Fungal endophthalmitis remains a significant cause of vision impairment and blindness. Moreover, the prognosis is poor, in part due to delay in diagnosis and to limited availability of effective antifungal agents with good ocular penetration.
KEYWORDS: Aspergillus, endophthalmitis, isavuconazole, retina, inflammation, ERG, ocular immunology
ABSTRACT
Fungal endophthalmitis remains a significant cause of vision impairment and blindness. Moreover, the prognosis is poor, in part due to delay in diagnosis and to limited availability of effective antifungal agents with good ocular penetration. Thus, it is imperative to evaluate the therapeutic efficacy in fungal endophthalmitis of newer antifungal agents. In this study, we assessed the efficacy of isavuconazole in treating Aspergillus fumigatus endophthalmitis in an exogenous mouse model of the disease. Briefly, endophthalmitis was induced by intravitreal (IVT) injection of A. fumigatus spores into immunocompetent C57BL/6 (B6) mouse eyes. Mice were randomized into five groups that received isavuconazole via (i) oral gavage, (ii) IVT injections, (iii) intravenous injection, (iv) IVT injection followed by oral gavage, and (v) IVT injection followed by intravenous injection. Our data showed that isavuconazole treatment via all routes reduced fungal burden in A. fumigatus-infected eyes. This coincided with the preservation of retinal structural integrity (histology analysis) and retinal function (electroretinography [ERG] analysis), resulting in significantly improved disease outcome. Furthermore, isavuconazole treatment reduced the levels of inflammatory cytokines (tumor necrosis factor α [TNF-α], interleukin 1β [IL-1β], and IL-6) and cellular infiltration in the eyes. Notably, oral administration of isavuconazole was as effective in ameliorating endophthalmitis as intravitreal injection of the drug. Collectively, our study demonstrates that isavuconazole is effective in treating A. fumigatus endophthalmitis in mice, indicating its potential use in human ocular infections.
INTRODUCTION
Infectious endophthalmitis remains a vision-threatening complication of ocular surgery and trauma (1–3). Exogenous endophthalmitis occurs due to postoperative or posttraumatic complications or because of problems with post-intravitreal injection, whereas endogenous endophthalmitis occurs due to the hematogenous spread of pathogens into the eye, primarily among immunocompromised individuals (4). The overall incidence of endophthalmitis ranges from 0.03% to 1.3% following cataract surgery and can be as high as 30 to 40% following open globe injuries. Among microbial pathogens, mycotic or fungal endophthalmitis accounts for 8.6% to 18.6% of culture-positive cases (2) and has steadily increased over the last 20 years, in part due to the growing number of immunosuppressed patients (5–7). Aspergillus species are the most common fungal organisms reported, representing 56 to 74% of all cases of fungal endophthalmitis after cataract surgery (2, 8) and 4 to 14% of posttraumatic fungal endophthalmitis cases, with Aspergillus fumigatus being the most common fungal pathogen (9).
The prognosis of fungal endophthalmitis depends on the magnitude of intraocular involvement, the virulence of the organism, and the timing and mode of interventions (10). Visual outcome is poor because of early macular involvement by Aspergillus, along with the vast retinal necrosis and choroidal damage that can occur and be seen on histopathology of these eyes (11). Treatment of endophthalmitis is more effective during the early course of the disease, when symptoms are mild. Therefore, early diagnosis of fungal endophthalmitis is important in order to prevent irreversible damage in the retina leading to vision loss (4).
Amphotericin B and the azole antifungals are the most commonly used antifungal drugs for treatment of Aspergillus endophthalmitis (8), with voriconazole being the most preferred because it can be delivered by oral, intravenous (i.v.), and intravitreal (IVT) routes (4) and reaches higher concentrations in the vitreous humor (12). However, the emergence of azole resistance among Aspergillus species (13–15) and voriconazole's interaction with other drugs, requiring close therapeutic monitoring (16), warrant evaluation of newer antifungal agents with fewer complications and better therapeutic efficacy for the management of fungal endophthalmitis.
Isavuconazole, an active moiety of the prodrug isavuconazonium sulfate (Cresemba; Astellas Pharma US, Inc., Northbrook, IL), is a newer azole agent with broad-spectrum antifungal activity (17). Both in vitro and in vivo studies have shown that isavuconazole is active against Candida spp. (both fluconazole-sensitive and fluconazole-resistant strains), Aspergillus spp., Cryptococcus spp., and other molds, including Scedosporium species and members of the order Mucorales (17). Isavuconazole has been approved by the United States Food and Drug Administration (FDA) for the treatment of invasive aspergillosis and mucormycosis (18). Being a water-soluble triazole, the prodrug is rapidly converted to the active component, isavuconazole, with an almost complete (99%) conversion rate (19). Hence, it is available in both intravenous and oral formulations (20).
Despite being safe and effective against aspergillosis, to the best of our knowledge, isavuconazole has not been used for the treatment of ocular infections either in human or animal models. Given the heterogeneity of fungal pathogens in causing ocular infection (4) and the extended-spectrum activity of isavuconazole against yeasts, molds, and dimorphic fungi, in this study, we assessed isavuconazole's therapeutic efficacy in a mouse model of Aspergillus endophthalmitis.
RESULTS
Isavuconazole treatment improves the outcome of disease in a mouse model of A. fumigatus endophthalmitis.
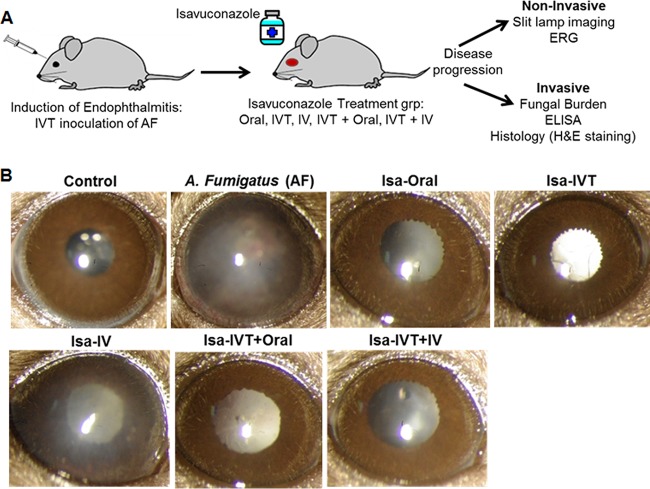
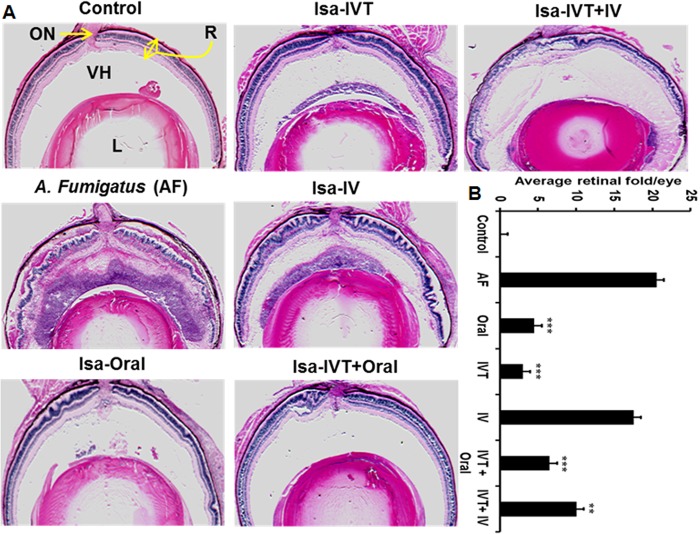
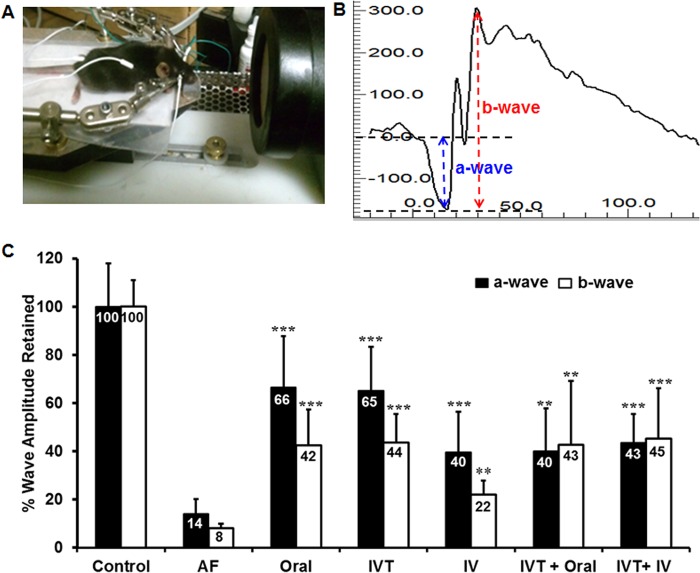
Using a mouse model of A. fumigatus endophthalmitis, we assessed the therapeutic efficacy of isavuconazole by investigating the effects of various routes of administration (oral, i.v., or IVT, alone or in combination) on disease outcomes (Fig. 1A). Following A. fumigatus spore injection and isavuconazole treatment, the gross ocular health of treated and untreated mouse eyes was examined using a slit lamp. A. fumigatus-infected eyes (n = 8) showed dense corneal opacity and a severe inflammatory response (i.e., cellular infiltration), whereas eyes treated with isavuconazole (n = 8/group) displayed clear/transparent corneas and/or anterior chambers (Fig. 1B). Moreover, histological analysis (Fig. 2A) revealed that isavuconazole treatment significantly reduced cellular infiltration into the posterior segment of the eye and diminished retinal folding/detachment (Fig. 2B). To assess whether isavuconazole treatment of A. fumigatus-infected mice improved visual function, we performed scotopic electroretinography (ERG) (Fig. 3A and B), a common test to assess retinal function in both humans and rodents (20, 21). Relative to those of the untreated group, eyes from A. fumigatus -infected mice retained only 14% and 8% of the a wave (the response generated from photoreceptors) and b wave (the response generated from the inner retina, mostly the bipolar cells) amplitudes, respectively, indicating a significant loss in retinal function. In contrast, all eyes from A. fumigatus-infected mice that received isavuconazole had significantly greater a wave and b wave amplitudes compared to those of untreated A. fumigatus-infected mice (range of a wave amplitudes, 40 to 66%; range of b wave amplitudes, 22 to 45%; Fig. 3C), indicating that isavuconazole-treated mice retained partial retinal function. Notably, the greatest retention of retinal function occurred in the groups where isavuconazole was administered via either oral or intravitreal routes.
FIG 1.
Isavuconazole is effective in treating A. fumigatus (AF) endophthalmitis. (A) Schematic showing the experimental model, isavuconazole treatment groups (n = 8/group), and methods to monitor the disease progression in fungal endophthalmitis. (B) Representative micrographs obtained by slit-lamp microscopy on day 4 postinfection/treatment. The white intraocular opacity in some eyes is due to cataract formation from anesthesia. IVT, intravitreal; IV, intravenous; Isa, isavuconazole.
FIG 2.
Isavuconazole treatment ameliorated mouse retinal tissue damage in A. fumigatus endophthalmitis. (A) Representative hematoxylin- and eosin-stained sections (n = 8/group) of eyes from C57BL/6 mice of both sexes that were infected with Aspergillus fumigatus (AF) and treated with isavuconazole (Isa) by the indicated routes (detailed in methods). Eyes were obtained on day 4 postinfection/treatment. (B) The degree and number of retinal folds were assessed in hematoxylin- and eosin-stained images described in panel A, with more folds being indicative of greater damage. **, P < 0.005; ***, P < 0.001 by Student's t test. R, retina; ON, optic nerve; VH, vitreous humor; L, lens, IV, intravenous.
FIG 3.
Isavuconazole treatment preserved retinal function in A. fumigatus (AF) endophthalmitis. Electroretinography (ERG) responses were recorded from uninfected control, A. fumigatus-infected untreated, and A. fumigatus-infected, isavuconazole-treated eyes (n = 8/group). (A) Experimental setup to record mouse ERG and (B) a representative ERG wave showing amplitudes of a waves (blue) and b waves (red) in a normal C57BL/6 mouse. (C) For comparative analysis amplitudes of both a- and b-wave from the uninfected control mice were set at 100%. The untreated and isavuconazole-treated groups were compared to the uninfected control group and results were graphed as a percentage of the normal retinal function retained ± SD. **, P < 0.005; ***, P < 0.001 by one-way analysis of variance (ANOVA). IV, intravenous.
Isavuconazole treatment reduces fungal burden and intraocular inflammation.
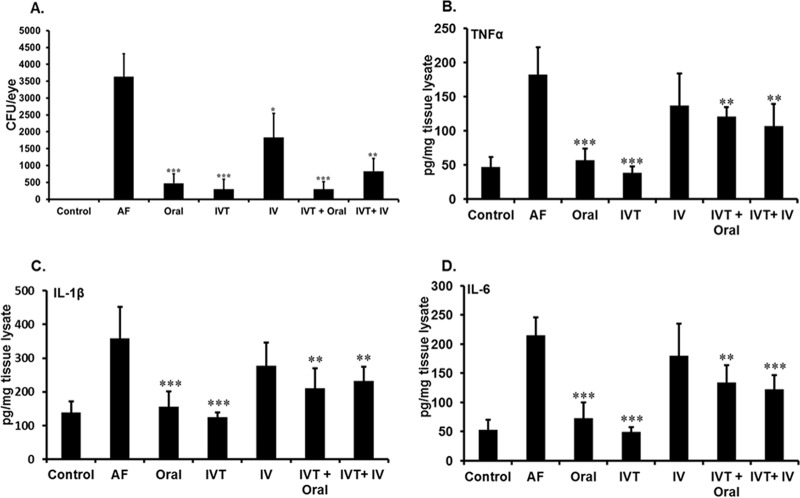
To determine the potential mechanisms by which isavuconazole improves disease outcomes, we assessed its impact on two hallmark characteristics of A. fumigatus endophthalmitis, fungal burden and inflammatory cytokines, in treated and untreated A. fumigatus-infected mice. All routes of treatment with isavuconazole reduced fungal burden in A. fumigatus-infected mice relative to that of those that were untreated; however, i.v. administration was the least effective route (Fig. 4A). We also assessed the effect of isavuconazole treatment on ocular inflammation using an enzyme-linked immunosorbent assay (ELISA) consisting of inflammatory mediators. While elevated levels of inflammatory cytokines (e.g., tumor necrosis factor α [TNF-α], interleukin 1β [IL-1β], and IL-6) were observed in the eyes of untreated A. fumigatus-infected mice, cytokine levels were greatly reduced in A. fumigatus-infected mice that received isavuconazole treatment (Fig. 4B to D). Compared with untreated A. fumigatus-infected mice, oral or IVT administration alone were most effective at significantly reducing levels of inflammatory cytokines, whereas the combination of both IVT + oral and IVT + i.v. significantly reduced cytokine levels, with somewhat modest effects.
FIG 4.
Isavuconazole treatment reduces fungal burden and inflammatory cytokines in A. fumigatus-infected eyes. The eyes (n = 8/group) of C57BL/6 mice were uninfected (control), infected with A. fumigatus and untreated (AF), or treated with isavuconazole (Isa) by the indicated routes (details in Materials and Methods). (A) Estimation of fungal burden as determined by the number of fungal CFU per eye. (B, C, and D) Indicated inflammatory cytokine levels as assessed by enzyme-linked immunosorbent assay (ELISA). The untreated and isavuconazole-treated groups were compared. For bacterial burden, the data represent mean ± SEM; for cytokines, data represent mean ± SD. *, P < 0.05; **, P < 0.005; ***, P < 0.001 by Student's t test. IV, intravenous.
DISCUSSION
While the occurrence of fungal endophthalmitis is rare, timely diagnosis and treatment is required to prevent vision impairment, including blindness (22). Treatment of fungal endophthalmitis is a challenge, as patients are often asymptomatic and the disease onset is delayed (4). An aggressive treatment regimen includes both systemic and intravitreal administration of antifungal drugs and typically results in good visual outcome. However, even though there are several antifungal agents available for treatment, the exact treatment outcomes are unclear, as data on fungal endophthalmitis are scarce and the overall ocular clinical experience is limited (2). Moreover, the adverse effects of current antifungal drugs (16), specifically their ocular toxicity (23), and the emergence of drug resistance (24, 25) make the treatment of these blinding infections challenging. Thus, novel antifungal agents are urgently needed for ocular use.
Using a mouse model of exogenous fungal endophthalmitis, our study, for the first time, supports the use of isavuconazole as a new therapeutic option for the treatment of Aspergillus endophthalmitis. Interestingly, our data show that oral administration of isavuconazole was as effective as intravitreal injection in reducing overall disease severity and preserving retinal/visual function. This is relevant, as oral administration of isavuconazole is a common method used for the treatment of invasive aspergillosis (26). Similarly, sufficient intravitreal concentrations of voriconazole have been shown in humans when administered orally (27). The oral dose of isavuconazole used in our study was similar to that used in the study of disseminated A. fumigatus infection, which found that isavuconazole treatment increased survival and decreased kidney fungal burden in mice (28). The reduction in fungal burden that we observed corroborates prior studies in a mucormycosis mouse model, which demonstrated the therapeutic efficacy of isavuconazole to decrease fungal load in the lung and brain (29). Based on our findings, it is reasonable to assume that, following oral administration in mice, isavuconazole crosses the blood-retinal barrier to exert antifungal properties. While a prior study demonstrated widespread tissue distribution of isavuconazole following oral administration (30), the pharmacokinetics and pharmacodynamics of the drug in the eye need to be studied further, specifically within the context of fungal endophthalmitis.
Vision loss in infectious endophthalmitis is due to both pathogen virulence factors and host-evoked inflammatory damage to the retina leading to the disruption of the normal visual axis (4, 20). Our histological analysis revealed that infection with A. fumigatus resulted in massive cellular infiltration in the vitreous cavity, along with retinal folding/detachment and significant damage to retinal architecture. These deleterious changes were drastically reduced upon treatment with isavuconazole. Moreover, isavuconazole treatment attenuated the A. fumigatus-induced inflammation as revealed by decreased levels of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in ocular tissue.
We observed that isavuconazole treatment resulted in lower fungal burden, diminished retinal tissue damage, and attenuated intraocular inflammation. However, to assess whether these findings are translated into better visual outcomes, we utilized ERG analysis. To this end, our data show that A. fumigatus infection reduced the amplitude of a waves (the response generated from photoreceptors) and b waves (the response generated from the inner retina, mostly the bipolar cells), suggesting the functional impairment of retinal neurons. Remarkably, isavuconazole-treated eyes showed better retention of ERG response, indicating the preservation of retinal function. Previous studies have shown that voriconazole causes visual disturbances by attenuating the b wave of the electroretinogram (23, 31). Therefore, our study suggests that isavuconazole may be a safer alternative to voriconazole in treating Aspergillus endophthalmitis. Indeed, several clinical studies support the notion that isavuconazole is well tolerated, with isavuconazole-treated patients having significantly reduced rates of common side effects, such as vision changes, photosensitivity, and skin disorders (18, 32, 33). Additional benefits of isavuconazole may include cost effectiveness in comparison with voriconazole (34) and amphotericin B (35) in managing endophthalmitis caused by molds, such as A. fumigatus, where the recommended duration of treatment is 6 to 12 weeks, accompanied by close ophthalmologic examination (8).
Infectious endophthalmitis can be caused by a variety of microbes (36). Among fungal pathogens, Candida spp. remain the leading cause of endogenous endophthalmitis among immunocompromised individuals, whereas Aspergillus spp. are the major causative agents of exogenous endophthalmitis following traumatic injury to the eye, especially in tropical regions of the world (4). However, the pathogenesis of Aspergillus endophthalmitis is not well studied, in part, due to unavailability of good animal models. To test the efficacy of therapeutic efficacy of isavuconazole, we developed the first immunocompetent murine model of A. fumigatus endophthalmitis (unpublished data), which mimics exogenous endophthalmitis. This model can be utilized to study the pathobiology of exogenous endophthalmitis due to drug-sensitive and resistant fungi and to potentially evaluate the therapeutic efficacy of existing and newer antifungal agents in the eye.
Although all routes of isavuconazole administration improved disease outcome, oral or IVT administration resulted in a better treatment response compared to i.v. administration. We believe that one possible explanation for this discrepancy is that the drug dosage was not high enough when given i.v., as the amount of drug delivered by i.v. route was limited due to severe toxicity and animal death when high concentrations were administered by this route.
In summary, we observed a significant improvement in Aspergillus endophthalmitis in mice treated with isavuconazole, with considerable reduction in both fungal burden and intraocular inflammation. Considering the limited availability of antifungal drugs for ocular use and the emergence of antifungal resistance, our proof-of-concept study supports the evaluation of this drug for the management of Aspergillus and of possibly other etiologies of fungal endophthalmitis. However, additional studies are needed for estimation of the intraocular concentration of isavuconazole administered via different routes. Nonetheless, present study demonstrates the utility of different markers (histologic, cytokine production, and retinal function) as outcome measures for the treatment of Aspergillus endophthalmitis.
MATERIALS AND METHODS
Fungal culture and spore preparation.
A clinical isolate of A. fumigatus was obtained from the Division of Infectious Diseases, Department of Internal Medicine, at Wayne State University School of Medicine. For spore preparation, A. fumigatus was grown on Sabouraud's dextrose agar (SDA) plates for 6 days at room temperature. Following sporulation, the fungal spores were harvested, and the fungal count was adjusted to 15,000 CFU in one μl of sterile phosphate-buffered saline (PBS).
Induction of Aspergillus endophthalmitis.
Immunocompetent C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) at 6 to 8 weeks of age, both male and female littermates, were used in all groups (n = 8/group). Endophthalmitis was induced by intravitreal injection of A. fumigatus spores (15,000 CFU/eye), similarly to previous methods for bacterial endophthalmitis (20, 21). Eyes from animals that were not infected or that were injected with sterile PBS served as controls. Disease progression was monitored by both noninvasive and invasive methods, as described previously (20) (Fig. 1A). All procedures were conducted in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University.
Isavuconazole drug treatment.
Mice were injected intravitreally with A. fumigatus spores and were randomly divided into five treatment groups to test different routes and combinations of isavuconazole (256 mg/kg, equivalent to 122.9 mg/kg active dose until indicated otherwise) administration, as described below. Doses of isavuconazole were chosen based on its efficacy in other animal models of A. fumigatus infections (28, 37). For all groups, treatment began 6 h following induction of A. fumigatus endophthalmitis. Isavuconazole was administration intravenously through the tail vein or orally via a sterilized oral gavage-feeding needle. Uninfected and PBS-injected mice were used as mock controls. All experiments were repeated at least twice.
Group I: i.v. administration.
Mice received daily i.v. injections of isavuconazole (5 mg/kg), for a total of four injections.
Group II: oral administration.
Mice received oral gavage (122.9 mg/kg) once daily, for a total of four doses.
Group III: IVT administration.
Mice received one IVT injection of isavuconazole (10 μg/eye) 6 h post-A. fumigatus infection and a second IVT injection of the same dose 24 h postinfection.
Group IV: IVT + oral.
Mice received a single IVT injection of isavuconazole (10 μg/eye), followed by daily oral gavage (122.9 mg/kg) for three subsequent days.
Group V: IVT + i.v.
Mice received a single IVT injection of isavuconazole (10 μg/eye), followed by a daily i.v. injection of isavuconazole (5 mg/kg), for a total of three doses.
Fungal burden estimation.
The fungal burden in control versus treatment groups was estimated by serial dilution and a plate count method, using SDA plates as described earlier (38). Briefly, 4 days postinfection and drug treatment, the eyes were enucleated and homogenized in sterile PBS by using a manual mortar and pestle. The homogenate was serially diluted in sterile PBS and plated on SDA plates. The results were expressed as the mean number of CFU/eye ± the standard error of the mean (SEM).
Enzyme-linked immunosorbent assay.
Following endophthalmitis induction and drug treatment, the levels of inflammatory cytokines were measured by ELISA (21, 38, 39). Briefly, the eyes were enucleated, and lysates were prepared as described above. Total protein was estimated using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Rockford, IL), and 15 to 20 μg total protein was used for cytokine measurements. The ELISA was performed using ELISA kits for TNF-α, IL-1β, and IL-6 (BD Biosciences, San Jose, CA) per the manufacturer's instructions. The data are presented as the mean pg/mg of the tissue lysates ± standard deviation (SD).
Histology analysis.
Eyes from the euthanized mice were enucleated 4 days postinfection for histopathological examination and fixed in 4% formalin. The embedding, sectioning, and hematoxylin and eosin stain (H&E) staining were performed by Excalibur Pathology, Inc. (Oklahoma City, OK). Histological sections were scanned using a PathScan Enabler IV scanner (Mayer Instruments, Houston, TX), and the retinal folds were counted manually by three masked observers.
Electroretinography.
Scotopic electroretinography (ERG) was used to determine retinal function in Aspergillus-infected and isavuconazole-treated groups, as described previously (21). The mice (control and infected) were anesthetized 4 days after injection. The temperature of the mice was maintained at 37°C using a heat pad. The pupils were dilated using 2.5% phenylephrine and 1% tropicamide ophthalmic solutions. Electroretinograms were recorded following bilateral mydriasis and 12 h of dark adaptation. Indifferent, silver-embedded thread eye electrodes (OcuScience LLC, Kansas City, MO) were used to record the electroretinogram. Reference needle electrodes (stainless steel subdermal electrodes) were placed in the anterior scalp and a ground needle electrode was placed in the tail (Fig. 3A). ERG responses were acquired using an ERG system (OcuScience LLC, Kansas City, MO) and analyzed using ERGVIEW 4.882. Ganzfeld light stimulus was used to present 10 10-ms flashes, with light intensities increasing from 0.0001 to 100 cd-s/m2. The amplitudes of the a waves and b waves were recorded. The ERG a wave amplitude was measured between the ERG baseline and the first negative peak, while the ERG b wave amplitude was measured between the first negative peak and the first positive peak (Fig. 3B).
Statistical analysis.
All data are expressed as the mean ± SD unless indicated otherwise. Statistical differences between experimental groups were determined using an unpaired Student's t test and one-way analysis of variance (ANOVA) with a post hoc test. All statistical analyses were performed using GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA).
ACKNOWLEDGMENTS
This study received funding and drug support from Astellas Pharma Global Development, Inc. (ISR001314). Research in our laboratory is also supported in part by NIH grants R01EY026964 and R01EY02738 (to A.K.) and by an unrestricted grant from Research to Prevent Blindness, Inc., to the Department of Ophthalmology, Wayne State University. The immunology resource core is supported by an NIH center grant (grant P30EY004068 to Linda D. Hazlett, Ph.D.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to the lab members for critical reading and reviewing of the manuscript.
We report no conflicts of interest. P.H.C. is in the speakers bureau for Astellas, Inc.
REFERENCES
- 1.Palioura S, Sivaraman K, Joag M, Sise A, Batlle JF Jr, Miller D, Espana EM, Amescua G, Yoo SH, Galor A, Karp CL. 2018. Candida endophthalmitis after descemet stripping automated endothelial keratoplasty with grafts from both eyes of a donor with possible systemic candidiasis. Cornea 37:515–518. doi: 10.1097/ICO.0000000000001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narang S, Gupta A, Gupta V, Dogra MR, Ram J, Pandav SS, Chakrabarti A. 2001. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol 132:609–617. doi: 10.1016/S0002-9394(01)01180-1. [DOI] [PubMed] [Google Scholar]
- 3.Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer AL, Hunt JJ, Novosad BD. 2007. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res 26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand ML. 2017. Bacterial and fungal endophthalmitis. Clin Microbiol Rev 30:597–613. doi: 10.1128/CMR.00113-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergoulidou M, Krause L, Foerster MH, Thiel E, Schwartz S. 2011. Endogenous filamentous fungal endophthalmitis–single-centre survey in patients with acute leukaemia or postallogeneic stem cell transplantation and review of the literature. Mycoses 54:e704–e711. doi: 10.1111/j.1439-0507.2010.02004.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, McMullan R, Hedderwick S, Mulholland DA, Best RM. 2006. Diagnosis and treatment of endogenous fungal endophthalmitis. Ophthalmologica 220:134–136. doi: 10.1159/000090580. [DOI] [PubMed] [Google Scholar]
- 7.Wong VK, Tasman W, Eagle RC Jr, Rodriguez A. 1997. Bilateral Candida parapsilosis endophthalmitis. Arch Ophthalmol 115:670–672. doi: 10.1001/archopht.1997.01100150672022. [DOI] [PubMed] [Google Scholar]
- 8.Wykoff CC, Flynn HW Jr, Miller D, Scott IU, Alfonso EC. 2008. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology 115:1501.e2–1507.e2. doi: 10.1016/j.ophtha.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Srinivasan R, Kaliaperumal S, Saha I. 2008. Post-traumatic fungal endophthalmitis—a prospective study. Eye (Lond) 22:13–17. doi: 10.1038/sj.eye.6702463. [DOI] [PubMed] [Google Scholar]
- 10.Essman TF, Flynn HW Jr, Smiddy WE, Brod RD, Murray TG, Davis JL, Rubsamen PE. 1997. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers 28:185–194. [PubMed] [Google Scholar]
- 11.Rao NA, Hidayat AA. 2001. Endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared with aspergillosis. Am J Ophthalmol 132:244–251. doi: 10.1016/S0002-9394(01)00968-0. [DOI] [PubMed] [Google Scholar]
- 12.Vilela RC, Vilela L, Vilela P, Vilela R, Motta R, Possa AP, de Almeida C, Mendoza L. 2014. Etiological agents of fungal endophthalmitis: diagnosis and management. Int Ophthalmol 34:707–721. doi: 10.1007/s10792-013-9854-z. [DOI] [PubMed] [Google Scholar]
- 13.Denning DW, Bowyer P. 2013. Voriconazole resistance in Aspergillus fumigatus: should we be concerned? Clin Infect Dis 57:521–523. doi: 10.1093/cid/cit321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma C, Kumar R, Kumar N, Masih A, Gupta D, Chowdhary A. 2018. Investigation of multiple resistance mechanisms in voriconazole-resistant Aspergillus flavus clinical isolates from a chest hospital surveillance in Delhi, India. Antimicrob Agents Chemother 62:e01928-. doi: 10.1128/AAC.01928-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJG, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine MT, Chandrasekar PH. 2016. Adverse effects of voriconazole: over a decade of use. Clin Transplant 30:1377–1386. doi: 10.1111/ctr.12834. [DOI] [PubMed] [Google Scholar]
- 17.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 18.Natesan SK, Chandrasekar PH. 2016. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist 9:291–300. doi: 10.2147/IDR.S102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-Ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Singh CN, Glybina IV, Mahmoud TH, Yu FS. 2010. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J Infect Dis 201:255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh PK, Donovan DM, Kumar A. 2014. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother 58:4621–4629. doi: 10.1128/AAC.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chee YE, Eliott D. 2017. The role of vitrectomy in the management of fungal endophthalmitis. Semin Ophthalmol 32:29–35. doi: 10.1080/08820538.2016.1228396. [DOI] [PubMed] [Google Scholar]
- 23.Zrenner E, Tomaszewski K, Hamlin J, Layton G, Wood N. 2014. Effects of multiple doses of voriconazole on the vision of healthy volunteers: a double-blind, placebo-controlled study. Ophthalmic Res 52:43–52. doi: 10.1159/000359952. [DOI] [PubMed] [Google Scholar]
- 24.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. 2018. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Cheng Y, Song X, Wang C, Su G, Liu Z. 2015. A comparative treatment study of intravitreal voriconazole and liposomal amphotericin B in an Aspergillus fumigatus endophthalmitis model. Invest Ophthalmol Vis Sci 56:7369–7376. doi: 10.1167/iovs.15-17266. [DOI] [PubMed] [Google Scholar]
- 27.Hariprasad SM, Mieler WF, Holz ER, Gao H, Kim JE, Chi J, Prince RA. 2004. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol 122:42–47. doi: 10.1001/archopht.122.1.42. [DOI] [PubMed] [Google Scholar]
- 28.Seyedmousavi S, Bruggemann RJ, Meis JF, Melchers WJ, Verweij PE, Mouton JW. 2015. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob Agents Chemother 59:2855–2866. doi: 10.1128/AAC.04907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo G, Gebremariam T, Lee H, Edwards JE Jr, Kovanda L, Ibrahim AS. 2014. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 58:2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt-Hoffmann A-H, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, Spickermann J, Schneidkraut MJ. 2017. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 61:e01292-. doi: 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong W-H, Brown RL, Reed B, Burke NS, Duvoisin RM, Morgans CW. 2015. Voriconazole, an antifungal triazol that causes visual side effects, is an inhibitor of TRPM1 and TRPM3 channels. Invest Ophthalmol Vis Sci 56:1367–1373. doi: 10.1167/iovs.14-15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledoux M-P, Toussaint E, Denis J, Herbrecht R. 2017. New pharmacological opportunities for the treatment of invasive mould diseases. J Antimicrob Chemother 72:i48–i58. doi: 10.1093/jac/dkx033. [DOI] [PubMed] [Google Scholar]
- 33.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 34.Harrington R, Lee E, Yang H, Wei J, Messali A, Azie N, Wu EQ, Spalding J. 2017. Cost-effectiveness analysis of isavuconazole vs. voriconazole as first-line treatment for invasive aspergillosis. Adv Ther 34:207–220. doi: 10.1007/s12325-016-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagshaw E, Enoch DA, Blackney M, Posthumus J, Kuessner D. Economic impact of treating invasive mold disease with isavuconazole compared with liposomal amphotericin B in the UK. Future Microbiol. doi: 10.2217/fmb-2018-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durand ML. 2013. Endophthalmitis. Clin Microbiol Infect 19:227–234. doi: 10.1111/1469-0691.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepak AJ, Marchillo K, VanHecker J, Andes DR. 2013. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57:6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talreja D, Singh PK, Kumar A. 2015. In vivo role of TLR2 and MyD88 signaling in eliciting innate immune responses in staphylococcal endophthalmitis. Invest Ophthalmol Vis Sci 56:1719–1732. doi: 10.1167/iovs.14-16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh PK, Kumar A. 2015. Retinal photoreceptor expresses toll-like receptors (TLRs) and elicits innate responses following TLR ligand and bacterial challenge. PLoS One 10:e0119541. doi: 10.1371/journal.pone.0119541. [DOI] [PMC free article] [PubMed] [Google Scholar]