Abstract
Direct oral anticoagulants (DOACs) have quickly become attractive alternatives to the long‐standing standard of care in anticoagulation, vitamin K antagonist. DOACs are indicated for prevention and treatment of several cardiovascular conditions. Since the first approval in 2010, DOACs have emerged as leading therapeutic alternatives that provide both clinicians and patients with more effective, safe, and convenient treatment options in thromboembolic settings. With the expanding role of DOACs, clinicians are faced with increasingly complex decisions relating to appropriate agent, duration of treatment, and use in special populations. This review will provide an overview of DOACs and act as a practical reference for clinicians to optimize DOAC use among common challenging scenarios. Topics addressed include (1) appropriate indications; (2) use in patients with specific comorbidities; (3) monitoring parameters; (4) transitioning between anticoagulant regimens; (5) major drug interactions; and (6) cost considerations.
Keywords: anticoagulation, oral direct thrombin inhibitor, oral factor Xa inhibitors, pharmacotherapy
Subject Categories: Vascular Disease, Thrombosis, Quality and Outcomes, Cardiovascular Disease, Anticoagulants
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- ASCVD
atherosclerotic cardiovascular disease
- CAD
coronary artery disease
- CKD
chronic kidney disease
- CrCl
creatinine clearance
- CYP
cytochrome P450
- DAPT
dual‐antiplatelet therapy
- DOAC
direct oral anticoagulant
- FDA
US Food and Drug Administration
- HR
hazard ratio
- INR
international normalized ratio
- LMWH
low molecular weight heparin
- NVAF
nonvalvular atrial fibrillation
- PCI
percutaneous coronary intervention
- VKA
vitamin K antagonists
- VTE
venous thromboembolism
Direct oral anticoagulants (DOACs)—dabigatran (Pradaxa), rivaroxaban (Xarelto), apixaban (Eliquis), edoxaban (Savaysa), and betrixaban (Bevyxxa) are anticoagulation pharmacotherapy used for the prevention of thrombosis in several cardiovascular contexts.1 DOACs are categorized into 2 main classes: oral direct factor Xa inhibitors (ie, rivaroxaban, apixaban, edoxaban, and betrixaban) and direct thrombin inhibitors (ie, dabigatran). In 2010, the US Food and Drug Administration (FDA) approved its first DOAC, dabigatran, followed by rivaroxaban, apixaban, edoxaban, and betrixaban in the following years. DOACs are relatively new agents demonstrating superiority or noninferiority to prior standards of care, anticoagulation with vitamin K antagonists (VKA; ie, warfarin), or low‐molecular‐weight heparins (LMWHs), in reducing risk of thromboembolic complications with similar or reduced bleeding risk.2, 3, 4, 5 Advantages of DOACs compared with VKAs include fewer monitoring requirements, less frequent follow‐up, more immediate drug onset and offset effects (important for periprocedural and acute bleeding management), and fewer drug and food interactions.6 As a result, DOAC prescriptions exceeded those for warfarin by 2013, with apixaban being the most frequently prescribed DOAC for patients with nonvalvular atrial fibrillation (NVAF).7
Over the past decade, DOACs have been the subject of extensive investigation in many clinical scenarios. Though guidelines and review articles have provided detailed and in‐depth analyses of the immense literature base, these can be too cumbersome and challenging to integrate into everyday clinical use. The purpose of this review is to be a practical reference or algorithm for the busy clinician to navigate key aspects of effective DOAC prescribing, with an emphasis on addressing key situations where clinical uncertainty exists. This review will provide recommendations to address special clinical situations to include indications, use in specific comorbidities, monitoring parameters, transitioning to or off of therapy, drug interactions, and cost.
Indications for Use
In general, FDA‐approved indications for each of the DOACs are comparable (see Table 1). Dabigatran, rivaroxaban, apixaban, and edoxaban are approved for the lowering the risk of stroke and embolism in NVAF as well as deep vein thrombosis and pulmonary embolism treatment/prophylaxis.8, 9, 10, 11 Unique indications include betrixaban for prophylaxis of venous thromboembolism (VTE) in hospitalized patients for an acute medical illness, and rivaroxaban in combination with aspirin to reduce major cardiovascular events in patients with chronic coronary artery disease (CAD) or peripheral artery disease.9, 12 However, there is still uncertainty in understanding safe and effective use of DOACs in the setting of patients with cardiovascular comorbidities, specifically atrial fibrillation (AF) with recent percutaneous coronary intervention (PCI), AF with concomitant artificial heart valves, stable atherosclerotic cardiovascular disease (ASCVD), and cancer‐associated thromboembolism. This section aims to clarify the use of DOACs within these patient subgroups.
Table 1.
Indications for DOAC Prescribing
| DOACs | Indications |
|---|---|
| Dabigatran | FDA‐approved indications |
| Stroke prevention in NVAF | |
| Treatment of deep vein thrombosis and pulmonary embolism | |
| Prevention of recurrent deep vein thrombosis and pulmonary embolism | |
| Prevention of thromboembolism after total hip replacement | |
| Off‐label indications | |
| Prevention of thromboembolism after total knee replacement | |
| Prevention of thromboembolism after PCI with NVAF | |
| Rivaroxaban | FDA‐approved indications |
| Stroke prevention in NVAF | |
| Treatment of deep vein thrombosis and pulmonary embolism | |
| Prevention of recurrent deep vein thrombosis and pulmonary embolism | |
| Prevention of thromboembolism after total knee replacement and after total hip replacement | |
| Prevention of thromboembolism in hospitalized acutely ill medical patients | |
| Prevention of major cardiovascular events in patients with chronic CAD/peripheral artery disease | |
| Off‐label indications | |
| Prevention of thromboembolism after PCI with NVAF | |
| Apixaban | FDA‐approved indications |
| Stroke prevention in NVAF | |
| Prevention of thromboembolism after total knee replacement and after total hip replacement | |
| Treatment of deep vein thrombosis and pulmonary embolism | |
| Prevention of recurrent deep vein thrombosis and pulmonary embolism | |
| Off‐label indications | |
| Treatment of heparin‐induced thrombocytopenia; | |
| Prevention and treatment of cancer‐associated deep vein thrombosis | |
| Prevention of thromboembolism in hospitalized acutely ill medical patients | |
| Prevention of thromboembolism after PCI with NVAF | |
| Edoxaban | FDA‐approved indications |
| Stroke prevention in NVAF | |
| Treatment of deep vein thrombosis and pulmonary embolism | |
| Off‐label indications | |
| Prevention and treatment of cancer associated deep vein thrombosis | |
| Prevention of thromboembolism after total knee replacement and after total hip replacement | |
| Prevention of thromboembolism after PCI with peripheral artery disease | |
| Betrixaban | FDA‐approved indications |
| Prevention of deep vein thrombosis and pulmonary embolism in adults hospitalized for an acute medical illness |
CAD indicates coronary artery disease; DOAC, direct oral anticoagulant; FDA, US Food and Drug Administration; NVAF, nonvalvular atrial fibrillation; and PCI, percutaneous coronary intervention.
AF and PCI
The combination of AF and CAD has often led to confusion regarding the optimal antithrombotic strategy, historically favoring atherothrombotic prevention to bleeding complications. Patients with CAD undergoing PCI are to receive dual‐antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 inhibitor (ie, clopidogrel, prasugrel, or ticagrelor) for prevention of recurrent atherosclerosis and stent thrombosis.13 When this occurs in patients with comorbid AF, the potential need for triple antithrombotic therapy consisting of DAPT and anticoagulation (historically VKA) arises.14 As several studies have shown, bleeding risk rises significantly with each successive antithrombotic agent added; however, trade‐offs regarding thrombosis are not as clear cut.15
Recent investigations shed light on this common clinical scenario. The PIONEER AF‐PCI (Prevention of Bleeding in Patients With Atrial Fibrillation Undergoing PCI) trial revealed that dual therapy with rivaroxaban dosed at nonstandardized AF dosing (15 mg daily, or 10 mg daily if renal impairment) and a P2Y12 inhibitor (predominantly clopidogrel) or triple therapy with rivaroxaban 2.5 mg twice daily plus DAPT was associated with lower bleeding risk in comparison to triple therapy (VKA at standard AF dosing, aspirin, and clopidogrel; hazard ratio [HR], 0.59; 95% CI, 0.47–0.76) but similar thrombotic events.16 The RE‐DUAL PCI (Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran Versus Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) trial showed that dual therapy with dabigatran (110 and 150 mg twice‐daily regimens) and a P2Y12 inhibitor (predominantly clopidogrel) revealed similar results, demonstrating a lower risk of bleeding and hospitalization compared with triple therapy (VKA dose adjusted to an international normalized ratio [INR] of 2–3, aspirin, clopidogrel). The magnitude of difference in bleeding events was dose dependent (HR, 0.52; 95% CI, 0.42–0.63; and HR, 0.72; 95% CI, 0.58–0.88 for the dabigatran 110 and 150 mg regimens, respectively). At the same time, dabigatran at both doses was noninferior with respect to the prevention of ischemic event rates.17 The AUGUSTUS (Antithrombotic Therapy After Acute Coronary Syndrome or PCI in Atrial Fibrillation) trial directly examined the benefit and risk of dropping aspirin with both warfarin and apixaban in combination with a P2Y12 inhibitor (clopidogrel). The AUGUSTUS trial keenly addressed a key missing component to the literature and criticisms of RE‐DUAL PCI and PIONEER AF‐PCI trials by comparing triple therapy to triple therapy and dual therapy to dual therapy. The trial demonstrated the antithrombotic regimens that included apixaban in combination with a P2Y12 inhibitor (without aspirin) resulted in less bleeding (HR, 0.69; 95% CI, 0.58–0.81) and fewer hospitalizations (HR, 0.83; 95% CI, 0.74–0.93) without significantly effecting the number of ischemic events than regimens that included a VKA, aspirin, or both.18 Clinicians should be confident that dropping aspirin according to clinical trial protocols when using apixaban, dabigatran, or rivaroxaban in combination with a P2Y12 inhibitor will substantially lower bleeding risk without increasing thrombotic risk, a sentiment endorsed by recent consensus documents.14, 19 When it comes to antithrombotic therapy in this scenario, less may be more.
Another important consideration is the use of add‐on therapy to further mitigate bleeding risk, specifically gastrointestinal bleeding risk in patients on dual therapy. As has been demonstrated in patients on DAPT, use of gastrointestinal protection with H2 antihistamine receptor blockers and proton pump inhibitors can reduce bleeding complications in patients on DAPT.20 Providers are encouraged to coprescribe gastrointestinal prophylaxis using a proton pump inhibitor for patients on dual‐antithrombotic regimens, especially those at high risk of gastrointestinal bleeding. This same rationale may be applied to patients on DOACs and antiplatelets or agents with antiplatelet properties (ie, NSAIDs, systemic corticosteroids, etc).
AF and Artificial Heart Valves
Valvular heart disease and AF commonly coexist and each contribute independently to thromboembolic events and mortality.21 Valvular AF refers to the presence of mechanical prosthetic heart valve or moderate to severe mitral stenosis, conditions that substantially increase thromboembolic risk and serve as a principal exclusion criterion for the major DOAC phase III AF trials.22, 23, 24, 25 More specifically, the RE‐ALIGN (Dabigatran Versus Warfarin in Patients With Mechanical Heart Valves) trial established the use of DOACs as contraindicated in patients with mechanical heart valves.26 The study was terminated early, as patients with mechanical prosthetic heart valves experienced excess thromboembolic and bleeding events. Therefore, VKA remains the preferred agent for this patient population as recommended in the current American College of Chest Physicians and American College of Cardiology/American Heart Association guidelines.27, 28, 29 Bioprosthetic heart valves are considered less thrombogenic compared with mechanical heart valves but nonetheless are associated with increased risk and require chronic anticoagulation in the setting of concurrent AF or mitral stenosis.30 Overall, the use of DOACs in patients with AF with bioprosthetic valves or other valve repairs still constitutes a gray area in clinical practice because of limited investigation and consensus about the definition of “valvular” AF. Despite the unfavorable outcomes of the RE‐ALIGN trial of mechanical valves, there is a growing interest to investigate use of DOACs in the setting of bioprosthetic valves. Subgroup analysis of the pivotal trials of selected DOACs (apixaban and edoxaban),31, 32 as well as small‐cohort studies of DOACs (apixaban, rivaroxaban, and dabigatran),33, 34, 35, 36 support their safe and effective use in patients with bioprosthetic valves. The most recent addition to this area evaluated 218 patients undergoing bioprosthetic aortic or mitral valve replacement or repair and randomized to edoxaban 60 mg daily or warfarin dose adjusted to an INR between 2 and 3 during the first 3 months after the procedure.37 Though the results are yet to be published, edoxaban was found to be noninferior for the primary efficacy (death, asymptomatic intracardiac thrombosis, and clinical thromboembolic event—defined as myocardial infarction, stroke, pulmonary embolism, symptomatic valve thrombosis, systemic non–central nervous system embolism or deep vein thrombosis) and safety (major bleeding) end points. Thus, the limited evidence supports the notion that bioprosthetic heart valves should not preclude DOAC use, a practice that is echoed by the European Heart Rhythm Association (see Table 2).38
Table 2.
DOAC Use in Valvular Heart Disease
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Betrixaban | |
|---|---|---|---|---|---|
| Mechanical prosthetic valve | Contraindicated | NAa | |||
| Moderate to severe mitral stenosis | Contraindicated | NAa | |||
| Bioprosthetic valveb | Acceptable | NAa | |||
NA indicates not applicable.
Betrixaban is used only as a prophylactic agent; it is not indicated for treatment.
Not advised for rheumatic mitral stenosis.
Reproduced in part from Steffel et al38 with permission. Copyright ©2018, Oxford University Press.
Stable Atherosclerotic Cardiovascular Disease
Aspirin use for secondary prevention of ASCVD is well established and is widely recommended by major clinical guidelines for indefinite use.39 In this setting, major ASCVD events are reduced ≈20% to 30%, but major bleeding increased ≈1.4 to 1.6‐fold.40, 41 In an effort to expand upon the ASCVD risk reduction witnessed with aspirin monotherapy, prior investigations evaluated more potent antiplatelets (ie, clopidogrel, ticagrelor, and vorapaxar) and VKA therapy as alternatives to aspirin and as add‐on therapy with aspirin.42 The recurring theme with the use of these strategies was a slight reduction in ASCVD events but at the expense of increased bleeding episodes and no effect on mortality. After promising results were hinted at with combining antiplatelet therapy with low‐dose rivaroxaban in patients with acute coronary syndrome undergoing PCI, investigations into use of this strategy for chronic ASCVD were sought.43 The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) study demonstrated that rivaroxaban plus aspirin had significant secondary prevention benefits for patients with ASCVD (ie, CAD and peripheral artery disease), which led to FDA approval for this indication in 2018.44 Patients were randomized to 3 treatment arms: rivaroxaban 2.5 mg twice daily with aspirin 100 mg daily; rivaroxaban 5 mg twice daily alone; or aspirin 100 mg daily alone.44 This is a drastic dose reduction for rivaroxaban from the recommended 20 mg daily dose approved for patients with NVAF and VTE, but the rationale for low doses was provided by previous trials.42 The benefits of therapy were slightly offset by the increased risk in major bleeding by 1.70‐fold (P<0.001). Nonetheless, the reduced rivaroxaban dose in combination with aspirin did not increase fatal or critical organ bleeding, and the net clinical benefit still favored the combination therapy.44 Overall, the COMPASS trial demonstrated significant benefit in reducing major cardiovascular events, a composite of cardiovascular death, stroke, and myocardial infarction by 24% (P<0.001), and major adverse limb events (including amputations) by 46% (P=0.0037) with low doses of rivaroxaban in combination with aspirin.44, 45 This novel indication for rivaroxaban provides an important new antithrombotic therapy regimen for a large group of high‐risk patients with established ASCVD and another pathway for reducing residual atherosclerotic risk.
A common clinical conundrum occurs in the setting of stable ASCVD and concomitant AF: What is the most effective long‐term antithrombotic regimen? This question was addressed in AFIRE (Antithrombotic Therapy for Atrial Fibrillation With Stable Coronary Disease).46 The trial randomized 2236 Japanese patients with AF who had undergone PCI or coronary artery bypass grafting >1 year earlier or angiographically confirmed CAD not requiring revascularization to rivaroxaban 15 mg daily (10 mg daily with creatinine clearance [CrCl] 15–49 mL/min) or combination therapy consisting of rivaroxaban plus an antiplatelet (aspirin or P2Y12 inhibitor). The doses of rivaroxaban were lower than the FDA‐approved doses (20 mg with normal and 15 mg with impaired renal function) because of pharmacokinetic modeling revealing that Japanese patients achieve similar plasma levels of rivaroxaban compared with 20 mg in Caucasian patients.47 The AFIRE trial was stopped early because of increased mortality in the combination therapy
group (1.85% versus 3.37% per patient year; HR, 0.55; 95% CI, 0.38–0.81). The rivaroxaban monotherapy arm was noninferior in cardiovascular efficacy measures (P<0.001) and superior in reducing major bleeding (P=0.01) compared with the combination therapy group. Thus, the results of the AFIRE study extended the concept of less intense antithrombotic for optimal risk‐benefit established in the acute‐care setting from PIONEER AF‐PCI,16 RE‐DUAL PCI,17 and AUGUSTUS,18 to the time period beyond a year from diagnosis and intervention.
Cancer‐Associated Thromboembolism
Patients with cancer are at increased risk for venous and arterial thromboembolism and bleeding events.48 Anticoagulation within the cancer population is complicated by comorbidities that can affect drug disposition (ie, renal insufficiency, high rates of nausea and vomiting), thrombotic risk (ie, concurrent AF), or bleeding risk (ie, thrombocytopenia); drug‐drug interactions between anticoagulants and oncology medications; malnourished/underweight states; and patient preferences (ie, burdensome laboratory testing, patient discomfort with injections, and medication cost).48 As such, the growing need for optimal anticoagulation strategies is of mounting importance. Historically, LMWHs have been the cornerstone of cancer‐associated VTE on the basis of 2 trials providing reduced thrombotic risk compared with warfarin.49, 50 Evidence comparing the use of DOACs to LMWH have emerged to provide an oral dosing option that may be more cost effective and does not require frequent laboratory monitoring. The Hokusai VTE Cancer (Edoxaban for the Treatment of Cancer‐Associated Venous Thromboembolism) trial compared edoxaban 60 mg daily with dalteparin 200 IU/kg subcutaneously daily×1 month, then 150 IU/kg daily thereafter.51 Edoxaban was noninferior to dalteparin with respect to the primary end point of recurrent VTE or major bleeding, displaying a nonsignificant reduction in recurrent VTE (23% relative risk reduction, P=0.09), but increasing major bleeding (1.77‐fold increase; P=0.04). The Select‐D (Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism) pilot trial compared rivaroxaban at standard VTE dosing (15 mg twice daily×21 days, then 20 mg daily thereafter) versus dalteparin (same dosing regimen used in the Hokusai VTE Cancer trial) and revealed reduced recurrent VTE (HR, 0.43; 95% CI, 0.19–0.99) but a 1.83‐fold (95% CI, 0.68–4.96) increased risk of major bleeding after 6 months of treatment with rivaroxaban.52 The ADAM VTE (Apixaban, Dalteparin, in Active Cancer Associated Venous Thromboembolism) trial was the first trial for apixaban to support active treatment of cancer‐associated VTE.53 Apixaban dosed at 10 mg twice daily×7 days, then 5 mg twice daily thereafter, was compared with dalteparin (dosed as above) over 6 months. Apixaban was associated with a noninferiority with regard to the primary end point of major bleeding (0% versus 2.1%; P=0.9956) and a robust 74% relative risk reduction in recurrent VTE (P=0.0182). Similar results were recently published in the Caravaggio (Apixaban for the Treatment of Venous Thromboembolism Associated With Cancer) trial, demonstrating noninferiority with respect to recurrent VTE (P<0.001) and comparable major bleeding (P=0.60).54 Thus, the current landscape for treatment of VTE in the cancer population seems to support apixaban over the current standard of care with LMWH and other DOACs, establishing the ideal balance of thrombosis prevention while not inducing excess bleeding. However, several other trials investigating DOAC to LMWH for treatment of cancer‐associated VTE are slated to be completed within the next 1 to 2 years. Subgroup analyses from phase III VTE and AF trials and retrospective observational cohorts have also demonstrated consistent efficacy and safety outcomes in the cancer population.48 In response, major guidelines now endorse the use of DOAC in cancer‐associated VTE treatment.55, 56 Trials investigating the use of DOACs for the prevention of VTE in patients with cancer have also been conducted and support the use of these agents in patients with cancer undergoing ambulatory chemotherapy with intermediate‐high VTE risk and low bleeding risk.48, 57, 58 As current practice heavily favors LMWH and VKAs, these novel and compelling trials supporting the use of DOACs will likely serve to ignite a paradigm shift in practice standards among this growing population of complex patients.
Comorbidities Affecting DOAC Pharmacokinetics
Patient‐specific comorbidities, particularly ones that affect systemic exposure of the medication, need to be taken into account when managing patients on DOAC therapy. Comorbidities may alter the DOAC elimination rate, therefore increasing the risk of a thromboembolic or bleeding events. Of the many factors that need to be considered, we will address the 3 most pertinent patient comorbidities: renal impairment, hepatic impairment, and extreme body weights.
Renal Insufficiency
Patients with chronic kidney disease (CKD) have an increased risk for thromboembolic and bleeding events. Historically, warfarin has been the preferred anticoagulant used in severe CKD, but robust data supporting its efficacy and safety are lacking, coupled with the concern for warfarin‐induced vascular calcifications and worsening nephropathy, more appropriate alternatives were eagerly sought.59 However, the presence of coexisting renal impairment can often lead to uncertainty in selecting the best DOAC. All DOAC therapies are eliminated by the kidneys to varying degrees, and alterations in renal clearance must be taken into account when dosing these agents. Dabigatran is the most renally eliminated, accountable for 80% of its clearance pathway, followed by edoxaban, rivaroxaban, apixaban, and betrixaban: 50%, 35%, 27%, and 11%, respectively.38, 59 Any clinical decisions on how to treat patients with DOACs requires the assessment of patient renal function, which should be monitored frequently, at least annually or more frequently if a patient has additional comorbidities and risk factors. Renal dose adjustments include a decreased dose or decreased frequency of the regimen, and for most DOACs are based on the Cockcroft‐Gault CrCl equation to estimate renal function.
Phase III trials involving DOACs have excluded patients with severe renal dysfunction (CrCl <30 mL/min) or dialysis.22, 23, 25 Apixaban exhibits minimal renal clearance, but the clinical significance of this is uncertain and there are varying recommendations regarding when to dose adjust.10, 38, 60 Patients with CrCl <25 mL/min were excluded in both AF and VTE trials using apixaban.24, 61, 62 Nonetheless, FDA‐approved prescribing information requires no dose adjustment for apixaban in patients with renal impairment alone, including patients with end‐stage renal disease and those on hemodialysis.10 To qualify for apixaban dose adjustment, one must meet at least 2 of the following characteristics; just remember your ABCs:
Age ≥80 years.
Body weight ≤60 kg.
Creatinine (serum) ≥1.5 mg/dL.
The Europeans provide an alternative guidance, recommending standard dosing for CrCl >30 mL/min, reduced dose for CrCl 15 to 30 mL/min, and avoidance for CrCl <15 m/min.38 The 2018 AF guidelines recommend both warfarin and apixaban for patients with end‐stage renal disease (CrCl <15 m/min or on dialysis).28 However, warfarin has little proven benefit and a large risk of harm in end‐stage renal disease. There is growing evidence that apixaban in patients with AF and advanced CKD or end‐stage renal disease may be associated with a lower risk of major bleeding compared with warfarin63, 64 or, at the very least, similar rates of bleeding.65
Rivaroxaban use in severe CKD, particularly in patients on dialysis, is quite contentious. The package labeling states that rivaroxaban can be used in patients on dialysis, albeit at a reduced dose. However, this is based on limited pharmacokinetic data.9 The limited observational data suggested varying effects on bleed risk compared with warfarin in patients with stage 5 CKD or on dialysis.59, 66 Regardless, major guidelines and consensus documents do not recommend use of rivaroxaban in patients with stage 5 CKD or on dialysis.59
Betrixaban is mainly eliminated via the hepatobiliary system, with limited renal involvement in clearance. However, betrixaban can neither be recommended nor contraindicated in patients with severe renal insufficiency, as this agent has not been evaluated in this setting.67
Contrary to other DOACs, edoxaban displays a decrease in efficacy compared with warfarin in patients with an intact renal clearance (CrCl >95 mL/min), which led to the FDA's issuing a warning about the use of this agent in individuals with normal‐high CrCl.11 However, a post hoc analysis of the ENGAGE AF (Edoxaban Versus Warfarin in Patients With Atrial Fibrillation) trial showed that edoxaban remained safe and effective in patients with normal renal function.68
Knowledge of when and how to renally dose adjust is imperative to appropriate DOAC prescribing. This is best highlighted in recent studies suggesting that up to 32% of patients experience inappropriate DOAC dosing,69, 70, 71 the most common form being subtherapeutic dosing due to renal insufficiency, the greatest risk factor influencing deviation from FDA‐approved doses.69 Inappropriate dosing of DOAC carries grim consequences, with increased risk for thrombotic and bleeding complications resulting from subtherapeutic and supratherapeutic dosing, respectively.72
In summary, DOACs are safe and effective in patients with moderate CKD (CrCl 30–50 mL/min). Dabigatran, rivaroxaban, and edoxaban should undergo dose adjustment for renal impairment and should be avoided for severe renal impairment (CrCl <30 mL/min). Edoxaban should be avoided in those with normal renal function (>95 mL/min). Apixaban and betrixaban undergo the least amount of renal elimination and may be the DOACs of choice in severe renal impairment. See Figure 1 for suggested DOAC use based on patient renal function.
Figure 1. DOAC use in renal insufficiency.

*Apixaban dose adjustments are based on patient serum creatinine, age, and body weight; **rivaroxaban dose adjustments are based on patient indication; ***betrixaban: no dose adjustments provided for hemodialysis patients (has not been studied). CrCl indicates Cockcroft‐Gault creatinine clearance; ESRD, end‐stage renal disease; NVAF, nonvalvular atrial fibrillation; and VTE, venous thromboembolism.
Hepatic Impairment
Similar to other disease states noted above, patients with hepatic impairment are at increased risk of bleeding complications and thrombotic events.73 Alterations in hepatic function affects DOAC biotransformation to varying extents. Apixaban is the most reliant on hepatic metabolism for drug elimination, accounting for 75% of its elimination pathway, followed by rivaroxaban, edoxaban, dabigatran, and betrixaban: 65%, 50%, 20%, and up to 18%, respectively.12, 73 Rivaroxaban and apixaban require cytochrome P450 (CYP) enzymes for metabolism, while dabigatran and edoxaban require no to minimal CYP metabolism.73 There was minimal hepatic elimination of betrixaban, and it is not metabolized by CYP enzymes, nor does it induce or inhibit CYP activity.67 As there is no great monitoring parameter to assess for safety, patients with hepatic dysfunction may not be ideal candidates for these agents. Restrictions for the use of DOACs in patients with hepatic impairment are based on the Child‐Pugh classification system and exclusion criteria applied in pivotal trials (see Table 3).38, 73, 74 The Child‐Pugh score uses the presence of clinical and biochemical abnormalities to assess the severity of the hepatic dysfunction. All DOACs are contraindicated in patients with severe hepatic disease in which warfarin is the only recommended anticoagulant in this patient population.38 Dabigatran, apixaban, and edoxaban are viable options in patients with moderate hepatic impairment and do not require dose adjustments.38 All DOACs could be considered in patients with mild hepatic impairment without any dose adjustments. With the lack of information, the optimal anticoagulation strategy for this patient population remains unclear and it is recommended to obtain blood tests to evaluate hepatic function and coagulation parameters before initiating and periodically throughout DOAC therapy. Summarized in Table 4 are recommendations for patients with hepatic impairment based on their Child‐Pugh classification.
Table 3.
Child‐Pugh Score for Classification of Hepatic Impairment
| Score | 1 | 2 | 3 |
|---|---|---|---|
| Bilirubin, mg/dL | <2 | 2–3 | >3 |
| Albumin, g/dL | >3.5 | 2.8–3.5 | <2.8 |
| Ascites | None | Mild | Moderate |
| Encephalopathy (grade) | None | 1 and 2 | 3 and 4 |
| INR | <1.7 | 1.7–2.3 | >2.3 |
Grade A, <7 points; Grade B, 7 to 9 points; Grade C, 10 to 15 points. INR indicates international normalized ratio.
Reproduced in part from Steffel et al38 with permission. Copyright ©2018, Oxford University Press.
Table 4.
DOAC Recommendations Based on Degree of Hepatic Impairment
| DOAC | Contraindication | Use With Caution | No Dose Reduction |
|---|---|---|---|
| Dabigatran | Child‐Pugh C | Child‐Pugh B | Child Pugh A |
| Rivaroxaban | Child‐Pugh B or C | NA | Child Pugh A |
| Apixaban | Child‐Pugh C | Child Pugh B | Child Pugh A |
| Edoxaban | Child‐Pugh C | Child Pugh B | Child Pugh A |
| Betrixaban | aChild‐Pugh B or C | NA | Child Pugh A |
DOAC indicates direct oral anticoagulant; and NA, not applicable.
Use is not recommended in patients with hepatic impairment (has not been studied).
Reproduced in part from Steffel et al38 with permission. Copyright ©2018, Oxford University Press.
Extreme Body Weights
Optimal anticoagulant agents and dosing strategy for patients with extreme body weights have not been established for DOACs. Concerns have arisen with the use of DOACs in patients with extremes of body weight attributable to physiological changes that affect clearance of the medication and may lead to adverse outcomes, as well as limited data to guide prescribers. Fixed drug doses may lead to decreased drug exposures in obese patients and increased drug exposures in underweight patients based on drug pharmacokinetic changes (see Figure 2).75, 76, 77, 78, 79, 80 Weight was not an exclusion criterion in any of the large randomized trials evaluating DOACs in AF or VTE populations. Subgroup analyses from these trials reveal no difference in efficacy or safety outcomes in obese patients, and these findings were reinforced by results of meta‐analyses; however, extreme‐body‐weight populations have been severely underrepresented in clinical trials.81 An analysis of trials conducted by the International Society on Thrombosis and Haemostasis suggests that DOACs are safe in patient ≤120 kg (body mass index ≤40 kg/m2) at standard doses and are not recommended in patients >120 kg (body mass index >40 kg/m2).82 Since these recommendations, several retrospective, single‐center studies have shed more light on this topic. Collectively, dabigatran and to a lesser extent rivaroxaban reveal suboptimal peak plasma concentrations (in 20%–28% of obese patients studied) compared with apixaban.83 When evaluating efficacy and safety outcomes, DOAC therapies compare similarly with warfarin; however, the majority of the data are for apixaban and rivaroxaban, and those that included dabigatran revealed higher rates of thrombosis and lower rates of bleeding suggesting impaired systematic exposure.84, 85, 86, 87, 88 The largest of these studies was that conducted by Coons et al,88 which evaluated 1840 patients with acute VTE to receive DOAC (rivaroxaban in 91.8%, apixaban in 5.2%, and dabigatran in 3%) versus dose‐adjusted warfarin to an INR goal of 2 to 3. This retrospective study found similar rates of recurrent VTE (6.5% versus 6.4%; P=0.93) and bleeding (1.7% versus 1.2%; P=0.31) in DOAC compared with warfarin‐treated patients, respectively. Interestingly, the mean weight of 115 kg, the fact that <50% of patients were >120 kg (body mass index >40 kg/m2), and the primary use of rivaroxaban (DOAC with the least amount of pharmacokinetic alterations among obese patients) makes this study less generalizable. Nevertheless, although these trials had several inherent limitations, predominantly being underpowered to fully elucidate whether DOACs are noninferior to warfarin in obese patients, since a randomized clinical trial in this population remains unlikely, they do provide important data to this field. A lack of information exists for edoxaban and betrixaban within this patient population. Within the obese population (patients >120 kg and/or >40 kg/m2), it is recommended that use of dabigatran, edoxaban, and betrixaban be avoided and that rivaroxaban and apixaban may be used with caution.
Figure 2. DOAC pharmacokinetic profiles in the extremes of body weight.

AUC indicates area under the curve; Cmax, maximal concentration; and NR, not reported.
Among low‐body‐weight patients (<60 kg), measuring renal function plays an important role in assessing use of DOACs, as renal function is commonly overestimated in this population because of lower muscle mass. In addition, low‐body‐weight patients commonly present with comorbid conditions (ie, elderly age, frailty, and renal impairment) that predispose to adverse outcomes.38 DOACs with recommended dose reductions based on low body weight (≤60 kg) include apixaban (in addition to age and renal function) and edoxaban—both as a result of pharmacokinetic evaluation demonstrating increased systemic exposure in this population (see Figure 2). Dabigatran may also represent a less‐than‐ideal agent for underweight patients because of increased systemic exposure (see Figure 2) and the likelihood of coexisting renal insufficiency in this patient population with observational studies demonstrating increased dabigatran‐induced bleeding at low body mass indexes (<23.9 kg/m2).89 There was no conclusive consensus for patients with weight <60 kg for rivaroxaban, but similar to other DOACs, this agent displays increased systemic exposure in cachectic patients (see Figure 2).38 Patient ethnicity should also be taken into consideration, especially in low‐body‐weight patients. In a nationwide study conducted in South Korea with AF, patients weighing <60 kg were treated with regular doses of dabigatran, rivaroxaban, apixaban, and edoxaban or warfarin.77 Findings showed that DOACs were not only more effective but safer than warfarin for patients even with extremely low body weight (<50 kg).77
Overall, pharmacokinetic data suggest extreme body weights affect DOAC disposition, but this has not been overtly confirmed in limited trial data. Recommendations for DOAC use in patients of extreme body weight are listed in Figure 3.38, 77, 82 Given the paucity of data and caution advised by international groups, DOAC use in this population should be accompanied by laboratory monitoring to assess drug effect and prevent adverse outcomes, see monitoring section below.
Figure 3. DOAC use in extremes of body weight.
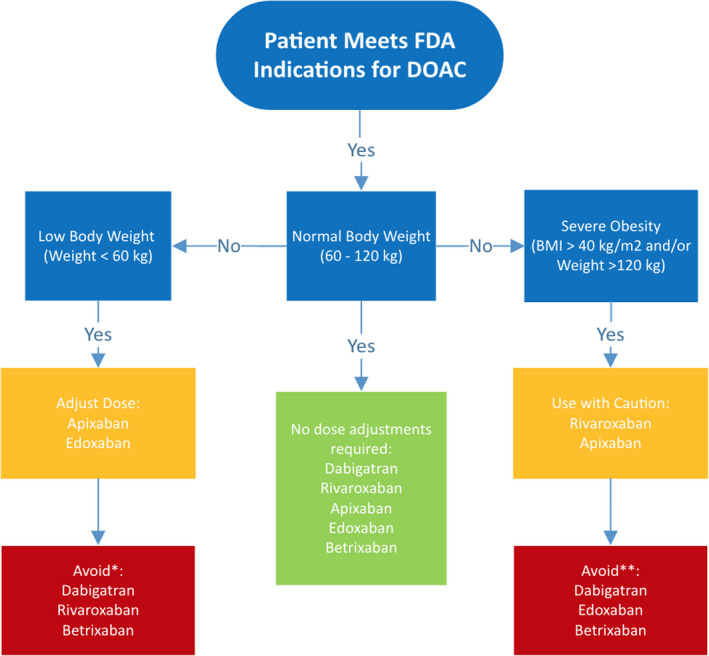
*Data not available for rivaroxaban and betrixaban; **avoid unless absolutely necessary; warrants specific laboratory monitoring (refer to Monitoring Parameters for more detail).
Monitoring Parameters
There are no FDA‐approved method to monitor the anticoagulant effect of DOACs. Qualitative coagulation assays such as activated partial thromboplastin time, thrombin time, and prothrombin time can be used as first‐line tests if evaluation for medication compliance is clinically important. However, these tests are insufficient to assess the degree of anticoagulant effect as seen with INR for management of VKA therapy. Quantitative measures exist such as anti–factor Xa levels, plasma drug concentrations, dilute thrombin time, and ecarin thrombin time to directly assess anticoagulation effects.90, 91 However, quantitative tests do not have an established clinical role, since standardized therapeutic ranges have not been established and quantitative test results have not been correlated with clinical outcomes. Other general monitoring parameters include signs and symptoms of bleeding, complete blood count, and a comprehensive metabolic panel specifically evaluating liver function tests, albumin, total bilirubin, and serum creatinine (see Table 5).These monitoring parameters should be performed in all patients before initiation of a DOAC and routinely monitored 1 to 3 months after initiation and then every 6 to 12 months thereafter, or more frequent follow‐ups based on patient‐specific characteristics is recommended in provider plans.
Table 5.
DOAC Laboratory Monitoring
| Drug Name | Qualitative | Quantitative | Other | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aPTT | TT | PT | Anti‐FXa Levels | Plasma Drug Concentration | dTT | ECT | CBC | CMP | |
| Dabigatran | x | x | x | x | x | x | x | x | |
| Rivaroxaban | x | x | x | x | x | ||||
| Apixaban | x | x | x | x | x | ||||
| Edoxaban | x | x | x | x | x | ||||
| Betrixaban | x | x | x | x | |||||
aPTT indicates activated partial thromboplastin time; CBC, complete blood count; CMP, comprehensive metabolic panel; dTT, dilute thrombin time; ECT, ecarin thrombin time; FXa, activated factor X; PT, prothrombin time; and TT, thrombin time.
In addition to laboratory monitoring parameters, a comprehensive DOAC monitoring approach should include the following items assessed at each follow‐up:
Current health status.
Adherence and cost.
Bleeding/thromboembolic events.
Adverse effects.
Complete medication review for drug interactions.
Reassessment of appropriateness of therapy.
Repeat warranted laboratory parameters.
Continuing follow‐up appointments for patients.
Anticoagulant Transitioning
As with any high‐risk medication such as anticoagulants, clinical situations may arise that require transitioning to or off of DOAC therapy. Two common transition scenarios include (1) switching between anticoagulants and (2) periprocedural management.
Switching Between Anticoagulants
It is not uncommon to require transition from one anticoagulant to another because of either cost, comorbid conditions, patient preference, hospitalization, thrombotic complications, bleeding complications, or procedures. Five scenarios exist that comprise DOAC therapy switching from/to another anticoagulant:
VKA to DOAC
DOAC to VKA
DOAC to DOAC
DOAC to parenteral anticoagulant
Parenteral anticoagulant to DOAC
For all scenarios, assessment of each drug's pharmacokinetic profile (ie, half‐life), pharmacodynamics profile (INR for VKA or activated partial thromboplastin time for unfractionated heparin, etc), as well as patients’ renal function, must be taken into account. The ultimate goal is to limit interruption of the therapeutic anticoagulation during the transition to minimize the risk of atherothrombosis. In certain cases, this requires overlap of the 2 anticoagulants. See Table 6.92
Table 6.
Algorithm for Switching Between Anticoagulants
| From | To | Action |
|---|---|---|
| VKA | DOAC |
Stop VKA and start DOAC once INR is <2 or lower INR limit of therapeutic range Measurement of INR before and after DOAC initiation is warranted as DOAC may falsely elevated INRs |
| Dabigatran | VKA |
CrCl >50 mL/min: start VKA and stop dabigatran 3 d later CrCl 31 to 50 mL/min: start VKA and stop dabigatran 2 d later CrCl 15 to 30 mL/min: start VKA and stop dabigatran 1 d later |
|
Rivaroxaban Apixaban |
VKA |
Start VKA and stop DOAC 3 d later OR for continuous anticoagulation: Stop DOAC and start LMWH and VKA at the time DOAC would have been due, then stop LMWH when INR is within therapeutic range |
| Edoxaban | VKA |
Start VKA and stop DOAC 3 d later OR for continuous anticoagulation: Patients taking 60 mg: reduce edoxaban to 30 mg and start warfarin concomitantly. Stop edoxaban when INR >2 Patients taking 30 mg: reduce edoxaban to 15 mg and start warfarin concomitantly. Stop edoxaban when INR ≥2 |
| Betrixaban | VKA | Start VKA and stop DOAC when INR > lower limit of therapeutic range |
| DOAC | DOAC | Stop current DOAC regimen and begin the new DOAC agent at the time next dose of DOAC is due |
| DOAC | Parental anticoagulanta | Stop DOAC and start parenteral anticoagulant at the same time that the next dose of DOAC would have been given |
| Parenteral anticoagulanta | DOAC |
Intravenous: Start DOAC 0 to 2 h after stopping UFH Subcutaneous: Stop LMWH and start DOAC at the same time that the next dose of LMWH would have been given |
CrCl indicates Cockcroft‐Gault creatinine clearance; DOAC, direct oral anticoagulant; INR, International Normalized Ratio; LMWH, low‐molecular‐weight heparin; UFH, unfractionated heparin; and VKA, vitamin K antagonist.
Parenteral anticoagulant: LMWH or UFH.
Source: American Heart Association, Inc.92
Transitioning between VKA and DOAC and vice versa requires additional explanation that takes into account the INR measurement. When transitioning from a VKA to a DOAC, the half‐life of the VKA compound is imperative to guide therapy. Since warfarin is the principle VKA utilized in the United States, with a half‐life of ≈36 to 48 hours, it is advisable to stop warfarin and check INR at least 3 days later. DOACs can be initiated once the INR is ≤2; see Table 7 for management strategies based on INR values.38 When converting from a DOAC to a VKA, providers should administer DOACs concomitantly with VKAs because of the VKAs’ known slow onset of action (≈5–10 days).38 An INR should be rechecked between days 3 and 5 of warfarin therapy. INRs should be checked just before the dose of the DOAC because of their ability to elevate INR values. The INRs should be monitored frequently for at least the first month until INR stability has been achieved (ie, ≥3 consecutive INR readings at goal).
Table 7.
INR Considerations When Transitioning Between VKA and DOAC
| VKA‐to‐DOAC Conversion | |
|---|---|
| INR ≤2 | Start DOAC immediately |
| INR 2–2.5 | Start DOAC immediately or preferably the next day |
| INR 2.5–3 |
Postpone DOAC Recheck INR in 1–3 d |
| INR ≥3 |
Postpone DOAC Recheck INR in 3–5 d |
| DOAC‐to‐VKA Conversion | |
|---|---|
| INR ≤2 |
Continue DOAC (half‐dose for edoxaban) while INR remains ≤2 Recheck INR in 1–3 d (before DOAC intake) |
| INR >2 |
Stop DOAC Recheck INR 1 d after discontinuing DOAC |
DOAC indicates direct oral anticoagulant; INR, International Normalized Ratio; and VKA, vitamin K antagonist.
Reproduced in part from Steffel et al38 with permission. Copyright ©2018, Oxford University Press.
Procedural Management
Past guidelines on perioperative and postoperative management for patients on DOACs have been primarily based on surgical risk (both thrombotic and bleeding), the DOAC's pharmacokinetic profile, concurrent medications, and patient characteristics (ie, comorbid diseases, history of bleeding complications, renal function, and age).92, 93 Because of DOACs’ fast onset and offset, periprocedural bridging is generally not required; however, it is important to note the length of time to hold regimens before and after procedures. Providers may find it useful to obtain specific coagulation measurements before procedures, especially in high‐risk interventions, to determine the level of hemostasis. However, for the majority of patients undergoing elective procedures, the interruption of DOAC therapy can be substantiated on a time‐based approach that takes into consideration bleeding risk of the procedure and renal function (see Table 8).38, 93 Furthermore, the results of the ongoing PAUSE (Perioperative Anticoagulant Use for Surgery Evaluation; NCT02228798) will help to further define a more conclusive standard‐of‐care approach for perioperative management.94
Table 8.
When to Interrupt and Restart DOAC Therapy During Elective Procedures
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Betrixaban | |
|---|---|---|---|---|---|
| Minor‐bleeding‐risk procedure | |||||
| Recommended to not stop in most minor surgical procedures | NAb | ||||
| STOP: 12–24 h before procedurea | |||||
| RESTART: 6 h after intervention | |||||
|
Low‐bleed‐risk procedure Stop 24–96 h before procedure | |||||
| CrCl ≥80 mL/min | STOP: ≥24 | STOP: ≥24 | STOP: ≥24 | STOP: ≥24 | STOP: ≥96 |
| CrCl ≤50–79 mL/min | STOP: ≥36 | STOP: ≥24 | STOP: ≥24 | STOP: ≥24 | STOP: ≥96 |
| CrCl ≤30–49 mL/min | STOP: ≥48 | STOP: ≥24 | STOP: ≥24 | STOP: ≥24 | Not indicated |
| CrCl ≤15–29 mL/min | Not indicated | STOP: ≥36 | STOP: ≥36 | STOP: ≥36 | Not indicated |
| CrCl ≤15 mL/min | Consider measuring drug activity to determine absence of drug affect | Not indicated | |||
| RESTART | ≥24 h after intervention | ||||
|
High‐bleed‐risk procedure Stop 48–96 h before procedure | |||||
| CrCl ≥80 mL/min | STOP: ≥48 | STOP: ≥48 | STOP: ≥48 | STOP: ≥48 | STOP: ≥96 |
| CrCl ≤50–79 mL/min | STOP: ≥72 | STOP: ≥48 | STOP: ≥48 | STOP: ≥48 | STOP: ≥96 |
| CrCl ≤30–49 mL/min | STOP: ≥96 | STOP: ≥48 | STOP: ≥48 | STOP: ≥48 | Not indicated |
| CrCl ≤15–29 mL/min | Not indicated | STOP: ≥48 | STOP: ≥48 | STOP: ≥48 | Not indicated |
| CrCl ≤15 mL/min | Consider measuring drug activity to determine absence of drug effect | Not indicated | |||
| RESTART | ≥48 to 72 h after intervention | ||||
Minor‐bleeding‐risk interventions: dental, cataract, glaucoma, endoscopy without biopsy or resection, superficial surgery; low‐bleeding‐risk interventions: endoscopy with biopsy, prostate biopsy, bladder biopsy, pacemaker or implantable cardioverter‐defibrillator implantation, noncoronary angiography, electrophysiological study/catheter ablation; high‐bleeding‐risk intervention: major surgery, spinal puncture or placement of spinal/epidural catheter, other situations in which complete hemostasis is required. CrCl indicates Cockcroft‐Gault creatinine clearance; and NA, not applicable.
Skip 1 dose of dabigatran or apixaban; no dose of edoxaban or rivaroxaban is skipped.
Has not been studied.
Reproduced in part from Steffel et al38 with permission. Copyright ©2018, Oxford University Press.
Major Drug Interactions
Drug‐drug interactions are an important concern for any patient managed on DOAC therapy. Concomitantly administered drugs that alter DOAC plasma concentrations can lead to serious complications, with increased DOAC concentrations potentially resulting in bleeding events, and decrease DOAC concentration placing the patient at risk for thrombus formation. Initially, DOACs were viewed as having minimal drug interactions, which has proven incorrect. When compared with VKAs, which are highly associated with substantial drug‐drug interactions, DOACs impart a lower risk; however, they still have significant risk of interactions. Three different types of drug interactions need to be evaluated when managing DOAC therapy: (1) agents affecting renal clearance, (2) agents affecting hepatic clearance, and (3) agents concurrently affecting hemostasis.
Agents Affecting Renal Clearance
As all DOACs used for treatment of atherothrombosis rely on the kidneys for elimination to varying degrees, agents that hinder this organ's ability to clear DOAC may place the patient at increased risk for bleeding complications. Common agents to monitor are NSAIDs, diuretics, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and immunosuppressants (ie, calcineurin inhibitors such as cyclosporine and tacrolimus, mammalian target of rapamycin inhibitors such as sirolimus and everolimus).38 No DOAC‐specific dose adjustments are required but warrant more frequent monitoring of renal function and possibly holding therapy or consideration for transition to an agent with less renal involvement if renal function is compromised.
Agents Affecting Hepatic Clearance
All DOACs are eliminated by either CYP metabolic enzymes or permeability glycoprotein transporters; thus, agents that inhibit or induce these enzyme systems can constitute major drug‐drug interactions and place the patient at undue risk for complications (see Table 9). A common drug‐drug interaction example includes CYP3A4 inhibitors with the use of apixaban, which as discussed above is the DOAC with the highest reliance of hepatic biotransformation for clearance. Regimens such as ketoconazole, ritonavir, and itraconazole significantly reduce apixaban drug concentrations and therefore should be avoided.10 Because of the likelihood of concomitant interacting medications, the risk of drug‐drug interactions increases when DOACs are prescribed in patients with the following comorbidities: HIV, organ transplantation, infection, malignancy, epilepsy, and arrhythmia. Refer to previously published reviews for a more comprehensive list of interacting medications and associated management strategies.38, 95
Table 9.
| Drug Interaction | Effect of DOAC | Recommendations | |
|---|---|---|---|
| Dabigatran | P‐gp inhibitors | Increase in concentration | Reduce dose or avoid depending on renal function |
| P‐gp inducers | Significant reduction in concentration | Avoid use | |
| Antacids | Moderate reduction in concentration | No dose adjustments required; consider spacing regiments by 2 h | |
| Apixaban | Strong CYP3A4 inhibitor+P‐gp inhibitor | Significant increase in concentration | Reduce dose or avoid use |
| Moderate CYP3A4 inhibitor+P‐gp inhibitor | Moderate increase in concentration |
No dose adjustments required; use with caution Avoid use in patient with severe renal insufficiency |
|
|
Strong CYP3A4 inducer or P‐gp inducer |
Significant reduction concentration | Avoid use | |
| Rivaroxaban | Strong CYP3A4 inhibitor+P‐gp inhibitor | Significant increase in concentration | Avoid use |
| Moderate CYP3A4 inhibitor+P‐gp inhibitor | Moderate increase in concentration |
No precaution necessary Avoid use in patient with severe renal insufficiency |
|
|
Strong CYP3A4 inducer or P‐gp inducer |
Significant reduction concentration | Avoid use | |
| Edoxaban | P‐gp inhibitors | Increase in concentration |
AF: Do not reduce dose VTE treatment: Reduce dose |
| P‐gp inducers | Significant reduction in concentration | Avoid use with rifampin | |
| Betrixaban | P‐gp inhibitors | Increase in concentration | Avoid: CrCl <30 mL/min |
| P‐gp inducers | Significant reduction in concentration | Not addressed |
| Drug Interaction Examples | |
|---|---|
| Strong CYP3A4 inhibitors+combined P‐gp inhibitor | Itraconazole, ketoconazole, ritonavir |
| Moderate CYP3A4 inhibitors+combined P‐gp inhibitor | Clarithromycin, diltiazem |
| Strong CYP3A4 inducer+combined P‐gp inducer | Carbamazepine, rifampin, St. John's wort |
| Strong CYP3A4 inducers | Phenytoin |
| P‐gp inhibitors | Amiodarone, clarithromycin, cyclosporine, dronedarone, erythromycin ivacaftor, ketoconazole, nifedipine, quinidine, ranolazine, ticagrelor, tolvaptan, verapamil |
| P‐gp inducers | Rifampin |
Drug examples cited in the US prescribing information. AF indicates atrial fibrillation; CrCl, Cockcroft‐Gault creatinine clearance; CYP, cytochrome P450; P‐gp, permeability glycoprotein; and VTE, venous thromboembolism.
Source: American Heart Association, Inc.92
Agents Concurrently Affecting Bleeding Risk
As mentioned above, bleeding complications increase when DOACs are coprescribed with antiplatelets (aspirin, P2Y12 inhibitors) or agents with antiplatelet properties (NSAIDs, systemic corticosteroids, etc), and these combinations should be minimized whenever possible and carefully balanced against improved thrombosis prevention when clinically indicated. If concomitant therapy cannot be avoided, addition of H2 antihistamine receptor blockers or proton pump inhibitors to mitigate gastrointestinal bleeding should be undertaken.
Cost Analysis
DOACs have quickly gained popularity as an alternative anticoagulation strategy and surpassed VKAs as first‐line agents because of improved clinical outcomes, simplicity of dosing, lack of monitoring requirements, and expanding indication list. All DOACs are currently branded medications; therefore, cost remains a significant barrier to access. Apixaban was recently granted generic approval at the end of 2019 but product availability (likely not for several more years), cost and generic status of the other DOACs remain unknown.96 While DOACs are substantially more expensive than VKA, laboratory monitoring costs are reduced since INR monitoring is not required. US cost analyses have shown that apixaban has the highest quality‐adjusted life year for stroke prevention in NVAF patients followed by dabigatran, edoxaban, rivaroxaban, and the lowest being warfarin.97, 98, 99A recent study demonstrated that dabigatran was associated with lower all‐cause costs and healthcare resource use compared with rivaroxaban, while having similar all‐cause costs and hospitalizations compared with apixaban, but higher outpatient and pharmacy healthcare resource use.100
In the United States, commercially insured patients are eligible for time‐limited programs to reduce out‐of‐pocket expense using manufacturer copayment cards (most limiting patient costs to ≤$10 per month). Federally funded patients (Medicare, Medicaid, etc) are ineligible for these programs (see Table 10).101 Despite the cost, from 2010 to 2017 there was a dramatic increase in the use of DOACs for NVAF.7 Analyses have shown that apixaban has become the most prescribed DOAC for NVAF and accounted for approximately half of new DOAC prescriptions by 2017. While the information in Table 10 demonstrates the cash price of DOACs, this does not take into account other factors that need to be considered for enhanced patient care, including clinical outcomes, quality of life, patient preference, and laboratory monitoring costs.
Table 10.
US Cost Comparison of DOACs
| Apixaban | Dabigatran | Rivaroxaban | Edoxaban | Betrixaban | |
|---|---|---|---|---|---|
| Cash price |
$300–$500/mo $8.38/tablet |
$300–$500/mo $8.01/tablet |
$350–$600/mo $8.38/tablet |
$350/mo $13.46 per tablet |
$450–$680/mo $18 per tablet |
| Copay carda | Yes | Yes | Yes | Yes | Yes |
| Copay | $10/mo | $5/mo | $10/mo | $4/mo | $75/mo reduction after patient pays initial $50 |
| Free 30‐d coupon | Yes | Yes | No | No | No |
| Patient assistance program | Bristol‐Myer Squibb | Boehringer‐Ingelheim | Johnson & Johnson | No program available | The Patient Advocate Foundation |
| Eligibility for patient assistance program |
US resident Limited or no prescription coverage Income requirements |
US resident Limited or no prescription coverage Income requirements |
US resident Limited or no prescription coverage Income requirements |
NA |
US resident Limited or no prescription coverage Income requirements |
DOAC indicates direct oral anticoagulant; and NA, not applicable.
Available to commercially insured patients only. Not valid if enrolled in state or federally funded prescription benefit program.
Conclusion
DOACs have revolutionized anticoagulant management and are becoming the cornerstone treatment for stroke prevention in AF and VTE prophylaxis and treatment, and the list of other indications is expanding. There are many factors that will affect appropriate efficacy and safety end points when prescribing DOAC therapy, and this review aims to address these scenarios. Patient comorbidities must be considered when selecting the most appropriate anticoagulant. It is now recognized that routine monitoring of renal and hepatic function, signs/symptoms of bleeding, and parameters of compliance should be considered for all patients. Clinicians should incorporate patient preferences, clinical outcomes data, patient characteristics, and quality‐of‐life considerations when recommending an anticoagulant. Cost can play a critical role in this decision, with DOAC affordability for individual patients varying based on medication benefit copayment tiers. As the future of anticoagulant therapies continues to evolve, DOACs will remain a critical therapy for preventing thrombotic events.
Sources of Funding
None.
Disclosures
BAW reports institutional grant from Akcea.
(J Am Heart Assoc. 2020;9:e017559 DOI: 10.1161/JAHA.120.017559.)
For Sources of Funding and Disclosures, see page 15.
References
- 1. Aronis KN, Hylek EM. Evidence gaps in the era of non‐vitamin K oral anticoagulants. J Am Heart Assoc. 2018;7:e007338 DOI: 10.1161/JAHA.117.007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 3. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:320–328. [DOI] [PubMed] [Google Scholar]
- 4. Kapoor A, Ellis A, Shaffer N, Gurwitz J, Chandramohan A, Saulino J, Ishak A, Okubanjo T, Michota F, Hylek E, et al. Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: a network meta‐analysis. J Thromb Haemost. 2017;15:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Hernandez AF, Gibson CM. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–544. [DOI] [PubMed] [Google Scholar]
- 6. Rose DK, Bar B. Direct oral anticoagulant agents: pharmacologic profile, indications, coagulation monitoring, and reversal agents. J Stroke Cerebrovasc Dis. 2018;27:2049–2058. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy. 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prescribing information. Pradaxa (dabigatran etaxilate). Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2018. [Google Scholar]
- 9. Prescribing information. Xarelto (rivaroxaban). Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019. [Google Scholar]
- 10. Prescribing information. Eliquis (apixaban). Princeton, NJ: Bristol‐Myers Squibb Company; 2018. [Google Scholar]
- 11. Prescribing information. Savaysa (edoxaban). Tokyo, Japan: Daiichi Sankyo Co., LTD; 2017. [Google Scholar]
- 12. Prescribing information. Bevyxxa (Betrixaban). San Francisco, CA: Portola Pharmaceuticals, Inc; 2017. [Google Scholar]
- 13. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 14. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, Cannon CP, Tanguay JF, Granger CB, Mauri L, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention. Circulation. 2018;138:527–536. [DOI] [PubMed] [Google Scholar]
- 15. Hansen ML, Sorensen R, Clausen MT, Fog‐Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1441. [DOI] [PubMed] [Google Scholar]
- 16. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 17. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 18. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 19. Lip GYH, Collet JP, Haude M, Huber K. Management of antithrombotic therapy in AF patients presenting with ACS and/or undergoing PCI: a summary of the Joint Consensus Document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Eur Heart J. 2018;39:2847–2850. [DOI] [PubMed] [Google Scholar]
- 20. Vaduganathan M, Bhatt DL, Cryer BL, Liu Y, Hsieh WH, Doros G, Cohen M, Lanas A, Schnitzer TJ, Shook TL, et al. Proton‐pump inhibitors reduce gastrointestinal events regardless of aspirin dose in patients requiring dual antiplatelet therapy. J Am Coll Cardiol. 2016;67:1661–1671. [DOI] [PubMed] [Google Scholar]
- 21. Renda G, Ricci F, Giugliano RP, De Caterina R. Non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol. 2017;69:1363–1371. [DOI] [PubMed] [Google Scholar]
- 22. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 23. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 24. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 25. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 26. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 27. Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e576S–e600S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 29. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–1201. [DOI] [PubMed] [Google Scholar]
- 30. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 31. Guimaraes PO, Pokorney SD, Lopes RD, Wojdyla DM, Gersh BJ, Giczewska A, Carnicelli A, Lewis BS, Hanna M, Wallentin L, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: insights from the ARISTOTLE trial. Clin Cardiol. 2019;42:568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carnicelli AP, De Caterina R, Halperin JL, Renda G, Ruff CT, Trevisan M, Nordio F, Mercuri MF, Antman E, Giugliano RP, et al. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135:1273–1275. [DOI] [PubMed] [Google Scholar]
- 33. Duraes AR, de Souza Roriz P, de Almeida Nunes B, Albuquerque FP, de Bulhoes FV, de Souza Fernandes AM, Aras R. Dabigatran versus warfarin after bioprosthesis valve replacement for the management of atrial fibrillation postoperatively: DAWA pilot study. Drugs R D. 2016;16:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yadlapati A, Groh C, Malaisrie SC, Gajjar M, Kruse J, Meyers S, Passman R. Efficacy and safety of novel oral anticoagulants in patients with bioprosthetic valves. Clin Res Cardiol. 2016;105:268–272. [DOI] [PubMed] [Google Scholar]
- 35. Russo V, Attena E, Mazzone C, Esposito F, Parisi V, Bancone C, Rago A, Nigro G, Sangiuolo R, D'Onofrio A. Nonvitamin K antagonist oral anticoagulants use in patients with atrial fibrillation and bioprosthetic heart valves/prior surgical valve repair: a multicenter clinical practice experience. Semin Thromb Hemost. 2018;44:364–369. [DOI] [PubMed] [Google Scholar]
- 36. Biase LD, Trivedi C, Mohanty P, Mohanty S, Gianni C, Bai R, Santangeli P, Sanchez J, Horton R, Hranitzky P, et al. Periprocedural and long term safety and feasibility of treatment with novel oral anticoagulants in patients with biological valve and atrial fibrillation. J Am Coll Cardiol. 2015;65:A355. [Google Scholar]
- 37. ENAVLE . Edoxaban noninferior to warfarin for thromboembolism, bleeding early after surgical valve implantation or repair. Available at: https://www.healio.com/cardiology/surgery/news/online/%7B8d5cf185-8f48-4c0b-8f68-5079aba93fa6%7D/edoxaban-noninferior-to-warfarin-for-thromboembolism-bleeding-early-after-surgical-valve-implantation-or-repair. Accessed March 31, 2020.
- 38. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan‐Schilling V, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 39. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 40. Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123:768–778. [DOI] [PubMed] [Google Scholar]
- 41. Antithrombotic Trialists Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coppens M, Weitz JI, Eikelboom JWA. Synergy of dual pathway inhibition in chronic cardiovascular disease. Circ Res. 2019;124:416–425. [DOI] [PubMed] [Google Scholar]
- 43. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook‐Bruns N, Fox KA, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 44. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 45. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 46. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113. [DOI] [PubMed] [Google Scholar]
- 47. Tanigawa T, Kaneko M, Hashizume K, Kajikawa M, Ueda H, Tajiri M, Paolini JF, Mueck W. Model‐based dose selection for phase III rivaroxaban study in Japanese patients with non‐valvular atrial fibrillation. Drug Metab Pharmacokinet. 2013;28:59–70. [DOI] [PubMed] [Google Scholar]
- 48. Mosarla RC, Vaduganathan M, Qamar A, Moslehi J, Piazza G, Giugliano RP. Anticoagulation strategies in patients with cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, et al. Low‐molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. [DOI] [PubMed] [Google Scholar]
- 50. Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677–686. [DOI] [PubMed] [Google Scholar]
- 51. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
- 52. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36:2017–2023. [DOI] [PubMed] [Google Scholar]
- 53. McBane RD 2nd, Wysokinski WE, Le‐Rademacher JG, et al. Apixaban and dalteparin in active malignancy‐associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost. 2020;18:411–421. DOI: 10.1111/jth.14662 [DOI] [PubMed] [Google Scholar]
- 54. Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, Cohen A, Bauersachs R, Brenner B, Torbicki A, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599–1607. [DOI] [PubMed] [Google Scholar]
- 55. Khorana AA, Noble S, Lee AYY, Soff G, Meyer G, O'Connell C, Carrier M. Role of direct oral anticoagulants in the treatment of cancer‐associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891–1894. [DOI] [PubMed] [Google Scholar]
- 56. National Comprehensive Cancer Network . NCCN guidelines for cancer‐associated venous thromboembolic disease. Available at: https://jnccn.org/view/journals/jnccn/16/11/article-p1289.xml. Accessed May 16, 2019. [DOI] [PubMed]
- 57. Khorana AA, Soff GA, Kakkar AK, Vadhan‐Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 58. Carrier M, Abou‐Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 59. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74:2204–2215. [DOI] [PubMed] [Google Scholar]
- 60. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 61. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 62. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 63. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, Kaewput W, Pachariyanon P, Cheungpasitporn W. Safety and efficacy of apixaban versus warfarin in patients with end‐stage renal disease: meta‐analysis. Pacing Clin Electrophysiol. 2018;41:627–634. [DOI] [PubMed] [Google Scholar]
- 64. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, et al. Outcomes associated with apixaban use in patients with end‐stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kumbhani D. RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation—RENAL‐AF. Oral Presentation at. American Heart Association Annual Scientific Sessions (AHA 2019) November 2019, Philadelphia, PA. [Google Scholar]
- 66. Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, Baker WL. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132:1078–1083. [DOI] [PubMed] [Google Scholar]
- 67. Huisman MV, Klok FA. Pharmacological properties of betrixaban. Eur Heart J Suppl. 2018;20:E12–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of renal function on outcomes with edoxaban in the ENGAGE AF‐TIMI 48 trial. Circulation. 2016;134:24–36. [DOI] [PubMed] [Google Scholar]
- 69. Sanghai S, Wong C, Wang Z, Clive P, Tran W, Waring M, Goldberg R, Hayward R, Saczynski JS, McManus DD. Rates of potentially inappropriate dosing of direct‐acting oral anticoagulants and associations with geriatric conditions among older patients with atrial fibrillation: the SAGE‐AF study. J Am Heart Assoc. 2020;9:e014108 DOI: 10.1161/JAHA.119.014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Murata N, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, Matsumoto M, Kojima T, Hanada S, et al. Clinical outcomes of off‐label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF Registry. Circ J. 2019;83:727–735. [DOI] [PubMed] [Google Scholar]
- 71. Ruiz Ortiz M, Muñiz J, Raña Míguez P, Roldán I, Marín F, Asunción Esteve‐Pastor M, Cequier A, Martínez‐Sellés M, Bertomeu V, Anguita M. Inappropriate doses of direct oral anticoagulants in real‐world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA Registry. Europace. 2018;20:1577–1583. [DOI] [PubMed] [Google Scholar]
- 72. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non‐vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]
- 73. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol. 2018;71:2162–2175. [DOI] [PubMed] [Google Scholar]
- 74. Steuber TD, Howard ML, Nisly SA. Direct oral anticoagulants in chronic liver disease. Ann Pharmacother. 2019;53:1042–1049. DOI: 10.1177/1060028019841582. [DOI] [PubMed] [Google Scholar]
- 75. Barsam SJ, Patel JP, Roberts LN, Kavarthapu V, Patel RK, Green B, Arya R. The impact of body weight on rivaroxaban pharmacokinetics. Res Pract Thromb Haemost. 2017;1:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59‐7939) in healthy subjects. J Clin Pharmacol. 2007;47:218–226. [DOI] [PubMed] [Google Scholar]
- 77. Lee SR, Choi EK, Park CS, Han KD, Jung JH, Oh S, Lip GYH. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol. 2019;73:919–931. [DOI] [PubMed] [Google Scholar]
- 78. Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non‐valvular atrial fibrillation from the RE‐LY trial. J Thromb Haemost. 2011;9:2168–2175. [DOI] [PubMed] [Google Scholar]
- 79. McCaughan GJB, Favaloro EJ, Pasalic L, Curnow J. Anticoagulation at the extremes of body weight: choices and dosing. Expert Rev Hematol. 2018;11:817–828. [DOI] [PubMed] [Google Scholar]
- 80. Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, LaCreta FP, Frost CE. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boonyawat K, Caron F, Li A, Chai‐Adisaksopha C, Lim W, Iorio A, Lopes RD, Garcia D, Crowther MA. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: a systematic review and meta‐analysis. J Thromb Haemost. 2017;15:1322–1333. [DOI] [PubMed] [Google Scholar]
- 82. Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Piran S, Traquair H, Chan N, Bhagirath V, Schulman S. Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: a retrospective study. Res Pract Thromb Haemost. 2018;2:684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kushnir M, Choi Y, Eisenberg R, Rao D, Tolu S, Gao J, Mowrey W, Billett HH. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single‐centre, retrospective analysis of chart data. Lancet Haematol. 2019;6:e359–e365. [DOI] [PubMed] [Google Scholar]
- 85. Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother. 2019;53:165–170. [DOI] [PubMed] [Google Scholar]
- 86. Safouris A, Demulder A, Triantafyllou N, Tsivgoulis G. Rivaroxaban presents a better pharmacokinetic profile than dabigatran in an obese non‐diabetic stroke patient. J Neurol Sci. 2014;346:366–367. [DOI] [PubMed] [Google Scholar]
- 87. Breuer L, Ringwald J, Schwab S, Kohrmann M. Ischemic stroke in an obese patient receiving dabigatran. N Engl J Med. 2013;368:2440–2442. [DOI] [PubMed] [Google Scholar]
- 88. Coons JC, Albert L, Bejjani A, Iasella CJ. Effectiveness and safety of direct oral anticoagulants versus warfarin in obese patients with acute venous thromboembolism. Pharmacotherapy. 2020;40:204–210. [DOI] [PubMed] [Google Scholar]
- 89. Lee CH, Lin TY, Chang SH, Chen CH, Hsu YJ, Hung KC, Wen MS. Body mass index is an independent predictor of major bleeding in non‐valvular atrial fibrillation patients taking dabigatran. Int J Cardiol. 2017;228:771–778. [DOI] [PubMed] [Google Scholar]
- 90. Conway SE, Hwang AY, Ponte CD, Gums JG. Laboratory and clinical monitoring of direct acting oral anticoagulants: what clinicians need to know. Pharmacotherapy. 2017;37:236–248. [DOI] [PubMed] [Google Scholar]
- 91. Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non‐vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Raval AN, Cigarroa JE, Chung MK, Diaz‐Sandoval LJ, Diercks D, Piccini JP, Jung HS, Washam JB, Welch BG, Zazulia AR, et al. Management of patients on non‐vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation. 2017;135:e604–e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL Jr, Ortel TL, Saxonhouse SJ, Spinler SA. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69:871–898. [DOI] [PubMed] [Google Scholar]
- 94. Douketis JD, Spyropoulos AC, Anderson JM, Arnold DM, Bates SM, Blostein M, Carrier M, Caprini JA, Clark NP, Coppens M, et al. The Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) study for patients on a direct oral anticoagulant who need an elective surgery or procedure: design and rationale. Thromb Haemost. 2017;117:2415–2424. [DOI] [PubMed] [Google Scholar]
- 95. Wiggins BS, Dixon DL, Neyens RR, Page RL II, Gluckman TJ. Select drug‐drug interactions with direct oral anticoagulants: JACC review topic of the week. J Am Coll Cardiol. 2020;75:1341–1350. [DOI] [PubMed] [Google Scholar]
- 96. FDA approves first generics of Eliquis. FDA Press Announcement. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-generics-eliquis. Accessed December 30 2019.
- 97. Shah A, Shewale A, Hayes CJ, Martin BC. Cost‐effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke. 2016;47:1555–1561. [DOI] [PubMed] [Google Scholar]
- 98. Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC. Cost‐effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. [DOI] [PubMed] [Google Scholar]
- 99. Mendoza‐Sanchez J, Silva F, Rangel L, Jaramillo L, Mendoza L, Garzon J, Quiroga A. Benefit, risk and cost of new oral anticoagulants and warfarin in atrial fibrillation; a multicriteria decision analysis. PLoS One. 2018;13:e0196361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gilligan AM, Franchino‐Elder J, Song X, Wang C, Henriques C, Sainski‐Nguyen A, Wilson K, Smith DM, Sander S. Comparison of all‐cause costs and healthcare resource use among patients with newly‐diagnosed non‐valvular atrial fibrillation newly treated with oral anticoagulants. Curr Med Res Opin. 2018;34:285–295. [DOI] [PubMed] [Google Scholar]
- 101. NeedyMeds . Patient assistance programs. Available at: https://www.needymeds.org. Accessed May 17, 2019.


