Key Points
Question
Does intensive blood pressure reduction decrease the risk of hematoma expansion and improve outcomes in patients with deep intracerebral hemorrhage?
Findings
In this exploratory analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 randomized clinical trial, intensive blood pressure reduction was associated with a decreased risk of hematoma expansion in deep intracerebral hemorrhage, and this association was driven by hemorrhages located in the basal ganglia. No association with outcome was found in this population.
Meaning
Intensive blood pressure reduction was associated with a decreased risk of hematoma expansion, an important neuroimaging marker of primary brain injury, in patients with intracerebral hemorrhage that compromises the basal ganglia; however, intensive blood pressure reduction was not associated with improved outcomes.
This exploratory analysis of Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 randomized clinical trial data seeks to determine whether intensive blood pressure reduction is associated with decreased risk of hematoma expansion in patients with intracerebral hemorrhage and if these associations are modified by the specific deep-brain nuclei involved.
Abstract
Importance
Hypertension is the strongest risk factor for spontaneous intracerebral hemorrhage (ICH) involving deep brain regions, but it appears to be unknown if intensive blood pressure reduction in the acute care setting decreases hematoma expansion or improves outcomes in patients with deep ICH.
Objective
To determine whether intensive blood pressure reduction is associated with decreased risk of hematoma expansion and changes in 90-day modified Rankin Scale scores and if these associations are modified by the specific deep-brain nuclei involved.
Design, Setting, and Participants
This study is an exploratory analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 international, multicenter randomized clinical trial, which was conducted from May 2011 to September 2015, enrolled eligible patients with primary ICH, and followed up with them for 90 days. Patients who had ICH and complete neuroimaging data were included in the analysis. Data analysis was completed from July 2018 to December 2018.
Exposures
Participants were randomized to either intensive treatment (with a systolic blood pressure target of 110-139 mm Hg) or standard treatment (with a systolic blood pressure target of 140-179 mm Hg).
Main Outcomes and Measures
The main outcome was hematoma expansion, defined as an increase greater than 33% in hematoma volume between baseline and 24 hours. Functional outcome was evaluated 90 days after the ICH via the modified Rankin Scale.
Results
Of 1000 trial participants, 870 (87.0%) had deep ICH, of whom 780 (89.7%) had complete neuroimaging data (of 336 thalamic and 444 basal ganglia hemorrhages). The baseline characteristics of the intensive and standard treatment groups remained balanced in this subgroup of the original study. Intensive treatment was associated with a decreased risk of hematoma expansion in univariable analysis (odds ratio [OR], 0.62 [95% CI, 0.43-0.87]; P = .006) and multivariable analysis (OR, 0.61 [95% CI, 0.42-0.88]; P = .009). This association was modified by the specific deep location of the ICH (OR, 0.44 [95% CI, 0.22-0.96]; interaction P = .02), with stratified analyses showing a reduction in risk of hematoma expansion with intensive vs standard treatment among basal ganglia ICH (OR, 0.44 [95% CI, 0.27-0.72]; P = .001) but not thalamic ICH (OR, 0.91 [95% CI, 0.51-0.64]; P = .76). Intensive treatment was not associated with an improvement in the modified Rankin Scale score distribution.
Conclusions and Relevance
Compared with standard treatment, intensive blood pressure treatment was associated with reduced hematoma expansion in deep ICH, specifically among basal ganglia hemorrhages.
Introduction
Spontaneous intracerebral hemorrhage (ICH) remains a devastating disease, with high mortality and limited treatment options.1,2 Cases of deep ICH, or hemorrhages involving the thalamus and basal ganglia, are the most common and severe form of ICH, with up to 60% of cases resulting in death or severe disability.3,4,5 Hypertension is the strongest risk factor for deep ICH, and mounting evidence indicates that factors contributing to ICH risk also influence the severity and outcome of this condition.6,7,8,9,10 However, it is unknown if intensive blood pressure (BP) reduction in the acute care setting decreases primary brain injury and improves outcomes in deep ICH.
Although several large randomized clinical trials have evaluated the effect of intensive BP reduction in the first few hours of ICH, the clinical benefit of this intervention remains unclear.11,12 The Antihypertensive Treatment of Acute ICH-2 (ATACH-2) trial evaluated the safety and efficacy of intensive BP reduction (to a systolic BP of 110 to 139 mm Hg within 2 hours of symptom onset) in comparison with standard therapy (to a systolic BP of 140 to 179 mm Hg).11 The ATACH-2 trial reported no significant effect of this intervention on hematoma expansion or poor outcome at 3 months, although an exploratory post hoc analysis of these data showed a linear increase in the risk of hematoma expansion with increasing mean minimum systolic BP achieved from 120 to 130 mm Hg.13 Of note, to our knowledge, no study to date has tested whether intensive BP reduction is associated with a decrease in hematoma expansion or improved outcomes specifically in deep ICH.
We therefore performed an exploratory analysis of the ATACH-2 trial data focusing on patients with deep ICH. The overarching hypothesis is that ICH caused by hypertension will be more sensitive to intensive BP treatment. To account for the loss of statistical power incurred by restricting the study population to deep ICH, the primary analysis sought to determine whether intensive BP reduction decreases hematoma expansion, a well-studied neuroimaging marker of primary brain injury and a factor associated with poor outcome.14,15,16 We then conducted interaction analyses to determine whether the specific deep-brain nuclei involved (basal ganglia or thalamus) modified any identified association.
Methods
Study Design and Inclusion Criteria
We performed an exploratory analysis of the ATACH-2 trial, the details of which are described elsewhere.17 In brief, ATACH-2 was an international, randomized, multicenter, 2-group, open-label trial that enrolled 1000 patients within 4.5 hours of onset of primary ICH of less than 60 mL who also had elevated systolic BP (>180 mm Hg). Participants were randomized to intensive treatment (target systolic BP, 110-139 mm Hg within 2 hours) or standard treatment (target systolic BP, 140-179 mm Hg within 2 hours) using intravenous nicardipine. Patients with an ICH volume greater than 60 mL or a Glasgow Coma Scale score less than 5 were excluded. Enrolled patients with supratentorial deep ICH and available neuroimaging data were included. The study protocols were approved by an ethics committee at each site, and written informed consent was obtained from each participant or his or her legal surrogate. The ATACH-2 study is registered with ClinicalTrials.gov (NCT01176565), and the data are publicly available.
Neuroimaging
Head computed tomographic scans were obtained at baseline and 24 hours after treatment initiation and processed centrally by a core imaging analysis center. Readers blinded to treatment assignment, clinical status, and time of scan determined hemorrhage location on the baseline scan and calculated hemorrhage volume using computerized image analysis. Following the criteria used in ATACH-2, hematoma expansion was defined as an increase greater than 33% in hematoma volume between baseline and 24-hour computed tomographic scans. In secondary analyses, we added an absolute increase of greater than 6 mL as a criterion to define hematoma expansion, because this cutoff has been suggested to have the highest positive predictive values for poor outcome.14,15
Functional Outcomes
The Modified Rankin Scale (mRS) was obtained via in-person clinical evaluation at 3 months. This 3-month mRS was dichotomized for the main analysis on outcome (with patients grouped by those with scores of 0-3 vs those with scores of 4-6), with sensitivity analyses using the full range of mRS categories as the dependent variable (shift analysis).
Statistical Methods
Discrete variables are presented as counts (with percentages) and continuous variables as means (SD) or medians (interquartile range [IQR]), as appropriate. Differences in baseline and imaging characteristics by treatment group (intensive vs standard) were compared using Fisher exact test (2-tailed), Kruskal-Wallis, or unpaired t test, as appropriate. We implemented univariable and multivariable regression modeling to evaluate the unadjusted and adjusted associations of intensive treatment with hematoma expansion and functional outcome. Multivariable model building proceeded in several steps: first, covariates with P < .10 in univariable analyses were included in the model; second, universal confounders (age and sex) and imbalanced variables between treatment groups (hypertension and smoking) were forced into the model; third, covariates with P > .10 were backward eliminated; and fourth, collinear covariates, as expressed by a variance inflation factor greater than 5, were identified and removed from the model. We tested for outcome modification by the specific deep structure involved (thalamus vs basal ganglia) by adding product terms to the regression models. We subsequently implemented the analysis after stratifying by specific deep location. For the primary analysis (intensive treatment and hematoma expansion), we used a Bonferroni-corrected P value of <.025 to account for the 2 hypotheses being evaluated (association with hematoma expansion and outcome). We used R version 3.5.1 (R Foundation for Statistical Computing) for all analyses. Data analysis was completed from July 2018 to December 2018.
Results
Study Population
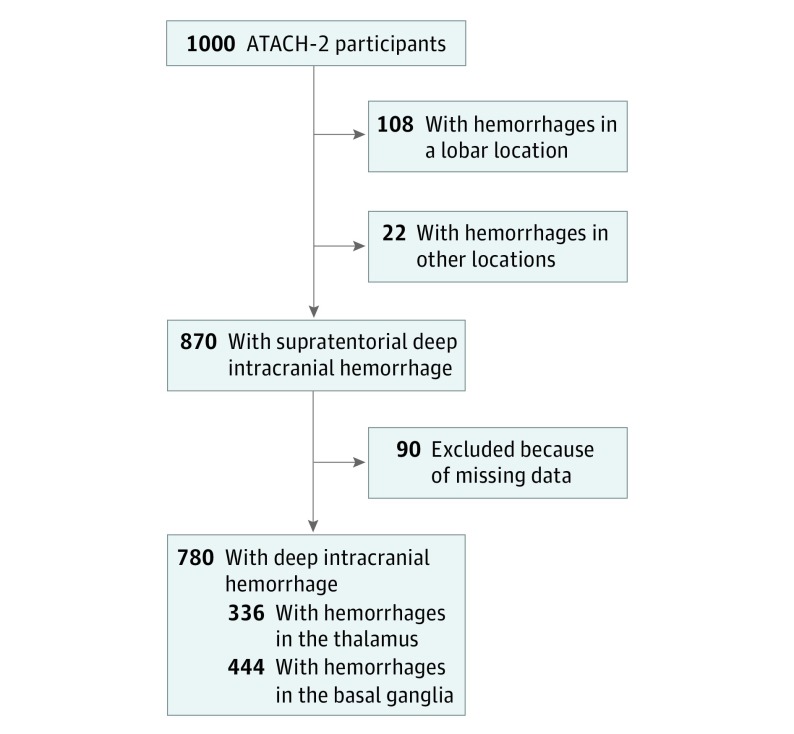
Among 1000 patients enrolled in ATACH-2, 870 (87.0%) had supratentorial, deep ICH. Of these, 780 (89.7%) had complete neuroimaging data and were included in the analysis (Figure). Among the included patients, 336 (43.0%) had thalamic ICH, 444 (56.9%) had basal ganglia ICH, and 405 (51.9%) were randomized to intensive BP reduction (mean [SD] age, 62 [13] years; 289 female patients [37.1%]). The characteristics of patients who were treated vs those who were untreated remained balanced, as it was in the original analysis, after restricting the study data to deep ICH and available neuroimaging data (Table 1). After stratification by deep location, patients in the intensive treatment group were more likely to be black (56 of 405 [13.8%] vs 31 of 375 [8.3%]; P = .02), have a history of hypertension (328 of 393 [83.4%] vs 278 of 362 [76.8%]; P = .03), and take antihypertensive medication (204 of 405 [50.4%] vs 157 of 375 [41.9%]; P = .02) before enrollment. Patients in the intensive treatment group were less likely to have a history of smoking (166 of 405 [41.0%] vs 183 of 375 [48.8%]; P = .03).
Figure. Flowchart of Patient Inclusion and Exclusion Criteria.
Flowchart of patient inclusion and exclusion criteria. Included patients from the Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 (ATACH-2) trial had supratentorial deep intracerebral hemorrhage and complete neuroimaging data.
Table 1. Baseline Demographic and Clinical Characteristics of Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 Trial Patients With Deep Intracerebral Hemorrhage and Available Neuroimaging Data.
| Variable | Patients, No. (%) | ||
|---|---|---|---|
| Standard Treatment (n = 375) | Intensive Treatment (n = 405) | P Value | |
| Age, mean (SD), y | 62 (13) | 62 (13) | .88 |
| Male | 245 (65.3) | 246 (60.7) | .21 |
| Race/ethnicity | |||
| Black | 31 (8.3) | 56 (13.8) | .02 |
| White | 91 (24.3) | 103 (25.4) | .77 |
| Hispanic | 25 (6.7) | 32 (7.9) | .80 |
| Hypertension, No./total No. (%) | 278/362 (76.8) | 328/393 (83.4) | .03 |
| Diabetes | 53 (14.1) | 76 (18.8) | .10 |
| Hyperlipidemia, No./total No. (%) | 81/359 (22.5) | 97/381 (25.4) | .40 |
| Congestive heart failure | 8 (2.1) | 12 (3.0) | .61 |
| Atrial fibrillation | 10 (2.7) | 13 (3.2) | .71 |
| Prior ischemic stroke | 65 (17.3) | 62 (15.3) | .52 |
| Smoker | 183 (48.8) | 166 (41.0) | .03 |
| Cocaine use | 7 (1.9) | 10 (2.5) | .74 |
| Antihypertensive medication | 157 (41.9) | 204 (50.4) | .02 |
| Admission | |||
| Glasgow Coma Scale score, median (interquartile range) | 15 (13-15) | 15 (13-15) | .70 |
| Blood pressure, mean (SD) | |||
| Systolic | 175 (25) | 176 (26) | .44 |
| Diastolic | 111 (20) | 113 (21) | .39 |
| International normalized ratio, mean (SD) | 1 (0.1) | 1 (0.1) | .23 |
Association Between Intensive BP Reduction and Hematoma Expansion
Mean (SD) baseline hematoma volume was 12.2 (10.8) mL, and the 24-hour hematoma volume was 14.7 (14.5) mL; these values were similar between treatment groups (Table 2). The mean (SD) volume of hematoma expansion was 2.4 (10) mL. Hematoma expansion greater than 33% was present in 165 of 790 patients (20.9%) overall and was less frequent in the intensive vs standard group (70 of 405 [17.3%] vs 95 of 375 [25.3%]; unadjusted P = .008) (Table 2). Hematoma expansion greater than 6 mL was present in 114 patients overall (14.6%) and was also less frequent in the intensive group vs the standard group (44 of 405 [10.9%] vs 70 of 375 [18.7%]; unadjusted P = .003). In multivariable analysis, intensive treatment was associated with a reduction in the risk of hematoma expansion greater than 33% (odds ratio [OR], 0.61 [95% CI, 0.42-0.88]; P = .009) and hematoma expansion greater than 6 mL (OR, 0.55 [95% CI, 0.35-0.87]; P = .01) after adjustment for age, sex, history of hypertension, history of smoking, baseline international normalized ratio, baseline ICH volume, and minutes to baseline scan (Table 3).
Table 2. Neuroimaging Characteristics in Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 Trial Patients With Deep Intracerebral Hemorrhages.
| Variable | Mean (SD) | P Value | |
|---|---|---|---|
| Standard Treatment (n = 375) | Intensive Treatment (n = 405) | ||
| Time from symptom onset to baseline scan, min | 98 (49) | 101 (53) | .36 |
| Intracerebral hemorrhage volume, mL | |||
| Baseline | 12.6 (11.1) | 12.0 (10.6) | .50 |
| At 24 h | 15.5 (15.4) | 14.0 (13.6) | .16 |
| Hematoma expansion volume, mL | 4.0 (9.1) | 3.0 (8.7) | .24 |
| Intraventricular hemorrhage present at baseline, No. (%) | 111 (29.6) | 105 (25.9) | .29 |
| Intraventricular hemorrhage volume, mL | |||
| Baseline | 2.4 (6.1) | 2.0 (5.5) | .42 |
| At 24 h | 3.0 (7.8) | 2.3 (5.4) | .16 |
| Hematoma expansion, No. (%) | |||
| >33% | 95 (25.3) | 70 (17.3) | .008 |
| >6 mL | 70 (18.7) | 44 (10.9) | .003 |
Table 3. Association Between Intensive Blood Pressure Reduction, Hematoma Expansion, and Functional Outcome by Deep Intracerebral Hemorrhage Location.
| Outcome | All Deep Locations (n = 780) | Basal Ganglia (n = 444) | Thalamus (n = 336) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Hematoma expansion >33%a | 0.62 (0.43-0.87) | .006 | 0.61 (0.42-0.88) | .009 | 0.44 (0.27-0.69) | .001 | 0.44 (0.27-0.72) | .001 | 0.97 (0.57-1.66) | .93 | 0.91 (0.51-1.64) | .76 |
| Hematoma expansion >6 mLa | 0.53 (0.35-0.79) | .002 | 0.55 (0.35-0.87) | .01 | 0.35 (0.21-0.59) | <.001 | 0.38 (0.21-0.66) | <.001 | 1.14 (0.57-2.31) | .72 | 1.21 (0.55-2.70) | .64 |
| Poor outcome b | 1.00 (0.74-1.34) | .98 | 1.22 (0.84-1.75) | .30 | 1.02 (0.68-1.53) | .92 | 1.39 (0.84-2.31) | .20 | 0.85 (0.56-1.30) | .46 | 1.02 (0.58-1.77) | .96 |
The multivariable model includes age, sex, history of hypertension, history of smoking, baseline international normalized ratio, baseline intracerebral hemorrhage volume (natural log transformed), and minutes to baseline scan.
Defined by a modified Rankin Scale score of 4 to 6 points. The multivariable model includes age, sex, Glasgow Coma Scale score, baseline intracerebral hemorrhage volume (natural log transformed), presence of intraventricular hemorrhage, and hematoma expansion.
Outcome Modification by Specific Deep Structure Involved
Specific deep location (thalamus vs basal ganglia) modified the association of treatment with hematoma expansion greater than 33% (interaction term: OR, 0.44 [95% CI, 0.22-0.96]; P = .02). Intensive BP reduction decreased the risk of hematoma expansion greater than 33% in basal ganglia ICH (OR, 0.44 [95% CI, 0.27-0.72]; P = .001) but not thalamic ICH (OR, 0.91 [95% CI, 0.51-1.64]; P = .76) (Table 3). The same association was seen when using hematoma expansion greater than 6 mL; intensive BP reduction decreased the risk of hematoma expansion greater than 6 mL in basal ganglia ICH (OR, 0.38 [95% CI, 0.21-0.66]; P < .001) but not thalamic ICH (OR, 1.21 [95% CI, 0.55-2.70]; P = .64) (Table 3). The mean absolute volume of hematoma expansion was smaller in the intensive vs standard group in patients with basal ganglia ICH (mean [SD], 4.6 [9.0] mL vs 2.6 [70] mL; P = .02) but not in patients with thalamic ICH (mean [SD], 3.2 [9.0] mL vs 3.5 [10.0] mL; P = .74).
Association Between Intensive Treatment and Outcome
A total of 754 of 780 patients (96.7%) had complete available outcome data. Overall, 714 patients (94.6%) with deep ICH were alive at 3 months and 470 of the 754 patients (62.3%) had a favorable functional outcome (mRS score, 0-3 points). There was no difference in the proportion of patients who died (21 of 405 [5.2%] vs 20 of 375 [5.3%]; P = .92) or who had a poor outcome at 3 months (148 of 405 [36.5%] vs 137 of 375 [36.5%]; P = .93) between intensive and standard treatment groups. In ordinal analysis, there was no difference in the distribution of 3-month mRS scores between patients in the intensive and the standard treatment groups in deep, basal ganglia, or thalamic ICH (Table 4). A post hoc power analysis assuming an α of .05 and a sample size of 444 patients indicated that the present analysis has 55% power to detect the absolute risk reduction of 10% that was originally stipulated by the trial.
Table 4. Distribution of Scores on the Modified Rankin Scale by Deep Intracerebral Hemorrhage Location.
| Modified Rankin Score at 3 mo | Patients, No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Deep Location (n = 754) | Basal Ganglia (n = 430) | Thalamus (n = 324) | |||||||
| Standard (n = 364) | Intensive (n = 390) | P Value | Standard (n = 211) | Intensive (n = 219) | P Value | Standard (n = 153) | Intensive (n = 171) | P Value | |
| 0 | 22 (6.0) | 17 (4.4) | .88 | 15 (7.1) | 10 (4.6) | .93 | 7 (4.6) | 7 (4.1) | .83 |
| 1 | 69 (19.0) | 83 (21.3) | 49 (23.2) | 59 (26.9) | 20 (13.1) | 24 (14.0) | |||
| 2 | 64 (17.6) | 79 (20.3) | 35 (16.6) | 41 (18.7) | 29 (19.0) | 38 (22.2) | |||
| 3 | 71 (19.5) | 64 (16.4) | 43 (20.4) | 37 (16.9) | 28 (18.3) | 27 (15.8) | |||
| 4 | 106 (29.1) | 106 (27.2) | 56 (26.5) | 55 (25.1) | 50 (32.7) | 51 (29.8) | |||
| 5 | 12 (3.3) | 20 (5.1) | 3 (1.4) | 9 (4.1) | 9 (5.9) | 11 (6.4) | |||
| 6 | 20 (5.5) | 21 (5.4) | 10 (4.7) | 8 (3.7) | 10 (6.5) | 13 (7.6) | |||
Discussion
We report an exploratory analysis of the ATACH-2 trial testing the hypothesis that intensive BP reduction decreases hematoma expansion specifically in hemorrhages located in deep structures of the brain. Importantly, baseline characteristics across the treatment and control groups remained balanced, as they were in the original trial, after restricting the analysis to patients with deep ICH, the subgroup of interest in this analysis. We found that intensive BP reduction is associated with a decreased risk of hematoma expansion in this specific type of ICH. We also found outcome modification by the specific deep-brain nucleus compromised by the hemorrhage. Stratified analyses evaluating each of these nuclei identified the basal ganglia as the deep-brain structure driving most of the association. Intensive BP reduction was not associated with improvements in the distribution of functional outcomes in this population, although this analysis was not appropriately powered.
The association between intensive BP reduction and decreased risk of hematoma expansion in deep ICH is consistent with the hypertensive cause of this disease. Genetic studies have shown that the burden of BP-associated alleles is associated both with the risk of developing deep ICH and severity of the disease through larger hematoma volumes.6,7,8,9 In this model, more severe hypertensive vasculopathy would predispose a patient to additional rupture of small vessels in the area of the ICH, probably triggered by a BP surge,18 resulting in larger hematoma volumes and increased risk of hematoma expansion. Therefore, the observed association between intensive BP reduction and decreased hematoma expansion in deep ICH could be explained by less rupture of surrounding diseased small vessels in the setting of reduced BP.
The finding that intensive treatment is associated with a reduced risk of hematoma expansion in the basal ganglia, but not the thalamus, raises important hypotheses about pathophysiological and clinical differences among ICH in deep structures. While ICH in both the thalamus and basal ganglia is thought to arise mostly from hypertension-associated cerebral small vessel disease,7 these structures are supplied by different vascular territories. There may be underlying biological differences in the response of these territories to short-term changes in BP that could be translated into different risks of hematoma expansion. Another explanation for this observation stems from the different anatomic structures surrounding each of these deep nuclei. Basal ganglia hemorrhages often dissect the surrounding parenchyma and expand into adjacent deep structures or white matter, whereas expansion in thalamic hemorrhages often results in decompression of the hematoma into the ventricular system with consequent intraventricular hemorrhage.19,20,21 Further research is needed to determine the specific biological differences that result in these analytical differences when evaluating each deep brain nuclei separately.
While deep ICH has often been grouped as a single phenotype in ICH studies, these results provide evidence of clinically significant differences in ICH by specific deep location. Basal ganglia ICH, specifically cases in the putamen, are believed to be almost exclusively the consequence of hypertension-associated pathophysiology.7 The clinical consequences of these hemorrhages are likely a direct result of parenchymal hematoma volume and expansion with minimal confounding from intraventricular hemorrhage, hydrocephalus, and other neuroanatomic factors.22 Basal ganglia ICH therefore represents an ideal population for proof-of-concept trials of new therapies targeting primary injury, in which interventions are likely to show the greatest treatment outcome.23 This approach of targeted patient selection has been successful in several recent ischemic stroke trials.24,25,26
Despite a robust association between intensive BP reduction and risk of hematoma expansion, this study failed to find an association between the intervention and functional outcome. This result was somewhat expected, because the analysis was not adequately powered to detect the 10% risk difference in outcome pursued by the trial after restricting the original study population to those with deep hemorrhages. It is also possible that the null result for the clinical outcome was driven by a statistically significant but biologically small effect of intensive BP treatment on hematoma expansion; this idea relies on the assumption that the observed association accurately represents a causal effect.27
Limitations
This study has limitations to consider. First, as a post hoc analysis of a randomized clinical trial, these results could represent a false-positive association caused by stratification of the study population and repeated testing. However, the preserved balance of baseline characteristics between the treatment groups after restricting the original study population to deep ICH and the use of a Bonferroni-corrected P value make this scenario unlikely. Second, with stratification by specific deep location, we had limited power to detect differences in outcome between the treatment groups. Finally, the results of the study are limited by the lack of replication. Further research is needed to determine if these observations can be reproduced in past and/or future trials of intensive BP treatment in ICH.
Conclusions
In conclusion, we report the results of an exploratory analysis of the ATACH-2 trial focused on deep ICH. We found that intensive BP reduction within 4.5 hours of symptom onset is associated with a decreased risk of hematoma expansion in patients with deep ICH. Interaction analysis followed by stratified analysis indicated that this association was driven by hemorrhages located in the basal ganglia. We did not see improved outcomes in this population. Whether this reduction in hematoma expansion can translate into clinical benefit warrants further study.
References
- 1.Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392(10154):1257-1268. doi: 10.1016/S0140-6736(18)31878-6 [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632-1644. doi: 10.1016/S0140-6736(09)60371-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167-176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 4.Sreekrishnan A, Dearborn JL, Greer DM, et al. Intracerebral hemorrhage location and functional outcomes of patients: a systematic literature review and meta-analysis. Neurocrit Care. 2016;25(3):384-391. doi: 10.1007/s12028-016-0276-4 [DOI] [PubMed] [Google Scholar]
- 5.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65(4):518-522. doi: 10.1212/01.wnl.0000172915.71933.00 [DOI] [PubMed] [Google Scholar]
- 6.Falcone GJ, Biffi A, Devan WJ, et al. ; GOCHA Investigators . Burden of blood pressure-related alleles is associated with larger hematoma volume and worse outcome in intracerebral hemorrhage. Stroke. 2013;44(2):321-326. doi: 10.1161/STROKEAHA.112.675181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982;32(8):871-876. doi: 10.1212/WNL.32.8.871 [DOI] [PubMed] [Google Scholar]
- 8.Falcone GJ, Biffi A, Devan WJ, et al. ; International Stroke Genetics Consortium . Burden of risk alleles for hypertension increases risk of intracerebral hemorrhage. Stroke. 2012;43(11):2877-2883. doi: 10.1161/STROKEAHA.112.659755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcone GJ, Biffi A, Brouwers HB, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70(8):988-994. doi: 10.1001/jamaneurol.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcone GJ, Woo D. Genetics of spontaneous intracerebral hemorrhage. Stroke. 2017;48(12):3420-3424. doi: 10.1161/STROKEAHA.117.017072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Palesch YY, Barsan WG, et al. ; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network . Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375(11):1033-1043. doi: 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson CS, Heeley E, Huang Y, et al. ; INTERACT2 Investigators . Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355-2365. doi: 10.1056/NEJMoa1214609 [DOI] [PubMed] [Google Scholar]
- 13.Toyoda K, Koga M, Yamamoto H, et al. ; ATACH-2 Trial Investigators . Clinical outcomes depending on acute blood pressure after cerebral hemorrhage. Ann Neurol. 2019;85(1):105-113. doi: 10.1002/ana.25379 [DOI] [PubMed] [Google Scholar]
- 14.Al-Shahi Salman R, Frantzias J, Lee RJ, et al. ; VISTA-ICH Collaboration; ICH Growth Individual Patient Data Meta-analysis Collaborators . Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885-894. doi: 10.1016/S1474-4422(18)30253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE; VISTA Collaboration . Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238-1244. doi: 10.1212/WNL.0b013e3182143317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35(3):195-201. doi: 10.1159/000346599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care. 2011;15(3):559-576. doi: 10.1007/s12028-011-9538-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer U, Cooney MT, Bull LM, et al. Acute post-stroke blood pressure relative to premorbid levels in intracerebral haemorrhage versus major ischaemic stroke: a population-based study. Lancet Neurol. 2014;13(4):374-384. doi: 10.1016/S1474-4422(14)70031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delcourt C, Sato S, Zhang S, et al. ; INTERACT2 Investigators . Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology. 2017;88(15):1408-1414. doi: 10.1212/WNL.0000000000003771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59(4):767-773. doi: 10.1227/01.NEU.0000232837.34992.32 [DOI] [PubMed] [Google Scholar]
- 21.Hanley DF. Intraventricular hemorrhage and ICH outcomes: Severity factor and treatment target. Stroke J Cereb Circ. 2009;40(4):1533-1538. doi: 10.1161/STROKEAHA.108.535419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheth KN, Rosand J. Targeting the immune system in intracerebral hemorrhage. JAMA Neurol. 2014;71(9):1083-1084. doi: 10.1001/jamaneurol.2014.1653 [DOI] [PubMed] [Google Scholar]
- 23.Mayer SA, Davis SM, Skolnick BE, et al. ; FAST trial investigators . Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke. 2009;40(3):833-840. doi: 10.1161/STROKEAHA.108.524470 [DOI] [PubMed] [Google Scholar]
- 24.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 26.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer SA, Brun NC, Begtrup K, et al. ; FAST Trial Investigators . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]