Abstract
Background
Spontaneous intracranial hypotension (SIH) is an underdiagnosed disease. Its incidence is estimated at 5 per 100 000 persons per year.
Methods
This review is based on a selective literature search in PubMed covering the years 2000–2019, as well as on the authors’ personal experience.
Results
The diagnostic and therapeutic methods discussed here are supported by level 4 evidence. SIH is caused by spinal leakage of cerebrospinal fluid (CSF) out of ventral dural tears or nerve root diverticula, or, in 2–5% of cases, through a fistula leading directly into the periradicular veins (CSF-venous fistula). In half of all patients, no CSF leak is demonstrable. A low CSF opening pressure on lumbar puncture is present in only one-third of patients; imaging studies are thus needed to confirm and localize a spinal CSF leak. Half of all patients in whom myelographic computed tomography (CT) reveals contrast medium reaching the epidural space have ventral dural tears, which tend to be located at upper thoracic spinal levels. Epidural blood patches applied under fluoroscopic or CT guidance can seal the CSF leak in 30–70% of patients, but 90% of patients with ventral dural tears will need operative closure. Some patients who have no visible epidural contrast medium on CT presumably do not have SIH, while others do, in fact, have a CSF leak from a diverticulum or a CSF-venous fistula and will need to have the site of the leak demonstrated with the aid of further studies, such as dynamic (subtraction) myelography in the lateral decubitus position.
Conclusion
The management of patients with SIH calls for complementary imaging studies to demonstrate the causative spinal CSF leak. Often, successful treatment requires surgical closure of the leak. In view of the sparse evidence available to date, controlled studies should be performed.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submissions is 5 July 2021.
Spontaneous intracranial hypotension was first described in 1938 by Georges Schaltenbrand, a neurologist in Würzburg, Germany, who named it “hypoliquorrhea” (1, 2). Isolated case reports appeared in the 1940s, and the syndrome has been described many times in print since the mid-1990s (3– 9).*
Schievink et al. carried out an observational study in the greater Los Angeles area to determine the incidence of SIH. 11 patients with SIH and 23 with spontaneous subarachnoid hemorrhage (SAH) were seen in an emergency department (10, 11). On the basis of an assumed incidence of 10 cases of SAH per 100 000 persons per year, an annual incidence of 5 SIH cases per 100 000 persons was derived.
In our neurosurgery department, the number of referrals of patients with SIH and the corresponding catchment area have grown steadily larger: 25 patients with SIH were treated in 2016 and another 25 in 2017, 35 in 2018, and 89 in 2019. Women are affected twice as commonly as men; the average age of onset is 40 years (7, 11– 12).
The condition is pathogenetically separated from that of postlumbar puncture headache and from postoperative cerebrospinal fluid (CSF) loss (13). Clinical differential diagnoses include postural orthostatic tachycardia syndrome (POTS), Chiari I malformation, and cervicogenic headache (12, 13).
Clinical features
The most prominent symptom is positionally dependent headache that is worse in the standing position and tends to increase in severity over the course of the day. Rapid onset is typical: most patients can identify “the day it all began” (14, 15). The positional dependence of the headache, and its being most pronounced in the back of the head, can be explained as the result of the the following causal sequence: low CSF volume—sagging of the brain—tension on the cranial nerves and dura mater. The dura mater of the posterior fossa is especially sensitive to tension (14).
* Georges Schaltenbrand (1897–1979) carried out experiments on human beings in 1940 that were illegal even according to the laws of the time and were in violation of ethical standards. To support his hypothesis that multiple sclerosis was an infectious illness, he injected cerebrospinal fluid from monkeys that were (supposedly) infected with MS, as well as directly from MS patients, into persons suffering from severe mental illness or dementia, without their consent. Legal proceedings against Schaltenbrand were terminated in 1948, and he was able to pursue his academic career without further impediment.
In patients who have been suffering from SIH for longer periods—the average latency from symptom onset to diagnosis is three months—the headaches can lose their positional dependence, or even worsen when the patient reclines (12, 14– 16). About half of all patients complain of auditory disturbances (“ringing in the ears,” tinnitus, a pressure sensation in the ear), and some are initially treated for sudden hearing loss or suspected Ménière’s disease (17). SIH can have a wide range of further presentations, ranging all the way to coma or apparent frontotemporal dementia (14– 18) (box).
BOX. ICHD-3 diagnostic criteria (13) and clinical manifestations of spontaneous intracranial hypotension.
-
Definition
Orthostatic headache of spontaneous onset that is due to low CSF pressure or a CSF leak, and is usually accompanied by nuchal pain, tinnitus, auditory disturbances, phonophobia, and nausea. Remission of symptoms after normalization of CSF pressure or closure of the CSF leak.
-
Diagnostic criteria
Any kind of headache in temporal relation to low CSF pressure or CSF leak.
CSF pressure <60 mm H2O and/or evidence of a CSF leak on an imaging study (head MRI, spinal MRI, myelography, myelographic CT, dynamic subtraction myelography)
No other ICHD-3 diagnosis is more suitable.
-
Clinical manifestations
Orthostatic headache (75–80% of patients) (14)
Tinnitus, auditory disturbance (“underwater feeling”), dizziness (approx. 50% of patients) (10, 17, 26)
Nausea, vomiting, photophobia, meningismus (approx. 20% of patients) (10, 26)
Other types of headache: position-independent, thunderclap, on exertion, on coughing, in second half of the day, worsening on lying down (approx. 20% of patients) (10, 14)
Diplopia: abducens, oculomotor nerve, or trochlear nerve palsy, visual field defect (<10% of patients) (5, 10)
The symptom complex of leptomeningeal hemosiderosis (sensorineural hearing loss, ataxia, dementia) (5–10% of patients) (16)
Facial pain, facial palsy, facial spasms (<5% of patients)
Galactorrhea, hyperprolactinemia (<5% of patients) (16)
(Position-dependent) Unconsciousness (<1% of patients) (10, 16)
Cervical myelopathy, tetraplegia, bulbar palsy (single case reports) (16)
Symptom complex (and MRI changes) of the posterior reversible encephalopathy syndrome (PRES) or the reversible cerebral vasoconstriction syndrome (RCVS) (single case reports) (16)
Dural sinus and/or bridging vein thrombosis (single case reports) (16)
Parkinson’s syndrome, position-dependent tremor, chorea, ataxia (single case reports) (16)
Unilateral hearing loss, acute hearing loss (single case reports) (16)
Benign peripheral positioning vertigo (single case reports) (16)
Non-convulsive status epilepticus (single case reports) (16)
Symptom complex of progressive paraparesis due to secondary spinal cord herniation (single case reports)
Diagnostic evaluation
As stated in the criteria of the International Classification of Headache Disorders (ICHD-3), the diagnosis of SIH is based on an opening pressure of less than 60 mm H2O that is measured in a lumbar puncture performed with the patient in the lying position, or else on the demonstration of a CSF leak by an imaging study (box) (13).
The CSF “loss” is nearly always situated in the spine, usually as the result of a ventral, vertical dural tear, a laterally located nerve root diverticulum, or (rarely) a CSF-venous fistula, i.e., a direct connection between a nerve root sheath and the periradicular veins (19– 21). Only approximately one-third of patients with SIH have a CSF opening pressure below 60 mm H2O. The opening pressure can, in fact, be normal or even elevated, particularly in patients with a long history of SIH, or with large abdominal girth (22). This fact only underscores the importance of demonstrating a spinal CSF leak (or the indirect consequences of such a leak) with imaging studies and further ancillary studies, in order to support the diagnosis of SIH.
At present, there is no single, absolutely reliable test or biomarker (no imaging study, blood test, or CSF test) that establishes the diagnosis of SIH. The diagnostic algorithm presented in the table 1 has been developed, and is currently used, in several centers with large case numbers.
Table. Diagnostic and therapeutic algorithms for SIH.
| Diagnostic algorithm | |
| Type of diagnostic study | Goal of study and pathological findings |
| Head MRI | Demonstration of SIH signs and potential complications (SIH: homogeneous, linear pachymeningeal contrast enhancement, subdural hematoma or hygroma, dilated dural venous sinuses, mamillopontine distance <6.5 mm, narrowing of the suprasellar cistern to <4 mm and of the prepontine csf space to <5 mm, kinking of the midbrain with respect to the pons, diminished interpeduncular angle, enlarged pituitary gland, low cerebellar tonsils,…) |
| Spinal MRI | Demonstration of epidural fluid and/or meningeal diverticula |
| Possibly, transorbital ultrasonography | Measurement of the optic nerve sheath diameter in the lying and standing positions (SIH: >5% decrease of diameter) |
| CSF pressure measurement | Measurement of the CSF opening pressure (SIH: <60 mm h2o) (beware: only in one-third of cases) |
| Possibly, CSF infusion test | Measurement of CSF outflow resistance, Rcsf (SIH: <5 mmhg/ml × min in the first 3 months) |
| Dynamic myelography | Demonstration of a CSF leak with contrast medium egress into the epidural space |
| Myelo-CT | Demonstration of epidural contrast medium |
| Possibly, dynamic CT myelography | Precise localization of the CSF leak
|
| Possibly, dynamic subtraction myelography | Demonstration of a CSF-venous fistula (study performed with patient in the lateral decubitus position) |
| Therapeutic algorithm | |
| Measure | Means of performance |
| Bed rest, hydration, caffeine, possibly theophylline | |
| Epidural blood patch | Under fluoroscopic or CT guidance; possibly, multiple blood patches |
| Surgery | Indicated if the site of the leak has been precisely localized and epidural blood patches have been ineffective; as an emergency procedure in case of rapid progression or life-threatening manifestations |
CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging; SIH, spontaneous intracranial hypotension
Magnetic resonance imaging of the head with contrast enhancement
Magnetic resonance imaging (MRI) of the head is performed in order to detect the sequelae and potential complications of spinal CSF loss that can be seen on this imaging modality. Spinal CSF loss leads to “sagging” of the brain, and consequently to compensatory dilatation of the intracranial venous structures, as a consequence of the so-called Monro–Kellie doctrine: the volume of the intracranial compartment being constant and composed of the three elements brain, blood, and CSF, CSF loss must be compensated for by increased blood volume. Sagging of the brain can cause a subdural hematoma or hygroma, kinking of the midbrain and pons toward the clivus, lessening of the distance (e.g.) from the optic chiasm to the pituitary gland, and tension on the cranial nerves. The compensatory dilatation of venous structures leads to increased dural contrast enhancement, enlargement of the dural venous sinuses and of the cranial nerves, and enlargement of the pituitary gland. Increased, homogenous contrast enhancement of the dura mater is the most sensitive sign.
Overall, the head MRI findings of SIH are specific, but only moderately sensitive (23). Space-occupying subdural hematomas are the main finding of immediate therapeutic significance. Approximately one-fourth of all subdural hematomas in persons under 60 years old are due to SIH, rather than trauma (24).
Magnetic resonance imaging of the spine
As the CSF leak is located in the spine in all but a few exceptional cases, T2-weighted MR images are obtained, in order to look for epidural fluid collections. Nonetheless, spinal MRI usually does not reveal the exact site of the causative SF leak.
Transorbital ultrasonography
In SIH, but not in POTS (an important differential diagnosis, see above), the width of the CSF-filled optic nerve sheaths diminishes as the patient stands up from a lying position. Decreases of more than 5% are considered pathological (25, 26). Transorbital ultrasonography is also suitable for follow-up examinations and for the differential diagnosis of idiopathic intracranial hypertension (IIH) (25).
CSF opening pressure measurement and lumbar infusion test
The subarachnoid space is entered with the tip of a 20-gauge needle, and the CSF opening pressure is measured manometrically. Then, 2 mL/min of Ringer’s lactate are injected intrathecally via perfusor, and a pressure curve is obtained. This enables calculation of the outflow resistance, which is markedly lower in patients with early-stage SIH than in persons without a CSF leak (27). The performance of this test and the interpretation of the findings require a certain degree of experience. It is mainly useful for differential diagnosis in patients who have had their symptoms for only a short time (27).
Dynamic myelography in the prone or lateral decubitus position
Boli of contrast medium are introduced into the subarachnoid space through an LP needle. The patient is prone at first, and the head of the table is then lowered while lateral fluoroscopic imaging of the back is performed. The goal is to visualize the egress of contrast medium as it runs in a cranial direction on the ventral side of the subarachnoid space.
If the spinal MRI arouses suspicion of CSF loss from meningeal diverticula, this study is performed in the lateral decubitus position with horizontal (i.e., anteroposterior) fluoroscopy.
Myelo–computed tomography
Myelo–computed tomography (myelo-CT) is subsequently performed to determine whether any constrast medium lies in the epidural space, and, if so, then at what level.
Dynamic CT myelography
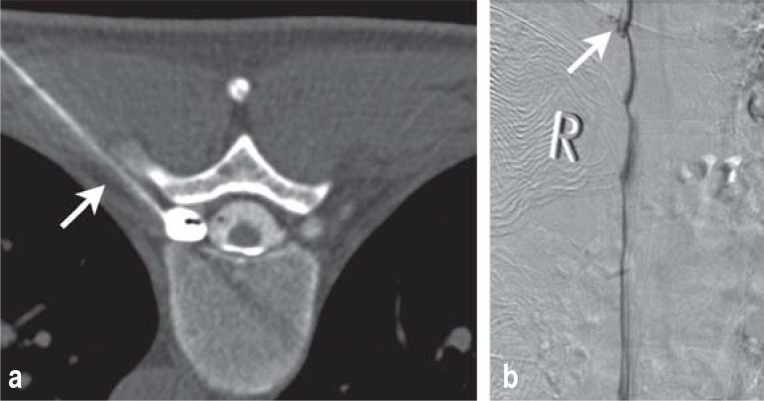
Dynamic myelography and subsequent myelo-CT fail to localize the spinal CSF leak precisely in half of all cases. If the presence of a leak has been demonstrated, a dynamic CT myelography is performed to localize it. A lumbar puncture is performed, and the patient is positioned in the CT scanner with the head down. The target area is repetitively imaged with one or more spirals while contrast medium is injected. Ideally, the site of the leak can then be seen as an uninterrupted connection between the subarachnoid and epidural spaces (Figure 1b).
Figure 1.
a ventral dural tear at the level of the T3/4 intervertebral disc
Drawing of a CSF leak caused by a disk spur tearing the dura mater.
Dynamic CT myelography showing contrast medium egress from the subarachnoid space into the epidural space at the level of the T3/4 intervertebral disc (open arrow). In this case, the contrast medium flows in a cranial direction in the epidural space beyond the site of the leak (arrows).
Drawing of the surgical field: after the dura mater has been opened, the vertical tear in the ventral dura mater and the penetrating disk spur can be seen.
Intraoperative image showing the tear and the disk spur, about the size of a kernel of rice.
(Illustrations in Figures 1a and 1c: Anja Giger, Bern)
This type of study delivers a large radiation load to the patient and should, therefore, only be performed after therapeutic measures such as epidural blood patches have failed (28).
Dynamic digital subtraction myelography
Dynamic digital subtraction myelography (DSM) is reserved for patients whose symptoms, history, and test findings imply probable SIH, but whose myelo-CT does not reveal any epidural contrast medium. DSM can then be used to demonstrate the direct movement of contrast medium from the subarachnoid space into a vein (CSF-venous fistula). In this type of study, the patient—who may be under general anesthesia—is positioned in the lateral decubitus position with the head down. Contrast medium is infused into the lumbar subarachnoid space at 1 mL/sec, and digital subtraction images are obtained 1 to 2 times per second (29) (Figure 2b).
Figure 2.
Spontaneous intracranial hypotension (SIH) without CSF egress into the epidural space
This 39-year-old man has a large meningeal diverticulum of the right T8 nerve root, which was filled with contrast medium before CT-guided injection of the patient’s own blood into it through a 20-gauge needle (arrow).
This 65-year-old man suffered from SIH due to a CSF-venous fistula of the right T9 nerve root, with direct entry of the contrast medium into the periradicular veins (arrow).
Treatment
The treatment options are based solely on descriptive studies, as no controlled studies have been published to date. Moreover, it remains unknown how many patients recover from SIH without any specific treatment (e.g., with self-directed bed rest) (10).
As a first step, bed rest is supplemented with hydration, caffeine (200 mg po tid to 300 mg po qid), and possibly also theophylline (30). Bed rest should be of short duration, a few days at most.
If the symptoms fail to improve, an epidural blood patch (or fibrin-glue patch) is applied. An epidural blood patch can be applied without any imaging with the aid of the loss-of-resistance method—the administering physician detects penetration into the epidural space as a loss of resistance to passage of the needle right after the tip has crossed the ligamentum flavum—or else under fluoroscopic or CT guidance, often after prior CT myelography (Figures 2a, 3) (31, 32).
Operative closure of the spinal CSF leak is indicated if the symptoms persist despite less invasive treatment measures and if the spinal CSF leak has been definitively localized (20) (Figure 1). At surgery, the leak can be closed microsurgically with simple suturing, or with an adhesive patch on the dura mater. With current neurosurgical methods, nerve root diverticula, direct CSF-venous fistulae, and laterally and ventrally located dural tears can be reached through a dorsal approach and closed safely and with minimal invasiveness through an interlaminar fenestration or hemilaminectomy (20, 33). Ventral dural tears require a transdural approach with detachment of the denticulate ligaments, so that the spinal cord can be mobilized out of the way under intraoperative neuromonitoring (20, 33).
Discussion
For many years, patients suffering from SIH were often incorrectly diagnosed. The symptoms of SIH are highly variable, and the characteristic orthostatic headache is sometimes present only at the onset of the disease. In the later course of SIH, the symptoms can change and become more diffuse. Headache, for example, can be present even with the patient recumbent. Moreover, SIH can manifest itself with chronic subdural hematomas, particularly in younger patients (24); headache during the second half of the day; or even dementia or coma (18). Patients are often unable to work for months at a time, and it is not at all uncommon for them to receive the diagnosis of burnout or a psychosomatic stress reaction (34).
The diagnosis of SIH can be firmly established through multiple types of study whose indications are complementary. The proof of a spinal CSF leak is contrast medium egress into the epidural space, which can be demonstrated in half of all cases (18). In turn, the site of egress is a ventral, vertically oriented dural tear in about half of such cases, a meningeal diverticulum in about 20%, and unidentifiable in 30%. In the other half of all cases, no contrast medium is seen exiting into the epidural space, either because the CSF directly enters a vein (2–5% of cases), or else because the volume of CSF loss may be too small to detect by CT myelography, by off-label MR myelography with intrathecally injected gadolinium, or by intrathecal 111In-DTPA radionuclide scintigraphy, a technique that is only rarely used today (35). The disadvantages of the latter two techniques compared to CT myelography are their poorer spatial resolution and, above all, their inadequate temporal resolution, which can make precise localization of the CSF leak difficult or impossible.
Finally, if no egress of contrast medium into the epidural space can be demonstrated, POTS and other types of headache (cervicogenic headache, chronic tension headache) should be reconsidered in the differential diagnosis (12, 13).
Once the diagnosis of SIH has been made clinically and on the basis of the imaging studies (box), the initial treatment can be conservative, with bed rest, hydration, caffeine, and perhaps also theophylline. Conservative treatment succeeds in far fewer than half of all patients (30).
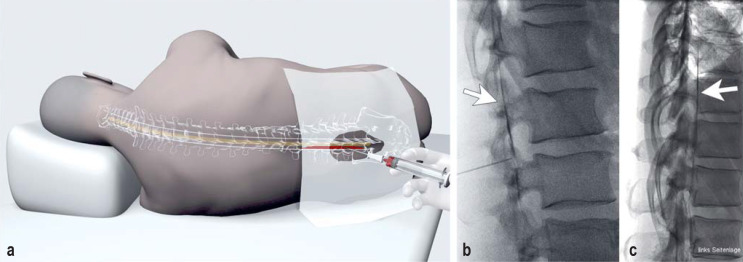
The application of one or more blood patches renders 30–70% of patients with SIH asymptomatic (32– 33). Patients often report an improvement of short duration, lasting from 1–2 days to 2 weeks. The patch can be applied in a non-targeted fashion or under fluoroscopic or CT guidance, and it may consist of either blood or fibrin glue. Contrast-medium–guided applications are superior to the loss-of-resistance method, in which no imaging study is performed (36, 37) (Figures 2a, 3).
Figure 3.
Fluoroscopically guided application of an epidural blood patch
With the patient in the lateral decubitus position, the epidural space is punctured precisely in the midline at the levels of the L2/3 to L5/S1 intervertebral discs (a, b). The physician feels the entry of the needle tip into the epidural space immediately just outside the dorsal dura mater of the lumbar theca as a sudden loss of resistance, when the tip passes through the ligamentum flavum. The loss-of-resistance sensation can be enhanced with the use of specially designed fluid or air injections. The correct position of the needle and the free flow from its tip are tested in a trial injection of contrast medium with the patient’s head slightly lowered (b, c: arrows); next, blood sterilely obtained from a forearm vein is injected through the needle. Our injection target volume is at least 30 mL. If the blood does not distribute itself freely, the patient reports buttock pain and sciatica a short time later. If this happens, the puncture can be continued at another level. (Illustration in Figure 3a: Anja Giger, Bern)
There is no consensus on whether the epidural blood patch must be placed precisely over the site of the spinal CSF leak, or whether, alternatively, it might suffice to raise the pressure in the epidural space with a non-targeted patch application (31, 36– 38). Punctures at multiple lumbar levels may be needed to deliver the epidural blood to the site of the leak; the technique involves demonstration of an uninterrupted flow of contrast medium (and thus, presumably, of the subsequently injected blood as well) epidurally in the cranial direction.
20–100 mL of blood are injected (32). Higher volumes are correlated with better therapeutic outcomes (39). Complications only rarely result from injections of contrast-medium and blood into the epidural space, even if they have to be performed repeatedly: among more than 300 patients who underwent diagnostic myelography and epidural blood patching in our institution from 2016 onward, only one developed clinically symptomatic aseptic meningitis, which lasted for two days.
Some patients report a sensation of pressure in the head during the injection. 17% of patients treated with either epidural blood patching or surgery develop symptoms of a transient conversion of SIH into intracranial hypertension (27, 33, 40). Idiopathic intracranial hypertension (IIH), a disease defined (among other criteria) by an elevated CSF opening pressure at lumbar puncture (for non-obese patients, >200 mm H2O; for patients with BMI >30, >250 mm H2O), can be regarded as the “opposite” of SIH in many ways. The main clinical features of IIH are headache, papilledema, and visual disturbances, and MRI reveals a lessening of volume in the intracranial CSF spaces, with widening of the CSF spaces in the optic nerve sheaths.
Headaches due to intracranial hypo- and hypertension may be difficult to tell apart on clinical grounds alone. Follow-up studies such as MRI and ocular ultrasonography can help. In rare cases, the symptoms of so-called rebound intracranial hypertension can persist for weeks or months and may need to be treated with drugs (acetazolamide, topiramate), or even lumbar punctures or a CSF shunting procedure.
The precise neuroradiological localization of the CSF leak is a prerequisite to its surgical closure. In doubtful cases, dynamic myelography and CT myelography may need to be repeated; even so, localization is faulty in 5% of cases (33). The preferential site of ventral dural tears is the upper portion of the thoracic spine: in our patient collective, 50% of the dural tears were at either T1/2 or T2/3 (33).
Less invasive surgical techniques for the repair of ventral dural tears have been developed in recent years. These can now be operated on through a dorsal approach, obviating the need for invasive ventral approaches with vertebrectomy (33). In particular, the introduction of transdural neurosurgical approaches with intraoperative neuromonitoring has led to very high closure rates (>95%), low recurrence rates (<5%), and low rates of permanent neurological morbidity, such as mild hypesthesia or subjective leg weakness (<5%) (20, 33). The closure of lateral nerve root diverticula by clipping regularly causes hypesthesia in a narrow thoracic dermatome.
More than 80% of the patients who undergo surgery feel markedly better, or are even asymptomatic, on the day after the procedure. 17% develop transient symptoms of intracranial hypertension, analogously to patients who have been successfully treated with a blood patch (27).
The time it takes for the patient’s symptoms to resolve completely after definitive treatment is determined to a large extent by their duration up to the time of surgery: the longer the preoperative duration, the more protracted the recovery. We see all patients in clinical follow-up three months after surgery. If the patient remains less than fully functional in everyday life or has other, new symptoms, we obtain new imaging studies (even invasive ones, if necessary).
Case presentation
A 29-year-old woman complained of severe headaches that arose immediately whenever she got up from a lying position and rapidly improved when she lay down again. The headaches had begun four weeks before presentation. She had been seen in two different emergency departments already, an MRI of the head had been obtained, SIH had been diagnosed, and two lumbar epidural blood patches had been applied. Nonetheless, she had developed increasing difficulty concentrating, diminished cognitive performance, gait instability, and persistent inability to work, and was therefore referred to us. Dynamic CT myelography performed by us then revealed a spinal CSF leak at T1/2. A third blood patch was applied, but the symptoms persisted. A microsurgical closure of the ventral spinal CSF leak was successfully performed through a minimally invasive dorsal transdural approach. The patient felt better within a few days of the procedure and was able to stop taking the supportive drugs that had been prescribed (caffeine, ibuprofen). Radiological follow-up two months after surgery documented the complete closure of the dural CSF leak. The patient was free of symptoms and had returned to work.
Key Messages.
The most prominent clinical manifestation of spontaneous intracranial hypotension (SIH) is orthostatic headache. Many patients complain of auditory disturbances.
The longer the disease has been present, the less positionally dependent the headaches become.
Only one-third of patients have a low opening CSF pressure on lumbar puncture. The key diagnostic finding is the egress of CSF from the spinal subarachnoid space into the epidural space.
Epidural blood patches applied under fluoroscopic or CT guidance render 30–70% of SIH patients asymptomatic. Ventral dural tears usually need surgery.
At present, all types of dural CSF fistula can be closed with a minimally invasive microsurgical procedure performed through a dorsal approach. The success rate of operative closure is >95%.
Questions on the article in Issue 27–28/2020:
Spontaneous Intracranial Hypotension—Presentation, Diagnosis, and Treatment
CME credit for this unit can be obtained via cme.aerzteblatt.de until 5 July 2021.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the sex distribution of spontaneous intracranial hypotension?
Men are affected twice as commonly as women.
Women are affected three times as commonly as men.
Men are affected three times as commonly as women.
Women are affected twice as commonly as men.
Men and women are equally affected.
Question 2
What is the mean age of symptom onset in spontaneous intracranial hypotension, in years?
40
60
90
70
80
Question 3
Which of the following are common clinical manifestations of spontaneous intracranial hypotension?
Frontotemporal dementia
Cervical myelopathy, tetraplegia, bulbar palsy
Non-convulsive status epilepticus
Progressive paraparesis due to secondary spinal cord herniation
Tinnitus, auditory disturbances, dizziness
Question 4
How do patients typically describe the headaches that they have at the onset of symptoms?
Position-independent headache, getting better over the course of the day
Position-dependent headache, getting worse over the course of the day
Position-independent headache of sudden onset
Position-dependent headache, especially strong at night
Position-independent headache, less strong at night
Question 5
In a patient with suspected spontaneous intracranial hypotension, what types of study are most suitable for the localization of the CSF leak?
ECG combined with an infusion test
An infusion test coupled with CSF pressure measurement
Spinal MRI with simultaneous CSF pressure measurement
Transcranial ultrasonography combined with an infusion test
Dynamic myelography and, if necessary, dynamic CT myelography
Question 6
What MRI finding can be considered a clue to the diagnosis of spontaneous intracranial hypotension?
Enlargement of the dural venous sinuses
Narrowing of venous structures
Decreased contrast enhancement of the dura mater
Lifting of the brain
Shrinking of the pituitary gland
Question 7
Approximately what fraction of patients with spontaneous intracranial hypotension have a measured CSF opening pressure <60 mm H2O?
One-eighth
One-fourth
Three-fourths
Half
One-third
Question 8
Which of the following is a component of the initial treatment of spontaneous intracranial hypotension once the diagnosis has been established?
Copious exercise, in the fresh air if possible
Reduced dietary intake and administration of sedating drugs
Bed rest, hydration, and caffeine administration
Laxative administration
Stable lateral decubitus positioning
Question 9
What can dynamic digital subtraction myelography demonstrate?
A subdural bleed
A CSF-venous fistula
An epidural bleed
A CSF-arterial fistula
Narrowing of the subdural space
Question 10
In the treatment of spontaneous intracranial hypotension with one or more blood patches, the patient’s problem may undergo transformation, in the short term, into intracranial hypertension.
What are the major clinical manifestations of intracranial hypertension?
Paresthesiae, itching, and headache
Ataxia, tremor, and headache
Tremor, aphasia, and tinnitus
Papilledema, visual disturbances, and headache
Rash, itching, and high blood pressure
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Acknowledgement
The authors thank Anja Giger of Bern for creating the illustrations in Figures 1a, 1c, and 3a.
Footnotes
Conflict of interest statement
The authors state that they have no conflict of interest.
References
- 1.Schaltenbrand G. Neuere Anschauungen zur Pathophysiologie der Liquorzirkulation [New observations on the pathological physiology of the cerebrospinal fluid circulation] Zentralbl Neurochir. 1938;3:290–300. [Google Scholar]
- 2.Schaltenbrand G. Normal and pathological physiology of the cerebrospinal fluid circulation. Lancet. 1953;1:805–808. doi: 10.1016/s0140-6736(53)91948-5. [DOI] [PubMed] [Google Scholar]
- 3.Puech P, Lhermitte J, Buvat JF, et al. Un cas d’hypotension intracranienne spontanee A case of spontaneous intracranial hypotension. Rev Neurol. 1942;74 [Google Scholar]
- 4.Jacobs MB, Wasserstein PH. Spontaneous intracranial hypotension: an uncommon and underrecognized cause of headache. West J Med. 1991;155:178–180. [PMC free article] [PubMed] [Google Scholar]
- 5.Berlit P, Berg-Dammer E, Kuehne D. Abducens nerve palsy in spontaneous intracranial hypotension. Neurology. 1994;44 doi: 10.1212/wnl.44.8.1552. [DOI] [PubMed] [Google Scholar]
- 6.Horton JC, Fishman RA. Neurovisual findings in the syndrome of spontaneous intracranial hypotension from dural cerebrospinal fluid leak. Ophthalmology. 1994;101:244–251. doi: 10.1016/s0161-6420(94)31340-6. [DOI] [PubMed] [Google Scholar]
- 7.Renowden SA, Gregory R, Hyman N, Hilton-Jones D. Spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatr. 1995;59:511–515. doi: 10.1136/jnnp.59.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schievink WI, Meyer FB, Atkinson JLD, Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. 1996;84:598–605. doi: 10.3171/jns.1996.84.4.0598. [DOI] [PubMed] [Google Scholar]
- 9.Schievink WI, Morreale VM, Atkinson JL, et al. Surgical treatment of spontaneous spinal cerebrospinal fluid leaks. J Neurosurg. 1998;88:243–246. doi: 10.3171/jns.1998.88.2.0243. [DOI] [PubMed] [Google Scholar]
- 10.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–2295. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI, Maya MM, Moser F, et al. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8:325–328. doi: 10.1007/s10194-007-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranz PG, Gray L, Amrhein TJ. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948–959. doi: 10.1111/head.13328. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia. (3) 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 14.Schievink WI. Misdiagnosis of spontaneous intracranial hypotension. Arch Neurol. 2003;60:1713–1718. doi: 10.1001/archneur.60.12.1713. [DOI] [PubMed] [Google Scholar]
- 15.Mea E, Chiapparini L, Savoiardo M, et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164–165. doi: 10.1007/s10072-008-0914-5. [DOI] [PubMed] [Google Scholar]
- 16.Urbach H. Intracranial hypotension: clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol. 2014;27:414–424. doi: 10.1097/WCO.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Hagiwara M, Roehm P. Spontaneous intracranial hypotension presenting with severe sensorineural hearing loss and headache. Otol Neurotol. 2012;33:e65–e66. doi: 10.1097/MAO.0b013e318254ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schievink WI, Maya MM, Moser FG, Jean-Pierre S, Nuño M. Coma: A serious complication of spontaneous intracranial hypotension. Neurology. 2018;90:e1638–e1645. doi: 10.1212/WNL.0000000000005477. [DOI] [PubMed] [Google Scholar]
- 19.Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology. 2016;87:673–679. doi: 10.1212/WNL.0000000000002986. [DOI] [PubMed] [Google Scholar]
- 20.Beck J, Ulrich CT, Fung C, et al. Diskogenic microspurs as a major cause of intractable spontaneous intracranial hypotension. Neurology. 2016;87:1220–1226. doi: 10.1212/WNL.0000000000003122. [DOI] [PubMed] [Google Scholar]
- 21.Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol. 2019;40:745–753. doi: 10.3174/ajnr.A6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia. 2016;36:1209–1217. doi: 10.1177/0333102415623071. [DOI] [PubMed] [Google Scholar]
- 23.Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019;76:580–587. doi: 10.1001/jamaneurol.2018.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck J, Gralla J, Fung C, et al. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J Neurosurg. 2014;121:1380–1387. doi: 10.3171/2014.6.JNS14550. [DOI] [PubMed] [Google Scholar]
- 25.Fichtner J, Ulrich CT, Fung C, et al. Sonography of the optic nerve sheath diameter before and after microsurgical closure of a dural CSF fistula in patients with spontaneous intracranial hypotension—a consecutive cohort study. Cephalalgia. 2019;39:306–315. doi: 10.1177/0333102418793640. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani D, Rodriguez B, Häni L, et al. Postural changes in optic nerve and optic nerve sheath diameters in postural orthostatic tachycardia syndrome and spontaneous intracranial hypotension: A cohort study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223484. e0223484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Häni L, Fung C, Jesse CM, et al. Insights into the natural history of spontaneous intracranial hypotension from lumbar infusion testing. Neurology. 2020;95:1–9. doi: 10.1212/WNL.0000000000009812. [DOI] [PubMed] [Google Scholar]
- 28.Dobrocky T, Mosimann PJ, Zibold F, et al. Cryptogenic cerebrospinal fluid leaks in spontaneous intracranial hypotension: role of dynamic CT myelography. Radiology. 2018;289:766–772. doi: 10.1148/radiol.2018180732. [DOI] [PubMed] [Google Scholar]
- 29.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine. 2019;13:1–4. doi: 10.3171/2019.6.SPINE19487. [DOI] [PubMed] [Google Scholar]
- 30.Kong DS, Park K, Nam DH, et al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91–96. doi: 10.1227/01.neu.0000163093.38616.35. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Lee MJ, Park HJ, et al. Clinical effect of the proximity of epidural blood patch injection to the leakage site in spontaneous intracranial hypotension. Br J Neurosurg. 2018;32:671–673. doi: 10.1080/02688697.2018.1519109. [DOI] [PubMed] [Google Scholar]
- 32.Martin R, Louy C, Babu V, Jiang Y, Far A Schievink W. A two-level large-volume epidural blood patch protocol for spontaneous intracranial hypotension: retrospective analysis of risk and benefit. Reg Anesth Pain Med. 2019 doi: 10.1136/rapm-2018-100158. pii: rapm-2018-100158 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 33.Beck J, Raabe A, Schievink WI, et al. Posterior approach and spinal cord release for 360° repair of dural defects in spontaneous intracranial hypotension. Neurosurgery. 2019;84:E345–E351. doi: 10.1093/neuros/nyy312. [DOI] [PubMed] [Google Scholar]
- 34.Rosebrock RE, Diehn FE, Luetmer PH, et al. Penetrating osseous spicules causing high-flow ventral CSF leaks in the setting of relatively low BMI: a preliminary study. Clin Neuroradiol. 2018;28:539–543. doi: 10.1007/s00062-017-0596-6. [DOI] [PubMed] [Google Scholar]
- 35.Dobrocky T, Winklehner A, Breiding PS, et al. Spine MRI in spontaneous intracranial hypotension for CSF leak detection; non superiority of intrathecal Gadolinium to heavily T2 weighted fat saturated sequences. Am J Neuroradiol AJNR (in press) doi: 10.3174/ajnr.A6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franzini A, Messina G, Nazzi V, et al. Spontaneous intracranial hypotension syndrome: a novel speculative physiopathological hypothesis and a novel patch method in a series of 28 consecutive patients. J Neurosurg. 2010;112:300–306. doi: 10.3171/2009.6.JNS09415. [DOI] [PubMed] [Google Scholar]
- 37.Cho KI, Moon HS, Jeon HJ, et al. Spontaneous intracranial hypotension: efficacy of radiologic targeting vs blind blood patch. Neurology. 2011;76:1139–1144. doi: 10.1212/WNL.0b013e318212ab43. [DOI] [PubMed] [Google Scholar]
- 38.Bartynski WS, Grahovac SZ, Rothfus WE. Incorrect needle position during lumbar epidural steroid administration: inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. AJNR Am J Neuroradiol. 2005;26:502–505. [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Hseu SS, Fuh JL, et al. Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain. 2017;140:344–352. doi: 10.1093/brain/aww328. [DOI] [PubMed] [Google Scholar]
- 40.Kranz PG, Amrhein TJ, Gray L. Rebound intracranial hypertension: a complication of epidural blood patching for intracranial hypotension. Am J Neuroradiol. 2014;35 doi: 10.3174/ajnr.A3841. [DOI] [PMC free article] [PubMed] [Google Scholar]