Summary
Meiotic synapsis and recombination ensure correct homologous segregation and genetic diversity. Asynapsed homologs are transcriptionally inactivated by meiotic silencing, which serves a surveillance function and in males drives meiotic sex chromosome inactivation. Silencing depends on the DNA damage response (DDR) network, but how DDR proteins engage repressive chromatin marks is unknown. We identify the histone H3-lysine-9 methyltransferase SETDB1 as the bridge linking the DDR to silencing in male mice. At the onset of silencing, X chromosome H3K9 trimethylation (H3K9me3) enrichment is downstream of DDR factors. Without Setdb1, the X chromosome accrues DDR proteins but not H3K9me3. Consequently, sex chromosome remodeling and silencing fail, causing germ cell apoptosis. Our data implicate TRIM28 in linking the DDR to SETDB1 and uncover additional factors with putative meiotic XY-silencing functions. Furthermore, we show that SETDB1 imposes timely expression of meiotic and post-meiotic genes. Setdb1 thus unites the DDR network, asynapsis, and meiotic chromosome silencing.
Keywords: meiotic silencing, MSCI, sex chromosomes, DNA damage response, mouse, H3K9me3
Graphical Abstract
Highlights
-
•
The histone methyltransferase SETDB1 is essential for male mouse meiosis
-
•
The meiotic DDR network recruits SETDB1 to the XY pair, where it induces H3K9me3
-
•
SETDB1 deletion perturbs meiotic sex chromosome remodeling and silencing
-
•
SETDB1 ensures timely expression of meiotic and post-meiotic genes
During male meiosis in mammals, the asynapsed regions of the X and Y chromosomes retain DNA double-strand breaks (DSBs), which triggers silencing of the sex chromosomes, a process essential for fertility. Hirota et al. show that meiotic DSB factors recruit the H3K9-methyltransferase SETDB1, inducing XY-chromatin remodeling and silencing.
Introduction
Defective synapsis or recombination can cause mutation and aneuploidy in offspring. To prevent these outcomes, surveillance mechanisms operate during prophase I to eliminate germ cells in which either process is defective. Current data support the existence in mice of two such surveillance mechanisms. The first is triggered by persistent DNA damage and transduces germ cell elimination via the CHK2/p53/p63 checkpoint pathway (Bolcun-Filas et al., 2014, Di Giacomo et al., 2005, Marcet-Ortega et al., 2017, Pacheco et al., 2015, Rinaldi et al., 2017). The second operates in the absence of persistent DNA damage and responds instead to asynapsis (Di Giacomo et al., 2005, Wojtasz et al., 2012). Homologs asynapsed at pachynema undergo meiotic silencing, a megabase-scale chromatin remodeling process that inactivates hundreds of genes (Inagaki et al., 2010). Evidence suggests that meiotic silencing eliminates germ cells with asynapsis by depriving them of critical gene products (Cloutier et al., 2015).
In addition to its surveillance role, meiotic silencing is responsible for the inactivation of the asynapsed XY chromosome regions during male meiosis. This process, meiotic sex chromosome inactivation (MSCI), affects most or all XY genes and results in the formation of the condensed XY body (Yan and McCarrey, 2009, McKee and Handel, 1993, Solari, 1964). Disturbances in MSCI lead to misexpression of toxic sex genes and midpachytene germ cell failure (Royo et al., 2010). MSCI defects have been invoked as a cause of meiotic infertility in intersubspecific hybrids (Bhattacharyya et al., 2013, Campbell et al., 2013) and mice exhibiting chromosome abnormalities, including X-autosome translocations (Homolka et al., 2007) and Double Y syndrome (Royo et al., 2010).
Mechanistically, meiotic silencing initiates from recombinational DNA double-strand breaks (DSBs) that are located within asynapsed chromosome axes (ElInati et al., 2017, Carofiglio et al., 2013, Schoenmakers et al., 2008). Supported by SYCP3 and HORMAD1/2, BRCA1-A complex components and ATR localize to these DSBs and thereafter spread along the full length of asynapsed chromosome axes (Lu et al., 2013, Royo et al., 2013, Wojtasz et al., 2012, Daniel et al., 2011, Kouznetsova et al., 2009, Sciurano et al., 2007, Turner et al., 2004, Xu et al., 2003). Subsequently, facilitated by MDC1 (Ichijima et al., 2011) and TOPBP1 (ElInati et al., 2017), ATR spreads into chromatin loops, catalyzing serine-139 phosphorylation of histone H2AX (Cloutier et al., 2015, Fernandez-Capetillo et al., 2003) (γH2AX; Figure 1A).
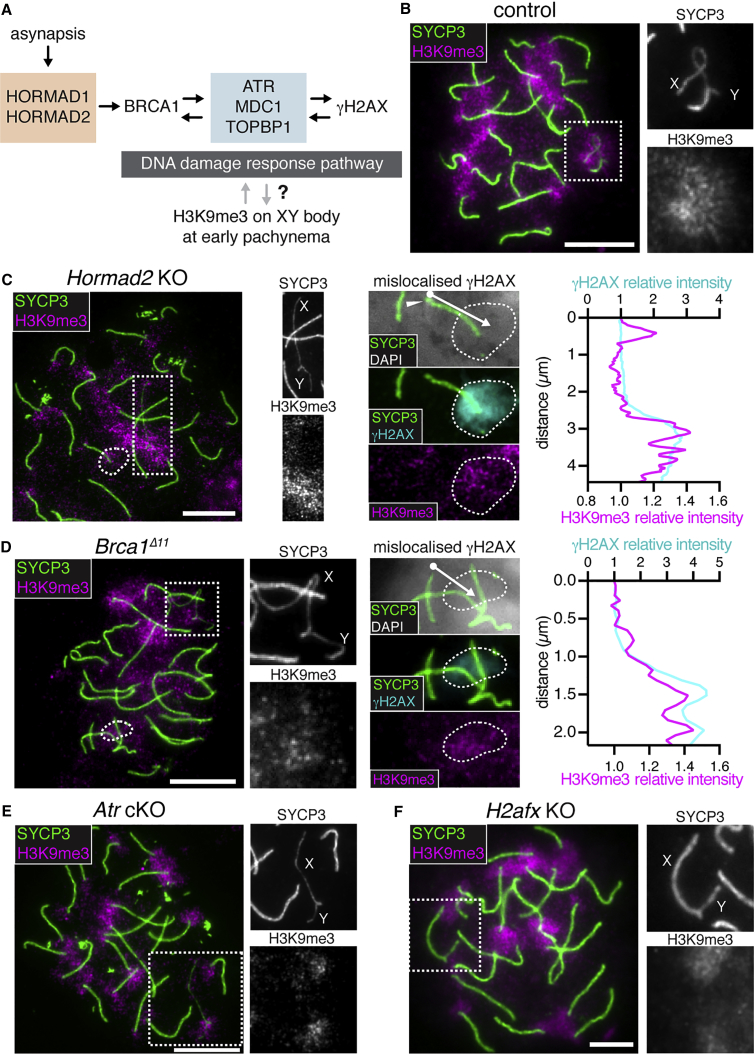
Figure 1.
DNA Damage Response Factors Direct H3K9me3 Acquisition on the X Chromosome
(A) Schematic of the MSCI pathway.
(B–F) Early pachytene spermatocytes from control (B, n = 38 cells), Hormad2 KO (C, n = 32 cells), Brca1Δ11 (D, n = 17 cells), Atr cKO (E, n = 20 cells), and H2afx KO (F, n = 34 cells) immunostained for SYCP3 (green) and H3K9me3 (magenta). Dashed rectangles highlight XY pair, which is magnified in right panels. Far right panels (C and D) show magnified images of mislocalized γH2AX (cyan, dashed circle) and plot profiles of relative fluorescence intensity of γH2AX (cyan) and H3K9me3 (magenta). Arrows show lines used for plot profile analysis. Arrowhead shows DAPI-dense pericentric heterochromatin. 8-week-old mice were used for analyses. Scale bars: 5 μm.
See also Figure S1.
While it is clear that the DNA damage response (DDR) network has a critical role in meiotic silencing, how it ultimately induces the inactive chromatin state is not known. One possibility is that DDR components direct acquisition of canonical repressive histone modifications at asynapsed chromosomes. Based on their localization to the XY body, a number of candidate modifications have been identified, but none has yet been shown to be essential for the initiation of silencing (van der Heijden et al., 2007, Namekawa et al., 2006, Khalil et al., 2004, Baarends et al., 1999). Among these candidates, we were drawn to Histone H3 lysine 9 (H3K9) methylation. H3K9 monomethylation does not exhibit preferential XY localization (Kato et al., 2015), and H3K9 dimethylation appears on the XY pair at the pachytene-to-diplotene transition, too late for a role in MSCI initiation (Namekawa et al., 2006, Khalil et al., 2004). However, H3K9 trimethylation (H3K9me3) is observed on the XY body at early pachynema (Kato et al., 2015, Page et al., 2012, van der Heijden et al., 2007, Khalil et al., 2004) and is downstream of the DDR in mitotic cells (Ayrapetov et al., 2014, Sun et al., 2009).
Here, we show that H3K9me3 enrichment on the asynapsed X chromosome is directed by DDR factors. Using a conditional knockout (cKO) approach, we demonstrate that H3K9me3 acquisition is dependent on the H3K9 methyltransferase (MTase) SETDB1 and that Setdb1 deletion disrupts XY body formation and XY gene silencing. We find that SETDB1 recruitment to the XY pair is dependent on H2AX and identify TRIM28 as a candidate bridging factor. Thus, our results identify SETDB1 as the link between the DDR network and meiotic chromosome silencing.
Results
DDR Factors Direct H3K9me3 Acquisition on the X Chromosome at Pachynema
Using SYCP3 immunostaining to label chromosome axial elements, we first confirmed previous reports (Kato et al., 2015, Page et al., 2012, van der Heijden et al., 2007, Khalil et al., 2004) that H3K9me3 is enriched on the XY bivalent, as well as on pericentric heterochromatin, at early pachynema (Figure 1B). H3K9me3 was not observed along chromosome axes or at persistent meiotic DSB sites (Figure S1A). We then investigated genetic interactions between XY-associated H3K9me3 and meiotic silencing components. We assayed sex chromosome H3K9me3 patterns in Hormad2 knockouts (KOs) (Wojtasz et al., 2012) (Figure 1C), Brca1 exon 11 deletion mutants (Turner et al., 2004, Xu et al., 2003) (Brca1Δ11; Figure 1D), Atr cKOs (Widger et al., 2018) (Figure 1E), and H2afx KOs (Celeste et al., 2002) (Figure 1F), all of which exhibit defective MSCI and resulting germ cell failure at midpachynema. We restricted our analysis at this stage to the X chromosome because the heterochromatic Y chromosome is constitutively positive for H3K9me3, even in spermatogonia (Baumann et al., 2008) (Figure S1B). Although preserved at pericentric heterochromatin, H3K9me3 was not observed on the X chromosome in these mutants. H3K9me3 enrichment to the X chromosome is therefore downstream of DDR factors.
Examination of Hormad2 KOs and Brca1Δ11 mutants revealed further information on the relationship between DDR factors and H3K9me3. As well as defective MSCI, these mutants exhibit another phenotype: ATR normally destined for the X chromosome mislocalizes to synapsed autosomes, where it induces ectopic domains of γH2AX (Wojtasz et al., 2012, Turner et al., 2004). We found that in Hormad2 KOs and Brca1Δ11 mutants, these ectopic γH2AX domains were also enriched for H3K9me3 (Figures 1C, 1D, and S1C). These findings suggest a close spatial relationship between γH2AX and H3K9me3 acquisition.
Meiotic Setdb1 Deletion Causes Midpachytene Apoptosis
The enzyme responsible for meiotic X chromosome-associated H3K9me3 is unknown. H3K9me3-catalyzing MTases SUV39H1 and its paralog SUV39H2 are dispensable for XY body-associated H3K9 methylation (Peters et al., 2001). To identify alternative H3K9me3 MTase candidates, we analyzed published RNA sequencing (RNA-seq) data from mouse spermatogenic subpopulations (Gan et al., 2013). Setdb1 caught our attention because its expression exceeded that of other candidate H3K9me3 MTases during pachynema (Figure 2A). SETDB1 localized to the XY pair at early pachynema (Figure 2B). Furthermore, testis immunoprecipitation (IP) followed by western blotting revealed that SEDTB1 and γH2AX form a complex by either direct or indirect interaction (Figure 2C). In mice, constitutive deletion of Setdb1 causes embryonic lethality (Dodge et al., 2004), and conditional ablation in early germ cells causes spermatogenic arrest before meiosis (An et al., 2014, Liu et al., 2014). We therefore sought to delete Setdb1 later in spermatogenesis, prior to pachynema.
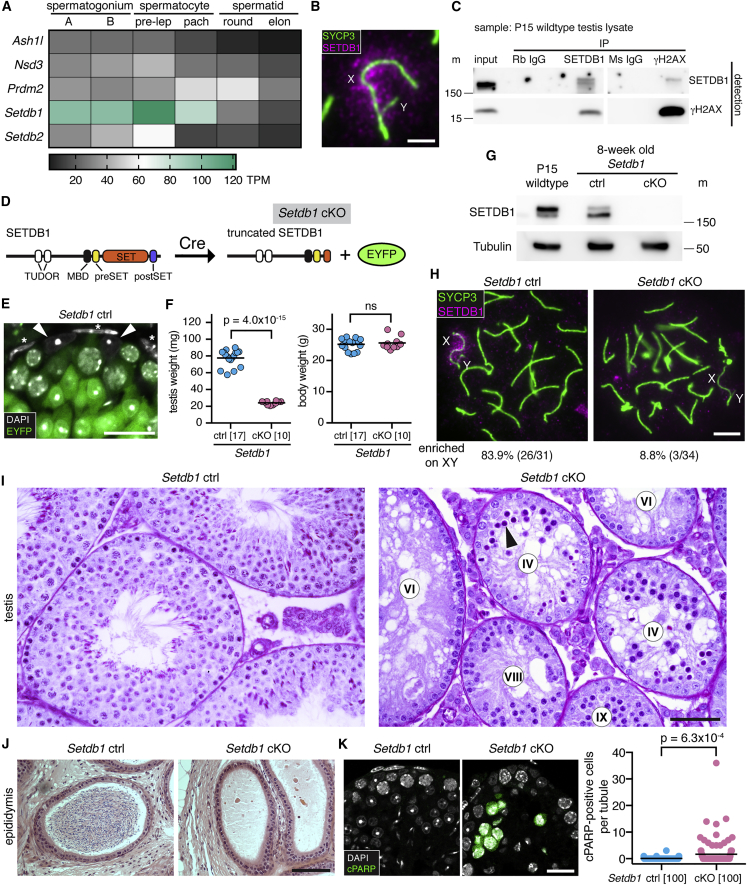
Figure 2.
Setdb1 Deletion Causes Midpachytene Apoptosis
(A) H3K9me3 MTase expression in male germ cells by RNA-seq. A: typeA; B: type B; pre-lep: pre-leptonema; pach: pachynema; elon: elongated; TPM: transcripts per million.
(B) Setdb1 control XY bivalent immunostained for SYCP3 (green) and SETDB1 (magenta). See quantitation in (H). Scale bar: 2 μm.
(C) Western blot of input and immunoprecipitated samples using P15 wild-type testis lysate treated with nuclease. Rb: rabbit; Ms: mouse; m: size marker. Expected size: 180 kDa (SETDB1), 17 kDa (γH2AX).
(D) Schematic of SETDB1 domain structure before and after Cre recombination. Setdb1 cKO also expresses EYFP from Gt(ROSA)26Sor Cre reporter locus.
(E) Testis section of Setdb1 control immunostained for EYFP (green, stained with GFP antibody). Note that EYFP is negative in Sertoli cells (arrowheads) and peritubular myoid cells (asterisks). Scale bar: 20 μm.
(F) Testis and body weights in Setdb1 control and cKO mice. Number of mice analyzed in brackets. ns: not significant. p value calculated using unpaired t test.
(G) Testis SETDB1 western blot of Setdb1 control and cKO. Tubulin was used as a loading control. 50 μg of protein per lane was loaded. m: size marker. Expected size: 180 kDa (SETDB1), 50 kDa (Tubulin). SETDB1 antibody used recognizes two SETDB1 bands, of which the upper band is considered to be ubiquitinated SETDB1 (Ishimoto et al., 2016).
(H) Early pachytene Setdb1 controls and Setdb1 cKOs immunostained for SYCP3 (green) and SETDB1 (magenta). Percentages of cells positive for SETDB1 signals on XY are shown below panels.
(I and J) Histology of Setdb1 control and cKO testes (I, periodic acid-Schiff staining) and epididymides (J, hematoxylin and eosin staining). Arrowhead: apoptotic pachytene cell. Number labels show tubule stages. Scale bars: 50 μm (I), 100 μm (J).
(K) Testis sections of Setdb1 control and cKO immunostained for cleaved PARP (green). Chart shows number of cells positive for cleaved PARP per tubule. Number of tubules analyzed in brackets. p value calculated using unpaired t test. Scale bar: 20 μm.
8-week-old mice were used for analyses except for P15 sample in (C) and (G).
See also Figure S2.
To achieve meiotic Setdb1 deletion, we generated Setdb1 cKO Setdb1flox/− male mice carrying a Ngn3-Cre transgene (Schonhoff et al., 2004). The Setdb1 cKO mutation deletes the core amino acids in the catalytic SET domain (Matsui et al., 2010) (Figure 2D). Ngn3-Cre is expressed in the gastrointestinal tract and pancreas, as well as in male germ cells from post-natal day (P) 7, and has been used to efficiently deplete Topbp1, Atr, and Mov10l1 during meiosis (ElInati et al., 2017, Widger et al., 2018, Zheng and Wang, 2012). We confirmed using a ROSA26-EYFP Cre reporter that Ngn3-Cre is active in germ cells from the spermatogonial stage and is not active in somatic cells of the testis (Figure 2E). This Cre transgene was superior to Stra8-Cre (Sadate-Ngatchou et al., 2008) at achieving efficient meiotic SETDB1 depletion (Figures S2A–S2C). The mean testis weight in 8-week-old Setdb1 cKO males was reduced relative to Setdb1 control (Setdb1flox/+; Ngn3-Cre) males, while the mean body weight was unaffected (Figure 2F). SETDB1 protein levels were reduced in Setdb1 cKO testes (Figure 2G), which was confirmed by immunofluorescence analysis of nuclear spreads (Figure 2H). Although the conditional mutation may have been expected to generate a truncated SETDB1, no such protein was identified by western blotting in Setdb1 cKO testis (Figure S2D). Germ cell progression in Setdb1 cKO males was unaffected up to stage IV, corresponding to midpachynema of meiosis. At this point, there was a complete block in germ cell development (Figure 2I). As a result, later germ cell types were absent, and sperm were not present in the cauda epididymides (Figure 2J). Setdb1 cKO tubule sections contained elevated numbers of cleaved-PARP stained spermatocytes (Figure 2K). Germ cell loss in Setdb1 cKOs therefore occurs via apoptosis.
Minor Effects of Setdb1 Deletion on Meiotic Recombination and Synapsis
Midpachytene germ cell failure can be caused by defects in homologous recombination, synapsis, or MSCI. We first established whether homologous recombination was affected in Setdb1 cKOs. For this purpose, we counted foci of the meiotic DSB markers RPA2 and RAD51 at leptonema and early pachynema. Relative to controls, at leptonema in Setdb1 cKOs, the mean RPA2 count was unchanged, and the mean RAD51 count was marginally decreased (Figures S3A and S3B). At early pachynema in Setdb1 cKOs, the mean RPA2 count was slightly higher while the mean RAD51 count was unaffected (Figures S3C and S3D). We also found that at early pachynema, RPA2 and RAD51 counts on the X chromosome and at the PAR were similar between Setdb1 cKOs and controls (Figures S3E and S3F). Thus, Setdb1 deletion had a minimal effect on the abundance of these recombination markers.
Next, we assayed synapsis in Setdb1 cKOs. We used antibodies to centromeres and to the asynapsis marker HORMAD2, localization of which was unaffected in Setdb1 cKOs (Figure 3A). Asynapsis was observed in 53% of Setdb1 cKO cells but only 3% of control cells at early pachynema (Figure S4A). Despite being more common, the severity of the asynapsis phenotype in Setdb1 cKOs was minor, most often affecting only the XY pair (Figures S4A and S4B) or a single autosomal bivalent (Figures S4A and S4C). XY and autosomal asynapsis were not always coincident in Setdb1 cKO cells. Interestingly, autosomal asynapsis in Setdb1 cKOs was observed exclusively at the centromeric end of the bivalent (100%; n = 27 asynapsed bivalents). In contrast, autosomal asynapsis in controls was not exclusively centromeric (77%; n = 31 asynapsed bivalents; p = 1.17 x 10−2; Fisher’s exact test). To assess whether the centromeric asynapsis associated with Setdb1 deletion preferentially affected smaller autosomes, we performed DNA-fluorescence in situ hybridization (FISH) for chromosomes 1 (a large chromosome) and 19 (a small chromosome) (Figure S4D). In control spermatocytes, asynapsis of chromosome 19 was more common than asynapsis of chromosome 1 (see legend for quantitation). In the Setdb1 cKO, the incidence of asynapsis was increased for both autosomes. However, the frequency ratio of chromosome 19 to chromosome 1 asynapsis was similar to that in the control. The role of SETDB1 in ensuring centromeric synapsis is not therefore specific for smaller autosomes.
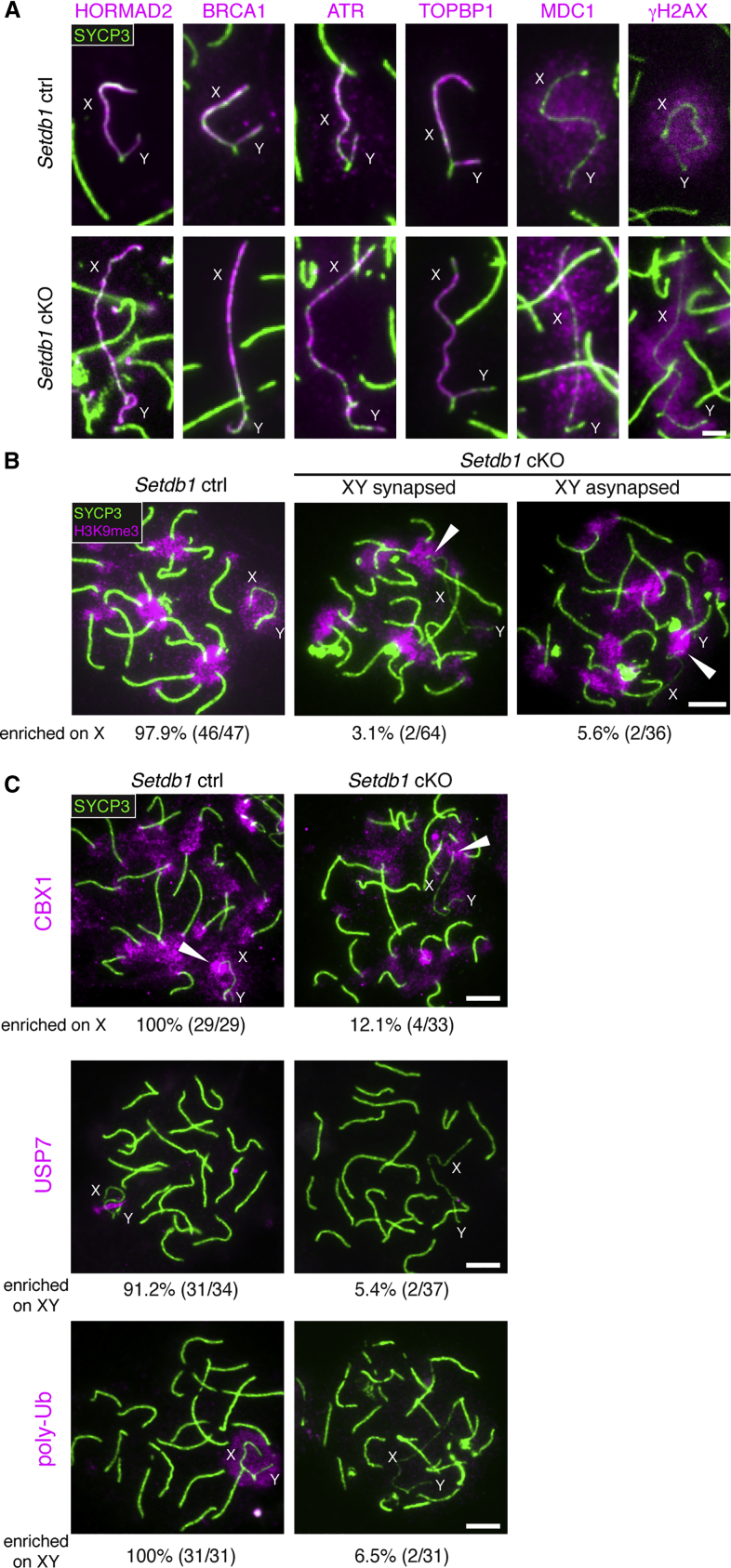
Figure 3.
SETDB1 Is Required for Epigenetic Remodeling of the XY Pair
(A) Early pachytene Setdb1 control and cKO XY bivalents immunostained for SYCP3 (green) and silencing factors (magenta; n ≥ 30 cells for each factor). Scale bar: 2 μm.
(B) Early pachytene Setdb1 controls and cKOs immunostained for SYCP3 (green) and H3K9me3 (magenta). Arrowheads: H3K9me3 on pericentric heterochromatin of X. Percentages of cells positive for H3K9me3 signals on non-pericentric X are shown below panels. Scale bar: 5 μm.
(C) Early (CBX1) and mid (USP7 and poly-Ub) pachytene Setdb1 controls and cKOs immunostained for SYCP3 (green) and indicated factors (magenta). Arrowheads: CBX1 on pericentric heterochromatin of X. Percentages of cells positive for signals on non-pericentric X (CBX1) or XY (USP7 and poly-Ub) are shown below panels. Scale bar: 5 μm.
8-week-old mice were used for analyses. See also Figures S3 and S4.
SETDB1 Is Required for Epigenetic Remodeling of the XY Pair
Given the mild defects in recombination and synapsis, we examined whether MSCI was perturbed in Setdb1 cKOs. We focused first on localization of silencing factors to the XY bivalent at early pachynema. Increasing numbers of asynapsed autosomes can indirectly antagonize γH2AX accumulation on the XY pair (Mahadevaiah et al., 2008). For this reason, we initially examined cells without autosomal asynapsis. XY localization of SYCP3, HORMAD2, BRCA1, ATR, TOPBP1, MDC1, and γH2AX occurred normally in these cells, whether the sex chromosomes were synapsed (Figure 3A) or asynapsed (Figure S4E). Also in Setdb1 cKO cells with autosomal asynapsis, XY γH2AX localization was unimpaired (Figure S4F). The magnitude of asynapsis in this mutant thus falls below that required to antagonize XY γH2AX accumulation (Mahadevaiah et al., 2008). Importantly, however, while in Setdb1 cKOs, H3K9me3 localization to pericentric heterochromatin and to the Y chromosome was unaffected, localization to the asynapsed X chromosome did not occur (Figure 3B).
We next assessed the impact of Setdb1 deletion on other XY chromatin associated factors. CBX1, also known as heterochromatin protein 1 (HP1) beta, directly binds to H3K9me3 (Bannister et al., 2001) and facilitates spreading of this mark during heterochromatin formation (Mozzetta et al., 2015). In controls, CBX1 was observed on the XY body not only at diplonema, as previously reported (Metzler-Guillemain et al., 2003, Turner et al., 2001, Motzkus et al., 1999), but also at early pachynema. As observed for H3K9me3, in Setdb1 cKOs, CBX1 was preserved at pericentric heterochromatin and at the Y chromosome but was lost at the asynapsed X chromosome (Figure 3C). Pathways regulating later stages of XY chromatin remodeling were also perturbed in this mutant. H2A lysine-119 deubiquitylating enzyme USP7, which is recruited by SCML2 (Hasegawa et al., 2015, Luo et al., 2015), was not observed on the XY pair (Figure 3C). Similarly, RNF8-dependent polyubiquitylation (poly-Ub), which is required for reactivation of sex chromosomes after meiosis (Sin et al., 2012), was absent (Figure 3C). Thus, SETDB1 acts downstream of the DDR pathway in XY chromatin remodeling by catalyzing H3K9me3.
SETDB1 Is Required for Condensation of the XY Pair
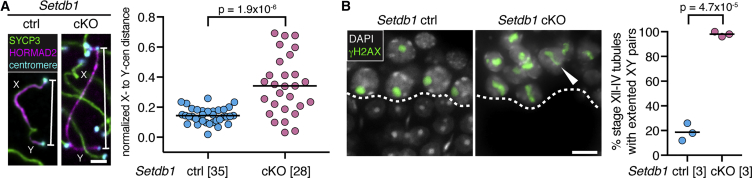
During MSCI, the extended XY pair condenses to form the XY body. We noted that at early pachynema, Setdb1 cKOs exhibited persistently extended XY bivalents. To quantitate this phenotype, we immunolabeled sex chromosomes for SYCP3, HORMAD2, and centromeres and used an established approach to measure the mean distance between the X and Y centromeres (Ichijima et al., 2011). In Setdb1 cKO early pachytene cells, the mean X-to-Y centromere distance was 2-fold higher than that in controls (Figure 4A), indicating that XY body formation in this mutant was defective. To confirm this phenotype, we analyzed the condensation state of γH2AX-immunolabeled XY pairs in stage-matched Setdb1 cKO and control testis sections. In controls, XY pairs were extended during stage XII (late zygonema) and condensed during stages I to IV (early-to-mid pachynema). However, in Setdb1 cKOs, XY pairs were extended during stage XII and thereafter remained extended from stage I to stage IV, when germ cell elimination takes place (Figure 4B). SETDB1 is therefore essential for sex chromosome condensation.
Figure 4.
SETDB1 Is Required for Condensation of the XY Pair
(A) Immunostaining and quantification of early pachytene X-Y centromere distance in Setdb1 controls and cKOs (SYCP3: green; HORMAD2: magenta; centromeres: cyan). Number of cells analyzed in brackets. p value calculated using Mann-Whitney test. Scale bar: 2 μm.
(B) Immunostaining and quantification of XY condensation in Setdb1 control and cKO testis sections (γH2AX: green; DAPI: white). Dashed line: boundary between spermatocytes and spermatids. In Setdb1 cKOs, spermatids are absent as a result of stage lV block. Arrowhead: example of extended XY pair. Chart represents percentage of stage Xll–lV tubules with extended XY pairs (50 tubules per mouse analyzed; three mice per genotype). p value calculated using unpaired t test. Scale bar: 10 μm. 8-week-old mice were used for analyses.
SETDB1 Is Required for XY Silencing at Pachynema
Condensation of the XY pair is associated with sex-gene silencing at pachynema (Inagaki et al., 2010, Yan and McCarrey, 2009). To establish effects of Setdb1 deletion on XY gene expression, we performed RNA-seq on fluorescence-activated cell sorting (FACS)-purified (Bastos et al., 2005) early-to-mid pachytene cells from Setdb1 cKOs and wild-type C57BL6/J males. An initial analysis of published RNA-seq data (Gan et al., 2013) showed that the mean X- and Y-gene transcripts per million (TPM) in purified pachytene cells are lower than in purified spermatogonia and preleptotene cells (Figure S5A). The mean X- and Y-gene TPMs in our wild-type purified pachytene population was similar to those observed in this published study. However, in purified Setdb1 cKO pachytene cells, the mean X- and Y-gene TPMs were elevated (Figure S5A), suggesting that XY silencing was defective.
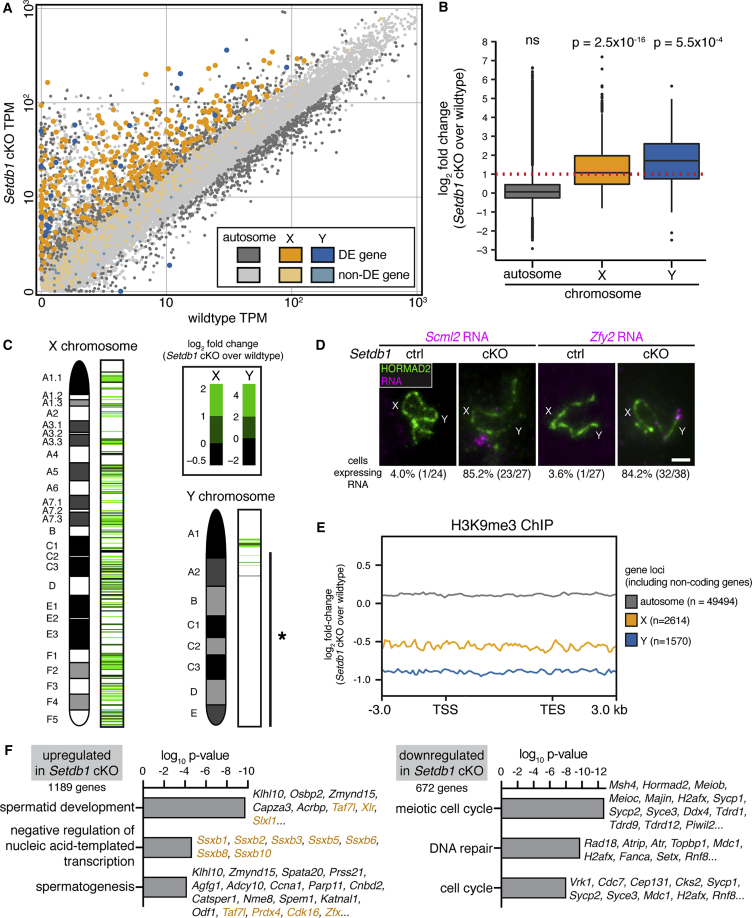
We next performed differential expression analysis. 63% (304/784) of X-protein-coding and 38% (29/105) of Y-protein-coding genes were differentially expressed between Setdb1 cKOs and wild-type pachytene cells. Furthermore, almost all of these XY genes were expressed more highly in the Setdb1 cKOs than in the wild-types (99.7% of X- and 93.1% of Y-encoded differentially expressed genes; Figure 5A and Table S1). Upregulated Y genes included Zfy1 and Zfy2, misexpression of which causes midpachytene germ cell elimination (Royo et al., 2010). As a result of this skewed XY expression change, median expression from the X and Y chromosomes in Setdb1 cKOs was increased 3.3- and 3.7-fold, respectively, relative to wild-types (Figure 5B). In contrast, only 9% of autosomal genes were differentially expressed, and of these genes, 56.2% were upregulated and 43.7% downregulated in Setdb1 cKOs compared to wild-types (n = 1,528 genes). Consequently, median expression from the autosomes was unchanged (Figure 5B). An elevation in median X and Y expression was not observed in published RNA-seq datasets derived from Setdb1 cKO embryonic stem cells (ESCs) or primordial germ cells (PGCs) and was thus a pachytene-specific effect (Figures S5B and S5C). The X-to-autosome (X:A) ratio in Setdb1 cKO pachytene cells was elevated compared to that in wild-types (Figure S5D), consistent with preferential overexpression of X versus autosomal genes in the former genotype. Genes upregulated in the Setdb1 cKO were found across the length of the X chromosome (Figure 5C; see legend for discussion of Y chromosome). Thus, Setdb1 deletion caused upregulation of sex-linked genes in pachytene cells but not in ESCs or PGCs.
Figure 5.
Defective MSCI in Setdb1 cKO Pachytene Spermatocytes
(A) Comparison of gene expression levels between wild-type ad Setdb1 cKO pachytene cells. DE: differentially expressed. DE genes: autosome (dark gray); X (dark orange); Y (dark blue). non-DE genes: autosome (light gray); X (light orange); Y (light blue). TPM: transcripts per million.
(B) Boxplot represents gene expression log2-fold change of Setdb1 cKO relative to wild-type. Box: 25th/75th percentiles. Line on box: median. Whisker: 1.5 times the interquartile range from the 25th/75th percentiles. Red dashed line: 2-fold change. ns: not significant. p value calculated using Welch’s t test.
(C) Heatmaps representing gene expression log2-fold change of Setdb1 cKO relative to wild-type across different chromosomal locations. Asterisk: long arm of the Y chromosome, which is occupied by multicopy genes (Ssty1, Ssty2, Sly, Asty). Note that the software used for these heatmaps excludes multi-mapped X and Y genes.
(D) X-linked Scml2 and Y-linked Zfy2 RNA-FISH (magenta) of Setdb1 controls and cKOs immunostained for HORMAD2 (green). Percentages of cells positive for RNA signals are shown below panels. Scale bar: 2 μm.
(E) H3K9me3 occupancy log2-fold change of Setdb1 cKO relative to wild-type. Gray: autosomal genes; orange: X genes; blue: Y genes. Analysis includes all coding and noncoding genes. TSS: transcription start site. TES: transcription end site.
(F) The top three ontology terms enriched in upregulated and downregulated genes in Setdb1 cKO cells. Example genes are listed on the right. Orange: X-linked genes.
See also Figures S5–S7 and Tables S1 and S2.
We complemented our transcriptomic analysis with RNA-FISH for the X gene Scml2 and the Y gene Zfy2. These genes are silenced at pachynema in control males but not in MSCI mutants (ElInati et al., 2017, Royo et al., 2010, Bhattacharyya et al., 2013, Royo et al., 2013). Following RNA-FISH, early pachytene cells were identified using HORMAD2 immunostaining, as described previously (Cloutier et al., 2015, Cloutier et al., 2016). Both Scml2 and Zfy2 were misexpressed at early pachynema in Setdb1 cKOs, and the proportion of cells exhibiting Scml2 and Zfy2 RNA-FISH signals was similar to that observed in established MSCI mutants (ElInati et al., 2017, Wojtasz et al., 2012) (Figure 5D). Thus, transcriptomic and RNA-FISH analyses demonstrate that SETDB1 is critical for MSCI.
Based on our immunostaining analysis (Figure 3B), we hypothesized that SETDB1 mediates silencing by directing H3K9me3 acquisition at X genes. We performed ultra-low-input native chromatin immunoprecipitation sequencing (ChIP-seq) (Brind’Amour et al., 2015) to compare H3K9me3 occupancy at all genes (coding and non-coding) in early-to-mid pachytene Setdb1 cKOs and wild-type cells. H3K9me3 levels at autosomal genes were similar between these two genotypes. However, H3K9me3 levels at X and Y genes were reduced in the Setdb1 cKO relative to the wild-type (Figure 5E). The reduction in H3K9me3 was observed both at XY genes upregulated in the Setdb1 cKO and at XY genes that were not upregulated (Figure S6). These findings indicate that in the Setdb1 cKO reduction of XY H3K9me3 occurs at a chromosomal level.
We examined putative functions of genes upregulated upon depletion of the repressive H3K9me3 mark (Figure 5F and Table S2). Genes upregulated in Setdb1 cKOs relative to wild-types were enriched most highly for the Gene Ontology (GO) category “spermatid development.” This finding was surprising because spermatids were absent in Setdb1 cKOs (Figures 2I and 2J). The “spermatid development” category included genes mapping to the X chromosome, where spermatid genes are abundant (Mueller et al., 2008), as well as autosomal genes. Essential functions in spermiogenesis have been described for several of these X genes, e.g., Taf7l (Cheng et al., 2007) and Slxl1 (Cocquet et al., 2010), and autosomal genes, e.g., Klhl10 (Yan et al., 2004), Osbp2 (Udagawa et al., 2014), Zmynd15 (Clark et al., 2004), Capza3 (Geyer et al., 2009), Acrbp (Kanemori et al., 2016), and Six5 (Sarkar et al., 2004). We also performed GO analysis on genes downregulated in Setdb1 cKOs relative to wild-types (Figure 5F). The most significantly enriched categories featured genes functioning in meiosis. These findings indicate that Setdb1 regulates the timely expression of spermatogenesis genes, promoting expression of meiosis genes and preventing premature expression of spermatid genes.
A Subset of ERVs Is Upregulated in the Setdb1 cKO
SETDB1 silences endogenous retroviruses (ERVs) in somatic cells (Kato et al., 2018) and PGCs (Liu et al., 2014). Using our RNA-seq data, we addressed whether SETDB1 performs a similar function in pachytene cells. 5% (38/836) of ERVs were upregulated in the Setdb1 cKO relative to the wild-type (Figure S7A and Table S3). Upregulated ERVs were derived from multiple families, with the most highly overexpressed ERV being MMERVK10C-int. A similar proportion of ERVs (43/836) was downregulated in the Setdb1 cKO relative to the wild-type. In the Setdb1 cKO, upregulated ERVs showed a more marked decrease in H3K9me3 occupancy than downregulated ERVs or non-differentially expressed ERVs (Figures S7B and S7C). In conclusion, the effect of Setdb1 deletion on ERV silencing in pachytene cells is milder than observed in other contexts (Karimi et al., 2011, Kato et al., 2018, Liu et al., 2014, Matsui et al., 2010).
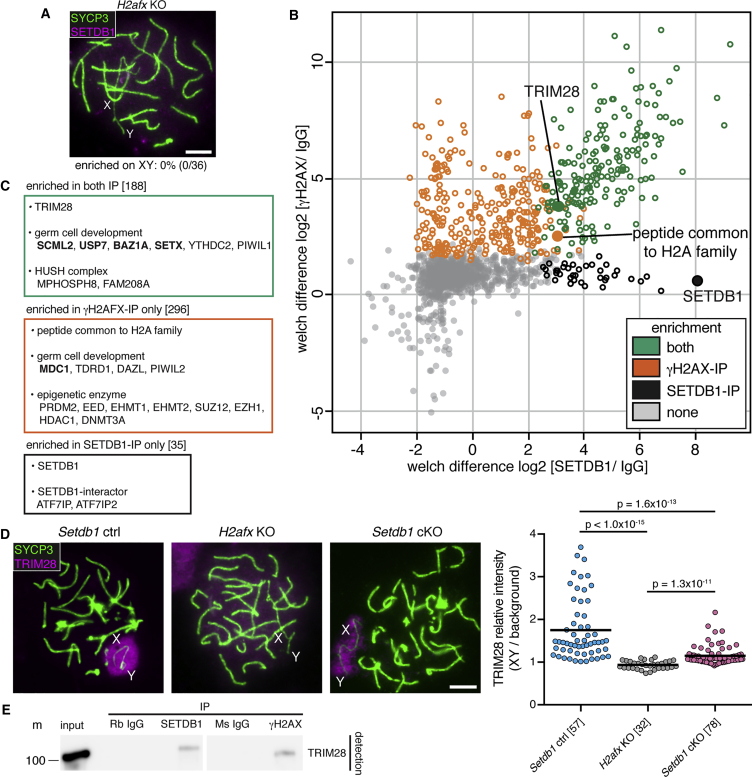
TRIM28 Is a Candidate DDR-SETDB1 Bridging Factor in MSCI
Our findings showed that SETDB1 acts downstream of the DDR network. Consistent with this conclusion, SETDB1 localization to the XY bivalent did not occur in H2afx KO pachytene cells (Figure 6A). However, the mechanism underlying sex chromosome SETDB1 recruitment was unclear. TRIM28, hnRNP K, and Krüppel-associated box zinc-finger proteins (KRAB-ZFPs) recruit SETDB1 in other contexts (Ecco et al., 2017, Iyengar and Farnham, 2011, Thompson et al., 2015), and TRIM28 is also involved in the DDR (White et al., 2006). To assess which of these cofactors could be important in XY-SETDB1 recruitment, we performed testis IP-mass spectrometry (MS) on P15 wild-type testis using both γH2AX and SETDB1 as bait (Figure 6B and Table S4). Proteins significantly enriched in both the γH2AX and SETDB1 IP-MS experiments included TRIM28 and hnRNP K but not KRAB-ZFPs. Both IP-MS experiments also pulled down known XY body-associated proteins (e.g., SCML2, USP7) (Hasegawa et al., 2015, Luo et al., 2015), as well as components of the HUSH complex, which promote SETDB1-mediated H3K9me3 spreading (Tchasovnikarova et al., 2015) (Figure 6C). Proteins observed only in the γH2AX IP-MS included MDC1 and several epigenetic enzymes, while those unique to the SETDB1 IP-MS included the SETDB1-stabilizing factors ATF7IP and ATF7IP2 (Ichimura et al., 2005, Timms et al., 2016) (Figure 6C). IP-MS can thus identify additional candidate XY-silencing factors.
Figure 6.
Genetic and Protein Interaction of SETDB1 with γH2AX and TRIM28
(A) Early pachytene H2afx KOs immunostained for SYCP3 (green) and SETDB1 (magenta). Percentages of cells positive for SETDB1 signals on XY are shown below panels.
(B) IP-MS analysis to identify SETDB1- and γH2AX-interactors in testes. Axis values show LFQ intensity log2-fold change of SETDB1-IP (X axis) or γH2AX-IP (Y axis) relative to control immunoglobulin G (IgG)-IP. Green: enriched in both γH2AX- and SETDB1-IP. Orange: enriched only in γH2AX-IP. Black: enriched only in SETDB1-IP. Gray: non-enriched. Detection of H2AX is limited due to sequence similarity among H2A family proteins.
(C) Examples of proteins enriched in γH2AX- and/or SETDB1-IP. Number of proteins enriched in brackets. Bold: known MSCI factors.
(D) Early pachytene Setdb1 controls, H2afx KOs, and Setdb1 cKOs immunostained for SYCP3 (green) and TRIM28 (magenta). Chart shows relative intensity of TRIM28 on XY relative to non-XY region in nucleus. Number of cells analyzed in brackets. p value calculated using Mann-Whitney test.
(E) Western blot of input and immunoprecipitated samples using P15 wild-type testis lysate treated with nuclease. Rb: rabbit; Ms: mouse; m: size marker. Expected TRIM28 size: 110 kDa.
8-week-old mice were used in (A) and (D); P15 mice were used in (B) and (E). Scale bars: 5 μm.
To further assess their candidacy, we analyzed TRIM28 and hnRNP K immunostaining in early pachytene cells. hnRNP K was present throughout autosomal chromatin but was notably excluded from the XY pair (Figure S7D). However, TRIM28 localized to the sex chromosomes, further supporting a role for this protein in linking the DDR to SETDB1 (Figure 6D). Testis IP followed by western blotting confirmed that both SETDB1 and γH2AX interact with TRIM28 (Figure 6E). In H2afx KO pachytene cells, localization of TRIM28 to the XY pair was abolished (Figure 6D), placing TRIM28 downstream of the DDR network in MSCI. TRIM28 was observed on the XY pair in the Setdb1 cKO (Figure 6D). However, the staining intensity of XY-associated TRIM28 was lower than that in the control (Figure 6D). Thus, while TRIM28 is upstream of SETDB1 in MSCI, SETDB1 may facilitate TRIM28 amplification on the sex chromosomes (see Discussion). Overall, our findings support a role for TRIM28 in bridging the DDR to SETDB1.
Discussion
A fundamental role for the DDR network in initiating meiotic silencing is well established. DDR proteins collaborate to induce phosphorylation of H2AX on asynapsed chromosomes. However, whether resulting γH2AX is sufficient to silence transcription has been unclear. Here, we show that the repressive histone mark H3K9me3, catalyzed by SETDB1, is an additional critical step downstream of γH2AX in MSCI, driving sex chromosome condensation and XY gene silencing. The midpachytene apoptosis in the Setdb1 cKO could be attributed to toxic sex-gene expression (Royo et al., 2010, Royo et al., 2015) and/or de-repression of a subset of ERVs. SETDB1 represses ERVs in various contexts (Collins et al., 2015, Karimi et al., 2011, Kato et al., 2018, Liu et al., 2014, Matsui et al., 2010) and has developmental functions that include oogenesis (Eymery et al., 2016, Kim et al., 2016), early development (Dodge et al., 2004), and neurogenesis (Tan et al., 2012). Our findings reveal a distinct role in male meiosis, where it links the meiotic DDR network to H3K9me3.
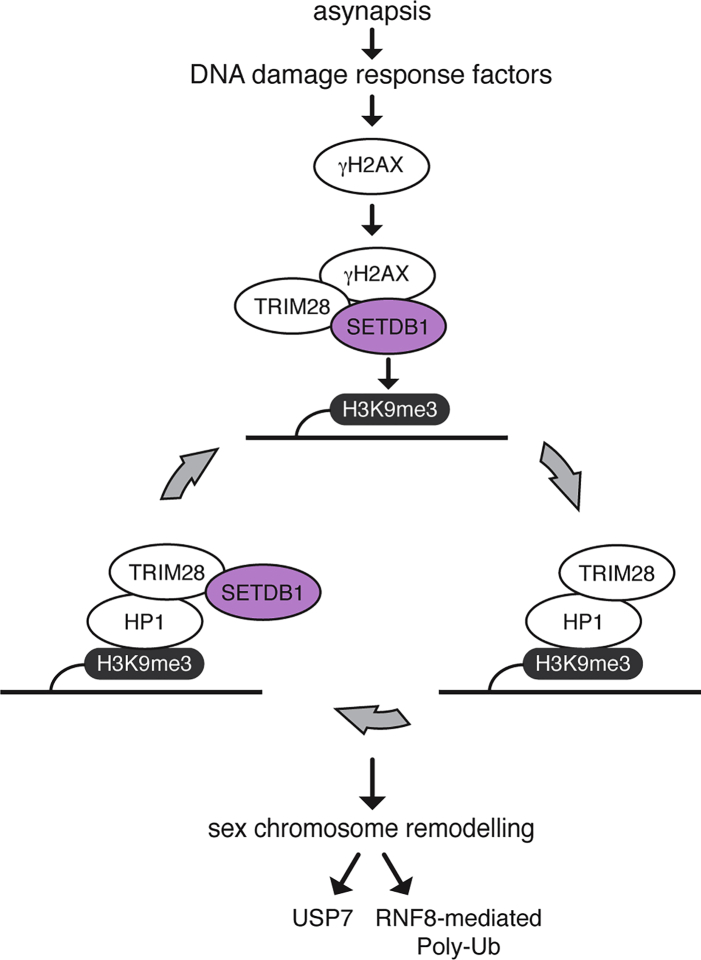
Our data suggest that SETDB1 interacts with the DDR network via TRIM28 (Figure 7). Localization of TRIM28 to the XY pair requires H2AX but not SETDB1. However, in the absence of SETDB1, levels of sex chromosome-associated TRIM28 are reduced relative to the control. We therefore propose that SETDB1 ensures optimal XY-associated TRIM28-enrichment. In mitotic cells, TRIM28 recruits SETDB1, with resulting H3K9me3 acting as a binding site for HP1 proteins (CBX1, CBX3, CBX5) (Bannister et al., 2001, Matsui et al., 2010, Rowe et al., 2010, Schultz et al., 2002). Because HP1 can directly bind to TRIM28 (Ryan et al., 1999), a rolling cycle amplification could then be initiated, recruiting more SETDB1. Similar positive-feedback systems are commonplace in MSCI. For example, accrual of BRCA1 and ATR at chromosome axes is interdependent (Royo et al., 2013, Turner et al., 2004), as is enrichment of ATR, MDC1, and γH2AX at chromatin loops (Royo et al., 2013).
Figure 7.
Model Explaining the Role of SETDB1 in MSCI
Asynapsis sensors and DNA damage response factors are recruited to XY pair, resulting in γH2AX accumulation. γH2AX recruits TRIM28 and SETDB1, which mediates H3K9me3 acquisition. HP1 (CBX1, CBX3, CBX5)-TRIM28 complex binds to H3K9me3 and recruits more SETDB1, facilitating the repressive chromatin state. USP7 and RNF8-mediated poly-Ub pathways act downstream of H3K9me3 for further sex chromosome remodeling.
Setdb1 deletion disrupts XY localization of CBX1 at early pachynema. CBX1 and other HP1 family proteins form dimers bridging two H3K9me3 nucleosomes in heterochromatin (Machida et al., 2018). It would be interesting to investigate the effect of meiotic perturbation of HP1 dimerization on chromosome silencing. At midpachynema in Setdb1 cKOs, XY pairs did not acquire USP7 and poly-Ub. Previous works indicate that SCML2-USP7 and RNF8-mediated poly-Ub act independently during XY chromatin remodeling for proper spermiogenesis (Hasegawa et al., 2015, Lu et al., 2010, Sin et al., 2012). Whether CBX1 acts upstream of one or both of these pathways warrants further investigation.
We observed in Setdb1 cKOs a mild defect in synapsis. This phenotype was observed preferentially at the PAR. Although we observed no difference between the Setdb1 cKO and control in PAR-localization of recombination factors, SETDB1 may have a specialized role at this chromosome region. Alternatively, the PAR phenotype may reflect a more general function for SETDB1 in synapsis. PAR asynapsis is a common finding in meiotic mutants (Fernandez-Capetillo et al., 2003, Ichijima et al., 2011, Turner et al., 2004, de Vries et al., 2005, Widger et al., 2018) and may occur because this chromosome region is small in length and thus more susceptible to synapsis defects. Consistent with this hypothesis, the smallest autosome, chromosome 19, is also more commonly asynapsed than chromosome 1 in the Setdb1 cKO. Although the mechanism by which SETDB1 regulates synapsis is unclear, our data suggest a specific role at centromeric chromosome ends. One possibility is that H3K9 methylation at pericentric regions is important for synapsis. Autosomal asynapsis, associated with defective pericentric H3K9 methylation, is also observed in mice doubly deficient for Suv39h1 and Suv39h2 (Peters et al., 2001). However, it is also noteworthy that autosomal centromeres, such as the XY pair, synapse later than other chromosome regions (Bisig et al., 2012, Kauppi et al., 2011). SETDB1 could therefore have a specific function in promoting synapsis at late-pairing regions.
Setdb1 deletion at pachynema unexpectedly causes premature upregulation of genes normally expressed in spermatids and downregulation of meiotic genes. Since these autosomal genes are not direct H3K9me3 targets (Figure 5E), we speculate that their dysregulation occurs as an indirect consequence of defective MSCI. The effect of MSCI on autosomal gene expression has not been well examined. In female somatic cells, a mechanistically distinct form of X chromosome inactivation (XCI) occurs, which functions to balance somatic X-gene expression levels with that of males. Recent work demonstrates that disrupting somatic XCI causes dysregulation of transcription at the genome-wide level (Borensztein et al., 2017, Sakata et al., 2017). By analogy, we suggest that as well as silencing XY genes, MSCI regulates autosomal gene expression patterns in the mammalian germline.
In addition to mammals, meiotic silencing has been described in other organisms, including C. elegans (Kelly and Aramayo, 2007). In both organisms, asynapsis is the trigger for silencing, but downstream molecular events that lead to gene inactivation were thought to be distinct. For instance, meiotic silencing in C. elegans relies not on DDR factors, as in mammals, but instead on components of the RNA interference machinery (Kelly and Aramayo, 2007). Interestingly, a report indicates that the Setdb1 homolog met-2 is essential for MSCI in C. elegans (Checchi and Engebrecht, 2011). Our current findings are therefore significant because they identify Setdb1 as a silencing factor conserved between these two highly diverged model organisms.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit ATR | Cell Signaling Technology | 2790; RRID:AB_2227860 |
| rabbit BRCA1 | gift from S. Namekawa | N/A |
| rat CBX1 | gift from P. B. Singh | N/A |
| human centromere (CREST serum) | gift from W. Earnshaw | N/A |
| rabbit cleaved-PARP | Abcam | ab32064; RRID:AB_777102 |
| rabbti GFP | Cell Signaling Technology | 2956; RRID:AB_1196615 |
| rabbit H3K9me3 (for ChIP) | Active Motif | 39161; RRID:AB_2532132 |
| rabbit H3K9me3 (for IF) | Millipore | 07-442; RRID:AB_310620 |
| rabbit hnRNP K | Abcam | ab52600; RRID:AB_880478 |
| guinea pig HORMAD2 | gift from A. Tóth | N/A |
| sheep MDC1 | Serotec | AHP799; RRID:AB_323725 |
| mouse poly-ubiquitylation (clone E6C5) | Millipore | 05-678; RRID:AB_11214408 |
| rabbit RAD51 | Millipore | PC130; RRID:AB_2238184 |
| rabbit RPA2 | Abcam | ab10359; RRID:AB_297095 |
| rabbit SETDB1 | Proteintech | 11231-1-AP; RRID:AB_2186069 |
| guinea pig SYCP3 | in house | N/A |
| rabbit TOPBP1 | Abcam | ab105109; RRID:AB_11129928 |
| mouse TRIM28 | Abcam | ab22553; RRID:AB_447151 |
| rabbit USP7 | Bethyl | A300-033A; RRID:AB_203276 |
| mouse α-Tubulin | Sigma | T9026; RRID:AB_477593 |
| mouse γH2AFX | Millipore | 05-636; RRID:AB_309864 |
| VeriBlot for IP Detection Reagent (HRP) | Abcam | ab131366 |
| Anti-mouse IgG VeriBlot for IP secondary antibody (HRP) | Abcam | ab131368 |
| Deposited Data | ||
| RNA-seq and ChIP-seq data | this study | GEO: GSE107671 |
| Experimental Models: Organisms/Strains | ||
| Hormad2 KO | Dr. A. Tóth | Wojtasz et al. (2012) |
| Brca1Δ11 | NCIMR | Brca1tm2.1Cxd |
| H2afx KO | Dr. A. Nussenzweig | Celeste et al. (2002) |
| Dmc1 KO | Jackson laboratory | Dmc1tm1Jcs |
| Atrflox | Jackson laboratory | Atrtm2Bal |
| Setdb1flox | Dr. Y. Shinkai | Matsui et al. (2010) |
| Gt(ROSA)26Sortm1(EYFP)Cos | Jackson laboratory | Gt(ROSA)26Sortm1(EYFP)Cos |
| Ngn3-Cre | Jackson laboratory | Tg(Neurog3-cre)C1Able |
| Stra8-Cre | Jackson laboratory | Tg(Stra8-icre)1Reb |
| Software and Algorithms | ||
| Fiji | http://fiji.sc/ | N/A |
| R | https://www.r-project.org/ | v3.3.2 |
| Prism | GraphPad | v7.0d |
| Kallisto | https://pachterlab.github.io/kallisto/ | v0.44.0 |
| Sleuth | https://pachterlab.github.io/sleuth/ | v0.30.0 |
| BiomaRt | https://bioconductor.org/packages/biomaRt/ | v2.36.1 |
| DAVID Bioinformatics Resources | https://david.ncifcrf.gov | v6.8 |
| ChromHeatMap | https://bioconductor.org/packages/ChromHeatMap/ | v1.34.0 |
| AnnotationDbi | https://bioconductor.org/packages/AnnotationDbi/ | v1.42.1 |
| org.Mm.eg.db | https://bioconductor.org/packages/org.Mm.eg.db/ | v3.6.0 |
| pairwiseCI | https://cran.r-project.org/web/packages/pairwiseCI/index.html | v0.1.26 |
| Hisat2 | https://ccb.jhu.edu/software/hisat2/index.shtml | v2.1.0 |
| deepTools | https://deeptools.readthedocs.io/en/develop/ | v3.1.1 |
| RepEnrich | https://github.com/nskvir/RepEnrich | v1.2 |
| DESeq2 | https://bioconductor.org/packages/DESeq2/ | v1.20.0 |
| MaxQuant | http://www.coxdocs.org/doku.php?id=:maxquant:start | v1.6.0.13 |
| Perseus | http://www.coxdocs.org/doku.php?id=perseus:start | v1.4.0.2 |
| Other | ||
| Published RNA-seq datasets reanalysed in this study | GEO | GSE35005, GSE60377, GSE29413 |
Contact for Reagent and Resource Sharing
Further information and requests for reagents should be directed to and will be fulfilled by the Lead Contact, James M.A. Turner (james.turner@crick.ac.uk).
Experimental Model and Subject Details
Mice
All animals were maintained with appropriate care according to the United Kingdom Animal Scientific Procedures Act 1986 and the ethics guidelines of the Francis Crick Institute. Mice were housed in individually ventilated cages and had free access to water and food. All studies were approved by local ethical review and UK Home Office. Male mice at age indicated in figures or legends were used for analyses. C57BL/6J strain was used as wildtype. Hormad2 KO mice (Wojtasz et al., 2012), Brca1Δ11 mice (Xu et al., 1999), H2afx KO mice (Celeste et al., 2002), and Dmc1 KO mice (Pittman et al., 1998) were generated on the C57BL/6J background. Atr cKO mice (Widger et al., 2018) were generated on the C57BL/6J background by mating Atrflox/flox females (Brown and Baltimore, 2003) with Atr+/- (Brown and Baltimore, 2003); Ngn3-Cre (Schonhoff et al., 2004) males. Setdb1 control and cKO mice were generated on the C57BL/6J background by mating Setdb1flox/flox; Gt(ROSA)26Sortm1(EYFP)Cos/tm1(EYFP)Cos (Matsui et al., 2010, Srinivas et al., 2001) females with Setdb1+/- (Matsui et al., 2010) males carrying either Ngn3-Cre or Stra8-Cre (Sadate-Ngatchou et al., 2008) transgene.
Method Details
Immunofluorescence Staining of Nuclear Spreads
Glass slides (ThermoFisher, AA00008032E00MNT10) were cleaned by boiling in water for ten minutes and dried before use. Testes were dissected in Roswell Park Memorial Institute (RPMI) medium and 100 μl of cell suspension were placed on slides. Slides were applied with 50 μl of 0.05% Triton X-100 in water and incubated at room temperature for 10 minutes. Cells were fixed in 2% paraformaldehyde (PFA), 0.02% sodium dodecyl sulfate (SDS) in phosphate-buffered saline (PBS) at room temperature for one hour, washed in water, and air-dried. Slides were blocked in blocking solution [0.15% bovine serum albumin (BSA) and 0.1% Tween 20 in PBS] at room temperature for one hour and incubated with primary antibodies (see list in Key Resources Table) in humidified chamber at 37°C overnight. For TRIM28 staining, slides were incubated at 4°C. Dilution of primary antibodies were 1:10 (MDC1, TRIM28), 1:50 (ATR, RAD51, RPA2), 1:100 (CREST, H3K9me3, hnRNP K, HORMAD2, SETDB1, SYCP3, TOPBP1), 1:200 (CBX1, poly-Ub, USP7), 1:250 (γH2AX), 1:500 (BRCA1). Secondary antibodies (1:250-500, AlexaFluor 488, 568, or 647, ThermoFisher) were applied in blocking buffer at 37°C for one hour. After wash in PBS at room temperature, specimens were mounted in Vectashield with 4',6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, H-1200).
Plot Profiles of H3K9me3 and γH2AX Staining
Captured images of nuclear spreads immunostained for SYCP3, γH2AX, H3K9me3 were analysed using Fiji (Rueden et al., 2017, Schindelin et al., 2012). Signal intensity at the origin was assigned a value of 1, and relative intensity was calculated.
Measurement of Normalized Distance between X- and Y-Centromeres
Captured images of nuclear spreads immunostained for SYCP3, HORMAD2 and centromere were analysed using Fiji (Rueden et al., 2017, Schindelin et al., 2012). Normalised distance was calculated by dividing measured distance between X- and Y-centromeres by largest diameter of nucleus.
RNA Fluorescence In Situ Hybridization (RNA-FISH) Followed by Immunofluorescence Staining
Bacterial artificial chromosome probes for Scml2 (RP24-204O18, Children's Hospital Oakland Research Institute) and Zfy2 (CITB-288D7, Research Genetics) were labelled using Abbott Nick Translation Kit. Testes were dissected in RPMI medium and 100 μl of cell suspension was placed on cleaned glass slides (kept cold hereafter). Cells were permeabilised in 0.5% Triton X-100, 2 mM Vanadyl Ribonucleoside in PBS for 10 minutes, fixed in 4% PFA in PBS for 10 minutes and washed in PBS. After dehydration in an ethanol series (2x 70, 80, 95, 100%), air-dried specimens were hybridised with the denatured probe mixed with 3 μg of mouse Cot-1 DNA (ThermoFisher, 18440016) in hybridisation buffer (50% formamide, 25% dextran sulphate, 5 mg/ml bovine serum albumin, 1mM Vanadyl Ribonucleoside in 2x SSC) at 37°C overnight. Slides were washed in 50% Formamide in 1x SSC at 45°C and in 2x SSC at 45°C, and then blocked in 1% BSA, 0.1% Tween in 4x SSC at 37°C for 30 minutes. Slides were incubated with HORMAD2 antibody at 37°C for 30 minutes. Secondary antibody (1:250, AlexaFluor 647, ThermoFisher) was applied in PBS at 37°C for 30 minutes. After wash in 0.1% Tween in 4x SSC at room temperature, specimens were mounted in Vectashield with DAPI.
DNA-FISH
DNA-FISH of nuclear spread specimens was performed as described in detail previously (Hirota et al., 2017). Briefly, specimens were washed in 2x SSC and denatured in 70% formamide in 2x SSC. After dehydration in an ethanol series, air-dried specimens were hybridised with the denatured probe in hybridisation buffer at 37°C for overnight. After washing in 2x SSC, 0.1x SSC, and 4xSSC, 0.1 % Tween20, specimens were mounted in Vectashield with DAPI. Bacterial artificial chromosome [RP24-502P5 (Sly)] or chromosome painting probes from MetaSystems (XMP 1 Green and XMP 19 Orange) were used as probes.
Immunofluorescence Staining of Testis Sections
Isolated testes were fixed in 4% PFA at 4°C overnight, washed in 70% ethanol, embedded in paraffin, and sectioned. Sections attached on glass slides were warmed at 60°C for 10 minutes and washed in Histo-Clear (National Diagnostics, HS-200) at room temperature for five minutes twice. Specimens were rehydrated in an ethanol series (2x 100, 2x 95, 75%) and then in water. Antigen retrieval was performed by boiling the slides in 0.01 M sodium citrate in water for 10 minutes and letting the solution cool for 20 minutes. Slides were blocked in blocking solution (5% normal goat serum and 0.1% Triton X-100 in PBS) at room temperature for one hour and incubated with primary antibodies (see list in Key Resources Table) in humidified chamber at 37°C for two hours. Dilution of primary antibodies were 1:250 (cleaved-PARP, γH2AX). Secondary antibodies (1:500, AlexaFluor 488, 568, ThermoFisher) were applied in blocking buffer at room temperature for one hour. After wash in PBS at room temperature, specimens were mounted in Vectashield with DAPI.
For EYFP staining, testes were fixed in 4% PFA at 4°C for two hours, washed in 0.2% Tween 20 in PBS, immersed successively in 10% and 30% sucrose in PBS, embedded in OCT compound (VWR), frozen, and sectioned at -20°C. Dried specimens were washed three times in PBS, blocked in blocking solution at room temperature for 30 minutes and incubated with GFP antibody (1:200) at 4°C overnight. Secondary antibody (1:500, AlexaFluor 488 anti-rabbit IgG, ThermoFisher) was applied in blocking buffer at room temperature for one hour. After wash in PBS, specimens were mounted in Vectashield with DAPI.
Histology
Isolated testes and epididymides were fixed in Bouin’s solution overnight, washed in 70% ethanol, embedded in paraffin, sectioned, and stained with Periodic Acid-Schiff staining (for testes) or hematoxylin and eosin (for epididymides).
Microscopy
Images of nuclear spreads, RNA-FISH, and DNA-FISH were captured using Deltavision Microscopy System (100x/1.35NA Olympus UPlanApo objective; GE Healthcare). Images of immunostained testis sections were captured using Leica SP5 confocal microscopy (63x/1.40NA objective). Histology images were captured using Olympus BH2 microscope with 20x/0.46NA (for epididymis) or 40x/0.70NA (for testis) Olympus SPlan objectives.
Isolation of Pachytene Spermatocytes
Early-to-mid pachytene cells were purified using fluorescence activated cell sorting following the published method (Bastos et al., 2005) with a modification from testes of wildtype C57BL/6J mice at postnatal day 15 (P15), when germ cells reach early-mid pachynema in the first wave of spermatogenesis. Briefly, seminiferous tubules were dissociated by two-step collagenase digestions and treated with Trypsin buffer (0.125% Trypsin, 500 μg/ml collagenase, 3.29 μg/ml DNase I in the incubation buffer described in Bastos et al., 2005) at 32°C for 20 minutes. Cell suspension was filtered (40 μm), stained with Hoechst 33342 (5 μg/ml) and propidium iodide (2 μg/ml), and used for sorting. As spermatocytes are arrested at midpachynema in Setdb1 cKOs, Setdb1 cKO mice at P15 or later were used for isolating early-to-mid pachytene cells. Immunofluorescence staining of nuclear spreads using test-sorted testicular cells from P15 wildtype mice confirmed purity of pachytene cells (82% and 76%, 50 cells analysed for each). 500 cells for each genotype were used for RNA isolation. 3268 cells of wildtype and 5203 cells of Setdb1 cKO were used for chromatin immunoprecipitation.
RNA Sequencing (RNA-Seq)
Total RNA was extracted from 500 pachytene cells using RNAqueous-Micro Total RNA Isolation Kit (ThermoFisher, AM1931) and eluted in 10 μl of elution solution. cDNA was amplified from 1 μl of total RNA by using Smart-seq2 protocol (Picelli et al., 2013, Picelli et al., 2014) (15 amplification cycles). 10 ng of cDNA was sheared using Covaris E220. Libraries of ∼300 bp were generated using Ovation Ultralow DR kit (NuGEN, 0330) with 14 PCR cycles. Sequencing was performed on the Illumina HiSeq 4000 system (single read, 75 bp).
Chromatin Immunoprecipitation Sequencing (ChIP-Seq)
Ultra-low-input native ChIP-seq libraries were prepared by following a published protocol (Brind’Amour et al., 2015) using sorted wildtype (3268 cells) and Setdb1 cKO (5203 cells) early-to-mid pachytene cells. Briefly, sorted cells were treated with micrococcal nuclease at 21°C for 7.5 minutes and used for H3K9me3 ChIP. 5% of the samples after micrococcal nuclease digestion were taken as input. 6.25 μl of 1:100 diluted H3K9me3 antibody (Active Motif, 39161) per reaction was used for ChIP. DNA from pulled-down chromatin was eluted, end-repaired, phosphorylated, and A-tailed. NEBNext Adapter for Illumina was used as adapters and adapter-ligated DNAs were amplified by 13 PCR cycles using NEBNext Index primers (New England Biolabs, included in E7335 and E7500). The libraries were size-selected (200-500 bp) using E-Gel (ThermoFisher). Sequencing was performed on the Illumina HiSeq 2500 system (single read, 75 bp).
Immunoprecipitation (IP)
Four testes of wildtype C57BL/6J mice at P15 were homogenised in 1 ml of IP buffer [20 mM Tris (pH 7.5), 150 mM sodium chloride, 1% Triton X-100, 25 U/ml Pierce Universal Nuclease (ThermoFisher, 88700), supplemented with protease inhibitor cocktail (Roche, 04693159001) and phosphatase inhibitor cocktail (Roche, 04906845001)] and rotated at room temperature for 30 minutes. Lysates were cleared by centrifugation (15000 g, 4°C for 10 minutes) twice and incubated with 20 μl of 1:1 mixture of protein A and protein G Dynabeads (ThermoFisher, 10002D and 10004D; pre-washed in IP buffer) at 4°C for 30 minutes. After removing beads, 400 μl of precleared lysates were mixed with 3 μg of antibodies and rotated at 4°C for 1.5 hours. Lysates were then mixed with 50 μl of 1:1 mixture of protein A and protein G Dynabeads (pre-washed in IP buffer) and rotated at 4°C overnight. Beads were washed five times in IP buffer without nuclease at 4°C and boiled in 1x SDS-PAGE sample buffer [80 mM Tris (pH6.8), 2% SDS, 10% Glycerol, 0.0006% Bromophenol blue, 5% 2-Mercaptoethanol] for 10 minutes.
Western Blot
For the data used in Figures 2G and S2B, testes were homogenised in lysis buffer [1mM Tris (pH 8.0), 150 mM sodium chloride, 1mM ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA), 1 mM magnesium chloride, 0.1% NP40, 1mM dithiothreitol (DTT), 1mM phenylmethanesulfonyl fluoride (PMSF), supplemented with protease inhibitor cocktail (Roche)], incubated on ice for 15 minutes, and the resulting lysates were cleared by centrifugation (6000 g, 4°C for 10 minutes).
Protein lysates were used for SDS-PAGE (Laemmli, 1970) and blotted onto polyvinylidene difluoride membranes (Millipore, IPVH00005) or nitrocellulose membranes (LI-COR, 926-31092; used only for γH2AX detection). Membranes were blocked in blocking buffer (5% skimmed milk in TBST) at room temperature for one hour and incubated with primary antibodies (see list in Key Resources Table) in blocking buffer at 4°C overnight. Dilution of primary antibodies were 1:1000 (SETDB1, γH2AX, TRIM28) and 1:5000 (α-Tubulin). Secondary antibody (VeriBlot for IP Detection Reagent or Anti-mouse IgG VeriBlot for IP secondary antibody, 1:3000, Abcam) was applied in blocking buffer at room temperature for 45 minutes. After wash in TBST, signals were detected using Clarity Western ECL Substrate (Bio-Rad, 1705060).
Trypsin Digestion
The immunoprecipitated proteins were electrophoresed for approximately 1 cm by SDS-PAGE. Whole lanes were then excised and proteins were subjected to in-gel trypsin digestion using a Perkin Elmer Janus liquid handling system. Briefly, the excised gel pieces were de-stained in 50% acetonitrile, 50 mM ammonium bicarbonate. Cysteines were reduced in 10 mM DTT, and alkylated in 55 mM iodoacetamide. After alkylation, the proteins were digested with 6 ng/μl trypsin at 37°C overnight. The resulting peptides were extracted in 2% formic acid, 1% acetonitrile.
Mass Spectrometry
The peptides were loaded on 50-cm Easy Spray column (75 μm inner diameter, 2 μm particle size, ThermoFisher), equipped with an integrated electrospray emitter. Reverse phase chromatography was performed using the RSLC nano U3000 (ThermoFisher) with a binary buffer system at a flow rate of 250 nl/minute. Solvent A was 0.1% formic acid, 5% DMSO, and solvent B was 80% acetonitrile, 0.1% formic acid, 5% DMSO. The in-gel digested samples were run on a linear gradient of solvent B (2- 35%) for 98 minutes. Total run time was 120 minutes including column conditioning. Each sample was analysed in triplicate. The nanoLC was coupled to an LTQ Orbitrap Velos Pro (ThermoFisher) operated in data-dependent mode acquiring one MS1 scan followed by 10 CID scans acquired in centroid mode. The CID normalized collision energy was set at 35 with 10 milliseconds activation time and a maximum ion injection time for MS2 scans at 50 milliseconds. The dynamic exclusion was set at 20 seconds and singly charged peptides and peptides with unassigned charge states were excluded from fragmentation.
Quantification and Statistical Analysis
Statistics and Reproducibility
R v3.3.2 and GraphPad Prism 7 were used for statistical tests. Lines on dot plots show mean values. Box plots are defined in figure legends. Two-tailed p-values were calculated using unpaired t-test (Figures 2F, 2K, 4B, and S2A), Mann-Whitney test (Figures 4A, 6D, and S3), Welch's t-test (Figures 5B, S5B, S5C, and S7B), or Fisher's exact test (Figures S4A and S4D). P-values <0.05 were regarded as significant. The experimental findings were reproduced successfully at least twice. Due to limited cell number, ChIP-seq experiment was performed on single wildtype and Setdb1 cKO samples though four technical replicates were performed for each sample.
Analysis of RNA-Seq Data
RNA-seq data were processed using an R script and transcript quantification was performed using the kallisto R package (Bray et al., 2016). Kallisto was run in single-end read mode using the mouse transcriptome index (GRCm38), with the estimated average fragment length set to 200, the average standard deviation of fragment length set to 20, and the number of bootstraps for estimating confidence intervals set to 150. Paired-end mode was selected for the PGC Setdb1 cKO dataset (Liu et al., 2014). Normalisation was performed using kallisto to generate transcript-level TPM values which were later aggregated to gene-level TPM values using sleuth (Pimentel et al., 2017). Protein-coding genes having an average TPM >0.1 in at least one condition were used in subsequent scatterplot and boxplot analyses. Differential expression analysis was performed using sleuth and genes with q-value <0.2 calculated by Wald test were considered as differentially expressed. All chromosome and gene biotype annotations were extracted from Ensembl using the R package BiomaRt (Durinck et al., 2009). Gene ontology analyses were performed using DAVID Bioinformatics Resources (https://david.ncifcrf.gov) (Huang et al., 2009a, Huang et al., 2009b).
Chromosome Heatmap
The R package ChromHeatMap (http://bioconductor.org/packages/ChromHeatMap/) was used to plot the log2 expression ratio of Setdb1 cKO relative to wildtype TPM values as a heatmap separately for both X and Y chromosomes. The location of each gene along the heatmap was precisely mapped to the genomic locus using the AnnotationDbi and org.Mm.eg.db packages (http://bioconductor.org/packages/AnnotationDbi/; https://www.bioconductor.org/packages/org.Mm.eg.db/).
X-Autosome Expression Ratio
X:autosome expression ratios with 95% confidence intervals were calculated using the pairwiseCI package (https://cran.r-project.org/web/packages/pairwiseCI/index.html) in R using ’median.ratio’ setting with 1,000,000 bootstrap replications. Genes that were expressed < 1 TPM were excluded from downstream calculations.
Analysis of ChIP-Seq Data
Sequences were aligned using Hisat2 v2.1.0 (Kim et al., 2015) to the mouse genome index (GRCm38). Coverage from each bam file was computed using the deepTools (Ramírez et al., 2014) and compared in 100 bp genomic bins after normalisation for sequencing depth with setting ‘reciprocal ratio’. Gene regions were scaled to the same size, and 3 kb upstream and downstream sequences were included for computing scores for each region in comparisons. This score matrix was used to generate profile plots for each set of genomic regions.
ERV Analysis Using RNA-Seq and ChIP-Seq Data
Sequences were aligned to the genome using Hisat2 (v2.1.0), and unique and multi-mapped reads were extracted separately. Spliced alignments were permitted on RNA-seq libraries, but disallowed for ChIP-seq libraries. Annotation for M. musculus repetitive LINE and LTR elements were obtained from UCSC genome Table Browser (mm10). The RepEnrich package (Criscione et al., 2014) was subsequently used to obtain fraction counts for each repetitive element, and used in further downstream analyses. Estimated read counts from the RNA-seq data were used to perform differential expression analysis between Setdb1 cKO and wildtype samples using DESeq2 (Love et al., 2014). A negative binomial distribution was applied to the count data using the parametric mode, and significance test was performed using the Wald test. Genes having a p-value < 0.01 were labelled significant on subsequent scatter plots comparing counts per million (CPM) between Setdb1 cKO and wildtype samples.
Processing and Analysis of Mass Spectrometry Data
Raw data files were analysed using MaxQuant software (version 1.6.0.13) (Cox and Mann, 2008). Parent ion and tandem mass spectra were searched against UniprotKB Mus musculus (July 2017) database. For the search the enzyme specificity was set to trypsin with maximum of two missed cleavages. The precursor mass tolerance was set to 20 ppm for the first search (used for mass re-calibration) and to 6 ppm for the main search. Product mass tolerance was set to 0.5 Da. Carbamidomethylation of cysteines was specified as fixed modification, oxidized methionines and N-terminal protein acetylation were searched as variable modifications. The datasets were filtered on posterior error probability to achieve 1% false discovery rate on protein level. For label free quantification the LFQ option within MaxQuant software was selected. The proteingroups.txt MaxQuant output table was imported into Perseus v1.4.0.2 software (Tyanova et al., 2016) for further downstream processing and statistical analysis. LFQ intensities were log2 transformed and missing values were imputed from a noise distribution generated using default Perseus settings. LFQ values were then used to determine proteins that were significantly enriched compared to the control (IgG) conditions. The test settings were Welch's t-test, S0=1.0 and a permutation-based FDR controlled at 0.01.
Data and Software Availability
The sequencing datasets generated during this study are available in the Gene Expression Omnibus (GEO) under the accession code GEO: GSE107671 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107671). GEO accession codes of the published RNA-seq datasets re-analysed during this study are GEO: GSE35005 (Figures 2A and S5A) (Gan et al., 2013), GEO: GSE29413 (Figure S5B) (Karimi et al., 2011), and GEO: GSE60377 (Figure S5C) (Liu et al., 2014).
Acknowledgements
This work was supported by the European Research Council (CoG 647971), the Kyoto University Foundation, and the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001193 to J.T. and FC001120 to K.N.), UK Medical Research Council (FC001193 and FC001120), and Wellcome Trust (FC001193 and FC001120). We thank the Francis Crick Institute Biological Research, Advanced Sequencing, Light Microscopy, and Experimental Histopathology facilities for their expertise; Y. Shinkai for the Setdb1flox mice; A. Tóth for Hormad2 KO testes and HORMAD2 antibody; A. Nussenzweig for H2afx KO mice; W. Earnshaw for the CREST antibody; P. Singh for the CBX1 antibody; S. Namekawa for the BRCA1 antibody; J. Lee and S. Ogushi for technical advice; J. Zohren for statistical advice; D. Barry for image analysis advice; V. Maciulyte for help coordinating NGS analysis; and members of the J.T. laboratory for comments on the manuscript.
Author Contributions
T.H. and J.M.A.T. conceived the project. T.H. and S.K.M. performed immunofluorescence and RNA-FISH. T.H. performed immunoprecipitation, western blotting, DNA-FISH, RNA-seq, and ChIP-seq library preparation. T.H. and E.E. performed FACS purification. T.H., P.B., M.N.S., and K.K.N. performed bioinformatics analyses. V.E. and A.P.S. performed MS analysis. D.G.d.R. analyzed testis histology. T.H. and O.A.O. performed genotyping. T.H. and J.M.A.T. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 1, 2018
Footnotes
Supplemental Information includes seven figures and four tables and can be found with this article online at https://doi.org/10.1016/j.devcel.2018.10.004.
Supplemental Information
References
- An J., Zhang X., Qin J., Wan Y., Hu Y., Liu T., Li J., Dong W., Du E., Pan C. The histone methyltransferase ESET is required for the survival of spermatogonial stem/progenitor cells in mice. Cell Death Dis. 2014;5:e1196. doi: 10.1038/cddis.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetov M.K., Gursoy-Yuzugullu O., Xu C., Xu Y., Price B.D. DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin. Proc. Natl. Acad. Sci. USA. 2014;111:9169–9174. doi: 10.1073/pnas.1403565111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarends W.M., Hoogerbrugge J.W., Roest H.P., Ooms M., Vreeburg J., Hoeijmakers J.H., Grootegoed J.A. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev. Biol. 1999;207:322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- Bannister A.J., Zegerman P., Partridge J.F., Miska E.A., Thomas J.O., Allshire R.C., Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Bastos H., Lassalle B., Chicheportiche A., Riou L., Testart J., Allemand I., Fouchet P. Flow cytometric characterization of viable meiotic and postmeiotic cells by hoechst 33342 in mouse spermatogenesis. Cytometry A. 2005;65:40–49. doi: 10.1002/cyto.a.20129. [DOI] [PubMed] [Google Scholar]
- Baumann C., Schmidtmann A., Muegge K., De La Fuente R. Association of ATRX with pericentric heterochromatin and the Y chromosome of neonatal mouse spermatogonia. BMC Mol. Biol. 2008;9:29. doi: 10.1186/1471-2199-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T., Gregorova S., Mihola O., Anger M., Sebestova J., Denny P., Simecek P., Forejt J. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA. 2013;110:E468–E477. doi: 10.1073/pnas.1219126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisig C.G., Guiraldelli M.F., Kouznetsova A., Scherthan H., Höög C., Dawson D.S., Pezza R.J. Synaptonemal complex components persist at centromeres and are required for homologous centromere pairing in mouse spermatocytes. PLoS Genet. 2012;8:e1002701. doi: 10.1371/journal.pgen.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E., Rinaldi V.D., White M.E., Schimenti J.C. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–536. doi: 10.1126/science.1247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztein M., Syx L., Ancelin K., Diabangouaya P., Picard C., Liu T., Liang J.B., Vassilev I., Galupa R., Servant N. Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat. Struct. Mol. Biol. 2017;24:226–233. doi: 10.1038/nsmb.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Brind’Amour J., Liu S., Hudson M., Chen C., Karimi M.M., Lorincz M.C. An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat. Commun. 2015;6:6033. doi: 10.1038/ncomms7033. [DOI] [PubMed] [Google Scholar]
- Brown E.J., Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P., Good J.M., Nachman M.W. Meiotic sex chromosome inactivation is disrupted in sterile hybrid male house mice. Genetics. 2013;193:819–828. doi: 10.1534/genetics.112.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carofiglio F., Inagaki A., de Vries S., Wassenaar E., Schoenmakers S., Vermeulen C., van Cappellen W.A., Sleddens-Linkels E., Grootegoed J.A., Te Riele H.P.J. SPO11-independent DNA repair foci and their role in meiotic silencing. PLoS Genet. 2013;9:e1003538. doi: 10.1371/journal.pgen.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A., Petersen S., Romanienko P.J., Fernandez-Capetillo O., Chen H.T., Sedelnikova O.A., Reina-San-Martin B., Coppola V., Meffre E., Difilippantonio M.J. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchi P.M., Engebrecht J. Caenorhabditis elegans histone methyltransferase MET-2 Shields the Male X chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation. PLoS Genet. 2011;7:e1002267. doi: 10.1371/journal.pgen.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Buffone M.G., Kouadio M., Goodheart M., Page D.C., Gerton G.L., Davidson I., Wang P.J. Abnormal sperm in mice lacking the Taf7l gene. Mol. Cell. Biol. 2007;27:2582–2589. doi: 10.1128/MCB.01722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.T., Firozi K., Justice M.J. Mutations in a novel locus on mouse chromosome 11 resulting in male infertility associated with defects in microtubule assembly and sperm tail function. Biol. Reprod. 2004;70:1317–1324. doi: 10.1095/biolreprod.103.020628. [DOI] [PubMed] [Google Scholar]
- Cloutier J.M., Mahadevaiah S.K., ElInati E., Nussenzweig A., Tóth A., Turner J.M.A. Histone H2AFX links meiotic chromosome asynapsis to prophase I oocyte loss in mammals. PLoS Genet. 2015;11:e1005462. doi: 10.1371/journal.pgen.1005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J.M., Mahadevaiah S.K., ElInati E., Tóth A., Turner J. Mammalian meiotic silencing exhibits sexually dimorphic features. Chromosoma. 2016;125:215–226. doi: 10.1007/s00412-015-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquet J., Ellis P.J.I., Yamauchi Y., Riel J.M., Karacs T.P.S., Rattigan A., Ojarikre O.A., Affara N.A., Ward M.A., Burgoyne P.S. Deficiency in the multicopy Sycp3 -Like X-linked genes Slx and Slxl1 causes major defects in spermatid differentiation. Mol. Biol. Cell. 2010;21:3497–3505. doi: 10.1091/mbc.E10-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.L., Kyle K.E., Egawa T., Shinkai Y., Oltz E.M. The histone methyltransferase SETDB1 represses endogenous and exogenous retroviruses in B lymphocytes. Proc. Natl. Acad. Sci. USA. 2015;112:8367–8372. doi: 10.1073/pnas.1422187112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Criscione S.W., Zhang Y., Thompson W., Sedivy J.M., Neretti N. Transcriptional landscape of repetitive elements in normal and cancer human cells. BMC Genomics. 2014;15:583. doi: 10.1186/1471-2164-15-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel K., Lange J., Hached K., Fu J., Anastassiadis K., Roig I., Cooke H.J., Stewart A.F., Wassmann K., Jasin M. Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat. Cell Biol. 2011;13:599–610. doi: 10.1038/ncb2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries F.A.T., de Boer E., van den Bosch M., Baarends W.M., Ooms M., Yuan L., Liu J.G., van Zeeland A.A., Heyting C., Pastink A. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 2005;19:1376–1389. doi: 10.1101/gad.329705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo M., Barchi M., Baudat F., Edelmann W., Keeney S., Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc. Natl. Acad. Sci. USA. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J.E., Kang Y.K., Beppu H., Lei H., Li E. Histone H3-K9 methyltransferase ESET is essential for early development. Mol. Cell. Biol. 2004;24:2478–2486. doi: 10.1128/MCB.24.6.2478-2486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S., Spellman P.T., Birney E., Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecco G., Imbeault M., Trono D. KRAB zinc finger proteins. Development. 2017;144:2719–2729. doi: 10.1242/dev.132605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElInati E., Russell H.R., Ojarikre O.A., Sangrithi M., Hirota T., de Rooij D.G., McKinnon P.J., Turner J.M.A. DNA damage response protein TOPBP1 regulates X chromosome silencing in the mammalian germ line. Proc. Natl. Acad. Sci. USA. 2017;114:12536–12541. doi: 10.1073/pnas.1712530114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymery A., Liu Z., Ozonov E.A., Stadler M.B., Peters A.H.F.M. The methyltransferase Setdb1 is essential for meiosis and mitosis in mouse oocytes and early embryos. Development. 2016;143:2767–2779. doi: 10.1242/dev.132746. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O., Mahadevaiah S.K., Celeste A., Romanienko P.J., Camerini-Otero R.D., Bonner W.M., Manova K., Burgoyne P., Nussenzweig A. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev. Cell. 2003;4:497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Gan H., Wen L., Liao S., Lin X., Ma T., Liu J., Song C.X., Wang M., He C., Han C. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat. Commun. 2013;4:1995. doi: 10.1038/ncomms2995. [DOI] [PubMed] [Google Scholar]
- Geyer C.B., Inselman A.L., Sunman J.A., Bornstein S., Handel M.A., Eddy E.M. A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice. Dev. Biol. 2009;330:142–152. doi: 10.1016/j.ydbio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Sin H.S., Maezawa S., Broering T.J., Kartashov A.V., Alavattam K.G., Ichijima Y., Zhang F., Bacon W.C., Greis K.D. SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev. Cell. 2015;32:574–588. doi: 10.1016/j.devcel.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden G.W., Derijck A.A.H.A., Pósfai E., Giele M., Pelczar P., Ramos L., Wansink D.G., van der Vlag J., Peters A.H.F.M., de Boer P. Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat. Genet. 2007;39:251–258. doi: 10.1038/ng1949. [DOI] [PubMed] [Google Scholar]
- Hirota T., Ohta H., Powell B.E., Mahadevaiah S.K., Ojarikre O.A., Saitou M., Turner J.M.A. Fertile offspring from sterile sex chromosome trisomic mice. Science. 2017;357:932–935. doi: 10.1126/science.aam9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolka D., Ivanek R., Capkova J., Jansa P., Forejt J. Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res. 2007;17:1431–1437. doi: 10.1101/gr.6520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Systematic and in tegrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ichijima Y., Ichijima M., Lou Z., Nussenzweig A., Camerini-Otero R.D., Chen J., Andreassen P.R., Namekawa S.H. MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 2011;25:959–971. doi: 10.1101/gad.2030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T., Watanabe S., Sakamoto Y., Aoto T., Fujita N., Nakao M. Transcriptional repression and heterochromatin formation by MBD1 and MCAF/AM family proteins. J. Biol. Chem. 2005;280:13928–13935. doi: 10.1074/jbc.M413654200. [DOI] [PubMed] [Google Scholar]
- Inagaki A., Schoenmakers S., Baarends W.M. DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics. 2010;5:255–266. doi: 10.4161/epi.5.4.11518. [DOI] [PubMed] [Google Scholar]
- Ishimoto K., Kawamata N., Uchihara Y., Okubo M., Fujimoto R., Gotoh E., Kakinouchi K., Mizohata E., Hino N., Okada Y. Ubiquitination of lysine 867 of the human SETDB1 protein upregulates its histone H3 lysine 9 (H3K9) methyltransferase activity. PLoS One. 2016;11:e0165766. doi: 10.1371/journal.pone.0165766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S., Farnham P.J. KAP1 protein: an enigmatic master regulator of the genome. J. Biol. Chem. 2011;286:26267–26276. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemori Y., Koga Y., Sudo M., Kang W., Kashiwabara S., Ikawa M., Hasuwa H., Nagashima K., Ishikawa Y., Ogonuki N. Biogenesis of sperm acrosome is regulated by pre-mRNA alternative splicing of Acrbp in the mouse. Proc. Natl. Acad. Sci. USA. 2016;113:E3696–E3705. doi: 10.1073/pnas.1522333113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M.M., Goyal P., Maksakova I.A., Bilenky M., Leung D., Tang J.X., Shinkai Y., Mager D.L., Jones S., Hirst M. DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell. 2011;8:676–687. doi: 10.1016/j.stem.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Takemoto K., Shinkai Y. A somatic role for the histone methyltransferase Setdb1 in endogenous retrovirus silencing. Nat. Commun. 2018;9:1683. doi: 10.1038/s41467-018-04132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Alavattam K.G., Sin H.S., Meetei A.R., Pang Q., Andreassen P.R., Namekawa S.H. FANCB is essential in the male germline and regulates H3K9 methylation on the sex chromosomes during meiosis. Hum. Mol. Genet. 2015;24:5234–5249. doi: 10.1093/hmg/ddv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi L., Barchi M., Baudat F., Romanienko P.J., Keeney S., Jasin M. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science. 2011;331:916–920. doi: 10.1126/science.1195774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W.G., Aramayo R. Meiotic silencing and the epigenetics of sex. Chromosome Res. 2007;15:633–651. doi: 10.1007/s10577-007-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A.M., Boyar F.Z., Driscoll D.J. Dynamic histone modifications mark sex chromosome inactivation and reactivation during mammalian spermatogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:16583–16587. doi: 10.1073/pnas.0406325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zhao H., Dan J., Kim S., Hardikar S., Hollowell D., Lin K., Lu Y., Takata Y., Shen J. Maternal Setdb1 is required for meiotic progression and preimplantation development in mouse. PLoS Genet. 2016;12:e1005970. doi: 10.1371/journal.pgen.1005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova A., Wang H., Bellani M., Camerini-Otero R.D., Jessberger R., Höög C. BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J. Cell Sci. 2009;122:2446–2452. doi: 10.1242/jcs.049353. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S., Brind’Amour J., Karimi M.M., Shirane K., Bogutz A., Lefebvre L., Sasaki H., Shinkai Y., Lorincz M.C. Setdb1 is required for germline development and silencing of H3K9me3-marked endogenous retroviruses in primordial germ cells. Genes Dev. 2014;28:2041–2055. doi: 10.1101/gad.244848.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.Y., Wu J., Ye L., Gavrilina G.B., Saunders T.L., Yu X. RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev. Cell. 2010;18:371–384. doi: 10.1016/j.devcel.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.Y., Xiong Y., Kuang H., Korakavi G., Yu X. Regulation of the DNA damage response on male meiotic sex chromosomes. Nat. Commun. 2013;4:2105. doi: 10.1038/ncomms3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Zhou J., Leu N.A., Abreu C.M., Wang J., Anguera M.C., de Rooij D.G., Jasin M., Wang P.J. Polycomb protein SCML2 associates with USP7 and counteracts histone H2A ubiquitination in the XY chromatin during male meiosis. PLoS Genet. 2015;11:e1004954. doi: 10.1371/journal.pgen.1004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S., Takizawa Y., Ishimaru M., Sugita Y., Sekine S., Nakayama J.I., Wolf M., Kurumizaka H. Structural basis of heterochromatin formation by human HP1. Mol. Cell. 2018;69:385–397.e8. doi: 10.1016/j.molcel.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah S.K., Bourc’his D., de Rooij D.G., Bestor T.H., Turner J.M.A., Burgoyne P.S. Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J. Cell Biol. 2008;182:263–276. doi: 10.1083/jcb.200710195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Ortega M., Pacheco S., Martínez-Marchal A., Castillo H., Flores E., Jasin M., Keeney S., Roig I. p53 and TAp63 participate in the recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2017;13:e1006845. doi: 10.1371/journal.pgen.1006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Leung D., Miyashita H., Maksakova I.A., Miyachi H., Kimura H., Tachibana M., Lorincz M.C., Shinkai Y. Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature. 2010;464:927–931. doi: 10.1038/nature08858. [DOI] [PubMed] [Google Scholar]
- McKee B.D., Handel M.A. Sex chromosomes, recombination, and chromatin conformation. Chromosoma. 1993;102:71–80. doi: 10.1007/BF00356023. [DOI] [PubMed] [Google Scholar]
- Metzler-Guillemain C., Luciani J., Depetris D., Guichaoua M.R., Mattei M.G. HP1beta and HP1gamma, but not HP1alpha, decorate the entire XY body during human male meiosis. Chromosome Res. 2003;11:73–81. doi: 10.1023/a:1022014217196. [DOI] [PubMed] [Google Scholar]
- Motzkus D., Singh P.B., Hoyer-Fender S. M31, a murine homolog of Drosophila HP1, is concentrated in the XY body during spermatogenesis. Cytogenet. Cell Genet. 1999;86:83–88. doi: 10.1159/000015418. [DOI] [PubMed] [Google Scholar]
- Mozzetta C., Boyarchuk E., Pontis J., Ait-Si-Ali S. Sound of silence: the properties and functions of repressive Lys methyltransferases. Nat. Rev. Mol. Cell Biol. 2015;16:499–513. doi: 10.1038/nrm4029. [DOI] [PubMed] [Google Scholar]
- Mueller J.L., Mahadevaiah S.K., Park P.J., Warburton P.E., Page D.C., Turner J.M.A. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat. Genet. 2008;40:794–799. doi: 10.1038/ng.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namekawa S.H., Park P.J., Zhang L.F., Shima J.E., McCarrey J.R., Griswold M.D., Lee J.T. Postmeiotic sex chromatin in the male germline of mice. Curr. Biol. 2006;16:660–667. doi: 10.1016/j.cub.2006.01.066. [DOI] [PubMed] [Google Scholar]
- Pacheco S., Marcet-Ortega M., Lange J., Jasin M., Keeney S., Roig I. The ATM signaling cascade promotes recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2015;11:e1005017. doi: 10.1371/journal.pgen.1005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page J., de la Fuente R., Manterola M., Parra M.T., Viera A., Berríos S., Fernández-Donoso R., Rufas J.S. Inactivation or non-reactivation: what accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma. 2012;121:307–326. doi: 10.1007/s00412-012-0364-y. [DOI] [PubMed] [Google Scholar]
- Peters A.H.F.M., O’Carroll D., Scherthan H., Mechtler K., Sauer S., Schöfer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Picelli S., Björklund Å.K., Faridani O.R., Sagasser S., Winberg G., Sandberg R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- Picelli S., Faridani O.R., Björklund A.K., Winberg G., Sagasser S., Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014;9:171–181. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Pimentel H., Bray N.L., Puente S., Melsted P., Pachter L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods. 2017;14:687–690. doi: 10.1038/nmeth.4324. [DOI] [PubMed] [Google Scholar]
- Pittman D.L., Cobb J., Schimenti K.J., Wilson L.A., Cooper D.M., Brignull E., Handel M.A., Schimenti J.C. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol. Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- Ramírez F., Dündar F., Diehl S., Grüning B.A., Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi V.D., Bolcun-Filas E., Kogo H., Kurahashi H., Schimenti J.C. The DNA damage checkpoint eliminates mouse oocytes with chromosome Synapsis Failure. Mol. Cell. 2017;67:1026–1036.e2. doi: 10.1016/j.molcel.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H.M., Jakobsson J., Mesnard D., Rougemont J., Reynard S., Aktas T., Maillard P.V., Layard-Liesching H., Verp S., Marquis J. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature. 2010;463:237–240. doi: 10.1038/nature08674. [DOI] [PubMed] [Google Scholar]
- Royo H., Polikiewicz G., Mahadevaiah S.K., Prosser H., Mitchell M., Bradley A., de Rooij D.G., Burgoyne P.S., Turner J.M.A. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr. Biol. 2010;20:2117–2123. doi: 10.1016/j.cub.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Royo H., Prosser H., Ruzankina Y., Mahadevaiah S.K., Cloutier J.M., Baumann M., Fukuda T., Höög C., Tóth A., de Rooij D.G. ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev. 2013;27:1484–1494. doi: 10.1101/gad.219477.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H., Seitz H., ElInati E., Peters A.H.F.M., Stadler M.B., Turner J.M.A. Silencing of X-linked microRNAs by meiotic sex chromosome inactivation. PLoS Genet. 2015;11:e1005461. doi: 10.1371/journal.pgen.1005461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T., Eliceiri K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R.F., Schultz D.C., Ayyanathan K., Singh P.B., Friedman J.R., Fredericks W.J., Rauscher F.J. KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Krüppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell. Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadate-Ngatchou P.I., Payne C.J., Dearth A.T., Braun R.E. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46:738–742. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Nagao K., Hoki Y., Sasaki H., Obuse C., Sado T. Defects in dosage compensation impact global gene regulation in the mouse trophoblast. Development. 2017;144:2784–2797. doi: 10.1242/dev.149138. [DOI] [PubMed] [Google Scholar]
- Sarkar P.S., Paul S., Han J., Reddy S. Six5 is required for spermatogenic cell survival and spermiogenesis. Hum. Mol. Genet. 2004;13:1421–1431. doi: 10.1093/hmg/ddh161. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers S., Wassenaar E., van Cappellen W.A., Derijck A.A., de Boer P., Laven J.S.E., Grootegoed J.A., Baarends W.M. Increased frequency of asynapsis and associated meiotic silencing of heterologous chromatin in the presence of irradiation-induced extra DNA double strand breaks. Dev. Biol. 2008;317:270–281. doi: 10.1016/j.ydbio.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Schonhoff S.E., Giel-Moloney M., Leiter A.B. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Schultz D.C., Ayyanathan K., Negorev D., Maul G.G., Rauscher F.J. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16:919–932. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciurano R., Rahn M., Rey-Valzacchi G., Solari A.J. The asynaptic chromatin in spermatocytes of translocation carriers contains the histone variant gamma-H2AX and associates with the XY body. Hum. Reprod. 2007;22:142–150. doi: 10.1093/humrep/del330. [DOI] [PubMed] [Google Scholar]
- Sin H.S., Barski A., Zhang F., Kartashov A.V., Nussenzweig A., Chen J., Andreassen P.R., Namekawa S.H. RNF8 regulates active epigenetic modifications and escape gene activation from inactive sex chromosomes in post-meiotic spermatids. Genes Dev. 2012;26:2737–2748. doi: 10.1101/gad.202713.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A.J. The morphology and ultrastructure of the sex vesicle in the mouse. Exp. Cell Res. 1964;36:160–168. doi: 10.1016/0014-4827(64)90169-7. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Jiang X., Xu Y., Ayrapetov M.K., Moreau L.A., Whetstine J.R., Price B.D. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 2009;11:1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.L., Nishi M., Ohtsuka T., Matsui T., Takemoto K., Kamio-Miura A., Aburatani H., Shinkai Y., Kageyama R. Essential roles of the histone methyltransferase ESET in the epigenetic control of neural progenitor cells during development. Development. 2012;139:3806–3816. doi: 10.1242/dev.082198. [DOI] [PubMed] [Google Scholar]
- Tchasovnikarova I.A., Timms R.T., Matheson N.J., Wals K., Antrobus R., Göttgens B., Dougan G., Dawson M.A., Lehner P.J. GENE SILENCING. Epigenetic silencing by the HUSH complex mediates position-effect variegation in human cells. Science. 2015;348:1481–1485. doi: 10.1126/science.aaa7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.J., Dulberg V., Moon K.-M., Foster L.J., Chen C., Karimi M.M., Lorincz M.C. hnRNP K coordinates transcriptional silencing by SETDB1 in embryonic stem cells. PLoS Genet. 2015;11:e1004933. doi: 10.1371/journal.pgen.1004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms R.T., Tchasovnikarova I.A., Antrobus R., Dougan G., Lehner P.J. ATF7IP-mediated stabilization of the histone methyltransferase SETDB1 is essential for heterochromatin formation by the HUSH complex. Cell Rep. 2016;17:653–659. doi: 10.1016/j.celrep.2016.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.M., Burgoyne P.S., Singh P.B. M31 and macroH2A1.2 colocalise at the pseudoautosomal region during mouse meiosis. J. Cell Sci. 2001;114:3367–3375. doi: 10.1242/jcs.114.18.3367. [DOI] [PubMed] [Google Scholar]
- Turner J.M.A., Aprelikova O., Xu X., Wang R., Kim S., Chandramouli G.V.R., Barrett J.C., Burgoyne P.S., Deng C.X. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 2004;14:2135–2142. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- Udagawa O., Ito C., Ogonuki N., Sato H., Lee S., Tripvanuntakul P., Ichi I., Uchida Y., Nishimura T., Murakami M. Oligo-astheno-teratozoospermia in mice lacking ORP4, a sterol-binding protein in the OSBP-related protein family. Genes Cells. 2014;19:13–27. doi: 10.1111/gtc.12105. [DOI] [PubMed] [Google Scholar]
- White D.E., Negorev D., Peng H., Ivanov A.V., Maul G.G., Rauscher F.J. KAP1, a novel substrate for PIKK family members, colocalizes with numerous damage response factors at DNA lesions. Cancer Res. 2006;66:11594–11599. doi: 10.1158/0008-5472.CAN-06-4138. [DOI] [PubMed] [Google Scholar]
- Widger A., Mahadevaiah S.K., Lange J., ElInati E., Zohren J., Hirota T., Pacheco S., Maldonado-Linares A., Stanzione M., Ojarikre O. ATR is a multifunctional regulator of male mouse meiosis. Nat. Commun. 2018;9:2621. doi: 10.1038/s41467-018-04850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasz L., Cloutier J.M., Baumann M., Daniel K., Varga J., Fu J., Anastassiadis K., Stewart A.F., Reményi A., Turner J.M.A. Meiotic DNA double-strand breaks and chromosome asynapsis in mice are monitored by distinct HORMAD2-independent and -dependent mechanisms. Genes Dev. 2012;26:958–973. doi: 10.1101/gad.187559.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wagner K.U., Larson D., Weaver Z., Li C., Ried T., Hennighausen L., Wynshaw-Boris A., Deng C.X. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Xu X., Aprelikova O., Moens P., Deng C.X., Furth P.A. Impaired meiotic DNA-damage repair and lack of crossing-over during spermatogenesis in BRCA1 full-length isoform deficient mice. Development. 2003;130:2001–2012. doi: 10.1242/dev.00410. [DOI] [PubMed] [Google Scholar]
- Yan W., McCarrey J.R. Sex chromosome inactivation in the male. Epigenetics. 2009;4:452–456. doi: 10.4161/epi.4.7.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Ma L., Burns K.H., Matzuk M.M. Haploinsufficiency of kelch-like protein homolog 10 causes infertility in male mice. Proc. Natl. Acad. Sci. USA. 2004;101:7793–7798. doi: 10.1073/pnas.0308025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Wang P.J. Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet. 2012;8:e1003038. doi: 10.1371/journal.pgen.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.