Abstract
Aims
The aim of this study was to assess the burden of heart failure (HF) patients with/without iron deficiency/iron deficiency anaemia (ID/A) from the health insurance perspective.
Methods and results
We conducted a retrospective claims database analysis using the Institut für angewandte Gesundheitsforschung Berlin research database. The study period spanned from 1 January 2012 to 31 December 2014. HF patients were identified by International Statistical Classification of Diseases and Related Health Problems, 10th revision, German Modification codes (I50.‐, I50.0‐, I50.00, I50.01, I50.1‐, I50.11, I50.12, I50.13, I50.14, I50.19, and I50.9). HF patients were stratified into HF patients without ID/A and HF patients with ID/A (D50.‐, D50.0, D50.8, D50.9, and E61.1). HF patients with ID/A were stratified into three subgroups: no iron treatment, oral iron treatment, and intravenous iron treatment. A matching approach was applied to compare outcomes for HF patients without ID/A vs. HF patient with untreated incident ID/A without iron treatment and for HF patients receiving no iron treatment vs. oral iron treatment vs. intravenous iron treatment. Matching parameters included exact age, sex, and New York Heart Association functional class. An optimization algorithm was used to balance total health care costs in the baseline period for the potential matched pairs without sample size reduction. In total, 172 394 (4537.4 per 100 000) HF patients were identified in the Institut für angewandte Gesundheitsforschung Berlin research database in 2013. Of these, 11.1% (19 070; 501.9 per 100 000) were diagnosed with ID/A and/or had a prescription for iron medication in 2013. The mean age of HF patients was 77.0 years (±12.0 years). Women were more frequently diagnosed with HF (54.6%). HF patients with untreated incident ID/A (1.77%) had a significantly higher all‐cause mortality than HF patients without ID/A (33.1% vs. 24.1%, P < 0.01). The analysis of health care utilization revealed significant differences in the rate of all‐cause hospitalization (72.9% vs. 50.5%, P < 0.01). The annual health care costs for HF patients with untreated incident ID/A amounted to €17 347 with incremental costs of €849 (P < 0.01) attributed to ID/A.
Conclusions
Heart failure is associated with a major burden for patients and the health care system in terms of health care resource utilization, costs, and mortality. Our findings suggest that there is an unmet need for treating more HF patients with ID/A with iron medication.
Keywords: Claims data, Heart failure, Iron deficiency anaemia, Statutory health insurance, Cost of illness
Introduction
Heart failure (HF) is a clinical syndrome presenting with typical symptoms like breathlessness, ankle swelling, and fatigue. These symptoms might be accompanied by signs of elevated jugular venous pressure, pulmonary crackles, and peripheral oedema. HF is caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or an elevated intracardiac pressure when the patients are at rest or during phases of stress.1 HF appears in different stages that are classified according to the New York Heart Association (NYHA) into four functional classes NYHA I to NYHA IV describing the severity of symptoms and exercise tolerance.2 Approximately 800 000 to 1 600 000 individuals of the German population are affected by HF3 with a prevalence of 3956.3 per 100 000 and an incidence of 270–655 per 100 0004 and differ among federal states in Germany.5, 6 HF is associated with an increased mortality and was the third most common reason for death in Germany in 2015 with 47 414 cases and 5.1% of all deceased.7 Iron deficiency anaemia (ID/A) has been reported as a frequent co‐morbidity in chronic HF in up to 50% and has been associated with impaired functional capacity, poor quality of life, and increased mortality,8, 9, 10, 11 whereas all‐cause anaemia alone has been reported in congestive HF patients in up to 17%.12 Treating HF patients with ID/A with intravenous iron supplement treatment improves symptoms, functional capacity, and quality of life.13, 14 For patients with systolic HF and ID receiving intravenous iron supplement treatment also a reduction of recurrent cardiovascular (CV) and HF‐related hospitalizations and all‐cause and CV mortality was reported.15
Nevertheless, little is known about the burden of ID/A in HF patients from a health care system/payer perspective. So far, health care utilization—in terms of hospitalizations and outpatient visits, as well as corresponding health care expenditures—has not been investigated for HF patients with and without ID/A, taking into account the broad spectrum of sectors in a health care system.
The aim of this study was to provide current information on the prevalence of HF in Germany and to further assess the incremental burden of ID/A in patients suffering from HF from the perspective of the statutory health insurance (SHI). Moreover, this study compared the effect of intravenous iron treatment vs. oral iron treatment or no iron treatment in HF patient suffering from ID/A.
Methods
Study design
We conducted a retrospective claims database analysis using the Institut für angewandte Gesundheitsforschung Berlin (InGef) research database (formerly known as Health Risk Institute research database) containing anonymized health care claims of approximately four million covered lives. The InGef research database is adjusted to the German population in terms of age and gender and is considered to be in good accordance with the German population for measures of morbidity, mortality, and drug usage.16 The study was conducted from the perspective of the SHI.
Data source
Legal basis/foundation
The InGef research database includes verified claims data of the participating insurance companies, which is originally used for reimbursement purposes. These claims data were used in this study for scientific research purposes in accordance with German Social Law.
Data protection
The InGef research database addresses all data protection regulations in Germany. All personal information of patients, physicians, and other health care providers is anonymized before the data are made available for research. Results will not be reported for groups with less than five patients.
Data flow
The InGef database consists of claims data from approximately 75 different health insurances corresponding to approximately two‐thirds of the overall number of health insurances in Germany. The InGef research database is an adjusted sample of the pooled claims data of the participating health insurances and covers approximately four million lives, structured to represent the German population in terms of age and gender as of 2013.
Study period
The study period encompasses the time frame from 1 January 2012 to 31 December 2014.
Study population
Heart failure patients were identified by using International Statistical Classification of Diseases and Related Health Problems, 10th revision, German Modification (ICD‐10‐GM) codes (Heart failure I50.‐, Right ventricular failure I50.0‐, Primary right ventricular failure I50.00, Secondary right ventricular failure I50.01, Left ventricular failure I50.1‐, Left ventricular failure, NYHA functional class I I50.11, Left ventricular failure, NYHA functional class III 50.12, Left ventricular failure, NYHA functional class III I50.13, Left ventricular failure, NYHA functional class IV I50.14, Left ventricular failure, unspecified I50.19, Heart failure, unspecified I50.9) in the inpatient (main or secondary diagnoses) and/or outpatient setting (verified diagnoses) from 1 January 2013 to 31 December 2013.
Subgroups
Heart failure patients were stratified into HF patients without ID/A and HF patients with ID/A using ICD‐10‐GM codes [Iron deficiency anaemia D50.‐, Iron deficiency anaemia secondary to blood loss (chronic) D50.0, Other iron deficiency anaemias D50.8, Iron deficiency anaemia, unspecified D50.9, Iron deficiency E61.1] for ID/A and/or Anatomical Therapeutic Chemical Classification System (ATC) codes for prescribed iron medication (B03A) in the time frame from 1 January 2013 to 31 December 2013.
An index quarter was defined for each group. For HF patients without ID/A, the index quarter was defined by the first HF diagnosis in 2013. For HF patients with ID/A, the first quarter in 2013 with either both an HF diagnosis and an iron prescription or both an HF diagnosis and an ID/A diagnosis determined the index quarter.
To assure that HF patients without ID/A were not misclassified, they additionally were screened for ID/A codes in the four quarters before the index quarter and in the three quarters after the index quarter.
Furthermore, HF patients with ID/A were stratified into three subgroups: no iron treatment, oral iron treatment, and intravenous iron treatment. Oral iron treatment was identified by ATC codes B03AA and B03AB for oral preparations and B03AD and B03AE for oral combinations of iron and other agents. Intravenous iron treatment was identified by ATC codes B03AC for parenteral iron preparations.
To ensure that patients in the no iron treatment group were treatment naïve and were not treated with iron throughout the entire individual observation period, patients having a prescription of iron in the four quarters before the index quarter or starting an iron treatment in the three quarters after the index without a concurrent HF diagnosis were excluded from this group.
To be able to compare HF patients with oral iron treatment and intravenous iron treatment, only patients who were incident on iron treatment and did not switch the iron treatment were included in the analysis. Therefore, patients who either had a prescription of iron in the four quarters before the index quarter or who switched between oral and intravenous iron in the index quarter or the three subsequent quarters were excluded from these two groups.
Patients were described in terms of age and sex at the index quarter. They were stratified into NYHA functional classes using the highest NYHA functional class coded by ICD‐10‐GM in the index quarter. NYHA functional class I was identified by ICD‐10‐GM code I50.11, NYHA functional class II was identified by ICD‐10‐GM code I50.12, NYHA functional class III was identified by ICD‐10‐GM code I50.13, and NYHA functional class IV was identified by ICD‐10‐GM code I50.14. HF patients with no specific NYHA functional class code in 2013 (I50, I50.0, I50.00, I50.01, I50.1, I50.19, and I50.9) were classified as NYHA functional class N/A.
Matching
An exact direct 1:1 matching approach was used to compare HF patients without ID/A with HF patients with untreated incident ID/A. Matching parameters were age, sex, and NYHA functional class. Total health care costs in the four quarters before the index quarter (baseline period) were balanced using an optimization algorithm. In cases where there were various matching partners available for an HF patient with incident ID/A, the optimization algorithm chose the individual that in total overall matched pairs minimized the costs difference of total health care costs in the four quarters before the index quarter.
An exact direct 1:1:1 matching approach was used to compare HF patients with ID/A receiving no iron treatment with HF patients with ID/A who were treated with oral iron medication and with HF patients with ID/A who were treated with intravenous iron medication. Matching parameters were age classes of 5 years, sex, and NYHA functional class. Total health care costs in the four quarters before the index quarter were balanced using an optimization algorithm. In cases where there were various matching partners available, the optimization algorithm chose the individual that in total overall matched pairs minimized the costs difference of total health care costs in the four quarters before the index quarter (baseline period).
Matching balance was measured by the standardized difference with a threshold of 10%17, 18, 19, 20 indicating an imbalance of the matching parameters if the standardized difference exceeds the threshold.
Patients with the need of dialysis in the baseline period were excluded before the matching as dialysis‐dependent ID/A was not the subject of this study.
Mortality
All‐cause mortality was analysed in an individual 1 year time frame of four quarters including the index and the following three quarters. All‐cause mortality was described as an annual rate, and Kaplan–Meier estimators were used to determine time to death. Differences in the all‐cause mortality between the groups were tested with log‐rank tests for statistical significance. All‐cause mortality was defined as any reason for death as the utilized data source contains no explicit reason for death for each patient.
Outcomes
Health care utilization and costs were assessed in an individual 1 year time frame including the index quarter and the following three quarters. Hospitalizations were analysed in terms of all‐cause hospitalizations and HF‐related hospitalization, defined as hospitalizations with an HF diagnosis recorded as the main or secondary diagnosis. Outpatient visits were considered to be HF related if an HF diagnosis was coded as a verified diagnosis in the same quarter. Sick leave was analysed in relation to HF, defined as all cases of sick leave with an HF diagnosis, and overall. Health care costs were analysed in total and for the cost domains inpatient care, outpatient care, pharmaceuticals, devices and aids, and sick leave payments. Health care costs were separately analysed for HF patients deceasing in the 1 year time frame and those surviving it.
Results
Descriptive unadjusted analyses
Study population
The InGef research database encompassed 3 799 392 individuals who were continuously observable in the years 2012 to 2014 with exception of individuals who deceased in 2013 or 2014. This sample served as the basis of the analysis.
Prevalence
In total, 172 394 (4537.4 per 100 000) HF patients were identified in the InGef research database in 2013. Extrapolated to the German population, this would result in a total of approximately 3 653 691 individuals and 3 159 716 individuals for the SHI population.21, 22 Of these, 11.1% (19 070; 501.9 per 100 000) were also diagnosed with ID/A and/or had a prescription for iron medication in 2013. ID alone was documented in only 0.9% of the HF patients (1529 patients).
Demographics and New York Heart Association functional classes
The mean age of HF patients was 77.0 years (±12.0 years) for both sexes and all NYHA functional classes. Patients with NYHA functional class IV were on average older (79.3 ± 10.8 years) than patients with other NYHA functional classes (NYHA I: 72.2 ± 13.0 years, NYHA II: 75.1 ± 11.6 years, NYHA III: 77.7 ± 11.2 years, and NYHA N/A: 77.2 ± 12.2 years). Women with HF (54.6%) were on average older than male HF patients overall NYHA functional classes, ranging from 74.8 ± 12.8 years in NYHA functional class I to 82.0 ± 9.8 years in NYHA functional class IV. In comparison, men with HF had a mean age of 74.0 ± 12.0 years, ranging from 69.9 ± 12.7 years in NYHA functional class I to 76.5 ± 11.0 years in NYHA functional class IV.
The mean age of HF patients with ID/A was 79.1 ± 10.9 years in contrast to 76.7 ± 12.1 years for HF patients without ID/A. The share of female patients was 58.5% for the group of HF patients with ID/A and 54.1% for the group of HF patients without ID/A. Female HF patients were on average older in both groups, but the difference was notably smaller in the group of HF patients with ID/A (women: 80.5 ± 11.0 years and men: 77.2 ± 10.5 years) than in the group of HF patients without ID/A (women: 79.3 ± 11.4 years and men: 73.6 ± 12.2 years).
Heart failure patients with ID/A presented with NYHA functional classes I (3%), II (13%), III (19%), and IV (20%). Most of the patients were classified as NYHA functional class N/A (45%) due to NYHA functional class unspecific ICD‐10‐GM diagnoses in the index quarter. In contrast, HF patients without ID/A presented with NYHA functional classes I (5%), II (15%), III (13%), IV (9%), and NYHA functional class N/A (58%).
Mortality
Heart failure patients with ID/A had a higher all‐cause mortality (17.8% vs. 10.8%, χ2 test P < 0.01) (Figure 1 ) in general, whereas for HF patients with NYHA functional class IV, all‐cause mortality was higher in the group of HF patients without ID/A (36.4% vs. 34.7%, χ2 test P < 0.01).
Figure 1.
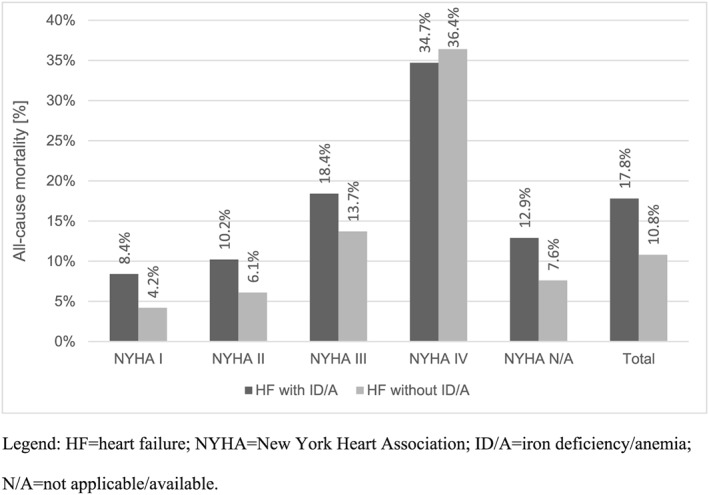
All‐cause mortality stratified by NYHA functional classes.
Iron treatment
The majority of HF patients with ID/A did not receive iron treatment (56.1%). Oral iron was administered in 37.9% and intravenous iron was administered in 6.0% of patients.
Health care utilization
The all‐cause hospitalization rate for HF patients with ID/A was 72.9% in contrast to 50.5% (χ2 test P < 0.01) for HF patients without ID/A. HF‐related hospitalization occurred in 50.4% of the HF patients with ID/A and in 31.1% (χ2 test P < 0.01) of the HF patients without ID/A. The average all‐cause hospitalization lasted 14.6 days for HF patients with ID/A and 14.0 days (t‐test P < 0.01) for HF patients without ID/A. The average length of stay per HF‐related hospitalization was 15.4 days for HF patients with ID/A compared with 13.6 days (t‐test P < 0.01) for HF patients without ID/A.
On average, HF patients with ID/A had 31.8 HF‐related outpatient visits compared with 19.3 (t‐test P < 0.01) in HF patients without ID/A. The most physician contacts were with a primary care physician (10.5 contacts), a nephrologist (6.1 contacts), or an internist working as primary care provider (3.9 contacts) for HF patients with ID/A. In the group of HF patients without ID/A, the most physician contacts were also with a primary care physician (7.8 contacts), an internist working as primary care provider (2.9 contacts), or a laboratory physician with 1.4 contacts, respectively.
Sick leave
A share of 5.3% of the HF patients without ID/A had at least one episode of sick leave during the study period. The proportion was 2.0% (χ2 test P < 0.01) in the group of HF patients with ID/A. HF‐related sick leave occurred in 0.7% of the HF patients without ID/A and in 0.3% (χ2 test P < 0.01) of the HF patients with ID/A.
Health care costs
The mean annual overall health care costs of the surviving patients amounted to €14 998 for HF patients with ID/A and amounted to €6916 (t‐test P < 0.01) for HF patients without ID/A. The cost driver in both groups was inpatient care. Mean annual overall health care costs of the deceasing HF patients were notably higher than those of the surviving HF patients. The main cost driver was also inpatient care. However, all other cost domains showed decreasing costs for the deceased HF patients (Table 1).
Table 1.
Annual mean health care costs per cost domain and in total—unmatched comparison
| HF patients with ID/A | HF patients without ID/A | |||
|---|---|---|---|---|
| Cost domain/group | Survivor | Deceased | Survivor | Deceased |
| Inpatient care | €8463 | €16 091 | €3946 | €12 088 |
| Outpatient care | €3082 | €1817 | €1075 | €721 |
| Pharmaceuticals | €2862 | €1717 | €1512 | €1224 |
| Devices and aids | €534 | €336 | €270 | €229 |
| Sick leave payments | €58 | €21 | €113 | €28 |
| Total | €14 998 | €19 983 | €6916 | €14 290 |
HF, heart failure; ID/A, iron deficiency/iron deficiency anaemia.
Matching analysis—incremental burden of iron deficiency anaemia
Matching heart failure patients without iron deficiency anaemia to heart failure patients with untreated incident iron deficiency anaemia
The exact matching using age, sex, and NYHA functional class resulted in 3048 HF patients without ID/A and 3048 HF patients with untreated incident ID/A. The standardized difference for baseline costs reached 3.5%, which is below the threshold of 10% as criteria for balanced comparison groups.
Demographics and New York Heart Association functional classes
The mean age of the matched cohorts of HF patients without ID/A and HF patients with untreated incident ID/A was 79.9 years (±10.1 years). More than half of the patients were women (58.1%). The proportion of the NYHA functional classes was NYHA I 2.9%, NYHA II 12.2%, NYHA III 20.1%, NYHA IV 20.1%, and NYHA N/A 44.6%.
Mortality
Heart failure patients with untreated incident ID/A had a significantly higher all‐cause mortality than HF patients without ID/A (33.1% vs. 24.1%, McNemar test P < 0.01). This was observed overall individual NYHA functional classes except for NYHA I where the same trend did not reach statistical significance (Table 2). When stratified by male and female patients, the all‐cause mortality rate was significantly higher for HF patients with untreated incident ID/A for the two NYHA functional classes III and IV for both sexes. Male patients had a higher all‐cause mortality than their female counterparts in both groups. However, the highest all‐cause mortality was observed in female HF patients with incident untreated ID/A and an NYHA functional class IV with 56.5% (Table 2).
Table 2.
All‐cause mortality rates for HF patients without ID/A and for HF patients with untreated incident ID/A—1:1 matched comparison
| NYHA (max. in 2013) | HF without ID/A | HF with untreated incident ID/A without iron treatment | McNemar test | |||
|---|---|---|---|---|---|---|
| n | % | n | % | P‐value | ||
| Number of deceased patients—male | NYHA N/A | 123 | 23.0 | 167 | 31.2 | <0.01 |
| NYHA I | 7 | 17.9 | 5 | 12.8 | 0.53 | |
| NYHA II | 19 | 10.9 | 46 | 26.3 | <0.01 | |
| NYHA III | 71 | 26.6 | 105 | 39.3 | <0.01 | |
| NYHA IV | 109 | 41.6 | 138 | 52.7 | 0.01 | |
| NYHA I–IV | 206 | 27.7 | 294 | 39.6 | <0.01 | |
| Total | 329 | 25.7 | 461 | 36.1 | <0.01 | |
| Number of deceased patients—female | NYHA N/A | 142 | 17.2 | 169 | 20.5 | 0.07 |
| NYHA I | <5 | 8.0 | 7 | 14.0 | 0.32 | |
| NYHA II | 36 | 18.4 | 45 | 23.0 | 0.24 | |
| NYHA III | 66 | 19.0 | 128 | 36.9 | <0.01 | |
| NYHA IV | 159 | 45.2 | 199 | 56.5 | <0.01 | |
| NYHA I–IV | 265 | 28.0 | 379 | 40.1 | <0.01 | |
| Total | 407 | 23.0 | 548 | 31.0 | <0.01 | |
| Number of deceased patients—both | NYHA N/A | 265 | 19.5 | 336 | 24.7 | <0.01 |
| NYHA I | 11 | 12.4 | 12 | 13.5 | 0.82 | |
| NYHA II | 55 | 14.8 | 91 | 24.5 | <0.01 | |
| NYHA III | 137 | 22.3 | 233 | 37.9 | <0.01 | |
| NYHA IV | 268 | 43.6 | 337 | 54.9 | <0.01 | |
| NYHA I–IV | 471 | 27.9 | 673 | 39.9 | <0.01 | |
| Total | 736 | 24.1 | 1.009 | 33.1 | <0.01 | |
HF, heart failure; ID/A, iron deficiency/iron deficiency anaemia; NYHA, New York Heart Association.
The time‐to‐death analysis also revealed significant differences (log‐rank test P < 0.01) between the two matched cohorts. Figure 2 shows the Kaplan–Meier curves for the overall population of HF patients without ID/A and those with HF and untreated incident ID/A. The stratification by NYHA functional classes also showed significant differences between the two matched cohorts for all NYHA functional classes except for NYHA I (Figure 2 ).
Figure 2.
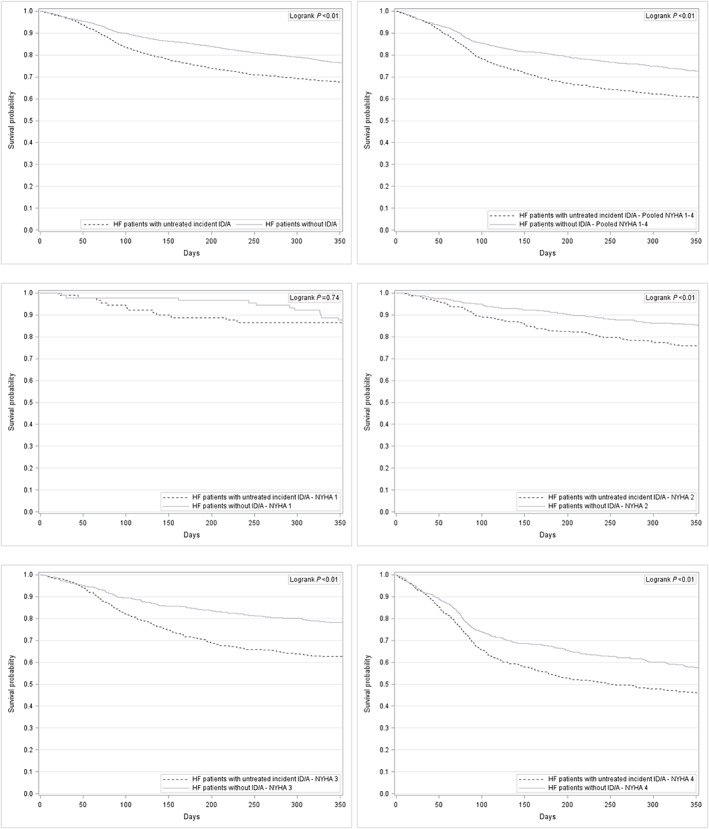
Kaplan–Meier curves for HF patients without ID/A and for HF patients with untreated incident ID/A—overall and stratified by NYHA functional class. HF, heart failure; ID/A, iron deficiency anaemia; NYHA, New York Heart Association.
Health care utilization
The analysis of health care utilization revealed significant differences in the rate of all‐cause hospitalized patients (HF patients without ID/A: 82.6%, HF patients with untreated incident ID/A: 83.8%, McNemar test P = 0.03). The rate of HF‐related hospitalized patients did not differ significantly between the two matched cohorts (HF patients without ID/A: 64.0%, HF patients with untreated incident ID/A: 63.0%, McNemar test P = 0.17). HF patients with untreated incident ID/A had significantly more all‐cause hospitalizations (mean 2.2, Wilcoxon signed rank test P < 0.01) and similar HF‐related hospitalizations (mean 1.2, Wilcoxon signed rank test P = 1) compared with HF without ID/A.
The average duration of an all‐cause hospitalization was 30.8 days for HF patients without ID/A and 31.6 days for HF patients with untreated incident ID/A (Wilcoxon signed rank test P = 0.04). HF‐related hospitalizations were of shorter duration in both groups (16.9 days for HF patients without ID/A and 17.8 days for HF patients with untreated incident ID/A) but significantly longer for HF patients with untreated incident ID/A (Wilcoxon signed rank test P = 0.01).
Health care costs
The analysis of annual all‐cause health care costs revealed incremental costs of €849 for the HF patients with untreated incident ID/A (Wilcoxon signed rank test P < 0.01) compared with HF patients without ID/A. In detail, HF patients with untreated incident ID/A surviving the study period had €385 higher total mean annual health care costs than HF patients without ID/A (Wilcoxon signed rank test P = 0.81), whereas the total mean annual health care costs of deceased HF patients were higher in HF patients without ID/A (Wilcoxon signed rank test P < 0.01). The cost drivers were the costs for inpatient care in both comparison groups and for all subgroups (Table 3).
Table 3.
Annual mean health care costs per cost domain and in total—1:1 matched comparison
| Cost domain/group | HF patients without ID/A | HF patients with untreated incident ID/A | ||||
|---|---|---|---|---|---|---|
| All patients | Survivor | Deceased | All patients | Survivor | Deceased | |
| Inpatient care | €12 420 | €10 245 | €19 251 | €13 833 | €10 932 | €19 696 |
| Outpatient care | €1102 | €1201 | €790 | €1063 | €1258 | €669 |
| Pharmaceuticals | €2391 | €2582 | €1792 | €1891 | €2217 | €1230 |
| Devices and aids | €472 | €543 | €247 | €496 | €613 | €258 |
| Sick leave payments | €114 | €140 | €34 | €65 | €75 | €44 |
| Total | €16 498 | €14 711 | €22 113 | €17 347 | €15 096 | €21 896 |
HF, heart failure; ID/A, iron deficiency/iron deficiency anaemia.
Matching analysis—comparison of treatment options for iron deficiency anaemia
Matching heart failure patients with iron deficiency anaemia receiving no iron treatment to heart failure patients with iron deficiency anaemia receiving oral iron treatment and to heart failure patients with iron deficiency anaemia receiving intravenous iron treatment
For the comparison of treatment options for ID/A, a total of 10 217 HF patients (5731 HF patients with ID/A receiving no iron treatment, 3870 HF patients with ID/A receiving oral iron treatment, and 616 HF patients with ID/A receiving intravenous iron treatment) were available in the database for the analysis. After the exclusion of dialysis‐dependent patients, 5645 HF patients with ID/A receiving no iron treatment, 3837 HF patients with ID/A receiving oral iron treatment, and 394 HF patients with ID/A receiving intravenous iron treatment remained in the comparison groups.
The exact match using age groups of 5 years, sex, and NYHA functional class resulted in 352 patients in each matched cohort. The groups were considered to be balanced with a standardized difference of 0% for sex and all NYHA functional classes. The standardized differences for exact age and baseline costs reached up to 1.4% and 4.92%, respectively, also indicating balanced matched cohorts.
Demographics and New York Heart Association classes
The majority of HF patients in the three matched cohorts was female with 58% of the population. The average age was 79.3 years (±8.7 years) for HF patients with ID/A receiving no iron treatment, 79.4 years (±8.6 years) for HF patients with ID/A receiving oral iron treatment, and 79.3 years (±8.5 years) for HF patients with ID/A receiving intravenous iron treatment. The proportion of NYHA functional classes was 1.4% NYHA I, 12.2% NYHA II, 16.8% NYHA III, 8.5% NYHA IV, and 61.1% NYHA N/A.
Mortality
A total of 23.6% of the HF patients with ID/A receiving no iron treatment deceased in the follow‐up period. HF patients with ID/A receiving oral iron treatment had a lower all‐cause mortality with 22.4%, and HF patients with ID/A receiving intravenous iron treatment had the lowest all‐cause mortality with 18.5%. Men had a higher risk of dying compared with women in all three matched cohorts with 27.9% vs. 20.5% for HF patients with ID/A receiving no iron treatment, 24.5% vs. 21.0% for HF patients with ID/A receiving oral iron treatment, and 21.1% vs. 16.6% for HF patients with ID/A receiving intravenous iron treatment (Table 4).
Table 4.
All‐cause mortality rates for HF patients with ID/A stratified by treatment option—1:1:1 matched comparison
| NYHA functional class | HF patients with ID/A receiving no iron treatment | HF patients with ID/A receiving oral iron treatment | HF patients with ID/A receiving intravenous iron treatment | No treatment vs. oral treatment | No treatment vs. intravenous treatment | Oral treatment vs. intravenous treatment | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | McNemar test | McNemar test | McNemar test | ||
| P‐value | P‐value | P‐value | ||||||||
| Number of deceased patients—male | NYHA N/A | 19 | 22.6 | 23 | 27.4 | 21 | 25.0 | 0.43 | 0.71 | 0.72 |
| NYHA I | — | — | — | — | — | — | — | — | — | |
| NYHA II | 6 | 28.6 | — | — | — | — | — | — | — | |
| NYHA III | 9 | 36.0 | — | — | 5 | 20.0 | — | 0.21 | — | |
| NYHA IV | 7 | 46.7 | 5 | 33.3 | 5 | 33.3 | 0.41 | 0.48 | 1.00 | |
| NYHA I–IV | 22 | 34.9 | 13 | 20.6 | 10 | 15.9 | 0.06 | 0.01 | 0.47 | |
| Total | 41 | 27.9 | 36 | 24.5 | 31 | 21.1 | 0.48 | 0.17 | 0.48 | |
| Number of deceased patients—female | NYHA N/A | 21 | 16.0 | 22 | 16.8 | 20 | 15.3 | 0.86 | 0.85 | 0.73 |
| NYHA I | — | — | — | — | — | — | — | — | — | |
| NYHA II | — | — | 5 | 22.7 | — | — | — | — | — | |
| NYHA III | 11 | 32.4 | 6 | 17.6 | — | — | 0.17 | — | — | |
| NYHA IV | 6 | 40.0 | 8 | 53.3 | 8 | 53.3 | 0.48 | 0.32 | 1.00 | |
| NYHA I–IV | 21 | 28.4 | 21 | 28.4 | 14 | 18.9 | 1.00 | 0.13 | 0.11 | |
| Total | 42 | 20.5 | 43 | 21.0 | 34 | 16.6 | 0.90 | 0.25 | 0.22 | |
| Number of deceased patients—both | NYHA N/A | 40 | 18.6 | 45 | 20.9 | 41 | 19.1 | 0.51 | 0.89 | 0.62 |
| NYHA I | — | — | — | — | — | — | — | — | — | |
| NYHA II | 10 | 23.3 | 9 | 20.9 | — | — | 0.76 | — | — | |
| NYHA III | 20 | 33.9 | 10 | 16.9 | 9 | 15.3 | 0.04 | 0.02 | 0.78 | |
| NYHA IV | 13 | 43.3 | 13 | 43.3 | 13 | 43.3 | 1.00 | 1.00 | 1.00 | |
| NYHA I–IV | 43 | 31.4 | 34 | 24.8 | 24 | 17.5 | 0.21 | 0.00 | 0.10 | |
| Total | 83 | 23.6 | 79 | 22.4 | 65 | 18.5 | 0.70 | 0.07 | 0.17 | |
HF, heart failure; ID/A, iron deficiency/iron deficiency anaemia; NYHA, New York Heart Association.
The time‐to‐death analysis revealed a significant advantage for HF patients with ID/A receiving intravenous iron treatment compared with HF patients with ID/A receiving no iron treatment in NYHA functional class II (log‐rank test P = 0.01), NYHA functional class III (log‐rank test P = 0.01), and pooled NYHA functional classes I–IV (log‐rank test P < 0.01), whereas oral iron treatment compared with no iron treatment only showed a significant advantage in NYHA functional class III (log‐rank test P = 0.02).
Direct comparison of oral iron treatment and intravenous iron treatment favoured intravenous iron treatment in NYHA functional class II patients (log‐rank test P = 0.02) and revealed the same trend for the pooled NYHA functional classes I–IV (log‐rank test P = 0.13). See Figure 3, 4, 5 for individual Kaplan–Meier curves for HF patients with ID/A stratified by treatment option and NYHA functional classes.
Figure 3.
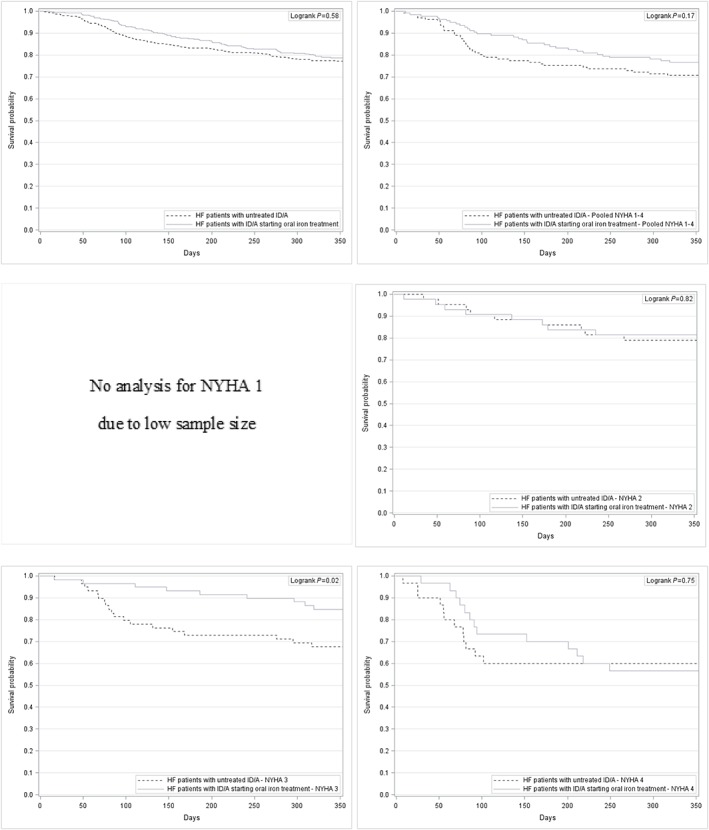
Kaplan–Meier curves for HF patients with untreated ID/A and for HF patients with ID/A starting oral iron treatment—overall and stratified by NYHA functional class. HF, heart failure; ID/A, iron deficiency anaemia; NYHA, New York Heart Association.
Figure 4.
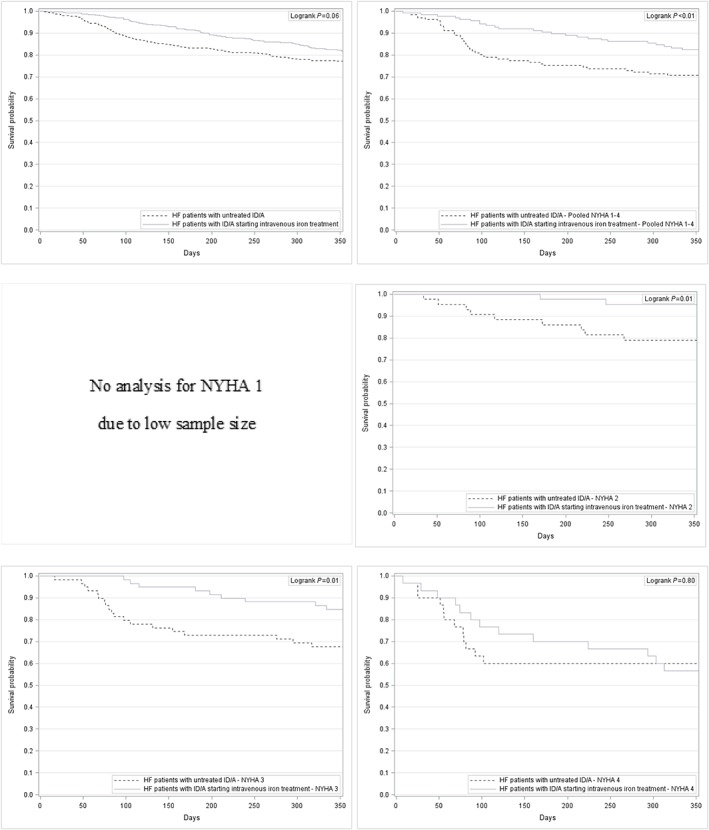
Kaplan–Meier curves for HF patients with untreated ID/A and for HF patients with ID/A starting intravenous iron treatment—overall and stratified by NYHA functional class. HF, heart failure; ID/A, iron deficiency anaemia; NYHA, New York Heart Association.
Figure 5.
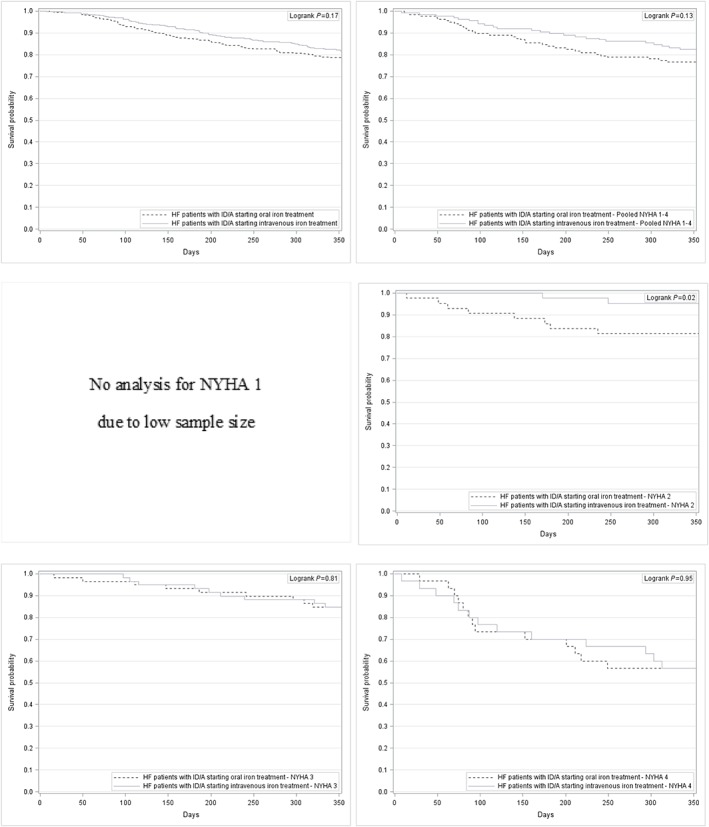
Kaplan–Meier curves for HF patients with ID/A starting oral iron treatment and for HF patients with ID/A starting intravenous iron treatment—overall and stratified by NYHA functional class. HF, heart failure; ID/A, iron deficiency anaemia; NYHA, New York Heart Association.
Health care utilization
Heart failure patients with ID/A receiving no iron treatment had the highest all‐cause hospitalization rate (78.1%) of the three matched cohorts compared with 76.4% for HF patients with ID/A receiving oral iron treatment and 70.7% for HF patients with ID/A receiving intravenous iron treatment. No significant difference was observed between the HF patients with ID/A receiving no iron treatment and those receiving oral iron treatment (McNemar test P = 0.32). In contrast, the all‐cause hospitalization rate of HF patients with ID/A receiving intravenous iron treatment differed significantly from those receiving no iron treatment (McNemar test P < 0.01) and those receiving oral iron treatment (McNemar test P < 0.01). More than half of the HF patients with ID/A receiving no iron treatment (53.7%) and of the HF patients with ID/A receiving oral iron treatment (51.4%) experienced HF‐related hospitalizations. HF patients with ID/A receiving intravenous iron treatment had a lower HF‐related hospitalization rate with 48.6% (χ2 test P < 0.01). HF‐related hospitalization rates increased with higher NYHA functional class from 31.2%–38.1% for NYHA functional class N/A up to 90.0%–93.3% for NYHA functional class IV.
Heart failure patients with ID/A receiving no iron treatment had on average 2.0 all‐cause hospitalizations and 0.9 HF‐related hospitalizations. HF patients with ID/A receiving oral or intravenous iron treatment had similar average hospitalizations (2.0 and 1.9 all‐cause hospitalizations; 0.9 and 0.9 HF‐related hospitalizations, respectively). The average duration of an all‐cause hospitalization was 30.1 days for HF patients with ID/A receiving no iron treatment, 26.6 days for HF patients with ID/A receiving oral iron treatment, and 24.9 days for HF patients with ID/A receiving intravenous iron treatment. HF‐related hospitalizations were of shorter duration in all three matched cohorts (14.0 days for HF patients with ID/A receiving no iron treatment, 13.0 days for HF patients with ID/A receiving oral iron treatment, and 12.0 days for HF patients with ID/A receiving intravenous iron treatment).
The main contact in the outpatient setting for HF patients with ID/A was the primary care physician with 13.5 visits for HF patients with ID/A receiving no iron treatment, 13.4 visits for HF patients with ID/A receiving oral iron treatment, and 13.9 visits for HF patients with ID/A receiving intravenous iron treatment. The second most contact was with an internist working as a primary care provider with 3.7, 4.7, and 5.9 visits, respectively. In contrast, a cardiologist was only seen 0.7, 0.9, and 1.4 times by the respective HF patient groups. All‐cause sick leave occurred in the group of HF patients with ID/A receiving no iron treatment with 2.6% of the HF patients having sick leave and in less than 1.4% for HF patients with ID/A receiving oral treatment and for HF patients with ID/A receiving intravenous iron treatment (less than five patients). The average length of sick leave in of HF patients with ID/A receiving no iron treatment was 2.3 days.
Health care costs
The comparison of all‐cause annual health care costs identified HF patients with ID/A receiving no iron treatment to have the highest mean costs (€15 144). The lowest all‐cause annual costs were observed in HF patients with ID/A receiving oral iron treatment (€14 130). The cost driver in all three matched cohorts was costs for inpatient care, accounting for 74.3% in HF patients with ID/A receiving no iron treatment, 71.3% in HF patients with ID/A receiving oral iron treatment, and 51.7% in HF patients with ID/A receiving intravenous iron treatment. The smaller share of costs for inpatient care in the intravenous iron group was compensated by a share of 33.9% of total costs for pharmaceuticals, which accounted in contrast for 14.5% in HF patients with ID/A receiving no iron treatment and for 16.2% in HF patients with ID/A receiving oral iron treatment. Deceased HF patients had 1.3, 1.3, and 1.4 times higher annual mean total health care costs (Table 5).
Table 5.
Annual mean health care costs per cost domain and in total—1:1:1 matched comparison
| HF patients with ID/A receiving no iron treatment | HF patients with ID/A receiving oral iron treatment | HF patients with ID/A receiving intravenous iron treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost domain/group | All patients | Survivor | Deceased | All patients | Survivor | Deceased | All patients | Survivor | Deceased |
| Inpatient care | €11 249 | €9734 | €16 160 | €10 078 | €8724 | €14 758 | €7806 | €6686 | €12 753 |
| Outpatient care | €1149 | €1253 | €811 | €1178 | €1278 | €830 | €1558 | €1636 | €1214 |
| Pharmaceuticals | €2193 | €2485 | €1247 | €2288 | €2564 | €1334 | €5114 | €5242 | €4548 |
| Devices and aids | €511 | €562 | €346 | €561 | €607 | €400 | €553 | €538 | €617 |
| Sick leave payments | €42 | €55 | €0 | €25 | €32 | €0 | €54 | €67 | €0 |
| Total | €15 144 | €14 089 | €18 564 | €14 130 | €13 206 | €17 321 | €15 085 | €14 169 | €19 131 |
HF, heart failure; ID/A, iron deficiency/iron deficiency anaemia.
Brief summary of main results
This study estimated the current prevalence of HF in Germany to be 4537.4 per 100 000 with a share of 11.1% also suffering from ID/A. HF was shown to be a disease of the elderly (mean age 77.0 ± 12.0 years). Most HF patients with ID/A did not receive iron treatment (56.1%).
Heart failure patients with untreated incident ID/A suffered from a significantly higher all‐cause mortality than matched HF patients without ID/A (33.1% vs. 24.1%, McNemar test P < 0.01), independently of NYHA functional class. The incremental health care costs of untreated incident ID/A were estimated at €849 with inpatient care being the cost driver.
The comparison of treatment options for ID/A revealed the highest all‐cause mortality for HF patients with untreated ID/A (23.6%), whereas patients receiving intravenous iron treatment had the lowest all‐cause mortality of 18.5% (McNemar test P = 0.07). A significant survival benefit was shown for the NYHA functional classes II (log‐rank test P = 0.01) and III (log‐rank test P = 0.01) as well as for the pooled NYHA functional classes I–IV (log‐rank test P < 0.01). For oral iron treatment, a significant survival benefit compared with no iron treatment was only shown for NYHA functional class III (log‐rank test P = 0.02). Total annual health care costs ranged from €14 130 for HF patients with ID/A receiving oral iron treatment to €15 144 for HF patients with ID/A receiving no iron treatment. The costs for inpatient care were the cost driver in all three cohorts.
Discussion
General results for prevalence and demographics
This study provides current data on the prevalence of HF in Germany (4537.4 per 100 000 individuals). Compared with DEGAM3 and Störk et al.4 (3956.3 per 100 000), our study shows that the prevalence of HF has significantly increased over the last years. It shows that about 11.1% of all HF patients are diagnosed with ID/A and that more than half of these HF patients (56.1%) receive no ID/A specific treatment. The proportion of HF patients diagnosed with ID/A is significantly lower than what is known from the literature with up to 50%. Moreover, the share of ID alone among HF patients was 32.5% as reported by Klip et al. whereas in our study, this proportion only reached 0.9%.11 This might be related to the findings from Wienbergen et al.,23 who reported that iron status is often not determined (37.8%) despite of European Society of Cardiology (ESC) guideline 2016 recommendation.1
Comparison results for heart failure patients without iron deficiency anaemia vs. heart failure patients with untreated incident iron deficiency anaemia
This study revealed that HF patients with untreated incident ID/A had a lower 1 year survival probability than HF patients without ID/A, irrespective of NYHA class. Similar implications are found in the literature that highlights the need to diagnose and treat ID/A among HF patients.9, 12, 24 Anker et al.13 and Ponikowski et al.14 showed that treating HF patients with ID/A with intravenous iron supplement (ferric carboxymaltose) improves symptoms, functional capacity, and quality of life. For patients with systolic HF and ID receiving intravenous iron supplement treatment also a reduction of recurrent CV and HF‐related hospitalizations and all‐cause and CV mortality was reported.15 From a cost perspective, the cost driver for the treatment of HF with and without ID/A is the inpatient costs. This was also shown by a review of Lesyuk et al. who identified 16 cost‐of‐illness studies from Germany, Greece, Ireland, Italy, Nigeria, Poland, South Korea, Spain, Sweden, and the USA. Most of the included studies found hospital admission to be the most expensive part of the total costs for HF patients. Total annual costs per HF patient were estimated lowest for South Korea with $868 and highest for Germany with $25 532.25 Out of the 16 studies, two studies reported annual costs for Germany. Zugck et al. reported annual costs ranging from €11 794 to €16 303 for 2002 that is similar to our findings of €16 498 for HF patients without ID/A and €17 347 for HF patients with untreated incident ID/A.26 Neumann et al. reported direct medical costs of €2879 for all HF patients in Germany in 2006.27 These costs are not comparable with our results as they refer to medical costs directly linked to HF treatment whereas our cost estimates include all health care expenditures of HF patients, as the focus of our study was to investigate the impact of ID/A in HF.
Comparison results for heart failure patients with iron deficiency anaemia and no evidence of iron treatment to heart failure patients with iron deficiency anaemia and oral iron treatment and to heart failure patients with iron deficiency anaemia and intravenous iron treatment
Heart failure patients with ID/A and no iron medication have the highest mortality rate in all study cohorts. HF patients with ID/A are mainly treated by a primary care physician and see a cardiologist on irregularly basis.
Recent studies have shown that treatment with intravenous iron can improve the functional capacity, symptoms, and quality of life in patients with HF and ID.13, 14, 28, 29 The use of oral iron failed to improve exercise capacity as was shown by Lewis et al.30
This study revealed that HF patients with untreated incident ID/A had a lower 1 year survival probability than HF patients without ID/A, irrespective of NYHA class. Additionally, the study showed that iron treatment is associated with an improved survival probability. This trend was particularly evident in HF patients with ID/A starting intravenous iron treatment. They had a significantly higher 1 year survival probability than untreated patients in most NYHA classes. Intravenous iron treatment was also associated with survival advantages when compared with oral iron treatment, especially among NYHA class II patients.
Internal validity and risk of bias
Between 45% and 61% of the HF patients in this study were classified as NYHA functional class N/A, meaning that they had no NYHA functional class specific ICD‐10‐GM code in the index quarter, but an ICD‐10‐GM diagnosis of I50, I50.0, I50.00, I50.01, I50.1, I50.19, and I50.9. An alternative algorithm or a more NYHA functional class specific coding practice might provide the opportunity to group these patients also into the four NYHA functional classes and thereby would enhance the statistical power of the results. Otherwise, it could also influence the results in the opposite direction.
German claims data contain no direct information about the reason of death. The present study therefore analysed the overall mortality of HF patients. Nevertheless, the data source provides sufficient information to analyse the overall mortality and the time to death as German claims data contain information on the status of an individual whether it is alive or dead and the corresponding insurance time. Moreover, the information recorded in the inpatient setting contains a reason for discharge from hospital that could be death and in combination with the end date of the inpatient stay provides the exact date of death. However, more disease specific information would be desirable to analyse disease specific mortality rates.
Strengths and weaknesses
This study has some weaknesses. First, German claims data contain no clinical information that is not necessary for the reimbursement purpose of the data collection. Therefore, the study had to rely on the information that is coded in the utilized coding systems namely the ICD‐10‐GM catalog. The ICD‐10‐GM catalog provides information about the NYHA functional classes I to IV but contains also non‐NYHA functional class specific codes. In cases where only these codes were recorded, no further clinical information was available to classify these HF patients into one of the NYHA functional groups.
Furthermore, the data source contains no information on the intention of the treating physicians. For a proportion of the 56.1% HF patients with ID/A, who received no iron treatment, the decision of not treating these patients with iron medication might have been an active and reasonable decision of the treating physician. Laboratory data to verify this assumption are not available in German claims data, but the degree of ID/A is supposed to have an impact on the treatment decision of the attending physician.
This study has some major strengths. First, the utilized data source allows to generalize the results of this study to a major part of the German population as approximately 85% of the German population are covered by the SHI. In contrast to registries and clinical trials where a selected population is investigated, this analysis might not be affected by a selection bias. Second, participants of the German SHI system benefit on nearly full coverage of all health care services. Only little copayments exist in the German SHI and these are furthermore limited to 2% of the annual income of the insured individuals (1% for chronically ill individuals) §62 SGB V. German claims data therefore provide not only the full picture of costs from the payer's perspective but also nearly the full picture of all direct health care costs and the corresponding health care utilization.
Generalizability
The InGef research database is based on claims data from the SHI system but is adjusted to the German overall population in terms of age and sex. This fact limits the generalizability of the result for the overall SHI population as the proportion of women is higher in this population than in the overall German population due to the fact that proportionately, more men choose a private health insurance in Germany. On the other hand, the generalizability of the results to the German population might be biased due to the fact that individuals with an annual income above a defined threshold (so called ‘Jahresarbeitsentgeltgrenze’) could choose a private health insurance instead of the SHI. These individuals tend to be healthier than the individuals that have to be insured by the SHI.31 Moreover, the prevalence of CV disease, for example, HF, shows regional differences among the federal states in Germany. The adjusted age and sex distribution of the InGef research database does not account for these regional differences.5
Limitations
In general, claims data analyses are subject to limitations, as they are primarily collected for accounting purposes, and therefore, clinical parameters are not covered. Furthermore, diagnoses in the outpatient setting are only recorded on a quarterly basis, which leads to an overestimation of HF‐related outpatient visits in this analysis as all visits within a quarter were considered as HF related if an HF diagnosis was recorded in that quarter. Another limitation of the study is due to the fact, that ‘over‐the‐counter’ (OTC) medication is not recorded in the database. The group of HF patients with ID/A and no iron treatment might be overestimated if patients buy OTC iron treatment on their own. From an analytical standpoint, our analysis therefore shows conservative results for this patient group as some of these patients might benefit from OTC iron treatment that was not captured in the comparison.
Conclusions
Heart failure is associated with a major burden for patients and the health care system in terms of health care resource utilization, costs, and mortality. ID/A is an independent predictor of unfavourable outcome, and a cost driver in HF patients and HF patients with ID/A has higher all‐cause mortality and causes higher health care utilization and costs. Compared with previous findings,9, 32 this claims database analysis suggests that ID/A is underdiagnosed in the German setting, which was also suggested elsewhere.23, 33 Especially ID alone is almost non‐existent according to our findings. The present study revealed that HF patients with ID/A are, on average, older and sicker in terms of NYHA classification, with a higher proportion of women than HF patients without ID/A. These findings are in line with evidence obtained in other observational studies.9, 12 In addition, the data suggest that ID/A in HF patients is undertreated and that intravenous iron is a rarely chosen treatment option in the German setting, despite clinical guidelines suggesting this treatment.1 The total health care costs of HF patients increase with the presence of ID/A. HF patients with ID/A receiving pharmaceutical treatment with iron medication present with lower inpatient costs compared with HF patients with untreated ID/A. The additional costs for iron medication are still compensated by lower costs for inpatient care.
Our findings suggest that there is an unmet need for treating more HF patients with ID/A with iron medication, resulting in costs savings for the SHI and reduced mortality for the affected HF patients. However, more research is needed to evaluate long‐time effects and to further investigate the cohort of undefined NYHA functional class.
Role of data owner
The data analysis was performed in cooperation between Xcenda GmbH and Elsevier Health Analytics.
Conflict of interest
I.B. and T.H. are employees of Vifor Pharma and Vifor Fresenius Medical Care Renal Pharma, Munich, Germany. The authors declare that no further conflict of interest exists.
Funding
This work was supported by Vifor Pharma and Vifor Fresenius Medical Care Renal Pharma, Munich, Germany.
Jacob C., Altevers J., Barck I., Hardt T., Braun S., and Greiner W. (2019) Retrospective analysis into differences in heart failure patients with and without iron deficiency or anaemia, ESC Heart Failure, 6, 840–855. 10.1002/ehf2.12485.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed. Boston, Mass: Little, Brown & Co; 1994. 253–256. [Google Scholar]
- 3. Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM) , Leitlinie Nr. 9: Herzinsuffizienz. Langfassung. Omikron Publ; 2006.
- 4. Störk S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, Hupfer S, Klebs S. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 2017; 106: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dornquast C, Kroll LE, Neuhauser HK, Willich SN, Reinhold T, Busch MA. Regional differences in the prevalence of cardiovascular disease. Dtsch Arztebl Int 2016; 113: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohlmeier C, Mikolajczyk R, Frick J, Prütz F, Haverkamp W, Garbe E. Incidence, prevalence and 1‐year all‐cause mortality of heart failure in Germany: a study based on electronic healthcare data of more than six million persons. Clin Res Cardiol 2015; 104: 688–696. [DOI] [PubMed] [Google Scholar]
- 7. Statistisches Bundesamt (Destatis) , Gesundheit Todesursachen in Deutschland 2015, Fachserie 12 Reihe 4, 2017.
- 8. Comin‐Colet J, Enjuanes C, Gonzalez G, Torrens A, Cladellas M, Merono O, Ribas N, Ruiz S, Gómez M, Verdú JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 10. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 11. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582. [DOI] [PubMed] [Google Scholar]
- 12. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes—insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation 2003; 107: 223–225. [DOI] [PubMed] [Google Scholar]
- 13. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P, Trial Investigators FAIR‐HF. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 16. Andersohn F, Walker J. Characteristics and external validity of the German health risk institute (HRI) database. Pharmacoepidemiol Drug Saf 2016; 25: 106–109. [DOI] [PubMed] [Google Scholar]
- 17. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007; 26: 734–753. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. Assessing balance in measured baseline covariates when using many‐to‐one matching on the propensity‐score. Pharmacoepidemiol Drug Saf 2008; 17: 1218–1225. [DOI] [PubMed] [Google Scholar]
- 19. Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat 1986; 40: 249–251. [Google Scholar]
- 20. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–398. [DOI] [PubMed] [Google Scholar]
- 21. Bundesministerium für Gesundheit , Gesetzliche Krankenversicherung, Kennzahlen und Faustformeln, Stand Januar 2018.
- 22. Statistisches Bundesamt , Bevölkerung und Erwerbstätigkeit, Bevölkerungsfortschreibung auf Grundlage des Zensus 2011, 2016.
- 23. Wienbergen H, Pfister O, Hochadel M, Michel S, Bruder O, Remppis BA, Maeder MT, Strasser R, von Scheidt W, Pauschinger M, Senges J, Hambrecht R, RAID‐HF (Registry Analysis of Iron Deficiency–Heart Failure) REGISTRY Study Group . Usefulness of iron deficiency correction in management of patients with heart failure [from the Registry Analysis of Iron Deficiency‐Heart Failure (RAID‐HF) registry]. Am J Cardiol 2016; 118: 1875–1880. [DOI] [PubMed] [Google Scholar]
- 24. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients: a systematic review and meta‐analysis. J Am Coll Cardiol 2008; 52: 818–827. [DOI] [PubMed] [Google Scholar]
- 25. Lesyuk W, Kriza C, Kolominsky‐Rabas P. Cost‐of‐illness studies in heart failure: a systematic review 2004‐2016. BMC Cardiovasc Disord 2018; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zugck C, Müller A, Helms TM, Wildau HJ, Becks T, Hacker J, Haag S, Goldhagen K, Schwab JO. Health economic impact of heart failure: an analysis of the nationwide German database. Dtsch Med Wochenschr 2010; 135: 633–638. [DOI] [PubMed] [Google Scholar]
- 27. Neumann T, Biermann J, Erbel R, Neumann A, Wasem J, Ertl G, Dietz R. Heart failure: the commonest reason for hospital admission in Germany: medical and economic perspectives. Dtsch Arztebl Int 2009; 106: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filippatos G, Farmakis D, Colet JC, Dickstein K, Luscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart Rothe B, Greenlaw N, Ford I, Ponikowski P, Anker SD. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. Eur J Heart Fail 2013; 15: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 30. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E, Heart Failure Clinical Research Network NHLBI. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grunow M, Nuscheler R. Public and private health insurance in Germany: the ignored risk selection problem. Health Econ 2014; 23: 670–687. [DOI] [PubMed] [Google Scholar]
- 32. Wexler D, Silverberg D, Sheps D, Blum M, Keren G, Iaina A, Schwartz D. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol 2004; 96: 79–87. [DOI] [PubMed] [Google Scholar]
- 33. von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, Dahm JB, Angermann CE. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP registry. Clin Res Cardiol 2017; 106: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]


