Abstract
Purpose:
To determine the risk factors for development of neovascular glaucoma (NVG) in patients after an acute central retinal vein occlusion (CRVO).
Design:
Retrospective cohort study
Methods:
Review of medical records of 646 patients with a diagnosis of CRVO between 2013 to 2017 at the Bascom Palmer Eye Institute. Inclusion criteria: 1) CRVO onset to presentation <90 days; 2) Absence of anterior segment neovascularization on presentation; 3) No intravitreal anti-VEGF injection before presentation. Patients meeting inclusion criteria were screened for potential risk factors for development of NVG. Risk of developing NVG was assessed with Kaplan-Meier survival analysis and Cox proportional hazards models.
Results:
Thirteen of 98 patients (13%) who met inclusion criteria developed NVG. The mean adjusted time to NVG diagnosis from onset of CRVO related symptoms was 212 days. Patients presenting with a worse initial visual acuity (p=0.034), a relative afferent pupillary defect (RAPD) (p=0.002), or a history of systemic hypertension (p=0.026) had an increased risk of NVG compared to those who did not. Age, BMI, history of glaucoma, history of diabetes, and central retinal thickness were not significantly associated with development of NVG.
Conclusions:
Risk factors for NVG development included history of systemic hypertension, worse visual acuity on presentation, and RAPD on presentation. Patients presenting with these findings should be followed at closer intervals and informed of the greater risk for neovascularization. Intravitreal anti-VEGF therapy delayed, but did not prevent NVG.
Introduction
Despite numerous natural history studies, the ability to predict which patients will develop neovascular glaucoma (NVG) after a central retinal vein occlusion (CRVO) remains limited.1,2 Central retinal vein occlusion has been classified into ischemic and non-ischemic variants depending on the area of retinal ischemia.1-3 The more common non-ischemic variant occurs in 81% of cases and poses little risk for neovascular complications.2,4-6 The criteria to separate ischemic from non-ischemic CRVO has been a topic of debate.1,2,7 In the Central Vein Occlusion Study (CVOS), an ischemic CRVO was classified as greater or equal to 10 disc diameter areas of non-perfusion observed on fluorescein angiography (FA). Neovascular glaucoma developed in 20% of patients with ischemic CRVO as defined by this criteria.1
Fundus findings have previously been reported to lack sensitivity in identifying ischemic CRVO, and FA is of limited utility in one-third of patients who present with dense intraretinal hemorrhage.2,3,8 Realizing the limitations of FA in patients with acute CRVO, Hayreh studied multiple clinical factors such as relative afferent pupillary defect (RAPD), visual acuity (VA), visual fields, and electroretinograms (ERG) to classify ischemic CRVO.2 This classification was recently used in the Rubeosis Anti-VEGF (RAVE) Trial to assess the ability of chronic vascular endothelial growth factor (VEGF) blockade in patients with ischemic CRVO for prevention of neovascular complications.9 Although ERG and kinetic visual field testing are performed routinely in large academic centers, many practices lack this modality to assess acute CRVO patients. Without proper risk stratification, patients presenting with CRVO remain difficult to assess for the risk of NVG. The goal of this study is to analyze the characteristics of patients presenting with central retinal vein occlusion, identify risk factors for the subsequent development of neovascular glaucoma, and determine who might benefit from early intervention to prevent neovascular glaucoma in high risk patients.
Patients and Methods
We identified patients presenting to the Bascom Palmer Eye Institute/Anne Bates Leach Eye Hospital (emergency department and outpatient visits) between January 2013 and December 2017 with an ICD-9 or ICD-10 diagnosis of CRVO. After screening, each patient’s clinical records starting from the date of CRVO diagnosis to the most recent clinical visit were carefully reviewed. All charts were reviewed by two ophthalmologists (AJR & SSS) using a standardized protocol to ensure an accurate CRVO diagnosis that was consistent with the ICD coding. All reasonable means were used to ensure that the data were reliable and any patients with inadequate or unreliable information were excluded from the study. The Institutional Review Board at the University of Miami School of Medicine approved this protocol before beginning the study. This study followed the tenets of the Declaration of Helsinki.
Inclusion and Exclusion Criteria
Only patients with clinical findings consistent with a diagnosis of CRVO (acute vision loss, diffuse intraretinal hemorrhage, venous tortuosity) were included in the study. We limited inclusion to patients presenting within the first 90 days of symptom onset as defined by day of subjective visual acuity decrease.
We excluded patients with a hemi-retinal vein occlusion, branch retinal vein occlusion, or any retinopathy that mimicked a CRVO; patients who received any form of intravitreal anti-VEGF or corticosteroid treatment prior to presentations; patients who exhibited any form of neovasculariation of the iris(NVI) or neovascularization of the angle (NVA) at time of presentation; patients younger than 18 years of age; patients lost to follow-up after their initial visit; and patients presenting with acute bilateral CRVOs.
Data collection
For the baseline visit, we recorded the date of presentation, probable estimated date of CRVO as determined by subtracting the subjective symptom onset from the date of clinical presentation, age, gender, body mass index (BMI), history of glaucoma, history of systemic hypertension, history of diabetes, and degree of diabetic retinopathy if present.
For the baseline visit on presentation and for an additional 3 clinical visits, we assessed the following variables at each visit: visual acuity (VA), intraocular pressure (IOP), relative afferent pupillary defect (RAPD), macular edema, central retinal thickness by optical coherence tomography (OCT), and whether an intravitreal anti-VEGF injection was administered at that visit. Retinal thickness was measured with either Cirrus HD-OCT (Carl Zeiss Meditec, Inc. Oberkochen, Germany) or Spectralis HRA+OCT (Heidelberg Engineering, Inc. Heidelberg, Germany) instruments.
If a patient was noted to have NVI/NVA and an elevated IOP >22mmHg, it was recorded as an NVG event. We did not record posterior segment neovascularization as a neovascular event in this study.
Statistical Analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means and standard deviations. Time to the occurrence of neovascular glaucoma was assessed with Kaplan-Meier survival analysis and Cox proportional hazards regression. All analyses were done using SAS version 9.4 (Cary, NC, USA). A p-value ≤ 0.05 was considered statistically significant.
Results
We examined the medical records of 646 patients over the 5 year period and 98 patients met the study inclusion criteria. Among the 646 patients, the majority of patients were excluded due to CRVO symptoms greater than 90 days (or without a clear date of initial symptomatic onset) or due to anti-VEGF injections by an outside provider prior to presentation. After review through the remaining charts, an additional 17 patients were excluded due to lack of follow-up after their initial visit.
Of the remaining 98 patients, the mean follow-up time was 17 months (range 0.4-66.6 months). Of note, all patients who developed anterior segment neovascularization (NVI or NVA) in our data set had pressures above 22mmHg and met our criteria for NVG. Clinical characteristics are presented in Table 1.
Table 1.
Clinical characteristics of the 98 patients meeting inclusion criteria
| Categorical Variables |
Value | All Patients (n = 98) |
No Neovascular (n = 85, 86.7%) |
Neovascular (n = 13, 13.3%) |
|||
|---|---|---|---|---|---|---|---|
| Gender | Female | 47 | (48%) | 40 | (47%) | 7 | (53.8%) |
| Male | 51 | (52%) | 45 | (53%) | 6 | (46.2%) | |
| Age less than or equal to 50 years | No | 78 | (79.6%) | 65 | (76.4%) | 13 | (100%) |
| Yes | 20 | (20.4%) | 20 | (23.6%) | 0 | (0%) | |
| Afferent Pupillary Defect | No | 84 | (85.7%) | 75 | (88.3%) | 9 | (69.2%) |
| Yes | 14 | (14.3%) | 10 | (11.7%) | 4 | (30.8%) | |
| Anti-VEGF at first visit? | No | 36 | (36.7%) | 30 | (35.2%) | 6 | (46.2%) |
| Yes | 62 | (63.3%) | 55 | (64.8%) | 7 | (53.8%) | |
| History of Primary Open Angle Glaucoma | No | 74 | (75.5%) | 65 | (76.4%) | 9 | (69.2%) |
| Yes | 24 | (24.4%) | 20 | (23.5%) | 4 | (30.8%) | |
| History Hypertension | No | 32 | (32.7%) | 30 | (35.2%) | 2 | (15.4%) |
| Yes | 66 | (67.4%) | 55 | (64.8%) | 11 | (84.6%) | |
| History Diabetes | No | 65 | (66.3%) | 58 | (68.2%) | 7 | (53.8%) |
| Yes | 33 | (33.7%) | 27 | (31.8%) | 6 | (46.2%) | |
| Diabetic Retinopathy (DR) | No | 21 | (63.6%) | 17 | (63%) | 4 | (66.6%) |
| Yes | 12 | (36.4%) | 10 | (37%) | 2 | (33.3%) | |
| Degree of DR | mild | 4 | (33.3%) | 4 | (40%) | 0 | (0%) |
| moderate | 4 | (33.3%) | 2 | (20%) | 2 | (100%) | |
| severe | 1 | (8.3%) | 1 | (10%) | 0 | (0%) | |
| PDR | 3 | (25%) | 3 | (30%) | 0 | (0%) | |
| Macular edema assessment | none | 31 | (31.6%) | 27 | (31.7%) | 4 | (30%) |
| OCT | 53 | (54.1%) | 45 | (52.9%) | 8 | (61.5%) | |
| clinical exam | 14 | (14.3%) | 13 | (15.4%) | 1 | (7.7%) | |
| Continuous Variables |
Mean | SD | Mean | SD | Mean | SD | |
| BMI | 28.5 | (4.9) | 28.7 | (4.8) | 27.0 | (5.8) | |
| Diastolic blood pressure (mmHg) | 79.1 | (12.5) | 79.1 | (11.9) | 79.3 | (17.3) | |
| Systolic blood pressure (mmHg) | 145.0 | (24.9) | 145.7 | (24.9) | 139.4 | (24.9) | |
| Age at First Visit (years) | 63.7 | (15.3) | 62.8 | (15.7) | 69.3 | (11.1) | |
| IOP at first visit (mmHg) | 16.9 | (4.1) | 16.7 | (3.8) | 18.3 | (5.4) | |
| LogMAR VA at first visit | 0.93 | (0.62) | 0.90 | (0.62) | 1.14 | (0.6) | |
| OCT thickness at first visit (μm) | 631.8 | (238.1) | 631.6 | (221.4) | 632.6 | (334.5) | |
Development of Neovascular Glaucoma
The cumulative probability of developing NVG was 13% (n=13). The mean time to development of NV was 421 days (median=198; range 21-1286 days). When accounting for the last intravitreal anti-VEGF injection given during the observational period (when the stimulus for neovascularization may be minimized and the day counter reset to Day 0),10 the mean time to development of NVG was 212 days after last anti-VEGF injection (median=150; range 21-990 days).
At the onset of NVG, the mean VA was count fingers, with a mean IOP of 41.9 ± 10.3 mmHg. Nine of 13 patients (69%) underwent subsequent pan-retinal photocoagulation (PRP). Eight of 13 patients (62%) underwent subsequent incisional surgery, with either valved or non-valved glaucoma drainage devices (n=4 for each respective group). One patient underwent cyclophotocoagulation (CPC). All but the one patient who underwent CPC had an anti-VEGF injection at the time of NVG diagnosis. After medical and surgical intervention to control IOP, the mean VA remained count fingers with a mean IOP of 22.5 ± 11.9 mmHg at an average follow up of 25± 13 months. For the non-NVG cohort, the mean VA was 20/90 with a mean IOP of 16.4 ± 4.9 mmHg at an average follow up of 15± 12 months.
Clinical findings
Gender, BMI, systolic or diastolic blood pressure at initial visit, history of diabetes, degree of diabetic retinopathy, or anti-VEGF injection at first visit were not significantly different in patients who did and did not develop NVG (Table 2). Of the patients who developed NVG, the average number of intravitreal anti-VEGF injections performed was 4 (range 0-18). Of the 27 patients with a history of glaucoma, 24 patients had a history of primary open angle glaucoma (POAG), 2 patients had unspecified glaucoma (undefined per chart review), and 1 patient had a history of chronic angle closure glaucoma (CACG). The one patient with CACG had previous glaucoma shunt surgery before presenting with a CRVO; this patient did not develop NVI/NVA. Neither history of glaucoma (POAG, unspecified, CACG), nor history of POAG alone were significantly different among patients who did and did not develop NVG.
Table 2:
Categorical Variables for Risk of Neovascular Glaucoma
| Categorical Variables |
Value with Greater Hazard |
Kaplan- Meier. p - value |
Cox Regression p - value |
Hazard Ratio (HR) |
HR 95% CI Low |
HR 95% CI High |
|---|---|---|---|---|---|---|
| Gender | Female | 0.4842 | 0.4868 | 0.68 | 0.22 | 2.04 |
| Afferent Pupillary Defect | Yes | 0.0022 ** | 0.0068 ** | 6.04 | 1.64 | 22.19 |
| Anti-VEGF at first visit? | No | 0.4506 | 0.4536 | 0.65 | 0.21 | 2.00 |
| History of POAG | No | 0.9506 | 0.9509 | 0.96 | 0.29 | 3.18 |
| History of Hypertension | Yes | 0.0255 * | 0.0406 * | 5.05 | 1.07 | 23.78 |
| History of Diabetes | Yes | 0.3788 | 0.3833 | 1.63 | 0.54 | 4.87 |
| Diabetic Retinopathy (DR) | No | 0.5682 | 0.5717 | 0.61 | 0.11 | 3.45 |
| Degree of DR | moderate | 0.1231 | (1) | |||
| Macular edema assessment | 0.5985 | |||||
| -Compare no macular edema to OCT | OCT | 0.3243 | 1.99 | 0.51 | 7.83 | |
| -Compare no macular edema to clinical exam | Clinical exam | 0.7575 | 1.44 | 0.14 | 14.65 |
p < 0.05,
p < 0.01
POAG= Primary open angle glaucoma. CI = confidence interval. HR=Hazard Ratio (1) No Cox regression was possible due to an absence of failures (eyes with a neovascular event) in one group.
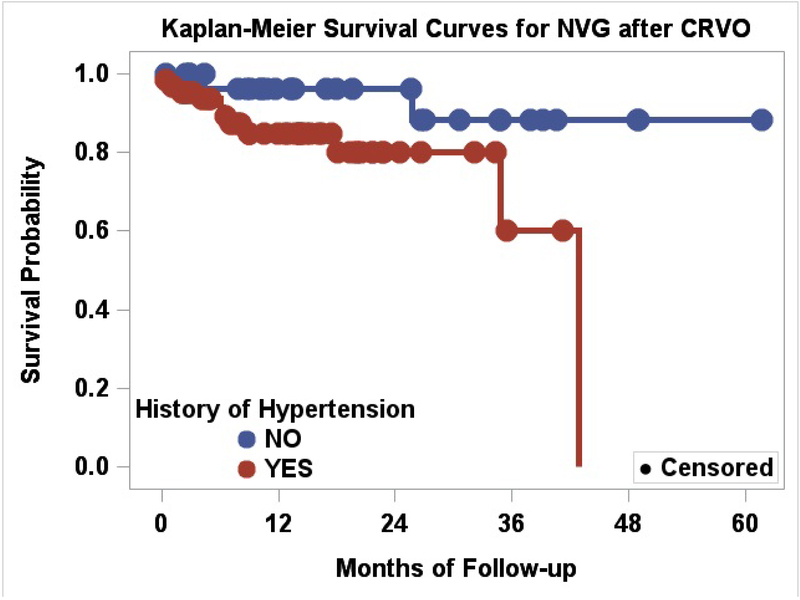
A history of systemic hypertension (p=0.026) was associated with the development of NVG (Figure 1). Although age on presentation for the NVG group versus non-NVG group differed (69± 11 years and 63± 16 years respectively), it was not found to be a significant risk factor (p=0.133). None of the patients younger than 50 years of age developed NVG. When patients were arbitrarily divided into older (n=78) versus younger (n=20) than 50 years of age, this relationship was non-significant (p=0.053).
Figure 1.
Kaplan-Meier Survival Curves for NVG after CRVO, Systemic Hypertension as a risk factor
Examination findings
The mean baseline visual acuity (Snellen equivalent) on presentation for the entire cohort was 20/170. For the non-NVG group, the mean initial VA was 20/160, while those who developed NVG had a mean VA of 20/275. There was an increased risk of NVG for every 0.5 logMAR visual acuity worse a patient presented with (hazard ratio [HR], 1.70; 95% confidence interval [CI], 1.04-2.77; p=0.034). Of the 25 eyes with an initial VA 20/50 or better, only 1 eye had NVG (representing 4% of eyes with initial VA of 20/50 or better and 7.7% of the 13 eyes with NVG) versus 24 eyes that did not (representing 96% of eyes with initial VA of 20/50 and 28.2% of the 85 eyes without NVG).
The mean IOP on presentation for the entire cohort was 17.0± 4.1 mmHg. For those who did not develop NVG, the mean IOP was 16.7± 3.8 mmHg, while the mean IOP on presentation for those who developed NVG was 18.4± 5.4 mmHg. Patients presenting with elevated IOP were not found to be at increased risk of developing NVG (p=0.090).
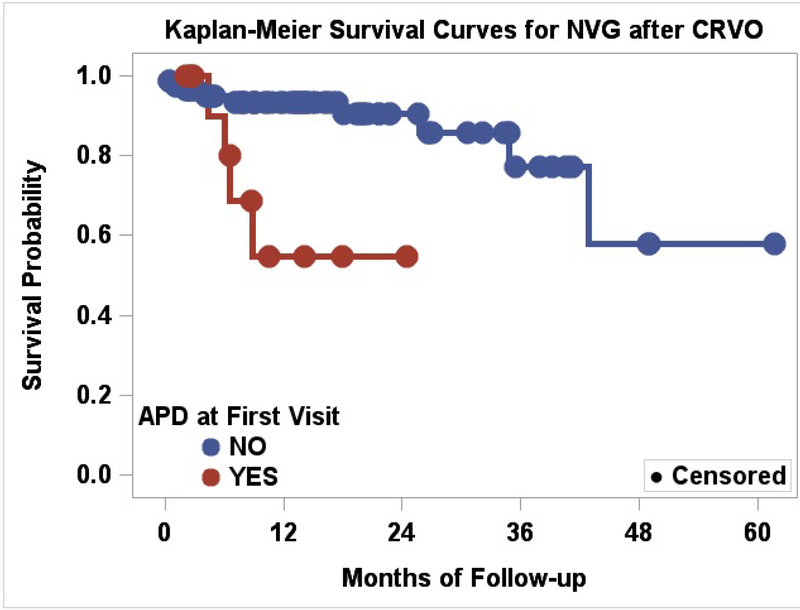
The risk of developing NVG was significantly greater in patients who had an initial RAPD (p=0.002) (Figure 2). Patients presenting with an RAPD in the setting of an acute CRVO had an elevated hazard ratio of 6.04 and relative risk increase of 2.15. Table 2 and 3.
Figure 2.
Kaplan-Meier Survival Curves for NVG after CRVO, Relative Afferent Pupillary Defect as a risk factor
Table 3:
Continuous Variables for Risk of Neovascular Glaucoma
| Continuous Variables |
HR units | Cox Regression p - value |
Hazard Ratio (HR) |
HR 95% CI Low |
HR 95% CI High |
|
|---|---|---|---|---|---|---|
| BMI | lower | 5 units | 0.3790 | 0.69 | 0.31 | 1.57 |
| Weight | lower | 10 pounds | 0.3427 | 0.91 | 0.75 | 1.11 |
| Height | lower | 3 inches | 0.7090 | 0.90 | 0.51 | 1.58 |
| Diastolic blood pressure | lower | 5 mmHg | 0.7934 | 0.97 | 0.74 | 1.26 |
| Systolic blood pressure | lower | 5 mmHg | 0.4680 | 0.95 | 0.82 | 1.10 |
| Age at First Visit | higher | 10 years | 0.1333 | 1.35 | 0.91 | 2.00 |
| IOP at first visit | higher | 5 mmHg | 0.0901 | 1.75 | 0.92 | 3.35 |
| LogMAR VA at first visit | higher (worse) | 0.5 units | 0.0342 * | 1.70 | 1.04 | 2.77 |
| OCT thickness at first visit | lower | 250 μm | 0.8365 | 1.09 | 0.50 | 2.37 |
p < 0.05
CI = confidence interval. HR=Hazard Ratio
Macular edema and OCT findings
Sixty seven patients (68%) had macular edema on initial presentation either by clinical exam (n=14) or OCT imaging (n=53). The presence of macular edema on presentation was not significantly associated with future development of NVG (p=0.5985). Among patients who received an OCT on presentation (n=54), the mean central retinal thickness was 632± 238 μm. When subdivided into NVG and non-NVG patients, mean central retinal thickness was 632± 221 μm and 632± 335 μm respectively. Increased central retinal thickness on presentation was not found to be a significant risk factor (p=0.8365).
Discussion
Comparison of the incidence of neovascular glaucoma in our study versus other studies is difficult given the variability of methods and inclusion criteria among studies. 6,7,11,12 In studies where the population was limited to ischemic CRVOs, the incidence of NVG was 22-50%,6,7,9,12-14 and in studies where ischemic status was undefined, the incidence of NVG was 5-21%.3,6,15,16 We included all CRVO subtypes presenting within 3 months of symptom onset with a NVG incidence rate of 13%.
We determined that systemic hypertension was associated with NVG; this has not been described in previous studies.7,12,16 One possible explanation may be chronic changes in the retinal arteriolar vasculature from hypertensive retinopathy.17 With these changes, the retinal vasculature would be less likely to adapt to acute ischemic events, such as a CRVO, thereby increasing the risk of neovascularization.11,16 In our series, none of the patients younger than 50 years of age developed NVG. Those with younger age may have a more robust and healthy vasculature, protecting them from the effects of retinal ischemia. If this hypothesis of chronic damage to vasculature were true however, then one would expect patients with diabetes and especially those with severe non-proliferative or proliferative diabetic retinopathy to be at equal if not higher risk for NVG. We did not observe this association, although the power to detect this was low, so this explanation does not fully account for the association between systemic hypertension and NVG.
Certain clinical signs such as worse VA and RAPD on CRVO presentation are associated with increased risk of NVG. Notably from our results, it is difficult to place an upper VA threshold on which to base NVG risk. A previous study reported that VA better than 20/400 was indicative of a non-ischemic CRVO, with these eyes less likely to develop NVG.2 However, approximately half of our patients (6/13) who developed NVG presented with VA better than 20/400. We found that of the 25 eyes with an initial VA of 20/50 or better, only 1 eye developed NVG. This suggests that patients with a VA 20/50 or better are at a lower risk for NVG. Additionally, we found that for every 0.5 logMAR VA worse on presentation, there was a corresponding 1.7 times increased risk of developing NVG (Table 3). Our findings are consistent with the results of the CVOS group, where a 3-line VA change translated to a 1.7 times increased risk of developing NVI/NVA.12 Though not directly translatable to an absolute Snellen equivalent for clinical use, these results are still consistent with the previous findings that patients presenting with worse visual acuities have a higher risk of developing NVG.12 An RAPD has also been strongly correlated with ischemic CRVO.2,9,18 We identified RAPD to be the factor with the greatest hazard ratio (HR = 6.04) for progression to NVG.
Additionally, the relative risk (RR 2.15) increase for eyes with a RAPD highlights that this finding should at least double the concern that the eye will develop NVG. We propose that a simple pupil exam be performed at each visit and if an RAPD is present, that this be given appropriate clinical consideration when determining follow-up intervals.
We did not determine an association between macular edema and risk of developing NVG. As shown in previous studies, presence of macular edema has not been found to be associated with ischemic CRVO.2,19 Although this finding was not a significant risk factor, we attempted to quantitatively compare central retinal thickness by OCT and its association with NVG. As central retinal thickness increased, we found no significant association with developing NVG, further supporting the observation that macular edema and NVG are independent, unrelated sequelae of CRVOs. Notably, no significant difference has been reported when central retinal thickness was measured using two different OCT instruments (Cirrus HD-OCT and Spectralis HRA+OCT) as was done in this present study.20
The highest risk for neovascular glaucoma conversion, conventionally known as “100 day glaucoma,”21-23 more accurately falls within the first 7 months of CRVO onset.2 In our study, the mean time to NVG onset was 421 days, significantly outside this window. However, the original CRVO natural history studies occurred before the era of anti-VEGF therapy, which has since changed the treatment paradigm for CRVO macular edema. By taking into account a patient’s last anti-VEGF injection, the mean adjusted time to NVG onset (from last anti-VEGF injection) was halved to 212 days, or around 7 months. The results of this “adjustment” highlight that anti-VEGF therapy may “reset the clock” to NVG onset, extending the risk beyond the 7 months. This extended timeline to NVG should be considered when following patients who receive serial injections. Mistakenly associating improved macular edema as a surrogate for decreased neovascular risk may lead to a false sense of security when extending follow-up visits. Instead, clinicians should realize that the highest risk for NVG conversion still persists into the 7 months following discontinuation of anti-VEGF therapy.
Despite anti-VEGF therapy for CRVO related macular edema, the value of this intervention in preventing neovascular glaucoma may be limited.9,24 We hypothesized that during the acute phase of a CRVO, a robust VEGF release secondary to retinal ischemia could be counter acted by anti-VEGF therapy, allowing time for new collateral vessel formation.25 However, we observed no difference in risk for patients who received an intravitreal anti-VEGF injection on presentation compared to those who did not. In light of these findings as well as those of the RAVE trial, these results indicate that despite our improved ability to calculate risk of NVG in patients with CRVO, our ability to prevent this complication remains inadequate. Despite these findings, we still recommend intravitreal anti-VEGF injection to the affected CRVO eye on presentation. Given the inherent subjectivity of CRVO symptom onset, this injection would standardize the CRVO eye to a known day 0, especially in cases when the subjective onset of symptoms is unclear. We realize that there may be some delay in developing macular edema after the actual occlusive event, and thus the true CRVO event may predate the date of vision loss. However, with a “day 0” date established, a follow-up schedule may be planned that minimizes risk for NVG conversion between interval visits. Additionally, serial anti-VEGF injections during the acute phase may allow for intraretinal hemorrhage to resolve, allowing for more reliable characterization of retinal ischemia by fluorescein angiography.
The presence of decreased vision, RAPD, or systemic hypertension should alert the clinician of a higher NVG conversion risk and the need for timely subspecialty referral. If a patient’s VA decreases or a new RAPD develops on a subsequent follow-up visit, conversion to an ischemic CRVO should be considered and a patient’s NVG risk should be re-evaluated with a series of closer follow-up intervals. Notably, although the presence of these factors portend a higher risk for conversion to NVG, the opposite does not necessarily hold true (i.e., the lack of RAPD does not decrease the risk of NVG compared to all eyes with CRVO). There was no appreciable decrease in NVG risk for patients in the absence of these risk factors. It should be stressed that these eyes should still be regularly followed and screened for neovascularization. Upon establishing care with a subspecialist, ancillary retinal testing such as FA, ERG, or visual fields may be used to further stratify a patient’s NVG risk. In patients with increased risk, therapies such as PRP may be considered, although this intervention is controversial and beyond the scope of this paper.1,4,26
Limitations to our study include the retrospective nature of the data set and the small sample size of NVG complications. The limited sample size, attributed to our stringent inclusion criteria, were carefully selected to limit any potential bias. We meticulously reviewed the complete medical records of 646 patients to generate our data set. We attempted to reduce the selection bias towards non-ischemic CRVOs by limiting our study to patients presenting within the first 90 days of symptom onset, thus eliminating patients with chronic CRVOs outside the range of greatest conversion to NVG.2 However, by eliminating chronic CRVOs, we eliminated from our sample set the 3-33% of eyes that would typically convert from a non-ischemic CRVO to ischemic CRVO.12,27,28 Given our limited sample size, a multivariate analysis could not be performed. As gonioscopy was not uniformly performed by clinicians each visit, patients with early NVG (NVA and normal IOP) could have been missed until they presented with an elevated IOP spike. This limitation would be expected to create a delayed time-to-NVG, but would not be expected to alter the underlying NVG risk factors. Another limitation is an assumption that patients not returning after three follow-up visits did not develop NVG. We attempted to limit this assumption by excluding any patient lost to follow-up after their initial visit. In addition, 80% of patients included in the data set resided in Miami-Dade County (where our institution is located). Given that our institute is the only tertiary care eye center in the region, we made an assumption that any patient who had already established care and had previously presented to our institute for acute CRVO, would return to our 24-hour emergency department if pain or sudden onset vision loss from neovascular glaucoma suddenly developed. This may not have been true of all patients.
Neovascular glaucoma is a complication of CRVO associated with poor visual outcomes. The use of OCT has become integral in the management of CRVO, but our results indicate that central retinal thickness measurement should not be used as a predictor for future risk of NVG. Clinical factors that are easily attainable on presentation such as history of systemic hypertension, poor visual acuity, and RAPD can assist the clinician in rapidly stratifying a patient in terms of risk of NVG development. Such a classification would help identify those who require more frequent or earlier follow-up, and may benefit from further interventions such as pan-retinal photocoagulation or anti-VEGF therapy. Anti-VEGF injections, though effective in improving macular edema, may functionally delay the onset of NVG and should not be seen as a curative therapy in patients presenting with CRVO.
Acknowledgements:
The authors would like to acknowledge Jayanth Sridhar MD and Alison Bozung OD for their help with editing this paper.
Research supported by a NEI Core Center Grant P30 EY014801
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Authors do not have any financial or proprietary interest in products, methods, or material mentioned in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102(10):1434–1444. [PubMed] [Google Scholar]
- 2.Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228(3):201–217. [DOI] [PubMed] [Google Scholar]
- 3.Chan CK, Ip MS, Vanveldhuisen PC, et al. SCORE Study report #11: incidences of neovascular events in eyes with retinal vein occlusion. Ophthalmology. 2011;118(7):1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson TH. Central retinal vein occlusion: what's the story? Br J Ophthalmol. 1997;81(8):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol. 1994;42(3):109–132. [PubMed] [Google Scholar]
- 6.McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1113–1123 e1115. [DOI] [PubMed] [Google Scholar]
- 7.Hayreh SS, Zimmerman MB. Ocular neovascularization associated with central and hemicentral retinal vein occlusion. Retina. 2012;32(8):1553–1565. [DOI] [PubMed] [Google Scholar]
- 8.Laatikainen L, Kohner EM. Fluorescein angiography and its prognostic significance in central retinal vein occlusion. Br J Ophthalmol. 1976;60(6):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DM, Wykoff CC, Wong TP, et al. Ranibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trial. Retina. 2014;34(9):1728–1735. [DOI] [PubMed] [Google Scholar]
- 10.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. 2007;18(6):502–508. [DOI] [PubMed] [Google Scholar]
- 11.Zegarra H, Gutman FA, Conforto J. The natural course of central retinal vein occlusion. Ophthalmology. 1979;86(11):1931–1942. [DOI] [PubMed] [Google Scholar]
- 12.Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115(4):486–491. [DOI] [PubMed] [Google Scholar]
- 13.Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Mansouri MR. Surgical induction of chorioretinal venous anastomosis in ischaemic central retinal vein occlusion: a non-randomised controlled clinical trial. Br J Ophthalmol. 2005;89(1):64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90(5):488–506. [DOI] [PubMed] [Google Scholar]
- 15.Recchia FM, Carvalho-Recchia CA, Hassan TS. Clinical course of younger patients with central retinal vein occlusion. Arch Ophthalmol. 2004;122(3):317–321. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair SH, Gragoudas ES. Prognosis for rubeosis iridis following central retinal vein occlusion. Br J Ophthalmol. 1979;63(11):735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89(10):1132–1145. [DOI] [PubMed] [Google Scholar]
- 18.Servais GE, Thompson HS, Hayreh SS. Relative afferent pupillary defect in central retinal vein occlusion. Ophthalmology. 1986;93(3):301–303. [DOI] [PubMed] [Google Scholar]
- 19.Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology. 1996;103(4):551–560. [DOI] [PubMed] [Google Scholar]
- 20.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50(7):3432–3437. [DOI] [PubMed] [Google Scholar]
- 21.Smith JL. Unilateral glaucoma in carotid occlusive disease. JAMA. 1962;182(6):683–684. [DOI] [PubMed] [Google Scholar]
- 22.May DR, Klein ML, Peyman GA, Raichand M. Xenon arc panretinal photocoagulation for central retinal vein occlusion: a randomised prospective study. Br J Ophthalmol. 1979;63(11):725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inouye T A case of obstruction of the central vein and glaucoma; with remarks. Roy Lond Ophthalmic Hosp Rep. 1910;18(1):24–36. [Google Scholar]
- 24.Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124–1133 e1121. [DOI] [PubMed] [Google Scholar]
- 25.Fuller JJ, Mason JO 3rd, White MF Jr., McGwin G Jr., Emond TL, Feist RM. Retinochoroidal collateral veins protect against anterior segment neovascularization after central retinal vein occlusion. Arch Ophthalmol. 2003;121(3):332–336. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS, Klugman MR, Podhajsky P, Servais GE, Perkins ES. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion. A 10-year prospective study. Graefes Arch Clin Exp Ophthalmol. 1990;228(4):281–296. [DOI] [PubMed] [Google Scholar]
- 27.Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118(1):119–133 e111-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf S, Arend O, Bertram B, et al. Hemodilution therapy in central retinal vein occlusion. One-year results of a prospective randomized study. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):33–39. [DOI] [PubMed] [Google Scholar]