Abstract
The ability to recover one’s bearings when lost is a skill that is fundamental for spatial navigation. We review the cognitive and neural mechanisms that underlie this ability, with the aim of linking together previously disparate findings from animal behavior, human psychology, electrophysiology, and cognitive neuroscience. Behavioral work suggests that reorientation involves two key abilities: first, the recovery of a spatial reference frame (a cognitive map) that is appropriate to the current environment; and second, the determination of one’s heading and location relative to that reference frame. Electrophysiological recording studies, primarily in rodents, have revealed potential correlates of these operations in place, grid, border/boundary, and head direction cells in the hippocampal formation. Cognitive neuroscience studies, primarily in humans, suggest that the perceptual inputs necessary for these operations are processed by neocortical regions such as the retrosplenial complex, occipital place area and parahippocampal place area, with the retrosplenial complex mediating spatial transformations between the local environment and the recovered spatial reference frame, the occipital place area supporting perception of local boundaries, and the parahippocampal place area processing visual information that is essential for identification of the local spatial context. By combining results across these various literatures, we converge on a unified account of reorientation that bridges the cognitive and neural domains.
In Brief:
An essential aspect of navigation is the ability to regain one’s bearings after disorientation. Julian, Keinath, Marchette and Epstein review the literature on the cognitive and neural mechanisms underlying spatial reorientation, synthesizing results from animal behavior, human psychology, electrophysiology, and cognitive neuroscience.
Introduction
At some point in our lives, all of us have had the unsettling experience of losing our spatial bearings. Perhaps we had come up from a subway station onto a busy street and did not know which way we were facing. Perhaps we had taken a walk in the woods and lost track of where we were. In situations like these, unless we are aided by another person or a navigational device such as a compass or global positioning system, we must look out at the world and use perceptual information to figure out where we are and which way we are facing. In other words, we must spatially reorient ourselves. How do we accomplish this? And what are the neural systems involved?
The experience of being lost underscores the fact that we are spatially oriented much of the time — but not always. This psychological distinction between orientation and disorientation implies the existence of an internal representation of large-scale navigable space that we use to keep track of our current spatial situation. Such a representation is referred to as a cognitive map. In its strongest form, a cognitive map could be a Euclidean coordinate system [1,2], although less rigid forms of spatial knowledge, such as graph-like representations [3–5], are also possible. When we are disoriented, we no longer know where we are or which way we are facing on the map, and when we are misoriented, we have plotted our map location or heading inaccurately.
There are two ways that an oriented navigator can update their map coordinates as they move around the world. Path integration, sometimes called dead reckoning, involves the use of idiothetic cues, such as vestibular information, motor efference copies, proprioceptive signals, and optic flow, to actively update position and heading as one travels from a known starting position, often a home or a nest [2,6,7]. Landmark-based piloting, on the other hand, involves the use of allothetic (external) cues for updating [2]. Reorientation comes into play when one’s spatial updating — either from path integration or from landmarks — becomes inaccurate. It is then necessary to re-establish one’s coordinates de novo using allothetic cues. This can involve recovery of heading direction, location, or both. In this review, we will use the term reorientation as it is commonly used in the colloquial sense to encompass both functions. Formally, however, one should distinguish between heading retrieval and self-localization and, as we will see, between self-localization in the local sense — for example, where am I within the room? — and the global sense — for example, which room am I in? (Figure 1).
Figure 1. Components of reorientation.
A lost navigator must solve three tasks in order to regain her bearings: recognize her current context (context recognition), determine where she is located in that context (self-location) and which way she is facing (heading retrieval).
Reorientation is only relevant for navigators using a cognitive-map-based wayfinding strategy. Navigators using more basic strategies, such as beaconing (moving directly to a goal) [8], view matching (moving to reduce the perceptual discrepancy between the current view and the view at the goal location) [9], or route following (a procedural strategy in which one implements a fixed series of actions in response to specific cues) [10], do not require reorientation, as in these cases there are no internal spatial coordinates to recover.
In this review, we will discuss the cognitive and neural mechanisms that underlie spatial reorientation. Our aim is to synthesize results from animal behavior, human psychology, electrophysiology, and cognitive neuroscience into a unified view of the topic. We will focus first on the behavioral literature, next on the neural correlates of reorientation within the brain’s spatial representation system, and finally on the brain systems that mediate the interaction between visual and spatial codes. We end with a brief assessment of prospects for future research.
Cognitive mechanisms of reorientation
Psychologists and ethologists have learned much about the cognitive mechanisms underlying spatial reorientation by observing the behavior of humans and animals. In this section, we describe how behavioral work has illuminated three central questions about spatial reorientation: what are the external cues are used for reorientation? What are the internal reference frames recovered? And is reorientation supported by a single cognitive mechanism or multiple mechanisms?
What are the external cues used for reorientation?
Although our surroundings provide us with many cues that could in principle be used for reorientation, such as nearby objects (for example, a mailbox), distal objects (for example, a mountain), and celestial bodies (for example, the North Star), laboratory experiments suggest that reorientation behavior is often guided primarily by the spatial geometry of the environment. This fact was first discovered by Cheng and Gallistel, who observed that after misorientation [11] or disorientation [12], rats in a rectangular chamber would often attempt to retrieve a buried reward by digging in the location that was diagonally opposite the correct location (Figure 2A). This behavior is notable because the correct location and the diagonally opposite location are indistinguishable if the only information the animal has to orient themselves is the geometry of the chamber as defined by the walls. The animals did not appear to use non-geometric features, such as odors, visual patterns, or wall colors, to resolve this geometric ambiguity, although they could learn an association between the reward and a feature that was co-located with it. This tendency to rely on the shape of space for reorientation has been subsequently observed in a number of species, including fish [13], human children [14], and human adults tested under conditions that place demands on language and working memory [15,16]. Experiments in avian species and monkeys, on the other hand, have observed more equal reliance on geometric and non-geometric features [17,18].
Figure 2. Cognitive mechanisms underlying reorientation.
(A) In the standard reorientation task, a navigator is allowed to locate a reward in one corner (R, correct corner) of a small rectangular chamber with polarizing features along the walls (for example, black stripes). They are then removed from the chamber and disoriented. When placed back in the chamber at test, navigators typically use boundary geometry to reorient, searching equally often at the rewarded and geometrically equivalent opposite (G) corners, while ignoring non-geometric features. (B) Judgment of relative direction (JRD) tasks reveal the reference frames underlying spatial representations. Memory for the relative locations of buildings on a college campus (map shown on left) was reported more accurately when imagining headings that were aligned to cardinal directions (N, W, S, E) compared to diagonal directions (NW, SW, SE, NE), with the best performance for imagined headings facing North (adapted with permission from [29]). (C) To test whether context recognition is dissociable from heading retrieval, mice were trained to locate a hidden reward in two rectangular chambers that had identical geometry but were distinguishable by vertical versus horizontal stripes along one wall. Mice dug more often in the corners that were geometrically appropriate for each chamber, indicating that they used the features to distinguish between the chambers (adapted with permission from [35]). However, they failed to use the features to distinguish between geometrically appropriate corners within each chamber, instead relying exclusively on geometry (data not shown).
On the basis of their results, Cheng and Gallistel hypothesized that reorientation is supported by a geometric module that exclusively uses the shape of surrounding space to re-establish heading after misorientation or disorientation, and is impenetrable to non-geometric featural cues. This idea has generated considerable discussion over the past 30 years, in part because of the broader theoretical significance of the modularity claim. It is now clear that non-geometric cues can have an important influence over behavior after disorientation in many species; for example, when 18–24-month-old children search for a hidden toy they exhibit the classic result of failing to use a visual feature (a single colored wall) to disambiguate between geometrically equivalent locations in a small (4 × 6 feet) rectangular room, but they will use the same feature to distinguish between the locations in a large (8 × 12 feet) room [19]. It remains debated, however, whether this use of non-geometric features reflects incorporation of non-geometric information into reorientation [20], or the operation of a separate post-reorientation mechanism that checks the features at the target location for consistency with visual memory [21]. For our purposes, it is not important to resolve this debate, but merely to note that the literature indicates that environmental geometry is a powerful cue for reorientation.
There are at least three reasons why geometry may be particularly important for reorientation. First, environmental shape — the topography of the landscape — is an inherently stable aspect of the environment [2]. Indeed, there is evidence that the navigational system distinguishes between stable and unstable objects, using only the former as spatial references [22]. Second, boundary geometry tends to cover a large field of view and hence is perceptually salient. Third, geometry may be especially useful for a disoriented navigator because the intrinsic shape of the environment can be used to define an orientational axis [23]. Discrete landmarks, on the other hand, only define a consistent direction if they are distal from the viewer [1] (which may explain why nongeometric features have a stronger effect in larger environments). Interestingly, discrete landmarks are useful for spatial updating in oriented animals [24], even if they are less useful for reorientation, because the bearing between the observer and the landmark defines a unique direction in space if the locations of both the observer and the landmark are known [25]. Moreover, it is possible that discrete landmarks might be more important for reorientation in natural environments, which tend to be more open than the restricted enclosures that are almost universally used in laboratory experiments [26].
What are the internal reference frames recovered?
Location and heading — the quantities recovered during reorientation — must be defined relative to a reference frame. Some insight into these reference frames has come from studies using the judgment-of-relative-direction task [27]. Subjects in these experiments first learn an environment containing several objects. They are then removed from the environment and asked to imagine that they are standing at one object while facing a second; they then point to a third object. To perform this task, subjects must mentally re-instantiate a location and heading on each trial — a task akin to reorientation. Because they do this in the absence of relevant perceptual cues — trials are performed outside the recalled environment — the task illuminates the internal representations used during reorientation.
A consistent result from these experiments is that performance is orientation-dependent; that is, accuracy varies as a function of imagined facing direction. In some experiments, one imagined direction is preferred, while in others, directions opposite or orthogonal to this direction are also preferred, but to a lesser extent [28]. For example, in one study examining judgment-of-relative-directions defined by buildings on a college campus with a North–South alignment, accuracy was greatest for North-facing views, and it was also greater for East, South, and West facing views than for views facing diagonal directions [29] (Figure 2B). The advantage for the preferred directions is observed across different imagined standing positions, thus indicating that the preference is for a direction rather than for a specific view.
These results have been interpreted as evidence that we assign spatial axes to environments when we first encounter them, which we then use to orient ourselves when we encounter them again, or (in this case) imagine encountering them. Spatial recall is more accurate for imagined views that are aligned with these axes than for imagined views that are misaligned. Notably, environmental geometry plays an important role in defining these axes, though other factors are also influential, including egocentric experience (the direction that one first enters an environment is often privileged, especially if it is aligned with local geometry) [30], the arrangement of objects within a room [31], and even the intrinsic alignment of these objects [32]. These results suggest that — in humans at least — these axes are established by a cognitive mechanism that is sensitive to several different kinds of spatial organization in the visually perceived environment, including the shape of local space, but also other factors.
Is reorientation supported by a single cognitive mechanism or multiple mechanisms?
We have been discussing reorientation as a single process; there is, however, some evidence that it might be divisible into separate subcomponents. Heading retrieval and self-localization are logically dissociable from each other: a compass indicates heading but not location, whereas a global positioning system indicates location but not heading. Under some circumstances, animals use different cues to solve each problem. In the Morris Water Maze, for example, when rodents are placed into a circular pool at random locations and must navigate to a hidden platform, they use distal cues provided by the surrounding experimental room (including, potentially, its shape) to determine their heading, while using proximal cues provided by distance to the wall of the pool to determine their location [33,34]. That is, they use the cues that are most informative to solve each component of the task.
Additional evidence for multiple reorientation mechanisms comes from a recent study from our lab, which focused on the distinction between heading retrieval and context recognition [35]. The idea here is that reorientation involves not only determining one’s heading and location on a cognitive map, but also recognizing which cognitive map to retrieve. To demonstrate a dissociation between these two functions, we trained mice on a novel version of the Cheng and Gallistel reorientation task in which there were two rectangular chambers, rather than one, each with a different reward location. Each chamber had a distinct visual pattern along one of the walls, which was potentially informative about both heading (because the location of the pattern broke the geometric symmetry of the chamber) and contextual identity (because the patterns in each chamber were different). Strikingly, the search behavior of the animals indicated that they used the visual pattern to distinguish between the chambers, but did not use the pattern to distinguish between geometrically equivalent headings within each chamber (Figure 2C). This demonstrates a dissociation between heading retrieval and context recognition, insofar as these functions are controlled by different cues.
Neural mechanisms for reorientation
As conceptualized above, reorientation involves recovery of location on a cognitive map and facing direction relative to the map’s coordinate system. To understand the neural basis of this phenomenon, we must consider how reorientation affects the neural systems that mediate the cognitive map.
Over forty years of neurophysiological research have identified neurons in the rodent brain that are believed to be crucial for cognitive-map based navigation [36,37], with recent evidence suggesting a similar organization in humans [38]. These neurons include: place cells in the hippocampus, which fire when the animal is in specific locations within the environment [39,40]; grid cells in medial entorhinal cortex (MEC), which fire in a regular hexagonal lattice of locations that tile the floor of the environment, [41,42]; head-direction cells in dorsal presubiculum, anterodorsal thalamus, MEC, retrosplenial complex (RSC), and other structures, which fire based on the orientation of the head in the navigational plane [43,44]; and border/boundary cells in (respectively) the MEC and subiculum, which fire when the animal is in proximity to navigational boundaries at particular allocentric directions [45,46]. Although the full range of functionality of these systems remains a topic of considerable debate, with much recent evidence suggesting that they do more than simply represent physical space [47–50], it is generally accepted that in the navigational realm, place cells allow different locations and environments to be distinguished from each other, grid cells allow distances between locations to be defined, head-direction cells allow the animal to represent a heading relative to a consistent reference frame, and border/boundary cells allow spatial codes to be referenced to fixed topographical elements such as chamber walls.
Reorienting the hippocampal map
Within a given environment, hippocampal place cells exhibit unique preferred firing locations, known as ‘place fields’, with reliable relationships between the preferred locations of different cells. Thus, one can speak of the orientation and translational position of this hippocampal map relative to the external environment. Reorientation then corresponds to the recovery of a previously held map orientation and translational position following disorientation.
What are the external cues that are relevant to the recovery of map orientation? Many studies have shown that objects at the extremities of the perceptible environment are strong controllers of the orientation of the hippocampal map. Place fields rotate their locations around the center of the experimental chamber when objects in the surrounding room or along the walls of the chamber are rotated between navigational episodes [34,51,52]. Moreover, when distal cues, chamber geometry, and the internal sense of direction of the animal are rotated relative to each other, thus placing them into conflict, place cells exhibit mixed behavior, sometimes rotating with the chamber, sometimes with the distal cues, and sometimes remapping entirely [53].
At first glance, these findings would seem to be at odds with the behavioral data, suggesting that reorientation behavior is primarily controlled by environmental geometry. However, an important feature of most of these recording experiments is the fact that the animals are typically not disoriented before being placed back in the chamber. Thus, most studies of the effect of environmental cues are studies of oriented navigation, not studies of reorientation. In one classic report that did examine place fields in rodents subjected to disorientation (during both environmental familiarization and subsequent recording sessions), Knierim and colleagues [54] found that a cue card along the wall of a cylindrical chamber did not control the locations of hippocampal place fields. This suggests that objects at extremities are only used for reorientation if the animal has previously experienced them to be stable while in an oriented state.
Because they used a cylindrical chamber, Knierim and colleagues [54] could not test the role of spatial geometry in reorientation. In a recent study [55], we addressed this issue by recording from hippocampal place cells when disoriented mice foraged in rectangular, square, and isosceles triangle shaped chambers, each containing a salient visual marking along one wall. Congruent with the classical behavioral results, we found that the shape of the chamber determined the alignment of the recovered hippocampal map, whereas the location of the visual marking had no effect (Figure 3A). For example, from trial-to-trial, each place cell recorded in the rectangular chamber tended to have two place field locations that were 180° rotations of each other. Similarly, place cells recorded in the square chamber tended to have four place field locations at rotational offsets of 90°.
Figure 3. Neural mechanisms for reorientation.
(A) Following disorientation, the hippocampal map reorients based on chamber geometry rather than featural cues (adapted with permission from [55]). Mice in this experiment were disoriented between each experimental trial. For each chamber shape (rectangle, square, and isosceles triangle), trial-by-trial place fields reflected the geometric symmetries of the chamber (2×, 4×, 1×), despite the presence of a disambiguating feature cue along one wall. Simultaneously recorded place cells had fields that aligned coherently. (B) When trained to perform a classic reorientation task, there was a strong correspondence between the hippocampal map alignment on each trial and the location that the animal searched (adapted with permission from [55]; note that counter to this example, geometrically aligning place fields were found throughout the chamber, not just at the goal location). (C) Like place cells, head-direction cells tend to exhibit two preferred firing directions in a rectangular chamber from block-to-block following disorientation, oriented 180 degrees from each other (adapted with permission from [63]).
The presence of the visual marking along the wall failed to break these geometric symmetries. Only in the isosceles triangle did cells exhibit unique firing locations, consistent with the fact that this shape has no rotational symmetry. When we trained mice to perform Cheng and Gallistel’s classic reorientation task in the rectangular chamber, we observed a strong correspondence between the hippocampal map alignment on each trial and the location that the animal searched (Figure 3B). This suggests that geometry-based reorientation behavior in rodents is driven by geometry-based reorientation of the hippocampal map. Lesion studies provide concordant evidence for the idea that the hippocampus is centrally involved in the use of boundary geometry to recall previously learned spatial locations [56,57]. It remains to be seen if a similar effect of hippocampal-map reorientation would be observed in larger chambers, where non-geometric features might have a larger effect on reorientation behavior.
The recovered orientation of the hippocampal map is likely determined by input from head direction cells. These cells fire when the animal is facing a specific direction in the environment. Crucially, the preferred firing directions of individual cells are fixed relative to each other [58]. For example, if head-direction cell A fires at a heading 45 degrees clockwise relative to the preferred heading of head-direction cell B, this relationship will be maintained across navigational episodes and even across different environments [44]. Thus, it is possible to think of the head-direction system as a ‘neural compass’ that has a definable orientation relative to the external world. Previous work has shown that the orientation of the head direction system is tightly coupled to the orientation of the hippocampal map [59], giving credence to the idea that the head direction cells set the orientation of the entire system.
As with hippocampal place cells, visual features such as cue cards along the wall are strong controllers of the head-direction cell firing fields in oriented animals, but not in disoriented animals [54]. In the latter case, environmental geometry comes into play. For example, Knight and colleagues [60] found that when rats were removed from an isosceles-triangle shaped chamber, disoriented, and then placed back into the chamber, the head-direction cell firing fields recovered their previous relationship to the chamber geometry. Head-direction cells have also been shown to shift preferred direction in conjunction with rotations of rectangular and trapezoidal shaped enclosures when rats are disoriented [61], and to align to T-mazes set at different orientations when animals are moved between experimental rooms [62]. But the control of these cells by boundary geometry in disoriented animals is not absolute, as some cells will align to non-geometric cues rather than boundary shape if these cues take the form of a constellation of multiple objects that is rotated coherently relative to chamber geometry [61].
In a recent study, Weiss and colleagues [63] examined the responses of head direction cells in a reorientation task performed by rats in a rectangular chamber. Consistent with the 180-degree rotational symmetry of the chamber, they found that these cells tended to exhibit two possible preferred firing directions, oriented 180 degrees from each other (Figure 3C). The preferred direction often flipped after the animal was disoriented (which happened every four to six trials, rather than every trial as in the classical paradigm), but remained stable across successive trials when the animal was not disoriented between them. Grid cells simultaneously recorded in the same session also exhibited two preferred orientations, which were strongly correlated with the flipping of the head-direction cells. Thus, like hippocampal place cells, headdirection and grid cells also tend to be controlled by geometry after disorientation.
The heading signal that reorients the cognitive map might be conveyed from head-direction cells to place cells via border cells. The firing fields of these cells typically rotate coherently with head-direction cell tuning curves [46]. This interpretation is consistent with the known anatomy: there are strong projections from the subiculum, including one from the head-direction-cell-rich dorsal presubiculum to the MEC [64], which then projects to the hippocampus [65]. Grid cells, whose orientation has also been shown to be strongly controlled by environmental boundaries [66,67], may mediate the border cell input to the hippocampal map, although this is uncertain, as the directionality of information flow between grid and place cells remains a matter of debate [68,69].
Boundaries and place/grid field locations
In addition to being oriented, the cognitive map must also be translationally aligned. Environmental boundaries play an important role in solving this problem by acting as references for the locations of place and grid cell firing fields [66,67,70–74]. For example, O’Keefe and Burgess [71] recorded from place cells in oriented rats while systematically varying the size and shape of the environment. They found that place cells tended to respond at a fixed distances and directions from the chamber walls across these environmental deformations. Complementary results have also been found for grid cells [75]. Consistent with these physiological observations, neuroimaging studies have found that the human hippocampus activates proportionally with the number of spatial boundaries in imagined scenes [76], and responds strongly during learning of environmental locations with respect to boundaries [77].
The importance of boundaries for establishing the translational offset of the hippocampal map is well characterized by the boundary-vector cell model, in which place cell firing fields are determined by their distance and direction to environmental boundaries [72]. This model nicely complements behavioral studies of reorientation. First, the model predicts search behavior like that observed in the standard spatial reorientation task [78]. Second, it predicts behavioral findings that location is recovered on the basis of boundaries at specific distances and directions from the navigator, not on the basis of boundary lengths or angles of intersection ([79], but see 80]). Third, border cells exhibit adult-like firing fields as soon as rats are able to freely explore their environment (at around 16–18 days old) [81], and may thus provide the first critical input to hippocampal place cells, consistent with the importance of boundaries for reorientation early in development. Finally, although this model hypothesizes the existence of border cells with a wide range of boundary-distance tunings, the large majority of cells recorded thus far have firing fields neighboring boundaries. The effect of environment size on the prepotency of geometry for reorientation [19] might thus be due to the fact that boundaries are more proximal to the navigator in smaller environments, subsequently activating border cells which in turn lead the navigator to recover their location relative to the geometry.
Despite these congruencies, however, the boundary-vector cell model is not a model of reorientation, at least as currently constituted. Computational implementations of the model assume the existence of a reliable head-direction signal, which is used to transform egocentric bearings of perceived boundaries (‘boundary to the right’, for example) into allocentric boundary vectors (‘boundary to the East’, for example) [82]. Thus, the model describes how boundaries can be used to calculate one’s location when heading is known, but does not describe how boundaries are used to recover the orientation of the hippocampal map when heading is unknown.
A recent paper [25] showed that a similar kind of spatial model can be used to solve the converse problem: computation of heading from perceptual inputs (in this case, visual markings along the boundary or other featural cues) when location is known. Thus, one possibility is that one’s bearings are recovered after disorientation through iterative refinement of the location and heading estimates. Alternatively, a separate mechanism may be used to recover heading from environmental geometry, which then resets the orientation tuning of head direction cells. Under the latter hypothesis, boundary information used for orienting and translating the cognitive map would be processed through different pathways.
Choosing a cognitive map from ‘the cognitive atlas’
As discussed above, an important aspect of reorientation is choosing which cognitive map to retrieve. Place fields are usually stable across navigational episodes in familiar environments. But when an animal changes navigational contexts, for example by being moved to a new experimental chamber, a new hippocampal map can be recruited in a process known as remapping [83]. There are two major types of remapping. Global remapping occurs when all simultaneously recorded place cells shift their fields to unpredictable locations (with some previously-silent cells becoming active, and vice versa), quickly forming a new and distinct representation of the environment [84,85]. Rate remapping, on the other hand, occurs when place cells fire in the same locations relative to chamber geometry, but with firing rates that reliably differ between different navigational contexts [86].
Remapping can be induced by changes to a range of external sensory cues, including changes to featural cues, such as replacement of a white intramaze cue card with a black one [84], or replacement of a familiar testing cylinder by a novel cylinder of a different color [87]. Remapping can also be induced by changes to environmental geometry, such as changing the chamber shape from a square to a circle [70,88]. The emergence of remapping depends upon how much prior experience an animal has with a particular navigational context, and remapping can be disrupted by inhibiting plasticity [84,87]. These mnemonic components indicate that remapping is more than just differential response to different sensory contents; rather, it reflects recruitment of a unique cognitive map (drawn from the ‘cognitive atlas’) for each navigational or episodic context.
From perceptual representations to spatial codes
Reorientation, by definition, involves an interplay between internal spatial codes and perceptual representations. In the previous section, we discussed how remapping affects internal spatial codes. Here we consider the neural systems that provide the perceptual inputs to the reorientation process. Of key interest here are three brain regions that respond strongly when subjects view navigationally-relevant stimuli, such as landscapes, urban scenes, buildings and rooms: the retrosplenial complex, the occipital place area, and the parahippocampal place area [89–91]. These ‘scene’ regions are part of a larger set of brain areas that activate during virtual or mental navigation [92–95], and thus are prime candidates for processing visual information useful for reorientation.
Retrosplenial complex: spatial transformations
We focus first on the retrosplenial complex. Considerable evidence from neuroimaging, neurophysiology, and neuropsychology suggests that the retrosplenial/medial parietal region is centrally involved in spatial reorientation. In humans and monkeys, this region encompasses retrosplenial cortex proper (BA 29/30) and the posterior cingulate (BA 23/31). In rodents, there is a homolog of retrosplenial cortex (areas 29 and 30), but no posterior cingulate. Functional magnetic resonance imaging (fMRI) activity during passive viewing of visual scenes is observed in posterior portion of this region, along the parietal-occipital sulcus [96–99]. A recent study [99] indicated that this scene-responsive locus may be anatomically distinct from BA29/30 and suggested the name medial place area to avoid confusion. Here we use retrosplenial complex as a general term to refer to the retrosplenial/medial parietal region, retrosplenial complex/medial place area to indicate the functionally-defined scene-responsive territory within retrosplenial complex, and BA29/30 to refer to retrosplenial cortex proper (Figure 4A).
Figure 4. The retrosplenial complex supports spatial transformations necessary for reorientation.
(A) Left: retrosplenial complex/medial place area (RSC/MPA) and retrosplenial cortex (BA 29/30) shown on the human cortical surface. Right: retrosplenial cortex shown on the rodent brain. (B) fMRI evidence that the retrosplenial complex represents heading in a local reference frame. During training before scanning, participants learned the locations of objects (denoted by circles) inside virtual reality ‘museums’ that were oriented at 90 degrees from each other within a larger navigable space. During scanning, participants performed a judgement-of-relativedirection task that required them to imagine facing each object encountered during training. Multivoxel activity patterns in RSC were similar for imagined views across the two museums that had the same heading as defined by the local (museum-centered) reference frame indicated by the red arrows (adapted with permission from [116]). (C) In rodents, retrosplenial cortex contains ‘bidirectional’ (BD) cells that have preferred firing directions that represent heading in a local reference frame tied to each subchamber (adapted with permission from [121]). These bidirectional cells were interspersed with classical head direction cells that represented heading in a global reference frame that was consistent across both subchambers.
Initial evidence that retrosplenial complex is crucial for spatial reorientation came from neuropsychological reports [91,100–102]. Patients with damage to this region often exhibit ‘heading disorientation’: an inability to use perceptual information to orient themselves in largescale space [101]. For example, one patient was able to identify landmarks, but “the landmarks that he recognized did not provoke directional information with respect to those landmarks” [103]. These patients exhibit impairments on tasks that require them to transform between different spatial reference frames; for example, reproducing an array of cards in their original locations on the floor after a 90° rotation of the body [104] or drawing an arrow on a floor plan to indicate the viewpoint from which a photograph was taken [105].
Neuroimaging studies provide further support for the role of retrosplenial complex in spatial reorientation. Early reports indicated this region was one of several brain areas engaged during wayfinding [94], spatial memory retrieval [106], and mental imagery of scenes [96]. Although retrosplenial complex/medial place area responds during scene perception [96,107], its response is enhanced when subjects attempt to use the scene to orient themselves within the larger environment — for example, when attempting to recover the facing direction of the depicted view [108]. Activity in retrosplenial complex is greater in familiar environments for which subjects have survey knowledge [97,109,110], and shows adaptation when images depicting the same allocentric facing direction, or taken from the same location, are presented sequentially [111–113]. Anterior to retrosplenial complex/medial place area, BA29/30 may play a possibly distinct role in reorientation, as it shows selective response to objects that are fixed in space [114,115].
More recent fMRI studies have used multivoxel pattern analysis (MVPA) to elucidate the specific role that retrosplenial complex plays in spatial reorientation. In one case, subjects were taught locations of objects in a virtual environment consisting of several rectangular ‘museums’ within a larger courtyard, and then scanned while they performed a judgment of relative direction task on objects within the museums [116] (Figure 4B). Participants’ imagined facing direction (and their imagined location) could be decoded from fMRI activity patterns elicited during this task. Notably, these spatial codes were linked to local geometry: similar patterns were elicited for geometrically equivalent directions — such as ‘facing away from the door, along the long axis of the museum’ — in different museums.
These results suggest that the retrosplenial complex can code heading and location relative to the local environmental features (in this case, the walls of each museum), which is a key initial step towards solving the spatial reorientation problem. Interestingly, when subjects performed a location memory task in the same environment, they confused geometrically-equivalent locations in different museums, just as one would predict based on these fMRI results [117]. A further study [118] on a real-world environment found that the directional codes elicited in retrosplenial complex during the judgment of relative direction task are also found when subjects oriented in response to visual rather than imagined stimuli, as they would do if they were actually reorienting in the world. In that study, as in a similar study using fMRI adaptation [112], heading codes were consistent across the entire environment rather than being tied to local geometry, suggesting that retrosplenial complex is flexible in the scope of the reference frames it can encode.
It is unclear what is driving these retrosplenial complex computations at a cellular level, although a possible candidate has emerged from neurophysiological studies. It has long been known that rodent retrosplenial cortex contains neurons that encode many different kinds of spatial information, including head-direction cells [119] and direction-dependent place cells [120]. Recently, Jacob and colleagues [121] reported the existence of a new class of neurons in dysgranular retrosplenial cortex (area 30) that are similar to head-direction cells, but with directional tuning curves that are anchored to a local reference frame (Figure 4C). When rats navigated in an environment consisting of two connected rectangular subchambers that were polarized in opposing directions by cue cards at the end of each compartment, these cells fired in a specific direction in one subchamber and reversed their firing by 180° when the rat crossed into the other subchamber. These ‘bidirectional’ cells were intermixed with classical head-direction cells, which exhibited a consistent firing direction across the entire environment, and a second group of bidirectional cells which exhibited two consistent 180°-offset firing directions within both subchambers. Thus, the firing of some cells reflected the heading of the animal relative to local cues, while the firing of other cells reflected the global heading of the animals, echoing the different reference frames observed in human fMRI results [112,116,118]. Interactions between bidirectional and head direction cells might provide a mechanism for aligning the head direction system to the local reference frame during reorientation. This hypothesis might be tested in future studies by recording from head direction and bidirectional cells simultaneously during reorientation (the animals in Jacob and colleagues’ study [121] were not disoriented).
Additional recording studies have reported a wide variety of spatially responsive cells in rodent retrosplenial cortex, including cells that represent turn direction, path position, allocentric location, and combinations of all three when running along a fixed track [122,123] (see also [124,125]), and cells that represent context-distinguishing features and events [126,127]. These results suggest that the role of the retrosplenial complex in mediating between local and global reference frames during reorientation may be a subset of a general role in mediating between different spatial reference frames and between different spatial and behavioral contexts [82,100,128,129].
The hypothesis that retrosplenial complex supports reorientation by mediating the relationship between local landmarks (including geometry) and the head-direction system is consistent with rodent lesion data. In rats, retrosplenial cortex provides input to dorsal presubiculum and hence to the head-direction system [130]. Similar connectivity is observed in monkeys and humans [131]. Consistent with this connectivity, lesions to retrosplenial cortex reduce the stability of head-direction cell firing fields and — crucially — disrupt landmark control of head-direction cells when animals are placed back into a familiar chamber after disorientation [132]. This disruption is only partial, suggesting that there may be an alternative path for landmark information to reach the head-direction system, perhaps through direct connections from early visual cortex to the presubiculum [133,134].
Of relevance when interpreting these lesion studies is the fact that they were performed in a round chamber with only a single orientational cue. It thus remains unclear how retrosplenial lesions would affect landmark control of head-direction cells in situations where the animals must reorient themselves relative to geometry, or other more complex cues such as a constellation of objects. We speculate that the effect of retrosplenial lesions might be even more dramatic in such cases.
Occipital place area: boundary perception
Where do the perceptual inputs to the retrosplenial complex coordinate-transformation process come from? One possibility is that they are processed within retrosplenial complex itself, based on visual information obtained from the adjacent calcarine cortex (peripheral V1). Consistent with this view, Silson et al. [99] demonstrated retinotopic visual responses in the posterior portion of retrosplenial complex/medial place area. Alternatively (or additionally), reorientation might rely on information obtained from other brain regions within the dorsal visual stream. The functional relationship between retrosplenial complex and its dorsal stream inputs is not yet well understood [135]. However, recent work suggests that occipital place area is an important source of perceptual information about the local environment, and in particular about its spatial structure.
The occipital place area is located near the transverse occipital and intraparietal sulci in humans (Figure 5A) [136]. A homologous scene-responsive region has been identified in monkeys [98]; whether an occipital place area homologue exists in rodents is an open question. The occipital place area is rarely impacted by neurological insult; however, its position close to the skull makes it an ideal target for transcranial magnetic stimulation (TMS). This technique allows researchers to create a ‘virtual lesion’ that temporarily disrupts normal information processing. Consistent with its scene-selective fMRI response, TMS of occipital place area leads to impairments in the ability to recognize the categories of scenes [137] and discriminate scenes based on their spatial layout [138].
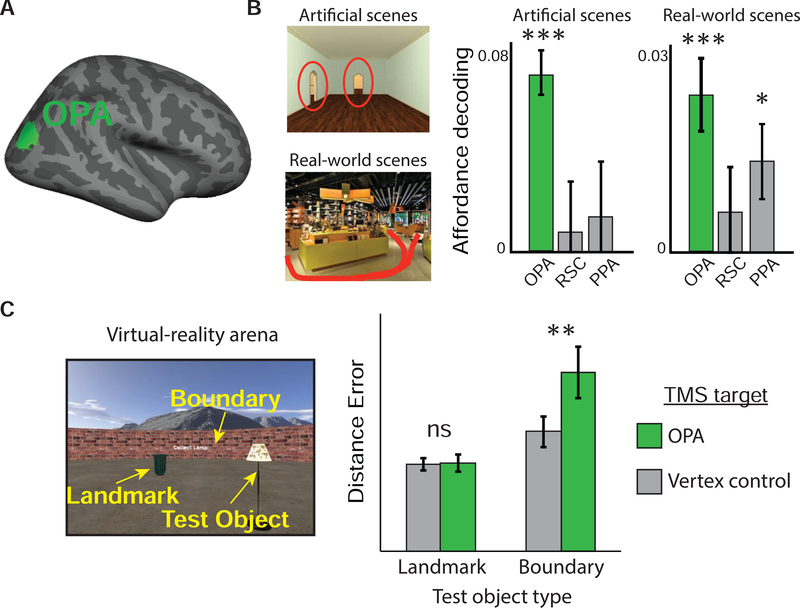
Figure 5. occipital place area is involved in perception of boundaries and local navigational affordances.
(A) Occipital place area (OPA) shown on the human cortical surface. (B) occipital place area multivoxel activity patterns reflect the locations of fine-grained navigational affordances in both artificial scenes (the position of doorways) and real-world scenes (locations of pathways), irrespective of other perceptual details present in the scenes [142]. (C) To test the causal role of occipital place area in boundary perception, participants learned the locations of test objects inside a virtual-reality arena containing a ‘landmark’ object and surrounded by a boundary wall. Half of the test objects had locations defined relative to the location of the ‘landmark’ object, and the other half had locations defined relative to a boundary. Compared to a vertex control stimulation site, TMS to the occipital place area impaired memory for the boundary-tethered object locations, but not the landmark-tethered objects (adapted with permission from [146]).
Results from fMRI studies indicate that the occipital place area may play an especially important role in extracting information about scenes’ spatial structure. The fMRI responses in the occipital place area are sensitive to spatial manipulations such as changes in the ‘sense’ (left versus right) and egocentric depth information of visual scenes [139,140]. Moreover, the occipital place area response is greater when subjects view sequences of scenes that depict egocentric motion through the environment than when they view jumbled sequences that do not depict a coherent path [141]. Multivoxel patterns in occipital place area contain fine-grained information about where a navigator can move in scenes, such as whether there is a door or a pathway for egress on the left or right (Figure 5B) [142]. These ‘navigational affordances’ can be thought of as the complement of navigational boundaries: whereas affordances are where one can go in the local environment, boundaries are where one’s movement is blocked. In both humans and monkeys, the occipital place area shows a bias for the lower visual field [143,144], suggesting it may be particularly important for determining paths, spaces, and boundaries that are defined along the ground plane [145].
A recent TMS study from our laboratory [146] directly linked the occipital place area to boundary perception (Figure 5C). Participants received TMS to either the right occipital place area or a control region before performing a spatial memory task that required them to learn the locations of several objects within a virtual reality arena. The locations of some of the objects were fixed relative to the arena boundary, whereas the locations of others were fixed relative to a proximal ‘landmark’ object that was always visible but could move to different locations within the arena. During test trials, participants were teleported into the arena and instructed to walk to the remembered location of each test object. Because participants started each trial in a random location and facing direction, they had to use visual information to reorient on each test trial. Strikingly, TMS to occipital place area selectively impaired the performance accuracy for the boundary-referenced objects, but not for the landmark-referenced objects, indicating that occipital place area has a specialized role in the perception of boundaries. Moreover, this effect was only found when the boundary was defined by a wall, not when it was defined by a surface texture on the ground. This last result is especially interesting, because previous behavioral work in young children had shown that two-dimensional markings on the ground are ineffective cues for reorientation compared to even subtle perturbations of three-dimensional surface layout [147].
These TMS results suggest that occipital place area may be the perceptual source for geometric information during reorientation. But there is one important caveat: the experiment was performed in a round arena, with distal cues at infinity to define the orientation of the space. Thus, the data do not speak directly to the occipital place area’s role in recovering heading relative to environmental boundaries, only its role in recovering position. Although it is parsimonious to hypothesize that boundary inputs from occipital place area might be crucial for both functions, this is a possibility that needs to be tested in future studies.
Parahippocampal place area: recognition of places and contexts
We now turn to the third scene-selective region. The parahippocampal place area is located near the parahippocampal and lingual boundary in humans [107,148] (Figure 6A), and two homologous scene-selective patches have been found near parahippocampal cortex in monkeys [98,149]. Although the parahippocampal place area appears to play a broad role in scene recognition [150], we speculate that its primary function in reorientation may be recognition of the overall navigational context — that is, choosing the right map from the cognitive atlas.
Figure 6. Parahippocampal place area is involved in identification of place and contexts.
(A) Parahippocampal place area (PPA) shown on the human cortical surface. (B) Postrhinal cortex (POR), a putative homologue of parahippocampal cortex shown on the rodent brain. (C) Lesions to POR result in context recognition impairments. Sham-operated control rats explore familiar objects appearing in incongruent but familiar contexts more than those appearing in congruent contexts. Postrhinal cortex lesions eliminate this preference. In a comparable noncontextual object recognition task, postrhinal cortex lesions had no effect (adapted with permission from [158]). (D) parahippocampal place area shows similar multivoxel activity patterns for images of the interior and exterior of the same buildings, thus reflecting the same navigational context, but only in participants who have navigational experience with the buildings (Penn students), not in participants who do not (Temple students) (adapted from ref. [163]).
Damage to the parahippocampal place area resulting from a stroke causes profound place recognition impairments, a deficit termed ‘landmark agnosia’. Individuals with damage to this region have relatively spared general perceptual abilities, and are able to navigate using selfmotion cues or small-scale visual details (such as house number). These individuals tend to become lost, however, because they are unable to recognize salient perceptual cues that define navigational context, such as buildings or landscapes, particularly when they must be recognized as ‘scenes’ rather than ‘objects’ [151]. Depending on the size of the lesion, these patients might also exhibit a more general problem with topographical learning [101,152,153].
On the basis of cytoarchitectonic characteristics and anatomical connectivity, the parahippocampal place area may be homologous with rodent postrhinal cortex (Figure 6B) [154,155]. Animal lesion studies of the posterior parahippocampal/ postrhinal region have confirmed the role of this region in context recognition [156–159] (Figure 6C). Moreover, the magnitude of navigation impairments following postrhinal lesions is not delay dependent, confirming that, like the human parahippocampal place area, the postrhinal cortex serves a perceptual function in rodents [160]. The postrhinal cortex is anatomically connected to the hippocampus via the medial entorhinal cortex, thus possibly providing navigational context information to the hippocampus [161]. Lesions of postrhinal cortex have little effect on the stability of place-cell location representations over time in a single navigational context in oriented animals [162], but a critical open question is whether damage to postrhinal cortex results in impairment of hippocampal remapping across contexts during reorientation.
Recent studies examining the parahippocampal place area’s pattern of fMRI response in humans provide convergent evidence for the importance of this region in context recognition. The parahippocampal place area response pattern is similar for scenes depicting different views of the same navigational context (such as the inside and outside of the same building), but only in participants that have learned the association between these views [163] (Figure 6D). The parahippocampal place area is sensitive to the presence of boundaries in scenes that form a navigable space [107] and represents the gross shape of the space defined by boundaries (for example, open versus closed) [164,165]. The parahippocampal place area is also sensitive to non-geometric features that could serve as useful indicators of context, such as texture and material properties [166] and visual summary statistics [167]. Further, the parahippocampal place area response to objects is greater if the objects have properties that would make them useful as landmarks [168], such being large and stable [169,170], distal [171], strongly associated with particular navigational contexts [172], or if they have been previously encountered at navigational decision points [173,174]. Together, these results are consistent with the idea that the parahippocampal place area is involved in context recognition on the basis of a variety of cues, including boundaries, objects, and visual features.
In contrast to its putative role in context recognition, a role for parahippocampal place area in the retrieval of heading is less likely for two reasons. First, the parahippocampal place area is insensitive to ‘sense’ (left-right) information in scenes, information which is necessary for computing the orientation conveyed by landmarks [139]. Second, lesions of postrhinal cortex in rodents do not disrupt featural control over head direction cell orientation representations, despite impairing context recognition [175]. The role of the parahippocampal place area in selflocalization has not been fully explored, though there is some evidence to suggest that it may have some involvement in this function (for example, see [176]).
Conclusion
In this review, we have attempted to provide a unified account of spatial reorientation. Long a topic of central concern in cognitive science, recent neuroscientific work makes it possible to link the cognitive operations behind spatial reorientation to possible neural mechanisms. Broadly, we conceptualize reorientation as involving the recovery of an appropriate cognitive map, and determination of one’s heading and location within the map’s coordinate system. As described above, neural correlates of these spatial functions are found in place, grid, border/boundary, and head-direction cells. Evidence for the perceptual processes underlying reorientation are observed in the retrosplenial complex, occipital place area, and parahippocampal place area, and indeed reorientation provides a powerful paradigm for examining the role of perceptual cues in shaping spatial representations. A particularly notable theme that recurs throughout this literature is the importance of environmental geometry; evidence for its use as a prepotent reorientation cue is found in behavioral, electrophysiological, and neuroimaging studies.
Our account is necessarily provisional. In many cases, the evidence we provide does not come from studies of reorientation per se, but from studies of oriented navigation or scene perception. It will be important for future work to fill this gap by studying reorientation more directly, and we hope that the ideas presented here might provide a guide for doing so. Another useful line of future investigation would be to understand reorientation in terms of the full range of spatial knowledge structures that the brain encodes. In the current account, we default to a traditional view of a cognitive map as a unified (perhaps Euclidean) coordinate system, but there is reason to believe that spatial knowledge in the real world might be fragmented [177,178], graph-like [179], or hierarchical [180]. It would also be of great interest to study reorientation in the real world. Geometry seems to dominate for organisms in small chambers or enclosed rooms, but the cues used for reorientation in the wild are not yet known. Finally, we note that there are differences between species that have been elided over in the current account, such as differences between the rodent and primate visual systems [181].
As a coda, we suggest that the study of reorientation might have consequences that go beyond spatial navigation. Humans ‘reorient’ in many different situations, not just spatial — consider, for example, the experience of picking up a book or a scientific article and mentally reorienting ourselves to the material within. There is now considerable evidence that the neural systems involved in spatial navigation are also involved in processing ‘spaces’ across a wide variety of domains [38,47–50]. This suggests the possibility that reorientation might be thought of not as a subset of spatial navigation, but rather as a general cognitive operation that applies under many situations.
Acknowledgements
Supported by U.S. NIH grants EY-022350 and EY-027047 (to R.A.E), NSF grant SBE-1041707, and NSF Graduate Research Fellowship (to J.B.J.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Keefe J, and Nadel L (1978). The hippocampus as a cognitive map (Clarendon Press Oxford; ). [Google Scholar]
- 2.Gallistel CR (1990). The organization of learning (The MIT Press; ). [Google Scholar]
- 3.Trullier O, Wiener SI, Berthoz A, and Meyer JA (1997). Biologically based artificial navigation systems: Review and prospects. Prog. Neurobiol 51, 483–544. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers B (2000). The spatial semantic hierarchy. Artif. Intell 119, 191–233. [Google Scholar]
- 5.Chrastil ER, and Warren WH (2014). From cognitive maps to cognitive graphs. PloS One 9, e112544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittelstaedt M-L, and Mittelstaedt H (1980). Homing by path integration in a mammal. Naturwissenschaften 67, 566–567. [Google Scholar]
- 7.Etienne AS, and Jeffery KJ (2004). Path integration in mammals. Hippocampus 14, 180–192. [DOI] [PubMed] [Google Scholar]
- 8.Leonard B, and McNaughton BL (1990). Spatial representation in the rat: Conceptual, behavioral, and neurophysiological perspectives. In Neurobiology of Comparative Cognition, Kesner RP and Olton DS, Eds., 363–422. [Google Scholar]
- 9.Collett M, Chittka L, and Collett TS (2013). Spatial memory in insect navigation. Curr. Biol 23, R789–R800. [DOI] [PubMed] [Google Scholar]
- 10.Cook D, and Kesner RP (1988). Caudate nucleus and memory for egocentric localization. Behav. Neural Biol 49, 332–343. [DOI] [PubMed] [Google Scholar]
- 11.Cheng K (1986). A purely geometric module in the rat’s spatial representation. Cognition 23, 149–178. [DOI] [PubMed] [Google Scholar]
- 12.Margules J, and Gallistel CR (1988). Heading in the rat: Determination by environmental shape. Anim. Learn. Behav 16, 404–410. [Google Scholar]
- 13.Sovrano VA, Bisazza A, and Vallortigara G (2002). Modularity and spatial reorientation in a simple mind: encoding of geometric and nongeometric properties of a spatial environment by fish. Cognition 85, B51–B59. [DOI] [PubMed] [Google Scholar]
- 14.Hermer L, and Spelke ES (1994). A geometric process for spatial reorientation in young children. Nature 370, 57–59. [DOI] [PubMed] [Google Scholar]
- 15.Hermer-Vazquez L, Spelke ES, and Katsnelson AS (1999). Sources of flexibility in human cognition: Dual-task studies of space and language. Cognit. Psychol 39, 3–36. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff KR, and Newcombe NS (2008). Is language necessary for human spatial reorientation? Reconsidering evidence from dual task paradigms. Cognit. Psychol 56, 142–163. [DOI] [PubMed] [Google Scholar]
- 17.Vallortigara G, Zanforlin M, and Pasti G (1990). Geometric modules in animals’ spatial representations: A test with chicks (Gallus gallus domesticus). J. Comp. Psychol 104, 248. [DOI] [PubMed] [Google Scholar]
- 18.Gouteux S, Thinus-Blanc C, and Vauclair J (2001). Rhesus monkeys use geometric and nongeometric information during a reorientation task. J. Exp. Psychol. Gen 130, 505. [DOI] [PubMed] [Google Scholar]
- 19.Learmonth AE, Nadel L, and Newcombe NS (2002). Children’s use of landmarks: Implications for modularity theory. Psychol. Sci 13, 337–341. [DOI] [PubMed] [Google Scholar]
- 20.Cheng K, Huttenlocher J, and Newcombe NS (2013). 25 years of research on the use of geometry in spatial reorientation: a current theoretical perspective. Psychon. Bull. Rev 20, 1033–1054. [DOI] [PubMed] [Google Scholar]
- 21.Lee SA, and Spelke ES (2010). Two systems of spatial representation underlying navigation. Exp. Brain Res 206, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biegler R, and Morris RG (1993). Landmark stability is a prerequisite for spatial but not discrimination learning. Nature 361, 631–633. [DOI] [PubMed] [Google Scholar]
- 23.Cheng K, and Gallistel CR (2005). Shape parameters explain data from spatial transformations: comment on Pearce et al.(2004) and Tommasi & Polli (2004). [DOI] [PubMed]
- 24.Suzuki S, Augerinos G, and Black AH (1980). Stimulus control of spatial behavior on the eight-arm maze in rats. Learn. Motiv 11, 1–18. [Google Scholar]
- 25.Bicanski A, and Burgess N (2016). Environmental Anchoring of Head Direction in a Computational Model of Retrosplenial Cortex. J. Neurosci 36, 11601–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lew AR (2011). Looking beyond the boundaries: time to put landmarks back on the cognitive map? Psychol. Bull 137, 484. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski LT, and Bryant KJ (1977). Sense of direction, spatial orientation, and cognitive maps. J. Exp. Psychol. Hum. Percept. Perform 3, 590. [Google Scholar]
- 28.McNamara TP (2002). How are the locations of objects in the environment represented in memory? In International Conference on Spatial Cognition (Springer; ), pp. 174–191. [Google Scholar]
- 29.Marchette SA, Yerramsetti A, Burns TJ, and Shelton AL (2011). Spatial memory in the real world: long-term representations of everyday environments. Mem. Cognit 39, 1401. [DOI] [PubMed] [Google Scholar]
- 30.Shelton AL, and McNamara TP (2001). Systems of spatial reference in human memory. Cognit. Psychol 43, 274–310. [DOI] [PubMed] [Google Scholar]
- 31.Mou W, and McNamara TP (2002). Intrinsic frames of reference in spatial memory. J. Exp. Psychol. Learn. Mem. Cogn 28, 162. [DOI] [PubMed] [Google Scholar]
- 32.Marchette SA, and Shelton AL (2010). Object properties and frame of reference in spatial memory representations. Spat. Cogn. Comput 10, 1–27. [Google Scholar]
- 33.Hamilton DA, Akers KG, Weisend MP, and Sutherland RJ (2007). How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J. Exp. Psychol. Anim. Behav. Process 33, 100. [DOI] [PubMed] [Google Scholar]
- 34.Knierim JJ, and Hamilton DA (2011). Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol. Rev 91, 1245–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julian JB, Keinath AT, Muzzio IA, and Epstein RA (2015). Place recognition and heading retrieval are mediated by dissociable cognitive systems in mice. Proc. Natl. Acad. Sci. USA 112, 6503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grieves RM, and Jeffery KJ (2017). The representation of space in the brain. Behav. Processes 135, 113–131. [DOI] [PubMed] [Google Scholar]
- 37.Hartley T, Lever C, Burgess N, and O’Keefe J (2014). Space in the brain: how the hippocampal formation supports spatial cognition. Phil Trans R Soc B 369, 20120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein RA, Patai EZ, Julian JB, and Spiers HJ (2017). The cognitive map in humans: spatial navigation and beyond. Nat. Neurosci 20, 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, and Fried I (2003). Cellular networks underlying human spatial navigation. Nature 425, 184–188. [DOI] [PubMed] [Google Scholar]
- 40.O’Keefe J, and Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. [DOI] [PubMed] [Google Scholar]
- 41.Hafting T, Fyhn M, Molden S, Moser M-B, and Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei X-X, Suthana N, Sperling MR, Sharan AD, and Fried I (2013). Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci 16, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube JS, Muller RU, and Ranck JB (1990). Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J. Neurosci 10, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taube JS (2007). The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30, 181–207. [DOI] [PubMed] [Google Scholar]
- 45.Solstad T, Boccara CN, Kropff E, Moser M-B, and Moser EI (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. [DOI] [PubMed] [Google Scholar]
- 46.Lever C, Burton S, Jeewajee A, O’Keefe J, and Burgess N (2009). Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci 29, 9771–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronov D, Nevers R, and Tank DW (2017). Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constantinescu AO, O’Reilly JX, and Behrens TE (2016). Organizing conceptual knowledge in humans with a gridlike code. Science 352, 1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nau M, Schröder TN, Bellmund JL, and Doeller CF (2018). Hexadirectional coding of visual space in human entorhinal cortex. Nat. Neurosci, 1. [DOI] [PubMed] [Google Scholar]
- 50.Julian JB, Keinath AT, Frazzetta G, and Epstein RA (2018). Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat. Neurosci, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’keefe J, and Conway DH (1978). Hippocampal place units in the freely moving rat: why they fire where they fire. Exp. Brain Res 31, 573–590. [DOI] [PubMed] [Google Scholar]
- 52.Cressant A, Muller RU, and Poucet B (1997). Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci 17, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeffery KJ, Donnett JG, Burgess N, and O’Keefe JM (1997). Directional control of hippocampal place fields. Exp. Brain Res 117, 131–142. [DOI] [PubMed] [Google Scholar]
- 54.Knierim JJ, Kudrimoti HS, and McNaughton BL (1995). Place cells, head direction cells, and the learning of landmark stability. J. Neurosci 15, 1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keinath AT, Julian JB, Epstein RA, and Muzzio IA (2017). Environmental geometry aligns the hippocampal map during spatial reorientation. Curr. Biol 27, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGregor A, Hayward AJ, Pearce JM, and Good MA (2004). Hippocampal lesions disrupt navigation based on the shape of the environment. Behav. Neurosci 118, 1011. [DOI] [PubMed] [Google Scholar]
- 57.Vargas JP, Petruso EJ, and Bingman VP (2004). Hippocampal formation is required for geometric navigation in pigeons. Eur. J. Neurosci 20, 1937–1944. [DOI] [PubMed] [Google Scholar]
- 58.Yoganarasimha D, Yu X, and Knierim JJ (2006). Head direction cell representations maintain internal coherence during conflicting proximal and distal cue rotations: comparison with hippocampal place cells. J. Neurosci 26, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoganarasimha D, and Knierim JJ (2005). Coupling between place cells and head direction cells during relative translations and rotations of distal landmarks. Exp. Brain Res 160, 344–359. [DOI] [PubMed] [Google Scholar]
- 60.Knight R, Hayman R, Ginzberg LL, and Jeffery K (2011). Geometric cues influence head direction cells only weakly in nondisoriented rats. J. Neurosci 31, 15681–15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark BJ, Harris MJ, and Taube JS (2012). Control of anterodorsal thalamic head direction cells by environmental boundaries: comparison with conflicting distal landmarks. Hippocampus 22, 172–187. [DOI] [PubMed] [Google Scholar]
- 62.Dudchenko PA, and Zinyuk LE (2005). The formation of cognitive maps of adjacent environments: evidence from the head direction cell system. Behav. Neurosci 119, 1511. [DOI] [PubMed] [Google Scholar]
- 63.Weiss S, Talhami G, Gofman-Regev X, Rapoport S, Eilam D, and Derdikman D (2017). Consistency of spatial representations in rat entorhinal cortex predicts performance in a reorientation task. Curr. Biol 27, 3658–3665. [DOI] [PubMed] [Google Scholar]
- 64.Witter MP, and Amaral DG (2004). The hippocampal region. Rat Nerv. Syst, 637–703. [Google Scholar]
- 65.Zhang S-J, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser M-B, and Moser EI (2013). Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science 340, 1232627. [DOI] [PubMed] [Google Scholar]
- 66.Krupic J, Bauza M, Burton S, Barry C, and O’Keefe J (2015). Grid cell symmetry is shaped by environmental geometry. Nature 518, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stensola T, Stensola H, Moser M-B, and Moser EI (2015). Shearing-induced asymmetry in entorhinal grid cells. Nature 518, 207–212. [DOI] [PubMed] [Google Scholar]
- 68.Solstad T, Moser EI, and Einevoll GT (2006). From grid cells to place cells: a mathematical model. Hippocampus 16, 1026–1031. [DOI] [PubMed] [Google Scholar]
- 69.Bush D, Barry C, and Burgess N (2014). What do grid cells contribute to place cell firing? Trends Neurosci. 37, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller RU, and Kubie JL (1987). The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Keefe J, and Burgess N (1996). Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. [DOI] [PubMed] [Google Scholar]
- 72.Hartley T, Burgess N, Lever C, Cacucci F, and O’Keefe J (2000). Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379. [DOI] [PubMed] [Google Scholar]
- 73.Burgess N, and Hartley T (2002). Orientational and geometric determinants of place and head-direction. Adv. Neural Inf. Process. Syst 1, 165–172. [Google Scholar]
- 74.Barry C, and Burgess N (2007). Learning in a geometric model of place cell firing. Hippocampus 17, 786–800. [DOI] [PubMed] [Google Scholar]
- 75.Barry C, Hayman R, Burgess N, and Jeffery KJ (2007). Experience-dependent rescaling of entorhinal grids. Nat. Neurosci 10, 682. [DOI] [PubMed] [Google Scholar]
- 76.Bird CM, Capponi C, King JA, Doeller CF, and Burgess N (2010). Establishing the boundaries: the hippocampal contribution to imagining scenes. J. Neurosci 30, 11688–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doeller CF, King JA, and Burgess N (2008). Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl. Acad. Sci 105, 5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barry C, Lever C, Hayman R, Hartley T, Burton S, O’Keefe J, Jeffery K, and Burgess Œ (2006). The boundary vector cell model of place cell firing and spatial memory. Rev. Neurosci 17, 71–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee SA, Sovrano VA, and Spelke ES (2012). Navigation as a source of geometric knowledge: Young children’s use of length, angle, distance, and direction in a reorientation task. Cognition 123, 144–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yousif SR, and Lourenco SF (2017). Are all geometric cues created equal? Children’s use of distance and length for reorientation. Cogn. Dev 43, 159–169. [Google Scholar]
- 81.Bjerknes TL, Moser EI, and Moser M-B (2014). Representation of Geometric Borders in the Developing Rat. Neuron 82, 71–78. [DOI] [PubMed] [Google Scholar]
- 82.Byrne P, Becker S, and Burgess N (2007). Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev 114, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colgin LL, Moser EI, and Moser M-B (2008). Understanding memory through hippocampal remapping. Trends Neurosci. 31, 469–477. [DOI] [PubMed] [Google Scholar]
- 84.Bostock E, Muller RU, and Kubie JL (1991). Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1, 193–205. [DOI] [PubMed] [Google Scholar]
- 85.Save E, Nerad L, and Poucet B (2000). Contribution of multiple sensory information to place field stability in hippocampal place cells. Hippocampus 10, 64–76. [DOI] [PubMed] [Google Scholar]
- 86.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, and Moser M-B (2005). Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309, 619–623. [DOI] [PubMed] [Google Scholar]
- 87.Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, and Muller RV (1998). Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280, 2121–2126. [DOI] [PubMed] [Google Scholar]
- 88.Lever C, Wills T, Cacucci F, Burgess N, and O’Keefe J (2002). Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416, 90–94. [DOI] [PubMed] [Google Scholar]
- 89.Julian JB, Fedorenko E, Webster J, and Kanwisher N (2012). An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage 60, 2357–2364. [DOI] [PubMed] [Google Scholar]
- 90.Malcolm GL, Groen II, and Baker CI (2016). Making sense of real-world scenes. Trends Cogn. Sci 20, 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Epstein RA (2008). Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci 12, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aguirre GK, Zarahn E, and D’esposito M (1998). Neural components of topographical representation. Proc. Natl. Acad. Sci 95, 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boccia M, Nemmi F, and Guariglia C (2014). Neuropsychology of environmental navigation in humans: review and meta-analysis of fMRI studies in healthy participants. Neuropsychol. Rev 24, 236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, and O’keefe J (1998). Knowing where and getting there: a human navigation network. Science 280, 921–924. [DOI] [PubMed] [Google Scholar]
- 95.Rosenbaum RS, Ziegler M, Winocur G, Grady CL, and Moscovitch M (2004). “I have often walked down this street before”: fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus 14, 826–835. [DOI] [PubMed] [Google Scholar]
- 96.O’Craven KM, and Kanwisher N (2000). Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J. Cogn. Neurosci 12, 1013–1023. [DOI] [PubMed] [Google Scholar]
- 97.Epstein RA, Higgins JS, Jablonski K, and Feiler AM (2007). Visual scene processing in familiar and unfamiliar environments. J. Neurophysiol 97, 3670–3683. [DOI] [PubMed] [Google Scholar]
- 98.Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, and Tootell RB (2011). Scene-selective cortical regions in human and nonhuman primates. J. Neurosci 31, 13771–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silson EH, Steel AD, and Baker CI (2016). Scene-selectivity and retinotopy in medial parietal cortex. Front. Hum. Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maguire E (2001). The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand. J. Psychol 42, 225–238. [DOI] [PubMed] [Google Scholar]
- 101.Aguirre GK, and D’Esposito M (1999). Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi N, Kawamura M, Shiota J, Kasahata N, and Hirayama K (1997). Pure topographic disorientation due to right retrosplenial lesion. Neurology 49, 464–469. [DOI] [PubMed] [Google Scholar]
- 103.Ino T, Doi T, Hirose S, Kimura T, Ito J, and Fukuyama H (2007). Directional disorientation following left retrosplenial hemorrhage: a case report with fMRI studies. Cortex 43, 248–254. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto R, and Nakano I (2014). The card placing test: a new test for evaluating the function of the retrosplenial and posterior cingulate cortices. Eur. Neurol 72, 38–44. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki K, Yamadori A, Hayakawa Y, and Fujii T (1998). Pure topographical disorientation related to dysfunction of the viewpoint dependent visual system. Cortex 34, 589–599. [DOI] [PubMed] [Google Scholar]
- 106.Burgess N, Maguire EA, Spiers HJ, and O’Keefe J (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14, 439–453. [DOI] [PubMed] [Google Scholar]
- 107.Epstein R, and Kanwisher N (1998). A cortical representation of the local visual environment. Nature 392, 598–601. [DOI] [PubMed] [Google Scholar]
- 108.Epstein RA, Parker WE, and Feiler AM (2007). Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J. Neurosci 27, 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolbers T, and Buchel C (2005). Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J. Neurosci 25, 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iaria G, Chen J-K, Guariglia C, Ptito A & Petrides M (2007). Retrosplenial and hippocampal brain regions in navigation: complementary functional contributions to the formation and use of cognitive maps. Eur. J. Neurosci 25, 890–899. [DOI] [PubMed] [Google Scholar]
- 111.Baumann O, and Mattingley JB (2010). Medial parietal cortex encodes perceived heading direction in humans. J. Neurosci 30, 12897–12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shine JP, Valdés-Herrera JP, Hegarty M, and Wolbers T (2016). The human retrosplenial cortex and thalamus code head direction in a global reference frame. J. Neurosci 36, 6371–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park S, and Chun MM (2009). Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. Neuroimage 47, 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Auger SD, Mullally SL, and Maguire EA (2012). Retrosplenial cortex codes for permanent landmarks. PloS One 7, e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Auger SD, Zeidman P, and Maguire EA (2015). A central role for the retrosplenial cortex in de novo environmental learning. Elife 4, e09031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchette SA, Vass LK, Ryan J, and Epstein R (2014). Anchoring the neural compass: coding of local spatial reference frames in human medial parietal lobe. Nat. Neurosci 17, 1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marchette SA, Ryan J, and Epstein RA (2017). Schematic representations of local environmental space guide goal-directed navigation. Cognition 158, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vass LK, and Epstein RA (2017). Common neural representations for visually guided reorientation and spatial imagery. Cereb. Cortex 27, 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen LL, Lin L-H, Green EJ, Barnes CA, and McNaughton BL (1994). Headdirection cells in the rat posterior cortex. Exp. Brain Res 101, 8–23. [DOI] [PubMed] [Google Scholar]
- 120.Cho J, and Sharp PE (2001). Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav. Neurosci 115, 3. [DOI] [PubMed] [Google Scholar]
- 121.Jacob P-Y, Casali G, Spieser L, Page H, Overington D, and Jeffery K (2016). An independent, landmark-dominated head-direction signal in dysgranular retrosplenial cortex. Nat. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alexander AS, and Nitz DA (2017). Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance traveled. Curr. Biol 27, 1551–1560. [DOI] [PubMed] [Google Scholar]
- 123.Alexander AS, and Nitz DA (2015). Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci 18, 1143. [DOI] [PubMed] [Google Scholar]
- 124.Sato N, Sakata H, Tanaka YL, and Taira M (2006). Navigation-associated medial parietal neurons in monkeys. Proc. Natl. Acad. Sci 103, 17001–17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mao D, Kandler S, McNaughton BL, and Bonin V (2017). Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat. Commun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith DM, Barredo J, and Mizumori SJ (2012). Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus 22, 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vedder LC, Miller AM, Harrison MB, and Smith DM (2016). Retrosplenial cortical neurons encode navigational cues, trajectories and reward locations during goal directed navigation. Cereb. Cortex 27, 3713–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vann SD, Aggleton JP, and Maguire EA (2009). What does the retrosplenial cortex do? Nat. Rev. Neurosci 10, 792–802. [DOI] [PubMed] [Google Scholar]
- 129.Miller AM, Vedder LC, Law LM, and Smith DM (2014). Cues, context, and longterm memory: the role of the retrosplenial cortex in spatial cognition. Front. Hum. Neurosci 8, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoder RM, Peck JR, and Taube JS (2015). Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. J. Neurosci 35, 1354–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kravitz DJ, Saleem KS, Baker CI, and Mishkin M (2011). A new neural framework for visuospatial processing. Nat. Rev. Neurosci 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark BJ, Bassett JP, Wang SS, and Taube JS (2010). Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J. Neurosci 30, 5289–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goodridge JP, and Taube JS (1997). Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J. Neurosci 17, 9315–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoder RM, Clark BJ, and Taube JS (2011). Origins of landmark encoding in the brain. Trends Neurosci. 34, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilber AA, Clark BJ, Forster TC, Tatsuno M, and McNaughton BL (2014). Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. J. Neurosci 34, 5431–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grill-Spector K (2003). The neural basis of object perception. Curr. Opin. Neurobiol 13, 159–166. [DOI] [PubMed] [Google Scholar]
- 137.Ganaden RE, Mullin CR, and Steeves JK (2013). Transcranial magnetic stimulation to the transverse occipital sulcus affects scene but not object processing. J. Cogn. Neurosci 25, 961–968. [DOI] [PubMed] [Google Scholar]
- 138.Dilks DD, Julian JB, Paunov AM, and Kanwisher N (2013). The occipital place area is causally and selectively involved in scene perception. J. Neurosci 33, 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dilks DD, Julian JB, Kubilius J, Spelke ES, and Kanwisher N (2011). Mirror-image sensitivity and invariance in object and scene processing pathways. J. Neurosci 31, 11305–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Persichetti AS, and Dilks DD (2016). Perceived egocentric distance sensitivity and invariance across scene-selective cortex. Cortex 77, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamps FS, Lall V, and Dilks DD (2016). The occipital place area represents firstperson perspective motion information through scenes. Cortex 83, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonner MF, and Epstein RA (2017). Coding of navigational affordances in the human visual system. Proc. Natl. Acad. Sci 114, 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silson EH, Chan AW-Y, Reynolds RC, Kravitz DJ, and Baker CI (2015). A retinotopic basis for the division of high-level scene processing between lateral and ventral human occipitotemporal cortex. J. Neurosci 35, 11921–11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arcaro MJ, and Livingstone MS (2017). Retinotopic organization of scene areas in macaque inferior temporal cortex. J. Neurosci 37, 7272–7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bonner MF, and Epstein RA (2018). Computational mechanisms underlying cortical responses to the affordance properties of visual scenes. PLoS Comp. Bio, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Julian JB, Ryan J, Hamilton RH, and Epstein RA (2016). The occipital place area is causally involved in representing environmental boundaries during navigation. Curr. Biol 26, 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee SA, and Spelke ES (2011). Young children reorient by computing layout geometry, not by matching images of the environment. Psychon. Bull. Rev 18, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aguirre GK, Zarahn E, and D’Esposito M (1998). An area within human ventral cortex sensitive to building stimuli: evidence and implications. Neuron 21, 373–383. [DOI] [PubMed] [Google Scholar]
- 149.Kornblith S, Cheng X, Ohayon S, and Tsao DY (2013). A network for scene processing in the macaque temporal lobe. Neuron 79, 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Epstein RA (2014). Neural systems for visual scene recognition. In Scene Vision, Kverga K and Bar M, Eds., pp. 105–134. [Google Scholar]
- 151.Mendez MF, and Cherrier MM (2003). Agnosia for scenes in topographagnosia. Neuropsychologia 41, 1387–1395. [DOI] [PubMed] [Google Scholar]
- 152.Habib M, and Sirigu A (1987). Pure topographical disorientation: a definition and anatomical basis. Cortex 23, 73–85. [DOI] [PubMed] [Google Scholar]
- 153.Epstein R, DeYoe EA, Press DZ, Rosen AC, and Kanwisher N (2001). Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cogn. Neuropsychol 18, 481–508. [DOI] [PubMed] [Google Scholar]
- 154.Burwell RD (2001). Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J. Comp. Neurol 437, 17–41. [DOI] [PubMed] [Google Scholar]
- 155.Furtak SC, Wei SM, Agster KL, and Burwell RD (2007). Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17, 709–722. [DOI] [PubMed] [Google Scholar]
- 156.Bussey TJ, Duck J, Muir JL, and Aggleton JP (2000). Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav. Brain Res 111, 187–202. [DOI] [PubMed] [Google Scholar]
- 157.Eacott MJ, and Gaffan EA (2005). The roles of perirhinal cortex, postrhinal cortex, and the fornix in memory for objects, contexts, and events in the rat. Q. J. Exp. Psychol. Sect. B 58, 202–217. [DOI] [PubMed] [Google Scholar]
- 158.Norman G, and Eacott MJ (2005). Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav. Neurosci 119, 557. [DOI] [PubMed] [Google Scholar]
- 159.Okudzhava VM, Natishvili TA, Gurashvili TA, Gogeshvili KS, Chipashvili SA, Bagashvili TI, Andronikashvili GT, Kvernadze GG, and Okudzhava NV (2009). Spatial recognition in cats: effects of parahippocampal lesions. Neurosci. Behav. Physiol 39, 613–618. [DOI] [PubMed] [Google Scholar]
- 160.Liu P, and Bilkey DK (2002). The effects of NMDA lesions centered on the postrhinal cortex on spatial memory tasks in the rat. Behav. Neurosci 116, 860. [DOI] [PubMed] [Google Scholar]
- 161.Ho JW, and Burwell RD (2014). Perirhinal and postrhinal functional inputs to the hippocampus In Space, Time and Memory in the Hippocampal Formation (Springer; ), pp. 55–81. [Google Scholar]
- 162.Nerad L, Liu P, and Bilkey DK (2009). Bilateral NMDA lesions centered on the postrhinal cortex have minimal effects on hippocampal place cell firing. Hippocampus 19, 221–227. [DOI] [PubMed] [Google Scholar]
- 163.Marchette SA, Vass LK, Ryan J, and Epstein RA (2015). Outside looking in: Landmark generalization in the human navigational system. J. Neurosci 35, 14896–14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kravitz DJ, Peng CS, and Baker CI (2011). Real-world scene representations in highlevel visual cortex: it’s the spaces more than the places. J. Neurosci 31, 7322–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park S, Brady TF, Greene MR, and Oliva A (2011). Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J. Neurosci 31, 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cant JS, and Goodale MA (2007). Attention to form or surface properties modulates different regions of human occipitotemporal cortex. Cereb. Cortex 17, 713–731. [DOI] [PubMed] [Google Scholar]
- 167.Cant JS, and Xu Y (2012). Object ensemble processing in human anterior-medial ventral visual cortex. J. Neurosci 32, 7685–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Troiani V, Stigliani A, Smith ME, and Epstein RA (2012). Multiple object properties drive scene-selective regions. Cereb. Cortex, 12, 883–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Konkle T, and Oliva A (2012). A real-world size organization of object responses in occipitotemporal cortex. Neuron 74, 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Julian JB, Ryan J, and Epstein RA (2016). Coding of Object Size and Object Category in Human Visual Cortex. Cereb. Cortex, 27, 3095–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amit E, Mehoudar E, Trope Y, and Yovel G (2012). Do object-category selective regions in the ventral visual stream represent perceived distance information? Brain Cogn. 80, 201–213. [DOI] [PubMed] [Google Scholar]
- 172.Bar M, and Aminoff E (2003). Cortical analysis of visual context. Neuron 38, 347–358. [DOI] [PubMed] [Google Scholar]
- 173.Janzen G, and van Turennout M (2004). Selective neural representation of objects relevant for navigation. Nat. Neurosci 7, 673–677. [DOI] [PubMed] [Google Scholar]
- 174.Schinazi VR, and Epstein R (2010). Neural correlates of real-world route learning. Neuroimage 53, 725–735. [DOI] [PubMed] [Google Scholar]
- 175.Peck JR, and Taube JS (2017). The postrhinal cortex is not necessary for landmark control in rat head direction cells. Hippocampus 27, 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, and Nadel L (1998). Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36, 1217–1238. [DOI] [PubMed] [Google Scholar]
- 177.Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser M-B, and Moser EI (2009). Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci 12, 1325. [DOI] [PubMed] [Google Scholar]
- 178.Meilinger T, Strickrodt M, and Bülthoff HH (2016). Qualitative differences in memory for vista and environmental spaces are caused by opaque borders, not movement or successive presentation. Cognition 155, 77–95. [DOI] [PubMed] [Google Scholar]
- 179.Warren WH, Rothman DB, Schnapp BH, and Ericson JD (2017). Wormholes in virtual space: From cognitive maps to cognitive graphs. Cognition 166, 152–163. [DOI] [PubMed] [Google Scholar]
- 180.Hirtle SC, and Jonides J (1985). Evidence of hierarchies in cognitive maps. Mem. Cognit 13, 208–217. [DOI] [PubMed] [Google Scholar]
- 181.Ekstrom AD (2015). Why vision is important to how we navigate. Hippocampus 25, 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]