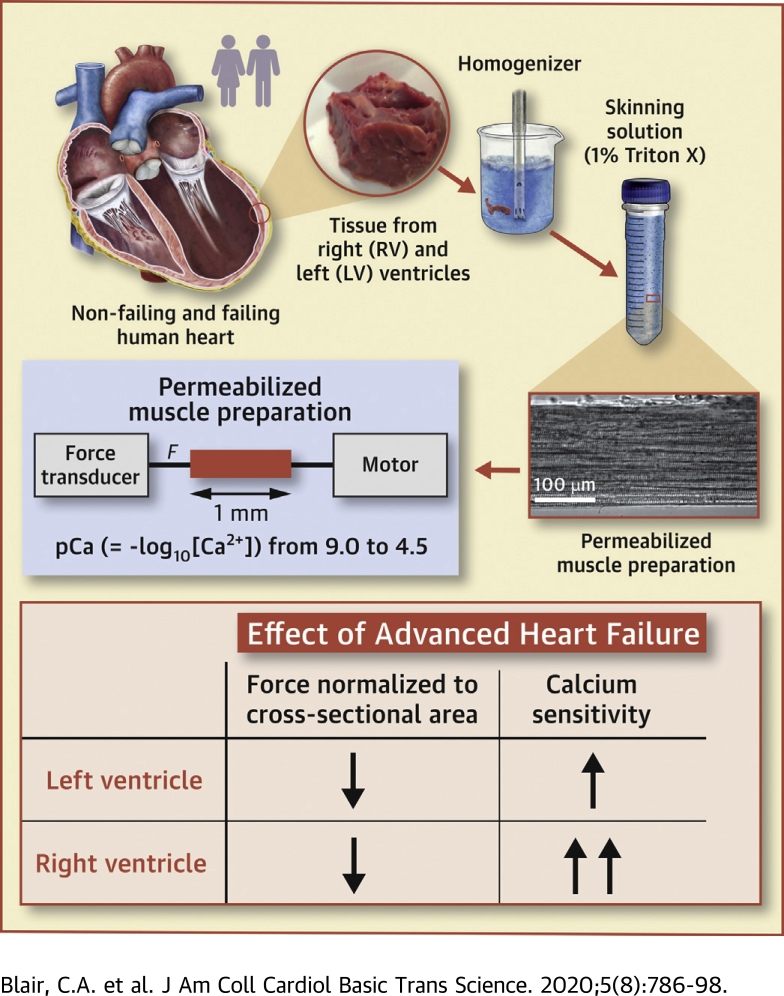
Visual Abstract
Key Words: Ca2+ sensitivity, heart failure, human myocardium, myofilament proteins, ventricular function
Abbreviations and Acronyms: Ca2+, calcium; Fpas, passive force; Fact, maximum Ca2+-activated force; ktr, rate of force recovery; LV, left ventricle; MyBP-C, myosin binding protein-C; nH, Hill coefficient; Pmax, maximum power output; PKA, protein kinase A; RLC, regulatory light chain; RV, right ventricle; TnI, troponin I; Vmax, maximum shortening velocity
Highlights
-
•
Contractile assays were performed using multicellular preparations isolated from the left and right ventricles of organ donors and patients with heart failure.
-
•
Heart failure reduced maximum force and power by approximately 30% in the myocardium from both ventricles.
-
•
Heart failure increased the Ca2+ sensitivity of contraction, but the effect was bigger in right ventricular tissue than in left ventricular samples.
-
•
The changes in Ca2+ sensitivity may reflect ventricle-specific post-translational modifications to sarcomeric proteins.
Summary
This study measured how heart failure affects the contractile properties of the human myocardium from the left and right ventricles. The data showed that maximum force and maximum power were reduced by approximately 30% in multicellular preparations from both ventricles, possibly because of ventricular remodeling (e.g., cellular disarray and/or excess fibrosis). Heart failure increased the calcium (Ca2+) sensitivity of contraction in both ventricles, but the effect was bigger in right ventricular samples. The changes in Ca2+ sensitivity were associated with ventricle-specific changes in the phosphorylation of troponin I, which indicated that adrenergic stimulation might induce different effects in the left and right ventricles.
In healthy humans, the left ventricle (LV) is a thick-walled, bullet-shaped chamber that generates approximately 120 mm Hg during systole. Conversely, the right ventricle (RV) is a thinner crescent-shaped structure that produces approximately 25 mm Hg (1,2). Laplace’s law (3) shows that wall thickness will contribute to the systolic pressure difference, but it is not known whether interventricular differences in contractile function augment the structural effect.
The impact of heart failure on cells from the LV and RV is also unclear. Although numerous studies have shown that heart failure reduces maximum force and increases the calcium (Ca2+) sensitivity of contraction of the permeabilized LV myocardium (4, 5, 6, 7, 8, 9, 10), few experiments have been performed using human RV samples. One recent study by Hsu et al. (11) demonstrated that the Ca2+ sensitivity of contraction was increased in the RV of patients who had systemic sclerosis-associated pulmonary arterial hypertension. However, these investigators were not able to study LV tissue. Previous experiments using rat models of heart failure have yielded apparently conflicting results. Belin et al. (12) demonstrated that heart failure reduced maximum force in myocytes from both ventricles. Conversely, Perreault et al. (13) showed that heart failure increased maximum force in RV tissue but had no effect on LV samples. Belin et al. (12) found that heart failure reduced Ca2+ sensitivity in the LV without affecting the RV, whereas Perreault et al. (13) observed no change in LV samples but did observe an increase in the sensitivity of RV tissue.
This paper extends these studies to the human myocardium. The data showed that heart failure reduced the maximum force and power generated by multicellular preparations from both ventricles without changing contractile kinetics. Ca2+ sensitivity was increased in failing myocardium from both ventricles, but the effect was greater for RV samples than that for LV tissue. Biochemical assays suggested that the sensitivity effects might reflect ventricle-specific modulation of the phosphorylation of cardiac troponin I (TnI).
Methods
Human samples
Myocardial samples were acquired at the University of Kentucky using a published protocol (14). Briefly, hearts from 12 patients who underwent cardiac transplantation (Supplemental Table S1) and from 5 organ donors who did not have heart failure (Supplemental Table S2) were handed to a researcher in the operating room, who then dissected samples from through-wall sections of the distal region of the LV and RV. LV samples were further dissected transmurally into sub-epicardial, mid-myocardial, and sub-endocardial specimens. RV samples were not separated transmurally because the RV wall is typically much thinner. Specimens were snap frozen in liquid nitrogen within approximately 30 min and stored in the vapor phase of liquid nitrogen until use. The University of Kentucky Institutional Review Board approved all procedures, and subjects or their legally authorized representatives gave informed consent.
Previous data from the laboratory (15) showed that heart failure had a greater impact on the contractile properties of the middle transmural region of the LV than on samples from the sub-epicardium or sub-endocardium. This study compared samples from the RV and the LV mid-myocardium to optimize the probability of detecting important effects.
Table 1 summarizes clinical characteristics and the use of medications for the patients and organ donors.
Table 1.
Summary of Clinical Characteristics and Medication Use for Patients and Organ Donors
| Heart Transplant | Organ Donor | p Value | |
|---|---|---|---|
| Age (yrs) | 48.0 ± 17.3 | 41.7 ± 15.7 | 0.494 |
| Male | 58.3 | 60.0 | 1.000 |
| Ejection fraction (%) | 24.3 ± 10.8 | 47.6 ± 15.6 | 0.006 |
| Percentage of patients/donors taking medication | |||
| β-blocker | 83.3 | 0.0 | 0.008 |
| ACE inhibitor | 83.3 | 0.0 | 0.008 |
| Statin | 66.7 | 25.0 | 0.262 |
| Aldosterone antagonist | 50.0 | 0.0 | 0.234 |
| Inotrope | 83.3 | 0.0 | 0.008 |
| Digitalis | 8.3 | 0.0 | 1.000 |
| Vasopressor | 0.0 | 50.0 | 0.050 |
| Aspirin | 75 | 25.0 | 0.118 |
Values are mean ± SD or %.
p Values for age and ejection fraction were calculated using unpaired Student's t-tests. All other p-values were calculated using Fisher’s exact tests. Data values that were unknown for a patient or organ donor were excluded from the analysis.
ACE = angiotensin-converting enzyme.
Preparations and experimental setup
Multicellular preparations were obtained by mechanical disruption of approximately 100 mg of tissue followed by chemical permeabilization (1% v/v Triton detergent). Preparations (860 ± 248 μm) from 17 patients (5 nonfailing and 12 failing hearts) were attached between a force transducer (resonant frequency, 600 Hz; Model 403, Aurora Scientific, Aurora, Ontario, Canada) and a motor (step time 0.6 ms; model 312B, Aurora Scientific) and stretched to a sarcomere length of 2.24 ± 0.01 μm in pCa (= −log10[Ca2+]) 9.0 solution. The cross-sectional area (4.05 ± 2.08 × 10−8 m2) was estimated from the width of the preparation, assuming a circular profile. Measurements were performed at 15 °C using SLControl software (16).
Force and Ca2+ sensitivity measurements
As described by Haynes et al. (15), preparations were initially activated in a solution with a saturating Ca2+ concentration of pCa 4.5. Maximum force was defined as the steady-state force measured during this trial. Subsequent trials measured force in solutions with higher pCa values. Force values were corrected for potential preparation-dependent run down using reference contractions measured in pCa 9.0 and pCa 4.5 solutions throughout the protocol.
Tension pCa curves were calculated by fitting a function of the form:
| (Eq. 1) |
to the data, where Fpas is the passive force, Fact is maximum Ca2+-activated force, nH is the Hill coefficient, and is the free Ca2+ concentration needed to develop half-maximum Ca2+-dependent force. Thirty-one of the 98 preparations studied were discarded from the analysis because the force measured in pCa 4.5 solution dropped by >30% during the protocol (n = 19), or the r2 value for the fit to the Hill curve was <0.95 (n = 12).
Power measurements
Myocardial power output was measured by allowing each preparation to shorten against pre-set loads for 80 ms in pCa 4.5 solution. Shortening velocity was calculated as the rate of change of fiber length during the final 50 ms of each force clamp. A hyperbolic equation of the form (F+a) (V+b) = (Fmax+a) b was then fitted to the dataset, where F is force, Fmax is the isometric force, and a and b are constants. Vmax, the preparation’s maximum shortening velocity, was estimated by extrapolating the equation to zero force.
Because power is the product of force and velocity, power-force curves were calculated by fitting the following function:
| (Eq. 2) |
to the experimental data. Maximum power, Pmax, was calculated as the greatest value of Equation 2. Data from the 43 preparations, in which the r2 value for the power-force fit was <0.9, were analyzed.
Biochemical assays
The phosphorylation statuses of TnI, myosin binding protein-C (MyBP-C), and myosin regulatory light chain (RLC) were analyzed using gel electrophoresis as described by Haynes et al. (15). The samples used in these gels were saved from the homogenate after the mechanical disruption and chemical permeabilization procedures were used to prepare samples for contractile assays. Phosphorylated proteins were assessed using Pro-Q Diamond (Invitrogen, Carlsbad, California). Total protein was measured using SYPRO Ruby (Invitrogen). Data from multiple gels were collated and normalized to values from a single reference control that was included in every experiment.
TnI Ser23/24 specific phosphorylation was determined by Western blot as described by Nixon et al. (17) and Salhi et al. (18) using the following antibodies: rabbit anti-phosphorylated TnI Ser-23/24 (Cell Signaling Technology, Inc., Danvers, Massachusetts), anti-rabbit Cy5-labeled secondary antibody (Jackson ImmunoResearch Laboratories, Inc, West Grove, Pennsylvania), mouse anti-cardiacT TnI antibody (clone 5; Fitzgerald, Acton, Massachusetts), and anti-mouse Cy2 labeled fluorescent secondary antibody (Jackson ImmunoResearch Laboratories).
Statistical analysis
Data were analyzed in SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) using linear mixed effects models that incorporated 2 main effects (heart failure status and ventricular region) and their interaction. Testing for the interaction determined whether the effect of 1 independent variable depended on the other. An example of an interaction is a value that decreases with heart failure in samples from the LV but increases with heart failure in samples from the RV, so that the effect of heart failure depends on the ventricle that is being studied.
As previously described (15), the linear mixed model accounted for the hierarchical nested structure of the data (values obtained from multiple preparations from different regions of the same hearts) and had greater statistical power than a standard 2-way analysis of variance test for this type of experimental design. Post hoc analyses were performed using Tukey-Kramer corrections that took into account the number of hearts and the number of samples from each heart. The p values <0.05 were considered significant. Data are reported as mean ± SEM, unless otherwise specified. Data could be clinically significant although statistical significance was not achieved, especially with smaller sample sizes.
Results
Heart failure reduces Ca2+-activated force in multicellular preparations from both ventricles
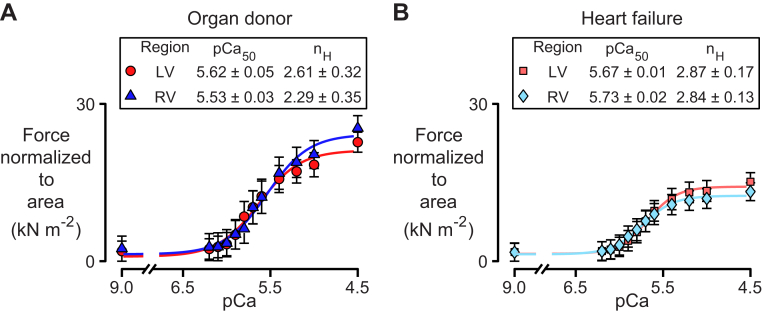
Figure 1 and Supplemental Figure 1 show force normalized to the cross-sectional area as a function of the activating Ca2+ concentration (pCa) for multicellular preparations from the LVs and RVs of organ donors and patients with heart failure. The solid lines are the best-fits of Equation 1 to the mean data values measured at each Ca2+ concentration for each type of preparation.
Figure 1.
Force Pca Curves
Data for multicellular preparations isolated from the left ventricle (LV) and right ventricle (RV) of (A) organ donors and (B) patients who had heart failure. Symbols and table entries show mean ± SEM. Data were acquired as follows: LV organ donor, 9 preparations from 4 hearts; RV organ donor, 12 preparations from 5 hearts; LV heart failure, 22 preparations from 10 hearts; RV heart failure, 24 preparations from 11 hearts. pCa = −log10[Ca2+]. nH = Hill coefficient.
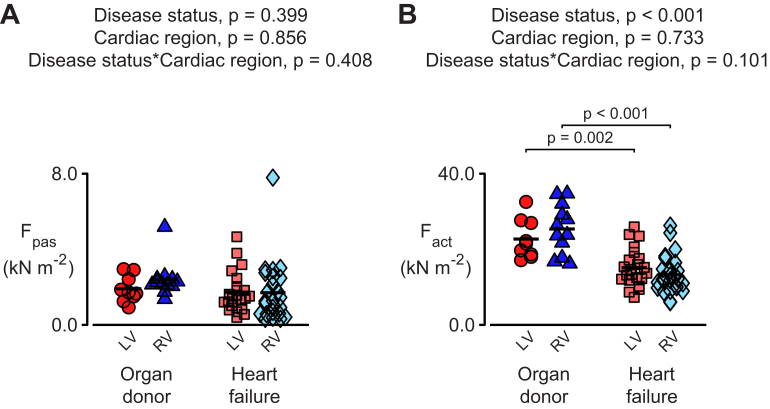
Figure 2 shows passive forces (Fpas) and Ca2+-activated forces (Fact) calculated by fitting Equation 1 to the data for each experimental preparation. The statistical analysis showed that Fpas did not depend on heart failure status or ventricular region. In contrast, Fact was reduced by >30% in samples from failing hearts (p < 0.001). Similar reductions were observed in the samples from the LVs and RVs (i.e., there was no effect of cardiac region).
Figure 2.
Heart Failure Reduces Ca2+-Activated Force in Multicellular Preparations From Both Ventricles
(A) Passive (Fpas) and (B) active (Fact) force values deduced by fitting Equation 1 to data from the individual preparations summarized in Figure 1. The total force in pCa 4.5 solution (F) is equal to Fpas+Fact. the mean r2 value for these fits was 0.979 ± 0.002. in this, and all similar Figures, data were analyzed using a linear mixed model that tested for 2 main effects (heart failure status and ventricular region) and their statistical interaction. Post hoc tests were performed using Tukey-Kramer corrections that took into account the number of hearts as well as the number of samples From each heart. Ca2+ = calcium; other abbreviations as in Figure 1.
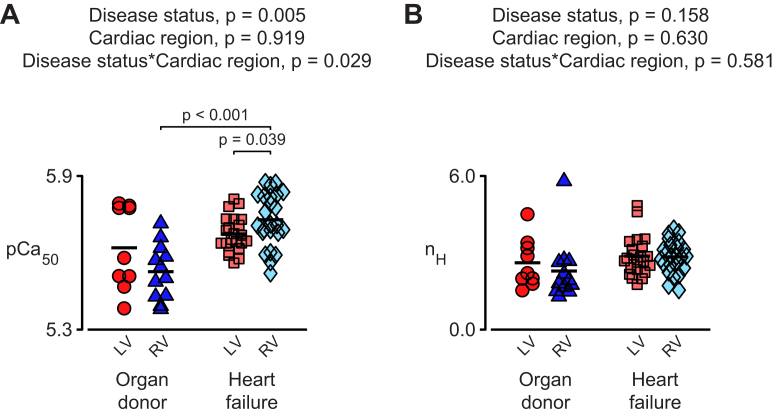
Heart failure has a greater effect on the Ca2+ sensitivity of the RV
Figure 3 shows the Ca2+ sensitivity (pCa50) and the cooperativity (nH) calculated for each multicellular preparation. The pCa50 values were higher for the failing samples than that for preparations from the organ donors (p = 0.005) but there was a statistical interaction between disease status and cardiac region (p = 0.029). The interaction meant that the increase in calcium sensitivity induced by heart failure was statistically greater in samples from the RV than in samples from the LV. In addition, the post hoc tests showed that in failing hearts, samples from the RV were more sensitive to Ca2+ than the samples from the LV (p = 0.039). No statistically significant effects were observed for the nH values.
Figure 3.
Heart Failure Has a Greater Effect on the Ca2+ Sensitivity of Myocardium From the RV
Values of (A) Ca2+sensitivity (pCa50) and (B) cooperativity (nH) deduced by fitting Equation 1 to data from the individual preparations summarized in Figure 1. Abbreviations as in Figure 1.
Heart failure reduces the power output of myocardium from both ventricles
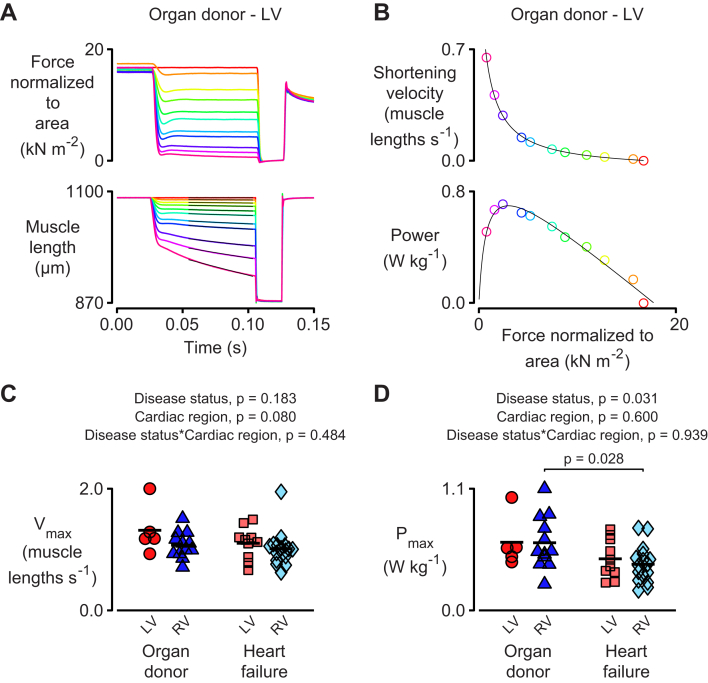
Power quantified the rate at which the myocardial preparations performed mechanical work and was calculated as the product of force and velocity. Figure 4A to 4C illustrates the experimental technique that was used to calculate the maximum shortening velocity (Vmax) and the maximum power output (Pmax) of a single representative preparation. Data from multiple preparations are shown in Figure 4D and 4E. As in the case of Fact (Figure 2B), Pmax was reduced by approximately 30% in samples from both the LVs and RVs of failing hearts. In contrast, no statistically significant effects were observed for Vmax.
Figure 4.
Heart Failure Reduces the Maximum Power Output of Multicellular Preparations From Both Ventricles
(A) Representative traces showing (top) force and (bottom) length for a multicellular preparation released to shorten against different loads. (B) Velocity and power plotted against force for these records. (C) Maximum shortening velocity and (D) maximum power for preparations from both ventricles. Data were acquired as follows: LV organ donor, 5 preparations from 3 hearts; RV organ donor, 12 preparations from 5 hearts; LV heart failure, 10 preparations from 7 hearts; RV heart failure, 16 preparations from 8 hearts. Pmax = maximum power output; Vmax = maximum shortening velocity; other abbreviations as in Figure 1.
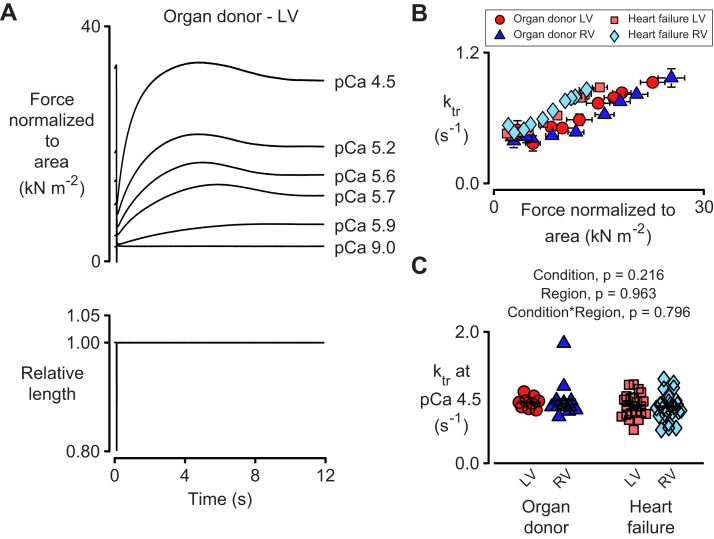
Vmax is believed to depend on cross bridge cycling kinetics (19), which can be estimated by measuring the rate of force recovery (ktr) after a quick shortening and/or re-stretch perturbation. Figure 5A shows representative records measured for a LV preparation isolated from an organ donor. Consistent with previous results from human myocardium (15), force overshot the steady-state isometric value during the recovery phase (20,21). The ktr values increased approximately linearly with steady-state isometric force in every experimental group (Figure 5B). The rates measured in maximally activating pCa 4.5 solution did not depend on heart failure status or ventricular region.
Figure 5.
Rates of Tension Recovery Do Not Depend on Heart Failure Status or Ventricular Region at Maximum Levels of Ca2+ Activation
(A) Representative traces showing (top) force normalized to cross-sectional area and (bottom) length for a preparation subjected to a rapid shortening/re-stretch protocol (20% muscle length, 20 ms hold). Note the large overshoots that are characteristic of force recovery in human myocardium. The rate of force recovery (ktr) was calculated by fitting a single exponential to the trace between the minimum force after re-stretch and the maximum force attained during the recovery. (B) ktr values plotted against isometric force. Symbols show mean ± SEM for trials in different pCa solutions. Data were acquired from the same preparations summarized in Figure 1. (C) Statistical analysis of ktr values measured in pCa 4.5 solution. Abbreviations as in Figure 1.
Data for ktr values measured at sub-maximal activation levels ranging from pCa 6.0 to pCa 5.2 are shown in Supplemental Figure S2. The kinetics were faster in the samples from patients with heart failure at some activation levels, with the difference being more pronounced in the RV. The physiological significance of these results is not yet clear.
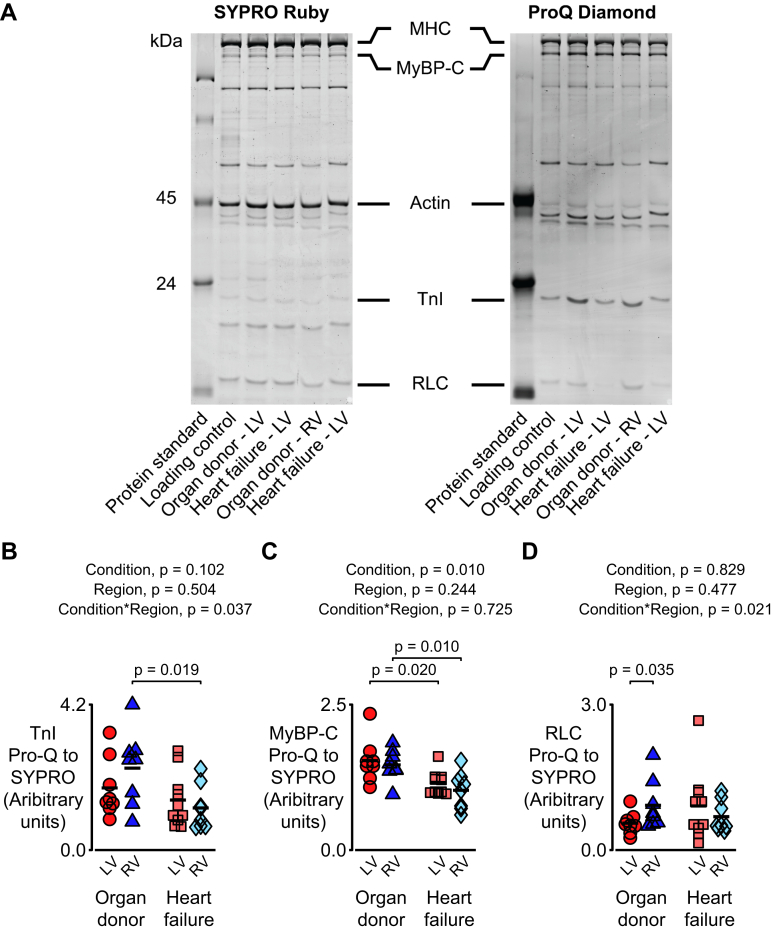
Heart failure modulates the phosphorylation of myofilament proteins
Figure 6A shows images from a representative gel that that was stained with Pro-Q Diamond to show phosphorylated proteins and then SYPRO Ruby to show total protein. Figure 6B to 6D shows data obtained from similar images quantifying the phosphorylation levels of TnI, MyBP-C, and RLC. Both TnI and RLC exhibited a statistical interaction (p = 0.037) between heart failure status and the cardiac region with the phosphorylation levels trending higher in RV samples from organ donors and lower in RV samples from patients with heart failure.
Figure 6.
Heart Failure Modulates the Phosphorylation of Myofilament Proteins
(A) Representative images from a gel stained with Pro-Q Diamond (to show phosphorylated proteins) and then SYPRO Ruby (to show total protein). Statistical analyses for (B) troponin I (TnI), (C) myosin binding protein-C (MyBP-C), and (D) regulatory light chain (RLC). Data were acquired as follows: LV organ donor, 8 samples from 4 hearts; RV organ donor, 8 samples from 4 hearts; LV heart failure, 10 samples from 9 hearts; RV heart failure, 8 samples from 8 hearts.
The data for MyBP-C showed a different pattern. No interaction was observed, but there was a main effect of heart failure status (p = 0.010). This implied that the phosphorylation of MyBP-C was lower in samples from both ventricles in failing hearts.
Supplemental Figures S3 to S7 show the relative phosphorylation of TnI, MyBP-C, and RLC plotted against pCa50, nH, Fpas, Fact, and the maximum value of ktr. Deming regression (which takes into account experimental uncertainty in both the x and y coordinates) showed that pCa50 was negatively correlated with the relative phosphorylation of both MyBP-C and RLC, and that Fact was positively correlated with the relative phosphorylation of MyBP-C.
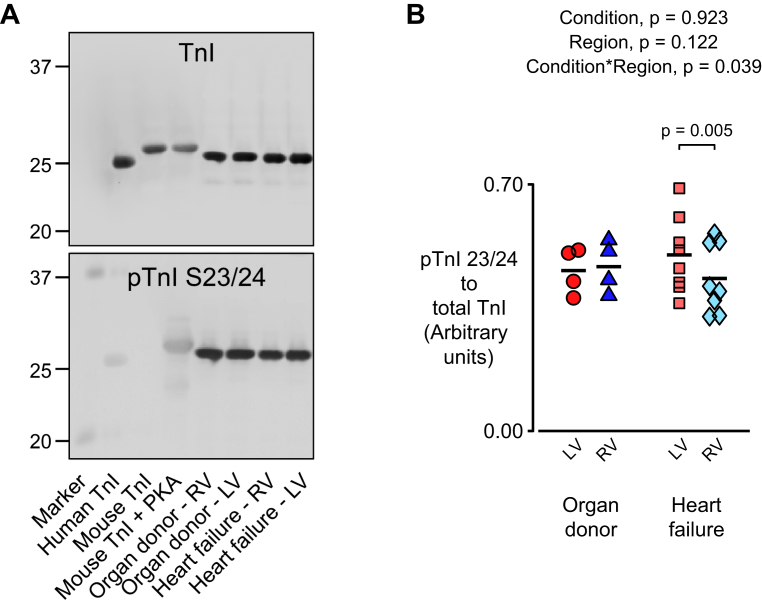
Pro-Q Diamond is commonly used to study sarcomeric proteins (22, 23, 24) but the stain is not completely specific to phosphorylated proteins and does not provide information about post-translational modifications to identified residues. Western blotting (Figure 7) was performed to address these potential limitations. As for the experiments performed using Pro-Q Diamond (Figure 6B), the phosphorylation status of TnI 23/24 exhibited a statistical interaction (p = 0.039) between heart failure status and cardiac region, which indicated that the effect of heart failure on TnI phosphorylation depends on the ventricle.
Figure 7.
Tn1 Phosphorylation Exhibits a Statistical Interaction Between Heart Failure Status and Ventricular Region
(A) Western blots probed with antibodies for total TnI (top) and (bottom) TnI phosphorylated at serine 23/24. (B) Data showing phosphorylated TnI to total TnI. Data were acquired as follows: LV organ donor, 4 samples from 4 hearts; RV organ donor, 4 samples from 4 hearts; LV heart failure, 8 samples from 8 hearts; RV heart failure, 8 samples from 8 hearts. Abbreviations as in Figures 1 and 6.
Discussion
To our knowledge, this is the first study that systematically investigated how heart failure in humans affects the contractile properties of chemically permeabilized myocardial samples from LVs and RVs. The main findings are that heart failure: 1) reduces isometric force and maximum power output in preparations from both ventricles; and 2) increases the calcium sensitivity (pCa50) of RV tissue more than that of LV tissue. Biochemical data suggested that the changes in calcium sensitivity might reflect ventricle-specific modulation of TnI phosphorylation.
Reduced isometric force and power in failing tissue may reflect ventricular remodeling
Figures 1 and 2B show that heart failure reduced maximum Ca2+-activated force by at least 30% in multicellular preparations from both ventricles. Maximum power was also reduced by a similar amount in both ventricles (Figure 4D). In principle, these effects could reflect a change in the fractional volume occupied by sarcomeres and/or alterations to cross-bridge kinetics that reduce the force-generating capacity of myosin heads in failing ventricles.
Figures 4E, 5B, and 5C show that neither Vmax nor ktr were affected by heart failure status or cardiac region at maximal levels of Ca2+ activation. Both parameters are indicative of cross-bridge kinetics and would be expected to change if the attachment and/or detachment rates of myosin were altered. Thus, the present data did not support a kinetic basis for the reduction in force. Instead, the mostly likely explanation for the approximate 30% reduction in force and power in failing samples was a decrease in the fractional volume occupied by contractile machinery.
This conclusion was consistent with a previous study by Haynes et al. (15) that compared the contractile properties of multicellular preparations from the sub-endocardial, mid-myocardial, and sub-epicardial regions of the LV free wall of organ donors and patients with heart failure. Histological data (staining with picrosirius red) included in that study showed that the collagen to tissue ratio fell from approximately 27% in mid-myocardial tissue from patients with heart failure to approximately 15% in corresponding samples from organ donors. Unfortunately, histological data from RV samples were not available for the present study. However, if the preparations had exhibited similar trends to those reported by Haynes et al., the increased fibrosis in the failing samples could account for approximately 50% of the reductions shown in Figures 1, 2B, and 4E. Other potential mechanisms that could reduce maximum contractile force in failing myocardium include disarray of myocytes and/or myofibrils (25) and changes in the fractional volume occupied by mitochondria (26). These possibilities could be tested in future work: 1) measuring the contractile function of single myocytes that do not have extracellular collagen (27); and 2) analyzing tissue samples with immunohistochemistry and electron microscopy (28).
Interventricular differences in Ca2+ sensitivity are associated with phosphorylation of TnI
Because myocardium is only transiently activated during each heart beat, its sensitivity to Ca2+ (quantified as a pCa50 value) is critically important for function. For example, all other things being equal, myocytes with higher pCa50 values will develop more contractile force in response to a given intracellular Ca2+ transient than myocytes with low Ca2+ sensitivities. Figure 3A confirmed previous work (7,29) that showed that myocardium from failing hearts is more sensitive to Ca2+ than tissue from organ donors (p < 0.005). One possibility is that the failing heart enhances Ca2+ sensitivity to try and compensate for its reduced force-generating capacity. Supplemental Figure S1 superposes force pCa curves measured with samples from organ donors and patients with heart failure. The curves nearly overlap at the sub-maximal activation levels where the heart normally operates.
The present data also revealed a new statistical interaction between heart failure status and cardiac ventricles. More specifically, heart failure increased the Ca2+ sensitivity of RV myocardium more than that of LV tissue. Figures 6 and 7 show data that provide insights into the molecular mechanisms that drive these effects. Two independent measures of TnI phosphorylation (Pro-Q Diamond staining [Figure 6B] and Western blotting with an antibody specific to Ser-23/24 phosphorylation [Figure 7B]) showed a statistical interaction between disease status and the cardiac ventricle. It is well known that phosphorylation of TnI (most notably at serine 23/24) reduces Ca2+ sensitivity (4,6,10,30, 31, 32, 33, 34, 35)); therefore, these statistical interactions are consistent with post-translational modifications to TnI contributing to the ventricle-specific effects.
Phosphorylation of RLC also exhibited a significant interaction (Figure 6C) with higher phosphorylation levels again being associated with reduced Ca2+ sensitivity. This effect is also evident in Supplemental Figure S3C, which shows the relative phosphorylation of RLC plotted against pCa50. This was a more complex relationship because increased phosphorylation of RLC was shown to enhance Ca2+ sensitivity (36,37). One possibility was that the phosphorylation of RLC changes during human heart failure partially compensated for the post-translational modifications to TnI and mitigated diastolic dysfunction. A similar conclusion was reached by van der Velden et al. (5), who observed changes in the phosphorylation of TnI and RLC in human heart failure that were similar to those shown here.
Figure 6D shows that MyBP-C was dephosphorylated in failing samples from both ventricles. This modification increased myofilament Ca2+ sensitivity (38, 39, 40, 41) and could therefore be driving the global increase in Ca2+ sensitivity observed in the failing samples (Figure 3A and Supplemental Figure S3B).
The physiological basis of the interventricular difference in Ca2+-sensitivity remains unclear. One possibility is that the ventricles are tuned to different mechanical loads. Another possibility is that they respond differently to β-adrenergic stimulation. Recent work that used Förster Resonance Energy Transfer (FRET) biosensors suggested that maximal inotropy required compartmentalized signaling responses (42). In addition, Surdo et al. (43) reported an enrichment in the expression of genes involved in adrenergic signaling in calls from the RV of healthy human donors in comparison to cells from the LV using single cell RNA-Seq. Building on this theme, Molina et al. (44) showed that in healthy dogs, the RV was more sensitive to β-adrenergic stimulation and exhibited increased activity of protein kinase A (PKA). This enzyme phosphorylates TnI (45) so differential responses to β-adrenergic stimulation could contribute to the effects shown in Figures 6B and 7B. Interestingly, Hsu et al. (11) showed that treatment with PKA equalized the Ca2+ sensitivity of RV myocardium from organ donors and in patients with systemic sclerosis-associated pulmonary hypertension, a finding that further reinforced the importance of PKA signaling in disease. However, the present data suggested that PKA was unlikely to be the only mechanism that contributed to interventricular differences because enhanced activity of the kinase in the RV should also have increased the phosphorylation of MyBP-C. This was not observed (Figure 6C).
Study limitations
Although experiments using human biospecimens sometimes have greater translational significance than studies that only use animals, they also have limitations. For example, it was exceedingly difficult to control for all of the potential confounders (including age, sex, genetic background, and comorbidities) that could have influenced the data (46). Some clinical data were also unavailable. This reduced the statistical power of tests that investigated potential relationships between cellular and organ-level function and was a particular concern for parameters related to the RV (Supplemental Table S3).
Sample processing was another concern. All of the hearts used were procured by the same personnel and cooled in ice cold saline within moments of being removed from the patient or donor (14). Nevertheless, the mean time from procurement to the sample being frozen in liquid nitrogen was approximately 30 min. As demonstrated by Walker et al. (47), this is clearly enough time to alter the post-translational status of sarcomeric proteins, which sometimes changes on a beat-to-beat basis (48).
Another issue was that that the patients and organ donors were treated with drugs that were intended to alter contractile properties. Catecholamine levels also rose during tissue procurement (46), which might have added additional complications. Again, it was difficult to know how to overcome these limitations in a translational research setting without having a negative impact on patient care.
Finally, although this study investigated the phosphorylation of several key myofilament proteins, the experiments could have missed other critical modifications. This could be addressed in future work using an unbiased approach (e.g., phosphoproteomics).
Conclusions
This study presented 3 important results. First, heart failure in humans depressed maximum contractile force and maximum power by similar amounts in myocardium from both ventricles. Second, human heart failure increased the Ca2+ sensitivity of RV myocardium more than the Ca2+ sensitivity of LV myocardium. Third, the differences in Ca2+ sensitivity were likely to involve interventricular differences in post-translational modifications to sarcomeric proteins, including TnI.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: This study showed that heart failure in humans reduced the force and power generated by myocardium from the LVs and RVs. The kinetics of the contraction (assessed as shortening velocity and the rate of force generation) were not markedly affected, so the contractile dysfunction was most likely to reflect remodeling (e.g., excess fibrosis and/or cellular disarray). In addition, heart failure increased the Ca2+ sensitivity of contraction, with the effect being greater in RV samples. These changes were associated with altered phosphorylation of TnI and MYP-C, which indicated that adrenergic signaling might induce different consequences in the LVs and RVs.
TRANSLATIONAL OUTLOOK: Although technically challenging, more studies are required to determine how the Ca2+ sensitivity of contraction varies in different regions of failing hearts. Abnormalities in these patterns might contribute to regional hypokinesia and/or hyperkinesia.
Acknowledgments
The authors acknowledge support from the Gill Cardiovascular Biorepository at the University of Kentucky and from the patients, organ donors, and families who donated samples.
Footnotes
Dr. Biesiadecki was supported by the National Institutes of Health (HL114940). Dr. Campbell was supported by the American Heart Association (GRNT25460003, TPA34860008), the National Institutes of Health (TR033173, HL133359, and HL146676. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Wang L., Yu P., Zhou B. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol. 2020;22:108–119. doi: 10.1038/s41556-019-0446-7. [DOI] [PubMed] [Google Scholar]
- 2.Friedberg M.K., Redington A.N. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 3.Kondo R.P., Dederko D.A., Teutsch C. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol. 2006;571:131–146. doi: 10.1113/jphysiol.2005.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z., Tendulkar A., Sun K. Comparison of the Young-Laplace law and finite element based calculation of ventricular wall stress: implications for postinfarct and surgical ventricular remodeling. Ann Thorac Surg. 2011;91:150–156. doi: 10.1016/j.athoracsur.2010.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velden J., de Jong J.W., Owen V.J., Burton P.B., Stienen G.J. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res. 2000;46:487–495. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 6.van der Velden J., Papp Z., Zaremba R. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 7.Kooij V., Saes M., Jaquet K. Effect of troponin I Ser23/24 phosphorylation on Ca2+-sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol. 2010;48:954–963. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papp Z., van der Velden J., Borbély A., Édes I., Stienen G.J.M. Altered myocardial force generation in end-stage human heart failure. ESC Heart Fail. 2014;1:160–165. doi: 10.1002/ehf2.12020. [DOI] [PubMed] [Google Scholar]
- 9.Knott A., Purcell I., Marston S. In vitro motility analysis of thin filaments from failing and non-failing human heart: troponin from failing human hearts induces slower filament sliding and higher Ca2+ sensitivity. J Mol Cell Cardiol. 2002;34:469–482. doi: 10.1006/jmcc.2002.1528. [DOI] [PubMed] [Google Scholar]
- 10.Brixius K., Savvidou-Zaroti P., Mehlhorn U., Bloch W., Kranias E.G., Schwinger R.H.G. Increased Ca2+-sensitivity of myofibrillar tension in heart failure and its functional implication. Basic Res Cardiol. 2002;97:I111–I117. doi: 10.1007/s003950200039. [DOI] [PubMed] [Google Scholar]
- 11.Bodor G.S., Oakeley A.E., Allen P.D., Crimmins D.L., Ladenson J.H., Anderson P.A.W. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96:1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 12.Hsu S., Kokkonen-Simon K.M., Kirk J.A. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137:2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belin R.J., Sumandea M.P., Sievert G.A. Interventricular differences in myofilament function in experimental congestive heart failure. Pflugers Arch. 2011;462:795–809. doi: 10.1007/s00424-011-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perreault C.L., Bing O.H., Brooks W.W., Ransil B.J., Morgan J.P. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ Res. 1990;67:707–712. doi: 10.1161/01.res.67.3.707. [DOI] [PubMed] [Google Scholar]
- 15.Blair C.A., Haynes P., Campbell S.G. A protocol for collecting human cardiac tissue for research. VAD J. 2016 doi: 10.13023/VAD.2016.12. Accessed August 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes P., Nava K.E., Lawson B.A. Transmural heterogeneity of cellular level power output is reduced in human heart failure. J Mol Cell Cardiol. 2014;72:1–8. doi: 10.1016/j.yjmcc.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell K.S., Moss R.L. SLControl: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol. 2003;285:H2857–H2864. doi: 10.1152/ajpheart.00295.2003. [DOI] [PubMed] [Google Scholar]
- 18.Nixon B.R., Walton S.D., Zhang B. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol. 2014;72:177–185. doi: 10.1016/j.yjmcc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salhi H.E., Walton S.D., Hassel N.C. Cardiac troponin I tyrosine 26 phosphorylation decreases myofilament Ca(2+) sensitivity and accelerates deactivation. J Mol Cell Cardiol. 2014;0:257–264. doi: 10.1016/j.yjmcc.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huxley A.F. Muscle structure and theories of contraction. Prog Biophys. 1957;7:255–318. [PubMed] [Google Scholar]
- 21.Campbell K.S. Tension recovery in permeabilized rat soleus muscle fibers after rapid shortening and restretch. Biophys J. 2006;90:1288–1294. doi: 10.1529/biophysj.105.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinett J.C., Hanft L.M., Geist J., Kontrogianni-Konstantopoulos A., McDonald K.S. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J Gen Physiol. 2019;151:645–659. doi: 10.1085/jgp.201812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei H., Jin J.P. A dominantly negative mutation in cardiac troponin I at the interface with troponin T causes early remodeling in ventricular cardiomyocytes. Am J Physiol Cell Physiol. 2014;307:C338–C348. doi: 10.1152/ajpcell.00053.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarigopula M., Davis R.T., 3rd, Mungai P.T. Cardiac myosin light chain phosphorylation and inotropic effects of a biased ligand, TRV120023, in a dilated cardiomyopathy model. Cardiovasc Res. 2015;107:226–234. doi: 10.1093/cvr/cvv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R., Correll R.N., Davis J. Cardiac-specific deletion of protein phosphatase 1beta promotes increased myofilament protein phosphorylation and contractile alterations. J Mol Cell Cardiol. 2015;87:204–213. doi: 10.1016/j.yjmcc.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houston B.A., Stevens G.R. Hypertrophic cardiomyopathy: a review. Clin Med Insights Cardiol. 2014;8:53–65. doi: 10.4137/CMC.S15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosca M.G., Hoppel C.L. Mitochondrial dysfunction in heart failure. Heart Fail Rev. 2013;18:607–622. doi: 10.1007/s10741-012-9340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanft L.M., Emter C.A., McDonald K.S. Cardiac myofibrillar contractile properties during the progression from hypertension to decompensated heart failure. Am J Physiol Heart Circ Physiol. 2017;313:H103–H113. doi: 10.1152/ajpheart.00069.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito T., Asai K., Sato S., Takano H., Mizuno K., Shimizu W. Ultrastructural features of cardiomyocytes in dilated cardiomyopathy with initially decompensated heart failure as a predictor of prognosis. Eur Heart J. 2015;36:724–732. doi: 10.1093/eurheartj/ehu404. [DOI] [PubMed] [Google Scholar]
- 30.van der Velden J., Stienen G.J.M. Cardiac disorders and pathophysiology of sarcomeric proteins. Physiol Rev. 2019;99:381–426. doi: 10.1152/physrev.00040.2017. [DOI] [PubMed] [Google Scholar]
- 31.Wolff M.R., Whitesell L.F., Moss R.L. Calcium sensitivity of isometric tension is increased in canine experimental heart failure. Circ Res. 1995;76:781–789. doi: 10.1161/01.res.76.5.781. [DOI] [PubMed] [Google Scholar]
- 32.Wolff M.R., Buck S.H., Stoker S.W., Greaser M.L., Mentzer R.M. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salhi H.E., Hassel N.C., Siddiqui J.K. Myofilament calcium sensitivity: mechanistic insight into TnI Ser-23/24 and Ser-150 phosphorylation integration. Front Physiol. 2016;7:567. doi: 10.3389/fphys.2016.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardswell S.C., Cuello F., Rowland A.J. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solaro R.J., Henze M., Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res. 2013;112:355–366. doi: 10.1161/CIRCRESAHA.112.268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaremba R., Merkus D., Hamdani N. Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomics Clin Appl. 2007;1:1285–1290. doi: 10.1002/prca.200600891. [DOI] [PubMed] [Google Scholar]
- 37.Chang A.N., Battiprolu P.K., Cowley P.M. Constitutive phosphorylation of cardiac myosin regulatory light chain in vivo. J Biol Chem. 2015;290:10703–10716. doi: 10.1074/jbc.M115.642165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kampourakis T., Sun Y.B., Irving M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc Natl Acad Sci U S A. 2016;113:E3039–E3047. doi: 10.1073/pnas.1602776113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamdani N., Borbely A., Veenstra S.P. More severe cellular phenotype in human idiopathic dilated cardiomyopathy compared to ischemic heart disease. J Muscle Res Cell Motil. 2010;31:289–301. doi: 10.1007/s10974-010-9231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kooij V., Boontje N., Zaremba R. Protein kinase C α and ε phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res Cardiol. 2010;105:289–300. doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamidi R., Gresham K.S., Li J., Stelzer J.E. Cardiac myosin binding protein-C Ser302 phosphorylation regulates cardiac β-adrenergic reserve. Science Advances. 2017;3 doi: 10.1126/sciadv.1602445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Copeland O., Sadayappan S., Messer A.E., Steinen G.J., van der Velden J., Marston S.B. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Surdo N.C., Berrera M., Koschinski A. FRET biosensor uncovers cAMP nano-domains at beta-adrenergic targets that dictate precise tuning of cardiac contractility. Nat Commun. 2017;8:15031. doi: 10.1038/ncomms15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina C.E., Johnson D.M., Mehel H. Interventricular differences in β-adrenergic responses in the canine heart: role of phosphodiesterases. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Layland J., Solaro R.J., Shah A.M. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Jweied E., deTombe P., Buttrick P.M. The use of human cardiac tissue in biophysical research: the risks of translation. J Mol Cell Cardiol. 2007;42:722–726. doi: 10.1016/j.yjmcc.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker L.A., Medway A.M., Walker J.S., Cleveland J.C., Jr., Buttrick P.M. Tissue procurement strategies affect the protein biochemistry of human heart samples. J Muscle Res Cell Motil. 2011;31:309–314. doi: 10.1007/s10974-010-9233-6. [DOI] [PubMed] [Google Scholar]
- 48.Solaro R.J., Warren C.M., Scruggs S.B. Why is it important to analyze the cardiac sarcomere subproteome? Exp Rev Proteomics. 2010;7:311–314. doi: 10.1586/epr.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.