Summary
Pathogenic bacteria taken up into the macrophage phagosome are the target of many anti-microbial mechanisms. Although mitochondria-derived antimicrobial effectors like reactive oxygen species (mROS) aid in bacterial killing, it is unclear how these effectors reach bacteria within the phagosomal lumen. We show here that endoplasmic reticulum stress triggered upon methicillin-resistant Staphylococcus aureus (MRSA) infection induces mROS that are delivered to bacteria-containing phagosomes via mitochondria-derived vesicles (MDVs). The endoplasmic reticulum stress sensor, IRE1α induces mROS, specifically hydrogen peroxide (mH2O2) upon MRSA infection. MRSA infection also stimulates the generation of MDVs, which require the mitochondrial stress-response factor Parkin, and contribute to mH2O2 accumulation in bacteria-containing phagosomes. Accumulation of phagosomal H2O2 requires Toll-like receptor signaling and the mitochondrial enzyme, superoxide dismutase-2 (Sod2), which is delivered to phagosomes by MDVs. Sod2 depletion compromises mH2O2 production and bacterial killing. Thus, mitochondrial redox capacity enhances macrophage antimicrobial function by delivering mitochondria-derived effector molecules into bacteria-containing phagosomes.
Introduction
Reactive oxygen species (ROS) play pivotal roles in signaling and defense of biological organisms. These highly reactive molecules oxidize lipids, proteins and other cellular constituents, leading to a spectrum of responses ranging from altered signaling to cell death. While ROS are constitutively generated and de-toxified during cellular metabolism, ROS levels acutely increase during cellular stress (Holmstrom and Finkel, 2014), and can be a potent weapon in the host arsenal to control invading pathogens. In cells of the innate immune system, like macrophages and neutrophils, ROS are primarily generated by the phagocyte NADPH oxidase and/or mitochondrial metabolism. Upon infection, the phagocyte oxidase multi-protein complex, also referred to as NOX2, can be recruited to phagosomal membranes to generate a burst of superoxide into the phagosome lumen (Winterbourn and Kettle, 2013). However, many bacterial pathogens, like Mycobacterium tuberculosis, Salmonella enterica serovar Typhimurium, Coxiella burnetti, and Francisella tularensis prevent the rapid NOX2-mediated burst either by avoiding or delaying complex recruitment or by utilizing detoxification mechanisms (Celli and Zahrt, 2013; Koster et al., 2017; Mertens and Samuel, 2012; Vazquez-Torres and Fang, 2001). Mitochondrial ROS (mROS) can also contribute to bacterial killing (West et al., 2011), but how these reactive molecules reach bacteria within the macrophage phagosome is ill-defined.
ROS induction can be driven by cellular stress response pathways (Bronner et al., 2015), like the endoplasmic reticulum unfolded protein response (UPR), activated through the three ER sensors, PERK, ATF6 and IRE1α (Zeeshan et al., 2016; Zhang and Kaufman, 2004). Indeed, many studies support an integral role for cellular stress pathways in modulating innate immune responses (Muralidharan and Mandrekar, 2013). We previously showed that IRE1α is critical for stimulating macrophage anti-microbial function (Abuaita et al., 2015). Specifically, IRE1α activation resulted in sustained macrophage ROS production required for killing MRSA. Notably, IRE1α-dependent killing in macrophages was only partially NOX2-dependent, despite the well-established role of NOX2 in neutrophil oxidative defenses against Staphylococcus aureus (Rigby and DeLeo, 2012). Since IRE1α signaling induces mROS production (Tufanli et al., 2017), we hypothesized that the mechanism by which IRE1α activation led to MRSA killing relied on mROS generation.
Here we show that infection of macrophages by MRSA stimulates IRE1α-dependent production of mROS, specifically hydrogen peroxide (mH2O2). Infection also triggers generation of Parkin-dependent mitochondrial-derived vesicles (MDVs), previously described as a pathway for mitochondrial quality control (Soubannier et al., 2012a). MDVs form in a dynamin related protein 1 (Drp1)-independent response to increased oxidative stress, and contain select mitochondrial proteins, like the outer mitochondrial membrane protein, Tom20, which is present on some, but not all, MDVs (Soubannier et al., 2012a). These vesicles may function as a quality control mechanism by delivering damaged mitochondrial components to the endolysosomal pathway (Sugiura et al., 2014) or as a mechanism for interorganelle communication (Neuspiel et al., 2008). In MRSA-infected macrophages, we find that MDVs deliver the mitochondrial peroxide-generating enzyme, Sod2, into the bacteria-containing phagosome, controlling bacterial burden. Our findings reveal a mechanism by which programmed cellular stress responses repurpose a mitochondrial quality control mechanism to enable anti-microbial defense.
Results
Induction of mROS by IRE1α promotes macrophage bactericidal function
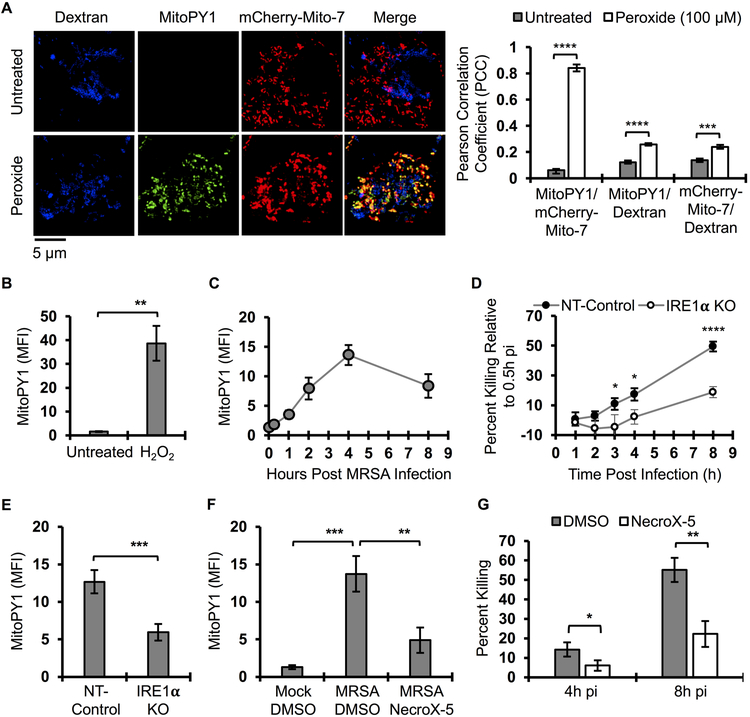
We previously showed that MRSA infection stimulates macrophages to produce global ROS via activation of the ER stress sensor IRE1α, which was required in vitro and in vivo for effective MRSA killing (Abuaita et al., 2015). Based on the extensive crosstalk between ER and mitochondrial stress pathways (Senft and Ronai, 2015), we hypothesized that IRE1α-dependent antimicrobial activity might rely on mROS, and indeed, previous reports indicated that mitochondrial ROS (mROS) enhance macrophage bactericidal function (Geng et al., 2015; West et al., 2011). To test this hypothesis, we assessed whether mROS was induced by macrophages in response to MRSA infection. First, we validated that MitoPY1, a mitochondrially-targeted probe that fluoresces in response to H2O2 (Dickinson and Chang, 2008; Dickinson et al., 2013), was indeed mitochondrially restricted in macrophages. RAW264.7 cells were transfected with mCherry-Mito-7 (Olenych et al., 2007) and loaded with MitoPY1. MitoPY1 fluorescence intensity increased when macrophages were stimulated with exogenous H2O2 (Fig. 1A). The MitoPY1 signal was highly co-localized with mCherry-Mito-7 but showed poor co-localization with a fluid phase marker, 10 kD Cascade Blue-Dextran, chased into the lysosomal network (Davis and Swanson, 2010). We also quantified the difference in mean fluorescence intensity (MFI) of MitoPY1 by flow cytometry (Fig. 1B). During MRSA infection, MitoPY1 fluorescence intensity increased over time, peaking at 4h pi (Fig. 1C). To determine whether IRE1α was required for mH2O2 induction by MRSA infection, we generated IREα-deficient RAW264.7 macrophages using CRISPR/Cas9 (Fig. S1A and S1B) and evaluated MRSA killing in these cells. We observed a significant decrease in MRSA killing in the IREα-deficient cells compared to the WT control cells (Fig. 1D), with kinetics similar to those observed those in NOX2-deficient macrophages (Fig. S1C). IRE1α deficiency suppressed the ability of MRSA-infected macrophages to induce mH2O2 compared to the non-targeted control (Fig. 1E). To test the requirement for mROS in killing MRSA, infected macrophages were treated with the ROS scavenger, NecroX-5, which is primarily localized to mitochondria (Kim et al., 2010; Thu et al., 2016). Infected macrophages treated with NecroX-5 exhibited significantly lower MitoPY1 fluorescence than control-treated cells (Fig. 1F), and decreased capacity to kill MRSA (Fig. 1G). These data indicate that IRE1α is critical for infection-induced mH2O2 and establishes a role for mROS in macrophage bactericidal function against MRSA.
Figure 1. MRSA infection stimulates bactericidal mH2O2 via IRE1α.
(A) Representative live fluorescence images of RAW264.7 macrophages transfected with mCherry-Mito-7 expressing plasmid, pulsed with Cascade Blue Dextran (molecular weight 10,000) for 24h, followed by a 1h pulse with MitoPY1. Cells were then treated with H2O2 (100 μM) or left untreated. Images were acquired on a Leica TCS SP8 confocal scanning microscope. Quantification of co-localization (Pearson Correlation Coefficient, PCC) between mCherry-Mito-7, Dextran and MitoPY1 was performed with Huygens Essential imaging software. Graph is presented as the mean of at least 150 cells for each condition from n≥3 independent experiments +/− standard error of the mean (SEM).
(B) Flow cytometric analysis of mean fluorescence intensity (MFI) of macrophages after a pulse with MitoPY1 for 1h and chased with unlabeled medium with or without H2O2.
(C) Time course measurement of MFI of macrophages pulsed with MitoPY1 for 1h, infected with MRSA and monitored over time by flow cytometry.
(D) Time course measurement of MRSA intracellular killing by NT-control and IRE1α-deficient macrophages. Percent killing was calculated using the following formula [1 - (CFUindicated time points / CFU0.5h pi)] X 100.
(E) Flow cytometric analysis of IRE1α KO macrophages and NT-control after 1 hr labeling with MitoPY1, followed by MRSA infection and analyzed at 4h pi.
(F) Flow cytometric analysis of macrophages labeled with MitoPY1 for 1h and infected with MRSA for 4h in the presence or absence of mROS scavenger, NecroX-5.
(G) Percent intracellular MRSA killing by macrophages in the presence and absence of NecroX-5. Percent killing was calculated using the following formula [1 - (CFUindicated time points / CFU1h pi)] X 100.
MFI of MitoPY1 quantification was determined using FlowJo software, representing the geometric mean. MFI obtained from unstained cells was subtracted from the MFI of all stained samples. Unless otherwise indicated, graphs are presented as the mean of n≥3 independent experiments +/− SD. pValue: *< 0.05, **< 0.01, ***< 0.001 and ****<0.0001.
Mitochondrial peroxide accumulation in phagosomes is TLR-dependent
We reasoned that mH2O2 could contribute to bactericidal function indirectly by signaling and/or by direct delivery to the phagosome. If direct delivery, we might expect to see mH2O2 accumulate in the phagosome. To monitor mH2O2 spatial localization during infection, we imaged live cells stimulated with viable MRSA, killed MRSA or latex beads. Macrophages were pulsed with MitoPY1 and imaged 4h post-phagocytosis (Fig. 2A). Hydrogen peroxide increased within the mitochondrial network during infection with live or fixed MRSA, but not with beads. We also observed smaller MitoPY1+ puncta throughout the cell. Notably, mH2O2 accumulated to significantly higher levels in MRSA-containing phagosomes than bead-containing phagosomes (Fig. 2B). To visualize the dynamic distribution of mH2O2 during infection, we performed time-lapse imaging of infected macrophages pre-loaded with MitoPY1. By 10 min pi, the MitoPY1 signal within the mitochondrial network had increased. MitoPY1+ puncta could be observed associated with the bacterial phagosome as early as 50 min pi, followed by accumulation of probe within the bacteria-containing phagosome (Fig. 2C and Movie S1). These data suggest that mitochondrially-derived hydrogen peroxide accumulates within phagosomes and reveal the possibility that mH2O2 may contribute to macrophage bactericidal effector function through a direct delivery mechanism.
Figure 2. TLR signaling controls mH2O2 accumulation in the bacterial phagosome.
(A) Representative fluorescence microscopy of RAW264.7 macrophages pulsed with MitoPY1 (green) for 1h and treated with beads (red), or infected with live MRSA-mCherry or fixed MRSA-mCherry for 4h. Images were acquired on an Olympus IX-70 inverted live-cell fluorescence microscope and analyzed with MetaMorph imaging software.
(B) Quantification of MitoPY1 MFI associated with macrophage phagosomes using ImageJ software. Phagosomes were defined by the area in the cell where red fluorescent beads or MRSA were localized.
(C) Time lapse imaging of macrophages pulsed with MitoPY1 for 1h (green) and then infected with MRSA-mCherry (red). Time shown as minutes:seconds.
(D) Live microscopy images of WT and TLR 2/4/9-deficient (TLR2/4/9 KO) bone marrow-derived macrophages pulsed with MitoPY1 (green) for 1h and then infected with MRSA-mCherry (red) for 4h.
(E) Quantification of MFI of MitoPY1 associated with phagosomes from WT and TLR2/4/9-deficient macrophages using similar criteria as in panel B.
Graphs represent averages of MitoPY1 MFI from n≥305 phagosomes for each group, pooled from at least 3 independent experiments +/−SEM.
pValue: *< 0.05 and ****< 0.0001.
Mitochondrial ROS induction in macrophages occurs when cells internalize beads conjugated to Toll-like receptor (TLR) ligands (TLR2 and TLR4), but not beads alone (West et al., 2011). To test whether TLR signaling was required for mH2O2 generation and accumulation within MRSA-containing phagosomes, we measured MitoPY1 fluorescence intensity in wild-type (WT) and TLR2/4/9-deficient bone marrow-derived macrophages (BMDMs) during MRSA infection. We observed increased overall mH2O2 in both WT and TLR2/4/9-deficient BMDMs during MRSA infection, indicating that mH2O2 induction is largely independent of TLR2/4/9 signaling (Fig. S2A). However, TLR2/4/9-deficient macrophages failed to kill MRSA (Fig. S2B) and MitoPY1 accumulation in their MRSA-containing phagosomes was decreased compared to WT macrophages (Fig. 2D and 2E), indicating that TLR signaling controls mH2O2 delivery to or accumulation within bacteria-containing phagosomes.
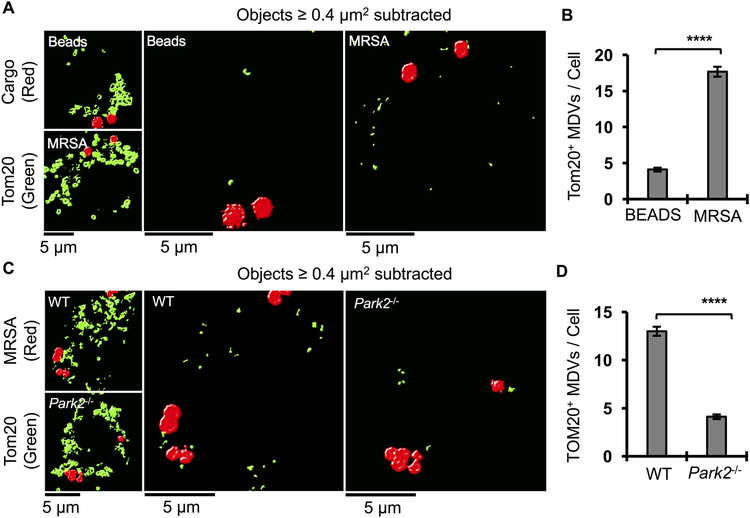
Bacterial infection triggers Parkin-dependent generation of MDVs
Recent studies have revealed that mitochondrial constituents can be selectively transported to other intracellular compartments by different populations of MDVs, often stimulated by oxidative stress (McLelland et al., 2014; Soubannier et al., 2012a). Since infection increases mH2O2, we reasoned that could trigger generation of MDVs, a potential mechanism to deliver antimicrobial cargo from the mitochondria to the phagosome. To test this hypothesis, we first assessed if MDVs are induced by MRSA infection. Macrophages were infected with MRSA and subjected to immunofluorescence analysis by high-resolution confocal microscopy. MRSA infection stimulated an increase of small particles positive for the mitochondrial outer membrane protein, Tom20, compared to bead-containing macrophages (Fig. 3A). These particles could be quantified by image analysis in which large mitochondrial network objects ≥ 0.4 μm2 were subtracted (Fig. 3A and 3B, Fig. S3A and S3B). The Parkinson’s Disease-associated protein, Parkin, regulates biogenesis of a subset of MDVs (McLelland et al., 2014). We therefore tested the requirement for Parkin in generating infection-induced Tom20+ MDVs by confocal microscopy and quantitative image analysis. MRSA-infected Park2−/− BMDM had significantly lower numbers of Tom20+ MDVs compared to WT BMDM (Fig. 3C and 3D). Collectively, these results show that MRSA infection induces formation of MDVs through a Parkin-dependent mechanism.
Figure 3. MRSA infection induces Parkin-dependent MDVs.
(A) Representative images of bone marrow-derived macrophages stimulated with beads (red) or infected with MRSA (red) for 4h and stained with anti-Tom20 (green) antibody. Images were acquired on a Leica TCS SP8 scanning confocal microscope and deconvoluted using Huygens Essential software. Right panels are processed images after subtraction of Tom20+ large objects (surface area ≥ 0.4 μm2).
(B) Quantification of the number of Tom20+ small objects (surface area < 0.4 μm2) per macrophage stimulated with beads or infected with MRSA. Deconvoluted confocal images were processed by Huygens Essential software using the following criteria; 10% threshold, 10% seed and garbage of 50. Tom20+ objects with surface area larger than 0.4 μm2 were filtered out and the remaining objects were enumerated per cell. Graph represents the mean of at least 107 cells for each group, pooled from 3 independent experiments +/− SEM.
(C) Representative confocal images of WT and Parkin deficient (Park2−/−) bone marrow-derived macrophages infected with MRSA for 4h. Images were processed using similar criteria as in panel A.
(D) Quantification of Tom20+ smaller objects (surface area < 0.4 μm2) from WT and Parkin-deficient macrophages infected with MRSA. Confocal microscopy images processed as in panel B and presented as mean +/− SEM from n≥135 cells for each group, pooled from 3 independent experiments. Macrophages were derived from ≥ 2 WT and 2 Park2−/− animals.
pValue: ****<0.0001.
Parkin controls phagosomal mH2O2 accumulation and promotes bactericidal function
Polymorphic alleles of Park2 can mediate susceptibility to microbial infection (Al-Qahtani et al., 2016; Manzanillo et al., 2013). We hypothesized that MDVs could enhance macrophage bactericidal function by facilitating mH2O2 accumulation within phagosomes. Upon measuring the requirement for Parkin in mH2O2 accumulation in phagosomes, we found that infected Parkin-deficient macrophages produced higher levels of mH2O2 than WT macrophages, indicating that Parkin is not necessary for mH2O2 induction per se (Fig. 4A). Notably, despite higher overall levels of mH2O2, Parkin-deficient macrophages displayed only minimal accumulation of MitoPY1 in phagosomes compared to WT macrophages (Fig. 4B and 4C). WT and Park2−/− BMDM were infected with MRSA to assess bactericidal capacity. Parkin-deficient macrophages were less capable of killing MRSA, Staphylococcus epidermidis and Salmonella enterica serovar Typhimurium compared to WT macrophages (Fig. 4D-F). Moreover, using a murine abscess model, we found that Park2−/− mice supported greater bacterial burden, and increased cytokine production in excised lesions compared to WT mice (Fig. 4G-I). These in vivo results are consistent with a role for Parkin in the innate immune response to MRSA infection, although given its key function in regulating mitochondrial stress, the contribution of Parkin to innate immunity is likely complex. Taken together, these data suggest that Parkin enables accumulation of bactericidal mH2O2 in the macrophage phagosome and contributes to control of anti-microbial defenses.
Figure 4. Parkin promotes bacterial killing and enhances immunity to MRSA infection.
(A) Flow cytometric analysis of WT and Parkin-deficient macrophages pulsed with MitoPY1 for 1h, followed by MRSA infection for 4h. Data are represented as geometric mean of n≥3 independent experiments +/− SD. Macrophages were derived from ≥ 2 WT and 2 Park2−/− animals.
(B) Representative live fluorescence wide-field microscopy images of WT and Parkin deficient macrophages pulsed with MitoPY1 (green) for 1h followed by infection with MRSA-mCherry (red). Images were acquired at 4h pi and processed with MetaMorph software.
(C) Ratiometric measurement of MitoPY1 fluorescent intensity of phagosomes relative to total cellular fluorescent intensity (MFI-phagosome/MFI-cell). Phagosomes were defined by the area of the cell where the red fluorescent MRSA-mCherry were located. Data presented are the mean from n≥384 phagosomes for each group, pooled from 3 independent experiments +/− SEM. Macrophages were derived from ≥ 2 WT and 2 Park2−/− animals.
(D) Time course measurement of MRSA intracellular killing by WT and Parkin-deficient bone marrow-derived macrophages. Percent killing indicates percent difference in CFU obtained at the indicated time point relative to 0.5h pi. Data presented are the mean of n≥3 independent experiments +/− SD. Macrophages were derived from ≥ 2 WT and 2 Park2−/− animals.
(E-F) Percent of Staphylococcus epidermidis or Salmonella Typhimurium killing by WT and Parkin-deficient macrophages. Percent killing indicates percent difference in CFU obtained at the indicated time point relative to 1h pi, and represents the mean of n≥3 independent experiments +/− SD. Macrophages were derived from ≥ 2 WT and 2 Park2−/− animals.
(G) Bacterial burden in abscesses excised from male and female wild-type (WT) and Parkin-deficient C57BL/6 mice (Park2−/−) infected subcutaneously with 107 CFU of MRSA for 3 days. Horizontal lines represent the mean of n=13 WT and n=12 Park2−/− mice. Data are pooled from 2 independent experiments.
(H-I) KC or IL1β cytokine levels in subcutaneous skin abscess homogenates of WT and Park2−/− mice. Cytokine levels were measured by ELISA. Data are presented as the mean of n=13 WT and n=12 Park2−/− mice pooled from 2 independent experiments. pValue: *< 0.05, **< 0.01, ***<0.001 and ****< 0.0001.
Parkin regulates the mitochondrial stress response through Pink1-dependent and -independent pathways. Upon oxidative stress, Pink1 accumulates on the mitochondrial membrane, acting as a signal to recruit Parkin, which can induce mitophagy (Matsuda et al., 2010), or formation of MDVs (McLelland et al., 2014). However, Parkin can also stimulate the NFκB pathway to maintain mitochondrial integrity and cell survival through activation of NEMO (Muller-Rischart et al., 2013). We found that Pink1-deficient BMDM exhibited diminished bactericidal capacity compared to WT BMDM, similar to Park2−/− BMDM (Fig. 5A and 5B). To determine if the Parkin-dependent killing we observed involved mitophagy, we used an RNAi approach to deplete Drp1 (Fig. 5C), which is required for mitophagy (Narendra et al., 2008), but not MDV scission (Soubannier et al., 2012a). Drp1 knockdown macrophages killed MRSA as efficiently as non-targeted control macrophages (Fig. 5D), suggesting that mitophagy is not required for bactericidal function. Treatment of WT or Park2−/− macrophages with a NEMO inhibitor also did not affect MRSA killing, even though NEMO-dependent NF-kB translocation was decreased by inhibitor treatment (Fig. 5E and S4A-B). Finally, MRSA infection of Parkin-deficient macrophages did not stimulate increased Caspase-3/7 activation (Fig. S4C) nor was activation of AMPK or SOCS, negative regulators of inflammatory signaling, altered (Fig. S5A and S5B). These data implicate the Parkin- and Pink1-dependent mitochondrial stress response pathway in bactericidal activity against MRSA in the phagosome.
Figure 5. Parkin and Pink1 contribute to MRSA killing independently from mitophagy.
(A-B) Percent of MRSA intracellular killing by WT and Park2−/− or WT and Pink1−/− bone marrow-derived macrophages.
(C) Immunoblot analysis of cell lysates from RAW264.7 macrophages expressing shRNA specific to mouse Drp1 (Drp1 KD) or non-target control (NT-Control). GAPDH was used as a loading control.
(D) Percent of MRSA intracellular killing by NT-Control and Drp1 KD RAW264.7 macrophages as calculated by the difference in CFU obtained at the indicated time point relative to 1h pi. Data shown represent the mean of n≥3 independent experiments +/− SD.
(E) Percent of MRSA intracellular killing by RAW264.7 macrophages when treated with 100 μM of control peptide (Ctrl peptide) or NEMO Binding Domain peptide inhibitor (NEMO inhibitor).
Percent killing was calculated with the following formula [1 - (CFU indicated time points / CFU 1h pi)] X 100 and presented as the mean of n≥3 independent experiments +/− SD. pValue: *< 0.05, **< 0.01 and ***< 0.001.
Under stress conditions, MDVs accumulate when cells are treated with Bafilomycin A1, an inhibitor of the vacuolar ATPase, likely by preventing their resolution into the lysosomal network and subsequent degradation (Soubannier et al., 2012a; Sugiura et al., 2014). We therefore hypothesize that treatment with BafA1 (Yoshimori et al., 1991) would increase MDV accumulation and thereby enhance bactericidal mROS delivery into MRSA-containing phagosomes. We first quantified MDV induction in infected macrophages treated with BafA1, and observed that BafA1 treatment increased Tom20+ MDV accumulation in MRSA-infected macrophages compared to controls (Fig. S6A and S6B). BafA1-treated macrophages exhibited higher levels of MitoPY1 in MRSA-containing phagosomes and killed MRSA more efficiently compared to DMSO-treated cells (Fig. S6C-E). To further investigate how MDVs associate with MRSA-containing phagosomes, we performed transmission electron microscopy (TEM) on MRSA-infected macrophages treated with or without BafA1 at 4h pi. We could readily observe MRSA-containing phagosomes containing double membrane-bound vesicles (Fig. S6F). When cells were treated with BafA1, MRSA phagosomes appeared more spacious and the double membrane-bound vesicles could be easily observed inside the phagosome. To determine if these vesicles were derived from mitochondria, we performed immunogold labeling using anti-Tom20 antibody, followed by TEM. Although immunogold fixation conditions decreased definition of phagosomal membranes, Tom20+ particles were observed in close proximity to the bacterial surface within the phagosomal space (Fig. S6G). These data are consistent with a model where infection triggers MDV generation, followed by accumulation of bactericidal mH2O2 in the phagosome.
Sod2 is required for generation of bactericidal mH2O2 and is delivered to bacteria-containing phagosomes
Hydrogen peroxide is constitutively generated in the mitochondria from superoxide produced by respiration that is converted by the mitochondrial matrix enzyme manganese superoxide dismutase (Sod2) (Murphy, 2009). We reasoned that Sod2 might be required to generate the bactericidal mH2O2 induced by MRSA infection. To define the spatial distribution of Sod2, we performed confocal immunofluorescence microscopy on untreated or infected macrophages. In untreated macrophages, Sod2 predominantly localized to the mitochondrial network (Fig. S7A). In macrophages infected with MRSA or beads, we used anti-Sod2 antibody, as well as an anti-LAMP-1 antibody to identify the boundaries of the phagosome and could observe large Sod2+ mitochondrial network objects juxtaposed to both bead- and MRSA-containing phagosomes (Fig. 6A). In contrast, small Sod2+ vesicles were induced during MRSA infection, and were found within MRSA-containing phagosomes. To quantify Sod2+ vesicle accumulation in MRSA-containing phagosomes, we subtracted Sod2+ mitochondrial network objects ≥ 0.4 μm2 (as described in Fig. S3), and enumerated Sod2+ MDV located within a 1 μm radius around bead- and MRSA-containing phagosomes. Bacteria-containing phagosomes contained significantly more Sod2+ MDV compared to bead-containing phagosomes (Fig. 6B and 6C). To determine whether Sod2 was required for bactericidal activity, we stably knocked down Sod2 in RAW264.7 macrophages (Sod2 KD) (Fig. 6D) and tested the requirement for Sod2 in mH2O2 (Fig. 6E) and superoxide (Fig. S7B and S7C) generation during MRSA infection. Compared to control cells, Sod2 KD cells failed to induce mH2O2 upon MRSA infection, while producing higher levels of superoxide regardless of MRSA infection. Although Sod2 depletion increased mitochondrial superoxide production, it did not alter Caspase-3/7 activation (Fig. S7D) or host cell death during MRSA infection (Fig. S7E). Notably, Sod2 depletion impaired macrophage killing of MRSA compared to NT-control cells (Fig. 6F), and in vitro exposure of MRSA to H2O2 confirmed that peroxide can be directly bactericidal (Fig. 6G). Collectively, these results suggest that Sod2 is delivered by MDVs to phagosomes and enhances MRSA killing through peroxide generation.
Figure 6. Sod2 is the mitochondrial payload delivered to the phagosome to promote MRSA killing.
(A) Representative confocal microscopy images of RAW264.7 macrophages infected with MRSA (blue) or stimulated with beads (blue) for 4h and stained for Lamp1 (red) and Sod2 (green). Images were acquired using a Leica TCS SP8 confocal scanning microscope and deconvoluted using Huygens Essential software.
(B) Confocal microscopy images of a magnified area of macrophages where bead or MRSA-containing phagosomes were observed. Large Sod2+ network objects with surface area larger than 0.4 μm2 were subtracted from the images as described in Fig. S3.
(C) Quantification of Sod2+ small objects (surface area < 0.4 μm2) within a 2 μm2 area around beads or MRSA. Large Sod2+ objects were filtered out of the deconvoluted images and the number of remaining Sod2+ objects were enumerated per 2 μm2 areas of the cell where MRSA or beads were localized. Data represent the mean of at least 60 phagosomes for each group from 3 independent experiments.
(D) Immunoblots of cell lysate from RAW264.7 macrophages stably transduced with lentivirus-encoded shRNA for non-target (NT-Control) or Sod2 (Sod2 KD), probed with anti-Sod2 antibody, or anti-Actin antibody as a loading control.
(E) Flow cytometric analysis of RAW264.7 Sod2 KD or NT-control macrophages after a 1h pulse with MitoPY1, followed by MRSA infection for 4h. Data were analyzed with FlowJo software. MFI represents geometric mean of n≥3 independent experiments +/− SD.
(F) Intracellular MRSA killing by Sod2 KD and NT-control macrophages was calculated by the difference in CFU obtained at the indicated time point relative to 1h pi. Graph bars represent the mean percent killing of n≥3 independent experiments +/− SD.
(G) MRSA susceptibility to different concentrations of hydrogen peroxide in vitro. Graph represents the mean percent killing from n≥3 independent experiments +/− SD.
pValue: *< 0.05, ***< 0.001 and ****< 0.0001.
Sod2+ vesicles define a distinct population of MDV
MDV populations are heterogenous and contain different mitochondrial proteins (Sugiura et al., 2014). To determine if Sod2+ vesicles induced by MRSA infection also contained Tom20, we performed confocal immunofluorescence microscopy on infected macrophages to assess whether these proteins co-localized within MDVs, using isotype matched antibodies as specificity controls (Fig. S8A). WT bone marrow-derived infected macrophages exhibited both Sod2+ and Tom20+ MDVs, but these populations were largely distinct (Fig. 7A-C), with relatively few Sod2+/Tom20+ vesicles observed. However, generation of both Sod2+ and Tom20+ MDV populations required Parkin and Pink1 (Fig. 7A-C and S8B), indicating that Sod2+ MDVs are also controlled by the mitochondrial stress machinery. We also interrogated co-localization of Sod2 with another matrix enzyme, citrate synthase (Cs), and a mitochondrial inner membrane protein, Complex I (Fig. S9A-D) (Vogtle et al., 2017). Generation of all MDVs was Parkin-dependent, but as with Tom20, Sod2+ vesicles were largely distinct from Cs+ and Complex I+ vesicles. Collectively, these data establish Sod2+ as cargo in a population of MDVs distinct from those previously reported (Sugiura et al., 2014), and hint at a rich diversity of MDV subtypes.
Figure 7. Parkin and Pink1 regulate MRSA-induced Tom20+ and Sod2+ MDV formation.
(A) Representative confocal microscopy images of WT and Park2−/− bone marrow-derived macrophages infected with MRSA for 4h (blue) and stained for Tom20 (green) and Sod2 (red). Images were acquired using a Leica TCS SP8 confocal scanning microscope and deconvoluted using Huygens Essential software. Right panels are processed images after subtraction of Tom20+ and Sod2+ large objects (surface area ≥ 0.4 μm2). (B) Quantification of different subsets of MDV formed by WT and Park2−/− macrophages infected with MRSA. Deconvoluted confocal images were processed by Huygens Essential software using the following criteria; 10% threshold, 10% seed and garbage of 50. Tom20+ and Sod2+ objects with surface area larger than 0.4 μm2 were filtered out and the remaining objects enumerated on a per cell basis for whether they were single or double positive for Tom20 and Sod2. Graphs are presented as the mean of n≥114 cells derived from ≥ 2 WT and 2 Park2−/− mice, and pooled from 3 independent experiments +/− SEM.
(C) Quantification of MDVs that are single and double positive for Tom20 and Sod2 in WT and Pink1−/− bone marrow-derived macrophages during MRSA infection. Data were acquired using the same criteria as in panel B and presented as the mean of n≥113 cells derived from 1 WT mouse or 1 Pink1−/− mouse, and pooled from 3 independent experiments +/− SEM.
pValue: ***< 0.001 and ****< 0.0001.
DISCUSSION
The generation of anti-microbial reactive oxygen intermediates is an integral weapon in the innate immune arsenal. We find that infection by MRSA stimulates the production of mitochondrial ROS, specifically mitochondrial hydrogen peroxide, and identify Sod2 as a key enzyme responsible for infection-induced mH2O2 generation. MRSA infection also triggers biogenesis of mitochondria-derived vesicles. Induction of mH2O2-containing MDV during infection required TLR signaling and employed the Parkin/Pink1-dependent mitochondrial stress pathway. Notably, these studies reveal delivery of a Sod2 payload to the bacteria-containing phagosome by MDVs as an intrinsic mechanism for anti-microbial killing. Taken together, our data support a model where Sod2-driven mH2O2 production and accumulation via MDV delivery establish a potent killing ground for bacterial pathogens within the macrophage phagosome.
Generation of MDV has been described as a quality control mechanism that could transport damaged respiratory chain complexes from mitochondria to the endolysosomal compartment for degradation, and thus the MDV population is increased when acidification and lysosomal fusion are decreased by treatment with BafA1 (Soubannier et al., 2012a). These data are consistent with our observations that BafA1 treatment increased MDV numbers, mH2O2 and bactericidal function in MRSA-infected cells. Of note, A549 lung epithelial cells infected with S. aureus Cowan under conditions that yield small colony variant (SCV) persisters also exhibited decreased numbers of SCV upon BafA1 treatment, which could potentially be explained by more effective killing (Leimer et al., 2016). In contrast, alveolar macrophages infected with opsonized S. aureus showed decreased ROS and decreased bactericidal activity upon BafA1 treatment (Bidani et al., 2000). These differences could be due to whether bacteria are opsonized or not, different macrophage types or different S. aureus strains, but overall suggest that the contribution of MDV in innate immune effector function may vary by context. Recent studies reveal that MDV can carry out diverse functions within the cell, and likely represent multiple vesicle populations with distinct cargo related to their function. Sugiura, et al, provided evidence for MDV generation as a critical step in peroxisome biogenesis, where MDV containing the peroxisomal proteins, Pex3 and Pex14, fuse with ER-derived vesicles, containing Pex16, resulting in import-competent organelles (Sugiura et al., 2017). This model of peroxisome biogenesis adds another dimension to our understanding of how MDVs carry out mitochondrial communication with other organelles. Our work further identifies the phagosome as a cryptic destination for MDVs that is revealed by infection.
Different proteins are incorporated in MDVs when mitochondria are stressed with different stimuli, implying the existence of mechanisms to package and transport specific cargos (Soubannier et al., 2012a; Soubannier et al., 2012b). For instance, exogenous ROS applied to isolated mitochondria results in MDVs enriched in the outer membrane protein, VDAC. However, when ROS is generated by isolated mitochondria through inhibition of complex III by Antimycin A, MDVs carried complex III subunit core2 without enrichment of VDAC (Soubannier et al., 2012b). A recent study showed that in response to heat shock or LPS, MDVs delivered mitochondrial antigens to antigen-loading compartment independently of Parkin (Matheoud et al., 2016). In contrast, when cells are treated with Antimycin A to stimulate mitochondrial ROS production, MDVs are targeted to lysosomes in a Parkin-dependent manner (McLelland et al., 2014). In the context of infection, our data demonstrate that MDVs are generated in a Parkin/Pink-dependent manner, similar to MDVs destined for lysosomes. Moreover, we show that infection-induced MDVs contain the mitochondrial matrix enzyme, Sod2. Collectively, our data and others support a model where specific stimuli define the packaging and destination of MDV subsets.
Within the mitochondrial matrix, Sod2 cooperates with other enzymes and anti-oxidant proteins to detoxify superoxide generated during respiration. Notably, dysregulation of Sod2 is associated with many human diseases, some of which are associated with increased inflammation (Flynn and Melov, 2013). Altering the spatial distribution of Sod2 could disrupt the coordinated ROS detoxification process, resulting in increased hydrogen peroxide production without the capacity for further detoxification. Previous studies provide some evidence to support this hypothesis. Overexpression of Sod2 increased the steady state of hydrogen peroxide in cancer cells and may contribute to tumor invasion and metastasis (Nelson et al., 2003; Ranganathan et al., 2001). In contrast, decreasing Sod2 activity in Sod2-heterozygous mice led to higher production of superoxide radical when measured by aconitase activity, which correlated with an increase in mitochondrial oxidative damage (Williams et al., 1998). Consistent with these data, our results showed that depleting macrophage Sod2 resulted in elevated steady state levels of mitochondrial superoxide and decreased hydrogen peroxide during MRSA infection. As a consequence, Sod2-deficient macrophages (Sod2 KD) failed to efficiently kill MRSA. Although we were not able to successfully generate live Sod2-deficient mice, studies of Sod2 knockdown in zebrafish demonstrate a protective role for Sod2 during infection by Pseudomonas aeruginosa (Peterman et al., 2015). Our studies have identified MDVs as a delivery mechanism by which antimicrobial effectors can be trafficked into the macrophage phagosome. We propose that relocalization of a subset of Sod2 protein from the coordinated redox environment of the mitochondria through packaging into MDVs allows repurposing of this enzyme from detoxification to anti-microbial defense.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Reagents and resources can be obtained by directing requests to the Lead Contact, Mary O’Riordan (oriordan@umich.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Wild-type C57BL/6, Park2−/−, Pink1−/− and Cybb-/y (NOX2-deficient) were purchased from Jackson Laboratory. Tlr2/4/9−/− have been described previously (Abuaita et al. 2015). All animals were housed in specific pathogen free facilities at the University of Michigan Medical School Unit for Laboratory Animal Medicine (ULAM). Mice were weaned at 22 days after birth, separated into same sex groups and housed in plastic cages in a ventilated rack. Food (Chow 5L0D) and water were provided ad libitum, and health checks were performed daily. Cages were changed every two weeks using the Micro-isolator Technique. Male and female mice were infected between the ages of 10-14 weeks. Infected mice were housed in biosafety level 2 containment and treated in accordance with an IACUC-approved protocol.
Cells
Bone marrow was isolated from male and female mice between the ages of 8-10 weeks. Primary bone marrow-derived macrophages (BMDMs) were prepared by flushing mouse femurs in DMEM supplemented with 100 units/ml of Pen/Strep. Cells were differentiated by incubation in BMDM medium (50% DMEM, 2 mM L-glutamine, 1 mM sodium pyruvate, 30% L929-conditioned medium, 20% heat-inactivated fetal bovine serum (FBS) and Pen/Strep). L-929 and HEK 293T cells were cultured in MEM supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 1mM Non-essential amino acid (NEAA), 10 mM HEPES, and 10% heat-inactivated FBS). RAW 264.7 cells were cultured in RPMI 1640 containing 2 mM L-glutamine and 10% heat-inactivated FBS. All cells were incubated at 37°C in 5% CO2.
Bacterial culture
USA300 LAC, a community associated methicillin-resistant Staphylococcus aureus strain (MRSA), its isogenic strain harboring pSarA-mCherry plasmid (MRSA-mCherry) (Boles and Horswill, 2008), Salmonella enterica subsp. enterica serovar Typhimurium SL1344 (Detweiler et al., 2001) and Staphylococcus epidermidis were stored at −80°C in LB medium containing 20% glycerol. Staphylococcus and Salmonella strains were cultured in tryptic soy medium (TSA, Becton Dickinson) and Luria-Bertani medium (LB, Fisher) respectively. Selected colonies were grown overnight at 37°C with shaking (240 rpm) in liquid broth. Bacteria were pelleted, washed and re-suspended in PBS. The bacterial inoculum was estimated based on OD600 and verified by plating serial dilutions on agar plates to determine colony forming units (CFU).
METHOD DETAILS
Macrophage infections
Macrophages were seeded in a 24-well tissue culture treated plate at a density of 1.5 X 105 cells/well. The next day, macrophages were infected at a multiplicity of infection (MOI) of 20 in culture medium (as described above for either BMDM or RAW264.7) without antibiotics for 45 minutes. Infected macrophages were washed 3 times with PBS, incubated in medium containing 100 μg/ml of gentamicin to kill extracellular bacteria for 15 minutes, then incubated in medium containing gentamicin (50 μg/ml for Staphylococcus strains or 10 μg/ml for Salmonella) for the duration of the experiment. In experiments monitoring early MRSA killing, bacteria (MOI of 2) were added to macrophages and infection was synchronized by centrifugation at 1500rpm for 5 min. Infected macrophages were incubated at 37°C for 10 minutes to complete phagocytosis and extracellular bacteria were killed by adding Lysostaphin (10 U/ml). The number of intracellular bacteria was determined by washing infected macrophages with PBS, lysing with 0.1% NP-40, and enumerating bacterial CFU via serial dilution and growth on agar plates at 37°C for 24 hours. The percentage of killed MRSA was calculated with the following formula [1 - (CFU indicated time points / CFU 0.5 or 1h pi)] X 100, which represents the percent difference between CFU at indicated time points relative to either 0.5 or 1h pi. Where indicated, macrophages were pre-incubated with NecroX-5 (10 μM), Bafilomycin A1 (100 nM), Control peptide (100 μM) or NEMO peptide inhibitor (100 μM) for 30 min prior to infection, and all inhibitors were maintained throughout the experiment.
Mouse infections
Subcutaneous MRSA infections were performed as previously described (Tseng et al., 2011). Sample-size estimation was performed by power analysis, with alpha set at 5% and beta set at 80%. Male and Female C57BL/6 mice and Park2−/− mice were shaved on the right flank. Mice were inoculated with 107 bacteria in 100 μl of PBS subcutaneously in the shaved area of the skin using a 27 gauge needle. Mice were sacrificed on day 3 pi and skin abscesses were excised, weighed and homogenized in PBS. Total CFU per mouse abscess were enumerated by serial dilution and plating on TSA agar. Total CFU was converted to CFU/mg of tissue weight. The University of Michigan Medical School ELISA core analyzed cytokine levels from samples blinded to identifiers, and resulting values were converted to pg/mg of tissue weight.
Cellular mROS measurement
Macrophages were plated in 60 mm non-treated dishes and treated with 10 μM MitoPY1 (TOCRIS) or 10 μM MitoSOX (Thermo Fisher) for 1 hour. Macrophages were washed 3 times with media and where indicated, macrophages were treated for 30 min with NecroX-5 (10 μM) or control solvent prior to infection with MRSA (MOI of 20). For positive controls, macrophages were treated with 100 μM hydrogen peroxide or 10 μM Antimycin A for 1 hour to induce oxidation of MitoPY1 or MitoSOX, respectively. Macrophages were subjected to flow cytometry and data were analyzed with FlowJo software. The mean fluorescence intensity for each condition was determined as the geometric mean.
Phagosomal mROS measurement
Macrophages were plated in 35 mm glass bottom dishes (MatTek). The next day, macrophages were treated with MitoPY1 (10 μM) for 1 hour, washed three times with media and stimulated with inert red fluorescent beads or infected with live or dead (inactivated by paraformaldehyde) red fluorescent MRSA harboring pSarA-mCherry (MOI of 20). Macrophages were imaged in Ringer buffer (155 mM NaCl, 5 mM KCl, 1 mM MgCl2.6H2O, 2 mM NaH2PO4.H2O, 10 mM HEPES, and 10 mM Glucose) with an Olympus IX70 inverted live-cell fluorescence microscope. Fluorescence images were further processed by MetaMorph imaging software. For quantification of mROS association with phagosomes, the mean fluorescence intensity of MitoPY1 at the phagosome area was measured by ImageJ. Phagosomal regions in the cell images were defined by the location of red fluorescent beads or bacteria. For BMDM experiments, the ratiometric MFI of the phagosomal area over the MFI of the cell was calculated. This was done because Park2−/− macrophages exhibit higher global MitoPY1 fluorescence intensity when compared to WT macrophages prior to infection.
Immunofluorescence staining and confocal microscopy
Macrophages were seeded onto microscope coverslips and infected with MRSA (MOI of 20) or stimulated with fluorescent beads. Cells were fixed at 4h pi with 3.7% paraformaldehyde at RT for 20 minutes and permeabilized with PBS+ 0.1% Triton X-100 for 15 min. MRSA were stained using chicken anti-protein A antibody conjugated to biotin (Abcam ab18598) in staining buffer (PBS, 0.1% Triton X-100, 5% BSA, and 10% normal goat serum). Host proteins were stained using mouse anti-Sod2 (Abcam ab110300, clone 9E2BD2), rabbit anti-Sod2 (Abcam ab13533), mouse anti-Complex I (Abcam ab109798, clone 18G12BC2), mouse anti-p65 (Santa Cruz, sc-8008), rabbit anti-Cs (Abcam ab96600), rat anti-Lamp1 (DSHB, clone 1D4B), and rabbit anti-Tom20 (Santa Cruz, sc-11415). For control staining, normal rabbit IgG (Santa Cruz, sc-2027) and mouse control IgG (Abcam ab81032) were used. Secondary antibodies; goat anti-mouse conjugated to Alexa-488, 594 or 647, goat anti-rat (Alexa-594), goat anti-rabbit conjugated to Alexa-488, 594 and 647 and Streptavidin (Alexa-405) were used according to manufacturer’s procedure (Thermo Fisher). Cover glasses were mounted on microscope slides using Prolong Diamond (Thermo Fisher). Cells were imaged using a Leica TCS SP8 or Nikon A1 confocal microscope and deconvoluted using Huygens Essential software by scientific volume imaging using the following criteria; Threshold (10%), Seed (10%) and Garbage Volume (50). To define MDVs, large objects (surface area larger than 0.4 μm2) were filtered out from the mitochondrial fluorescence labeled channel and the number of remaining objects per cell was recorded.
Live confocal microscopy
Macrophages were transfected with mCherry-Mito-7 plasmid (Olenych et al., 2007) by electroporation using Amaxa cell line nucleofector kit V according to manufacturer’s protocol (Lonza), seeded in 35 mm glass bottom dishes (MatTek) for several hours and then incubated overnight in medium containing 100 μg/mL Cascade Blue Dextran, Molecular weight 10,000 (Thermo Fisher). The next day, macrophages were treated with MitoPY1 (10 μM) for 1h, washed 3 times with media and exposed to 100 μM hydrogen peroxide for 1h to induce oxidation of MitoPY1. Cells were imaged on a Leica TCS SP8 confocal scanning laser microscope. Images were deconvoluted and analyzed using Huygens Essential software.
Transmission electron microscopy
Macrophages were infected with MRSA (MOI of 20) in the presence of Bafilomycin A1 (100 nM) or control DMSO. Infected macrophages were fixed at 4h pi with 2.5% glutaraldehyde for at least 1h at room temperature, then overnight at 4°C. For immuno-gold staining, infected macrophages were stained for Tom20 prior to fixation with glutaraldehyde according to manufacturer’s procedure (AURION). Briefly, cells were fixed with 3.7% paraformaldehyde at room temperature for 20 minutes and permeabilized with Sorenson’s buffer containing 0.1% Triton X-100 for 15 minutes. Cells were blocked with the AURION blocking solution (AURION-BSA-c) and stained using primary anti-Tom20 antibody (Santa Cruz) and secondary goat anti-rabbit ultra-small gold antibody (AURION). Silver stain enhancement was carried out by using the AURION R-GENT SE-EM according to reagent protocol (AURION). Glutaraldehyde fixed samples were washed with Sorenson’s buffer 3-times before post-fixing in 2% osmium tetroxide in Sorenson’s buffer for 1h at room temperature. Samples were washed again 3-times with Sorenson’s buffer, then dehydrated through ascending concentrations of ethanol, treated with propylene oxide, and embedded in EMbed 812 epoxy resin. Semi-thin sections were stained with toluidine blue for tissue identification. Selected regions of interest were ultra-thin sectioned to 70 nm and post stained with uranyl acetate and Reynolds lead citrate. Sections were examined using a JEOL JEM-1400 Plus transmission electron microscope (TEM) at 80 kV.
Generation of RAW264.7ΔIre1-α and RAW264.7 shRNA stable knockdown cells
The generation of lentivirus for CRISPR-Cas9 knockout and shRNA knockdown was done by using HEK293T packaging cells grown in DMEM with 10% FBS. The virus particles were produced by transfecting the cells with the TRC shRNA encoded plasmid (pLKO.1) or guide RNA (gRNA) encoded plasmid lentiCRISPR v2 (Sanjana et al., 2014) along with the packaging plasmids (pHCMV-G, and pHCMV-HIV-1) (Kulpa et al., 2013) using FUGENE-HD transfection reagent (Promega). Media was changed after 24h and virus particles were collected after 72h post-transfection. A total of 2 ml of medium containing virus were concentrated ten-fold by ultracentrifugation at 24,000 rpm for 2h at 4°C and used to transduce RAW264.7 cells. Transduced cells were selected with puromycin (3 μg/ml). The mouse Sod2-specific shRNA plasmid with the sense sequence of (GCTTACTACCTTCAGTATAAA), the mouse Drp1-specific shRNA (GGCAATTGAGCTAGCGTATA) and the non-target control shRNA plasmid were purchased from Sigma-Aldrich. The efficiency of knockdown was monitored by immunoblot analysis using anti-Sod2 antibody (Santa Cruz) and anti-Drp1 (Cell Signaling). Actin and GAPDH were used as loading controls and were detected by anti-Actin (Fisher) and Anti-GAPDH (Santa Cruz). The mouse IRE1-α specific gRNA sequence (CTTGTTGTTTGTCTCGACCC) and the non-target gRNA control sequence (TCCTGCGCGATGACCGTCGG) were cloned into lentiCRISPRv2 according to the Zhang lab protocol (Sanjana et al., 2014). Single clones of RAW264.7ΔIre1-α were isolated and confirmed by immunoblot using anti-IRE1-α antibody (clone 14C10, Cell Signaling). RAW264.7ΔIre1-α clone was also confirmed by absence of endonuclease activity when cells were treated with endoplasmic reticulum stress inducer thapsigargin (5 μM) using an xbp1 splicing assay (Figure S1) as previously described (Abuaita et al., 2015).
Cell death and Caspase-3/7 activity
Cell death and Caspase-3/7 were measured by flow cytometry using SYTOX green dead cell stain and CellEvant Caspase-3/7 green flow cytometry assay kit according to manufacturer’s protocol (Thermo Fisher). Briefly, macrophages were incubated with SYTOX green dead cell stain (30 nM) in HBSS for 20 minutes at room temperature or CellEvant Caspase-3/7 Green detection reagent (2 μM) in media for 30 minutes at 37°C. For positive controls, digitonin (0.01%, Sigma Aldrich) was used to permeabilize the plasma membrane and staurosporine (1 μM, Cayman) used to induce Caspase-3/7 activation. The percent of SYTOX+ or Caspase-3/7+ cells was determined by gating against mock unstained cells.
QUANTIFICATION AND STATISTICAL METHODS
No data were excluded from experimental analysis. Data were graphed using Microsoft Excel 2016 and statistically analyzed using Graphpad Prism 7. Where indicated, the mean of at least 3 independent experiments was presented with error bars showing standard deviation (SD) or standard error of the mean (SEM), as described in the figure legends. The value of sample size (n) and number of animals differ between experiments, and are indicated in figure legends. SD between groups were similar and the data were normally distributed in MDV quantification assays. Other assays were performed and tested under the assumption that the data would be normally distributed. Differences between two groups were tested using the Mann-Whitney Test. Differences between three or more groups were tested using One-way ANOVA and followed up by Tukey’s multiple comparisons test. P values of less than 0.05 were considered significant and designated by: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. All statistically significant comparisons within experimental groups are marked.
DATA AND SOFTWARE AVAILABILITY
Raw data are available upon request, which should be directed to the Lead Contact. There was no proprietary software used in this study.
ADDITIONAL RESOURCES
The mCherry-Mito-7 plasmid from the Davidson Lab (Olenych et al., 2007) was used under a material transfer agreement (MTA) with Addgene.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial strains | ||
| A community-associated methicillin-resistance Staphylococcus aureus (MRSA) strain USA300 | Nebraska Tn Mutant Library (NTML) | MOR427 |
| MRSA-mCherry; USA300 isolate harboring pSarA-mCherry | Boles and Horswill, 2008 | MOR380 |
| Salmonella enterica subsp. enterica serovar Typhimurium SL1344 | Detweiler et al., 2001 | MOR23 |
| Staphylococcus epidermidis | O’Riordan Lab Collection | MOR602 |
| Mice and cell lines | ||
| RAW 264.7 macrophage; Abelson murine leukemia virus transformed | ATCC | Cat# TIB-71 |
| Hek293T embryonic epithelial kidney cells | ATCC | Cat# CRL-3216 |
| L929 mouse fibroblast | ATCC | Cat# CCL-1 |
| C57BL/6 | Jackson Laboratory | Stock No: 000664 |
| TLR2/4/9−/− | Merkel Laboratory (FDA) | N/A |
| Park2−/− | Jackson Laboratory | Stock No: 006582 |
| Pink1 −/− | Jackson Laboratory | Stock No: 017946 |
| Cybb-/y | Jackson Laboratory | Stock No: 002365 |
| Chemicals | ||
| MitoPY1 | Tocris Bioscience | Cat# 4428 |
| MitoSOX | Thermo Fisher | Cat# M36008 |
| NecroX-5 | Cayman Chemical | Cat# 17278 |
| Hydrogen Peroxide | Sigma-Aldrich | Cat# 216763 |
| FluoSpheres red | Thermo Fisher | Cat# F8821 |
| Microspheres blue | Thermo Fisher | Cat# F8815 |
| Bafilomycin A1 | Cayman Chemical | Cat# 11038 |
| Antimycin A | Cayman Chemical | Cat# 19433 |
| Thapsigargin | Tocris Bioscience | Cat# 1138 |
| Gentamicin | Thermo Fisher | Cat# 15750060 |
| Puromycin | Sigma-Aldrich | Cat# P8833 |
| Sytox green | Thermo Fisher | Cat# S34860 |
| Silver enhancement reagent | AURION | Cat# 704.000 |
| Lysostaphin | Sigma-Aldrich | Cat# L7386 |
| NEMO binding domain (NBD) inhibitor peptide and control peptide | Novus Biologicals | Cat# NBP2-26504 |
| Dextran, cascade blue, molecular weight 10,000, lysine fixable | Thermo Fisher | Cat# D1976 |
| CellEvant Caspase-3/7 green flow cytometry assay kit | Thermo Fisher | Cat# C10740 |
| Staurosporine | Cayman Chemical | Cat# 81590 |
| Plasmids, enzymes and kits | ||
| mCherry-Mito-7 | Olenych et al., 2007 | Addgene Cat# 55102 |
| plentiCRISPR v2 | Sanjana et al., 2014 | Addgene Cat# 52961 |
| pHCMV-G | Kulpa et al., 2013 | MOR372 |
| pHCMV-HIV-1 | Kulpa et al., 2013 | MOR373 |
| plentiCRISPR v2 non-target gRNA | O’Riordan Lab Collection | MOR523 |
| plentiCRISPR v2 IRE1α gRNA | O’Riordan Lab Collection | MOR519 |
| pLKO.1 non-target shRNA control | Sigma-Aldrich | Cat# SHC002 |
| pLKO.1 Sod2 shRNA | Sigma-Aldrich | TRCN0000311411 |
| pLKO.1 Drp1 shRNA | Sigma-Aldrich | TRCN0000321169 |
| M-MLV Reverse Transcriptase | Thermo Fisher | Cat# 28025013 |
| Taq DNA polymerase | Promega | Cat# M3001 |
| FUGENE-HD transfection kit | Promega | Cat# E2311 |
| Amaxa cell line nucleofector kit V | Lonza | Cat# VACA-1003 |
| Antibodies | ||
| Biotin chicken anti-protein A | Abcam | Cat# ab18598 |
| Mouse anti-Sod2 | Abcam | Cat# ab110300 |
| Rabbit anti-Sod2 | Abcam | Cat# ab13533 |
| Mouse anti-Complex I | Abcam | Cat# ab109798 |
| Mouse anti-p65 | Santa Cruz | Cat# sc-8008 |
| Rabbit anti-Cs | Abcam | Cat# ab96600 |
| Rat-anti-Lamp1 | DSHB | Clone 1D4B |
| Rabbit anti-Tom20 | Santa Cruz | Cat# sc-11415 |
| Rabbit anti-IRE1α | Cell Signaling | Cat# 3294 |
| Rabbit anti-GAPDH | Santa Cruz | Cat# sc-25778 |
| Rabbit anti-Sod2 | Santa Cruz | Cat# sc-30080 |
| Rabbit anti-Drp1 | Cell Signaling | Cat# 8570 |
| Rabbit anti-Beta Actin | Thermo Fisher | Cat# MA5-15739 |
| Normal rabbit IgG | Santa Cruz | Cat # sc-2027 |
| Mouse control IgG | Abcam | Cat# ab81032 |
| Alexa Fluor-488 goat anti-Mouse | Thermo Fisher | Cat# A-11001 |
| Alexa Fluor-594 goat anti-Mouse | Thermo Fisher | Cat# A-11005 |
| Alexa Fluor-647 goat anti-Mouse | Thermo Fisher | Cat# A-21235 |
| Alexa Fluor-488 goat anti-Rabbit | Thermo Fisher | Cat# A-11008 |
| Alexa Fluor-594 goat anti-Rabbit | Thermo Fisher | Cat# A-11012 |
| Alexa Fluor-647 goat anti-Rabbit | Thermo Fisher | Cat# A-21244 |
| Alexa Fluor-594 goat anti-Rat | Thermo Fisher | Cat# A-11007 |
| Alexa Fluor-405 Streptavidin | Thermo Fisher | Cat# S32351 |
| Ultra small-gold Goat anti-Rabbit | AURION | Cat# 800.011 |
| IRDye 800CW Goat anti-Mouse | LI-COR | Cat# 925-32210 |
| IRDye 680RD Goat anti-Rabbit | LI-COR | Cat# 925-68071 |
| Software and Algorithms | ||
| Excel 2016 | Microsoft Office | microsoft.com |
| FlowJo version 10 | FlowJo, LLC | flowjo.com |
| Huygens Essential | Scientific Volume Imaging | svi.nl |
| ImageJ | NIH | imagej.nih.gov |
| MetaMorph for Olympus Version | Molecular Devices, | moleculardevices.com |
| 7.0.0 | LLC | |
| Prism 7 | GraphPad | graphpad.com |
Acknowledgments
This work was supported by NIH award R21 AI101777 (M.X.O). B.H.A. was supported by NIH T32 AI007538, T32 HLL007517 and an American Heart Association Post-doctoral fellowship. We thank O’Riordan lab members for many helpful discussions. We gratefully acknowledge the University of Michigan Medical School Center for Live Cell Imaging (CLCI), Microscopy and Image Analysis Laboratory (MIL) and the Cancer Center Immunology Core, as well as the Center for Statistics, Computing and Analytics Research at the University of Michigan. We thank Dr. T. Merkel (FDA) for providing cells from Tlr2/4/9−/− mice.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- Abuaita BH, Burkholder KM, Boles BR, and O’Riordan MX (2015). The Endoplasmic Reticulum Stress Sensor Inositol-Requiring Enzyme 1alpha Augments Bacterial Killing through Sustained Oxidant Production. mBio 6, e00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani AA, Al-Anazi MR, Al-Zoghaibi FA, Abdo AA, Sanai FM, Al-Hamoudi WK, Alswat KA, Al-Ashgar HI, Khan MQ, Albenmousa A, et al. (2016). PARK2 polymorphisms predict disease progression in patients infected with hepatitis C virus. Ann Hepatol 15, 824–833. [DOI] [PubMed] [Google Scholar]
- Bidani A, Reisner BS, Haque AK, Wen J, Helmer RE, Tuazon DM, and Heming TA (2000). Bactericidal activity of alveolar macrophages is suppressed by V-ATPase inhibition. Lung 178, 91–104. [DOI] [PubMed] [Google Scholar]
- Boles BR, and Horswill AR (2008). Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS pathogens 4, e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM, and O’Riordan MX (2015). Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity 43, 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, and Zahrt TC (2013). Mechanisms of Francisella tularensis intracellular pathogenesis. Cold Spring Harb Perspect Med 3, a010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, and Swanson JA (2010). Technical advance: Caspase-1 activation and IL-1beta release correlate with the degree of lysosome damage, as illustrated by a novel imaging method to quantify phagolysosome damage. J Leukoc Biol 88, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler CS, Cunanan DB, and Falkow S (2001). Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc Natl Acad Sci U S A 98, 5850–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, and Chang CJ (2008). A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J Am Chem Soc 130, 9638–9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, Lin VS, and Chang CJ (2013). Preparation and use of MitoPY1 for imaging hydrogen peroxide in mitochondria of live cells. Nat Protoc 8, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, and Melov S (2013). SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, et al. (2015). Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nature immunology 16, 1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom KM, and Finkel T (2014). Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15, 411–421. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Koo SY, Ahn BH, Park O, Park DH, Seo DO, Won JH, Yim HJ, Kwak HS, Park HS, et al. (2010). NecroX as a novel class of mitochondrial reactive oxygen species and ONOO(−) scavenger. Arch Pharm Res 33, 1813–1823. [DOI] [PubMed] [Google Scholar]
- Koster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, Grigsby SJ, Mittal E, Park HS, Jones V, et al. (2017). Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci U S A 114, E8711–E8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa DA, Del Cid N, Peterson KA, and Collins KL (2013). Adaptor protein 1 promotes cross-presentation through the same tyrosine signal in major histocompatibility complex class I as that targeted by HIV-1. J Virol 87, 8085–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimer N, Rachmuhl C, Palheiros Marques M, Bahlmann AS, Furrer A, Eichenseher F, Seidl K, Matt U, Loessner MJ, Schuepbach RA, et al. (2016). Nonstable Staphylococcus aureus Small-Colony Variants Are Induced by Low pH and Sensitized to Antimicrobial Therapy by Phagolysosomal Alkalinization. J Infect Dis 213, 305–313. [DOI] [PubMed] [Google Scholar]
- Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, and Cox JS (2013). The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, et al. (2016). Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell 166, 314–327. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland GL, Soubannier V, Chen CX, McBride HM, and Fon EA (2014). Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens K, and Samuel JE (2012). Defense mechanisms against oxidative stress in Coxiella burnetii: adaptation to a unique intracellular niche. Adv Exp Med Biol 984, 39–63. [DOI] [PubMed] [Google Scholar]
- Muller-Rischart AK, Pilsl A, Beaudette P, Patra M, Hadian K, Funke M, Peis R, Deinlein A, Schweimer C, Kuhn PH, et al. (2013). The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol Cell 49, 908–921. [DOI] [PubMed] [Google Scholar]
- Muralidharan S, and Mandrekar P (2013). Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. Journal of leukocyte biology 94, 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP (2009). How mitochondria produce reactive oxygen species. Biochem J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, Pumiglia K, Bennett JA, and Melendez JA (2003). Elevated sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin Cancer Res 9, 424–432. [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, and McBride HM (2008). Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 18, 102–108. [DOI] [PubMed] [Google Scholar]
- Olenych SG, Claxton NS, Ottenberg GK, and Davidson MW (2007). The fluorescent protein color palette. Curr Protoc Cell Biol Chapter 21, Unit 21 25. [DOI] [PubMed] [Google Scholar]
- Peterman EM, Sullivan C, Goody MF, Rodriguez-Nunez I, Yoder JA, and Kim CH (2015). Neutralization of mitochondrial superoxide by superoxide dismutase 2 promotes bacterial clearance and regulates phagocyte numbers in zebrafish. Infection and immunity 83, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Huang TT, Epstein CJ, Jeffrey JJ, et al. (2001). Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. The Journal of biological chemistry 276, 14264–14270. [DOI] [PubMed] [Google Scholar]
- Rigby KM, and DeLeo FR (2012). Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34, 237–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, and Zhang F (2014). Improved vectors and genome-wide libraries for CRISPR screening. Nature methods 11, 783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft D, and Ronai ZA (2015). UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, and McBride HM (2012a). A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol 22, 135–141. [DOI] [PubMed] [Google Scholar]
- Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, and McBride HM (2012b). Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One 7, e52830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Mattie S, Prudent J, and McBride HM (2017). Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542, 251–254. [DOI] [PubMed] [Google Scholar]
- Sugiura A, McLelland GL, Fon EA, and McBride HM (2014). A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 33, 2142–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu VT, Kim HK, Long le T, Nyamaa B, Song IS, Thuy TT, Huy NQ, Marquez J, Kim SH, Kim N, et al. (2016). NecroX-5 protects mitochondrial oxidative phosphorylation capacity and preserves PGC1alpha expression levels during hypoxia/reoxygenation injury. Korean J Physiol Pharmacol 20, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Sanchez-Martinez M, Arruda A, and Liu GY (2011). Subcutaneous infection of methicillin resistant Staphylococcus aureus (MRSA). J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufanli O, Telkoparan Akillilar P, Acosta-Alvear D, Kocaturk B, Onat UI, Hamid SM, Cimen I, Walter P, Weber C, and Erbay E (2017). Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc Natl Acad Sci U S A 114, E1395–E1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A, and Fang FC (2001). Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect 3, 1313–1320. [DOI] [PubMed] [Google Scholar]
- Vogtle FN, Burkhart JM, Gonczarowska-Jorge H, Kucukkose C, Taskin AA, Kopczynski D, Ahrends R, Mossmann D, Sickmann A, Zahedi RP, et al. (2017). Landscape of submitochondrial protein distribution. Nat Commun 8, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, and Ghosh S (2011). TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, and Richardson A (1998). Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. The Journal of biological chemistry 273, 28510–28515. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, and Kettle AJ (2013). Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal 18, 642–660. [DOI] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, and Tashiro Y (1991). Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. The Journal of biological chemistry 266, 17707–17712. [PubMed] [Google Scholar]
- Zeeshan HM, Lee GH, Kim HR, and Chae HJ (2016). Endoplasmic Reticulum Stress and Associated ROS. Int J Mol Sci 17, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, and Kaufman RJ (2004). Signaling the unfolded protein response from the endoplasmic reticulum. The Journal of biological chemistry 279, 25935–25938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available upon request, which should be directed to the Lead Contact. There was no proprietary software used in this study.