Abstract
Cellular metabolism is at the foundation of all biological activities. The catabolic processes that support cellular bioenergetics and survival have been well studied. By contrast, how cells alter their metabolism to support anabolic biomass accumulation is less well understood. During the commitment to cell proliferation, extensive metabolic rewiring must occur in order for cells to acquire sufficient nutrients such as glucose, amino acids, lipids and nucleotides that are necessary to support cell growth, and to deal with the redox challenges that arise from the increased metabolic activity associated with anabolic processes. Defining the mechanisms of this metabolic adaptation for cell growth and proliferation is now a major focus of research. Understanding the principles that guide anabolic metabolism may ultimately enhance ways to treat diseases that involve deregulated cell growth and proliferation, such as cancer.
eTOC
Cell proliferation is associated with substantial rewiring of metabolism to support extensive need for macromolecule biosynthesis. Better understanding of how cells acquire and utilize nutrients for biosynthetic pathways and how they cope with metabolic challenges linked to high rates of proliferation will lead to improved cancer treatments.
Introduction
Cell proliferation requires the accumulation of intracellular biomass, such as proteins and lipids, in order to produce daughter cells. At the same time DNA replication must occur to pass along the genetic information. Accordingly, net production of proteins, lipids and nucleic acids is essential for a successful replicative cell division. The biosynthesis of these macromolecules is achieved mainly through a network of cellular metabolic pathways that direct the acquisition and utilization of various sources of nutrients. Characterizing the principles underlying these cellular biosynthetic pathways can thus provide important insights into the understanding of cell growth and proliferation.
Unlike unicellular organisms which directly sense and scavenge nutrients from the environment1–3, metazoan cells do not generally take up nutrients in a cell autonomous manner. In animal cells, nutrient acquisition is directed primarily by growth factor signalling (Box 1). The directed uptake of nutrients then stimulates intracellular nutrient-sensing kinases, establishing signalling cascades that redirect nutrients from catabolic pathways (aimed at molecule breakdown for energy production) to anabolic pathways (aimed at biosynthesis of cellular components). Recent studies have shed new light on how cells reprogramme their metabolism from catabolism to anabolism to fuel cell proliferation.
Box 1. Growth factor signalling.
Growth factors are signalling molecules that promote cell growth, proliferation or differentiation. Typical examples of growth factors include insulin, epidermal growth factor (EGF), fibroblast growth factor (FGF), erythropoietin (EPO), platelet-derived growth factor (PDGF), transforming growth factors (TGFs) and cytokines. Growth factors are usually secreted by specific organs and cell types to promote particular physiological processes. For example, insulin is produced by the β-pancreatic islet cells when the body nutrient levels are high, and it stimulates glucose uptake by liver, fat and skeletal muscle cells where glucose can be converted to glycogen or triglycerides for energy storage8. Upon tissue injury, EGF, FGF and a number of other growth factors are secreted by local immune cells and fibroblasts to promote tissue regeneration that involves the proliferation of keratinocytes and endothelial cells6.
Growth factor-stimulated cells often display increased ability to take up nutrients. Receptor tyrosine kinase (RTK) signalling is a typical downstream mediator of growth factor stimulation. Most growth factors that regulate metabolism bind to cognate RTKs, and result in the auto-phosphorylation of the receptor to initiate a series of kinase-mediated signalling events. Members of the phosphoinositide 3-kinase (PI3K) family are major targets of RTKs. Upon interaction with the auto-phosphorylated form of RTK, PI3K gets activated and converts the plasma membrane lipid phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3). Local enrichment of PtdIns(3,4,5)P3 serves as the subsequent signalling cue to bring together kinases including phosphoinositide-dependent kinase 1 (PDK1) and AKT (also known as protein kinase B, PKB)155. PDK1 can phosphorylate the regulatory loop of AKT and promote AKT activation. AKT is a serine/threonine kinase, for which a variety of downstream targets have been identified, including glucose uptake through glucose transporter 1(GLUT1) and induction of glycolysis by activation of hexokinase (HK), which converts glucose to glucose 6-phosphate (G6P) (see figure). Together, activation of AKT targets supports an anabolic growth phenotype156,157. This process is under tight control as PtdIns(3,4,5)P3 can be dephosphorylated and converted back to PtdIns(4,5)P2 through the lipid phosphatase activity of phosphatase and tensin homolog deleted on chromosome 10 (PTEN)158. This negative regulatory mechanism, among others, dampens the PI3K–AKT pathway activity and ensures that normal cells can only commit themselves to proliferation when persistent growth factor signalling is present.

In this Review, we examine the regulation of nutrient uptake and utilization when cells are instructed to proliferate by physiological cues such as in the context of tissue regeneration, and when cells acquire oncogenic mutations that stimulate cell proliferation. We highlight the regulation of glucose uptake, amino acid acquisition, and lipid and nucleotide synthesis during homeostatic cell proliferation, and then consider how cells maintain growth in nutrient scarce environments such as wound beds or in poorly-vascularized tumour regions.
Regulation of glucose uptake
In addition to the use of glucose as an energy source for ATP generation, proliferating cells often display enhanced uptake of glucose that provides an important source of carbon to support lipid production and the biosynthesis of nucleotides and non-essential amino acids [G] (NEAAs), which occurs via redirection of metabolites of glycolysis and the tricarboxylic acid (TCA) cycle (Figure 1 and Supplementary Box 1). To replenish TCA cycle intermediates that are used for the production of biomass, cells use anaplerosis [G], and an important anaplerotic substrate is glutamine4. Characterization of how metazoan cells regulate glucose uptake and its utilization is important to the understanding of how cellular metabolism is rewired to support anabolic growth.
Figure 1. Glucose uptake and utilization.
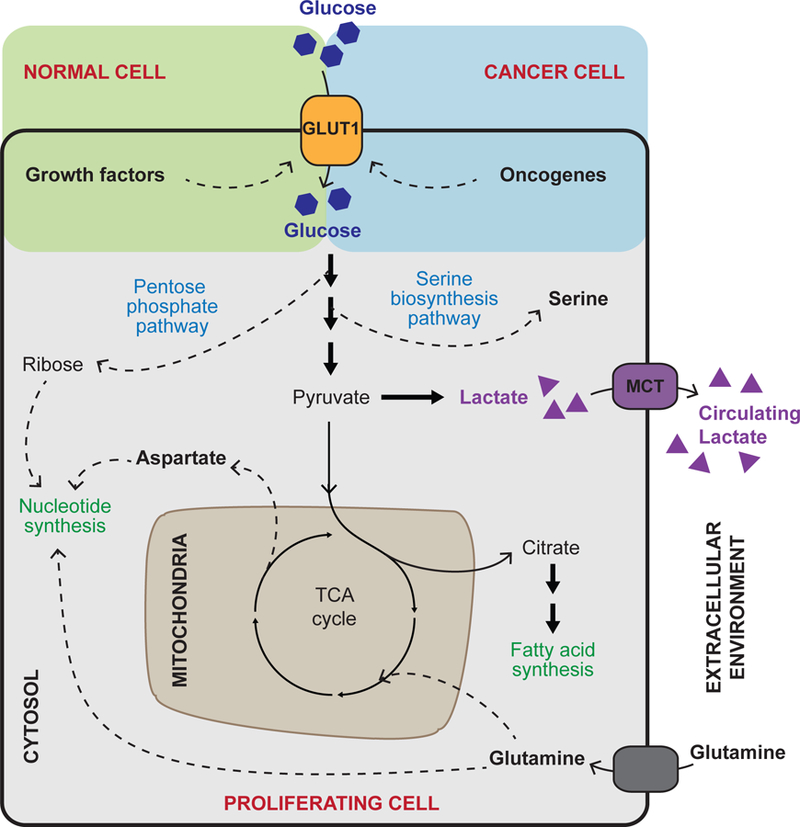
In order to support cell growth and proliferation, glucose uptake is promoted by the growth factor signalling pathways in normal cells or by oncogenic mutations in cancer cells, which may render growth signalling pathways constantly active. Glucose is an important carbon source in energy-generating pathways of oxidative metabolism, fuelling the tricarboxylic acid (TCA) cycle and subsequent oxidative phosphorylation in mitochondria (not shown). In proliferating cells, intermediates of glucose metabolism are often diverted from glycolysis and the TCA cycle (dashed arrows) and used for biosynthetic purposes such as the production of nucleotides, amino acids and fatty acids. The TCA cycle is kept active by anaplerotic reactions involving glutamine, which — beyond supporting anabolic reactions — replenishes TCA cycle intermediates that are diverted from the cycle to support anabolism. As a result of aerobic glycolysis, a large portion of glucose carbon is also converted to lactate and secreted. Cancer associated adaptations are highlighted in red.
GLUT1, glucose transporter 1; MCT, monocarboxylate transporter.
Signalling cues instruct glucose uptake.
In animal cells, glucose uptake is triggered non-cell-autonomously. Metazoan cell growth is primarily stimulated by growth factor signalling (Box 1), and cells can only acquire large quantities of glucose as a result of the activation of these signalling pathways. Various growth factors, such as insulin, vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), serve as the primary signalling cues that direct glucose uptake. In insulin-responsive cells such as those in the muscle, liver and fat tissues, most of this glucose is converted for storage into glycogen and fats (primarily triglycerides stored in the adipose tissue). However, in most other cells, increase in glucose metabolism following growth factor signalling leads to accumulation of biomass5–8. Glucose uptake is often increased in growth factor-stimulated cells. This is largely achieved through a series of kinase-mediated signalling events, including the activation of receptor tyrosine kinases (RTKs) and the downstream phosphoinositide 3-kinase (PI3K) and AKT (also known as protein kinase B, PKB) signalling cascade (Box 1).
Studies of T cell activation have provided a good example of how PI3K and AKT signalling informs a cell to take up and utilize glucose in support of cell growth. In the context of T cells, activation of T-cell receptor (TCR) upon antigen stimulation leads to the tyrosine phosphorylation of the cytoplasmic tail region of co-stimulatory receptor CD28, which — analogous to RTKs — is able to initiate a number of signalling cascades including the PI3K–AKT axis9,10. The activation of AKT promotes expression and plasma membrane localization of the glucose transporter 1 (GLUT1)11–13. In addition, AKT increases the cellular activity of the glycolytic enzyme hexokinase such that the imported glucose is captured by glycolysis as well as by other pathways to support amino acid, nucleotide and fatty acid biosynthesis12 (Box 1 and Supplementary Box 1). Upon withdrawal of growth factor or glucose, T cells rapidly undergo apoptosis as a result of reduction in mitochondrial membrane potential and cellular ATP level. However, cell survival can be rescued by ectopic expression of GLUT1 and hexokinase14. These studies demonstrate that glucose uptake and utilization are direct results of extracellular stimuli and that maintaining glucose influx and metabolism is essential to sustain cell survival and growth.
Utilizing glucose for cell growth.
Stimulation of resting T cells by TCR and CD28 induces a drastic increase in glucose uptake and this uptake supports the clonal expansion of antigen-stimulated T cells15. Augmented glucose influx leads to a high glycolytic rate. However, only a small fraction of glucose is fully oxidized in the TCA cycle (Supplementary Box 1) and serves as a source for ATP production via oxidative phosphorylation. In fact, the majority of carbon in glucose is converted to lactate and secreted. This has long been considered paradoxical. For the past 100 years, cellular nutrient uptake has been considered a demand-driven system based on biochemical studies performed primarily in bacteria grown in minimal medium [G]. Under such conditions, glycolysis is regulated by a declining level of ATP, and oxidative phosphorylation is stimulated primarily by a rise in ADP. However, the glucose uptake in metazoans is a supply-driven system, in which the cellular glucose uptake is primarily stimulated by growth factor signalling. When growth factor stimulated cells take up more glucose than they require, excess glycolytic intermediates are diverted into pathways that support the production of NEAAs, nucleotides and lipids required for cell growth. Any excess glycolytic end products are dealt with by converting pyruvate to lactate and secreting lactate back into the extracellular environment (Figure 1).
The metabolic phenomenon where rapidly proliferating cells in a nutrient-rich environment continue to take up and metabolize glucose in excess of their anabolic requirements is known as aerobic glycolysis (also known as the Warburg effect). In fact, this phenomenon has been known for almost a century, with initial observations that tumour cells display a much higher rate of glucose consumption when compared to their normal counterparts, and that in tumour cells glucose is mainly utilized via glycolysis even in the presence of adequate oxygen16,17. Unlike normal cells that are instructed to proliferate by extracellular signals, most cancer cells have acquired the ability to take up glucose cell-autonomously through the activation of oncogenes (Figure 1) and hence, acquire much more glucose than they require for their oxidative metabolism. Cancer genome sequencing has revealed that a large portion of human cancers harbour genetic alterations in the genes encoding members of the RTK family that increase their kinase activities18. For example, EGF receptor mutation or amplification occurs in 50% of non-small cell lung cancer (NSCLC) patients in Asia and 15% NSCLC patients in Western countries19. HER2 (human epidermal growth factor receptor 2, also known as ERBB2) is amplified in up to 20% of breast cancer patients20. In addition to RTKs, the PIK3CA gene, encoding the catalytic subunit of PI3K, is one the most frequently mutated genes in cancer, particularly in breast, colon and liver cancers21,22. Loss-of-function mutations in the PTEN gene — a negative regulator of PI3K signalling — are also highly prevalent in prostate and endometrial cancers23. These genetic events are selected, at least in part, to allow tumour cells to constantly acquire glucose and other nutrients from the environment, independent of growth factor stimulation.
Deregulated glucose uptake has emerged as a hallmark of cancer metabolism24. In fact, positron emission tomography-based imaging [G] of 18F-fluorodeoxyglucose uptake (FDG-PET) is widely used in the clinic for tumour monitoring25. When studied in tissue culture, cancer cells utilize glucose to generate glycolytic and TCA cycle intermediates in order to support biomolecular synthesis. Despite this, most cultured cancer cell lines secrete the majority of the glucose they take up as lactate, which accumulates in the tissue culture medium26. Quantitative metabolic flux analyses at the organismal level have led to new insights of glucose metabolism in tumours in vivo27,28. It has been shown that lactate produced by tumour cells via aerobic glycolysis can be consumed by non-proliferating tumour cells and the surrounding stromal cells in the tumour microenvironment, which direct lactate to the TCA cycle, using it as a primary source of carbon. Similar observations have also been made in normal tissues and it has been suggested that in some tissues the input of glucose into the TCA is mostly indirect — via circulating lactate produced by other cells28.
Acquisition of amino acids
In addition to the uptake of glucose as a carbon source, proliferating cells also need a substantial amount of amino acids in order to increase protein content, which makes up more than half of a cell’s dry mass29. Through a network of nutrient responsive pathways, cells maintain a battery of amino acids to charge tRNAs for protein translation, to support the biosynthesis of macromolecules including nucleotides, as well as to fuel bioenergetics such as through the catabolism of certain amino acids to produce acetyl-CoA. In proliferating cells, metabolic programmes are often rewired to actively acquire amino acids from the extracellular space to sustain biomass accumulation. In addition, opportunistic pathways can be utilized to scavenge protein when free amino acids are limiting in the extracellular environment.
Sensing and importing amino acids.
Similar to glucose, growth factor signalling can also directly promote amino acid uptake and utilization (Figure 2). The mechanistic target of rapamycin complex 1 (mTORC1) is a central coordinator of amino acid availability and allocation30. Increased AKT activity downstream of growth factor signalling results in phosphorylation of tuberous sclerosis complex, subunit 2 (TSC2), and subsequently dislocation of the TSC complex from the lysosome31–33. Dissociation of TSC from its lysosomal-resident target, Ras homolog enriched in brain (RHEB), leads to the derepression of RHEB activity and results in the activation of mTORC1 kinase activity34 (Figure 2). A primary functional output of mTORC1 activation is the increase in protein synthesis, which is achieved largely through direct phosphorylation of p70S6 kinase 1 [G] (S6K1) and eIF4E binding protein 1 [G] (4EBP1) by mTORC130. To complement the demand for increased protein translation, concerted AKT and mTORC1 activation induces and sustains cell surface expression of amino acid transporters35. In addition, through S6K1 kinase activity, mTORC1 can induce ribosome biogenesis in order to sustain the expanded protein translation capacity36.
Figure 2. Amino acid sensing and acquisition.

Cellular levels of amino acids can be increased by increased expression and cell membrane retention of amino acid transporters, which is downstream of receptor tyrosine kinase signalling mediated by growth factors. In addition, mechanistic target of rapamycin complex 1 (mTORC1) senses growth factor signalling and the presence of amino acids to promote the utilization of amino acids by activating protein translation. CASTOR1, Sestrin2, and SAMTOR are amino acid sensor proteins for arginine, leucine, and methionine (through S-adenosylmethionine (SAM)), respectively. These sensors control the activity of the GATOR2–GATOR1 complexes, negatively regulating the activity of RAG GTPases and hence inhibiting mTORC1 localization to the lysosome and activation. The suppressive functions of these sensors are diminished in the presence of the amino acids that they sense, leading to the activation of mTORC1. When amino acid levels are low, general control nonderepressible (GCN2) is activated by the increase of uncharged tRNAs and results in the inhibition of global protein translation (not shown). Paradoxically, GCN2 activation leads to increased expression of activating transcription factor 4 (ATF4). ATF4 mediates integrated stress response that includes the upregulation of genes that mediate amino acid uptake, synthesis and utilization (translation) to eventually support cell adaptation and recovery from stress. Note that ATF4 increases the expression of a variety of tRNA synthetases that allows the cell to better capture the limited amount of cellular amino acid for protein translation, which facilitates the production of proteins that are critical in stress adaptation and recovery. Cells can also acquire amino acids through macropinocytosis of extracellular proteins followed by their lysosomal degradation. Macropinocytic utilization is inhibited by mTORC1 and is promoted in cancer via oncogenic RAS and phosphoinositide 3-kinase (PI3K) pathway. Certain cancers have also been associated with upregulation of phosphoglycerate dehydrogenase (PHGDH), leading to increased serine synthesis. These cancers depend on PHGDH for growth and interference with de novo serine biosynthesis could be explored as a potential strategy in cancer treatment. Cancer associated adaptations are highlighted in red.
ASNS, asparagine synthetase; PSAT1, phosphoserine aminotransferase 1; RHEB, Ras homolog enriched in brain; TSC, tuberous sclerosis complex; x(c)(−), cystine–glutamine antiporter.
Besides growth factor signalling inputs, mTORC1 activity is tightly controlled by the availability of amino acids. When sufficient amounts of amino acids are present in the cells, the heterodimeric RAG GTPases promote the localization of mTORC1 to the lysosome, where it is in proximity of RHEB37. Regulation of the RAG GTPases and hence mTORC1 activity by amino acid levels is achieved through specific amino acid sensor proteins. For example, Sestrin2 (encoded by SESN2) was identified as a leucine sensor38,39. When the cellular leucine level decreases, Sestrin2 can bind and inhibit GATOR2, leading to the activation of the GATOR1 complex that represses the RAG GTPases and hence precludes full mTORC1 activation37 (Figure 2). In addition, CASTOR1 was identified as a sensor of cytosolic arginine40, and SAMTOR (encoded by BMT2) was shown to be a sensor of S-adenosyl methionine and hence indicative of the cellular methionine level41 (Figure 2). Similarly to Sestrin2, both CASTOR1 and SAMTOR function as negative regulators of the RAG GTPases through the GATOR2–GATOR1 axis. Overall, the intracellular amino acid sensors directly influence amino acid consumption by decreasing mTORC1 stimulation of translation when these amino acids become limiting40,41.
In addition to the suppression of mTORC1 activity, reduction in amino acid levels also provokes the integrated stress response [G]. As a considerable amount of tRNA becomes uncharged upon amino acid deficiency, general control nonderepressible 2 (GCN2) kinase is activated, which phosphorylates eukaryotic initiation factor 2α (eIF2α) to attenuate global protein translation. Paradoxically, during integrated stress response, translation initiation can still occur at alternative open reading frames [G], present in certain genes, such as ATF4 (activating transcription factor 4), leading to the upregulation of their protein products42 (Figure 2). ATF4 functions as a transcription factor and induces the expression of a cohort of genes that together promote cell survival upon metabolic stress. An important group of genes downstream of ATF4 encodes proteins that constitute amino acid transporters. For example, expression of both subunits of the x(c)(−) system: SLC7A11 (encoding cystine [G] –glutamine antiporter xCT) and SLC3A2 (encoding common 4F2 heavy chain) are strongly induced upon ATF4 activation, promoting cystine uptake at the expense of cellular glutamate (Figure 2) and to maintain redox homeostasis43–45 (see also below). In addition, expression of a variety of other amino acid transporter genes, including SLC1A5 (encoding ASCT2), SLC7A5 (encoding LAT1), SLC7A1 (encoding CAT-1), SLC6A9 (encoding GLYT1), are elevated by ATF4, which in turn facilitate uptake of amino acids including glutamine, leucine, arginine, lysine and glycine43,46–48. ATF4 also increases the expression of almost all cytosolic aminoacyl tRNA synthetases, enhancing the capture of the acquired amino acid for maintaining protein synthesis43,49. In the meantime, through the induction of Sestrin2, ATF4 prevents excessive amino acid consumption by mTORC1 until intracellular amino acid levels are restored50 (Figure 2).
Synthesizing non-essential amino acids.
Apart from direct amino acid uptake, cells are capable of synthesizing a variety of NEAAs when needed. Many NEAAs have critical roles in cell growth beyond serving as building blocks for protein translation, including acting as substrates for nucleotide synthesis as well as supporting cellular redox homeostasis (see also below). As a consequence, proliferating cells often demonstrate metabolic adaptations that favor the synthesis of NEAAs. However, production of NEAAs generally requires re-allocation of nutrients and energy, and is therefore rigorously regulated.
When cells sense a decrease in amino acid levels through GCN2, ATF4 activation can directly lead to the upregulation of enzymes involved in NEAA synthesis, such as ASNS in asparagine synthesis51, and PHGDH and PSAT1 in serine biosynthesis52 (Figure 2). Proliferating cancer cells often engage NEAA production in a more proactive manner. For example, recurrent genetic amplifications of the PHGDH gene have been identified in melanoma and breast tumours, which correlate with increased glycolytic flux into the serine biosynthetic pathway53,54. In addition to serine and glycine production, the transamination reaction in the serine biosynthesis pathway also generates α-ketoglutarate (αKG) from glutamate, which was shown to be critical for replenishing the TCA cycle while metabolic intermediates of the TCA cycle can leave the cycle and act as precursors for biosynthesis of macromolecules such as nucleotides and fatty acids (Supplementary Box 1)54. De novo serine synthesis appears essential to cancer cells with PHGDH overexpression, as they are exquisitely sensitive to genetic or pharmacological interference of this pathway54–56. Furthermore, beyond PHGDH overexpressed tumours, targeting serine and glycine in general holds promise for controlling cancer cell growth, as serine and glycine metabolism is critically involved in the regulation of redox balance — which is often disturbed in cancer cells — and in support of nucleotide synthesis57 (see also below). Restriction of dietary serine and glycine has been shown to reduce tumour growth in xenograft models as well as in genetically engineered mouse models of intestinal cancer and lymphoma58,59.
Aspartate is another NEAA that is important for proliferating cells because it is an essential substrate for nucleotide synthesis60 (Figure 1). However, the extracellular free aspartate level is low and most cells express the aspartate transporter SLC1A3 at low levels61. Cellular aspartate is mostly derived from the amination of the TCA cycle intermediate oxaloacetate (Figure 1; see also Supplementary Box 1) and flux through the TCA cycle has been shown to be important in supporting aspartate synthesis. A major function of the mitochondrial electron transport chain (ETC) is to allow access to oxygen as the terminal electron acceptor in order to regenerate NAD+ and sustain TCA cycle activity. Remarkably, in cancer cells, reduced cell proliferation caused by ETC inhibition can be rescued with direct aspartate supplementation62,63. However, restoration of cell growth by aspartate upon ETC inhibition does not occur in all cell types. In endothelial cells, ETC inhibition leads to reduced cell proliferation as well as decreased aspartate levels, but aspartate supplementation is not sufficient to rescue the impaired cell growth caused by ETC deficiency64. Moreover, regulatory T cells and haematopoietic stem cells are able to proliferate in the absence of ETC activity, although the abilities to suppress T cell function and to initiate erythropoiesis, respectively, are considerably impaired65,66. Nonetheless, these findings highlight that by supporting aspartate production mitochondrial respiration is important for anabolic metabolism beyond its conventional ATP-generating role. Because of the dependency of certain cancer cell types on ETC activity, associated TCA flux and consequent robust aspartate production, ETC inhibition as well as therapeutic aspartate deprivation may be explored as potential strategies to impede cancer growth67–69.
Protein scavenging as an amino acid source.
As described above, cells can specifically adjust the levels of certain amino acids by modulating the expression of selective transporters and/or by regulating de novo biosynthetic pathways. In addition, mammalian cells can use a non-selective endocytic pathway, termed macropinocytosis, to take up nutrients in bulk from the extracellular space. Cells initiate macropinocytosis through protrusions of the plasma membrane that fold back to the membrane forming macropinosomes, which then are trafficked to fuse with lysosomes for cargo degradation70,71 (Figure 2). In principle, cells can recover various types of nutrients from macropinocytosis. Macropinocytic scavenging of proteins has been demonstrated to be a crucial amino acid source to support cell proliferation, especially in cancer71,72.
Macropinocytosis is evolutionarily conserved. Both unicellular and multicellular eukaryotes can acquire nutrients through macropinocytosis73. Similar to other nutrient acquisition strategies, mammalian cells regulate macropinocytosis at multiple levels. The initiation of macropinocytosis is characterized by membrane ruffling and macropinosome formation, and is normally stimulated by growth factor signalling and PI3K activation74,75. Importantly, oncogenic RAS is a major regulator of the rate and volume of macropinosome uptake (Figure 2). Macropinosome cargo can sustain the proliferation of RAS mutant cancer cells by providing nutrients in poorly vascularized tumour regions76–78.
As part of the cellular nutrient acquisition network, macropinocytosis is coordinated with other nutrient sensing and nutrient responsive pathways in a concerted manner. The AMP-activated protein kinase (AMPK) monitors energy stress by sensing an increase in AMP to ATP ratio, and via its kinase activity, restores metabolic homeostasis by suppressing anabolism and enhancing catabolic processes such as macroautophagy (hereafter referred to as autophagy)79. Beyond its role in promoting lysosomal degradation of self-constituents during autophagy, AMPK can also regulate macropinocytic degradation of extracellular fluids and necrotic cell debris80. In addition, as a master sensor of certain amino acids, mTORC1 acts as a negative regulator of macropinocytic trafficking to the lysosome (Figure 2). Suppression of mTORC1 activity limits the utilization of extracellular free amino acids, but greatly enhances the catabolism of extracellular proteins via macropinocytosis78,81. This paradoxical role of mTORC1 may explain the fact that while genes encoding RTK, PI3K and PTEN are most frequently altered in many types of cancer, activating mutations in mTORC1 are rare. Constitutive mTORC1 activation would lead to increased dependencies on the utilization of extracellular free amino acids, and hence would limit the flexibility of nutrient acquisition strategies in the face of fluctuations in extracellular environment during tumour progression.
Elevation of fatty acid synthesis
Fatty acids are the building blocks for all lipids and hence are key constituents of all biological membrane structures. As a result, proliferating cells need to acquire sufficient fatty acids to support membrane growth and integrity.
Synthesizing fatty acids.
Most normal cells, even when they are in proliferative state, take up much of their required fatty acids from the circulation via the activity of lipoprotein lipase (LPL) and fatty acid translocases such as CD36 (ref. 82–84). However, it has been known since the 1950s that tumour cells have elevated fatty acid synthesis activity, which is further exacerbated as cancer progresses. De novo fatty acid synthesis makes a major contribution to the intracellular fatty acid pool of tumour cells83–85.
Elevated endogenous fatty acid synthesis is supported by the increased glycolytic flux observed in most cancer cells. As glucose carbon enters the TCA cycle, a considerable amount is diverted from the cycle and being exported back to the cytosol as citrate (Figure 3; see also Supplementary Box 1). The ATP citrate lyase (ACLY) functions to cleave citrate into oxaloacetate while releasing acetyl coenzyme A (CoA), which is the primary substrate for fatty acid chain elongation. Through the activity of acetyl-CoA carboxylase (ACC), acetyl-CoA is converted to malonyl-CoA and is committed to fatty acid synthesis. Fatty acid synthase (FASN) carries out the stepwise condensation of malonyl-CoA, elongating the fatty acid chain until palmitate is generated, which serves as the precursor for production of various other fatty acid species83 (Figure 3). Enzymes in the fatty acid synthesis pathway, FASN in particular, are found to have increased expression levels across various cancer types and this increased expression correlates with worse clinical outcomes 86–88. In fact, many cancer cells become dependent on the fatty acid synthesis pathway and targeting key enzymes of de novo fatty acid synthesis has emerged as a potential therapeutic option in cancer treatment83,89.
Figure 3. Fatty acid synthesis.

Fatty acids can be taken up from the environment (for example, from the circulation) through the activity of lipoprotein lipase (LPL) and fatty acid translocase. In addition, fatty acid synthesis can occur de novo. Growth factor signalling promotes utilization of glucose for fatty acid synthesis via redirecting citrate (dashed arrow) away from the tricarboxylic acid (TCA) cycle. AKT activation results in the increase in fatty acid synthesis, partly by promoting the activity of ATP citrate lyase (ACLY). Acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN) are both involved in the early steps of fatty acid synthesis leading to the formation of palmitate. These enzymes have been shown to be elevated in cancers and hence are potential targets in cancer treatment (highlighted in red boxes). The production of saturated fatty acids needs to be balanced with that of unsaturated fatty acids to avoid endoplasmic reticulum (ER) stress, which occurs in response to accumulation of saturated fatty acids in cellular (including ER) membranes and consequent disruption of membrane homeostasis. This balance is partly achieved through the regulation of oxygen-dependent and iron-dependent enzyme, stearoyl-CoA desaturase (SCD). Note that acetyl-CoA acts as the substrate for acetylation of histones as well as a variety of non-histone proteins. This link has been demonstrated to be an important mechanism by which cells coordinate metabolic status with gene expression and protein activities.
GLUT1, glucose transporter 1.
Upregulated enzymatic activity, together with the associated cellular metabolic changes, helps to explain how cancer cells achieve increased levels of fatty acid synthesis. It remains controversial what advantages tumour cells obtain by relying more on de novo fatty acid synthesis, despite their access to environmental lipids. One possibility is that by actively elevating fatty acid synthesis and stocking up on carbon and energy, cancer cells can better prepare themselves for regional nutrient scarcity when the tumour expands, or when cancer cells experience adverse environments during metastasis and recolonization. For example, metastasis in certain cancer types was shown to depend on the ability of tumour cells to access and metabolize lipids90,91. Moreover, it was found that de novo fatty acid synthesis is essential for cancer cells to survive antiangiogenic therapy, and allows tumour cells to rapidly regrow and metastasize once the treatment is withdrawn92. Another potential benefit that cancer cells may gain from fatty acid synthesis may be related to the fact fatty acid synthesis converts a considerable amount of the reducing equivalent of NADPH to NADP+, therefore contributing to cellular redox balance by serving as an electron acceptor (see also below). This was proposed to be particularly important in hypoxic tumour cells where oxygen, a major electron acceptor, is limited93. Nonetheless, increased fatty acid synthesis may assume different roles under distinct malignant contexts and during different cancer stages. Further mechanistic delineation of the role of fatty acid synthesis in supporting cell growth and tumorigenesis is needed.
Balancing saturated and unsaturated fatty acids.
In addition to increasing fatty acid synthesis, it is equally important for proliferating cells to balance the content of saturated and unsaturated fatty acids. Accumulation of excess saturated fatty acids is known to trigger endoplasmic reticulum (ER) stress [G], partly by impairing ER membrane fluidity94. In mammalian cells, fatty acid desaturation is carried out by stearoyl-CoA desaturases (SCDs) that function primarily at the ER, converting saturated fatty acids to monounsaturated fatty acids in an oxygen-dependent and iron-dependent manner (Figure 3). Given the critical role of SCDs in maintaining cellular unsaturated fatty acid levels, development of inhibitors to SCD1, the major form of SCDs in most human cells, has been an active area of research in treating cancer as well as metabolic syndromes associated with increased lipid accumulation95.
Because of the dependence on oxygen for desaturation reactions, the activity of SCD1 is strongly reduced under hypoxic conditions94,96. Because the progression of tumours often involves periods of oxygen limitation, cancer cells need to employ alternative means to acquire unsaturated fatty acids and therefore become less dependent on SCD1. For example, it was shown that cells transformed by oncogenic RAS have enhanced ability to scavenge extracellular lysophospholipids [G] to supplement cellular unsaturated fatty acids under hypoxia or SCD1 inhibition97. In the context of clear cell renal cell carcinoma, triglycerides stored in cellular lipid droplets are able to release unsaturated fatty acids into phospholipid pools upon hypoxia-induced SCD1 inhibition98. Moreover, in certain liver and lung carcinomas, it was suggested that palmitate can be desaturated to produce sapienate [G], thereby acting as an alternative means to generate unsaturated fatty acids, relieving the dependence on SCD199.
Production of nucleotides
In comparison with other nutrients, nucleotide production through endogenous mechanisms is of particular importance to proliferating cells, because the amount of nucleotides directly taken up from the extracellular space is negligible. During cell proliferation, the demand for nucleotides increases owing to the need to synthesize ribosomal RNA (rRNA), duplicate the genome (synthesize DNA) and maintain the transcriptome (produce large amounts of mRNA). Nucleotide biosynthesis is also uniquely positioned in the cellular metabolic network, because pathways that lead to ribose, pyrimidine and purine production require substrate and energy input from multiple metabolic processes, including the pentose phosphate pathway [G] (PPP), one carbon unit cycle [G], electron transport chain (ETC) and the TCA cycle (see Figure 1 and Supplementary Box 1).
Generating ribose-5-phosphate.
Ribose-5-phosphate is a phosphorylated form of the pentose sugar, ribose, on which nitrogenous bases are built to form nucleotides. Cellular ribose-5-phosphate is normally derived from the oxidative phase of PPP, where the rate-limiting enzyme glucose-6-phosphate dehydrogenase (G6PD) diverts glucose away from glycolysis to power ribose-5-phosphate production as well as NADPH generation (Supplementary Box 1). The phosphoribosyl pyrophosphate synthetase (PRPS) can subsequently convert ribose-5-phosphate to phosphoribosyl pyrophosphate (PRPP), which activates ribose-5-phosphate and commits it to nucleotide biosynthesis (Figure 4).
Figure 4. Synthesis of pyrimidine and purine nucleotides.

Nucleotide synthesis is key for cell proliferation as it is required for DNA replication, gene transcription and ribosome biogenesis, and the uptake of nucleotides from extracellular sources is negligible. Nucleotide biosynthesis interrogates multiple metabolic pathways and requires the utilization of various substrates as the carbon and nitrogen sources, including amino acids and metabolites of the folate cycle (the colours of carbon and nitrogen in the chemical structure of pyrimidines and purines correspond to the contributing source). Pyrimidine synthesis intersects with amino acid metabolism via positive regulation of carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD) downstream of mTROC1 as well as with ATP production and energy metabolism (electron transport chain (ETC) via the activity of dihydroorotate dehydrogenase (DHODH); DHODH, which is highlighted in red, has been implicated as a potential target for cancer treatment). Purine synthesis is indirectly regulated by mechanistic target of rapamycin complex 1 (mTORC1) via its stimulation of the mitochondrial folate cycle. In addition, the key energy sensor AMP-activated protein kinase (AMPK) negatively regulates purine biosynthesis by inhibiting the activity of phosphoribosyl pyrophosphate synthetase (PRPS). This allows fine tuning of nucleotide synthesis in accordance with cellular energy metabolism.
IMPDH, inosine monophosphate dehydrogenase; PRPP, phosphoribosyl pyrophosphate; QH2, dihydroxyquinone; Ribose-5-P, Ribose-5-phosphate; RPA, phosphoribosyl amine; THF, tetrahydrofolate.
The production of nucleotides is energy demanding. For example, during purine synthesis, seven molecules of ATP are consumed in order to build a molecule of inosine monophosphate (IMP) on PRPP, and an additional molecule of ATP or GTP is required to convert IMP to AMP or GMP. As a result, nucleotide synthesis must be tightly coordinated with cellular energy status. Indeed, enzymatic activity of PRPS is diminished as ADP and GDP levels increase, which reflect the accumulation of nucleotide products, as well as indicate a drop in cellular energy level100. Moreover, it was found that AMPK activation upon energy stress can directly phosphorylate PRPS and convert the enzyme from its active hexamer form to the inactive monomer form101 (Figure 4). These mechanisms reduce nucleotide synthesis when the cellular energy status is not optimal.
Building pyrimidine nucleotides.
Pyrimidine nucleotide synthesis is initiated in the cytosol by carbamoyl phosphate synthetase-II (CPS-II), which utilizes ATP, HCO3- and the amide group of glutamine to form carbamoyl-phosphate. Through the activity of aspartate transcarbamoylase (ATCase), carbamoyl-phosphate is then condensed with aspartate to produce carbamoyl aspartate. Dihydroorotase subsequently circularizes carbamoyl aspartate to form the ring-structured dihydroorotate. In human cells, CPS-II, ATCase and dihydroorotase activities are all contained in the trifunctional protein CAD (Figure 4). Consistent with the hypothesis that normal cell growth is dependent upon growth factor stimulation, CAD is known to be regulated by growth factor signalling, partly through the action of mitogen-activated protein kinase (MAPK)102,103. Furthermore, downstream of mTORC1, S6K1 was found to directly phosphorylate CAD and induce its activity to provide pyrimidine nucleotides for ribosomal biogenesis and hence supporting protein translation104,105. By positioning CAD under the regulation of mTORC1, the initiation of pyrimidine synthesis is only favored in the presence of sufficient amino acid substrates, as monitored by mTORC1 activity (Figure 4). As a result, nucleotide production is coordinated with amino acid availability, which together, support cell proliferation.
A unique feature of the next step in pyrimidine synthesis is that the enzyme, dihydroorotate dehydrogenase (DHODH), is localized on the outer surface of inner mitochondrial membrane, oxidizing dihydroorotate to produce orotate (Figure 4). Unlike many other dehydrogenases that require NAD+ or NADP+ as electron acceptor, DHODH instead transfers electrons to ubiquinone [G], which then passes the electrons on to respiratory complex III and ultimately to oxygen106. As a result, the production of orotate in pyrimidine synthesis is coupled to ETC and energy generation, which exerts an additional control over nucleotide synthesis and ensures that it is coupled to cellular energy status. The unique metabolic positioning of this reaction also makes DHODH a vulnerable node within pathways of growth regulation that has the potential to be a cancer treatment target. In line with this, it was found that combinatorial inhibition of oncogenic BRAFV600E (B-Raf proto-oncogene, V600E mutation) and DHODH can lead to a reduction in melanoma tumour growth107. Similarly, high-throughput screening identified DHODH as a drug target, which when inhibited, induces differentiation of a wide range of acute myeloid leukemia (AML) cells108.
Subsequently, to complete pyrimidine synthesis, orotate is combined with PRPP to form uridine monophosphate (UMP), through the activity of uridine monophosphate synthetase (UMPS). All other pyrimidine nucleotide species are derived from UMP. Notably, in order to produce cytidine triphosphate (CTP), an additional molecule of glutamine is required to provide the amide group (Figure 4). Moreover, for DNA synthesis, deoxy UMP (dUMP) is converted to deoxythymidine monophosphate (dTMP) by thymidylate synthase (TYMS). This reaction requires the folate cycle [G] intermediate 5,10-methylene-tetrahydrofolate (5,10-methylene-THF) as the source of methyl group (Figure 4), and is a target for the antimetabolite [G] 5-fluorouracil [G] in cancer treatment109.
Assembling purine nucleotides.
Synthesis of purine nucleotides also utilizes various carbon and nitrogen sources and integrates a number of metabolic pathways (Figure 4). The entire purine synthesis pathway takes place in the cytosol. PRPP-amidotransferase (PPAT) first displaces the pyrophosphate group in PRPP by the amide group from glutamine to produce phosphoribosyl amine (PRA). PRA is then converted to the ring-structured phosphoribosylaminoimidazole (AIR), utilizing carbon and nitrogen from glycine, glutamine and 10N-formyl-THF. Subsequently, the HCO3- carbon and aspartate nitrogen join to form phosphoribosylaminoimidazole succinocarboxamide (SAICAR), which is further cleaved by adenylosuccinate lyase (ADSL) to release aminoimidazole carboxamide ribonucleotide (AICAR) and the TCA cycle intermediate fumarate. Finally, the common purine precursor IMP is generated byAICAR formyltransferase, IMP cyclohydrolase (ATIC), with the addition of a second one-carbon unit from 10N-formyl-THF.
The other species of purine nucleotides are derived from IMP. To synthesize AMP, the adenylosuccinate synthase (ADSS) takes an additional molecule of aspartate, and the resulting adenylosuccinate is cleaved to release AMP and fumarate. Production of GMP involves first the oxidation of IMP by IMP dehydrogenase (IMPDH) to form xanthosine monophosphate (XMP), and subsequently amination of XMP to GMP using the amide group from glutamine.
The production of purine nucleotides requires the direct contribution of carbon and nitrogen from glycine and 10N-formyl-THF (Figure 4). As a result, the folate cycle and the metabolism of serine and glycine are intimately connected to the regulation of purine synthesis. In human cells, folate metabolism occurs in both the cytosolic and mitochondrial compartments. When studied in cell culture, the mitochondrial serine hydroxymethyltransferase 2 (SHMT2) favours the generation of glycine from serine, while transferring the β-carbon of serine to tetrahydrofolate (THF) to produce 5,10-methylene-THF. Methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) subsequently catalyzes the production of 10N-formyl-THF, which is mostly exported to the cytosol in the form of formate. The cytosolic arm of folate metabolism then regenerates 10N-formyl-THF from formate to support purine biosynthesis57. Similar to pyrimidine synthesis, purine production is also highly coordinated with the cellular nutrient status and growth signalling pathways. For example, mTORC1 activity was demonstrated to promote purine synthesis, partly through the regulation of enzymes involved in serine biosynthesis and folate metabolism110.
Taken together, in order to build a full battery of pyrimidine and purine nucleotides, cells need a continuous supply of carbon and nitrogen derived from a variety of amino acids and metabolic intermediates. The nucleotide synthesis pathways are intimately coupled with multiple metabolic processes. Because of these extensive metabolic interactions, nucleotide synthesis is under rigorous cellular control. Many of the antimetabolites currently used in cancer therapy exploit the process of nucleotide biosynthesis as a way to selectively impair proliferating cells.
Interactions with microenvironment
Beyond the cellular metabolic network, extensive metabolic interactions also occur between a proliferating cell and the extracellular environment within which it resides. Multiple components are present in this microenvironment, including the vasculature, extracellular matrix (ECM), and various other cell types such as fibroblasts and immune cells (Figure 5). The interactions between proliferating cells and their surroundings are bi-directional and multifaceted. Here we consider how the extracellular environment influences the metabolism of proliferating cells.
Figure 5. Metabolic interactions with the extracellular environment.

Extensive metabolic interactions exist between proliferating cells and the microenvironment. In the case of cancer cells and the tumour microenvironment, cancer cells receive metabolic signalling cues such as growth factors, which can be provided by infiltrating immune cells. These cues rewire their metabolic programmes to support biosynthesis and growth (see Figs. 1 to 4). Hypoxia (low O2 levels), which is a hallmark of solid tumours further promotes metabolic rewiring by activating hypoxia-inducible factor-1α (HIF-1α), which, among other functions, promotes aerobic glycolysis and lactate production. Lactate secreted by cancer cells can have effects on the surrounding environment. For example, cancer cell-derived lactate has been shown to induce secretion of vascular endothelial growth factor (VEGF), thereby promoting angiogenesis and increasing the potential of cancer dissemination. Cancer cells also acquire nutrients directly from the environment, including glucose, amino acids and macromolecules, such as components of the extracellular matrix (ECM) via integrin receptor-mediated endocytosis as well as other extracellular proteins and lysophospholipids via macropinocytosis. Specialized fibroblasts, known as cancer-associated fibroblasts (CAFs), have a particularly important role in cancer. CAFs have the ability to support cancer cell growth by mechanisms involving ECM deposition and autophagy. Autophagy in CAFs and other stromal cells present in cancer environment can result in the release of nutrients, such as amino acids, which can be taken up and utilized by cancer cells (for example, as building blocks for protein synthesis or for bioenergetics).
GLUT1, glucose transporter 1; MCT, monocarboxylate transporter; RTK, receptor tyrosine kinase; TGFβ, transforming growth factor beta.
Receiving metabolic stimulation from the niche.
Wound healing is a paradigm process where cell proliferation is enhanced in a physiological context. A healing wound represents a typical milieu where crosstalk occurs between the parenchymal cells that must expand in number to repair damaged tissue, and the associated stromal and immune cells that provide environmental support. Wound healing is a highly coordinated process which involves complex metabolic interactions between the cell types involved. The process is initiated by inflammation at the injury site, which is in part achieved when innate immune cells are recruited by the damage-associated molecular patterns [G] (DAMPs) — including uric acid, nucleotides, and hyaluronan fragments — that are released as a consequence of tissue damage111–113. These molecules act as signals to elicit inflammatory responses preceding tissue repair114. During epithelial regeneration, the enrichment of growth factors released by immune cells and fibroblasts into the wound bed serves as a signal to allow nutrient uptake and proliferation by the remaining cells to expand and establish re-epithelialization. Meanwhile, immune cell-derived transforming growth factor beta (TGFβ) induces cell fate change in local fibroblasts, which in turn produce and deposit ECM proteins to support tissue remodelling114,115. Tissue damage is also often associated with disruptions of blood supply and oxygen delivery. The local hypoxic environment stimulates the production of VEGF by macrophages, which promotes endothelial cell proliferation for neovascularization114,116 (Figure 5).
The development of a tumour resembles the process of wound healing in many aspects. In fact, it was proposed that tumours can be studied as “wounds that do not heal”116. From the metabolic perspective, interactions between cancer cells and the tumour microenvironment (TME) not only co-opt features of wound healing, but also manifest novel characteristics, which likely result from the heterogeneous nature of oncogenic transformation, and the stromal environment of the organ in which a tumour arises. For example, the presence of hyaluronan in the TME was shown to serve as a metabolic instruction that promotes glycolysis in cancer cells by dampening thioredoxin-interacting protein (TXNIP) levels, which promotes GLUT1 internalization, thereby enriching GLUT1 at the plasma membrane117. Cancer-associated fibroblasts [G] (CAFs) have long been recognized to have a deterministic role during tumour progression118,119. CAFs undergo substantial metabolic changes in response to growth factors and the hypoxic environment, and support the anabolic growth of cancer cells115. One recent example indicates that upon TGFβ signalling, the p38 MAPK pathway is activated in CAFs, which promotes the cytokine production and release from CAFs to stimulate the usage of glycogen stored in adjacent cancer cells as a source of glucose120. In addition, the hypoxic environment in the tumour niche signals to cancer cells to reinforce aerobic glycolysis through stabilization of hypoxia-inducible factor-1α (HIF-1α) and induction of its downstream gene targets121 (Figure 5). The resultant increase in lactate production, in turn, was shown to enhance VEGF secretion by immune cells and stimulate angiogenesis to supply nutrient and oxygen for the progressive development of tumours as they grow in size122. Overall, cancer cells harness properties of the wound healing process during the prolonged interaction with TME to adjust their metabolic programmes in support of proliferation.
Acquiring nutrients from opportunistic sources.
To support the high rates of proliferation, transformed cancer cells often deplete their microenvironment of a variety of nutrients, such as NEAAs that are critical for anabolic growth123,124. As a result, cancer cells often need to turn to opportunistic modes of nutrient acquisition from the TME to maintain tumour growth and to adapt to the changes in their environment. As discussed previously, RAS-transformed cancer cells are able to scavenge extracellular protein and lysophospholipids to use as a source of amino acids and fatty acids77,97. In addition to utilizing extracellular fluids, cancer cells are able to catabolize ECM components deposited by fibroblasts, such as laminin and collagen that are present in abundance in TME, to retrieve amino acids under nutrient starvation125,126 (Figure 5).
In contrast to proliferating cells, most stromal cells in the TME turn to autophagic degradation of intracellular constituents to survive nutrient limitation. Counterintuitively, autophagic cells release some of their recycled metabolic intermediates into the TME. This release of nutrients can in turn support the growth of neighbouring cancer cells127 (Figure 5). For example, in the context of pancreatic ductal adenocarcinoma (PDAC), stroma-associated pancreatic stellate cells (PSCs) release alanine as a major product of autophagy, and this released alanine can fuel the TCA cycle in PDAC cells128.
Beyond taking up catabolic products from stromal cells, cancer cells may adapt to actively utilize a variety of surrounding nutrients, particularly during metastasis as they invade new territories that are metabolically challenging. For example, omentum [G] invasion is a major form of ovarian cancer metastasis. It was suggested that upregulation of the fatty acid-binding protein 4 (FABP4) in ovarian cancer cells is one of the determinants of their homing at the omentum as it enables cancer cells to acquire lipids directly from omental adipocytes — in this case, omental adipocytes serve as an important source of energy and fatty acids to support growth of metastases129.
Adaptations to metabolic stress
Cell growth and proliferation expose cells to a new set of stresses associated with meeting the supply demands of anabolic growth while retaining sufficient bioenergetics and redox regulation to maintain viability. In addition, despite extensive metabolic rewiring, proliferating cells are constantly encountering metabolic stresses. This is particularly true for cancer cells as their proliferation exceeds the ability of the existing vasculature to supply sufficient oxygen and nutrients to support both growth and survival.
Surviving nutrient limitations.
When nutrient supply is compromised, mammalian cells are able to engage a number of mechanisms in order to sustain bioenergetics and support viability. Autophagy is a critical catabolic process whereby cells salvage intracellular constituents under nutrient scarce conditions. Nutrient limitations often result in a reduction in mTORC1 activity and its dissociation from the unc-51 like autophagy activating kinase (ULK) complex. As a consequence, the ULK complex becomes activated and initiates the formation of the double-membrane autophagosomes. Through the coordination of a series of autophagy-related proteins (ATGs), the autophagosome membranes encapsulate cargo and deliver the substrates to lysosome for degradation130,131. Autophagy can also be a selective process which involves a variety of cargo receptors that specifically target distinct substrates in a signal-dependent manner. Selective autophagic substrates include proteins, lipids as well as ribosomes130–132. As a result, autophagy pathways repurpose the limited intracellular resources and enable cell to survive nutrient and energy crisis. In addition to the recycling of nutrients, autophagy is also an important mechanism that mediates the degradation of selected (for example, damaged or superfluous) organelles to maintain cellular integrity and functionality130,131.
Autophagy is typically associated with the inhibition of cell growth. Notably however, the role of autophagy in cancer progression is context-dependent. On the one hand, it was suggested in mouse models that autophagy deficiency results in increased susceptibility to cancer initiation. It is, worth noting that a defect in autophagy can lead to genomic instability as well as the activation of oncogenic signalling cascades, and hence autophagy may limit tumorigenesis through mechanisms that are not directly linked to metabolism133. On the other hand, the nutrient scavenging function of autophagy has been increasingly associated with its role in sustaining cancer cell growth and tumour progression. For example, in a mouse model of RAS-driven NSCLC, autophagy was shown to have a critical role in maintaining the cellular nucleotide pool and energy balance to support cancer cell survival134,135. Pancreatic cancer cells were found to have elevated basal autophagy activity when compared to normal pancreatic cells, and autophagy was demonstrated to be a required component for pancreatic tumour growth136–138. In addition, as discussed above, cancer cell-extrinsic autophagy pathways — occurring in the host liver or in stromal cells in the tumour microenvironment — can also affect the nutrient status and hence the survival and growth of cancer cells128,139. Understanding the role of autophagy in supporting tumour cell growth and proliferation has the potential to reveal metabolic vulnerabilities and provide new therapeutic options in cancer treatments.
Maintaining redox homeostasis.
Maintaining redox homeostasis is essential to all metabolic states. One important indicator of cellular redox balance is the maintenance of the NADH to NAD+ ratio. In mammalian cells, electrons can be captured from fuels, such as glucose, converting NAD+ to NADH, and eventually accepted by oxygen via ETC in the mitochondria to drive ATP production. Whether glycolytic products are catabolized in the mitochondrial TCA cycle or diverted into anabolic synthesis, the cells are left with NADH that must be reconverted to NAD+ to maintain redox homeostasis. Just as in resting cells, in cancer cells with a high glycolytic rate as a result of PI3K mutation, the malate–aspartate shuttle [G] is essential to promote the translocation of cytosolic electrons into the mitochondria for consumption in oxidative phosphorylation140,141. The malate–aspartate shuttle, therefore, serves as a mechanism to maintain redox homeostasis in the cytosolic compartment to allow for NAD+ regeneration and its continued supply to support constant glucose utilization (Figure 6).
Figure 6. Maintaining redox homeostasis in proliferating cells.

Owing to high glycolytic rate and associated biosynthetic reactions, proliferating cells face the problem of an elevated NADH to NAD+ ratio and thus, maintaining redox homeostasis is critical during cell proliferation. Mechanisms to maintain NADH to NAD+ ratio include the malate–aspartate shuttle, which regenerates cytosolic NAD+ to sustain constant flow through biosynthetic pathways. Also, an increase in lactate production supports regeneration of NAD+. At the same time, proliferating cells face the problem of increased generation of reactive oxygen species (ROS), primarily derived from electron transport chain in mitochondria and NADPH oxidases (NOXs). Excessive ROS cause oxidative stress, which curbs cell proliferation and viability. Thus, proliferating cells require efficient antioxidant systems to counteract the destructive activity of ROS. The two key cellular antioxidant defense systems include the thioredoxin system (not shown) and the glutathione system. Glutathione is synthesized from glutamate, cysteine and glutamine. Glutamine importantly regulates this process by supplying glutamate and promoting cystine uptake. Additionally, NADPH is required for efficient ROS detoxification as it is involved in generating reduced glutathione — which is active against ROS — from its oxidized form (not shown). NADPH is supplied by the conversion of glycolysis intermediates in the pentose phosphate pathway as well as by mitochondrial serine metabolism in the folate pathway. Antioxidant defense has been shown to be important for cancer cell survival and tumour growth. For example, cancer cells were demonstrated to rely on glutathione synthesis and to upregulate mitochondrial folate pathway (highlighted in red).
MCT, monocarboxylate transporter; THF, tetrahydrofolate; x(c)(−), cystine–glutamine antiporter.
Not all nutrient usage in proliferating cells is for anabolic synthesis. For example, many cancer cells were shown to have an increased demand for serine57. Apart from its anabolic role in protein translation and nucleotide synthesis, it was shown that the catabolism of serine in mitochondria is critical to support NADPH production and redox balance142. In addition, besides the role as a TCA cycle anaplerotic substrate, glutamine is important in maintaining the cellular glutathione [G] pool for redox regulation (see next paragraph), by providing glutamate and sustaining the uptake of extracellular cystine143,144 (Figure 6).
As a result of their high glycolytic rate and the progressive depletion of oxygen in the local environment as the cell mass accumulates, proliferating cells face the problem of an elevated NADH to NAD+ ratio, which results in redox imbalance and consequently an increase in the generation of reactive oxygen species (ROS). The mitochondrial ETC is one of the main sources of cellular ROS (Figure 6). When electrons are being passed to oxygen, a fraction of them escape from the ETC and directly react with oxygen to produce superoxide, which is the precursor of many other ROS such as hydrogen peroxide and hydroxyl radicals145. Another source of ROS are the NADPH oxidases (NOXs), which are membrane-bound enzymes that catalyze the production of a superoxide free radical by transferring one electron to oxygen from NADPH (Figure 6). In order to prevent ROS accumulation that can lead to irreversible damage to cellular components, cells possess multiple antioxidant mechanisms such as the thioredoxin [G] system and the glutathione system. Furthermore, when ROS level rises, the master regulator of antioxidant response nuclear factor, erythroid 2 like 2 (NRF2) is stabilized as its negative regulator kelch-like ECH associated protein 1 (KEAP1) gets oxidized and loses its ability to sequester NRF2 in the cytosol for proteasomal degradation. Nuclear NRF2 acts as a transcription factor that activates a collection of genes involved in the synthesis and utilization of cellular antioxidant systems to clear excessive cellular ROS.
Interestingly, a modest increase in ROS level can stimulate cell growth, partly because hydrogen peroxide can oxidize cysteine residues of a number of protein phosphatases including PTEN and MAPK phosphatases, and inhibit their activities146. Physiological level of ROS is essential for many biological processes. For example, ROS generation by ETC complex III is indispensable during the differentiation of mesenchymal stem cells into adipocytes147. ROS that originate from mitochondrial metabolism are also required to promote cell proliferation and mediate tumorigenesis in KRAS-driven cancer cells148. However, excessive ROS generation can exceed physiological cellular antioxidant capacity. Proliferating cells frequently upregulate their antioxidant defense mechanisms. In cancer cells, this is most clearly demonstrated by the frequent genomic alterations in the KEAP1–NRF2 axis that lead to increased antioxidant defense and are associated with a poor cancer prognosis18. Increased antioxidant capacity resulting from NRF2 activation as well as other mechanisms was found to promote tumorigenesis in various cancer settings149–151. Furthermore, oxidative stress has been demonstrated to be a major inhibitory factor for cancer metastasis. Successful metastatic cancer cells have been shown to undergo reversible metabolic reprogramming to enhance antioxidant capability by modulating the mitochondria folate pathway to overcome this barrier152. Targeting the antioxidant system has become an area of intense research in cancer biology.
Conclusions and perspectives
Studies over the past decades have greatly advanced our understanding of cellular metabolism. Metabolic regulation is critically involved in various cellular processes, including cell growth and proliferation. Through the investigation of physiological as well as pathological cell proliferation, the knowledge of how cells acquire and utilize various types of nutrients for anabolic growth has been greatly expanded. With this growing knowledge of anabolic metabolism, we have also seen the development of metabolic interventions in disease treatment. In cancer clinics, the metabolic characteristics of cancer cells are now used to both diagnose and stage many common tumours, as exemplified through the wide spread use of FDG-PET scanning in cancer evaluation in the clinic. The use of antimetabolites as well as the development of small molecule inhibitors targeting metabolic enzymes has led to improved treatment outcomes153. For example, nucleoside analogues gemcitabine and cytarabine are components of chemotherapies that are applied to the treatment of various types of malignancies154. Metabolic interventions have had a long and successful history in cancer treatment and these approaches are only likely to expand in coming years.
Supplementary Material
Acknowledgement
We thank members of the Thompson laboratory for critical discussions during manuscript preparation. J.Z. is supported by the Leukemia and Lymphoma Society postdoctoral fellowship.
Glossary terms
- Non-essential amino acids
Amino acids that can be synthesized endogenously and often participate in processes beyond protein translation, such as nucleotide synthesis.
- Anaplerosis
Biochemical reactions that replenish the intermediates of metabolic pathways.
- Minimal medium
Medium that contains the minimum nutrients possible for the growth of cells or microorganisms.
- Positron emission tomography-based imaging
An imaging technique used clinically to observe metabolic processes for disease diagnosis or monitoring.
- p70S6 kinase 1 (S6K1))
A kinase downstream of mTORC1 signalling pathway, activation of which can lead to increased protein synthesis.
- eIF4E binding protein 1(4EBP1)
A protein translation repressor. Its activity is inhibited by mTORC1-mediated phosphorylation in order to increase protein synthesis.
- Integrated stress response
A mechanism that usually suppresses general protein synthesis but promotes the expression of specific transcription factors to mediate cellular stress response.
- Alternative open reading frame
Different open reading frames within a gene, transcription of which can lead to different gene products that may assume different biological roles.
- Cystine
The oxidized dimer form of the amino acid cysteine.
- Endoplasmic reticulum (ER) stress
Cellular stress in the endoplasmic reticulum (ER) that often results from the accumulation of misfolded proteins or a failure to maintain membrane integrity of the ER.
- Lysophospholipids
Derivatives of phospholipids in which one of the two acyl chains is lost.
- Sapienate
A fatty acid that is usually a component of the secretion from sebaceous glands in the skin. Sapienate can be synthesized from palmitate by desaturation.
- Pentose phosphate pathway
A metabolic pathway parallel to glycolysis, which involves the generation of various pentoses including the ribose 5-phosphate that functions as a precursor for nucleotide synthesis.
- One carbon unit cycle
A group of biochemical reactions that involve the transfer of the one-carbon groups among various molecules such as tetrahydrofolate, S-adenosylmethionine and vitamin B12.
- Ubiquinone
A coenzyme that functions as an electron carrier in various biological processes, including the electron transport chain as part of aerobic cellular respiration.
- Folate cycle
A group of biochemical reactions occurring in both the cytosolic and mitochondrial compartments that involve the metabolism of folate and its congeners. Folate cycle can provide critical metabolic intermediates for the biosynthesis of macromolecules such as nucleotides.
- Antimetabolite
A chemical used in cancer treatment that often interferes with cellular nucleotide synthesis and DNA replication.
- 5-Fluorouracil
A medication used in cancer treatment primarily by targeting the thymidylate synthase to block thymidine synthesis.
- Damage-associated molecular patterns
A group of biomolecules that are enriched upon non-infectious inflammatory responses such as during the process of wound healing.
- Cancer-associated fibroblasts (CAFs)
A cell type derived from normal fibroblasts within the tumour microenvironment that promotes cancer development.
- Omentum
Layers of peritoneum that surround abdominal organs.
- Malate–aspartate shuttle
A biochemical system that translocates electrons from the cytosol into the mitochondria for oxidative phosphorylation, and the process involves the exchange of malate and aspartate between the two compartments.
- Glutathione
A tripeptide of glutamate, cysteine and glycine that together with glutathione-utilizing enzymes functions to maintain cellular redox homeostasis and detoxify electrophilic compounds such as reactive oxygen species.
- Thioredoxin
A class of small proteins encoded by the TXN and TXN2 genes that mainly function as cellular antioxidants
Footnotes
Competing interests
C.B.T. is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. He is also a former member of the Board of Directors and stockholder of Merck and Charles River Laboratories. He has patents related to cellular metabolism.
References
- 1.Galdieri L, Mehrotra S, Yu S & Vancura A Transcriptional regulation in yeast during diauxic shift and stationary phase. OMICS 14, 629–638, doi: 10.1089/omi.2010.0069 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marijuan PC, Navarro J & del Moral R On prokaryotic intelligence: strategies for sensing the environment. Biosystems 99, 94–103, doi: 10.1016/j.biosystems.2009.09.004 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Schaller GE, Shiu SH & Armitage JP Two-component systems and their co-option for eukaryotic signal transduction. Curr Biol 21, R320–330, doi: 10.1016/j.cub.2011.02.045 (2011). [DOI] [PubMed] [Google Scholar]
- 4.DeBerardinis RJ et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104, 19345–19350, doi: 10.1073/pnas.0709747104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheinwald JG & Green H Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 265, 421–424 (1977). [DOI] [PubMed] [Google Scholar]
- 6.Martin P Wound healing--aiming for perfect skin regeneration. Science 276, 75–81 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Nissen NN et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 152, 1445–1452 (1998). [PMC free article] [PubMed] [Google Scholar]
- 8.Saltiel AR & Kahn CR Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806, doi: 10.1038/414799a (2001). [DOI] [PubMed] [Google Scholar]
- 9.Frauwirth KA et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Esensten JH, Helou YA, Chopra G, Weiss A & Bluestone JA CD28 Costimulation: From Mechanism to Therapy. Immunity 44, 973–988, doi: 10.1016/j.immuni.2016.04.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthel A et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem 274, 20281–20286 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Rathmell JC et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23, 7315–7328 (2003).This work demonstrates that growth factor signalling is necessary for mammalian cells to maintain glucose uptake in support of bioenergetics and cell survival.
- 13.Wieman HL, Wofford JA & Rathmell JC Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18, 1437–1446, doi: 10.1091/mbc.e06-07-0593 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA & Thompson CB In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell 6, 683–692 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Fox CJ, Hammerman PS & Thompson CB Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5, 844–852, doi: 10.1038/nri1710 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Warburg O, Wind F & Negelein E The Metabolism of Tumors in the Body. J Gen Physiol 8, 519–530 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warburg O On the origin of cancer cells. Science 123, 309–314 (1956). [DOI] [PubMed] [Google Scholar]
- 18.Kandoth C et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339, doi: 10.1038/nature12634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recondo G, Facchinetti F, Olaussen KA, Besse B & Friboulet L Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol, doi: 10.1038/s41571-018-0081-4 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Arteaga CL et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol 9, 16–32, doi: 10.1038/nrclinonc.2011.177 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Samuels Y et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554, doi: 10.1126/science.1096502 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Engelman JA, Luo J & Cantley LC The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7, 606–619, doi: 10.1038/nrg1879 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MS et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501, doi: 10.1038/nature12912 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlova NN & Thompson CB The Emerging Hallmarks of Cancer Metabolism. Cell Metab 23, 27–47, doi: 10.1016/j.cmet.2015.12.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almuhaideb A, Papathanasiou N & Bomanji J 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 31, 3–13, doi: 10.4103/0256-4947.75771 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vander Heiden MG, Cantley LC & Thompson CB Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033, doi: 10.1126/science.1160809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faubert B et al. Lactate Metabolism in Human Lung Tumors. Cell 171, 358–371 e359, doi: 10.1016/j.cell.2017.09.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui S et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118, doi: 10.1038/nature24057 (2017).References 27 and 28 provide the first evidence that lactate in the circulation can act as the major carbon source to fuel the TCA cycle in vivo.
- 29.Palm W & Thompson CB Nutrient acquisition strategies of mammalian cells. Nature 546, 234–242, doi: 10.1038/nature22379 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxton RA & Sabatini DM mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976, doi: 10.1016/j.cell.2017.02.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoki K, Li Y, Zhu T, Wu J & Guan KL TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4, 648–657, doi: 10.1038/ncb839 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Manning BD, Tee AR, Logsdon MN, Blenis J & Cantley LC Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10, 151–162 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Menon S et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156, 771–785, doi: 10.1016/j.cell.2013.11.049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long X, Lin Y, Ortiz-Vega S, Yonezawa K & Avruch J Rheb binds and regulates the mTOR kinase. Curr Biol 15, 702–713, doi: 10.1016/j.cub.2005.02.053 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Edinger AL & Thompson CB Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell 13, 2276–2288, doi: 10.1091/mbc.01-12-0584 (2002).This study shows that AKT promotes cell survival and growth in part by maintaining nutrient transporter levels on the plasma membrane.
- 36.Hannan KM et al. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol 23, 8862–8877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfson RL & Sabatini DM The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Cell Metab 26, 301–309, doi: 10.1016/j.cmet.2017.07.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxton RA et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351, 53–58, doi: 10.1126/science.aad2087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfson RL et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48, doi: 10.1126/science.aab2674 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chantranupong L et al. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 165, 153–164, doi: 10.1016/j.cell.2016.02.035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu X et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813–818, doi: 10.1126/science.aao3265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu PD, Harding HP & Ron D Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 167, 27–33, doi: 10.1083/jcb.200408003 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding HP et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Sato H et al. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun 325, 109–116, doi: 10.1016/j.bbrc.2004.10.009 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Lewerenz J & Maher P Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 284, 1106–1115, doi: 10.1074/jbc.M807325200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez AB et al. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J 402, 163–173, doi: 10.1042/BJ20060941 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicklin P et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534, doi: 10.1016/j.cell.2008.11.044 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang N et al. Increased Amino Acid Uptake Supports Autophagy-Deficient Cell Survival upon Glutamine Deprivation. Cell Rep 23, 3006–3020, doi: 10.1016/j.celrep.2018.05.006 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Shan J et al. The C/ebp-Atf response element (CARE) location reveals two distinct Atf4-dependent, elongation-mediated mechanisms for transcriptional induction of aminoacyl-tRNA synthetase genes in response to amino acid limitation. Nucleic Acids Res 44, 9719–9732, doi: 10.1093/nar/gkw667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye J et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 29, 2331–2336, doi: 10.1101/gad.269324.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siu F, Bain PJ, LeBlanc-Chaffin R, Chen H & Kilberg MS ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 277, 24120–24127, doi: 10.1074/jbc.M201959200 (2002). [DOI] [PubMed] [Google Scholar]
- 52.DeNicola GM et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet 47, 1475–1481, doi: 10.1038/ng.3421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locasale JW et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 43, 869–874, doi: 10.1038/ng.890 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Possemato R et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350, doi: 10.1038/nature10350 (2011).References 53 and 54 provide early evidence that the serine synthesis pathway is upregulated in cancer and that it is critical in mediating tumorigenesis.
- 55.Pacold ME et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat Chem Biol 12, 452–458, doi: 10.1038/nchembio.2070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullarky E et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc Natl Acad Sci U S A 113, 1778–1783, doi: 10.1073/pnas.1521548113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang M & Vousden KH Serine and one-carbon metabolism in cancer. Nat Rev Cancer 16, 650–662, doi: 10.1038/nrc.2016.81 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Maddocks OD et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546, doi: 10.1038/nature11743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddocks ODK et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376, doi: 10.1038/nature22056 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Lane AN & Fan TW Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 43, 2466–2485, doi: 10.1093/nar/gkv047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Bermudez J et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol 20, 775–781, doi: 10.1038/s41556-018-0118-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birsoy K et al. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551, doi: 10.1016/j.cell.2015.07.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan LB et al. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell 162, 552–563, doi: 10.1016/j.cell.2015.07.017 (2015).References 62 and 63 demonstrate that a major function of the mitochondrial electron transport chain is to support aspartate production.
- 64.Diebold Lauren P., H. J. G., Gao Peng, Martinez Carlos A., Weinberg Samuel E. & Chandel Navdeep S. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nature Metabolism 1, 158–171, doi: 10.1038/s42255-018-0011-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat Cell Biol 19, 626–638, doi: 10.1038/ncb3527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinberg SE et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature 565, 495–499, doi: 10.1038/s41586-018-0846-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alistar A et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 18, 770–778, doi: 10.1016/S1470-2045(17)30314-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molina JR et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med 24, 1036–1046, doi: 10.1038/s41591-018-0052-4 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Sullivan LB et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat Cell Biol 20, 782–788, doi: 10.1038/s41556-018-0125-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swanson JA Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 9, 639–649, doi: 10.1038/nrm2447 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Recouvreux MV & Commisso C Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front Endocrinol (Lausanne) 8, 261, doi: 10.3389/fendo.2017.00261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finicle BT, Jayashankar V & Edinger AL Nutrient scavenging in cancer. Nat Rev Cancer 18, 619–633, doi: 10.1038/s41568-018-0048-x (2018). [DOI] [PubMed] [Google Scholar]
- 73.Bloomfield G & Kay RR Uses and abuses of macropinocytosis. J Cell Sci 129, 2697–2705, doi: 10.1242/jcs.176149 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Haigler HT, McKanna JA & Cohen S Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol 83, 82–90 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palm W, Araki J, King B, DeMatteo RG & Thompson CB Critical role for PI3-kinase in regulating the use of proteins as an amino acid source. Proc Natl Acad Sci U S A 114, E8628–E8636, doi: 10.1073/pnas.1712726114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bar-Sagi D & Feramisco JR Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 (1986). [DOI] [PubMed] [Google Scholar]
- 77.Commisso C et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637, doi: 10.1038/nature12138 (2013).This study shows that macropinocytosis promotes the use of extracellular protein as an amino acid source in RAS-transformed cells.
- 78.Palm W et al. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell 162, 259–270, doi: 10.1016/j.cell.2015.06.017 (2015).This work demonstrates that mTORC1 activity suppresses the use of extracellular protein as an amino acid source through macropinocytosis.
- 79.Garcia D & Shaw RJ AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell 66, 789–800, doi: 10.1016/j.molcel.2017.05.032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SM et al. PTEN Deficiency and AMPK Activation Promote Nutrient Scavenging and Anabolism in Prostate Cancer Cells. Cancer Discov 8, 866–883, doi: 10.1158/2159-8290.CD-17-1215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nofal M, Zhang K, Han S & Rabinowitz JD mTOR Inhibition Restores Amino Acid Balance in Cells Dependent on Catabolism of Extracellular Protein. Mol Cell 67, 936–946 e935, doi: 10.1016/j.molcel.2017.08.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldberg IJ, Eckel RH & Abumrad NA Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res 50 Suppl, S86–90, doi: 10.1194/jlr.R800085-JLR200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menendez JA & Lupu R Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7, 763–777, doi: 10.1038/nrc2222 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Zaidi N et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res 52, 585–589, doi: 10.1016/j.plipres.2013.08.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medes G, Thomas A & Weinhouse S Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 13, 27–29 (1953). [PubMed] [Google Scholar]
- 86.Gansler TS, Hardman W 3rd, Hunt DA, Schaffel S & Hennigar RA Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28, 686–692 (1997). [DOI] [PubMed] [Google Scholar]
- 87.Sebastiani V et al. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol Oncol 92, 101–105 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Visca P et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res 24, 4169–4173 (2004). [PubMed] [Google Scholar]
- 89.Mashima T, Seimiya H & Tsuruo T De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer 100, 1369–1372, doi: 10.1038/sj.bjc.6605007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pascual G et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45, doi: 10.1038/nature20791 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Zhang M et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov 8, 1006–1025, doi: 10.1158/2159-8290.CD-17-1371 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sounni NE et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after antiangiogenic therapy withdrawal. Cell Metab 20, 280–294, doi: 10.1016/j.cmet.2014.05.022 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Hochachka PW, Rupert JL, Goldenberg L, Gleave M & Kozlowski P Going malignant: the hypoxia-cancer connection in the prostate. Bioessays 24, 749–757, doi: 10.1002/bies.10131 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Ackerman D & Simon MC Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol 24, 472–478, doi: 10.1016/j.tcb.2014.06.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, Dales NA & Winther MD Opportunities and challenges in developing stearoyl-coenzyme A desaturase-1 inhibitors as novel therapeutics for human disease. J Med Chem 57, 5039–5056, doi: 10.1021/jm401516c (2014). [DOI] [PubMed] [Google Scholar]
- 96.Igal RA Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim Biophys Acta 1861, 1865–1880, doi: 10.1016/j.bbalip.2016.09.009 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Kamphorst JJ et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A 110, 8882–8887, doi: 10.1073/pnas.1307237110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ackerman D et al. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep 24, 2596–2605 e2595, doi: 10.1016/j.celrep.2018.08.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vriens K et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406, doi: 10.1038/s41586-019-0904-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nosal JM, Switzer RL & Becker MA Overexpression, purification, and characterization of recombinant human 5-phosphoribosyl-1-pyrophosphate synthetase isozymes I and II. J Biol Chem 268, 10168–10175 (1993). [PubMed] [Google Scholar]
- 101.Qian X et al. Conversion of PRPS Hexamer to Monomer by AMPK-Mediated Phosphorylation Inhibits Nucleotide Synthesis in Response to Energy Stress. Cancer Discov 8, 94–107, doi: 10.1158/2159-8290.CD-17-0712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graves LM et al. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature 403, 328–332, doi: 10.1038/35002111 (2000). [DOI] [PubMed] [Google Scholar]
- 103.Sigoillot FD et al. Nuclear localization and mitogen-activated protein kinase phosphorylation of the multifunctional protein CAD. J Biol Chem 280, 25611–25620, doi: 10.1074/jbc.M504581200 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Ben-Sahra I, Howell JJ, Asara JM & Manning BD Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328, doi: 10.1126/science.1228792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robitaille AM et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323, doi: 10.1126/science.1228771 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Wang Y & Hekimi S Understanding Ubiquinone. Trends Cell Biol 26, 367–378, doi: 10.1016/j.tcb.2015.12.007 (2016). [DOI] [PubMed] [Google Scholar]
- 107.White RM et al. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471, 518–522, doi: 10.1038/nature09882 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sykes DB et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell 167, 171–186 e115, doi: 10.1016/j.cell.2016.08.057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ducker GS & Rabinowitz JD One-Carbon Metabolism in Health and Disease. Cell Metab 25, 27–42, doi: 10.1016/j.cmet.2016.08.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM & Manning BD mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733, doi: 10.1126/science.aad0489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi Y, Evans JE & Rock KL Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521, doi: 10.1038/nature01991 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Boeynaems JM & Communi D Modulation of inflammation by extracellular nucleotides. J Invest Dermatol 126, 943–944, doi: 10.1038/sj.jid.5700233 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Scheibner KA et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177, 1272–1281 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Singer AJ & Clark RA Cutaneous wound healing. N Engl J Med 341, 738–746, doi: 10.1056/NEJM199909023411006 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 16, 582–598, doi: 10.1038/nrc.2016.73 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Dvorak HF Tumors: wounds that do not heal-redux. Cancer Immunol Res 3, 1–11, doi: 10.1158/2326-6066.CIR-14-0209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sullivan WJ et al. Extracellular Matrix Remodeling Regulates Glucose Metabolism through TXNIP Destabilization. Cell 175, 117–132 e121, doi: 10.1016/j.cell.2018.08.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Camps JL et al. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci U S A 87, 75–79 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olumi AF et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59, 5002–5011 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Curtis M et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab, doi: 10.1016/j.cmet.2018.08.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keith B & Simon MC Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472, doi: 10.1016/j.cell.2007.04.019 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trabold O et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen 11, 504–509 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Kamphorst JJ et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 75, 544–553, doi: 10.1158/0008-5472.CAN-14-2211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan M et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat Cell Biol 18, 1090–1101, doi: 10.1038/ncb3410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muranen T et al. Starved epithelial cells uptake extracellular matrix for survival. Nat Commun 8, 13989, doi: 10.1038/ncomms13989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olivares O et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun 8, 16031, doi: 10.1038/ncomms16031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Katheder NS & Rusten TE Microenvironment and tumors-a nurturing relationship. Autophagy 13, 1241–1243, doi: 10.1080/15548627.2017.1310361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sousa CM et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483, doi: 10.1038/nature19084 (2016).This work shows that autophagic degradation and release of cellular constituents in pancreatic stellate cells can support the growth of pancreatic cancer cells.
- 129.Nieman KM et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17, 1498–1503, doi: 10.1038/nm.2492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rabinowitz JD & White E Autophagy and metabolism. Science 330, 1344–1348, doi: 10.1126/science.1193497 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaur J & Debnath J Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16, 461–472, doi: 10.1038/nrm4024 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Wyant GA et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751–758, doi: 10.1126/science.aar2663 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amaravadi R, Kimmelman AC & White E Recent insights into the function of autophagy in cancer. Genes Dev 30, 1913–1930, doi: 10.1101/gad.287524.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karsli-Uzunbas G et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 4, 914–927, doi: 10.1158/2159-8290.CD-14-0363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo JY et al. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev 30, 1704–1717, doi: 10.1101/gad.283416.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang S et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev 25, 717–729, doi: 10.1101/gad.2016111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang A et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov 4, 905–913, doi: 10.1158/2159-8290.CD-14-0362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang A et al. Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov 8, 276–287, doi: 10.1158/2159-8290.CD-17-0952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Poillet-Perez L et al. Autophagy maintains tumour growth through circulating arginine. Nature 563, 569–573, doi: 10.1038/s41586-018-0697-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Allen EL et al. Differential Aspartate Usage Identifies a Subset of Cancer Cells Particularly Dependent on OGDH. Cell Rep 17, 876–890, doi: 10.1016/j.celrep.2016.09.052 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Ilic N et al. PIK3CA mutant tumors depend on oxoglutarate dehydrogenase. Proc Natl Acad Sci U S A 114, E3434–E3443, doi: 10.1073/pnas.1617922114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ye J et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov 4, 1406–1417, doi: 10.1158/2159-8290.CD-14-0250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Muir A et al. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife 6, doi: 10.7554/eLife.27713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shin CS et al. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun 8, 15074, doi: 10.1038/ncomms15074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kong H & Chandel NS Regulation of redox balance in cancer and T cells. J Biol Chem 293, 7499–7507, doi: 10.1074/jbc.TM117.000257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tonks NK Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670, doi: 10.1016/j.cell.2005.05.016 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Tormos KV et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14, 537–544, doi: 10.1016/j.cmet.2011.08.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weinberg F et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107, 8788–8793, doi: 10.1073/pnas.1003428107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schafer ZT et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113, doi: 10.1038/nature08268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.DeNicola GM et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109, doi: 10.1038/nature10189 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harris IS et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222, doi: 10.1016/j.ccell.2014.11.019 (2015). [DOI] [PubMed] [Google Scholar]
- 152.Piskounova E et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191, doi: 10.1038/nature15726 (2015).This work demonstrates that melanoma cells need to overcome the oxidative stress barrier in order to successfully metastasize.
- 153.Luengo A, Gui DY & Vander Heiden MG Targeting Metabolism for Cancer Therapy. Cell Chem Biol 24, 1161–1180, doi: 10.1016/j.chembiol.2017.08.028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parker WB Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem Rev 109, 2880–2893, doi: 10.1021/cr900028p (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cantley LC The phosphoinositide 3-kinase pathway. Science 296, 1655–1657, doi: 10.1126/science.296.5573.1655 (2002). [DOI] [PubMed] [Google Scholar]
- 156.Manning BD & Cantley LC AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274, doi: 10.1016/j.cell.2007.06.009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manning BD & Toker A AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405, doi: 10.1016/j.cell.2017.04.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vivanco I & Sawyers CL The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2, 489–501, doi: 10.1038/nrc839 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


