Highlights
-
•
Invasive adenocarcinoma of the vulva arising from extramammary Paget’s disease is possible in women of child-bearing age.
-
•
Radical vulvectomy, sentinel lymph node biopsy, and inguinal lymphadenectomy are safe and feasible during pregnancy.
-
•
Chemotherapy may be used as adjuvant therapy for vulvar adenocarcinoma during pregnancy when radiation is contraindicated.
-
•
Primary cesarean delivery may be considered for pregnant women with recent vulvar surgery.
-
•
Trastuzumab may be considered for maintenance therapy of Her2/Neu positive vulvar cancer in postpartum women.
1. Introduction
Extramammary Paget’s disease (EMPD) of the vulva is a rare condition with limited data to direct management. Case series from major cancer centers document fewer than 100 cases in 45 years (Onaiwu et al., 2017). Vulvar EMPD is most common in postmenopausal Caucasian women and is often associated with synchronous or metachronous cancers including colon, cervical, urinary tract, breast, endometrial, and vulvar cancers. Many cases are multifocal and associated with one or more recurrences over time. Prognosis is generally favorable, but survival is negatively affected by invasive disease, underlying adenocarcinoma, coexisting cancers, and involved lymph nodes. Few case reports include premenopausal women, with the youngest patients reported in their thirties (Onaiwu et al., 2017). There are no existing reports of pregnant women with invasive vulvar EMPD. We describe the case of a young woman diagnosed with invasive vulvar EMPD during pregnancy and include a discussion of her antepartum considerations and postpartum management.
2. Case report
A 37-year-old G1P0 female of mixed Asian descent presented for her first prenatal visit in December 2018 with vulvar irritation at 7 weeks gestation. Examination revealed an erythematous, edematous left labia. Cephalexin was prescribed for suspected bacterial infection and cultures were sent. At Dermatology’s recommendation, treatment was switched to clotrimazole plus amoxicillin/clavulanic acid without resolution. A punch biopsy was obtained in January 2019, revealing EMPD with depth of invasion 3 mm and readily identifiable angiolymphatic invasion (Fig. 1).
Fig. 1.
Punch biopsy of vulvar lesion showing invasive extramammary Paget’s disease, H&E stain at (a) 20× and (b) 200×. Tumor cells expressed pancytokeratin, CK7, and breast mix (BRST2 + mammaglobin). S100 and SOX10 were negative.
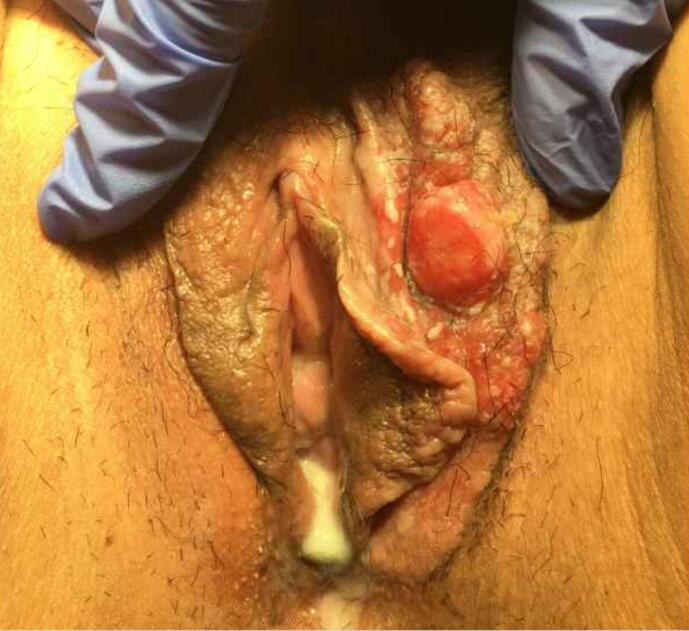
The patient’s past medical history was remarkable for spontaneous coronary artery dissection (SCAD) at age 32 with subsequent coronary artery stent placement. Family history was significant for breast cancer in her mother and stomach cancer in her maternal grandmother. The patient had a remote history of low-grade cytology on pap smear, but her recent cervical cancer screening was unremarkable. Physical exam revealed a 5 × 6 cm erythematous and white lesion (Fig. 2).
Fig. 2.
Left vulvar lesion prior to excision. Final pathology was consistent with invasive adenocarcinoma in association with extramammary Paget’s disease.
The patient had consultations with gynecologic oncology and maternal fetal medicine (MFM), at which time she elected to continue her viable first trimester pregnancy. She underwent mammography and colonoscopy without evidence of concomitant cancer. In February 2019, at 16w5d, she underwent left radical vulvectomy. Pathology was consistent with invasive adenocarcinoma of the vulva in association with EMPD, 15 mm in greatest dimension with 10.5 mm depth of invasion and positive lymphovascular space invasion. Margins were negative for invasive carcinoma but positive for EMPD. Receptor expression was as follows: weakly ER positive (25%), moderately PR positive (60%), and Her2 positive (80%).
Due to the finding of invasive adenocarcinoma on final pathology, the lymph nodes were evaluated with pelvic MRI. PET was avoided in the setting of pregnancy. A single enlarged left inguinal lymph node concerning for metastasis measured 1.5 cm. The patient underwent bilateral sentinel lymph node biopsy with evidence of carcinoma in the left inguinal node on frozen section. Thus, concurrent completion left inguinal lymphadenectomy was performed. Right sentinel lymph node biopsy was negative for carcinoma, thus sparing the patient a right completion lymphadenectomy. The gravid uterus did not interfere with the ability to adequately preform lymphadenectomy. Final pathology revealed that the left sentinel node had focal extracapsular extension. One additional node was involved within the left lymphadenectomy specimen.
Staging was completed with CT of the chest and MRI of the abdomen/pelvis, revealing stage IIIC vulvar adenocarcinoma with no evidence of further metastatic disease. The patient desired continuation of her viable second trimester pregnancy. She was treated with carboplatin and paclitaxel with the intention to augment to chemoradiation after delivery. MFM recommended scheduled preterm delivery at 34–35 weeks gestation to facilitate maternal treatment, although the patient desired earlier delivery. Through shared decision making, delivery at 33 weeks was planned.
The patient underwent two cycles of chemotherapy prior to delivery. She received corticosteroids to promote fetal lung maturity and underwent uncomplicated primary cesarean delivery at 33w0d with bilateral salpingectomy and oophoropexy. She was discharged home on postoperative day three. Placental and fallopian tube pathology showed no evidence of carcinoma. The viable female neonate was hospitalized for 4 weeks, after which she was discharged home in stable condition.
Five weeks after delivery, surgical recovery was complete and PET CT showed no evidence of metastatic disease. The patient ceased breastfeeding and began chemoradiation therapy with weekly cisplatin and 33 fractions of 180 cGy for a total of 5940 cGy of pelvic radiation. Chemoradiation concluded in September 2019. Given Her2 receptor expression, the patient opted for trastuzumab therapy. An initial 8 mg/kg loading dose was administered following the conclusion of radiation therapy, which was followed by 6 mg/kg doses every 21 days. Therapy is planned for a total of 1 year. At the time of authorship, the patient is recovering well with no evidence of disease.
3. Comment
We report the case of a 37-year-old female diagnosed with extramammary Paget’s disease (EMPD) of the vulva during pregnancy, later found to have stage IIIC adenocarcinoma of the vulva, who underwent primary surgical treatment with unilateral radical vulvectomy followed by bilateral sentinel lymph node biopsy and unilateral inguinal lymphadenectomy. The patient received antepartum chemotherapy, underwent scheduled preterm cesarean delivery, and was subsequently treated with chemoradiation and targeted biologic therapy.
EMPD and vulvar cancer during pregnancy are rare, with no cases of vulvar EMPD and approximately 38 cases of vulvar cancer reported in the literature (Amant et al., 2019). Our patient identified vulvar irritation at her initial prenatal visit. There was a 1-month delay in diagnosis during empiric therapy with antibiotics and antifungals. A systematic review of vulvar cancer cases diagnosed during pregnancy documented a mean delay in diagnosis of 3 months (Matsuo et al., 2014). There is a significant survival disadvantage for patients whose diagnosis is delayed >8 weeks (Matsuo et al., 2014). Given that delay in diagnosis is detrimental to survival, the threshold for biopsy of vulvar lesions should be low.
There are insufficient data to support a preferred treatment of EMPD in pregnancy. Although some case series report non-surgical therapies such as topical imiquimod, laser therapy, and phototherapy for EMPD outside of pregnancy, primary surgical management is generally accepted (Edey et al., 2019, van der Linden et al., 2016). The need for lymphadenectomy remains controversial. Lymph node interrogation for EMPD is generally supported when the histologic depth of invasion is >1 mm (van der Linden et al., 2016); however, some algorithms support avoiding lymphadenectomy if no clinically enlarged nodes are present (Tsutsumida et al., 2003). Expert opinion supports primary surgical treatment of vulvar cancer in pregnancy with radical local excision plus sentinel node procedure and/or unilateral or bilateral lymph node dissection (Amant et al., 2019). Our patient underwent local radical vulvectomy for EMPD followed by a second procedure for sentinel lymph node biopsy with left completion lymphadenectomy after the diagnosis of adenocarcinoma was discovered.
Our patient elected to continue her pregnancy. Given the contraindication to radiation therapy during pregnancy, she was offered expectant management vs adjuvant treatment with chemotherapy alone. Case reports of vulvar adenocarcinoma with underlying EMPD have shown relative chemoresistance (Yamamoto et al., 2001); however, effective chemotherapy with paclitaxel plus carboplatin has been reported in cases of advanced squamous cell vulvar cancer (Amant et al., 2018). Considering the poor prognosis of her stage IIIC disease, the patient opted for adjuvant therapy. Use of paclitaxel plus carboplatin was extrapolated from studies of squamous cell vulvar and cervical cancers (Amant et al., 2018, Tewari et al., 2017). Following delivery, our patient underwent chemoradiation. Case reports and retrospective reviews suggest efficacy of radiotherapy, especially when administered at sufficient doses (van der Linden et al., 2016).
Our patient desired trastuzumab therapy. Her2/Neu overexpression may be present in 70–100% of EMPD cases, although the clinical significance is unknown (van der Linden et al., 2016). Case reports have shown response of EMPD to treatment with trastuzumab with and without concurrent cytotoxic therapy (Ichiyama et al., 2017). In this case, trastuzumab therapy was thought to be reasonable given the rarity and aggressiveness of her disease. The dosing and duration were extrapolated from breast cancer literature.
In addition to oncologic treatment decisions, consideration was given to the timing and mode of delivery for the continuing pregnancy. Expert opinion notes that the timing of delivery should be individualized based on pregnancy factors and aggressiveness of the cancer. In our patient, delivery was planned preterm to facilitate cancer treatment based on a tumor with aggressive characteristics and patient preference. Prior reports suggest that pregnant patients with vulvar cancer and nodal involvement should undergo pregnancy termination or planned delivery to allow for radiation treatment. While data extrapolated from breast cancer research suggest that a delay in radiotherapy by 6–8 weeks is within safety limits for epithelial cancers (Amant et al., 2019), the effect of a delay in therapy for adenocarcinoma is unknown, and prolonged delay to allow for term delivery may be detrimental to maternal health. While some pregnant patients with gynecologic cancers are candidates for vaginal delivery, a planned cesarean delivery is often recommended for patients with vulvar cancer after surgical treatment to prevent vulvar wound dehiscence (Amant et al., 2019). We offered our patient vaginal delivery, though she opted for cesarean delivery.
Vulvar EMPD in pregnancy is an exceedingly rare presentation given that this disease typically occurs in women outside of reproductive age. Care should be taken to report in detail any future cases to gain a better understanding of EMPD in pregnancy.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
5. Disclosures
The authors have no disclosures to report. ICMJE disclosure forms are included in the submission.
Author Contributions
Dr. Tran was the primary gynecologic oncologist caring for the patient, developed treatment plans with the support of an oncology team, and performed all surgical interventions. He reviewed and edited the information presented in this manuscript.
Dr. Bruce was a resident physician involved in the postoperative care of the patient. She performed the background literature review and drafted the above manuscript.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
Histopathology images and analyses were kindly provided by Danielle Cronin, MD, of the Department of Pathology at Kaiser Permanente Santa Clara Medical Center.
References
- Amant F., Nooij L., Annibali D., van Rompuy A.S., Han S., van den Bulck H. Brief report on 3-weekly paclitaxel carboplatin efficacy in locally advanced or metastatic squamous vulvar cancer. Gynecol. Obstet. Invest. 2018;83(6):620–626. doi: 10.1159/000487435. [DOI] [PubMed] [Google Scholar]
- Amant F., Berveiller P., Boere I.A., Cardonick E., Fruscio R., Fumagalli M. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann. Oncol. 2019;30(10):1601–1612. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- Edey K.A., Allan E., Murdoch J.B., Cooper S., Bryant A. Interventions for the treatment of Paget's disease of the vulva. Cochrane Database Syst. Rev. 2019;6:CD009245. doi: 10.1002/14651858.CD009245.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama T., Gomi D., Fukushima T., Kobayashi T., Sekiguchi N., Sakamoto A. Successful and long-term response to trastuzumab plus paclitaxel combination therapy in human epidermal growth factor receptor 2-positive extramammary Paget's disease: A case report and review of the literature. Mol. Clin. Oncol. 2017;7(5):763–766. doi: 10.3892/mco.2017.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Whitman S.A., Blake E.A., Conturie C.L., Ciccone M.A., Jung C.E. Feto-maternal outcome of pregnancy complicated by vulvar cancer: a systematic review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;179:216–223. doi: 10.1016/j.ejogrb.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Onaiwu C.O., Salcedo M.P., Pessini S.A., Munsell M.F., Euscher E.E., Reed K.E. Paget's disease of the vulva: A review of 89 cases. Gynecol. Oncol. Rep. 2017;19:46–49. doi: 10.1016/j.gore.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390(10103):1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumida A., Yamamoto Y., Minakawa H., Yoshida T., Kokubu I., Sugihara T. Indications for lymph node dissection in the treatment of extramammary Paget's disease. Dermatol. Surg. 2003;29(1):21–24. doi: 10.1046/j.1524-4725.2003.29001.x. [DOI] [PubMed] [Google Scholar]
- van der Linden M., Meeuwis K.A., Bulten J., Bosse T., van Poelgeest M.I., de Hullu J.A. Paget disease of the vulva. Crit. Rev. Oncol. Hematol. 2016;101:60–74. doi: 10.1016/j.critrevonc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Sakuragi N., Shirato H., Shimizu M., Fujimoto S. Radiotherapy with concurrent chemotherapy for vulvar adenocarcinoma associated with extramammary Paget's disease. Gynecol. Oncol. 2001;80(2):267–271. doi: 10.1006/gyno.2000.6054. [DOI] [PubMed] [Google Scholar]