, and bone surrounding the spiral ganglia was opened with fine forceps on a cold block (4–8°C). Spiral ganglia were removed, incubated in 0.25% trypsin for 20 min at 37°C, and then dissociated by gentle pipetting. Cells were pelleted by centrifugation (5 min at 5,000 rpm, 4°C), resuspended in culture medium made with Neurobasal medium with N1 supplement (Sigma-Aldrich, St. Louis, MO), plated in glass-bottom, poly-d-lysine-coated 35-mm culture dishes (P35GC-1.5-1.4-C; MatTek, Ashland, MA), and incubated at 37°C, 5% CO2. Fresh culture medium with 100 nM brain-derived neurotrophic factor (BDNF) was added the day after plating, after which half the medium was exchanged with fresh medium with growth factor every 4 days.
Spiral ganglion neurons were identified by their elongated, bipolar (spindle) shape. Responses to IR stimulation were studied 5–30 days after plating, in Tyrode solution, a bicarbonate-buffered saline solution (the time in vitro had no evident effect on recorded responses). A constant gas flow maintained the CO2 concentration above the cultures at ~5%. In most of the illustrated experiments, the initial bath temperature was maintained at 24–30°C by a flow of warm air, which also heated the microscope stage and specimen holder. These conditions preserved the response of the in vitro preparation to repeated bouts of stimulation. In a few experiments (e.g., Fig. 4), warmer (35–37°C) and cooler (15–21°C) initial temperatures were tested. In these cases, the room temperature was adjusted to be within 5°C of the desired bath temperature (using space heaters or air conditioning), to enhance temperature control.
Fig. 4.
Response to infrared radiation (IR) stimulation increases with irradiation magnitude and initial temperature. A: peak change in average intracellular [Ca2+] (ΔCa2+) as a function of the temperature increase (°C) produced by different laser intensities (W/mm2) in neurons loaded with fluo-5F or fluo-5N in medium containing no added Ca2+. Most data were collected at an initial temperature of 24°C (284 points from 98 neurons), with additional data collected at initial temperatures of 15–19 and 35–37°C. Inset shows fluorescence changes (ΔF/F0) produced by 2 different IR intensities applied at an initial temperature of 35°C. In the plot, each initial temperature is denoted by a different symbol (Δ, 35–37°C; ●, 24°C; ▼, 15–19°C); larger circled symbols indicate the mean for each intensity. Lines indicate the least-squares fit for each initial temperature (3rd-order polynomial; MATLAB). The temperature increase produced in the IR beam by each irradiation power was measured using a microthermistor and sulforhodamine B fluorescence changes as in Fig. 1. The Spearman correlation test indicated that the [Ca2+] response increased with increasing laser power when the initial temperature was 35–37°C (P < 0.01) and when the initial temperature was 24°C (P < 0.05); the correlation between the [Ca2+] response and laser power was not significant when the initial temperature was 15–19°C. Welch’s nonparametric unpaired test indicated that the [Ca2+] responses for initial temperatures of 35–37°C were significantly greater than those at 24°C (P < 0.05). B: traces in a–c superimpose responses recorded from 2 neighboring fluo-5F-loaded neurons. Sequential responses show that the IR-induced fluorescence increase recorded when the initial temperature was 21°C (a and c) changed (reversibly) into a fluorescence decrease when the initial temperature was reduced to 15°C (b). The cooler temperature increased the magnitude of the negative-going artifact during the stimulus train, suggesting an increase in resting [Ca2+] (see text). Findings in B were repeated in 3 experiments (≥5 neurons in each).
Imaging and IR stimulation.
Cultures were mounted on the stage of an inverted microscope (Nikon TE2000). Confocal images were taken using a Yokogawa spinning disk confocal head (Semrock, Rochester, NY) with the following Nikon objectives: ×10, numerical aperture (NA) 0.4; ×40 water, NA 1.2; or ×40 oil, NA 1.3. Images were collected and analyzed using ImageJ software. Fluo indicators were excited using an argon 488 laser (Laser Physics, West Jordan, UT), and emission at 520–540 nm was collected with a Cascade512B charge-coupled device camera (Roper Scientific/Photometrics, Tucson, AZ).
IR stimulation was delivered to cells via a fiber optic cable (usually 200-µm diameter) coupled to an 1,863-nm laser (Capella Aculight; Lockheed-Martin, Bothell, WA). The fiber was held and controlled with a micromanipulator, allowing IR to be focused ~60–100 μm away from the target cells. A pilot beam was used as a guide to position the IR beam. Unless otherwise specified, trains of 200-µs pulses were delivered at 200–250 Hz for 2–5 s, using laser powers ranging from 0.1 to 0.9 W/mm2 (usually ~0.6 W/mm2; see Fig. 4A). The laser power used during most experiments was 10% or more above the threshold power needed to evoke a detectable response. Under these conditions, neurons continued to respond to IR stimulation for up to 6 h with moderate laser powers; higher laser powers produced more rapid rundown of the preparation. During the experiments, cells were challenged with various inhibitors and blockers as described in results.
Loading and calibration of fluorescent Ca2+ indicators.
Fluorescent Ca2+ indicators in the fluo family were loaded into cells in their membrane-permeable acetoxymethyl ester (AM) forms (ThermoFisher Scientific, Waltham, MA). Intracellular esterases cleave off the AM moiety, thereby trapping the indicator within the cell (or cellular compartment). Fluo indicators had Kd values that ranged from high Ca2+ affinity (fluo-4), to intermediate affinity (fluo-5F), to relatively low affinity (fluo-5N; see values below). Unless otherwise noted, the loading protocol was a 1-h incubation with a 5 µM concentration of the AM form of the indicator, after which the medium was replaced with indicator-free medium. These indicators act as Ca2+ buffers and thus can reduce the amplitude and prolong the time course of the [Ca2+] changes being measured. This buffering effect is greater for the high-affinity indicator (fluo-4), whose Kd value (400 nM) is closer to the range of resting cytosolic [Ca2+]. To minimize this distorting artifact, most measurements used fluo-5F or -5N, reserving the higher affinity fluo-4 for detecting small changes in [Ca2+] close to the resting level. In the resting cell, the fluorescence of low-affinity fluo-5N is confined mainly to ER (and lysosomes) because the [Ca2+] in ER (200–1,000 µM; reviewed in Verkhratsky 2005) exceeds the Kd value (60–90 µM) of fluo-5N, whereas [Ca2+] values in resting nucleus, cytosol, and mitochondria are much less than fluo-5N’s Kd.
Imaging began 30 min after indicator washout in experiments focusing on cytosolic [Ca2+], and ~5 h after indicator washout for experiments focusing on signals from fluo-5N within the ER. Based on results in Fig. 2, most records were collected in medium containing no added Ca2+; measurements indicated that such solutions usually contained <5 µM contaminant Ca2+. Ca2+ buffers (EGTA or BAPTA, 1 mM) were added in some cases to achieve even lower bath [Ca2+] (see e.g., Fig. 2, C and E). Bath [Ca2+] in EGTA was calculated using Maxchelator (http://maxchelator.stanford.edu/CaMgATPEGTA-NIST.htm). Ca2+ was occasionally added to the bath for 10 min to facilitate refilling of intracellular stores.
Fig. 2.
Infrared radiation (IR)-induced changes in fluorescence (ΔF/F0; and thus intracellular [Ca2+]) do not require extracellular Ca2+ or action potentials. Traces show fluorescence responses plotted as ΔF/F0 recorded in spiral ganglion neurons loaded for 15 min with fluo-5F AM. One neuron was exposed to a succession of solutions (A–D): first in 2 mM Ca2+ (A), then in medium containing no added [Ca2+] (B; labeled 0 mM Ca2+, but measurements indicate that such solutions usually contain up to ~5 µM contaminant Ca2+). EGTA (1 mM) was added to this low-[Ca2+] solution (C; reducing bath [Ca2+] to ~4 nM), and then 25 µM tetrodotoxin (TTX; D). E: record from a different neuron bathed in 10 mM Na+/140 mM K-gluconate with 1 mM BAPTA. Records were collected after >5-min incubation in each solution, and fluorescence was averaged over the cell body. Calculation of bath [Ca2+] in EGTA was via Maxchelator (http://maxchelator.stanford.edu/CaMgATPEGTA-NIST.htm). Laser power was ~0.6 W/mm2.
Most data are plotted as fluorescence (F) or the IR-induced change in fluorescence (ΔF) normalized to the fluorescence before stimulation (F0), using ImageJ (NIH, Bethesda, MD) and MATLAB (The MathWorks, Natick, MA). Some records (e.g., Fig. 5A) were filtered using a three-point median filter (MATLAB).
Fig. 5.
Infrared radiation (IR) stimulation increases [Ca2+] in both cytosol and nucleus. A: time course of calculated change in [Ca2+] (ΔCa2+) in response to IR stimulation in a neuron loaded with fluo-5N. Superimposed are the response in the nucleus (dotted line), the average response in non-nuclear regions (cytoplasm; dashed line), and the response averaged over the whole neuron (solid line). B: fluorescence micrographs of this neuron before (30 s) and at the peak response to IR stimulation (41 s). The analyzed nuclear and cytosolic regions are outlined. The medium contained no added Ca2+. Calibration, 10 µm.
Calculation of intracellular [Ca2+] changes from Ca2+ indicator dye fluorescence.
Ca2+ concentrations were calculated from fluorescence measurements (F) using the equation
where Fmin is the minimal fluorescence (no Ca2+) and Fmax is the maximal fluorescence (saturated with Ca2+). F/Fmin was calculated from (F/F0) × (F0/Fmin), where F0 is the fluorescence before stimulation, and
assuming that resting [Ca2+] is 40 nM. The value of F0/Fmin is relatively insensitive to the assumed value of [Ca2+]rest for the lowest affinity indicator (fluo-5N), but considerably more sensitive for the high-affinity indicator fluo-4. The change in [Ca2+] caused by stimulation is the calculated [Ca2+] minus [Ca2+]rest.
Fmin and Fmax for the fluo indicators were measured in several representative spiral ganglion neurons bathed in an intracellular-like medium (145 mM K-gluconate, 10 mM NaCl, 10 mM glucose, 1 mM MgCl2), which allowed the cells to remain intact when 100 µM ionomycin was added to permeabilize the cell membrane to ions, including Ca2+. In this medium Fmin was measured after 30 min in the Ca2+ buffer BAPTA (1 mM), and Fmax was determined after a 30-min equilibration in 1 mM Ca2+. Measured Fmax/Fmin values were ~150 for fluo-4, fluo-5F, and fluo-5N, in agreement with published values (Molecular Probes Handbook).
To measure Kd, F was measured from z-series stacks of images (0.2-µm increments, Olympus ×40 oil objective) as medium [Ca2+] was changed [calculated using MAXCHELATOR (http://maxchelator.stanford.edu/) from the total calcium and buffer in each solution], using the fluorescence of the z plane in best focus. These data were plotted as (F/Fmax − Fmin/Fmax)/(1 − F/Fmax) vs. [Ca2+], and Kd was measured from the slope. Measured Kd values were 0.4 µM for fluo-4, ~2 µM for fluo-5F, and 60–90 µM for fluo-5N, similar to values published by the manufacturer (https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/tables/comparison-of-in-vitro-and-in-situ-kd-values-for-various-ca2-indicators.html). Calibrations were complicated by the presence of extensive ER.
Transient temperature changes produced by IR pulse trains.
The temperature increase produced by IR pulse trains was measured using the fluorescence of a sulforhodamine B solution. Rhodamine B fluorescence decreases with increasing temperature (Barigelletti 1987; Kan et al. 2013). Figure 1A shows the fluorescence changes produced by IR pulse trains applied in bathing medium (no cells) at five different laser powers. Calibration data in Fig. 1B indicate that the relationship between the peak changes in sulforhodamine B fluorescence and bath temperature (monitored by thermocouples; Omega, Norwalk, CT) was approximately linear. These fluorescence changes have a higher spatial and temporal resolution than thermistor-based measurements, and thus were the preferred method for monitoring IR-induced temperature changes around cells. Measurements on expanded versions (not shown) of the fluorescence changes in Fig. 1A indicated that the fluorescence (and hence temperature) change reached ~80% of its final value within 2 s after the onset or offset of the IR radiation. The rapid decay suggests that the narrow diameter of the IR beam allowed rapid collapse of the temperature gradient via both thermal conduction and convection currents in the medium. Based on the calibration curve in Fig. 1B, the peak magnitude of this temperature change for a cell in the IR beam was 3–9°C in this experiment.
Fig. 1.
Infrared radiation (IR) pulse trains increase temperature and decrease fluorescence of sulforhodamine and fluo indicators. A: transient decreases in sulforhodamine B fluorescence during a series of 5 IR pulse trains. Sulforhodamine B (20 µM) was imaged using confocal optical sectioning to isolate a region of the culture medium in the IR beam. IR pulse trains (200-µs pulses at 200 Hz for 4 s) were applied at increasing laser power from 0.2 to 0.9 W/mm2; stimulus duration is indicated by horizontal bars. Fluorescence (F) is normalized to the initial fluorescence (F0). B: change in sulforhodamine B fluorescence (ΔF/F0) as a function of radiant exposure (RE; bottom x-axis) and the change in bath temperature (ΔT; top x-axis). Similar results were obtained with sulforhodamine B labeling of gelatin or cells (not shown). C: change in fluo-4 fluorescence (ΔF/F0) induced by 2 bouts of IR irradiation in a spiral ganglion cell that was incubated with 5 µM fluo-4 AM for 30 min, fixed in 4% paraformaldehyde for 5 min, and washed with the normal medium containing 2 mM Ca2+. D and E: pictorial and graphical representations, respectively, of the spatial distribution of the change in bath sulforhodamine B fluorescence during IR stimulation. In D and E, X indicates the tip of the optical fiber, and white pixels in D indicate the fluorescence (temperature) change. Graph in E plots ΔF (left axis; arbitrary units) and the calculated ΔT (right axis) during IR stimulation along the dashed line shown in D. The fluorescence decrease (indicating a temperature increase) was relatively uniform for distances 20–60 µm from the end of the optical fiber. Calibration in D, 200 µm.
Fluo family indicators also exhibit decreased fluorescence with increasing temperature (Oliver et al. 2000; Shuttleworth and Thompson 1991). This effect of temperature is greater for the more fluorescent Ca2+-bound forms of the indicators (Shuttleworth and Thompson 1991). This was consistent with our finding that the fluorescence decrease during IR irradiation was greater for high-affinity indicators like fluo-4 (where resting fluorescence is higher) than for low-affinity indicators like fluo-5N. The fluorescence decrease was evident even in cells loaded with fluo-4 and subsequently fixed in paraformaldehyde before imaging (Fig. 1C). Thus the IR-induced fluorescence decrease is a property of the indicator and complicates readings of the cellular response to IR pulses. A previous paper from this laboratory had erroneously attributed a part of this fluorescence decrease during IR stimulation to a decrease in intracellular [Ca2+] (see appendix).
Figure 1D uses bath sulforhodamine B fluorescence to illustrate the spatial extent of the temperature change produced by IR stimulation (white indicates warmest temperature). Figure 1E plots this spatial extent, demonstrating that the temperature increase was maximal and nearly uniform within ~20–60 µm of the tip of the fiber optic cable, similar to the typical distance between imaged neurons and the tip.
Immunostaining.
Cultures were washed with Tyrode medium and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature (22°C). They were then incubated for 1–2 h in 2% goat serum with 0.1% Triton in PBS for 1–2 h (to block nonspecific binding), after which primary antibody (1:200 dilution) was added, followed by incubation for 1–2 days at 4°C. Primary antibodies were rabbit anti-TRPV4 (lot no. ACC034AN1950; Alomone Laboratories), rabbit-anti-TRPM2 (lot no. GR303073-6; Abcam), and rabbit anti-GRP78 (ER marker, conjugated to Alexa 488; Invitrogen). Cultures were then washed with PBS and incubated with the secondary antibody (1:400 dilution) in 2% goat serum and 0.1% Triton for 1–2 h at room temperature (22°C). The secondary antibody for TRPV4 and TRPM2 staining was tetramethylrhodamine goat anti-rabbit (lot no. 1275897; Life Technologies). For tetramethylrhodamine secondary antibody, the free rhodamine was removed by running 0.5 ml through a very small ion exchanger column (12 mg of AG-1-X2 anion exchange resin in the filter-plugged tip of a pipette preequilibrated with PBS). The resin bound the free dye and allowed the labeled antibody to pass through unbound. Cultures were then washed with PBS, stored at 4°C, and imaged at room temperature. In control experiments, no staining was detected when the primary antibody was omitted.
Drug sources and concentrations.
Inhibitors of IP3 [2-aminoethoxydiphenyl borate (2-APB), IC50 42 µM; xestospongin, IC50 358 nM] and TRPV4 [HC-067047, IC50 17–133 nM; GSK-2193874, IC50 2–40 nM) were obtained from Tocris (IC50 values from Tocris website). 2-APB also blocks TRPM2-mediated thermal responses in preoptic neurons (30 µM; Song et al. 2016). Because the response we studied arose from the ER in intact cells, we had to use higher antagonist concentrations and longer exposure times than those required for isolated receptors or receptors on the plasma membrane surface.
Statistical analysis.
Statistical comparisons were performed using PRISM software (GraphPad) and MATLAB. Paired comparisons were performed using the t-test or Wilcoxon rank test; comparison of multiple groups was performed using the Mann-Whitney test. Polynomials were fit to the relationship between [Ca2+] changes and laser power by using a least-squares polynomial fit (MATLAB). Spearman nonparametric correlation analysis was used to assess the effect of laser power on the amplitude of cytosolic [Ca2+] responses. Differences between Ca2+ responses at different initial temperatures were analyzed using Welch’s nonparametric unpaired test.
RESULTS
The temperature increase produced by IR irradiation causes a transient increase in intracellular [Ca2+] in spiral ganglion neurons.
Figure 2, A–D, shows fluorescence changes (ΔF, normalized to the initial fluorescence F0) recorded following 1,863-nm IR irradiation in a representative spiral ganglion neuron loaded with the Ca2+ indicator fluo-5F. Figure 2A was recorded in the normal 2 mM extracellular Ca2+ medium, whereas Fig. 2B is the evoked response after this medium was exchanged for medium containing no added Ca2+. The ratio of the average peak responses recorded in these two media (Ca2+-free/Ca2+) was 0.88 ± 0.22 (mean ± SE; 15 neurons in 6 different cultures, not significantly different by paired t-test), and the responses in the nominally Ca2+-free solution remained stable with repeated stimulation. The IR-induced fluorescence increase in this neuron continued after addition of the Ca2+ buffer EGTA (1 mM; Fig. 2C) and after addition of tetrodotoxin (TTX; 25 µM; Fig. 2D) to block most Na+-dependent action potentials. In all cases, the fluorescence (and thus [Ca2+]) increased rapidly to a peak following the IR irradiation and then decayed more slowly back to baseline levels. The peak [Ca2+] increase calculated from the fluorescence change in Fig. 2, A and B, was ~0.8 µM (see methods; see summed data in Fig. 4A). Decay times varied widely: the mean decay half-time in fluo-5F-filled neurons was 30 ± 19 s (range 3–70 s, n = 30).
Because some voltage-dependent Na+ channels are not blocked by TTX, we also blocked any Na+-dependent action potentials using high-[K+] medium containing only 10 mM Na+. Figure 2E shows that an IR-induced fluorescence increase also occurred when the Ca2+ buffer BAPTA (1 mM) was added to this low-[Na+] medium. Results like that shown in Fig. 2E were observed in all five cultures tested in similar media, although the responses recorded in this “extreme” medium became smaller over time. The results in Fig. 2 thus indicate that the IR-induced intracellular [Ca2+] elevation occurs and persists with minimal extracellular [Ca2+] and does not require Na+-dependent action potentials. These findings suggest that the elevation of intracellular [Ca2+] is due to release from an intracellular store. Unless otherwise indicated, subsequent results were obtained in medium containing no added Ca2+ ([Ca2+] < 5 µM).
Figure 3A, a–c, presents evidence that the IR-induced photothermal effect drives the fluorescence increase. Perfusion of extracellular medium over the target neuron, which would minimize the IR irradiation-induced temperature increase, reversibly eliminated the response. The fluorescence decrease observed at the onset of irradiation, likely due to an effect of temperature on the indicator itself (see methods and Fig. 1C), was also reversibly eliminated by local perfusion.
Fig. 3.
The temperature increase that elevates intracellular [Ca2+] can be produced by infrared radiation (IR) stimulation (A) or by brief perfusion with a warm solution (B). A: reduction of the IR-induced temperature increase by local perfusion reversibly decreases fluo-5F fluorescence changes (ΔF/F0) evoked by 2 bouts of IR irradiation. Responses were recorded before (a), during (b) and after (c) local perfusion with the same medium. B: the apparatus used to perfuse neurons with a pulse of warm solution consisted of an outer glass tube and a shorter inner stainless steel tube with a small hole in the side. Insulated magnet wire was wrapped around the inner tube to form an electrically insulated heating coil. Solution warmed via the heating coil was either perfused over the cells (b) or sucked back through the outer tube (a, “holding”). The holding configuration was used throughout the records shown in c–f, except when the perfusion pulse was applied (horizontal bar). The fluo-5F fluorescence response evoked by the heated perfusion pulse (d) was absent when the perfusing solution was not heated (c). The temperature change produced by heated (f) and nonheated (e) perfusion pulse was calculated using the decrease in sulforhodamine B fluorescence. The initial bath temperature was 26°C, and the peak temperature during the pulse was 33°C (7°C temperature increase). The relationship between sulforhodamine B fluorescence and temperature was calculated by measuring the fluorescence changes when the temperature (measured by a thermocouple) was changed more slowly by addition of medium at different temperatures. Fluo-5F and sulforhodamine B records (which utilize different wavelengths) were collected on separate trials that used the same heat pulse settings. Sulforhodamine (20 µM) was present throughout in both bath and perfusing solution.
The fact that heating decreases the fluorescence of fluo family Ca2+ indicators (especially their Ca-bound forms, see methods) probably accounts for the observation that in most neurons studied (including Figs. 2 and 3A), the peak fluorescence increase occurred after, rather than during, the IR stimulation. The effect of warming on the indicator itself can mask all or part of the Ca2+-induced increase in fluorescence until the local temperature returns to baseline. Although the mechanisms underlying the decrease in fluorescence during irradiation (temperature effect on indicator) and the increase in fluorescence following irradiation (increased intracellular [Ca2+]) are different, both phenomena were caused by the IR-irradiation-induced temperature increase.
Figure 3B shows that a transient temperature increase produced by other means can also produce a transient increase in intracellular [Ca2+]. Solution warmed by a heating coil (using the apparatus diagrammed in Fig. 3B, a and b) was transiently perfused over fluo-5F-preloaded spiral ganglion neurons. Perfusion with heated solution produced a transient increase in fluo-5F fluorescence (Fig. 3Bd), whereas perfusion with nonheated solution did not (Fig. 3Bc). More than 50% of the cells analyzed (k = 3 culture plates, n = 21 cells) elicited twofold or greater increase in fluo-5F fluorescence. The magnitude of the illustrated temperature increase was 7–8°C (baseline 24°C), assessed using the temperature-dependent decrease in the fluorescence of sulforhodamine B (as in Fig. 1, A and B; compare Fig. 3Bf with heat to Fig. 3Be without). The perfusion rate and heater coil settings were adjusted to yield a temperature increase comparable in magnitude and duration to those induced by IR stimulation in records like those in Fig. 2. These perfusion results are consistent with the hypothesis that the IR-induced increase in cytosolic [Ca2+] was due to the associated temperature increase. Furthermore, it appears that the exact time course of the temperature increase was not critical: the temperature increase produced by IR pulse trains in Figs. 1, 2, and 3A was likely composed of many small steps (200/s) summing to give an approximately steady-state temperature change by 1–2 s, whereas the time course of the perfusion-induced temperature increase was likely smoother. Results in Fig. 3Bd also suggest that the abolition of the IR-induced [Ca2+] elevation by local perfusion in Fig. 3Ab was not due to washout of some IR-produced substance, since in Fig. 3Bd perfusion with a pulse of warm solution produced a fluorescence/[Ca2+] increase.
Figure 4A plots the peak Δ[Ca2+] as a function of laser energy measured in spindle-shaped neurons in medium containing no added Ca2+. The temperature increases in the IR beam (bottom x-axis) had an approximately linear relationship to radiation intensity (top x-axis). Most data in Fig.4A were collected at an initial temperature of ~24°C. The relationship between laser energy and average peak Δ[Ca2+] was nonlinear, curving upward at energy levels above 0.6 W/mm2. Some neurons exhibited a poststimulation decrease, rather than an increase, in average peak Δ[Ca2+], suggesting that removal of Ca2+ from the cytosol (e.g., due to Ca2+ uptake into ER or Ca2+ extrusion from the cell) exceeded addition of Ca2+ to cytosol. At all tested laser powers, the increase in cytosolic [Ca2+] was greater when the initial temperature was higher (35–37°C, closer to normal mammalian temperatures; Fig. 4A, inset). Traces in Fig. 4B show that [Ca2+] increases produced by IR stimulation were reversibly inhibited by reducing the initial temperature. Figure 4B, a and c, shows that the fluorescence increases recorded in two neighboring neurons when the bath temperature was 21°C were reversibly blocked when the bath temperature was reduced to 15°C (Fig. 4Bb). This low-temperature block of the IR-induced increase in cytosolic [Ca2+] occurred in 10 of 10 tested neurons. IR-induced [Ca2+] increases occurred when the initial medium temperature was between 21 and 37°C.
On the basis of these results, the records shown in subsequent figures were all obtained using irradiation intensities of at least 0.5 W/mm2, with an initial bath temperature of 24–30°C. At temperatures higher than 30°C, the IR-induced [Ca2+] increase was harder to sustain in zero-[Ca2+] solutions, suggesting a greater need to recharge internal Ca2+ stores by Ca2+ influx across the plasma membrane (e.g., via store-operated channels).
Cellular compartments exhibit different [Ca2+] responses to stimulation.
Records in Fig. 2 averaged stimulation-induced fluorescence changes from the entire soma. In some neurons, such as the fluo-5N-labeled cell in Fig. 5B, it was possible to identify a clear nuclear region, enabling separate analysis of stimulation-induced fluorescence changes in nucleus and cytosol. Cytosolic fluorescence was heterogeneous (see below), but on average, cytosolic fluorescence exceeded nuclear fluorescence in the resting cell. IR stimulation increased both cytosolic and nuclear fluorescence to approximately the same peak level (compare 30 and 41 s in Fig. 5B). Because average resting fluorescence was lower in the nucleus, the stimulation-induced increases in fluorescence (ΔF/F0, and thus Δ[Ca2+]) were greater in the nucleus than in the cytosol (Fig. 5A). In this and some other neurons, the nuclear [Ca2+] elevation also persisted longer than the cytosolic elevation (see nuclear record in Fig. 6D). Stimulation-induced elevations in nuclear [Ca2+] were almost always evident when the spatial resolution of the image was adequate to identify a nuclear region.
Fig. 6.
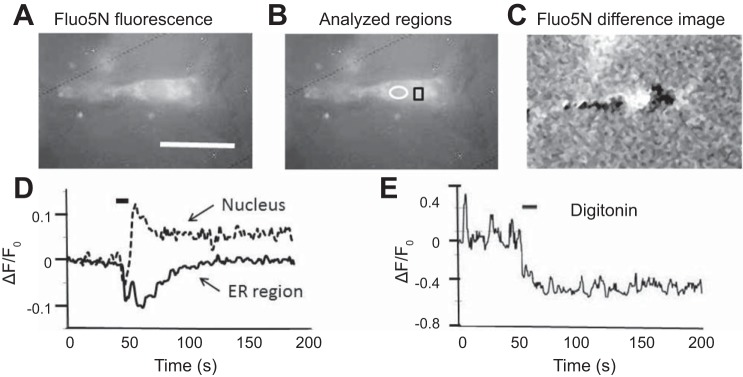
Infrared radiation (IR) stimulation reduces [Ca2+] in endoplasmic reticulum (ER). A: fluorescence micrograph of spiral ganglion neuron imaged 5–10 h after a 1-h incubation with fluo-5N AM. B: same cell with the analyzed regions of interest outlined in the nucleus (white oval) and (presumed) ER (black square). ER has a high [Ca2+] in the resting cell and thus should be one of the most fluorescent organelles at rest with a low-affinity indicator like fluo-5N. C: difference image, calculated by subtracting the image of the resting cell from the poststimulation image (averaged from images at 55–80 s). Brighter regions in C indicate regions where stimulation increased fluorescence/[Ca2+], and darker (black) regions indicate regions where stimulation reduced fluorescence/[Ca2+]. Calibration in A–C, 10 µm. D: time course of normalized fluorescence changes (ΔF/F0) induced in nucleus and presumed ER by IR stimulation. Stimulation increased fluorescence (and thus [Ca2+]) in the nucleus but decreased fluorescence in presumed ER. Data in A–D all came from one neuron. E: stimulation-induced decrease in [Ca2+] in a different neuron filled with fluo-5N and then exposed for 1 min to 5 µM digitonin to deplete cytosolic fluo-5N, so most of the remaining fluo-5N was within the ER. For all records the medium was nominally Ca2+ free ([Ca2+] < 5 µM). Horizontal bars in D and E indicate IR stimulation.
Both resting and stimulated fluorescence in Fig. 5B show marked nonuniformity of fluorescence in cytosolic (non-nuclear) regions. With low-affinity, negatively charged indicators such as fluo-5N, it is likely that the regions exhibiting the highest fluorescence in the resting neuron represent ER, whose lumen is expected to have a high resting [Ca2+]. In Fig. 5B these brightly labeled regions were punctate and disperse, but in some neurons, such as that in Fig. 6A, there were large juxtanuclear bright regions likely to be ER. Separate analysis of stimulation-induced changes in nuclear and (presumptive) ER regions (outlined in Fig. 6B) showed a long-lasting [Ca2+] increase in the nucleus but a decrease in the presumptive ER region (Fig. 6D). The differential response of nuclear and ER regions to stimulation is also apparent in the difference image of Fig. 6C, which was calculated by subtracting the fluorescence image of the resting neuron (Fig. 6A) from that recorded following stimulation. In this difference image, the nuclear region becomes brighter, indicating a stimulation-induced increase in [Ca2+], whereas the juxtanuclear regions (which were most fluorescent in the resting image) become darker, indicating a stimulation-induced loss of Ca2+. A similar stimulation-induced decrease in fluorescence was also seen in neurons preloaded with fluo-5N, in which cytosolic fluo-5N was depleted using digitonin (Fig. 6E). Brief exposure to digitonin permeabilizes the plasma membrane, but not ER membranes, so that most of the fluo-5N remaining in the cell is within the ER. These data are consistent with a stimulation-induced efflux of Ca2+ from the ER.
This study did not explicitly test for a mitochondrial contribution to the IR-induced increase in cytosolic [Ca2+], but the pattern of the Ca2+ increase, a rapid increase followed by a slow decrease, is more characteristic of ER (rapid passive release, slower active uptake) than of mitochondria (rapid passive uptake followed by slower active extrusion; reviewed in Barrett 2001). Also, the mitochondrial matrix is not expected to have a high [Ca2+] in resting cells.
Maintenance of IR-evoked [Ca2+] elevations requires Ca2+ uptake by ER.
The ability of spiral ganglion neurons to continue to release Ca2+ from (presumed) ER during repeated stimulation in medium lacking Ca2+ suggests that much of the Ca2+ released into the cytosol is efficiently resequestered by the ER Ca2+-ATPase. This hypothesis predicts that inhibition of ER sequestration will reduce the amplitude and slow the decay of subsequent poststimulation [Ca2+] elevations. To test this idea, neurons were incubated in zero-Ca2+ medium containing 50 µM cyclopiazonic acid (CPA), which inhibits the ER Ca2+-ATPase (reviewed by Verkhratsky 2005). Comparison of the dark and light traces in Fig. 7A shows that, as predicted, the peak amplitude of the IR-evoked Ca2+ increase was reduced with addition of CPA (light traces), which also slowed the falling phase of the response. When [CPA] was increased to 150 µM (Fig. 7B), intracellular [Ca2+] remained elevated long (minutes) after the end of the IR train, resulting in a progressive increase in cytosolic [Ca2+] with repeated stimulation. These results suggest that the ER Ca2+-ATPase has a major role in removing Ca2+ from the cytosol and pumping it back into the ER, and thus that maintenance of the IR-induced Ca2+ elevation in zero-Ca2+ medium requires internal recycling of the released Ca2+.
Fig. 7.

Maintenance of stimulation-induced [Ca2+] elevations involves endoplasmic reticulum (ER) sequestration of released Ca2+. A: superimposed responses to 2 trains of infrared radiation (IR) stimulation before (dark trace) and shortly after (light trace) addition of cyclopiazonic acid (CPA; 50 µM). CPA reduced the amplitude and slowed the falling phase of IR-induced fluorescence (ΔF/F0)/[Ca2+] elevations. B: response to repeated stimulus trains in the same neuron after [CPA] was increased to 150 µM. The responses to repeated stimulation became prolonged, with a progressive increase in cytosolic [Ca2+]. The indicator was fluo-4, and the medium contained zero added Ca2+ and 2 mM EGTA. Note in A that CPA did not change the magnitude of the decrease in fluorescence during stimulation, which is due to a direct effect of temperature on fluo-4 (see Fig. 1C). These findings were replicated in 2 experiments (≥5 neurons in each). C: filling the neuron with BAPTA inhibits the response to IR stimulation. Control response is superimposed on responses recorded after a fluo-5F-loaded neuron was loaded with the Ca2+ buffer BAPTA, which attenuated (22 min) and then eliminated (28 min) the response.
Figure 7C shows results consistent with this hypothesis in a neuron preloaded with fluo-5F and then filled with BAPTA by exposure to its membrane-permeable form (BAPTA-AM; 25 µM). After 22 min, the amplitude of the Δ[Ca2+] response to IR stimulation was reduced and its time course was prolonged, as expected from an increase in the Ca2+ buffering capacity. After 28 min, IR stimulation evoked practically no response. Note, however, that this result has several alternative explanations. Perhaps the IR stimulation continued to release Ca2+ from the ER, but BAPTA bound most of this Ca2+ so that the elevation of cytosolic [Ca2+] detected by the indicator was minimal, or perhaps BAPTA blocked most Ca2+ release from the ER by keeping the stimulation-induced elevation of cytosolic [Ca2+] below the level required to induce Ca2+-induced Ca2+ release (CICR). It is also possible that BAPTA blocked the response by competing for Ca2+ with the ER Ca2+-ATPase (thereby preventing refilling of ER Ca2+ stores) and/or by buffering Ca2+ within the ER.
A recent study also concluded that the IR-stimulated (1,470 nm) increase in cytosolic [Ca2+] produced in HT22 hippocampal and glioblastoma cells was due to release from ER, based on persistence of the [Ca2+] increase in low-[Ca2+] medium and inhibition of the increase when ER Ca2+ was depleted by thapsigargin (Moreau et al. 2018).
Mechanisms of IR-induced release of Ca2+ from ER.
The two types of channels most frequently associated with release of Ca2+ from the ER are ryanodine (RyR) and IP3 receptors (IP3R; reviewed by Morales-Tlalpan et al. 2005; Verkhratsky 2005). Both types of receptor can open in response to elevations in local cytosolic [Ca2+] and thus mediate CICR. Glutamate stimulation of spiral ganglion neurons releases Ca2+ from ER by a mechanism involving CICR, and RyR1–RyR3 are expressed in adult rat spiral ganglion neurons (Morton-Jones et al. 2006, 2008). Figure 8 shows results obtained with three pharmacological agents that influence RyR activation. High concentrations of ryanodine are reported to inhibit RyR (reviewed in Verkhratsky 2005), and Fig. 8A, a and b, shows that in some neurons 20 µM ryanodine did indeed block the IR-induced Ca2+ increase. This inhibition by high ryanodine concentration might also be due to depletion of ER Ca2+, because ryanodine both increases Ca2+ leak from the ER and inhibits Ca2+ reuptake into ER (reviewed in Mukherjee et al. 2014). Figure 8Ac shows that the ryanodine-induced inhibition of the response to IR stimulation could be overcome by adding 1 mM Ca2+ and 2 mM caffeine to the bathing solution. Caffeine increases activation of RyRs (reviewed in Verkhratsky 2005), and Fig. 8B, a and b, shows that caffeine alone enhanced the IR-induced fluorescence increase even in (nominally) Ca2+-free solution (see also Morton-Jones et al. 2006). Dantrolene inhibits activation of certain RyRs (Zhao et al. 2001), but Fig. 8C, a–d, shows that, surprisingly, even prolonged exposure to 25 µM dantrolene failed to reduce the peak response to IR stimulation, although dantrolene did reversibly slow the time course of the response. These results suggest that RyR-induced CICR contributes to the IR-induced response in at least some spiral ganglion neurons. Perhaps RyR activation is not always essential for the response, or perhaps the response is mediated by a dantrolene-insensitive form of RyR (e.g., RyR2; Zhao et al. 2001).
Fig. 8.
Responses to infrared radiation (IR) stimulation were reduced by ryanodine, increased by caffeine, and slowed by dantrolene. A: the [Ca2+] elevation in nominally Ca2+-free solution (a) was inhibited by 20 µM ryanodine over a period of 20 min (b). Addition of 1 mM Ca2+ and 2 mM caffeine reversed this block and prolonged the response to IR stimulation (c). B: modest response to IR stimulation (ΔF/F0) in nominally Ca2+-free solution (a) was enhanced by a 10-min exposure to 10 mM caffeine (b). C: sequential records (a–d) show that 25 µM dantrolene gradually and reversibly slowed the fluorescence increase in response to stimulation. The illustrated record was made in medium containing 2 mM Ca2+, but dantrolene had similar effects in medium containing zero added Ca2+. Indicator was fluo-5N for all records. Inhibition of the IR-induced fluorescence increase by ryanodine (Ab) was observed in 3 experiments (≥5 neurons in each), but the blocking effect of high ryanodine concentration was variable. In some preparations, even prolonged exposure to 50 µM ryanodine did not completely block the response.
Figure 9 shows the effects of two drugs that inhibit (directly or indirectly) IP3R-mediated Ca2+ release: xestospongin C (e.g., Gafni et al. 1997; Ozaki et al. 2002; Tumelty et al. 2011) and 2-APB (e.g., Kapoor et al. 2015). Figure 9A, a–c, shows that xestospongin C (50 µM) inhibited the IR-induced [Ca2+] elevation. This inhibitory effect was long lasting, with partial inhibition persisting even after a 1-h washout, and even after 2 mM Ca2+ was added to the nominally Ca2+-free solution (not shown). 2-APB (100 µM) also inhibited the IR-induced fluorescence increase (Fig. 9B, a–c) in the cell shown in Fig. 9Bf, an effect that was fully reversible after prolonged drug washout (Fig. 9B, d and e). The effects of xestospongin C and 2-APB are not limited to inhibition of IP3R activity (see below for additional actions of 2-APB), but the extent and reproducibility of the inhibitory effects illustrated in Fig. 9 are consistent with the hypothesis that CICR resulting from activation of IP3R plays an important role in the response of spiral ganglion neurons to IR stimulation.
Fig. 9.
Responses to infrared radiation (IR) stimulation were inhibited by xestospongin C and 2-aminoethoxydiphenyl borate (2-APB). A: the IR-induced fluorescence (ΔF/F0)/[Ca2+] elevation in nominally Ca2+-free solution (a) was inhibited by 50 µM xestospongin C (b and c). This inhibition was very slow to reverse (not shown). A possible mechanism for the long-lasting decrease in fluorescence observed in c is considered in results, Temperature dependence and involvement of TRP channels. B: sequential records (a–e) demonstrate reversible inhibition of the IR-induced fluorescence increase by 100 µM 2-APB in nominally Ca2+-free solution. The fluorescence image of the neuron is shown in f (calibration, 20 µm). Indicator in all records was fluo-5F. Xestospongin findings were repeated in 2 experiments and 2-APB findings in 3 experiments (≥5 neurons in each).
Another approach to inhibiting CICR is to introduce an exogenous Ca2+ buffer into the cytosol to bind the released Ca2+, thereby keeping cytosolic [Ca2+] below the threshold required to initiate CICR. Figure 7C shows that filling neurons with the Ca2+ buffer BAPTA did indeed inhibit the IR-induced fluorescence increase.
Temperature dependence and involvement of TRP channels.
Evidence presented thus far is consistent with the hypothesis that CICR initiated by activation of ER IP3Rs and/or RyRs plays an important role in the response of spiral ganglion neurons to IR stimulation. A complication, however, is that evidence suggests that the effects of IR stimulation are mediated by the resulting 6–12°C temperature increase (Figs. 1, 3, and 4), and with this temperature change neither RyRs nor IP3Rs are sufficiently temperature sensitive (Dickinson and Parker 2013a, 2013b; Fu et al. 2005) to account for the initiation of CICR following IR stimulation. One possible mechanism underlying the temperature-sensitive initiation of CICR is Ca2+ efflux from ER via warmth-sensitive TRP channels (e.g., reviewed in Ferrandiz-Huertas et al. 2014; Voets 2014; Wang and Siemens 2015), many of which are Ca2+ permeable and present in ER membranes (Dong et al. 2010). TRPV3 is present in many inner ear cells (Ishibashi et al. 2008) and activates over the temperature range studied here (Wang and Siemens 2015) but seems an unlikely candidate because it is opened by 2-APB (Chung et al. 2004), whereas the response studied here was inhibited by 2-APB. A more likely candidate is TRPV4, whose reported activation spans the range 24–40°C (Chung et al. 2003; Ferrandiz-Huertas et al. 2014; Güler et al. 2002; Todaka et al. 2004; Vizin et al. 2015; Watanabe et al. 2002). Activation of TRPV4 is increased by IP3 (García-Elias et al. 2008), and in certain endothelial cells, the gating of clustered TRPV4 channels exhibits positive cooperativity, which would amplify the channels’ ability to promote CICR (Sonkusare et al. 2014).
Figures 10 and 11A present evidence that TRPV4 contributes to the IR-stimulated increase in cytosolic [Ca2+] in spiral ganglion neurons. Figure 10A, a–c, demonstrates that HC-067047 (3 µM), an inhibitor of TRPV4 channels (e.g., Everaerts et al. 2010; Jo et al. 2016), reversibly blocked the IR-induced increase in fluorescence. Figure 10B, a–c, shows that another TRPV4 inhibitor, GSK-2193874 (1 µM; Cheung et al. 2017), also reversibly inhibited the response. These inhibitors are thought to be specific for TRPV4 (Cheung et al. 2017; Dalsgaard et al. 2016; Everaerts et al. 2010). However, the high drug concentrations and long exposure times needed for these drugs to reach ER membranes raise the possibility of off-target effects, as well.
Fig. 10.
Evidence that a warmth-sensitive transient receptor potential channel (possibly TRPV4) contributes to the infrared radiation (IR)-stimulated increase in intracellular [Ca2+]. A: 20-min exposure to HC-067047 (3 µM), a TRPV4 channel inhibitor, blocked the IR-induced fluorescence increase (ΔF/F0; compare b with a). c, Partial recovery of response 1 h following drug washout. HC-067047 (1–3 µM) similarly inhibited the IR-evoked response in 10 neurons in a different preparation. B: another TRPV4 inhibitor, GSK-2193874 (1 µM), also blocked the IR-induced fluorescence increase within 20 min. Findings in B were repeated in 2 experiments (≥5 neurons in each). In all records the medium was nominally Ca2+ free and the indicator was fluo-5F. Note that in HC-067047 and GSK-2193874, the magnitude of the negative-going artifact during the stimulus train was increased, and the IR train was followed by a decrease in fluorescence (see text).
Fig. 11.
Fluorescence micrographs demonstrate overlapping immunostaining for endoplasmic reticulum marker GRP78 and transient receptor potential (TRP) channels. A: GRP78 immunostaining (green; left), TRPV4 immunostaining (red; middle), and merged image (right); yellow indicates overlap). B: similar to A, except that middle image shows TRPM2 immunostaining. Calibration, 10 µm.
Previous work showed that TRPV4 is expressed in mouse spiral ganglion neurons (Ishibashi et al. 2008), and most cultured spiral ganglion neurons (81% of 83 spindle-shaped cells) stained with an antibody against TRPV4 (Fig. 11A). The pattern of TRPV4 staining resembles that shown by Albert et al. (2012) in rat vestibular ganglion neurons. TRPV4 staining colocalized with staining for GRP78, an ER marker (Park et al. 2017). TRPV4 coprecipitates with IP3R in HEK-293 cells (García-Elias et al. 2008).
TRPM2 is also present in spiral ganglion neurons (Takumida et al. 2009). It activates over temperature ranges around 40°C (Kamm and Siemens 2016; Song et al. 2016; Tan and McNaughton 2016) and thus could contribute to IR-stimulated responses initiated from baseline temperatures ≥30°C. Most cultured spiral ganglion neurons (95% of 154 spindle-shaped cells) stained with an antibody against TRPM2, and the somatic staining overlapped with that for ER marker GRP78 (Fig. 11B). For both TRPV4 staining in Fig. 11A and TRPM2 staining in Fig. 11B, the overlap with GRP78 ER staining was especially marked in regions on either side of the centrally located nucleus. Like the response to IR stimulation studied here (Fig. 9B), TRPM2 channels are inhibited by 2-APB (Song et al. 2016). It is not yet resolved whether 2-APB blocks activation of IP3Rs directly and/or via their association with TRP channels. In the present study, 2-APB’s inhibition of the response to IR stimulation was more effective at acidic than at alkaline pH (not shown), similar to the pH dependence reported for 2-APB’s inhibitory effects on other TRP channels (Gao et al. 2016).
Results in Figs. 10 and 11 thus support the hypothesis that opening of warmth-sensitive channels TRPV4 and possibly TRPM2 in ER contributes importantly to initiating IR-induced CICR.
In Figs. 4Bb, 10Ab, and 10Bb, the decrease in fluorescence following the IR train in cells exposed to cold, HC-067047, or GSK-2193874 resembles the decrease also observed in xestospongin C (Fig. 9Ac). A possible explanation for these findings is that the IR-induced temperature elevation also stimulates Ca2+ uptake into ER, an effect unmasked when CICR is inhibited (see Fig. 12 and discussion).
Fig. 12.

Mechanisms likely to be involved in infrared radiation (IR)-induced changes in cytosolic [Ca2+] in spiral ganglion neurons. The temperature increase produced by IR stimulation activates warmth-sensitive receptors such as transient receptor potential channel TRPV4 in endoplasmic reticulum (ER), thereby elevating cytosolic [Ca2+] in local domains near inositol 1,4,5-trisphosphate (IP3R) and ryanodine receptors (RyR). The resulting Ca–induced Ca2+ release (CICR) accounts for the observed rapid [Ca2+] elevation. The subsequent slow decay of cytosolic [Ca2+] is mediated by the ER Ca2+-ATPase, whose activation is increased (possibly) via STIM1, whose temperature-dependent phosphorylation of phospholamban (PLN) reduces PLN’s inhibition of Ca2+-ATPase activity.
Another interesting feature of these results is that certain treatments enhanced the rapid decrease in fluorescence measured during the IR train: caffeine (Fig. 8Bb), dantrolene washout (Fig. 8Cd), xestospongin C (Fig. 9A, b and c), cold (Fig. 4Bb), HC-067047 (Fig. 10Ab), and GSK-2193874 (Fig. 10Bb). As shown in Fig. 1C, part of this negative-going signal is due to a direct effect of increased temperature on the fluorescence of fluo indicators, an effect that is greater for the Ca2+-bound form of the indicator (Shuttleworth and Thompson 1991). Thus an increase in the magnitude of this negative-going signal suggests that the treatment increased resting cytosolic [Ca2+]. Inhibition of the activity of Ca2+-ATPases by cold temperatures is expected to increase cytosolic [Ca2+], and caffeine is expected to increase resting [Ca2+] by increasing Ca2+ efflux from ER. The increased magnitude of the fluorescence decrease in xestospongin C, HC-067047, and GSK-2193874 suggests that at rest, noninhibited IP3Rs and TRPV4 channels may help reduce Ca2+ leakage from ER.
DISCUSSION
Results presented in this article demonstrate that trains of IR irradiation (1,863 nm, 200-µs pulses at 200–250 Hz for 2–5 s, ~0.6 W/mm2) cause elevation of cytosolic and nuclear [Ca2+] in cultured mouse spiral ganglion neurons. These [Ca2+] increases, measured using the fluo family of Ca2+ indicators, had a peak amplitude of up to 1.4 µM and a duration of 10–200 s following the IR pulse train. This finding is consistent with previous reports that IR pulse trains increase [Ca2+] in cultured cardiomyocytes (Dittami et al. 2011), in neurons and glia in the rat brain in vivo (Cayce et al. 2014a), and in neural and glial cell lines (Moreau et al. 2018). We present evidence that the [Ca2+] increase in spiral ganglion neurons is caused by the IR photothermal effect and that much of this [Ca2+] increase represents CICR from the ER. Additional evidence suggests that the [Ca2+] increase that initiates CICR results from activation of warmth-sensitive TRP channels (TRPV4, possibly TRPM2) in the ER.
IR-induced increase in cytosolic [Ca2+] depends on the IR-induced temperature increase and does not require action potentials or Ca2+ influx across the plasma membrane.
The IR stimulation elevated the local bath temperature by 6–12°C (above an initial temperature of 24–30°C), as measured by the temperature-dependent fluorescence of sulforhodamine B (Fig. 1B). This temperature increase decayed rapidly (within ~2 s) after the end of the IR pulse train. A comparable temperature increase produced by perfusion with a warm solution produced an increase in cytosolic [Ca2+] (Fig. 3Bd) comparable to that produced by IR stimulation, supporting the hypothesis that the effects of IR stimulation are mediated by the associated temperature increase. This finding also argues against the idea that this effect of IR pulse trains was mediated by changes in membrane capacitance (e.g., Shapiro et al. 2012), because perfusion with warm solution is unlikely to change capacitance fast enough to produce substantial depolarization. Also arguing against a major role for capacitance changes is the finding that the effect of a given magnitude of IR stimulation on the [Ca2+] response was greater when the initial temperature was warmer (Fig. 4A), because initial temperature would be expected to have minimal effect on IR-induced capacitance changes.
Several studies have reported that rapid IR-induced temperature changes can change cellular membrane potentials (Albert et al. 2012; Carvalho-de-Souza et al. 2018; Liu et al. 2014; Romanenko et al. 2014; Shapiro et al. 2012). Spiral ganglion neurons can generate action potentials (reviewed by Reijntjes and Pyott 2016), and IR stimulation initiates action potentials in many neurons (reviewed by Thompson et al. 2014). However, the IR-induced [Ca2+] increases measured in this study did not require action potentials, because responses persisted in intracellular-like medium (145 mM K+, 10 mM Na+, and low [Ca2+] <5 nM, BAPTA buffered) that depolarized the cell membrane potential and would prevent the depolarizing currents needed for Na- and/or Ca2+-dependent action potentials (Fig. 2). Our findings do not of course rule out possible effects of IR stimulation on the membrane potential across the plasma membrane. Perhaps very rapid and brief temperature changes produced by brief, intense IR pulses favor plasma membrane depolarization by a transient increase in membrane capacitance (Carvalho-de-Souza et al. 2018), whereas longer IR pulses or trains of pulses such as those used in the present study favor effects due to the net temperature change, such as activation of TRP channels in the ER.
Evidence for IR-induced Ca2+ release from ER.
The finding that the IR-induced increase in cytosolic [Ca2+] could be evoked repeatedly in medium containing little or no Ca2+ indicates that the response is mediated by Ca2+ release from an intracellular source capable of efficiently resequestering the released Ca2+. The most likely such compartment is the ER, which contains a high concentration of Ca2+ in resting cells. In a healthy cell filled with a low-affinity Ca2+ indicator (fluo-5N), ER (and possibly lysosomes) are expected to exhibit a bright fluorescence at rest, and these bright compartments decreased their fluorescence following IR stimulation (Fig. 6, A–D). This IR-induced decrease was also prominent when cytosolic (but not ER) fluo-5N was depleted by permeabilizing the plasma membrane with a brief exposure to digitonin (Fig. 6E). Taken together, these results strongly suggest that the IR-induced elevations in cytosolic (and nuclear) [Ca2+] are mediated by Ca2+ release from the ER. Consistent with this idea, inhibition of ER Ca2+ sequestration with CPA reduced the amplitude and prolonged the falling phase of the IR-induced [Ca2+] elevation, eventually elevating resting cytosolic [Ca2+] and abolishing the IR-induced response (Fig. 7, A and B).
Mechanisms underlying IR-induced changes in ER Ca2+ release.
CICR from ER can be mediated by activation of RyRs and/or IP3Rs (Wiltgen et al. 2014). Our findings are consistent with contributions from both sources to the IR-induced increase in cytosolic [Ca2+] in spiral ganglion neurons. RyRs are present in the spiral ganglion (Liang et al. 2009; Morton-Jones et al. 2006), and the IR-induced [Ca2+] elevations were enhanced by 2–10 mM caffeine and (usually) reduced by 20 µM ryanodine. IP3Rs are present in the spiral ganglion (Liu and Yang 2015), and IR-induced responses were reduced by two IP3R antagonists, xestospongin C and 2-APB. Note, however, that each of these drugs can have additional effects on other cellular Ca2+ regulatory mechanisms, including TRP channels and store-operated Ca2+ channels (e.g., Saleem et al. 2014). Cell-to-cell variability in the positive-feedback nature of the various processes influencing CICR might contribute to the observed variability in the amplitude and duration of IR-induced [Ca2+] responses. Variability was also reported for “Ca-puffs” from the ER of neuroblastoma cells (Dickinson and Parker 2013b).
Because neither RyRs nor IP3Rs are sufficiently temperature sensitive to account for the IR-stimulated Ca2+ release studied here (Dickinson and Parker 2013b; Fu et al. 2005), we tested whether activation of warmth-sensitive TRP channels might cause the initial Ca2+ release that initiates CICR following IR-stimulation. A candidate of special interest is TRPV4, which is activated over the temperature ranges studied (6–12°C IR-induced temperature increase above an initial temperature of 24–30°C). In these cultured spiral ganglion neurons, the IR-induced [Ca2+] elevation was reversibly inhibited by cooling to 15°C (Fig. 4B) and by two TRPV4 inhibitors, HC-067047 and GSK-2193874 (Fig. 10, A and B). Somatic immunostaining for TRPV4 colocalized with immunostaining for an ER marker GFP78 (Fig. 11A). IP3Rs bind to TRPV4 channels and promote TRPV4 activation (García-Elias et al. 2008). Clusters of TRPV4 channels in vascular smooth muscle can gate cooperatively, which might amplify their contribution to CICR (Sonkusare et al. 2012). Taken together, these findings suggest that TRPV4 channels play an important role in the response to IR stimulation. TRPV4 channels are also likely to be important for hearing, because TRPV4 knockout mice exhibit earlier development of age-related hearing loss and increased susceptibility to noise-induced hearing loss (Tabuchi et al. 2005).
Increases in intracellular [Ca2+] were also evident in the smaller number of experiments when the IR-induced temperature increase was applied from an initial temperature of 35–37°C (Fig. 4A). This increase in intracellular [Ca2+] occurred in medium containing no added Ca2+ and thus also represented release from intracellular stores. This finding opens the possibility for involvement of additional TRP channels whose activation by heat usually requires temperatures greater than 37°C (e.g., Wang and Siemens 2015). One such channel is TRPM2, whose somatic immunostaining in cultured spiral ganglion neurons colocalized with immunostaining for an ER marker (Fig. 11B). Additional work is needed to test the relative contributions of TRPV4 and TRPM2 to the effects of IR stimulation applied to spiral ganglion neurons at physiological temperatures, including studies of neurons lacking these TRP channels. Targets for future study include other heat-activated TRP channels such as TRPV1, TRPV3, and TRPM3 as well as possible heteromeric TRP channels (Du et al. 2014; Ferrandiz-Huertas et al. 2014; Ishibashi et al. 2008; Planells-Cases et al. 2011; Vriens et al. 2011). The temperature ranges of activation for TRP channels might vary depending on the cellular environment.
Figure 12 diagrams a scenario consistent with our and others’ findings, in which the temperature increase produced by IR stimulation activates TRP channels, possibly associated with IP3Rs. The resulting elevation of cytosolic [Ca2+] in microdomains near IP3Rs and/or RyRs then initiates CICR. IP3-dependent CICR can be activated by very small increases in [Ca2+] in local domains near the ER in neuroblastoma cells (Dickinson and Parker 2013a).
A recent study (Moreau et al. 2018) offered a different mechanism for the IR-stimulated release of Ca2+ from ER that they recorded in neural and glial cell lines, namely, that ER Ca2+ release results from IR-induced activation of phosphoinositide signaling. Their conclusion is based on observations that the Ca2+ release was blocked by U-73122 (inhibitor of phospholipase C) and by 2-APB (inhibitor of IP3R). It is possible that IR-induced Ca2+ release involves CICR initiated by both the phospholipase C/IP3 mechanism they proposed and the TRP channel/IP3R mechanism we propose in this article. However, it is likely that at least some aspects of the phenomenon studied by Moreau et al. (2018) differ from those of the response we studied, because the temperature increase that produced a half-maximal [Ca2+] increase in their study was 19°C, whereas the maximal temperature increase in our study was 12°C. Part of this difference might be due to which TRP channels are present in the different cells.
IR-induced temperature increases also appear to increase Ca2+ uptake by ER, because under conditions that inhibit IR-stimulated CICR, the IR stimulation often evoked a slowly decaying decrease in cytosolic [Ca2+] (see e.g., Fig. 9Ac with an IP3R antagonist, Fig. 4B with cooling, and Fig. 10Bb with a TRPV4 antagonist). The activity of the Ca2+-ATPase in ER is increased by an ER Ca2+ sensor, STIM1, which is activated by increasing temperature (Xiao et al. 2011). STIM1 increases ER Ca2+-ATPase activity via interactions with ER phospholamban (PLN in Fig. 12; Zhao et al. 2015). In medium containing normal [Ca2+], STIM1 activation following a temperature pulse might also contribute to refilling of ER via store-activated channels such as Orai (Moccia et al. 2015; Xiao et al. 2011).
The effects of this IR-induced increase in Ca2+ on spiral ganglion excitability have not yet been determined. Albert et al. (2012) found that IR pulse trains increased excitability in rodent (mouse, rat) retinal and vestibular ganglion cells, whereas Rabbitt et al. (2016) found that in chinchilla vestibular hair cells, IR pulse trains produced a brief period of increased excitability followed by a longer period of decreased excitability. It is as yet unclear how the IR-induced increase in intracellular [Ca2+] studied here might contribute to increased electrical excitability, but the decreased excitability might involve activation of Ca2+-activated K+ channels, consistent with the finding of Rabbitt et al. (2016) that the decreased excitability was blocked by iberiotoxin, an inhibitor of the BK (large conductance) type of Ca2+-activated K+ channels. Inability to decrease excitability might contribute to the increased susceptibility to noise-induced hearing loss evident in TRPV4 knockout mice (Tabuchi et al. 2005).
In summary, the present work demonstrates that stimulation with IR pulses produces a transient increase in cytosolic [Ca2+] in cultured spiral ganglion neurons. This effect is triggered by the IR-induced temperature increase, does not require extracellular Ca2+, and is likely amplified by CICR from ER via both RyRs and IP3Rs. The temperature-sensitive Ca2+ release that triggers CICR likely involves TRPV4 (and perhaps TRPM2) channels in ER. The temperature increase also appears to increase the rate of Ca2+ uptake into ER. It is not yet clear how the IR-induced temperature increases and resultant changes in cytosolic [Ca2+] affect the electrical excitability and discharge of spiral ganglion neurons, but our findings indicate that the temperature at which these experiments are conducted has a major effect on the results. Our findings also introduce IR stimulation as an experimental tool to induce rapid release of intracellular Ca2+ stores.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant 1R01DC013798 (to S. M. Rajguru).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N.B., E.F.B., and S.M.R. conceived and designed research; J.N.B., S.R., J.S., C.M., J.P., and S.M.R. performed experiments; J.N.B., S.R., J.S., C.M., J.P., E.F.B., and S.M.R. analyzed data; J.N.B., S.R., J.S., C.M., J.P., E.F.B., and S.M.R. interpreted results of experiments; J.N.B., S.R., J.S., C.M., E.F.B., and S.M.R. prepared figures; J.N.B., S.R., J.S., C.M., J.P., E.F.B., and S.M.R. drafted manuscript; J.N.B., S.R., J.S., C.M., J.P., E.F.B., and S.M.R. edited and revised manuscript; J.N.B., S.R., J.S., C.M., J.P., E.F.B., and S.M.R. approved final version of manuscript.
APPENDIX: COMPARISON WITH PREVIOUS STUDY
A previous study from this laboratory on the effects of IR stimulation on spiral ganglion neurons (Lumbreras et al. 2014) reported findings that differ from those in the present study in two key respects. First, that study interpreted the decrease in indicator (Ca420) fluorescence observed during IR stimulation as being caused by a decrease in intracellular [Ca2+]. At that time the authors were not aware of the work of Shuttleworth and Thompson (1991), which demonstrated that the fluorescence of many Ca2+ indicators decreases in response to an increase in temperature and that this temperature dependence is greater for the Ca2+-bound form of the indicator. Figures 1, A–C, and 3A in the present article show evidence that the fluorescence decrease of fluo indicators during IR stimulation is temperature dependent and can be demonstrated even in a paraformaldehyde-fixed neuron. Given these findings, it seems likely that at least part of the decrease in fluorescence during IR stimulation reported previously (Lumbreras et al. 2014) was due to an effect of temperature on the Ca2+ indicator rather than to a decrease in cytosolic [Ca2+].
A second major difference is that Lumbreras et al. (2014) reported that after IR stimulation, the indicator fluorescence usually fell (suggesting a decrease in cytosolic [Ca2+]), whereas the present study finds that for higher laser powers (≥0.6 W/mm2), the dominant poststimulation response is a transient increase in cytosolic [Ca2+] (Fig. 2). This difference is likely due to the different initial temperatures used in the two studies: the temperature of the room in which the Lumbreras et al. (2014) study was conducted was ~20°C, whereas the room temperature in the present study was ~24°C, closer to (albeit still colder than) mammalian temperatures. Figure 4A plots the effects of modifying the initial temperature (by heating or cooling both the medium and the room temperature), showing that cooler initial temperatures inhibit the fluorescence/cytosolic [Ca2+] elevation (Fig. 4B) and warmer initial temperatures increase the response (Fig. 4A, inset). When the cytosolic [Ca2+] elevation is inhibited, the dominant response observed after IR stimulation is a decrease in florescence/cytosolic [Ca2+], due perhaps to a heat-induced increase in the rate of Ca2+ reuptake into ER (see also Figs. 9Ac and Fig. 10, Ab and Bb).
Another difference from the previous study is that the present study used an improved culture technique that resulted in longer survival of the dissociated spiral ganglion neurons.
REFERENCES
- Albert ES, Bec JM, Desmadryl G, Chekroud K, Travo C, Gaboyard S, Bardin F, Marc I, Dumas M, Lenaers G, Hamel C, Muller A, Chabbert C. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J Neurophysiol 107: 3227–3234, 2012. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- Barigelletti F. Effect of temperature on the photophysics of rhodamine 101 in a polar solvent. Chem Phys Lett 140: 603–606, 1987. doi: 10.1016/0009-2614(87)80495-5. [DOI] [Google Scholar]
- Barrett EF. Contrasting contributions of endoplasmic reticulum and mitochondria to Ca2+ handling in neurons. J Gen Physiol 118: 79–82, 2001. doi: 10.1085/jgp.118.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-de-Souza JL, Pinto BI, Pepperberg DR, Bezanilla F. Optocapacitive generation of action potentials by microsecond laser pulses of nanojoule energy. Biophys J 114: 283–288, 2018. doi: 10.1016/j.bpj.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayce JM, Bouchard MB, Chernov MM, Chen BR, Grosberg LE, Jansen ED, Hillman EM, Mahadevan-Jansen A. Calcium imaging of infrared-stimulated activity in rodent brain. Cell Calcium 55: 183–190, 2014a. doi: 10.1016/j.ceca.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayce JM, Friedman RM, Chen G, Jansen ED, Mahadevan-Jansen A, Roe AW. Infrared neural stimulation of primary visual cortex in non-human primates. Neuroimage 84: 181–190, 2014b. doi: 10.1016/j.neuroimage.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayce JM, Wells JD, Malphrus JD, Kao C, Thomsen S, Tulipan NB, Konrad PE, Jansen ED, Mahadevan-Jansen A. Infrared neural stimulation of human spinal nerve roots in vivo. Neurophotonics 2: 015007, 2015. doi: 10.1117/1.NPh.2.1.015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Bao W, Behm DJ, Brooks CA, Bury MJ, Dowdell SE, Eidam HS, Fox RM, Goodman KB, Holt DA, Lee D, Roethke TJ, Willette RN, Xu X, Ye G, Thorneloe KS. Discovery of GSK2193874: an orally active, potent, and selective blocker of transient receptor potential vanilloid 4. ACS Med Chem Lett 8: 549–554, 2017. doi: 10.1021/acsmedchemlett.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 278: 32037–32046, 2003. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci 24: 5177–5182, 2004. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Sonkusare SK, Teuscher C, Poynter ME, Nelson MT. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Sci Rep 6: 33841, 2016. doi: 10.1038/srep33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Parker I. Factors determining the recruitment of inositol trisphosphate receptor channels during calcium puffs. Biophys J 105: 2474–2484, 2013a. doi: 10.1016/j.bpj.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Parker I. Temperature dependence of IP3-mediated local and global Ca2+ signals. Biophys J 104: 386–395, 2013b. doi: 10.1016/j.bpj.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittami GM, Rajguru SM, Lasher RA, Hitchcock RW, Rabbitt RD. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J Physiol 589: 1295–1306, 2011. doi: 10.1113/jphysiol.2010.198804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J Neurochem 113: 313–328, 2010. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J 28: 4677–4685, 2014. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089, 2010. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz-Huertas C, Mathivanan S, Wolf CJ, Devesa I, Ferrer-Montiel A. Trafficking of thermoTRP channels. Membranes (Basel) 4: 525–564, 2014. doi: 10.3390/membranes4030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhang GQ, Hao XM, Wu CH, Chai Z, Wang SQ. Temperature dependence and thermodynamic properties of Ca2+ sparks in rat cardiomyocytes. Biophys J 89: 2533–2541, 2005. doi: 10.1529/biophysj.105.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997. doi: 10.1016/S0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gao L, Yang P, Qin P, Lu Y, Li X, Tian Q, Li Y, Xie C, Tian JB, Zhang C, Tian C, Zhu MX, Yao J. Selective potentiation of 2-APB-induced activation of TRPV1–3 channels by acid. Sci Rep 6: 20791, 2016. doi: 10.1038/srep20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Elias A, Berna-Erro A, Rubio-Moscardo F, Pardo-Pastor C, Mrkonjić S, Sepúlveda RV, Vicente R, González-Nilo F, Valverde MA. Interaction between the linker, pre-S1, and TRP domains determines folding, assembly, and trafficking of TRPV channels. Structure 23: 1404–1413, 2015. doi: 10.1016/j.str.2015.05.018. [DOI] [PubMed] [Google Scholar]
- García-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem 283: 31284–31288, 2008. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M. Expression of transient receptor potential vanilloid (TRPV) 1, 2, 3, and 4 in mouse inner ear. Acta Otolaryngol 128: 1286–1293, 2008. doi: 10.1080/00016480801938958. [DOI] [PubMed] [Google Scholar]
- Jenkins MW, Duke AR, Gu S, Chiel HJ, Fujioka H, Watanabe M, Jansen ED, Rollins AM. Optical pacing of the embryonic heart. Nat Photonics 4: 623–626, 2010. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Frye AM, Phuong TT, Redmon SN, Roberts R, Berkowitz BA, Yarishkin O, Križaj D. Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci USA 113: 3885–3890, 2016. doi: 10.1073/pnas.1515895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm GB, Siemens J. The TRPM2 channel in temperature detection and thermoregulation. Temperature (Austin) 4: 21–23, 2016. doi: 10.1080/23328940.2016.1258445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan T, Aoki H, Binh-Khiem N, Matsumoto K, Shimoyama I. Ratiometric optical temperature sensor using two fluorescent dyes dissolved in an ionic liquid encapsulated by Parylene film. Sensors (Basel) 13: 4138–4145, 2013. doi: 10.3390/s130404138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor N, Tran A, Kang J, Zhang R, Philipson KD, Goldhaber JI. Regulation of calcium clock-mediated pacemaking by inositol-1,4,5-trisphosphate receptors in mouse sinoatrial nodal cells. J Physiol 593: 2649–2663, 2015. doi: 10.1113/JP270082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Huang L, Yang J. Differential expression of ryanodine receptor in the developing rat cochlea. Eur J Histochem 53: 249–260, 2009. doi: 10.4081/ejh.2009.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Frerck MJ, Holman HA, Jorgensen EM, Rabbitt RD. Exciting cell membranes with a blustering heat shock. Biophys J 106: 1570–1577, 2014. doi: 10.1016/j.bpj.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Yang J. Developmental expression of inositol 1, 4, 5-trisphosphate receptor in the post-natal rat cochlea. Eur J Histochem 59: 2486, 2015. doi: 10.4081/ejh.2015.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbreras V, Bas E, Gupta C, Rajguru SM. Pulsed infrared radiation excites cultured neonatal spiral and vestibular ganglion neurons by modulating mitochondrial calcium cycling. J Neurophysiol 112: 1246–1255, 2014. doi: 10.1152/jn.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, D’Angelo E. Stim and Orai proteins in neuronal Ca2+ signaling and excitability. Front Cell Neurosci 9: 153, 2015. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Tlalpan V, Arellano RO, Díaz-Muñoz M. Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium 37: 203–213, 2005. doi: 10.1016/j.ceca.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Moreau D, Lefort C, Pas J, Bardet SM, Leveque P, O’Connor RP. Infrared neural stimulation induces intracellular Ca2+ release mediated by phospholipase C. J Biophotonics 11: e201700020, 2018. doi: 10.1002/jbio.201700020. [DOI] [PubMed] [Google Scholar]
- Morton-Jones RT, Cannell MB, Housley GD. Ca2+ entry via AMPA-type glutamate receptors triggers Ca2+-induced Ca2+ release from ryanodine receptors in rat spiral ganglion neurons. Cell Calcium 43: 356–366, 2008. doi: 10.1016/j.ceca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Morton-Jones RT, Cannell MB, Jeyakumar LH, Fleischer S, Housley GD. Differential expression of ryanodine receptors in the rat cochlea. Neuroscience 137: 275–286, 2006. doi: 10.1016/j.neuroscience.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Thomas NL, Williams AJ. Insights into the gating mechanism of the ryanodine-modified human cardiac Ca2+-release channel (ryanodine receptor 2). Mol Pharmacol 86: 318–329, 2014. doi: 10.1124/mol.114.093757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AE, Baker GA, Fugate RD, Tablin F, Crowe JH. Effects of temperature on calcium-sensitive fluorescent probes. Biophys J 78: 2116–2126, 2000. doi: 10.1016/S0006-3495(00)76758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Hori M, Kim YS, Kwon SC, Ahn DS, Nakazawa H, Kobayashi M, Karaki H. Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle. Br J Pharmacol 137: 1207–1212, 2002. doi: 10.1038/sj.bjp.0704988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Eun Kim G, Morales R, Moda F, Moreno-Gonzalez I, Concha-Marambio L, Lee AS, Hetz C, Soto C. The endoplasmic reticulum chaperone GRP78/BiP modulates prion propagation in vitro and in vivo. Sci Rep 7: 44723, 2017. doi: 10.1038/srep44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv Exp Med Biol 704: 491–515, 2011. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Brichta AM, Tabatabaee H, Boutros PJ, Ahn J, Della Santina CC, Poppi LA, Lim R. Heat pulse excitability of vestibular hair cells and afferent neurons. J Neurophysiol 116: 825–843, 2016. doi: 10.1152/jn.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajguru SM, Matic AI, Robinson AM, Fishman AJ, Moreno LE, Bradley A, Vujanovic I, Breen J, Wells JD, Bendett M, Richter CP. Optical cochlear implants: evaluation of surgical approach and laser parameters in cats. Hear Res 269: 102–111, 2010. doi: 10.1016/j.heares.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajguru SM, Richter CP, Matic AI, Holstein GR, Highstein SM, Dittami GM, Rabbitt RD. Infrared photostimulation of the crista ampullaris. J Physiol 589: 1283–1294, 2011. doi: 10.1113/jphysiol.2010.198333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes DOJ, Pyott SJ. The afferent signaling complex: regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear Res 336: 1–16, 2016. doi: 10.1016/j.heares.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Romanenko S, Siegel PH, Wagenaar DA, Pikov V. Effects of millimeter wave irradiation and equivalent thermal heating on the activity of individual neurons in the leech ganglion. J Neurophysiol 112: 2423–2431, 2014. doi: 10.1152/jn.00357.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem H, Tovey SC, Molinski TF, Taylor CW. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol 171: 3298–3312, 2014. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun 3: 736, 2012. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Effect of temperature on receptor-activated changes in [Ca2+]i and their determination using fluorescent probes. J Biol Chem 266: 1410–1414, 1991. [PubMed] [Google Scholar]
- Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353: 1393–1398, 2016. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett 382: 304–308, 2005. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M. Expression of transient receptor potential channel melastin (TRPM) 1-8 and TRPA1 (ankyrin) in mouse inner ear. Acta Otolaryngol 129: 1050–1060, 2009. doi: 10.1080/00016480802570545. [DOI] [PubMed] [Google Scholar]
- Tan CH, McNaughton PA. The TRPM2 ion channel is required for sensitivity to warmth. Nature 536: 460–463, 2016. doi: 10.1038/nature19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Stoddart PR, Jansen ED. Optical stimulation of neurons. Curr Mol Imaging 3: 162–177, 2014. doi: 10.2174/2211555203666141117220611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Wei Y, Lu J, Xu A, Xia M. Short-wavelength infrared laser activates the auditory neurons: comparing the effect of 980 vs. 810 nm wavelength. Lasers Med Sci 32: 357–362, 2017. doi: 10.1007/s10103-016-2123-4. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem 279: 35133–35138, 2004. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Tumelty J, Hinds K, Bankhead P, McGeown NJ, Scholfield CN, Curtis TM, McGeown JG. Endothelin 1 stimulates Ca2+-sparks and oscillations in retinal arteriolar myocytes via IP3R and RyR-dependent Ca2+ release. Invest Ophthalmol Vis Sci 52: 3874–3879, 2011. doi: 10.1167/iovs.10-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279, 2005. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Vizin RC, Scarpellini CS, Ishikawa DT, Correa GM, de Souza CO, Gargaglioni LH, Carrettiero DC, Bícego KC, Almeida MC. TRPV4 activates autonomic and behavioural warmth-defence responses in Wistar rats. Acta Physiol (Oxf) 214: 275–289, 2015. doi: 10.1111/apha.12477. [DOI] [PubMed] [Google Scholar]
- Voets T. TRP channels and thermosensation. Handb Exp Pharmacol 223: 729–741, 2014. doi: 10.1007/978-3-319-05161-1_1. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70: 482–494, 2011. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Walsh AJ, Tolstykh GP, Martens S, Ibey BL, Beier HT. Action potential block in neurons by infrared light. Neurophotonics 3: 040501, 2016. doi: 10.1117/1.NPh.3.4.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Siemens J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature (Austin) 2: 178–187, 2015. doi: 10.1080/23328940.2015.1040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Binder P, Fang Q, Wang Z, Xiao W, Liu W, Wang X. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br J Pharmacol 175: 1293–1304, 2018. doi: 10.1111/bph.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Wiltgen SM, Dickinson GD, Swaminathan D, Parker I. Termination of calcium puffs and coupled closings of inositol trisphosphate receptor channels. Cell Calcium 56: 157–168, 2014. doi: 10.1016/j.ceca.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Coste B, Mathur J, Patapoutian A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nat Chem Biol 7: 351–358, 2011. doi: 10.1038/nchembio.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem 276: 13810–13816, 2001. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Li T, Brochet DX, Rosenberg PB, Lederer WJ. STIM1 enhances SR Ca2+ content through binding phospholamban in rat ventricular myocytes. Proc Natl Acad Sci USA 112: E4792–E4801, 2015. doi: 10.1073/pnas.1423295112. [DOI] [PMC free article] [PubMed] [Google Scholar]