Abstract
For young adults with acute lymphoblastic leukemia, pediatric-based regimens are likely to provide the following when compared to hyper-CVAD regimens: better disease control, less hospitalization time, diminished acute toxicities, decreased financial cost, more quality-adjusted life years, and fewer adverse late effects, such as infertility, myelodysplasia, and second malignant neoplasms. There are also reasons to expect less cardiac and cognitive dysfunction after pediatric regimens. The improved quality and quantity of life associated with pediatric regimens renders them preferable to hyper-CVAD regimens for the treatment of Philadelphia-negative B-precursor or T-cell acute lymphoblastic leukemia and lymphoblastic lymphoma in young adults.
1 |. INTRODUCTION
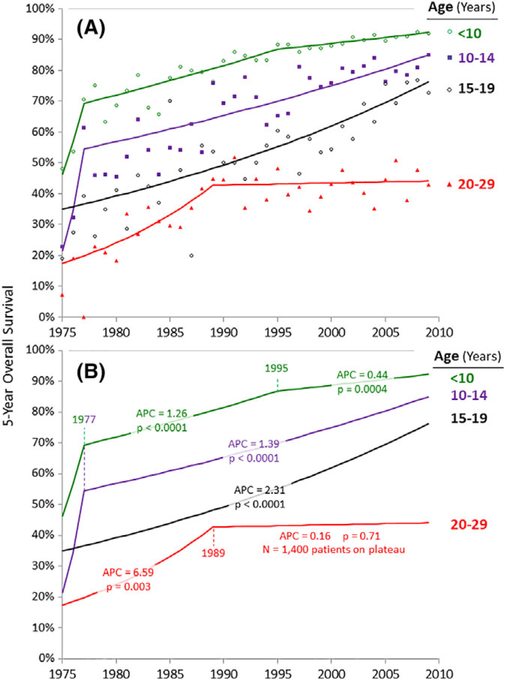
Although younger patients in the United States (US) with acute lymphoblastic leukemia (ALL) have had continuous survival improvement since the 1970s, 20–29 years old have had no evidence for progress since 1989 (Figure 1). As of 2009, the last year of available National Cancer Institute (NCI) Surveillance, Epidemiology and End-Results (SEER) data, their 5-year overall survival (OS) was an estimated 44% in comparison with 78% for 15–19 years old, who have had progressive improvement (Figure 1).1 Joinpoint analysis identifies no statistically significant change in OS of 1400 20–29 years old patients treated since 1989 (Figure 1B), whereas all of the phases of OS increase leading up to 2009 in younger patients were statistically significant (age 15–19, P < .0001; age 10–14, P < .0001; age < 10, P < .0004) (Figure 1B). Also, the incidence of ALL/LBL in increasing in the US, with more than 1300 AYAs expected to be diagnosed with these acute lymphoid malignancies in 2018.2
FIGURE 1.
Annual 5-year overall survival of children and AYAs <30 years of age, diagnosed with ALL (ICD-O-3 9801, 9826, 9827, 9831, 9835–9837) during 1975–2009, SEER9|13|18, by age.2 A, Individual data points and Joinpoint regressions.3 B, Joinpoint regressions and APC (APC, average annual percent change)/P values
Treatment regimens for ALL developed in pediatric patients should be considered preferable for AYAs to regimens historically developed in adult patients.4 Over the last half century, pediatric cooperative groups conducted >160 randomized trials to both improve efficacy and reduce toxicity of ALL therapy.4 These trials were focused on reducing acute, subacute and delayed adverse sequelae without compromising OS, as successively indicated by the continued statistically significant improvement in OS among all ALL patients <20 years of age (Figure 1B). Here we review the specific differences in survival and quality of life during and after treatment between pediatric-based and “hyper-CVAD” regimens,5 the most widely used ALL treatment in routine practice for AYAs and older adults in the US.
2 |. COMPARISON OF PEDIATRIC AND HYPER-CVAD REGIMENS
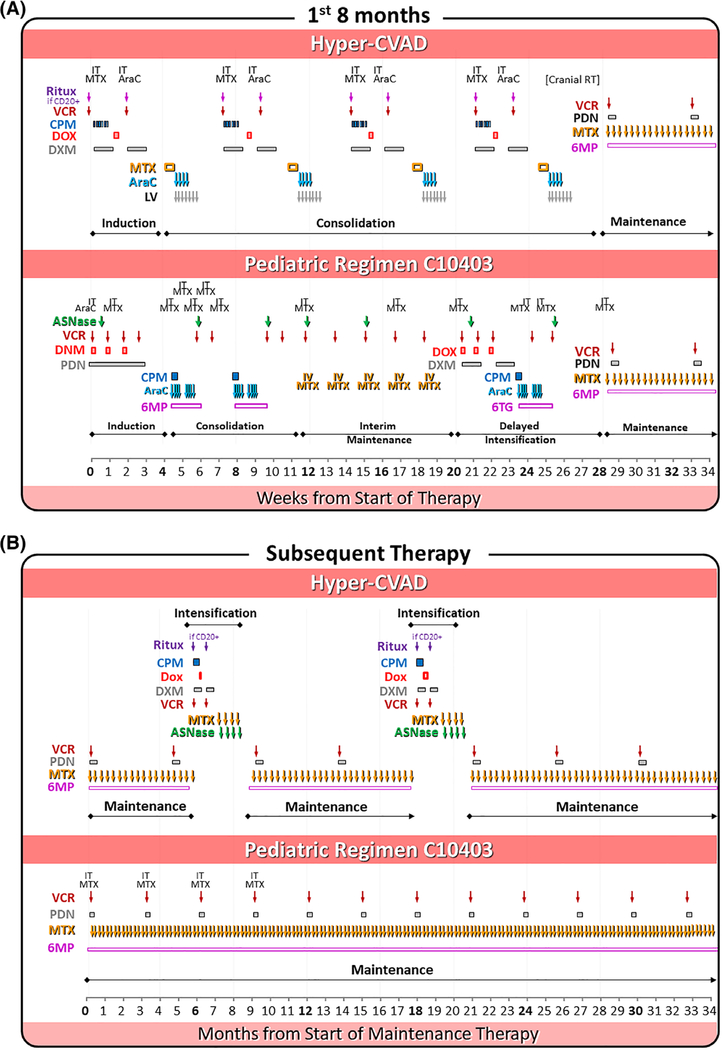
Hyper-CVAD for ALL was developed at the University of Texas M.D. Anderson Cancer Center (UTMDACC) 25 years ago5] by extrapolating from a Burkitt lymphoma regimen developed in the 1980s by the Pediatric Oncology Group and St. Jude Children’s Research Hospital.6 The upper panels of Figure 2A and 2B depict the regimen for ALL. It derives its name from combination “A”: high-dose cyclophosphamide, vincristine, doxorubicin (Adriamycin), and dexamethasone.5 Following neutrophil recovery, patients receive “B”: high-dose cytarabine and high-dose methotrexate. A and B are then repeated for a total of 8 cycles (4A + 4B) over an average of 6 months. Maintenance therapy after the 4A-B cycles consists of 2.5 years of daily oral 6-mercaptopurine, weekly oral methotrexate, and monthly vincristine and steroid pulses. In patients with ALL but not in patients with LBL, maintenance is prescribed to be interrupted during months 6–7 and 18–19 with late intensification phases:7,8 A, as previously administered, followed by 4 weekly infusions of methotrexate and asparaginase. Central nervous system (CNS) prophylaxis consists of 4–6 intrathecal doses of methotrexate and cytarabine during the A-B cycles if classified as low CNS risk and from 6 to 8 if high risk.7 Patients with overt CNS leukemia or cranial-nerve root involvement at diagnosis receive 24–30 Gy of radiation in 10–12 fractions directed to the whole brain or, for cranial nerve involvement, to the base of the skull.5 The delayed intensification and intrathecal phases are variably applied by some groups, such as withholding one or both of the delayed intensifications or administering up to 16 doses of intrathecal chemotherapy.
FIGURE 2.
Phases of therapy on hyper-CVAD (upper panel) and the pediatric C10403 (lower panel) regimens during the first 8 months (A) and subsequent therapy (B)
Hyper-CVAD has been modified over the years for different types of ALL and with attempts to either intensify therapy (including adding asparaginase)7,9,10 or reduce intensity.11 Table 1 compares the dose intensity and total cumulative doses of the basic hyper-CVAD adult regimen for Ph-ALL with the pediatric AALL0232 regimen that was applied by the intergroup (CALGB [now The Alliance], SWOG, ECOG) to adults 16–39 years of age with T-cell or B-precursor Ph-ALL in the C10403 trial.13 Total doses boldfaced and underlined identify the greatest difference between the two regimens.
TABLE 1.
Comparison of dose intensity and total cumulative dosage in pediatric andhyper-CVAD therapy regimens, with substantially greater dosages boldfaced and underlinedd
| Chemotherapy agent | Dose Unit | Pediatric (C10403) | Hyper-CVAD5,6 |
|---|---|---|---|
| Induction (4 weeksa) | Dose intensity/week | ||
| Cyclophosphamide | mg/m2 | 0 | 450 |
| Anthracycline | mg/m2 | 23 | 13 |
| (doxorubicin equiv.) | |||
| Steroid (dexamethasone equiv.) | mg/m2 | 70 | 47g |
| Vincristine | mgd | 2 | 1 |
| Asparaginase (pegylated E. coli) | IU/m2 | 625 | 0 |
| Rituximab (if CD20+) | mg/m2 | 0 | 94 |
| Intrathecal methotrexate | mg | 6 | 3 |
| Intrathecal cytarabine | mg | 18 | 25 |
| Consolidation (21 weeksb) | Dose intensity/week | ||
| Cyclophosphamide | mg/m2 | 143 | 257 |
| Anthracycline (dox equivalent) | mg/m2 | 4 | 7 |
| Steroid (dexamethasone equiv.) | mg/m2 | 7 | 33g |
| Cytarabine | mg/m2 | 86 | 2286 |
| Methotrexate IV | mg/m2 | 24 | 191 |
| 6-Mercaptopurine | mg/m2 | 80 | 0 |
| 6-Thioguanine | mg/m2 | 40 | 0 |
| Vincristine | mgd | 1.3 | 0.6 |
| Asparaginase, pegylated E. coli | IU/m2 | 714 | 0 |
| Rituximab (if CD20+) | mg/m2 | 0 | 89 |
| Intrathecal methotrexate | mg | 5 | 1.7–2.3f |
| Intrathecal cytarabine | mg | 0 | 14–29f |
| All phases (including maintenancec) | Total cumulative dose | ||
| Cyclophosphamide | mg/m2 | 3000 | 10,800 |
| Anthracycline | mg/m2 | 158 | 300 |
| (doxorubicin equiv.) | |||
| Steroid | mg/m2d | 5400 | 3400g |
| (dexamethasone equiv.) | |||
| Cytarabine | mg/m2 | 1800 | 72,000 |
| Methotrexate IV | mg/m2 | 500 | 4800 |
| Methotrexate PO | mg/m2 | 2160 | 2080 |
| 6-Mer cap to purine | mg/m2 | 58,380 | 69,900e |
| 6-Thioguanine | mg/m2 | 840 | 0 |
| Vincristine | mgd | 90 | 76 |
| Asparaginase, pegylated E. coli | IU/m2 | 17,500 | 0 |
| Asparaginase, native Erwinia | IU/m2 | 0 | 160,000* |
| Intrathecal methotrexate | mg | 132 | 48–96f |
| Intrathecal cytarabine | mg | 70 | 400–800f |
| Cranial radiation | Gy | 0–12f | 0–30f |
| Rituximab (if CD20+) | mg/m2 | 0 | 4500 |
Hyper-CVAD induction may be shorter than 4 weeks, but was assumed to be 4weeks and equivalent to the pediatrics regimen.
Pediatric: consolidation + interim maintenance + delayed intensification, Hyper-CVAD: cycles B-A-B-A-B-A-B.
Including two late intensification phases during maintenance on hyper-CVAD.
Assuming the all patients had a BSA >1.3 m2 and received the 2 mg capped dose.
No asparaginase for LBL patients.
Depending on CNS status at diagnosis.
Hyper-CVAD dose for this agent was independent of body surface area (BSA); the values are based on the assumption that the average AYA cancer patient has a BSA of 1.70 m2 based on 3613 adult cancer patients evaluated during 2005 with an average BSA of 1.79 m2 who were considerably older12; AYAs are to have an average BSA that is 5% lower.
During induction, hyper-CVAD has an alkylating agent (high-dose cyclophosphamide) and, for CD20+ patients, rituximab, but not asparaginase, which is limited to the pediatric regimen. Dose intensities for anthracycline, steroid, vincristine and intrathecal methotrexate are greater on the pediatric regimen and for intrathecal cytarabine greater on hyper-CVAD regimens. For consolidation therapy (defined in the table footnote), the dose intensity is distinctly greater on hyper-CVAD regimens, not only for the myelosuppressive agents cytarabine and methotrexate, but also for alkylating agent and anthracycline therapy. For the entire therapy, the total dose of the alkylating, anthracycline, and antimetabolite agents are all substantially greater on hyper-CVAD therapy, especially cyclophosphamide, doxorubicin, cytarabine, and methotrexate. Hyper-CVAD regimens do not include thioguanine and does include rituximab if the patient’s ALL is CD20+, the effectiveness of which has been demonstrated for hyper-CVAD14 and also in a randomized trial in adults when added to a pediatric ALL regimen.15 The lower total alkylating, anthracycline, and cytarabine exposure in the pediatric regimen derives from decades of successful attempts by pediatric oncologists to reduce the adverse subacute, delayed and late effects of their regimens in regard to gonadotoxic, cardiotoxic, neurotoxic and carcinogenic potential, the impact of each of which has been confirmed by long-term follow-up results.16–18
Hyper-CVAD regimens for ALL have been developed and applied at single- but not multi-institution investigations. Generalizing the results of multi-institutional studies is challenging19 but single institution studies are particularly vulnerable to be impacted by referral patterns, the socioeconomic status and racial/ethnicity mix of patients treated, intensity of treatment protocol application, and anticipation, intervention and management of adverse events.
3 |. DISEASE CONTROL
Six comparisons of disease control with hyper-CVAD and pediatric regimens have been published in peer-reviewed journals, all of which were retrospective (and nonrandomized). Two were reports of comparable outcome at single institutions and four reported inferior outcome with hyper-CVAD, of which two were conducted at two centers and two at single centers.
3.1 |. Similar outcomes with pediatric and hyper-CVAD regimens
One comparison was from UTMDACC where hyper-CVAD was developed. The pediatric regimen (“augmented BFM”) there was administered in a nonrandomized manner during 2006–2014 to 106 13–39 years old patients with newly diagnosed Ph-ALL. The comparison group was 102 15–40 year old patients treated with hyper-CVAD or R-hyper-CVAD at the same institution during 2002–2015 who were retrospectively selected for comparable characteristics. Among the hyper-CVAD treated patients, 40% also received rituximab (R-hyper-CVAD), since their ALL was characterized as CD20-positive, and six others received ofatumumab. A “final result” of the two patient groups and outcomes was published in 2016, with 5-year OS rates of 60% reported for both groups.20
The only reported difference in prognostic factors evaluated in the UTMDACC comparison was age distribution, with the pediatric regimen group having a median age that was 5 years younger (22 vs. 27 years). There are however several differences in the reported data that suggest the hyper-CVAD-treated patients had an overall better prognosis than the pediatric regimen group. The proportion of patients transplanted in first remission because of high-risk cytogenetic/molecular findings or high levels of minimal residual disease was 5.9% on a hyper-CVAD regimen and 10.4% on the pediatric regimen. Only one of the six transplanted hyper-CVAD patients had died at the time of analysis, vs. 4 of 11 transplanted patients on the pediatric regimen. As described in the “final results” report, the hyper-CVAD-treated patients had a lower CNS relapse rate (6.9% isolated and 10.8% concurrent with marrow relapse) than did the pediatric regimen group (8.5% isolated and 14.3% concurrent with marrow relapse) but neither of these differences were statistically significant (χ2 P = .66 and.46, respectively). Also, the “final results” report described the [concurrent] rate as similar to “published pediatric and adult series”. This comparison is inaccurate since CNS relapse in AYA ALL patients have been reported to be ≤5% on multiple pediatric-based regimens.21–24
Without censoring of the transplanted patients, there was no significant difference in the continuous complete remission rate or OS, whether the age group was less or greater than 21 years. The investigators concluded that the pediatric regimen resulted in similar efficacy but was associated with different toxicity profiles, asparaginase-induced with the pediatric regimen and myelosuppression-related with hyper-CVAD regimens. Had rituximab also been provided to CD20-positive patients treated with the pediatric regimen, the efficacy of the two regimens may have been more comparable.
A subsequent report from UTMDACC25 appears to be discrepant with the “final results” in that the 5-year OS rate of 15–39 years old with Ph-ALL treated with hyper-CVAD regimens at UTMDACC was 58% for AYAs treated during 2000–2009 and a projected 49% for those treated during 2010–2016, rates that are lower than the 60% in the final report. In addition, the report shows AYAs treated at UTMDACC for Ph-ALL to not have benefitted from hyper-CVAD regimens, in contrast to older patients. Since 1990, AYAs were the only age group among patients treated for Ph-ALL with hyper-CVAD regimens in whom survival did not improve, with overlapping OS curves out to 15 years for those treated during 1990–1999 and 2000–2009, and identical curves during 4.5 years of follow-up when AYAs diagnosed during 2000–2016 are included.25 This report is consistent with the lack of improvement in 20–29 years old shown in Figure 1.
3.2 |. Noncomparative reports in AYAs with ALL
The results of the C10403 study are consistent with the higher DFS in the pediatric regimen. In 2014, when first reported, 296 evaluable 16–39 years old AYAs with pre-B and T-cell ALL had a projected 5-year OS of 68% (95% CI 60%−74%) in a combined cooperative group setting of 36 centers in 29 states.26 These results allowed rejection of the null hypothesis of this Phase II trial that the true median EFS was, at most, 32 months, and historical control data that AYAs treated on adult cooperative group regimens had a 2-year EFS of 35%−40%.26 The updated results document the progress and were submitted for publication in June 2018. Given that 32% of the C10403 patients were obese,26 the C10403 results can be interpreted to be better than expected.
At the Princess Noorah Oncology Center in Saudi Arabian, 67 consecutive patients aged 14–35 years with Ph-ALL treated with a modified Children’s Oncology Group (COG) protocol.27 Their 4-year EFS and OS were 71.4 ± 6.0% and 81.8 ± 5.0%, respectively. In patients negative for minimal residual disease on day 29, the EFS and OS were 78.6 ± 6.8% and 90.5 ± 4.5%, respectively.
3.3 |. Statistically significant worse outcomes with hyper-CVAD
Among 73 patients <50 years of age at the King Abdullah Medical City in Saudi Arabia, 43 received a pediatric regimen and 30 were treated with a hyper-CVAD regimen. The 3-year OS was 72.6% and 48.5%, respectively (P = .04).28 In Turkey, 20 adults of 18–50 years of age treated at two centers with a pediatric Berlin-Frankfurt-Munster (BFM) regimen during 2006–2012 were compared with 30 adults of age 18–59 years of age treated with hyper-CVAD, 30% of whom were < 25 years of age.29 The mean OS was 41.5 ±6.4 months in the hyper-CVAD group and 55.1 ±4.9 months in the pediatric regimen (P = .012) and the relapse-free survival was 39.1 ±6.8 and 53.9 ±5.4 months, respectively (P = .009). Similarly, at the Beirut Medical Center in Lebanon, 38 adults were treated with hyper-CVAD and 24 adults received a pediatric BFM-like regimen.30 At a median follow-up time of 2.4 years and with 39% and 42% of the patients having undergone allogeneic stem cell transplantation in the hyper-CVAD and pediatric regimen groups, respectively, the corresponding 3-year OS and disease-free survival (DFS) rates were 71.9% vs. 76.9% (P = 0.81) and 54.7% vs. 76.4% (P = .44), respectively.
Another two-center comparison was in consecutive adult patients with ALL treated with a pediatric regimen in Pueblo, Mexico. Serving patients of low socioeconomic status, the oncologists there used a simplified St. Jude Total Therapy XII pediatric regimen with four doses of native E. coli asparaginase during induction and three doses every 3 months during maintenance.31 Eighty consecutive patients with a median age of 31 and who were as old as 86 years and treated between 1999 and 2009 had a median OS of 2.3 years and 12-year OS and DFS rates of 27% and 35%, respectively. The authors compared these results with those concurrently obtained with hyper-CVAD at a large hospital in Mexico City in 36 adult patients who had a median OS of 15 months and 5-year rate of 10%. They concluded that in comparison with hyper-CVAD, the pediatric regimen was more effective, less toxic, deliverable as an outpatient regimen, and comprised of more affordable and less expensive drugs.31 A subsequent report from Mexico City of 559 adults with ALL primarily treated with hyper-CVAD, in whom the median age was 28% and 67% were AYAs, the 3-year overall survival was 22%.32
3.4 |. T-cell ALL and LBL
T-ALL/T-LBL and B-precursor ALL are usually evaluated separately. The largest reported series of adults with T-ALL/T-LBL treated with hyper-CVAD were at Emory University, University of Alabama-Birmingham and H. Lee Moffitt Cancer Center.33 In 64 patients with a median (range) age of 30 (17–74) years, hyper-CVAD was the primary induction regimen in 56, 3 were switched to hyper-CVAD after starting another regimen, and 5 had asparaginase added to hyper-CVAD. Overall, remission was achieved in 43 (70%) patients, and at the time of analysis the median relapse-free and OS rates were 13 and 18 months, respectively, 70% had relapsed and the 2-year survival was 35%. The authors concluded that the outcomes with hyper-CVAD were poor.
In Sweden, hyper-CVAD was administered to 19 T-ALL patients of median (range) age of 32 (18–72) years.34 Hyper-CVAD was modified with betamethasone replacing dexamethasone and different late intensifications during maintenance. Of 15 patients who did not undergo transplant in first remission, 12 (80%) relapsed within 2 years, despite all but 1 completing protocol therapy. The authors recommended that hyper-CVAD not be used in Sweden for T-cell ALL.34
In contrast, the pediatric regimen has had superior results for AYAs with T-ALL in several COG studies. On CCG-1961, CCG-1882, and AALL0434, 173, 88 and 87 patients ≥16 years of age with T-ALL had 5-year EFS rates of 73%, 83% and 85%, respectively.21,35,36 The COG AALL0434 trial demonstrated that even early thymic-precursor T-ALL, historically one of the worst types of T-ALL with 5-year survival rates of 20% in children and adolescents, had a 5-year EFS >80%.37 On C10403, 71% of AYAs with T-ALL had a 68% 2-year EFS, which was comparable to the results with B-precursor ALL.26 The Princess Noorah Saudi Arabia study cited above also found no difference in T- and B-precursor ALL outcomes: EFS: 69.2 ± 7.6% vs. 75.6 ± 9.5%; P = .57; OS: 79.8 ± 6.5% vs. 85.7 ± 7.6%; P = .98; respectively.27 On the most recent COG T-ALL clinical trial (AALL0434), the overall 4-year DFS rate in children and AYAs was 89.3% (SE 1.5%), and those randomized to an outpatient methotrexate/asparaginase regimen had a superior DFS (P = .0173) compared to those randomized to a high-dose methotrexate regimen that required hospitalization for each course of treatment.36
UTDMACC investigators reported the results of 33 untreated LBL patients, 39% of whom were <26 years of age, 80% had the T-cell immunophenotype, and 70% had stage III-IV, were treated with hyper-CVAD. The projected 3-year progression-free and OS rates were 66% and 70%, respectively.8 Investigators in France treated 148 18- to 59-year-old LBL patients with a pediatric ALL protocol, >50% of whom were >34 years of age, 89% had T-lineage LBL38 and 73% had stage III-IV. The mean (95% CI) 3-year EFS and OS were 63.3% (54.2–71.0%) and 69.2% (60.0–76.7%), respectively, leading the investigators to conclude that in adults with LBL, a pediatric-like ALL regimen provide a good response rate and outcome.38
3.5 |. Hematopoeitic stem-cell transplantation
Finally, and one of the most important differences, is that the pediatric-inspired regimen usually reduces the need for hematopoietic stem-cell transplant39 and has been reported to achieve better outcomes than allografting in first remission.40 This may be due to the delayed intensification during early remission that in concept is similar to the intensification of therapy with the transplant approach.4 Being able to avoid the toxicities, late adverse effects, and financial cost of transplant substantially favors the pediatric regimen. Further, young AYA recipients are reported to be more susceptible to allogenic transplant-induced acute graft-vs.-host disease than either younger or older patients.41
4 |. ACUTE AND DELAYED TOXICITIES, TREATMENT-RELATED MORTALITY, AND ADVERSE LATE EFFECTS
Table 2 summarizes the differences in rates of adverse events and potential complications between hyper-CVAD regimens and the pediatric regimen, the former from UTMDACC and five other groups of investigators including SWOG5,20,42,44,48,50,52 and the latter based mainly on 318 AYAs treated on the C10403 trial of which 296 were fully evaluable.13 For toxicities not reported for C10403, other sources were used, including the Fertility Risk Calculator.16,17,43,45–47,49 Each of the major categories are described below.
TABLE 2.
Comparison of potential adverse events and complications of the pediatric regimen (primarily C10403) and hyper-CVAD in AYAs with ALL
| Pediatric (eg, C10403) | Hyper-CVAD | |||||
|---|---|---|---|---|---|---|
| Hospitalization after initial diagnosis | Not required | Required for 8 cycles | ||||
| Prolonged cytopenia | ± | +++ | [42] | |||
| Readmission for fever and neutropenia | ++ | [13, 43] | ++++ | [5, 44] | ||
| Infections grade 3–4 | ++ | [13] | ++++ | [20, 44] | ||
| Blood product support and ICU admissions | + | +++ | ||||
| Hypersensitivity reactions (Asparaginase) | ++ | [13] | ++ | [20] | ||
| Pancreatitis | +/++ | [13, 43, 45] | + | [20] | ||
| Hepatic dysfunction | +++ | [13, 43, 45] | +++ | [20] | ||
| Hyperbilirubinemia | ++/+++ | [13, 43, 45] | ++ | [20] | ||
| Hemorrhage | ± | [13, 43] | + | [20] | ||
| Thrombosis | +/++ | [13, 43, 45] | ++ | [20] | ||
| Neurotoxi city-central | + | [13] | ++ | |||
| Neurotoxicity-peripheral | +/++ | [13] | +/++ | [20] | ||
| Infertility | ± | [46, 47, 49] | ++++ | [48, 49] | ||
| Osteonecrosis | + | [13, 43] | + | [20] | ||
| Cardiomyopathy potential | + | [16] | ++ | ? | ||
| Secondary malignancy potential | ± | [16, 17] | ++ | [42, 50–52] | ||
| Myelodysplasia and AML | ||||||
| Financial cost | Outpatient therapyall maintenance drugs oral except vincristine | 13+ hospitalizations I drug costsonly 2 oral drugs | ||||
± rare, <1%; +, 1%-9%; ++, 10%-25%; +++, 25%-50% or moderate; ++++, >50% or severe.
4.1 |. Myelosuppression, immunosuppression, and infection
Hyper-CVAD regimens have a higher dose of all the major myelosuppressive agents (cytarabine, mercaptopurine, methotrexate, cyclophosphamide) and a greater dose of corticosteroid during consolidation with hyper-CVAD (Table 1). Thus, hospitalization for neutropenic fever and for overt infection is more common with hyper-CVAD regimens,23 originally reported to be 63% during induction therapy and 60% during consolidation (42% for B cycles and 18% for A cycles).5 In contrast, AYA patients treated with the pediatric regimen on C10403 had a 19% grade 3+ neutropenic fever rate during induction and 46% during the rest of 2.5 years of treatment.5 For AYA patients treated at COG institutions on the same regimen used in C10403, the grade 3+ neutropenic fever rate was 7% during induction and 41% during the remaining 2–3 years of therapy.13 Two attempts to superimpose asparaginase on the hyper-CVAD cycles both led to an unacceptable increase in the grade 3–5 infection rate,53,54 with 4 deaths among 11 patients entered in one trial and early termination of the trial.54
4.2 |. Therapy completion and treatment-related mortality
Less than 5% of the patients on the aforementioned C10403 pediatric trial were reported to be removed from protocol therapy because of toxicity.13,26 In contrast, 16% of Ph-ALL patients <60 years of age treated with hyper-CVAD regimens, either with or without rituximab, at UTMDACC received ≤4 of the planned 8 A-B cycles7 (Chi-square P value <.001). In two Australian centers, 29% of 58 adult patients achieving complete remission had to cease therapy prematurely.55 Unacceptable rates of treatment-related mortality (TRM) with the hyper-CVAD regimen have also occurred. A multicenter report from Turkey describes a nonrelapse mortality rate of 30% in 166 adults <65 years of age who were treated with hyper-CVAD, in comparison with a rate of 6% in 65 adults treated with another adult treatment regimen, CALGB-8811 (P = .003).56 During the 1990s, the UTMDACC-based NCI Community Clinical Oncology Program used hyper-CVAD at their sites and had to discontinue it due to a high rate of TRM (~15%, Winn R, Brown T, personal communication]. Onsite at UTMDACC, 6 of 102 (6%) patients died of myelosuppression complications while in complete remission.20 At the two Australian centers cited above, 63 adult patients treated with hyper-CVAD had an 8% induction mortality.55 In contrast, C10403 had a 2% TRM during induction in 318 AYAs13 and a 3% TRM rate overall in 296 evaluable AYAs26 and the COG regimen upon which C10403 was based had a 4.4% TRM among 601 AYAs 16–30 years of age.57 C10403s OS and low TRM is particularly impressive since 32% of the patients were obese (BMI > 30)26 and their 2-year survival rate on C10403 was 11 absolute percentage points lower than who were not obese [Stock W, personal communication, submitted for publication].
4.3 |. Hepatic dysfunction, hypercoagulability, pancreatitis
Hepatic and pancreatic dysfunction are additional risks during asparaginase therapy on pediatric regimens, especially in obese patients45 or those who concurrently consume alcohol.58 In adults aged 18–50 treated with the most intensive pegaspargase regimen (10 doses in 30 weeks), the grade III/IV hyperbilirubinemia, alanine transferase and thrombosis rates were 7%, 29% and 16%, respectively.23 In another review, 60%, 17% and 19% of 48 adults treated with pegaspargase had grade III/IV hepatic, pancreatic and thrombotic toxicities, respectively.45 In a trial that administered 6 doses of pegaspargase regardless of prior hepatotoxicity, 31.4% of 18–57 years old developed high-grade hyperbilirubinemia but 45.5% of these patients who were successfully rechallenged without recurrence of high-grade hyperbilirubinemia.59 In adults 21–55 years old, 29% of whom were >45 years of age, 13% of those who received the highest doses of pegaspargase experienced grade III/IV hepatotoxicity.60
The incidence of asparaginase-associated thrombotic complications is age dependent, with one study finding 5% of pediatric patients and 42% of adults 30 years of age or older treated with intensive asparaginase had venous thromboembolic events, and age was the only significant predictor of these events.61 In 152 adults, 18–60 years old treated with 522 doses of pegaspargase, 11.2% had venous thromboses.62 Even 11–16 years old have a greater risk of thrombosis than younger patients.63 Lower rates of coagulopathy in adult patients treated with the pegaspargase have also been reported.64 On C10403, the incidence of grade III-V thrombosis was 7.6%.13 Thus, asparaginase in the pediatric regimen significantly increases hypercoaguabilty, but most thromboses are related to central venous catheters and not life-threatening,64 treatable with 3 months of anticoagulant (eg, fondaparinux), and not recurrent with repeated pegaspargase dosing.62 A current COG cancer control study (ACCL1333) is evaluating apixiban for prevention of thrombosis during Induction, in AYAs and younger patients with central venous catheters.65
4.4 |. Central nervous system (CNS) toxicity
The amount of CNS-active therapy is also greater with hyper-CVAD regimens, including intrathecal cytarabine, intravenous high-dose methotrexate, and intravenous high-dose cytarabine (Table 1). For ~5% of adult ALL patients with CNS leukemia at diagnosis,66 cranial radiation is prescribed5 on both pediatric and hyper-CVAD regimens. However, the radiation dose on the pediatric regimen is 12–18 Gy lower than prescribed on the hyper-CVAD regimens (Table 1). CNS radiation is rarely a component of current pediatric regimens. Although there have been no reported evaluations of cognitive function in patients previously treated with hyper-CVAD regimens, its higher cumulative doses of known neurotoxic therapies may increase the risk for a significantly worse neurologic outcome. The lack of data on long-term CNS outcomes data after hyper-CVAD regimens also represents a deficiency of this treatment approach, compared with the careful monitoring and reporting of same in the pediatric regimens.
4.5 |. Infertility
Hyper-CVAD contains more than twice the cumulative dose of cyclophosphamide than the pediatric AALL0232 regimen (10.8 vs. 3.0 g/m2; Table 1). The hyper-CVAD dose is in the high potential range for rendering males with prolonged azoospermia.49 For women, the predicted amenorrhea risk post-treatment with 5 g/m2 total cyclophosphamide dose is ≥80% age 40+ and 30% to 70% aged 30–40; hyper-CVAD delivers >2× this dose.49 Of all the premenopausal women who have been treated with hyper-CVAD, only three are reported to have had successful pregnancies.48 In contrast, among AYAs 15–20 years of age at diagnosis of ALL, live births occurred in 205 of 270 females and 330 of 443 males fathered children;46 Fifteen to 20 years old are not known to be more resistant to chemotherapy infertility than older AYAs, and thus these data are likely relevant for the entire AYA age range. In another study, 135 successful pregnancies occurred in 225 females with ALL who were 15–20 years of age at diagnosis when treated with a pediatric regimen and had survived at least 5 years.47 Fertility preservation is strongly recommended for the higher total cyclophosphamide dose on hyper-CVAD regimens and not for the dose on the pediatric regimen.47
4.6 |. Cardiotoxicity
Hyper-CVAD has a total of 300 mg/m2 of doxorubicin in comparison with a cardiotoxic equivalent dose of 158 mg/m2 of doxorubicin (100 mg/m2 of daunorubicin and 75 mg/m2 of doxorubicin) on the pediatric regimen (Table 1). Although long-term comparisons of cardiomyopathies after the two regimens have not been reported, improvements in the pediatric regimen have resulted in a reduction of the cardiac mortality rate 15 years after surviving ALL for 5 years from 0.6% for children diagnosed in the 1970s to 0.1% for those diagnosed in the 1990s.16 In an attempt to reduce cardiomyopathy risk, doxorubicin in the hyper-CVAD regimens is administered over 24 h, but the effectiveness of this approach in long-term ALL survivors has not been reported.
4.7 |. Myelodysplasia and carcinogenesis
Myelodysplasia (MDS) and secondary acute myelogenous leukemia (MDS/AML) occurs more frequently after hyper-CVAD than with the pediatric type of regimen; 16 (2.5%) of 641 adults with ALL treated with hyper-CVAD at the UTMDACC, most of whom were older than AYAs, developed secondary AML or MDS in a median of 32 months after diagnosis.51 Of 125 adults consecutively treated in Australia with hyper-CVAD for ALL or non-Hodgkin lymphoma, 4 (4.4%) developed MDS/AML within 4 years.42 EBV-positive lymphoproliferative disease has occurred 2–3 years after hyper-CVAD therapy.67 Thus far, myelodysplasia and carcinogenesis have not yet been reported to be increased in AYAs treated with a pediatric regimens, with follow-up times as long as 8 years.22 Although the pediatric regimen may not have applied to AYAs for a sufficient number of years to know yet whether MDS/AML will be a problematic for them, the fact that most of the cases after hyper-CVAD occurred within 3–4 years suggests that MDS/AML after pediatric therapy should have been observed and reported by now.
Nor is there a biologic reason to expect children to be less vulnerable to MDS/AML; on the contrary this complication was the primary reason a pediatric ALL regimen was amended.68 Also, pediatric oncologists have endeavored for more than a half century to track and reduce the carcinogenicity of their ALL regimens.4 All of the alkylating, topoisomerase inhibitor and nitrosourea components of pediatric regimens during the 1970s and 1980s were subsequently removed. Of 8500 children and adolescents diagnosed with ALL during 1965–1994 and followed on the Childhood Cancer Survivor Study for at least 20 years, 153 (1.8%) died of a second malignant neoplasms.16 As the treatment regimen was modified over time to be less oncogenic, the current rate cancer is predicted to be even lower despite some evidence that 1520 years old have a higher rate than younger patients. Among 2169 children treated during 1962–1998 at St. Jude Children’s Research Hospital for newly diagnosed ALL, the 30-year cumulative secondary myeloid malignancy rate was 2.2% (SE 0.3%).69 With subsequent lower exposures to anthracyclines and exclusion of the leukemogenic topoisomerase etoposide,70 secondary MDS/AML is expected to be reduced further with current pediatric ALL regimens.71
4.8 |. Overall quality of life
One study compared total life-years and quality-associated life years (QALYs) up to 10 years after diagnosis among adults treated with either a hyper-CVAD or pediatric-inspired regimen.72 Health-state utilities were estimated based on treatment stage, with an assumption that the pediatric protocol had 0.10 disutility compared with hyper-CVAD before the maintenance phase of treatment. By year 10 after diagnosis, the pediatric regimen was associated with a 24% greater number of life-years and 32% greater number of QALYs relative to hyper-CVAD. The difference between the QALYs and life-years implies a greater quality of life during the additional years of life. The authors do not attribute this advantage of the pediatric regimen to any specific treatment factor but did conclude that, compared to the hyper-CVAD regimen, pediatric-inspired protocols led to an overall increase both in life-years and QALYs following the initial stages of treatment.
In a subsequent similar study by different investigators,73 the pediatric-inspired protocol was not only associated with improved life-years and QALYs at 5 and 10 years after diagnosis, the improvement increased with further follow-up (described as a “lifetime horizon”). The “lifetime” mean (95% CI) life-years were 11.4 (11.3–11.5) for the pediatric-inspired regimen and 6.1 (5.5–6.8) for hyper-CVAD. For QALYs, the corresponding values were 5.8 (5.4–6.1) and 3.7 (3.3–4.1) years. The 36% greater life years and 46% greater QALYs for the pediatric inspired regimen were both greater than the observed corresponding 25% and 20% differences at 10 years and 16% and 9% at 5 years in the updated outcomes. All of the differences were statistically significant.
Another concern is that the report of “final results” of the hyper-CVAD regimens20 imply that there is no plan to evaluate quality or quantity of life beyond the time of evaluation for the report. Yet, with a majority of the patients still alive when the results were tabulated, continued follow-up is necessary to address and compare the many potential life-impairing complications and consequences.
4.9 |. Hospitalization
Due to the acknowledged risk for neutropenic fever and sepsis, hyper-CVAD regimens require prolonged hospitalization for the each of the 8A and B treatments and the 2A treatments during months 6 and 18 of maintenance,7 a minimal total of 66 days for treatment administration per se. For the conventional pediatric regimens used in AYAs (eg, C10403), the only scheduled admission time is at the onset of the 29-day remission induction phase. In 4986 children treated on five ALL treatment regimens, the initial hospitalization averaged 10 days,74 which indicates that most AYAs would not need to stay in the hospital for the entire duration of induction on the pediatric regimen. In a high-risk ALL pediatric protocol, 100 1- to 17-year-old patients spent a median of 19, 0 and 0 days hospitalized during induction, consolidation, and maintenance, respectively.75 A recent effort in 24 adult patients to conduct the A phase of hyper-CVAD in the outpatient setting resulted in an average of 8 hospital days avoided.76 Hyper-CVAD regimen’s intensive myelosuppression requires most patients to be readmitted for neutropenic fever or overt infection, as described above in the Myelosuppression, Immunosuppression, Infection section.
Over decades of effort, pediatric oncologists transitioned their ALL regimens to the outpatient setting, such that with one recent exception (described below), none of their therapy for Ph-ALL and LBL requires hospitalization following initiation of induction therapy. The most intensive pediatric regimens developed by the Children’s Cancer Group for high-risk ALL required a median of <20 hospital days during 2–3 years of therapy after remission induction in 492 children and adolescents.74 An additional 155 patients who received augmented therapy because of a slow early response during induction (also performed on the C10403 trial, but without hospitalization required) required a mean of 10 more hospital days.74 In another high risk CCG trial, the median was 19, 0 and 0 days for induction, consolidation and maintenance phases, respectively.75 The exception is the current version of the COG therapy for high-risk B-precursor Ph-ALL that includes 4 high-dose methotrexate infusions during interim maintenance39 and on the average add 18 hospital days.71 In the randomized COG AALL0232 trial of the high-dose methotrexate infusions in high-risk pre-B Ph-ALL patients, the benefit was inversely proportional to age, with AYAs having had equivocal benefit from high-dose methotrexate (Stock W, Advani A, Luger S; personal communication). As described above, the reverse was true in T-ALL patients randomized to high-dose methotrexate on COG AALL0434, with the outpatient methotrexate/asparaginase regimen concluded to be better.37 The successor, adult cooperative group phase III trial (A041501) that uses the same pediatric regimen does not include inpatient high-dose methotrexate therapy.77
Although the low number of hospital days on a pediatric regimen may not be as achievable in AYAs, experience in adult patients with a pediatric regimen in the Nordic/Baltic countries suggests that hospitalization time on a pediatric regimen would be more comparable to that achieved in children on a pediatric regimen than with hyper-CVAD regimens.78 Except for thrombosis, pancreatitis and osteonecrosis, the risk of 19 specified toxicities was not enhanced by age above 10 years in 221 18- to 45-year-old and 266 10- to 17-year-old.78 There is an obvious motivation to keep children at home as much as possible, but the need for AYAs to be able to continue marital and parental responsibilities, school, employment, and access to their friends and support network must also be regarded.
The hospitalization time required by hyper-CVAD regimens is also inconsistent with the outpatient infrastructure model favored in medical oncology, with the vast majority of treatment regimens in adult oncology not requiring hospitalization for administration. In this sense, the pediatric regimen is more consistent with emphasis in general medical oncology to conduct as much of cancer therapy as feasible in the clinic and outpatient departments.
4.10 |. Financial cost
Multiple aspects of the hyper-CVAD regimen render it more expensive than the pediatric regimens. The most significant factor is the hospitalization requirement for most phases of treatment and more frequent readmissions for neutropenic fever. Except for the initial hospitalization all of the C10403 pediatric regimen can be administered in the outpatient setting and all of the maintenance therapy is oral except for vincristine and intrathecal chemotherapy. For pediatric patients treated on the AALL0232 version of C10403, the median cost of protocol therapy was calculated to be $109,000.79 The cost for adult patients would be less since pediatric patients are more expensive to treat. To hospitalize adult patients for leukemia therapy, the estimated charge, according to Avalere Health average sales pricing, is $43,500 per patient per episode80 to which the cost of hospitalizations for fever and neutropenia and other complications have to be added. The higher cumulative chemotherapy doses of hyper-CVAD add ~$56,000 to the total charge.81 An early attempt to reduce the financial cost of hyper-CVAD by administering A treatments in the outpatient clinic is estimated to have reduced gross charges by 13% (average of $2,541 per cycle).76
4.11 |. Complexity and ease of administration
Over more than a half century of hundreds of clinical trials,4 the pediatric ALL regimens have evolved into more complex, intricate, multiple-phasic, and risk-based regimens than hyper-CVAD which has remained relatively simple and easier to comprehend and administer.4 The pediatric regimens also evolved to be delivered in the outpatient setting, allowing children to be at home as much as possible. This patient-centered strategy requires a robust clinic infrastructure to support the care of outpatients who require frequent interaction with the medical system. The time for the treating medical team to arrange and administer the largely inpatient hyper-CVAD regimen is considerably less than the time required to administer the nearly all-outpatient pediatric regimen. Also, the hyper-CVAD regimens provide oncologists with more control over therapy adherence, particularly for AYAs who are independent of family support and generally less compliant with oral medications and outpatient appointments. One advantage of treating AYAs in comparison with older adults is their ability to tolerate therapy with less frequent toxicities and need to adjust dosage, interrupt therapy, and be hospitalized for complications.82
5 |. NATIONAL COMPREHENSIVE CANCER NETWORK ( NCCN) GUIDELINES
Since 2012, the NCCN has recommended either a clinical trial or a pediatric-inspired regimen for newly diagnosed Ph-ALL in AYAs.83 The clinical trial recommendation for AYAs was based, in part, on the likelihood that a clinical trial would be based on pediatric therapy.83 In 2016, the NCCN added hyper-CVAD with rituximab (R-hyperCVAD) to its AYA ALL Guidelines but specified that it was for CD20-positive ALL only and that pediatric regimens for all forms of Ph-ALL were “preferred”. In 2017, the NCCN Guidelines expanded hyper-CVAD to all Ph-AYA and added a pediatric-inspired University of Southern California regimen with the specification that both were based on data from single institutions as opposed to the pediatric regimens that were based on data from multi-institutional or cooperative group studies.84
6 |. CONCLUSIONS AND RECOMMENDATIONS
The challenge in the US is to convince those using hyper-CVAD in AYAs to adopt a pediatric regimen, as has been applied to adult ALL patients in Spain, France, Germany, the Netherlands, Sweden, Finland, Denmark, Italy, United Kingdom, Czech Republic, and Turkey,4 or to enter the patient on a pediatric-inspired clinical trial such as Alliance A041501 that opened in 2017.85 Ideally, a prospective randomized controlled trial comparing hyper-CVAD with a pediatric-inspired regimen should be conducted to help resolve the remaining controversy. Meanwhile, based on the data presented here, the lack of survival improvement in AYAs in the US with ALL (Figure 1) can be explained, in part, by adult-inspired treatment delivered on medical oncology services and at community cancer treatment sites.86
Oncologists who care for adults are less familiar with risk stratification, administration of complicated ALL regimens, and management of these regimens’ toxicities compared with pediatric oncologists.86 AYAs are often more challenging to manage than older adults, given their psychosocial situations, student or parental responsibilities, and economic limitations.87 ALL therapy in AYAs disrupts school, work and family life, which in turn results in nonadherence to treatment and follow-up appointments.86,88
Since the majority of hyper-CVAD treated patients are in community cancer centers and private practices that treat most of the adult ALL patients, the primary challenge will be to target practicing oncologists and hematologists. Whether in a private practice or at an academic medical center, patients considering hyper-CVAD should be informed of the potentially greater risk of infertility, myelodysplasia, second malignant neoplasm, hospitalization requirement, and financial encumbrance. We conclude that, in absence of a randomized trial, the pediatric regimen is preferable to a hyper-CVAD regimen for AYA ALL patients when critical factors including quality and quantity of life, as well economic impact, are considered. We agree with the most recently published algorithm85 that recommends 18–40 years old adults with newly diagnosed B-precursor or T-cell ALL either be enrolled on Alliance A041501 or treated with another pediatric regimen. On balance, the pediatric approach is superior for AYAs from the standpoint of both superior survival and fewer late effects, as well as lower burden of therapy.
ACKNOWLEDGMENTS
S. Siegel and A. Bleyer created the original drafts and integrated recommendations from Anjali Advani, Nita Seibel, Lori Muffly, Wendy Stock, Selina Luger, David Freyer, Dan Douer, Rebecca Johnson, Daniel DeAngelo, Brandon Hayes-Lattin, Mark Lewis, Jerry Jaboin, Bijal Shah, and Peter Coccia.
CONFLICT OF INTERESTS
Drs. Muffly has research support from Shire Pharmaceuticals. Dr. Advani has research support and honoraria from Pfizer and Amgen, and prior consulting remuneration from Jazz and Sigma-Tau Pharmaceuticals. Dr. Johnson serves as a consultant and on the speaker bureau of Shire Pharmaceuticals and Jazz Pharmaceuticals, which produce asparaginase products. Dr. DeAngelo received funding from Shire Pharmaceuticals. Dr. Douer had served as consultant to Shire Pharmaceuticals. Dr. Hayes-Lattin was on a speaker’s bureau for Sigma-Tau Pharmaceuticals. Dr. Shah has research grant support from Incyte, Rosetta Genomics, and the DeBartolo institute for Personalized Medicine and has received reimbursement from Amgen, Baxalta, Bayer, Celgene, Clonoseq, Jazz and Pfizer pharmaceutical companies. Dr. Bleyer served as a consultant to Enzon and Sigma-Tau Pharmaceuticals when they had an asparaginase product, which is one of the agents included in this review. The following (in alphabetical order) are not aware of any conflicts of interest with the contents or messaging of the manuscript: Drs. Coccia, Freyer, Jaboin, Lewis, Reed, Siegel.
Footnotes
SWOG, Children’s Oncology Group, The Alliance, ECOG-ACRIN, and NRG Oncology Group belong to National Cancer institute-Sponsored National Clinical Trials Network Cooperative Group
REFERENCES
- 1.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission. [Google Scholar]
- 2.McNeer JL, Bleyer A. Acute lymphoblastic leukemia and lymphoblastic lymphoma in adolescents and young adults. Pediatr Blood Cancer. 2018;65(6):e269892 10.1002/pbc.26989. [DOI] [PubMed] [Google Scholar]
- 3.Joinpoint Regression Program, Version 4.1.1. August 2014; Statistical Research and Applications Branch, National Cancer Institute. [Google Scholar]
- 4.Siegel S, Stock W, Johnson R, et al. Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia-negative acute lymphoblastic leukemia: a review of advantages and challenges. JAMA Oncol. 2018;15:725–734. 10.1002/pbc.26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. [DOI] [PubMed] [Google Scholar]
- 6.Hutchison RE, Murphy SB, Fairclough DL, et al. Diffuse small non-cleaved cell lymphoma in children, Burkitt’s versus non-Burkitt’s types. Results from the pediatric oncology group and St. Jude Children’s Research Hospital. Cancer. 1989;64(1):23–28. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104(6):1624–1630. [DOI] [PubMed] [Google Scholar]
- 9.Abaza Y,M, Kantarjian H, Faderl S, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol. 2018;93(1):91–99. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Park JH, Yoon SY, Cho YH, Lee MH. A pilot study of daunorubicin-augmented hyper-CVAD induction chemotherapy for adults with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2018;81(2):393–398. [DOI] [PubMed] [Google Scholar]
- 11.Jalaeikhoo H, Rajaeinejad M, Keyhani M, Zokaasadi M, Dehghani Firoozabadi MM. Effectiveness of modified hyper-CVAD chemotherapy regimen in the treatment of adult acute lymphoblastic leukemia: a retrospective experience. Cancer Med. 2018;7(3):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS One. 2010;5(1):e8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Advani AS, Sanford B, Luger S, et al. Frontline-treatment of acute lymphoblastic leukemia in older adolescents and young adults using a pediatric regimen is feasible: toxicity results of the prospective US intergroup trial C10403 (Alliance). Am Soc Hematol: Blood. 2013; 122(121):3903. [Google Scholar]
- 14.Thomas DA, Rytting ME, O’Brien SM, et al. Outcome for adolescents and young adults with the hyper-CVAD (with or without rituximab) regimens for de novo acute lymphoblastic leukemia or lymphoblastic lymphoma. Blood. 2009;114:2037 (American Society of Hematology Annual Meeting Abstract 3084).19567878 [Google Scholar]
- 15.Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016;375(11): 1044–1053. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of children with cancer. N Engl J Med. 2016; 364(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia S Late effects among survivors of leukemia during childhood and adolescence. Blood Cells Mol Dis. 2003;31(1):84–92. [DOI] [PubMed] [Google Scholar]
- 18.Gibson TM, Mostoufi-Moab SM, Stratton K, et al. Temporal trends in chronic disease among survivors of childhood cancer diagnosed across three decades: a report from the childhood cancer survivor study (CCSS). J Clin Oncol. 2017;35(Suppl):abstr LBA10500. [Google Scholar]
- 19.Bleyer A. In and out, good and bad news, of generalizability of SWOG treatment trial results. JNCI. 2014;106(3):dju027 Epub 2014 Mar 13. [DOI] [PubMed] [Google Scholar]
- 20.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M. Swedish adult acute lymphocytic leukemia group; Swedish childhood leukemia group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer. 2006;107(7):1551–1561. [DOI] [PubMed] [Google Scholar]
- 22.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29(3): 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. [DOI] [PubMed] [Google Scholar]
- 24.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the program Espanol de Tratamiento en Hematologia pediatric-based protocol ALL-96. J Clin Oncol. 2008;26: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki K, Jabbour EJ, O’Brien SM, et al. Outcome of patients with Philadelphia chromosome-negative acute lymphoblastic leukemia by age group over 35 years: a single institution experience. Blood. 2016;128: 3975 American Society of Hematology Annual Meeting Abstract 3975. [Google Scholar]
- 26.Stock W, Luger SM, Advani AS, et al. Favorable outcomes for older adolescents and young adults with acute lymphoblastic leukemia: early results of U.S. intergroup trial C10403. Blood. 2014;124(21):796 American Society of Hematology Annual Meeting Abstract 796. [Google Scholar]
- 27.Eldadah S, Jastaniah W, Alsaeed A, et al. Treatment of adolescents and young adults with Philadelphia negative acute lymphoblastic leukemia using a risk and response based protocol with extended intensification and high dose methotrexate: a report from a single center in Saudi Arabia. J Clin Oncol. 2017;35(Suppl):abstr e18527. [Google Scholar]
- 28.Alabdulwahab AS, Elsayed HG, Sherisher MA, Zeeneldin A, Alghamdi K, Elbjeirami WM. The Dana Farber consortium protocol (DFCP) vs. classic hyper-CVAD for treatment of acute lymphoblastic leukemia in patients <50 years. Single institution experience. Leuk Res. 2017;60:58–62. [DOI] [PubMed] [Google Scholar]
- 29.Alacacioglu I, Medeni SS, Ozsan GH, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? A retrospective analysis of the outcomes of BFM and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60(4):219–22325. [DOI] [PubMed] [Google Scholar]
- 30.El-Cheikh J, El Dika I, Massoud R, et al. Hyper-CVAD compared with BFM-like chemotherapy for the treatment of adult acute lymphoblastic leukemia. A retrospective single-center analysis. Clin Lymphoma Myeloma Leuk. 2017;17(3):179–185. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Delgado GJ, Macías-Gallardo J, Lutz-Presno JA, et al. Outcome of adults with acute lymphoblastic leukemia treated with a pediatric-inspired therapy: a single institution experience. Leuk Lymphoma. 2011;52(2):314–316. [DOI] [PubMed] [Google Scholar]
- 32.Crespo-Solis E, Espinosa-Bautista K, Alvarado-Ibarra M, et al. Survival analysis of adult patients with ALL in Mexico City: first report from the acute leukemia workgroup (ALWG) (GTLA). Cancer Med. 2018;7: 2423–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kota VK, Hathaway AM, Shah BD, et al. Poor outcomes with hyper CVAD induction for T-cell lymphoblastic leukemia/lymphoma. Blood. 2015;126:3762 American Society of Hematology Annual Meeting Abstract 3762. [Google Scholar]
- 34.Kozlowski P, Astrom M, Ahlberg L, Bernell P, et al. High relapse rate of T cell acute lymphoblastic leukemia in adults treated with hyper-CVAD chemotherapy in Sweden. Eur J Haematol. 2014;92: 377–381. [DOI] [PubMed] [Google Scholar]
- 35.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s oncology group. Blood. 2008;111(5):2548–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter SS, Devidas M, Chen S, et al. Capizzi-style methotrexate with pegasparagase is superior to high-dose methotrexate in T-lineage acute lymphoblastic leukemia: results from Children’s oncology group (COG) AALL0434. Blood. 2015;126:794 American Society of Hematology Annual Meeting Abstract. [Google Scholar]
- 37.Wood BL, Winter SS, Dunsmore KP, et al. T-ALL shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype, and validation of the prognostic value of end-induction minimal residual disease (MRD) in COG AALL0434. Blood. 2014;124:1 American Society of Hematology Annual Meeting Abstract.24993873 [Google Scholar]
- 38.Lepretre S, Touzart A, Vermeulin T, et al. Pediatric-like acute lymphoblastic leukemia therapy in adults with lymphoblastic lymphoma: the GRAALL-LYSA LL03 study. J Clin Oncol. 2016;34(6): 572–580. [DOI] [PubMed] [Google Scholar]
- 39.Isakoff MS, Freyer DF, Bleyer A. Young adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen do not need a bone marrow transplant in first remission. Blood. 2013;121: 5253–5255. [DOI] [PubMed] [Google Scholar]
- 40.Seftel MD, Neuberg D, Zhang MJ, et al. Pediatric-inspired therapy compared to allografting for Philadelphia chromosome-negative adult ALL in first complete remission. Am J Hematol. 2016;91(3): 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vignon M, Andreoli A, Dhédin N, et al. Graft-versus-host disease in adolescents and young adults (15–24 years old) after allogeneic hematopoietic stem cell transplantation for acute leukemia in first complete remission. J Adolesc Young Adult Oncol. 2016;6:299–306. [DOI] [PubMed] [Google Scholar]
- 42.Gill S, Lane SW, Crawford J, et al. Prolonged haematological toxicity from the hyper-CVAD regimen: manifestations, frequency, and natural history in a cohort of 125 consecutive patients. Ann Hematol. 2008; 87:727–734. [DOI] [PubMed] [Google Scholar]
- 43.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s oncology group study AALL0232. J Clin Oncol. 2016;34(20): 2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol. 2012;24: 1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract. 2017;24:299–308. [DOI] [PubMed] [Google Scholar]
- 46.Childhood Cancer Survivor Study. Pregnancy Outcomes: All Pregnancies in Female Survivors or Female Partners of Male Survivors https://ccss.stjude.org/content/dam/en_US/shared/ccss/documents/data/data-bk-pregnancy-base-m.pdf. Accessed August 1, 2017.
- 47.Chow EJ, Stratton KL, Leisenring, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the childhood cancer survivor study cohort. Lancet Oncol. 2016;17(5):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shehadri T, Hourigan MJ, Wolf M, Mollee PN, Seymour JF. The effect of the hyper-CVAD chemotherapy regimen on fertility and ovarian function. Leuk Res. 2006;30(4):483–485. [DOI] [PubMed] [Google Scholar]
- 49.Fertility Risk Tool. https://www.teamlivestrong.org/we-can-help/fertility-services/risks. Accessed February 15, 2018.
- 50.Buyukasik Y, Acar K, Kelkitli E. Hyper-CVAD regimen in routine management of adult acute lymphoblastic leukemia: a retrospective multicenter study. Acta Haematol. 2013;130:199–205. [DOI] [PubMed] [Google Scholar]
- 51.Verma D, O’Brien S, Thomas D, et al. Therapy-related acute myelogenous leukemia and myelodysplastic syndrome in patients with acute lymphoblastic leukemia treated with the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimens. Cancer. 2009;115(1):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romaguera JE, Fayad L, Rodriguez M, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23: 7013–7023. [DOI] [PubMed] [Google Scholar]
- 53.Faderl S, Thomas DA, O’Brien S, et al. Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy. Clin Lymph Myeloma Leuk. 2011;11(1):54–59. [DOI] [PubMed] [Google Scholar]
- 54.Pegaspargase and combination chemotherapy in treating patients with newly diagnosed acute lymphoblastic leukemia. https://clinicaltrials.gov/ct2/show/results/NCT01005914?term=01005914&rank=1. Accessed August 4, 2018.
- 55.Morris K, Weston H, Mollee P, Marlton P, Gill D, Kennedy G. Outcome of treatment of adult acute lymphoblastic leukemia with hyperfractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone/methotrexate, cytarabine: results from an Australian population. Leuk Lymphoma. 2011;52(1):85–91. [DOI] [PubMed] [Google Scholar]
- 56.Alacacioglu I, Medeni SS, Ozsan GH, et al. Is the BFM regimen feasible for the treatment of adult acute lymphoblastic leukemia? A retrospective analysis of the outcomes of BFM and hyper-CVAD chemotherapy in two centers. Chemotherapy. 2014;60(4):219–223. [DOI] [PubMed] [Google Scholar]
- 57.Larsen EC, Salzer W, Nachman J, et al. Treatment toxicity in adolescents and young adult patients compared with younger patients treated for high risk B-precursor acute lymphoblastic leukemia a report from the Children’s oncology group study AALL0232. Blood. 2011;118:1510 American Society of Hematology Annual Meeting Abstract 1510. [Google Scholar]
- 58.Stock W, Douer D, Deangelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymph. 2011;52(12):2237–2253. [DOI] [PubMed] [Google Scholar]
- 59.Burke PW, Aldoss I, Lunning MA, Devlin SM. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res. 2018;66:49–56. 10.1016/j.leukres.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Minetto P, Bisso N, Guolo F, et al. Patient and therapy-related factors affecting the toxicity of pegylated-asparaginase for the treatment of adult acute lymphoblastic leukemia. Am Soc Hematol: Blood. 2017;130: 1297 Abstr 1297. [Google Scholar]
- 61.Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aldoss I, Douer D, Behrendt CE, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol. 2016;96(4): 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appel IM, Hop WC, van Kessel-Bakvis C, Stigter R, Pieters R. L-Asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost. 2008; 100(2):330–337. [PubMed] [Google Scholar]
- 64.Piatkowska-Jakubas B, Krawczyk-Kulís M, Giebel S, et al. Use of L-asparaginase in acute lymphoblastic leukemia: recommendations of the group. Pol Arch Med Wewn. 2008;118(11):664–669. [PubMed] [Google Scholar]
- 65.A study of the safety and effectiveness of apixaban in preventing blood clots in children with leukemia who have a central venous catheter and are treated with asparaginase. https://clinicaltrials.gov/ct2/show/NCT02369653?term=apixaban&recrs=ab&cond=leukemia&cntry=US&rank=1. Accessed August 4, 2018.
- 66.Ko SY, Chi HS, Jang S, Park CJ. Morphologic detection of blast cells in the cerebrospinal fluid at diagnosis of adult acute lymphoblastic leukemia appears to be associated with adverse prognosis. Int J Lab Hematol. 2014;36(4):451–458. [DOI] [PubMed] [Google Scholar]
- 67.Luskin MR, Roy DB, Wasik MA, Loren AW. Development of lymphomas containing Epstein-Barr virus after therapy with hyper-CVAD regimen. Clin Lymph Myeloma Leuk. 2014;14(2):e55–e58. [DOI] [PubMed] [Google Scholar]
- 68.Winick NJ, McKenna RW, Shuster JJ, et al. Secondary acute myeloid leukemia in children with acute lymphoblastic leukemia treated with etoposide. J Clin Oncol. 1993;11(2):209–217. [DOI] [PubMed] [Google Scholar]
- 69.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297(11):1207–1215. [DOI] [PubMed] [Google Scholar]
- 70.Smith MA, Rubinstein L, Ungerleider RS. Therapy-related acute myeloid leukemia following treatment with epipodophyllotoxins: estimating the risks. Med Pediatr Oncol. 1994;23(2):86–98. [DOI] [PubMed] [Google Scholar]
- 71.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL consortium. Eur J Cancer. 2011;47(9):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guzauskas GF, Villa KF, Vanhove GF, Fisher VL, Veenstra DL. Risk-benefit analysis of pediatric-inspired versus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone protocols for acute lymphoblastic leukemia in adolescents and young adults. J Adolesc Young Adult Oncol. 2017;6(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hooper E, Hu X, Lin PL, DeAngelo D. QoL of pediatric-inspired compared to hyper-CVAD regimens for newly diagnosed AYA patients with Ph-ALL: a modeling analysis. 2017 American Society of Clinical Oncology annual meeting. J Clin Oncol. 2017;35(Suppl):abstr e22002. [Google Scholar]
- 74.Gaynon PS, Bostrom BC, Hutchinson RJ, et al. Duration of hospitalization as a measure of cost on Children’s cancer group acute lymphoblastic leukemia studies. J Clin Oncol. 2001;19(7):1916–1925. [DOI] [PubMed] [Google Scholar]
- 75.Steinherz PG, Gaynon P, Miller DR, et al. Improved disease-free survival of children with acute lymphoblastic leukemia at high risk for early relapse with the New York regimen: a new intensive therapy protocol: a report from the Childrens’ cancer study group. J Clin Oncol. 1986;4(5):744–752. [DOI] [PubMed] [Google Scholar]
- 76.Kubal TE, Salamanca CR, Chavez JC, et al. Transition of intensive chemotherapy for acute lymphoblastic leukemia to the outpatient setting: analysis of safety and cost savings. Blood. 2015;126:5604. [Google Scholar]
- 77.A phase III trial to evaluate the efficacy of the addition of inotuzumab ozogamicin (a conjugated anti-CD22 monoclonal antibody) to frontline therapy in young adults (ages 18–39 years) with newly diagnosed precursor B-cell ALL. NCT03150693 https://clinicaltrials.gov/ct2/show/NCT03150693?term=alliance&recrs=ab&cond=acute+lymphoblastic+leukemia&cntry=US&rank=2. Accessed August 4, 2018.
- 78.Toft N, Birgens H, Abrahamsson, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2017. 32, 606–615. [DOI] [PubMed] [Google Scholar]
- 79.DiNofia AM, Seif AE, Devidas M, et al. Cost comparison by treatment arm and center-level variations in cost and inpatient days on the phase III high-risk B acute lymphoblastic leukemia trial AALL0232. Cancer Med. 2018;7(1):3–12. https://onlinelibrary.wiley.com/doi/10.1002/cam4.1206. Accessed August 4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Total Cost of Cancer Care by Site of Service: Physician office vs outpatient hospital, March 2012. Prepared by Avalere Health. https://www.communityoncology.org/pdfs/avalere-cost-of-cancer-care-study.pdf; Accessed August 4, 2018.
- 81.Chemotherapy Average Sales Price, Medicare. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2016ASPFiles.html. Accessed August 4, 2018.
- 82.Schwandt A, Harris P, Hunsberger S, et al. The role of age on dose-limiting toxicities in phase I dose-escalation trials. Clin Cancer Res. 2014;20(18):4768–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.How BA. NCCN guidelines can help young adults and older adolescents with cancer and the professionals who care for them. J Natl Compr Canc Netw. 2012;10(9):1065–1071. [DOI] [PubMed] [Google Scholar]
- 84.Brown PA, Shah B, Fathi A, et al. NCCN guidelines insights. Acute lymphoblastic leukemia. Version 1.2017. J Natl Compr Canc Netw. 2017; 15(9):1091–1102. [DOI] [PubMed] [Google Scholar]
- 85.Muffly LS, Reizine N, Stock W. Management of acute lymphoblastic leukemia in young adults. Clin Adv Hematol Oncol. 2018;16(2):138–146. [PubMed] [Google Scholar]
- 86.Wolfson JA, Richman J, Sunn C-L, et al. Understanding causes of inferior outcome in AYAs with ALL in a real-world setting. Am Soc Hematol Blood. 2017;130:352 Abstr 300. [Google Scholar]
- 87.Boissel N, Baruchel A. Acute lymphoblastic leukemia in adolescent and young adults: treat as adults or as children? Blood. 2018;132: 351–361. [DOI] [PubMed] [Google Scholar]
- 88.Aldoss IT, Marcucci G, Pullarkat V. Treatment of acute lymphoblastic leukemia in adults: applying lessons learned in children. Oncology (Williston Park). 2016;30(12):1080–1091. [PubMed] [Google Scholar]