Abstract
Mounting evidence indicates that the nervous system plays a central role in cancer pathogenesis. In turn, cancers and cancer therapies can alter nervous system form and function. This Commentary seeks to describe the burgeoning field of “cancer neuroscience” and encourage multidisciplinary collaboration for the study of cancer-nervous system interactions.
A growing appreciation that nervous system activity regulates development, homeostasis, plasticity and regeneration in diverse tissues has prompted investigations of similar roles for dictating cancer formation and progression. Numerous examples have now come to light that reveal mechanistic parallels in the way the nervous system regulates normal and neoplastic cellular function across a range of tissue types. As such, nervous system-cancer crosstalk - both systemically and within the local tumor microenvironment - is now emerging as a crucial regulator of cancer initiation and progression. However, much remains to be learned. The finding that neurons constitute an important non-neoplastic cell type in a broad range of cancers galvanized a recent Banbury meeting on the Nervous System and Cancer (December 10–13, 2019), engaging members of the neuroscience and cancer biology communities. We have written this Commentary in an effort to elucidate emerging principles, identify pressing unanswered questions, and define the scope of this burgeoning new field of “cancer neuroscience”.
Nervous system activity controls cancer initiation and progression
The nervous system branches as extensively as the circulatory system and this dense innervation of nearly all tissues – from bone marrow (Katayama et al., 2006) to salivary glands (Knox et al., 2010) - is essential to regulate normal tissue function. Analogous to its role in organogenesis, tissue homeostasis, plasticity and regeneration, the nervous system can also control malignant tumor initiation, growth and metastasis. While the molecular mechanisms by which neural cells influence cancer cells vary by tissue type, one unifying principle is that the functional effect of the neural-cancer interaction can typically be predicted by the influence of nervous system elements on the normal cellular counterpart of a given cancer. This principle is illustrated by the parallel influences of neuronal activity on normal and neoplastic glial cell proliferation. In the central nervous system (CNS), where glutamatergic neuronal activity promotes glial precursor cell proliferation (Gibson et al., 2014), the activity of glutamatergic neurons similarly drives the growth of malignant gliomas in experimental model systems (Venkatesh et al., 2015; Venkatesh et al., 2019). The underlying mechanisms involve both paracrine signaling and direct electrochemical communication (Figure 1A–B). Neuronal activity-dependent secretion of growth factors from neurons and from activity-sensing glial cells promotes glioma progression (Venkatesh et al., 2015). In addition, malignant cells can electrically integrate into neural circuitry through bona fide neuron-to-glioma synapses (Venkataramani et al., 2019; Venkatesh et al., 2019). Malignant glioma cells are themselves coupled by gap junctions, such that neuronal activity-dependent currents propagate through an extensively interconnected neural-glioma network (Venkataramani et al., 2019; Venkatesh et al., 2019). Post-synaptic electrical signaling promotes cancer progression through glioma cell membrane potential depolarization (Venkatesh et al., 2019) and consequent voltage-sensitive mechanisms that remain to be elucidated.
Interactions between the nervous system and cancer.
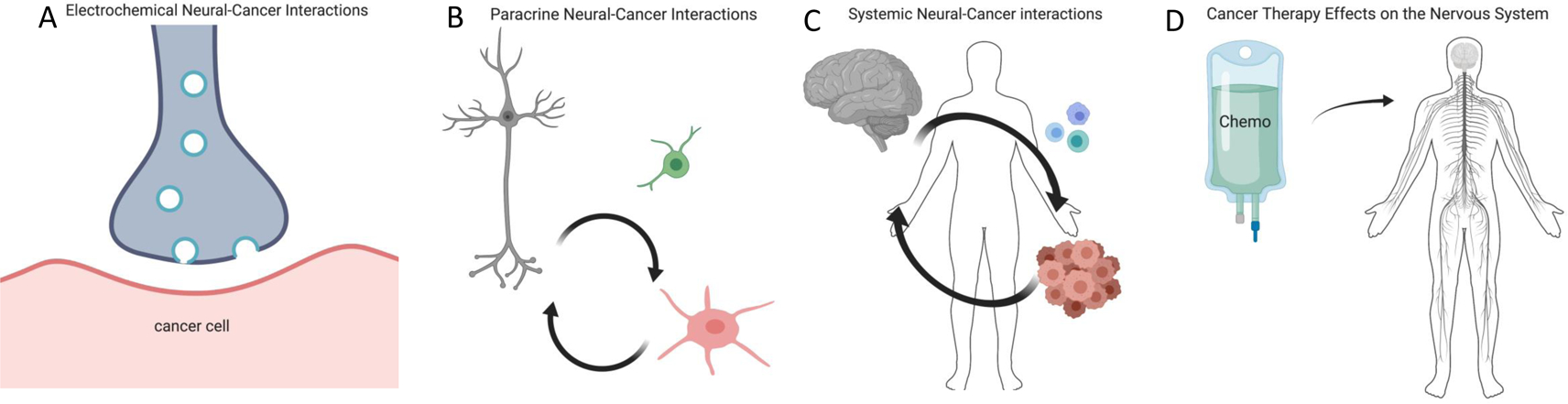
A) Synaptic communication between neurons and brain cancer cells (e.g. malignant glioma, red) can regulate cancer growth through neurotransmitter and voltage-regulated mechanisms. Whether synaptic interactions occur between peripheral nervous system axons and cancer cells outside of the central nervous system remains to be explored. B) Paracrine signaling between neurons/nerves (grey) and cancer cells (red), for example neuronal activity-dependent release of neurotransmitters or growth factors, regulates cancer growth in a wide range of tissues. The influence of neurons on malignant cells may be direct or may be mediated through effects on other cell types (green) in the tumor microenvironment. Cancer-derived paracrine factors remodel the nervous system to promote increased neural activity in the tumor microenvironment. C) Circulating factors from cancer (red) can influence nervous system (grey) functions such as sleep, while the nervous system can influence cancer progression through circulating factors such as hormones and progenitor cells or through altered immune system (blue) function. D) Cancer therapies frequently cause nervous system toxicities, from peripheral neuropathy to cognitive impairment. Molecular and cellular mechanisms of nervous system toxicities and the putative role that such disruption in nervous system function may play in cancer treatment efficacy require further study.
The cancer-promoting effect of excitatory neurotransmission extends to brain metastases as well. Breast cancer cells that have metastasized to the brain upregulate neurotransmitter receptor expression and extend perisynaptic processes to receive neuronal activity-dependent neurotransmitter signals that trigger a receptor-mediated signaling cascade, induce inward currents in the malignant cells and drive growth of breast cancer brain metastases (Zeng et al., 2019). How other types of metastatic cancer may interact with CNS neurons remains to be determined.
Outside of the CNS, peripheral nerve-derived neurotransmitter and growth factor signaling similarly regulate the progression of diverse cancers including pancreatic, gastric, colon, prostate, breast, oral and skin cancers in experimental model systems (Figure 1B; Magnon et al., 2013; Hayakawa et al., 2017; Renz et al., 2018). Signaling between sympathetic, parasympathetic or sensory nerves in the tumor microenvironment and malignant cells may regulate cancer initiation, progression or metastasis, often through neurotransmitter-dependent signaling cascades. The function of a given neuronal/nerve type must be understood in a context-specific manner. For example, parasympathetic (i.e., cholinergic) nerves may exert opposite effects in different tumor tissue types, such as promoting growth in the cancer of one organ and inhibiting growth in the cancer of another. In this regard, cholinergic signaling inhibits the growth and progression of pancreatic adenocarcinoma (Renz et al., 2018), but strongly promotes adenocarcinoma of the stomach (Hayakawa et al., 2017), an organ in which parasympathetic innervation is dominant. It is not yet known whether peripheral nerve-cancer cell interactions exclusively reflect paracrine signaling events, or whether nerve-to-cancer cell synapses, synapse-like structures or electrical coupling exist outside of the CNS that enable peripheral nerve to cancer communication. Moreover, the roles of diverse peripheral glial cells in nerve-cancer interactions outside of the central nervous system are largely unexplored.
Nervous system-cancer crosstalk occurs both through direct nerve-cancer interactions and via nervous system regulation of other cell types within the tumor microenvironment (e.g., immune cells, endothelial cells). These neural-cancer interactions may occur between neurons or nerves in the local microenvironment (Figure 1B), or through systemic signaling (Figure 1C), such as through elevated circulating catecholamines (neurotransmitters). Neural regulation of angiogenesis via endothelial cell metabolism (Zahalka et al., 2017) or immune system function (Borovikova et al., 2000) represent distinct mechanisms through which the nervous system may exert a systemic effect on the tumor environment, and interdisciplinary efforts involving oncology, immunology and neuroscience are needed to fully dissect these important neural-immune-cancer interactions.
Cancers influence nervous system function
Nervous system-cancer crosstalk is bidirectional, and cancers may induce profound nervous system remodeling and dysfunction. Secreted signals from brain tumors (gliomas) influence the function of invaded neural circuits by inducing aberrant synaptogenesis, increasing neuronal excitability and causing seizures (Yu et al., 2020). This pathological increase in neuronal activity promotes the activity-dependent signals that drive glioma growth (Venkatesh et al., 2015; Venkatesh et al., 2019; Venkataramani et al., 2019). Similarly, cancers outside of the CNS can act at a distance to disrupt normal brain function (e.g., sleep) (Figure 1C; Borniger et al., 2018). In the peripheral nervous system (PNS), cancers induce axonal ingrowth (axonogenesis) into the tumor microenvironment (Figure 1B; Hayakawa et al., 2017), where nerve density strongly correlates with cancer aggressiveness in many tumor types. Axonogenesis has been shown in several tumor types to be promoted by cancer cell secretion of neurotrophins (such as nerve growth factor), often through a feed-forward mechanism triggered by increased adrenergic or cholinergic signaling (Hayakawa et al., 2017). Beyond axonogenesis, recent studies have described neurogenesis within the tumor microenvironment from neural precursor cells detected only in the circulation of subjects with cancer (Mauffrey et al., 2019). Cancers also exhibit a propensity to invade nerve fibers (“perineural invasion”), causing remodeling of these peripheral nerves and chronic pain syndromes. In both central and peripheral cancers, this structural and functional remodeling of the nervous system amplifies neuron-cancer interactions and contributes to cancer growth and to cancer-related symptoms.
Influence of cancer therapies on the nervous system
Elucidating the mechanisms by which cancer therapy alters nervous system function (Figure 1D) is central to understanding the bidirectional interactions between neural and malignant cells. Traditional cancer therapies, such as radiation and chemotherapies, exert long-lasting deleterious effects on nervous system function, evident as cancer therapy-related cognitive impairment (colloquially known as “chemobrain” or “chemofog”, a syndrome characterized by impaired attention, memory, multi-tasking and sometimes increased anxiety) and as peripheral neuropathies (sensory loss, motor weakness or pain). Similar long-term nervous system effects of newer targeted therapies and cancer immunotherapies are incompletely understood and only now beginning to come to light. Cancer therapies differentially affect cognition, as well as the types of nerves predominantly affected in chemotherapy-associated peripheral neuropathy. The underlying cellular and molecular etiologies of cancer therapy-induced neural toxicity are becoming better understood, and therapeutic strategies aimed at neuroprotection or neural regeneration are now beginning to emerge (Gibson et al., 2019; Pease-Raissi et al., 2017). However, to what extent chemotherapy-induced neuropathy modulates nerve-cancer interactions to limit malignant growth is not yet clear, and the potentially beneficial role that therapy-induced neurotoxicity may play in the anti-neoplastic efficacy of radiation and chemotherapy remains to be explored. A more complete elucidation of both the mechanisms and implications of cancer therapy-induced neurotoxicity are needed in order to develop optimized therapeutic strategies aimed at both effectively treating cancer and minimizing the debilitating neurological side effects.
Pressing questions and a call for interdisciplinary collaboration
Much remains to be discovered with respect to the fundamental biology of the peripheral nervous system and its role in normal tissue development, homeostasis, plasticity and regeneration. The resulting knowledge from developmental and regenerative biology will be synergistic to understanding these interactions in cancer. Analogous to circuit-mapping efforts of the central nervous system over the past decade, similar mapping of the cranial, peripheral and enteric nervous systems is warranted, as their complex anatomy remains poorly characterized. Moreover, single cell analyses, coupled with the development of new tools for lineage-analysis and pluripotent stem cell modeling, will be required to define and associate the myriad nerve types with specific cancer phenotypes.
We are only beginning to uncover how the nervous system contributes to the initiation, growth, spread, recurrence and therapeutic resistance of cancers. The powerful tools of modern neuroscience, from electrophysiology to optogenetics, should be leveraged towards an understanding of cancer pathophysiology. Tissue and tumor type-specific differences underscore the need for careful investigation of each type of cancer over the course of its progression to elucidate the ways in which malignancy and cancer-induced nervous system remodeling co-evolve.
A more complete understanding will require true interdisciplinary study and collaboration between the disciplines of neuroscience, developmental biology, immunology, and cancer biology. Attention should be given not only to direct neuron-cancer cell interactions, but also to the influence of the nervous system on other cells of the local stromal, immune and systemic tumor environment. At this intersection of fields, exciting opportunities exist for cancer biologists to complement the great strides made in cancer genomics, immuno-oncology and precision therapeutics with a new dimension in the armamentarium, and for neuroscientists to take full advantage of sophisticated modern neuroscience approaches for the benefit of millions of individuals suffering from cancer and the effects of its current therapies. While much remains to be learned about neural regulation of tumor growth, early phase clinical trials are already underway targeting neural mechanisms that modulate tumor growth in specific tumor types. Precise targeting of neural-cancer interactions will ultimately provide new opportunities for improving outcomes of many difficult-to-treat malignancies.
Acknowledgements:
The authors would like to thank the National Cancer Institute (NCI) and Dr. Chamelli Jhappan for early appreciation of nervous system-cancer interactions and organization of the foundational “Nerves in Cancer” meeting in 2015. We also thank Rebecca Leshan at Banbury Center and Pearl Huang at Cygnal Therapeutics for their support in organizing the 2019 Banbury meeting.
Footnotes
Declarations of Interests: M.M., P.S.F., T.C.W, H.H., E.K.S. and D.A.T. are on the SAB for Cygnal Therapeutics. J.B.H. is an employee of Cygnal Therapeutics. F.W. is co-founder of Divide & Conquer (DC Europa Ltd).
References
- Borniger JC, Walker Ii WH, Surbhi, Emmer KM, Zhang N, Zalenski AA, Muscarella SL, Fitzgerald JA, Smith AN, Braam CJ, et al. (2018). A Role for Hypocretin/Orexin in Metabolic and Sleep Abnormalities in a Mouse Model of Non-metastatic Breast Cancer. Cell Metab 28, 118–129 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, and Tracey KJ (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, et al. (2017). Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, and Frenette PS (2006). Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421. [DOI] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, and Hoffman MP (2010). Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, and Frenette PS (2013). Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361. [DOI] [PubMed] [Google Scholar]
- Mauffrey P, Tchitchek N, Barroca V, Bemelmans AP, Firlej V, Allory Y, Romeo PH, and Magnon C (2019). Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678. [DOI] [PubMed] [Google Scholar]
- Pease-Raissi SE, Pazyra-Murphy MF, Li Y, Wachter F, Fukuda Y, Fenstermacher SJ, Barclay LA, Bird GH, Walensky LD, and Segal RA (2017). Paclitaxel Reduces Axonal Bclw to Initiate IP3R1-Dependent Axon Degeneration. Neuron 96, 373–386 e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, et al. (2018). Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov 8, 1458–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Korber C, Kardorff M, Ratliff M, Xie R, et al. (2019). Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538. [DOI] [PubMed] [Google Scholar]
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, et al. (2015). Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 161, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, et al. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Lin CJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, et al. (2020). PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, and Frenette PS (2017). Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galvan JA, Robinson HPC, Zlobec I, et al. (2019). Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]


