Key Points
Question
Does administration of a recombinant human soluble thrombomodulin reduce mortality of critically ill patients with sepsis-associated coagulopathy?
Findings
In this randomized clinical trial that included 800 patients with sepsis-associated coagulopathy, treatment with thrombomodulin, compared with placebo, did not significantly reduce 28-day all-cause mortality (26.8% in the thrombomodulin group vs 29.4% in the placebo group).
Meaning
Use of a recombinant human soluble thrombomodulin did not significantly reduce 28-day all-cause mortality in critically ill patients with sepsis-associated coagulopathy.
Abstract
Importance
Previous research suggested that soluble human recombinant thrombomodulin may reduce mortality among patients with sepsis-associated coagulopathy.
Objective
To determine the effect of human recombinant thrombomodulin vs placebo on 28-day all-cause mortality among patients with sepsis-associated coagulopathy.
Design, Setting, and Participants
The SCARLET trial was a randomized, double-blind, placebo-controlled, multinational, multicenter phase 3 study conducted in intensive care units at 159 sites in 26 countries. All adult patients admitted to one of the participating intensive care units between October 2012 and March 2018 with sepsis-associated coagulopathy and concomitant cardiovascular and/or respiratory failure, defined as an international normalized ratio greater than 1.40 without other known etiology and a platelet count in the range of 30 to 150 × 109/L or a greater than 30% decrease in platelet count within 24 hours, were considered for inclusion. The final date of follow-up was February 28, 2019.
Interventions
Patients with sepsis-associated coagulopathy were randomized and treated with an intravenous bolus or a 15-minute infusion of thrombomodulin (0.06 mg/kg/d [maximum, 6 mg/d]; n = 395) or matching placebo (n = 405) once daily for 6 days.
Main Outcome and Measures
The primary end point was 28-day all-cause mortality.
Results
Among 816 randomized patients, 800 (mean age, 60.7 years; 437 [54.6%] men) completed the study and were included in the full analysis set. In these patients, the 28-day all-cause mortality rate was not statistically significantly different between the thrombomodulin group and the placebo group (106 of 395 patients [26.8%] vs 119 of 405 patients [29.4%], respectively; P = .32). The absolute risk difference was 2.55% (95% CI, −3.68% to 8.77%). The incidence of serious major bleeding adverse events (defined as any intracranial hemorrhage; life-threatening bleeding; or bleeding event classified as serious by the investigator, with administration of at least 1440 mL [typically 6 units] of packed red blood cells over 2 consecutive days) was 23 of 396 patients (5.8%) in the thrombomodulin group and 16 of 404 (4.0%) in the placebo group.
Conclusions and Relevance
Among patients with sepsis-associated coagulopathy, administration of a human recombinant thrombomodulin, compared with placebo, did not significantly reduce 28-day all-cause mortality.
Trial Registration
ClinicalTrials.gov Identifier: NCT01598831
This randomized trial compares the effect of recombinant human soluble thrombomodulin vs placebo in patients with sepsis-associated coagulopathy on 28-day all-cause mortality.
Introduction
Sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, remains the most common cause of death in critically ill patients.1 Numerous therapies intended to target the various biological pathways of sepsis have been tested in large randomized studies, but none have shown a significant benefit on mortality.2,3,4
Sepsis-associated coagulopathy, defined by a prolonged international normalized ratio (INR) and reduced platelet count, is highly predictive of 28-day mortality.5 The most notable benefit on mortality by an anticoagulant agent for patients with sepsis with overt disseminated intravascular coagulation was observed with drotrecogin α (activated), a recombinant human activated protein C,6 which was withdrawn from the market in 2011.
ART-123 is a recombinant human soluble thrombomodulin (rhsTM; thrombomodulin α) composed of 498 amino acids (64 kDa) from the soluble and active extracellular domains of thrombomodulin. The primary mechanism of protection afforded by rhsTM is derived from its capacity to bind circulating thrombin molecules and serve as an activation complex to convert protein C to activated protein C.7,8 In addition, rhsTM inhibits inflammation and organ injury caused by damage-associated molecular patterns, such as high mobility group box protein 1 and histones.9,10,11
Post hoc analysis of a phase 2b randomized clinical trial of patients with sepsis and suspected disseminated intravascular coagulation suggested that reduced mortality associated with rhsTM administration was best predicted when the following 3 factors were present: infection, at least 1 sepsis-associated organ dysfunction (cardiovascular and/or respiratory), and coagulopathy indicated by prolongation of the INR and reduction of platelet count.12 The hypothesis was that rhsTM can reduce 28-day mortality in patients with sepsis-associated coagulopathy. This double-blind, placebo-controlled, multinational, multicenter phase 3 study was conducted to determine the adverse effects and efficacy of rhsTM in addition to standard care in patients with sepsis-associated coagulopathy.
Methods
Study Design
The SCARLET (Sepsis Coagulopathy Asahi Recombinant LE Thrombomodulin) trial was a randomized, double-blind, placebo-controlled, multinational, multicenter, parallel-group phase 3 study with 319 sites in 27 countries (enrollment by 159 sites) in North America, Europe (including Israel), South America, Asia-Pacific, and Russia. The study protocol (Supplement 1) and informed consent form were reviewed and approved by the institutional review board or independent ethics committee at each participating site. Informed consent was obtained, as detailed in the protocol (Supplement 1), before any study-specific procedures were performed in accordance with all applicable ethical, regulatory, and local requirements. Patients were enrolled from October 2012 to March 2018.
Study Patients
Critically ill patients who were aged at least 18 years with clinical objective evidence of bacterial infection and a known site of infection, systemic inflammatory response syndrome criteria of white blood cell count and temperature, coagulopathy due to sepsis, and a concurrent diagnosis of cardiovascular and/or respiratory dysfunction requiring mechanical ventilation for hypoxemia were eligible for inclusion (Supplement 1). Cardiovascular dysfunction was defined as a requirement for vasopressors to maintain mean arterial pressure of at least 65 mm Hg after adequate fluid resuscitation. Adequate fluid resuscitation was defined as intravenous administration of at least 20 mL/kg crystalloid or 10 mL/kg colloid infusion within 6 hours and/or a central venous pressure greater than 8 mm Hg or a pulmonary artery occlusion pressure greater than 12 mm Hg. Respiratory dysfunction was defined as an acute need for (invasive) mechanical ventilation and partial pressure of oxygen/fraction of inspired oxygen (Pao2/Fio2) ratio of less than 250 (or <200 when the lung was the site of infection). Coagulopathy was characterized by (1) an INR greater than 1.40 without other known etiology (eg, anticoagulant therapy, chronic liver disease) and (2) a platelet count in the range of 30 to 150 × 109/L or a decrease in platelet count greater than 30% within 24 hours. Patients with a platelet count of 30 × 109/L or less were not eligible for inclusion.
The time limit for the confirmation of inclusion criteria was modified during the study after protocol version 4.0 was implemented, substantially lengthening the maximum time between the first qualifying INR measure and the first dose of rhsTM or placebo from 15 hours to 40 hours. The purpose of this change was to provide more time for investigators to enroll participants because the 15-hour restriction affected the ability to identify and enroll patients in a timely manner. The study protocol including amendments is available in Supplement 1. The eligibility of each patient was discussed by telephone with a physician from one of the 2 regional clinical coordinating centers to confirm the presence of infection, organ dysfunction, coagulopathy, and all other inclusion and exclusion criteria before randomization.
Randomization and Intervention
Randomization was stratified by site with a block size of 4 patients using an interactive voice/web response system, which linked patient randomization numbers to treatment codes. At randomization, patients who met all inclusion criteria and no exclusion criteria were assigned randomly, in a 1:1 ratio, to receive rhsTM at a dose of 0.06 mg/kg/d (maximum dose of 6 mg/d) or matching placebo once daily for 6 consecutive days. These interventions were administered by a dedicated study nurse or trained intensive care unit nurse as an intravenous bolus injection or diluted in 50 mL of 0.9% saline for a 15-minute infusion. Placebo and rhsTM were supplied in identically labeled, individual glass ampules.
Study Outcomes
The primary efficacy end point was all-cause mortality 28 days after the start of the intervention. Secondary efficacy end points were all-cause mortality at 3 months and resolution of organ dysfunction through day 28, measured by shock-free and alive days, ventilator-free and alive days, and dialysis-free and alive days. Follow-up was complete on February 28, 2019, so analyses for secondary end points have not yet been completed and results are therefore not reported.
The primary safety end points included adverse events and major bleeding events through day 28 (defined as any intracranial hemorrhage; life-threatening bleeding; or bleeding event classified as serious by the investigator, with administration of at least 1440 mL [typically 6 units] of packed red blood cells over 2 consecutive days). The secondary safety end point was antidrug antibodies to rhsTM.
Prespecified exploratory laboratory tests were performed to assess differences in coagulation parameters (the plasma concentrations of D-dimer, prothrombin fragment F1.2, and thrombin-antithrombin complex), inflammation parameters (C-reactive protein level, the presence of microparticles or C5a), rhsTM plasma concentration, organ dysfunction (hepatic dysfunction, bilirubin concentration ≥2.0 mg/dL; renal dysfunction, creatinine concentration ≥2.0 mg/dL), and adverse event parameters between baseline (ie, after randomization and before receiving the intervention) and day 28.
Post hoc analyses were performed among subgroups of patients based on baseline thrombin-antithrombin complex concentration, baseline protein C, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, number of baseline organ dysfunctions, arterial lactate concentration, and maintenance of the protocol-specified coagulopathy criteria (INR >1.4 and platelet count >30 × 109/L) at baseline.
Statistical Analysis
The study was powered for the 28-day all-cause mortality end point. For sample size and power calculations, a χ2 test was used. A sample size of 800 patients provided 80% power at a 5% 2-sided α level based on an assumption of an absolute risk reduction of 8% in mortality (24% in the placebo group vs 16% in the rhsTM group). This sample size assumption was based on the results of the previous phase 2b study.12
The efficacy end points were analyzed in the full analysis set, which included all patients who consented and were randomly assigned to treatment and received at least 1 dose of the study drug. Patients were analyzed according to the treatment group they were randomized to, regardless of the study drug received. The adverse event end points were analyzed in the safety population, which included all patients who received at least 1 dose of the study treatment. Patients were analyzed based on the study intervention received. One patient received both rhsTM and placebo and was included in the rhsTM group for the adverse event analysis.
The primary efficacy analysis for this study was based on a Cochran-Mantel-Haenszel test stratified by pooled sites at a 5% 2-sided α level. Individual sites that enrolled fewer than 8 patients were pooled together by region (North America, Western Europe [including Israel] combined with Australia and New Zealand, and Eastern Europe combined with the rest of world). Descriptive statistics were used to summarize. Survival time was summarized using Kaplan-Meier methods. Analysis of the difference between the treatment groups has not yet been conducted because, at the time of writing this article, the 12-month end point had not been reached. Prespecified subgroup analyses were performed per the statistical analysis plan (Supplement 2). Post hoc sensitivity analyses were conducted using generalized linear mixed models with pooled site as a random effect. Post hoc subgroup analyses of the patients who maintained the protocol-specified coagulopathy criteria (INR >1.4 and platelet count >30 × 109/L) at baseline and, according to baseline thrombin-antithrombin complex concentration, baseline protein C, APACHE II score, number of baseline organ dysfunctions, and arterial lactate concentration were conducted in the same way as for the prespecified subgroup analyses. The change of the plasma concentrations of D-dimer, prothrombin fragment F1.2, and thrombin-antithrombin complex from baseline were compared between the 2 groups using a Wilcoxon rank sum test in a post hoc analysis. No adjustment for multiple comparisons was made and thus results of secondary analyses should be interpreted as exploratory.
Patients with unknown mortality status at day 28 were imputed for testing of the primary end point. Investigators assessed whether, at the last point they had contact with the patient, the patient’s health was such that it was unlikely that they would be alive at day 28. If so, it would be assumed the patient was dead at day 28. Otherwise, the patient was classified as alive for the primary analysis.
This study included unblinded interim analyses performed by an independent data monitoring committee. The futility analyses were conducted using a lower O’Brien-Fleming–type boundary, setting minimum acceptable efficacy to an absolute risk reduction of 6%. This boundary was constructed using a 1-sided α of .20. Early termination of the study for efficacy was not actively pursued. However, the primary efficacy results surpassed an O’Brien-Fleming–type boundary using a 2-sided α of .001. The effect on the final α level at the 0.1% level was very small but was nonzero. The α spent at the interim analysis was approximately .00005. Because of the small size of this α spent, no adjustment to the final α level was made.
Statistical analyses were conducted using SAS version 9.3 or 9.4 (SAS Institute). A 2-sided P value less than .05 was considered statistically significant. The statistical analysis plan is available in Supplement 2.
Results
Patient Characteristics
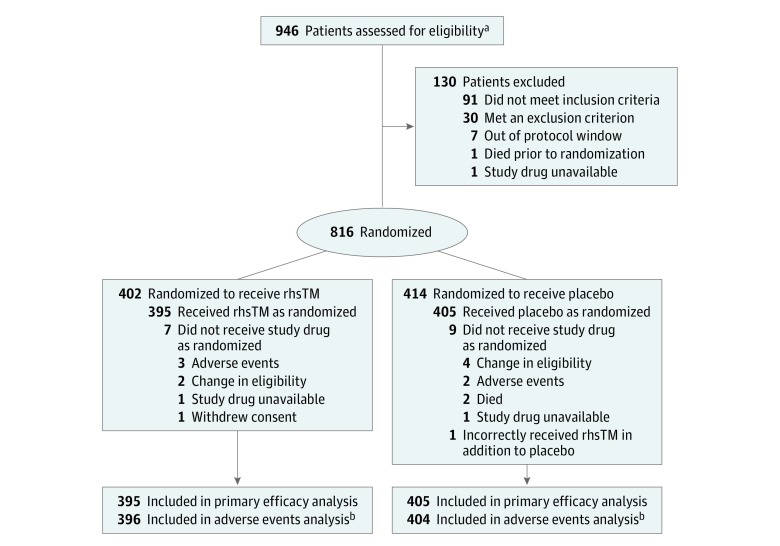
Informed consent was obtained from 946 patients, and 816 were authorized as eligible to participate by the clinical coordinating centers. These 816 patients were randomized in a 1:1 ratio to receive rhsTM (n = 402) or a placebo (n = 414). Among these randomized patients, 800 (mean age, 60.7 years; 437 [54.6%] men) received either rhsTM (n = 395) or placebo (n = 405). Sixteen patients did not receive the assigned intervention because of adverse events (n = 5), death (n = 2), withdrawal of consent (n = 1), lack of availability of the study drug (n = 2), or change in eligibility before receiving the intervention (n = 6). One patient randomized to receive the placebo was incorrectly also given rhsTM (Figure 1). This patient was included in the placebo group for the efficacy analyses and in the rhsTM group for the safety analyses, as described in the Methods section.
Figure 1. Flow of Patients in a Study of the Effect of Thrombomodulin on Patients With Sepsis.
aData were not collected on how many patients were approached.
bOne patient randomized to the placebo group also was inadvertently given recombinant human thrombomodulin (rhsTM). This patient was analyzed in the placebo group for the efficacy analysis and in the rhsTM group for the adverse events analysis.
Baseline demographics and characteristics were well balanced between the rhsTM and placebo groups (Table). In both groups, the mean baseline INR was 1.8 and the mean baseline platelet count was approximately 120 × 109/L. The mean APACHE II score (range, 0-71; a higher score indicates greater severity of illness and higher risk of death) was approximately 22 in both groups. The rate of cardiovascular dysfunction was 90.9%; respiratory dysfunction, 67.1%; renal dysfunction, 32.8%; and hepatic dysfunction, 21.3%; 604 patients (75.5%) had more than 1 organ dysfunction, with cardiovascular and respiratory dysfunction presenting together in 484 patients (60.5%).
Table. Demographic and Baseline Characteristics of Patients in a Study of the Effect of Thrombomodulin on Patients With Sepsis.
| Characteristics | No. (%) | |
|---|---|---|
| rhsTM (n = 395) | Placebo (n = 405) | |
| Patients per ICU, median (IQR) | 2.0 (1.0-4.0) | 2.0 (1.0-4.0) |
| ICUs | 125 | 127 |
| Age, median (IQR), y | 63 (50-73) | 62 (51-73) |
| Sex | ||
| Men | 216 (54.7) | 221 (54.6) |
| Women | 179 (45.3) | 184 (45.4) |
| INRa | ||
| Mean (SD) | 1.8 (0.6) | 1.8 (0.6) |
| <1.20 | 7 (1.8) | 8 (2.0) |
| 1.20-1.39 | 45 (11.4) | 46 (11.4) |
| 1.40-1.59 | 126 (31.9) | 135 (33.3) |
| ≥1.60 | 217 (54.9) | 216 (53.3) |
| Platelet count, x109/La | ||
| Mean (SD) | 123.3 (74.1) | 121.3 (65.1) |
| >150 | 92 (23.3) | 96 (23.7) |
| 150-101 | 144 (36.5) | 136 (33.6) |
| 100-81 | 48 (12.2) | 66 (16.3) |
| ≤80 | 111 (28.1) | 107 (26.4) |
| APACHE II scoreb | (n = 356) | (n = 366) |
| Mean (SD) | 22.3 (8.1) | 22.1 (8.0) |
| <25 | 220 (61.8) | 219 (59.8) |
| ≥25 | 136 (38.2) | 147 (40.2) |
| Organ dysfunctionc | ||
| Cardiovascular | 361 (91.4) | 369 (91.1) |
| Respiratory | 247 (62.5) | 290 (71.6) |
| Renal | 125 (31.6) | 137 (33.8) |
| Hepatic | 87 (22.0) | 83 (20.5) |
| Arterial lactate, mmol/Ld | (n = 306) | (n = 331) |
| Mean (SD) | 4.2 (3.6) | 3.8 (2.8) |
| ≤6 | 253 (82.7) | 277 (83.7) |
| >6 | 53 (17.3) | 54 (16.3) |
| Serum creatinine, mg/dL | (n = 390) | (n = 400) |
| Mean (SD) | 1.8 (1.2) | 1.8 (1.2) |
| <2 | 265 (67.9) | 263 (65.8) |
| ≥2 | 125 (32.1) | 137 (34.3) |
| Renal replacement therapy | ||
| Yes | 44 (11.1) | 49 (12.1) |
| No | 351 (88.9) | 356 (87.9) |
| Primary site of infection | ||
| Intra-abdominal | 123 (31.1) | 119 (29.4) |
| Lung | 94 (23.8) | 110 (27.2) |
| Urinary tract | 82 (20.8) | 75 (18.5) |
| Blood | 38 (9.6) | 36 (8.9) |
| Skin/skin structure | 27 (6.8) | 30 (7.4) |
| Other | 31 (7.8) | 35 (8.6) |
| Diabetes | ||
| Yes | 90 (22.8) | 97 (24.0) |
| No | 305 (77.2) | 308 (76.0) |
| Heparin use | ||
| Yes | 203 (51.4) | 213 (52.6) |
| No | 192 (48.6) | 192 (47.4) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; IQR, interquartile range; INR, international normalized ratio.
SI conversion: To convert serum creatinine to μmol/L, multiply by 88.4.
INR and platelet count were categorized according to Lyons et al.5
APACHE II scores range from 0 to 71; 0 indicates the lowest prediction of mortality and 71 indicates the highest. Predicted mortality for a patient with an APACHE II score of 25 is 35% to 55%.
Cardiovascular dysfunction was defined as a requirement for vasopressors to maintain mean arterial pressure of at least 65 mm Hg after adequate fluid resuscitation; respiratory dysfunction, an acute need for (invasive) mechanical ventilation and partial pressure of oxygen/fraction of inspired oxygen ratio of less than 250; hepatic dysfunction, a bilirubin concentration of at least 2.0 mg/dL; renal dysfunction, a creatinine concentration of at least 2.0 mg/dL. Total percentages exceed 100% because some patients had more than 1 organ dysfunction.
A normal blood lactate range was considered 0.5 to 1.6 mmol/L.
The primary sites of infection at baseline were comparable in the 2 groups (Table). The most common site of infection was intra-abdominal. New infection after enrollment occurred in 99 of 395 patients (25.1%) in the rhsTM group and 100 of 405 patients (24%) in the placebo group.
As a concomitant medication, heparin (low dose for deep vein thrombosis prophylaxis or heparin flushes) was administered to about half of the patients in both groups.
Primary Outcome for Efficacy
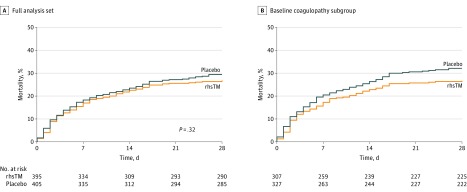
The 28-day all-cause mortality rate was 106 of 395 patients (26.8%) in the rhsTM group vs 119 of 405 (29.4%) in the placebo group (P = .32, Cochran-Mantel-Haenszel test), a difference of 2.55% (95% CI, −3.68% to 8.77%). The Kaplan-Meier survival curve for the full analysis set is shown in Figure 2A. Two patients (1 in the rhsTM group and 1 in the placebo group) had unknown mortality status at day 28 and were assessed as “assumed alive” by the investigators. In a post hoc sensitivity analysis for the primary outcome that accounted for pooled site as a random effect, the adjusted 28-day all-cause mortality rate was 24.8% in the rhsTM group vs 27.5% in the placebo group (P = .31).
Figure 2. Kaplan-Meier Plots of Survival Time in Patients With Sepsis in a Study of the Effect of Thrombomodulin vs Placebo.
Kaplan-Meier plots of survival time for the 2 treatment groups in A, the full analysis set population and B, in a subgroup (post hoc analysis) of patients with baseline INR greater than 1.4 and platelet counts greater than 30 × 109/L (n = 634) . Patients whose status was alive or unknown were censored at last available date. In the full analysis set population, the median (interquartile range) observation time was 28 (18-28) days for the recombinant human thrombomodulin (rhsTM) group and 28 (16-28) days for the placebo group. In the baseline coagulopathy subgroup, the median (interquartile range) observation time was 28 (17-28) days for the rhsTM group and 28 (13-28) days for the placebo group.
Subgroup Analyses
In prespecified subgroup analyses (Supplement 2), the difference in 28-day all-cause mortality rates between the rhsTM and placebo group was 4.66% (95% CI, −3.07% to 12.38%) in the subgroup of patients with baseline APACHE II scores less than 25 (n = 439) and −1.45% (95% CI, −12.69% to 9.80%) in the subgroup of patients with APACHE II scores greater than or equal to 25 (n = 283) (eFigure 1 in Supplement 3). The difference in 28-day all-cause mortality between the rhsTM and placebo group was −0.87% (95% CI, −9.52% to 7.77%) in the subgroup of patients who received heparin (n = 416) and 6.25% (95% CI, −2.72% to 15.22%) in the subgroup of patients who did not receive heparin (n = 384) (eFigure 1 in Supplement 3).
Coagulation Markers
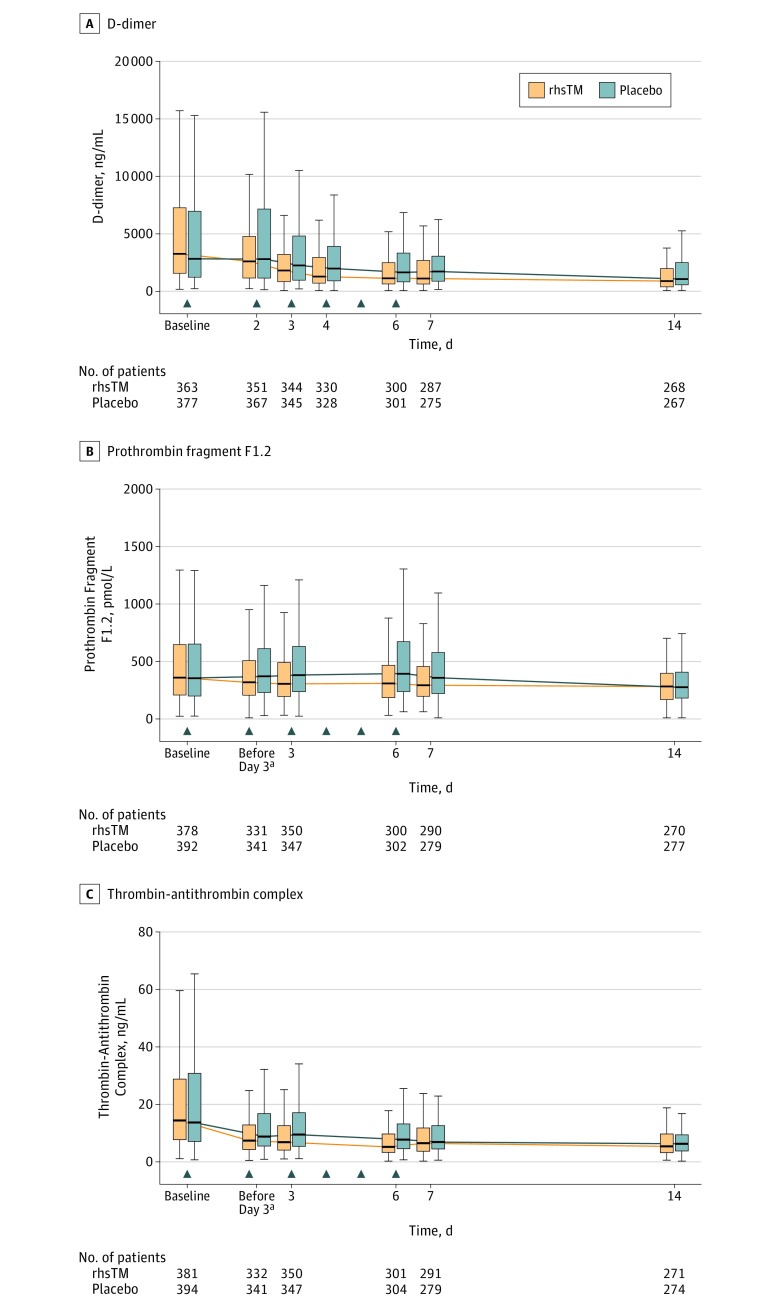
At day 6, the median plasma concentration of D-dimer was 1129.5 ng/mL in the rhsTM group and 1649 ng/mL in the placebo group, the median plasma concentration of prothrombin fragment F1.2 was 309.6 pmol/L in the rhsTM group and 393.2 pmol/L in the placebo group, and the median plasma concentration of thrombin-antithrombin complex was 5.2 ng/mL in the rhsTM group and 7.8 ng/mL in the placebo group. In the post hoc analysis, the change from baseline of each marker during the treatment period (6 days) was statistically significantly greater (P < .05) in the rhsTM group than in the placebo group (Figure 3).
Figure 3. Coagulation Parameters Over Time in Patients With Sepsis in a Study of the Effect of Thrombomodulin vs Placebo.
The box represents the 25% to 75% interquartile range (IQR). Whiskers indicate minimum and maximum values up to 1.5 times the interquartile range from the quartiles. The data for outliers are not shown. The arrows indicate study drug administration. rhsTM indicates recombinant human soluble thrombomodulin.
aThe value prior to dosing on day 3.
Post Hoc Subgroup Analyses
Approximately 80% of patients in each of the study groups had an INR greater than 1.4 at baseline, but the baseline INR in the remaining approximately 20% of patients was less than or equal to 1.4 after initial confirmation of eligibility (Table). In post hoc analyses, 28-day all-cause mortality rates in the subgroup of patients with coagulopathy at baseline (n = 634; INR >1.4 and platelet count >30 × 109/L) were 82 of 307 patients (26.7%) in the rhsTM group and 105 of 327 (32.1%) in the placebo group, a difference of 5.40% (95% CI, −1.68% to 12.48%) (eFigure 1 in Supplement 3). For patients with an INR less than or equal to 1.4 (n = 146), mortality rates were 15 of 76 patients (19.7%) in the rhsTM group and 10 of 70 (14.3%) in the placebo group. For patients with baseline platelet counts less than or equal to 30 × 109/L (n = 24), mortality rates were 9 of 13 patients (69.2%) in the rhsTM group and 4 of 11 (36.4%) in the placebo group. Four patients had an INR less than or equal to 1.4 and platelet counts less than or equal to 30 × 109/L at baseline. The Kaplan-Meier survival curve for the baseline coagulopathy subgroup is shown in Figure 2B.
In the subgroup of patients with baseline thrombin-antithrombin complex concentration greater than or equal to 10 ng/mL (n = 489), the 28-day mortality rate was 74 of 248 patients (29.0%) in the rhsTM group vs 82 of 241 (35.7%) in the placebo group, a difference of 6.65% (95% CI, −1.62% to 14.93%) (eFigure 1 in Supplement 3). In the subgroup of patients with baseline thrombin-antithrombin complex concentration less than 10 ng/mL (n = 286), the 28-day mortality rate was 31 of 133 patients (23.3%) in the rhsTM group vs 28 of 153 (18.3%) in the placebo group, a difference of −5.01% (95% CI, −14.45% to 4.44%).
The transition of cumulative 28-day mortality rates throughout the trial was analyzed because of the protracted enrollment (approximately 5.5 years). The cumulative 28-day mortality rate in the full analysis set ranged from 20.0% to 27.9% in the rhsTM group and 26.7% to 33.3% in the placebo group after 2014 (eFigure 2A in Supplement 3). The results for the subgroup of patients with coagulopathy at baseline are shown in eFigure 2B in Supplement 3.
Adverse Event Analyses
Overall, 754 patients (94.3%) experienced at least 1 treatment-emergent adverse event (377 patients [95.2%] in the rhsTM group and 377 patients [93.3%] in the placebo group) (eTable 1 in Supplement 3). Overall, 408 patients (51.0%) experienced at least 1 treatment-emergent serious adverse event, including 206 patients (52.0%) in the rhsTM group and 202 patients (50.0%) in the placebo group. A serious major bleeding event occurred in 23 patients (5.8%) in the rhsTM group and 16 patients (4.0%) in the placebo group, which included 2 patients with intracerebral bleeding in the rhsTM group and 1 in the placebo group (eTable 2 in Supplement 3). No antidrug antibodies to rhsTM were reported.
Discussion
In this randomized, double-blind, placebo-controlled, multinational, multicenter phase 3 study, rhsTM did not significantly reduce 28-day all-cause mortality in patients with sepsis-associated coagulopathy. Patient heterogeneity has been and continues to be a hallmark of sepsis populations within clinical trials. Therefore, this study was designed to select patients with sepsis-associated coagulopathy based on well-defined markers of coagulation; however, the primary efficacy end point was not achieved.
There are several possible reasons for this finding. First, approximately 20% of patients did not meet the criteria for coagulopathy as defined by INR and platelet count at baseline. A protocol amendment modifying the inclusion criteria was implemented midway through the study, which substantially lengthened (from 15 to 40 hours) the allowable time from first qualifying INR measurement to receiving the intervention. Thus, although all of the patients included in the study met the criteria of INR greater than 1.4 at some point during screening, some no longer did by the time they received the intervention. Second, the mortality rate in the placebo group was higher than the assumed value used for the sample size calculation prior to the study (24%). Third, the heparin used for prophylaxis of deep vein thrombosis may have attenuated the efficacy of rhsTM because heparin also has anticoagulation activity. Fourth, although the randomization was stratified by site, 55 of 159 clinical sites enrolled only 1 patient, which may have influenced the efficacy results.
The enrolled patient population represented a typical sepsis patient population in its clinical heterogeneity. The greater proportion of patients with intra-abdominal infection in this study is consistent with previous observations that coagulopathy is more prevalent with intra-abdominal than respiratory infection in sepsis.13
No new adverse event concerns were identified in this study. The incidence of serious major bleeding events was similar to the incidence in the previous phase 2b study12 and was numerically higher in the rhsTM than in the placebo group.
Limitations
The study has several limitations. First, the sample size may not have been sufficient for the statistical evaluation of study drug efficacy. The 95% CI is consistent with an effect anywhere between a small harm and a benefit exceeding that used as the basis for the sample size calculation. Second, post hoc analyses performed to investigate the population most likely to respond favorably to rhsTM treatment were not preplanned and should be interpreted with caution. Third, the final follow-up has been completed but data collection is still ongoing; therefore, effects on some of the end points, such as a long-term mortality, have not yet been analyzed.
Conclusions
Among patients with sepsis-associated coagulopathy, administration of a recombinant human thrombomodulin, compared with placebo, did not significantly reduce 28-day all-cause mortality.
Trial protocol
Statistical analysis plan
eResults
Data sharing statement
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554. doi: 10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366(22):2122-2124. doi: 10.1056/NEJMe1203412 [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Thompson BT, Barie PS, et al. ; PROWESS-SHOCK Study Group . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. doi: 10.1056/NEJMoa1202290 [DOI] [PubMed] [Google Scholar]
- 4.Opal SM, Dellinger RP, Vincent JL, Masur H, Angus DC. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C? Crit Care Med. 2014;42(7):1714-1721. doi: 10.1097/CCM.0000000000000325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med. 2018;46(5):736-742. doi: 10.1097/CCM.0000000000002997 [DOI] [PubMed] [Google Scholar]
- 6.Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924-1933. doi: 10.1111/j.1538-7836.2004.00955.x [DOI] [PubMed] [Google Scholar]
- 7.Mohri M. ART-123: Recombinant human soluble thrombomodulin. Cardiovasc Drug Rev. 2006;18(4):312-325. doi: 10.1111/j.1527-3466.2000.tb00055.x [DOI] [Google Scholar]
- 8.Mohri M, Sugimoto E, Sata M, Asano T. The inhibitory effect of recombinant human soluble thrombomodulin on initiation and extension of coagulation--a comparison with other anticoagulants. Thromb Haemost. 1999;82(6):1687-1693. [PubMed] [Google Scholar]
- 9.Ito T, Kawahara K, Okamoto K, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28(10):1825-1830. doi: 10.1161/ATVBAHA.107.150631 [DOI] [PubMed] [Google Scholar]
- 10.Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets. 2012;13(3):421-431. doi: 10.2174/138945012799424606 [DOI] [PubMed] [Google Scholar]
- 11.Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One. 2013;8(9):e75961. doi: 10.1371/journal.pone.0075961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled, phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(9):2069-2079. doi: 10.1097/CCM.0b013e31828e9b03 [DOI] [PubMed] [Google Scholar]
- 13.Volakli E, Spies C, Michalopoulos A, Groeneveld ABJ, Sakr Y, Vincent JL. Infections of respiratory or abdominal origin in ICU patients: what are the differences? Crit Care. 2010;14(2):R32. doi: 10.1186/cc8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eResults
Data sharing statement