Abstract

HIV in the central nervous system (CNS) contributes to the development of HIV-associated neurological disorders (HAND), even with chronic antiretroviral therapy. In order for antiretroviral therapy to be effective in protecting the CNS, these drugs should have the ability to localize in brain areas known to be affected by HIV. Consequently, this study aimed to investigate the localization patterns of three first-line antiretroviral drugs, namely, efavirenz, tenofovir, and emtricitabine, in the rat brain. Liquid chromatography–tandem mass spectrometry (LC–MS/MS) and matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) were utilized to assess the pharmacokinetics and brain spatial distribution of the three drugs. Each drug was administered (50 mg/kg) to healthy female Sprague–Dawley rats via intraperitoneal administration. LC–MS/MS results showed that all three drugs could be delivered into the brain, although they varied in blood–brain barrier permeability. MALDI-MSI showed a high degree of efavirenz localization across the entire brain, while tenofovir localized mainly in the cortex. Emtricitabine distributed heterogeneously mainly in the thalamus, corpus callosum, and hypothalamus. This study showed that efavirenz, tenofovir, and emtricitabine might be a potential drug combination antiretroviral therapy for CNS protection against HAND.
1. Introduction
The combination antiretroviral therapy (cART)1 has transformed human immunodeficiency virus acquired immunodeficiency syndrome (HIV/AIDS) from a deadly incurable disease to a well-managed chronic disease. This transition has granted the opportunity to focus on other severe complications associated with HIV, including renal, cardiovascular, and neurological diseases, among others, with HIV of the central nervous system (CNS) being the most severe. Atripla is a fixed-dose regimen containing one non-nucleoside reverse transcriptase inhibitor (efavirenz (EFV)) and two nucleoside reverse transcriptase inhibitors (tenofovir disoproxil fumarate (TFV-DF) and emtricitabine (FTC)).2 Atripla is recommended for the treatment of HIV type 1.
The CNS is considered a viral reservoir for HIV where the virus replicates independently from circulating drug levels.3 HIV invades the brain soon after infection and replicates rapidly leading to various neurological complications.4 The virus crosses the blood–brain barrier (BBB) via a mechanism known as the “Trojan horse” hypothesis. Briefly, infected monocyte-derived macrophages carry the virus into the CNS.5,6 The viral infection spreads further within the brain when the virus attacks the resident perivascular microglial cells,7 T-lymphocytes, and astrocytes.8 HIV often targets the neural and microglial cells by expressing chemokine receptors (e.g., C-X-C chemokine receptor type 4) responsible for the infection of brain cells.9 Consequently, the brain can serve as an important sanctuary for HIV to continuously replicate even after the systematic viral suppression has been achieved.
Despite the extensive use of cART, the treatment of HIV-associated neurological disorders (HAND)10 remains a challenge. Nearly half of HIV-infected individuals have developed different forms of HAND.7,11 These various forms of HAND include asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and the most severe form known as HIV-associated dementia (HAD), which primarily targets patients with low CD4 T-lymphocyte counts.12 The mechanisms underlying HAND are not fully understood; however, several studies suggest that most patients display extensive neurodegeneration in specific brain regions. Molecular imaging techniques were previously used to study the brain regions most affected by the virus including the subcortical regions (e.g., thalamus, basal ganglia, and hippocampus)13 and the cerebral cortex (CTX).14 The cART regimens undoubtedly reduce plasma viral load, yet their potential to treat HAND remains unknown.15
Antiretroviral drugs must penetrate through the BBB and reach the infected brain cells for effective viral suppression in the CNS.16 Therefore, it is essential to investigate the spatial distribution and localization of current antiretroviral drugs in different brain regions and within the HIV-infected cells.
The characterization of antiretroviral drug distribution in biological tissues has solely relied on quantifications from tissue homogenates using liquid chromatography–tandem mass spectrometry (LC–MS/MS) techniques. LC–MS/MS has been used to study the pharmacokinetics, tissue distribution, and metabolism of most antiretroviral drugs including EFV, FTC, TFV, and more.17−19 Mass spectrometry imaging (MSI) is a powerful technology used to map the distribution of drugs and their metabolites within tissue sections.20,21 The key advantages of using MSI include a label-free analysis approach, high resolution, and high throughput per single experiment. Matrix-assisted laser desorption ionization (MALDI) ionization is often used for MSI experiments among other ionization techniques.
In this study, LC–MS/MS was used to quantify EFV, FTC, and TFV in plasma and brain homogenate samples following intraperitoneal (IP) administration. Subsequently, MALDI-MSI was used to show the localization patterns of the three antiretroviral drugs in rat brain tissues. The results from this study provide information about the BBB permeability toward the three drugs and to further help understand their role in neuroprotection.
2. Results and Discussion
In this study, we developed an LC–MS/MS method to quantify three first-line antiretroviral drugs in rat plasma and brain homogenate samples. The optimized LC–MS/MS method was validated in accordance with the European Medicines Agency (EMA) Bioanalytical Method Validation Guidelines.22 Furthermore, we developed and optimized an MALDI-MSI method that was utilized to map the CNS exposure of the three antiretroviral drugs in rat brain tissues.
2.1. LC–MS/MS Method Development and Optimization
The precursor and product ions of the three target compounds and their internal standards were obtained by direct injection of each freshly prepared standard into the mass spectrometer operated in the MRM positive ion mode (Table 1). The proposed MS fragmentation pathways are shown in Figure S2A–C. In positive ionization mode, acidifying the mobile phase with FA was used to further enhance the detection of [M + H] + ions.23
Table 1. MRM Transitions for EFV, TFV, FTC, and Their Respective Internal Standards CBB, ADV, and ZDV.
| analyte | MRM transitions (m/z) |
|---|---|
| EFV | 316.500 → 298.468 |
| TFV | 288.152 → 270.012 |
| FTC | 248.401 → 130.072 |
| CBBa | 284.047 → 135.079 |
| ADVb | 502.025 → 442.189 |
| ZDVc | 268.103 → 127.093 |
Internal standard for EFV.
Internal standard for TFV.
Internal standard for FTC.
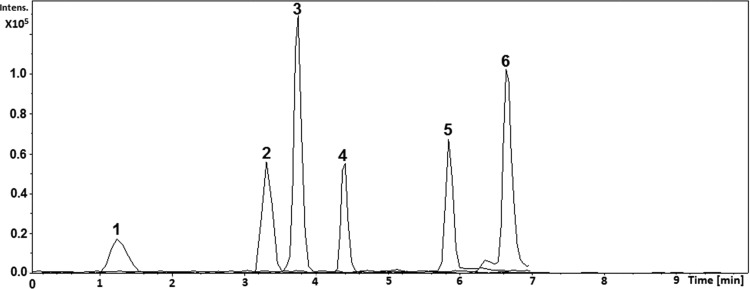
Chromatographic conditions were assessed, including the LC column selection, column temperature, mobile phase selection, and the flow rate. The polar nature of FTC and TFV resulted in poor retention in several commercially available chromatography columns tested. An Ascentis Express Amide analytical column (5 cm × 2.1 mm, a particle size of 2.7 μm, and pore size of 90 Å) resulted in satisfactory separation compared to other columns tested when operated at room temperature. Acetonitrile and methanol were tested with different acid additives such as formic acid and acetic acid in varying strengths. Methanol (0.1% v/v FA) and ultrapure water (0.1% v/v FA) resulted in better sensitivity, efficiency, and peak shape for all analytes. After several trial runs starting from 5, 10, 20, 30, 40, 50, and 60% methanol, we observed that starting the LC method at 30% methanol resulted in all analytes being separated and retained in a shorter runtime of 10 min. The LC–MS/MS method explained above was validated following procedures described in our previous study.24 Chromatogram and validation parameters are shown in Figure 1. The calculated LOD and LLOQ values are shown in Table 2.
Figure 1.
MRM chromatogram: (1) TFV (tR: 1.3 min), (2) FTC (tR: 3.3 min), (3) ZDV (tR: 3.7 min), (4) ADV (tR: 4.4 min), (5) CBB (tR: 5.8 min), and (6) EFV (tR: 6.6 min).
Table 2. Limit of Detection (LOD) and Quantification (LLOQ) for Analysis of the Three Antiretroviral Drugs by LC–MS/MS.
| plasma |
brain |
|||
|---|---|---|---|---|
| drug | LOD (ng/mL) | LLOQ (ng/mL) | LOD (ng/g) | LLOQ (ng/g) |
| EFV | 5.0 | 15.0 | 1.0 | 5.0 |
| TFV | 5.0 | 20.0 | 0.5 | 2.5 |
| FTC | 5.5 | 10.0 | 5.0 | 10.0 |
2.2. Quantitative Analysis by LC–MS/MS
A validated LC–MS/MS method was utilized for the quantification of EFV, TFV, and FTC in rat plasma and brain homogenates following IP administration of 50 mg/kg each drug to healthy female SD rats. The method validation data is presented in the Supporting Information including extraction recoveries, matrix effect (Table S2), and accuracy and precision (Table S3A–C).
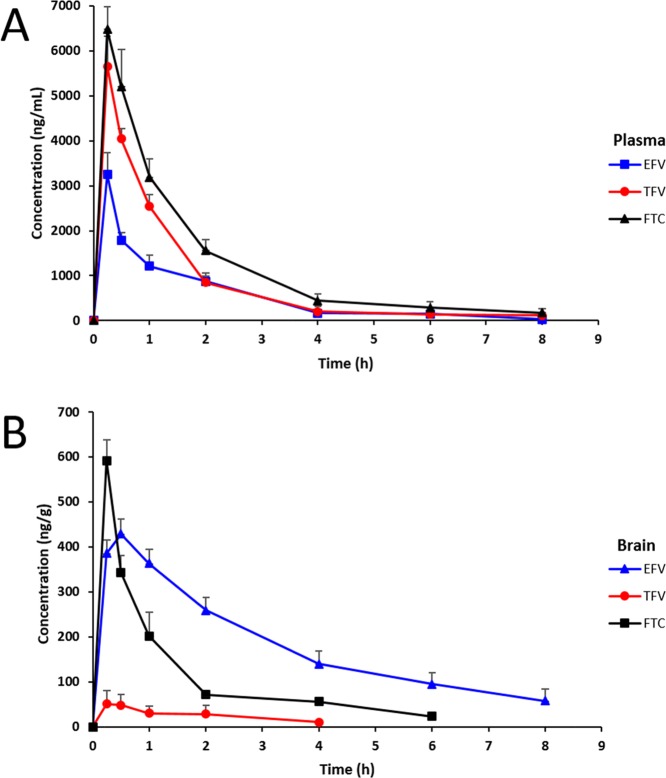
The plasma and brain concentration–time profiles are shown in Figure 2, with their corresponding pharmacokinetic parameters summarized in Table 3. The three drugs were detected from 0.25 to 8 h, while no traces of each drug were detected after 24 h. The mean highest concentrations reached (Cmax) for EFV, TFV, and FTC in plasma were 3246.07 ± 480.54, 5651.72 ± 672.87, and 6470.33 ± 500.57 ng/mL, respectively. All three drugs had plasma Cmax at a Tmax of 0.25 h. The terminal half-lives (t1/2) for EFV, TFV, and FTC in plasma were 0.6, 0.9, and 0.96 h, respectively. From the plasma concentration–time profile, the estimated area under the curve (AUC0–t) values for EFV, TFV, and FTC were 4255.38, 6807.64, and 9796.38 ng h/mL respectively.
Figure 2.
Concentration–time profiles of EFV, TFV, and FTC in plasma (A) and brain (B) homogenates following a single dose of 50 mg/kg to the rats via IP administration (n = 3, mean ± SEM).
Table 3. Pharmacokinetic Parameters of EFV, TFV, and FTC after a Single IP Administration to Healthy Female SD Rats.
| EFV |
TFV |
FTC |
||||
|---|---|---|---|---|---|---|
| parameter | plasma | brain | plasma | brain | plasma | brain |
| Cmax ± SEM (ng/mL or ng/g) | 3246.07 ± 480.54 | 428.54 ± 33.34 | 5651.72 ± 672.87 | 51.06 ± 29.23 | 6470.33 ± 500.57 | 591.57 ± 46.28 |
| Tmax (h) | 0.25 | 0.50 | 0.25 | 0.25 | 0.25 | 0.25 |
| t1/2 (h) | 0.60 | 2.80 | 0.90 | 1.89 | 0.96 | 0.65 |
| AUC0–t (ng h/mL or ng h/g) | 4255.38 | 1509.14 | 6807.64 | 538.50 | 9796.38 | 666.53 |
The quantitative analysis of homogenates of the dissected one-half rat brain hemisphere was performed to estimate absolute concentrations of each drug in the brain. EFV was only detected from 0.25 to 8 h. TFV was only detected from 0.25 to 4 h and FTC from 0.25 to 6 h, while no traces of each drug were detected after 24 h. The mean Cmax’s for EFV, TFV, and FTC reached in the brain were 428.54 ± 33.34, 51.06 ± 29.23, and 591.57 ± 46.28 ng/g, respectively. EFV had Cmax occurring at Tmax of 0.5 and 0.25 h for TFV and FTC, respectively. The t1/2’s for EFV, TFV, and FTC in the brain were 2.8, 1.89, and 0.65 h, respectively. The estimated brain AUC0–t values for EFV, TFV, and FTC were 1509.14, 538.50, and 666.53 ng h/g, respectively.
In the comparison of plasma to brain concentration levels, all three drugs had higher concentrations in plasma than in brain homogenates. In both plasma and brain homogenates, no drug was detected at 24 h post-dose. The plasma concentration levels were in the order of FTC > > TFV > EFV. Overall, the three drugs had a different BBB penetration affinity, with the brain concentration levels in the order of FTC ≫ EFV > TFV. These results are in accordance with previous studies, which have demonstrated that TFV has a low CNS penetration when compared to other antiretroviral drugs. Best et al. found that TFV concentrations in the cerebrospinal fluid (CSF) were only 5% of the total plasma concentrations.25 In our study, we found that TFV brain concentrations were 0.9% TFV of the plasma concentrations, which is even lower than the reported CSF percentage. Anthonypillai et al. demonstrated the ability of TFV to cross the blood-CSF barrier and reach the CSF but at low concentrations.26 The poor BBB permeability makes it difficult for TFV to reach the CNS. EFV was previously reported to have a high BBB penetration with the CSF unbound above the wild-type in vitro IC50 (0.51 ng/g) in lymphocytes, and CSF total concentrations exceeded this IC50 value by a ratio of 26.27 The IC50 values of 0.51, 11.5, and 1.73 ng/g were for EFV, TFV, and FTC, respectively.
Central nervous system penetration effectiveness (CPE) scores have been previously used to summarize and rank the CNS penetrability of various therapeutic drugs.28 The CPE scores are based on physiochemical pharmacodynamic and pharmacokinetic properties of drugs and CSF concentration from clinical studies.28 While these reported values reflect the extents of CNS penetration for different drugs, they do not convey any information regarding their concentration levels, distribution, and localization patterns in the brain.
The drug penetration through the BBB depends on various factors such as molecular weight, lipophilicity (measured as log P), plasma protein binding affinity, ionization, and membrane transporters including the influx and efflux transporters.29Table 4 shows the physiochemical and biopharmaceutical properties of EFV, TFV, and FTC. The influence of the molecular size on BBB penetration is inversely related to the square root of the molecular weight.29 The Lipinski rule states that the molecular weight should be less than 500 Da.1 Banks et al.29 proposed that the molecular weight of drugs should be less than 450 Da to easily penetrate the BBB.10 The three antiretroviral drugs have molecular weights less than 450 Da; therefore, other factors define their BBB penetration. Veber’s drug-like filter is another approach for predicting the BBB drug transit. Veber’s drug-like filter states that molecules, with a polar surface area less or equal to 140 Å2 and no more than 10 rotatable bonds, will have a better chance of crossing the biological barriers.31,32 From Table 4, it is clear that both EFV and FTC satisfy these criteria. Lipophilicity has commonly been used in the prediction of BBB penetration of drugs. A study by Honarparvar showed that lipophilic molecules are likely to partition into the lipid interior of membranes.32 They found that molecules with log P values from 1.5 to 2.7 have higher chances of penetration through the BBB,33 with an average log P value of 2.2.
Table 4. Physiochemical and Biopharmaceutical Properties of EFV, TFV, and FTC.
| physiochemical and biopharmaceutical properties |
|||||
|---|---|---|---|---|---|
| drug | molecular weight | log P | protein binding % | Å2 | rotatable bond count |
| EFV | 315.7 | 4.6 | 99.5 | 38.3 | 1.0 |
| TFV | 287.2 | –1.6 | <0.7 | 260.0 | 19.0 |
| FTC | 247.2 | 0.6 | <4.0 | 113.0 | 2.0 |
Even though it is anticipated that lipophilic drugs easily cross the BBB and enter the brain, this is not always the case, as other factors also govern the CNS deposition of drugs as shown by the variation in drug delivery observed in this study. The binding of drugs to plasma proteins can significantly reduce the free fraction of drugs in the system available for CNS absorption.34 The high protein binding of EFV35 can possibly explain the observation that FTC concentrations are higher than EFV in both plasma and brain, while EFV has a higher log P value of 4.6 than 0.6 for FTC (Table 4). FTC has a log P value of 0.6 and is considered to have low lipophilicity; however, the low protein binding of these drugs allows for increased levels available for CNS absorption. TFV is extremely non-lipophilic with a log P value of −1.6. Although the protein binding of TFV is low (<0.7),36 it suggests that lipophilicity is the main factor leading to low levels of TFV in the brain. Moreover, TFV does not comply with Veber’s drug-like filter (Table 4). Among the BBB transporters, multidrug-resistance-associated proteins (MRP4 and MRP5) are known to transport TFV from the brain to the bloodstream (strong efflux transporters).37 This could be another possible explanation for the poor BBB penetrability.
2.3. Spatial Distribution of Antiretroviral Drugs in the Rat Brain
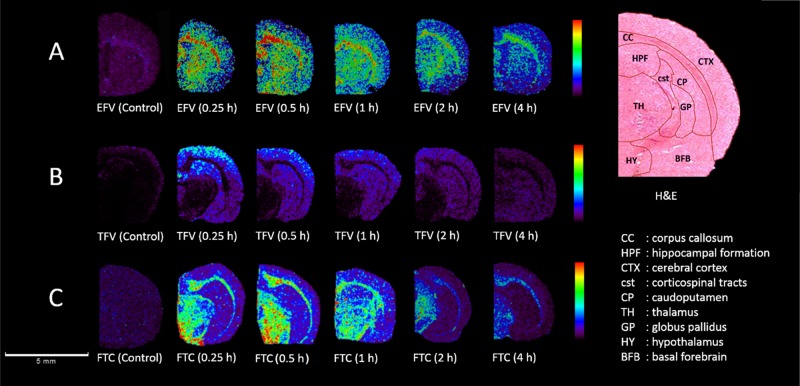
MALDI-MS was optimized for the detection of the three antiretroviral drugs in rat brain tissue sections in reflectron positive ion mode. For each drug, a protonated precursor ion was monitored for imaging purposes. The precursor ions for EFV, TFV, and FTC have m/z 316.673, m/z 288.121, and m/z 248.463, respectively (Figures S4–S6). A minimum of three brain sections was analyzed from each biopsy to confirm the reproducibility of the method. The estimated CHCA matrix applied to each slide had an average weight of 3.82 ± 0.57 mg (n = 3). The matrix application method was highly reproducible across all slides. Figure 3 shows the coronal rat brain images showing the localization patterns for EFV, TFV, and FTC at 0.25, 0.5, and 1 h post-dose.
Figure 3.
MALDI-MS images of coronal rat brain sections showing a time-dependent spatial distribution of three antiretroviral drugs. (A) Ion images for EFV (m/z 316.673) generated from 0 to 20% of maximum intensity, (B) for TFV (m/z 288.121) generated from 0 to 20% of maximum intensity, and (C) for FTC (m/z 248.463) generated from 0 to 50% of maximum intensity. Ion intensities for the three drugs were normalized against the TIC. Spatial resolution of 100 μm and a scale bar of 5 mm. The H&E staining with regional labels and the regions of interest (ROIs) were confirmed using the rat brain atlas.
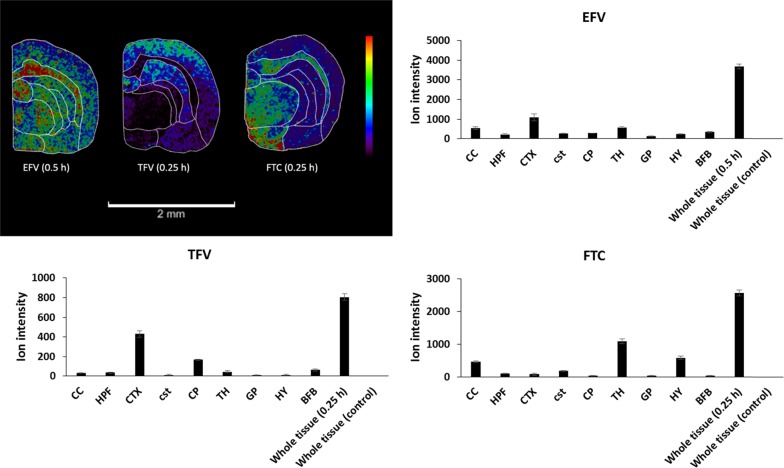
The images in Figure 3A clearly show that EFV is widespread throughout the brain; overall, the drug is highly distributed in the cerebral cortex (CTX) and corpus callosum (CC). EFV was also highly abundant in the basal forebrain (BFB), globus pallidus (GP), and hippocampal formation (HPF) after 0.5 h post-dose. Figure 3B shows that TFV localizes in a different way. At 0.25 h post-dose, high intensity is observed in the striatum in a caudoputamen (CP), corticospinal tracts (cst), GP, and high in the CTX. In contrast, Figure 3C shows a high degree of localization for FTC, mainly in the thalamus (TH), hypothalamus (HY), and CC. Figure 4 shows the relative abundance of the three drugs in various brain regions presented as bar graphs.
Figure 4.
Relative ion abundance of the three drugs in different brain regions. Images were obtained at a spatial resolution of 100 μm and a scale bar of 2 mm. The sum ion intensities (a.u.) are presented for each drug at 0.5 h post-dose for EFV (m/z 316.673) and 0.25 h post-dose for both TFV (m/z 288.121) and FTC (m/z 248.463).
Quantifying antiretroviral drugs in the CSF to measure of their potential to combat CNS-HIV is not enough. There are advanced imaging techniques such as magnetic resonance imaging, positron-emission tomography, MSI, and many others; however, not enough research has been done utilizing these techniques to visualize and understand the regional brain distribution of antiretroviral drugs. Several studies have shown that the presence of HIV in the brain leads to cortical atrophy resulting in different manifestations of HAND.14,38 Significant evidence suggests that the neurotic effects associated with HIV also originate from other brain regions including the basal ganglia located in the BFB,13 CC,39 and HPF.40 It has been documented that the cortical regions are mostly affected at later stages of the disease;14 however, Ragin et al. evaluated the occurrence and the extent of brain injury using quantitative magnetic resonance imaging in patients infected within a year. They observed reductions in both total and cortical gray matter.41 HAND is associated with various brain regions,13,14,38−41 and cART regimens are expected to cross the BBB and distribute homogeneously across the brain for improved viral suppression and better CNS protection. CTX is the main region often associated with HAND, and our results show that EFV and TFV reached this region with an exception of FTC.
Despite the benefits of using cART1,33 for treatment of HIV, HAND9,30 remain a threat to HIV-infected people. The brain is an HIV sanctuary where the virus replicates independently from the blood.3 To the best of our knowledge, there are no published studies on antiretroviral drug distribution in rat brain tissues or brain of any species for that matter.
3. Conclusions
We demonstrated that MALDI-MSI is a versatile platform to study the spatial distribution and localization of EFV, TFV, and FTC in situ. The three drugs reached the brain at different levels and localized in the same brain regions associated with HAND. The coadministration of these drugs could offer CNS protection against HAND to a certain extent. Furthermore, this work generally demonstrated a mass spectrometric approach that can be applied to directly detect and visualize these clinically important analytes more broadly in biological tissues in order to create an index that better represents drug penetration across the BBB. Future developments in MSI will enable imaging of these drugs at cellular and subcellular levels.
4. Experimental Section
4.1. Materials, Chemicals, and Reagents
TFV-DF, TFV, FTC, and EFV were purchased from Sigma-Aldrich, Mylan (South Africa), and Adcock Ingram (South Africa). The internal standards, namely, adefovir dipivoxil (ADV), zidovudine (ZDV), and 4-(4-carboxybenzyl)-2H-1,4-benzoxazin-3(4H)-one (CBB), were all purchased from Sigma-Aldrich. LC–MS-grade methanol and acetonitrile were purchased from Sigma-Aldrich. Analytical-grade formic acid (FA) was purchased from Merck Millipore (Merck, South Africa). The MALDI matrix, α-cyano-4-hydroxycinnamic acid (CHCA) was purchased from Bruker (Bremen, Germany). A MilliQ purification system (Bedford, MA) was used to generate ultrapure water. All other chemicals and reagents used in this study were of analytical grade.
4.2. Animals
Twenty-seven female Sprague–Dawley (SD) rats aged 4–5 weeks were supplied by the Biomedical Resource Unit (University of KwaZulu-Natal, Durban, South Africa). The 27 animals were randomly divided into nine groups (n = 3 for each group). All animals were housed under air-conditioned rooms at a constant temperature of 22 °C with a 12 h light/dark cycle and given access to water and food ad libitum. The protocols followed for the rat experiments were approved by the Institutional Animal Research Ethics Committee of the University of KwaZulu-Natal (Approval reference: AREC/007/017D).
4.2.1. Drug Administration and Sampling
Each rat received a single dose (50 mg/kg) of each drug prepared in 10% (v/v) aqueous solution of dimethyl sulfoxide via IP injection. Three rats were euthanized via halothane inhalation at each time point (0.25, 0.5, 1, 2, 4, 6, 8, 24 h). Blood samples were collected via the cardiac puncture technique, transferred into K3-EDTA-coated tubes, and centrifuged at 10000 × g for 10 min. Consequently, the plasma samples were collected and stored at −80 °C. After blood collection, animals were perfused with saline.42 Following perfusion, rat brain tissues were rapidly dissected after the termination, washed with saline to avoid any possible blood contamination, and snap-frozen in liquid nitrogen (−80 °C). All collected brains were stored at −80 °C until further treatment. Each frozen brain was cut into two hemispheres where one-half was allocated for MALDI-MSI and the other one was for LC–MS/MS analysis, respectively.
4.3. Sample Preparation for LC–MS/MS
4.3.1. Plasma Samples
5 μL of internal standard (500 ng/mL) was added to 100 μL of the plasma homogenate sample. The mixture was briefly vortexed, and 895 μL of methanol was added to extract target analytes while inducing the precipitation of proteins. The mixture was further vortexed for 30 s and centrifuged at 10000 × g for 10 min at 4 °C to remove the precipitate. The supernatant was filtered through a Supel-Select HLB (30 mg, 1 mL) (Supelco, Sigma, St. Louis, MO) solid-phase extraction (SPE) cartridge. The selection of SPE cartridges was based on analyte percentage recovery after filtration (Table S1). The extract was collected into autosampler vials and vortexed briefly before injecting 5 μL into the LC–MS/MS system.
4.3.2. Brain Samples
Half of each brain (hemisphere) was weighed and homogenized in three volumes of ultrapure water (3 mL/g tissue). The same extraction procedure used for plasma samples was followed for brain homogenate samples.
The quality control (QC) samples were prepared at different concentration levels (low, mid, and high) following the same protocol described above. Each QC sample was prepared by spiking the sample matrix (plasma or brain) with varying amounts of target compounds and constant amounts of their respective internal standards. The limit of detection (LOD) and the lower limit of quantification (LLOQ) were determined using signal-to-noise ratios of ≤3 and ≤5, respectively. The validation parameters including the extraction recovery, matrix effect, and accuracy and precision (intraday and interday) were assessed as previously described by Bratkowska et al.24
4.4. LC–MS/MS Analysis
Liquid chromatography (LC) was performed on a Thermo Scientific Dionex UltiMate 3000 instrument equipped with a binary solvent delivery system and an autosampler (Dionex Softron GmbH, Germany). Chromatographic separation was achieved by gradient elution at room temperature on a 5 cm Ascentis Express Amide analytical column with an internal diameter of 2.1 mm, particle size of 2.7 μm, and pore size of 90 Å. The mobile phases were ultrapure water with 0.1% v/v FA (A) and methanol with 0.1% v/v FA (B). The gradient LC method was initially increased from 30 to 100% B in 6.5 min. It was then held at 100% for 0.5 min before returning to the initial composition over 0.5 min. The injection volume was 5 μL, the flow rate was set at 0.35 mL/min, and the equilibration time was 2.5 min.
Mass spectrometric detection was performed on an Amazon speed ion trap (Bruker Daltonics, Bremen, Germany) with an electrospray ionization ion source. The detection of all the analytes was performed in positive ion mode where the [M + H] + ion was selected as a precursor ion. The multiple reaction monitoring (MRM) mode was selected to monitor the mass transitions (precursor ions → product ions) of EFV, TFV, FTC, and their internal standards (CBB, ADV, and ZDV). Other mass spectrometric conditions are as follows: nebulizer: 1.5 bar, dry gas: 1.8 L/min, dry temperature: 180 °C, scan range: m/z 50 → 600. Data were analyzed using Data Analysis 4.0 SP 5 and Quant Analysis 2.1 (Bruker Daltonics, Bremen, Germany).
4.5. Sample Preparation for MALDI-MS Imaging
A Leica Microsystems CM1100 (Wetzlar, Germany) cryostat was set at −20 °C and used for all the tissue sectioning. All brain sections (12 μm thickness) were sectioned at −1.13 mm anterior bregma. Once sliced, the sections were carefully thawed-mounted onto indium titanium oxide (ITO)-coated glass slides (Bruker Daltonics, Bremen, Germany). The glass slides were stored in a deep freezer at −80 °C until further sample treatment. The slides were removed from the freezer (prior analysis) and placed into a desiccator for 20 min at room temperature to avoid air water condensation on the sample surface. Optical images were acquired using an HP LaserJet 3055 all-in-one printer prior matrix application. The CHCA matrix solution was prepared at 7 mg/mL in 50% acetonitrile and 0.1% TFA. ImagePrep (Bruker Daltonics, Bremen, Germany) was used to uniformly deposit the matrix onto the slides following a standardized previously described method.23 Briefly, it is a five-cycle method, and each cycle is composed of three steps including spraying, incubation, and drying. Matrix deposition was in the form of an aerosol. The fine droplets (size of ∼20 μm) were generated using a vibrating spray generator. The CHCA matrix crystal size ranged from 5 to 15 μm with an average of 10 μm. The average total thickness of the matrix layer was 1.5 V. ImagePrep was operated at controlled conditions under nitrogen (N2). Each glass slide was weighed before and after matrix deposition to determine the total amount of matrix standard applied by difference.
4.6. MALDI-MS Image Acquisition
The acquisition of the mass spectra was achieved using an Autoflex III MALDI-time-of-flight (TOF) mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with a Smartbeam Nd:YAG laser (355 nm). The MALDI-MS method was optimized for the detection of the three compounds by spotting freshly prepared standards on a ground steel MALDI target plate and on an untreated brain tissue section. External calibration was performed using CHCA matrix ions and a Peptide Calibration Standard Kit II (Bruker Daltonics). Three batches of brain tissue sections were analyzed for each drug. All experiments were carried out in the full-scan reflectron positive mode within the mass range of m/z 100–550. The optimum laser power was kept at 65% for all the compounds. Each pixel was collected with 500 shots per spectrum in 10 random walk shot steps at a digitization rate of 0.5 GS s–1 and a spatial resolution of 100 μm. The LIFT MS/MS experiments were performed in brain tissues to further confirm each analyte, and the data is reported in Table S6.
4.7. Hematoxylin and Eosin Staining
After MALDI-MSI analysis, the matrix was removed with 70 and 100% EtOH, and the slides were stained with hematoxylin & eosin (H&E). The H&E images of the stained slides were generated using a scanner (Leica SCN 400, Leica Microsystems).
4.8. Image Processing
For detailed analysis, the raw MALDI-MS data was exported as an imzML file from FlexImaging 4.1 software (Bruker Daltonics, Bremen, Germany) and imported into MSIQuant analysis Lab software (Uppsala University, Sweden).43 MSIQuant was then used to process the data. All images were normalized against the total ion current (TIC). The relative intensities were adjusted for each analyte to improve the quality of images.
4.9. Statistical Analysis
Pharmacokinetics and statistical calculations were computed using Stata 13 (StataCorp, College Station, TX). Graphical presentation of the data was plotted using Microsoft Excel. All values were presented as mean ± standard error of the mean (SEM) from three independent experiments (n = 3).
Acknowledgments
This work was supported by the National Research Foundation, Medical Research Council, Aspen Pharmacare, and the University of KwaZulu-Natal, Durban. The authors wish to thank Patrik Källback and Per Andrén (Uppsala University, Sweden) for the MSIQuant software. The authors also wish to thank Annapurna Pamreddy for assisting with the animal experiments.
Glossary
Abbreviations
- (CNS)
Central nervous system
- (HAND)
HIV-associated neurological disorders
- (EFV)
Efavirenz
- (TFV)
Tenofovir
- (FTC)
Emtricitabine
- (LC–MS/MS)
Liquid chromatography–tandem mass spectrometry
- (MALDI-MSI)
Matrix-assisted laser desorption ionization mass spectrometry imaging
- (SD)
Sprague–Dawley
- (IP)
Intraperitoneal administration
- (BBB)
Blood–brain barrier
- (CTX)
Cortex
- (TH)
Thalamus
- (CC)
Corpus callosum
- (HY)
Hypothalamus
- (cART)
Combination antiretroviral therapy
- (HIV)
Human immunodeficiency virus
- (NNRTI)
Non-nucleoside reverse transcriptase inhibitor
- (NRTIs)
Nucleoside reverse transcriptase inhibitors
- (TFV-DF)
Tenofovir disoproxil fumarate
- (MSI)
Mass spectrometry imaging
- (MALDI)
Matrix-assisted laser desorption ionization
- (ADV)
Adefovir dipivoxil
- (ZDV)
Zidovudine
- (CBB)
4-(4-Carboxybenzyl)-2H-1,4-benzoxazin-3(4H)-one
- (CHCA)
α-Cyano-4-hydroxycinnamic acid
- (SPE)
Solid-phase extraction
- (CSF)
Cerebrospinal fluid
- (CPE)
Central nervous system penetration effectiveness
- (BFB)
Basal forebrain
- (GP)
Globus pallidus
- (HPF)
Hippocampal formation
- (cst)
Corticospinal tracts
- (ROIs)
Regions of interest
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b02582.
MS spectra; MS fragmentation pathways; sample extraction method development and optimization; LC–MS/MS method validation data; pharmacokinetics and brain distribution data MS imaging data (PDF)
Author Contributions
S.N., T.G., S.B., and S.M. designed the study. S.N. and S.M. performed all experiments. S.N., S.M., and S.B. wrote the manuscript. S.D.S., T.N., H.G.K., T.G., and S.B. supervised and acquired funding for this work. All authors have read and reviewed the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Levine A. M. Therapeutic approaches to neoplasms in AIDS. Rev. Infect. Dis. 1990, 12, 938–943. 10.1093/clinids/12.5.938. [DOI] [PubMed] [Google Scholar]
- Deeks E. D.; Perry C. M. Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate Single-Tablet Regimen (Atripla®). Drugs 2010, 70, 2315–2338. 10.2165/11203800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fois A. F.; Brew B. J. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr. HIV/AIDS. Rep. 2015, 12, 299–303. 10.1007/s11904-015-0257-9. [DOI] [PubMed] [Google Scholar]
- Davis L. E.; Hjelle B. L.; Miller V. E.; Palmer D. L.; Llewellyn A. L.; Merlin T. L.; Young S. A.; Mills R. G.; Wachsman W.; Wiley C. A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992, 42, 1736–1739. 10.1212/WNL.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Ghafouri M.; Amini S.; Khalili K.; Sawaya B. E. HIV-1 associated dementia: symptoms and causes. Retrovirology 2006, 3, 28–39. 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult B.; Chana G.; Masliah E.; Everall I. Neurobiology of HIV. Int. Rev. Psychiatry. 2008, 20, 3–13. 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Gaskill P. J.; Calderon T. M.; Luers A. J.; Eugenin E. A.; Javitch J. A.; Berman J. W. Human Immunodeficiency Virus (HIV) Infection of Human Macrophages Is Increased by Dopamine : A Bridge between HIV-Associated Neurologic Disorders and Drug Abuse. Am. J. Pathol. 2009, 175, 1148–1159. 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. H.; Henderson L.; Nath A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016, 14, 373–381. 10.2174/1570162X14666161006121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I.; Campbell L. A.; Harry G. J.; Avdoshina V. When Human Immunodeficiency Virus Meets Chemokines and Microglia: Neuroprotection or Neurodegeneration?. J. Neuroimmune Pharmacol. 2013, 8, 118–131. 10.1007/s11481-012-9353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R.; Brouwers P.; Spitzer A. R.; Grafman J.; Safai B.; Perno C.; Larson S.; Berg G.; Fischl M.; Wichman A.; Thomas R. Response of human-immunodeficiency-virus-associated neurological disease to 3′-azido-3′-deoxythymidine. Lancet 1987, 329, 132–135. 10.1016/S0140-6736(87)91968-4. [DOI] [PubMed] [Google Scholar]
- Rumbaugh J. A.; Tyor W. HIV-associated neurocognitive disorders: Five new things. Neurol. Clin. Pract. 2015, 5, 224–231. 10.1212/CPJ.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur J. C.; Steiner J.; Sacktor N.; Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010, 67, 699–714. 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Aylward E. H.; Henderer J. D.; McArthur J. C.; Brettschneider P. D.; Harris G. J.; Barta P. E.; Pearlson G. D. Reduced basal ganglia volume in HIV-1-associated dementia Results from quantitative neuroimaging. Neurology 1993, 43, 2099–2099. 10.1212/WNL.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Thompson P. M.; Dutton R. A.; Hayashi K. M.; Toga A. W.; Lopez O. L.; Aizenstein H. J.; Becker J. T. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4 T lymphocyte decline. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15647–15652. 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. K.; Clifford D. B.; Franklin D. R.; Woods S. P.; Ake C.; Vaida F.; Ellis R. J.; Letendre S. L.; Marcotte T. D.; Atkinson J. H.; Rivera-Mindt M.; Vigil O. R.; Taylor M. J.; Collier A. C.; Marra C. M.; Gelman B. B.; McArthur J. C.; Morgello S.; Simpson D. M.; McCutchan J. A.; Abramson I.; Gamst A.; Fennema-Notestine C.; Jernigan T. L.; Wong J.; Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 2010, 75, 2087–2096. 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf W. H. Blood-Brain Barrier Permeability to Drugs. Annu. Rev. Pharmacol. Toxicol. 1974, 14, 239–248. 10.1146/annurev.pa.14.040174.001323. [DOI] [Google Scholar]
- Huang J.; Gautam N.; Bathena S. P. R.; Roy U.; McMillan J.; Gendelman H. E.; Alnouti Y. UPLC–MS/MS quantification of nanoformulated ritonavir, indinavir, atazanavir, and efavirenz in mouse serum and tissues. J. Chromatogr., B 2011, 879, 2332–2338. 10.1016/j.jchromb.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft J. C.; McConnachie L. A.; Koehn J.; Kinman L.; Sun J.; Collier A. C.; Collins C.; Shen D. D.; Ho R. J. Y. Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation. J. Controlled Release 2018, 275, 229–241. 10.1016/j.jconrel.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathipati P. K.; Mandal S.; Destache C. J. Simultaneous quantification of tenofovir, emtricitabine, rilpivirine, elvitegravir and dolutegravir in mouse biological matrices by LC–MS/MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2016, 129, 473–481. 10.1016/j.jpba.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prideaux B.; Stoeckli M. Mass spectrometry imaging for drug distribution studies. J. Proteomics 2012, 75, 4999–5013. 10.1016/j.jprot.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Sun N.; Walch A. Qualitative and quantitative mass spectrometry imaging of drugs and metabolites in tissue at therapeutic levels. Histochem. Cell Biol. 2013, 140, 93–104. 10.1007/s00418-013-1127-4. [DOI] [PubMed] [Google Scholar]
- E.M.A . Guideline on bioanalytical method validation. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (accessed 15 March).
- Ntshangase S.; Shobo A.; Kruger H. G.; Asperger A.; Niemeyer D.; Arvidsson P. I.; Govender T.; Baijnath S. The downfall of TBA-354 – a possible explanation for its neurotoxicity via mass spectrometric imaging. Xenobiotica 2017, 48, 938–944. 10.1080/00498254.2017.1375168. [DOI] [PubMed] [Google Scholar]
- Bratkowska D.; Shobo A.; Singh S.; Bester L. A.; Kruger H. G.; Maguire G. E.; Govender T. Determination of the antitubercular drug PA-824 in rat plasma, lung and brain tissues by liquid chromatography tandem mass spectrometry: Application to a pharmacokinetic study. J. Chromatogr., B 2015, 988, 187–194. 10.1016/j.jchromb.2015.02.041. [DOI] [PubMed] [Google Scholar]
- Best B. M.; Letendre S. L.; Koopmans P.; Rossi S. S.; Clifford D. B.; Collier A. C.; Gelman B. B.; Marra C. M.; McArthur J. C.; McCutchan J. A.; Morgello S.; Simpson D. M.; Capparelli E. V.; Ellis R. J.; Grant I.; LOW CSF Concentrations of the Nucleotide HIV Reverse Transcriptase Inhibitor, Tenofovir. J. Acquir. Immune Defic. Syndr. 2012, 59, 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonypillai C.; Gibbs J. E.; Thomas S. A. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006, 3, 1–10. 10.1186/1743-8454-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best B. M.; Koopmans P. P.; Letendre S. L.; Capparelli E. V.; Rossi S. S.; Clifford D. B.; Collier A. C.; Gelman B. B.; Mbeo G.; McCutchan J. A.; Simpson D. M.; Haubrich R.; Ellis R.; Grant I.; Grant I.; McCutchan J. A.; Ellis R. J.; Marcotte T. D.; Franklin D.; Ellis R. J.; McCutchan J. A.; Alexander T.; Letendre S.; Capparelli E.; Heaton R. K.; Atkinson J. H.; Woods S. P.; Dawson M.; Wong J. K.; Fennema-Notestine C.; Taylor M. J.; Theilmann R.; Gamst A. C.; Cushman C.; Abramson I.; Vaida F.; Marcotte T. D.; von Jaeger R.; McArthur J.; Smith M.; Morgello S.; Simpson D.; Mintz L.; McCutchan J. A.; Toperoff W.; Collier A.; Marra C.; Jones T.; Gelman B.; Head E.; Clifford D.; Al-Lozi M.; Teshome M. Efavirenz concentrations in CSF exceed IC(50) for wild-type HIV. J. Antimicrob. Chemother. 2011, 66, 354–357. 10.1093/jac/dkq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld C.; Reichelt D.; Evers S.; Husstedt I. CSF Penetration by Antiretroviral Drugs. CNS Drugs 2013, 27, 31–55. 10.1007/s40263-012-0018-x. [DOI] [PubMed] [Google Scholar]
- Banks W. A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9, S3–S3. 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L.; diMarzo-Veronese F.; Schüpbach J.; Tourtellotte W. W.; Ho D. D.; Müller F.; Shapshak P.; Vogt M.; Groopman J. E.; Markham P. D. Intra-blood–brain-barrier synthesis of HTLV-III-specific IgG in patients with neurologic symptoms associated with AIDS or AIDS-related complex. N. Engl. J. Med. 1985, 313, 1498–1504. 10.1056/NEJM198512123132402. [DOI] [PubMed] [Google Scholar]
- Veber D. F.; Johnson S. R.; Cheng H.-Y.; Smith B. R.; Ward K. W.; Kopple K. D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- Honarparvar B.; Govender T.; Maguire G. E.; Soliman M. E.; Kruger H. G. Integrated Approach to Structure-Based Enzymatic Drug Design: Molecular Modeling, Spectroscopy, and Experimental Bioactivity. Chem. Rev. 2014, 114, 493–537. 10.1021/cr300314q. [DOI] [PubMed] [Google Scholar]
- Groopman J. E. The challenge of combination antiretroviral therapy for AIDS: An introduction. Am. J. Med. 1991, 90, S1. 10.1016/0002-9343(91)90403-K. [DOI] [PubMed] [Google Scholar]
- Smith Q. R.; Fisher C.; Allen D. D.. The Role of Plasma Protein Binding in Drug Delivery to Brain. In Blood-Brain Barrier: Drug Delivery and Brain Pathology, Kobiler D.; Lustig S.; Shapira S. Eds. Springer US: Boston, MA, 2001; pp 311–321. [Google Scholar]
- Boffito M.; Back D. J.; Blaschke T. F.; Rowland M.; Bertz R. J.; Gerber J. G.; Miller V. Protein Binding in Antiretroviral Therapies. AIDS Res. Hum. Retroviruses 2003, 19, 825–835. 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- Droste J. A. H.; Verweij-van Wissen C. P. W. G. M.; Kearney B. P.; Buffels R.; vanHorssen P. J.; Hekster Y. A.; Burger D. M. Pharmacokinetic Study of Tenofovir Disoproxil Fumarate Combined with Rifampin in Healthy Volunteers. Antimicrob. Agents Chemother. 2005, 49, 680–684. 10.1128/AAC.49.2.680-684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G.; Wielinga P.; Zelcer N.; de Haas M.; van Deemter L.; Wijnholds J.; Balzarini J.; Borst P. Characterization of the Transport of Nucleoside Analog Drugs by the Human Multidrug Resistance Proteins MRP4 and MRP5. Mol. Pharmacol. 2003, 63, 1094–1103. 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- Kallianpur K. J.; Kirk G. R.; Sailasuta N.; Valcour V.; Shiramizu B.; Nakamoto B. K.; Shikuma C. Regional Cortical Thinning Associated with Detectable Levels of HIV DNA. Cereb. Cortex 2012, 22, 2065–2075. 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. M.; Dutton R. A.; Hayashi K. M.; Lu A.; Lee S. E.; Lee J. Y.; Lopez O. L.; Aizenstein H. J.; Toga A. W.; Becker J. T. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. NeuroImage 2006, 31, 12–23. 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Masliah E.; Ge N.; Achim C. L.; Hansen L. A.; Wiley C. A. Selective Neuronal Vulnerability in HIV Encephalitis. J. Neuropathol. Exp. Neurol. 1992, 51, 585–593. 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Ragin A. B.; Du H.; Ochs R.; Wu Y.; Sammet C. L.; Shoukry A.; Epstein L. G. Structural brain alterations can be detected early in HIV infection. Neurology 2012, 79, 2328–2334. 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntshangase S.; Mdanda S.; Naicker T.; Kruger H. G.; Baijnath S.; Govender T. Spatial distribution of elvitegravir and tenofovir in rat brain tissue: Application of MALDI-MSI and LC-MS/MS. Rapid Commun. Mass Spectrom. 2019, 1643. 10.1002/rcm.8510. [DOI] [PubMed] [Google Scholar]
- Källback P.; Nilsson A.; Shariatgorji M.; Andrén P. E. msIQuant – Quantitation Software for Mass Spectrometry Imaging Enabling Fast Access, Visualization, and Analysis of Large Data Sets. Anal. Chem. 2016, 88, 4346–4353. 10.1021/acs.analchem.5b04603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.