Abstract
The use of flow diverting stents for wide based, intracranial aneurysms has become an invaluable treatment option. While intracranial hemorrhage and ischemic stroke from dislodged atherosclerotic emboli are common adverse events, the potential for delayed granulomatous inflammation from possible hydrophilic polymer emboli is rarely recognized. We present a unique case in which visible chipping of the pusher wire for stent placement was observed, followed by clinical and radiographic evidence suggestive of a delayed foreign body reaction to intracranial hydrophilic polymer emboli. A 55-year-old woman underwent placement of a Pipeline embolization device for a left-sided, broad-based aneurysm at the base of the internal carotid artery and posterior communicating artery. Two months later she developed right-sided focal neurological deficits. Imaging showed ipsilateral focal edema and enhancing lesions with contrast. Although not confirmed with biopsy and histopathology, clinical and radiographic evidence suggests that this patient probably experienced a delayed foreign body reaction to hydrophilic polymer emboli from compromised procedural equipment during flow diverting stent placement. Although previously described, this is the first instance to our knowledge in whichvisible chipping of the pusher wire was observed on a Pipeline embolization device.
Keywords: Granulomatous inflammation, foreign body reaction, hydrophilic polymer embolization, flow diverting stent, cerebral aneurysm
Introduction
Endovascular equipment, such as sheaths, catheters, microcatheters, and guidewires, is commonly coated with hydrophilic material to increase lubricity and facilitate equipment navigation during endovascular procedures.1 However, this hydrophilic coating may become dislodged and cause hydrophilic polymer emboli (HPE). HPE may lead to complications, such as incitement of a foreign body inflammatory reaction.2,3 HPE during neurointerventional procedures may result in cerebral foreign body reactions that result in neurological deficits, and the optimal management of these reactions remains to be determined.
Here we describe our experience with a patient who experienced a probable cerebral foreign body reaction due to HPE following treatment of a cerebral aneurysm with a Pipeline embolization device (PED) (Medtronic Inc., Irvine, CA, USA). The imaging appearance of cerebral foreign body reactions and treatment options for this uncommon complication are discussed.
Case description
A 55-year-old woman with hypercholesterolemia, coronary artery disease, prior ischemic stroke, and previous subarachnoid hemorrhage due to rupture of a right posterior communicating artery aneurysm presented to our neurointerventional clinic with a second cerebral aneurysm. The patient had previously experienced subarachnoid hemorrhage due to rupture of a right posterior communicating artery aneurysm that was treated by endovascular coil embolization. The patient made a remarkable clinical recovery, but she was also found to have an unruptured, broad-based aneurysm arising from the contralateral supraclinoid left internal carotid artery (ICA). Given her history of cerebral aneurysm rupture, she elected to undergo treatment of this left ICA aneurysm by placement of a PED, which is a flow diverting stent designed for treatment of cerebral aneurysms with broad necks. The patient was loaded with aspirin and clopidogrel in anticipation of PED placement.
On the day of the procedure, the patient was placed under general anesthesia, and neurophysiological monitoring was performed to detect any evidence of a procedural complication. Arterial access was obtained via the right common femoral artery with a 6 French shuttle sheath (Cook Medical LLC, Bloomington, IN, USA). A 5 French Berenstein angiographic catheter (Cordis, Milpitas, CA, USA) was introduced into the shuttle sheath and used to select the proximal cervical left ICA. Digital subtraction angiography (DSA) identified a broad-based aneurysm that originated from the supraclinoid left ICA at the origin of the posterior communicating artery (Figure 1). The aneurysm measured 8 mm × 6 mm in diameter with a 7.6 mm neck, and this anatomy was deemed favorable for treatment with a PED.
Figure 1.
Digital subtraction angiography (DSA) following left internal carotid artery (ICA) injection during Pipeline embolization device (PED) placement. DSA in the anteroposterior (a) and lateral (b) projections demonstrate an 8 mm × 6 mm aneurysm with a 7.6 mm neck arising near the origin of the posterior communicating artery (a, b thick arrows). (c) A non-digital subtraction angiogram of the PED stent shortly after deployment crossing the span of the aneurysm base (single bracket). Distal to the stent is the guidewire tip coil (solid thin arrow) and proximal to the stent is the proximal marker/pusher wire (dashed thin arrow).
The shuttle sheath was advanced over the Berenstein catheter into the cervical left ICA, and the Berenstein was exchanged for a 5 French Navien distal access catheter (Medtronic Inc.). Next, a Marksman microcatheter (Medtronic, Inc.) was then introduced into the Navien over a Traxcess microwire (Microvention, Aliso Viejo, CA, USA), and the M1 segment of the left middle cerebral artery (MCA) was selected with the microcatheter and microwire without difficulty. The Navien distal access catheter was advanced over the microcatheter and positioned within the cavernous ICA. The microwire was removed, and a 4.75 mm × 16 mm PED was introduced into the microcatheter and deployed within the left supraclinoid ICA across the neck of the posterior communicating artery aneurysm (Figure 1).
During PED deployment, there was a transient 30% decrease in right upper extremity motor evoked potentials detected by neurophysiological monitoring. DSA performed immediately after these changes did not identify a filling defect, vessel cut-off, or other thromboembolic complication. The patient's mean arterial pressure was increased from 65 to 80 mmHg, and the motor evoked potentials rapidly returned to baseline. No other changes were detected by neurophysiological monitoring during the procedure.
After deployment of a single PED, all catheters and wires were removed from the patient. Inspection of the PED pusher wire demonstrated multiple areas where the hydrophilic coating was chipped and friable. The pusher wire was saved for inspection by the company that manufactured the device. Hemostasis in the right groin was achieved by placement of a closure device and manual pressure. The patient awoke at her baseline and was free of neurological deficits. She was discharged from the hospital the day after treatment at her normal neurological baseline with plans for clinical follow-up in one month.
One month after the procedure, the patient complained of new intermittent bitemporal headaches. The benign description of these headaches was felt to be consistent with expected interval thrombosis of the treated left posterior communicating artery aneurysm following PED placement. The patient had no focal neurological deficits, fevers, or other concerning symptoms, and she was reassured. However, 2 months after PED placement, the patient experienced a mechanical fall following the sudden onset of right-sided numbness and weakness that persisted for several seconds before resolving. She was brought to an outside hospital and, on further questioning, she endorsed several days of bilateral lower extremity weakness that preceded her fall. She was compliant with her dual antiplatelet regimen of aspirin 325 mg and clopidogrel 75 mg daily.
On physical exam, the patient responded slowly to questions and exhibited a subtle right-sided facial droop, 4/5 strength in the right upper extremity and bilateral lower extremities, and mild dysmetria on bilateral finger to nose testing. The remainder of the physical exam was normal.
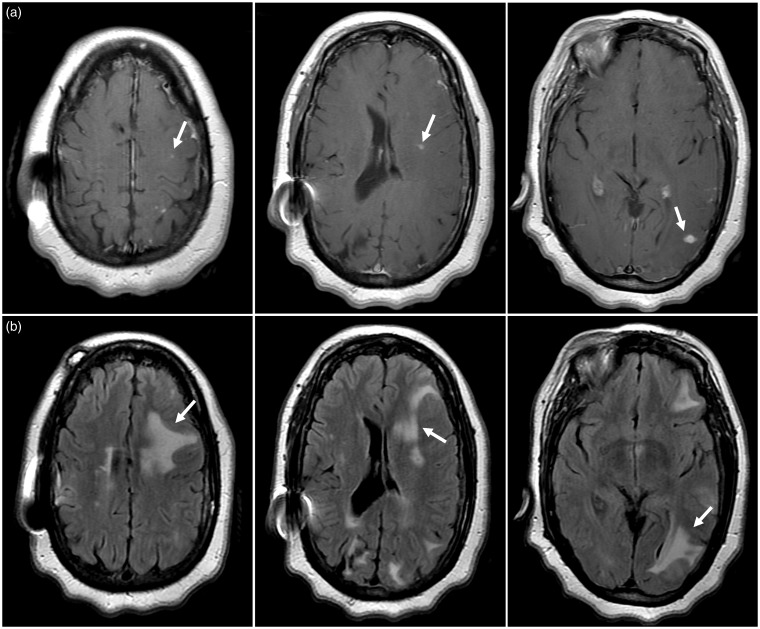
The patient underwent brain imaging by magnetic resonance imaging (MRI), which demonstrated multiple T1 post-contrast enhancing foci imaging and surrounding T2 hyperintense signal abnormality within the left cerebral hemisphere (Figure 2). There was significant mass effect associated with the regions of T2 signal abnormality that resulted in partial effacement of the left lateral ventricle. These imaging findings were thought to be most consistent with mycotic aneurysms, septic emboli, or multifocal foreign body reactions.
Figure 2.
Brain magnetic resonance imaging findings following new neurological symptoms 2 months after Pipeline embolization device (PED) treatment. (a) Axial post-contrast T1-weighted images show multiple small enhancing foci (arrows) within the left cerebral hemisphere. (b) Axial fluid-attenuated inversion recovery (FLAIR) images identify increased signal abnormality surrounding the regions of contrast enhancement that is most consistent with vasogenic edema (arrows). Regions of FLAIR signal abnormality in the right cerebral hemisphere represent baseline non-specific white matter disease based on comparison with pre-PED treatment neuroimaging studies. Regions of T2 signal abnormality in the right cerebral hemisphere did not demonstrate associated enhancement and were felt to be most consistent with non-specific periventricular white matter changes.
The patient was transferred to our hospital for additional evaluation. A DSA was performed, which showed no evidence of a mycotic aneurysm, vascular filling defect, vascular irregularity, or other new abnormality. The PED was in stable position and was free of any filling defects or stenosis. Interval near complete occlusion of the left posterior communicating artery aneurysm after PED placement was noted (Figure 3).
Figure 3.
Digital subtraction angiography (DSA) following new symptom development 2 months after Pipeline embolization device (PED) placement. DSA in the anteroposterior (a) and lateral (b) projections demonstrate minimal residual filling (arrows) of the left posterior communicating artery aneurysm previously treated by flow diversion. No other vascular abnormality is identified to account for the patient's symptoms.
Additional evaluation was performed with a lumbar puncture and laboratory studies. Cerebrospinal fluid (CSF) identified a leukocyte count of 6 white blood cells/µL (corrected for red blood cells to three white blood cells/µL). CSF protein was mildly elevated at 142 mg/dL (normal range <45 mg/dL), and CSF glucose was 64 mg/dL (normal range >40 mg/dL). Blood and CSF cultures were negative for bacterial or fungal growth. Blood cultures were negative. Estimated sedimentation rate was 19 mm/hour (normal range 0–30) and C-reactive protein was 0.8 mg/dL (normal range <0.9 mg/dL). All remaining laboratory values were within normal limits.
The absence of abnormal systemic inflammatory or infectious markers in the blood was felt to be most consistent with cerebral granulomatous inflammation based on prior studies that did not find evidence of a systemic immune response.1,2 A presumptive diagnosis of a foreign body reaction due to HPE was made based on the MRI appearance of the brain that was isolated to the hemisphere treated by the PED and the evidence of hydrophilic coating shearing on the pusher wire following the procedure.
The patient was treated with oral steroids to reduce cerebral inflammation. Over the course of several days the patient began to show clinical improvement of her symptoms, and she was discharged. The patient was seen in follow-up as an outpatient 2weeks later (12 weeks after aneurysm treatment) and, at that time, her only complaint was continued headaches that were unchanged in character. Her neurological deficits had resolved. A follow-up brain MRI performed at this time showed an interval reduction in the conspicuity and number of enhancing foci in the left cerebral hemisphere and reduced T2 signal abnormality surrounding these lesions. The mass effect within the left cerebral hemisphere was likewise reduced (Figure 4). The patient was subsequently lost to follow-up in our clinic, but her outside hospital medical records demonstrate no further focal neurological deficits one and a half years after treatment. She did continue to experience more mild headaches, which were treated with over-the-counter acetaminophen.
Figure 4.
Brain magnetic resonance imaging (MRI) after treatment for presumed hydrophilic polymer emboli (HPE) using steroids. MRI was obtained 2 weeks after treatment with oral steroids, and she was symptom free at the time of this MRI. (a) Axial post-contrast T1-weighted images show an interval reduction in the number and conspicuity of small enhancing foci (arrows) within the left cerebral hemisphere. (b) Axial fluid-attenuated inversion recovery (FLAIR) images demonstrate an interval reduction in the degree of edema surrounding the enhancing foci following steroid treatment (arrows).
Discussion
Here we have described the first report, to our knowledge, of cerebral HPE due to hydrophilic coating chipping of a PED pusher wire. Although a histopathological diagnosis in our patient was not obtained, our imaging findings and patient presentation coincide with similar reports with histopathological confirmation of foreign body reaction and granulomatous inflammation from HPE.1,2,11,18
Flow diverting stents have become an invaluable tool for the treatment of large, wide-necked intracranial aneurysms.4 Previous trials have shown the use of PED placement for intracranial aneurysms to be both safe and effective,5,6 but HPE following PED placement have been described.3,7 Lorentzen et al. recently reported a patient 3-month status post-PED placement who presented with global aphasia and right-sided hemiparesis and was found to have white matter edema and focal post-contrast enhancement on imaging.3 Microscopic examination of brain tissue revealed signs of foreign body reaction to non-polarizable material characteristic of dislodged hydrophilic coating. In both of these prior cases of HPE after PED placement, the source of the HPE was either uncertain or was associated with other equipment.3,7
Cerebral HPE is not isolated to the PED, and the phenomenon was first reported in 1997 by Barnwell and colleagues.8 Since this initial report, HPE has been described after several different types of cerebrovascular procedures, which include diagnostic cerebral angiography, cerebral artery stenting, aneurysm coil embolization, thrombectomy, and cerebral arterythrombolysis.1–3,7–12 HPE is also not isolated to the brain, and there are several reports of HPE to various organs following endovascular procedures.13–17 Therefore, HPE remains a risk of any endovascular procedure during which hydrophilic coating material may be dislodged from endovascular equipment.
The cause of HPE is not completely understood, but several explanations for its occurrence have been proposed. Histological evidence of coating material found at the radial artery access site of two patients suggests that the hydrophilic coating may dislodge during introducer sheath insertion.10 In vitro experiments have shown that polyvinylpyrrolidone, which is a common hydrophilic coating, can be released from shuttle sheath catheters with minimal manipulation.7 Similarly, HPE was demonstrated during in vitro manipulation of a microcatheter that was introduced through a diagnostic catheter.8 As neurointerventional procedures have increased in complexity with triaxial and quadraxial endovascular access constructs, there may be an increased risk of friction between multiple guide, intermediate, and micro catheters and microwires, which may lead to an increased risk of HPE.1,2,8 Endovascular navigation of more tortuous vascular anatomy has also been suggested to contribute to dislodgement of the hydrophilic coating.8 In response to these HPE concerns, some authors advocate techniques to reduce friction between endovascular devices, such as using bilateral access points, reduced use of multiaxial endovascular constructs, avoidance of quick removal of catheters and wires from access sites, avoidance of tight-fitting axial configurations, proper storage of endovascular equipment, and on-label use of endovascular equipment.1,2 Whether advancements in equipment coating technology can reduce or obviate the risk of HPE remains to be determined.
Our patient presented with headaches that progressed to neurological deficits, but the clinical presentation of brain HPE is variable. Other case reports and small series have described focal neurological deficits, cognitive deficits, headaches, seizures, and fatal cerebral hemorrhage.6,11–13,15,17 By contrast, a series of seven patients with imaging findings suggestive of HPE after endovascular therapy found four of the patients (57%) to be asymptomatic.9 In a review of 32 patients with documented intracranial polymer reactions, 97% had symptoms including focal neurological deficits (41%), headache (22%), constitutional symptoms (19%), meningitis (16%), seizure and/or involuntary movements (9%), coma (6%), and syncope (3%).1 The first patient fatality secondary to brain HPE was reported in 2009 after the coiling of a giant intracranial aneurysm.18 The timing of HPE symptom onset also varies. Symptoms secondary to cerebral HPE have been reported as early as one day and as late as 9 months after an endovascular procedure.10 Our patient first developed headaches one month after PED placement, which may have been due to HPE in retrospect. Therefore, neurointerventionalists should have a high degree of suspicion for HPE if unusual symptoms develop following neuroendovascular procedures.
The neuroimaging and pathological evaluation of HPE is characteristically suggestive of a foreign body granulomatous reaction. Brain MRI typically identifies enhancing foci with surrounding edema that develop following cerebral endovascular procedures, as was the case in our patient. Histological analysis of brain tissue following biopsy or autopsy has identified granulomatous inflammation, ischemic infarction, parenchymal hemorrhage, abscess formation, and chemical meningitis.1,2,10,11 A presumptive diagnosis of HPE may be made based on brain MRI with an appropriate level of suspicion.
Cerebrospinal fluid and blood laboratory evaluation did not find evidence of a systemic inflammatory or infectious response in our patient, which is similar to a prior report.2 Although laboratory evaluation in patients with HPE is not consistently reported, our findings and those of others suggest that these costly studies may have limited utility in establishing the HPE diagnosis in the absence of an associated clinical meningitis.
The optimal treatment of HPE has not been determined, although effective treatment regimens have been reported in several case reports and series. Oral methylprednisolone dosed at 16 mg four times daily resulted in significant clinical improvements.3 Additional corticosteroid regimens that led to symptomatic improvement include pulse dose methylprednisolone followed by a prednisone taper over 60 days or a 2-month regimen of dexamethasone.2,11 In one case, a symptomatic relapse after prior steroid treatment was successfully treated with azathioprine, an immunosuppressive medication.3 Our patient was treated with a short oral steroid taper, which was felt to be the optimal treatment for the symptomatic cerebral edema surrounding the enhancing foci. Her symptoms improved, which was commensurate with a reduction in cerebral edema on brain MRI after treatment. As the patient was subsequently lost to follow-up we were unfortunately unable to evaluate further the long-term outcome of our treatment regimen.
Due to low clinical suspicion, the need for targeted histopathological analysis, and declining rates of post mortem analysis of organs and vasculature, HPE is likely to be under recognized and under reported.13 In addition, there have been significant barriers to investigating, publishing, and reporting of suspected HPE episodes,13 but proper device investigation and reporting may result in improved endovascular technology and patient safety.13 In 2015, the US Food and Drug Administration (FDA) released a safety communication after retrospectively reviewing the medical literature and medical device reports providing recommendations and safe clinical practices.19 This retrospective analysis was prompted after several publications of post mortem HPE frequencies.14,20 Improved pre and post-market testing, surveillance, and reporting has been suggested to improve the safety and efficacy of future devices.13
Conclusions
HPE may occur following PED embolization and result in symptomatic cerebral granulomatous inflammation and edema. HPE should be suspected in patients who develop unexpected neurological symptoms following cerebral endovascular procedures, and recognition of typical brain imaging findings are critical for the diagnosis of HPE in the absence of brain biopsy.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mehta RI, Mehta RI. Polymer-induced central nervous system complications following vascular procedures: spectrum of iatrogenic injuries and review of outcomes. Hum Pathol 2016; 53: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro M, Ollenschleger MD, Baccin C, et al. Foreign body emboli following cerebrovascular interventions: clinical, radiographic, and histopathologic features. Am J Neuroradiol 2015; 36: 2121–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorentzen AO, Nome T, Bakke SJ, et al. Cerebral foreign body reaction after carotid aneurysm stenting. Interv Neuroradiol 2016; 22: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briganti F, Leone G, Marseglia M, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 2015; 28: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becske T, Kallmes DF, Saataci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 6.Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline embolization device. J Neurosurg 2014; 120: 365–374. [DOI] [PubMed] [Google Scholar]
- 8.Barnwell SL, D'Agostino AN, Shapiro SL, et al. Foreign bodies in small arteries after use of an infusion microcatheter identified to the manufacturer. Am J Neuroradiol 1997; 18: 1886–1889. [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz JP, Marotta T, Kelly CO, et al. Enhancing brain lesions after endovascular treatment of aneurysms. Am J Neuroradiol 2014; 35: 1954–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fealey ME, Edwards WD, Giannini C, et al. Complications of endovascular polymers associated with vascular introducer sheaths and metallic coils in 3 patients, with literature review. Am J Surg Pathol 2008; 32: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 11.Meiers C, Abebe Y, Alberto N, et al. Cerebral granulomatous inflammation secondary to hydrophilic polymer embolization following thrombectomy. Am J Case Rep 2017; 18: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari SA, Anderson RR, Caron MJ, et al. Hydrophilic polymer embolic complication during diagnostic cerebral angiography presenting with delayed intracranial hemorrhage: case report and literature review. J NeuroIntervent Surg 2018; 19: 1–4. [DOI] [PubMed] [Google Scholar]
- 13.Mehta RI, Mehta RI. Hydrophilic polymer embolism: implications for manufacturing, regulation, and postmarket surveillance of coated intravascular medical devices. J Patient Safety 2018, pp. 1–11. Epub ahead of print, 19 March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RI, Mehta RI, Choi JM, et al. Hydrophilic polymer embolism and associated vasculopathy of the lung: prevalence in a retrospective autopsy study. Hum Pathol 2016; 46: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez JA, Chen W, Frankel WL, et al. Hydrophilic polymer-associated ischemic enterocolitis. Am J Surg Pathol 2017; 41: 271–276. [DOI] [PubMed] [Google Scholar]
- 16.Sequeira A, Parimoo N, Wilson J, et al. Polymer embolization from minimally invasive interventions. Am J Kidney Dis 2013; 61: 984–987. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AK, Peters MS, Rokea A, et al. Cutaneous microemboli from hydrophilic polymer after endovascular procedures. J Am Dermatol 2015; 73: 666–671. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RI, Mehta RI, Fishbein MC, et al. Intravascular polymer material after coil embolization of a giant cerebral aneurysm. Hum Pathol 2009; 40: 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. Intravascular Medical Devices: FDA Safety Communication – Lubricious Coating Separation. FDA, 23 November 2015. Available at: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm610630.pdf.
- 20.Grundeken MJ, Li X, Kurpershoek EC, et al. Distal embolization of hydrophilic-coating material from coronary guidewires after percutaneous coronary interventions. Circ Cardiovasc Interv 2015; 8: 1–7. [DOI] [PubMed] [Google Scholar]