Abstract
Patient: Female, 48
Final Diagnosis: Spinal schawnnoma
Symptoms: Dysuria • leg pain
Medication: —
Clinical Procedure: —
Specialty: Orthopedics and Traumatology
Objective:
Challenging differential diagnosis
Background:
Spinal schwannomas are benign tumors arising from Schwann cells. Although they have been well described, tumor movement in the spinal canal is an extremely rare finding, and entirely cystic spinal schwannomas have rarely been reported. This is the first report of a spinal schwannoma that simultaneously exhibited both these unusual features.
Case Report:
A 48-year-old female presented with dysuria and right leg pain. Initial magnetic resonance imaging (MRI) revealed a well-delineated intradural cystic lesion at the level of L4–S1 vertebrae that was isointense with cerebrospinal fluid on both T1- and T2-weighted images. A follow-up MRI 6 months later showed that the tumor had moved to the level of L2–L4; it also revealed tortuous configuration of nerve roots of the cauda equina. The tumor was resected, and a diagnosis of schwannoma with extensive cystic degeneration was pathologically confirmed.
Conclusions:
Various possible mechanisms have been suggested for the mobility of extramedullary tumors. In the present case, MRI findings indicated the cause of the tumor movement might be attributed to the laxity of nerve roots. Besides, it is highly atypical for a schwannoma to present an entirely cystic appearance, and the combination of the 2 extraordinary features made preoperative diagnosis difficult. However, 16 out of 22 (73%) of previously reported mobile spinal tumors were schwannomas, so the differential diagnosis for a mobile spinal tumor should include schwannoma, even when the lesion seems entirely cystic on MRI. To minimize the risk of complications and additional surgical dissection, physicians should acknowledge that spinal tumors can migrate.
MeSH Keywords: Cauda Equina, Magnetic Resonance Imaging, Neurilemmoma, Orthopedics, Radiology, Spinal Cord Neoplasms
Background
Spinal schwannomas are the most common intradural extramedullary spinal tumors that arise from spinal nerve root sheaths. Mobile spinal schwannomas have been described at various vertebral levels since 1978 [1]. Although most of them have been reported within the lumbar spine [2], their occurrence is rare, and limited data is available to date. Besides, spinal schwannomas are mostly solid or heterogeneously solid tumors [3]; entirely cystic intradural extramedullary spinal schwannomas are uncommon. Here, we report an extremely rare case of an intradural schwannoma that was mobile and presented an entirely cystic appearance, which are 2 highly atypical features.
Case Report
A 48-year-old Japanese female presented to our hospital with the chief complaint of dysuria and right leg pain, which had gradually worsened over the previous 3 weeks. Her motor function was normal. She had a 7-year history of uterine myoma. Magnetic resonance imaging (MRI) was acquired using a 1.5T scanner with a spine coil (Ingenia, Philips Medical Systems, Best, The Netherlands). Unenhanced MRI revealed a well-delineated tumor in the spinal canal at the level of L4–S1 (Figure 1). The tumor was 51 mm in diameter and showed isointensity with the cerebrospinal fluid on both T1- and T2-weighted turbo spin-echo images.
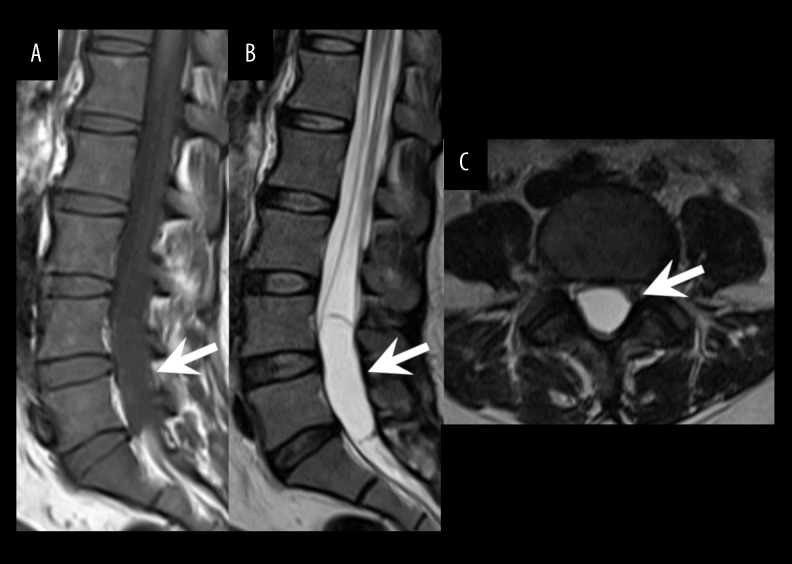
Figure 1.
Initial MR imaging. The initial sagittal T1-weighted (A), sagittal T2-weighted (B), and axial T2-weighted (C) images showed an intradural extramedullary cystic mass at the level of the L4–S1 vertebrae with homogeneous intensity identical to that of cerebrospinal fluid. The cystic mass had a smooth margin, and no solid component was observed.
Differential diagnoses from the MRI findings included arachnoid and ependymal cysts. A lumbar puncture was performed; the cerebrospinal fluid cytology was negative. Surgery was planned but postponed because of severe anemia due to menorrhagia. The patient was then followed up as an outpatient because her bladder function improved after the lumbar puncture. She could perform activities of daily living independently, but her right leg pain remained.
A follow-up MRI at 6 months after the initial scan showed that the tumor had relocated to the level of L2–L4 (Figure 2). No restricted diffusion was noted on diffusion-weighted image (b=1000 s/mm2), and no solid component was observed on any of the sequences, including coronal thin-section (1.5 mm) constructive interference in steady-state images.
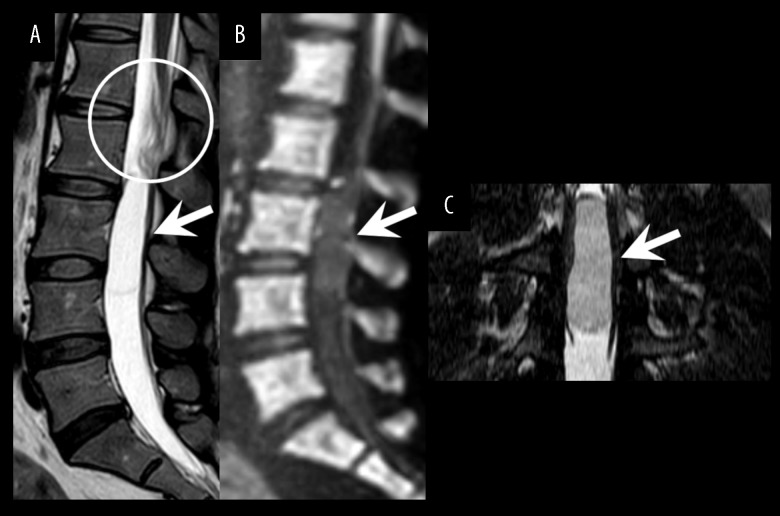
Figure 2.
Follow-up MRI at 6 months after the initial scan. The sagittal T2-weighted image (A) revealed that the tumor had moved to the level of L2–L4 (arrow) and showed the wrinkled appearance of nerve roots (circle). The sagittal diffusion-weighted image (B) showed no abnormal signal intensity. The thin-section coronal constructive interference in steady state image (C) demonstrated no solid component.
The tumor was resected via a posterior approach at 8 months after the initial MRI. The pathological examination confirmed a diagnosis of schwannoma with extensive cystic degeneration (Figure 3). Her symptoms resolved postoperatively, and at 1 month after the operation, she was entirely free from previous symptoms.
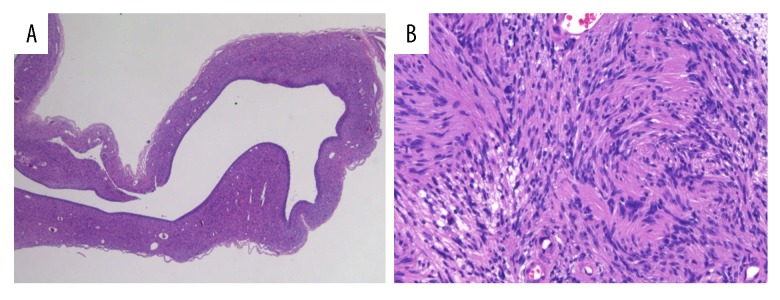
Figure 3.
Hematoxylin and eosin (H&E) staining of the resected specimen. (A) A low-power field (H&E stain, 20×) showed a large cystic space within the tumor. (B) A high-power field (H&E stain, 400×) revealed the proliferation of spindle cells with elongated nuclei, which exhibited a nuclear palisade arrangement.
Discussion
Previous reports have proposed possible mechanisms for the mobility of extramedullary tumors, including elongation of the nerve root due to tension resulting from the weight of the tumor [4,5], a cranial shift caused by the injection of intrathecal contrast medium during myelography [6], and the intentional use of a Valsalva maneuver [4,7,8]. In the present case, the tumor movement may have been caused by laxity of the nerve roots [9,10]; surgery confirmed the adhesion of the cauda equina nerves to the tumor, and the second MRI revealed tortuous configuration of the nerve roots.
Postural changes could be another possible cause of tumor mobility [11]. Kotani et al. demonstrated the movement of a spinal schwannoma using cine MRI during a change in spinal posture [7]. We attempted the same technique for our case, but the tumor demonstrated no movement during the scan.
Mobile spinal tumors are rare, and a literature search revealed only 22 reported cases that comprised schwannomas, ependymomas, neurenteric cysts, and a hemangioblastoma [7–9,12–16]. The majority (approximately 70%) of the mobile spinal tumors were found to be schwannoma, and all the tumors were solid or heterogeneously solid. In addition, only 14 cases of spinal schwannomas have been reported to be cystic [3,17–23]. To the best of our knowledge, this is the first report of a mobile spinal schwannoma with a completely cystic appearance. The presence of these 2 features, both highly unusual for schwannomas in the spinal canal, made preoperative diagnosis difficult.
Some studies have indicated that contrast-enhanced MRI helps differentiate cystic schwannomas from other cystic lesions such as epidermoid, arachnoid, and neurenteric cysts [3,21,22]. However, our MRI protocol did not include contrast-enhanced sequences.
To minimize the risk of complications and unnecessary additional surgical dissection, spinal surgeons and radiologists should keep in mind that tumors of the cauda equina, particularly schwannomas, may migrate. Some authors have reported the usefulness of perioperative or intraoperative imaging studies, such as MRI, myelography, or ultrasonography, to confirm the precise location of the tumor [4,5,24]. In the past, myelography was regarded as the gold standard for the diagnosis of spinal diseases [25]. It enables a real-time assessment of the spatial relationship between the spinal canal structure and the spinal tumor; however, the intradural injection of contrast medium itself can induce the movement of a mobile spinal tumor [6]. MRI provides excellent contrast resolution, and repeated preoperative MRI allows visualization of the presence and extent of the mobility of a spinal tumor [26]. However, in light of cost-effectiveness in healthcare, it is imperative to carefully consider the indication for the use of such costly imaging studies. Intraoperative MRI provides real-time imaging guidance, but it is not available in most hospitals. Intraoperative ultrasound can help visualize the real-time location of the spinal tumor before the dural opening [27], and previous reports have indicated its usefulness for the determination and modification of the dural incision site [2,24,26]. However, ultrasonography has some disadvantages: it is operator dependent, offers only a limited field of view, and can only be used after dural exposure [27]; thus, additional laminectomy may be needed if the tumor has moved a substantial distance from the location indicated by preoperative imaging [24]. Surgeons should plan perioperative imaging studies based on the advantages and disadvantages of each imaging modality.
Conclusions
This case exhibited 2 rare features of a spinal schwannoma simultaneously: the schwannoma presented an entirely cystic appearance, and the tumor location changed between repeated MRI scans. MRI findings suggested that the tumor movement may have been caused by the laxity of the cauda equina. The tumor’s entirely cystic appearance on MRI made preoperative diagnosis difficult; however, previous reports have indicated that the schwannoma is the most frequent mobile spinal tumor, so the differential diagnosis for a mobile spinal tumor should include schwannoma even when the tumor seems to be cystic. To prevent unnecessary additional procedures, physicians should be aware of the possibility of movement of a spinal tumor.
Footnotes
Conflicts of interest
None.
References:
- 1.Hollin SA, Drapkin AJ, Wancier J, Huang YP. Mobile schwannoma of the cauda equina. Case report. J Neurosurg. 1978;48(1):135–37. doi: 10.3171/jns.1978.48.1.0135. [DOI] [PubMed] [Google Scholar]
- 2.Toscano DT, Felbaum DR, Ryan JE, et al. Mobile schwannoma of the lumbar spine: A case report and review of the literature. Cureus. 2016;8(7):e715. doi: 10.7759/cureus.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parmar H, Patkar D, Gadani S, Shah J. Cystic lumbar nerve sheath tumours: MRI features in five patients. Australas Radiol. 2001;45(2):123–27. doi: 10.1046/j.1440-1673.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Marin-Sanabria EA, Sih IM, Tan KK, Tan JS. Mobile cauda equina schwannomas. Singapore Med J. 2007;48(2):e53–56. [PubMed] [Google Scholar]
- 5.Iizuka H, Iida T, Akai T. Mobile neurinoma of the cervicothoracic junction. Surg Neurol. 1998;50(5):492–93. doi: 10.1016/s0090-3019(98)00125-6. [DOI] [PubMed] [Google Scholar]
- 6.Tavy DL, Kuiters RR, Koster PA, Hekster RE. Elusive tumor of the cauda equina. Case report. J Neurosurg. 1987;66(1):131–33. doi: 10.3171/jns.1987.66.1.0131. [DOI] [PubMed] [Google Scholar]
- 7.Kotani T, Okawa A, Akazawa T, Sakuma T. Mobile ependymoma diagnosed with cine MRI. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-202984. bcr2013202984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon K, Filis AK, Cohen AR. Mobile spinal ependymoma. J Neurosurg Pediatr. 2010;5(1):85–88. doi: 10.3171/2009.7.PEDS09244. [DOI] [PubMed] [Google Scholar]
- 9.Wortzman G, Botterell EH. A mobile ependymoma of the filum terminale. J Neurosurg. 1963;20:164–66. doi: 10.3171/jns.1963.20.2.0164. [DOI] [PubMed] [Google Scholar]
- 10.Varughese G, Mazagri R. Mobile tumours in the lumbar spinal canal: A diagnostic problem. Can J Surg. 1997;40(1):59–63. [PMC free article] [PubMed] [Google Scholar]
- 11.Isu T, Iwasaki Y, Akino M, et al. Mobile schwannoma of the cauda equina diagnosed by magnetic resonance imaging. Neurosurgery. 1989;25(6):968–71. doi: 10.1097/00006123-198912000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Stuart S, Jones B, Mankad K, et al. Migrating tumour of the cauda equina. Postgrad Med J. 2010;86(1015):313. doi: 10.1136/pgmj.2010.097147. [DOI] [PubMed] [Google Scholar]
- 13.Kojima S, Yoshimura J, Takao T, Tamura T, Nishiyama K, Maruyama S, et al. Mobile spinal enterogenous cyst resulting in intermittent paraplegia in a child: Case report. J Neurosurg Pediatr. 2016;18(4):448–51. doi: 10.3171/2016.4.PEDS15666. [DOI] [PubMed] [Google Scholar]
- 14.Kothari A, Singh N, Anjum R. Mobile schwannomas of lumbar spine: A diagnostic dilemma. J Clin Orthop Trauma. 2017;8(2):197–200. doi: 10.1016/j.jcot.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najjar M, Elias E, Skaf G. Mobile lumbar spine ependymoma: Case report and review of literature. J Neurosurg Sci. 2017;61(6):677–79. doi: 10.23736/S0390-5616.16.03278-1. [DOI] [PubMed] [Google Scholar]
- 16.Luo H, Xiao J, Lv S, et al. A mobile hemangioblastoma of the cauda equina: case report and review of the literature. J Spinal Cord Med. :2018. doi: 10.1080/10790268.2018.1547855. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karatas A, Is M, Yildirim U, et al. Thoracic intradural cystic schwannoma: A case report. Turk Neurosurg. 2007;17(3):193–96. [PubMed] [Google Scholar]
- 18.Kasliwal MK, Kale SS, Sharma BS, Suri V. Totally cystic intradural extramedullary schwannoma. Turk Neurosurg. 2008;18(4):404–6. [PubMed] [Google Scholar]
- 19.Akhaddar A, Ajja A, Albouzidi A, et al. Cystic schwannoma of the cauda equina mimicking hemangioblastoma. Neurochirurgie. 2008;54(2):101–3. doi: 10.1016/j.neuchi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Vikram M, Pande A, Vasudevan MC, Ravi R. Cervical solitary long segment cystic Schwannoma. Br J Neurosurg. 2010;24(2):208–10. doi: 10.3109/02688690903301557. [DOI] [PubMed] [Google Scholar]
- 21.Albert AF, Kirkman MA, du Plessis D, et al. Giant solitary cystic schwannoma of the cervical spine: a case report. Clin Neurol Neurosurg. 2012;114(4):396–98. doi: 10.1016/j.clineuro.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Savardekar A, Singla N, Mohindra S, et al. Cystic spinal schwannomas: A short series of six cases. Can we predict them preoperatively? Surg Neurol Int. 2014;5(Suppl. 7):S349–53. doi: 10.4103/2152-7806.139666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Gupta R, Handa A, Sinha R. Totally cystic intradural schwannoma in thoracic region. Asian J Neurosurg. 2017;12(1):131–33. doi: 10.4103/1793-5482.145570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman JA, Wetjen NM, Atkinson JL. Utility of intraoperative ultrasound for tumors of the cauda equina. Spine (Phila Pa 1976) 2003;28(3):288–90. doi: 10.1097/01.BRS.0000042271.75392.E4. discussion 291. [DOI] [PubMed] [Google Scholar]
- 25.Bakhsh A. Role of conventional lumbar myelography in the management of sciatica: An experience from Pakistan. Asian J Neurosurg. 2012;7(1):25–28. doi: 10.4103/1793-5482.95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terada Y, Toda H, Yokote A, Iwasaki K. A mobile schwannoma of the cervical spinal cord: case report and review of the literature. Neurosurgery. 2016;78(1):E156–59. doi: 10.1227/NEU.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 27.Prada F, Vetrano IG, Filippini A, et al. Intraoperative ultrasound in spinal tumor surgery. J Ultrasound. 2014;17(3):195–202. doi: 10.1007/s40477-014-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]