Soluble forms of HIV-1 envelope trimers exhibit conformational heterogeneity and undergo CD4-induced (CD4i) exposure of epitopes of non-neutralizing antibodies that can potentially hinder induction of broad neutralizing antibody responses. These limitations have been mitigated through recent structure-guided approaches and include trimer-stabilizing mutations that resist trimer conformational transition and exposure of CD4i epitopes. The use of small-molecule viral inhibitors that allosterically block CD4 binding represents an alternative strategy for stabilizing Env trimer in the pre-CD4-triggered state of both soluble and membrane-bound trimers. In this study, we report that the viral entry inhibitor BMS-626529 restricts trimer conformational transition and improves the immunogenicity of select Env trimer immunogens.
KEYWORDS: BMS 626529, envelope trimer, SOSIP trimer, antigenicity, human immunodeficiency virus, immunogenicity, viral entry inhibitor
ABSTRACT
Small-molecule viral entry inhibitors, such as BMS-626529 (BMS-529), allosterically block CD4 binding to HIV-1 envelope (Env) and inhibit CD4-induced structural changes in Env trimers. Here, we show that the binding of BMS-529 to clade C soluble chimeric gp140 SOSIP (ch.SOSIP) and membrane-bound trimers with intact transmembrane domain (gp150) prevented trimer conformational transitions and enhanced their immunogenicity. When complexed to BMS-529, ch.SOSIP trimers retained their binding to broadly neutralizing antibodies (bNAbs) and to their unmutated common ancestor (UCA) antibodies, while exposure of CD4-induced (CD4i) non-bNAb epitopes was inhibited. BMS-529-complexed gp150 trimers in detergent micelles, which were isolated from CHO cells, bound to bNAbs, including UCA and intermediates of the CD4 binding site (bs) CH103 bNAb lineage, and showed limited exposure of CD4i epitopes and a glycosylation pattern with a preponderance of high-mannose glycans. In rabbits, BMS-529-complexed V3 glycan-targeting ch.SOSIP immunogen induced in the majority of immunized animals higher neutralization titers against both autologous and select high mannose-bearing heterologous tier 2 pseudoviruses than those immunized with the noncomplexed ch.SOSIP. In rhesus macaques, BMS-529 complexed to CD4 bs-targeting ch.SOSIP immunogen induced stronger neutralization against tier 2 pseudoviruses bearing high-mannose glycans than noncomplexed ch.SOSIP trimer immunogen. When immunized with gp150 complexed to BMS-529, rhesus macaques showed neutralization against tier 2 pseudoviruses with targeted glycan deletion and high-mannose glycan enrichment. These results demonstrated that stabilization of Env trimer conformation with BMS-529 improved the immunogenicity of select chimeric SOSIP trimers and elicited tier 2 neutralizing antibodies of higher potency than noncomplexed trimers.
IMPORTANCE Soluble forms of HIV-1 envelope trimers exhibit conformational heterogeneity and undergo CD4-induced (CD4i) exposure of epitopes of non-neutralizing antibodies that can potentially hinder induction of broad neutralizing antibody responses. These limitations have been mitigated through recent structure-guided approaches and include trimer-stabilizing mutations that resist trimer conformational transition and exposure of CD4i epitopes. The use of small-molecule viral inhibitors that allosterically block CD4 binding represents an alternative strategy for stabilizing Env trimer in the pre-CD4-triggered state of both soluble and membrane-bound trimers. In this study, we report that the viral entry inhibitor BMS-626529 restricts trimer conformational transition and improves the immunogenicity of select Env trimer immunogens.
INTRODUCTION
Induction of high titers of neutralizing antibody (nAb) responses against both autologous and heterologous HIV-1 viruses is the desired goal of current HIV envelope (Env) immunogen designs (1, 2). Recent development of HIV-1 immunogens include germ line-targeting Env trimers that present epitopes that are more avidly bound by unmutated common ancestors (UCAs) of broadly neutralizing antibodies (bnAbs) (3–6). However, non-neutralizing epitope targets that include the CD4-induced (CD4i) and V3 (third variable) loop epitopes on Env gp120 can pose hindrance to the induction and development of autologous nAb responses (7–10). We previously described a clade C, the globally predominant HIV-1 subtype, transmitted/founder (TF) Env protein (CH505TF), isolated from an HIV-1-infected subject (CH505) from Africa. CH505TF Env proteins bind to the UCAs of the developed CD4 binding site CH103 and CH235 bnAb lineages and are potential immunogens for initiating autologous nAb responses in small animals and rhesus macaques (5, 11–13). Antigenicity and immunogenicity were improved with the inclusion of trimer-stabilizing mutations (E64K and A316W) that block exposure of both CD4i and V3 loop epitopes (14) in the CH505TF chimeric SOSIP (ch.SOSIP) that includes BG505 gp41 (5). However, ch.SOSIP trimers with the above-described stabilizing mutations are not resistant to CD4-induced conformational exposure of CD4i and V3 loop epitopes, and such non-bnAb epitopes could potentially be exposed in vivo following immunization. Thus, in addition to the inclusion of additional amino acid substitutions to more stably prevent exposure of non-bnAb epitopes, other strategies may improve the immunogenicity of Env trimer immunogens and enhance autologous nAb responses (10, 15, 16).
One alternate strategy for stabilizing Env trimer conformation and suppression of CD4-induced conformational changes is the use of low-molecular-weight viral entry inhibitors (17–19). The Bristol Myers Squibb (BMS) viral entry inhibitors are a class of small-molecule entry inhibitors that potently neutralize HIV-1 variants of diverse clades (20, 21). BMS-626529 (BMS-529 in the text here) is the active component of the prodrug BMS-663068 (22). Unlike CD4, BMS-529 does not induce large conformational changes in gp120 (18). The conformations of both intact Env trimers on the viral surface and soluble stabilized trimers, as defined in terms of single-molecule fluorescence resonance energy transfer (smFRET) states, has been reported to be stabilized in the low FRET (pretriggered) state 1 conformation by binding to both bnAbs and the BMS-529 molecule (23). Binding of CD4 lowers the activation barrier of the transition from the pretriggered Env to an intermediate and the CD4-bound conformations (24, 25). However, BMS-529 and related compounds bind to an induced pocket that is distinct from the CD4-induced Phe43 (F43) cavity (20). Structural data revealed that of three gp120 aromatic residues (W69, W112, and W427) that contribute to CD4 binding (26), W427 adopts different conformations in the CD4- and BMS-529-bound states (20), this suggests that BMS-529 can allosterically hinder CD4-induced conformational changes. Furthermore, the binding of bnAbs, including CD4 binding site (bs) bnAbs are not precluded on SOSIP trimers when complexed to BMS-529 (20, 27). Thus, the use of small-molecule viral entry inhibitors such as BMS-529 to stabilize Env trimers in a state that is resistant to CD4-induced conformational changes could improve immunogenicity of trimers and favor induction of nAb responses.
Here, we studied two clade C Env trimers (CH505TF ch.SOSIP and CH848.10.17.DT ch.SOSIP) (5, 28) to determine whether BMS-529 can stabilize the conformation of trimers and enhance antigenicity for binding to bnAbs and key UCAs. Second, we investigated whether BMS-529-induced stabilization of either soluble ch.SOSIP trimers or gp150 trimers in detergent micelles isolated from CHO cell membranes would improve immunogenicity in both rabbits and rhesus macaques. We report that both CH505TF and CH848.10.17.DT ch.SOSIP trimers, when complexed to BMS-529, retained binding to bnAbs and UCAs but resisted soluble CD4 (sCD4)-induced exposure of non-bnAb epitopes. In both rabbits and macaques, the BMS-529-complexed ch.SOSIP immunogens showed improved immunogenicity in eliciting nAbs against both tier 2 autologous and select heterologous pseudoviruses.
RESULTS
Stabilization of Env SOSIP trimers by BMS-529.
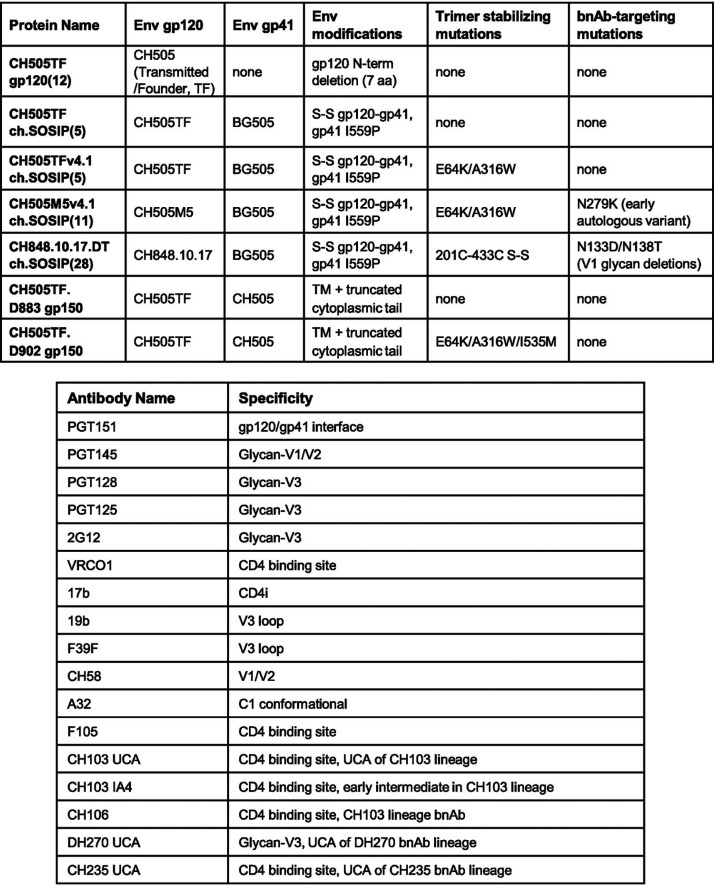
As a strategy to inhibit sCD4 binding and exposure of CD4i/V3 epitopes, we used CH505TF gp140 ch.SOSIP trimers with (CH505TFv4.1) and without (CH505TF) stabilizing mutations (Table 1) and studied the effect of BMS-529 binding to both ch.SOSIP trimers. At high concentrations of BMS-529 (30 μM, 50-fold molar excess of BMS-529 to gp140), sCD4 binding to ch.SOSIP gp140 trimers was completely inhibited (Fig. 1A). As previously reported for BG505 gp140 SOSIP (20), BMS-529 strongly inhibited the binding of non-bnAbs, including the V3 loop and CD4i-binding monoclonal antibodies (MAbs), to CH505TF ch.SOSIP (Fig. 1B). However, the binding of sCD4 resulted in the complete exposure of V3/CD4i epitopes in ch.SOSIP trimers with stabilizing mutations (Fig. 1C). Following a titration of BMS-529 concentrations, we determined that BMS-529 at 10 μM completely blocked V3/CD4i epitope exposure, even in the presence of sCD4 at a concentration that fully triggered gp120 conformational changes (Fig. 1C). The BMS-SOSIP complex was stable and retained the antigenicity profile favoring bnAb binding for at least 7 days at 4°C. Thus, CH505TFv4.1 ch.SOSIP, when complexed to BMS-529, is resistant to sCD4-induced conformational changes and shows diminished binding of non-bnAbs.
TABLE 1.
Protein constructs and antibodies used in the study
FIG 1.
Exposure of CD4-induced epitopes on Env ch.SOSIP trimers complexed to BMS-529. (A) Binding of soluble CD4 (sCD4) to CH505 ch.SOSIP trimers with or without BMS-529. Overlaid SPR sensorgrams show binding of unstabilized (CH505TF, top) and stabilized (CH505TFv4.1, bottom) ch.SOSIP trimers to sCD4. SOSIP trimers (at concentrations of 200 nM or 600 nM) were preincubated with 50-fold molar excess of BMS-529 and injected over sCD4 immobilized to the SPR sensor chip. (B) Binding of CH505TF ch.SOSIP trimers to indicated non-bnAbs (MAb gp120 epitopes used were 17b-CD4i, 19b-V3 loop, F39F-CD4-bs, CH58-V2, and A32-C1) with (solid bars) or without (hatched bars) BMS-529. Binding ratio was calculated by BLI measurements of each MAb binding and normalized to that of PGT151 (binding ratio = binding of MAb [nM]/binding of PGT151 [nM]). (C) Binding of CH505TFv4.1 ch.SOSIP trimers complexed to BMS-529 (10 μM) to immobilized CD4i MAb 17b (top) or to V3 loop MAb 19b (bottom). Binding of ch.SOSIP trimers was also measured in the presence of saturating concentration (600 nM) of sCD4 (TFv4.1+sCD4) to measure full exposure of CD4i epitopes and compared to trimers preincubated with 10 μM BMS-529 (TFv4.1+CD4+BMS). SPR maximal binding responses in RU (response units) plotted are after a 300-s injection of ch.SOSIP trimers at 200 nM.
Using a panel of bnAbs and non-bnAbs, we studied two clade C Env ch.SOSIP gp140 proteins (Table 1) with trimer-stabilizing mutations (29), CH505TFv4.1 and CH848.10.17.DT, and with each trimer complexed to BMS-529. The two ch.SOSIP proteins were designed to target the CD4 binding site (CH505TFv4.1) or the V3 glycan (CH848.10.17.DT) bnAb epitopes and bound to the UCAs of their respective bnAb lineages with high nanomolar affinities (12, 28, 30). Binding of BMS-529 to either ch.SOSIP trimer did not disrupt the binding of any of the classes of bnAbs tested (Table 1), while the masking of several non-bnAb epitopes (CD4i, V3, CD4 binding site [bs], C1 conformational) was retained (Fig. 2A and B). Next, we tested three different ch.SOSIP trimers (CH505TFv4.1, CH505M5v4.1, and CH848.10.17.DT) (Table 1) complexed to BMS-529 for binding to UCAs of CD4 bs (CH103 and CH235 bnAbs) and V3 glycan DH270 bnAb lineages that we have previously described (12, 13, 31). The binding of each of the UCAs to their respective ch.SOSIP trimer when complexed to BMS-529 was retained (Fig. 2C to F). However, the CD4 bs UCAs showed varied results. While the binding of CH103 UCA (Fig. 2E) was reduced compared to that of the CH505TFv4.1 ch.SOSIP without BMS-529, a slight improvement in the binding of CH235 UCA (Fig. 2C) to the BMS-SOSIP complex was observed. Compared to that of the untreated ch.SOSIP trimer, binding of DH270 UCA to the CH848.10.17.DT trimer when complexed to BMS-529 remained unchanged (Fig. 2F). Similarly, the binding of CH235 UCA to CH505M5v4.1, which is a higher-affinity interaction than CH235 binding to CH505TFv4.1 ch.SOSIP trimers (6), was fully retained when the trimer was complexed to BMS-529 (Fig. 2D). Together, these results showed that ch.SOSIP trimers, when complexed to BMS-529, are resistant to sCD4-induced conformational changes and exposure of non-neutralizing V3/CD4i epitopes, while the binding of the bnAbs and UCAs of bnAb lineages was retained.
FIG 2.
Antigenicity of CH505TFv4.1 and CH848.10.17.DT ch.SOSIP trimers. (A) Binding of bnAbs and non-bnAbs to CH505TFv4.1 ch.SOSIP with or without BMS-529. SOSIP trimers at 240 nM were preincubated with 100 μM BMS-529 prior to measuring SOSIP binding to the indicated MAbs immobilized on BLI sensor tips. Binding ratio of each MAb is normalized to the maximal binding observed with the trimer specific bnAb PGT151 (binding ratio = binding of MAb [nM]/binding of PGT151 [nM]) and measured by BLI analysis. (B) Binding ratio of bnAbs and non-bnAbs to CH848.10.17.DT ch.SOSIP with and without BMS-529 and measured as described above. (C to F) Overlaid SPR curves show binding of the UCAs of CD4-bs and glycan V3 bnAb lineages to ch.SOSIP trimers complexed (solid curves) or noncomplexed (dashed curves) to BMS-529. Binding curves shown are of CH235 UCA (CD4-bs) to CH505TFv4.1 and CH505M5v4.1 ch.SOSIP trimers (C and D, respectively), CH103 UCA (CD4-bs) to CH505TFv4.1 ch.SOSIP (E), and DH270 UCA to CH848.10.17.DT ch.SOSIP (F). Each UCA MAb was immobilized onto a CM5 SPR sensor chip, and ch.SOSIP trimers at 475 nM preincubated with 100 μM BMS-529 were injected over the MAbs for an association length of 300 s. Data shown are representative of two independent experiments.
Antigenicity of membrane-bound gp150.
To study the effect of BMS-529 binding to membrane-bound trimers, we purified detergent-solubilized CH505TF trimers (CH505TF.D883 gp150) from CHO cell membrane by detergent solubilization of the membrane fraction from cell lysate and trimer-specific PGT145 bnAb affinity chromatography. While biochemical and biophysical analyses of the membrane-bound CH505TF gp150 showed it to be in a micelle-associated trimeric configuration and able to bind to trimer-specific bnAbs (Fig. 3A to D), binding of non-nAbs, including those to the V3 loop, was also observed (Fig. 3D). N-Linked glycan analysis of CH505TF.D883 gp150 showed a predominance of high-mannose over processed glycans (Fig. 3E), notably, in the V1V2 and V3 regions, compared to that of CH505TF gp120. These results are consistent with the reported glycan profiles of both soluble SOSIP and membrane-anchored Env trimers (32, 33).
FIG 3.
Biochemical properties and antigenicity of CH505TF gp150 transmembrane (TM) trimers. (A) Coomassie blue-stained native PAGE analysis of detergent-solubilized CH505TF.D883 gp150. Thyroglobulin (669 kD) and ferritin (440 kD) size standards are included for comparison. (B) Size exclusion profile of CH505TF.D883 gp150 trimers run on Superdex 200 increase 10/300 GL column and with size standards and gp120 monomer peak indicated by arrows. (C) A representative negative-stain electron microscopy (EM) micrograph of CH505TF.D883 gp150. (D) Binding of different classes of bnAbs and non-bnAbs to CH505TF gp150 by BLI analysis (top). The bottom panel shows binding of CH103_UCA and CH106 bnAb and binding of CD4i-MAb 17b with and without soluble CD4. (E) Site-specific glycan profiles of CH505TF gp120 and CH505TF gp150 showing relative abundance of high mannose (red bars) versus processed (blue bars) glycans at specified sites. (F) Antigenicity of CH505TF.D902 gp150 binding to bnAbs with or without BMS-529. CH505TF gp150 at 240 nM were preincubated with 100 μM BMS-529 prior to measuring gp150 binding to the indicated MAbs immobilized on BLI sensor tips. Binding ratio of each MAb is normalized to the maximal binding observed with the trimer specific bnAb PGT145 (binding ratio = binding of MAb [nM]/binding of PGT145 [nM]) and measured by BLI analysis. (G to J) Exposure of CD4-induced epitopes on Env gp150 trimers complexed to BMS-529. SPR curves show binding of CH505TF.D883 and CH505TF.D902 gp150 trimers (100 nM) with (black curves) or without (red curves) sCD4 (600 nM) to the CD4i MAb 17b (G) or to the V3 loop MAb 19b (H). Binding of CH505TF.D883 and CH505TF.D902 gp150 trimers (10 nM) complexed to (green curves) or without (black curves) BMS-529 (100 μM) to the CD4i MAb 17b (I) or to the V3 loop MAb 19b (J). CH505TF.D902 gp150 trimers were expressed with E64K/A316W/I535M mutations and CH505TF.D883 gp150 without any mutation.
Stabilization of membrane-bound gp150 with BMS-529.
Next, we used two approaches for the stabilization of gp150 trimer conformational flexibility that previously gave favorable results with CH505TFv4.1 ch.SOSIP trimers: (a) inclusion of trimer-stabilizing (E64K/A316W/I535M) mutations and produced CH505TF.D902 gp150, and (b) formation of a complex of CH505.D902 gp150 with the small-molecule entry inhibitor (BMS-529).
Unlike CH505TF.D883 gp150, CH505TF.D902 gp150 with stabilizing mutations (Table 1) showed markedly diminished binding to CD4i MAb 17b (Fig. 3G). The binding of the V3 MAb 19b was also reduced in CH505TF.D902 compared to that in CH505TF.D883 gp150 (Fig. 3H). As observed with CH505TFv4.1 ch.SOSIP, preincubation with sCD4 resulted in complete exposure of the 17b CD4i MAb epitope on both gp150 trimers (Fig. 3G). Unlike the CH505TFv4.1 ch.SOSIP trimer, the V3 loop was not completely locked down in the gp150 trimer counterpart with the same trimer-stabilizing mutations. However, no further increase in 19b binding to CH505TF.D902 gp150 was observed following incubation with sCD4 (Fig. 3H).
To test whether the CH505TF gp150 could be made resistant to the exposure of CD4i epitopes, gp150 proteins were preincubated with BMS-529 and the antigenicity of the complexes analyzed. Binding to the CD4i MAb 17b was either strongly inhibited or remained completely blocked in the BMS-complexed form of CH505TF.D883 or CH505TF.D902 gp150 proteins, respectively (Fig. 3I). In contrast, BMS-529 did not completely block V3 loop exposure in the gp150 trimer forms (Fig. 3J). Binding of several bnAbs was retained in the BMS-bound CH505TF.D902 gp150 (Fig. 3F). These results showed that the CD4i epitope and V3 loop exposure in membrane-bound gp150 proteins can be restricted without impacting bnAb binding when the Env trimer is complexed to the small-molecule inhibitor BMS-529. However, unlike that observed for CH505TFv4.1 ch.SOSIP, the V3 lockdown as assessed by 19b MAb binding was not complete in the BMS-529-complexed CH505TF.D902 gp150 proteins.
Affinities of CD4 binding site bnAb lineage antibodies to CH505TF trimers complexed to BMS-529.
To further examine the conformation of gp150 trimers, we compared binding affinities of CH103 bnAb lineage members to CH505TF gp150 and ch.SOSIP gp140 trimers. Using surface plasmon resonance (SPR) single-cycle kinetics, we measured CH103 lineage (12) Fab affinities to both CH505TFv4.1 ch.SOSIP and to CH505TF.D902 gp150, with each trimer either complexed to BMS-529 or noncomplexed (Fig. 4). The CH103 UCA Fab bound to both ch.SOSIP and gp150 trimers with similar fast association (>1 × 104 M−1s−1) and dissociation (∼10−2 s−1) rates, resulting in affinities of 449.3 and 661.8 nM, respectively (Fig. 4A; Table 2). The affinities of the intermediate IA4 Fab for both trimers were approximately an order of magnitude stronger than that of the UCA. IA4 gave a 3-fold weaker affinity for gp150 (dissociation constant [KD] = 50.4 nM) than the ch.SOSIP trimer (KD = 16.1 nM), largely due to a difference in dissociation rates (Fig. 4A; Table 2). Unlike the binding to CH505TFv4.1 ch.SOSIP, CH106 Fab bound to CH505TF.D902 gp150 with a biphasic dissociation phase that gave an order of magnitude difference in the measured rates (Fig. 4A; Table 2) and indicated likely heterogeneity in the bnAb epitope presentation on the membrane-bound form of the trimer. The affinities of CH106 Fab to CH505TF.D902 gp150 trimer and CH505TFv4.1 ch.SOSIP trimer were similar (KD < 1 nM and KD = 4.4 nM, respectively) (Fig. 4; Table 2).
FIG 4.
Affinities of CH103 lineage antibodies to stabilized CH505TFv4.1 ch.SOSIP and CH505TF.D902 gp150 trimers. (A) SPR single-cycle kinetic rate measurements of CD4 binding site bNAb (CH103 lineage and VRC01) antibodies (Fabs) against CH505TFv4.1 ch.SOSIP and CH505TF.D902 gp150 trimers. Six sequential injections of each Fab at concentrations ranging from 5 to 100 μg/ml were performed against trimers complexed to (right) or without (left) BMS-529 and bound to streptavidin (biotinylated CH505TFv4.1 ch.SOSIP) or immobilized 2G12 MAb (CH505TF.D902 gp150) sensor surfaces. Curve fitting analysis was performed using a 1:1 Langmuir binding model (CH103 UCA) or the heterogeneous ligand model. KDs show affinity values for Fabs that bound with biphasic dissociation and derived using the high and low dissociation rate components. (B) Affinity (KD) plots of CH103 UCA, intermediate IA4, CH106, and VRC01 bnAbs to CH505TFv4.1 ch.SOSIP (left) or CH505TF.D902 gp150 (right) trimers complexed to (▲) or without (●) BMS-529. KD values derived from lower dissociation rates (○ and △) of CH106 Fab to CH505TF.D902 gp150 and derived from the heterogeneous ligand model curve fitting analysis are plotted.
TABLE 2.
Kinetic rates and dissociation constants of CH103 lineage MAbs and VRC01 bnAb to CH505TFv4.1 ch.SOSIP and CH505TF.D902 gp150 trimers with and without BMS-529a
| Group | Lineage | ka (×104 ms−1) | kd (×10−4 s−1) | KD (nM) |
|---|---|---|---|---|
| CH505TF.D902 gp150 | CH103 UCA | 2.68 | 177.5 | 661.8 |
| CH103 IA4 | 4.31 | 21.72 | 50.4 | |
| CH106b | 4.23 | 61.26 | 145.0 | |
| 0.58 | <0.1 | <1.0 | ||
| VRC01 | 3.16 | 7.08 | 22.4 | |
| CH505TFv4.1 ch.SOSIP | CH103 UCA | 2.86 | 128.6 | 449.3 |
| CH103 IA4 | 6.42 | 10.33 | 16.1 | |
| CH106 | 1.99 | 0.88 | 4.4 | |
| VRC01 | 3.27 | 9.24 | 28.2 | |
| CH505TF.D902 gp150+BMS | CH103 UCA | 1.18 | 227.6 | 1934 |
| CH103 IA4 | 3.65 | 203.3 | 557.4 | |
| CH106b | 1.47 | 56.26 | 382.0 | |
| 0.40 | 1.13 | 28.3 | ||
| VRC01 | 3.42 | 11.81 | 34.5 | |
| CH505TF v4.1 ch.SOSIP+BMS | CH103 UCA | 2.87 | 605.9 | 2110 |
| CH103 IA4 | 3.30 | 184.4 | 559.1 | |
| CH106 | 1.18 | 0.45 | 3.8 | |
| VRC01 | 1.84 | 3.60 | 19.6 |
Data are representative of two independent experiments.
Data with a biphasic curve, and both fast and slow phases are reported.
When CH505TFv4.1 ch.SOSIP or CH505TF.D902 gp150 trimers were complexed to BMS-529, CH103 UCA binding was approximately 3- to 5-fold weaker than that for the respective noncomplexed trimers (Table 2; Fig. 4A and B). Similarly, complexing both trimers to BMS-529 reduced their affinities for IA4, primarily due to weakened dissociation rates. In contrast, affinities of the bnAb CH106 to BMS-529 complexed trimers were similar to those of the respective trimers without BMS-529 (Fig. 4; Table 2). The binding of VRC01 Fab, a different class of CD4 binding site bnAb that also bound with nanomolar affinities to both CH505TF ch.SOSIP and gp150 trimers, was unaffected when either trimer form was complexed to BMS-529 (Fig. 4A and B). These affinity data indicate that BMS-529 locks the CH505TF trimers (both ch.SOSIP and gp150) in a conformational state that retains the strong affinities (nM) of CD4-bs bnAbs (CH106 and VRC01) but shows weakened affinities to the UCA or the germ line-proximal intermediate IA4 of the CH103 bnAb lineage. These results are consistent with a model in which the UCA and the earliest intermediate of the CH103 lineage bind more avidly to CH505TF trimer conformations that differ from the BMS-529-bound conformation; the latter presumably corresponds to the closed conformation recognized by most bnAbs. We note that our previous negative-stain electron microscopy results indicated that the CH103 Fab either bound to or induced an open conformation of BG505 SOSIP (11).
Immunogenicity of SOSIP trimer-BMS complex in rabbits.
Based on the observed antigenicity of BMS-529-complexed trimers for binding to bnAbs, we hypothesized that such stabilized trimer immunogens will disfavor antibody responses to non-bnAb epitopes and show improved immunogenicity in autologous tier 2 neutralization relative to that observed with the uncomplexed immunogen. Therefore, we tested the immunogenicity of CH505TFv4.1 and CH848.10.17.DT ch.SOSIP trimers in two rabbit immunization groups, with each trimer either complexed to (SOSIP+BMS) or without (SOSIP) the BMS-529 molecule. In either group immunized with CH505TFv4.1 ch.SOSIP with or without BMS-529, the immunogen trimer binding titers reached nearly peak level after three immunizations (postimmunization-3 [PI-3]) and were higher in magnitude than the binding to the linear V3 peptide (SOSIP/V3 median log area under the concentration-time curve [AUC] ratio = 1.4 and 1.6 for SOSIP and SOSIP+BMS groups, respectively) (Fig. 5A). Following further boosting (PI-6), the animals in the BMS group showed an approximately 4-fold higher magnitude of SOSIP to V3 binding titers, while the V3 peptide titers remained relatively higher in the noncomplexed SOSIP group (SOSIP/V3 median log AUC ratio = 1.2 and 3.5 for SOSIP and SOSIP+BMS groups, respectively) (Fig. 5A). Thus, the Env binding data showed that the V3 linear peptide binding response was relatively less favored in the animal group immunized with CH505TFv4.1 ch.SOSIP immunogen complexed to BMS-529.
FIG 5.
Serology and neutralization titers of antibodies induced in rabbits immunized with CH505TFv4.1 ch.SOSIP immunogens with or without BMS-529. (A) enzyme-linked immunosorbent assay (ELISA) binding titers (log AUC) of pre- and postbleed serum samples to CH505TFv4.1 ch.SOSIP trimers (left) or CH505 V3 peptide (right) from rabbits immunized with SOSIP alone (●) and SOSIP+BMS (▲). Each symbol represents a data point from one animal, and the best fit line traces the median values at each time point. (B) Neutralization titers (ID50 reciprocal dilution from a TZM-bl pseudovirus assay) of postimmunization 3 (PI-3) sera from each immunized animal against tier 2 autologous CH505 or tier 1 CH505w4.3 pseudoviruses. (C) Neutralization titers of postimmunization 6 sera from each immunized animal against tier 2 autologous CH505 or Tier 1 CH505w4.3 pseudoviruses. Median values of neutralization ID50s are marked with solid lines. ID50s of <20 are plotted with a given value of 10.
Neutralization titers against tier 1 CH505w4.3 pseudoviruses in both earlier (PI-3) and later (PI-6) time point sera were similar in both immunized groups (Fig. 5B and C). Weak neutralization against autologous CH505TF tier 2 pseudoviruses was inconsistently observed in a few animals in both groups (Fig. 5B). With further boosting, more animals showed autologous CH505TF neutralization (50% infective dose [ID50] of ≥100 in 4/6 and 2/4 in SOSIP+BMS and SOSIP groups, respectively), but the median autologous tier 2 neutralization ID50 titers were similar in both groups (Fig. 5C), and no heterologous neutralization was observed in either group (data not shown). Thus, BMS-529-complexed CH505TFv4.1 ch.SOSIP immunogen in rabbits showed improvement in trimer versus V3 linear peptide binding titers, but not in autologous tier 2 neutralization potency.
A second clade C Env CH848.10.17 (31) was expressed as ch.SOSIP with V1 glycan (N133D.N138T) deletion (CH848.10.17.DT) that enhances the affinity to the UCA of the glycan V3 DH270 bnAb lineage and allows the UCA to neutralize the V1 glycan mutant of the autologous CH848 pseudovirus (28). In rabbits immunized with CH848.10.17.DT ch.SOSIP immunogens, both immunization groups showed similar and markedly higher binding titers to the SOSIP immunogen (PI-4 median log AUC = 13.1 and 14.1 for SOSIP and SOSIP+BMS groups, respectively) than to V3 linear epitope (PI-4 median log AUC = 2.9 and 3.4 for SOSIP and SOSIP+BMS groups, respectively) (Fig. 6A). Thus, the favored induction of CH848.10.17.DT ch.SOSIP binding titers over linear V3 peptide responses did not require BMS-529-induced trimer stabilization. Immunized animals in both groups showed weak or no neutralization against tier 1 pseudoviruses (SF162.LS and MW965.26) (Fig. 6B). There was no enhancement in the neutralization breadth in the BMS-SOSIP group, with sera from both immunization groups showing neutralization against the same tier 2 pseudoviruses (Fig. 6C). Sera from both groups of immunized animals exhibited strong neutralization (ID50 > 103 in PI-3) against the CH848.10.17 pseudoviruses with V1 glycan deletion (CH848.10.17.DT) but not against the viruses with V1 glycans intact (CH848.10.17) (Fig. 6C). However, a notable difference in the neutralization potency was observed in the two immunized groups. In PI-3 sera, 5/6 animals in the SOSIP+BMS group gave strong neutralization (ID50 reciprocal dilution > highest dilution at 43,740), while 1/6 in the SOSIP group had similar higher titers against the glycan-deleted CH848.10.17.DT tier 2 pseudoviruses (Fig. 6C). Neutralization assays repeated at higher dilutions gave median ID50 titers of 5,960 (±16,530) and 24,057 (±52,840) for SOSIP and SOSIP+BMS immunized groups, respectively (Fig. 6D). Following three additional boosts, the majority of PI-6 sera (5/6) in the SOSIP+BMS group showed sustained higher neutralization titers (ID50 reciprocal dilution, >104) in both PI-5 and PI-6 time points (Fig. 6D). In contrast, the median titers in the SOSIP group were consistently lower than those in the SOSIP+BMS group at each immunization time point (median ID50 titers of SOSIP and SOSIP+BMS groups at PI-6 were 41,526 ± 74,488 and 10,812 ± 22,900, respectively) (Fig. 6D). The difference in neutralization titers at three postimmunization time points (Fig. 6D) in animals immunized with CH848.10.17.DT ch.SOSIP immunogen complexed to BMS-529 compared to the titers induced by the uncomplexed immunogen was significant (two-sided P = 0.0411, Wilcoxon rank). The PI-6 sera of both groups also showed weak neutralization against the tier 2 CH848.10.17 (with V1 glycans) pseudoviruses and were similar (IC50 < 100) in the two groups except for two animals (ID50 = 161 and 118) in the SOSIP+BMS group (Table 3). Neutralization titers were higher in the BMS-complexed group against the heterologous tier 2 JR-FL pseudoviruses but only following high-mannose N-glycan (Man9GlcNAc2) enrichment with kifunensine treatment (median titers of SOSIP and SOSIP+BMS groups = 4,425 ± 1,911 and 13,080 ± 7,845, respectively) (Fig. 6D; Table 3). Thus, the V3-glycan targeting the CH848.10.17.DT ch.SOSIP immunogen complexed to BMS-529 showed improved immunogenicity by inducing higher neutralization titers against the tier 2 glycan-deleted pseudoviruses in the majority of immunized animals than in the animal group immunized with the uncomplexed ch.SOSIP immunogen.
FIG 6.
Serology and neutralization titers of antibodies induced in rabbits immunized with CH848.10.17.DT ch.SOSIP immunogens with or without BMS-529. (A) ELISA binding titers of prebleed and postimmunization (PI) serum samples to CH848.10.17.DT ch.SOSIP trimers (left) and CH505 V3 peptide (right) from rabbits immunized with SOSIP alone (red) and with SOSIP+BMS (blue). Each symbol represents one animal, and the best fit line traces the median value at each time point. (B) Neutralization ID50 titers induced in rabbits after 6 immunizations with CH848.10.17.DT ch.SOSIP with or without BMS-529. Neutralization data against tier 1 pseudoviruses are shown. (C) Neutralization titers (ID50 reciprocal dilution) of PI-3 sera from each immunized animal in SOSIP and SOSIP+BMS groups against the listed pseudoviruses, including the autologous CH848.10.17.DT. ID50 values are color coded based on the range of values shown on the right. (D) Neutralization ID50 titers of PI sera 3, 5, and 6 from each immunized animal against the autologous CH848.10.17.DT pseudoviruses. Each symbol represents one animal, and the lines mark the median ID50s.
TABLE 3.
Neutralization ID50 titers induced in rabbits after 6 immunizations with CH848.10.17.DT ch.SOSIP with or without BMS-529a
Maximum dilution titers reached in the assay are indicated as >43,740.
Immunogenicity in rhesus macaques of Env trimers complexed to BMS-529.
Finally, we studied immunogenicity in rhesus macaques of both CH5050TFv4.1 ch.SOSIP and CH505TF.D902 gp150 trimers; each immunogen was prepared either complexed or noncomplexed to BMS-529. In animals immunized with ch.SOSIP trimers, the autologous trimer binding responses peaked after the second immunization and were of similar magnitudes in both SOSIP and SOSIP+BMS groups (Fig. 7A). While the median SOSIP immunogen binding titers were sustained after two additional boosts in all animals in the BMS group, the SOSIP-alone animals showed a declining trend in SOSIP trimer binding titers with subsequent boosting (Fig. 7A). Moreover, the gp120 binding titers in the SOSIP group peaked at approximately 2-fold higher log AUC value than in the BMS group (median log AUC = 4.8 and 2.7 for SOSIP and SOSIP+BMS groups, respectively) (Fig. 7A). Thus, the BMS-complexed CH505TFv4.1 ch.SOSIP immunogen induced antibody responses that showed improvement in sustained trimer binding titers while showing weaker responses to gp120 binding than the noncomplexed ch.SOSIP immunogen.
FIG 7.
Serology and neutralization titers of antibodies induced in rhesus macaques immunized with CH505TFv4.1 ch.SOSIP immunogens with or without BMS-529. (A) ELISA binding titers of postimmunization serum samples to CH505TF gp120 (●) or CH505TFv4.1 ch.SOSIP (▲) from macaques immunized with SOSIP alone (left) and with SOSIP+BMS (right). Each symbol represents a data point from one animal, and the best fit line traces the median values at each time point. (B) SPR binding response (response units [RU]) to CH505 Env proteins of purified IgG Fabs from postimmunization 2, 3, and 4 serum samples of macaques immunized with SOSIP alone or with SOSIP+BMS. Each symbol represents Fab sample from one animal, and the solid lines represent median values. (C) SPR dissociation rates of CH505TFv4.1 ch.SOSIP binding to IgG Fabs from PI-2, -3, and -4 sera from macaques immunized with SOSIP alone (○) and with SOSIP+BMS (△). Each symbol represents one animal, and the lines trace the median ID50 values. (D) SPR analysis of fast-phase (●) and slow-phase (□) dissociation rates of CH505TFgp120, CH505TFv4.1 ch.SOSIP, and CH505M5v4.1 ch.SOSIP binding to IgG Fabs from PI-2, -3, and -4 sera from macaques immunized with CH505TF ch.SOSIP (left) and the same ch.SOSIP complexed to BMS-529 (SOSIP+BMS; right). Each symbol represents one immunized animal, and the lines represent the median Fab binding dissociation at the indicated PI time point. (E) Neutralization titers of PI-4 (left) and PI-6 (right) sera from macaques immunized with SOSIP alone (●) and with SOSIP+BMS (▲) against autologous CH505TF and variant pseudoviruses. Median values of neutralization ID50s are marked with solid lines. ID50s of <20 are plotted with a given value of 10.
To further resolve the differences in the binding titers and to quantitate the relative average affinities of Env binding responses in the two immunized rhesus groups, we prepared polyclonal Fabs following purification of total IgG from the serum samples at different immunization time points and performed SPR binding analysis. Overall, the purified IgG samples showed neutralization against the same pseudoviruses and with similar relative potencies as the corresponding serum samples (Table 4). A few samples with low serum ID50 (<100) showed no neutralization at an IgG concentration of >100 μg/ml (Table 4). SPR analysis showed that the purified Fab binding responses were markedly stronger to CH505TFv4.1 ch.SOSIP trimers than to CH505TF gp120 (Fig. 7B), indicating that the induced antibodies in both immunized groups bound with higher affinities to either conformational epitopes that likely include high-mannose glycans on the trimer and/or to gp41 epitopes at the base of the trimer (34). The median Fab binding responses were similar in the two immunized groups against both the autologous CH505TFv4.1 and the variant CH505M5v4.1 ch.SOSIP trimers (Fig. 7B). The SPR dissociation phases of the polyclonal Fabs were biphasic for all samples, indicating heterogeneity in binding affinities of the polyclonal Fab samples. To differentiate between relatively weaker and stronger binding in each polyclonal Fab sample, dissociation phases were resolved into fast and slow dissociation components using curve fitting analysis. The dissociation of Fabs bound to CH505TF gp120 monomers were 5- to 10-fold faster than their binding to ch.SOSIP trimers. Furthermore, the faster dissociation component was predominant for gp120 binding, and the median values were lower at each time point than the corresponding dissociation rates of ch.SOSIP trimer binding (Fig. 7D). In contrast, the predominance of lower dissociation rates was observed at each time point for binding to ch.SOSIP trimers (Fig. 7C and D). Fabs from both groups gave similar dissociation rates at the early immunization time point (PI-2), while the PI-3 Fabs gave relatively lower dissociation rates from the SOSIP+BMS group (median dissociation rates of PI-3 Fabs were 3.17 × 10−4 and 1.65 × 10−4 s−1 for SOSIP and SOSIP+BMS groups, respectively) (Fig. 7C). Thus, the SOSIP+BMS group showed a relatively higher average affinity responses at an earlier time point (PI-3), while the SOSIP-alone group required an additional boost to reach similar relative affinities, indicating a difference in the kinetics of the induced responses in the two immunized groups.
TABLE 4.
Serum and purified IgG neutralization titers for macaques immunized with CH505TF.v4.1 ch.SOSIP immunogen with (SOSIP+BMS) or without (SOSIP) BMS-529
Neutralization titers against the tier 1 CH505w4.3 pseudoviruses were similar in both immunized groups (median ID50 = 272 and 173 in the SOSIP and SOSIP+BMS groups, respectively) (Fig. 7E). While no autologous CH505TF pseudovirus neutralization was observed in the uncomplexed SOSIP group at PI-4, weak and inconsistent neutralization against autologous tier 2 pseudoviruses was observed in one or two animals in the SOSIP+BMS group (Table 5). Weak neutralization (ID50<100) against the tier 2 autologous CH505TF viruses was observed after multiple boosts (PI-6) in the uncomplexed ch.SOSIP-immunized animals as in the SOSIP+BMS group (Fig. 7E). Neither group showed neutralization against heterologous viruses (data not shown), and the median neutralization titers against the glycan-deleted variant and Man5 glycan-enriched CH505TF pseudoviruses (CH505TF.gly4/GnT1−, tier 1A) were similar in the two groups (Fig. 7E). We also tested neutralization against mutant pseudoviruses that include changes in loop D (N279K) and V5 (G458Y) and are neutralized by CH235/ANC131 bNAb class precursor antibodies (6). Compared to the uncomplexed SOSIP group, the SOSIP+BMS group showed higher median neutralization ID50 titers against the tier 2 CH505TF.G458Y.N279K/GnT1− pseudoviruses (median ID50 = 283.5 and 661 in the SOSIP and SOSIP+BMS groups, respectively) (Fig. 7E). Overall, compared to the noncomplexed immunogen, the BMS-complexed CH505TFv4.1 ch.SOSIP trimers induced antibodies in rhesus monkeys with similar neutralization profiles but induced higher titers (4/4 with >median ID50 in the SOSIP group) against the mutant tier 2 pseudoviruses capable of being neutralized by CH235 precursor antibodies.
TABLE 5.
Serum neutralization titers for macaques immunized with CH505TFv4.1 ch.SOSIP with BMS-529
| Animal IDa | Wk | ID50 (dilution) in TZM-bl cells |
|
|---|---|---|---|
| CH505TF replicate 1 | CH505TF replicate 2 | ||
| T677 | 0 | <20 | NTb |
| T677 | 10 | <20 | NT |
| T677 | 14 | <20 | NT |
| T677 | 16 | <20 | NT |
| BZ83 | 0 | <20 | <20 |
| BZ83 | 10 | <20 | <20 |
| BZ83 | 14 | 29 | <20 |
| BZ83 | 16 | <20 | <20 |
| T284 | 0 | <20 | NT |
| T284 | 10 | <20 | NT |
| T284 | 14 | <20 | NT |
| T284 | 16 | <20 | NT |
| 7077 | 0 | <20 | <20 |
| 7077 | 10 | <20 | 27 |
| 7077 | 14 | 31 | <20 |
| 7077 | 16 | 26 | <20 |
ID, identifier.
NT, not tested.
The membrane-bound gp150 immunogen CH505TF.D902 elicited similar binding responses to CH505TF gp140 in both immunized rhesus groups (Fig. 8A). The median neutralization titer, however, was an order of magnitude higher against tier 1 CH505w4.3 pseudoviruses in the gp150 group than those in the gp150+BMS group (median ID50 = 3,453 and 344 for gp150 and gp150+BMS groups, respectively) (Fig. 8B). Neutralization titers against other tier 1 pseudoviruses (SF162.LS and MN.3) were even weaker and infrequent in the gp150+BMS-immunized group (Fig. 8B). Unlike those observed in the SOSIP+BMS group, no neutralization against the CH505TF tier 2 pseudoviruses was observed in the gp150+BMS-immunized animals. Weak neutralization was detected against the Man5 glycan-enriched CH505TF variant gly4 pseudoviruses grown in GnT1− cells (Fig. 8C). Overall stronger neutralization titers were observed against pseudoviruses either treated with kifunensine (heterologous JR-FL+kifunensine) or against CH505TF variant pseudoviruses (CH505TF.G458Y.N279K and CH505TF.N279K.N280D) with both targeted glycan deletion and Man5 glycan enrichment (GnT1− grown) (Fig. 8C). Thus, compared to the ch.SOSIP+BMS immunogen, the membrane-bound gp150 complexed to BMS-529 induced rhesus antibodies with a similar neutralization profile and showed neutralization against Man5 glycan-enriched tier 2 pseudoviruses, with the exception of CH505TF pseudoviruses with intact glycans.
FIG 8.
Serology and neutralization titers of antibodies induced in rhesus macaques immunized with CH505TF.D902 gp150 immunogen with or without BMS-529. (A) ELISA binding titers of postimmunization serum samples to CH505TF gp140 from macaques immunized with gp150 alone (●) or with gp150+BMS (▲). Each symbol represents one animal, and the best fit lines represent the medians. (B) Neutralization titers (ID50 reciprocal dilution) of PI-4 sera from each immunized animal in SOSIP and SOSIP+BMS groups against the listed tier 1 and autologous tier 2 CH505TF pseudoviruses. Median values of neutralization ID50s are marked with solid lines, and ID50s of <20 are plotted with a given value of 10. (C) Neutralization titers (ID50 reciprocal dilution) of PI-4 sera from each immunized animal in SOSIP+BMS group against the autologous CH505TF and heterologous JRFL pseudoviruses and variants (targeted glycan deletion) grown in either 293T (complex, hybrid and oligomannose glycans) or GnT1− (Man5–9GlcNAc2 oligomannose glycans) cells. Median values of neutralization ID50s are marked with solid lines, and ID50s of <20 are plotted with a given value of 10. Treatment with kifunensine, a mannosidase I inhibitor, limits glycan processing on Env to high mannose (Man9GlcNAc2). The tier classification of the pseudoviruses are as follows: JRFL, JRFL+kifunensine (tier 2), CH505TF. Gly4/GNT1− (tier 1B), CH505TF.G458Y.N279K/293T (tier 1A), CH505TF.G458Y.N279K/GnT1− (tier 2), CH505TF.G458Y.N279K.N280D/GnT1− (tier 2).
DISCUSSION
Several recently designed Env gp140 SOSIP trimer constructs with stabilizing mutations can prevent exposure of both the V3 loop and CD4i epitopes (35). However, CD4 binding to several of these stabilized trimers can trigger conformational changes in gp120 that result in complete unmasking of both V3 loop and CD4i epitopes and in the induction of non-nAb responses without improvement in autologous nAb responses (7, 10, 16, 36). With the introduction of additional changes that stabilize the closed Env conformation and block CD4-induced exposure of non-nAb epitopes on SOSIP trimers, enhancement of immunogenicity has been reported (7, 8, 14, 37). Current structure-guided designs, however, require careful redesigning of the Env protein to accommodate disruption of the Env allostery network (38). In this study, we show that the use of the small-molecule viral entry inhibitor BMS-529 can be an alternative and simpler strategy to stabilize conformations of both SOSIP and membrane-bound forms of Env trimers. Using two clade C Env trimeric forms, we showed that the BMS-529 compound can stabilize the Env conformational flexibility and inhibit the unmasking of epitopes induced by CD4 binding. Overall, the antigenicity of all three trimeric forms, which included both SOSIP and membrane-bound trimers, showed favorable binding to the major classes of bnAbs, including the UCAs of targeted bnAb lineages.
A key test of the BMS-529 complexed trimer immunogens was to determine whether we would observe an enhancement in immunogenicity for autologous neutralization. We previously reported that the development of autologous neutralization in rabbits required multiple boosting with the CD4 bs-targeting CH505TFv4.1 ch.SOSIP immunogen (5). In this study, we observed that after repeated boosting, the CH505TFv4.1 ch.SOSIP immunogen complexed to BMS-529 did not enhance the overall potency of autologous neutralization. While the majority of the animals (5/6) in the BMS group showed similar neutralization ID50 values, the noncomplexed immunogen induced a much wider range of ID50 titers against the autologous pseudoviruses. Thus, one modest effect of conformational stabilization of CH505TFv4.1 ch.SOSIP with BMS-529 was to reduce the variance in autologous neutralizing antibody responses in the immunized animals. A notable observation was the enhancement in neutralization potency with the BMS-stabilized V3 glycan-targeting CH848.10.17.DT immunogen, a ch.SOSIP trimer designed to bind to the UCA of the V3 glycan bnAb DH270 and a potential prime to initiate the V3-glycan lineage (28). Compared to CH505TFv4.1 ch.SOSIP, the CH848.10.17.DT ch.SOSIP trimer induced much stronger autologous neutralization with fewer immunizations in all immunized animals. In both earlier and later time points, the BMS-complexed CH848.10.17.DT ch.SOSIP immunogen gave higher autologous neutralization than in animals immunized with the noncomplexed immunogen. Therefore, the effect of BMS-529-induced conformational stabilization on immunogenicity was more pronounced with CH848.10.17.DT than with the CH505TFv4.1 ch.SOSIP immunogen. It was recently reported that relatively higher-affinity and multimerized immunogens are required to overcome low germ line precursor frequency and germinal center competition (39). In this study, we have used SOSIP trimeric antigens, and future studies will test whether the effect of BMS stabilization can be further enhanced on multimeric immunogens. The need for multimerization could be more important for CH505TF ch.SOSIP, since we observed that BMS-529 stabilization results in the lowering of affinities of the UCA and an early intermediate of CH103 bnAb lineage but not the CD4 bs-bnAbs (CH106 and VRC01). The loss of binding affinities of the above-described CH103 lineage antibodies was due to an increase in dissociation rates that potentially could be overcome with the use of multimeric forms of the SOSIP immunogen.
In addition to ch.SOSIP immunogens, we studied the immunogenicity in rhesus macaques of membrane-bound gp150 forms of CH505TF Env following its purification from the CHO cell surface. The majority of the bnAbs we tested bound to CH505TF gp150, including the CD4 bs-bnAbs CH106 and VRC01 that bound with affinities similar to those when binding to the CH505TF ch.SOSIP proteins. One exception was PGT151, which bound to CH505TF ch.SOSIP trimers but not to its gp150 forms; this result was not unexpected, since the bnAb preferentially binds to cleaved trimers (40). Furthermore, unlike in ch.SOSIP trimers, the V3 MAb 19b epitope exposure could only be inhibited partially in CH505TF gp150 when complexed to BMS-529. The observed differences in antigenicity of the two trimeric forms of CH505TF were likely due to gp150 being expressed with a defective furin cleavage site, as recently reported when comparing glutaraldehyde-fixed cleaved versus uncleaved membrane-bound Env trimers (41). Although the deletion of the Env cytoplasmic tail has been reported to impact antigenicity (42, 43), maintaining the cytoplasmic tail may not be a strict requirement (41), and the truncation of the cytoplasmic tail was included to improve gp150 Env expression (44–46). In contrast to the observed immunogenicity of the ch.SOSIP trimers, the CH505TF gp150 when complexed to BMS-529 did not induce CH505TF tier 2 neutralization but neutralized tier 2 autologous variants and select heterologous pseudoviruses with high-mannose glycans. Overall, the enhancement in immunogenicity by BMS-529 stabilization of trimer conformational flexibility was more pronounced for stabilized SOSIP trimer immunogens than for gp150 trimer immunogens. Thus, our studies showed that the use of the viral entry inhibitor BMS-529 can be an alternative strategy to restrict trimer conformational flexibility and enhance immunogenicity of select SOSIP trimer immunogens.
MATERIALS AND METHODS
Expression and purification of proteins.
Chimeric SOSIP (ch.SOSIP) gp140 proteins were produced as previously described (28, 29). Briefly, ch.SOSIP gp140 was expressed in Freestyle 293 cells by transient transfection. CH505TF ch.SOSIP was purified from cell culture supernatant with PGT145 antibody affinity chromatography on an AKTA Pure (GE Healthcare). CH848.10.17.DT SOSIP gp140 was purified by PGT151 antibody affinity chromatography. Trimeric ch.SOSIP gp140 was purified by Superose6 (GE Healthcare) size exclusion chromatography in 10 mM Tris, 500 mM NaCl and snap-frozen.
Membrane-bound gp150 trimers.
Using lentivirus vectors comprising a tetracycline-inducible promoter, Chinese hamster ovary (CHO) cells were engineered to express membrane-bound CH505TF gp150 proteins as described previously (33), generating CH505TF.D883 gp150. The furin protease cleavage site between gp120 and gp41 was mutated by substituting serine residues for Arg508 and Arg511 (REKR to SEKS), and the cytoplasmic tail of gp150 was truncated by replacing the codon for Lys with a TAA stop codon. CH505TF gp150 was further modified by introducing trimer-stabilizing mutations (E64K/A316W/I535M) and then used to generate CHO cells expressing CH505TF.D902 gp150 protein. The recombinant CH505TF gp150 proteins were purified from CHO expressor cells using trimer-specific PGT145 affinity chromatography (33, 47). Cells were lysed in a 1% Brij-58 solution and homogenized and then incubated in a 1% Cymal-5 solution (33). PGT145 was coupled to CNBr-activated Sepharose 4 FF (GE Healthcare, Pittsburgh, PA) and columns packed for affinity purification using an NGC Quest 100 Plus chromatography system (Bio-Rad). The affinity column was washed to baseline in phosphate-buffered saline (PBS) plus 0.25% Cymal-5, and then the protein was eluted in 0.1 M glycine, 0.25% Cymal-5 elution buffer. The eluted protein was buffer exchanged back into PBS plus 0.25% Cymal-5, concentrated, and quantified by the bicinchoninic acid (BCA) assay. An aliquot (1 mg) of the final sample was buffer exchanged into PBS plus 0.01% Cymal-6 for glycan analysis.
SPR and BLI analysis.
Antigenicity of gp150 proteins was assessed against a panel of bnAbs and non-nAbs by surface plasmon resonance (SPR) and bio-layer interferometry (BLI) as described earlier (48, 49). SPR measurements of CH103 lineage Fabs binding to CH505TF.D902 gp150 and CH505TFv4.1 ch.SOSIP were obtained using a Biacore S200 instrument (GE Healthcare) in HBS-N (10 mM HEPES [pH 7.4], 150 mM NaCl) 1× running buffer. Differing methods of immobilization were used for the CH505TF Env constructs. CH505TF.D902 gp150 was diluted down to 100 μg/ml, injected over a 2G12 immobilized CM5 chip surface for 300 to 480 s at 5 μl/min, and captured to a level of 250 to 350 response units (RU). Protein capture was followed by a long dissociation period of 30 min or until the gp150 response stabilized. CH505TFv4.1 ch.SOSIP was biotinylated and immobilized via streptavidin to a level of 300 to 350 RU. Both CH505TF.D902 gp150 and CH505TFv4.1 ch.SOSIP were analyzed after incubation with the small-molecule inhibitor BMS-626529. Both proteins were mixed with 25 μM BMS and immobilized following the same procedures described above. For antibody-protein interactions, CH103 lineage Fabs were diluted to 4 μg/ml from 100 μg/ml and injected over the immobilized proteins and protein-BMS complexes using the single-cycle kinetics injection type at a flow rate of 50 μl/min. There were six 120-s injections of each Fab at increasing concentrations followed by a dissociation length of 600 s after the final injection. Results were analyzed using the BIAcore S200 evaluation software (GE Healthcare). A negative-control antibody surface (Ab82) or a blank streptavidin surface along with blank buffer binding were used for double reference subtraction to account for nonspecific protein binding and signal drift. Subsequent curve fitting analyses were performed using a heterogeneous ligand model with local Rmax for CH106 and CH103 IA4 Fabs and a 1:1 Langmuir model with a local Rmax for CH103UCA Fab. The reported binding curves are representative of 2 data sets.
Surface plasmon resonance measurements of purified plasma IgG avidity.
IgG avidity to a panel of HIV-1 antigens (CH505TF gp120 and biotinylated CH505M5v4.1 ch.SOSIP, CH505TF ch.SOSIP, and CH505TFv4.1 ch.SOSIP) was measured by surface plasmon resonance (BIAcore 4000; BIAcore/GE Healthcare, Pittsburgh, PA) analysis. Using a multiplex array format (2 × 8), binding response was measured by SPR following immobilization by amine coupling of envelope protein or capture of biotinylated antigens to immobilized streptavidin on Series S CM5 sensor chips (BIAcore/GE Healthcare, Pittsburgh, PA). Purified plasma IgG samples at 200 μg/ml were flowed (2.5 min) over spots (chip surfaces) of antigen followed by a dissociation phase (postinjection buffer wash) of 10 min and a regeneration with glycine, pH 2.0. Nonspecific binding of a preimmune (zero time point) sample was subtracted from each postimmunization IgG sample binding data. Data analyses were performed with BIA-evaluation 4000 and BIA-evaluation 4.1 software (BIAcore/GE Healthcare, Pittsburgh, PA). Binding responses were measured by averaging postinjection response units (RU) over a 10-s window, and dissociation rate constant, kd (per second) was measured during the postinjection phase after the stabilization of signal. Positive response was defined when both replicates had an RU value of ≥10. Relative avidity binding score is calculated as follows: avidity score (RU·s) = (binding response units/kd) (50, 51).
Fab preparation from purified IgG samples.
Polyclonal Fab preparation was performed using a modified protocol previously described (34). Purified IgG samples were initially dialyzed into 20 mM sodium phosphate, 10 mM EDTA, pH 7.0, and then concentrated to approximately 20 mg/ml by centrifugal filtration (Amicon/EMD Millipore). IgGs were digested in 20 mM sodium phosphate, 10 mM EDTA, 20 mM cysteine, pH 7.4, with papain-agarose resin (Thermo Fisher Scientific) for 5 h at 37°C. The Fab fragments were then separated from nondigested IgG and Fc fragments by a 1-h incubation with rProtein A Sepharose Fast Flow (GE Healthcare). Postincubation, cysteine was removed by centrifugal filtration and buffer exchanged into 1× PBS, pH 7.4 (Amicon/EMD Millipore).
Fab quality was determined by SDS-PAGE and size exclusion chromatography (SEC). For SDS-PAGE, 3 μg protein/lane was loaded on a 4% to 15% TGX stain-free gel (Bio-Rad) under both reducing and nonreducing conditions and run at 300 V in Tris-glycine-SDS buffer. Bands were visualized using a Gel Doc EZ imager (Bio-Rad), and the size of the fragments assessed by a protein standard ladder (Bio-Rad). For SEC, 25 μg protein was loaded on a Superdex 200 increase 10/300 column using a 100-μl loop and run at 0.5 ml/min using an Äkta Pure system (GE Healthcare). Fab peaks were analyzed with the system’s Unicorn 7.0.2 software. The size of the fragments was estimated using a linear regression calculated by running a mix of known-molecular-weight (GE Healthcare) protein standards on the same column.
Fab SPR affinity and kinetics measurements.
IgG Fab binding to HIV-1 Env antigens (CH505TF gp120 and biotinylated CH505M5v4.1 ch.SOSIP and CH505TFv4.1 ch.SOSIP) was measured by surface plasmon resonance (BIAcore 3000; BIAcore/GE Healthcare) analysis. Binding response was measured by SPR following immobilization by amine coupling of envelope protein or capture of biotinylated antigens to immobilized streptavidin on CM3 sensor chips (BIAcore/GE Healthcare). Purified IgG Fab samples were injected over antigen surfaces for 3 min at 30 μl/min followed by a 6-min dissociation period with buffer and then a 24-s injection of glycine, pH 2.0, for regeneration. Nonspecific binding of a preimmune (zero time point) sample was subtracted from each postimmunization IgG Fab sample binding data. Data analyses were performed with BIA-evaluation 4.1 software (BIAcore/GE Healthcare). Binding responses were measured by averaging postinjection response units (RU) over a 10-s window, and the dissociation rate constant, kd (per second), was measured during the postinjection phase after the stabilization of signal.
Subsequently, titrations were performed with select IgG Fabs against the same antigen panel. Rate constants (kon and koff) and KD were measured using the BIAcore 3000 (BIAcore/GE Healthcare). Fabs were injected over the immobilized surfaces for 3 min at 30 μl/min followed by a 10-min dissociation period and then a 24-s injection of glycine, pH 2.0. Kinetics results were analyzed using the BIA-evaluation 4.1 software (BIAcore/GE Healthcare). A blank surface subtraction was used to account for any nonspecific binding or signal drift. Subsequent curve fitting analysis was performed using a 1:1 Langmuir model with a local Rmax (51).
Site-specific glycan analysis.
The glycoproteins CH505TF gp120 and CH505TF gp150 were proteolytically digested prior to mass spectrometric analysis, as described previously (33). Briefly, 7 M urea was added to a 100 mM Tris solution (pH 8.0) containing 25 μg of the glycoprotein. The sample was reduced with Tris(2-carboxyethyl)phosphine hydrochloride (TCEP; 5 mM) at room temperature for 1 h followed by alkylation with iodoacetamide (20 mM) for an additional 1 h in the dark. The reaction was then quenched with excess iodoacetamide and 20 mM dithiothreitol for 15 min. The proteins were buffer exchanged using a 30-kDa-molecular-weight-cutoff (MWCO) filter prior to protease digestion with trypsin alone and a combination of trypsin and chymotrypsin. Proteins were digested overnight using protein/enzyme ratios of 30:1 and 25:1 for trypsin and chymotrypsin, respectively. The reactions were quenched with acetic acid, stored at −20°C, and subsequently analyzed by liquid chromatography-mass spectrometry (LC-MS). Results from the analysis of two data sets, from tryptic digest and the combination of enzymes, were combined to produce a complete glycan profile for these proteins.
High-resolution LC-MS experiments were performed out using an LTQ-Orbitrap Velos Pro (Thermo Scientific) mass spectrometer equipped with electron transfer dissociation (ETD) that is coupled to an Acquity M-class system (Waters). A 5-μl aliquot of the digest solution, ∼7 μM, was injected at 5 μl per minute onto a C18 PepMap 300 column using a linear gradient starting with 3% B (optima-grade acetonitrile in 0.1% formic acid) and ramping up to 90% B. Mass spectrometry data acquisition parameters included a source voltage of 3.0 kV, a capillary temperature of 250°C, and a resolution of 30,000 at m/z 400. Data-dependent acquisition was carried out by dynamic selection of the five most intense ions from each survey scan for alternating collision-induced dissociation (CID) and ETD followed by a high-resolution full MS scan. Glycopeptide compositions were manually identified in the LC-MS data files and were confirmed by a combination of high-resolution MS data and CID and ETD data.
Rabbit and nonhuman primate immunization.
In rabbit immunization studies, each animal in two groups (SOSIP and SOSIP+BMS) was immunized with 100 μg of ch.SOSIP trimer immunogen mixed with 25 μg glucopyranosyl lipid A stable emulsion (GLA-SE) adjuvant. Each animal was immunized intramuscularly (i.m.) at two sites with the same immunogen-adjuvant six times every month either with (6 animals in the SOSIP+BMS group) or without (4 animals in the SOSIP group) BMS-529 and prebleed and postimmunization sera collected at time zero and at a regular monthly interval.
Indian-origin rhesus macaques were housed and treated at AAALAC-accredited institutions. The study protocol and all veterinarian procedures were approved by the Duke University IACUC and were performed based on standard operating procedures developed by Bioqual (Rockville, MD). Two groups of macaques with 4 animals in each were immunized with 300 μg of ch.SOSIP trimer mixed with 25 μg of GLA-SE adjuvant and immunized i.m. in the quadriceps six times with a 1-month interval, and prebleed and postimmunization sera were collected at regular intervals. Using a similar regimen, three rhesus macaques were immunized with CH505TF gp150 trimers complexed to BMS-529 and mixed with GLA-SE adjuvant.
Neutralization assay.
Neutralization assays were performed in TZM-bl cells (NIH AIDS Research and Reference Reagent Program, contributed by John Kappes and Xiaoyun Wu) as described previously (52). Briefly, a pretitrated dose of Env-pseudotyped virus was incubated with serial 3-fold or 5-fold dilutions of test sample in duplicates in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottom culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 20 μg/ml DEAE dextran) were added to each well. One set of control wells received cells plus virus (virus control), and another set received cells only (background control). After 48 h of incubation, the cells were lysed by the addition of Britelite (PerkinElmer Life Sciences), and three-quarters of the cell lysate was transferred to a 96-well black solid plate (Costar) for measurement of luminescence. Neutralization titers were either the serum dilution (ID50) or antibody concentration (IC50) at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLUs. Assay stocks of Env-pseudotyped viruses were prepared by transfection in 293T/17 or 293S/GnT1− cells (American Type Culture Collection) and titrated in TZM-bl cells as described previously (52). For all reported results, the average RLU of virus control wells was >10 times the average RLU of cell control wells, the percent coefficient of variation (%CV) between RLU in the virus control wells was ≤30%, the percent difference between duplicate wells was ≤30%, and neutralization curves crossed the 50% neutralization cutoff 0 to 1 time. Unless otherwise stated or illustrated, the values and neutralization curves are from a single assay (duplicate wells). Additional information on the assay and all supporting protocols may be found at http://www.hiv.lanl.gov/content/nab-reference-strains/html/home.htm.
Statistical significance in differences in neutralization IC50 values was calculated by measuring the area under the curve (AUC) for each animal and comparing the AUCs in each immunogen group (with and without BMS) using an exact Wilcoxon test to derive a two-sided P value.
ACKNOWLEDGMENTS
We thank James Alin (Duke HVI) and Nathan Nicely (Duke HVI) for their contributions to the optimization of purification of membrane-bound trimers and James Alin (Duke HVI) for testing of antigenicity of SOSIP and gp150 Env trimers. We also thank Qifeng Han (Duke HVI) for nonhuman primate data analysis and Cynthia Bowman, Laura Sutherland, and Richard Scearce (Duke HVI) for veterinary technical assistance.
This work was supported by NIH, NIAID, Division of AIDS UM1 grant AI100645 for the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery (CHAVI-ID; to B.F.H.), the Duke Consortia for HIV/AIDS Vaccine Development (CHAVD; to B.F.H.) UM1-AI144371 and AI125093 (to H.D. and J.K.) and U19 AI067854 (M. Saag and J.K.).
REFERENCES
- 1.Haynes BF, Mascola JR. 2017. The quest for an antibody-based HIV vaccine. Immunol Rev 275:5–10. doi: 10.1111/imr.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Burton DR. 2017. Developing an HIV vaccine. Science 355:1129–1130. doi: 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire AT, Glenn JA, Lippy A, Stamatatos L. 2014. Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D. J Virol 88:2645–2657. doi: 10.1128/JVI.03228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders KO, Verkoczy LK, Jiang C, Zhang J, Parks R, Chen H, Housman M, Bouton-Verville H, Shen X, Trama AM, Scearce R, Sutherland L, Santra S, Newman A, Eaton A, Xu K, Georgiev IS, Joyce MG, Tomaras GD, Bonsignori M, Reed SG, Salazar A, Mascola JR, Moody MA, Cain DW, Centlivre M, Zurawski S, Zurawski G, Erickson HP, Kwong PD, Alam SM, Levy Y, Montefiori DC, Haynes BF. 2017. Vaccine induction of heterologous tier 2 HIV-1 neutralizing antibodies in animal models. Cell Rep 21:3681–3690. doi: 10.1016/j.celrep.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaBranche CC, Henderson R, Hsu A, Behrens S, Chen X, Zhou T, Wiehe K, Saunders KO, Alam SM, Bonsignori M, Borgnia MJ, Sattentau QJ, Eaton A, Greene K, Gao H, Liao HX, Williams WB, Peacock J, Tang H, Perez LG, Edwards RJ, Kepler TB, Korber BT, Kwong PD, Mascola JR, Acharya P, Haynes BF, Montefiori DC. 2019. Neutralization-guided design of HIV-1 envelope trimers with high affinity for the unmutated common ancestor of CH235 lineage CD4bs broadly neutralizing antibodies. PLoS Pathog 15:e1008026. doi: 10.1371/journal.ppat.1008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR. 2017. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46:1073.e6–1088.e6. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torrents de la Pena A, Julien JP, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens AJ, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW. 2017. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep 20:1805–1817. doi: 10.1016/j.celrep.2017.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, Jardine J, Menis S, Scheid JF, West AP, Schief WR, Stamatatos L. 2013. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski G, Georgeson E, Kalyuzhniy O, Willis JR, Kubitz M, Adachi Y, Reiss SM, Shin M, de Val N, Ward AB, Crotty S, Burton DR, Schief WR. 2017. Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nat Commun 8:1655. doi: 10.1038/s41467-017-01549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang G-Y, Schramm CA, Wiehe K, Alam SM, Bradley T, Gladden MA, Hwang K-K, Iyengar S, Kumar A, Lu X, Luo K, Mangiapani MC, Parks RJ, Song H, Acharya P, Bailer RT, Cao A, Druz A, Georgiev IS, Kwon YD, Louder MK, Zhang B, Zheng A, Hill BJ, Kong R, Soto C, NISC Comparative Sequencing Program, Mullikin JC, Douek DC, Montefiori DC, Moody MA, Shaw GM, Hahn BH, Kelsoe G, Hraber PT, Korber BT, Boyd SD, Fire AZ, Kepler TB, Shapiro L, Ward AB, Mascola JR, Liao H-X, Kwong PD, Haynes BF. 2016. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, Lynch RM, Alam SM, Moody MA, Ferrari G, Berrong M, Kelsoe G, Shaw GM, Hahn BH, Montefiori DC, Kamanga G, Cohen MS, Hraber P, Kwong PD, Korber BT, Mascola JR, Kepler TB, Haynes BF. 2014. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauthner MG, Nkolola JP, Havenar-Daughton C, Murrell B, Reiss SM, Bastidas R, Prevost J, Nedellec R, von Bredow B, Abbink P, Cottrell CA, Kulp DW, Tokatlian T, Nogal B, Bianchi M, Li H, Lee JH, Butera ST, Evans DT, Hangartner L, Finzi A, Wilson IA, Wyatt RT, Irvine DJ, Schief WR, Ward AB, Sanders RW, Crotty S, Shaw GM, Barouch DH, Burton DR. 2019. Vaccine-induced protection from homologous tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50:241.e6–252.e6. doi: 10.1016/j.immuni.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Taeye SW, de la Pena AT, Vecchione A, Scutigliani E, Sliepen K, Burger JA, van der Woude P, Schorcht A, Schermer EE, van Gils MJ, LaBranche CC, Montefiori DC, Wilson IA, Moore JP, Ward AB, Sanders RW. 2018. Stabilization of the gp120 V3 loop through hydrophobic interactions reduces the immunodominant V3-directed non-neutralizing response to HIV-1 envelope trimers. J Biol Chem 293:1688–1701. doi: 10.1074/jbc.RA117.000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin PF, Blair W, Wang T, Spicer T, Guo Q, Zhou N, Gong YF, Wang HG, Rose R, Yamanaka G, Robinson B, Li CB, Fridell R, Deminie C, Demers G, Yang Z, Zadjura L, Meanwell N, Colonno R. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci U S A 100:11013–11018. doi: 10.1073/pnas.1832214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si Z, Madani N, Cox JM, Chruma JJ, Klein JC, Schon A, Phan N, Wang L, Biorn AC, Cocklin S, Chaiken I, Freire E, Smith AB 3rd, Sodroski JG. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci U S A 101:5036–5041. doi: 10.1073/pnas.0307953101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madani N, Perdigoto AL, Srinivasan K, Cox JM, Chruma JJ, LaLonde J, Head M, Smith AB 3rd, Sodroski JG. 2004. Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J Virol 78:3742–3752. doi: 10.1128/JVI.78.7.3742-3752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pancera M, Lai YT, Bylund T, Druz A, Narpala S, O'Dell S, Schon A, Bailer RT, Chuang GY, Geng H, Louder MK, Rawi R, Soumana DI, Finzi A, Herschhorn A, Madani N, Sodroski J, Freire E, Langley DR, Mascola JR, McDermott AB, Kwong PD. 2017. Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat Chem Biol 13:1115–1122. doi: 10.1038/nchembio.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meanwell NA, Krystal MR, Nowicka-Sans B, Langley DR, Conlon DA, Eastgate MD, Grasela DM, Timmins P, Wang T, Kadow JF. 2018. Inhibitors of HIV-1 attachment: the discovery and development of temsavir and its prodrug fostemsavir. J Med Chem 61:62–80. doi: 10.1021/acs.jmedchem.7b01337. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Zhou N, Sun Y, Ray N, Lataillade M, Hanna GJ, Krystal M. 2013. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother 57:4172–4180. doi: 10.1128/AAC.00513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu M, Ma X, Castillo-Menendez LR, Gorman J, Alsahafi N, Ermel U, Terry DS, Chambers M, Peng D, Zhang B, Zhou T, Reichard N, Wang K, Grover JR, Carman BP, Gardner MR, Nikic-Spiegel I, Sugawara A, Arthos J, Lemke EA, Smith AB III, Farzan M, Abrams C, Munro JB, McDermott AB, Finzi A, Kwong PD, Blanchard SC, Sodroski JG, Mothes W. 2019. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568:415–419. doi: 10.1038/s41586-019-1101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herschhorn A, Ma X, Gu C, Ventura JD, Castillo-Menendez L, Melillo B, Terry DS, Smith AB 3rd, Blanchard SC, Munro JB, Mothes W, Finzi A, Sodroski J. 2016. Release of gp120 restraints leads to an entry-competent intermediate state of the HIV-1 envelope glycoproteins. mBio 7:e01598-16. doi: 10.1128/mBio.01598-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro JB, Nath A, Farber M, Datta SA, Rein A, Rhoades E, Mothes W. 2014. A conformational transition observed in single HIV-1 Gag molecules during in vitro assembly of virus-like particles. J Virol 88:3577–3585. doi: 10.1128/JVI.03353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, Torres JL, Copps J, Stanfield RL, Cupo A, Pugach P, Moore JP, Wilson IA, Ward AB. 2017. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547:360–363. doi: 10.1038/nature23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders KO, Wiehe K, Tian M, Acharya P, Bradley T, Alam SM, Go EP, Scearce R, Sutherland L, Henderson R, Hsu AL, Borgnia MJ, Chen H, Lu X, Wu NR, Watts B, Jiang C, Easterhoff D, Cheng HL, McGovern K, Waddicor P, Chapdelaine-Williams A, Eaton A, Zhang J, Rountree W, Verkoczy L, Tomai M, Lewis MG, Desaire HR, Edwards RJ, Cain DW, Bonsignori M, Montefiori D, Alt FW, Haynes BF. 2019. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science 366:eaay7199. doi: 10.1126/science.aay7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders KO, Nicely NI, Wiehe K, Bonsignori M, Meyerhoff RR, Parks R, Walkowicz WE, Aussedat B, Wu NR, Cai F, Vohra Y, Park PK, Eaton A, Go EP, Sutherland LL, Scearce RM, Barouch DH, Zhang R, Von Holle T, Overman RG, Anasti K, Sanders RW, Moody MA, Kepler TB, Korber B, Desaire H, Santra S, Letvin NL, Nabel GJ, Montefiori DC, Tomaras GD, Liao HX, Alam SM, Danishefsky SJ, Haynes BF. 2017. Vaccine elicitation of high mannose-dependent neutralizing antibodies against the V3-glycan broadly neutralizing epitope in nonhuman primates. Cell Rep 18:2175–2188. doi: 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson R, Watts BE, Ergin HN, Anasti K, Parks R, Xia SM, Trama A, Liao HX, Saunders KO, Bonsignori M, Wiehe K, Haynes BF, Alam SM. 2019. Selection of immunoglobulin elbow region mutations impacts interdomain conformational flexibility in HIV-1 broadly neutralizing antibodies. Nat Commun 10:654. doi: 10.1038/s41467-019-08415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, Wiehe K, Alam SM, Aussedat B, Walkowicz WE, Hwang KK, Saunders KO, Zhang R, Gladden MA, Monroe A, Kumar A, Xia SM, Cooper M, Louder MK, McKee K, Bailer RT, Pier BW, Jette CA, Kelsoe G, Williams WB, Morris L, Kappes J, Wagh K, Kamanga G, Cohen MS, Hraber PT, Montefiori DC, Trama A, Liao HX, Kepler TB, Moody MA, Gao F, Danishefsky SJ, Mascola JR, Shaw GM, Hahn BH, Harrison SC, Korber BT, Haynes BF. 2017. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci Transl Med 9:eaai7514. doi: 10.1126/scitranslmed.aai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go EP, Herschhorn A, Gu C, Castillo-Menendez L, Zhang S, Mao Y, Chen H, Ding H, Wakefield JK, Hua D, Liao HX, Kappes JC, Sodroski J, Desaire H. 2015. Comparative analysis of the glycosylation profiles of membrane-anchored HIV-1 envelope glycoprotein trimers and soluble gp140. J Virol 89:8245–8257. doi: 10.1128/JVI.00628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go EP, Ding H, Zhang S, Ringe RP, Nicely N, Hua D, Steinbock RT, Golabek M, Alin J, Alam SM, Cupo A, Haynes BF, Kappes JC, Moore JP, Sodroski JG, Desaire H. 2017. Glycosylation benchmark profile for HIV-1 envelope glycoprotein production based on eleven Env trimers. J Virol 91:e02428-16. doi: 10.1128/JVI.02428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi M, Turner HL, Nogal B, Cottrell CA, Oyen D, Pauthner M, Bastidas R, Nedellec R, McCoy LE, Wilson IA, Burton DR, Ward AB, Hangartner L. 2018. Electron-microscopy-based epitope mapping defines specificities of polyclonal antibodies elicited during HIV-1 BG505 envelope trimer immunization. Immunity 49:288.e8–300.e8. doi: 10.1016/j.immuni.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torrents de la Pena A, Sanders RW. 2018. Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies. Retrovirology 15:63. doi: 10.1186/s12977-018-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina-Ramirez M, Sanders RW, Sattentau QJ. 2017. Stabilized HIV-1 envelope glycoprotein trimers for vaccine use. Curr Opin HIV AIDS 12:241–249. doi: 10.1097/COH.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson R, Lu M, Zhou Y, Mu Z, Parks R, Han Q, Hsu A, Carter E, Blanchard SC, Edwards RJ, Wiehe K, Saunders KO, Borgnia M, Bartesaghi A, Mothes W, Haynes BF, Acharya P, Alam SM. 2019. Disruption of the HIV-1 Envelope allosteric network blocks CD4-induced rearrangements. Nature Comm 11:520. doi: 10.1038/s41467-019-14196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, Schief WR, Nemazee D, Crotty S. 2018. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV Vaccine immunogens. Immunity 48:133.e6–146.e6. doi: 10.1016/j.immuni.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Pena AT, Cupo A, Julien JP, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. 2014. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castillo-Menendez LR, Witt K, Espy N, Princiotto A, Madani N, Pacheco B, Finzi A, Sodroski J. 2018. Comparison of uncleaved and mature human immunodeficiency virus membrane envelope glycoprotein trimers. J Virol 92:e00277-18. doi: 10.1128/JVI.00277-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA, Doms RW, Baribaud F. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J Virol 76:2683–2691. doi: 10.1128/jvi.76.6.2683-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Kovacs JM, Peng H, Rits-Volloch S, Lu J, Park D, Zablowsky E, Seaman MS, Chen B. 2015. HIV-1 envelope. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein. Science 349:191–195. doi: 10.1126/science.aaa9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaBranche CC, Sauter MM, Haggarty BS, Vance PJ, Romano J, Hart TK, Bugelski PJ, Marsh M, Hoxie JA. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J Virol 69:5217–5227. doi: 10.1128/JVI.69.9.5217-5227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bultmann A, Muranyi W, Seed B, Haas J. 2001. Identification of two sequences in the cytoplasmic tail of the human immunodeficiency virus type 1 envelope glycoprotein that inhibit cell surface expression. J Virol 75:5263–5276. doi: 10.1128/JVI.75.11.5263-5276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egan MA, Carruth LM, Rowell JF, Yu X, Siliciano RF. 1996. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol 70:6547–6556. doi: 10.1128/JVI.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao Y, Wang L, Gu C, Herschhorn A, Xiang SH, Haim H, Yang X, Sodroski J. 2012. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat Struct Mol Biol 19:893–899. doi: 10.1038/nsmb.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol 87:1554–1568. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennison SM, Reichartz M, Seaton KE, Dutta S, Wille-Reece U, Hill AVS, Ewer KJ, Rountree W, Sarzotti-Kelsoe M, Ozaki DA, Alam SM, Tomaras GD. 2018. Qualified biolayer interferometry avidity measurements distinguish the heterogeneity of antibody interactions with Plasmodium falciparum circumsporozoite protein antigens. J Immunol 201:1315–1326. doi: 10.4049/jimmunol.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynch HE, Stewart SM, Kepler TB, Sempowski GD, Alam SM. 2014. Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J Immunol Methods 404:1–12. doi: 10.1016/j.jim.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montefiori DC. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]