Abstract
Bone marrow (BM) as an active hematopoietic organ is highly sensitive to changes in body microenvironments and responds to external physical stimuli from the surrounding environment. In particular, BM tissue responds to several cues related to infections, strenuous exercise, tissue/organ damage, circadian rhythms, and physical challenges such as irradiation. These multiple stimuli affect BM cells to a large degree through a coordinated response of the innate immunity network as an important guardian for maintaining homeostasis of the body. In this review, we will foc++us on the role of purinergic signaling and innate immunity in the trafficking of hematopoietic stem/progenitor cells (HSPCs) during their egression from the BM into peripheral blood (PB), as seen along pharmacological mobilization, and in the process of homing and subsequent engraftment into BM after hematopoietic transplantation. Innate immunity mediates these processes by engaging, in addition to certain peptide-based factors, other important non-peptide mediators, including bioactive phosphosphingolipids and extracellular nucleotides, as the main topic of this review. Elucidation of these mechanisms will allow development of more efficient stem cell mobilization protocols to harvest the required number of HSPCs for transplantation and to accelerate hematopoietic reconstitution in transplanted patients.
Keywords: Purinergic signaling, Innate immunity, Extracellular ATP, Adenosine, Hematopoietic stem cells, Stem cell mobilization and homing
Introduction
Hematopoietic stem/progenitor cells (HSPCs) isolated from bone marrow (BM), mobilized peripheral blood (mPB), and umbilical cord blood (UCB) have been successfully employed to treat numerous malignant and non-malignant disorders, including leukemia, lymphoma, selected inherited genetic diseases, and certain types of immune deficiencies [1]. This requires, on the one hand, a sufficient number of HSPCs in the graft to be transplanted and, on the other hand, strategies to ensure smooth homing and engraftment of these cells after infusion into the patient [1–3].
Based on aforementioned, the successful transplantation requires a sufficient number of harvested HSPCs per kg body weight of the patient and a fast, consistent, and long-term multilineage HSPC engraftmentis achieved after intravenous infusion of a minimum of 2 × 106 CD34+ stem cells/kg recipient body weight; however, a higher dose of 5 × 106 CD34+ cells/kg is considered preferable [4]. Therefore, to assure satisfaction of this requirement, when obtaining cells from mobilized peripheral blood (mPB) for grafting, it is important to apply efficient pharmacological mobilization protocols [5–7]. Unfortunately, some patients are resistant to standard mobilization protocols that employ granulocyte colony-stimulating factor (G-CSF) or the CXCR4-blocking small molecule AMD3100 as pro-mobilizing agents, and thus, there is a need to further optimize/improve existing strategies by enhancing this procedure [4, 5]. Therefore, studies are needed to further elucidate this process in order to identify crucial targets for pharmacological interventions.
A successful outcome for transplantation also requires that HSPCs rapidly home to BM and subsequently engraft to establish post-transplantation hematopoiesis [1, 3]. This requirement can be problematic with an insufficient number of HSPCs in the graft as seen (i) after poor harvesting of these cells from BM, (ii) poor mobilization efficiency into PB, or (iii) a low number of HSPCs in the UCB unit. Therefore, crucial for a positive transplantation outcome is achieving fast hematopoietic reconstitution, which, besides improving overall survival, significantly cuts costs related to post-transplantation care.
For several years, our group has been elucidating the role of innate immunity in regulating the trafficking of HSPCs [7–11]. In this review, we will provide an update on the role of purinergic signaling as modulators of innate immunity as an orchestrator of purinergic signaling in the mobilization and homing/engraftment of HSPCs [7]. Moreover, in light of new discoveries, the BM will be presented as a dynamic organ responsive to several cues related to infections, strenuous exercise, tissue/organ damage, the circadian rhythm clock, and certain physical challenges [7]. Specifically, when no systemic inflammation occurs, pharmacological mobilization or conditioning for transplantation by radio/chemotherapy induces a state of “sterile” inflammation in BM tissue that is triggered and coordinated by the innate immune system [11, 12]. Based on this mechanism, BM is an important guardian of homeostasis of the body, and, based on the published literature, we will present the current view of how innate immunity orchestrates the responses of BM tissue to these challenges.
Innate immunity and induction of the sterile inflammation state in BM
The immune system is based on inborn or innate and acquired adaptive immunity [13]. Innate immunity, which is the topic of this review, consists of both cellular and humoral arms. The cellular arm involves several types of cells, including neutrophils, monocytes, basophils, eosinophils, mast cells, natural killer cells, and dendritic cells, which respond to changes in the microenvronment and secrete soluble mediators [13]. The humoral arm consists of proteins of the complement cascade (ComC), which are activated in the (i) classical, (ii) mannan-binding lectin, and (iii) alternative pathways [13]. Moreover, to the humoral arm also belongs molecular pattern-recognition receptors (PRR), such as collectins, ficolins, and pentraxins, which circulate in PB. While pentraxins behave as the functional ancestors of antibodies (Abs), collectins, and in particular one of the ficolins known as mannan-binding lectin (MBL), play an important role in triggering the MBL pathway of ComC activation [13]. As we will discuss below, this pathway, in turn, plays an important role in co-operation with purinergic signaling in triggering the mobilization of HSPCs [14].
The main task of innate immunity is immune surveillance by its cellular and humoral arms to detect specific molecular patterns present in invading microorganisms or, what is important for this review, the host organism’s own damaged released mediators or stress-exposed epitopes that are not present in healthy tissues [13]. Such changes occur after challenging BM by administration of pro-mobilizing drugs or conditioning for transplantation by radio/chemotherapy and result in the innate immunity-induced state of “sterile” inflammation. This is evidenced by the fact that administration of pro-mobilizing drugs and conditioning for transplantation lead to activation of cells belonging to the innate immunity network and to activation of the purinergic signaling and ComC [11, 12, 15–17]. In this review, we will present the current concept of how innate immunity regulates both of these processes.
Retention of HSPCs in BM niches
HSPCs reside in BM in defined areas known as stem cell niches, and recent research indicates that these niches are perivascular (stromal derived factor-1, SDF-1+, and kit ligand, KL+), created partially by mesenchymal stromal cells and endothelial cells and often, but not always, located near trabecular bone in bone marrow cavities [18–21]. In addition, the existence of distinct niches, including quiescent nestinbright NG2+ arteriolar and proliferative nestindimLepr+ sinusoidal niches, for distinct subpopulations of HSCs has also been proposed. On the other hand, evidence also indicates significant involvement of osteoblastic niches located along trabecular bones, in which osteoblasts provide cellular support for HSPCs [18, 19]. Importantly, HSPCs are retained in these niches mainly due to two anchorage axes involving the receptor CXCR4 and the integrin VLA-4, which are present on the surface of HSPCs, and their corresponding ligands, SDF-1 and vascular cell adhesion molecule-1 (VCAM-1), which are expressed in cells that form the cellular support for the niche [7, 18–21]. It is important for this review that proteolytic enzymes released from innate immunity cells activated during mobilization degrade these peptide-based interactions. However, so far, no single proteolytic enzyme has been identified that, if selectively depleted, confers a poor-mobilizer phenotype [22]. This absence of an effect is explained by the existence of functional redundancy among proteolytic enzymes [7].
Importantly, both CXCR4 and VLA-4 receptors are associated with membrane lipid rafts on the outer cell membranes of HSPCs. These structures are enriched in cholesterol and ensure the connection of clustered receptors with downstream signaling pathways [23–25]. A crucial role in the integrity and stabilization of membrane lipid rafts is played by glycosylphosphatidylinositol (GPI-A). As reported, GPI-A is susceptible to digestion by the lipolytic enzyme phospholipase C-β2 (PLC-β1) [26–28]. This enzyme is released during mobilization from innate immunity cells, including granulocytes and monocytes, and plays an important role by disrupting the structure of membrane lipid rafts and promoting detachment of HSPCs from their BM niches [20, 29].
The importance of both anchor axes, CXCR-4–SDF-1 and VLA4–VCAM-1, in the retention of HSPCs in BM niches suggests that they can be mobilized into peripheral blood by administration of a specific antagonist of the CXCR4 receptor (AMD3100) or a blocking molecule of VLA-4 (BIO5192) [4, 5, 30]. Both of these compounds activate the innate immune response in the BM microenvironment, and the mobilization effect induced by these antagonists is based on the detachment of HSPCs from stem cell niches, indicating that a significant chemotactic gradient for HSPCs is continuously present within BM blood sinusoids [31–36]. This gradient depends on an elevated concentration of the potent HSPC chemoattractant sphingosine-1-phosphate (S1P), which is several times higher in concentration in PB than in the BM microenvironment [37].
A limited number of chemoattractants for HSPCs—important role of extracellular ATP as chemoattractant for HSPCs
Both during mobilization and in the homing process, HSPCs navigate from BM into PB and from PB into BM, respectively, following chemotactic gradients. Several chemokines, cytokines, and growth factors have been described that chemoattract different types of normal and malignant cells, including those involved in normal and pathological haemato-lymphopoiesis. Interestingly, of all the peptide-based factors studied so far, only the mentioned above β-chemokine stromal-derived factor 1 (SDF-1) is a chemoattractant for HSPCs [38, 39]. No other peptide-based chemokine, cytokine, or growth factor is able to chemoattract HSPCs, while several of these factors attract all other types of mature hematopoietic and lymphopoietic cells. Importantly, even if SDF-1 has this unique property among peptide-based factors, it is usually employed in in vitro chemotaxis assays at supra-physiological concentrations—up to 100 times higher than those measured in vivo in biological fluids [17]. Interestingly, the responsiveness of HSPCs to SDF-1 gradients may be increased by certain antimicrobial cationic peptides released during activation of the ComC, such as the C3 cleavage fragment C3a, or released from activated innate immunity cells (e.g., cathelicidin or β2-defensin) [17, 31, 40, 41]. Based on such findings, we conclude that innate immunity released mediators positively modulate and enhance the responsiveness of HSPCs to SDF-1 gradients.
The other significant chemoattractants for HSPCs belong to the family of bioactive phosphosphingolipids mentioned in previous paragraph (spingosine-1-phosphate, S1P; ceramide-1-phosphate, C1P) and what is a main topic of this review to the family of extracellular nucleotides (EXNs, e.g., adenosine triphosphate, ATP) [42, 43]. Importantly, these factors are much more potent chemoattractants for HSPCs than SDF-1 when employed at physiological doses in chemotaxis assays.
It is also important to mention that during BM sterile inflammation, enzymes that degrade these chemotactic factors are upregulated in the BM microenvironment, as are inhibitors of these enzymes. However, if expression of CD39 and CD73 ectonucleotidases that metabolize ATP increases in stressed BM requires further studies. The interplay and timing in release and biological activity of these enzymes and their inhibitors and the rate of release of chemotactic factors affects the in vivo trafficking of HSPCs. This important regulatory network requires further studies.
Extracellular nucleotides and purinergic signaling in innate immunity and sterile inflammation
Purinergic signaling is a primordial form of extracellular signaling mediated by EXNs, including the abovementioned purine, ATP, and its nucleoside metabolite, adenosine. This process is regulated by a family of cell surface-expressed ectonucleotidases (E-NTPDase, CD39) family (NTPDase1, 2, 3, and 8) and CD73 [42, 44–46]. As will be discussed later, ATP and adenosine exert opposing effects on the trafficking of HSPCs. Purinergic signaling also involves certain rare extracellular pyrimidines, such as UTP and UDP [42, 44–46].
EXNs stimulate target cells by engaging several families of purinergic receptors, including P2X, P2Y, and P1 subtypes, which are among the most abundant receptors in living organisms. Both HSPCs and innate immunity cells express several receptors that belong to purinergic receptor families. The P2X ionotropic channel receptor family consists of seven members (P2X1, 2, 3, 4, 5, 6, and 7), whereas the P2Y family includes a total of eight receptors (P2Y1, 2, 4, 6, 11, 12, 13, and 14) identified so far, which are also G protein-coupled receptors and respond to stimulation by ATP, ADP, UTP, UDP, or UDP glucose. The P1 receptor family consists of four G protein-coupled receptor subtypes (A1, A2A, A2B, and A3), which are activated by adenosine [42, 44–46].
As has been reported, P2X receptors enhance the response to ATP-induced migration and at the same time inhibit adhesion of HSPCs. Of the seven P2X receptors, P2X7 and P2X4 subtypes as we recently reported are both highly expressed on the surface of HSPCs [47]. These receptors activate the Nlrp3 inflammasome inside hematopoietic cells after stimulation by ATP, which is an important member of the class of danger-associated molecular pattern molecules (DAMPs or alarmines) and is released by activated innate immunity cells during the mobilization process or by BM cells exposed to radio/chemotherapy [48–50].
Inflammasomes are multiprotein oligomer complexes and are important components of the innate immunity network [48, 50, 51]. They are triggered during “sterile” inflammation in response to DAMPs, for example, ATP released by activated or damaged cells and during infections in response to pathogen-associated molecular pattern molecules (PAMPs). The NLRP3 inflammasome is expressed by myeloid cells, including Gr-1+ granulocytes, monocytes, and dendritic cells, and is currently the best-characterized member of this family operating in hematopoietic cells [48, 50, 51]. The Nlrp3 inflammasome, activated after the binding of ATP to P2X7 or P2X4 receptors, activates, in turn, intracellular caspase1. Activation of this enzyme promotes maturation and secretion of pro-inflammatory interleukin 1β (IL-1β) and interleukin 18 (IL-18). This process depends on caspase 1-mediated proteolytic cleavage of the cytokine pro-forms (pro-IL-1β and pro-IL-18). Mature cleaved IL-1β and IL-18 are subsequently released into the extracellular space [48, 50, 52]. Our most recent data indicate that these cytokines released in Nlrp3-caspase-1-dependent manner are important endogenous mediators amplifying mobilization process (manuscript in preparation).
In addition to P2X receptors, a role of metabotropic P2Y receptors in trafficking of HSPCs requires further studies as signaling from P2Y receptors also participates in innate immune signaling in response to DAMPs and PAMPs. As example, P2Y2, P2Y4, and P2Y6 receptors participate in innate immune responses to prevent macrophage infection by Toxoplasma gondii [53]. Moreover, however, the P2Y6 receptor has not been described so far as part of the Nlrp3 inflammasome; it is important for chemotaxis and promotion of inflammation [54]. This receptor is expressed by immune cells, including basophils, where it regulates IgE-dependent degranulation [55] as well as by endothelial cells [56] playing a role in expression of adhesion molecules that participate in vascular inflammation [54, 57]. Interestingly, liposaccharide (LPS) that upregulates expression of Nlrp3-inflammasome components in cells selectively increases expression of the P2Y6 receptor. Based on this observation, it would be interesting to address a potential role of P2Y6 receptor in trafficking of HSPCs.
Upon activation of the inflammasome in an ATP–P2X7 receptor- and ATP–P2X4 receptor-dependent manner, innate immunity cell release, in addition to IL-1β and IL-18, several other DAMPs, including high mobility group box 1 protein (Hmgb1) and S100 calcium-binding protein A9 (S100a9), which promote the state of sterile inflammation in the BM microenvironment. Innate immunity cells also release reactive oxygen species (ROS), which expose neoepitopes on the surface of cells in the BM microenvironment [48, 50]. Neoepitope antigens exposed by ROS are recognized by naturally occurring IgM antibodies, and neoepitope–IgM complexes become targets for mannan-binding lectin (MBL) and thereby activate the ComC in the MBL-dependent pathway [14, 58].
Overall, innate immunity triggers sterile inflammation in the BM microenvironment, and subsequently, this process becomes auto-amplified by autocrine and paracrine interactions. However, there are mechanisms that limit this process, and an intracellular anti-inflammatory enzyme, heme oxygenase 1 (HO-1), here plays an important role [59–61]. The biological effects of the abovementioned components of innate immunity and purinergic signaling in the trafficking of HSPCs will be discussed later in this review and are depicted at Figs. 1 and 2.
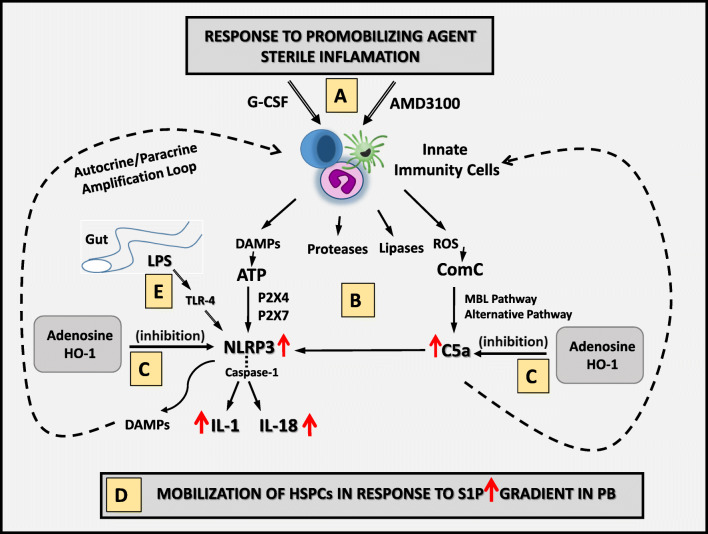
Fig. 1.
Innate immunity triggers mobilization of HSPCs. a Pro-mobilizing agents (e.g., G-CSF or AMD3100) activate innate immunity cells (granulocytes, monocytes, and dendritic cells) in the BM microenvironment to release danger-associated molecular pattern molecules (DAMPs), including extracellular ATP; proteolytic and lipolytic enzymes and ROS. b ATP released from innate immunity cells activates in autocrine/paracrine manner the Nlrp3 inflammasome after binding to P2X7 and P2X4 receptors. This event leads to caspase-1 activation and release from the innate immunity cells of active forms of IL-1β and IL-18, which, together with other DAMPs (e.g., HMGB1 and S100A9), amplify the mobilization process. Proteolytic enzymes and the lipolytic enzyme PLC-β2 disrupt the SDF-1–CXCR and VCAM-1–VLA4 anchoring mechanisms for HSPCs in BM niches and the structure of lipid rafts, respectively. In parallel on the surface of cells in the BM microenvironment, released ROS exposes neoepitope antigens, which, after the binding of IgM naturally occurring antibodies, activate the MBL pathway of ComC activation. c These innate immune responses amplified by purinergic signaling potentiate a mutual interaction between cells and crucial pathways involved in the mobilization process and are negatively regulated/controlled at Nlrp3 inflammasome level and ComC activation by extracellular adenosine (a degradation product of ATP) and the intracellular anti-inflammatory enzyme HO-1. d HSPCs are released from BM niches by a steep gradient of S1P in PB. e Also shown in this scheme, by releasing LPS, Gram-negative bacteria in the gut positively prime in innate immunity cells the Nlrp3 inflammasome complex in an LPS–TLR4-dependent manner, and increase synthesis of pro-IL-1β and pro-IL-18
Fig. 2.
Innate immunity facilitates homing of HSPCs after transplantation to BM. a As shown, myeloablative conditioning for transplantation by radio/chemotherapy upregulates HSPC chemoattractants (SDF-1, S1P, and extracellular ATP) in the BM microenvironment. At the same time, the ComC becomes activated on both sides of the BM–PB endothelial barrier. b ComC cleavage fragments promote homing of HSPCs by employing different mechanisms. C3a enhances the responsiveness of HSPCs to an SDF-1 homing gradient by increasing incorporation of the CXCR4 receptor into membrane lipid rafts. In addition, C3a, together with C5a, (i) upregulates homing-related molecules on the surface of BM endothelial cells, (ii) increases expression of metalloproteinases in HSPCs, and (iii) permeabilizes the endothelial barrier. c We propose that HSPCs migrating to BM niches are guided by activation of the Nlrp3 inflammasome, as Nlrp3-KO HSPCs show defective migration in in vitro assays in response to SDF-1, S1P, and ATP gradients and engraft more poorly after transplantation. Finally, migration of HSPCs is negatively regulated by extracellular adenosine and HO-1, and both C3a and C5a downregulate HO-1 expression in HSPCs
Bioactive phosphosphingolipids as potent chemoattractants for HSPCs
Both phosphosphingolipids and extracellular nucleotides are non-peptide regulators of HSPCs trafficking. S1P and C1P are produced by phosphorylation of sphingosine by two sphingosine kinases (Sphk1 and Sphk2). Genetic knockout of both kinases is lethal, indicating the important role of S1P in development and perturbation in S1P or S1P receptor expression in mice has an impact on the trafficking of HSPCs [62, 63].
While S1P activates five G protein-coupled receptors (S1PR1–5), receptors for C1P are not known. S1P is transported in the blood by erythrocytes, albumin, and high-density lipoprotein (HDL) and degraded by S1P lyase [64–66]. This explains how a high concentration of S1P in PB creates a steep gradient between BM and PB for HSPCs. It is also why S1P gradients play a crucial role in the egress of HSPCs from bone marrow into PB. During mobilization, the concentration of S1P in blood may be additionally increased due to activation of the ComC, whose final product, C5b–C9 (also known as membrane attack complex, MAC), facilitates release of S1P from erythrocytes and platelets into the blood [32, 67]. S1P is also upregulated in BM after conditioning for hematopoietic transplantation and is involved in the homing of HSPCs after transplantation [17, 62].
What is important for a topic of this review is data indicating molecular co-operation of phosphosphingolipids and extracellular nucleotides in regulating sterile inflammation in BM, activation of innate immunity cells, and regulating HSPCs trafficking. We noticed that S1P similarly like ATP activates Nlrp3 inflammasome in innate immunity cells (manuscript in preparation). Therefore, further studies are needed to elucidate mutual interaction of these signaling molecules in biological processes.
Innate immunity and purinergic signaling as trigger and orchestrator of HSPC mobilization
Based on aforementioned data, evidence has accumulated that the activation of innate immunity triggers egress of HSPCs from BM into PB. This effect depends on activation of purinergic signaling and the role of purinergic signaling in hematopoiesis and involvement in this phenomenon of P2 receptors was a subject of an excellent recent review published in Purinergic Signaling [68]. Figure 1 depicts this process during pharmacological mobilization induced by granulocyte colony-stimulating factor (G-CSF) or the CXCR4 receptor antagonist AMD3100. These drugs are common therapeutics employed in the clinic to mobilize donors to obtain HSPCs for transplantation.
As shown in this scheme, innate immunity cells are first responders to administration of pro-mobilizing drugs. Several elegant reports have demonstrated that granulocytes, monocytes, and dendritic cells are required to initiate mobilization. Specifically, mice deficient in neutrophils are poor mobilizers [69]. As a result of activation by pro-mobilizing drugs, innate immunity cells secrete several DAMPs/alarmines, including ATP and HMGB1, ROS, and proteolytic and lipolytic enzymes. Importantly, in in vivo animal models, a decrease in ATP release by pannexin 1 [43] or connexin 43 [70] channels, inhibition of ROS [36], and a lack of phospholipase C-β2 [71] all result in poor mobilization. As mentioned above, in contrast to the lipolytic enzyme phospholipase C-β2, not a single extracellular proteolytic enzyme has been identified whose deficiency results in defective mobilization due to a functional redundancy among proteolytic enzymes released from activated innate immunity cells.
All these factors secreted from activated innate immunity cells affect the initiation of mobilization in different ways. While ATP triggers activation of the Nlrp3 inflammasome after binding to P2X7 and P2X4 receptors on the surface of innate immunity cells in both an autocrine and paracrine manner, released from cells phospholipase C-β2 disrupts the structure of membrane lipid rafts on the surface of HSPCs in BM niches and frees HSPCs from anchoring by the SDF-1–CXCR4 and VCAM-1–VLA4 axes [20, 23, 31, 72, 73]. These retention axes are additionally affected by the proteolytic action of enzymes secreted by activated granulocytes. At the same time, mitochondria-released ROS activates the Nlrp3 inflammasome inside cells and in addition exposes neoepitopes on the surface of endothelial cells, which triggers activation of the ComC. From the reasons mentioned above, it would be interesting to see if P2Y6 receptor playing a role in vascular inflammation plays here a role as well [54, 57]. The ComC is also activated in response to HMGB1 and other alarmines. Activation of the ComC in an MBL- or alternative pathway-dependent manner leads to generation of C5a, which via C5aR again activates innate immunity cells and Nlrp3 inflammasome. Taking into consideration all aforementioned mechanisms, mice deficient in (i) innate immunity cells (e.g., neutrophils), (ii) pannexin 1 and connexin 43 channels, (iii) P2X7 and P2X4 receptors, (iv) MBL and factor B (FB), which are required for activation of the MBL and alternative pathways of ComC, respectively, and (v) C5, which is required for activation of the distal pathway of the ComC, are poor mobilizers [14, 43, 69, 70, 74, 75].
Interestingly, by using appropriate knockout (KO) mice, we previously demonstrated that, of the three pathways of ComC activation, the C1q-mediated classical pathway, the MBL-mediated mannan-binding lectin pathway, and the factor B (FB)-regulated alternative pathway, the latter two play the most important roles in pharmacological mobilization of HSPCs [14, 74]. In support of this conclusion, MBL-KO mice, which have a defective MBL pathway [14] of ComC activation and FB-KO mice [74], which do not activate the alternative pathway, are poor mobilizers but not C1q-KO mice [76], which do not activate the classical ComC pathway,
Nlrp3-KO animals are also poor mobilizers [51]. The Nlrp3 inflammasome seems to play an important and central role in the mobilization process and becomes activated both by the ATP-P2X7/P2X4 purinergic signaling pathway, S1p exposure, and by ComC-derived C5a. Activation of the Nlrp3 inflammasome potentiates release form innate immunity cells of several other DAMPs and, as mentioned above, activates caspase 1, which cleaves pro-IL-1β and pro-IL18, yielding their mature active forms secreted from cells [77, 78]. As we recently demonstrated, both IL-1β and IL-18 are strong endogenous pro-mobilizing mediators [51]. Moreover, both cytokines represent as mentioned important endogenous mechanisms facilitating the mobilization process in an Nlrp3 inflammasome-dependent manner, as mice with caspase 1 deficiency, which impairs cleavage of pro-forms and secretion of mature IL-1β and IL-18, are poor mobilizers (manuscript in preparation).
After HSPCs are released from their niches, they respond to a chemotactic gradient in the blood within the BM sinusoids. It has been demonstrated that the major chemoattractant in PB is mentioned above S1P [62]. The gradient of S1P across the BM–PB endothelial barrier is steep, even under steady-state conditions, and steep enough to chemoattract HSPCs released from their BM niches. This gradient may additionally increase during mobilization in response to activation of the distal part of the ComC, in which C5b–C9 (the MAC complex) potentiates release of S1P from erythrocytes and platelets [32, 67].
It is obvious that, once initiated, the process of HSPC mobilization must be tightly controlled through negative regulation. Recent research indicates that negative regulators of this process are the adenosine, metabolite of ATP, and intracellular heme oxygenase 1 (HO-1) [11, 79, 80]. As shown in Fig. 1, both adenosine and HO-1 negatively regulate the Nlrp3 inflammasome and the pro-mobilizing effects of ComC-released C5a. Therefore, as discussed below, both downregulation of the expression of adenosine in the BM microenvironment and inhibition of HO-1 enhance the mobilization of HSPCs [11, 79, 80].
Is this scheme complete? Probably not, but it may serve as a useful roadmap to developing new strategies that enhance the mobilization process. Further studies are needed to determine whether other inflammasomes, such as Aim2, are involved in the mobilization process in addition to Nlrp3. It is suggestive as Nlrp3-KO mice are still able to mobilize some HSPCs [51]. What is not shown in this scheme is that, in addition to the ComC, other proteolytic enzyme cascades, such as the coagulation and fibrinolytic cascades, also potentiate the egress of HSPCs [9]. In the case of the coagulation cascade, it has been demonstrated that thrombin activated during mobilization has crosstalk with the ComC, has C5 convertase activity, and generates C5a [75].
In addition to other member inflammsome family besides Nlrp3, the question of the importance of toll-like receptors remains. As shown in Fig. 1, TLR-4 is important in priming innate immunity cells for synthesis of IL-1β and IL-18 in response to gut-derived LPS [48]. Specifically, germ-free mice or mice that are depleted by employing antibiotics against gut bacteria producing LPS are poor mobilizers [81]. These observations are now explained by the effect of LPS on priming inflammasome elements and the synthesis of IL-1β and IL-18 in innate immunity cells. The role of LPS-mediated upregulation of P2Y6 receptor [54] in mobilization process requires further studies.
On the other hand, while TLRs are an important part of the innate immunity network and may have crosstalk with C3aR and C5aR, they do not promote, but instead even seem to prevent, mobilization of HSPCs into PB. This is supported by our observation that TLR signaling-deficient (MyD88-KO) mice are easy mobilizers [82]. This negative effect of TLRs on the mobilization of HSPCs may somewhat balance the mobilization process and may correlate with the intracellular expression of HO-1, as TLR-KO mice display a lower level of HO-1 in response to pro-mobilizing agents as compared to wild-type normal mobilizers. Since HO-1 is a negative regulator of HSPC trafficking, TLRs negatively affect the migration of HSPCs by enhancing intracellular HO-1 expression [82]. These results are somewhat supported by a recent report from another group demonstrating enhanced trafficking of HSPCs from BM to spleen in MyD88-KO, TLR2-KO, and TLR4-KO mice. Moreover, by comparing differences in the mobilization of HSPCs in mice treated with antibiotics to eliminate gut commensal microbiota as an endogenous source of a low level of LPS in PB with these mutant mice, the authors concluded that trafficking of HSPCs in these mice is TLR-independent [41].
Innate immunity coordinates homing and subsequent engraftment of HSPCs
The reverse phenomenon to HSPC mobilization is homing of intravenously injected HSPCs into circulation during hematopoietic transplantation, followed by their engraftment. As mentioned above, there are three chemotactic factors for HSPCs identified so far: (i) the chemokine SDF-1, (ii) the bioactive phosphosphingolipid S1P, and (iii) the extracellular nucleotide ATP. All these factors are upregulated in the BM microenvironment after conditioning for transplantation by radio- or chemotherapy and play an important role in the seeding efficiency of HSPCs into BM niches [32, 83, 84]. It has been demonstrated that deficiency of these factors in the BM microenvironment or a lack of corresponding receptors on the surface of HSPCs results in impaired homing of transplanted HSPCs to BM niches.
A crucial role in this process is played by SDF-1, which chemoattracts CXCR4+ HSPCs to the BM microenvironment. However, the fact that CXCR4−/− HSPCs still show some level of homing and engraftment to the BM of normal wild-type animals prompted us to investigate the other homing factors besides SDF-1 that may support the homing function of the SDF-1–CXCR4 axis [85–87]. To address the potential role of S1P and ATP, we transplanted HSPCs from CXCR4fl/flCreTg/− mice, in which CXCR4 had been eliminated by a Cre hematopoietic driver strategy, or wild-type HSPCs into sphingosine kinase 1-deficient (Sphk1−/−) mice, which are deficient for S1P expression in BM. These experiments revealed a compensatory role for S1P in the homing of CXCR4−/− HSPCs but since Sphk1−/−mice still engrafted with CXCR4−/− BM cells, indicated the involvement of still other factor/s in the homing process [62]. Our recent results demonstrated that, in the case of perturbation of the SDF-1–CXCR4 and S1P–S1P1R axes, ATP plays a role and compensates for their deficiency in the BM homing process. For example, we found that CM from BM cells of mice conditioned for transplantation by irradiation chemoattract HSPCs in an ATP-dependent manner. Moreover, following conditioning for transplantation with lethal irradiation, inhibition of ATP expression in the BM microenvironment by blockade of the ATP-releasing pannexin 1 channel negatively affected homing of normal HSPCs (manuscript in preparation).
Figure 2 depicts the mechanism of activation of the innate immune system in BM conditioned by radio/chemotherapy for transplantation of HSPCs. It is shown that the SDF-1, ATP, and S1P concentrations increase in the BM microenvironment after conditioning for transplantation. An important role in homing and engraftment is played again by activation of innate immunity and the ComC. Specifically, mice deficient in the C5 element of the ComC as well as mice which do not activate the MBL (MBL-KO) or the alternative pathways of the ComC (FB-KO) show decreased homing of HSPCs (manuscript in preparation). Moreover, while C3 cleavage fragments (C3a and desArgC3a) sensitize the responsiveness of HSPCs to an SDF-1 gradient by the abovementioned priming effect [88, 89], the C5 cleavage fragment C5a along with C3a inhibits intracellular activation of HO-1 by engaging C3aR and C5aR [90]. C3 and C5 cleavage fragments also upregulate expression of adhesion molecules on the surface of endothelial cells in BM sinusoids, which trap HSPCs circulating in blood, and in addition upregulate expression of metalloproteinases (e.g., MMP-9), which help HSPCs to pave the way across the endothelial barrier between BM and PB [91].
We also envision that HSPCs, which express the Nlrp3 inflammasome, may have an intrinsic “auto-navigation” system leading them to BM niches, based on the positive migration effect of the Nlrp3 inflammasome and the negative effect of intracellular HO-1. Specifically, inhibition of Nlrp3 in HSPCs leads to a decrease in their homing and engraftment (manuscript in preparation), while HSPCs having an HO-1 deficiency engraft faster in BM than do normal WT cells [91]. This auto-navigation system is negatively regulated by the extracellular ATP degradation metabolite adenosine, which inhibits Nlrp3 and upregulates HO-1 [11, 43].
The mechanisms presented in Fig. 2 need further study. It is still an open question whether, in addition to SDF-1, S1P, and ATP, there are other unidentified yet chemoattractants for HSPCs that are upregulated in the BM microenvironment during conditioning for hematopoietic transplantation. As reported, HSPCs have a Ca2+-sensing mechanism that directs them to migrate to bone. Moreover, conditioning for transplantation also upregulates several enzymes in the BM microenvironment that may degrade and inactivate chemotactic factors for HSPCs. On the other hand, several inhibitors of proteolytic and lipolytic enzymes are upregulated. Therefore, the interplay between these enzymes during sterile inflammation induced by conditioning for transplantation in BM requires further study.
Finally, HSPCs that lodge to BM niches need to expand and establish proper hematopoiesis. Evidence accumulated that this is also regulated by purinergic signaling. As reported, extracellular adenosine maintains the pool of HSPCs in zebra fish, murine embryos [92], and adult mice [93]. Moreover, as recently reported, ATP-mediated Nlrp3 inflammasome activation may regulate the pool of HSPCs and seems to be an integrator of metabolic activity in promoting HSPC formation and the development of the myeloid and lymphoid lineages in vivo and in vitro [94].
Novel strategies to enhance mobilization and homing/engraftment of HSPCs
Several interesting strategies have been proposed to enhance the mobilization and homing of HSPCs, but here, we will address strategies that are related to modulation of innate immunity and purinergic signaling. Thus, based on the fact that a significant number of HSPC donors are poor mobilizers of HSPCs in response to standard mobilization protocols employing G-CSF or AMD3100, new supportive strategies are needed to facilitate this process [95, 96]. According to what we have learned about the role of innate immunity and purinergic signaling, it is important to target expression of adenosine and HO-1 [11, 43], which are limiting factors in the egress of HSPCs from BM into PB. Since the cell-surface ectonucleotidases CD39 and CD73 play a role in increasing the metabolism of ATP to adenosine in the BM microenvironment, small-molecule inhibitors of these enzymes should facilitate G-CSF- or AMD3100-induced stem cell-mobilization efficacy. In fact, this has already been demonstrated in a mouse model [80]. Inhibition of HO-1 could also be achieved by using small-molecule inhibitors of this intracellular enzyme, and this possibility has also been demonstrated to be efficient in a murine model of HSPC mobilization [79].
On the other hand, it is important to increase homing and engraftment of HSPCs after transplantation. Here again, it is important to inhibit expression of HO-1, both in BM conditioned for transplantation and in HSPCs, as this anti-inflammatory enzyme impairs the BM-seeding efficiency of the transplanted cells [92]. The engraftment of HSPCs could also be boosted by exposure of the grafted cells before infusion into the patient to certain innate immunity-derived cationic antimicrobial peptides, such as C3a, cathelicidin, or β2-defensin [75, 97]. Based on the observation that HSPCs have an intrinsic navigation mechanism for homing to BM that is based on a positive effect of the Nlrp3 inflammasome and a negative effect of HO-1, their behavior could be modulated before infusion into the patient by exposure of HSPCs to ATP [43] or a small-molecule inhibitor of HO-1 [92], respectively. All these promising strategies, which have been tested in experimental animal models, await validation in well-controlled clinical studies.
Purinergic signaling and innate immunity as triggers of graft versus host disease
A dangerous complication of HSPC transplantation is immunological attack of immune cells present in the graft against recipient tissues known as graft versus host disease (GvHD). Evidence accumulated that this process is triggered and later on potentiated by purinergic signaling affecting immune cells present in the graft. This unwanted complication requires further studies as new pharmacological interventions could be proposed to improve clinical outcomes. For example, the importance of adenosine signaling through P1 receptors for immunosuppressive cues as a possible treatment target of GvHD was extensively discussed in a recent excellent review [98].
To support this as recently reported in response to ATP stimulation, a subset of innate lymphoid cells expressing CD39 and CD73 ectonucleotidases produces in extracellular space adenosine and secretes IL-22, which both have immunosuppressive properties. Interestingly, this subset of cells is missing in patients suffering from GvHD [99]. Moreover, as reported, Treg cell-mediated innate immunity effects are regulated by CD39, which decreases IL-2 expression by cytotoxic T cells. Thus, CD39 and CD73 enzymatic activities in degrading ATP into adenosine seem to be a major regulators of Treg-induced immunosuppressive behavior [98]. There are also proposed paracrine feed-back loops, through which adenosine receptors expressed by Treg cells would increase the number of CD39- and CD73-expressing cells [100]. In agreement, chronic treatment with a stable adenosine A2A receptor agonist was effective in a mouse model of graft-versus-host disease, probably due to an expansion of adenosine receptor- and ectonucleotidases expressing Treg cells [101–103].
These are reported some examples for new potential pharmacological interventions to prevent onset or ameliorate existing GvHD in transplanted patients. One of the potential targets occurred to be the P2X7 receptor. Nevertheless, while preclinical trials based on P2X7 receptor antagonism were reported to be promising for the treatment of this post-transplant complication [104], unfortunately inflammation-reducing effects with P2X7 receptor antagonists could not be reproduced in patients. To support this failure, phase II clinical trials based on oral application of the P2X7 receptor inhibitor AZD9056 did not show any benefits of conditions of sterile inflammation, such as Crohn’s disease, making further investigations necessary prior to therapeutic applications ([105]; reviewed in [106]). Combination therapies may be proposed for treating GvHD, such as employing Vedolizumab to block α4β7 integrin with anti-inflammatory treatment. Moreover, the antiviral drug tenofovir (inhibiting ATP release through Pannexin-1 channel [107]) may be also interesting option for controlling aberrant ATP release and activation of P2X7 receptor. Moreover, some already approved therapeutic compounds acting on purinergic signaling, like for example suramin that inhibits P2 receptor activity, await to be tested as immunosuppressive treatment in GvHD. Finally, since ATP activates via P2X7 receptor Nlrp3 inflammasome, a promising strategy has been proposed to inhibit directly Nlrp3 inflammasome by employing a small molecular inhibitor MCC950 [108]. Nevertheless, this promising data obtained in animal model require further conformation in the patients. Possible pharmacological applications of purinergic signaling for ameliorating GvHD are illustrated in Fig. 3.
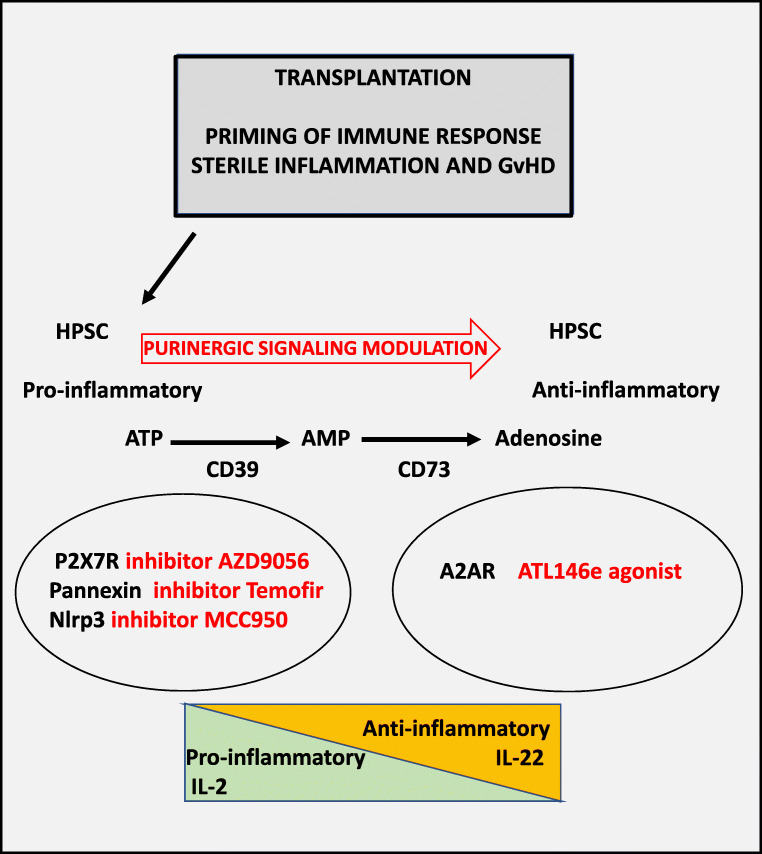
Fig. 3.
Possible targets of purinergic signaling for the treatment of GvHD. Transplantation of hematopoietic cells followed by antigen recognition results in sterile inflammation and GvHD. Transplanted HSPC are specified towards a pro-inflammatory phenotype. Pro-inflammatory cytokines, such as, i.e., IL-2 are secreted following P2X7 receptor activation and Pannexin-1 channels release large amounts of ATP that triggers cytotoxic responses in mechanism of pyroptosis via P2X7 receptor. On the other hand, ectonucleotidases (CD39 and CD73) convert extracellular ATP into anti-inflammatory adenosine that together with the release of anti-inflammatory IL-22 modulates GvHD. Pharmacological intervention of ATP-mediated purinergic signaling or increase in expression of CD39 and CD73 favors the conversion of pro-inflammatory into anti-inflammatory phenotypes
Conclusions
Evidence has accumulated that innate immunity modulates trafficking of HSPCs. It triggers the mobilization process and plays a role in homing and engraftment of these cells after transplantation. These multiple effects of innate immunity are mediated by its cellular and soluble components and involve purinergic signaling that triggers innate immunity response, as recently shown. There are several possibilities for modulating the activity of innate immunity and purinergic signaling in order to improve the clinical results of hematopoietic transplantations. The knowledge gained in animal preclinical models awaits testing and verification in clinical settings.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mariusz Z. Ratajczak, Email: mzrata01@louisville.edu
Henning Ulrich, Email: henning@iq.usp.br.
References
- 1.Huang X, Guo B, Capitano M, Broxmeyer HE. Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation. F1000Res. 2019;31:8. doi: 10.12688/f1000research.20002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Suszynska M. Emerging strategies to enhance homing and engraftment of hematopoietic stem cells. Stem Cell Rev Rep. 2016;12(1):121–128. doi: 10.1007/s12015-015-9625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpova D, Rettig MP, DiPersio JF. Mobilized peripheral blood: an updated perspective. F1000Research. 2019;8:2125. doi: 10.12688/f1000research.21129.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelus LM, Broxmeyer HE. Peripheral blood stem cell mobilization: a look ahead. Curr Stem Cell Rep. 2018;4(4):273–281. doi: 10.1007/s40778-018-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Supakorndej T, Krambs JR, Rao M, Abou-Ezzi G, Ye RY, Li S, Trinkaus K, Link DC. Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking. J Clin Invest. 2019;129(7):2920–2931. doi: 10.1172/JCI124829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratajczak MZ. A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia. 2015;29(4):776–782. doi: 10.1038/leu.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Front Immunol. 2013;4:204. doi: 10.3389/fimmu.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borkowska S, Suszynska M, Mierzejewska K, Ismail A, Budkowska M, Salata D, Dolegowska B, Kucia M, Ratajczak J, Ratajczak MZ. Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) Leukemia. 2014;28(11):2148–2154. doi: 10.1038/leu.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TS, Lapidot T, Ruf W. Extravascular coagulation in hematopoietic stem and progenitor cell regulation. Blood. 2018;132(2):123–131. doi: 10.1182/blood-2017-12-768986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Adamiak M, Plonka M, Abdel-Latif A, Ratajczak J. Mobilization of hematopoietic stem cells as a result of innate immunity-mediated sterile inflammation in the bone marrow microenvironment-the involvement of extracellular nucleotides and purinergic signaling. Leukemia. 2018;32(5):1116–1123. doi: 10.1038/s41375-018-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bujko K, Cymer M, Adamiak M, Ratajczak MZ. An overview of novel unconventional mechanisms of hematopoietic development and regulators of hematopoiesis - a roadmap for future investigations. Stem Cell Rev Rep. 2019;15(6):785–794. doi: 10.1007/s12015-019-09920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol. 2017;18:1288–1298. doi: 10.1038/ni.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamiak M, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ. Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades. Leukemia. 2017;31(1):262–265. doi: 10.1038/leu.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, Nowlan B, Cisterne A, Bendall LJ, Sims NA, Lévesque JP. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, Kucia M, Ratajczak J, Ratajczak MZ. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26(1):106–116. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24(12):1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 21.Doan PL, Chute JP. The vascular niche: home for normal and malignant hematopoietic stem cells. Leukemia. 2012;26(1):54–62. doi: 10.1038/leu.2011.236. [DOI] [PubMed] [Google Scholar]
- 22.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104(1):65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 23.Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27(1):24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25(2):211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 25.Alomari M, Almohazey D, Almofty SA, Khan FA, Al Hamad M, Ababneh D. Role of lipid rafts in hematopoietic stem cells homing, mobilization, hibernation, and differentiation. Cells. 2019;8(6):630. doi: 10.3390/cells8060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szpurka H, Schade AE, Jankowska AM, Maciejewski JP. Altered lipid raft composition and defective cell death signal transduction in glycosylphosphatidylinositol anchor-deficient PIG-A mutant cells. Br J Haematol. 2008;142(3):413–422. doi: 10.1111/j.1365-2141.2008.07203.x. [DOI] [PubMed] [Google Scholar]
- 27.Zurzolo C, Simons K. Glycosylphosphatidylinositol-anchored proteins: membrane organization and transport. Biochim Biophys Acta. 2016;1858(4):632–639. doi: 10.1016/j.bbamem.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Borkowska S, Poniewierska-Baran A, Schneider G, Suszynska M, Ratajczak J, Kucia M, Ratajczak MZ. Novel evidence that, in addition to proteolytic enzymes, lipolytic enzymes are involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) - an important pro-mobilizing role identified for hematopoietic-specific phospholipase C (PLCβ2) Blood. 2014;124:S711. doi: 10.1182/blood.V124.21.711.711. [DOI] [Google Scholar]
- 29.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 30.Karpova D, Rettig MP, Ritchey J, Cancilla D, Christ S, Gehrs L, Chendamarai E, Evbuomwan MO, Holt M, Zhang J, Abou-Ezzi G, Celik H, Wiercinska E, Yang W, Gao F, Eissenberg LG, Heier RF, Arnett SD, Meyers MJ, Prinsen MJ, Griggs DW, Trumpp A, Ruminski PG, Morrow DM, Bonig HB, Link DC, DiPersio JF. Targeting VLA4 integrin and CXCR2 mobilizes serially repopulating hematopoietic stem cells. J Clin Invest. 2019;129(7):2745–2759. doi: 10.1172/JCI124738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24(10):1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 32.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24(5):976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitz G, Boehmler AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 34.Massberg S, von Andrian UH. Novel trafficking routes for hematopoietic stem and progenitor cells. Ann N Y Acad Sci. 2009;1176:87–93. doi: 10.1111/j.1749-6632.2009.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, Pitson SM, Rettig M, DiPersio JF, Bradstock KF, Bendall LJ. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119(3):707–716. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 36.Golan K, Vagima Y, Ludin A, Itkin T, Cohen-Gur S, Kalinkovich A, Kollet O, Kim C, Schajnovitz A, Ovadya Y, Lapid K, Shivtiel S, Morris AJ, Ratajczak MZ, Lapidot T. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood. 2012;119(11):2478–2488. doi: 10.1182/blood-2011-06-358614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratajczak MZ, Kim CH, Abdel-Latif A, Schneider G, Kucia M, Morris AJ, Laughlin MJ, Ratajczak J. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26(1):63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagasawa T. A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol. 2000;72(4):408–411. [PubMed] [Google Scholar]
- 39.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19(2):257–267. doi: 10.1016/S1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 40.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 41.Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC. G-CSF regulates hematopoietic stem cell activity, in part, through activation of toll-like receptor signaling. Leukemia. 2014;28(9):1851–1860. doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012;120(12):2365–2375. doi: 10.1182/blood-2012-04-422378. [DOI] [PubMed] [Google Scholar]
- 43.Adamiak M, Bujko K, Cymer M, Plonka M, Glaser T, Kucia M, Ratajczak J, Ulrich H, Abdel-Latif A, Ratajczak MZ. Novel evidence that extracellular nucleotides and purinergic signaling induce innate immunity-mediated mobilization of hematopoietic stem/progenitor cells. Leukemia. 2018;32(9):1920–1931. doi: 10.1038/s41375-018-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2016;104:4–17. doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 45.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;25(8):661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burnstock G. Blood cells: an historical account of the roles of purinergic signalling. Purinergic Signal. 2015;11(4):411–434. doi: 10.1007/s11302-015-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bujko K, Adamiak M, Thapa A, Kucia M, Ratajczak J, Ratajczak MZ. Novel evidence that extracellular adenosine triphosphate (ATP), as a purinergic signaling mediator, activates mobilization by engaging a P2X4 ligand-gated cation channel receptor expressed on the surface of hematopoietic and innate immunity cells. Blood. 2019;134(Supplement_1):4472. doi: 10.1182/blood-2019-125858. [DOI] [Google Scholar]
- 48.Groslambert M, Py BF. Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res. 2018;25(11):359–374. doi: 10.2147/JIR.S141220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Place DE, Kanneganti TD. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenkiewicz AM, Adamiak M, Thapa A, Bujko K, Pedziwiatr D, Abdel-Latif AK, Kucia M, Ratajczak J, Ratajczak MZ. The Nlrp3 inflammasome orchestrates mobilization of bone marrow-residing stem cells into peripheral blood. Stem Cell Rev Rep. 2019;15(3):391–403. doi: 10.1007/s12015-019-09890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. The P2X7 receptor-interleukin-1 liaison. Front Pharmacol. 2017;16(8):123. doi: 10.3389/fphar.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreira-Souza AC, Marinho Y, Correa G, Santoro GF, Coutinho CM, Vommaro RC, Coutinho-Silva R. Pyrimidinergic receptor activation controls toxoplasma gondii infection in macrophages. PLoS One. 2015;10(7):e0133502. doi: 10.1371/journal.pone.0133502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riegel AK, Faigle M, Zug S, Rosenberger P, Robaye B, Boeynaems JM, Idzko M, Eltzschig HK. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117(8):2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakano M, Ito K, Yuno T, Soma N, Aburakawa S, Kasai K, Nakamura T, Takami H. UDP/P2Y6 receptor signaling regulates IgE-dependent degranulation in human basophils. Allergol Int. 2017;66(4):574–580. doi: 10.1016/j.alit.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–123. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Stachon P, Peikert A, Michel NA, Hergeth S, Marchini T, Wolf D, Dufner B, Hoppe N, Ayata CK, Grimm M, Cicko S, Schulte L, Reinöhl J, von zur Muhlen C, Bode C, Idzko M, Zirlik A. P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34(10):2237–2245. doi: 10.1161/ATVBAHA.114.303585. [DOI] [PubMed] [Google Scholar]
- 58.Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human neutrophils in auto-immunity. Semin Immunol. 2016;28(2):159–173. doi: 10.1016/j.smim.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Pae HO, Chung HT. Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune Netw. 2009;9(1):12–19. doi: 10.4110/in.2009.9.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alcaraz MJ, Fernández P, Guillén MI. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des. 2003;9(30):2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 61.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80(12):1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Adamiak M, Borkowska S, Wysoczynski M, Suszynska M, Kucia M, Rokosh G, Abdel-Latif A, Ratajczak J, Ratajczak MZ. Evidence for the involvement of sphingosine-1-phosphate in the homing and engraftment of hematopoietic stem cells to bone marrow. Oncotarget. 2015;6(22):18819–18828. doi: 10.18632/oncotarget.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115(4):501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 64.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682(1–3):48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352 Pt 3:809–815. doi: 10.1042/bj3520809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thuy AV, Reimann CM, Hemdan NY, Gräler MH. Sphingosine 1-phosphate in blood: function, metabolism, and fate. Cell Physiol Biochem. 2014;34(1):158–171. doi: 10.1159/000362992. [DOI] [PubMed] [Google Scholar]
- 67.Mierzejewska K, Klyachkin YM, Ratajczak J, Abdel-Latif A, Kucia M, Ratajczak MZ. Sphingosine-1-phosphate-mediated mobilization of hematopoietic stem/progenitor cells during intravascular hemolysis requires attenuation of SDF-1-CXCR4 retention signaling in bone marrow. Biomed Res Int. 2013;2013:814549–814545. doi: 10.1155/2013/814549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Filippin KJ, de Souza KFS, de Araujo Júnior RT, Torquato HFV, Dias DA, Parisotto EB, Ferreira AT, Paredes-Gamero EJ (2019) Involvement of P2 receptors in hematopoiesis and hematopoietic disorders, and as pharmacological targets. Purinergic Signal. 10.1007/s11302-019-09684-z [DOI] [PMC free article] [PubMed]
- 69.Wysoczynski M, Adamiak M, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ. Poor mobilization in T-cell-deficient nude mice is explained by defective activation of granulocytes and monocytes. Cell Transplant. 2017;26(1):83–93. doi: 10.3727/096368916X692221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez-Nieto D, Li L, Kohler A, Ghiaur G, Ishikawa E, Sengupta A, Madhu M, Arnett JL, Santho RA, Dunn SK, Fishman GI, Gutstein DE, Civitelli R, Barrio LC, Gunzer M, Cancelas JA. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119(22):5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adamiak M, Poniewierska-Baran A, Borkowska S, Schneider G, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Kucia M, Ratajczak J, Ratajczak MZ. Evidence that a lipolytic enzyme--hematopoietic-specific phospholipase C-β2--promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes. Leukemia. 2016;30(4):919–928. doi: 10.1038/leu.2015.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verí MC, DeBell KE, Seminario MC, DiBaldassarre A, Reischl I, Rawat R, Graham L, Noviello C, Rellahan BL, Miscia S, Wange RL, Bonvini E. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol Cell Biol. 2001;21(20):6939–6950. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ratajczak MZ, Adamiak M. Membrane lipid rafts, master regulators of hematopoietic stem cell retention in bone marrow and their trafficking. Leukemia. 2015;29(7):1452–1457. doi: 10.1038/leu.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adamiak M, Lenkiewicz AM, Cymer M, Kucia M, Ratajczak J, Ratajczak MZ. Novel evidence that an alternative complement cascade pathway is involved in optimal mobilization of hematopoietic stem/progenitor cells in Nlrp3 inflammasome-dependent manner. Leukemia. 2019;33(12):2967–2970. doi: 10.1038/s41375-019-0530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, Ratajczak J, Ratajczak MZ. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23(11):2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jalili A, Marquez-Curtis L, Shirvaikar N, Wysoczynski M, Ratajczak M, Janowska-Wieczorek A. Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells. Transfusion. 2010;50(9):2002–2010. doi: 10.1111/j.1537-2995.2010.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47(1):15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 78.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wysoczynski M, Ratajczak J, Pedziwiatr D, Rokosh G, Bolli R, Ratajczak MZ. Identification of heme oxygenase 1 (HO-1) as a novel negative regulator of mobilization of hematopoietic stem/progenitor cells. Stem Cell Rev Rep. 2015;11(1):110–118. doi: 10.1007/s12015-014-9547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adamiak M, Bujko K, Brzezniakiewicz-Janus K, Kucia M, Ratajczak J, Ratajczak MZ. The inhibition of CD39 and CD73 cell surface Ectonucleotidases by small molecular inhibitors enhances the mobilization of bone marrow residing stem cells by decreasing the extracellular level of adenosine. Stem Cell Rev Rep. 2019;15(6):892–899. doi: 10.1007/s12015-019-09918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velders GA, van Os R, Hagoort H, Verzaal P, Guiot HF, Lindley IJ, Willemze R, Opdenakker G, Fibbe WE. Reduced stem cell mobilization in mice receiving antibiotic modulation of the intestinal flora: involvement of endotoxins as cofactors in mobilization. Blood. 2004;103:340–346. doi: 10.1182/blood-2002-07-2270. [DOI] [PubMed] [Google Scholar]
- 82.Adamiak M, Abdelbaset-Ismail A, Kucia M, Ratajczak J, Ratajczak MZ. Toll-like receptor signaling-deficient mice are easy mobilizers: evidence that TLR signaling prevents mobilization of hematopoietic stem/progenitor cells in HO-1-dependent manner. Leukemia. 2016;30(12):2416–2419. doi: 10.1038/leu.2016.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basu S, Ray NT, Atkinson SJ, Broxmeyer HE. Protein phosphatase 2A plays an important role in stromal cell-derived factor-1/CXC chemokine ligand 12-mediated migration and adhesion of CD34+ cells. J Immunol. 2007;179(5):3075–3085. doi: 10.4049/jimmunol.179.5.3075. [DOI] [PubMed] [Google Scholar]
- 84.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10(4):529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10(4):463–471. doi: 10.1016/S1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 86.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 87.Onai N, Zhang YY, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96(6):2074–2080. doi: 10.1182/blood.V96.6.2074. [DOI] [PubMed] [Google Scholar]
- 88.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 89.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18(9):1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 90.Abdelbaset-Ismail A, Borkowska-Rzeszotek S, Kubis E, Bujko K, Brzeźniakiewicz-Janus K, Bolkun L, Kloczko J, Moniuszko M, Basak GW, Wiktor-Jedrzejczak W, Ratajczak MZ. Activation of the complement cascade enhances motility of leukemic cells by downregulating expression of HO-1. Leukemia. 2017;31(2):446–458. doi: 10.1038/leu.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, Turner AR, Ratajczak MZ, Janowska-Wieczorek A. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38(4):321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jing L, Tamplin OJ, Chen MJ, Deng Q, Patterson S, Kim PG, Durand EM, McNeil A, Green JM, Matsuura S, Ablain J, Brandt MK, Schlaeger TM, Huttenlocher A, Daley GQ, Ravid K, Zon LI. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J Exp Med. 2015;212:649–663. doi: 10.1084/jem.20141528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofer M, Pospisil M, Weiterova L, Hoferova Z. The role of adenosine receptor agonists in regulation of hematopoiesis. Molecules. 2011;16:675–685. doi: 10.3390/molecules16010675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frame JM, Long T, Schuster-Kubaczka C, Esain V, Lim SE, Daley GQ, North T. Inflammasome-mediated regulation of hematopoiesis in the vertebrate embryo. Blood. 2018;132:330. doi: 10.1182/blood-2018-99-117076. [DOI] [Google Scholar]
- 95.Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, Shaughnessy P, Snyder E, Bensinger W, Copelan E, Hosing C, Negrin R, Petersen FB, Rondelli D, Soiffer R, Leather H, Pazzalia A, Devine S. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308. doi: 10.1016/j.bbmt.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 96.Herbert KE, Demosthenous L, Wiesner G, Link E, Westerman DA, Came N, Ritchie DS, Harrison S, Seymour JF, Prince HM. Plerixafor plus pegfilgrastim is a safe, effective mobilization regimen for poor or adequate mobilizers of hematopoietic stem and progenitor cells: a phase I clinical trial. Bone Marrow Transplant. 2014;49(8):1056–1062. doi: 10.1038/bmt.2014.112. [DOI] [PubMed] [Google Scholar]
- 97.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, Ratajczak J, Laughlin MJ, Ratajczak MZ. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26(4):736–745. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apostolova P, Zeiser R. The role of purine metabolites as DAMPs in acute graft-versus-host disease. Front Immunol. 2016;7:439. doi: 10.3389/fimmu.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hazenberg MD, Haverkate NJE, van Lier YF, Spits H, Krabbendam L, Bemelman WA, Buskens CJ, Blom B, Shikhagaie MM. Human ectoenzyme-expressing ILC3: immunosuppressive innate cells that are depleted in graft-versus-host disease. Blood Adv. 2019;3(22):3650–3660. doi: 10.1182/bloodadvances.2019000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol. 2012;23(9):1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han KL, Thomas SV, Koontz SM, Changpriroa CM, Ha SK, Malech HL, Kang EM. Adenosine A2a receptor agonist-mediated increase in donor-derived regulatory T cells suppresses development of graft-versus-host disease. J Immunol. 2013;190(1):458–468. doi: 10.4049/jimmunol.1201325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lappas CM, Liu PC, Linden J, Kang EM, Malech HL. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol. 2010;87(2):345–354. doi: 10.1189/jlb.0609388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodrigues RJ, Tomé AR, Cunha RA. ATP as a multi-target danger signal in the brain. Front Neurosci. 2015;9:148. doi: 10.3389/fnins.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E, Zerweck A, Gärtner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat Med. 2010;12:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 105.Eser A, Colombel JF, Rutgeerts P, Vermeire S, Vogelsang H, Braddock M, Persson T, Reinisch W. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis. 2015;21(10):2247–2253. doi: 10.1097/MIB.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 106.Adamiak M, Moore JB, 4th, Zhao J, Abdelbaset-Ismail A, Grubczak K, Rzeszotek S, Wysoczynski M, Ratajczak MZ. Downregulation of heme oxygenase 1 (HO-1) activity in hematopoietic cells enhances their engraftment after transplantation. Cell Transplant. 2016;25(7):1265–1276. doi: 10.3727/096368915X688957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feig JL, Mediero A, Corciulo C, Liu H, Zhang J, Perez-Aso M, Picard L, Wilder T, Cronstein B. The antiviral drug tenofovir, an inhibitor of Pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PLoS One. 2017;12(11):e0188135. doi: 10.1371/journal.pone.0188135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jankovic D, Ganesan J, Bscheider M, Stickel N, Weber FC, Guarda G, Follo M, Pfeifer D, Tardivel A, Ludigs K, Bouazzaoui A, Kerl K, Fischer JC, Haas T, Schmitt-Gräff A, Manoharan A, Müller L, Finke J, Martin SF, Gorka O, Peschel C, Ruland J, Idzko M, Duyster J, Holler E, French LE, Poeck H, Contassot E, Zeiser R. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J Exp Med. 2013;210(10):1899–1910. doi: 10.1084/jem.20130084. [DOI] [PMC free article] [PubMed] [Google Scholar]