Abstract
Anthracycline-based chemotherapy can result in the development of a cumulative and progressively developing cardiomyopathy. Doxorubicin (DOX) is one of the most highly prescribed anthracyclines in the USA, due to its broad spectrum of therapeutic efficacy. Interference with different mitochondrial processes is chief among the molecular and cellular determinants of DOX cardiotoxicity, contributing to the development of cardiomyopathy. The present review provides the basis for the involvement of mitochondrial toxicity in the different functional hallmarks of anthracycline toxicity. Our objective is to understand the molecular determinants of a progressive deterioration of functional integrity of mitochondria that establishes an historic record of past drug treatments (“mitochondrial memory”) and renders the cancer patient susceptible to subsequent regimens of drug therapy. We focus on the involvement of DOX-induced mitochondrial oxidative stress, disruption of mitochondrial oxidative phosphorylation and in the permeability transition, contributing to altered metabolic and redox circuits in cardiac cells, ultimately culminating in disturbances of autophagy/mitophagy fluxes, and increased apoptosis. We also suggest some possible pharmacological and non-pharmacological interventions that can reduce mitochondrial damage. Understanding the key role of mitochondria in DOX-induced cardiomyopathy is essential to reduce the barriers that so dramatically limit the clinical success of this essential anticancer chemotherapy.
Keywords: Cardiovascular research, cardiovascular pathophysiology, cardiotoxicity, cardiomyopathy, mitochondria, oxidative stress, doxorubicin, Cell Biology/Structural Biology, oxidant stress, pathophysiology
1 –. Introduction: Pathophysiology of Doxorubicin Cardiotoxicity
Anthracylines are potent cytotoxic antibiotics with broad clinical use as anti-cancer agents. The first anthracycline to be identified was daunorubicin, from which several derivatives were developed for clinical use, including epirubicin, idarubicin, doxorubicin (DOX) and mitoxantrone1. One of the most dose-limiting side effects of anthracycline therapy is the development of a characteristic cardiomyopathy. The present review explores the mitochondrial determinants of DOX cardiotoxicity, the most widely prescribed of anthracyclines, emphasizing mitochondrial toxicity as a key factor in the cardiotoxicity.
Doxorubicin (or adriamycin®) was first isolated from colonies of Streptomyces peucetius caesius in 1967 in laboratories on the Adriatic coast 2, 3. Although other derivatives have since been produced, DOX remains the most widely prescribed clinical anti-cancer agent, being effective at low doses against a broad group of cancers, including leukemias, lymphomas, and several solid tumors, namely breast, gynecological, urogenital, endocrine, brain tumors, stomach cancer, as well as Ewing and Kaposi’s sarcomas 3, 4. Similar to its first-generation ancestor, DOX inhibits tumor progression by interfering with DNA replication and transcription. DOX inhibits DNA replication through multiple mechanisms, including intercalation into DNA, interference with DNA unwinding or DNA strand separation and helicase activity4, 5, histone eviction from open chromatin 6 and topoisomerase II inhibition 7, 8.
Side effects include including nausea, vomiting and fever3. Notably, the most clinically-limiting adverse feature of DOX is increased incidence of cardiovascular toxicity, namely hypotension, tachycardia, arrhythmias, and ultimately congestive heart failure, the latter of which is the most serious and dose-limiting adverse event 9–11.
Acute DOX toxicity follows a single dose or course of therapy, with transient electrophysiological abnormalities observed in some patients, the incidence being as high as 60% and occurring from within a few minutes to a week after treatment 12, 13. Although rare, pericarditis or myocarditis syndrome and acute left ventricle (LV) failure have been described in fatal cases following single treatments 14–16. Damage-associated molecular patterns, including those mitochondrial in origin, from cardiomyocytes or vascular cells, could be one explanation 17. Furthermore, DOX is a potent activator of NF-κB in cultured cardiomyoblasts 18, while the key pro-inflammatory mediator interferon-γ contributes to DOX effects in metabolic pathways 19.
The most difficult to predict and manage clinically is the development of cardiomyopathy and ultimately congestive heart failure. The incidence of DOX cardiomyopathy depends on the cumulative life-time dose, increasing to 36% when the dose exceeds 600 mg/m2 DOX 20. Heart failure incidence affects 26% of patients receiving DOX when the cumulative dose exceeds 550 mg/m2 10. Early-onset and progressive anthracycline cardiotoxicity can occur within a year or may not become apparent until years after cessation of treatment and can manifest as chronic dilated cardiomyopathy in adult patients or as restrictive cardiomyopathy in younger patients 21, 22, associated with decreased left ventricular ejection fraction (LVEF), occasionally leading to fatal events 22, 23. Early detection of sub-clinical cardiovascular alterations within the first-year post-treatment can lead to a total or partial recovery of cardiac function, as demonstrated by Cardinale et al. 24.
Several risk factors are associated with DOX cardiac toxicity including those that are treatment- (total cumulative dose, treatment schedule or association with radiotherapy or with other cardiotoxic agents) or patient-associated (age, gender, background of cardiovascular disorders, hypertension, liver disease, and electrolyte imbalance) factors 25, 26. Children, adolescents and elderly patients are most susceptible to the cardiotoxic effects of DOX chemotherapy 27, 28.
Macroscopically, DOX cardiotoxicity is characterized by enlarged hearts with dilation of all chambers and mural thrombi visible in both ventricles 29. At a microscopic level, early observations showed interstitial fibrosis, myofibrillar loss and cardiomyocytes showing microvacuolization are observed in fatal cases 30. Of importance, more recent observations in a cohort of adult cancer patients showed a reduction in left ventricle (LV) mass after DOX chemotherapy, even without significant alterations in LVEF 31. A similar result was observed in two separate studies. Willis et al. measured a subacute decrease of cardiac mass in both mice and humans, persisting for 6 months in the latter 32, while McLean et al. measured reduced heart mass and bi-ventricular dysfunction in adult female patients treated with a combination of DOX and trastuzumab. Importantly, these observations were not accompanied by decreased body weight or cachexia that normally accompanies catabolic presentations in patients undergoing aggressive chemotherapy 33.
2 –. Mitochondrial Determinants of Doxorubicin Cardiotoxicity
Several experimental models demonstrate that DOX inhibits mitochondrial respiration, including mouse induced pluripotent stem cells-derived cardiomyocytes 34 or rat permeabilized cardiac fibers 35. Importantly, cardiac mitochondria isolated from DOX-treated rats exhibit increased state 4 respiration, inhibition of ADP-stimulated state 3 respiration, and a decrease in respiratory control with no change in the oxidative phosphorylation efficiency 36; This pattern is characteristic of chemicals that accept and redirect electrons from the respiratory chain, i.e., redox cycling agents. Pioneering research by Doroshow and Davies using specific electron transport chain (ETC) reducing substrates and inhibitors demonstrated that DOX redox cycles primarily on complex I (NADH dehydrogenase) of the mitochondrial respiratory chain 37, 38. The result is the generation of superoxide anion and reactive oxygen species (ROS) (Figure 1). In fact, univalent reduction of DOX to form the semiquinone free radical has been demonstrated in vitro by isolated NADH oxidoreductases, microsomal membranes, the nuclear envelope, and isolated mitochondria. Being highly unstable, the unpaired electron is rapidly donated to alternate electron acceptors, molecular oxygen being an abundant substrate with a favorable redox potential (c.a. −300 mV in water at neutral pH) 39. Since DOX accumulates primarily in mitochondria and nuclei 40, the abundance of the former may account for the cardio-selective toxicity of the drug, combined with a less active antioxidant network in the heart compared with other tissues such as the liver 41–44.
Figure 1 –
Bioactivation of Doxorubicin (DOX) (A) by mitochondrial complex I (mitochondrial respiratory chain) at the expense of NADH or by the cytochrome P450 system, using reducing equivalents from NADPH. NAD(P) H acts as an electron donor for the reduction of DOX producing a semiquinone radical (B) which rapidly re-oxidizes in the presence of molecular oxygen to generate superoxide anion free radicals and the parent DOX. This process is known as DOX redox-cycle and increases the production of superoxide anion which can directly damage lipids and proteins or converted to other ROS, including hydrogen peroxide. Alternatively, in the absence of molecular oxygen, DOX semiquinone can be simply converted to secondary metabolism doxorubicinol (C) or can undergo aglycosylation (D) forming a C7 radical (DNA alkylation) (E) which can form dimmers (F) or be converted to a 7-deoxyaglycone (G) (DNA intercalation). Legend: SOD: Superoxide dismutase; O2•−: superoxide anion, H2O2: hydrogen peroxide, HO•: hydroxyl radical, ROOH: lipid peroxides, ROO•: peroxy radical.
Very likely contributing to increased mitochondrial oxidative stress and direct effects of the drug, iron overload in mitochondria can contribute to DOX mitochondrionopathy. Ichikawa et al. measured an increased accumulation of iron in mitochondria from isolated cardiomyocytes and hearts from patients specifically suffering from DOX-induced cardiomyopathy. The potential damaging role of increased mitochondrial iron accumulation was confirmed by the protective effect of dexrazoxane or the over-expression of ABCB8, a mitochondrial protein that exports iron 45.
It is possible that the inhibition of oxidative phosphorylation may also stem from multiple factors, such as a decrease in adenine nucleotide translocator (ANT) expression or a decrease in cardiolipin and cytochrome c content in the heart of sub-chronically treated rats 46, 47. Interestingly, a common allosteric effector for both proteins is cardiolipin, a four-acyl chain phospholipid, which in cells exists exclusively in the mitochondrial inner membrane. Cardiolipin serves as an anchor point for cytochrome c 48 and is necessary for the maximal activity of the ANT 49. It has been reported that DOX has a strong affinity for cardiolipin 50, 51. When DOX-cardiolipin complexes are formed, cardiolipin is not available for anchoring cytochrome c or lipid-protein interfaces for several other critical mitochondrial proteins 52. Furthermore, oxidized cardiolipin can contribute to activate mitochondria-initiated cell death 53.
DOX affects subsarcolemmal or interfibrillar cardiac mitochondria differently, with the latter being more affected as measured by the respiratory control ratio54. Since these mitochondrial populations are thought to differ in terms of their structure and activity 55, it is likely that differential DOX accumulation and direct effects in each mitochondrial population may trigger different outcomes in terms of bioenergetic and contractile dysfunction.
DOX also causes a dose-dependent induction of the mitochondrial permeability transition (MPT), a phenomenon by which mitochondria lose their ability to develop and maintain electrochemical gradients across the inner membrane, due to the “opening” of MPT pores (mPTP). Because mPTP opening is both calcium and redox regulated, pro-oxidant agents are known to increase pore opening 56, while the immune-suppressant cyclosporin-A (CyA) is a mPTP desensitizer 57. In fact, both cardiac myocytes 58, 59 and mitochondria isolated 59–61 from rats sub-chronically treated with DOX exhibit increased susceptibility to calcium-dependent loss of function 36, 58, 59 that is prevented by CyA and attributed to increased mPTP opening activity. This is an early cardiac-specific and thiol-dependent effect following sub-chronic treatment of Sprague-Dawley rats with DOX 60, 62. mPTP opening has also been identified in human atrial trabeculae, in which decreased mitochondrial respiration and transmembrane electric potential was measured together with decreased calcium loading capacity and contractile dysfunction. These effects were reversed by CyA suggesting that in the human myocardium, mPTP opening leads to inhibition of respiration and contractile alterations by DOX 63. Also, in vivo treatment with CyA, but not FK-506, another immuno-suppressant which lacks mPTP inhibitory activity, partly limited the disruption of mitochondrial bioenergetics and mitochondrial fragmentation caused by DOX treatment 64, again supporting the role of the mPTP in DOX cardiotoxicity in vivo.
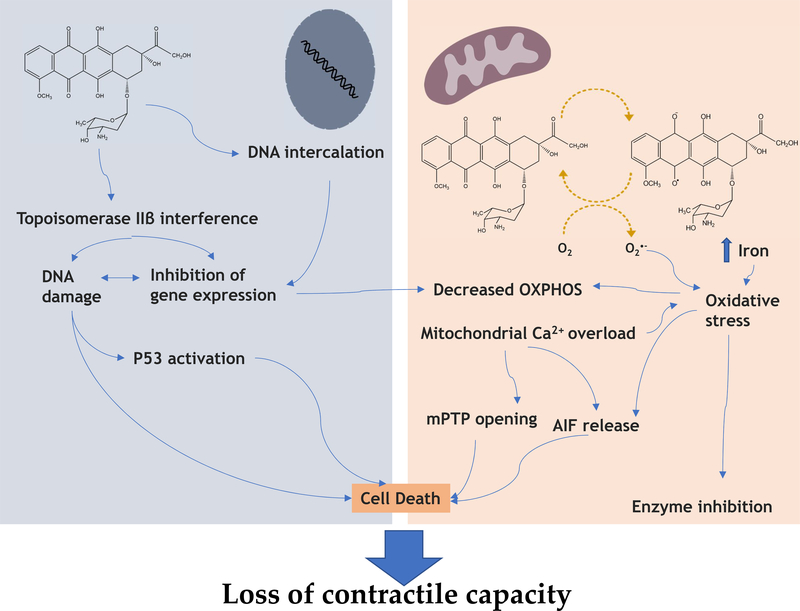
Indirect interference with mitochondrial function can occur also through nuclear-mediated effects, namely through inhibition of topoisomerase 2β in cardiomyocytes. In a seminal paper, Zhang et al demonstrated that interference by DOX with topoisomerase in cardiomyocytes is a main initiator of the cardiotoxic cascade, resulting in nuclear damage, p53 activation and downstream inhibition of mitochondrial function and defective mitochondrial biogenesis. Cardiomyocyte-specific deletion of topoisomerase 2β led to a sharp decrease in cardiotoxicity 65. Jean et al. 66 observed that by treating H9c2 cardiomyoblasts (incubation times up to 72h) or mice for 5 days with a mitochondria-targeted DOX, not only in vitro nuclear toxicity was averted, but also in vivo cardiotoxicity was decreased. Interestingly, the expression of mitochondrial proteins was not altered, which suggests that at least some of the mitochondrial toxicity is secondary to effects of the drug on the cell nucleus (Figure 2).
Figure 2 –
Summary of published data demonstrating the interplay between nuclear and mitochondrial effects of Doxorubicin (DOX). DOX directly inhibit mitochondrial function by direct interaction with Complex I and other complexes of the respiratory chain, as well as other proteins involved in oxidative phosphorylation, and by inactivation through increased reactive oxygen species (ROS) generation. ROS can play a dual role, depending on the species produced or magnitude or oxidative stress. Excessive ROS production, also resulting from mitochondrial free iron accumulation caused by DOX, if not counteracted by antioxidant defenses, can lead to cell death, while milder production can lead to an oxidative disruption of enzyme activity in cardiomyocytes. Excessive ROS production, combined with augmented cytosolic calcium leads to the opening of the mitochondrial permeability transition pore, which can result in cell death. Increased mitochondrial permeability is also responsible for the release of the apoptosis-inducing factor (AIF) which is involved in caspase-independent cell death. On the other hand, DOX accumulates in the nuclei of cardiac cells and intercalates into DNA and interferes with topoisomerase IIβ. Interaction of the complex DOX/ topoisomerase IIβ can lead to inhibition of mitochondrial biogenesis and gene expression resulting in secondary OXPHOS inhibition. DNA damage caused by DOX can also lead to overexpression of p53, a consequence of which can be an increased expression of downstream pro-apoptotic targets, activating cell death.
Acute inhibition of mitochondrial oxidative phosphorylation and oxidative stress may trigger downstream cardiomyocyte adaptations. Insight into what may account for the primary mode of DOX delayed cardiac toxicity may be gained by examining the compensatory changes that occur in immediate responses to drug administration. Transcriptionally, this might be reflected by activation of the Keap1/Nrf2/ARE pathway or altered regulation of MAP kinase and/or the PPAR nuclear receptor mediated pathways. In the oxidized state, Keap1 fails to bind to Nrf2, thereby permitting this transcription factor to accumulate in the nucleus and activate the transcriptional antioxidant response element (ARE). Net oxidation of Keap1 and activation of this Nrf2-mediated antioxidant response in rat cardiomyocytes is strong evidence for oxidative stress in response to DOX 67. The observation that modulation of the Keap1/Nrf2 pathway in mice alters both hepatotoxicity and nephrotoxicity suggests that DOX induces oxidative damage in these tissues as well 68, 69. Another possible mediator of redox stress response to DOX toxicity is the adapter protein p66Shc. DOX treatment induces p66Shc protein up-regulation specifically in nuclear fractions, leading to the activation of nuclear expression of FoxO3a, which occurs upstream of target genes for cell death 70.
A second prominent response to DOX treatment is the reprogramming of metabolic flux and altered regulation of mitochondrial biogenesis, both of which are consistent with an attempt to restore bioenergetic capacity lost in the course of drug-induced mitochondrial toxicity. Tokarska-Schlattner et al. demonstrated inhibition by DOX of in vitro activity of sarcomeric/cardiac mitochondrial creatine kinase at concentrations of 5–10 μM 71, a result later confirmed in intact perfused hearts 72, although for supra-physiological concentrations. Still, the latter work demonstrated acute inhibition of AMPK at 2 μM DOX, suggesting that master regulation of energy metabolism may be affected by DOX. This was later confirmed by isotopomeric analysis of cardiac tissue following subchronic DOX treatment of rats that demonstrated a net shift in metabolic flux from fatty acid oxidation to glucose oxidation and lactic acid production 73. Subsequent metabolomic studies provide substantiating evidence for cardiac metabolic remodeling following both acute and sub-chronic in vivo dosing with DOX 19, 74–78. This change in metabolic flux is consistent with the previously reported altered global gene expression profile 79 and suggests that the well-established metabolic reprogramming in cardiac tissue in response to DOX is regulated at the transcriptional level, possibly through regulation of PPAR nuclear receptors 80. In rodents, DOX administration results in the down-regulation of PPARα, PPARγ, and PPARσ in cardiac tissue, each of which has been implicated to be a significant factor in the metabolomic response to the drug 80–84. PPARγ-coactivating factor 1 α (PGC1α) is suggested to play a principal role in mediating this response 80, 84.
PGC1α likely plays a dual role in the compensatory response to mitochondrial dysfunction caused by DOX treatment, and possibly several other metabolic states. Not only is PGC1α involved in modulating the PPAR-mediated shift in metabolic flux 85, 86, it is also central in regulating mitochondrial proliferation (biogenesis), which is an important second arm in the compensatory metabolic response to bioenergetic stress 87–91. PGC1 α acts as a transducing element for sensing both high-energy phosphates and pyridine nucleotide status in the cell 88, 90 and is vital to the adjustment of cellular bioenergetics in response to changing metabolic demands. This includes both the remodeling of metabolic flux by co-activating PPAR nuclear receptors and the regulation of mitochondrial biogenesis by co-activating Nrf2 and its down-stream transcription factors.
As already introduced in section 1, the recent finding from Ni et al. showing that DOX-induced interferon-γ-mediated signaling in cardiomyocytes results in disruption of mitochondrial respiration and fatty acid oxidation, also decreasing AMPK signaling, links inflammation-related signaling and metabolic remodeling is treated hearts 19.
3 –. Disruption of Autophagy and Apoptosis Resulting from DOX treatment
DOX -induced mitochondrial toxicity can trigger autophagic (including mitophagic) responses, in an attempt to remove damaged mitochondrial and cellular structures. In fact, induction of autophagy by DOX has been reported in multiple studies. The formation of autophagosomes and an increased conversion of LC3A to LC3B markers of autophagy activation occurs in the hearts of C57BL/6J male mice treated with DOX 92. Markers of autophagy 93–95 and mitophagy 92, 96–98 are observed in cultured cardiac cells after DOX treatment. In fact, an accumulation of autophagosomes, autolysosomes and excessive elimination of intracellular organelles are characteristic of autosis 99, a type of cell death induced by excessive autophagy. In cellular models in which DOX over-stimulates autophagy, protection has been achieved by inhibitors of autophagy initiation 92, 100–102.
In contrast, other studies reported an inhibition of the autophagic flux in cardiac cells after DOX exposure 103, 104. In fact, some authors suggested that DOX-induced cardiotoxicity could reflect inhibition of lysosome acidification, resulting in an accumulation of autophagosomes and autolysosomes 105, 106. DOX treatment of neonatal rat cardiomyocytes inhibits basal autophagy, which could be detrimental to protecting the heart against DOX-induced toxicity 107. Inhibition of Parkin-mediated mitophagy induced by DOX treatment was also observed in the heart of C57BL/6J mice and in cultured HL-1 cardiomyocytes, leading to accumulation of dysfunctional mitochondria and subsequently cardiac cell death 108. According to this study, the inhibition of mitophagy is mediated by cytosolic p53, which binds to Parkin and inhibits its translocation to mitochondria to initiate the mitophagic process 108. The inhibition of autophagy/mitophagy induced by DOX treatment would promote the accumulation of damaged cellular organelles and oxidized proteins that could be deleterious for cardiac cells, further stimulating cell death. In this context, activation of autophagy before DOX exposure appears to be protective against DOX-induced cardiotoxicity 109.
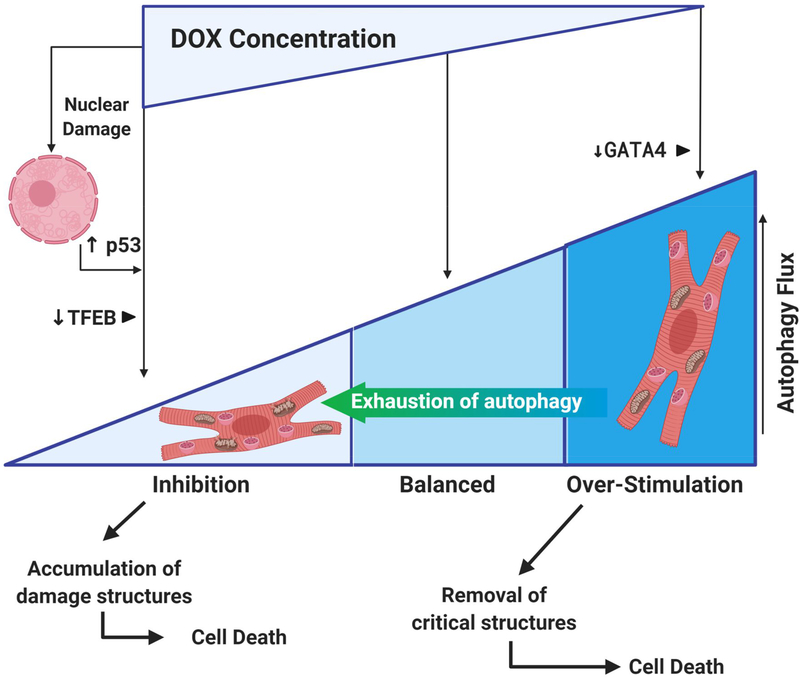
The mixed results observed (Figure 3) may depend on the model used, DOX concentration and time of incubation/treatment, and when using cells in culture, the type of cell line used. The degree of cell damage including the extension of oxidative damage, may also determine the degree of inhibition or hyper-activation of autophagy/mitophagy. Moreover, several positive and negative regulators of autophagy can be activated or inhibited, depending on the factors above. For example, Bartlett et al demonstrated that DOX can inhibit transcription factor EB (TFEB), which governs lysosomal function 110. Under the conditions and models used, loss of TFEB inhibits lysosomal-dependent autophagy, leading to DOX-induced cardiac injury 110. Another important player is the transcription factor GATA4. In this case, DOX-induced depletion of GATA4 in cultured neonatal cardiomyocytes leads to autophagy over-stimulation. Likewise, in the same model, when GATA4 was over-expressed, autophagy and DOX toxicity were decreased 102, which could also be related to a positive regulation by that transcription factor on Bcl-2 111. Chronic over-stimulation of the autophagy and/or mitophagy process resulting from DOX treatments may be followed by a suppression phase resulting from exhaustion of the autophagic reserve capacity, previously described in the context of Parkinson disease models 112.
Figure 3 –
Anthracyclines alter autophagic fluxes in cardiac cells. The literature reports apparent contradictory observations, with some indicating that anthracyclines stimulate autophagy/mitophagy, while others suggesting the opposite. The apparent contradictory results may stem not only from different experimental conditions used in in vitro studies, but also from the cumulative DOX concentration used, and the different oxidative damage caused. Extensive oxidative and DNA damage may lead to block of mitophagy/autophagy mediated by p53 over-expression, which binds to Parkin and inhibits its translocation to mitochondria. Arrest of autophagic fluxes may follow protective autophagy if the insult continues and autophagy capacity is exhausted. The accumulation of damaged structures can lead to cardiomyocyte death, which is also a consequence of anthracycline activation of the intrinsic and extrinsic cell death pathways amplified by stress responses following nuclear DNA damage. Alterations induced by DOX on different autophagy regulators TFEB and GATA4 can also drive autophagy fluxes towards inhibition or hyper-activation, respectively.
On another hand, DOX mitochondrial cardiotoxicity is related with the induction of apoptosis, which ultimately can contribute to cardiomyopathy 113–118. The mitochondrial-dependent apoptotic (or intrinsic) pathway being the most thoroughly described mechanism for the loss of cardiomyocytes 118–120. Damage to mitochondria and activation of mitochondrial-dependent apoptotic pathway in cardiac cells after DOX-induced oxidative stress has been reported by several groups, including our own 119, 120. Induction of the CyA-sensitive mPTP after DOX treatment (see previous section) may lead to the rupture of the mitochondrial outer membrane and release of pro-apoptotic factors, entrapped in the mitochondrial intermembrane space, promoting the activation of mitochondrial dependent apoptotic pathway 121. Still, the bioenergetic failure associated to mPTP opening and ATP depletion may be more closely associated with necroptosis than apoptosis 122. Supporting the pro-apoptotic role of DOX, accumulation of pro-apoptotic proteins, such as BAX, in the OMM as well as a reduction in anti-apoptotic proteins such as Bcl-2 and caspase-9 and −3 activation also occurs in cardiac cells after DOX treatment 120, 123. However, activation of a caspase-independent apoptotic pathway involving the release of mitochondrial AIF partly through cathepsin-B-dependent cleavage was also reported in H9c2 cardiomyoblasts treated with DOX 119, 124. Up-regulation of pro-apoptotic proteins such as Bax can also be dependent of p53 over-expression. In fact, up-regulation of p53 in cardiac cells has been demonstrated after DOX treatment, with its inhibition by either pharmacological or genetic means resulting in partial protection 34, 120, 125. In an apparent contradiction with the involvement of p53 in the pro-apoptotic effects of DOX in the myocardium, Li et al recently demonstrated that mice with homozygous removal of the p53 R172H (p53 172H/H ) mutation, which removes p53 tumor suppressor activity but maintains mitochondrial biogenesis activity, showed decreased DOX-induced cardiotoxicity. This can be explained by the protection of mtDNA afforded by the mutated p53, which limits the extent of mtDNA and mitochondrial damage caused by DOX. In another example of anterograde communication to mitochondria, induction of cardiomyocyte necrosis induced by DOX and respiratory defects were linked to expression of the BH3-only protein Bcl-2-like protein 19KDa interacting protein 3 (Bnip3) and to its translocation to mitochondria. Dhingra et al. showed that Bnip 3(−/−) mice are resistant to DOX-induced cardiotoxicity, showing preserved mitochondrial function and mortality rates similar to saline-treated animals 126.
The different susceptibility to DOX cardiotoxicity in children vs. adults may stem from age-specific differences to apoptotic stimuli. Several reports demonstrated that the expression of the apoptotic protein machinery is low or absent in the adult heart, while being very active in younger hearts, possibly contributing to decrease cardiac mass (as reported in Section 1), disrupt cardiac maturation, and result in progressive heart failure and later cardiomyopathy. Decreased heart mass has also been observed in adult patients as well, which suggest that in this group of patients, alternative cell death pathways, including necroptosis, may be triggered by DOX treatment.
In the context of autophagy/mitophagy alterations caused by DOX, mitochondrial disruption is likely involved not only a primer event, but also as consequence of irregular removal of damaged structures, resulting in cardiomyocyte death. This would be one explanation for the loss of ventricular mass observed in a significant cohort of patients, with later functional consequences.
4 –. The Nature of Mitochondrial Memory in DOX Cardiotoxicity
Alterations of redox and metabolic pathways, as well as mitochondrial inhibition, persist well-beyond the half-life of the drug, namely one to five weeks following the last of six drug treatments 73, 79. The same is true for various indicators of DOX-induced oxidative damage, wherein cardiac tissue demonstrated accelerated rates of ROS generation, induction of the permeability transition and decreased calcium loading capacity, and 8-hydroxyguanodine adducts in mitochondrial DNA (mtDNA) for as long as 5 weeks following the last of 6 or 8 weekly drug treatments 61, 127, 128; mtDNA was preferentially oxidized following DOX compared to nuclear DNA 129. Five weeks represents more than 25 drug half-lives; thus, the continuing generation of ROS and decrement in mitochondrial function are not the result of presence of the drug itself. Furthermore, the fact that the estimated half-life for the turnover of cardiac mitochondria proteins is 16–18 days 130, 131 suggests that whatever the drug-related change is, it is propagated to successive generations of mitochondria and not effectively eliminated by mitophagy (section 3). This cumulative dose-dependent and progressive mitochondrial dysfunction observed in rats is consistent with what is observed clinically in survivors of pediatric cancer chemotherapy. Patients successfully treated in childhood with DOX have a lower threshold and greater morbidity to the drug later in life, the degree to which is proportional to the previous cumulative dose of the drug 132.
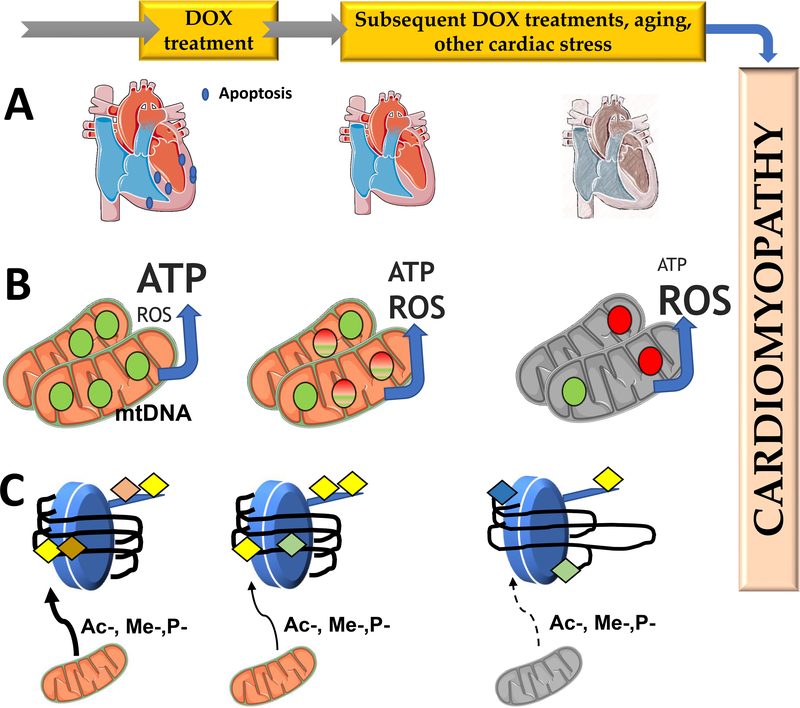
But what is the nature of DOX cardiotoxicity memory (Figure 4)? Increased removal of cardiomyocytes in children or adults treated with DOX, leading to decrease heart mass may account for a decreased resistance of the cardiac muscle to subsequent DOX treatments, or to undergo further cardiac insults that would further decrease contractile activity.
Figure 4 –
Possible mechanisms for DOX cardiotoxicity memory. The figure shows three proposed mechanisms by which a cardiac memory of DOX toxicity results in delayed and cumulative nature of DOX cardiotoxicity. Panel A represents removal of cardiomyocytes by cell death processes, which is more extensive in pediatric patients, to the higher activity of the apoptotic machinery in young hearts, when compared with adults. In panel B, DOX treatment results in oxidation of mtDNA, inhibiting its ability for replication and expression. With time, this leads to a reduction in mtDNA copy number and bioenergetic collapse with decrease mitochondrial ATP production and increased reactive oxygen species (ROS) production due to reduced transcription of critical OXPHOS subunits which can develop into cardiomyopathy. In panel C, DOX alters the nuclear epigenetic landscape in cardiomyocytes, either from direct interaction with DNA and histones, or indirectly by disturbing mitochondrial metabolism, altering the availability of methyl, acetyl or phosphate donors for epigenetic regulation. This persistent alteration of the epigenetic landscape would result in different profiles of gene expression, which can cause disruption of metabolism in cardiomyocytes and increase the susceptibility of the cardiomyocyte to subsequent DOX treatments or cardiac stress events.
A second possibility lies in the preferential oxidation of mtDNA 129. Progressive deterioration of mtDNA integrity may lead to an age- and/or second hit- decrease in mitochondrial fitness, similarly to what occurs in several other mitochondrial diseases 133, 134. In this model, because of the lower bioenergetic reserves of the heart, a metabolic threshold is crossed earlier in time, leading to the development of cardiac alterations, including congestive heart failure. We demonstrated in a rat model of DOX sub-chronic toxicity, treatment-related alterations in global flux curves for Complex III in all analyzed tissues and in Complex IV activity solely in heart. However, all mitochondrial threshold curves remained unchanged, suggesting that progressive deterioration of mitochondrial activity leads to a threshold crossing later in life 46. Also, DOX intercalates in mtDNA, leading to aggregation of mtDNA nucleoids and altering their distribution and contributing also to mtDNA depletion 135. The fact that different patients may have different cardiac mitochondrial capacity, hence different metabolic thresholds, may account for the different susceptibility to contractile alterations in patients.
A third possibility involves persistent alteration of epigenetic regulation of critical redox and metabolic genes after DOX treatment, discussed in the previous section, which would serve as a memory of redox and metabolic disturbances. Our laboratories demonstrated that subchronic administration of DOX leads to a long-standing decrease in mtDNA copy number and global methylation of DNA in cardiac, but not liver, tissue 136. Associated with this was an enhanced activity of histone deacetylase enzymes, an altered pattern of protein acetylation, and a differential pattern of gene expression. DNA-methyl transferase activity of cardiac myoblasts was inhibited when exposed to nM DOX concentrations in cell culture 137. In the same model, recent data showed also alterations in histone deacetylases (SIRT1 and HDAC2), histone lysine demethylases (KDM3A and LSD1) and histone lysine methyltransferases (SET7 and SMYD1), as well as decreased histone 3 acetylation 138. In mice, chronic treatment with DOX leads to the transcriptional activation of most all deacetylase enzymes in heart, Hdac2 being the exception 139. Because changes in the epigenome are also associated with the failing heart 140, this begs the question whether these alterations are specific to the drug or are secondary to the cardiomyopathy caused by DOX, a question that remains unanswered at this time. Epigenetic modification of gene expression is widely implicated as an early event key to the remodeling of cardiac structure and transcriptional reprogramming of metabolic flux in the failing heart 141, 142.
Persistent alterations in mitochondrial energy metabolism and gene expression caused by DOX 79 may indeed be related to epigenetic changes, but the opposite may also be true. It has been demonstrated that DOX treatment leads to a decrease in the expression of genes coding for the beta-oxidation of fatty acids, as well as expression of mitochondrial ATP-producing enzymes 79. Although not yet directly demonstrated, this suggests possible decrease in production of mitochondrial metabolites such as acetyl CoA, acetylcarnitine and ATP, each of which are epigenetic modifiers 143. The fact that changes to the epigenomic landscape are long-lived could implicate epigenetics as a key factor in the cumulative and persistent mitochondrial dysfunction associated with DOX treatment; providing an historical register of drug-induced loss of mitochondrial function.
5 –. Strategies to Mitigate DOX-induced Mitochondrial Disruption and Cardiotoxicity
Potential interventions aimed to reduce DOX cardiotoxicity revolved around the pro-oxidant nature of the side effects. As described above, dexrazoxane is the only FDA-approved protective therapy against DOX toxicity. Although the mechanism of cardioprotection by dexrazoxane was thought to be mediated by iron chelation, and thus indirectly through an antioxidant effect, equally or more potent iron chelators such as desferrioxamine 144 or deferasirox 145, fail to achieve the same degree of cardioprotection. Inhibition of cardiac topoisomerase 2-beta and inhibition of DOX-induced DNA breaks are also possible mechanisms for cardioprotection by dexrazoxane 146.
A limiting factor in understanding the role of cardiac mitochondrial disruption in DOX cardiotoxicity is that only a few studies described strategies to specifically prevent DOX-induced mitochondrial dysfunction. One particular study involved co-treatment of Sprague-Dawley rats with DOX and carvedilol, a nonselective beta-adrenergic receptor antagonist which displayed potent antioxidant protection of cardiac mitochondria 147–150. Carvedilol improved cardiac mitochondrial function, including augmenting calcium loading capacity, during sub-chronic DOX treatment 151. The effect was not mimicked by atenolol, a nonselective beta-adrenergic receptor antagonist lacking antioxidant activity 60. Accordingly, carvedilol may act as a multi-target agent, not only sparing the cardiac muscle due to its beta-adrenergic blockade properties, but also through iron chelation 152 or even to complex I-mediated effects 149.
Still, the role of mitochondrial oxidative damage as one main contributing factor for DOX cardiotoxicity was challenged, since a diet enriched in alpha-tocopherol succinate, an antioxidant Vitamin E derivative 153, prevented cardiac oxidative damage in Sprague-Dawley rats, but did not inhibit mitochondrial dysfunction 154. On the other hand, Chandran et al. demonstrated that co-administration of MitoQ, a triphenylphosphonium-conjugated analog of coenzyme Q, to rats treated with DOX for 12 weeks resulted in improved LV function as measured by two-dimensional echocardiography and increased cytochrome c activity 155. Because MitoQ is a mitochondria-targeted antioxidant, enrichment of mitochondrial membranes with the active antioxidant is beneficial against DOX toxicity. These effects were further confirmed with other molecules that also accumulate in mitochondria, including berberine 94, 156, although it also interferes with other pathways including p38 MAPK-mediated NF-κB signaling 157. In cardiac progenitor cells mitochondria-targeted resveratrol decreased cardiomyopathy, suggesting antioxidant protection is important to the improvement of mitochondrial function following DOX 158.
Humanin is a 24 amino-acid polypeptide that is coded by an open reading frame present in 16S rRNA of mtDNA and to which protective effects have been attributed to binding to different receptors 159. Lue et al demonstrated that a synthetic humanin analogue increases the protective effect of dexrazoxane in vivo by inhibiting DOX-induced decrease in ejection fraction, cardiac fibrosis, and by protecting mitochondrial function 160.
Non-pharmaceutical interventions with the potential to decrease DOX cardiac toxicity include physical activity, which may also work by multiple pathways. These include decreased drug delivery to the cardiac tissue 161, 162, decreased activation of the mitochondrial permeability transition pore and mitophagy 98, activation of PGC-1α expression with consequent increase in FOxO target genes 163, improvement of mitochondrial oxidative phosphorylation 164, up-regulation of antioxidant defenses, ultimately inhibiting mitochondrial damage 161, 165–167and triggering ofapoptotic signaling 41, 168, among others. The improved mitochondrial function observed in physical activity models can be mimicked by stimulating OXPHOS in cell models. In fact, by forcing H9c2 cardiomyoblasts to produce most ATP by OXPHOS, instead of glycolysis, the toxicity of DOX is decreased 95. Interestingly, mitochondrial amplification by troglitazone or a similar compound was able to selectively prime tumor cells to the cytotoxic effects of DOX 169.
Because physical activity and caloric restriction (CR) share similar profiles of cardioprotection, it is reasonable to think that CR may afford protection against DOX cardiotoxicity by way of altering the metabolome. Interestingly, CR combined with voluntary wheel running was demonstrated to decrease cardiac DOX accumulation almost 4-fold, while CR alone improved left ventricular developed pressure (LVDP), end systolic pressure (ESP), and left ventricular maximal rate of pressure development (dP/dtmax) in rats 170. In the same rodent model, in combination with CR (20% less food from a 125% fortified diet), resveratrol up-regulated protective autophagy in animals treated with a single injection of 10 mg/kg DOX, most likely through activation of AMPK and SIRT1171. Intermittent fasting also restores autophagic flux in a mouse model of acute and chronic DOX toxicity, exacerbated by the silencing of the ultra-violet radiation resistance-associated gene 172.
The above studies provide an important contribution not only to the mechanisms involved in DOX cardiotoxicity, but also strategies that can be used clinically to decrease the acute and delayed cardiotoxicity associated with DOX anticancer chemotherapy (Figure 5).
Figure 5:
Interventions aimed to decrease DOX cardiotoxicity through mitochondrial direct or indirect effects. Represented are compounds that prevent mitochondrial oxidative stress (including by chelating excess iron, as in the case for carvedilol), including MitoQ, carvedilol and berberine, with secondary protection of DNA damage. Dexrazoxane, the only Food and Drug Administration agent approved to decrease anthracycline toxicity which may act through two different mechanisms, iron chelation and inhibition of topoisomerase 2-beta. Both caloric restriction and physical activity are described to act through similar mechanisms, namely by activating metabolic modulators such as AMPK, SIRT1, PGC-1α, and in the case of physical activity by increasing cell defenses such as heat-shock proteins and antioxidant networks. It has also been described that physical activity decreases the amount of DOX accumulated by cardiomyocytes, thus indirectly reducing cardiac damage.
5 –. Final Conclusions
The present review highlights critical aspects of the pathophysiology of anthracycline, and specifically DOX, cardiotoxicity. We consider mitochondrial dysfunction memory as a critical point for the major consequences of DOX cumulative and progressively developing heart failure. We argue that DOX causes immediate and direct effects on the mitochondrial oxidative phosphorylation system, which are likely a result from oxidative stress or direct interference with mitochondrial structures, which add up to nuclear-mediated effects of the drug, altering gene expression and contributing to a downstream domino effect. This results in cardiomyocyte metabolic and redox remodeling, the magnitude of which may determine the resilience and possibly the recovery of the cardiac tissue. In addition, disturbance of mitochondrial oxidative phosphorylation and several signaling processes can lead further to cellular damage leading to an alteration of autophagy/mitophagy fluxes that further compromises cardiomyocyte metabolism. Ultimately, cardiomyocyte death can follow, which can explain the decreased heart mass measured in pediatric and adult patients. The mechanisms involved in the decrease of heart mass are not entirely known. As described above, loss of cardiac cells in pediatric patients may be better explained by their higher expression and activity of apoptotic machinery. The fact that the same decrease in cardiac mass occurs in adult patients suggests non-apoptotic processes leading to cell death.
The cumulative and progressive nature of DOX cardiotoxicity is the greatest challenge in oncology treatments and may stem from a persistent memory of the drug, unrelated to its presence in the heart. In this review we described some possibilities, including stronger pro-apoptotic effects on pediatric patients due to more active apoptotic machinery, acute oxidation of mtDNA associated with a progressive and aging-dependent loss of mitochondrial capacity, and persistent epigenetic alterations leading to long-lasting alterations in gene expression. The basal cardiac mitochondrial capacity and mtDNA “fitness” (copy number and replicative/transcription activity) may determine the progression of cardiac functional deterioration and/or the resistance to cumulative DOX treatments 173–175. This may also explain, at least in part, why some patients are more susceptible to anthracycline toxicity than others. This piece of reasoning adds on to the puzzling question is who will develop cardiac structural disease and the time elapsed before that occurs.
Epigenetic alterations are associated with disruption of mitochondrial function 176–178, correlating disturbances of oxidative phosphorylation with DOX-induced epigenetic remodeling, which again may depend on a host (epi)genetic background. Not excluding other non-mitochondrial targets, therapies or interventions aimed at directly or indirectly improving mitochondrial function (or at least decreasing the rate at which mitochondria become dysfunctional) are important considerations in improving cardiac outcomes associated with DOX chemotherapy. An outstanding question is when and where to act in order to improve and preserve mitochondrial function. Current biomarkers for detecting sub-clinical mitochondrial alterations that lead to DOX-induced delayed cardiomyopathy are still missing and should be an active field of research. Finally, another open question is how mitochondrial dysfunction positions itself in the cardiac toxicity caused by anthracyclines when synergizing with other anti-cancer agents, including ErbB2-targeted therapies.
The answer to all these questions will contribute to a safer use of DOX and related anthracyclines in oncology and will contribute to highlight more the role of mitochondria in the cardiotoxicity of clinically used drugs.
6 –. Acknowledgements
Work in PJO/VAS laboratory is partly funded by FEDER funds through the Operational Programme Competitiveness Factors - COMPETE and national funds by FCT - Foundation for Science and Technology (FCT), grants PTDC/BTM-SAL/29297/2017, POCI-01-;0145-FEDER-029297, UIDB/04539/2020, and PTDC/DTP-FTO/1180/2012. VAS is supported by FCT grant IF/01182/2015. Work in KBW laboratory is supported in-part by grants from the 3M Co., the American Heart Association, and the National Heart, Lung and Blood Institute, NIH. The authors are thankful to Gonçalo Pereira, Ph.D. and Luciana Ferreira, Ph.D. for their contribution to figures.
List of abbreviations
- AIF
Apoptosis-inducing factor
- AMPK
Adenosine monophosphate activated protein kinase
- ANT
Adenine nucleotide translocator
- ARE
Antioxidant response element
- ATP
Adenosine triphosphate
- CR
Caloric restriction
- CyA
Cyclosporin-A
- DOX
Doxorubicin (or adriamycin)
- dP/dtmax
Left ventricular maximal rate of pressure development
- Em
Two electron reduction potential
- ER
Endoplasmic reticulum
- ESP
End-systolic pressure
- GSH
Reduced form of glutathione
- HK1
Hexokinase 1
- IMM
Inner mitochondrial membrane
- Keap1
Kelch-like ECH-associated protein 1
- LV
Left ventricle
- LVDP
Left ventricular developed pressure
- LVEF
left ventricle ejection fraction
- MitoQ
Triphenylphosphonium-conjugated analog of coenzyme Q
- MPT
Mitochondrial permeability transition
- mPTP
Mitochondrial permeability transition pore
- mtDNA
mitochondrial DNA
- NADH
Nicotinamide adenine dinucleotide, reduced form
- NADPH
Nicotinamide adenine dinucleotide phosphate, reduced form
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- OMM
Outer mitochondrial membrane
- OXPHOS
Oxidative phosphorylation
- PDH
Pyruvate dehydrogenase
- PGC1α
PPARγ-coactivating factor 1 a
- PINK1
PTEN-induced kinase 1
- PPAR
Peroxisome proliferator-activated receptor
- ROCK1
Rho-associated protein kinase 1
- ROS
Reactive oxygen species
- tBid
Cleaved Bid
- UPS
Ubiquitin-proteasome system
- VDAC
Voltage-dependent anion channel
8 – References
- 1.Weiss RB. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol 1992;19:670–686 [PubMed] [Google Scholar]
- 2.Bonadonna G, Monfardini S, De Lena M, Fossati-Bellani F. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br. Med. J 1969;3:503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonadonna G, Monfardini S, De Lena M, Fossati-Bellani F, Beretta G. Phase i and preliminary phase ii evaluation of adriamycin (nsc 123127). Cancer Res. 1970;30:2572–2582 [PubMed] [Google Scholar]
- 4.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs 1997;54 Suppl 4:1–7 [DOI] [PubMed] [Google Scholar]
- 5.Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physicochemical and biological review. Biochimie. 1984;66:333–352 [DOI] [PubMed] [Google Scholar]
- 6.Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nature communications. 2013;4:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarr M, van Helden PD. Inhibition of transcription by adriamycin is a consequence of the loss of negative superhelicity in DNA mediated by topoisomerase ii. Mol. Cell. Biochem 1990;93:141–146 [DOI] [PubMed] [Google Scholar]
- 8.de Jong S, Zijlstra JG, de Vries EG, Mulder NH. Reduced DNA topoisomerase ii activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990;50:304–309 [PubMed] [Google Scholar]
- 9.Villani F, Meazza R, Materazzo C. Non-invasive monitoring of cardiac hemodynamic parameters in doxorubicin-treated patients: Comparison with echocardiography. Anticancer Res. 2006;26:797–801 [PubMed] [Google Scholar]
- 10.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879 [DOI] [PubMed] [Google Scholar]
- 11.Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer. 1987;60:1213–1218 [DOI] [PubMed] [Google Scholar]
- 12.Kilickap S, Barista I, Akgul E, Aytemir K, Aksoy S, Tekuzman G. Early and late arrhythmogenic effects of doxorubicin. Southern medical journal. 2007;100:262–265 [DOI] [PubMed] [Google Scholar]
- 13.Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. The American journal of cardiology. 1992;70:73–77 [DOI] [PubMed] [Google Scholar]
- 14.Gaudin PB, Hruban RH, Beschorner WE, Kasper EK, Olson JL, Baughman KL, Hutchins GM. Myocarditis associated with doxorubicin cardiotoxicity. American journal of clinical pathology. 1993;100:158–163 [DOI] [PubMed] [Google Scholar]
- 15.Wortman JE, Lucas VS Jr., Schuster E, Thiele D, Logue GL. Sudden death during doxorubicin administration. Cancer. 1979;44:1588–1591 [DOI] [PubMed] [Google Scholar]
- 16.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am. J. Med 1978;65:823–832 [DOI] [PubMed] [Google Scholar]
- 17.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC, Working Group on DiCD. Mitochondrial damage-associated molecular patterns and vascular function. European heart journal. 2014;35:1172–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo RM, Xu WM, Lin JC, Mo LQ, Hua XX, Chen PX, Wu K, Zheng DD, Feng JQ. Activation of the p38 mapk/nf-kappab pathway contributes to doxorubicin-induced inflammation and cytotoxicity in h9c2 cardiac cells. Molecular medicine reports. 2013;8:603–608 [DOI] [PubMed] [Google Scholar]
- 19.Ni C, Ma P, Wang R, Lou X, Liu X, Qin Y, Xue R, Blasig I, Erben U, Qin Z. Doxorubicin-induced cardiotoxicity involves ifngamma-mediated metabolic reprogramming in cardiomyocytes. J. Pathol. 2019;247:320–332 [DOI] [PubMed] [Google Scholar]
- 20.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314 [DOI] [PubMed] [Google Scholar]
- 21.Buzdar AU, Marcus C, Smith TL, Blumenschein GR. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55:2761–2765 [DOI] [PubMed] [Google Scholar]
- 22.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677 [PubMed] [Google Scholar]
- 23.Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, Nagai R. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum ca(2+)-atpase gene transcription. Circ Res. 2000;86:8–14 [DOI] [PubMed] [Google Scholar]
- 24.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988 [DOI] [PubMed] [Google Scholar]
- 25.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. New Engl. J. Med 1995;332:1738–1743 [DOI] [PubMed] [Google Scholar]
- 26.Danesi R, Fogli S, Gennari A, Conte P, Del Tacca M. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin Pharmacokinet. 2002;41:431–444 [DOI] [PubMed] [Google Scholar]
- 27.Kremer LC, van Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann. Oncol 2002;13:503–512 [DOI] [PubMed] [Google Scholar]
- 28.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8597–8605 [DOI] [PubMed] [Google Scholar]
- 29.Fitter W, deSa DJ, Pai KR. Adriamycin cardiotoxicity: Report of an unusual case with features resembling endomyocardial fibrosis. J. Clin. Pathol 1981;34:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kajihara H, Yokozaki H, Yamahara M, Kadomoto Y, Tahara E. Anthracycline induced myocardial damage. An analysis of 16 autopsy cases. Pathology, research and practice 1986;181:434–441 [DOI] [PubMed] [Google Scholar]
- 31.Jordan JH, Castellino SM, Melendez GC, Klepin HD, Ellis LR, Lamar Z, Vasu S, Kitzman DW, Ntim WO, Brubaker PH, Reichek N, D’Agostino RB Jr., Hundley WG. Left ventricular mass change after anthracycline chemotherapy. Circulation. Heart failure. 2018;11:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willis MS, Parry TL, Brown DI, Mota RI, Huang W, Beak JY, Sola M, Zhou C, Hicks ST, Caughey MC, D’Agostino RB Jr., Jordan J, Hundley WG, Jensen BC. Doxorubicin exposure causes subacute cardiac atrophy dependent on the striated muscle-specific ubiquitin ligase murf1. Circulation. Heart failure. 2019;12:e005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean BA, Hansen R, Paterson DI, White JA, Oudit GY. Breast cancer patients receiving anthracycline chemotherapy and trastuzumab have biventricular dysfunction and reduced heart mass. J. Am. Coll. Cardiol 2018;72:1872–1873 [DOI] [PubMed] [Google Scholar]
- 34.Cunha-Oliveira T, Ferreira LL, Coelho AR, Deus CM, Oliveira PJ. Doxorubicin triggers bioenergetic failure and p53 activation in mouse stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol 2018;348:1–13 [DOI] [PubMed] [Google Scholar]
- 35.Studneva I, Palkeeva M, Veselova O, Molokoedov A, Ovchinnikov M, Sidorova M, Pisarenko O. Protective effects of a novel agonist of galanin receptors against doxorubicin-induced cardiotoxicity in rats. Cardiovasc. Toxicol 2019;19:136–146 [DOI] [PubMed] [Google Scholar]
- 36.Solem LE, Henry TR, Wallace KB. Disruption of mitochondrial calcium homeostasis following chronic doxorubicin administration. Toxicol. Appl. Pharmacol 1994;129:214–222 [DOI] [PubMed] [Google Scholar]
- 37.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by nadh dehydrogenase. J. Biol. Chem 1986;261:3060–3067 [PubMed] [Google Scholar]
- 38.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. Ii. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J. Biol. Chem 1986;261:3068–3074 [PubMed] [Google Scholar]
- 39.Wood PM. The potential diagram for oxygen at ph 7. Biochem. J 1988;253:287–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cova D, De Angelis L, Monti E, Piccinini F. Subcellular distribution of two spin trapping agents in rat heart: Possible explanation for their different protective effects against doxorubicin-induced cardiotoxicity. Free Radic Res Commun. 1992;15:353–360 [DOI] [PubMed] [Google Scholar]
- 41.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am. J. Physiol. Heart Circ. Physiol 2005;289:H722–731 [DOI] [PubMed] [Google Scholar]
- 42.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: Alterations produced by doxorubicin. J. Clin. Invest 1980;65:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiserova H, Simunek T, Sterba M, den Hartog GJ, Schroterova L, Popelova O, Gersl V, Kvasnickova E, Bast A. New iron chelators in anthracycline-induced cardiotoxicity. Cardiovasc. Toxicol 2007;7:145–150 [DOI] [PubMed] [Google Scholar]
- 44.Quiles JL, Huertas JR, Battino M, Mataix J, Ramirez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95 [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest 2014;124:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira GC, Pereira SP, Tavares LC, Carvalho FS, Magalhaes-Novais S, Barbosa IA, Santos MS, Bjork J, Moreno AJ, Wallace KB, Oliveira PJ. Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion. 2016;30:95–104 [DOI] [PubMed] [Google Scholar]
- 47.Oliveira PJ, Wallace KB. Depletion of adenine nucleotide translocator protein in heart mitochondria from doxorubicin-treated rats--relevance for mitochondrial dysfunction. Toxicology. 2006;220:160–168 [DOI] [PubMed] [Google Scholar]
- 48.Sinibaldi F, Howes BD, Droghetti E, Polticelli F, Piro MC, Di Pierro D, Fiorucci L, Coletta M, Smulevich G, Santucci R. Role of lysines in cytochrome c-cardiolipin interaction. Biochemistry. 2013;52:4578–4588 [DOI] [PubMed] [Google Scholar]
- 49.Duncan AL, Ruprecht JJ, Kunji ERS, Robinson AJ. Cardiolipin dynamics and binding to conserved residues in the mitochondrial adp/atp carrier. Biochim Biophys Acta Biomembr. 2018;1860:1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker MA, King V, Howard KP. Nuclear magnetic resonance study of doxorubicin binding to cardiolipin containing magnetically oriented phospholipid bilayers. Biochim. Biophys. Acta 2001;1514:206–216 [DOI] [PubMed] [Google Scholar]
- 51.Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys. Chem 1990;35:247–257 [DOI] [PubMed] [Google Scholar]
- 52.Goormaghtigh E, Huart P, Brasseur R, Ruysschaert JM. Mechanism of inhibition of mitochondrial enzymatic complex i-iii by adriamycin derivatives. Biochim. Biophys. Acta 1986;861:83–94 [DOI] [PubMed] [Google Scholar]
- 53.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: A kiss of death. Free Radic Biol Med 2009;46:1439–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kavazis AN, Morton AB, Hall SE, Smuder AJ. Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion. 2017;34:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollander JM, Thapa D, Shepherd DL. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Heart Circ. Physiol 2014;307:H1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vercesi AE, Castilho RF, Kowaltowski AJ, de Oliveira HCF, de Souza-Pinto NC, Figueira TR, Busanello ENB. Mitochondrial calcium transport and the redox nature of the calcium-induced membrane permeability transition. Free Radic Biol Med 2018;129:1–24 [DOI] [PubMed] [Google Scholar]
- 57.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin a is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem 1989;264:7826–7830 [PubMed] [Google Scholar]
- 58.Zhou S, Heller LJ, Wallace KB. Interference with calcium-dependent mitochondrial bioenergetics in cardiac myocytes isolated from doxorubicin-treated rats. Toxicol. Appl. Pharmacol 2001;175:60–67 [DOI] [PubMed] [Google Scholar]
- 59.Solem LE, Heller LJ, Wallace KB. Dose-dependent increase in sensitivity to calcium-induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J Mol Cell Cardiol 1996;28:1023–1032 [DOI] [PubMed] [Google Scholar]
- 60.Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, Wallace KB. Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol. Appl. Pharmacol 2004;200:159–168 [DOI] [PubMed] [Google Scholar]
- 61.Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res. 2001;61:771–777 [PubMed] [Google Scholar]
- 62.Pereira GC, Pereira SP, Pereira CV, Lumini JA, Magalhaes J, Ascensao A, Santos MS, Moreno AJ, Oliveira PJ. Mitochondrionopathy phenotype in doxorubicin-treated wistar rats depends on treatment protocol and is cardiac-specific. PLoS One. 2012;7:e38867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montaigne D, Marechal X, Preau S, Baccouch R, Modine T, Fayad G, Lancel S, Neviere R. Doxorubicin induces mitochondrial permeability transition and contractile dysfunction in the human myocardium. Mitochondrion. 2011;11:22–26 [DOI] [PubMed] [Google Scholar]
- 64.Marechal X, Montaigne D, Marciniak C, Marchetti P, Hassoun SM, Beauvillain JC, Lancel S, Neviere R. Doxorubicin-induced cardiac dysfunction is attenuated by ciclosporin treatment in mice through improvements in mitochondrial bioenergetics. Clin Sci (Lond). 2011;121:405–413 [DOI] [PubMed] [Google Scholar]
- 65.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med 2012;18:1639–1642 [DOI] [PubMed] [Google Scholar]
- 66.Jean SR, Tulumello DV, Riganti C, Liyanage SU, Schimmer AD, Kelley SO. Mitochondrial targeting of doxorubicin eliminates nuclear effects associated with cardiotoxicity. ACS Chem Biol. 2015;10:2007–2015 [DOI] [PubMed] [Google Scholar]
- 67.Nordgren KK, Wallace KB. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol. Appl. Pharmacol 2014;274:107–116 [DOI] [PubMed] [Google Scholar]
- 68.Lin EY, Bayarsengee U, Wang CC, Chiang YH, Cheng CW. The natural compound 2,3,5,4’-tetrahydroxystilbene-2-o-beta-d glucoside protects against adriamycin-induced nephropathy through activating the nrf2-keap1 antioxidant pathway. Environ. Toxicol 2018;33:72–82 [DOI] [PubMed] [Google Scholar]
- 69.Zhao X, Jin Y, Li L, Xu L, Tang Z, Qi Y, Yin L, Peng J. Microrna-128–3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting sirtuin-1. Pharmacol. Res 2019;146:104276. [DOI] [PubMed] [Google Scholar]
- 70.Sampaio SF, Branco AF, Wojtala A, Vega-Naredo I, Wieckowski MR, Oliveira PJ. P66shc signaling is involved in stress responses elicited by anthracycline treatment of rat cardiomyoblasts. Arch. Toxicol 2016;90:1669–1684 [DOI] [PubMed] [Google Scholar]
- 71.Tokarska-Schlattner M, Wallimann T, Schlattner U. Multiple interference of anthracyclines with mitochondrial creatine kinases: Preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol. Pharmacol 2002;61:516–523 [DOI] [PubMed] [Google Scholar]
- 72.Tokarska-Schlattner M, Zaugg M, da Silva R, Lucchinetti E, Schaub MC, Wallimann T, Schlattner U. Acute toxicity of doxorubicin on isolated perfused heart: Response of kinases regulating energy supply. Am. J. Physiol. Heart Circ. Physiol 2005;289:H37–47 [DOI] [PubMed] [Google Scholar]
- 73.Carvalho RA, Sousa RP, Cadete VJ, Lopaschuk GD, Palmeira CM, Bjork JA, Wallace KB. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology. 2010;270:92–98 [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Tang Y, Zhang YC, Huang XH, Xie YQ, Xiang Y. A metabolomic study of rats with doxorubicin-induced cardiomyopathy and shengmai injection treatment. PLoS One. 2015;10:e0125209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, Corton JC, Abbott BD. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niu QY, Li ZY, Du GH, Qin XM. (1)h nmr based metabolomic profiling revealed doxorubicin-induced systematic alterations in a rat model. J. Pharm. Biomed. Anal 2016;118:338–348 [DOI] [PubMed] [Google Scholar]
- 77.Schnackenberg LK, Pence L, Vijay V, Moland CL, George N, Cao Z, Yu LR, Fuscoe JC, Beger RD, Desai VG. Early metabolomics changes in heart and plasma during chronic doxorubicin treatment in b6c3f1 mice. J. Appl. Toxicol. 2016;36:1486–1495 [DOI] [PubMed] [Google Scholar]
- 78.Tan G, Lou Z, Liao W, Zhu Z, Dong X, Zhang W, Li W, Chai Y. Potential biomarkers in mouse myocardium of doxorubicin-induced cardiomyopathy: A metabonomic method and its application. PLoS One. 2011;6:e27683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berthiaume JM, Wallace KB. Persistent alterations to the gene expression profile of the heart subsequent to chronic doxorubicin treatment. Cardiovasc. Toxicol 2007;7:178–191 [DOI] [PubMed] [Google Scholar]
- 80.Yang Y, Zhang H, Li X, Yang T, Jiang Q. Effects of pparalpha/pgc-1alpha on the energy metabolism remodeling and apoptosis in the doxorubicin induced mice cardiomyocytes in vitro. Int J Clin Exp Pathol 2015;8:12216–12224 [PMC free article] [PubMed] [Google Scholar]
- 81.Arunachalam S, Kim SY, Kim MS, Yi HK, Yun BS, Lee DY, Hwang PH. Adriamycin inhibits adipogenesis through the modulation of ppargamma and restoration of adriamycin-mediated inhibition of adipogenesis by ppargamma over-expression. Toxicol. Mech. Methods 2012;22:540–546 [DOI] [PubMed] [Google Scholar]
- 82.Arunachalam S, Tirupathi Pichiah PB, Achiraman S. Doxorubicin treatment inhibits ppargamma and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS Lett. 2013;587:105–110 [DOI] [PubMed] [Google Scholar]
- 83.Chen ZC, Chen LJ, Cheng JT. Doxorubicin-induced cardiac toxicity is mediated by lowering of peroxisome proliferator-activated receptor delta expression in rats. PPAR Res. 2013;2013:456042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y, Zhang H, Li X, Yang T, Jiang Q. Effects of pparalpha/pgc-1alpha on the myocardial energy metabolism during heart failure in the doxorubicin induced dilated cardiomyopathy in mice. Int J Clin Exp Med 2014;7:2435–2442 [PMC free article] [PubMed] [Google Scholar]
- 85.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578 [DOI] [PubMed] [Google Scholar]
- 86.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the ppars and pgc1 alpha: Implications for metabolic disease. Appl. Physiol. Nutr. Metab 2007;32:874–883 [DOI] [PubMed] [Google Scholar]
- 87.Di W, Lv J, Jiang S, Lu C, Yang Z, Ma Z, Hu W, Yang Y, Xu B. Pgc-1: The energetic regulator in cardiac metabolism. Curr. Issues Mol. Biol 2018;28:29–46 [DOI] [PubMed] [Google Scholar]
- 88.Komen JC, Thorburn DR. Turn up the power - pharmacological activation of mitochondrial biogenesis in mouse models. Br. J. Pharmacol 2014;171:1818–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leone TC, Kelly DP. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb Symp Quant Biol 2011;76:175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the pgc-1 family regulatory network. Biochim. Biophys. Acta 2011;1813:1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012;23:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu ZM, Li CB, Liu QL, Li P, Yang H. Ginsenoside rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci 2018;19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H, Wang H, Liang EY, Zhou LX, Dong ZL, Liang P, Weng QF, Yang M. Thrombopoietin protects h9c2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol Lett. 2018;15:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coelho AR, Martins TR, Couto R, Deus C, Pereira CV, Simoes RF, Rizvanov AA, Silva F, Cunha-Oliveira T, Oliveira PJ, Serafim TL. Berberine-induced cardioprotection and sirt3 modulation in doxorubicin-treated h9c2 cardiomyoblasts. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2904–2923 [DOI] [PubMed] [Google Scholar]
- 95.Deus CM, Zehowski C, Nordgren K, Wallace KB, Skildum A, Oliveira PJ. Stimulating basal mitochondrial respiration decreases doxorubicin apoptotic signaling in h9c2 cardiomyoblasts. Toxicology. 2015;334:1–11 [DOI] [PubMed] [Google Scholar]
- 96.Yin J, Guo J, Zhang Q, Cui L, Zhang L, Zhang T, Zhao J, Li J, Middleton A, Carmichael PL, Peng S. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the pink1/parkin pathway. Toxicol. In Vitro. 2018;51:1–10 [DOI] [PubMed] [Google Scholar]
- 97.Luo P, Zhu Y, Chen M, Yan H, Yang B, Yang X, He Q. Hmgb1 contributes to adriamycin-induced cardiotoxicity via up-regulating autophagy. Toxicol. Lett 2018;292:115–122 [DOI] [PubMed] [Google Scholar]
- 98.Marques-Aleixo I, Santos-Alves E, Torrella JR, Oliveira PJ, Magalhaes J, Ascensao A. Exercise and doxorubicin treatment modulate cardiac mitochondrial quality control signaling. Cardiovasc. Toxicol 2018;18:43–55 [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Levine B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015;22:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Wang XL, Chen HL, Wu D, Chen JX, Wang XX, Li RL, He JH, Mo L, Cen X, Wei YQ, Jiang W. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through ampk and p38-mapk. Biochem. Pharmacol 2014;88:334–350 [DOI] [PubMed] [Google Scholar]
- 101.Gharanei M, Hussain A, Janneh O, Maddock H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: A mitochondrial division/mitophagy inhibitor. PLoS One. 2013;8:e77713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor gata4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J. Biol. Chem. 2010;285:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zilinyi R, Czompa A, Czegledi A, Gajtko A, Pituk D, Lekli I, Tosaki A. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: The role of autophagy. Molecules. 2018;23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park JH, Choi SH, Kim H, Ji ST, Jang WB, Kim JH, Baek SH, Kwon SM. Doxorubicin regulates autophagy signals via accumulation of cytosolic ca(2+) in human cardiac progenitor cells. Int J Mol Sci. 2016;17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdullah CS, Alam S, Aishwarya R, Miriyala S, Bhuiyan MAN, Panchatcharam M, Pattillo CB, Orr AW, Sadoshima J, Hill JA, Bhuiyan MS. Doxorubicin-induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Scientific reports. 2019;9:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A, Xie M, Jiang N, May H, Kyrychenko V, Schneider JW, Gillette TG, Hill JA. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 2016;133:1668–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pizarro M, Troncoso R, Martinez GJ, Chiong M, Castro PF, Lavandero S. Basal autophagy protects cardiomyocytes from doxorubicin-induced toxicity. Toxicology. 2016;370:41–48 [DOI] [PubMed] [Google Scholar]
- 108.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, Matoba S. Cytosolic p53 inhibits parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nature communications. 2013;4:2308. [DOI] [PubMed] [Google Scholar]
- 109.Koleini N, Kardami E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget. 2017;8:46663–46680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartlett JJ, Trivedi PC, Yeung P, Kienesberger PC, Pulinilkunnil T. Doxorubicin impairs cardiomyocyte viability by suppressing transcription factor eb expression and disrupting autophagy. Biochem. J. 2016;473:3769–3789 [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, Liang Q. Transcription factor gata4 regulates cardiac bcl2 gene expression in vitro and in vivo. FASEB J. 2006;20:800–802 [DOI] [PubMed] [Google Scholar]
- 112.Juarez-Flores DL, Gonzalez-Casacuberta I, Ezquerra M, Bano M, Carmona-Pontaque F, Catalan-Garcia M, Guitart-Mampel M, Rivero JJ, Tobias E, Milisenda JC, Tolosa E, Marti MJ, Fernandez-Santiago R, Cardellach F, Moren C, Garrabou G. Exhaustion of mitochondrial and autophagic reserve may contribute to the development of lrrk2 (g2019s) -parkinson’s disease. Journal of translational medicine. 2018;16:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann. N. Y. Acad. Sci 1999;874:156–168 [DOI] [PubMed] [Google Scholar]
- 114.Jing X, Yang J, Jiang L, Chen J, Wang H. Microrna-29b regulates the mitochondria-dependent apoptotic pathway by targeting bax in doxorubicin cardiotoxicity. Cell Physiol Biochem. 2018;48:692–704 [DOI] [PubMed] [Google Scholar]
- 115.Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Scientific reports. 2017;7:44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang XL, Wang X, Xiong LL, Zhu Y, Chen HL, Chen JX, Wang XX, Li RL, Guo ZY, Li P, Jiang W. Salidroside improves doxorubicin-induced cardiac dysfunction by suppression of excessive oxidative stress and cardiomyocyte apoptosis. J Cardiovasc Pharmacol. 2013;62:512–523 [DOI] [PubMed] [Google Scholar]
- 117.Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, Steinmetz N, van Eck-Smit BL. Annexin v imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45:842–848 [PubMed] [Google Scholar]
- 118.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and bcl-2:Bax ratio. Cancer Res. 2002;62:4592–4598 [PubMed] [Google Scholar]
- 119.Moreira AC, Branco AF, Sampaio SF, Cunha-Oliveira T, Martins TR, Holy J, Oliveira PJ, Sardao VA. Mitochondrial apoptosis-inducing factor is involved in doxorubicin-induced toxicity on h9c2 cardiomyoblasts. Biochim. Biophys. Acta. 2014;1842:2468–2478 [DOI] [PubMed] [Google Scholar]
- 120.Sardao VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in h9c2 cardiomyoblasts. Cancer Chemother Pharmacol. 2009;64:811–827 [DOI] [PubMed] [Google Scholar]
- 121.Rasola A, Bernardi P. Mitochondrial permeability transition in ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233 [DOI] [PubMed] [Google Scholar]
- 122.Zhu H, Sun A. Programmed necrosis in heart disease: Molecular mechanisms and clinical implications. J Mol Cell Cardiol. 2018;116:125–134 [DOI] [PubMed] [Google Scholar]
- 123.Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through sirt1-mediated deacetylation of p53. Cardiovasc Res. 2011;90:538–545 [DOI] [PubMed] [Google Scholar]
- 124.Bae S, Siu PM, Choudhury S, Ke Q, Choi JH, Koh YY, Kang PM. Delayed activation of caspase-independent apoptosis during heart failure in transgenic mice overexpressing caspase inhibitor crma. Am. J. Physiol. Heart Circ. Physiol 2010;299:H1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM. Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol. Cell. Biochem 2005;273:25–32 [DOI] [PubMed] [Google Scholar]
- 126.Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW 2nd, Kirshenbaum LA. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5537–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Serrano J, Palmeira CM, Kuehl DW, Wallace KB. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim. Biophys. Acta. 1999;1411:201–205 [DOI] [PubMed] [Google Scholar]
- 128.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol. Lett 2001;121:151–157 [DOI] [PubMed] [Google Scholar]
- 129.Palmeira CM, Serrano J, Kuehl DW, Wallace KB. Preferential oxidation of cardiac mitochondrial DNA following acute intoxication with doxorubicin. Biochim. Biophys. Acta 1997;1321:101–106 [DOI] [PubMed] [Google Scholar]
- 130.Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim. Biophys. Acta 2011;1813:1295–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J. Biol. Chem 1971;246:2425–2429 [PubMed] [Google Scholar]
- 132.Bansal N, Amdani SM, Hutchins KK, Lipshultz SE. Cardiovascular disease in survivors of childhood cancer. Curr Opin Pediatr. 2018;30:628–638 [DOI] [PubMed] [Google Scholar]
- 133.Faustin B, Rossignol R, Rocher C, Benard G, Malgat M, Letellier T. Mobilization of adenine nucleotide translocators as molecular bases of the biochemical threshold effect observed in mitochondrial diseases. J. Biol. Chem 2004;279:20411–20421 [DOI] [PubMed] [Google Scholar]
- 134.Mazat JP, Rossignol R, Malgat M, Rocher C, Faustin B, Letellier T. What do mitochondrial diseases teach us about normal mitochondrial functions...That we already knew: Threshold expression of mitochondrial defects. Biochim. Biophys. Acta 2001;1504:20–30 [DOI] [PubMed] [Google Scholar]
- 135.Ashley N, Poulton J. Anticancer DNA intercalators cause p53-dependent mitochondrial DNA nucleoid re-modelling. Oncogene. 2009;28:3880–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ferreira A, Cunha-Oliveira T, Simoes RF, Carvalho FS, Burgeiro A, Nordgren K, Wallace KB, Oliveira PJ. Altered mitochondrial epigenetics associated with subchronic doxorubicin cardiotoxicity. Toxicology. 2017;390:63–73 [DOI] [PubMed] [Google Scholar]
- 137.Ferreira LL, Cunha-Oliveira T, Veloso CD, Costa CF, Wallace KB, Oliveira PJ. Single nanomolar doxorubicin exposure triggers compensatory mitochondrial responses in h9c2 cardiomyoblasts. Food Chem. Toxicol 2019;124:450–461 [DOI] [PubMed] [Google Scholar]
- 138.Hanf A, Oelze M, Manea A, Li H, Munzel T, Daiber A. The anti-cancer drug doxorubicin induces substantial epigenetic changes in cultured cardiomyocytes. Chem Biol Interact. 2019;313:108834. [DOI] [PubMed] [Google Scholar]
- 139.Piotrowska I, Isalan M, Mielcarek M. Early transcriptional alteration of histone deacetylases in a murine model of doxorubicin-induced cardiomyopathy. PLoS One. 2017;12:e0180571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosa-Garrido M, Chapski DJ, Schmitt AD, Kimball TH, Karbassi E, Monte E, Balderas E, Pellegrini M, Shih TT, Soehalim E, Liem D, Ping P, Galjart NJ, Ren S, Wang Y, Ren B, Vondriska TM. High-resolution mapping of chromatin conformation in cardiac myocytes reveals structural remodeling of the epigenome in heart failure. Circulation. 2017;136:1613–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berezin A Epigenetics in heart failure phenotypes. BBA Clin. 2016;6:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Warren JS, Oka SI, Zablocki D, Sadoshima J. Metabolic reprogramming via pparalpha signaling in cardiac hypertrophy and failure: From metabolomics to epigenetics. Am. J. Physiol. Heart Circ. Physiol 2017;313:H584–H596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10:12–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Voest EE, van Acker SA, van der Vijgh WJ, van Asbeck BS, Bast A. Comparison of different iron chelators as protective agents against acute doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol 1994;26:1179–1185 [DOI] [PubMed] [Google Scholar]
- 145.Hasinoff BB, Patel D, Wu X. The oral iron chelator icl670a (deferasirox) does not protect myocytes against doxorubicin. Free Radic Biol Med. 2003;35:1469–1479 [DOI] [PubMed] [Google Scholar]
- 146.Deng S, Yan T, Jendrny C, Nemecek A, Vincetic M, Godtel-Armbrust U, Wojnowski L. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both topoisomerase ii isoforms. BMC Cancer. 2014;14:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kametani R, Miura T, Harada N, Shibuya M, Wang R, Tan H, Fukagawa Y, Kawamura S, Matsuzaki M. Carvedilol inhibits mitochondrial oxygen consumption and superoxide production during calcium overload in isolated heart mitochondria. Circ J. 2006;70:321–326 [DOI] [PubMed] [Google Scholar]
- 148.Oliveira PJ, Esteves T, Rolo AP, Palmeira CM, Moreno AJ. Carvedilol inhibits the mitochondrial permeability transition by an antioxidant mechanism. Cardiovasc. Toxicol. 2004;4:11–20 [DOI] [PubMed] [Google Scholar]
- 149.Cocco T, Cutecchia G, Montedoro G, Lorusso M. The antihypertensive drug carvedilol inhibits the activity of mitochondrial nadh-ubiquinone oxidoreductase. J. Bioenerg. Biomembr. 2002;34:251–258 [DOI] [PubMed] [Google Scholar]
- 150.Oliveira PJ, Rolo AP, Palmeira CM, Moreno AJ. Carvedilol reduces mitochondrial damage induced by hypoxanthine/xanthine oxidase: Relevance to hypoxia/reoxygenation injury. Cardiovasc. Toxicol 2001;1:205–213 [DOI] [PubMed] [Google Scholar]
- 151.Santos DL, Moreno AJ, Leino RL, Froberg MK, Wallace KB. Carvedilol protects against doxorubicin-induced mitochondrial cardiomyopathy. Toxicol. Appl. Pharmacol 2002;185:218–227 [DOI] [PubMed] [Google Scholar]
- 152.Bertrand JT, Brown LF, Kinzonzi M, Mansilu M, Djunghu B. Aids knowledge in three sites in bas-zaire. AIDS Educ. Prev 1992;4:251–266 [PubMed] [Google Scholar]
- 153.Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch Biochem Biophys. 1998;352:229–236 [DOI] [PubMed] [Google Scholar]
- 154.Berthiaume JM, Oliveira PJ, Fariss MW, Wallace KB. Dietary vitamin e decreases doxorubicin-induced oxidative stress without preventing mitochondrial dysfunction. Cardiovasc. Toxicol. 2005;5:257–267 [DOI] [PubMed] [Google Scholar]
- 155.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: Cardioprotection by mito-q. Biophys. J 2009;96:1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiong C, Wu YZ, Zhang Y, Wu ZX, Chen XY, Jiang P, Guo HC, Xie KR, Wang KX, Su SW. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol Lett. 2018;15:5721–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao Y, Tian X, Liu G, Wang K, Xie Y, Qiu Y. Berberine protects myocardial cells against anoxia-reoxygenation injury via p38 mapk-mediated nf-kappab signaling pathways. Exp Ther Med 2019;17:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abe J, Yamada Y, Takeda A, Harashima H. Cardiac progenitor cells activated by mitochondrial delivery of resveratrol enhance the survival of a doxorubicin-induced cardiomyopathy mouse model via the mitochondrial activation of a damaged myocardium. J Control Release. 2018;269:177–188 [DOI] [PubMed] [Google Scholar]
- 159.Lee C, Yen K, Cohen P. Humanin: A harbinger of mitochondrial-derived peptides? Trends Endocrinol. Metab 2013;24:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lue Y, Gao C, Swerdloff R, Hoang J, Avetisyan R, Jia Y, Rao M, Ren S, Atienza V, Yu J, Zhang Y, Chen M, Song Y, Wang Y, Wang C. Humanin analog enhances the protective effect of dexrazoxane against doxorubicin-induced cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol 2018;315:H634–H643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang F, Iskra B, Kleinerman E, Alvarez-Florez C, Andrews T, Shaw A, Chandra J, Schadler K, Aune GJ. Aerobic exercise during early murine doxorubicin exposure mitigates cardiac toxicity. J Pediatr Hematol Oncol. 2018;40:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol. 2013;62:263–269 [DOI] [PubMed] [Google Scholar]
- 163.Kavazis AN, Smuder AJ, Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced foxo transcription in cardiac and skeletal muscle. J Appl Physiol (1985). 2014;117:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ascensao A, Lumini-Oliveira J, Machado NG, Ferreira RM, Goncalves IO, Moreira AC, Marques F, Sardao VA, Oliveira PJ, Magalhaes J. Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin Sci (Lond). 2011;120:37–49 [DOI] [PubMed] [Google Scholar]
- 165.Pfannenstiel K, Hayward R. Effects of resistance exercise training on doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. 2018;71:332–339 [DOI] [PubMed] [Google Scholar]
- 166.Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, Virani SA, McKenzie DC, Stohr EJ, Waburton DER, Campbell KL. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: A rct. Breast Cancer Res Treat. 2018;167:719–729 [DOI] [PubMed] [Google Scholar]
- 167.Kirkham AA, Shave RE, Bland KA, Bovard JM, Eves ND, Gelmon KA, McKenzie DC, Virani SA, Stohr EJ, Warburton DER, Campbell KL. Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: A proof-of-concept rct. Int J Cardiol. 2017;245:263–270 [DOI] [PubMed] [Google Scholar]
- 168.Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol (1985). 2006;100:519–527 [DOI] [PubMed] [Google Scholar]
- 169.Skildum A, Dornfeld K, Wallace K. Mitochondrial amplification selectively increases doxorubicin sensitivity in breast cancer cells with acquired antiestrogen resistance. Breast Cancer Res Treat. 2011;129:785–797 [DOI] [PubMed] [Google Scholar]
- 170.Hall SE, Smuder AJ, Hayward R. Effects of calorie restriction and voluntary exercise on doxorubicin-induced cardiotoxicity. Integr Cancer Ther. 2019;18:1534735419843999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dutta D, Xu J, Dirain ML, Leeuwenburgh C. Calorie restriction combined with resveratrol induces autophagy and protects 26-month-old rat hearts from doxorubicin-induced toxicity. Free Radic Biol Med. 2014;74:252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.An L, Hu XW, Zhang S, Hu X, Song Z, Naz A, Zi Z, Wu J, Li C, Zou Y, He L, Zhu H. Uvrag deficiency exacerbates doxorubicin-induced cardiotoxicity. Scientific reports. 2017;7:43251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yue P, Jing S, Liu L, Ma F, Zhang Y, Wang C, Duan H, Zhou K, Hua Y, Wu G, Li Y. Association between mitochondrial DNA copy number and cardiovascular disease: Current evidence based on a systematic review and meta-analysis. PLoS One. 2018;13:e0206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Foote K, Reinhold J, Yu EPK, Figg NL, Finigan A, Murphy MP, Bennett MR. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell. 2018;17:e12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pereira CV, Oliveira PJ, Will Y, Nadanaciva S. Mitochondrial bioenergetics and drug-induced toxicity in a panel of mouse embryonic fibroblasts with mitochondrial DNA single nucleotide polymorphisms. Toxicol. Appl. Pharmacol. 2012;264:167–181 [DOI] [PubMed] [Google Scholar]
- 176.Lozoya OA, Martinez-Reyes I, Wang T, Grenet D, Bushel P, Li J, Chandel N, Woychik RP, Santos JH. Mitochondrial nicotinamide adenine dinucleotide reduced (nadh) oxidation links the tricarboxylic acid (tca) cycle with methionine metabolism and nuclear DNA methylation. PLoS Biol. 2018;16:e2005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aon MA, Cortassa S, Juhaszova M, Sollott SJ. Mitochondrial health, the epigenome and healthspan. Clin Sci (Lond). 2016;130:1285–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Minocherhomji S, Tollefsbol TO, Singh KK. Mitochondrial regulation of epigenetics and its role in human diseases. Epigenetics. 2012;7:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]