Abstract
Prostate cancer is the second most common cancer in men worldwide. The clinical behaviour of prostate cancer ranges from low-grade indolent tumours that never develop into clinically significant disease to aggressive, invasive tumours that may progress rapidly to metastatic disease and death. Therefore, there is an urgent clinical need to detect high-grade cancers and to differentiate them from the indolent, slow-growing tumours. Conventional methods of cancer detection—such as levels of prostate-specific antigen (PSA) in serum, digital rectal examination, and random biopsies—are limited in their sensitivity, specificity, or both. The combination of conventional anatomical MRI and functional magnet resonance sequences—known as multiparametric MRI (mp-MRI)—is emerging as an accurate tool for identifying clinically relevant tumours owing to its ability to localize them. In this Review, we discuss the value of mp-MRI in localized and metastatic prostate cancer, highlighting its role in the detection, staging, and treatment planning of prostate cancer.
Introduction
Prostate cancer is the second most common cancer in men worldwide, and in the USA alone it has been estimated that 239,000 cases and 29,700 deaths have ocurred in 2013.1,2 The clinical behaviour of prostate cancer ranges from low-grade indolent tumours that never develop into clinically significant disease to aggressive, invasive tumours that may rapidly progress into metastatic disease and, ultimately, death. Largely as a result of increased prostate-specific antigen (PSA) screening since the 1980s, prostate cancer incidence has risen, and more men are diagnosed with early-stage disease.3 However, most of these prostate cancers grow slowly and 10–20 years can go by between diagnosis and death. Therefore, health issues related to prostate cancer treatment—such as cardiovascular disease or hypertension—must be considered when deciding treatment. Currently, about one in six men are diagnosed with prostate cancer in their lifetime, but only one in 33 will die of it.4 The prevalence of ‘silent’ disease is enormous; an autopsy study of 1,056 men who died from causes other than prostate cancer detected undiagnosed and asymptomatic prostate cancer in 68–77% of men aged 60–79, which suggests that many prostate tumours grow so slowly that patients often die from other causes before the prostate cancer ever becomes clinically apparent.5 Another autopsy study of 212 men aged 30–98 revealed an age-dependent increase in prostate cancer incidence, with 55% of tumours found in men aged >70 years.6
Because as many as 60% of cancers are low volume and low grade (Gleason score 3 + 3), PSA screening has been criticized as it can lead to the overdiagnosis of these low-grade prostate tumours that have little potential for causing death.7 It has been suggested that tumours smaller than 0.5 cm in diameter are not clinically significant once the long doubling time of prostate cancer is considered (>24 months in 79% of patients).8,9 In light of these statistics, it is easy to overlook the fact that many men continue to die of prostate cancer. Those men presenting with advanced-stage disease often progress to metastatic disease and ultimately to castration-resistant prostate cancer, which is incurable. Therefore, there is an urgent clinical need to detect high-grade cancers and to differentiate them from those that are indolent, slow-growing tumours. Conventional methods of cancer detection, such as serum PSA, digital rectal examination, and random biopsies are limited in their sensitivity, specificity, or both. However, MRI is a promising method of localizing prostate tumours and determining their size, aggressiveness and invasiveness, thereby predicting their future biological behaviour.10 The combination of conventional anatomical and functional MRI is known as multiparametric MRI (mp-MRI). This method is emerging as an accurate tool for identifying clinically relevant tumours owing to its ability to establish their precise location and the additional information provided by functional MRI sequences, such as diffusion-weighted (DW) MRI, dynamic contrast-enhanced (DCE) MRI, and MR spectroscopy (MRS), which significantly improve the ability of mp-MRI to predict the behaviour of tumours. Herein, we review the value of mp-MRI in localized and metastatic prostate cancer and will highlight its role in the detection, staging, and treatment planning of prostate cancer.
Multiparametric MRI
Mp-MRI is an accurate and noninvasive imaging method that is increasingly being used in the management of patients with prostate cancer. In addition to conventional anatomical MRI provided by T2-weighted (T2W)–MRI, mp-MRI uses additional parameters such as DW–MRI, DCE–MRI, and MRS, that significantly improve the ability of mp-MRI to predict behaviour of tumours. T2W–MRI provides superior soft-tissue resolution compared with other imaging techniques—such as CT—allowing detection and biopsy guidance as well as staging of prostate cancer.11,12 Mp-MRI has also been used to detect residual or recurrent disease after local treatment, such as surgery or radiation.13 A consensus publication by Dickinson et al.14 suggested that the MRI data should include T1-weighted (T1W)–MRI, T2W–MRI, DW–MRI, and DCE–MRI at a minimum, with optimal data sets also including MRS. In the following sections, these four components of mp-MRI will be discussed in detail.
Anatomical MRI: T1W and T2W
Anatomical MRI of the prostate consists of T1W and T2W–MRI sequences. The guidelines composed by the European Society of Urogenital Radiology (ESUR) state that T2 sequences should include the prostate, seminal vesicles, and external sphincter with ≤3 mm section thickness and in-plane resolution of 0.7 mm or better.15 T1W images are used mainly for the identification of post-biopsy haemorrhage, as haemorrhage can interfere with the diagnostic value of mp-MRI. On T1W images, biopsy-related haemorrhage demonstrates high signal intensity relative to normal prostate tissue.16 Haemorrhage may result in artefacts making the interpretation of the MRI challenging and ultimately leading to inaccurate results on T2W–MRI and dynamic DCE–MRI. Therefore, haemorrhage identification by T1W images is critical to confirm the accuracy of image interpretation. In one study the accuracy of T2W–MRI decreased from 83% if performed less than 3 weeks after biopsy to 46% if performed more than 3 weeks after biopsy, which allows time for haemorrhage to resolve.17 T2W images of capsular irregularity, thickening, and retraction due to injury after biopsy can be similar to extracapsular extension, which will lead to over-staging.18 For these reasons, a delay of at least 6–10 weeks after biopsy is recommended before obtaining MRI of the prostate to allow residual h aemorrhage to resolve before imaging studies.18–20
T2W–MRI allows anatomical visualization of the transitional and peripheral zones, where 30% and 70% of tumours are located, respectively.21 On T2W images, the normal peripheral zone demonstrates uniform high signal intensity due to the water content of glandular structures in the peripheral zone. Tumours are typically low in signal intensity compared with the glandular peripheral zone (Figure 1). Low signal intensity (hypo-intensity) in the peripheral zone, however, is not a specific finding for prostate cancer in itself and its differential diagnosis includes haemorrhage, prostatitis, benign prostatic hyperplasia (BPH).22 Although tumour detection is more difficult in the transitional zone of the prostate—due to the coexistence of BPH and the heterogeneous nodularity—tumours in this area can be discerned as lenticular or irregular regions of homogenous hypo-intensity with ill-defined margins in comparison to well circumscribed nodules with distinct rims found in BPH.23
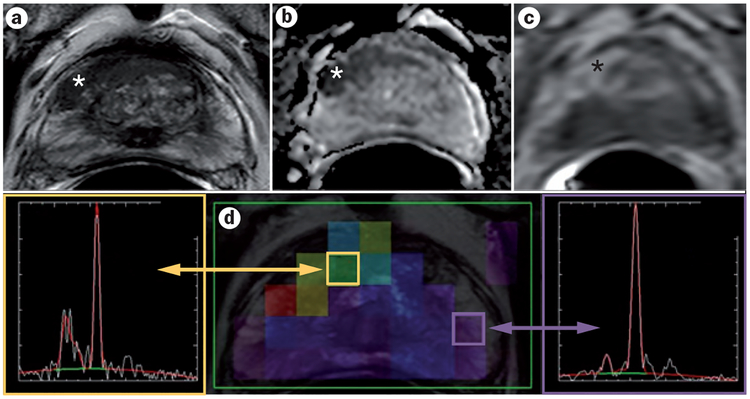
Figure 1 |.
A 61-year-old man with serum PSA of 23.85 ng/ml. a | Axial T2W–MRI, b | apparent diffusion coefficient map of diffusion-weighted MRI, and c | raw DCE–MRI demonstrate a 1 cm right apical mid-peripheral zone lesion (asterisk). d | Magnetic resonance spectroscopy shows an elevated choline-to-citrate ratio in the right mid-base anterior transitional zone compared with the normal left peripheral zone. Subsequent TRUS–MRI fusion-guided biopsy revealed a Gleason 4 + 4 tumour within that lesion. Abbreviations: DCE–MRI, dynamic contrast-enhanced MRI; PSA, prostate-specific antigen; T2W–MRI, T2-weighted MRI; TRUS, transrectal ultrasound.
T2W–MRI is the most useful MRI sequence in determining whether tumours are confined to the prostate or extending beyond the capsule. The presence of extra-capsular extension (ECE) has implications for risk stratification of patients with prostate cancer because ECE automatically increases the radiological stage to T3a and could change the treatment plan and prognosis.24 On T2W–MRI, ECE appears as a direct extension of the tumour into the surrounding periprostatic fat, or it can be identified as an asymmetric tumour capsular bulge, capsular abutment, obliteration of the rectoprostatic angle, broad capsular base or asymmetric neurovascular bundles.15,25–28 Seminal vesicle invasion (SVI) upstages the patient to clinical stage T3b, and is associated with an increased risk of lymph-node metastasis.29 On T2W–MRI, SVI appears as focal low signal intensity lesions within the seminal vesicles (Figure 2).30
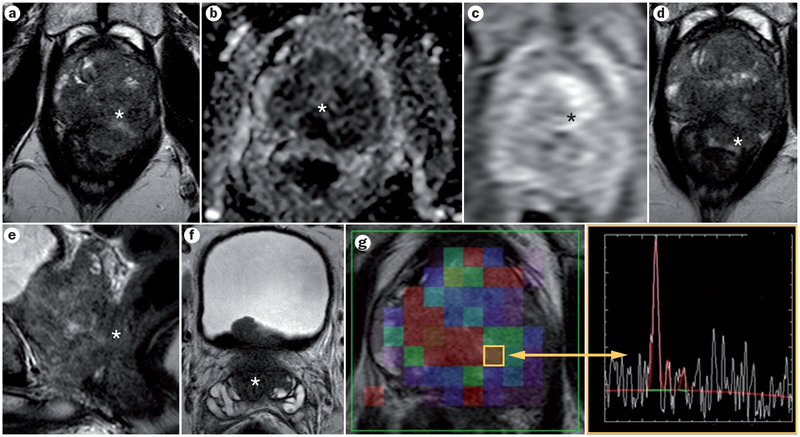
Figure 2 |.
A 59-year-old man with serum PSA of 24.7 ng/ml. a | Axial T2W–MRI, b | apparent diffusion coefficient map of DW–MRI, and c | raw DCE–MRI demonstrate a large 5 cm lesion, which affects almost the entire prostate (asterisk). d,e | The lesion has extracapsular extension and invades the rectum (asterisk). f | Seminal vesicles are invaded bilaterally (asterisk). g | Magnetic resonance spectroscopy shows an elevated choline-to-citrate ratio (asterisk). Subsequent TRUS/MRI fusion-guided biopsy revealed a Gleason 5 + 5 tumour within the prostate. Abbreviations: DCE–MRI, dynamic contrast-enhanced MRI; DW–MRI, diffusion-weighted MRI; PSA, prostate-specific antigen; T2W–MRI, T2-weighted MRI; TRUS, transrectal ultrasound.
When used alone, the accuracy of T2W–MRI for the detection of prostate cancer imaging is highly variable, with reported sensitivities ranging from 51% to 91%, and specificities of 27–91%.31–35 The sensitivity ranges for local prostate cancer staging and detecting ECE are 14.4–100%, and the specificity ranges from 67% to 100%.27,36–47 These results are highly dependent on field strength, technique, verification standards, and patient selection. When using MRI with strength of 3 Teslas (3T), lesions as small as 3 mm in diameter can generally be detected with high sensitivity. Sensitivities do not reach 100% due to the microscopic nature of some tumours that are not visible on MRI, whereas specificities do not reach 100% because other conditions—such as BPH, post-biopsy haemorrhage, and prostatitis—also display low signal intensity on T2W–MRI, similar to prostate tumours.39 Therefore, although anatomic MRI is a useful modality in prostate cancer imaging, it achieves optimal accuracy when combined with other functional sequences in mp-MRI.
Diffusion-weighted MRI
DW–MRI quantifies the Brownian motion of free water protons within a tissue by applying a series of magnetic gradients, known as ‘b’ values.48 For prostate cancer, b values between 0 and 800 sec/mm2 are commonly used during DW–MRI scans. Because molecular diffusion is inversely proportional to tissue cellularity and cell membrane integrity, water diffusion measurements provide key information about tissue architecture in benign and malignant tissues.49 Normal, well-structured glandular prostate tissue allows free diffusion of water molecules and, therefore, displays a low intensity on high b-value DW–MRI.50 Malignant tissue has abundant stroma with diminished extracellular space and is more densely packed than normal tissue, resulting in restricted free water motion within the tumour, therefore, reducing the diffusion of water and resulting in high intensity foci on high b-value DW–MRI.50 Often, DW–MRI is displayed as the apparent diffusion coefficient (ADC) map. ADC represents a quantitative assessment of water diffusion; lower values are associated with a higher rate of malignancy. Prostate cancer, therefore, is identified as a low signal region on ADC maps against a background of normal tissue with higher signal intensity (Figure 1).51 DW–MRI is a rapidly evolving technique and recent studies have demonstrated that ‘ultrahigh’ b values of 1,000 to 2,000 sec/mm2 further increase the specificity of prostate cancer detection.52–55 Particularly within the transitional zone, ultrahigh b values help differentiate benign conditions such as BPH from cancer.56
DW–MRI is an important component of prostate mp-MRI protocols and improves diagnostic accuracy, planning of targeted biopsies, and staging.34,57–64 A meta-analysis of ten studies that included data from 586 patients with suspected prostate cancer, showed a DW–MRI sensitivity of 76% and a specificity of 86%.65 Notably, in six studies that analysed data from 260 men with pathologically confirmed prostate cancer, the sensitivity of DW–MRI was 88% with a specificity of 84%, and this specificity was higher in patients with both suspected and confirmed cancer, suggesting that DW–MRI is useful to confirm prostate cancer for high-risk patients.65
DW–MRI is considered a quantitative technique because ADC values can be calculated from conventional DW–MRI. The addition of DW–MRI to T2W–MRI significantly improves the accuracy of prostate tumour volume measurements when compared with T2W–MRI alone, and it has been suggested that DW–MRI in combination with other components of mp-MRI—such as T2W–MRI and DCE–MRI—most accurately predicts prostate tumour volume in lesions larger than 0.5 cm.66 In addition to diagnostic utility, ADC values may be useful in predicting tumour aggressiveness as ADC values have been correlated with Gleason scores.67–69 Jung et al.68 demonstrated that use of DW–MRI, in combination with T2W–MRI, improved prostate cancer detection in the transitional zone, and also confirmed that tumour ADC values inversely correlated with Gleason scores in 156 patients scanned at 1.5T. A recent study of 20 patients by Turkbey et al.70 showed that T2W–MRI and DW–MRI obtained at 3T with the use of combined endorectal coil and 16-channel surface coil had a higher sensitivity of 76% for detecting prostate lesions than without an endorectal coil, which had a sensitivity of 45%; however, it is unclear whether this has an impact on patient outcomes. There are some limitations to the use of DW–MRI. Because it is highly sensitive to increased motion artefacts, DW–MRI is not always diagnostic. Moreover, DW–MRI is less useful in staging because of the lower resolution and greater image distortion than traditional T2W–MRI, making accurate assessment of ECE difficult with this image sequence. However, the role of DW–MRI in this regard is evolving, and with the use of high b values in conjunction with 3T MRI it is possible to achieve better signal-to-noise ratios sufficient to visualize the prostate capsule. Soylu et al.71 examined 131 men aged 43–75 with a known diagnosis of prostate cancer and found that the use of DW–MRI in conjunction with T2W–MRI improved the specificity from 93.1% to 96.6% and the positive predictive value from 93.6% to 98.3% from both experienced and relatively inexperienced radiologists. The noninvasive use of DW–MRI to predict tumour behaviour and its spread is highly promising and needs further investigation.
Magnetic resonance spectroscopic imaging
Proton magnetic resonance spectroscopic imaging (MRSI) has been used for several decades and represents a functional method that assesses the biochemical characteristics of prostate lesions, specifically the intracellular concentrations of choline and citrate. Normally, the prostate is a highly efficient producer of citrate whereas its production of choline is much less robust. Owing to the increased cell membrane turnover of rapidly dividing cancer cells, tumours display increased choline levels and higher choline-to-citrate ratios, whereas benign tissue normally expresses high citrate and low choline levels, resulting in a low choline-to-citrate ratio.72 MRSI takes advantage of the different intracellular metabolic profiles of cancer cells versus normal cells; the areas under metabolite peaks represent metabolite concentrations that can quickly be analysed by comparing the height of the choline peak to the height of the citrate peak; the more negative the slope of the line connecting the choline peak to the citrate peak, the more suspicious the lesion is for cancer (Figure 1). Unfortunately, benign prostate hyperplasia can also demonstrate an increased choline-to-citrate ratio. However, because MRSI is carried out after T2W anatomic MR imaging, overlaying the spectral data with anatomic images can help differentiate tumours from normal tissue.
One useful application of MRSI is in the detection of cancer in patients with rising PSA levels and prior negative biopsy. Ganie et al.73 performed MRSI in 87 patients with PSA >5 ng/ml and prior transrectal ultrasound (TRUS)-guided biopsy, and found that the addition of MRSI to conventional endorectal coil MRI improved detection of cancerous lesions, with a sensitivity of 87.3% and a specificity of 81.3%. A study by Kobus et al.74 examined the use of MRSI alone versus MRSI plus DW–MRI at 3T in 54 patients with biopsy-proven cancer before undergoing prostatectomy. They found a significant correla tion between aggressiveness and choline-to-citrate ratio in both the peripheral zone and transitional zone, however, further validation is needed.71 In addition, other metabolic resonances are of interest, particularly polyamines such as spermine. Similar to citrate, polyamine levels are drastically decreased in prostate cancer cells.74 Jung et al.75 developed a 5-point standardized scoring system for the analysis of spectral data, in which a score of 1 is probably benign and a score of 5 is probably malignant.75 This scoring system demonstrates an accuracy of 74.2–85.0% and excellent interobserver agreement.75
Limitations of MRSI include the longer acquisition times and the increased technical skill required compared with other mp-MRI techniques. Proper magnetic resonance shimming (that is, a device used to adjust the homogeneity of a magnetic field) is critical, and experienced readers are necessary to correctly interpret partial volume effects. Furthermore, MRSI demands increased time for post-processing the data, which requires trained employees and decreases clinical output. For these reasons, most users have decided that MRSI does not have a favourable cost-benefit ratio and other mp-MRI sequences such as T2W–MRI, DW–MRI, and DCE–MRI are more commonly used instead. Finally, because MRSI is not actually an anatomic pulse sequence, it has a limited role in determining anatomic location of a tumour or extension beyond the prostate.
Dynamic contrast-enhanced MRI
DCE–MRI evaluates the vascularity of tumours through the use of fast T1W–MRI scanning sequences before, during, and after the rapid administration of gadolinium-based MRI contrast agents, such as gadolinium chelates.76 DCE–MRI provides an assessment of perfusion and vascular permeability throughout the prostate and specifically within a potential tumour.77 Owing to neoangiogenesis, tumours have more permeable, heterogeneous, and disorganized vessels than normal tissue, causing prostate cancers to typically exhibit early and rapid enhancement as well as early washout, which are associated with tumour aggressiveness.78
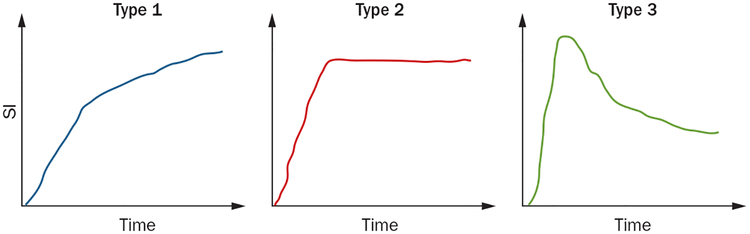
The ESUR guidelines recommend a bolus injection at 3 ml/sec with a standard dose of contrast agent and suggest a minimum slice thickness of 4 mm of the MR image.15 The easiest and most common method of evaluating DCE–MRI images is to qualitatively detect focal early enhancement with early washout as compared to normal prostate tissue (Figure 1). After a rapid rise in signal following contrast media injection, the lesion can have one of three enhancement patterns: progressively enhancing (type 1), plateauing (type 2), and washing out (type 3) (Figure 3). Type 1 curves are characteristic of benign tissue, although low-grade malignancies can also enhance in this manner. Type 2 curves demonstrate an increase in contrast followed by a plateau, and are suspicious for cancer—but not definitive—as many benign lesions exhibit this type of enhancement. Type 3 curves demonstrate rapid early enhancement as well as fast washout and are characteristic of tumours. Of the curve types, type 3 curves are most commonly associated with tumours, although heterogeneous mixtures of all three curves are often found within a single cancerous lesion (Figure 3).79 The gadolinium concentration over time can also be used to measure the area under the plasma concentration time curve (AUC), the time to peak enhancement, and the initial slope. Another method of analysing DCE–MRI is to use pharmacokinetic parameters Ktrans (transfer of gadolinium contrast from the vasculature to the tumour, representing forward vascular perfusion and permeability) and Kep (reverse transfer of contrast agent from the extra cellular space back to the plasma, representing backward leakage) to quantify tumour enhancement.80,81 DCE–MRI has higher sensitivity than T2W–MRI alone, with an overall sensitivity of 73% and specificity of 81%,82,83 and its diagnostic capabilities are even more improved in lesions of >0.5 cm, with a sensitivity of 90% and a specificity of 88%.82,84 This high sensitivity of DCE–MRI makes it a useful functional imaging modality in the detection of tumours as well as the assessment of treatment response.
Figure 3 |.
Curve types representing enhancement patterns on DCE–MRI. Type 1 represents progressive enhancement, type 2 rapid enhancement with plateauing, and type 3 represents rapid enhancement followed by a rapid wash out of the contrast material. Abbreviations: DCE–MRI, dynamic contrast-enhanced MRI; SI, signal intensity.
A recent study of 45 patients with prostate cancer examined the ability of DCE–MRI at 3T to predict prostate cancer aggressiveness when compared with the gold-standard prostatectomy pathology results.85 They found that both quantitative (Ktrans and Kep) and semi-quantitative (wash-in and wash-out) DCE parameters were helpful in assessing prostate cancer aggressiveness in the peripheral zone.85 Another study by Li et al.86 found that the combination of diffusion tensor imaging with DCE–MRI improved the diagnostic performance of detecting prostate cancer in the peripheral zone compared with either technique alone.
Limitations in the interpretation of DCE–MRI data include overlap in enhancement properties between benign and malignant regions in the transitional zone, as benign prostatic hyperplasia and other benign inflammatory conditions within the transitional zone also exhibit substantial hypervascularity.87,88 DCE–MRI is typically of lower spatial resolution than other sequences, especially when DCE–MRI is performed rapidly in a short period of time and for pharmacokinetic analysis. In such cases, DCE–MRI should be combined with T2W–MRI to provide anatomic information for local staging of a tumour.
Detection of prostate cancer
The traditional method of prostate cancer detection includes serum PSA measurements, routine digital rectal examinations, and a systematic TRUS-guided biopsy, which provides 12 random cores of tissue from the posterior part of the gland. However, the 12–14 core TRUS-guided biopsy has only a cancer detection rate of 27–44% and leads to overdiagnosis of clinically insignificant tumours while missing or under sampling clinically relevant tumours, particularly in the anterior area of the prostate gland.89–91 The anterior prostate gland accounts for up to 20% of the largest tumours in patients with suspected prostate cancer, and is a difficult area to biopsy using the traditional 12-core random TRUS-guided technique currently used by most urologists because the needle has to travel further to the anterior part of the gland.92 Adding mp-MRI to this traditional biopsy strategy or using mp-MRI as a substitute for a biopsy in selected patients with low-risk lesions detected on mp-MRI can improve prostate cancer detection (Figure 4).57,93–95 Performing mp-MRI before biopsy is based on the principle of guiding biopsies according to lesions identified on imaging, rather than systematically sampling the posterior region of the prostate. TRUS–MRI fusion biopsy can be used to direct biopsy needles into abnormal regions of the prostate, similar to virtually all other cancer biopsies.93 Targeting biopsies to a suspicious lesion identified by mp-MRI may detect high-grade tumours in an equivalent or higher percentage of patients than random biopsies while using fewer biopsy cores with fewer complications and lower diagnosis rates of insignificant tumours.96 A study of 555 patients with suspicion of prostate cancer demonstrated that targeted biopsies as determined by pre-biopsy mp-MRI had increased detection accuracy of significant prostate cancer than extended systematic biopsies.97 In another study, 1,448 patients suspected of having prostate cancer underwent targeted and systematic biopsies; the cancer detection rate was higher in the targeted biopsy group with a positive predictive value for mp-MRI of 70.1–90.1%.98 A study of 582 patients by Siddiqui et al.99 showed that targeted biopsy detected 67% more Gleason ≥4 + 3 tumours than 12-core biopsy alone and missed 36% of Gleason ≤3 + 4 tumours, thus minimizing the detection of lower grade disease. It should be noted that the economic implications of performing a pre-biopsy mp-MRI are not trivial, with an estimated $2,000–3,000 charge in the USA, although real costs are considerably lower.100
Figure 4 |.
Flowchart showing the utility of mp-MRI in various clinical scenarios of prostate cancer. MRI can be used as a cancer staging tool after a positive biopsy before definitive treatment, to identify target lesions before a targeted biopsy, as a way to monitor active surveillance patients and as a guide for patients with prior negative biopsies but rising serum PSA levels. MRI also has a role in the follow up of patients with a PSA recurrence after treatment. Abbreviations: DRE, digital-rectal examination; mp-MRI, multiparametric MRI; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy; TRUS, transrectal ultrasound.
Prostate cancer staging
Mp-MRI can assess the risk of prostate cancer based on the appearance of the lesions. Mp-MRI is recommended before a patient is considered for active surveillance, as it allows detection of poor prognostic features—such as a large tumour volume or high-grade tumours, particularly in the anterior prostate—that would be unsuitable for active surveillance. In an effort to standardize the level of detection of suspicious lesions on mp-MRI, Dickinson et al.101 reported a 5-point scale for scoring mp-MRI sequences, with a score of 1 indicating a low risk of clinically significant disease (Gleason ≥4 + 3 and/or lesions ≥0.5 cm), and a score of 5 indicating that clinically significant disease is highly likely to be present. In a study of 800 patients who underwent 3T mp-MRI of the prostate, patients with low suspicion lesions according to mp-MRI were more likely to have negative biopsies or low-grade tumours. These results suggest that the risk of clinically significant disease in patients with low-risk lesions on a pre-biopsy mp-MRI is sufficiently small to justify deferring biopsy or pursuing active surveillance if a cancer is identified after biopsy. In patients with intermediate-risk or high-risk disease, the follow-up after focal therapy should include a mp-MRI after 6 months and, again, on a yearly basis thereafter.102
Treatment planning
In patients with a new diagnosis of prostate cancer, the role of mp-MRI is to help to determine the best treatment option for the patient. For patients with low-risk disease (PSA<10 ng/ml with biopsy Gleason score ≤6 and clinical stage T1–T2a), treatment is with curative intent by radical prostatectomy, radiation therapy, or active surveillance.15 In these patients, mp-MRI can be used to confirm eligibility for active surveillance when significant disease is absent, and to help with surgical and radiation treatment planning.15 In patients with intermediate-risk disease (PSA 10–20 ng/ml, biopsy Gleason score 7, or clinical stage T2b or T2c), mp-MRI is most helpful in staging disease to detect ECE.15 In patients with high-risk disease (PSA >20 ng/ml, biopsy Gleason score 8–10, or clinical stage >T2c), mp-MRI can be used to detect skeletal or nodal metastases, although lymph-node staging can be unreliable because 70% of metastatic lymph nodes in men with prostate cancer are <8 mm and, therefore, difficult to detect with mp-MRI.15,103 Finally, in patients with a PSA recurrence after focal therapy mp-MRI is a useful tool to evaluate the prostatic fossa as other techniques such as PET imaging or a TRUS-guided biopsy have a low sensitivity for recurrent disease.
Conclusions
Because prostate cancer often grows slowly with a long latency time before becoming clinically significant, there is a great need to accurately assess the size and invasiveness of detected cancers. The use of imaging to noninvasively identify prostate cancer and evaluate the extent of disease burden enables optimal treatment planning. Mp-MRI can help to differentiate clinically significant tumours from benign lesions by combining anatomical and functional imaging techniques. Mp-MRI is also clinically useful in prostate cancer staging by detecting ECE and seminal vesicle invasion. Mp-MRI is currently most widely used for tumour localization and cancer staging in patients with prostate cancer, however, it is increasingly being used in patients undergoing active surveillance as a monitoring tool as well as to monitor recurrence in patients after definitive therapy. Future development of novel imaging techniques that provide biological information about tumour behaviour may further guide diagnosis and treatment options.
Key points.
T2-weighed MRI allows anatomical visualization of both the transitional and peripheral zones of the prostate, where 30% and 70% of tumours are located, respectively
A delay of at least 6–10 weeks after a biopsy procedure is recommended before obtaining MRI of the prostate to allow residual haemorrhage to resolve
The addition of diffusion-weighted (DW) MRI significantly improves the accuracy of prostate tumour volume measurements when compared with T2-weighted MRI alone
There is a significant negative correlation between tumour apparent diffusion coefficient (ADC) values and Gleason scores, suggesting that ADC values are useful in predicting the aggressiveness of tumours
Dynamic contrast-enhanced MRI provides an assessment of perfusion and vascular permeability of the tumour; semiquantitative parameters in this approach (peak enhancement and washout gradient) are associated with tumour aggressiveness
MR spectroscopy compares the metabolic profiles of cancer cells with those of normal cells; increased levels of choline and high choline:citrate ratios can identify different types and grades of tumours
Review criteria.
We searched for original articles focusing on prostate cancer in PubMed published between 1990 and 2014. The search terms we used were “multiparametric MRI” and “prostate cancer”. All papers identified were English-language full-text papers. We also searched the reference lists of identified articles for further papers.
Acknowledgements
The authors would like to thank the NIH intramural funding programme for financial support.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Siegel R, Naishadham D & Jemal A Cancer statistics, 2013. CA Cancer J. Clin 63, 11–30 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Jemal A et al. Global cancer statistics. CA Cancer J. Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy M, Konety B & Warlick C Prostate cancer screening: issues and controversies. Minn. Med 93, 39–44 (2010). [PubMed] [Google Scholar]
- 4.Weissbach L & Altwein J Active surveillance or active treatment in localized prostate cancer? Dtsch Arztebl. Int 106, 371–376 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell IJ, Bock CH, Ruterbusch JJ & Sakr W Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol 183, 1792–1796 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamatiou K, Alevizos A, Agapitos E & Sofras F Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcinomatous lesions in Greek male population: an autopsy study. Prostate 66, 1319–1328 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Welch HG & Black WC Overdiagnosis in cancer. J. Natl Cancer Inst 102, 605–613 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Schmid HP, McNeal JE & Stamey TA Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer 71, 2031–2040 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Stamey TA et al. Localized prostate cancer. Relationship of tumour volume to clinical significance for treatment of prostate cancer. Cancer 71, 933–938 (1993). [DOI] [PubMed] [Google Scholar]
- 10.Mullerad M et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J. Urol 174, 2158–2163 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Rastinehad AR et al. Improving detection of clinically significant prostate cancer: MRI/TRUS fusion-guided prostate biopsy. J. Urol 10.1016/j.juro.2013.12.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habchi H et al. Value of prostate multiparametric magnetic resonance imaging for predicting biopsy results in first or repeat biopsy. Clin. Radiol 69, e120–e128 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Schiavina R et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Curr. Radiopharm 6, 92–95 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Dickinson L et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur. Urol 59, 477–494 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Barentsz JO et al. ESUR prostate MR guidelines 2012. Eur. Radiol 22, 746–757 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenkrantz AB et al. Impact of delay after biopsy and post-biopsy haemorrhage on prostate cancer tumour detection using multi-parametric MRI: a multi-reader study. Clin. Radiol 67, e83–e90 (2012). [DOI] [PubMed] [Google Scholar]
- 17.White S et al. Prostate cancer: effect of postbiopsy haemorrhage on interpretation of MR images. Radiology 195, 385–390 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Thompson J, Lawrentschuk N, Frydenberg M, Thompson L & Stricker P The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. BJU Int. 112 (Suppl. 2), 6–20 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Ikonen S et al. Optimal timing of post-biopsy MR imaging of the prostate. Acta Radiol. 42, 70–73 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Kirkham AP et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin. Radiol 68, 1016–1023 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Kundra V, Silverman PM, Matin SF & Choi H Imaging in oncology from the University of Texas M. D. Anderson Cancer Centre: diagnosis, staging, and surveillance of prostate cancer. AJR Am. J. Roentgenol 189, 830–844 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Verma S & Rajesh A A clinically relevant approach to imaging prostate cancer: review. AJR Am. J. Roentgenol 196 (Suppl. 3), S1–S10 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Akin O et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology 239, 784–792 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Montironi R, Bostwick DG, Lopez-Beltran A & Berney DM Staging of prostate cancer. Histopathology 60, 87–117 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Roethke MC et al. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J. Urol 31, 1111–1116 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Zhang J et al. Clinical stage T1c prostate cancer: evaluation with endorectal MR imaging and MR spectroscopic imaging. Radiology 253, 425–434 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloch BN et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging--initial results. Radiology 245, 176–185 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Lee HW, Seo SI, Jeon SS, Lee HM & Choi HY Can we predict real T3 stage prostate cancer in patients with clinical T3 (cT3) disease before radical prostatectomy? Yonsei Med. J 51, 700–707 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masterson TA, Pettus JA, Middleton RG & Stephenson RA Isolated seminal vesicle invasion imparts better outcomes after radical retropubic prostatectomy for clinically localized prostate cancer: prognostic stratification of pt3b disease by nodal and margin status. Urology 66, 152–155 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Chandra RV et al. Endorectal magnetic resonance imaging staging of prostate cancer. ANZ J. Surg 77, 860–865 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Hricak H et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal--pelvic phased-array coils. Radiology 193, 703–709 (1994). [DOI] [PubMed] [Google Scholar]
- 32.Casciani E et al. Contribution of the MR spectroscopic imaging in the diagnosis of prostate cancer in the peripheral zone. Abdom. Imaging 32, 796–802 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Costouros NG et al. Diagnosis of prostate cancer in patients with an elevated prostate-specific antigen level: role of endorectal MRI and MR spectroscopic imaging. AJR Am. J. Roentgenol 188, 812–816 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Haider MA et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am. J. Roentgenol 189, 323–328 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Tamada T et al. Prostate cancer: relationships between postbiopsy haemorrhage and tumour detectability at MR diagnosis. Radiology 248, 531–539 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Scheidler J et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging--clinicopathologic study. Radiology 213, 473–480 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Ekici S et al. A comparison of transrectal ultrasonography and endorectal magnetic resonance imaging in the local staging of prostatic carcinoma. BJU Int. 83, 796–800 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Wefer AE et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J. Urol 164, 400–404 (2000). [PubMed] [Google Scholar]
- 39.Ikonen S et al. Prostatic MR imaging. Accuracy in differentiating cancer from other prostatic disorders. Acta Radiol. 42, 348–354 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Akin O et al. Local staging of prostate cancer with endorectal surface coil MR imaging in a mid-field magnetic system. Clin. Imaging 27, 47–51 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Kwek JW et al. Phased-array magnetic resonance imaging of the prostate with correlation to radical prostatectomy specimens: local experience. Asian J. Surg 27, 219–224 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Nakashima J et al. Endorectal MRI for prediction of tumour site, tumour size, and local extension of prostate cancer. Urology 64, 101–105 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi T et al. Prostate cancer: a comparative study of 11C-choline PET and MR imaging combined with proton MR spectroscopy. Eur. J. Nucl. Med. Mol. Imaging 32, 742–748 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Cirillo S et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur. Radiol 19, 761–769 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Sala E et al. Endorectal MR imaging before salvage prostatectomy: tumour localization and staging. Radiology 238, 176–183 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Tan JS et al. Local experience of endorectal magnetic resonance imaging of prostate with correlation to radical prostatectomy specimens. Ann. Acad. Med. Singapore 37, 40–43 (2008). [PubMed] [Google Scholar]
- 47.Futterer JJ et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging before radical prostatectomy: experienced versus less experienced readers. Radiology 237, 541–549 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Stejskal EO & Tanner JE Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys 42, 288–292 (1965). [Google Scholar]
- 49.Schmid-Tannwald C, Oto A, Reiser MF & Zech CJ Diffusion-weighted MRI of the abdomen: current value in clinical routine. J. Magn. Reson. Imaging 37, 35–47 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Issa B In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J. Magn. Reson. Imaging 16, 196–200 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Esen M, Onur MR, Akpolat N, Orhan I & Kocakoc E Utility of ADC measurement on diffusion-weighted MRI in differentiation of prostate cancer, normal prostate and prostatitis. Quant. Imaging Med. Surg 3, 210–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim CK, Park BK & Kim B High-b-value diffusion-weighted imaging at 3T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am. J. Roentgenol 194, W33–W37 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Rosenkrantz AB et al. Computed diffusion-weighted imaging of the prostate at 3T: impact on image quality and tumour detection. Eur. Radiol 23, 3170–3177 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Ueno Y et al. Computed diffusion-weighted imaging using 3-T magnetic resonance imaging for prostate cancer diagnosis. Eur. Radiol 23, 3509–3516 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Quentin M et al. Increased signal intensity of prostate lesions on high b-value diffusion-weighted images as a predictive sign of malignancy. Eur. Radiol 24, 209–213 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Katahira K et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur. Radiol 21, 188–196 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Delongchamps NB et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 107, 1411–1418 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Kim CK, Park BK, Lee HM & Kwon GY Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest. Radiol 42, 842–847 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Kitajima K et al. Prostate cancer detection with 3T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J. Magn. Reson. Imaging 31, 625–631 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Lim HK, Kim JK, Kim KA & Cho KS Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection—a multireader study. Radiology 250, 145–151 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Morgan VA, Kyriazi S, Ashley SE & DeSouza NM Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 48, 695–703 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Tanimoto A, Nakashima J, Kohno H, Shinmoto H & Kuribayashi S Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J. Magn. Reson. Imaging 25, 146–152 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Vargas HA et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumour detection and assessment of aggressiveness. Radiology 259, 775–784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimitsu K et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J. Magn. Reson. Imaging 27, 132–139 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Wu LM et al. Usefulness of diffusion-weighted magnetic resonance imaging in the diagnosis of prostate cancer. Acad. Radiol 19, 1215–1224 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Turkbey B et al. Correlation of magnetic resonance imaging tumour volume with histopathology. J. Urol 188, 1157–1163 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hambrock T et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259, 453–461 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Jung SI et al. Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumour detection and assessment of aggressiveness. Radiology 269, 493–503 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Turkbey B et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 258, 488–495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turkbey B et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J. Magn. Reson. Imaging 10.1002/jmri.24317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soylu FN et al. Seminal vesicle invasion in prostate cancer: evaluation by using multiparametric endorectal MR imaging. Radiology 267, 797–806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sciarra A et al. Modern role of magnetic resonance and spectroscopy in the imaging of prostate cancer. Urol. Oncol 29, 12–20 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Ganie FA et al. Endorectal coil MRI and MR-spectroscopic imaging in patients with elevated serum prostate specific antigen with negative trus transrectal ultrasound guided biopsy. Urol. Ann 5, 172–178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kobus T et al. Prostate cancer aggressiveness: in vivo assessment of MR spectroscopy and diffusion-weighted imaging at 3T. Radiology 265, 457–467 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Jung JA et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology 233, 701–708 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Feng Y, Jeong EK, Mohs AM, Emerson L & Lu ZR Characterization of tumour angiogenesis with dynamic contrast-enhanced MRI and biodegradable macromolecular contrast agents in mice. Magn. Reson. Med 60, 1347–1352 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verma S et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am. J. Roentgenol 198, 1277–1288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicholson B, Schaefer G & Theodourescu D Angiogenesis in prostate cancer: biology and therapeutic opportunities. Cancer Metastasis Rev. 20, 297–319 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Jackson AS et al. Dynamic contrast-enhanced MRI for prostate cancer localization. Br. J. Radiol 82, 148–156 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Tofts PS et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J. Magn. Reson. Imaging 10, 223–232 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Grant K et al. Functional and molecular imaging of localized and recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40 (Suppl. 1), S48–S59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Talab SS, Preston MA, Elmi A & Tabatabaei S Prostate cancer imaging: what the urologist wants to know. Radiol. Clin. North Am 50, 1015–1041 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Kim JK et al. Wash-in rate on the basis of dynamic contrast-enhanced MRI: usefulness for prostate cancer detection and localization. J. Magn. Reson. Imaging 22, 639–646 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Jager GJ et al. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology 203, 645–652 (1997). [DOI] [PubMed] [Google Scholar]
- 85.Vos EK et al. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3T. Eur. Urol 64, 448–455 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Li C et al. Detection of prostate cancer in peripheral zone: comparison of MR diffusion tensor imaging, quantitative dynamic contrast-enhanced MRI, and the two techniques combined at 3.0 T. Acta Radiol. 55, 239–247 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Deering RE, Bigler SA, Brown M & Brawer MK Microvascularity in benign prostatic hyperplasia. Prostate 26, 111–115 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Padhani AR et al. Dynamic contrast enhanced MRI of prostate cancer: correlation with morphology and tumour stage, histological grade and PSA. Clin. Radiol 55, 99–109 (2000). [DOI] [PubMed] [Google Scholar]
- 89.Babaian RJ et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J. Urol 163, 152–157 (2000). [PubMed] [Google Scholar]
- 90.Presti JC Jr, O’Dowd GJ, Miller MC, Mattu R & Veltri RW Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J. Urol 169, 125–129 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Eskew LA, Bare RL & McCullough DL Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J. Urol 157, 199–202; discussion 202–203 (1997). [PubMed] [Google Scholar]
- 92.Ouzzane A et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology 78, 1356–1362 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Lemaitre L et al. Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur. Radiol 19, 470–480 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Villers A et al. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumour volume: correlation with radical prostatectomy findings. J. Urol 176, 2432–2437 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Puech P et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology 74, 1094–1099 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Moore CM et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur. Urol 63, 125–140 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Haffner J et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 108, E171–E178 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Watanabe Y et al. Detection and localization of prostate cancer with the targeted biopsy strategy based on ADC map: a prospective large-scale cohort study. J. Magn. Reson. Imaging 35, 1414–1421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siddiqui MM et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur. Urol 64, 713–719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ouzzane A, Puech P & Villers A MRI and surveillance. Curr. Opin. Urol 22, 231–236 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Dickinson L et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur. Urol 59, 477–494 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Muller B et al. The role of multiparametric magnetic resonance imaging in focal therapy for prostate cancer: a delphi consensus project. BJU Int. 10.1111/bju.12548 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Hovels AM et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol 63, 387–395 (2008). [DOI] [PubMed] [Google Scholar]