Abstract
Hepatic Encephalopathy (HE) is associated with abnormalities in brain metabolism of glucose, oxygen and amino acids. In patients with acute liver failure, cortical lactate to pyruvate ratio is increased, which is indicative of a compromised cerebral oxidative metabolism. In this meta-analysis we have reviewed the published data on cerebral blood flow and metabolic rates from clinical studies of patients with HE. We found that hepatic encephalopathy was associated with reduced cerebral metabolic rate of oxygen, glucose, and blood flow. One exemption was in HE type B (shunt/by-pass) were a tendency towards increased cerebral blood flow was seen. We speculate that HE is associated with a disturbed metabolism—cytopathic hypoxia—and that type specific differences of brain metabolism is due to differences in pathogenesis of HE.
Abbreviations: ALF, Acute Liver Failure; CBF, Cerebral Blood Flow; CMR, Cerebral Metabolic Rate; HE, Hepatic Encephalopathy; ICH, Intracranial Hypertension; MHE, Minimal Hepatic Encephalopathy; MRI, Magnetic Resonance Imaging; OHE, Overt Hepatic Encephalopathy; pcMRI, Phase-Contrast MRI; PCS, Portocaval Shunt
Keywords: hepatic encephalopathy, liver failure, cerebral blood flow, cerebral metabolism
During Hepatic Encephalopathy (HE) the deterioration of brain function is accompanied by alterations in brain perfusion and energetics. Over several decades, several studies have focused on this pathophysiological aspect. The regulation of cerebral perfusion is essential in supporting the metabolic requirements for normal brain function. Under normal physiological conditions the global Cerebral Blood Flow (CBF) is kept almost constant during variations in systemic blood pressure—the cerebral autoregulation.1 Furthermore, during normal brain function the regional changes in neuronal activity are matched with a tight regulation of the regional CBF in the so called neurovascular unit.2 Several mechanisms are involved in this, including the myogenic response (the Bayliss effect)3 that couples hydrostatic pressure with vascular wall tone as well as the neurovascular coupling of metabolic supply and demand that acts through numerous and redundant mediators.4 Given the multifactorial pathophysiology of HE and the involvement several mechanisms that are directly or indirectly associated with perfusion and energy utilization, it is not surprising that patients with HE suffer from dysmetabolism and dysperfusion of the brain. For example, cerebral ammonium toxicity has experimentally been shown to affect extracellular potassium buffering,5 adenosine tone,6 glutamate signalling,7 lactate homeostasis,8 and microglia activation,9 which all represent potential points of interference with normal CBF regulation. With this literature review we summarize the published data on CBF and brain metabolism in patients with HE with special focus on: (1) the effect of HE on the global absolute CBF (mL of blood/100 g brain tissue * min) and (2) the cerebral metabolic rates of oxygen (CMRO2 (mL of oxygen/100 g brain tissue * min)) and glucose (CMRglc (µmol of glucose/100 g brain tissue * min)).
Methods
We performed our literature search using the terms “hepatic encephalopathy” and “cerebral blood flow”, “brain blood flow”, “fulminant liver failure”, or “cerebral metabolic rate”. The final search was completed in January 2018, using the following search engines: MEDLINE, Scopus, Web of Science, and Embase. From the publications, we extracted estimates of CBF and cerebral metabolic rates. Our inclusion criteria were: human data on absolute measurements of CBF in patients with HE. Analyses were done in the statistical software R ver. 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) using the package ‘metafor’. We calculated standardised mean differences of CBF and metabolic rates and fitted random-effects models as a conservative effect measure. I2 was used as a marker of heterogeneity. We undertook subgroup analyses based on the aetiology of HE and the method of CBF measurements. The majority of the studies reported results expressed in means ± standard deviation. Where median (range) values were used we accepted the median as an approximation of the sample mean and estimated the standard deviation as (maximum–minimum)/4.
Results
Our literature search resulted in 345 individual publications. Of these, 80 papers contained human original data. After exclusion of publications with case reports or only relative changes in CBF, we finally included 27 papers with absolute measurements of CBF in patients with HE. Of these, 16 described comparisons between patients with HE (minimal and/or overt) and controls (healthy subjects and/or cirrhotic patients without HE) allowing us to estimate the standardized mean differences. 14 studies also reported data on CMRO2 and CMRglc. The 27 included studies are listed in Table 1 and the CBF data from each study in Table 2.
Table 1.
The 27 Included Studies in the Meta-Analysis.
| Author | Year | Journal | HE type | Method |
|---|---|---|---|---|
| Zheng18 | 2017 | European Journal of Radiology | Cirrhosis OHE | pcMRI |
| Zheng19 | 2013 | European Journal of Radiology | MHE | MRI Arterial Spin |
| Strauss20 | 2001 | Gastroenterology | ALF | Kety-Schmidt 133-Xe |
| Almdal21 | 1989 | Scand J Gastroenterology | ALF | Kety-Schmidt 133-Xe |
| Aggarwal22 | 1991 | Transpl Proc | ALF | Kety-Schmidt 133-Xe |
| Wendon23 | 1994 | Hepatology | ALF | Kety-Schmidt 133-Xe |
| Durham24 | 1995 | JCBF | ALF | Kety-Schmidt 133-Xe |
| Jalan25 | 2001 | Hepatology | ALF-ICH | N2O |
| Jalan26 | 2004 | Gastroenterology | ALF-ICH | N2O |
| Dam27 | 2013 | Hepatology | Cirrhosis OHE | PET 15-O |
| Iversen28 | 2009 | Gastroenterology | Cirrhosis OHE | PET 15-O |
| Porro29 | 1969 | Gut | PCS | N2O |
| Zheng30 | 2012 | European Journal of Radiology | After TIPS | MRI Arterial Spin |
| Jalan31 | 2010 | Journal of Hepatology | 1 h after TIPS | N2O |
| Ahl32 | 2004 | Hepatology | MHE | PET 15-O |
| Larsen33 | 2000 | Critical Care Medicine | ALF | Kety-Schmidt 133-Xe |
| Larsen34 | 2000 | Critical Care Medicine | ALF | Kety-Schmidt 133-Xe |
| Larsen35 | 1999 | Transplantation | ALF | Kety-Schmidt 133-Xe |
| Philips36 | 1998 | Hepatology | Cirrhosis OHE | N2O |
| Larsen37 | 1996 | Livertransplantation Surgical | ALF | Kety-Schmidt 133-Xe |
| Larsen38 | 1995 | Hepatology | Cirrhosis OHE | Kety-Schmidt 133-Xe |
| Lockwood39 | 1991 | JCBF | Liver disease | PET 15-O |
| Testa40 | 1989 | Ital J Neu Sci | MHE | Kety-Schmidt 133-Xe |
| Rodriguez41 | 1987 | JCBFM | MHE | Kety-Schmidt 133-Xe |
| James42 | 1971 | Gut | Cirrhosis OHE | Kety-Schmidt 133-Xe |
| Posner43 | 1960 | J Clin Invest | Cirrhosis OHE | Kety-Schmidt |
| Fazekas44 | 1956 | American Journal of Medicine | Cirrhosis OHE | Kety-Schmidt |
ALF: Acute Liver Failure; ICH: Intracranial Hypertension; MHE: Minimal Hepatic Encephalopathy; MRI: Magnetic Resonance Imaging; OHE: Overt Hepatic Encephalopathy; pcMRI: Phase-Contrast MRI; PCS: Portocaval Shunt.
Table 2.
Cerebral Blood Flow Estimates From the Included Studies.
| Author | Year | OHE |
ALF |
Shunt (TIPS/PCS) |
MHE |
Cirrhotic controls |
Healthy controls |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | CBF mean ± s.d. | N | CBF mean ± s.d. | N | CBF mean ± s.d. | N | CBF mean ± s.d. | N | CBF mean ± s.d. | N | CBF mean ± s.d. | ||||||||
| Zheng | 2017 | 8 | 45.3 | ±16.0 | 11 | 55.6 | ±7.9 | 14 | 67.8 | ±7.3 | 31 | 57.1 | ±9.8 | ||||||
| Zheng | 2013 | 16 | 39.0 | ±8.0 | 16 | 67.4 | ±11.5 | 20 | 52.2 | ±12.0 | 25 | 48.8 | ±8.9 | ||||||
| Strauss | 2001 | 16 | 39.0 | ±8.0 | 5 | 62.0 | ±11.0 | 8 | 63.0 | ±9.0 | |||||||||
| Almdal | 1989 | 12 | 31.0 | ±4.0 | |||||||||||||||
| Aggarwal | 1991 | 33 | 42.0 | ±19.8 | |||||||||||||||
| Wendon | 1994 | 30 | 30.0 | ±14.3 | |||||||||||||||
| Durham | 1995 | 24 | 42.0 | ±13.0 | 24 | 55.0 | ±10.0 | ||||||||||||
| Jalan | 2001 | 9 | 111.0 | ±16.3 | |||||||||||||||
| Jalan | 2004 | 14 | 78.0 | ±9.7 | |||||||||||||||
| Dam | 2013 | 10 | 29.1 | ±9.4 | 9 | 44.7 | ±6.2 | ||||||||||||
| Iversen | 2009 | 6 | 30.2 | ±2.5 | 6 | 48.9 | ±5.1 | 7 | 51.0 | ±7.6 | |||||||||
| Porro | 1969 | 8 | 97.6 | ±26.8 | 8 | 63.8 | ±22.1 | 12 | 54.3 | ±9.1 | |||||||||
| Zheng | 2012 | 9 | 65.5 | ±17.9 | 9 | 60.0 | ±11.0 | ||||||||||||
| Jalan | 2010 | 9 | 67.2 | ±9.6 | 9 | 53.2 | ±7.2 | ||||||||||||
| Ahl | 2004 | 5 | 42.4 | ±6.2 | 3 | 52.8 | ±11.1 | ||||||||||||
| Larsen | 2000 | 8 | 34.0 | ±16.3 | |||||||||||||||
| Larsen | 2000 | 12 | 30.0 | ±9.8 | |||||||||||||||
| Larsen | 1999 | 13 | 43.8 | ±10.4 | |||||||||||||||
| Philips | 1998 | 14 | 44.0 | ±16.3 | |||||||||||||||
| Larsen | 1996 | 6 | 34.0 | ±10.8 | |||||||||||||||
| Larsen | 1995 | 6 | 60.8 | ±11.9 | 6 | 67.5 | ±13.3 | ||||||||||||
| Lockwood | 1991 | 11 | 51.0 | ±24.0 | 11 | 54.0 | ±20.0 | ||||||||||||
| Testa | 1989 | 20 | 42.3 | ±6.9 | 10 | 47.5 | ±5.4 | ||||||||||||
| Rodriguez | 1987 | 18 | 42.0 | ±7.0 | 18 | 50.0 | ±6.0 | ||||||||||||
| James | 1971 | 6 | 56.0 | ±18.4 | |||||||||||||||
| Posner | 1960 | 18 | 39.3 | ±10.9 | 11 | 53.0 | ±8.0 | ||||||||||||
| Fazekas | 1956 | 16 | 39.6 | ±8.0 | 20 | 47.1 | ±8.1 | ||||||||||||
Data expressed as means ± standard deviation in the unit of mL blood/100 g*min. ALF: Acute sLiver Failure; MHE: Minimal Hepatic Encephalopathy; OHE: Overt Hepatic Encephalopathy; PCS: Portocaval Shunt; TIPS: Transjugular Intrahepatic Portosystemic Shunt.
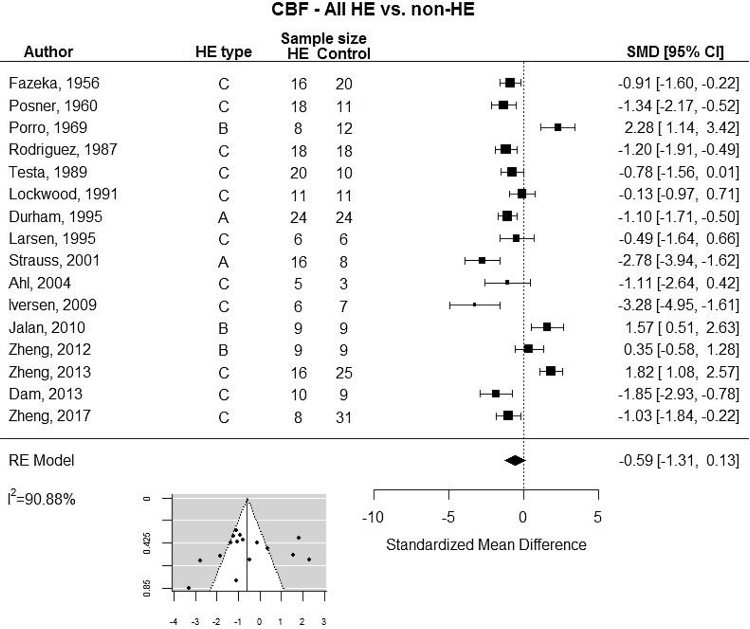
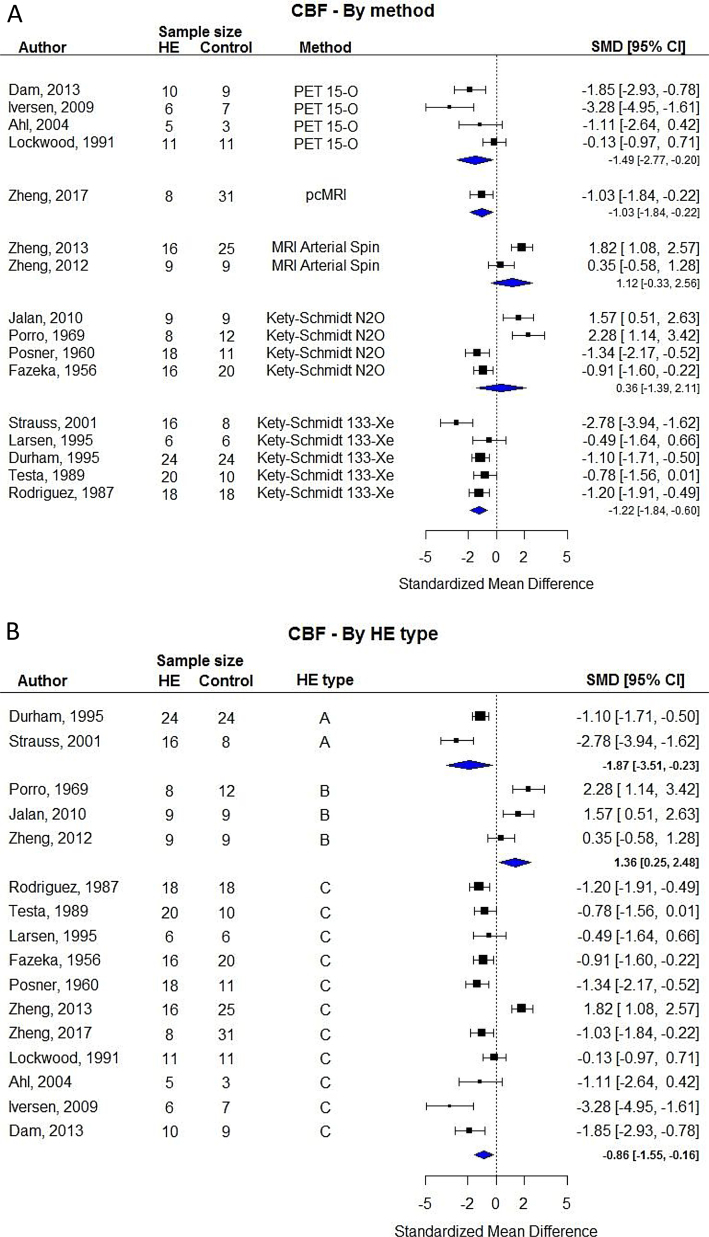
Our meta-analysis revealed a large degree of variation in CBF results across studies (Table 2). By limiting our analysis to the 16 studies that included comparisons between groups we found no clear difference between patients with HE compared with controls (Figure 1). The random-effects analysis showed substantial heterogeneity (I2 = 90.88%). We therefore undertook subgroup analyses based on the type of HE and modality of CBF measurement. These analyses showed that MRI and N2O based methods resulted in higher CBF during HE (Figure 2A) and that HE type B in contrast to type A and C was more likely to results in increased CBF (Figure 2B).
Figure 1.
Random-effects meta-analysis of studies with estimates of standardized mean differences (SMD). Insert: funnel plot. ALF: Acute Liver Failure; Minimal HE: Minimal Hepatic Encephalopathy; OHE: Overt Hepatic Encephalopathy; PCS: Portocaval Shunt; TIPS: Transjugular Intrahepatic Portosystemic Shunt.
Figure 2.
Random-effects subgroup analysis based on the modality of cerebral blood flow measurement (A) and aetiology of hepatic encephalopathy (B).
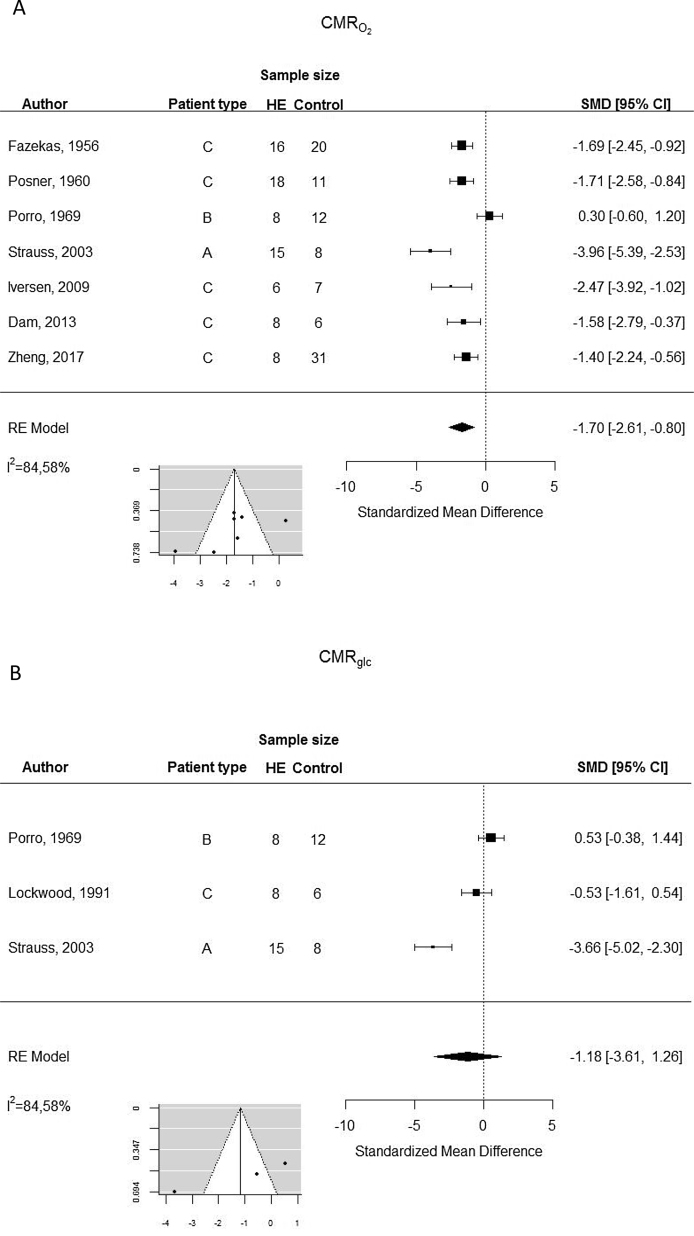
The metabolic rates of oxygen were significantly lower in patients with HE (Figure 3), but the analysis demonstrated substantial heterogeneity. The metabolic rate of glucose tended to be lower during HE, but only three studies were available for this comparison.
Figure 3.
Random-effects meta-analysis of cerebral metabolic rates of oxygen (A) and glucose (B).
Discussion
Our findings support the current understanding of HE as a metabolic encephalopathy with reduced neuronal activity and hence a reduced CBF and delivery of oxygen and glucose. Generally, we found that the results were characterised by a rather large degree of variation and substantial heterogeneity between studies. Of interest we found that HE due portacaval shunting (type B) was associated to an increased CBF, in contrast to studies of patients with HE of type A and C. This could indicate that the pathogenesis in HE is type specific and that the isolated hyperammonia that is often seen in type B HE leads to vasodilation and hyperperfusion, which also has been observed in animal studies.10 It is also noteworthy that the cerebral metabolic rates tended to be unaltered in type B HE in contrast to the reduced metabolic rates that were seen in type A and C. Since type A and C HE often is seen in patients with multi-organ failure, infections and electrolyte disturbances it is likely that the brain metabolism and blood flow is affected in a multifactorial fashion that leads to reduced neuronal activity and hence reduced blood flow. It is important to stress the fact that we found substantial heterogeneity in our meta-analysis and that most of the studies were done with rather small sample sizes and at different time-points in the disease course. It should also be noted that we were not able to account for the individual arterial partial pressures of carbon dioxide, haemoglobin levels or the type and depth of sedation, which all could represent potential confounding factors.
One interesting aspect observed in several experimental and clinical studies is cerebral accumulation of lactate8,11, 12, 13, 14, 15, 16 and we have previously proposed that the brain is suffering from some kind of cytopathic hypoxia13—e.g. a condition with anaerobe metabolism in spite of sufficient delivery of oxygen. This is based on the circumstance that the metabolic abnormalities seem too extensive to simply reflect reduced neuronal activity. Conditions associated with hypoxic metabolism include: (1) ischemia hypoxia due to low perfusion pressure, (2) low oxygen extraction due to low pO2, anaemia, or increased haemoglobin oxygen affinity, (3) arteriovenous shunting, (4) increased diffusion distance or reduced endothelial diffusion area, (5) impaired mitochondrial function, and (6) hypermetabolism. Most of these types of hypoxia can be excluded in HE based on published experimental and clinical data cited above. However dysperfusion in the cerebral microcirculation—for example due to shunting—might be a possible explanation, that to our knowledge has not been thoroughly studied although the hypothesis is far from new.17
In conclusion, we have found substantial heterogeneity in the published results on CBF and metabolic rates for oxygen and glucose in patients with HE. However, a general tendency towards reduced flow and metabolism was found.
Conflicts of interest
The authors have none to declare.
References
- 1.Lassen NA. Autoregulation of cerebral blood flow. Circ Res. 1964;15(suppl 4) [PubMed] [Google Scholar]
- 2.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28(3):220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangroo Thrane V, Thrane AS, Wang F. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013;19(12):1643–1648. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjerring PN, Dale N, Larsen FS. Acute hyperammonemia and systemic inflammation is associated with increased extracellular brain adenosine in rats: a biosensor study. Neurochem Res. 2015;40(2):258–264. doi: 10.1007/s11064-014-1357-4. [DOI] [PubMed] [Google Scholar]
- 7.Monfort P, Munoz MD, El Ayadi A, Kosenko E, Felipo V. Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab Brain Dis. 2002;17(4):237–250. doi: 10.1023/a:1021993431443. [DOI] [PubMed] [Google Scholar]
- 8.Bosoi CR, Rose CF. Elevated cerebral lactate: implications in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2014;29(4):919–925. doi: 10.1007/s11011-014-9573-9. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10(9):522–528. doi: 10.1038/nrgastro.2013.99. [DOI] [PubMed] [Google Scholar]
- 10.Bjerring PN, Bjerrum EJ, Larsen FS. Impaired cerebral microcirculation induced by ammonium chloride in rats is due to cortical adenosine release. J Hepatol. 2018 doi: 10.1016/j.jhep.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Witt AM, Larsen FS, Bjerring PN. Accumulation of lactate in the rat brain during hyperammonaemia is not associated with impaired mitochondrial respiratory capacity. Metab Brain Dis. 2017;32(2):461–470. doi: 10.1007/s11011-016-9934-7. [DOI] [PubMed] [Google Scholar]
- 12.Bosoi CR, Zwingmann C, Marin H. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol. 2014;60(3):554–560. doi: 10.1016/j.jhep.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Bjerring PN, Larsen FS. Changes in cerebral oxidative metabolism in patients with acute liver failure. Metab Brain Dis. 2013;28(2):179–182. doi: 10.1007/s11011-012-9346-2. [DOI] [PubMed] [Google Scholar]
- 14.Bjerring PN, Hauerberg J, Jorgensen L. Brain hypoxanthine concentration correlates to lactate/pyruvate ratio but not intracranial pressure in patients with acute liver failure. J Hepatol. 2010;53(6):1054–1058. doi: 10.1016/j.jhep.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth RF. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology. 2003;125(3):815–824. doi: 10.1016/s0016-5085(03)01054-0. [DOI] [PubMed] [Google Scholar]
- 16.Zwingmann C, Chatauret N, Leibfritz D, Butterworth RF. Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [H–C] nuclear magnetic resonance study. Hepatology. 2003;37(2):420–428. doi: 10.1053/jhep.2003.50052. [DOI] [PubMed] [Google Scholar]
- 17.Baldy-Moulinier M, Bories P. CBF and metabolism in hepatic encephalopathy: effects of acute hyperammoneamia and of L. dopa. Acta Neurol Scand Suppl. 1977;64:348–349. [PubMed] [Google Scholar]
- 18.Zheng G, Lu H, Yu W. Severity-specific alterations in CBF, OEF and CMRO2 in cirrhotic patients with hepatic encephalopathy. Eur Radiol. 2017;27(11):4699–4709. doi: 10.1007/s00330-017-4809-9. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Zhang LJ, Cao Y, Lu GM. Venous blood ammonia can be associated with cerebral blood flow in hepatic encephalopathy. Hepatology. 2013;58(2):832–833. doi: 10.1002/hep.26148. [DOI] [PubMed] [Google Scholar]
- 20.Strauss GI, Knudsen GM, Kondrup J, Moller K, Larsen FS. Cerebral metabolism of ammonia and amino acids in patients with fulminant hepatic failure. Gastroenterology. 2001;121(5):1109–1119. doi: 10.1053/gast.2001.29310. [DOI] [PubMed] [Google Scholar]
- 21.Almdal TP, Sorensen TI. Incidence of parenchymal liver diseases in Denmark, 1981 to 1985: analysis of hospitalization registry data. The Danish Association for the Study of the Liver. Hepatology. 1991;13(4):650–655. [PubMed] [Google Scholar]
- 22.Aggarwal S, Yonas H, Kang Y. Relationship of cerebral blood flow and cerebral swelling to outcome in patients with acute fulminant hepatic failure. Transplant Proc. 1991;23(3):1978–1979. [PubMed] [Google Scholar]
- 23.Wendon JA, Harrison PM, Keays R, Williams R. Cerebral blood flow and metabolism in fulminant liver failure. Hepatology. 1994;19(6):1407–1413. [PubMed] [Google Scholar]
- 24.Durham S, Yonas H, Aggarwal S, Darby J, Kramer D. Regional cerebral blood flow and CO2 reactivity in fulminant hepatic failure. J Cereb Blood Flow Metab. 1995;15(2):329–335. doi: 10.1038/jcbfm.1995.38. [DOI] [PubMed] [Google Scholar]
- 25.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Restoration of cerebral blood flow autoregulation and reactivity to carbon dioxide in acute liver failure by moderate hypothermia. Hepatology. 2001;34(1):50–54. doi: 10.1053/jhep.2001.25386. [DOI] [PubMed] [Google Scholar]
- 26.Jalan R, Olde Damink SW, Hayes PC, Deutz NE, Lee A. Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia and cerebral blood flow. J Hepatol. 2004;41(4):613–620. doi: 10.1016/j.jhep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Dam G, Keiding S, Munk OL. Hepatic encephalopathy is associated with decreased cerebral oxygen metabolism and blood flow, not increased ammonia uptake. Hepatology. 2013;57(1):258–265. doi: 10.1002/hep.25995. [DOI] [PubMed] [Google Scholar]
- 28.Iversen P, Sorensen M, Bak LK. Low cerebral oxygen consumption and blood flow in patients with cirrhosis and an acute episode of hepatic encephalopathy. Gastroenterology. 2009;136(3):863–871. doi: 10.1053/j.gastro.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi Porro G, Maiolo AT, Della Porta P. Cerebral blood flow and metabolism in hepatic cirrhosis before and after portacaval shunt operation. Gut. 1969;10(11):894–897. doi: 10.1136/gut.10.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng G, Zhang LJ, Wang Z. Changes in cerebral blood flow after transjugular intrahepatic portosystemic shunt can help predict the development of hepatic encephalopathy: an arterial spin labeling MR study. Eur J Radiol. 2012;81(12):3851–3856. doi: 10.1016/j.ejrad.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Jalan R, Olde Damink SW, Ter Steege JC. Acute endotoxemia following transjugular intrahepatic stent-shunt insertion is associated with systemic and cerebral vasodilatation with increased whole body nitric oxide production in critically ill cirrhotic patients. J Hepatol. 2011;54(2):265–271. doi: 10.1016/j.jhep.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Ahl B, Weissenborn K, van den Hoff J. Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology. 2004;40(1):73–79. doi: 10.1002/hep.20290. [DOI] [PubMed] [Google Scholar]
- 33.Larsen FS, Strauss G, Knudsen GM, Herzog TM, Hansen BA, Secher NH. Cerebral perfusion, cardiac output, and arterial pressure in patients with fulminant hepatic failure. Crit Care Med. 2000;28(4):996–1000. doi: 10.1097/00003246-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Larsen FS, Strauss G, Moller K, Hansen BA. Regional cerebral blood flow autoregulation in patients with fulminant hepatic failure. Liver Transplant. 2000;6(6):795–800. doi: 10.1053/jlts.2000.18705. [DOI] [PubMed] [Google Scholar]
- 35.Larsen FS, Ejlersen E, Strauss G. Cerebrovascular metabolic autoregulation is impaired during liver transplantation. Transplantation. 1999;68(10):1472–1476. doi: 10.1097/00007890-199911270-00007. [DOI] [PubMed] [Google Scholar]
- 36.Philips BJ, Armstrong IR, Pollock A, Lee A. Cerebral blood flow and metabolism in patients with chronic liver disease undergoing orthotopic liver transplantation. Hepatology. 1998;27(2):369–376. doi: 10.1002/hep.510270209. [DOI] [PubMed] [Google Scholar]
- 37.Larsen FS, Ejlersen E, Clemmesen JO, Kirkegaard P, Hansen BA. Preservation of cerebral oxidative metabolism in fulminant hepatic failure: an autoregulation study. Liver Transpl Surg. 1996;2(5):348–353. doi: 10.1002/lt.500020504. [DOI] [PubMed] [Google Scholar]
- 38.Larsen FS, Olsen KS, Ejlersen E, Hansen BA, Paulson OB, Knudsen GM. Cerebral blood flow autoregulation and transcranial Doppler sonography in patients with cirrhosis. Hepatology. 1995;22(3):730–736. [PubMed] [Google Scholar]
- 39.Lockwood AH, Yap EW, Wong WH. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab. 1991;11(2):337–341. doi: 10.1038/jcbfm.1991.67. [DOI] [PubMed] [Google Scholar]
- 40.Testa R, Rodriguez G, Arvigo F. Cerebral blood flow and plasma free tryptophan in cirrhotics with and without hepatic encephalopathy. Ital J Neurol Sci. 1989;10(4):415–421. doi: 10.1007/BF02334946. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez G, Testa R, Celle G. Reduction of cerebral blood flow in subclinical hepatic encephalopathy and its correlation with plasma-free tryptophan. J Cereb Blood Flow Metab. 1987;7(6):768–772. doi: 10.1038/jcbfm.1987.132. [DOI] [PubMed] [Google Scholar]
- 42.James IM, Garassini M. Effect of lactulose on cerebral metabolism in patients with chronic portosystemic encephalopathy. Gut. 1971;12(9):702–704. doi: 10.1136/gut.12.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posner JB, Plum F. The toxic effects of carbon dioxide and acetazolamide in hepatic encephalopathy. J Clin Invest. 1960;39:1246–1258. doi: 10.1172/JCI104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alman RW, Ehrmantraut WR, Fazekas JF, Ticktin HE. Cerebral metabolism in hepatic insufficiency. Am J Med. 1956;21(6):843–849. doi: 10.1016/0002-9343(56)90098-5. [DOI] [PubMed] [Google Scholar]