Short abstract
Background
Pain is one of the most common and distressing symptoms suffered by patients with progression of bone cancer; however, the mechanisms responsible for hyperalgesia are not well understood. The purpose of our current study was to determine contributions of the sensory signaling pathways of inflammatory tumor necrosis factor-α and interleukin-6 and downstream transient receptor potential ankyrin 1 (TRPA1) to neuropathic pain induced by bone cancer. We further determined whether influencing these pathways can improve bone cancer pain.
Methods
Breast sarcocarcinoma Walker 256 cells were implanted into the tibia bone cavity of rats to induce mechanical and thermal hyperalgesia. ELISA and western blot analysis were used to examine (1) the levels of tumor necrosis factor-α and interleukin-6 in dorsal root ganglion and (2) protein expression of tumor necrosis factor-α and interleukin-6 receptors (TNFR1 and IL-6R) and TRPA1 as well as intracellular signals (p38-MAPK and JNK).
Results
Tumor necrosis factor-α and interleukin-6 were elevated in the dorsal root ganglion of bone cancer rats, and expression of TNFR1, IL-6R, and TRPA1 was upregulated. In addition, inhibition of TNFR1 and IL-6R alleviated mechanical and thermal hyperalgesia in bone cancer rats, accompanied with downregulated TRPA1 and p38-MAPK and JNK.
Conclusions
We revealed specific signaling pathways leading to neuropathic pain during the development of bone cancer, including tumor necrosis factor-α-TRPA1 and interleukin-6-TRPA1 signal pathways. Overall, our data suggest that blocking these signals is beneficial to alleviate bone cancer pain.
Keywords: Bone cancer, mechanical hyperalgesia, thermal hyperalgesia, cytokines, TRPA1
Introduction
Pain is one of the most common and distressing symptoms suffered by patients with progression of cancer.1 Cancer pain mainly arises from a tumor compressing or infiltrating tissue, from nerve and other changes caused by a hormone imbalance or immune response, and so on.2 Of note, cancerous cells can originate in a number of different tissues such as prostate, breast, and lung. Many types of cancers have a propensity to metastasize to the bone microenvironment.2,3 Tumor burden within the bone causes excruciating breakthrough pain with properties of ongoing pain that is inadequately managed with current analgesics. Treatment options for bone cancer pain have been limited, partly due to our poor understanding of the underlying mechanisms responsible for pain.
Transient receptor potential ankyrin 1 (TRPA1) plays a functional role in regulating pain and neurogenic inflammation resulting from channel activation to a variety of compounds including pungent agents, irritant chemicals, reactive oxygen, and products of oxidative stress-induced lipid peroxidation.4 TRPA1 is presented in dorsal root ganglion (DRG) neurons5 and is engaged in the development of mechanical hypersensitivity and temperature sensitive pain.6,7 TRPA1 has also been reported to mediate mechanical hyperalgesia and thermal hypersensitivity in numerous models of neuropathic pain.4–6 Thus, in this report, we postulated that sensory TRPA1 plays a role in regulating mechanical and thermal sensitivity in bone cancer rats induced by implanting breast sarcocarcinoma Walker 256 cells into the tibia bone cavity. We hypothesized that bone cancer amplifies protein expression of TRPA1 in the DRG, and this thereby results in mechanical hyperalgesia and thermal hypersensitivity. We further hypothesized that blocking TRPA1 attenuates mechanical hyperalgesia and thermal hypersensitivity observed in bone cancer.
Moreover, chronic neuro-inflammation is one of the hallmarks in regulating neuropathic pain.8,9 Studies in neuropathic pain of human patients and experimental animals show that activation of glial cells and elevation of pro-inflammatory cytokines (PICs; i.e., tumor necrosis factor-α (TNF-α) and interleukin (IL)-6) are common features of neuropathic pain.10–12 The releases of PICs by stimulated astrocytes and microglia lead to the exacerbation of neuronal cells in the DRG and pain regulation-related central regions.10–12 Infiltration and accumulated immune cells from the periphery are also identified in and around the affected peripheral nerves and central regions of animal models with neuropathic pain.9
In particular, TNF-α mediates mechanical and thermal hyperalgesia in the development of inflammation.13 It has also been reported that TNF-α induces pain through the release of inflammatory mediators, such as prostaglandins sensitizing ion channels.14 A direct sensitization effect of TNF-α on voltage-gated sodium channels has been observed in neuronal cells.15 TNF-α treatment also results in an upregulation of TRPA1 expression in sensory neurons.16 In addition, evidence suggests that endogenous activation of peripheral TRPA1 receptors plays a critical role in the development of TNFα‐induced mechanical hyperalgesia and in sustaining the mechanical hyperalgesia observed after intra-articular injection of Freund’s complete adjuvant in rats.17 Moreover, it has been reported that IL-6 can cause mechanical hyperalgesia via increased PIC production (i.e., TNF-α).18 The release of hyperalgesic mediators, that is, prostaglandins, occurs subsequent to the release of cytokines. Mechanical hyperalgesia induced by IL-6 is reduced by a pre-inhibition of prostaglandin production.18 Also, IL-6 has been shown to upregulate the expression or activation of TRPA1 and knockdown of IL-6 signal transducing receptor subunits glycoprotein-130 (gp130) decreases mRNA expression of TRPA1 in sensory neurons.19,20
Thus, in this study, we postulated that TNF-α and IL-6 signal pathways are upregulated in the DRG of bone cancer rats. We further examined the effects of inhibition of TNF-α and IL-6 signals on protein expression of TRPA1 in the DRG and on mechanical and thermal hypersensitivity in bone cancer rats. We hypothesized that inhibition of TNF-α attenuates intracellular p38-MAPK and JNK signals in the DRG and thereby decreases the protein levels of TRPA1, which further alleviates bone cancer-induced mechanical hyperalgesia and thermal hyperalgesia. Likewise, blocking IL-6 attenuates expression levels of TRPA1 via p38-MAPK and JNK in the DRG, thereby improving bone cancer-induced mechanical and thermal pain.
Materials and methods
Animals
All animal protocols were in accordance with the guidelines of the International Association for the Study of Pain and approved by the Institutional Animal Care and Use Committee of Jilin University. Adult male Wistar rats (200–250 g) were housed in individual cages with free access to food and water and were kept in a temperature-controlled room (25°C) on a 12/12 h light/dark cycle.
A model of bone cancer pain
Wister rat breast sarcocarcinoma Walker 256 cells were prepared as described previously.21 Briefly, Walker 256 cells (2 × 107 in 0.5 ml) were injected into the abdominal cavity of the rats. Seven to 14 days later, the produced ascites (approximately 50–150 ml) were collected and centrifuged at 1000 g for 5 min. The cells in the ascites were washed three times with 10 ml D-Hank’s solution and diluted to a final concentration of 2 × 107 cells/ml. The cells were then kept on ice before being used.
The bone cancer pain model was established by inoculating Walker 256 cells to the tibia of the rats as described previously.21 Briefly, the rats were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneal (i.p.)) and the lower one-third of the tibia was exposed. Each of rats was injected with 1 × 105 Walker 256 cells in 5 μl Hank’s solution into the right tibia of the hind paw, and the injection site was closed using bone wax to prevent cell leakage. Rats that underwent the same surgical procedures and received the same volume of vehicle served as the sham controls.
Administration of drugs
Two weeks after inoculation of cancer cells, a TNF-α synthesis inhibitor, pentoxifylline (PTX, 10, 20, and 40 mg/kg body weight; Sigma), and SC144, an inhibitor to complexed IL-6R-gp130 (5, 10, and 20 mg/kg body weight; Sigma), were given i.p., individually, each day for three consecutive days. In the same manner, TRPA1 antagonist HC030031 (1, 3, 10, mg/kg body weight; Sigma) was administrated i.p. each day for the following three consecutive days.
Behavioral test
To quantify the mechanical sensitivity of the hindpaw, rats were placed in individual plastic boxes and allowed to acclimate for >30 min. Mechanical paw withdrawal threshold (PWT) of rat hindpaw in response to the stimulation of von Frey filaments was determined. A series of calibrated von Frey filaments (ranging from 0.5 to 15.0 g) were applied perpendicularly to the plantar surface of the hindpaw with an appropriate force to bend the filaments until paw withdrew. Note that the filaments were bent for 5–10 s in our protocols, and in general, they can be bent sufficiently for ∼60 s. In the presence of a response, the filament of next lower force was applied. In the absence of a response, the filament of next greater force was applied. To avoid injury during tests, the cutoff strength of the von Frey filament was 15 g. The tactile stimulus producing a 50% likelihood of withdrawal was determined using the “up-down” method.22–24 Each trial was repeated two times at approximately 2-min intervals. The mean value was used as the force produced a withdrawal response.
To determine thermal hyperalgesia, rat paw withdrawal latency (PWL) to a radiant heat was measured.24,25 Rats were placed individually in plastic cages on an elevated glass platform and allowed for 30-min acclimation. Each hind paw received three stimuli with a 10-min interval, and the mean of the three withdrawal latencies was defined as PWL. The heat was maintained at a constant intensity. To prevent tissue damage, the cutoff latency was set at 20 s. Note that all the behavioral tests were performed in a blind manner in this report to prevent experimental bias. In all the behavioral tests, the experimenter had no knowledge about the treatments that the rats had received.
No significant mechanical and thermal hyperalgesia were observed in control rats. As compared with controls, significant mechanical and thermal hyperalgesia were developed within a week after implantation of Walker 256 cells into the tibial canal of rats and lasted for four weeks. Previous studies also showed that the tumor occupies >90% of the intramedullary space on the day 14 following inoculation.21 Accordingly, the rats were subjected to the next experiments two weeks after inoculation of cancer cells.
ELISA measurements
All the tissues from individual rats were sampled for the analysis. In brief, DRG tissues (L4–L6) of the rats were removed. Total protein was then extracted by homogenizing the sample in ice-cold immunoprecipitation assay buffer with protease inhibitor cocktail kit (Promega Co., Madison, WI). The lysates were centrifuged, and the supernatants were collected for measurements of protein concentrations using a bicinchoninic acid assay reagent kit.
The levels of TNF-α were examined using an ELISA assay kit (Wuhan Fine Biotech Co., Wuhan, China) according to the provided description. Briefly, polystyrene 96-well microtitel immunoplates were coated with affinity-purified rabbit anti-TNF-α antibodies. Parallel wells were coated with purified rabbit IgG for evaluation of nonspecific signal. After overnight incubation, plates were washed. The diluted samples and TNF-α standard solutions were distributed in each plate. The plates were washed and incubated with anti-TNF-α galactosidase. The plates were washed and incubated with substrate solution. After incubation, the optical density was measured using an ELISA reader with 575 nm of wavelength. In the similar way, the levels of IL-6 were examined using an ELISA assay kit (Wuhan Fine Biotech Co.)
Western blot analysis
Briefly, DRG tissues (L4–L6) were removed, and total protein was extracted. The lysates were centrifuged, and the supernatants were collected. After being denatured, the supernatant samples containing 20 μg of protein were loaded onto gels and electrically transferred to a polyvinylidene fluoride membrane. The membrane was incubated overnight with primary antibodies (diluted at 1:500): rabbit anti-TRPA1 (1:500, Novus Bio, NB100-91319), anti-TNFR1 (1:500; Abcam #ab90463), anti-IL-6R (1:500; Abcam#ab103798), anti-p-p38-MAPK (1:500; USBio, USB#403230)/p38-MAPK (1:500; USBio, USB#403226), anti-p-JNK1(1:500; Abcam #ab47337)/JNK1 (1:500; Abcam #ab213521). The membranes were washed and incubated with an alkaline phosphatase conjugated antirabbit secondary antibody (1:1000). The primary and secondary antibodies were obtained from Abcam Co. or Antibodies online Com. The immunoreactive proteins were detected by enhanced chemiluminescence. The bands recognized by the primary antibody were visualized by exposure of the membrane onto an X-ray film. The membrane was stripped and incubated with anti-β-actin to show equal loading of the protein. The film was then scanned, and the optical density of TRPA1/TNFR1/IL-6R//p-p38-MAPK/p38-MAPK/p-JNK1/JNK1/β-actin bands was analyzed using the NIH Scion Image software.
Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze data for ELISA and western blots and two-way repeated ANOVA was used to analyze data for mechanical and thermal pain responses, and Tukey’s post hoc tests were used as appropriate. Values were presented as means ± standard error of mean. For all analyses, differences were considered significant at P < 0.05. All statistical analyses were performed by using SPSS for Windows version 13.0 (SPSS Inc.).
Results
Pain responses to mechanical and thermal stimuli after bone cancer
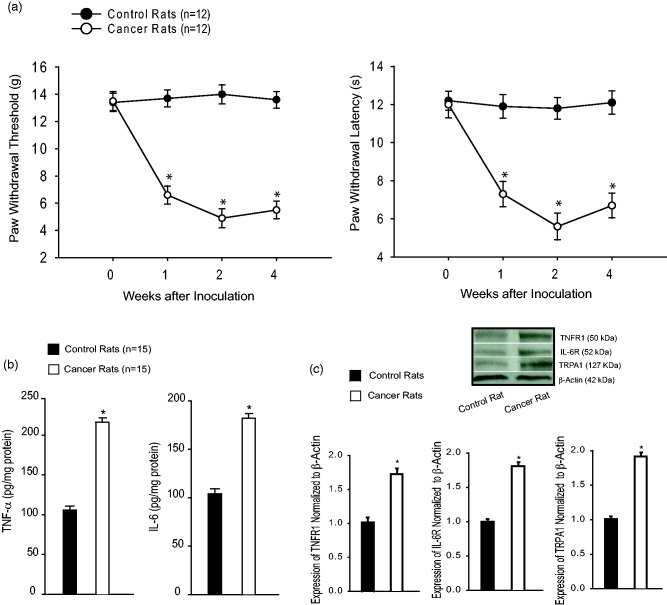
Bone cancer induced long-lasting pain behaviors in rats that were indicated by significantly increased mechanical and thermal sensitivity (Figure 1(a)). Mechanical allodynia and thermal hyperalgesia were observed within a week after injection of Walker 256 cells and lasted for four weeks (P < 0.05 vs. control animals, n = 12 in each group). Note that no behavioral test was performed more than four weeks after the cell implantation in this experiment. Thus, in the next experiments, the rats with two weeks of inoculation of cancer cells were used to examine the effects of TNF-α-TRPA1 and IL-6-TRPA1 signal pathways on neuropathic pain.
Figure 1.
(a) Mechanical and thermal sensitivity in control rats and bone cancer rats. With the development of bone cancer, PWT and PWL were decreased in cancer rats as compared with control rats. Significant mechanical and thermal hyperalgesia appeared one week after inoculation of cancer cells (*P < 0.05 vs. control rats, n = 12 in each group). (b) The levels of TNF-α and IL-6 in the DRG were amplified in bone cancer rats after two weeks of inoculation of cancer cells as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 15 in each group). (c) Bone cancer also increased protein expression of TNFR1, IL-6R, and TRPA1 as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 6–8 in each group). Top panel is typical bands and bottom panels are averaged data. IL-6: interleukin-6; IL-6R: IL-6 receptor; TNF-α: tumor necrosis factor-α; TNFR1: TNF-α receptor; TRPA1: transient receptor potential ankyrin 1.
Expression of TNF-α/IL-6/TRPA1 signal pathways in sensory neurons
In this experiment, DRG tissues were removed two weeks after inoculation of cancer cells. Figure 1(b) demonstrates that bone cancer amplified the levels of TNF-α and IL-6 in the DRG as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 15 in each group). Figure 1(c) further shows that bone cancer increased protein expression of TNFR1 (a subtype TNF-α receptor), IL-6R, and TRPA1 as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 6–8 in each group).
Effects of blocking TNF-α on mechanical and thermal sensitivity
We further examined the role played by inhibition of TNF-α in modifying mechanical and thermal sensitivity. Figure 2 shows that PWT and PWL appeared to be less in cancer rats without treatment than them in control rats. Injection of PTX attenuated mechanical and thermal hypersensitivity in cancer rats by showing increases of PWT and PWL as compared to the group with no treatment (P < 0.05, PTX treatment vs. no treatment). The inhibitory effects of PTX (10, 20, and 40 mg/kg) on mechanical and thermal sensitivity appeared in a dose-dependent way. The accumulated effects of PTX were also observed two to three days after administration of PTX.
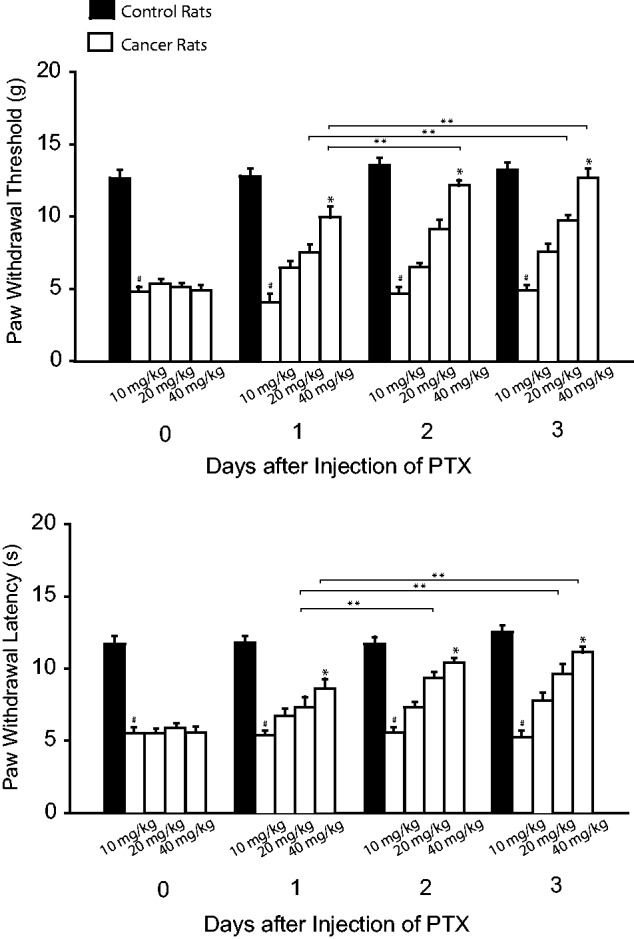
Figure 2. Effects of blocking TNF-α on mechanical and thermal sensitivity. TNF-α was inhibited by PTX (10, 20, and 40 mg/kg body weight; i.p. each day over three consecutive days). PWT and PWL were smaller in bone cancer rats without treatment. As PTX was given, PWT and PWL were increased in a dose-dependent way. #P < 0.05 versus control rats. *P < 0.05 versus no treatment and other dosages. **P < 0.05, indicated as the same dose of PTX among different days. The number of animals is 8 in control rats and 12 in bone cancer rats without treatment. The number of bone cancer rats with injection of PTX is 8 (10 mg/kg), 6 (20 mg/kg), and 9 (40 mg/kg). PTX: pentoxifylline.
Effects of blocking IL-6 signal on mechanical and thermal sensitivity
We also examined the role played by inhibition of IL-6 in mechanical and thermal sensitivity. Figure 3 shows that PWT and PWL appeared to be smaller in cancer rats with no treatment than them in control rats. This figure further shows that injection of SC144 (5, 10, 20 mg/kg) attenuated mechanical and thermal hypersensitivity in cancer rats as compared with the group of no treatment (P < 0.05, SC144 treatment vs. no treatment). The inhibitory effects of SC144 on mechanical and thermal sensitivity appeared in a dose-dependent way. The accumulated effects of SC144 were also observed two to three days after administration of SC144.
Figure 3.
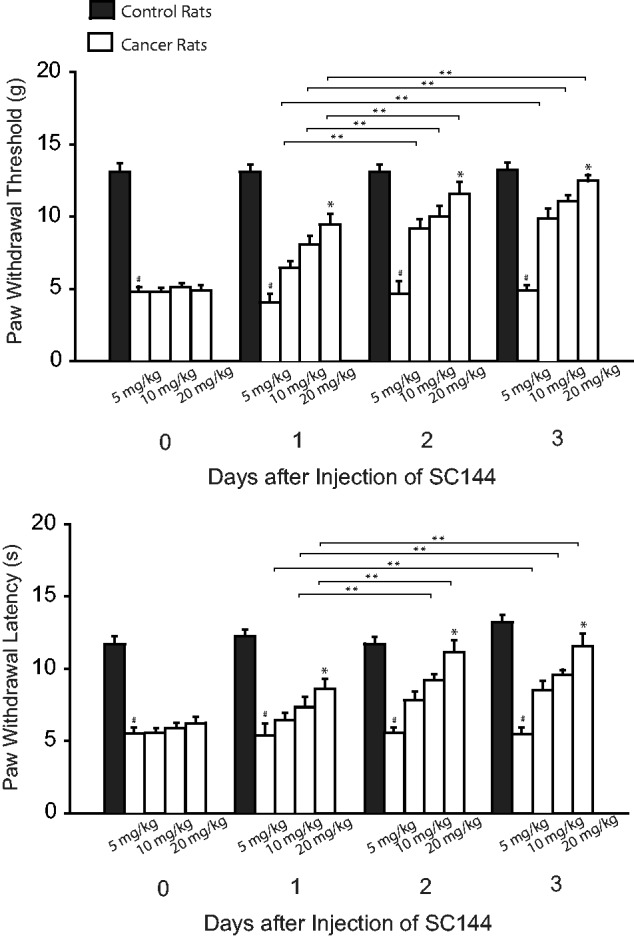
Effects of blocking IL-6 signal on mechanical and thermal sensitivity. IL-6R was inhibited by SC144 (5, 10, and 20 mg/kg body weight; i.p. each day over three consecutive days). PWT and PWL were smaller in bone cancer rats without treatment than in control rats. SC144 increased PWT and PWL in bone cancer rats as compared with no treatment. The effects of SC144 appeared in a dose-dependent way. #P < 0.05 versus control rats. *P < 0.05 versus no treatment and other dosages. **P < 0.05, indicated as the same dose of SC144 among different days. The number of animals is 10 in control rats and 12 in bone cancer rats without treatment. The number of bone cancer rats with injection of SC144 is 6 (5 mg/kg), 8 (10 mg/kg), and 8 (20 mg/kg).
Effects of blocking TRPA1 on mechanical and thermal sensitivity
Figure 4 shows that PWT and PWL appeared to be smaller in cancer rats without treatment than them in control rats. This figure also demonstrates that PWT and PWL were increased in a dose-dependent way after injection of HC030031 (1, 3, and 10 mg/kg) in bone cancer rats (P < 0.05, HC030031 treatment vs. no treatment). The accumulated effects of HC030031 were also observed after administration of HC030031.
Figure 4.
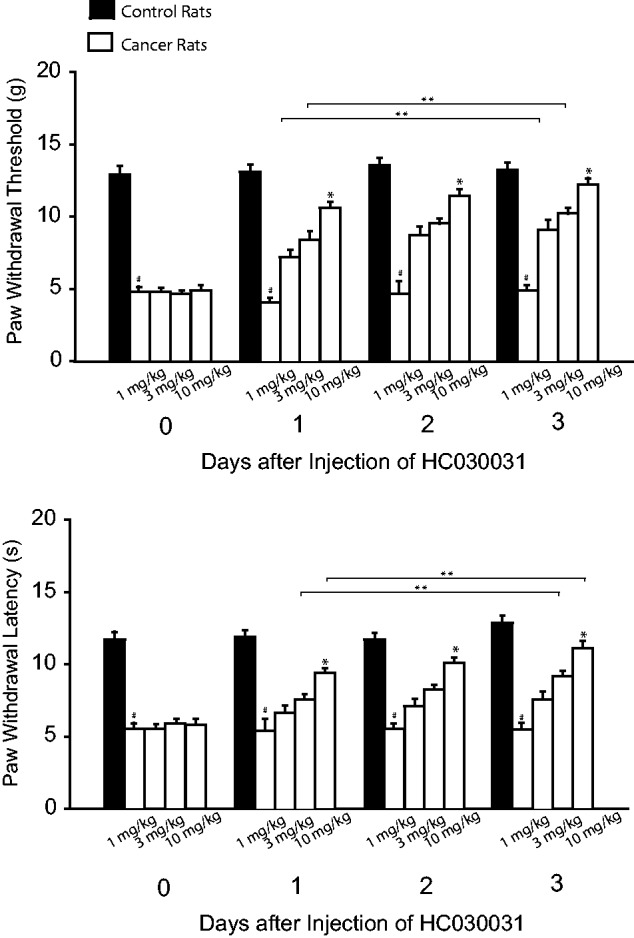
Effects of blocking TRPA1 on mechanical and thermal sensitivity. TRPA1 was blocked by administration of HC030031 (1, 3, and 10 mg/kg body weight; i.p. each day over three consecutive days). PWT and PWL decreased in bone cancer rats with no treatment. Injection of HC030031 increased PWT and PWL in bone cancer rats as compared to the group with no treatment. #P < 0.05 versus control rats. *P < 0.05 versus no treatment and other dosages. **P < 0.05, indicated as the same dose of HC030031 among different days. The number of animals is 12 in each group for control rats and for bone cancer rats without treatment. The number of bone cancer rats with injection of HC030031 is 8 in each group for three dosages (1 mg/kg, 3 mg/kg, and 10 mg/kg).
Effects of blocking TNF-α/IL-6 on TRPA1 signal leading to neuropathic pain
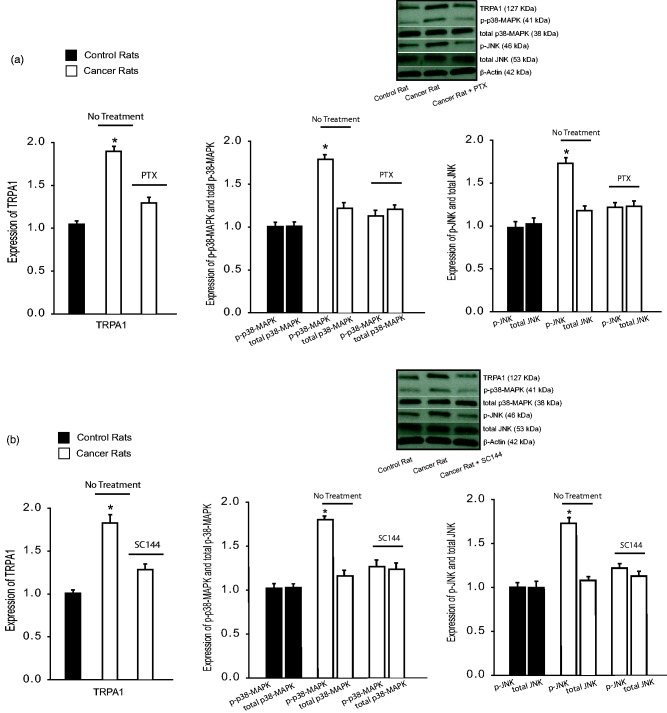
The effects of TNF-α and IL-6 on TRPA1 signal pathway were examined in additional groups. In this experiment, DRG tissues were removed three days after the beginning injection of PTX and SC144. Figure 5(a) demonstrates that bone cancer amplified TRPA1 as compared with control rats (P < 0.05, cancer rats vs. control rats, n = 6–8 in each group). This figure further shows that PTX attenuated upregulation of TRPA1 induced by bone cancer (P < 0.05 vs. cancer rats without PTX). Moreover, intracellular signal pathways of DRG neurons, namely, p38-MAPK and JNK, were examined. Bone cancer upregulated phosphorylated p38-MAPK and JNK, and these amplifications were inhibited by administration of PTX. It is noted that total protein levels of p38-MAPK and JNK were not increased significantly by bone cancer. In addition, Figure 5(b) demonstrates that TRPA1 as well as phosphorylated p38-MAPK and JNK were upregulated by bone cancer and SC144 attenuated upregulation of TRPA1 and phosphorylated p38-MAPK and JNK induced by cancer (P < 0.05 vs. cancer rats without SC144, n = 8 = 10 in each group).
Figure 5.
The effects of TNF-α and IL-6 inhibition on signal pathways leading to neuropathic pain. PTX (40 mg/kg body weight, i.p. each day for three consecutive days) was given to inhibit TNF-α. SC144 (20 mg/kg body weight, i.p. each day for three consecutive days) was given to inhibit IL-6 signal pathways. Three days after the beginning of respective injection of PTX and SC144, DRG tissues were removed for examination of TRPA1 and p38-MAPK and p-JNK signal pathways. (a)Averaged data and typical bands, without treatment bone cancer increased TRPA1 as well as intracellular signal p-p38-MAPK and p-JNK (phosphorylated form) in the DRG as compared with control rats. Furthermore, administration of PTX attenuated increases of TRPA1 and signal pathways in bone cancer rats. Note that the total protein levels of p38-MAPK and JNK were not elevated significantly by bone cancer. *P < 0.05 versus control rats and bone cancer rats with PTX. n = 6–8 in each group. (b) Averaged data and typical bands showing the effects of SC144. Bone cancer increased the protein levels of TRPA1 and intracellular signal p-p38-MAPK and p-JNK (phosphorylated form) in the DRG as compared with control animals. SC144 attenuated amplification of TRPA1 and these signal pathways in bone cancer rats. Total protein levels of p38-MAPK and JNK were not elevated significantly by bone cancer. *P < 0.05 versus control rats and bone cancer rats with SC144. n = 8–10 in each group. PTX: pentoxifylline; TRPA1: transient receptor potential ankyrin 1.
Discussion
TNF-α and IL-6 are PICs playing a critical role in the development and maintenance of inflammatory pain.9 TNF-α and IL-6 induce pain through the release of inflammatory mediators sensitizing ion channels.9 Data of our current study provided the evidence that (1) systemic administration of individual inhibitor of TNF-α, IL-6, and TRPA1 attenuated mechanical hyperalgesia and thermal hypersensitivity induced by bone cancer and (2) inhibition of TNF-α and IL-6 also attenuated expression of TRPA1, p38-MAPK, and JNK signals in the DRG. Taken together, results suggest that TNF-α and IL-6 play a role in modifying TRPA1 signal pathway by which it mediates mechanical hyperalgesia and thermal hypersensitivity induced by bone cancer.
It is noted that TNF-α plays an important role in regulating neuropathic pain in vivo experiments using various animal models.17,26–29 The mechanisms by which TNF-α mediates mechanical and thermal hyperalgesia include a direct sensitization effect of TNF-α and/or sensitization of inflammatory mediators evoked by TNF-α on ion channels in sensory neurons.14,15 TNF-α activates multiple signaling pathways, including the p38-MAPK and JNK pathways,9 which are recognized as important regulators of inflammatory pain. Also, TNF-α treatment has been observed to result in an upregulation of TRPA1 expression in sensory neurons.16 In this prior study using the cultured cells, it was found that TNF-α amplified TRPA1 via intracellular p38-MAPK signal.16 On the basis of those results, in the current study, we designed our in vivo experiments. Indeed, we observed that inhibition of TNF-α by PTX decreased expression of TRPA1, p38-MAPK, and JNK in the DRG of cancer rats, and this further attenuated neuropathic pain induced by bone cancer. Nonetheless, our results demonstrated that p38-MAPK and JNK signal pathways are stimulated by bone cancer and likely mediate upregulation of TRPA1 in sensory nerves. We demonstrated, for the first time, a novel mechanism by which TNF-α contributes to enhanced TRPA1 expression likely via p38-MAPK and JNK signals, which are involved in mechanical hyperalgesia and thermal hypersensitivity induced by bone cancer.
Calcium is a key regulator of major cellular processes. Its cytosolic concentration is determined mainly by extracellular calcium influx, release of calcium from internal stores, and mitochondrial uptake. The levels of calcium and its channel activity contribute to pathophysiological process of pain.30 It has been reported that mitochondrial-mediated dysregulation of calcium homeostasis or dysregulation of neurotrophins is involved in the mechanism of neuropathic pain.31,32 A prior study suggests that calcium influx is a part of process for upregulation of TRPA1 expression by TNF-α.33 Also, as TRPA1 receptor in sensory neurons is activated, calcium influx occurs in the involvement of pain response.30 In the current study, we observed that blocking TNF-α attenuated TRPA1 expression and decreased mechanical and thermal sensitivity. Thus, we speculated that the effects of blocking TNF-α would improve dysregulation of calcium homeostasis in attenuating neuropathic pain. In addition, increases in neurotrophins (such as nerve growth factor) can amplify pain response and upregulation of TRPA1 expression, and increases of TRPA1 activity are partly involved in the effects of neurotrophins.34,35 Thus, there is a possibility that enhancement of TRPA1 observed in this study is linked to dysregulation of neurotrophins in bone cancer-induced neuropathy.
IL-6 complexes with membrane-bound or soluble IL6R to activate cells expressing the gp130.36–38 Most cells are devoid of membrane-bound IL-6R and are thus unresponsive to IL-6; however, they can still react to IL-6 complexed with a soluble form of the IL-6R (sIL-6R) to activate gp130, a pathway called “trans-signaling.”39 A combination of all these receptors is the essential requirement of at least one gp130 subunit for signal transduction. After activation, gp130 acts via stimulation of the MAPK pathway and classical JAK-STAT signaling.40 In addition to transcriptional regulation, IL-6 has been shown to control protein synthesis of DRG neurons and thereby alter mechanical allodynia.41 Evidence further suggests that knockdown of IL-6 transducing receptor subunits gp130 decreases mRNA expression of TRPA1 in sensory neurons and IL-6 is engaged in the expression and/or activation of TRPA1.19,20
Thus, in the current study, we used SC144, a gp130 inhibitor, to block IL-6-mediated signal transduction in order to examine engagement of the IL-6R in mechanical and thermal hyperalgesia induced by bone cancer. Our data showed that systemic administration of SC144 significantly amplified PWT and PWL in bone cancer animals. Interestingly, SC144 attenuated expression of TRPA1 in the DRG of cancer rats, suggesting the role played by IL-6 in modifying TRPA1 during the development of neuropathic pain in cancer rats. Intracellular p38-MAPK and JNK signals are engaged in the effects of IL-6. Accordingly, our data indicate that bone cancer activates IL-6 signal, which subsequently amplifies expression of TRPA1 in the DRG via intracellular p38-MAPK and JNK thereby resulting in mechanical hyperalgesia and thermal hypersensitivity.
Conclusions
The protein expression levels of TRPA1 receptor in peripheral sensory neurons are upregulated after bone cancer; and inhibition of TRPA1, TNF-α, and IL-6 signals antagonizes mechanical hyperalgesia and thermal hypersensitivity in bone cancer rats. TRPA1 pathways play a role in TNF-α engagement of bone cancer-induced neuropathic pain via intracellular p38-MAPK and JNK signal. Also, the role of IL-6-TRPA1 signal in bone cancer-induced neuropathic pain is likely via p38-MAPK and JNK pathways. Results of our study provide a base for the mechanisms responsible for bone cancer-induced neuropathic pain. In particular, targeting one or more of these signaling molecules involved in activation of TNF-α-TRPA1 and IL-6-TRPA1 evoked by bone cancer may present new opportunities for treatment and management of neuropathic pain in patients with bone cancer.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the First Hospital of Jilin University (JDYY52015016), Norman Bethune Program of Jilin University (No. 2015335), and Science and Technology Development Program of Jilin Province (20160520160JH).
References
- 1.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007; 18: 1437–1449. [DOI] [PubMed] [Google Scholar]
- 2.Urch CE, Suzuki R. Pathophysiology of somatic, visceral, and neuropathic cancer pain In: Sykes N, Bennett MI, Yuan C-S. (eds) Clinical pain management: cancer pain. London: Hodder Arnold, 2008, pp. 3–12. [Google Scholar]
- 3.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013; 154: S54–S62. [DOI] [PubMed] [Google Scholar]
- 4.Koivisto A, Pertovaara A. Transient receptor potential ankyrin 1 (TRPA1) ion channel in the pathophysiology of peripheral diabetic neuropathy. Scand J Pain 2013; 4: 129–136. [DOI] [PubMed] [Google Scholar]
- 5.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427: 260–265. [DOI] [PubMed] [Google Scholar]
- 6.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50: 277–289. [DOI] [PubMed] [Google Scholar]
- 7.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112: 819–829. [DOI] [PubMed] [Google Scholar]
- 8.Skaper SD, Facci L, Zusso M, Giusti P. Neuroinflammation, mast cells, and glia: dangerous liaisons. Neuroscientist 2017; 23: 478–498. [DOI] [PubMed] [Google Scholar]
- 9.Miller RJ, Jung H, Bhangoo S, White FA. Cytokine and chemokine regulation of sensory neuron function In: Canning BJ, Spina DV. (eds) Handbook of experimental pharmacology. Berlin: Springer, 2009, pp. 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwak YS, Hulsebosch CE, Leem JW. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast 2017; 2017: 2480689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lees JG, Makker PGS, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer 2017; 73: 22–29. [DOI] [PubMed] [Google Scholar]
- 12.Ortmann KL, Chattopadhyay M. Decrease in neuroimmune activation by HSV-mediated gene transfer of TNFα soluble receptor alleviates pain in rats with diabetic neuropathy. Brain Behav Immun 2014; 41: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci 2005; 6: 521–532. [DOI] [PubMed] [Google Scholar]
- 14.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 2004; 361: 184–187. [DOI] [PubMed] [Google Scholar]
- 15.Jin X, Gereau R. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006; 26: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Karim I, McCrudden MTC, Linden GJ, Abdullah H, Curtis TM, McGahon M, About I, Irwin C, Lundy FT. TNF-α-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol 2015; 185: 2994–3002. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum 2011; 63: 819–829. [DOI] [PubMed] [Google Scholar]
- 18.Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain 2010; 151: 345–355. [DOI] [PubMed] [Google Scholar]
- 19.Malsch P, Andratsch M, Vogl C, Link AS, Alzheimer C, Brierley SM, Hughes PA, Kress M. Deletion of interleukin-6 signal transducer gp130 in small sensory neurons attenuates mechanonociception and down-regulates TRPA1 expression. J Neurosci 2014; 34: 9845–9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nummenmaa E, Hamalainen M, Moilanen LJ. Transient receptor potential ankyrin 1 (TRPA1) is functionally expressed in primary human osteoarthritic chondrocytes. Arthritis Res Ther 2016; 18: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao Y, Hou W, Yang L, Liu R, Gao Y, Kong X, Shi Z, Li W, Zheng H, Jiang S, Hua B. Increased expression of protease-activated receptor 2 and 4 within dorsal root ganglia in a rat model of bone cancer pain. J Mol Neurosci 2015; 53: 706–714. [DOI] [PubMed] [Google Scholar]
- 22.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 23.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 2017; 10: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience 2012; 211: 39–50. [DOI] [PubMed] [Google Scholar]
- 25.Cheah M, Fawcett JW, Andrews MR. Assessment of thermal pain sensation in rats and mice using the Hargreaves test. Bio Protoc 2017; 7: e2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Tang C, Tai LW, Ouyang Y, Li N, Hu Z, Chen X. Pro-resolving mediator maresin 1 ameliorates pain hypersensitivity in a rat spinal nerve ligation model of neuropathic pain. J Pain Res 2018; 11: 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magar S, Nayak D, Mahajan UB, Patil KR, Shinde SD, Goyal SN, Swaminarayan S, Patil CR, Ojha S, Kundu CN. Ultra-diluted Toxicodendron pubescens attenuates pro-inflammatory cytokines and ROS-mediated neuropathic pain in rats. Sci Rep 2018; 8: 13562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Wang B, Zhang G, Yang M, Liu N, Li YX, Ma H, Ma L, Sun T, Tan H, Yu J. Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochem Res 2018; 43: 2404–2422. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Xiao Y, Lv P, Teng Z, Dong Y, Qi Q, Liu Z. Rapamycin alleviates proinflammatory cytokines and nociceptive behavior induced by chemotherapeutic paclitaxel. Neurol Res 2018; 40: 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev 2014; 94: 81–140. [DOI] [PubMed] [Google Scholar]
- 31.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008; 112: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 32.Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg 2006; 102: 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng J, Wang J, Steinhoff M, Dolly JO. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci Rep 2016; 6: 21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devesa I, Ferrer-Montiel A. Neurotrophins, endocannabinoids and thermo-transient receptor potential: a threesome in pain signalling. Eur J Neurosci 2014; 39: 353–362. [DOI] [PubMed] [Google Scholar]
- 35.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 2007; 86: 550–555. [DOI] [PubMed] [Google Scholar]
- 36.Hoge J, Yan I, Janner N, Schumacher V, Chalaris A, Steinmetz OM, Engel DR, Scheller J, Rose-John S, Mittrucker H-W. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J Immunol 2013; 190: 703–711. [DOI] [PubMed] [Google Scholar]
- 37.Ruwanpura SM, McLeod L, Dousha LF, Seow HJ, Alhayyani S, Tate MD, Deswaerte V, Brooks GD, Bozinovski S, MacDonald M, Garbers C, King PT, Bardin PG, Vlahos R, Rose-John S, Anderson GP, Jenkins BJ. Therapeutic targeting of the IL-6 trans-signaling/mechanistic target of rapamycin complex 1 axis in pulmonary emphysema. Am J Respir Crit Care Med 2016; 194: 1494–1505. [DOI] [PubMed] [Google Scholar]
- 38.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 2014; 70: 11–20. [DOI] [PubMed] [Google Scholar]
- 39.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J 1994; 300: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003; 374: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci 2010; 30: 15113–15123. [DOI] [PMC free article] [PubMed] [Google Scholar]