Abstract
Purpose of Review
Spinal deformity is a common issue in pediatric patients with an underlying neurological diagnosis or syndrome. Management of neuromuscular scoliosis (NMS) is a major part of the orthopedic care of such patients, as the deformity is often progressive, and may affect gait, seating and positioning. In addition, untreated large spinal deformities may be associated with pain and/or cardiopulmonary issues over time.
Recent Findings
Recent changes in medical management of the underlying disease process appears to alter the natural history of certain neuromuscular conditions, and in the case of patients with Duchenne’s muscular dystrophy significantly diminish the incidence of spinal deformity. In the most common diagnosis associated with NMS, cerebral palsy, there is evidence that despite a high complication rate, surgical management of spinal deformity is associated with measurable improvements in validated health-related quality-of-life measures.
Summary
Spinal deformity is a common finding in patients with neurological diagnoses. It is important for those involved in the care of these patients to understand the natural history of NMS, as well as the potential risks and benefits to the patient and caregivers, of surgical and non-surgical interventions.
Keywords: Neuromuscular scoliosis, Cerebral palsy, Caregiver/patient outcome studies
Introduction
The term neuromuscular scoliosis (NMS) describes a non-congenital spinal deformity that occurs in patients with any type of pre-existing neuromuscular diagnosis (Table 1). NMS may present at any age; and in many cases progresses relentlessly, particularly in patients with more severe neurologic and systemic involvement. Progressive deformity in these patients may limit mobility and seating status, and may be associated with primary or secondary effects on cardiopulmonary function over time.
Table 1.
Common diagnoses responsible for neuromuscular scoliosis
| Cerebral palsy | |
| Duchenne muscular dystrophy | |
| Myelomeningocele | |
| Spinal muscular atrophy | |
| Friedrich ataxia | |
| Spinal cord injury |
Management of NMS includes observation, non-operative interventions such as bracing, specialized seating systems, and surgery. The risks of operative and non-operative treatments of spinal deformity in this patient population are well-documented in the literature, but there are few studies with high levels of evidence. In addition, the potential benefits of intervention, as well as the effects on quality of life, both for the patient and caregivers, remain under investigation at this time. The purpose of this monograph is to review the existing literature, with a focus on newer reports, regarding this complicated subject, and by doing so generate a description of the current state of management of NMS in pediatric patients.
Natural History
It is important to understand that the term NMS includes a large number of underlying diagnoses and pathologies. While the orthopedic manifestations of many of these patients may be similar, the nuances and complexity of the various underlying medical conditions limits complete generalization. As such, natural history data generated from patients with more common diagnoses (such as cerebral palsy) tends to be generalized to patients with less common pathologic situations, but some subtle differences do exist.
Data from two Swedish studies provides some of the most complete epidemiologic information in the literature regarding the incidence of NMS [1]. These authors assessed the incidence of NMS in 666 patients with cerebral palsy. They found that the risk of developing NMS increased with increasing patient age and GMFCS (gross motor function classification scale) level [1]. A larger follow-up population study published in 2018 confirmed the findings of the previous study and demonstrated that approximately 8% of GMFCS V patients had clinical evidence of scoliosis before 5 years of age [2]. By age 20, up to 75% of patients at that functional level had a spinal deformity with a Cobb angle greater than or equal to 40°.
While limited in number, studies do exist that assess the natural history of curve progression in patients with untreated NMS. Fujak et al. [3] reported on patients with untreated spinal deformity secondary to spinal muscular atrophy type II and III. They found early incidence and rapid progression of spinal deformity, along with simultaneous deterioration of pulmonary function. Gu et al. [4] reviewed 110 pediatric patients with a diagnosis of spastic quadriplegic CP. The authors determined that those patients with a deformity > 40° by the age of 12 were more likely to progress than those with a curve of ≤ 40° by the same age. Natural history studies regarding spinal deformity also exist for patients with Duchene’s muscular dystrophy [5, 6] and pediatric spinal cord injuries [7, 8], both of which have a known high incidence of spinal deformity and risk of progression.
Regardless of the diagnosis, the result of a progressive NMS deformity is similar. In many cases, the deformity may be associated with difficulties in daily care and positioning, as well as ambulation (in ambulatory patients) and wheelchair positioning or sitting balance in those unable to walk or stand. Secondary effects of these issues on family and caregivers may become evident. Over time, the deformity may contribute to pain, alterations in skin integrity (Fig. 1), as well as pulmonary and cardiac compromise secondary to mechanical effects on thoracic volume and compliance.
Fig. 1.
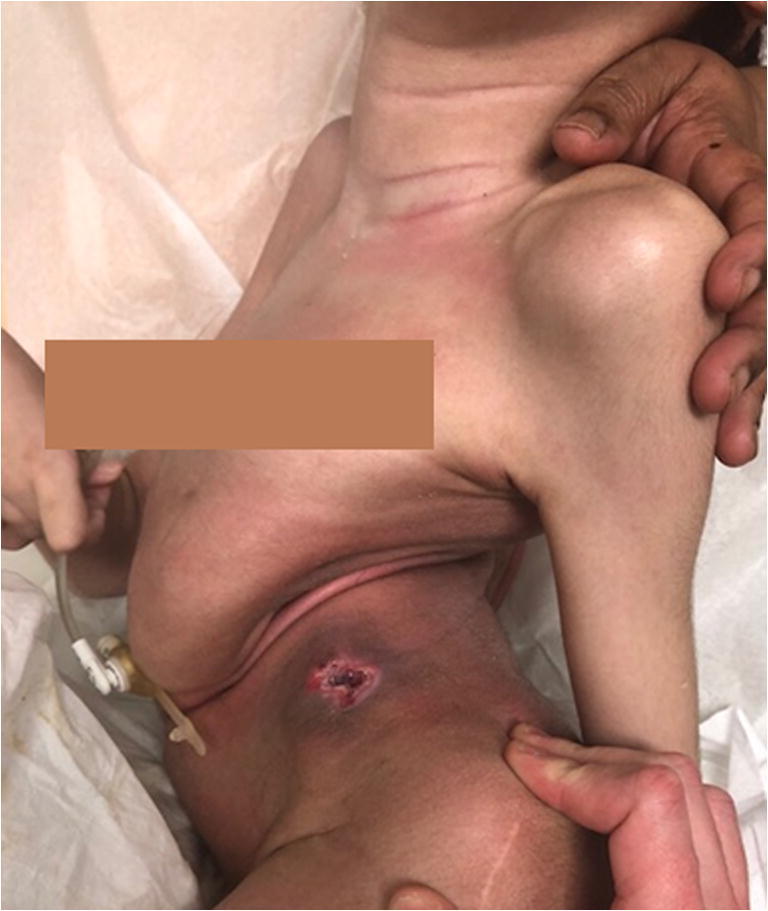
NMS patient with CP and severe curve leading to the ribs resting on the iliac crest. This has resulted in ulceration
Non-operative Management
The initial intervention in most patients with NMS is non-operative. However, the efficacy of such interventions has proven to be very limited. Bracing appears to have little effect on the progression of NMS, acting primarily as an external support for sitting balance in those patients with flexible curves. [9, 10]As the deformity progresses, the brace becomes less effective at either maintaining in-brace correction or aiding with upright positioning. Olafsson et al. [10] showed some limitation on curve progression in ambulatory patients with smaller, lumbar curves. Nakamura et al. [11] demonstrated improvements in Cobb angle, as well as sitting stability and caregiver satisfaction in a group of patients treated with a dynamic 3-point molded brace. Overall, long-term bracing for NMS is rarely effective.
Custom seating systems are utilized in an attempt to compensate for a pre-existing NMS deformity, and to allow better upright positioning. Unfortunately, these systems are costly, and often require significant time to design, fashion and procure. In many cases, the delivered product no longer fits adequately due to progression of the deformity during the time between measurement and delivery.
The most direct method of non-operative management of NMS in some conditions may be the minimization of occurrence through forms of medical treatment of the underlying disease process. This has been evident in patients with Duchenne’s muscular dystrophy (DMD) treated with steroids. Prior data on the natural history of patients with DMD was almost universal development of scoliosis once the patient becomes non-ambulatory [12]. In 2013, Lebel et al. [13] provided long-term follow-up of steroid treatment in DMD, which appeared to alter the natural history for these patients. The authors demonstrated a significantly increased survival rate, as well as a significant decrease in the incidence of scoliosis, in the steroid treatment group versus an untreated cohort.
Spinal deformity is a common finding in patients with spinal muscular atrophy (SMA) [14], and is generally associated with the more severe types of the disease. Non-operative management with bracing is rarely used due to potential constrictive effects on an already compromised respiratory system, as well as minimal efficacy. Early surgical treatment may be indicated in an attempt to control progression of the deformity [15]. Recent studies have shown significant improvement of motor function in SMA patients treated with intrathecal injections of an antisense oligonucleotide medication (Nusinersen) versus a group receiving sham injections [16••]. While no long-term studies of this medical treatment currently exist, it seems reasonable to believe that changing the natural history of SMA through medical treatment may have an effect on the incidence and progression of NMS associated with the untreated disease process.
Operative Treatment
Indications for operative intervention for patients with NMS many vary with the disease etiology and individual clinical circumstances of each patient and family. However, the main indications for surgical treatment in this patient population are progressive deformity with unacceptable truncal shift or pelvic obliquity that affects standing or sitting balance/positioning. These patients may have had attempts at non-operative intervention, but often have received no prior treatment. In the non-ambulatory patient population, skin ulcerations, difficulty with hygiene and inability to be positioned adequately in a wheelchair are among common sources of caregiver dissatisfaction, which may lead families to seek surgical care (Fig. 1).
As in cases of idiopathic and congenital scoliosis, the goals of surgery for NMS are to obtain a solid bony arthrodesis of the spine to prevent further deformity progression, to correct the deformity of the spine and pelvis, and to restore overall standing or seated balance. As the majority of NMS surgery is performed in non-ambulatory patients, the fundamental end goal is the creation and long-term maintenance of upright posture. Technical considerations in this patient population include the surgical approach used for the procedure, as well as the proximal and distal extent of the instrumentation and fusion.
Because of the need for a long and extensive instrumentation and fusion, most NMS patient undergo a posterior spinal fusion. There are limited reports of isolated anterior instrumentation procedures for the management of NMS [17]. Indications for the use of combined anterior and posterior procedures for NMS have changed over time. In the past, combined procedures were common in this patient population. Indications for the anterior portion of the intervention included rigid deformities, severe pelvic obliquity, poor posterior bone for fusion or a historically high risk of instrument failure and pseudarthrosis with posterior-only surgery. Recent technical advances in spinal implants (greater use of pedicle-based implants, greater rod size/stiffness), as well as more wide-spread use of intra-operative skeletal traction or traction-like devices to aid in correction of the deformity, have led to a significant decrease in the use of anterior spinal procedures in the NMS population [18, 19•].
Most NMS patients undergoing deformity correction and fusion, instrumentation is extended to the proximal thoracic spine (frequently T1 or T2) to aid in the correction of any existing thoracic kyphosis. In addition, this proximal extension may minimize the risks of post-surgical proximal junctional kyphosis. In 2003, Sink et al. [20] reviewed the sagittal profile of 41 CP patients who underwent spinal arthrodesis. These authors found a proximal loss of correction or implant failure in 32% of patients, and found that pre-existing thoracic hyperkyphosis was a risk factor for failure.
Due to the high incidence of significant pelvic obliquity in NMS patients, the instrumentation and fusion is often extended distally to include the pelvis. There is some evidence that the distal extent of the instrumentation can be limited to the lower lumbar spine in patients with minimal pre-existing pelvic obliquity. Patients with flaccid, or non-spastic NMS (e.g., DMD, SMA) may not develop the degree of pelvic obliquity found in spastic (e.g., CP) NMS patient. Takaso et al. [21] reviewed 27 patients with flaccid CP with underwent fusion with distal extent to L5. These authors noted no increase in pelvic obliquity with no major complications at 2-year minimum follow-up. McCall and Hayes [22] reported their results comparing fusing to L5 or the sacrum in NMS patients with a unit rod. These authors found lower rates of blood loss and complications with stopping the distal construct at L5, with satisfactory rates of correction of scoliosis and pelvic obliquity.
However, the vast majority of neuromuscular patients, particularly those who are non-ambulatory, appear best served by distal extension of the instrumentation and fusion to include the pelvis [23]. This allows maximum correction of what is often severe and rigid pelvic obliquity. Spinopelvic fixation has evolved over time. In addition, some types of pelvic stabilization have applications to patients with NMS associated with specific diagnoses. In cases of NMS in patients with upper lumbar or lower thoracic myeolodysplasia, the Warner-Fackler rod modification may provide an attractive, low profile alternative for distal fixation [24]. This method of fixation is amenable only to patients with NMS associated with myelodysplasia, due to the unique anatomic and neurologic features of this population. The unit rod, used with either sublaminar wiring or hybrid hook/wire/screw construct, has a long history of success in patients with NMS. However, this device requires significant skills with complex rod bending, and has been largely replaced with other types of segmental spinopelvic fixation. The Dunn-McCarthy method of spino-sacral fixation has been used in some centers with success, but is of limited use at this time [25].
Once segmental spinal instrumentation became the norm for management of pediatric spinal deformities, use of iliac screws or bolts and connection of these implants to the main construct directly or with offset connectors was commonplace [26]. However, these bolts are placed into the ilium through a posterior superior iliac spine entry point, which is often an area of relative prominence in many patients. Coupled with frequent malnutrition and thin habitus in NMS patients, these implants may become painful, and may lead to skin breakdown and infection (Fig. 2a,b).
Fig. 2.
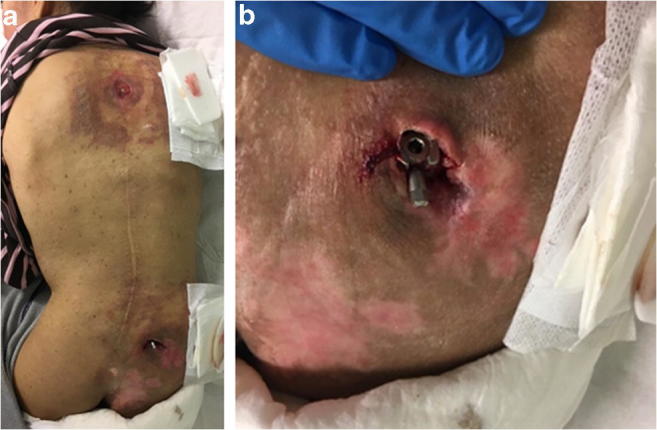
a and b NMS patient with low body mass index 10 years following PSF with placement of PSIS starting point iliac bolts. Note the prominent distal hardware and the exposure of the screw tulip and set screw outside the wound
In an effort to minimize this potential complication, the S2AI (sacral alar iliac screw) has been proposed and validated as a low profile option for pelvic fixation [27, 28]. This screw is started adjacent to the S2 foramen, crosses the SI joint, and enters the ilium. This starting point affords two main benefits when compared to traditional implants entering the pelvis through the posterior superior iliac spine. As it is more in line with the remainder of the lumbar implants, there is generally no need for a separate connector, which increases the overall stability of the construct. (Fig. 3) In addition, the screw is placed in an area of the pelvis with a larger covering soft tissue envelope, thus minimizing the risk of prominent implants. In a recent review of 38 patients with NMS and 5-year follow-up after S2AI screw placement, the authors reported 57% correction in pelvic obliquity. Only eight patients demonstrated screw lucency, and four patients developed a deep infection [29•].
Fig. 3.
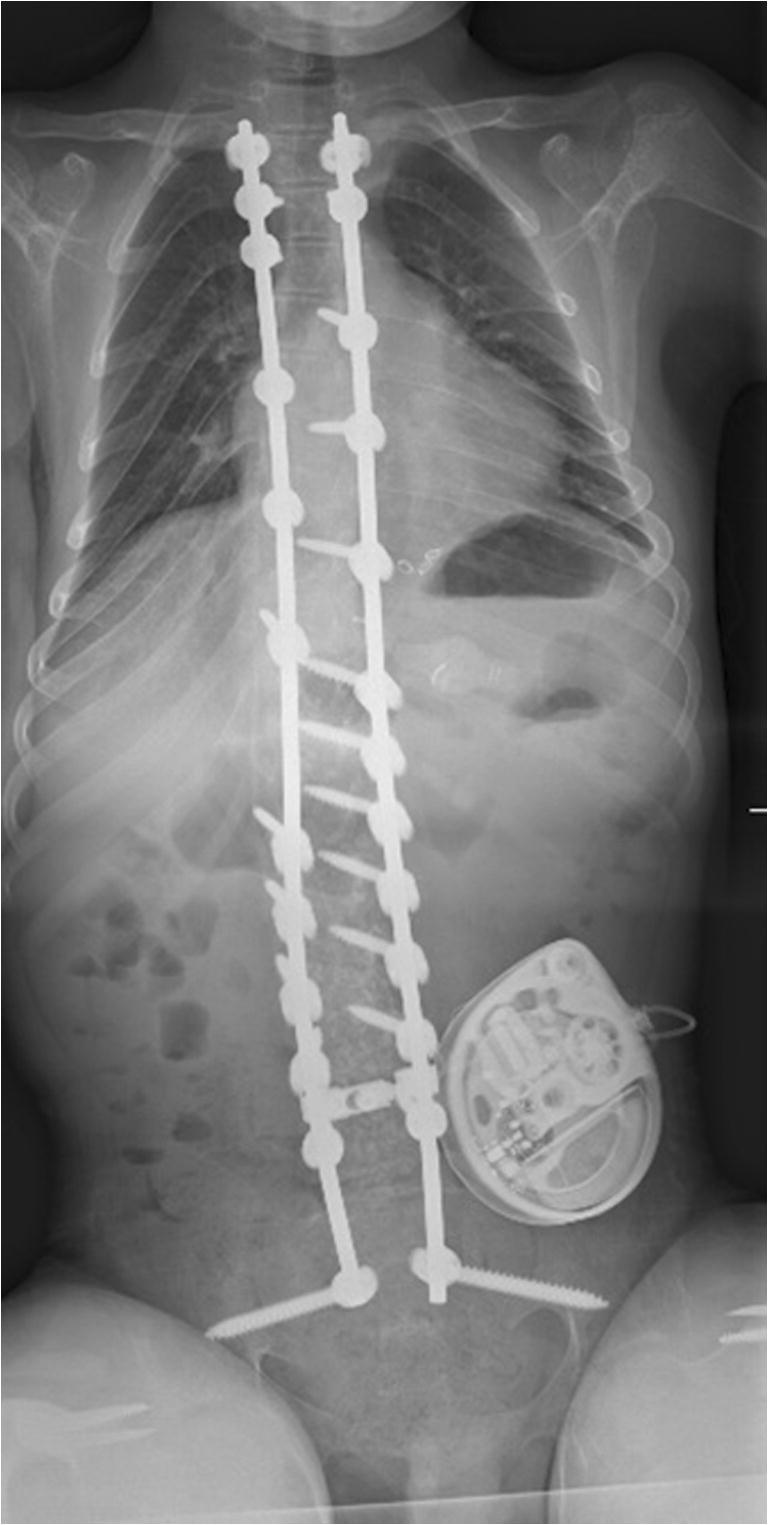
Upright AP full-length radiograph of a NMS patient who underwent distal fixation with S2AI screws. Note that the screws are in line with the remainder of the fusion construct
Lee et al. [30] recently compared traditional iliac screws to S2AI screws in NMS patients. There were no differences between the groups in regard to postoperative complications or infections. S2AI screws were noted to have a significantly lower rate of implant failure than traditional iliac screws; however, the rates of complications were equivalent for the two patient cohorts.
Complications
It is well documented that the risk of intraoperative and postoperative complications is higher in patients treated surgically for NMS when compared to idiopathic scoliosis patients managed with surgery [31]. The most common complications of operative treatment of NMS patients are blood loss, infection, respiratory distress, gastrointestinal distress, implant-related complications, and death [32, 33].
Several large series of patients with NMS and rates of complications have been published recently. Rumalla et al. [34] reviewed 2154 NMS fusion cases from 2002 to 2011 from a national inpatient database, and reported an overall complication rate of 40.1%.Turturro et al. [35] reviewed a single center consecutive series of 185 NMS patients over a 30-year time period. These authors reported a rate of adverse events that approached 30%, but specified that the rate of complications that affected the final care of the patient was approximately 9%. Cognetti et al [36•] reviewed the Scoliosis Research Society Morbidity and Mortality Database from 2004 to 2015 and reported 1385 complications in 29,019 patients, a rate of 6%. These authors noted that over the study period, there was a 3.5-fold decrease in the rate of complications, with a notable decrease in wound infections, respiratory complications, and implant-related complications. Toll et al. [37] reviewed 102 patients and found a rate of complications of 27%. Eighty percent of those were considered major complications. These authors found that patients with preexisting pulmonary conditions and higher intraoperative blood loss were at higher risk of developing complications.
There is frequently a risk of considerable blood loss associated with NMS surgery due to the lengthy dissection needed for the extensive fusion, as well as other complicating patient-related issues. Patients with NMS (especially CP) are known to have the highest risk of blood loss in posterior spinal fusion surgery [38]. Several strategies can be utilized to manage intraoperative blood loss in NMS patients. These include use of intraoperative hypotension to diminish perfusion to the dissection field, use of autologous return of intraoperative blood loss to the patient [39] (CellSaver, Haemonetics, Braintree, MA) and the use of anti-fibrinolytic medications [40] during the surgical procedure. In a recent report by Hardesty et al [41•], the use of a bipolar sealant device (Aquamantys, Medtronic, Minneapolis, MN) can diminish intraoperative blood loss and decrease rates of transfusion for NMS patients.
Rates of postoperative infection in neuromuscular scoliosis patients in some series can exceed 20%. These risks are increased due to the inherently fragile nature of the patient, as well the extensive surgery and physiologic load on the patient postoperatively Furthermore, many of these patients are susceptible to post-operative infection with different (and in some cases more virulent) organism than idiopathic patients. Strategies to diminish risk of infection may include restoration of adequate nutritional status of the patient prior to surgery [42] and use of aggressive intravenous and local antibiotics at the time of surgery [43].
In a large 30-year review of a single center experience with infection following spinal fusion for neuromuscular scoliosis, Ramo et al. [44] reported a deep infection rate of 10% in a cohort of 428 patients. Seventy-three percent of the infections were diagnosed within 12 months. Risk factors for infection included myelomeningocele, high body mass index, and incontinence. Modifiable surgical factors associated with risk of infection included inadequate prophylactic antibiotics and drain usage. If a postoperative infection occurs, it should be treated aggressively with a combination of operative debridements and parenteral antibiotics. Use of negative pressure wound suction systems can also be a beneficial adjunct in the care of NMS patients with a deep wound infection [45]. While a post-operative infection may be a lengthy ordeal, recent data has demonstrated that the risk of subsequent or recurrent deep infection is low in those patients who undergo comprehensive management of a post-operative spinal infection [46•].
Outcomes
Determining the outcomes of care for patients with NMS has proven to be challenging. In light of the risk of complications, both surgical and medical, multiple authors have attempted to assess the benefits of surgical stabilization of NMS [47, 48••]. As the vast majority of NMS treated surgically involves minimally or non-ambulatory CP patients, almost all efforts to determine outcomes have been performed on this cohort of patients. Difficulties with direct patient communication have led to use of the parents or other caregivers as surrogates in many studies.
Cassidy et al. [49] compared two groups of institutionalized patients with CP in 1994. One group was managed surgically, and a similar group was managed non-operatively. The authors found no difference in pain or pulmonary issues, decubiti, or time for daily care. They did find that the majority of the patient caregivers felt that those patients treated surgically were more comfortable than those who were not.
In 2003, Jones et al. [50] published a prospective evaluation of 20 CP patients with NMS who underwent spinal deformity correction and fusion. They utilized a validated questionnaire that was given to parents and permanent caregivers of the patients preoperatively, at 6 months and then at 1 year postoperatively. They found that while there were no differences between pre- and postoperative assessments of physical function, comorbidities or parental health, there were significant improvements in apparent patient pain, happiness, and parental satisfaction at one-year post surgery as reported by the caregivers. Bohtz et al. [51] reviewed 50 consecutive patients with CP who had a minimum 2-year follow-up after spinal arthrodesis. They administered modified version of the “Caregiver Priorities and Child Health Index of Life with Disabilities” to the caregivers of the patients, and found a satisfaction rate of 92% with the procedure.
More recently, multiple reports have been published that focus on the pre- and postoperative evaluation of this population utilizing the CPCHILD instrument given to the parents or caregivers. Jain et al [52••] queried a multicenter registry of GMFCS level IV and V CP/NMS patients managed with surgical correction and fusion whose caregivers had completed preoperative and 2-year postoperative CPCHILD evaluations. They found that most caregivers felt that the overall quality of life, health, and comfort had improved with surgical management. In addition, spinal stabilization was ranked second only to gastrostomy tube placement as the most beneficial intervention in the patients’ lives by greater than 70% of the respondents.
Finally, Miyanji et al [53••] reported on the 5-year follow-up of a cohort of severely involved NMS patients managed surgically for whom the caregivers completed the CPCHILD questionnaire. Reports from the caregivers of 69 patients were analyzed. While they found a high overall complication rate (> 50%), there was no significant correlation between complications and scores postoperatively at any point in the follow-up period. In addition, there were significant improvements in personal care, positioning, and comfort throughout the period of evaluation.
It appears that there is evidence to support a positive effect of surgical treatment on patient outcomes in NMS studied, despite a known high rate of complications. While the data is indirect, as the patients themselves are often unable to participate in the evaluation process, at 5 years follow-up there is statistically significant evidence generated through validated instruments that supports surgical intervention. Longer follow-up will be necessary to determine whether these results persist over time, and questions regarding the effect of surgical management on patient life expectancy will remain.
Conclusion
Neuromuscular scoliosis (NMS) in children may occur in association with multiple diagnoses. The vast majority of the literature reports on patients with NMS associated with cerebral palsy, although NMS occurs with a high incidence in patients with Duchenne’s muscular dystrophy and spinal muscular atrophy as well. In general, a greater level of neurologic involvement correlates with the incidence, severity, and progression of the deformity.
In some diagnoses, medical management to limit the neurologic involvement and progression may limit the onset and progression of NMS (e.g., DMD and SMA). However, for severely involved patients with cerebral palsy and associated NMS, surgical management is often the only option other than supportive care. The risks of complications in patients after surgical intervention for NMS remain high, despite maximal prophylactic measures. Long-term assessment of the benefits of spinal fusion and instrumentation for NMS is ongoing. Recent studies have shown that use of validated questionnaire instruments, such as the CPCHILD, demonstrates positive effects of surgery for the patient and caregivers in most cases.
Compliance with Ethical Standards
Conflict of Interest
Robert F. Murphy and James F. Mooney, III each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pediatric Orthopedics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Persson-Bunke M, Hagglund G, Lauge-Pedersen H, Wagner P, Westbom L. Scoliosis in a total population of children with cerebral palsy. Spine. 2012;37:E708–E713. doi: 10.1097/BRS.0b013e318246a962. [DOI] [PubMed] [Google Scholar]
- 2.Hagglund G, Pettersson K, Czuba T, Persson-Bunke M, Rodby-Bousquet E. Incidence of scoliosis in cerebral palsy. Acta Orthop. 2018;89:443–447. doi: 10.1080/17453674.2018.1450091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujak A, Raab W, Schuh A, Richter S, Forst R, Forst J. Natural course of scoliosis in proximal spinal muscular atrophy type II and IIIa: descriptive clinical study with retrospective data collection of 126 patients. BMC Musculoskelet Disord. 2013;14:283. doi: 10.1186/1471-2474-14-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Y, Shelton JE, Ketchum JM, Cifu DX, Palmer D, Sparkman A, Jermer-Gu MK, Mendigorin M. Natural history of scoliosis in nonambulatory spastic tetraplegic cerebral palsy. PM R. 2011;3:27–32. doi: 10.1016/j.pmrj.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro F, Zurakowski D, Bui T, Darras BT. Progression of spinal deformity in wheelchair-dependent patients with Duchenne muscular dystrophy who are not treated with steroids: coronal plane (scoliosis) and sagittal plane (kyphosis, lordosis) deformity. Bone Joint J. 2014;96-B:100–105. doi: 10.1302/0301-620X.96B1.32117. [DOI] [PubMed] [Google Scholar]
- 6.Roberto R, Fritz A, Hagar Y, Boice B, Skalsky A, Hwang HS, Beckett L, McDonald C, Gupta M. The natural history of cardiac and pulmonary function decline in patients with Duchenne muscular dystrophy. Spine. 2011;36:E1009–E1017. doi: 10.1097/BRS.0b013e3181fea1ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulcahey MJ, Gaughan JP, Betz RR, Samdani AF, Barakat N, Hunter LN. Neuromuscular scoliosis in children with spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19:96–103. doi: 10.1310/sci1902-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zidek K, Srinivasan R. Rehabilitation of a child with a spinal cord injury. Semin Pediatr Neurol. 2003;10:140–150. doi: 10.1016/S1071-9091(03)00022-6. [DOI] [PubMed] [Google Scholar]
- 9.Saito N, Ebara S, Ohotsuka K, Kumeta H, Takaoka K. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687–1692. doi: 10.1016/S0140-6736(98)01302-6. [DOI] [PubMed] [Google Scholar]
- 10.Olafsson Y, Saraste H, Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop. 1999;19:376–379. [PubMed] [Google Scholar]
- 11.Nakamura N, Uesugi M, Inaba Y, Machida J, Okuzumi S, Saito T. Use of dynamic spinal brace in the management of neuromuscular scoliosis: a preliminary report. J Pediatr Orthop B. 2014;23:291–298. doi: 10.1097/BPB.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 12.Archer JE, Gardner AC, Roper HP, Chikermane AA, Tatman AJ. Duchenne muscular dystrophy: the management of scoliosis. J Spine Surg. 2016;2:185–194. doi: 10.21037/jss.2016.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebel DE, Corston JA, McAdam LC, Biggar WD, Alman BA. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am. 2013;95:1057–1061. doi: 10.2106/JBJS.L.01577. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DP, Roye DP, Jr, Farcy JP, Leet A, Shelton YA. Surgical treatment of scoliosis in a spinal muscular atrophy population. Spine. 1990;15:942–945. doi: 10.1097/00007632-199009000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Chandran S, McCarthy J, Noonan K, Mann D, Nemeth B, Guiliani T. Early treatment of scoliosis with growing rods in children with severe spinal muscular atrophy: a preliminary report. J Pediatr Orthop. 2011;31:450–454. doi: 10.1097/BPO.0b013e31821722b1. [DOI] [PubMed] [Google Scholar]
- 16••.Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 17.Tokala DP, Lam KS, Freeman BJ, Webb JK. Is there a role for selective anterior instrumentation in neuromuscular scoliosis? Eur Spine J. 2007;16:91–96. doi: 10.1007/s00586-006-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keeler KA, Lenke LG, Good CR, Bridwell KH, Sides B, Luhmann SJ. Spinal fusion for spastic neuromuscular scoliosis: is anterior releasing necessary when intraoperative halo-femoral traction is used? Spine. 2010;35:E427–E433. doi: 10.1097/BRS.0b013e3181d9527e. [DOI] [PubMed] [Google Scholar]
- 19•.Jackson TJ, Yaszay B, Pahys JM, et al. Intraoperative traction may be a viable alternative to anterior surgery in cerebral palsy scoliosis >/=100 degrees. J Pediatr Orthop. 2018;38:e278–ee84. doi: 10.1097/BPO.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 20.Sink EL, Newton PO, Mubarak SJ, Wenger DR. Maintenance of sagittal plane alignment after surgical correction of spinal deformity in patients with cerebral palsy. Spine. 2003;28:1396–1403. doi: 10.1097/01.BRS.0000067088.99346.73. [DOI] [PubMed] [Google Scholar]
- 21.Takaso M, Nakazawa T, Imura T, Fukuda M, Takahashi K, Ohtori S. Segmental pedicle screw instrumentation and fusion only to L5 in the surgical treatment of flaccid neuromuscular scoliosis. Spine. 2018;43:331–338. doi: 10.1097/BRS.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 22.McCall RE, Hayes B. Long-term outcome in neuromuscular scoliosis fused only to lumbar 5. Spine. 2005;30:2056–2060. doi: 10.1097/01.brs.0000178817.34368.16. [DOI] [PubMed] [Google Scholar]
- 23.Allen BL, Jr, Ferguson RL. The Galveston technique for L rod instrumentation of the scoliotic spine. Spine. 1982;7:276–284. doi: 10.1097/00007632-198205000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Warner WC, Jr, Fackler CD. Comparison of two instrumentation techniques in treatment of lumbar kyphosis in myelodysplasia. J Pediatr Orthop. 1993;13:704–708. doi: 10.1097/01241398-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy RE, Bruffett WL, McCullough FL. S rod fixation to the sacrum in patients with neuromuscular spinal deformities. Clin Orthop Relat Res. 1999;364:26–31. doi: 10.1097/00003086-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Arlet V, Marchesi D, Papin P, Aebi M. The ‘MW’ sacropelvic construct: an enhanced fixation of the lumbosacral junction in neuromuscular pelvic obliquity. Eur Spine J. 1999;8:229–231. doi: 10.1007/s005860050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang TL, Sponseller PD, Kebaish KM, Fishman EK. Low profile pelvic fixation: anatomic parameters for sacral alar-iliac fixation versus traditional iliac fixation. Spine. 2009;34:436–440. doi: 10.1097/BRS.0b013e318194128c. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien JR, Yu WD, Bhatnagar R, Sponseller P, Kebaish KM. An anatomic study of the S2 iliac technique for lumbopelvic screw placement. Spine. 2009;34:E439–E442. doi: 10.1097/BRS.0b013e3181a4e3e4. [DOI] [PubMed] [Google Scholar]
- 29.Jain A, Sullivan BT, Kuwabara A, Kebaish KM, Sponseller PD. Sacral-alar-iliac fixation in children with neuromuscular scoliosis: minimum 5-year follow-up. World. 2017;108:474–478. doi: 10.1016/j.wneu.2017.08.169. [DOI] [PubMed] [Google Scholar]
- 30.Lee MC, Jarvis C, Solomito MJ, Thomson JD. Comparison of S2-alar and traditional iliac screw pelvic fixation for pediatric neuromuscular deformity. Spine J. 2018;18:648–654. doi: 10.1016/j.spinee.2017.08.253. [DOI] [PubMed] [Google Scholar]
- 31.Reames DL, Smith JS, Fu KM, Polly DW Jr, Ames CP, Berven SH, Perra JH, Glassman SD, McCarthy R, Knapp RD Jr, Heary R, Shaffrey CI, Scoliosis Research Society Morbidity and Mortality Committee Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the Scoliosis Research Society Morbidity and Mortality database. Spine. 2011;36:1484–1491. doi: 10.1097/BRS.0b013e3181f3a326. [DOI] [PubMed] [Google Scholar]
- 32.Lovell WW, Weinstein SL, Flynn JM. Lovell and Winter’s pediatric orthopaedics. 7. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 33.Brooks JT, Sponseller PD. What’s new in the management of neuromuscular scoliosis. J Pediatr Orthop. 2016;36:627–633. doi: 10.1097/BPO.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 34.Rumalla K, Yarbrough CK, Pugely AJ, Koester L, Dorward IG. Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications, and in-hospital outcomes. J Neurosurg Spine. 2016;25:500–508. doi: 10.3171/2016.2.SPINE151377. [DOI] [PubMed] [Google Scholar]
- 35.Turturro F, Montanaro A, Calderaro C, Labianca L, Di Sanzo V, Ferretti A. Rate of complications due to neuromuscular scoliosis spine surgery in a 30-years consecutive series. Eur Spine J. 2017;26:539–545. doi: 10.1007/s00586-017-5034-6. [DOI] [PubMed] [Google Scholar]
- 36•.Cognetti D, Keeny HM, Samdani AF, et al. Neuromuscular scoliosis complication rates from 2004 to 2015: a report from the Scoliosis Research Society Morbidity and Mortality database. Neurosurg Focus. 2017;43:E10. doi: 10.3171/2017.7.FOCUS17384. [DOI] [PubMed] [Google Scholar]
- 37.Toll BJ, Samdani AF, Janjua MB, Gandhi S, Pahys JM, Hwang SW. Perioperative complications and risk factors in neuromuscular scoliosis surgery. J Neurosurg Pediatr 2018;22(2):207–213. 10.3171/2018.2.PEDS17724. [DOI] [PubMed]
- 38.Jain A, Njoku DB, Sponseller PD. Does patient diagnosis predict blood loss during posterior spinal fusion in children? Spine. 2012;37:1683–1687. doi: 10.1097/BRS.0b013e318254168f. [DOI] [PubMed] [Google Scholar]
- 39.Michelet D, Julien-Marsollier F, Hilly J, Diallo T, Vidal C, Dahmani S. Predictive factors of intraoperative cell salvage during pediatric scoliosis surgery. Cell saver during scoliosis surgery in children. Anaesth Crit Care Pain Med. 2018;37:141–146. doi: 10.1016/j.accpm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Thompson GH, Florentino-Pineda I, Poe-Kochert C, Armstrong DG, Son-Hing J. Role of Amicar in surgery for neuromuscular scoliosis. Spine. 2008;33:2623–2629. doi: 10.1097/BRS.0b013e318187c046. [DOI] [PubMed] [Google Scholar]
- 41.Hardesty CK, Gordon ZL, Poe-Kochert C, Son-Hing JP, Thompson GH. Bipolar sealer devices used in posterior spinal fusion for neuromuscular scoliosis reduce blood loss and transfusion requirements. J Pediatr Orthop. 2018;38:e78–e82. doi: 10.1097/BPO.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 42.Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am. 1993;75:880–884. doi: 10.2106/00004623-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Glotzbecker MP, Riedel MD, Vitale MG, Matsumoto H, Roye DP, Erickson M, Flynn JM, Saiman L. What’s the evidence? Systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop. 2013;33:479–487. doi: 10.1097/BPO.0b013e318285c507. [DOI] [PubMed] [Google Scholar]
- 44.Ramo BA, Roberts DW, Tuason D, McClung A, Paraison LE, Moore HG, IV, Sucato DJ. Surgical site infections after posterior spinal fusion for neuromuscular scoliosis: a thirty-year experience at a single institution. J Bone Joint Surg Am. 2014;96:2038–2048. doi: 10.2106/JBJS.N.00277. [DOI] [PubMed] [Google Scholar]
- 45.van Rhee MA, de Klerk LW, Verhaar JA. Vacuum-assisted wound closure of deep infections after instrumented spinal fusion in six children with neuromuscular scoliosis. Spine J. 2007;7:596–600. doi: 10.1016/j.spinee.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 46•.Jain A, Modhia UM, Njoku DB, et al. Recurrence of deep surgical site infection in cerebral palsy after spinal fusion is rare. Spine Deform. 2017;5:208–212. doi: 10.1016/j.jspd.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Whitaker AT, Sharkey M, Diab M. Spinal fusion for scoliosis in patients with globally involved cerebral palsy: an ethical assessment. J Bone Joint Surg Am. 2015;97:782–787. doi: 10.2106/JBJS.N.00468. [DOI] [PubMed] [Google Scholar]
- 48••.DiFazio RL, Miller PE, Vessey JA, Snyder BD. health-related quality of life and care giver burden following spinal fusion in children with cerebral palsy. Spine. 2017;42:E733–E7E9. doi: 10.1097/BRS.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 49.Cassidy C, Craig CL, Perry A, Karlin LI, Goldberg MJ. A reassessment of spinal stabilization in severe cerebral palsy. J Pediatr Orthop. 1994;14:731–739. doi: 10.1097/01241398-199414060-00008. [DOI] [PubMed] [Google Scholar]
- 50.Jones KB, Sponseller PD, Shindle MK, McCarthy ML. Longitudinal parental perceptions of spinal fusion for neuromuscular spine deformity in patients with totally involved cerebral palsy. J Pediatr Orthop. 2003;23:143–149. [PubMed] [Google Scholar]
- 51.Bohtz C, Meyer-Heim A, Min K. Changes in health-related quality of life after spinal fusion and scoliosis correction in patients with cerebral palsy. J Pediatr Orthop. 2011;31:668–673. doi: 10.1097/BPO.0b013e318221093c. [DOI] [PubMed] [Google Scholar]
- 52••.Jain A, Sponseller PD, Shah SA, et al. Subclassification of GMFCS Level-5 cerebral palsy as a predictor of complications and health-related quality of life after spinal arthrodesis. J Bone Joint Surg Am. 2016;98:1821–1828. doi: 10.2106/JBJS.15.01359. [DOI] [PubMed] [Google Scholar]
- 53••.Miyanji F, Nasto LA, Sponseller PD, et al. Assessing the risk-benefit ratio of scoliosis surgery in cerebral palsy: surgery is worth it. J Bone Joint Surg Am. 2018;100:556–563. doi: 10.2106/JBJS.17.00621. [DOI] [PubMed] [Google Scholar]


