Abstract
N6-methyladenosine (m6A) RNA modification emerges in recent years as a new layer of regulatory mechanism controlling gene expression in eukaryotes. As a reversible epigenetic modification found not only in messenger RNAs (mRNAs), but also in non-coding RNAs (ncRNAs), m6A affects the fate of the modified RNA molecules and plays important roles in almost all vital bioprocesses, including cancer development. Here we review the up-to-date knowledge of the pathological roles and underlying molecular mechanism of m6A modifications (in both coding and non-coding RNAs) in cancer pathogenesis and drug response/resistance, and discuss the therapeutic potential of targeting m6A regulators for cancer therapy.
Keywords: RNA modification, N6-methyladenosine (m6A), cancer epigenetics, epitranscriptome, cancer stem cells, immune therapy, drug resistance, prognosis, non-coding RNA, targeted therapeutics
The regulation and function of RNA m6A modification
RNA m6A modification in eukaryotic RNA
Nucleotides, composed of nucleosides and phosphate groups, are the molecular building blocks of DNA and RNA. Accumulating number of chemical modifications on adenosine (A), guanosine (G), cytidine (C), and uridine (U) nucleosides on RNA have been identified since 1950s, adding additional complexity into the RNA world by broadly affecting the structure, biogenesis and function of RNA. N6-methyladenosine (m6A), methylated adenosine at the N6 position, is a widespread and abundant modification in messenger RNA (mRNA) and non-coding RNAs (ncRNAs), and represents one of the well-studied RNA modifications thus far. Since the first discovery in 1970s (Desrosiers et al., 1974; Perry and Kelley, 1974), m6A has been identified as the most prevalent internal modification in mRNA in most eukaryotic species including mammals (Adams and Cory, 1975; Desrosiers et al., 1974; Perry and Kelley, 1974; Perry et al., 1975), insects (Levis and Penman, 1978), plants (Nichols, 1979), yeast (Clancy et al., 2002), as well as in some viruses (Aloni et al., 1979; Beemon and Keith, 1977). However, this field did not move forward too much in several decades due to the lack of molecular biology, quantification and sequencing methodologies to comprehensively study m6A modifications in the transcriptome (Deng et al., 2018a; Deng et al., 2018c; Huang et al., 2020b).
The identification of the fat mass and obesity-associated protein (FTO) as the first m6A demethylase in 2011 suggested that m6A modification is reversible and dynamic and thus is likely functionally important (Jia et al., 2011). Thereafter, the functional importance of m6A modifications has been reported in virtually all major bioprocesses, normal development and diseases (including cancers) (reviewed in (Deng et al., 2018c; Frye et al., 2018; Roundtree et al., 2017a)). The landscape of m6A in the transcriptome (so called “epitranscriptome”) was first delineated by next generation sequencing (NGS) in 2012 (Dominissini et al., 2012; Meyer et al., 2012). m6A were identified in approximately one third of the mammalian mRNAs, with an average of 3-5 m6A modifications in each mRNA, and many m6A sites are evolutionally conserved between humans and mice. The deposition of m6A modification in the transcriptome is not random. The m6A modification sites have a typical consensus sequence DRACH (D=G, A or U; R= G or A; H= A, C or U), and are enriched in the coding sequence (CDS) and 3’ untranslated region (3’ UTR), with a particularly high enrichment around the stop codon area (Dominissini et al., 2012; Meyer et al., 2012). Thus far, several antibody-dependent (such as methylated RNA immunoprecipitation sequencing (MeRIP-seq) and m6A individual-nucleotide-resolution cross-linking and immunoprecipitation (miCLIP)) (Dominissini et al., 2012; Linder et al., 2015; Meyer et al., 2012) and -independent (such as MAZTER-seq, m6A-sensitive RNA-endoribonuclease–facilitated sequencing (m6A-REF-seq), and deamination adjacent to RNA modification targets sequencing (DART-seq)) (Garcia-Campos et al., 2019; Meyer, 2019; Zhang et al., 2019e) NGS methods have been developed for m6A sequencing, which make high-resolution detection of m6A epitrancriptome in diverse cell contexts a reality (reviewed by Huang et al., 2020a). The advent of such NGS-based methods advanced our understanding of this epigenetic mark. It has now become clear that m6A exists on almost every type of RNAs, including mRNAs, ribosomal RNAs (rRNAs), long non-coding RNAs (lncRNAs), microRNAs (miRNAs), small nulear RNAs (snRNAs), circular RNAs (circRNAs), and is dynamically regulated during many physiological and pathological processes, including tumorigenesis.
Dynamic regulation of m6A by ‘writers’ and ‘erasers’
Similar to DNA and protein, RNA can be methylated and demethylated by dedicated methyltransferases (also known as ‘writers’) and demethylases (also known as ‘erasers’), respectively. As the first known reversible mRNA modification, m6A has become a main focus in the epitranscriptomics field and a set of ‘writers’ and ‘erasers’ proteins of m6A have been characterized (Figure 1).
Figure 1.
Reversible m6A modification on mRNA. The adenosine (A) bases reside in mRNA could be methylated to form N6-methyladenosine (m6A) by the large MTC writer complex comprised of the METTL3-METTL14-WTAP core component and other regulatory cofactors or by METTL16 alone. This enzymatic reaction uses S-Adenosyl methionine (SAM) as a methyl donor. m6A could be recognized by m6A binding proteins (readers) to affect mRNA fate, or could be reversibly removed by m6A eraser proteins (i.e., FTO and ALKBH5). The demethylatio process requires α-Ketoglutaric acid (α-KG) and molecular oxygen (O2) as co-substrates and ferrous iron (Fe2+) as a cofactor.
The deposition of m6A modifications in mRNAs is executed by a multicomponent m6A methyltransferase complex (MTC), which is comprised of a core component, the methyltransferase-like 3 (METTL3)/methyltransferase-like 14 (METTL14) heterodimer, and other regulatory factors including WTAP, KIAA1429, ZC3H13, and RBM15/RBM15B (Knuckles et al., 2018; Liu et al., 2014; Ping et al., 2014; Schwartz et al., 2014; Wen et al., 2018; Yue et al., 2018). Within this complex, METTL3 is the sole catalytic subunit that binds to the methyl donor S-adenosyl methionine (SAM) and catalyzes methyl group transfer (Sledz and Jinek, 2016; Wang et al., 2016a; Wang et al., 2016b), whereas METTL14 is critical for m6A deposition by stabilizing METTL3 conformation and recognizing substrate RNAs (Sledz and Jinek, 2016; Wang et al., 2016a; Wang et al., 2016b). Recently, it was reported that METTL14 could recognize histone H3 lysine 36 trimethylation (H3K36me3) modification and mediate the selectivity of m6A deposition in mRNA (Huang et al., 2020a; Huang et al., 2019a). Distinct from the regulatory role of H3K36me3 in universal m6A modification, some transcription factors, such as ZFP217, SMAD2/3, and CEBPZ, could regulate m6A deposition in certain mRNAs in some special cell contexts by recruiting or repelling MTC (Aguilo et al., 2015; Barbieri et al., 2017; Bertero et al., 2018). Although MTC catalyzes most of the m6A methylation in poly(A) RNA, methyltransferase like 16 (METTL16), zinc finger CCHC-type containing 4 (ZCCHC4), and methyltransferase like 5 (METTL5) have also been identified as m6A methyltransferases, which could function alone and catalyze m6A on some structured RNAs, such as U6 snRNA, 28S rRNA, and 18S rRNA, respectively (Brown et al., 2016; Ma et al., 2019; Mendel et al., 2018; Pendleton et al., 2017; Tran et al., 2019; Warda et al., 2017).
RNA m6A modification could be removed by alpha-ketoglutarate (αKG)- and Fe(II)-dependent demethylases, specifically, the fat mass and obesity-associated protein (FTO) and alkB homolog 5 (ALKBH5) (Jia et al., 2011; Zheng et al., 2013), thus conferring dynamic regulation of m6A methylation under physiological and pathological conditions. As the first reported human demethylase of m6A, FTO could mediate demethylation of both internal m6A and 5’ cap m6Am (N6,2'-O-dimethyladenosine) in mRNA (Jia et al., 2011; Linder et al., 2015). However, internal m6A in mRNA is the major substrate of FTO in many cell types studied so far, including acute myeloid leukemia (AML) cells (Su et al., 2018; Wei et al., 2018; Zhang et al., 2019d). FTO plays oncogenic roles by promoting proliferation and inhibiting differentiation in AML cells (Li et al., 2017d), in which FTO is extensively expressed in cytoplasm and dramatically demethylates cytoplasmic m6A in mRNA, linking the oncogenic function of FTO to its cytoplasmic m6A demethylation activity (Wei et al., 2018). In other cases, functional roles of FTO inside nucleus still need to be addressed by additional studies. Unlike FTO, ALKBH5 seems to be an m6A-specific demethylase.
The writers and erasers are important for maintaining proper m6A level and gene expression across human tissues and cells. The dynamic balance between the deposition and removal of m6A modification is essential for normal bioprocesses and development. Therefore, mutation or dysregulation of writers and erasers is usually associated with diseases, such as cancers, by aberrantly adding or removing of m6A in RNA transcripts with critical biological functions.
Multifunction of m6A on mRNA fate decision
Gene expression is tightly controlled at different levels, including transcription, post-transcription, translation and post-translation. m6A is deposited on native RNA transcripts during transcription and influences gene expression post-transcriptionally through altering RNA structure or specific recognition by m6A-binding proteins (also known as ‘readers’) (Figure 2A).
Figure 2.
Functions of m6A modifications on coding and non-coding RNAs. m6A marks are deposited cotranscriptionally into nascent mRNAs, which undergo alternative splicing with the recruitment of splicing factors to m6A sites or flanking sequences. After splicing, m6A-containing mRNAs are recognized by YTHDC1 and exported into cytoplasm. Existence of m6A on mature mRNAs affects mRNA stability (stabilized by IGF2BP1/2/3 proteins and destabilized by YTHDF2/3 proteins), translation initiation, and translation elongation. m6A marks on lncRNAs play roles in RNA-RNA interaction, RNA-protein interaction, and chromatin remodeling. Anti-sense lncRNAs could modulate m6A abundance on the sense mRNA by recruiting m6A eraser (i.e., ALKBH5). Presence of m6A on pri-miRNA facilitates miRNA processing. In circRNAs, m6A could promote protein synthesis or inhibit circRNA immunity.
The m6A writers and erasers are located in nuclear speckles, where they are associated with mRNA splicing factors, implying the functional relevance of m6A with mRNA splicing (Bartosovic et al., 2017; Jia et al., 2011; Ping et al., 2014; Zheng et al., 2013). Indeed, m6A on precursor mRNAs (pre-mRNAs) could recruit splicing factor heterogeneous nuclear ribonucleoproteins A2/B1 (hnRNPA2B1) or increase the accessibility of flanking RNA sequence to splicing factors heterogeneous nuclear ribonucleoproteins C (hnRNPC) and hnRNPG by changing local structure, a mechanism known as “m6A switch” (Alarcon et al., 2015a; Liu et al., 2015; Liu et al., 2017; Zhou et al., 2019). On the other hand, m6A could also be bound by the reader protein YTH domain-containing protein 1 (YTHDC1), which recruits the splicing factor serine/arginine-rich splicing factor 3 (SRSF3) but repels SRSF10 to promote exon inclusion in targeted mRNAs (Xiao et al., 2016). YTHDC1 also affects the exportation m6A-modified mRNA transcripts from nucleus to cytoplasm (Roundtree et al., 2017b).
Cytoplasmic RNA could be loaded on ribosome for active translation or be sorted to messenger ribonucleoprotein (mRNP) foci, such as processing bodies (P-bodies) and stress granules, for degradation or storage. The YT521-B homology (YTH) domain-containing family proteins (YTHDFs) tend to accelerate metabolism of m6A-modified mRNAs in the cytoplasm. YTHDF1 selectively recognizes m6A and interacts with initiation factor eIF3 to promote translation initiation and protein synthesis (Wang et al., 2015). In contrast, YTHDF2 brings m6A-modified translatable mRNAs to mRNA decay sites (e.g., P-bodies), and recruits CCR4-NOT deadenylase complex to trigger deadenylation and degradation of the transcripts (Du et al., 2016; Wang et al., 2014a). YTHDF3 promotes mRNA translation in synergy with YTHDF1 and accelerated decay of m6A-containing mRNAs through interaction with YTHDF2 (Li et al., 2017a; Shi et al., 2017). In contrast to the destabilizing function of YTHDF2/3, the insulin-like growth factor-2 mRNA-binding protein (IGF2BP) family, including IGF2BP1, IGF2BP2, and IGF2BP3, protect m6A-modified mRNAs in P-bodies and stress granules from degradation through interacting with ELAV like RNA binding protein 1 (ELAVL1, also known as HuR), matrin 3 (MATR3), and poly(A) binding protein cytoplasmic 1 (PABPC1), and facilitate mRNA translation (Huang et al., 2018). Interestingly, METTL3 can also act as a reader in cytoplasm to promote translation of a subset of m6A-modified mRNAs independently of its methyltransferase activity (Choe et al., 2018; Lin et al., 2016). Since m6A is usually enriched around stop codon and far away from the translation initiation site, the role of m6A in promoting translation initiation is achieved by a closed-loop model, where mRNA circularization is mediated through the interaction between the eukaryotic translation initiation factors (eIFs) subunit at the 5’end of the mRNA and METTL3 or YTHDF1 bound to m6A sites near stop codon (Choe et al., 2018; Wang et al., 2015). It was also reported that m6A resided in 5’UTR could promote cap-independent translation through recruiting eIF3a to nearby translation initiation sites (Meyer et al., 2015). During translation elongation, although m6A-modified codon is same with unmodified codon in terms of amino acid coding, m6A modification on mRNA acts as a barrier to delay tRNA accommodation and thereby perturbs translation elongation dynamics (Choi et al., 2016).
m6A modification in ncRNAs
ncRNAs are a group of endogenous RNA molecules that are not translated into proteins but have specialized functions in the regulation of gene expression, which can be divided into lncRNAs with length exceeding 200 nucleotides (nt) and small ncRNAs less than 200 nt in length. In addition to protein-coding mRNAs, m6A modifications were found in ncRNAs including lncRNAs and small ncRNAs (such as miRNAs and snRNAs), and were shown to be important for their expression and functions (Brown et al., 2016; Linder et al., 2015; Liu et al., 2013; Meyer et al., 2012; Pendleton et al., 2017; Warda et al., 2017) (Figure 2B).
The m6A methylation in lncRNAs was observed when mapping m6A on poly(A) RNAs (Dominissini et al., 2012; Meyer et al., 2012). Without coding ability, lncRNAs are involved in gene expression regulation via interaction with RNA binding proteins, crosstalk with other RNA species, or chromatin remodeling. It was reported that m6A modification on lncRNA could affect RNA-protein interaction. For instance, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a conserved lncRNA whose mutation or upregulation has been consistently associated with tumorigenesis and metastasis. MALAT1 is often highly methylated with m6A, with multiple m6A sites detected by MeRIP-seq and site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET) (Dominissini et al., 2012; Liu et al., 2013; Meyer et al., 2012). Two of these m6A residues were reported to prevent formation of RNA local secondary structures and increase the recognition and binding of hnRNPC to a U5-tract in the MALAT1 hairpin through an ‘m6A switch’ mechanism (Liu et al., 2015). It was recently reported that m6A also plays a role on the RNA-RNA interaction function of lncRNAs, where internal m6A modification of the large intergenic coding RNA 1281 (linc1281) is required for sequestering pluripotency-related let-7 family miRNAs to ensure mouse embryonic stem cell identity (Yang et al., 2018). On the other hand, lncRNAs could also interact with m6A regulators to facilitate their function. For example, FOXM1-AS, a lncRNA antisense to FOXM1, promotes the interaction of ALKBH5 with FOXM1 nascent transcripts (Zhang et al., 2017c). Depleting FOXM1-AS phenocopied depletion of ALKBH5 in regulating FOXM1 methylation and expression (Zhang et al., 2017c). Similarly, GAS5-AS, a lncRNA antisense to GAS5, could enhanced GAS5 stability by interacting with ALKBH5 and regulating m6A modifications of GAS5 (Wang et al., 2019c). It appears that the m6A modulating function might be common for antisense lncRNAs.
miRNAs are a class of small and highly abundant non-coding RNAs involved in gene silencing or post-transcriptional gene expression regulation. Transcribed from DNA, the primary transcripts of miRNAs (pri-miRNAs) undergo a series of cleavage to form hair-pin precursor, precursor miRNAs (pre-miRNAs), and mature miRNAs. The canonical m6A motif GGAC are enriched in pri-miRNAs, but not in pre-miRNAs and mature miRNAs, and most of them can be methylated by METTL3 in cellulo (Alarcon et al., 2015b). The pri-miRNAs with m6A marks could be recognized by hnRNPA2B1, which interacts with DGCR8 and promotes miRNA processing (Alarcon et al., 2015a). Therefore, alternation of m6A level by METTL3 knockdown or overexpression could result in significant changes in the mature miRNA pool (Alarcon et al., 2015b).
CircRNAs are a new type of ncRNAs which, unlike linear RNAs, form a covalently closed continuous loop by back splicing. Recently, it was found that m6A modifications were also widespread in circRNAs and were written and read by the same machinery as did mRNAs (Zhou et al., 2017). However, the m6A enrichment pattern in circRNAs is distinct from that of mRNAs, exhibiting enrichment at the translation start site of their corresponding mRNAs (Zhou et al., 2017). It appeared that recognition of m6A-modified circRNAs by YTHDF2 did not promote degradation of circRNAs but instead played a role in regulating mRNA stability and circRNA immunity (Chen et al., 2019c; Zhou et al., 2017). m6A could also drive protein synthesis from circRNAs through recruiting YTHDF3 and initiation factor eIF4G2 (Yang et al., 2017a).
Aberrant m6A regulation in cancers
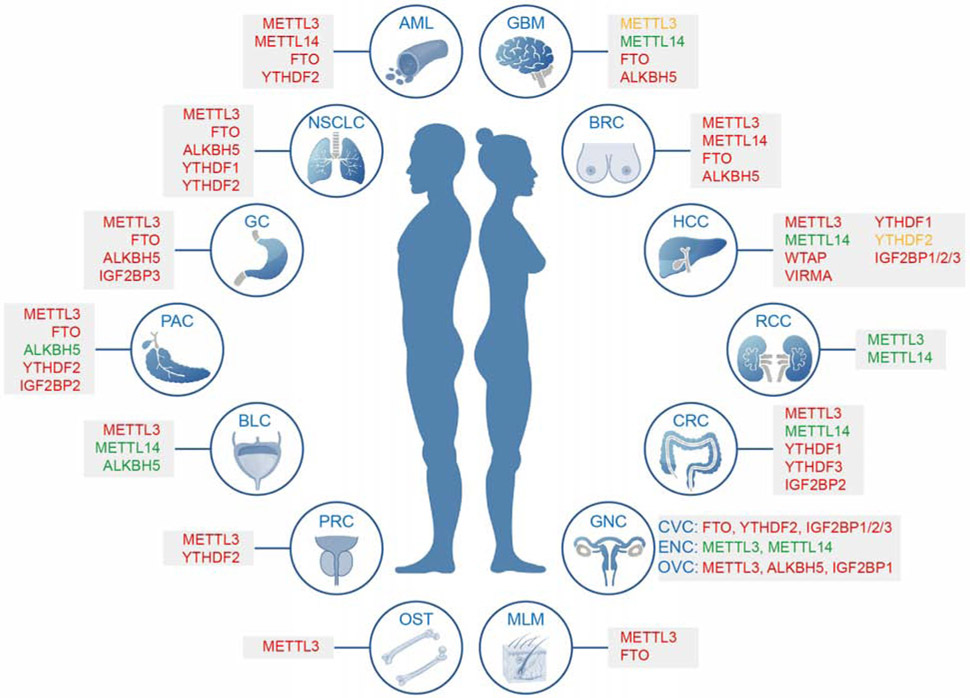
Although m6A modification does not change base pairing and coding, it broadly affects gene expression in multiple levels through interacting with diverse reader proteins and associated complexes. Therefore, dynamic m6A modification is critical for many normal bioprocesses, including self-renewal and differentiation of embryonic stem cells and hematopoietic stem cells, tissue development, circadian rhythm, heat shock or DNA damage response, and sex determination (Alarcon et al., 2015b; Chen et al., 2015; Deng et al., 2018c; Geula et al., 2015; Huang et al., 2020a; Wang et al., 2014b; Weng et al., 2019; Weng et al., 2018; Xiang et al., 2017; Zhang et al., 2017a; Zhao et al., 2017a; Zhao et al., 2017b; Zhao et al., 2014; Zheng et al., 2013; Zhou et al., 2015). Emerging data have suggested that the global abundance of m6A and expression levels of its regulators, including writers, erasers, and readers, are often dysregulated in various types of cancers and are critical for cancer initiation, progression, metastasis, as well as drug resistance and cancer relapse (Table 1 and Figure 3) (Huang et al., 2020b). Other intracellular events (such as mutations that cause gain or loss of m6A sites) and extracellular stimulations could also affect cellular m6A modification and are therefore associated with human cancers (Figure 4).
Table 1.
Roles of m6A writers, erasers and readers in human cancers.
| m6A regulator |
Role in cancer |
Cancer type | Target genes | References |
|---|---|---|---|---|
| Writer | ||||
| METTL3 | Oncogene | Acute myeloid leukemia | MYC, BCL2, PTEN, SP1, SP2 | (Barbieri et al., 2017; Vu et al., 2017) |
| Glioblastoma | SRSFs, SOX2 | (Li et al., 2019a; Visvanathan et al., 2018) | ||
| Hepatocellular carcinoma | SOCS2, SNAIL, LINC00958 | (Chen et al., 2018; Lin et al., 2019b; Zuo et al., 2020) | ||
| Hepatoblastoma | CTNNB1 | (Liu et al., 2019) | ||
| Bladder cancer | AFF4, IKBKB, RELA, MYC, CDCP1, miR-221/222, ITGA6 | (Cheng et al., 2019a; Han et al., 2019b; Jin et al., 2019b; Yang et al., 2019a) | ||
| Breast cancer | HBXIP, BCL2 | (Cai et al., 2018; Wang et al., 2020) | ||
| Gastric cancer | ZMYM1, HDGF, SEC62, ARHGAP5-AS1 | (He et al., 2019; Lin et al., 2019a; Liu et al., 2020b; Wang et al., 2019b; Yue et al., 2019; Zhu et al., 2019b) | ||
| Pancreatic cancer | pri-miR-25 | (Taketo et al., 2018; Xia et al., 2019; Zhang et al., 2019b) | ||
| Colorectal cancer | SOX2, pri-miR-1246 | (Li et al., 2019c; Peng et al., 2019) | ||
| Colon cancer | TP53(R273H,G>A) | (Uddin et al., 2019) | ||
| Melanoma | (Dahal et al., 2019) | |||
| Osteosarcoma | LEF1 | (Miao et al., 2019) | ||
| Prostate | GLI1 | (Cai et al., 2019) | ||
| Non-small cell lung cancer | YAP, MALAT1 | (Jin et al., 2019a) | ||
| Tumor suppressor | Glioblastoma | ADAM19 | (Cui et al., 2017) | |
| Endometrial cancer | PHLPP2, MTORC2 | (Liu et al., 2018a) | ||
| Renal cell carcinoma | (Li et al., 2017c) | |||
| METTL14 | Oncogene | Acute myeloid leukemia | MYB, MYC | (Weng et al., 2018) |
| Breast cancer | TGF–β signaling pathway genes | (Panneerdoss et al., 2018) | ||
| Tumor suppressor | Endometrial cancer | PHLPP2, MTORC2 | (Liu et al., 2018a) | |
| Glioblastomas | ADAM19 | (Cui et al., 2017) | ||
| Hepatocellular carcinoma | pri-miR-126 | (Ma et al., 2017) | ||
| Bladder cancer | NOTCH1 | (Gu et al., 2019) | ||
| Renal cell carcinoma | P2RX6 | (Gong et al., 2019) | ||
| Gastric cancer | (Zhang et al., 2019a) | |||
| Colorectal cancer | pri-miR-375 | (Chen et al., 2019a) | ||
| WTAP | Oncogene | Hepatocellular carcinoma | ETS1 | (Chen et al., 2019b) |
| VIRMA (KIAA1429) | Oncogene | Hepatocellular carcinoma |
ID2, GATA3 | (Cheng et al., 2019b; Lan et al., 2019) |
| Eraser | ||||
| FTO | Oncogene | Acute myeloid leukemia | ASB2, RARA, MYC, CEBPA | (Li et al., 2017d; Su et al., 2018) |
| Glioblastomas | (Cui et al., 2017) | |||
| Non-small cell lung cancer | MZF1, USP7 | (Li et al., 2019b; Liu et al., 2018b) | ||
| Pancreatic cancer | MYC | (Tang et al., 2019) | ||
| Cervical squamous cell carcinoma | β-catenin, E2F1, MYC | (Zhou et al., 2018; Zou et al., 2019) | ||
| Breast cancer | BNIP3 | (Niu et al., 2019) | ||
| Melanoma | PDCD1, CXCR4, SOX10 | (Yang et al., 2019c) | ||
| Gastric cancer | (Zhang et al., 2019a) | |||
| ALKBH5 | Oncogene | Glioblastomas | FOXM1 | (Zhang et al., 2017 c) |
| Breast cancer | NANOG | (Fry et al., 2018; Wu et al., 2019; Zhang et al., 2016a; Zhang et al., 2016b) |
||
| Non-small cell lung cancer | UBE2C, FOXM1 | (Chao et al., 2020; Guo et al., 2018) | ||
| Gastric cancer | NEAT1 | (Zhang et al., 2019c) | ||
| Ovarian carcinoma | BCL2 | (Zhu et al., 2019a) | ||
| Oral squamous cell carcinoma | FOXM1, NANOG | (Shriwas et al., 2020) | ||
| Tumor suppressor | Bladder cancer | ITGA6 | (Jin et al., 2019b) | |
| Pancreatic cancer | KCNK15-AS1, WIF1 | (He et al., 2018; Tang et al., 2020) | ||
| Readers | ||||
| YTHDF2 | Oncogene | Acute myeloid leukemia | TNFRSF2 | (Paris et al., 2019) |
| Hepatocellular carcinoma | (Yang et al., 2017b) | |||
| Prostate cancer | (Li et al., 2018) | |||
| Pancreatic cancer | YAP | (Chen et al., 2017) | ||
| Gastric cancer | (Zhang et al., 2017b) | |||
| Non-small cell lung cancer | 6PGD | (Sheng et al., 2019) | ||
| Cervical cancer | GAS5 | (Wang et al., 2019c) | ||
| Tumor suppressor | Hepatocellular carcinoma | EGFR, IL11, SRPINE2 | (Hou et al., 2019; Zhong et al., 2019) | |
| YTHDF1 | Oncogene | Colorectal cancer | (Bai et al., 2019; Nishizawa et al., 2018) | |
| Hepatocellular carcinoma | (Lin et al., 2019b; Zhao et al., 2018) | |||
| Non-small cell lung cancer | KEAP1 | (Shi et al., 2019) | ||
| Markel cell carcinoma | (Orouji et al., 2020) | |||
| YTHDF3 | Oncogene | Colorectal cancer | GAS5 lncRNA | (Ni et al., 2019) |
| YTHDC2 | Oncogene | Colon cancer | HIF1A | (Tanabe et al., 2016) |
| IGF2BP1/2/3 | Oncogene | Hepatocellular carcinoma and cervical cancer | MYC, FSCN1, TK1, MARCRSL1 | (Huang et al., 2018) |
| IGF2BP1 | Oncogene | Hepatocellular carcinoma and ovarian cancer | SRF | (Muller et al., 2019) |
| IGF2BP2 | Oncogene | Colorectal cancer | SOX2 | (Li et al., 2019c) |
| Pancreatic cancer | DANCR | (Hu et al., 2019) | ||
| IGF2BP3 | Oncogene | Gastric cancer | HDGF | (Wang et al., 2019b) |
| METTL3 | Oncogene | Non-small cell lung cancer | EGFR, TAZ, BRD4 | (Choe et al., 2018; Lin et al., 2016) |
| Ovarian carcinoma | AXL | (Hua et al., 2018) | ||
Figure 3.
Deregulation of m6A modifiers in human cancers. Modifiers in red indicate an oncogenic role, modifiers in green indicate a tumor suppressive role, while those in yellow have controversial roles reported, in the specific cancer type. AML, acute myeloid leukemia; BRC, breast cancer; NSCLC, non-small cell lung cancer; GC, gastric cancer; BLC, bladder cancer; PRC, prostate cancer; OST, osteosarcoma; GBM, glioblastoma; RCC, renal cell carcinoma; HCC, hepatocellular carcinoma; PAC, pancreatic cancer; CRC, colorectal cancer; GNC, gynecological cancer; CVC, cervical cancer; ENC, endometrial cancer; OVC, ovarian cancer; MLM, melanoma.
Figure 4.
Causes of aberrant m6A regulation in cancer. Deregulation of the m6A machinery could result in upregulation of oncogenes or downregulation of tumor suppressive genes, leading to cancer development. On the other hand, mutations from other nucleosides to A on mRNA render mRNA to m6A-mediated regulation and can also contribute to cancer. Environmental factors could reprogram the epitranscriptome and lead to cell immortalization.
Aberrant global m6A abundance in cancer
Aberrant decrease or elevation of global m6A abundance has been reported recently in some types of cancers, and the dysregulation could be associated with cancer progression and clinical outcome. For example, it was reported that the global m6A abundance in mRNA or total RNA (as detected by m6A dot blots or colorimetric ELISA-like assays) was significantly increased in human gastric cancer tissues compared to normal control tissues (Wang et al., 2019b). Another group found that human HCC exhibited a gain of global m6A abundance in mRNA and increased mRNA expression due to the reduction of YTHDF2 (Hou et al., 2019). In contrast, Gu et al. reported that the global m6A abundance was significantly decreased in human bladder cancer tissues, especially in more advanced bladder cancer, as detected by m6A dot blot assays and by bladder cancer tissue array and immunohistochemistry. The authors also showed that decreased m6A abundance is associated with poor prognosis in bladder cancer patients (Gu et al., 2019). In addition, it has been indicated that m6A is associated with drug response and could be an epigenetic driver of chemo-resistance in leukemia cells, where hypomethylation of m6A in response to elevation of FTO during tyrosine kinase inhibitor (TKI) treatment are required for obtaining TKI tolerance and growth advantage (Yan et al., 2018).
Dysregulation of m6A writers in cancer
METTL3 was first identified as an m6A methyltransferase more than 2 decades ago and found to be widely expressed in human tissues later (Bokar et al., 1994; Bokar et al., 1997; Tuck, 1992). However, it was until 2017 that the role of METTL3, as an m6A methyltransferase, was started to be implicated in human cancers. It was reported that METTL3 acts as an essential oncogene in AML cells, where it could be recruited to chromatin by the CAATT-box binding protein CEBPZ to induce m6A deposition on the associated mRNA transcripts, such as SP1 and SP2 (Barbieri et al., 2017). Vu et al. also reported that METTL3 is highly expressed in AML and plays a critical role in AML cell survival and leukemia progression through promoting translation of target mRNAs, including MYC, BCL2, and PTEN, in an m6A dependent manner (Vu et al., 2017). Since then the overexpression and oncogenic functions of METTL3 were reported in many other types of cancer (Table 1), including hepatocellular carcinoma (HCC) (Chen et al., 2018; Lin et al., 2019b), hepatoblastoma (Liu et al., 2019), gastric cancer (GC) (He et al., 2019; Lin et al., 2019a; Liu et al., 2020b; Wang et al., 2019b; Yue et al., 2019; Zhu et al., 2019b), colorectal cancer (CRC) (Li et al., 2019c; Peng et al., 2019), non-small cell lung cancer (NSCLC) (Choe et al., 2018), and bladder cancer (BLC) (Han et al., 2019b). For instance, METTL3 was shown to promote HCC growth and contribute to HCC progression by repressing SOCS2 expression through an YTHDF2-dependent mechanism (Chen et al., 2018), and was also involved in regulation of epithelial-mesenchymal transition (EMT) of HCC cells through YTHDF1-mediated promotion of Snail mRNA translation (Lin et al., 2019b). Both studies suggested upregulation of METTL3 as an adverse prognostic factor in patients with HCC. In GC, elevated METTL3 level could also serve as an independent predictor of poor prognosis (Wang et al., 2019a; Yue et al., 2019). Higher METTL3 expression was found in CRC metastatic tissues and was associated with a poor prognosis, with SOX2 being identified as a downstream target which could be stabilized by IGF2BP2 (Li et al., 2019c). The correlation of METTL3 expression with CRC metastasis was confirmed by another group, which reported that METTL3 methylated pri-miR-1246 to promote its maturation (Peng et al., 2019). The overexpression of METTL3 and its adverse prognostic impacts have also been reported in BLC, in which it promotes methylation of target mRNAs (e.g., AFF4, MYC, CDCP1, ITGA6) or miRNAs (i.e., pri-miR221/222) (Cheng et al., 2019a; Han et al., 2019b; Jin et al., 2019b; Yang et al., 2019a). Interestingly, the oncogenic function of METTL3 in NSCLC is complicated, where METTL3 could methylate YAP mRNA and recruit YTHDF1/3 and eIF3b to promote translation of YAP mRNA (Jin et al., 2019a), or could interact with eIF3h to promote translation of target mRNAs (e.g., BRD4) independent of its enzymatic activity (Choe et al., 2018; Lin et al., 2016). The enzyme activity-independent oncogenic role of METTL3 was also reported in ovarian cancer (OVC) (Hua et al., 2018).
Similar to METTL3, METTL14 also contains a MT-A70 domain (i.e., a methyltransferase domain, MTD) and forms a heterodimer complex with METTL3 (Liu et al., 2014). Recent structural studies suggested that METTL14's catalytic center is degenerated and is thus not involved in the catalyzing reaction; instead, METTL14 is required for stabilizing METTL3 conformation and substrate RNA binding (Sledz and Jinek, 2016; Wang et al., 2016a; Wang et al., 2016b). As a critical component of the MTC, METTL14 has also been shown to be overexpressed and play important roles in various types of cancers. METTL14 was first reported to be overexpressed and play a critical oncogenic role in the initiation and progression of AML (Weng et al., 2018). Depletion of METTL14 inhibited self-renewal of leukemia stem/initiating cells (LSCs/LICs) and promoted myeloid differentiation of AML cells, mainly through reducing m6A abundance on its target mRNAs MYB and MYC and therefore negatively regulating their mRNA stability and translation (Weng et al., 2018). In normal hematopoiesis, Mettl14 was also found to be highly expressed in murine hematopoietic stem cells and down-regulated during myelopoiesis, especially in mature myeloid cells; knockout of Mettl14 moderately inhibited self-renewal of mouse hematopoietic stem cells (HSCs) and promoted myeloid differentiation (Weng et al., 2018). In breast cancer (BRC) cells, METTL14 and ALKBH5 were shown to control each other’s expression and play pro-tumorigenic function by controlling the m6A levels in key EMT and angiogenesis-associated transcripts (Panneerdoss et al., 2018).
WTAP was shown to be upregulated and act as an oncogene in AML before it was identified as a component of the m6A methyltransferase complex (Bansal et al., 2014). Recently, an m6A related function of WTAP was reported in HCC, where WTAP was upregulated and promoted liver cancer development via the huR-ETS1-p21/p27 axis (Chen et al., 2019b). Another component of the MTC, VIRMA, was also shown to have higher expression in HCC than in normal liver tissues and enhance migration and invasion of HCC, through regulation on the ID2 mRNA or GATA3 pre-mRNA in an m6A dependent manner (Cheng et al., 2019b; Lan et al., 2019).
In contrast, a tumor suppressive role of METTL3 was suggested in renal cell carcinoma (RCC) (Li et al., 2017c) and endometrial cancer (ENC) (Liu et al., 2018a), where METTL3 expression was lower in tumor samples compared to adjacent non-tumor samples, leading to the activation of the AKT signaling pathway and the increased proliferation and tumorigenicity of such tumor cells. It should be noted that a controversial role of METTL3 in glioblastoma (GBM) was reported by different groups (Cui et al., 2017, Li et al., 2019a; Visvanathan et al., 2018), which may be caused by the different origin of primary samples used and hence reflect the heterogeneity of GBM. Similarly, in several types of cancer, METTL14 was also shown to be downregulated and exhibit a tumor suppressive role. A hotspot R298P mutation was present in METTL14 in human ENC, resulting in the reduction m6A mRNA methylation in the patient endometrial tumor samples and could promote the tumorigenicity of endometrial cancer cells (Liu et al., 2018a). The level of METTL14 as well as m6A was reduced in HCC, accounting for high metastatic capacity of HCC (Ma et al., 2017). The down-regulation of METTL14 and decreased global m6A abundance were also observed in human BLC, and were associated poor prognosis and more advanced disease stages in patients with BLC (Gu et al., 2019). By post-transcriptional suppression of NOTCH1 expression via an m6A-dependent mechanism, METTL14 inhibits bladder tumor-initiating cell (TIC) self-renewal and bladder tumorigenesis (Gu et al., 2019). In addition, the down-regulation and tumor-suppressive role of METTL14 (similar to METTL3) has also been reported in GBM (Cui et al., 2017).
Dysregulation of m6A erasers in cancer
FTO is the first identified RNA m6A demethylase (Jia et al., 2011), although it had been shown to also exhibit catalytic activity towards other types of modification on RNA and DNA (Jia et al., 2008). The pathological function of FTO in cancer as an m6A demethylase was first reported in AML, where FTO was found to be highly expressed (Li et al., 2017b). It was demonstrated that FTO acts as an oncogene to promote leukemogenesis and inhibit all-trans-retinoic acid (ATRA)-mediated leukemia cell differentiation by destabilizing ASB2 and RARA mRNA via reducing m6A abundance on these mRNA transcripts (Li et al., 2017b). Further studies revealed FTO as a direct target of R-2-hydroxyglutarate (R-2HG), a metabolite produced to high levels by mutant isocitrate dehydrogenase 1/2 (IDH1/2) enzymes (Su et al., 2018). Activity of FTO could be inhibited by R-2HG, leading to the increase of m6A abundance and decreased stability of MYC and CEBPA mRNAs, which subsequently caused growth inhibition, cell cycle arrest and apoptosis of leukemia cells; as a result, R-2HG displays an intrinsic anti-leukemia activity (Su et al., 2018). The oncogenic function of FTO was also reported in solid tumors, such as GBM (Cui et al., 2017), BRC (Niu et al., 2019), and melanoma (MLM) (Yang et al., 2019c). Inhibition of FTO by an FTO inhibitor MA2 phenocopied effects of METTL3 overexpression on suppressing GBM stem cell (GSC) growth and self-renewal, and on suppressing tumor progression in xenograft mice (Cui et al., 2017). In BRC, FTO promotes cancer cell proliferation and metastasis via negative regulation on BNIP3 mRNA, and high level of FTO is associated with poor prognosis (Niu et al., 2019). Recently, it was shown that FTO could be induced by metabolic stress through the autophagy and NF-κB pathway in melanoma; as a result, FTO expression was elevated in human MLM compared to normal skin tissues, and high level of FTO promoted growth of MLM cells in nude mice (Yang et al., 2019c).
ALKBH5, belonging to the same AlkB subfamily of the Fe(II)/ αKG dioxygenases as FTO, was the second identified RNA m6A demethylase (Zheng et al., 2013). Soon after its identification as an m6A eraser, ALKBH5 was found to be involved in the enrichment of breast cancer stem cells (BCSCs) in the hypoxic tumor microenvironment (Zhang et al., 2016a; Zhang et al., 2016b). Expression of ALKBH5 could be induced in a hypoxia-inducible factors-dependent manner in breast cancer cells under hypoxia, leading to the decreased m6A abundance in NANOG mRNA and the increased protein level of this pluripotency factor, which allowed for the enrichment of BCSCs (Zhang et al., 2016a; Zhang et al., 2016b). Another study showed that ALKBH5 was highly expressed in GSCs and that its high expression level correlated with poor clinical outcome in GBM patients (Zhang et al., 2017c). The in vitro and in vivo assays using cultured human GSCs and xenograft models suggested that ALKBH5 is required for GSC proliferation and selfrenewal. Interestingly, FOXM1-AS, a chromatin-bound lncRNA transcribed in the antisense orientation of FOXM1, facilitated the recruitment of ALKBH5 to its nascent target transcript FOXM1. Demethylation of FOXM1 pre-mRNA by ALKBH5 promoted HuR binding and resulted in increased stability of the FOXM1 pre-mRNA and enhanced FOXM1 protein expression, which is critical for the maintenance of GSC tumorigenicity (Zhang et al., 2017c). A more recent study investigated the function of m6A RNA modification in bladder cancer and found that ALKBH5, in opposite to METTL3, inhibited bladder cancer cell growth and progression repressing ITGA6 expression through m6A-dependent post-transcriptional regulation (Jin et al., 2019b). In pancreatic ductal adenocarcinoma (PDAC), ALKBH5 was also shown to exhibit a tumor suppressive role by decreasing m6A modification on WIF-1 RNA and mediating Wnt signaling, and overexpression of ALKBH5 sensitized PDAC cells to chemotherapy (Tang et al., 2020).
Dysregulation of m6A readers in cancer
The m6A reader proteins mediate effects of m6A modification by controlling the fate of the modified RNAs. Thus, deregulation of reader proteins may lead to misinterpretation of modified RNAs and the subsequent disorder of RNA metabolism. As the first reported m6A reader, YTHDF2 has been extensively studied in various cancer types, and was shown to play an oncogenic role in most cancer types. In prostate cancer (PRC), YTHDF2 was upregulated in cancer tissues compared to the adjacent normal tissues; miR-493-3p was found to be a negative regulator of YTHDF2, and inhibition of miR-493-3p could abrogate the inhibitory effect of YTHDF2 knockdown on PRC cell proliferation and migration (Li et al., 2018). In agreement with the upregulation of METTL3 and METTL14 in AML, YTHDF2 was expressed higher in AML samples compared to healthy controls and in AML cells with LSC activity compared to those without LCS activity (Paris et al., 2019). In addition, YTHDF2 was critical for AML initiation and propagation by shortening the half-life of diverse m6A-containing transcripts important for LSC function (Paris et al., 2019). However, YTHDF2 has been reported to play both oncogenic and tumor suppressive roles in HCC (Yang et al., 2017; Zhong et al., 2019). By examining expression of YTHDF2 in clinical HCC tissues using immunohistochemistry staining, Yang et al. showed that YTHDF2 expression is associated with the malignance of HCC, and is negatively regulated by miR-145, a miRNA that is downregulated in HCC patients (Yang et al., 2017). In contrast, Zhong et al. showed that YTHDF2 expression was suppressed in HCC under hypoxic environment, and forced expression of YTHDF2 inhibited HCC cell proliferation and growth in vitro and in vivo by directly binding to the m6A modification site of EGFR 3 ’ UTR to promote the degradation of EGFR mRNA (Zhong et al., 2019).
Among all YTH family proteins, YTHDF1 was the only one showing increased expression in CRC samples than in normal portions according to the TCGA database. CRC patients with high YTHDF1 expression had poor overall survival, suggesting YTHDF1 as a prognostic factor (Nishizawa et al., 2018). DNA copy number amplification or potential transcriptional regulation by the oncogenic MYC protein may account for the overexpression of YTHDF1 in CRC (Bai et al., 2019; Nishizawa et al., 2018). Similarly, YTHDF1 was highly expressed in liver cancer and acted as an adverse prognosis factor in liver cancer patients (Lin et al., 2019b; Zhao et al., 2018). A very recent study identified YTHDF1 as one of the genes evolved rapidly in hypoxia adaptation, whose expression decreased in Tibetan domestic mammals compared to lowlanders (Shi et al., 2019). Furthermore, YTHDF1 was shown to be amplified in NSCLC and critical for NSCLC cell proliferation, xenograft tumor formation and lung cancer progression; surprisingly, it was also found that low expression of YTHDF1 rendered cancer cells resistant to cisplatin treatment and resulted in a worse clinical outcome (Shi et al., 2019). This study highlights the important roles of YTHDF1 in both hypoxia adaptation and NSCLC pathogenesis.
Deregulation of other YTH family proteins in cancer was also reported. YTHDC2 expression was found to be positively correlated with the colon tumor stage, including metastasis; high expression of YTHDC2 contributed to colon tumor metastasis by promoting translation initiation of HIF-1α (Tanabe et al., 2016). A GAS5-YAP-YTHDF3 negative feedback loop was recently uncovered in CRC (Ni et al., 2019). The YAP-interacting lncRNA GAS5 could directly bound with YAP to facilitate its phosphorylation and ubiquitin-mediated degradation, leading to attenuated YAP-mediated transcription of YTHDF3 and the subsequent relief of GAS5 from YTHDF3-mediated m6A-dependent decay. As YTHDF3 was expressed at significant higher level in CRC tissues than in counterpart normal tissues, this negative feedback loop sustained YTHDF3 expression and established a key role of YTHDF3 in CRC progression (Ni et al., 2019). Although IGF2BP1/2/3 proteins were just recently identified as m6A readers (Huang et al., 2018), their elevated expression had been previously observed in various cancers (Bell et al., 2013; Degrauwe et al., 2016). Among them, IGF2BP1 and IGF2BP3 are oncofetal proteins which are produced by tumors as well as by fetal tissues but are downregulated in adult tissues (Lederer et al., 2014). All the IGF2BP proteins were shown to promote cell proliferation, colony formation, migration, and invasion of HCC cells and cervical cancer (CVC) cells through binding to m6A sites on target transcripts, including MYC, to stabilize these mRNAs and promote their translation (Huang et al., 2018). Recently, it was shown that IGF2BP1 promoted the expression of SRF in an m6A-dependent manner by impairing miRNA-directed decay of the SRF mRNA, leading to enhanced SRF-dependent transcriptional activity and tumor cell growth and invasion (Muller et al., 2019). Significant elevation of IGF2BP3 expression was found in gastric cancer compared to paired normal gastric mucosa, and the binding of IGF2BP3 to the m6A site in HDGF mRNA enhanced its stability, leading to the increased expression and secretion of HDGF and the subsequent gastric cancer growth and liver metastasis (Wang et al., 2019b). In addition, IGF2BPs have also been shown to bind to ncRNAs, such as lncRNAs (Hu et al., 2019; Lim et al., 2019). It was reported that IGF2BP2, which is upregulated and associated with poor outcomes in pancreatic cancer patients, stabilizes lncRNA DANCR by binding to m6A-modified DANCR (Hu et al., 2019). Whether IGF2BPs recognize other ncRNAs through m6A-dependent manners in cancer and what are the functional consequences have yet to be investigated.
Mutations in m6A sites in cancers
It has now been realized that dysregulation of m6A regulators, especially of writers and erasers, causes abnormal m6A modifications in critical transcripts and aberrantly regulates expression of these cancer-associated genes post-transcriptionally. Therefore, it is reasonable to speculate that mutations in the m6A sites of such transcripts may disturb m6A deposition and thus play a role in cancer. Indeed, a G>A mutation in codon 273 (resulting in the R273H mutation) of TP53 pre-mRNA was reported to confer drug resistance of colon cancer cells in an m6A dependent manner (Uddin et al., 2019). The transited adenosine in codon 273 was methylated by METTL3, resulted in promoted preferential pre-mRNA splicing and the production of R273H mutant p53 protein, which led to acquired multidrug resistance in colon cancer cells (Uddin et al., 2019). More recently, a large-scale population study identified a missense variant, the rs8100241 variant located in the exon of ANKLE1 with a G>A change (resulting in Ala>Thr change) in CRC, and showed that it was associated with decreased risk of CRC (Tian et al., 2019). Mechanistically, compared to the rs8100241[G] allele, the rs8100241[A] allele could be methylated by the m6A MTC and recognized by YTHDC1, which increased protein expression of ANKLE1, a potential tumor suppressor that inhibited cell proliferation by maintaining genomic stability (Tian et al., 2019). Besides the gain of de novo m6A sites due to mutations in cancers, mutations resulting in loss of m6A modification may also exist and contribute to cancer development and drug response. Some online tools are available that incorporate m6A site information and SNP information to facilitate the investigation of functional m6A site mutations in cancers (Jiang et al., 2018; Zheng et al., 2018). It is also of great importance that high-resolution m6A sequencing methods are available to obtain more accurate and comprehensive view of the m6A methylome in each individual cancer types and corresponding normal tissues.
m6A epitranscriptome change upon external stimulations
It was estimated that exposure to a wide variety of environmental factors, including smoke, alcohol, radiation, environmental chemicals, and viruses, accounts for at least two-thirds of all cancer cases in the United States according to a report from the NIH/NCI/NIEHS (https://www.niehs.nih.gov/health/materials/cancer_and_the_environment_508.pdf). In addition to the intracellular events such as dysregulation of m6A regulators and mutations in m6A sites, external environmental stimulations have also been shown to regulate m6A modification and contribute to cancer development.
Chemical carcinogens can cause genetic mutations in cancer-susceptibility genes and/or induce epigenetic changes to promote oncogenic transformation (Clark and Molloy, 2017; Luch, 2005; Vaz et al., 2017). Human bronchial epithelial (HBE) cells chronically treated with arsenite sodium showed significantly elevated m6A modification and were transformed with malignant phenotypes, which could be dramatically reversed by silencing of METTL3 (Gu et al., 2018). In another study, different types of human epithelial cells transformed by chemical carcinogens, including cadmium (Cd), 3-methylcholanthrene, and nickel (Ni), showed dynamic changes in m6A abundance (Yang et al., 2019b). Mechanistic studies identified CDCP1, an oncogene, as a critical m6A target in chemical-induced malignant transformation and suggested that the METTL3-m6A-CDCP1 axis could be a potential therapeutic target for the treatment of chemical-induced cancers (Yang et al., 2019b).
The involvement of m6A modification in cigarette smoke-induced cancer development was recently reported (Zhang et al., 2019b). Exposure of immortalized human pancreatic duct epithelial cells or pancreatic cancer cells to cigarette smoke condensate caused hypomethylation in the promoter of METTL3 and the recruitment of NFIC transcription factor to induce METTL3 expression. High level of METTL3 significantly catalyzed m6A formation in pri-miR-25 and resulted in the increased level of mature miR-25-3p, which suppressed PHLPP2 mRNA expression and evoked the oncogenic AKT-p70S6K signaling to promote the initiation and progression of PDAC (Zhang et al., 2019b).
Epstein–Barr virus (EBV) is the first human oncogenic virus discovered and contributes to approximately 2% of all human cancers. It was recently shown that EBV latent protein EBNA3C could activate transcription of METTL14 and promote METTL14 protein stability by directly interacting with METTL14 in the host cells. Increased expression of METTL14 in EBV latently infected cells reprogrammed the epitranscriptome and upregulated essential EBV latent antigens, including EBNA3C, via an m6A-dependent mechanism, and therefore contributed to EBV-mediated tumorigenesis (Lang et al., 2019).
Implications for cancer therapy
Targeting m6A regulators as cancer therapies
The identification of FTO as the first m6A demethylase revived research in this field, and since then FTO has been the most attractive target for developing inhibitors against m6A regulators for cancer treatment (Deng et al., 2018b). MO-I-500 was developed to selectively inhibit the m6A demethylase activity of FTO and was shown to inhibit the survival and/or colony formation of a triple-negative inflammatory breast cancer cell line (Singh et al., 2016; Zheng et al., 2014). Meclofenamic acid (MA), a nonsteroidal anti-inflammatory drug, was demonstrated to specifically inhibit FTO over ALKBH5 and further proved to inhibit GBM cell growth and survival (Cui et al., 2017; Huang et al., 2015). R-2HG, a major metabolic product of mutant IDH1/2, was found to be an inhibitor of FTO that binds directly to FTO and inhibits its m6A demethylase activity, leading to an inhibition of leukemic cell growth/survival and leukemia progression (Su et al., 2018). Very recently, a more potent FTO inhibitor FB23-2 was developed, which significantly inhibited AML progression in xeno-transplanted mice (Huang et al., 2019b).
Cancer therapeutic resistance occurs as cancer cells develop resistance to treatments including chemotherapy, radiotherapy, and targeted therapies, and is responsible for treatment failure and/or cancer recurrence. Mechanisms underlying therapy resistance vary, including genetic and/or epigenetic changes in cancer cells, the microenvironment that cancer cells resides, and the existence of a small population of self-renewal, drug resistant cancer stem cells (CSCs). The findings that m6A regulators are dysregulated and play important roles in various types of cancer suggest that m6A modification could be involved in therapy resistance development. Indeed, it was reported that FTO enhanced chemo-radiotherapy resistance in cervical squamous cell carcinoma through reducing m6A abundance in β-catenin mRNA (Zhou et al., 2018), while enhanced m6A modification in FZD10 mRNA in BRCA-mutated epithelial ovarian cancers owing to downregulation of FTO and ALKBH5 could result in reduced PARPi sensitivity (Fukumoto et al., 2019). In addition, leukemia cells with FTO upregulation and m6A hypomethylation showed increased TKI tolerance and growth advantage due to enhanced mRNA stability of proliferation/survival-related transcripts harboring m6A (Yan et al., 2018). Such data suggest that m6A RNA modification signatures may serve as predictive markers for personalized cancer treatment and shed light on the overcome of therapy resistance in cancer through modulating the m6A-related pathways (Figure 5). Encouragingly, proof-of-concept studies have emerged to support such a notion. In AML, depletion of METTL14 or FTO could sensitize leukemia cells to the differentiation-inducing agent ATRA (Hsu et al., 2017; Weng et al., 2018). By suppressing FTO signaling, R-2HG treatment could sensitize AML cells to a variety of first-line therapeutic agents such as ATRA, 5-azacytidine (5-Aza), decitabine (DAC), daunorubicin (DNR), and cytosine arabinoside (Ara-C), and sensitize GBM cells to temozolomide (TMZ) (Su et al., 2018). In addition, silencing of METTL3, which is elevated in GSC and GBM tumors and predicts poor survival in GBMs, led to enhanced sensitivity of GSCs to γ-irradiation (Visvanathan et al., 2018). Similarly, METTL3-depleted pancreatic cancer cells were more sensitive to anticancer reagents or therapy, including gemcitabine, 5-fluorouracil (5-FU), cisplatin, and irradiation (Taketo et al., 2018).
Figure 5.
Therapeutic implications of m6A modification. Considering the roles of FTO, ALKBH5, and METTL3 in glioblastoma stem cells (GSCs) and of METTL14 and FTO in leukemia stem cells (LSCs), inhibitors of m6A modifiers holds the potential to be combined with chemotherapy or radiotherapy to erase cancer stem cells (CSCs) and achieve complete remission. In addition, m6A regulators play a role in the immune system, and thus are promising targets for enhancing anti-tumor immunity when combined with immune therapies.
m6A and cancer immunotherapy
The immune system is a host defense system that protects against infections and diseases. In recent years, studies have shown that RNA m6A modification is involved in the development of the immune system and the induction of immune response. A dramatic alteration of lymphocyte homeostasis was observed, where T cells were prevented from homeostatic expansion and kept in a naive state, when Mettl3 was depleted (Li et al., 2017b). Mechanistically, Mettl3 deletion resulted in reduced m6A level and increased mRNA stability and protein level of Socs1, Socs3 and Cish, which negatively regulated IL-7 signaling in CD4+ T cells (Li et al., 2017b). Another study revealed the role of m6A in dendritic cell activation, in which Mettl3-mediated m6A modification on CD40, CD80, and TLR4 signaling adaptor Tirap transcripts enhanced their translation in dendritic cells for strengthening TLR4/NF-κB signaling-induced cytokine production and stimulating T cell activation (Wang et al., 2019a). Recently, two groups independently reported the role of m6A modification on the control of innate immune response to dsDNA or viruses. Rubio et al. found that METTL14 depletion, in opposite to ALKBH5 depletion, reduced human cytomegalovirus (HCMV) reproduction in host cells and stimulated dsDNA- or HCMV-induced interferon beta 1 (IFNB1) mRNA production and stabilization (Rubio et al., 2018). Similarly, Winkler et al. reported that deletion of METTL3 or YTHDF2 following viral infection stabilized IFNB1 in an m6A dependent manner, resulting in the subsequent suppression of viruses propagation in the host cells (Winkler et al., 2019). Interestingly, endogenous human circRNAs were m6A-modified and could be recognized by YTHDF2, which sequestered m6A-circRNA and was essential for suppression of innate immunity, while foreign circRNAs were unmodified and could induce antigen-specific T cell activation, antibody production, and anti-tumor immunity (Chen et al., 2019c).
Immunotherapy is a new strategy of cancer therapy that stimulates and improves the immune system's natural ability to fight cancer cells. YTHDF1 was recently found to control anti-tumor immunity and improve immunotherapy through regulating expression of lysosomal proteases in an m6A dependent manner (Han et al., 2019a). YTHDF1 could recognize m6A-marked transcripts encoding lysosomal proteases to increase their translation in dendritic cells, and loss of Ythdf1 enhanced the cross-presentation of tumor antigens and the cross-priming of CD8+ T cells in vivo. Moreover, Ythdf1 deletion enhanced the therapeutic efficacy of PD-L1 checkpoint blockade (Han et al., 2019a). In melanoma, increased level of FTO promoted tumor growth by reducing m6A methylation in PD-1 (PDCD1), CXCR4, and SOX10 and preventing their RNA decay mediated by YTHDF2. Knockdown of FTO in melanoma cells sensitized tumor cells to interferon gamma (IFNγ) in vitro and promoted melanoma response to anti-PD-1 antibody in mice (Yang et al., 2019c). These data together implicate that modulation of m6A regulators could be combined with anti-PD-1/PD-L1 blockade to improve anticancer immunotherapy (Figure 5).
Conclusions and Perspectives
Within the past few years, extensive efforts have been devoted to study m6A modification in cancers, leading to extensive accumulation of experimental data and increased knowledge on the roles of m6A modification and the associated machinery in various types of cancers. It is now clear that both global m6A levels and expression of m6A regulators (writers, erasers, and readers) are dysregulated in various types of cancers, and their dysregulation could be associated with drug resistance and prognosis in cancer patients. Evidence is emerging that m6A regulators play important pathological (more often tumor-promoting) roles in various types of cancers by modulating the epitranscriptome in cancers. Notably, in certain types of cancers such as AML, breast cancer, lung cancer and gastric cancer, both m6A writer and eraser genes are aberrantly overexpressed and play important oncogenic roles. While the underlying molecular mechanisms have yet to be fully understood, such phenomena suggest that dysregulation of the epitranscriptome by aberrantly expressed either writers or erasers could cause similar phenotypes (Deng et al., 2018c). Since both m6A writers and erasers are overexpressed in such cancers, the clinical implications of the global m6A abundance may not be very informative. Instead, m6A profiles (or signatures) of some particular transcripts or transcript loci could be better biomarkers for cancer diagnosis, classification and prognosis. However, currently available transcriptome-wide m6A-seq methods often need a large amount of RNA material (e.g., >20 μg total RNA), thus it is difficult to conduct m6A-seq for a large cohort of patients due to the limitation in primary patient samples. Therefore, much improved m6A-seq technologies that need much less RNA material and provide base-resolution m6A profiles are urgently needed. With such improved m6A-seq technologies, we can conduct m6A profiling with precious primary patient material or even with limited primary CSCs or TICs; the m6A profiles/signatures of certain particular transcripts or transcript loci could be identified as biomarkers for early cancer diagnosis, cancer classification, outcome prediction, and risk stratification. Besides cancer cells, cell-free circulating RNA (cfRNA) collected from plasma of cancer patients could also be used for m6A-seq, and particular m6A signatures could be used as biomarkers in clinic application. Thus far, more efforts have been focusing on identification of mRNA targets of m6A modification in cancers, and m6A modification on ncRNAs has been less studied and thus represents one of the future directions. Studying how m6A modification affects the production, cellular location, function of ncRNAs and how these processes are linked to cancer will greatly improve our understanding of the cellular function of m6A as well as the currently unappreciated functions of ncRNAs. More recently, m6A modifications were found on chromosome-associated regulatory RNAs (carRNAs), including promoter-associated RNAs, enhancer RNAs, and repeats RNAs, and were shown to regulate chromatin state and transcription (Liu et al., 2020a). Whether these carRNA species play a role in cancers remains an interesting topic that warrants further studies.
Given the functional importance of m6A modification and regulators in various types of cancers, targeting dysregulated m6A regulators represents an attractive strategy for cancer therapy. Indeed, some proof-of-concept studies already suggest that targeting dysregulated m6A regulators by small-molecule inhibitors holds therapeutic potential for cancer treatment. A few small pharmaceutic or biotech companies (e.g., STORM Therapeutics, Accent Therapeutics, Gotham Therapeutics and Genovel Biotech Corp.) have started to develop highly potent and selective small-molecule inhibitors targeting m6A regulators such as METTL3, METTL14, and FTO. Besides small-molecule compounds that target m6A regulators directly, PROTAC (proteolysis targeting chimera)-based inhibitors could also be developed to selectively degrade dysregulated m6A regulatory proteins for cancer therapy. In addition, because m6A modification also plays important roles in mediating responses of cancers to chemotherapy, radiotherapy, and immunotherapy, targeted treatment against m6A regulators can also be applied in the clinic together with chemotherapy, radiotherapy, or immunotherapy to achieve much improved cancer therapy in the near future. In addition, similar to genome editing, epitranscriptome editing can also be developed to restore or remove functional essential m6A sites that are mutated or dysregulated in cancers, and such editing may also be applicable in the clinic for cancer therapy in the future. Overall, study of m6A modification in cancer is a new frontier in cancer research, which not only reveals a new layer of epigenetic regulation in cancer and thereby provide novel insights into the molecular mechanisms underlying tumorigenesis, immune response, and drug resistance, but will also lead to the development of effective novel therapeutics. Targeting dysregulated m6A regulators by effective inhibitors (or targeting mutated or dysfunctional m6A sites by targeted epitranscriptome editing) alone or in combination with other therapeutics likely holds potent therapeutic potential for the treatment of various types of cancers, especially those resistant to currently available treatments.
ACKNOWLEDGEMENTS:
This work was supported in part by the National Institutes of Health (NIH) Grants R01 CA214965 (J.C.), R01 CA243386 (J.C.), R01 CA236399 (J.C.), and R01 CA211614 (J.C.). J.C. is a Leukemia & Lymphoma Society (LLS) Scholar. We apologize to colleagues whose work could not be cited due to space constraints.
Abbreviates:
- m6A
N6-methyladenosine
- mRNA
messenger RNA
- ncRNA
non-coding RNA
- A
adenosine
- G
guanosine
- C
cytidine
- U
uridine
- NGS
next generation sequencing
- CDS
coding sequence
- 3’ UTR
3’ untranslated region
- MeRIP-seq
methylated RNA immunoprecipitation sequencing
- miCLIP
m6A individual-nucleotide-resolution cross-linking and immunoprecipitation
- m6A-REF-seq
m6A-sensitive RNA-endoribonuclease–facilitated sequencing
- DART-seq
deamination adjacent to RNA modification targets sequencing
- rRNA
ribosomal RNA
- lncRNA
long non-coding RNA
- miRNA
microRNA
- snRNA
small nulear RNA
- circRNA
circular RNA
- MTC
methyltransferase complex
- SAM
S-adenosyl methionine
- H3K36me3
histone H3 lysine 36 trimethylation
- αKG
alpha-ketoglutarate
- m6Am
N6,2'-O-dimethyladenosine
- AML
acute myeloid leukemia
- pre-mRNA
precursor mRNA
- mRNP
messenger ribonucleoprotein
- P-body
processing body
- YTH
YT521-B homology
- eIF
eukaryotic translation initiation factor
- nt
nucleotides
- SCARLET
site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography
- pri-miRNAs
the primary transcripts of miRNAs
- pre-miRNAs
precursor miRNAs
- TKI
tyrosine kinase inhibitor
- HCC
hepatocellular carcinoma
- GC
gastric cancer
- CRC
colorectal cancer
- NSCLC
non-small cell lung cancer
- BLC
bladder cancer
- EMT
epithelial-mesenchymal transition
- GBM
glioblastoma
- MTD
methyltransferase domain
- LSC
leukemia stem cells
- LIC
leukemia initiating cells
- HSC
hematopoietic stem cells
- TIC
tumor initiating cells
- ATRA
all-trans-retinoic acid
- R-2HG
R-2-hydroxyglutarate
- BRC
breast cancer
- MLM
melanoma
- GSC
glioblastoma stem cell
- BCSC
breast cancer stem cell
- HBE
human bronchial epithelial
- Cd
cadmium
- Ni
nickel
- PDAC
pancreatic ductal adenocarcinoma
- EBV
Epstein–Barr virus
- MA
meclofenamic acid
- CSC
cancer stem cell
- 5-Aza
5-azacytidine
- DAC
decitabine
- DNR
daunorubicin
- Ara-C
cytosine arabinoside
- TMZ
temozolomide
- 5-FU
5-fluorouracil
- HCMV
human cytomegalovirus
- PROTAC
proteolysis targeting chimera
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS:
J.C. is the scientific founder of Genovel Biotech Corp. and holds equities with the company.
References Cited:
- Adams JM, and Cory S (1975). Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature 255, 28–33. [DOI] [PubMed] [Google Scholar]
- Aguilo F, Zhang F, Sancho A, Fidalgo M, Di Cecilia S, Vashisht A, Lee DF, Chen CH, Rengasamy M, Andino B, et al. (2015). Coordination of m(6)A mRNA Methylation and Gene Transcription by ZFP217 Regulates Pluripotency and Reprogramming. Cell Stem Cell 17, 689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, and Tavazoie SF (2015a). HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon CR, Lee H, Goodarzi H, Halberg N, and Tavazoie SF (2015b). N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y, Dhar R, and Khoury G (1979). Methylation of nuclear simian virus 40 RNAs. J Virol 32, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Yang C, Wu R, Huang L, Song S, Li W, Yan P, Lin C, Li D, and Zhang Y (2019). YTHDF1 Regulates Tumorigenicity and Cancer Stem Cell-Like Activity in Human Colorectal Carcinoma. Front Oncol 9, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal H, Yihua Q, Iyer SP, Ganapathy S, Proia DA, Penalva LO, Uren PJ, Suresh U, Carew JS, Karnad AB, et al. (2014). WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia 28, 1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, et al. (2017). Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, and Vanacova S (2017). N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res 45, 11356–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K, and Keith J (1977). Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol 113, 165–179. [DOI] [PubMed] [Google Scholar]
- Bell JL, Wachter K, Muhleck B, Pazaitis N, Kohn M, Lederer M, and Huttelmaier S (2013). Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci 70, 2657–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los Mozos IR, Sadee C, et al. (2018). The SMAD2/3 interactome reveals that TGFbeta controls m(6)A mRNA methylation in pluripotency. Nature 555, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, and Rottman F (1994). Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269, 17697–17704. [PubMed] [Google Scholar]
- Bokar JA, Shambaugh ME, Polayes D, Matera AG, and Rottman FM (1997). Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna 3, 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Kinzig CG, DeGregorio SJ, and Steitz JA (2016). Methyltransferase-like protein 16 binds the 3'-terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci U S A 113, 14013–14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, and Luo Y (2019). RNA m(6)A Methyltransferase METTL3 Promotes The Growth Of Prostate Cancer By Regulating Hedgehog Pathway. Onco Targets Ther 12, 9143–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, Liu Y, Zhang X, Zhang W, and Ye L (2018). HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett 415, 11–19. [DOI] [PubMed] [Google Scholar]
- Chao Y, Shang J, and Ji W (2020). ALKBH5-m(6)A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem Biophys Res Commun 521, 499–506. [DOI] [PubMed] [Google Scholar]
- Chen J, Sun Y, Xu X, Wang D, He J, Zhou H, Lu Y, Zeng J, Du F, Gong A, and Xu M (2017). YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle 16, 2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al. (2018). RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 67, 2254–2270. [DOI] [PubMed] [Google Scholar]
- Chen T, Hao YJ, Zhang Y, Li MM, Wang M, Han W, Wu Y, Lv Y, Hao J, Wang L, et al. (2015). m(6)A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell stem cell 16, 289–301. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu M, Xu X, Zeng K, Liu X, Sun L, Pan B, He B, Pan Y, Sun H, et al. (2019a). METTL14 Suppresses CRC Progression via Regulating N6-Methyladenosine-Dependent Primary miR-375 Processing. Mol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, et al. (2019b). WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer 18, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, et al. (2019c). N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol Cell 76, 96–109 e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H, Wu M, Liang Y, Zhu F, Zhang Y, Zhang X, et al. (2019a). The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-kappaB/MYC signaling network. Oncogene. [DOI] [PubMed] [Google Scholar]
- Cheng X, Li M, Rao X, Zhang W, Li X, Wang L, and Huang G (2019b). KIAA1429 regulates the migration and invasion of hepatocellular carcinoma by altering m6A modification of ID2 mRNA. Onco Targets Ther 12, 3421–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. (2018). mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561, 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ieong KW, Demirci H, Chen J, Petrov A, Prabhakar A, O'Leary SE, Dominissini D, Rechavi G, Soltis SM, et al. (2016). N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat Struct Mol Biol 23, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy MJ, Shambaugh ME, Timpte CS, and Bokar JA (2002). Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res 30, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, and Molloy PL (2017). Smoke-Induced Changes to the Epigenome Provide Fertile Ground for Oncogenic Mutation. Cancer Cell 32, 278–280. [DOI] [PubMed] [Google Scholar]
- Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, et al. (2017). m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell reports 18, 2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal U, Kang L, and Gupta M (2019). RNA m6A methyltransferase METTL3 regulates invasiveness of melanoma cells by matrix metallopeptidase 2. Melanoma research. [DOI] [PubMed] [Google Scholar]
- Degrauwe N, Suva ML, Janiszewska M, Riggi N, and Stamenkovic I (2016). IMPs: an RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev 30, 2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Su R, Feng X, Wei M, and Chen J (2018a). Role of N(6)-methyladenosine modification in cancer. Curr Opin Genet Dev 48, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Su R, Stanford S, and Chen J (2018b). Critical Enzymatic Functions of FTO in Obesity and Cancer. Front Endocrinol (Lausanne) 9, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Su R, Weng H, Huang H, Li Z, and Chen J (2018c). RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res 28, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R, Friderici K, and Rottman F (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proceedings of the National Academy of Sciences of the United States of America 71, 3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, and Wu L (2016). YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 7, 12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry NJ, Law BA, Ilkayeva OR, Carraway KR, Holley CL, and Mansfield KD (2018). N(6)-methyladenosine contributes to cellular phenotype in a genetically-defined model of breast cancer progression. Oncotarget 9, 31231–31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Harada BT, Behm M, and He C (2018). RNA modifications modulate gene expression during development. Science 361, 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T, Zhu H, Nacarelli T, Karakashev S, Fatkhutdinov N, Wu S, Liu P, Kossenkov AV, Showe LC, Jean S, et al. (2019). N(6)-Methylation of Adenosine of FZD10 mRNA Contributes to PARP Inhibitor Resistance. Cancer Res 79, 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, Viukov S, Winkler R, Nir R, Lasman L, Brandis A, et al. (2019). Deciphering the "m(6)A Code" via Antibody-Independent Quantitative Profiling. Cell 178, 731–747 e716. [DOI] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. (2015). Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006. [DOI] [PubMed] [Google Scholar]
- Gong D, Zhang J, Chen Y, Xu Y, Ma J, Hu G, Huang Y, Zheng J, Zhai W, and Xue W (2019). The m(6)A-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res 38, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wang Z, Zhou N, Li G, Kou Y, Luo Y, Wang Y, Yang J, and Tian F (2019). Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer 18, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Sun D, Dai H, and Zhang Z (2018). N(6)-methyladenosine mediates the cellular proliferation and apoptosis via microRNAs in arsenite-transformed cells. Toxicol Lett 292, 1–11. [DOI] [PubMed] [Google Scholar]
- Guo J, Wu Y, Du J, Yang L, Chen W, Gong K, Dai J, Miao S, Jin D, and Xi S (2018). Deregulation of UBE2C-mediated autophagy repression aggravates NSCLC progression. Oncogenesis 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Liu J, Chen C, Dong L, Liu Y, Chang R, Huang X, Liu Y, Wang J, Dougherty U, et al. (2019a). Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, et al. (2019b). METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer 18, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Wu W, Sun Z, and Chai L (2019). MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A-caused stabilization of SEC62. Biochem Biophys Res Commun 517, 581–587. [DOI] [PubMed] [Google Scholar]
- He Y, Hu H, Wang Y, Yuan H, Lu Z, Wu P, Liu D, Tian L, Yin J, Jiang K, and Miao Y (2018). ALKBH5 Inhibits Pancreatic Cancer Motility by Decreasing Long Non-Coding RNA KCNK15-AS1 Methylation. Cell Physiol Biochem 48, 838–846. [DOI] [PubMed] [Google Scholar]
- Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu Z, Hu B, Zhou J, Zhao Z, Feng M, et al. (2019). YTHDF2 reduction fuels inflammation and vascular abnormalization in hepatocellular carcinoma. Mol Cancer 18, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J, et al. (2017). Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell research 27, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Peng WX, Zhou H, Jiang J, Zhou X, Huang D, Mo YY, and Yang L (2019). IGF2BP2 regulates DANCR by serving as an N6-methyladenosine reader. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua W, Zhao Y, Jin X, Yu D, He J, Xie D, and Duan P (2018). METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol Oncol 151, 356–365. [DOI] [PubMed] [Google Scholar]
- Huang H, Weng H, and Chen J (2020a). The Biogenesis and Precise Control of RNA m6A Methylation. Trends in Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Deng X, and Chen J (2020b). RNA modifications in cancer: functions, mechanisms, and therapeutic implications. Annual Review of Cancer Biology 4, 221–240. [Google Scholar]
- Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. (2018). Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nature cell biology 20, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Weng H, Zhou K, Wu T, Zhao BS, Sun M, Chen Z, Deng X, Xiao G, Auer F, et al. (2019a). Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567, 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, Ni T, Zhang ZS, Zhang T, Li C, et al. (2019b). Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell 35, 677–691 e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, and Yang CG (2015). Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 43, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, and He C (2011). N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7, 885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou Z, and He C (2008). Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett 582, 3313–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Xie Y, He Z, Zhang Y, Zhao Y, Chen L, Zheng Y, Miao Y, Zuo Z, and Ren J (2018). m6ASNP: a tool for annotating genetic variants by m6A function. Gigascience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, Di W, Hu B, An J, Kong L, et al. (2019a). m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol 12, 135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jin H, Ying X, Que B, Wang X, Chao Y, Zhang H, Yuan Z, Qi D, Lin S, Min W, et al. (2019b). N(6)-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine 47, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villasenor R, Hess D, et al. (2018). Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev 32, 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, Yan X, Liao H, Chen X, Xie K, et al. (2019). KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer 18, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Singh RK, Pei Y, Zhang S, Sun K, and Robertson ES (2019). EBV epitranscriptome reprogramming by METTL14 is critical for viral-associated tumorigenesis. PLoS Pathog 15, e1007796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer M, Bley N, Schleifer C, and Huttelmaier S (2014). The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Semin Cancer Biol 29, 3–12. [DOI] [PubMed] [Google Scholar]
- Levis R, and Penman S (1978). 5'-terminal structures of poly(A)+ cytoplasmic messenger RNA and of poly(A)+ and poly(A)− heterogeneous nuclear RNA of cells of the dipteran Drosophila melanogaster. J Mol Biol 120, 487–515. [DOI] [PubMed] [Google Scholar]
- Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. (2017a). Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell research 27, 444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Yi Y, Miao Y, Long W, Long T, Chen S, Cheng W, Zou C, Zheng Y, Wu X, et al. (2019a). N6-methyladenosine Modulates Nonsense-mediated mRNA Decay in Human Glioblastoma. Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al. (2017b). m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, and Li B (2019b). The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun 512, 479–485. [DOI] [PubMed] [Google Scholar]
- Li J, Meng S, Xu M, Wang S, He L, Xu X, Wang X, and Xie L (2018). Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget 9, 3752–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, Chen ZH, Zeng ZL, Wang F, Zheng J, et al. (2019c). METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer 18, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tang J, Huang W, Wang F, Li P, Qin C, Qin Z, Zou Q, Wei J, Hua L, et al. (2017c). The M6A methyltransferase METTL3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget 8, 96103–96116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. (2017d). FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer cell 31, 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LJ, Wong SYS, Huang F, Lim S, Chong SS, Ooi LL, Kon OL, and Lee CG (2019). Roles and Regulation of Long non-coding RNAs in Hepatocellular Carcinoma. Cancer Research. [DOI] [PubMed] [Google Scholar]
- Lin S, Choe J, Du P, Triboulet R, and Gregory RI (2016). The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 62, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu J, Jiang W, Wang P, Sun C, Wang X, Chen Y, and Wang H (2019a). METTL3 Promotes the Proliferation and Mobility of Gastric Cancer Cells. Open medicine 14, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chai G, Wu Y, Li J, Chen F, Liu J, Luo G, Tauler J, Du J, Lin S, et al. (2019b). RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun 10, 2065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, and Jaffrey SR (2015). Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM, Zhao S, Shen B, Gao Y, Han D, and He C (2020a). N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al. (2018a). m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol 20, 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ren D, Du Z, Wang H, Zhang H, and Jin Y (2018b). m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun 502, 456–464. [DOI] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang J, Sun G, Wu Q, Ma J, Zhang X, Huang N, Bian Z, Gu S, Xu M, et al. (2019). m(6)A mRNA methylation regulates CTNNB1 to promote the proliferation of hepatoblastoma. Mol Cancer 18, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Dai Q, Zheng G, He C, Parisien M, and Pan T (2015). N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Parisien M, Dai Q, Zheng G, He C, and Pan T (2013). Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. Rna 19, 1848–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, and Pan T (2017). N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45, 6051–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yang S, Sui J, Xu SY, Cheng YP, Shen B, Zhang Y, Zhang XM, Yin LH, Pu YP, and Liang GY (2020b). Dysregulated N6-methyladenosine methylation writer METTL3 contributes to the proliferation and migration of gastric cancer. J Cell Physiol 235, 548–562. [DOI] [PubMed] [Google Scholar]
- Luch A (2005). Nature and nurture - lessons from chemical carcinogenesis. Nat Rev Cancer 5, 113–125. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, Chen K, Lu Z, Chen H, Shi YG, et al. (2019). N(6-)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol 15, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, and Sun SH (2017). METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology 65, 529–543. [DOI] [PubMed] [Google Scholar]
- Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, McCarthy AA, and Pillai RS (2018). Methylation of Structured RNA by the m(6)A Writer METTL16 Is Essential for Mouse Embryonic Development. Mol Cell 71, 986–1000 e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K (2019). DART-seq: an antibody-free method for global m6A detection. Nature methods 16, 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, and Jaffrey SR (2015). 5' UTR m(6)A Promotes Cap-Independent Translation. Cell 163, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, and Jaffrey SR (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W, Chen J, Jia L, Ma J, and Song D (2019). The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem Biophys Res Commun 516, 719–725. [DOI] [PubMed] [Google Scholar]
- Muller S, Glass M, Singh AK, Haase J, Bley N, Fuchs T, Lederer M, Dahl A, Huang H, Chen J, et al. (2019). IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res 47, 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, and Li J (2019). Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer 18, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JL (1979). 'Cap' structures in maize poly(A)-containing RNA. Biochim Biophys Acta 563, 490–495. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, Takahashi H, Nishida N, Haraguchi N, Sakai D, et al. (2018). Oncogene c-Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget 9, 7476–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L, Wang Y, Li X, Xiong XF, Wei B, et al. (2019). RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer 18, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orouji E, Peitsch WK, Orouji A, Houben R, and Utikal J (2020). Oncogenic Role of an Epigenetic Reader of m(6)A RNA Modification: YTHDF1 in Merkel Cell Carcinoma. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerdoss S, Eedunuri VK, Yadav P, Timilsina S, Rajamanickam S, Viswanadhapalli S, Abdelfattah N, Onyeagucha BC, Cui X, Lai Z, et al. (2018). Cross-talk among writers, readers, and erasers of m(6)A regulates cancer growth and progression. Sci Adv 4, eaar8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, Mapperley C, Lawson H, Wotherspoon DA, Sepulveda C, et al. (2019). Targeting the RNA m(6)A Reader YTHDF2 Selectively Compromises Cancer Stem Cells in Acute Myeloid Leukemia. Cell stem cell 25, 137–148 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, and Conrad NK (2017). The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 169, 824–835e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, Ji D, Wang Q, Zhang Z, Tang J, and Sun Y (2019). Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res 38, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RP, and Kelley DE (1974). Existence of methylated messenger RNA in mouse L cells. Cell 1, 37–42. [Google Scholar]
- Perry RP, Kelley DE, Friderici K, and Rottman F (1975). The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell 4, 387–394. [DOI] [PubMed] [Google Scholar]
- Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. (2014). Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, and He C (2017a). Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. (2017b). YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio RM, Depledge DP, Bianco C, Thompson L, and Mohr I (2018). RNA m(6) A modification enzymes shape innate responses to DNA by regulating interferon beta. Genes Dev 32, 1472–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. (2014). Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep 8, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, Li J, Liu S, Lu B, Zhang S, and Shan C (2019). YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, and He C (2017). YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Fan S, Wu M, Zuo Z, Li X, Jiang L, Shen Q, Xu P, Zeng L, Zhou Y, et al. (2019). YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat Commun 10, 4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriwas O, Priyadarshini M, Samal SK, Rath R, Panda S, Das Majumdar SK, Muduly DK, Botlagunta M, and Dash R (2020). DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m(6)A-demethylation of FOXM1 and NANOG. Apoptosis. [DOI] [PubMed] [Google Scholar]
- Singh B, Kinne HE, Milligan RD, Washburn LJ, Olsen M, and Lucci A (2016). Important Role of FTO in the Survival of Rare Panresistant Triple-Negative Inflammatory Breast Cancer Cells Facing a Severe Metabolic Challenge. PLoS ONE 11, e0159072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledz P, and Jinek M (2016). Structural insights into the molecular mechanism of the m(6)A writer complex. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, et al. (2018). R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell 172, 90–105 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, Doki Y, Mori M, Ishii H, and Ogawa K (2018). The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol 52, 621–629. [DOI] [PubMed] [Google Scholar]
- Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, Konno J, Torigoe T, Maeda H, Kutomi G, Okita K, et al. (2016). RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1alpha mRNA is translated. Cancer Lett 376, 34–42. [DOI] [PubMed] [Google Scholar]
- Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi Y, He S, and Shimamoto F (2020). m(6)A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation and mediating Wnt signaling. Mol Cancer 19, 3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tang X, Liu S, Chen D, Zhao Z, and Zhou J (2019). The role of the fat mass and obesity-associated protein in the proliferation of pancreatic cancer cells. Oncol Lett 17, 2473–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NV, Ernst FG, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffery SR, Graille M, and Lafontaine DL (2019). The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res 47, 7719–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck MT (1992). Partial purification of a 6-methyladenine mRNA methyltransferase which modifies internal adenine residues. Biochem J 288 (Pt 1), 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MB, Roy KR, Hosain SB, Khiste SK, Hill RA, Jois SD, Zhao Y, Tackett AJ, and Liu YY (2019). An N(6)-methyladenosine at the transited codon 273 of p53 pre-mRNA promotes the expression of R273H mutant protein and drug resistance of cancer cells. Biochem Pharmacol 160, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz M, Hwang SY, Kagiampakis I, Phallen J, Patil A, O'Hagan HM, Murphy L, Zahnow CA, Gabrielson E, Velculescu VE, et al. (2017). Chronic Cigarette Smoke-Induced Epigenomic Changes Precede Sensitization of Bronchial Epithelial Cells to Single-Step Transformation by KRAS Mutations. Cancer Cell 32, 360–376 e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, and Somasundaram K (2018). Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene 37, 522–533. [DOI] [PubMed] [Google Scholar]
- Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. (2017). The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hu X, Huang M, Liu J, Gu Y, Ma L, Zhou Q, and Cao X (2019a). Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun 10, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu B, and Shi J (2020). N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene 722, 144076. [DOI] [PubMed] [Google Scholar]
- Wang P, Doxtader KA, and Nam Y (2016a). Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell 63, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, et al. (2019b). METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. (2016b). Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014a). N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J, and Wang Y (2019c). Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. American journal of translational research 11, 4909–4921. [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, and He C (2015). N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, and Zhao JC (2014b). N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hobartner C, Sloan KE, and Bohnsack MT (2017). Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18, 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, Shi H, Cui X, Su R, Klungland A, et al. (2018). Differential m(6)A, m(6)Am, and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol Cell 71, 973–985 e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, et al. (2018). Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell 69, 1028–1038 e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H, Huang H, and Chen J (2019). RNA N (6)-Methyladenosine Modification in Normal and Malignant Hematopoiesis. Adv Exp Med Biol 1143, 75–93. [DOI] [PubMed] [Google Scholar]
- Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. (2018). METTL14 Inhibits Hematopoietic Stem/Progenitor Differentiation and Promotes Leukemogenesis via mRNA m(6)A Modification. Cell stem cell 22, 191–205 e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler R, Gillis E, Lasman L, Safra M, Geula S, Soyris C, Nachshon A, Tai-Schmiedel J, Friedman N, Le-Trilling VTK, et al. (2019). m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol 20, 173–182. [DOI] [PubMed] [Google Scholar]
- Wu L, Wu D, Ning J, Liu W, and Zhang D (2019). Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer 19, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Wu X, Cao M, Zhang P, Shi G, Zhang J, Lu Z, Wu P, Cai B, Miao Y, and Jiang K (2019). The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathology, research and practice 215, 152666. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Laurent B, Hsu CH, Nachtergaele S, Lu Z, Sheng W, Xu C, Chen H, Ouyang J, Wang S, et al. (2017). RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. (2016). Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell 61, 507–519. [DOI] [PubMed] [Google Scholar]
- Yan F, Al-Kali A, Zhang Z, Liu J, Pang J, Zhao N, He C, Litzow MR, and Liu S (2018). A dynamic N(6)-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res 28, 1062–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X, Xi J, Ye D, Zhu S, Chen W, et al. (2018). N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res 46, 3906–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Jin H, Que B, Chao Y, Zhang H, Ying X, Zhou Z, Yuan Z, Su J, Wu B, et al. (2019a). Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Jin H, Que B, Chao Y, Zhang H, Ying X, Zhou Z, Yuan Z, Su J, Wu B, et al. (2019b). Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene 38, 4755–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Wei J, Cui YH, Park G, Shah P, Deng Y, Aplin AE, Lu Z, Hwang S, He C, and He YY (2019c). m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun 10, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, et al. (2017a). Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res 27, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Li J, Feng G, Gao S, Wang Y, Zhang S, Liu Y, Ye L, Li Y, and Zhang X (2017b). MicroRNA-145 Modulates N(6)-Methyladenosine Levels by Targeting the 3'-Untranslated mRNA Region of the N(6)-Methyladenosine Binding YTH Domain Family 2 Protein. J Biol Chem 292, 3614–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, and Zhao G (2019). METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer 18, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, Cheng T, Gao M, Shu X, Ma H, et al. (2018). VIRMA mediates preferential m(6)A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell discovery 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, et al. (2017a). m(6)A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273–276. [DOI] [PubMed] [Google Scholar]
- Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, and Semenza GL (2016a). Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proceedings of the National Academy of Sciences of the United States of America 113, E2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, Gong J, and Shen L (2019a). Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med 8, 4766–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, and Semenza GL (2016b). Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget 7, 64527–64542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, Li S, Tan L, Mai D, Li G, et al. (2019b). Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun 10, 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY, Yang D, Zheng ZC, and Zhao Y (2019c). ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. Journal of physiology and biochemistry 75, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Pi J, Liu Y, Yu J, and Feng T (2017b). [Knockdown of YTH N(6)-methyladenosine RNA binding protein 2 (YTHDF2) inhibits proliferation and promotes apoptosis in MGC-803 gastric cancer cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 33, 1628–1634. [PubMed] [Google Scholar]
- Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bogler O, et al. (2017c). m(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer cell 31, 591–606 e596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wei LH, Wang Y, Xiao Y, Liu J, Zhang W, Yan N, Amu G, Tang X, Zhang L, and Jia G (2019d). Structural insights into FTO's catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci U S A 116, 2919–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen LQ, Zhao YL, Yang CG, Roundtree IA, Zhang Z, Ren J, Xie W, He C, and Luo GZ (2019e). Single-base mapping of m(6)A by an antibody-independent method. Sci Adv 5, eaax0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Roundtree IA, and He C (2017a). Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, Wang X, Beadell AV, Lu Z, Shi H, Kuuspalu A, Ho RK, and He C (2017b). m(6)A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen Y, Mao Q, Jiang X, Jiang W, Chen J, Xu W, Zhong L, and Sun X (2018). Overexpression of YTHDF1 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer biomarkers : section A of Disease markers 21, 859–868. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. (2014). FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24, 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Cox T, Tribbey L, Wang GZ, Iacoban P, Booher ME, Gabriel GJ, Zhou L, Bae N, Rowles J, et al. (2014). Synthesis of a FTO inhibitor with anticonvulsant activity. ACS Chem Neurosci 5, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. (2013). ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Nie P, Peng D, He Z, Liu M, Xie Y, Miao Y, Zuo Z, and Ren J (2018). m6AVar: a database of functional variants involved in m6A modification. Nucleic Acids Res 46, D139–D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, Ma H, and Kang T (2019). YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett 442, 252–261. [DOI] [PubMed] [Google Scholar]
- Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, Xing Y, Giallourakis CC, and Mullen AC (2017). Genome-Wide Maps of m6A circRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns that Are Distinct from mRNAs. Cell Rep 20, 2262–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, and Qian SB (2015). Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, He C, Parisien M, and Pan T (2019). Regulation of Co-transcriptional Pre-mRNA Splicing by m(6)A through the Low-Complexity Protein hnRNPG. Mol Cell 76, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R, Wang YY, and Zhe H (2018). FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta-catenin through mRNA demethylation. Molecular carcinogenesis 57, 590–597. [DOI] [PubMed] [Google Scholar]
- Zhu H, Gan X, Jiang X, Diao S, Wu H, and Hu J (2019a). ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res 38, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhu Y, Han S, Chen M, Song P, Dai D, Xu W, Jiang T, Feng L, Shin VY, et al. (2019b). Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis 10, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Dong L, Li C, Yin Z, Rao S, and Zhou Q (2019). The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int 19, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J, Cao M, Cai J, Wu J, and Wang X (2020). M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]