Abstract
Substance use disorders (SUDs) are marked by heterogeneity in clinical symptomatology and high relapse rates following treatment. Here, we describe specific peripheral and central stress responses associated with the pathophysiology of SUDs. We outline potential stress response measures including HPA axis markers, autonomic responses and central structural and functional brain alterations that could be exploited as putative biomarkers in SUDs. We posit that stress responses can be predictive of both the development of SUDs and their high relapsing nature. We examine their potential as candidate biomarkers, as well as the remaining challenges in developing and implementing them as biomarkers for the prevention and treatment of SUDs.
Central and Peripheral Stress System Alterations as Potential Biomarkers in Substance Use Disorders
Substance Use Disorders (SUDs) present a major public health burden to patients and society in the US, costing more than $400 billion annually in crime, poor health outcomes, and lost productivity [1]. Patients with SUDs have significant heterogeneity in clinical symptomatology and vast variability in response to treatment. In SUDs, unlike other psychiatric conditions, certain diagnostic biomarkers exist which may allow for detection and/or quantification of drugs in the body; this has clear clinical benefit in determining whether a patient has recently used a drug or has been abstinent [2]. While these objectively assessed markers of recent drug use are critical for the assessment of substance use, abuse and also current diagnosis of SUDs, they are not as informative in identifying who is at risk for development of SUDs or those who may be susceptible to a severe clinical course and be at risk of treatment failure or relapse [2, 3]. Hence, there is an urgent need for identification of objectively measured biomarkers that identify the neurobiological and behavioral processes that lead to the development and the SUD disease symptomatology, as well as those that might predict clinical outcome [4]. In turn, identification of these biomarkers could lead to significant improvements in treatment approaches for substance abuse [3]. Here, we present an overview of current knowledge regarding stress-related peripheral and central nervous system responses in d individuals with SUDs, as well as those at risk for SUDs and also assess whether any of these measures may be of benefit in developing as SUD biomarkers. We conclude with a discussion on the potential utility as well as the accompanying challenges of putative biomarkers of stress-related altered responses.
In the following sections, we describe emerging new findings on the role of the peripheral and central brain response to stress in addiction risk and drug motivation, and also highlight novel research identifying select stress response markers that may serve as potential biomarkers in SUD risk and relapse.
Stress and Addiction Risk
Many of the major theories of addiction identify an important role of stress in addiction. These include psychological models that view drug use and abuse as a coping strategy to deal with stress, reduce tension, self-medicate, or decrease withdrawal-related distress, as well as neurobiological models that propose incentive sensitization and stress allostasis concepts to explain how neuroadaptations in stress, reinforcement learning and reward pathways may enhance the key features of addiction, such as, craving, compulsion as well as loss of control over drug intake [5]. Growing evidence points to the critical role that stress plays in increasing addiction vulnerability. For instance, population-based and clinical studies indicate a significant association between psychosocial adversity, traumatic exposure, negative affect and chronic distress and addiction vulnerability [6–10]. This previous evidence linking specific type of adverse life events, chronic stressors, traumatic events and negative affective and internal deprivation states that have been associated with risk of addiction are presented in Table 1. Findings showing the impact of chronic and repeated exposure to stress and adversity on peripheral and central stress responses that increase an individual’s vulnerability to drug use and abuse are specifically addressed below.
Table 1:
Types of Adverse Life Events, Trauma, Chronic Stressors and Individual-level Variables Predictive of Addiction Risk (updated and adapted from Sinha 2008)
| Adverse Life Events | Childhood and Life Trauma | Chronic Stressors | Stressful Internal States |
|---|---|---|---|
| - Loss of parent - Parental divorce and conflict - Isolation & abandonment - Single parent family structure - Forced to live apart from parents - Loss of child by death or removal - Unfaithfulness of significant other - Loss of home to natural disaster - Death of significant other/close family member |
- Physical neglect; - Physical abuse by parent/caretaker/family member/spouse/significant other; - Emotional abuse and neglect; - Sexual abuse; - Rape; - Victim of gun shooting or other violent acts; - Observing violent victimization; |
- Being overwhelmed; - Unable to manage life problems; - Difficulties with job, living situation; - Financial problems; - Interpersonal conflicts, loneliness; - Unfulfilled desires; - Problems with children; - Illness of loved ones; - Negative emotionality; - Poor behavioral control; - Poor emotional control; |
- Hunger or food deprivation; - Food insecurity; - Extreme thirst; - Sleep deprivation, insomina; - Extreme hypo- or hyper-thermia; - Excessive drug use; - Drug withdrawal states; - Chronic illness |
Autonomic Nervous System (ANS) and Hypothalamic-Pituitary-Adrenal (HPA) axis Responses to Stress
The stress response is triggered by a challenging, threatening, overwhelming or aversive stimuli and is characterized by neural, physiological and hormonal changes that allow the organism to cope with the stressor (“fight or flight”), then return to baseline and maintain homeostasis. This adaptive process is called allostasis [11]. One aspect of the physiological response to stress is mediated by the HPA axis, which consists of the paraventricular nucleus of the hypothalamus, the pituitary gland, and the adrenal gland. Neurons in the paraventricular nucleus synthesize and release corticotropin-releasing factor (CRF) into the portal blood vessels that enter the anterior pituitary gland. Here the CRF binds to the CRF1 receptors on pituitary corticotropes, which in turn induce the release of adrenocorticotropic hormone (ACTH) into circulation. ACTH then stimulates the adrenal glands to synthesize and secrete glucocorticoids (corticosterone in rats, cortisol in humans), which mobilize and regulate the body’s stress response (for a review, see [12]). The second pathway involved in the biological response to stress is created by the autonomic nervous system, namely the sympathetic and the parasympathetic components. The sympathetic component mobilizes arousal by increasing heart rate and blood pressure; the parasympathetic component enforces the “brakes” for sympathetic arousal and functions to decrease and regulate the autonomic function [7]. The autonomic pathways also regulate and influence cortisol secretion directly via the splanchnic nerve innervation of the adrenal glands as well as in interaction with the central component of glucocorticoid activation [3, 13].
Importantly, for the current topic, growing evidence indicates that use and abuse of psychoactive substances actively involve these stress arousal pathways, stimulating and activating the HPA and autonomic axis in the case of psychostimulants (cocaine, nicotine, amphetamine), alcohol, cannabis and also with certain types of opiates[7]. There may be also significant variation in these responses as assessed by concentrations in plasma/serum for ACTH, plasma/serum and saliva for cortisol, salivary alpha amylase(measure of autonomic adrenergic arousal), and physiological assessments of heart rate, heart rate variability, as a function of extent of chronic stress or trauma exposure [7, 14, 15]. These responses to stress and to drugs have been reported to be modulated by genes encoding HPA axis stress response markers as well as the epigenetic changes in glucocorticoid signaling genes that might result from chronic exposure to stress and adversity [16]. While a number of studies have linked greater stress reactivity in plasma/salivary cortisol responses as a risk factor for co-morbidity of mood disorders and addiction [17–19], research has also shown that blunted salivary cortisol responses to stress in at-risk children with a family history of substance abuse also represented an addiction risk factor [20, 21]. Specifically, one study evaluated at-risk pre-pubertal (ages 10–12) boys with substance-abusing fathers, and found that high-risk boys secreted significantly less salivary cortisol in response to an anticipated stressor compared to controls [20]. In a recent study, our laboratory group demonstrated that 14–17 year old adolescents prenatally exposed to cocaine exhibited elevated basal salivary concentrations of cortisol relative to non-exposed youths; in contrast, they exhibited a blunted salivary cortisol response to a social stressor compared to controls [22]. Furthermore, this study showed that sex differences were associated with predicting future substance use, where girls’ self-reported sadness in response to the stressor predicted future drug use whereas in boys blunted salivary alpha-amylase (an ANS measure) response to the same social stressor predicted future drug use [22, 23], suggesting distinct physiological and emotional stress response risk profiles for boys and girls.
In another series of studies, impaired neuroendocrine responses to alcohol have also been associated with an increased motivation for binge/heavy alcohol intake, thereby serving as a potential risk marker for the progression from heavy drinking to alcohol dependence [24]. Specifically, in a longitudinal study of heavy drinkers and light drinkers that were exposed to an oral alcohol challenge and followed for six years, heavy drinkers showed greater sensitivity to stimulating effects and lower sensitivity to the sedative effects of alcohol compared to light drinkers; moreover, heavy drinkers demonstrated lower salivary cortisol release in response to the alcohol challenge, and presented with a greater number of AUD symptoms six years later relative to light drinkers [24, 25].
The possible mechanisms underlying the increased risk for problematic substance use documented in children of parents with SUDs, have also been examined by measuring HR response to psychosocial stress in participants (ages 11–20) who were children on parents with SUD and those who were not [26]. Findings showed that at-risk children had a blunted HR recovery to the stress exposure, suggesting that children who are at high risk for developing an SUD are marked by dysregulated autonomic nervous system (ANS) responding [26]. In another study, the same group examined the relationship of salivary cortisol levels in response to a social stress task and age of onset of alcohol use in adolescents aged 14–20 years [27]. The findings showed that those who began drinking at an earlier age showed lower salivary cortisol levels at the onset of and during the stressful task [27], suggesting that blunted activity of the HPA axis at the in response to stress is present in adolescents who begin drinking at an early age.
In non-human primates chronic, moderate alcohol exposure in cynomolgus monkeys led to decreased heart rate variability; however, when the animals were exposed to an acute stressor of removal from home cage to a novel environment, those with a history of alcohol exposure presented higher heart rates than controls [28].
We do not present a comprehensive review above, but rather a select set of studies with longitudinal follow-up and predictive validity to illustrate that moderate levels of drug use exposure, as well as certain types of chronic stress or prenatal drug/stress exposure can impair physiological HPA axis and emotional stress responses in ways that influence future risk of SUDs. Such adaptations or changes related to drug exposure or chronic stress exposure suggest altered central regulation of peripheral stress pathways. For example, one such pathway by which altered central regulation may occur is via altered GABA receptor functioning and presumably, other mechanisms known to regulate HPA axis and autonomic responses, possibly increasing addiction risk [29–31]. We posit that these peripheral alterations reflect alterations in central stress regulators and may serve as “readouts” of central function and may be developed further as risk biomarkers for the development of SUDs. However, it is clear that future research is needed to robustly assess the sensitivity and specificity of the stress response markers identified in the above sections in separate at-risk groups in their ability to accurately predict drug use initiation, and progression to SUDs. On the basis of the select review above, Table 2 (Key Table) includes specific central and peripheral stress response measures that may be explored as risk biomarkers for further development.
Table 2. Stress Response Disruptions Implicated in SUD Risk and in High Relapse Risk and Treatment Failure:
These measures may serve as potential biomarkers in acute, binge, and chronic/relapse use phases of SUDs and potential neurobiological targets for treatment development.
| Acute | Binge | Chronic/Relapse | Treatment Targets |
|---|---|---|---|
|
Increase in Cortisol Response Increase in ACTH Response |
Decreases in Cortisol Decreases in ACTH |
High Basal Cortisol Blunted Phasic Cortisol High Basal ACTH Blunted Phasic ACTH High Cortisol/ACTH Ratio as Relapse Predictor |
Pexacerfont Mifepristone Naltrexone Neuroactive Steroids Progesterone |
| Increase in HR Response | Blunted Phasic HR | High Basal HR Blunted Phasic HR Blunted HR Variability |
Doxazosin Prazosin |
| vmPFC Activation | VmPFC Hypoactivity in Stress and Cue States | VmPFC Hyperactivity in Neutral States | Guanfacine Progesterone |
 | |||
ACTH: adrenocorticotropic hormone; CRF: corticotropin-releasing factor; HR: heart rate; vmPFC: ventromedial prefrontal cortex. Treatment Targets: Pexacerfont- CRF1 receptor antagonist [84]; Mifepristone- glucocorticoid receptor antagonist [86]; Naltrexone-opioid receptor antagonist [83]; Neuroactive Steroids- GABAA receptor agonists [91]; Progesterone [90]; Doxazosin- alpha-1 adrenergic antagonist [88]; Prazosin- alpha-1 adrenergic antagonist [87]; Guanfacine- alpha-2 receptor antagonist [89].
Central Brain Response to Stress and Risk of SUDs
A number of previous human studies suggest that trauma, adversity and chronic stress alter the activity and structure of the prefrontal cortical, limbic and striatal brain networks involved in regulating stress, emotions, reward and higher cognitive or executive control functions [7]. These functions can include the regulation of distress and emotions, such as controlling and inhibiting impulses, refocusing and shifting attention, working memory, monitoring conflict and behavior, linking behaviors to possible future consequences, and flexible consideration of alternatives for response selection and decision making [32]. Recent evidence from human brain structural and magnetic resonance imaging (MRI) shows that recent life stressors such as those listed in Table 1 (e.g. death in family, divorce, relationships ending, being assaulted, financial crises, robberies), trauma (physical, emotional and/or sexual abuse) and chronic stress (subjective experience of continuous stressors or ongoing life problems) are associated with lower gray matter volume in medial prefrontal, amygdala, hippocampus and insula regions of the brain [33, 34]. Similarly, recent life stress and acute stress exposure (such as those listed in Table 1 and also above) may decrease responses in the prefrontal regions such as the dorsolateral prefrontal cortex (DLPFC) and ventromedial prefrontal cortex (VmPFC) associated with working memory, reward processing and resilient coping, and might be associated with at-risk drug use and emotional dysregulation, such as binge alcohol intake, emotional eating, and frequency of arguments and fights [35]. Thus, with increasing levels of stress, there can be decreased prefrontal functioning and increased limbic-striatal activation, as measured by functional magnetic resonance imaging (fMRI), a brain pattern associated with low behavioral and cognitive control [35]. In another study, prenatally cocaine-exposed 14–17 year old adolescents exhibited lower gray matter volume in limbic and frontal regions of the brain as assessed by MRI and whole-brain voxel-based morphometry relative to non-cocaine exposed adolescent controls, and lower gray matter volume in these brain regions was associated with substance use initiation [36], thereby suggesting that changes in brain volume may serve as biological risk markers for substance use. Indeed, low behavioral and cognitive control linked to the reduced prefrontal and insular cortex, and high activation of limbic-emotional and striatal-motivation brain regions under stress suggests one specific pattern underlying risk of addictive behaviors where there is a decreased ability to control rewarding behaviors [37]. Thus, motivational brain pathways appear to be key targets of disrupted central stress activity that suggest a potentially important mechanism by which stress might affect susceptibility to addiction vulnerability. Nevertheless, these potential pathways and targets need further assessment in future studies in order to be able to identify specific measures that upon validation, might be used as candidate risk biomarkers of SUD risk.
Chronic Drug Abuse Alters Stress Responses: Potential Predictors of Increased Drug Motivation and Candidate Biomarkers
The HPA Axis: Cortisol and ACTH in Drug Abuse Motivation
In humans, enhanced stress-system activity has been associated with chronic smoking as well as with cocaine and alcohol consumption [38, 39]. Elevated basal plasma and salivary cortisol levels have been observed with active binge alcohol intake and recent withdrawal from alcohol in alcohol dependent individuals [40–43] and lower basal plasma ACTH levels in individuals with a high risk for alcoholism based on family history compared to low risk individuals. [44]. Moreover, markedly reduced ACTH and cortisol concentrations in response to both pharmacological stimulation of the HPA axis with human corticotrophin-releasing hormone (CRH) and psychosocial stressors, such as mental arithmetic, cold pressor test, interpersonal conflict, have also been reported in chronic alcohol abusers compared to controls[41, 45–49]. Similarly, cigarette smoking has been shown to elevate circulating plasma ACTH and cortisol in moderate smokers [50, 51]; furthermore, decreased plasma and salivary ACTH and cortisol in response to psychosocial stress (public speaking, mental arithmetic) has been reported in smokers in withdrawal relative to sham smoking [52]. Moreover, in protracted abstinence, our group characterized the stress-induced and drug or alcohol cue-induced craving state in SUD patients; the findings indicated that these cravings were accompanied by enhanced negative emotion and anxiety as well as by altered plasma cortisol and ACTH concentrations in early abstinent cocaine abusers [53, 54], comorbid cocaine and alcohol abusers [55], as well as in early abstinent alcoholics relative to controls [56–58].
Together, these findings suggest that regular, binge use of psychoactive substances like nicotine, alcohol and cocaine alter the physiological stress pathways and such alterations are accompanied by greater motivation for drug and higher levels of drug use. Thus, we posit that these chronic drug-related stress changes in peripheral stress neuroendocrine function may be assessed further as putative biomarkers of the transition from controlled to compulsive drug seeking across a range of substances.
The Autonomic System: Heart rate and heart rate variability in SUDs in Drug Use Motivation
In addition to HPA axis dysregulation, chronic drug use can also lead to dysregulation of the autonomic nervous system. In our laboratory, inpatient treatment-engaged, recovering cocaine- and alcohol-dependent individuals completed research participation in experimental studies where in 3-day controlled experiments, they were exposed to stress, drug cues and an active neutral relaxing control cue using a standardized personalized guided imagery procedure [59]. We then assessed drug craving, anxiety, heart rate, blood pressure, and a number of neurochemicals including plasma Cortisol and ACTH, serum Brain Derived Neurotrophic Factor (serum BDNF), plasma neuropeptide Y, and immune system cytokines. These studies reported that early abstinent individuals with SUDs displayed persistent high basal heart rates [57], and reduced heart rate responses to the stress imagery conditions relative to healthy controls [3, 57, 60, 61]. One of the first studies to experimentally examine the role of autonomic reactivity in stress induced craving in nicotine dependent 15-hour abstinent smokers found that blunted stress-induced high frequency heart rate variability (HF-HRV) was associated with less time to initiate smoking and increased craving relief and reinforcement from smoking[62]. Recent work examined heart rate variability (HRV) in nicotine and alcohol dependent individuals compared to age-matched controls, and found that HRV was globally decreased in the addicted subjects [63]. Collectively, these data suggest that alterations in HR and HRV can result from chronic drug abuse and it is possible that – pending further testing -- these measures may serve as candidate prognostic biomarkers of treatment course, severity of illness and relapse risk in SUDs. Furthermore, they might also serve as predictive biomarkers of treatment outcome; by assessing treatments that aim to normalize tonic and phasic vagal reactivity in early abstinent individuals with SUDs, to possibly improve cessation and abstinence outcomes. However, these possibilities remain to be investigated in future research.
Central Brain Response in Drug Abuse Motivation
Chronic stress states and substance abuse each result in altered neuroadaptive function in the prefrontal–striatal limbic circuits [37]. Neuroimaging studies using fMRI have shown that hyperactivity in the limbic–striatal regions are not only associated with elevated levels of emotional distress, but also with stress-induced drug craving, specifically related to the striatal region (right caudate and thalamus) and with decreased neuronal activity in the right anterior cingulate cortex (ACC) [64, 65]. Additionally, subjective experience of emotional distress has been associated with heightened limbic activity in conditions of social isolation stress [66] and with hyperactivity in the amygdala during the experience of negative emotion, such as fear and sadness [67]. These findings suggest that heightened or sensitized striatal responses under stress and drug cues might underlie increased drug-craving states in addiction.
As previous work demonstrated that stress-induced and drug cue-induced craving is significantly greater in addicted individuals than in controls [57], our laboratory group also assessed brain correlates of stress and cue-induced alcohol craving in abstinent, treatment-engaged alcohol dependent individuals [68]. The findings indicated a robust brain hyperactivity in fMRI during the neutral relaxed state in the ventral striatum and the VmPFC/ACC, which correlated with provoked personalized, guided imagery of stress-induced and cue-induced alcohol craving [68]. Furthermore, the data identified neuroadaptations in the VmPFC, ventral striatum, and insula networks, showing disrupted functioning in the relaxed state as well as reduced activity during provoked or challenge conditions, such as stress and alcohol cue, in addicted individuals relative to healthy controls [68]. These findings suggest that prefrontal brain regions that are important in controlling emotions and reward/pleasures circuits are altered in chronic alcohol and cocaine use relative to controls. Whether these measures can serve as predictive biomarkers is discussed below.
Altered Stress Responses Predicting SUD Relapse
SUDs are chronic and relapsing in nature and clinical treatment studies suggest that more than two thirds of individuals with SUD relapse within weeks to months of initiating treatment [69, 70]. As relapse rates are high, research in the recent past has focused on whether there is a biology underlying relapse susceptibility, and if so, whether there are specific biobehavioral markers of relapse risk that may be targeted to develop new treatments for relapse prevention. While clinical studies have repeatedly shown that stress is associated with relapse [61], the underlying mechanism in this relationship is not clearly understood. Below, we present growing evidence that alterations and dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis and other neuropeptides as well as autonomic nervous system changes in individuals with SUDs may be able to predict drug relapse.
The HPA Axis: Cortisol and Cortisol/ACTH Ratio in SUD Relapse
Enhanced stress-related plasma cortisol levels have been associated with relapse factors in cocaine abusers [71], and reduced cortisol production in response to stress has been associated with a shorter time to future relapse in alcoholics [72] and in male smokers [73]. Inpatient treatment-engaged, recovering cocaine- and alcohol-dependent individuals completed research participation in experimental studies during which they were exposed to personalized, guided stress, drug cues, and neutral relaxing scenarios and assessed for drug craving, anxiety and stress responses, as assessed by autonomic nervous system and HPA axis markers; individuals with SUDs in early abstinence up to 4 weeks, were marked by persistent high basal levels of ACTH and cortisol as well as higher basal heart rates [57] relative to healthy controls, and these individuals also displayed blunted plasma ACTH, cortisol and heart rate responses to stress challenges compared to controls [3, 57, 60]. Moreover, after completion of the laboratory study, the patients were discharged from inpatient treatment and observed repeatedly for 90 days to assess future relapse outcomes. For the cocaine group, where altered stress responses were noted compared with controls (see above [60]), higher stress-induced ACTH and cortisol concentrations were not associated with time to relapse, but these responses were predictive of greater amounts of cocaine consumed during follow up [71]. Abstinent, treatment engaged, alcohol dependent individuals with high cortisol/ACTH ratios (a measure of sensitivity of the adrenal glands to release cortisol in response to the ACTH signal) were more likely -- more than double the risk-- to relapse more quickly than those with low cortisol/ACTH ratios after discharge from inpatient treatment [3]. In nicotine dependent individuals, in early abstinence, other research has also shown that blunted cortisol responses predicts early relapse [52, 74].
Growth Factors in SUD Relapse: The role of BDNF
Evidence from preclinical studies shows chronic drug-related central nervous system changes in brain-derived neurotropic factor (BDNF) and other growth factors during abstinence that have been associated with reinstatement of drug seeking in animal models of relapse [75]. In our laboratory, we found morning serum BDNF levels to be significantly higher in abstinent cocaine abusers [76]. Moreover, higher serum BDNF levels were highly predictive of shorter time to cocaine relapse and higher amounts of cocaine used, as well as greater number of days of cocaine use over a 90-day follow-up period in the cocaine dependent subjects studied [76].
Central Brain Response in Drug Abuse Relapse
Previous work has revealed that cocaine dependent individuals, as compared to healthy controls, display altered fMRI activation in prefrontal–limbic–striatal circuitry involved in stress, emotion, and reward processing as well as in the regulation of stress responses [64]. Moreover, altered function in these brain regions in cocaine dependent individuals has been associated with stress-induced and drug cue-induced craving and with an increased risk for future relapse [77]. Specifically, increased activity in the medial prefrontal cortex (PFC) has been associated with a shorter time before cocaine relapse occurs, correlating with higher numbers of days of cocaine use during the 90-day period in cocaine dependent individuals [77].
In addition an fMRI study in abstinent alcohol dependent individuals reported robust hyperactivity during the neutral relaxed state in the ventral striatum and the VmPFC/ACC relative to controls, correlating with provoked stress-induced and cue-induced drug craving [68]. Noteworthy, the stress- and drug cue-induced craving triggers were also associated with blunted responses in these brain regions in subjects under the stress and drug-cue conditions; and, both hyperactivation of the VmPFC in the neutral, relaxed state and hypoactivation of the VmPFC and the insula during stress were predictive of the amount of future time before alcohol relapse, as well as of the severity of alcohol relapse during the subsequent recovery period [68]. This work was furthered by assessing the relationship between central and peripheral nervous system markers in stress responsivity, and their combined effects on relapse risk [78]. The Findings showed that craving, high basal sympathetic activity and HPA adrenal sensitivity were significantly associated with VmPFC hypoactivity following exposure to stress [78]. This disrupted VmPFC activation in neutral and stress conditions most sensitively and specifically was able to predict a shorter time to future relapse. Furthermore, the disrupted neutral state VmPFC function mediated the relationship between adrenal sensitivity and future relapse risk [78].
Other studies have shown that structural alterations in brains of individuals with SUD exist and may serve as potential markers of relapse risk. For example, in a combined structural and functional MRI study with 46 detoxified alcohol dependent patients, subsequent relapsers displayed greater atrophy in the bilateral orbitofrontal cortex (OFC), the right medial PFC, and ACC compared to healthy controls and alcohol dependent patients who remained abstinent [79]. In another MRI study with 75 treatment seeking alcohol dependent subjects, relapsers showed global reduction in cortical thickness in the majority of brain regions compared to healthy controls [80]. These structural imaging studies suggest that gray matter atrophy in specific stress regulatory brain regions may play an important role in alcohol relapse risk.
Based on these above findings, we present a heuristic model in Figure 1 to illustrate the notion that with higher levels of drug use there are progressive alterations in stress and cue-related peripheral and central responses, including greater levels of drug motivation and craving and the drive for compulsive drug use. Parallel brain adaptations in stress circuits with increasing level of drug history and exposure are also shown with specific types of structural changes and disrupted function being predictive of future drug use and relapse (Figure 1) [81].
Figure 1. Progressive changes in behavioral, peripheral and central nervous system stress response in the progression to addiction.
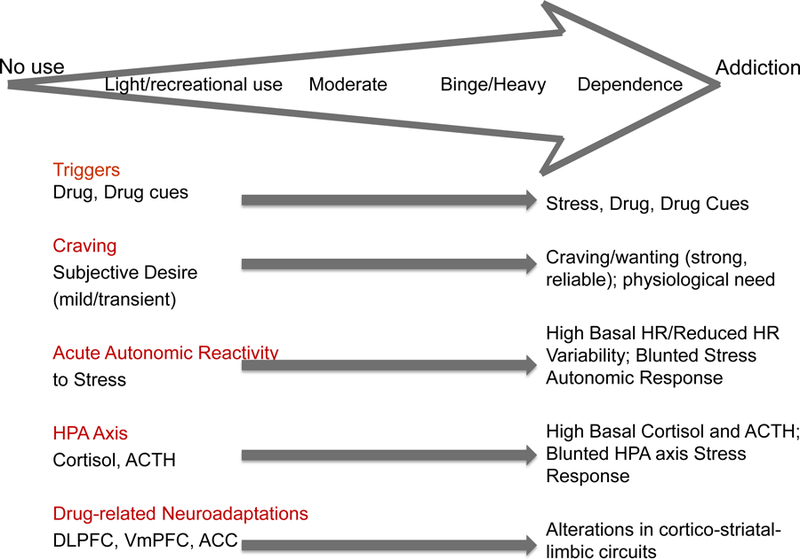
A schematic diagram representing progressive changes in stress responses and drug motivation and craving, altered stress and drug cue reactivity and parallel brain responses with increasing level of drug history and exposure. DLPFC=dorsolateral prefrontal cortex; VmPFC=ventromedial prefrontal cortex; ACC=anterior cingulate cortex. (based on heuristic model figure in Sinha, 2013 [81])
In summary, the findings in the section highlight that chronic drug use alters peripheral and central brain stress pathways. There is now growing evidence that such changes are not non-specific consequences of chronic drug use, but rather that specific alterations may play a role in compulsive motivation and drug craving and in predicting relapse and treatment outcome. The specific markers are also listed in Table 2 (Key Table) to identify those that have been shown to predict future drug use and relapse and may be developed further as putative biomarkers for different phases of the risk and relapse to SUDs.
Potential Predictive Biomarkers Relating to Treatment Targets
As previously discussed, recent findings are aiming to identify objective and sensitive peripheral and central stress response measures that may serve as biomarkers of addiction risk and relapse. Evidence from population-based and clinical studies suggest that trauma, adversity and chronic stress can increase addiction vulnerability, and that chronic psychoactive drug use is associated with dysregulation of the HPA axis and decreased prefrontal executive control over stress and reward pathways. Such altered stress responses may be predictive of increased drug craving and higher risk for future relapse. However, developing and validating these disrupted stress-related biobehavioral measures as biomarkers requires specific and robust attention. Table 2, Key Table, summarizes the nature of select stress disruptions that have been related to SUD risk and relapse outcomes and the potential for biomarker development for specific stage of SUD. It remains to be determined which of these measures may serve as valid biomarkers with high specificity and sensitivity to predict SUD risk and of relapse and treatment failure; their use may also vary based on sex, race, age and also individual genetic and environmental vulnerabilities, and these need to be assessed and established. Of note, individual differences in the noted adaptations that result in the disruptions listed in Table 2, Key Table, are expected, and indeed have been found and further validate the need to explore these as potential biomarkers. For example, those with high previous trauma and post-traumatic stress with cigarette smoking and history of alcohol dependence may show reduced heart rate variability while those without these synergistic risk factors might not [14]. This suggests that multilevel diagnostic, clinical history, biological responses and genetic and epigenetic vulnerabilities using Big Data approaches may be needed in order to develop multifactorial risk groupings that link to specific biomarkers of SUD risk.
On the basis of the evidence presented in previous section, it is clear that drug abuse alters peripheral and central stress responses. This also leads to the question of whether these measures may serve as prognostic biomarkers in treatment development. Given the heterogeneity of stress- and drug-related adaptations, it would be important to identify subgroups of SUD patients with specific disrupted stress measures or biomarkers that are predictive of drug intake or relapse. Once identified, these biomarkers may be utilized to test different treatment strategies to normalize these biomarkers and assess improvement in disease outcome. Such an approach has been used in the treatment development for several types of cancers, but it is in its infancy in SUD treatment development. For example, medications or behavioral interventions that improve VmPFC function and also normalize HPA axis function, i.e. reduce basal overactivity and re-instate normal phasic stress responses, may improve treatment outcome in individuals with SUDs and reduce relapse risk [82]. For example, Naltrexone treatment increased cortisol levels at baseline and decreases drug-related alcohol craving, while increasing alcohol stimulated ACTH and cortisol responses compared to placebo in non-treatment seeking individuals with alcohol use disorder (AUD) [83]. As such, Naltrexone reduced the alcohol-related neuroendocrine tolerance and improved alcohol’s effects in stimulating the HPA axis, thereby reducing the blunted alcohol response on the HPA axis. However, the findings also showed that Naltrexone increased basal HPA axis tone and tonic cortisol levels relative to placebo, an effect that might be detrimental to AUD individuals with higher subclinical and clinical HPA axis alcohol withdrawal/abstinence pathophysiology. It is possible that this aspect of Naltrexone may make it unsuitable for all AUD individuals, which may also contribute to its modest efficacy in AUDs. Moreover, CRF antagonists such as Pexacerfont, which directly acts on the stress axis, was found to reduce ACTH levels and increase cortisol responses to stress in anxious alcoholic patients [84]. Interestingly, negative results in this study and with other CRF receptor antagonists was observed with regard to efficacy in reducing alcohol craving responses in the laboratory [84, 85]. It is important to note however, that the patient samples were not selected because of a specific biomarker or alteration in stress responses as discussed in the previous sections. Thus, future research is needed to understand whether CRF antagonists may be targeted to specific subgroups of patients with the targeted prognostic biomarkers of high relapse risk.
Recent evidence also showed that manipulating central glucocorticoids with Mifepristone, that may also normalize peripheral HPA axis responses, was useful in decreasing alcohol intake in alcohol dependent individuals [86]. Noradrenergic compounds that have central effects on autonomic, HPA axis and prefrontal stress pathways have also been examined. For example, the alpha1-antagonist Prazosin was found to reduce stress-induced alcohol craving and negative emotions, while reducing basal cortisol response and increasing stress-induced cortisol responses in inpatient alcohol dependent patients in early abstinence [87]. Similarly, alpha1-adrenergic antagonist Doxazosin reduced cocaine use and improved abstinence outcomes in treatment seeking cocaine dependent individuals [88]. The alpha2-agonist Guanfacine in early abstinent cocaine and alcohol dependent individuals was also found to reduce cue-induced craving, decrease baseline cortisol response and normalize stress-induced cortisol responses [89]. Of note, progesterone versus placebo in treatment seeking cocaine dependent men and women have also been examined, indicating that progesterone can lead to reduced cue-induced cocaine craving and cortisol responses and improved prefrontal inhibitory function as measure by the Stroop task [90]. Similar effects with GABAergic neuroactive steroids have been documented, where cocaine and alcohol dependent individuals who received progesterone were grouped by their baseline levels of the progesterone-derived neuroactive steroid allopregnanolone (ALLO). The high ALLO group compared to the low ALLO group showed reductions in craving, improved cognitive performance, and both reduced basal cortisol and increased cortisol in response to stress [91]. Table 2, Key Table, provides a layout of the putative stress biomarkers and their potential neurobiological treatment targets. However, whether normalizing these specific stress disruptions and stress-induced craving may lead to improved treatment outcomes is yet to be established. In summary, this section illustrates how new pharmacological molecules that have been tested in preclinical studies of addiction, are active on the peripheral and central stress responses but their assessment thus far has not incorporated utilizing stress response biomarkers for sample selection and we suggest here the potential benefit and clinical utility of these putative biomarkers in the targeted treatment development to improve SUD clinical outcomes.
Concluding Remarks
This opinion paper provides a targeted summary of altered peripheral and central stress arousal responses in those at-risk for addiction and those with SUDs relative to control volunteers, with specific emphasis on whether these measures are predictive of future drug use, and abuse and of relapse. We suggest that positive findings of such links to SUD risk and relapse suggests further development of specific measure as biomarkers. Future studies need to focus on validation of stress biomarkers that specifically relate to loss of control drug intake (risk) and relapse (predictive), and then target those to assess SUD prevention and treatment outcomes to develop prognostic biomarkers that reduce risk of SUDs.
Trends Box.
The ratio of Cortisol/ACTH, a measure of sensitivity of the adrenal glands to release cortisol in response to the ACTH signal, may emerge as a potent peripheral predictive biomarker of dependence and particularly of relapse.
Changes in brain-derived neurotrophic factor (BDNF) in individuals with a Substance Use Disorder (SUD) during abstinence have been shown to predict future relapse.
Immune system markers, such as interleukin-6 (IL6), interleukin-10 (IL-10), interleukin-1 receptor antagonist (IL-1ra), and tumor necrosis factor alpha (TNFα) have been found to be altered in plasma levels of individuals with SUD compared to healthy controls, and thus may present potentially novel biomarkers in SUD.
Neuropeptide Y (NPY) expression was found to be lower in response to stress in the plasma of individuals with SUD compared to healthy controls, and the lower stress-related NPY was predictive of greater relapse severity, and thus may serve as another potential biomarker in SUD.
Clinician’s Corner.
Stressful events and stress biology play a critical role in addiction, contributing to its development and high relapsing nature.
Both stress and a various drugs of abuse stimulate sympathetic arousal and activate the HPA axis and elicit adrenocorticotropic hormone (ACTH) and cortisol responses, and specific patterns of responses are predictive of addiction risk.
Other neurochemicals such Brain Derived Neurotrophic Factor (BDNF) and Neuropepetide Y (NPY) in plasma have been associated with SUD relapse measures.
With both chronic stress and chronic drug use, these peripheral stress systems become dysregulated, with simultaneous disruptions in brain cortico-striatal-limbic circuits, and select changes are predictive of future addiction relapse risk.
Individuals with substance use disorders (SUDs) display basal hyperactivity of central brain and peripheral stress markers and hypoactivity in response to stress and drug cue provocation.
Acknowledgments
Authors do not have direct or indirect financial interests or relationships related to the subject of the manuscript. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics. This research was supported by grants from the NIH National Institute of Alcohol Abuse and Alcoholism (NIAAA) grant R01-AA020504.
REFERENCES
- 1.U.S. Department of Health and Human Services (HHS), Office of the Surgeon General, Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health Washington, DC: HHS, November 2016. [PubMed] [Google Scholar]
- 2.Bough KJ, et al. , Biomarkers for the development of new medications for cocaine dependence. Neuropsychopharmacology, 2014. 39(1): p. 202–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R, et al. , Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry, 2011. 68(9): p. 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Koob G, and Baler R, Biomarkers in substance use disorders. ACS Chem Neurosci, 2015. 6(4): p. 522–5. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF and Volkow ND, Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 2016. 3(8): p. 760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meaney MJ, Brake W, and Gratton A, Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology, 2002. 27(1–2): p. 127–38. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R, Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci, 2008. 1141: p. 105–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laucht M, et al. , Impact of psychosocial adversity on alcohol intake in young adults: moderation by the LL genotype of the serotonin transporter polymorphism. Biol Psychiatry, 2009. 66(2): p. 102–9. [DOI] [PubMed] [Google Scholar]
- 9.Carliner H, et al. , Trauma Exposure and Externalizing Disorders in Adolescents: Results From the National Comorbidity Survey Adolescent Supplement. J Am Acad Child Adolesc Psychiatry, 2017. 56(9): p. 755–764 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwabe L, Dickinson A, and Wolf OT, Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol, 2011. 19(1): p. 53–63. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS, Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 1998. 840: p. 33–44. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF, A role for brain stress systems in addiction. Neuron, 2008. 59(1): p. 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engeland WC, et al. , Zone-specific cell proliferation during compensatory adrenal growth in rats. Am J Physiol Endocrinol Metab, 2005. 288(2): p. E298–306. [DOI] [PubMed] [Google Scholar]
- 14.Dennis PA, et al. , Posttraumatic stress, heart rate variability, and the mediating role of behavioral health risks. Psychosom Med, 2014. 76(8): p. 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinnant JB, Erath SA, and El-Sheikh M, Harsh parenting, parasympathetic activity, and development of delinquency and substance use. J Abnorm Psychol, 2015. 124(1): p. 137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyrka AR, Ridout KK, and Parade SH, Childhood adversity and epigenetic regulation of glucocorticoid signaling genes: Associations in children and adults. Dev Psychopathol, 2016. 28(4pt2): p. 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao U, Comorbidity between Depressive and Addictive Disorders in Adolescents: Role of Stress and Hpa Activity. US Psyc, 2010. 3: p. 39–43. [PMC free article] [PubMed] [Google Scholar]
- 18.Rao U, et al. , Contribution of hypothalamic-pituitary-adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology, 2009. 34(13): p. 2721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao U and Morris MC, Cortisol Responses to Psychosocial Stress: The Role of Childhood Maltreatment and Depression. Int J Public Ment Health Neurosci, 2015. 2(1). [PMC free article] [PubMed] [Google Scholar]
- 20.Moss HB, Vanyukov MM, and Martin CS, Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry, 1995. 38(8): p. 547–55. [DOI] [PubMed] [Google Scholar]
- 21.Moss HB, et al. , Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol Psychiatry, 1999. 45(10): p. 1293–9. [DOI] [PubMed] [Google Scholar]
- 22.Chaplin TM, et al. , Prenatal cocaine exposure differentially affects stress responses in girls and boys: associations with future substance use. Dev Psychopathol, 2015. 27(1): p. 163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaplin TM, et al. , Parent-adolescent conflict interactions and adolescent alcohol use. Addict Behav, 2012. 37(5): p. 605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AC, et al. , A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biol Psychiatry, 2016. 79(6): p. 489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AC, et al. , Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry, 2014. 75(10): p. 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans BE, et al. , Blunted Heart Rate Response as a Potential Endophenotype of Substance Use Disorders: Evidence from High-Risk Youth. Front Pediatr, 2015. 3: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans BE, et al. , The relation between hypothalamic-pituitary-adrenal (HPA) axis activity and age of onset of alcohol use. Addiction, 2012. 107(2): p. 312–22. [DOI] [PubMed] [Google Scholar]
- 28.Shively CA, et al. , Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates (Macaca fascicularis). Psychopharmacology (Berl), 2007. 192(2): p. 183–91. [DOI] [PubMed] [Google Scholar]
- 29.Doyon WM, et al. , Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron, 2013. 79(3): p. 530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holly EN and Miczek KA, Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl), 2016. 233(2): p. 163–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostroumov A, et al. , Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron, 2016. 92(2): p. 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnsten A, Mazure CM, and Sinha R, This is your brain in meltdown. Sci Am, 2012. 306(4): p. 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansell EB, et al. , Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry, 2012. 72(1): p. 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dam NT, et al. , Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry, 2014. 71(8): p. 917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha R, et al. , Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A, 2016. 113(31): p. 8837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rando K, et al. , Prenatal cocaine exposure and gray matter volume in adolescent boys and girls: relationship to substance use initiation. Biol Psychiatry, 2013. 74(7): p. 482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li CS and Sinha R, Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev, 2008. 32(3): p. 581–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mello NK, Hormones, nicotine, and cocaine: clinical studies. Horm Behav, 2010. 58(1): p. 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaine SK and Sinha R, Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, 2017. 122: p. 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adinoff B, et al. , Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry, 1991. 148(8): p. 1023–5. [DOI] [PubMed] [Google Scholar]
- 41.Costa A, et al. , An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology, 1996. 21(3): p. 263–75. [DOI] [PubMed] [Google Scholar]
- 42.Kutscher S, et al. , Concomitant endocrine and immune alterations during alcohol intoxication and acute withdrawal in alcohol-dependent subjects. Neuropsychobiology, 2002. 45(3): p. 144–9. [DOI] [PubMed] [Google Scholar]
- 43.Adinoff B, et al. , Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res, 2003. 27(9): p. 1420–7. [DOI] [PubMed] [Google Scholar]
- 44.Dai X, Thavundayil J, and Gianoulakis C, Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology, 2002. 27(3): p. 442–52. [DOI] [PubMed] [Google Scholar]
- 45.Errico AL, et al. , Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol, 1993. 54(4): p. 393–8. [DOI] [PubMed] [Google Scholar]
- 46.Inder WJ, et al. , The acute effects of oral ethanol on the hypothalamic-pituitary-adrenal axis in normal human subjects. Clin Endocrinol (Oxf), 1995. 42(1): p. 65–71. [DOI] [PubMed] [Google Scholar]
- 47.al’Absi M, Bongard S, and Lovallo WR, Adrenocorticotropin responses to interpersonal stress: effects of overt anger expression style and defensiveness. Int J Psychophysiol, 2000. 37(3): p. 257–65. [DOI] [PubMed] [Google Scholar]
- 48.Lovallo WR, et al. , Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res, 2000. 24(5): p. 651–8. [PubMed] [Google Scholar]
- 49.Junghanns K, et al. , Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol, 2003. 38(2): p. 189–93. [DOI] [PubMed] [Google Scholar]
- 50.Seyler LE Jr., et al. , The effects of smoking on ACTH and cortisol secretion. Life Sci, 1984. 34(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 51.Pomerleau OF and Pomerleau CS, Cortisol response to a psychological stressor and/or nicotine. Pharmacol Biochem Behav, 1990. 36(1): p. 211–3. [DOI] [PubMed] [Google Scholar]
- 52.al’Absi M, Hatsukami D, and Davis GL, Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl), 2005. 181(1): p. 107–17. [DOI] [PubMed] [Google Scholar]
- 53.Fox HC, et al. , Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl), 2006. 185(3): p. 348–57. [DOI] [PubMed] [Google Scholar]
- 54.Sinha R, et al. , Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl), 2003. 170(1): p. 62–72. [DOI] [PubMed] [Google Scholar]
- 55.Fox HC, et al. , Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology, 2005. 30(9): p. 880–91. [DOI] [PubMed] [Google Scholar]
- 56.Sinha R, et al. , Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl), 2000. 152(2): p. 140–8. [DOI] [PubMed] [Google Scholar]
- 57.Sinha R, et al. , Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 2009. 34(5): p. 1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox HC, et al. , Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res, 2007. 31(3): p. 395–403. [DOI] [PubMed] [Google Scholar]
- 59.Sinha R, Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol, 2009. 14(1): p. 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox HC, et al. , Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology, 2008. 33(4): p. 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinha R, How does stress lead to risk of alcohol relapse? Alcohol Res, 2012. 34(4): p. 432–40. [PMC free article] [PubMed] [Google Scholar]
- 62.Ashare RL, et al. , Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology (Berl), 2012. 220(2): p. 259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuksel R, et al. , Autonomic Cardiac Activity in Patients with Smoking and Alcohol Addiction by Heart Rate Variability Analysis. Clin Invest Med, 2016. 39(6): p. 27519. [PubMed] [Google Scholar]
- 64.Sinha R, et al. , Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci, 2004. 1032: p. 254–7. [DOI] [PubMed] [Google Scholar]
- 65.Sinha R, et al. , Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl), 2005. 183(2): p. 171–80. [DOI] [PubMed] [Google Scholar]
- 66.Panksepp J, Nelson E, and Bekkedal M, Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries. Ann N Y Acad Sci, 1997. 807: p. 78–100. [DOI] [PubMed] [Google Scholar]
- 67.Phan KL, et al. , Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 2002. 16(2): p. 331–48. [DOI] [PubMed] [Google Scholar]
- 68.Seo D, et al. , Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry, 2013. 70(7): p. 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paliwal P, Hyman SM, and Sinha R, Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend, 2008. 93(3): p. 252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyman SM, et al. , Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend, 2008. 92(1–3): p. 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinha R, et al. , Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry, 2006. 63(3): p. 324–31. [DOI] [PubMed] [Google Scholar]
- 72.Adinoff B, et al. , Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res, 2005. 29(7): p. 1351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.al’Absi M, Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol, 2006. 59(3): p. 218–27. [DOI] [PubMed] [Google Scholar]
- 74.McKee SA, et al. , Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol, 2011. 25(4): p. 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoenbaum G, Stalnaker TA, and Shaham Y, A role for BDNF in cocaine reward and relapse. Nat Neurosci, 2007. 10(8): p. 935–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Sa C, et al. , Serum and plasma brain-derived neurotrophic factor (BDNF) in abstinent alcoholics and social drinkers. Alcohol, 2012. 46(3): p. 253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinha R and Li CS, Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev, 2007. 26(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 78.Blaine SK, Seo D, and Sinha R, Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol, 2017. 22(2): p. 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beck A, et al. , Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry, 2012. 69(8): p. 842–52. [DOI] [PubMed] [Google Scholar]
- 80.Durazzo TC, et al. , Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res, 2011. 35(6): p. 1187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinha R, The clinical neurobiology of drug craving. Curr Opin Neurobiol, 2013. 23(4): p. 649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milivojevic V and Sinha R, Targeting Stress Pathophysiology to Improve Alcoholism Relapse Outcomes. Neuropsychopharmacology, 2017. 42(5): p. 987–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Malley SS, et al. , Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl), 2002. 160(1): p. 19–29. [DOI] [PubMed] [Google Scholar]
- 84.Kwako LE, et al. , The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology, 2015. 40(5): p. 1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwandt ML, et al. , The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology, 2016. 41(12): p. 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vendruscolo LF, et al. , Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest, 2015. 125(8): p. 3193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fox HC, et al. , Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res, 2012. 36(2): p. 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shorter D, Lindsay JA, and Kosten TR, The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: A pilot study. Drug Alcohol Depend, 2013. 131(1–2): p. 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox HC, et al. , Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol, 2012. 26(7): p. 958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox HC, et al. , The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology, 2013. 38(9): p. 1532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milivojevic V, et al. , Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology, 2016. 65: p. 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


