Abstract
Alzheimer’s disease (AD) is the neurodegenerative disorder characterized by impairment of higher intellectual dysfunctions associated with changes in the cognitive, behavioral, and social activities. Aim of the study: The current study was designed to evaluate the potential of aldosterone antagonist in the treatment of AD. Methodology: The study was conducted on albino mice of either sex (n = 60). Mice were subcategorized into six groups, each group having 10 mice. Group I—normal control (CMC 1 mL/kg), group II—diseased [streptozotocin (STZ), 3 mg/kg, intracerebroventricular (i.c.v.)], group III—standard (piracetam, 200 mg/kg, i.p.), and groups IV–VI designated as the treatment group (eplerinone at dose levels of 4, 8, and 16 mg/kg, orally), respectively. The study was carried out for 14 consecutive days. STZ was administered through the i.c.v. route on first and third days of the study for memory impairment. The molecular docking was performed to investigate the chemical behavior of compounds to inhibit the AChE. Anti-Alzheimer’s effect was assessed by using the behavioral paradigms such as passive avoidance, elevated plus maze, Morris water maze, open field, and balance beam. Various endogenous antioxidants such as SOD, GSH, nitrite, MDA, CAT, and AChE were identified in brain tissues of treated mice to assess the oxidative stress index. Biochemical markers for AD such as norepinephrine, dopamine, and serotonin, Aβ 1–40, Aβ 1–42, NF-κB, and tumor necrosis factor alpha were analyzed in brain tissues of mice. Expression of beta amyloid was observed by PCR. Results: The in silico study indicated the distinct mechanism of eplerinone to inhibit the AChE. The outcomes of the in vivo study manifested that eplerinone at the highest dose was found to be more effective in the treatment of AD. Conclusion: It may be concluded from the research work that eplerinone can be effective for cognitive improvement which proposes its therapeutic effect in many neurodegenerative disorders such as AD.
1. Introduction
Alzheimer’s disease (AD) is the neurodegenerative disease characterized by impairment of higher intellectual dysfunctions associated with changes in the cognitive, behavioral, and social activities.1 AD is caused by the alteration in neuronal connections. Two major types of protein aggregates (extracellular and intracellular) are involved in AD. Extracellular, neuritic plaques consist of beta amyloid (Aβ) peptides that are obtained from the enzymatic proteolysis of amyloid precursor proteins (APP). Aβ peptides have beta-plated sheets, thioflavin, and Congo red that can be neurotoxic. α-Secretase causes the cleavage of APP to release the soluble form of APP. This type of cleavage cannot generate Aβ fragments. However, β-secretase and γ-secretase cause the cleavage of N-terminal to the start and within the transmembrane domain, respectively, causing the release of less soluble Aβ peptides which ultimately aggregate into amyloid fibrils. Intracellular aggregates known as neurofibrillary tangles contain microtubule-associated tau proteins. APP itself and presenilins cause the breakdown of APP which is responsible for AD.2 Aβ imparts a dominant role in inflammation of neuron by activation of microglia, which leads to the generation of proinflammatory mediators or cytokines such as tumor necrosis factor alpha (TNF-α) and reactive oxygen species (ROS) for instance superoxide and NO. These mediators contribute to the degeneration of neurons, resulting into cognitive deficit and pathogenesis of AD.3 TNF-α exerts its effects by stimulating TNFR1 and TNFR2 receptors, which will activate the NF-κB pathway.4 NF-κB is responsible for neuroinflammation.5 Major areas of the brain that are involved in AD are basal forebrain, hippocampus, and cerebral cortex. A major neurotransmission system involved in AD is the cholinergic system. Acetylcholine (ACh) imparts an essential role in cognition. Cholinergic input is received by the basal forebrain to the neocortex area, suggesting its role in cognition. Deficient cortical cholinergic neurotransmission causes the cognitive impairment, which is due to decreased activity of enzyme, choline acetyltransferase to synthesize ACh, and reduced synaptic reuptake of choline. However, receptors for ACh are not affected in AD. Memory retention and thinking abilities are due to the different signaling pathways arbitrated by glutamate between the neocortex and hippocampus. The difference in the concentration of glutamate at synapse between the peak signal and resting conditions is reduced, resulting into deficit long-term potentiation. In AD, glutamate concentration is increased continuously in synapse that causes the displacement of magnesium from NMDA receptor calcium channels at resting conditions.6 In addition to this, other neurotransmitters are also involved in memory and attention. Noradrenergic neuronal loss is also associated with early progression of AD before the inception of memory impairment.7 The abnormal function of dopamine occurred in AD, which is the main cause of Aβ generation. Exposure to Aβ for longer time can interfere with the release of glutamate and GABA, which ultimately leads to declined dopamine release and play a role in the cognitive impairment.8 There is a close association between the serotonergic system and cognition. Serotonin (5-HT) regulates the ACh activity and proteins that are involved in the memory enhancement.9 Aldosterone is responsible for oxidative stress. Aldosterone-associated induction of oxidative stress is due to the increased level of NADPH oxidase (leading to the increased ROS) and 3-nitrotyrosin expression. 3-Nitrotyrosine is responsible for the nitrosative stress.10 Aldosterone has the ability to enter the brain and stimulates the renin angiotensin system (RAAS). RAAS is responsible for the increased levels of renin and angiotensin-converting enzymes that ultimately leads to the increased angiotensin II responsible for memory impairment.11 Aldosterone is the major contributing factor for oxidative stress and inflammation because of its ability to shorten the leukocyte telomere length.12 There are a number of binding sites available in the brain for aldosterone. It was suggested that high aldosterone levels can lead to the endothelial dysfunction that ultimately may have negative effects on cognition.13 The current study was designed to evaluate the anti-Alzheimer effect of eplerinone, an aldosterone antagonist, in mice.
2. Results and Discussion
2.1. Results
2.1.1. In Silico Modeling
The molecular docking was performed to investigate the chemical behavior of compounds to inhibit the AChE. The cognate redocking generated the pose with a 0.39 Å rmsd to cocrystalized conformation, and the docking protocol was validated (Figure 1). The compounds were found to bind with the negative binding energy (ΔG) that highlighted the binding affinity toward the AChE active site. The piracetam docked with −5.057 ΔG (kcal/mol) and served as a standard’s threshold. Interestingly, the eplerenone complexation was stabilized with −5.43 ΔG (kcal/mol), which exceeded the standard’s threshold and revealed its comparable binding affinity toward AChE (Table 1). The compounds were found to efficiently penetrate the active site and established the important interactions with vital residues. The AChE binding pocket has the narrow active site gorge that, with the lining of hydrophobic or aromatic residues, facilitates the diffusion of ACh into the wider vicinity of active site’s catalytic triad (i.e., HIS-SER-GLU). This phenomenon is called as aromatic guidance, and it also governs the entrance of small molecules with suitable size and physicochemical properties (REF1). The conformational analysis revealed that piracetam efficiently passed through this active site gorge and disrupted the catalytic triad of AChE (Figure 2). However, eplerenone preferably oriented itself into the aromatic lining of active site gorge and thus blocked the active site’s entrance and inhibited the aromatic guidance of ACh at the binding pocket. This may highlight the distinct mechanism of eplerenone to inhibit the AChE. The piracetam complex was stabilized by the π–alkyl interaction with TRP86 and H-bond with TYR124 and thus blocked the aromatic lining. However, it penetrated deep into the binding pocket and established the π–alkyl contact with HIS447 of the catalytic triad and interrupted the AChE catalytic activity (Figure 3). On the other hand, orientation of eplerenone in aromatic lining resulted into hydrophobic and hydrophilic interactions at the pocket entrance. Eplerenone absorbed into the binding pocket by hydrophobic interaction with TRP286 at the peripheral anionic binding site and thus blocked the AChE active site’s entrance. Moreover, this complex was further stabilized by π–alkyl and H-bond with LEU76 and VAL34, which further reinforced the blockade of active site’s aromatic lining.
Figure 1.

Validation of induced fit docking protocol by cognate redocking; the apo conformation of the cocrystalized native ligand (orange) aligned against the redocked conformation (green).
Table 1. Parameters for Compounds Docked at the Active Site of AChE.
| compounds | binding energy (ΔG) kcal/mol | interacting residues | interaction type |
|---|---|---|---|
| piracetam | –5.057 | TRP86, HIS447, TYR124 | H-bonding, π–alkyl |
| eplerenone | –5.43 | TRP286, LEU76, VAL34 | H-bonding, alkyl, π–alkyl |
Figure 2.
Conformational analysis of docked compounds at the active site of AChE; simulated best binding mode of piracetam (A) and eplerenone (B) in the three-dimensional (3D) space of the AChE binding pocket.
Figure 3.
Piracetam (A) and eplerenone (B) interaction behavior at the active site of AChE; the two-dimensional perspective of docked compounds interacting with active site residues depicted as balls colored by type of interaction.
2.1.2. Effect of Eplerinone on Transfer Latency in Mice That Received i.c.v. STZ Using Elevated Plus Maze
Mice were treated with eplerinone at three dose levels (4, 8, and 16 mg/kg) for 14 consecutive days. On day 11, their cognitive performance was assessed by following elevated plus maze.
Table 2 shows that eplerinone-treated mice at doses of 8 and 16 mg/kg showed moderately significant (P ≤ 0.001) and highly significant (P ≤ 0.001), (F = 3.9) improvement in transfer latency (TL), respectively, on the day of assessment. However, animals in group IV (4 mg/kg) did not show any significant improvement in TL.
Table 2. Effect of Eplerinone of TL in Mice That Received i.c.v. STZ Using Elevated Plus Mazea.
| sr. no. | Groups | dose (mg/kg) | TL (sec.) on day 14th (day 1) | TL (sec.) on day 15th (day 2) |
|---|---|---|---|---|
| 1 | normal control | CMC 1 mL/kg | 11.20 ± 0.8*** | 8.8 ± 1.02*** |
| 2 | diseased (STZ) | 3.0 | 39.60 ± 0.74 | 45.20 ± 1.42 |
| 3 | standard (piracetam) | 200 | 17.4 ± 1.03*** | 14.20 ± 0.73** |
| 4 | eplerinone | 4 | 34.20 ± 14.22 | 21.4 ± 5.77*** |
| 5 | 8 | 22.60 ± 4.55** | 13.4 ± 15.33** | |
| 6 | 16 | 30.60 ± 1.28 | 15.20 ± 4.37*** |
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the STZ-treated group.
2.1.3. Effect of Eplerinone on Step Down Latency in Mice That Received i.c.v. STZ Following Passive Avoidance
Long-term memory or episodic memory was assessed by recording the step down latency (SDL) of mice using a passive avoidance model on day 15. Groups IV, V, and IV (4, 8, and 16 mg/kg) showed marked increase (P ≤ 0.001) (F = 4.193) in SDL, which is an indication of cognitive improvement. The data are presented in Table 3.
Table 3. Effect of Eplerinone on SDL in Mice That Received i.c.v. STZ Following Passive Avoidancea.
| sr. no. | groups | dose (mg/kg) | SDL (sec.) on day 14th (day 1) | SDL (sec.) on day 15th (day 2) |
|---|---|---|---|---|
| 1 | normal control | CMC 1 mL/kg | 16.76 ± 0.37*** | 20.6 ± 0.4*** |
| 2 | diseased (STZ) | 3.0 | 7.8 ± 0.71 | 2.10 ± 0.36 |
| 3 | standard (piracetam) | 200 | 15.10 ± 0.33*** | 26 ± 0.54*** |
| 4 | eplerinone | 4 | 9.2 ± 2.39 | 31.2 ± 9.64*** |
| 5 | 8 | 5.8 ± 1.74 | 29.60 ± 9.57*** | |
| 6 | 16 | 5.4 ± 1.28 | 38.60 ± 10.34*** |
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the STZ-treated group.
2.1.4. Effect of Eplerinone on Escape Latency in Mice That Received i.c.v. STZ Following Morris Water Maze
Spatial learning and memory were evaluated by observing the escape latency (EL) following Morris water maze paradigm on the 9th day of the study protocol prior to 3 consecutive trial days. Data presented in Table 4 showed that the EL was decreased evidently in animals treated with eplerinone (4 and 8 mg/kg), whereas animals in group VI did not show marked improvement in EL when compared with training days. However, the animals treated with eplerinone (4 and 16 mg/kg) showed highly significant (P ≤ 0.001) (F = 1.96) EL on assessment days when compared with the disease control group on the assessment day.
Table 4. Effect of Eplerinone on EL in Mice That Received i.c.v. STZ Following Morris Water Mazea.
| sr. no. | groups | dose (mg/kg) | EL (sec.) training day | EL (sec.) assessment day |
|---|---|---|---|---|
| 1 | normal control | CMC 1 mL/kg | 21.60 ± 0.50*** | 15.80 ± 0.86*** |
| 2 | diseased (STZ) | 3.0 | 35.80 ± 1.11 | 47.80 ± 1.82 |
| 3 | standard (piracetam) | 200 | 24.20 ± 0.37** | 18.60 ± 1.02*** |
| 4 | eplerinone | 4 | 22.80 ± 3.02*** | 14.60 ± 3.02*** |
| 5 | 8 | 28.00 ± 1.76* | 23.40 ± 1.86** | |
| 6 | 16 | 24.80 ± 4.53** | 26.00 ± 3.09*** |
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the STZ-treated group.
2.1.5. Effect of Eplerinone on Procedural Memory in Mice That Received i.c.v. STZ Following Balance Beam
Procedural memory (long-term memory) was assessed by following the balance beam model on day 15. Results presented in Table 5 indicated that mice treated with eplerinone at all selected dose levels (4, 8, or 16 mg/kg) took less time (P ≤ 0.001) (F = 0.60) to reach the end point by crossing the beam.
Table 5. Effect of Eplerinone on Procedural Memory in Mice That Received i.c.v. STZ Following Balance Beama.
| sr. no. | groups | dose (mg/kg) | training | retention |
|---|---|---|---|---|
| 1 | normal control | CMC 1 mL/kg | 31.60 ± 3.17 | 12.00 ± 0.83*** |
| 2 | diseased (STZ) | 3.0 | 35.80 ± 1.68 | 53.80 ± 1.71 |
| 3 | standard (piracetam) | 200 | 34.60 ± 2.03 | 11.20 ± 1.15*** |
| 4 | eplerinone | 4 | 39.80 ± 11.38 | 18.40 ± 1.96*** |
| 5 | 8 | 34.00 ± 1.48 | 20.80 ± 2.57*** | |
| 6 | 16 | 50.00 ± 1.44* | 18.40 ± 5.18*** |
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the STZ-treated group.
2.1.6. Effect of Eplerinone on Cognitive Performance in Mice That Received i.c.v. STZ Following the Open Field Model
The open field model was followed to assess the behavior of animals treated with either 4, 8, or 16 mg/kg. Various parameters observed using the open field model are given in Table 6. It is evident from the data that animals in groups V and IV showed highly significant (P ≤ 0.001) improvement in scratching, time spent in peripheral and central areas of field, and crossing the field (F = 0.205). However, the same groups showed a moderately significant decrease in latency (P ≤ 0.01). The animals treated with a selected drug at a dose of 4 mg/kg showed highly significant (P ≤ 0.001) improvement in freezing (F = 0.375), scratching (F = 6.096), central field area visited time, and number of crossings. However, there was only significant (P ≤ 0.05) decrease in latency time, and the time of peripheral field area visited was observed in the same group animals. All results were compared with the disease control group.
Table 6. Effect of Eplerinone on Cognitive Performance in Mice That Received i.c.v. STZ Following the Open Field Modela.
| parameters | normal control | diseased (STZ) | standard (piracetam) | eplerinone |
||||
|---|---|---|---|---|---|---|---|---|
| dose mg/kg | CMC 1 mL/kg | 3.0 | 200 | 4 | 8 | 16 | ||
| whole body movement | latency (sec.) | 2.8 ± 0.20 | 23.8 ± 5.35*** | 2.20 ± 0.25 | 8.4 ± 0.74* | 3.20 ± 0.73** | 2.60 ± 0.4** | |
| rearing (no.) | 9.00 ± 0.35 | 0.64 ± 0.26 | 6.46 ± 0.33 | 13.4 ± 1.96 | 9.00 ± 2.44 | 12.20 ± 2.33 | ||
| freezing (sec.) | 71.60 ± 0.99 | 16.8 ± 1.24*** | 82.30 ± 0.96 | 270.0 ± 4.47*** | 84.4 ± 8.65 | 68.20 ± 12.20 | ||
| part body movement | scratching (sec.) | 1.00 ± 0.63*** | 5.8 ± 0.66*** | 1.20 ± 0.48 | 2.00 ± 1.22*** | 0.60 ± 0.4*** | 1.00 ± 0.63*** | |
| teeth chattering | – | + | − | + | − | − | ||
| digging | − | + | − | + | − | − | ||
| location | field area visited | central (sec.) | 116.8 ± 4.35 | 166.0 ± 1.37* | 117.2 ± 1.39 | 8.60 ± 1.07*** | 6.20 ± 0.37*** | 3.20 ± 0.37*** |
| peripheral (sec.) | 184.0 ± 10.77 | 76.0 ± 4.84*** | 117.20 ± 1.39 | 59.4 ± 12.70* | 19.4 ± 1.77*** | 208.4 ± 32.20*** | ||
| crossing (no.) | 4.0 ± 1.70*** | 15.50 ± 0.50 | 29.30 ± 0.8 | 237 ± 18.54*** | 186 ± 11.66*** | 220.4 ± 34.51*** | ||
| ANS | defecation (no.) | 0.20 ± 0.20 | 3.4 ± 0.92 | 0.0 ± 0.0 | 0.8 ± 0.4** | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| urination (no.) | – | + | − | − | − | − | ||
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the STZ-treated group.
2.1.7. Effect of Eplerinone on Oxidative Stress in Mice Treated with i.c.v STZ
Neurochemical markers such as catalase (CAT), glutathione (GSH), malondialdehyde (MDA), nitrite, and superoxide dismutase (SOD) were identified in the brain tissues of mice after complete assessment of cognitive functions. The results are given in Table 7, clearly indicating the highly significant (P ≤ 0.001) elevated levels of GSH, SOD, and CAT while significant decreased levels of MDA in the treated mice of groups IV and V. However, the animals treated with 16 mg/kg of eplerinone showed moderately significant (P ≤ 0.01) variation in GSH and SOD levels and highly significant (P ≤ 0.001) raised levels of CAT and nitrite while reduced levels of MDA. The acetylcholinestrase level was markedly reduced in the animals treated with eplerinone at a dose of 16 mg/kg (F = 0. 142).
Table 7. Effect of Eplerinone on Oxidative Stress in Mice Treated with i.c.v STZa.
| sr. no. | treatment groups | dose (mg/kg) | GSH (μg/mg of brain tissue) | SOD (μg/mg of brain tissue) | CAT (μg/mg of brain tissue) | MDA (μmol/mg of brain tissue) | nitrite (nmol/mg of brain tissue | AChE (μmol/mg of brain tissue) |
|---|---|---|---|---|---|---|---|---|
| 1 | normal control | CMC (1 mL/kg) | 34.00 ± 1.04*** | 36.20 ± 0.96*** | 196.8 ± 1.06*** | 15.70 ± 0.62*** | 3.0 ± 0.35 | 2.0 ± 0.15*** |
| 2 | diseased (STZ) | 3.0 | 13.4 ± 0.92 | 20.62 ± 0.18 | 95.90 ± 0.33 | 89.0 ± 11.44 | 4.62 ± 0.18 | 7.15 ± 0.12 |
| 3 | standard (piracetam) | 200 | 35.20 ± 0.25*** | 22.8 ± 0.020 | 204.2 ± 1.90*** | 16.20 ± 0.58*** | 3.4 ± 0.18 | 3.99 ± 0.02*** |
| 4 | eplerinone | 4 | 46.05 ± 0.51*** | 39.4 ± 0.18*** | 359.7 ± 0.30*** | 17.72 ± 0.09*** | 2.74 ± 0.07 | 3.10 ± 0.16*** |
| 5 | 8 | 24.4 ± 0.18*** | 53.20 ± 0.25*** | 195.6 ± 0.36*** | 10.65 ± 0.06*** | 2.32 ± 0.09 | 2.30 ± 0.06*** | |
| 6 | 16 | 23.70 ± 0.37** | 30.10 ± 0.18** | 231.5 ± 0.44*** | 16.44 ± 0.166*** | 11.02 ± 0.24*** | 2.02 ± 0.05*** |
Data are represented as man ± SEM (n = 10), ***(P ≤ 0.001), **(P ≤ 0.01) and *(P ≤ 0.05) symbolizes highly significant, moderately significant and significant, respectively, as compared to STZ treated group.
2.1.8. Effect of Eplerinone on Oxidative Stress Index Ratio in Mice That Received i.c.v STZ
Peroxidant/antioxidant ratio is considered to be the oxidative stress index ratio. The oxidative stress index ratio is estimated in all treatment groups of mice. The ratio is displayed in Table 8. There is a sharp decline in oxidative stress (F = 911.8) in mice when treated with either 4, 8,or 16 mg/kg of eplerinone in comparison to the mice treated with intracerebroventricular (i.c.v) streptozotocin (STZ).
Table 8. Effect of Eplerinone on the Oxidative Stress Index Ratio in Mice That Received i.c.v STZa.
| sr. no. | groups | dose (mg/kg) | oxidative stress index ratio |
|---|---|---|---|
| 1 | normal control | CMC (1 mL/kg) | 0.73 ± 0.009*** |
| 2 | diseased (STZ) | 3.0 | 2.42 ± 0.073 |
| 3 | standard (piracetam) | 200 | 0.62 ± 0.009*** |
| 4 | eplerinone | 4 | 0.42 ± 0.005*** |
| 5 | 8 | 0.38 ± 0.004*** | |
| 6 | 16 | 0.56 ± 0.004*** |
Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the diseased group.
2.1.9. Effect of Eplerinone on Various Neurotransmitter Levels in Mice That Received i.c.v STZ
The brain tissues of all selected mice were analyzed for various neurotransmitters such as noradrenaline, serotonin, and dopamine levels on the last day of the study. Data are shown in Figure 4. Animals treated with eplerinone 4 and 8 mg/kg showed a dose-dependent increase in noradrenaline (F = 139.8) and dopamine (F = 528.8). However, greater increase of serotonin (F = 510.3) was observed in the group treated with 8 mg/kg and moderate and lesser increase of serotonin in mice treated with 4 and 16 mg/kg, respectively, compared to STZ-treated mice.
Figure 4.
Effect of eplerinone on various neurotransmitter levels in mice that received i.c.v STZ.
2.1.10. Levels of Aβ, TNF-α NF-κB in Brain Tissues of Mice
Results indicated (Figure 5) that protein levels of Aβ 1–40 (F = 27.94) and Aβ 1–42 (F = 24.36) and NF-κB (F = 32.62) were significantly (P < 0.05) decreased in animals treated with eplerinone 8 and 16 mg/kg dose levels when compared with the diseased groups. However, in the case of TNF-α (F = 3.08), a significant difference was seen only with eplerinone 16 mg/kg dose animals and other groups failed to decrease the TNF-α levels in the brain tissues.
Figure 5.
(a) Effects of eplerinone treatment on Aβ 1–40 levels, (b) effects of eplerinone treatment on Aβ 1–42 levels, (c) effects of eplerinone treatment on NF-κB levels, (d) effects of eplerinone treatment on the TNF-α level. Data are represented as man ± SEM (n = 10); ***(P ≤ 0.001), **(P ≤ 0.01), and *(P ≤ 0.05) symbolize highly significant, moderately significant, and significant, respectively, as compared to the diseased group.
2.1.11. PCR Analysis
The expression analysis of polymerase chain reaction (PCR) revealed that eplerinone at 16 mg/kg significantly decreased the beta amyloid precursor protein accumulation as shown by the band intensity graph (Figure 6).
Figure 6.
Band intensity of APP and beta actin.
3. Discussion
In this study, the anti-Alzheimer’s effect of eplerinone was evaluated in mice that received i.c.v STZ. The compounds were computationally modeled to understand their mechanism to inhibit the AChE. These compounds were in silico docked into the binding pocket of AChE by the induced fit docking. Molecular docking has been widely utilized in rational drug design to simulate the ligand binding mode and its complexation within the active site of receptors. The narrow active site gorge of AChE restricts the entrance of ligands by flexible aromatic lining. The induced fit docking is a unique approach that allows the simulation of the flexible binding site and thus considers the governance of flexible aromatic lining during compound docking. Herein, the conformational energy of eplerenone exceeded the standard’s threshold, which highlighted its comparable binding affinity for AChE. Unlike piracetam, it did not penetrate into the catalytic triad but oriented its interactions with aromatic lining of the active site gorge. In addition, eplerenone interacted with a distinct mechanism of inhibition that entailed the potential blockade of entrance and aromatic guidance of ACh at the active site of AChE. However, these insights may delineate the cognitive effects of eplerenone, which may facilitate its repurposing for AD.
Different types of memory such as spatial memory, procedural memory, long-term memory, and episodic memory of mice were tested by following the behavioral paradigms. Spatial learning and memory were assessed by employing the Morris water maze and elevated plus maze models. The TL of mice was noticeably decreased on the assessment day in the elevated plus maze. However, the results of the Morris water maze model indicated the appreciable decrease in EL in trial days and expanded time in the target area during assessment. The outcomes of both paradigms indicated a discernible improvement in memory.14 Passive avoidance, balance beam, and open-field models were employed for the assessment of long-term memory in mice. The results of these three models revealed augmented improvement of cognitive functions on the assessment day. Previous studies clearly indicate that aldosterone has an inimical effect on memory. The mineralocorticoid receptors of type-I are present in hippocampus, exhibiting its role in memory, as aldosterone is highly selective for type-I receptors thus mediating the cognition. Aldosterone receptor antagonist has a pivotal role in memory enhancement. Eplerinone ameliorates the blood flow in the cerebral area of the brain that attenuates the oxidative damage, thus suggesting its role in diminishing cerebral ischemia for cognitive enhancement.15 Oxidative damage occurred when excessive ROS are produced and the defense system of the body (involving endogenous enzymes) is incapable to eliminate the ROS. These ROS contribute to the number of neurodegenerative disorders.16 The adrenal gland secretes aldosterone as a result of angiotensin-II. Aldosterone levels cause the oxidative damage owing to its ability to penetrate in the brain and production of ROS which is involved in the activation of the RAAS and sympathetic system.11 The levels of biochemical markers for oxidative stress such as MDA SOD, GSH, and CAT were quantified in whole brain tissues of mice treated with eplerinone (either 4, 8, or 16 mg/kg). Antioxidant enzymes, that is, GSH, SOD, and CAT, were markedly increased, while MDA was decreased in treatment groups that had received i.c.v. STZ. The increased levels of glutathione peroxidase may be owing to its decreased metabolism, increased synthesis, or decreased utilization.17 The mice that were treated with i.c.v STZ revealed significant low levels of endogenous antioxidants (SOD, CAT, and GSH) and high levels of MDA, causing oxidative stress. STZ is responsible for impaired cognitive ability, oxidative stress, and cholinergic loss and reduced metabolism of glucose in the brain.18 The reduced energy metabolism leads to the memory impairment because of the dwindled acetyl co-A or ATP formation that proposes the cholinergic deficit. The decreased cholinergic activity is due to the increased AChE enzyme that causes the excessive breakdown of ACh, reducing its concentration in cholinergic synapses. ACh is a major neurotransmitter involved in memory enhancement.19 AChE levels were identified in all groups of mice. The animals treated with eplerinone (4, 8, and 16 mg/kg) exhibited low levels of AChE, which is an indication of increased cholinergic activity. Increased concentration of nitric oxide is associated with the oxidative stress, which was found in mice treated with STZ. Superoxides are produced during respiration that reacts with nitric oxide and generates peroxy-nitrite which is highly reactive, leading to oxidative damage and cytotoxicity.20 STZ is also responsible for neuroinflammation because of the increased concentration of various inflammatory mediators such as TNF-α, inflammatory mediators, and NF-kB. These mediators are responsible for neurodegeneration which is a possible mechanism of Alzheimer’s.21 The eplerinone treatment is effective to obviate the inflammation in brains of mice. The anti-inflammatory effect is due to the antagonistic effect of mineralocorticoid receptors in the brain and decreased expression of inflammatory cytokines and adhesion molecules. Aldosterone also induces the formation of superoxide.22 Aβ levels were determined owing to the fact that these proteins were aggregated and abnormally expressed in brains of Alzheimer’s patients. Deposition of beta amyloid in the brain is responsible for triggering cascade of events, leading to chronic inflammation which stimulates more and more production of beta amyloid.23 Aβ1-40 and Aβ1-42 are the most abundant form of Aβ protein in Alzheimer’s. Aβ1-42 is of great importance because it is early deposited in the form of plaque in AD. Quantification of Aβ1-40 and Aβ1-42 showed significant reduction in the mice brains treated with eplerinone. Previous studies clearly indicated the role of adrenergic neurons in the cognitive decline. The levels of neurotransmitters such as noradrenaline, serotonin, and dopamine are reduced during the progression of AD.24 Administration of STZ (i.c.v) in mice resulted in the decrease of concentration of all monoamines, that is, noradrenaline, dopamine, and 5-HT which were reversed after treating the animals with eplerinone. Decreased levels of noradrenaline are associated with various psychological problems including anxiety and difficulty for attention that leads to the advancement of Alzheimer’s and Parkinson’s and so forth.25 Previous studies indicated that a balance should be maintained between the pairs of neurotransmitters. Increased concentration of monoamines resulted to alleviate the AD.26
4. Conclusions
The outcomes of this research work revealed that eplerinone was effective in reversing STZ-induced memory impairment in mice. The current study suggests that the eplerinone may be effective in improving memory in AD and dementia.
5. Materials and Methods
Eplerenone, trichloroacetic acid solution (TCA), DTNB reagent, phosphate buffer solution, pyrogallol solution, dipotassium hydrogen phosphate, potassium hydroxide, potassium dihydrogen phosphate, and hydrogen peroxide were from Sigma Aldrich, and diazepam was from Pfizer. ELISA kits were used.
5.1. Molecular Docking
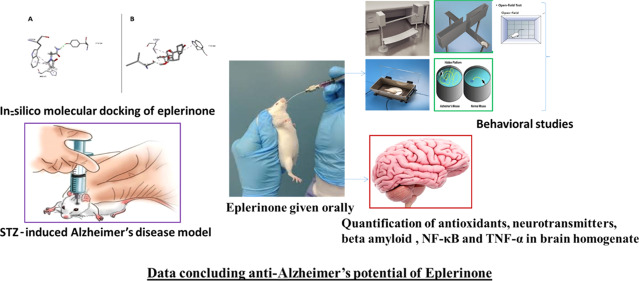
The compounds were in silico modeled to investigate their anticholinesterase mechanism by the induced fit docking protocol in a molecular operating environment (MOE) 2015.10. The X-ray crystallized 3D structure of AChE (PDB ID: 4EY6) was obtained from RSCB protein data bank repository (http://www.rscb.org). The 3D conformers of eplerenone (CID: 4438) and piracetam (CID: 4843) were retrieved from PubChem Database. These structures were modeled in Amber10: EHT molecular force field and prepared by Quick Prep application in MOE. The structures were corrected and optimized for missing residues, H-counts, termini capping, and alternates. The protonation state of these structures was optimized to withstand the molecular refinement of the docked pose. The binding site was defined in the vicinity of cocrystalized inhibitor at the active site of AChE. The compounds were docked by the Triangular Matcher placement method and scored with London dG. The generated poses were further refined by the induced fit method and ranked with the GBVI/WSA dG scoring function. The pose with the lowest binding score (ΔG) was used to model the ligand interaction profile in Discovery Studio Visualizer v17.2. The cocrystallized ligand was docked in its apo conformation, and root-mean-square deviation was computed to validate the docking protocol.
5.2. Experimental Animals
Before commencement of the study protocol, the protocol was approved by the Research Ethical Committee of Riphah institute of Pharmaceutical Sciences, Lahore, with the authentication number of REC/RPS-LHR/2019/031. These guidelines ruled under the regulations of National Institute of Health guide for the care and use of laboratory animals (NIH publication number 8023, revised 1978) and following ARRIVE guidelines. Albino mice (n = 60) of either sex were received from the University of veterinary and animal Sciences (UVAS), Lahore. All mice were almost 2 to 3 months old, having a weight of 30 to 40 g at the start of the study. All animals were housed at the Riphah International University for 15 days under day and night cycle, 4–55% humidity, and 22 ± 2 °C temperature to habituate them before the commencement of the study. They were provided with water and food ad libitum.
5.3. Study Design
Sixty experimental animals were taken and categorized into six groups, each containing 10 mice. Groups I, II, and III were designated as the control group (CMC, 1 mL/kg, orally), disease group (STZ 3 mg/kg i.c.v), and standard (piracetam 200 mg/kg. i.p.) groups, respectively. Groups IV, V, and VI served as treatment groups (eplerenone at doses of 4.0, 8.0, and 16 mg/kg, respectively). Eplerinone was administered through the oral route. The dose of eplerinone was selected by converting the available human dose into animal dose.27 STZ was administered on first and third days of treatment to all groups of animals except for the control group. The intracerebroventriular (i.c.v) route was chosen to administer the STZ in both right and left ventricles (bilaterally) of mice brains by using the stereotaxic apparatus. The groups were treated for 14 successive days with their respective treatments. Cognitive functions were evaluated on the last day (14th day) of treatment.
5.4. Assessment of Cognitive Functions
Cognitive performance was evaluated by the following paradigm.
5.4.1. Elevated Plus Maze
An elevated plus model is used to assess the spatial memory. All treated animals were tested for the cognitive function at 10th and 11th days of treatment. The 10th day was considered as the training day (day 1), while retention of memory was examined 24 h after the training (day 2) or 11th day of the treatment. The model is made up of four arms: two exposed 16 × 5 cm) and two closed arms (16 × 5 × 15 cm) that are elongated from center (5 × 5 cm). The height of the apparatus is 25 cm. The animals are tested for TL by placing each animal at the end of the open arm opposite to the center. TL is the time that the animal takes to enter from exposed arm into covered arm with all its four paws. The TL of each animal was observed for 90 s. If the animal did not enter into a closed arm within the assigned time that is 90 s, it was propelled into a closed arm and TL was considered as 90 s. The animal was permitted to explore the area for 120 s before returning to the cage. Decreased TL was considered as the cognitive improvement.28
5.4.2. Passive Avoidance Model
This is a fear-motivated test. This model is used to assess the episodic memory. The forebrain is involved in episodic memory. The test apparatus consists of a box with a grid floor. Three walls of the apparatus are made up of wood (25 × 25 × 25), and one wall is made up of glass. A wooden piece (8 × 5 × 1.5 cm) is fixed at the center of the floor. The test was conducted in three sessions starting from the last day of the treatment, that is, 14th day that was considered as the trial day. The first session was carried out by placing each animal on the wooden piece and SDL was recorded. As the animal steps down the floor, an electric current of 20 V was applied. SDL is the time when the animal steps down the floor with all four paws. The animals that showed the SDL within 2–15 s were selected for the second test that was conducted after 90 min of the first session. The retention test was carried out after 24 h, that is, on 15th day of treatment by selecting the animals that showed SDL less than 60 s. Increased in SDL was a sign of the cognitive enhancement.29
5.4.3. Morris Water Maze
A Morris water maze test is established to evaluate the spatial learning and memory. The apparatus consists of a pool with a diameter of 60 cm and height of 25 cm. The pool is filled with water up to the depth of 20 cm. The temperature of water is maintained at 25 °C. The pool has four poles, that is, east, west, south, and north. A hidden platform is placed in the middle of the pool, 1 inch above the level of water. Each mice was subjected to the training session for 4 consecutive days (6th to 9th day of treatment) to record the EL. EL is considered as the time that mice take to locate the hidden platform from the starting pole. The starting pole was changed on each day, but the position of the target platform remained constant. Retention of memory was assessed on 10th day of treatment (5th day of training) by removing the platform from the water. The animal was placed in water from any one of the pole and allowed to reach the target area to find the missing platform. Decreased EL was an indication of memory enhancement.30
5.4.4. Balance Beam
This apparatus is made up of a beam of 1 mm length and 12 mm wide plan surface, which is positioned 50 cm high above the floor fixed with two vertical bars. A black color box is placed at the end point containing some food material for the attraction of animal. Start point was illuminated to repel the mice. On the last day (14th day) of the study protocol 1 h post dose, each animal was trained to walk over the beam and time to reach end point was recorded. After 24 h (15th day), the animals were assessed for memory by repeating the same procedure.31
5.4.5. Open Field Model
This apparatus is composed of a white floor, which is divided into 36 (5 × 5) squares surrounded by black Plexiglas walls. The 32 squares alongside the walls are designated as the peripheral area or periphery, while the remaining 4 squares in the center are designated as the central field. The peripheral area is the protected area. The test was carried out in red light for 2 consecutive days after administration of the last dose on day 14 (training day). Memory retention was assessed on day 15.
Mice were examined for behavior, location, and autonomic nervous system (ANS) to assess the cognitive functions. Various parameters were observed to assess the behavior, that is, latency (initial time to start the movement), freezing (no movement), rearing, and grooming. The location was examined by observing the time spent in the central and peripheral areas of the field and number of crossing squares of field by each mouse. Urination and defecations were noted for the assessment of ANS.32
5.5. Neurochemical Studies
Animals were anesthetized by using the isoflurane. Intact brain tissues were isolated by cervical dislocation of mice. The isolated brain tissues were then homogenized in phosphate buffer (pH 7.4, 1/10 w/v. The homogenate was centrifuged at 4 °C and 6000 rpm for 10 min.33
5.5.1. Quantification of Glutathione Peroxidase
Precipitates are formed by the addition of 10% TCA (1 mL) in tissue homogenate (1 mL). An aliquot of the supernatant was mixed with sodium phosphate (4 mL) and DTNB (0.5 mL). The spectrophotometric analysis was done at 412 nm.
GSH was measured by the equation mentioned below
DF symbolizes the dilution factor, VU designated as aliquot volume, Y is taken as absorbance, and BT denotes brain tissue homogenate.34
4.5.2. Estimation of SOD
Solution of pyrogallol (0.1 mL) and 2.8 mL of potassium phosphate buffer (0.1 M, and pH 7.4) were mixed with 0.1 mL of homogenate. Absorbance was noted by a UV–visible spectrophotometer at 312 nm.35
Standard line of regression was made and used to measure SOD.
5.5.3. Quantification of CAT
Phosphate buffer (1.95 mL, pH 7, 50 mM) and H2O2 solution (1 mL, 30 mM) were mixed with 0.05 mL of tissue homogenate to note the absorbance, and the level of CAT was estimated by using the following formula.
δ.O.D represents the change in absorbance per minute and E symbolizes the extinction coefficient of H2O2 with a value of 0.071 mmol cm–1.36
The lowery method was used to measure the protein contents.37
Regression line used to estimate the contents of protein is as follows
5.5.4. Quantification of MDA
A thiobarbituric acid reagent (3 mL) was added to an aliquot of the supernatant (1 mL). The mixture was shaken well. After 15 min, the reaction mixture was cooled and centrifuged at 3500g for 10 min. Absorbance was noted at 532 nm.38
The following formula was employed for the measurement of MDA.
5.5.5. Quantification of Nitrite
Griess reagent was used for the determination of the nitrite level. Griess reagent and brain tissue homogenate were mixed in equal quantity and incubated for 10 min. After incubation, the absorbance of the sample was noted at 546 nm.39
The following regression line was used to measure the level of sodium nitrite.
5.5.6. Determination of the Oxidative Stress Index Ratio
The oxidative stress index ratio was determined by applying the following formula40
5.5.7. Estimation of the AChE Activity
Homogenate of brain tissues (0.4 mL) was taken, and it was mixed with 100 μL of DTNB ((0.1 mM) and 2.6 mL of phosphate buffer (pH = 8). The sample was quantified at the absorbance of 412 nm. After the stabilization of value, 20 μL of acetylthiocholin iodide was added in the cuvette, and the reading was taken for 10 min after every 20 min.
The mean of all readings was measured as follows
where A represents the absorbance change in 1 min. The rate of mole of acetylthiocholiniodide hydrolyzed/min/gm. of homogenate. Co = initial concentration.41
5.5.8. Determination of Norepinephrine, Serotonin, and Dopamine
5.5.8.1. Preparation of Tissue Homogenate
Mice were dissected, and their brains were removed and weighed. Brain tissue homogenate was prepared in HCl-butanol (5 mL) and then centrifuged at 2000 rpm for 10 min. The supernatant (1 mL) was taken, and 2.5 mL of heptane and 0.31 mL of HCl (0.1 M) were added in it, and it was shaken for 10 min. The mixture is then centrifuged under the above-mentioned conditions. Two layers were separated after centrifugation. The temperature is maintained at 0 °C during the procedure. The organic layer was dropped, and the aqueous layer (0.2 mL) was taken for the estimation of serotonin, dopamine, and norepinephrine.
5.5.8.2. Determination of Norepinephrine and Dopamine
HCl (0.05 mL, 0.4 M) and sodium acetate/EDTA buffer (0.1 mL, pH 6.9) were mixed with 0.2 mL of aqueous phase. After mixing, the oxidation reaction was carried out by adding 0.1 mL of solution of Na2SO3. The reaction was carried out for 1.5 min, after which acetic acid (0.1 mL) was mixed. The reaction mixture was heated for 6 min at 100 °C. The mixture was then cooled to 25 °C, and absorbance was noted at 350 nm for dopamine and 450 nm for norepinephrine.
Regression line for norepinephrine is as follows
Regression line for dopamine is as follows
5.5.8.3. Determination of Serotonin
O-Phthaldialdehyde (0.25 mL) was added in 0.2 l of the aqueous phase. The mixture is then heated up to 100 °C to obtain the fluorophore. The mixture was cooled to room temperature, and absorbance was noted. The emission wavelength is 340 nm and the excitation wavelength is 305 nm for the fluorescence method.42
Regression line for serotonin is as follows
5.6. ELISA Analysis
ELIZA kits of company Thermo USA for Aβ 1–40(CAT # KHB3441), Aβ 1–42 (CAT # KHB3481), NF-κB (CAT # 85-86081-11), and TNF-α (CAT # EK0527-CAP) were used. Aβ 1–40, Aβ 1–42, NF-κB, and TNF-α were complexed with the HRP-enzyme to form an antigen–antibody complex. TBM solution was added in the respective wells, and after that, sulfuric acid was added for cessation of reaction. Blue color changes to yellow color, and absorbance was measured at 450 nm. The quantities of the selected proteins were measured with their respective regression line equation of standard proteins.
5.7. Polymerase Chain Reaction
Gene expression analysis of APP was carried out by conventional PCR. Briefly, total RNA was separated using Triazole from frozen brain tissues of mice according to the protocol of manufacturers. Complementary DNA library was synthesized using 0.5 μg RNA using First strand kit (revertaid first strand cDNA synthesis kit, Thermo Fischer). APP2 mRNA expression was evaluated using APP2 specific primers; (5′→3′) forward primer: GTACCCACTGATGGCAACG, reverse primer AGGAACTTGCACTTGTCGG (Product size 350bp). Beta Actin mRNA expression was used as internal control: (5′→3′) forward primers GGAGATTACTGCTCTGGCTCC, reverse Primer; GTCGCCTTCACCGTTCCAG (Product size 105 bp). PCR conditions were as follows: the annealing temperature was 53 °C for 30 s and the extension temperature was 72 °C for 45 s and total of 36 cycles were performed. The PCR product (5 μL) was resolved by on 1.5% Agarose gel. PCR product sizes were verified using a DNA ladder (thermo fisher scientific).
5.8. Statistical Analysis
The results were expressed as mean ± SEM (n = 10) by applying the Graph Pad Prism software version 5, San Diego, CA, USA. The parametric test was applied to the animal behavioral analysis. Data are analyzed by applying the one way ANOVA followed by post hoc Dennett’s test. p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 were considered as significant (*), moderately significant (**), and highly significant (***), respectively, compared to the disease group.
Acknowledgments
This research was technically and financially supported by Riphah International University, Pakistan.
Author Contributions
S.H. and F.A. participated in the study design, acquisition, analysis, interpretation of data and drafting the article. Z.R. participated in the software editing. U.S. and A.U.R. participated in revising the article. Supervision and final approval of the manuscript for submission were done by the B.A..
The authors declare no competing financial interest.
References
- Rajesh V.; Riju T.; Venkatesh S.; Babu G. Memory enhancing activity of Lawsonia inermis Linn. leaves against scopolamine induced memory impairment in Swiss albino mice. Orient. Pharm. Exp. Med. 2017, 17, 127–142. 10.1007/s13596-017-0268-8. [DOI] [Google Scholar]
- Vinay K.; Abbas A. K.; Fauston N.. Robbins and Cotran Pathologic Basis of Disease; Saunders, ElSevier: China: 2005; Vol. 8, pp 208–221. [Google Scholar]
- Shi S.; Liang D.; Chen Y.; Xie Y.; Wang Y.; Wang L.; Wang Z.; Qiao Z. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer’s disease. Eur. J. Immunol. 2016, 46, 665–676. 10.1002/eji.201545855. [DOI] [PubMed] [Google Scholar]
- McCoy M. K.; Tansey M. G. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 2008, 5, 45. 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. V.; Kounatidis I. Nuclear factor-kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 2017, 8, 1805. 10.3389/fimmu.2017.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P. T. Neuroanatomy/pathology and the interplay of neurotransmitters in moderate to severe Alzheimer disease. Neurology 2005, 65, S5–S9. 10.1212/wnl.65.6_suppl_3.s5. [DOI] [Google Scholar]
- Szot P. Common factors among Alzheimer’s disease, Parkinson’s disease, and epilepsy: possible role of the noradrenergic nervous system. Epilepsia 2012, 53, 61–66. 10.1111/j.1528-1167.2012.03476.x. [DOI] [PubMed] [Google Scholar]
- Martorana A.; Koch G. Is dopamine involved in Alzheimer’s disease?. Front. Aging Neurosci. 2014, 6, 252. 10.3389/fnagi.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldenhuys W. J.; Van der Schyf C. J. Role of serotonin in Alzheimer’s disease. CNS Drugs 2011, 25, 765–781. 10.2165/11590190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Zhang J.; Lu L.; Chen S. S.; Quinn M. T.; Weber K. T. Aldosterone-Induced Inflammation in the Rat Heart. Am. J. Pathol. 2002, 161, 1773–1781. 10.1016/s0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-H.; Yu Y.; Kang Y.-M.; Wei S.-G.; Felder R. B. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1067–H1074. 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- Hajjar I.; Hart M.; Mack W.; Lipsitz L. A. Aldosterone, cognitive function, and cerebral hemodynamics in hypertension and antihypertensive therapy. Am. J. Hypertens. 2014, 28, 319–325. 10.1093/ajh/hpu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indumathy S.; Kavimani S.; Raman K. Role of angiotensin antagonists in memory enhancement. Int. J. Pharma Bio Sci. 2010, 1, 1–4. [Google Scholar]
- Dhingra D.; Kumar V. Memory-enhancing activity of palmatine in mice using elevated plus maze and Morris water maze. Adv. Pharmacol. Sci. 2012, 2012, 357368. 10.1155/2012/357368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S.; Akaike M.; Ise T.; Ueda Y.; Iwase T.; Sata M. Renin-angiotensin-aldosterone system has a pivotal role in cognitive impairment. Hypertens. Res. 2013, 36, 753. 10.1038/hr.2013.51. [DOI] [PubMed] [Google Scholar]
- Mariani E.; Polidori M. C.; Cherubini A.; Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J. Chromatogr. B: Biomed. Sci. Appl. 2005, 827, 65–75. 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Kim J.; Keum Y.-S. NRF2, a key regulator of antioxidants with two faces towards cancer. Oxid. Med. Cell. Longevity 2016, 2016, 2746457. 10.1155/2016/2746457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Suzanne M.; Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 548–559. 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Salkovic-Petrisic M.; Knezovic A.; Hoyer S.; Riederer P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeutic strategies in Alzheimer’s research. J. Neural Transm. 2013, 120, 233–252. 10.1007/s00702-012-0877-9. [DOI] [PubMed] [Google Scholar]
- Tiwari V.; Kuhad A.; Bishnoi M.; Chopra K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol., Biochem. Behav. 2009, 93, 183–189. 10.1016/j.pbb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Balez R.; Ooi L. Getting to NO Alzheimer’s disease: neuroprotection versus neurotoxicity mediated by nitric oxide. Oxid. Med. Cell. Longevity 2016, 2016, 3806157. 10.1155/2016/3806157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola M. S.; Aleisa A. M.; Al-Rejaie S. S.; Abuohashish H. M.; Parmar M. Y.; Alhomida A. S.; Ahmed M. M. Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol. Sci. 2014, 35, 1003–1008. 10.1007/s10072-014-1628-5. [DOI] [PubMed] [Google Scholar]
- Dinh Q. N.; Young M. J.; Evans M. A.; Drummond G. R.; Sobey C. G.; Chrissobolis S. Aldosterone-induced oxidative stress and inflammation in the brain are mediated by the endothelial cell mineralocorticoid receptor. Brain Res. 2016, 1637, 146–153. 10.1016/j.brainres.2016.02.034. [DOI] [PubMed] [Google Scholar]
- Cai Z.; Yan Y.; Wang Y. Minocycline alleviates beta-amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin. Interv. Aging 2013, 8, 1089. 10.2147/cia.s46536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinikainen K. J.; Soininen H.; Riekkinen P. J. Neurotransmitter changes in Alzheimer’s disease: implications to diagnostics and therapy. J. Neurosci. Res. 1990, 27, 576–586. 10.1002/jnr.490270419. [DOI] [PubMed] [Google Scholar]
- Chalermpalanupap T.; Kinkead B.; Hu W. T.; Kummer M. P.; Hammerschmidt T.; Heneka M. T.; Weinshenker D.; Levey A. I. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 21. 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari A.; Amenta F.; Silvestrelli G.; Tomassoni D.; Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech. Ageing Dev. 2006, 127, 158–165. 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Nair A.; Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K.; Miyazaki S.; Imaizumi M.; Stark H.; Schunack W. Improvement by FUB 181, a novel histamine H3-receptor antagonist, of learning and memory in the elevated plus-maze test in mice. N. Schmied. Arch. Pharmacol. 1998, 357, 508–513. 10.1007/pl00005200. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; Jeon S. J.; Jung J. W.; Lee S.; Yoon B. H.; Shin B. Y.; Son K. H.; Cheong J. H.; Kim Y. S.; Kang S. S.; Ko K. H.; Ryu J. H. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur. J. Pharmacol. 2007, 574, 140–147. 10.1016/j.ejphar.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V.; Williams M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848. 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong T. N.; Carlisle H. J.; Southwell A.; Patterson P. H. Assessment of motor balance and coordination in mice using the balance beam. J. Visualized Exp. 2011, 49, e2376. 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R. N.; Cummins R. A. The open-field test: a critical review. Psychol. Bull. 1976, 83, 482. 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- Hira S.; Saleem U.; Anwar F.; Ahmad B. Antioxidants Attenuate Isolation-and L-DOPA-Induced Aggression in Mice. Front. Pharmacol. 2018, 8, 945. 10.3389/fphar.2017.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rahman I.; Kode A.; Biswas S. K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159. 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]; b Hira S.; Saleem U.; Anwar F.; Sohail M. F.; Raza Z.; Ahmad B. β-Carotene: A Natural Compound Improves Cognitive Impairment and Oxidative Stress in a Mouse Model of Streptozotocin-Induced Alzheimer’s Disease. Biomolecules 2019, 9, 441. 10.3390/biom9090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado-Matsumoto A.; Fridovich I. Subcellular Distribution of Superoxide Dismutases (SOD) in Rat Liver. J. Biol. Chem. 2001, 276, 38388–38393. 10.1074/jbc.m105395200. [DOI] [PubMed] [Google Scholar]
- Saleem U.; Ahmad B.; Ahmad M.; Hussain K.; Bukhari N. I. Investigation of in vivo antioxidant activity of Euphorbia helioscopia latex and leaves methanol extract: a target against oxidative stress induced toxicity. Asian Pac. J. Trop. Med. 2014, 7, S369–S375. 10.1016/s1995-7645(14)60260-1. [DOI] [PubMed] [Google Scholar]
- Waterborg J. H., The Lowry method for protein quantitation. The Protein Protocols Handbook; Springer, 2002; pp 7–9. [Google Scholar]
- Bhangale J. O.; Acharya S. R. Anti-Parkinson activity of petroleum ether extract of Ficus religiosa (L.) leaves. Adv. Pharmacol. Sci. 2016, 2016, 9436106. 10.1155/2016/9436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais S.; Gill N.; Kumar N. Neuroprotective effect of Juniperus communis on chlorpromazine induced Parkinson disease in animal model. Chin. J. Biol. 2015, 2015, 542542. 10.1155/2015/542542. [DOI] [Google Scholar]
- Jaiswal S. K.; Sharma A.; Gupta V. K.; Singh R. K.; Sharma B. Curcumin mediated attenuation of carbofuran induced oxidative stress in rat brain. Biochem. Res. Int. 2016, 2016, 7637931. 10.1155/2016/7637931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V. Jr; Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Rahman H.; Eswaraiah M., Simple spectroscopic Methods for estimating Brain Neurotransmitters, Antioxidant Enzymes of Laboratory animals like Mice: A review. PharmaTutor Art, 2008; p 1244.