Introduction
Patients with end-stage cardiomyopathy undergoing left ventricular assist device (LVAD) implant frequently exhibit high-epicardial scar burden that correlates with the risk of postimplant ventricular arrhythmias.1 Radiofrequency (RF) ablation of critical circuit components via a percutaneous subxyphoid approach2 improves freedom from ventricular arrhythmias3,4 but is complicated in patients post-LVAD owing to difficulty accessing the pericardium. Mapping and ablation of arrhythmia circuits during LVAD implant is an area of growing interest; however, substrate-based methods for identifying critical ventricular tachycardia (VT) circuits in this setting are not well defined. We present a case where epicardial, high-density electroanatomic (EA) substrate mapping using the HD-Grid catheter (Abbott Park, IL) identified critical components of a VT circuit during sinus rhythm in a patient with a history of VT storm undergoing LVAD implant.
Key Teaching Points.
-
•
Mapping and ablation of ventricular tachycardia during left ventricular assist device implant is a growing field with important clinical implications.
-
•
Mapping software and hardware need to account for challenges from the open chest environment.
-
•
High-density electroanatomic mapping during sinus rhythm can identify critical components of a ventricular tachycardia circuit that can be targeted with focal ablation.
Case report
A 72-year-old man with ischemic cardiomyopathy (ejection fraction 10%–15%), 4-vessel coronary artery bypass graft in 2004, recurrent VT, and prior endocardial and epicardial ablations presented with VT storm and cardiogenic shock. Previous ablation details were not available. Electrocardiogram (ECG) was consistent with slow VT emanating from a basal, anterolateral, epicardial exit (inferior axis, positive precordial concordance, pseudodelta wave >75 ms, and maximal deflection index >0.59) (Figure 1, top).5,6 Transthoracic echo revealed ejection fraction 10%–15% with global hypokinesis that made it difficult to assess particular akinetic/hypokinetic regions. Amiodarone was administered intravenously. Extracorporeal membrane oxygenation and Impella (Abiomed, Danvers, MA) were placed for recurrent VT and progressive cardiogenic shock and the patient was referred for LVAD implantation with intraoperative epicardial VT mapping and ablation.
Figure 1.
Ventricular tachycardia (VT) electrocardiograms (ECG). Top: Twelve-lead ECG of clinical VT. Axis and morphology consistent with epicardial, basal, anterolateral focus. Bottom: Twelve-lead ECG of VT post–left ventricular assist device (LVAD) implant. Inferior apical septum exit site is consistent with an LVAD cannula origin.
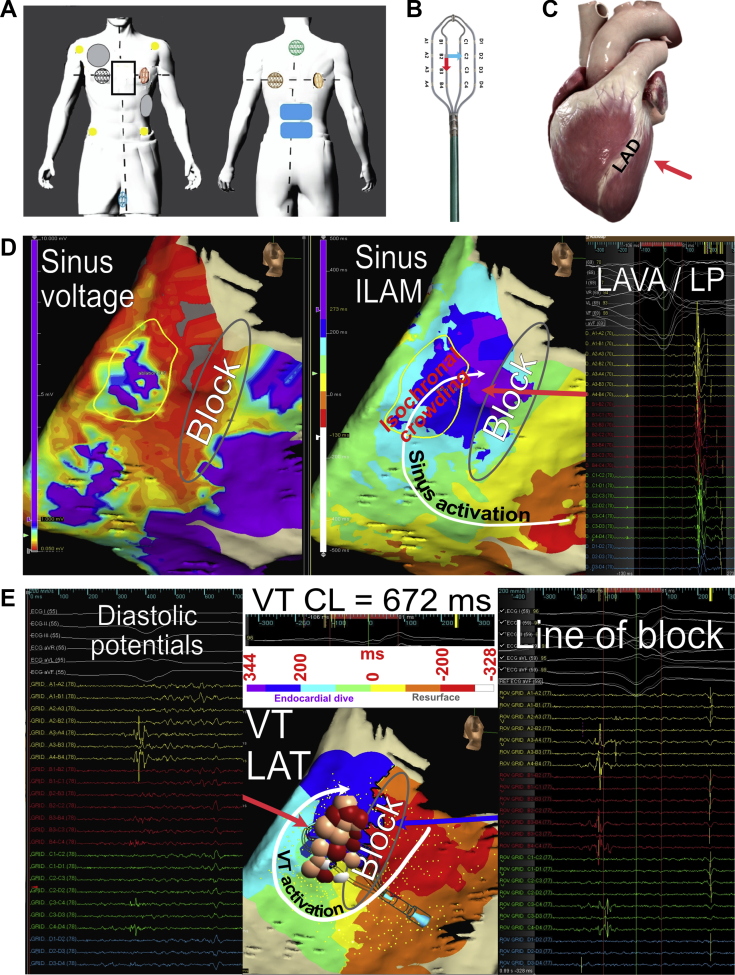
The EnSite Precision system (Abbott, Minneapolis, MN) was utilized in an impedance-only mode. Magnetic-based mapping was not used because the mapping magnet could not be positioned underneath the patient owing to operating table constraints. The RecordConnect was connected from the EnSite amplifier to an ECG cable and pin boxes in order to obtain electrograms, while the end connecting to the recording system was left unattached. The Ampere RF generator, CoolPoint pump, and TactiSys contact force module were utilized with the TactiCath SE for ablation. Pooling of blood in the area of interest provided an electrolyte-rich medium to permit impedance-based mapping. However, heparinized saline was available to fill the chest if mapping of the anterior regions of the heart was needed. Precordial leads were not placed owing to the sternotomy, and limb leads were used as EA reference points. The EnSite mapping patches and grounding patches were positioned as depicted in Figure 2A.
Figure 2.
Electroanatomic (EA) mapping. A: Placement of pads. Yellow circles: electrocardiogram limb electrodes; gray circles: defibrillation pads; black rectangle: sternotomy; colored circles: impedance mapping pads; blue rectangles: grounding pads. B: HD-Grid catheter (Abbott, Minneapolis, MN) depicting electrode and bipole orientation. Reprinted with permission from Abbott. C: Schematic of heart with red arrow identifying region that was mapped and ablated. Printed and modified with permission from Complete Heart Version 1.2, 3D4D medical. D: Sinus EA map. Left panel: Color scheme represents voltages from 0.05 to 1 mV. Region with scar (yellow circle) and region of conduction block (gray oval) are highlighted. Middle panel: Sinus isochronal late activation map (ILAM) depicting zones with conduction block (gray oval) and isochronal crowding (slow activation). Right panel: Example of electrical signals from each bipole on the HD-Grid demonstrating local abnormal ventricular activations (LAVA) and late potentials (LP) in the area with isochronal crowding. E: Ventricular tachycardia (VT) EA map. Left panel: HD-Grid positioned across the slow activation zone revealing fractionated, low-voltage, diastolic potentials, consistent with VT isthmus. Middle panel: Isochronal local activation time map (LAT) with arrow depicting a macroreentrant circuit ending with an endocardial dive. Gray oval corresponds to region of block observed during sinus activation mapping. Red and pink dots correspond to ablation lesions color coded by impedance drop (0–5, 5–10, >10 ohms). Right panel: HD-Grid positioned across the superior portion of the VT circuit revealing widely spaced doubles, consistent with conduction block. CL = cycle length.
The surgeons meticulously dissected the pericardium and exposed the vein grafts. Afterwards, cardiopulmonary bypass was initiated and the surgeon visually positioned the HD-Grid catheter along the lateral surface of the heart (Figure 2C). Sinus voltage and activation maps (Figure 2D) were built. A total of 20,204 points were collected in 10.4 minutes, with 2120 points over the 8 × 8-cm area of interest (supplementary video 1). EA sinus voltage map revealed a low-voltage area (Figure 2D, left panel, yellow circle) with local abnormal ventricular activation (LAVA) signals, which coincided with a zone of relatively slow, late conduction observed during isochronal late activation mapping of late potentials (LP) (Figure 2D, middle panel, right panel). Epicardial activation discontinuity was observed along the area of dense scar, possibly representing an area of conduction slowing or block (Figure 2D, gray oval).
Burst ventricular pacing from the patient’s implantable cardioverter-defibrillator at 375 ms induced monomorphic VT, which was mapped and targeted. Unconventional lead placement made localization by ECG criteria difficult. A total of 7837 points were collected in 4.4 minutes, with 985 points over a 5 × 6-cm area of interest (supplementary video 2). Activation mapping revealed a clockwise reentrant pattern around the line of conduction block, encompassing the region of slow conduction (Figure 2E, middle panel, white arrow). Conduction block with widely split double potentials was observed, coinciding with the region of block observed during sinus mapping (Figure 2E, middle panel, gray oval; and right panel). VT perpetuated through this area, suggesting wavefront breakthrough via mid- or endocardial connections, which would account for the missing cycle length (Figure 2E, middle panel, gray and purple arrows, gray oval). Diastolic potentials were observed across the deceleration zone (Figure 2E, left panel, and middle panel, red arrow). The possibility of a critical isthmus was suspected based on the activation characteristics in both sinus rhythm and VT and used to guide what would be a time-limited ablation strategy. VT terminated during mapping and was not easily induced. Attempts to induce were limited by operating room time constraints.
An irrigated TactiCath SE bidirectional catheter (Abbott) was placed on the epicardium by the surgeon under direct visualization. A region that included the suggested VT isthmus and LAVA area was targeted with RF ablation (Figure 2E, middle panel). We empirically chose 20 W with a maximum temperature set to 45°C, for 30 seconds for all ablation lesions, based on limited experience ablating under these circumstances. Fifteen ablation lesions were delivered with a total ablation time of 7.5 minutes. VT was not reinduced owing to time constraints in the operating room and decision to proceed with the LVAD implant procedure. The procedure added 45 minutes to the case (organizing, induction attempts, mapping, ablating).
At 60 days of follow-up, 2 episodes of VT were observed, both of which were terminated with antitachycardia pacing. A 12-lead ECG obtained during 1 of these events demonstrated slow VT exiting from the inferior apical septum, which is different than the preprocedural VT (Figure 1, bottom).
Discussion
EA mapping and RF ablation of epicardial VT during LVAD implant is an area of growing interest and investigation.1,7 Protocols for expeditious substrate mapping in an open-chest setting are needed for minimizing cardiopulmonary bypass time.
A previously published study1 used closely spaced multipolar catheters to identify low-voltage tissue and late potentials in sinus rhythm. These areas were then targeted for homogenization/ablation. The current study expands on this by combining sinus and VT mapping to correlate electrical features on the sinus map (ie, zones of slow conduction, late potentials, LAVAs) with critical components of the arrhythmia circuit.
In this case we used the HD-Grid catheter together with the EnSite Best Duplicate and Last Deflection algorithms to expeditiously build a high-density (>27,000 points) EA map during sinus rhythm and VT. The mapping catheter’s fixed 3-3-3-mm dimensions (Figure 2B) was also useful for field linearization and scaling, an algorithm that corrects distortions introduced into the impedance-based EA map from the presence of high-impedance air in between the mapping electrodes and the surface mapping patches.
The mapping catheter’s design enables bipolar recordings across and along splines (Figure 2B), which when used together with the mapping system’s Best Duplicate algorithm improves detection of low-amplitude LPs and LAVAs.8 Substrate-based ablation strategies that eliminate LAVAs9 and LPs during sinus rhythm improve long-term freedom from VT.
The Last Deflection sensing algorithm automatically annotates the latest deflections of the local electrogram during sinus rhythm, which when organized into isochronal activation maps identifies deceleration zones.10 Deceleration zones are areas of slow conduction during sinus rhythm (<0.6 m/s) that correlate with critical isthmuses of VT circuits.11,12
The intraoperative VT localized to the lateral wall as predicted by the preprocedural ECG. However, atypical ECG lead positioning limited direct morphologic comparison of the intraoperative VT to the clinical VT. VT spontaneously terminated during mapping and could not be easily induced. Ablation was performed in sinus rhythm and classic VT entrainment maneuvers could not be performed. Critical arrhythmia circuit elements were presumed based on diastolic potentials in VT that correlated with areas of slow conduction in sinus rhythm. The clinical VT was not observed post-procedure, suggesting the clinical VT had been eliminated.
Rapid mapping of LPs and LAVAs and identification of deceleration zones may eliminate the need for inducing and mapping VT, thereby reducing procedure time. Clinical outcomes using this technical approach should be evaluated with prospective trials.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Kristin H. Pallister is an employee of Abbott.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.06.023.
Appendix. Supplementary data
Sinus activation map.
VT activation map.
References
- 1.Moss J.D., Oesterle A., Raiman M. Feasibility and utility of intraoperative epicardial scar characterization during left ventricular assist device implantation. J Cardiovasc Electrophysiol. 2019;30:183–192. doi: 10.1111/jce.13803. [DOI] [PubMed] [Google Scholar]
- 2.Shirai Y., Liang J.J., Santangeli P. Comparison of the ventricular tachycardia circuit between patients with ischemic and nonischemic cardiomyopathies. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007249. [DOI] [PubMed] [Google Scholar]
- 3.Gokoglan Y., Mohanty S., Gianni C. Scar homogenization versus limited-substrate ablation in patients with nonischemic cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol. 2016;68:1990–1998. doi: 10.1016/j.jacc.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S., Baldinger S.H., Romero J. Substrate-based ablation versus ablation guided by activation and entrainment mapping for ventricular tachycardia: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1437–1447. doi: 10.1111/jce.13088. [DOI] [PubMed] [Google Scholar]
- 5.Josephson M.E., Callans D.J. Using the twelve-lead electrocardiogram to localize the site of origin of ventricular tachycardia. Heart Rhythm. 2005;2:443–446. doi: 10.1016/j.hrthm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Valles E., Bazan V., Marchlinski F.E. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71. doi: 10.1161/CIRCEP.109.859942. [DOI] [PubMed] [Google Scholar]
- 7.Moss J.D., Raiman M.J., Flatley E.E., Ota T., Jeevanandam V., Uriel N. Open chest epicardial voltage mapping during left ventricular assist device implant in patients with end-stage heart failure. J Heart Lung Transpl. 2016;35:S39. [Google Scholar]
- 8.Okubo K., Frontera A., Bisceglia C. Grid mapping catheter for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007500. [DOI] [PubMed] [Google Scholar]
- 9.Sacher F., Lim H.S., Derval N. Substrate mapping and ablation for ventricular tachycardia: The LAVA approach. J Cardiovasc Electrophysiol. 2015;26:464–471. doi: 10.1111/jce.12565. [DOI] [PubMed] [Google Scholar]
- 10.Raiman M., Tung R. Automated isochronal late activation mapping to identify deceleration zones: Rationale and methodology of a practical electroanatomic mapping approach for ventricular tachycardia ablation. Comput Biol Med. 2018;102:336–340. doi: 10.1016/j.compbiomed.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Aziz Z., Shatz D., Raiman M. Targeted ablation of ventricular tachycardia guided by wavefront discontinuities during sinus rhythm: A new functional substrate mapping strategy. Circulation. 2019;140:1383–1397. doi: 10.1161/CIRCULATIONAHA.119.042423. [DOI] [PubMed] [Google Scholar]
- 12.Irie T., Yu R., Bradfield J.S. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia. Circ Arrhythm Electrophysiol. 2015;8:390–399. doi: 10.1161/CIRCEP.114.002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sinus activation map.
VT activation map.