Abstract
The contribution of nerves to the pathogenesis of malignancies has emerged as an important component of the tumour microenvironment. Recent studies have shown that peripheral nerves (sympathetic, parasympathetic, and sensory) interact with tumour and stromal cells to promote the initiation and progression of a variety of solid and haematological malignancies. Furthermore, new evidence suggests that cancers may reactivate nerve-dependent developmental and regenerative processes to promote their growth and survival. Here, we review emerging concepts and discuss the therapeutic implications of manipulating nerves and neural signalling for the prevention and treatment of cancer.
Table of Contents Summary
This Review discusses the role that nerves play in the initiation and progression of cancers focussing on the evidence that tumours may reactivate nerve-mediated developmental and regenerative pathways to promote their own growth and survival.
INTRODUCTION
A network of autonomic and sensory nerves in the periphery, termed the peripheral nervous system (PNS), helps coordinate the molecular, cellular, and organ level processes that enable the body to maintain homeostatic control. These coordinated systems continuously adjust internal variables including blood pressure, pH, and metabolism, among many others, in order to adapt to internal and external pressures. Similarly, tumours must regulate these processes to ensure their survival1. A growing body of evidence suggests that just as healthy tissues recruit and maintain innervation [G] by the PNS, tumours co-opt neural programs to promote their growth and progression2–5.
Within the PNS, the autonomic nervous system (ANS), so called because it is classically considered to be outside of conscious control6, is further divided into the sympathetic nervous system [G] (SNS) and parasympathetic nervous system [G] (PSNS). On the cellular level, nerves in the SNS and PSNS are organized as two neurons in series; the first originating in the spinal cord or brain stem (central nervous system; CNS) and the second in a ganglion [G] in the periphery. These two neurons in series are known as pre-ganglionic and post-ganglionic, respectively. While anatomically defined by their origin, sympathetic and parasympathetic nerves are similar in that their post-ganglionic cell bodies reside outside of the CNS and sit in relative proximity to the glands and organs that they innervate (Figure 1). Thus, these post-ganglionic nerves are influenced by the microenvironment that they innervate, and in turn can rapidly respond to changes in the tumour microenvironment (TME). Since the neuronal cell bodies are in close proximity to the TME, their response to stimuli is not limited to the classical release of preformed neurotransmitters, but extends to altering transcription, translation, and cytoskeletal dynamics. Emerging studies suggest that sensory nerves, classically considered to act as information relays to the CNS, similarly interact with and influence the TME7–11. However, it is important to note that as the autonomic neuronal cell bodies are located near to—but outside—the organs that they innervate, their genetic material is often not captured by tumour profiling studies and therefore, their contributions to the TME may be underappreciated in such studies.
Figure 1. Autonomic innervation of major primary sites of cancer formation in the mouse: anatomical targets for pre- and post-ganglionic surgical denervation.
This schematic depicts pre- and post-ganglionic innervation by the parasympathetic nervous system (PSNS), in purple, and the sympathetic nervous system (SNS), in green, of several well-studied organs where nerves have been implicated in disease progression. Anatomical landmarks are highlighted to indicate where pre- and post-ganglionic denervation of exocrine glands can occur. SNS outflow is depicted originating in the thoracic and lumbar regions of the spinal cord. The cell bodies of these preganglionic nerves (depicted by dotted lines) either project to the paravertebral sympathetic chain (the column of ganglia depicted just outside the spinal column) including the superior cervical and stellate ganglia, or project to distant ganglia including the celiac and hypogastric (pelvic) ganglia. Postganglionic nerves are depicted by solid lines. The salivary glands are a collection of three bilateral glands found in the mandibular region and consist of (1) the parotid gland, (2) the sublingual gland, and (3) the submaxillary gland. PSNS outflow is depicted originating in the brainstem and sacral region of the spinal cord. As depicted for the SNS, dotted lines represent preganglionic nerves, and solid lines represent postganglionic nerves.*, indicates sensory nuclei (nodose ganglion) whose projections are carried by the vagus nerve, along with parasympathetic fibers.
Since the 16th century, it has been observed that nerves and blood vessels travel in tandem12, and that both structures are necessary for organ maintenance. As tumours require these same structures for growth and survival, it was thus reasoned that transection of these structures may provide cancer control. Almost two centuries ago, this hypothesis was tested for the first time when surgeons attempted to treat tumours of the lip by transecting the trigeminal nerve and its accompanying vessels13. While transection of nerves and vessels achieved symptomatic control (reduced ulceration and pain), all four patients in the study ultimately required surgical removal of the tumour for cure. However, it has only been in the past decade that the molecular mechanisms and contributions of nerves to the TME have been elucidated. This is largely due to technological advances that have enabled precise imaging and manipulation of neuronal input to the TME to study its effects on cancer initiation and progression. Similarly, these tools have helped to elucidate how tumours reactivate developmental neurogenic pathways common to organogenesis. Driven by evidence from clinical studies, novel translational therapeutic techniques are under development to target the interactions between nerves and the TME for the treatment of cancer. In this Review, we provide an overview of recent insights into the roles nerves play in regulating the TME, and how the TME regulates the recruitment of nerves to promote cancer development and progression. We also discuss potential therapeutic implications for the development of novel cancer treatments.
Innervation of cancer
In the same way as increased blood vessel formation is necessary for tumour growth, an increase in vascular density is one of the first histological changes observed in neoplastic tissues. Moreover, as cancer progresses from pre-neoplastic lesions to overt cancer, nerve density nearly doubles compared with age-matched non-neoplastic tissue controls2,14. Furthermore, an increase in nerve density has been found to correlate consistently with more aggressive disease, including for cancers of the prostate, colon and rectum, head and neck, breast, pancreas, stomach, and lung3,5,14–19. A variety of tools have been employed to explore the mechanistic importance of cancer innervation to disease initiation and progression.
Denervation studies
Like wires providing current to a circuit, nerves can be thought of as providing regulatory input to tissues. Therefore, loss of nerves (known as denervation), terminates this communication enabling investigation of the role of neural innervation on its target tissue. While conceptually simple, specific targeting of the nerves innervating a tissue of interest requires intimate understanding of neuroanatomy, as transecting a major nerve before it gives rise to its tissue-specific branch can cause major morbidity and mortality20. A variety of techniques have been developed to trace the innervation pattern of tissues (Table 1), most of which rely on the retrograde axonal transport of injected dyes or infection with fluorescent protein-expressing neurotropic viruses21,22. Thus, using these innervation maps (Figure 1), researchers have been able to study the contributions of nerves to cancer initiation, progression, and metastasis. Nevertheless, it is notable that construction of these innervation maps is based on studies in normal (wild type) organs, not in the setting of cancer. As such, further studies are needed to ascertain precise innervation maps of cancer tissues.
Table 1:
Tools for the study and manipulation of innervation of the tumour microenvironment
| Tool | Mechanism of action | Application | Ref |
|---|---|---|---|
| Mouse line | |||
| Advillin-Cre transgenic mouse | Peripheral sensory nerve-specific Cre recombinase (not expressed in CNS) | Enables specific deletion of genes in sensory nerves, or nerve depletion when crossed with DTA or DTR knock-in mice | 192,193 |
| Ngf knock-in mouse | Ngf gene preceded by a stop codon inserted into the ROSA26 locus | When crossed to a Cre-expressing mouse strain (e.g. epithelial or stromal expressed Cre), increases NGF production and tissue innervation | 4,59 |
| Nav1.8 Cre knock-in mouse | Express Cre recombinase from the endogenous sodium channel Scn10a locus. | Enables selective deletion of genes in nociceptive nerves, or nociceptive neuron depletion when crossed with DTA or DTR knock-in mice. | 194 |
|
TrkAF592A or TrkBF616A knock-in mice |
Trk knock-in alleles with modified kinase domains allowing pharmacological inhibition with nanomolar concentrations of the ligands 1NMPP1 or 1NaPP1 | Temporal pharmacological inhibition of nerve recruitment in the TME | 118,195 |
| Trkb-CreERT2 knock-in mouse | Tamoxifen-inducible sensory nerve-specific Cre recombinase | Enables temporal control over gene expression specifically in sensory nerves or nerve depletion when crossed with DTA or DTR knock-in mice. | 82 |
| Neurotoxin | |||
| 6-hydroxy dopamine (6OHDA) |
Polar monoamine mimetic that is taken up in the periphery by post-synaptic adrenergic nerves causing free radical destruction of nerve terminals | When given neonatally, it induces permanent global sympathectomy (as well as off-target CNS effects), whereas in the adult, it only causes temporary peripheral sympathectomy (as it is unable to cross the BBB) | 2,5,30,31 |
| RTX | A super agonist of sensory neuron-expressed calcium channel TRPV1, causing calcium-mediated neuronal death | When given neonatally, it induces permanent global sensory denervation, whereas in the adult, it causes temporary peripheral sensory denervation | 10,196 |
| Capsaicin | TRPV1 agonist causing cytotoxicity at high doses | Less potent than RTX requiring repeated injections or incorporation into daily mouse feeds to induce lasting sensory denervation. | 7,11,38,197 |
| Botulinum toxin | Bacterium-derived protease that is taken up by endocytosis in the axonal terminal, and which selectively cleaves synaptic proteins preventing acetylcholine release | When injected into a tissue of interest causes temporary denervation of cholinergic synapses | 3,18,32,198 |
| Surgical denervation | |||
| Mechanical neurectomy | Mechanical transection of axonal process, or removal of ganglionic cell body | Physical disruption of tissue innervation | 3–5,8,9,16,18,43 |
| Genetic vector | |||
| AAV | Replication inactivated viral vector that stably and predictably integrates desired engineered genetic information into neurons with minimal neurotoxicity | When injected peripherally in a tissue of interest, it travels in a retrograde manner to an innervating ganglion with the neuronal cell type selectivity determined by an engineered promoter (e.g. tyrosine hydroxylase for adrenergic specific expression) | 26,199 |
| Neuro modulation | |||
| Optogenetic ion channels | Light activated microbial ion channels with high fidelity spatial and temporal activation and inactivation | When expressed in peripheral nerves in vivo, it enables precise spatiotemporal control of nerve firing and neurotransmitter release | 200 |
| Chemogenetic receptors | Engineered G-protein coupled receptors activated by biologically inert drugs that modulate neuronal firing via altering intracellular levels of second messengers such as cAMP and calcium | When expressed in nerves in vivo, it enables temporal (and to a degree spatial) control of nerve firing and neurotransmitter release | 199,201 |
| Long-acting ion channels (NaChBac) | Voltage-gated prokaryotic sodium ion channel with prolonged inactivation kinetics | When expressed in nerves in vivo, it enables increased nerve firing and neurotransmitter release | 26,202 |
AAV, adeno-associated virus; BBB, blood-brain barrier; CNS, central nervous system; DTA, diphtheria toxin antigen; DTR, diphtheria toxin receptor; NGF, nerve growth factor; RTX, resiniferatoxin; Trk, tropomyosin receptor kinase; TRPV1, transient receptor potential cation channel subfamily V member 1.
Among the organs of the body, innervation of the prostate is one of the best studied due to its anatomically distinct (and thus easily manipulated) SNS and PSNS inputs. In the rodent, all sympathetic innervation comes from bilateral paired hypogastric nerves, and all parasympathetic innervation derives from bilateral paired pelvic nerves23. Transection of the hypogastric nerves was found to arrest age-related gland growth, and disrupt glandular epithelial morphology24,25, suggesting that these nerves provide necessary trophic factors for maintaining tissue homeostasis. To test whether sympathetic nerves have a similar effect in prostate cancer, surgical transection of the bilateral hypogastric nerves was found to cause tumour regression in an orthotopic human PC-3 xenograft mouse model, and inhibition of tumour growth in an autochthonous transgenic Hi-MYC prostate cancer model (in which mice express MYC specifically in the prostate)5. Similarly, sympathectomy [G] of the tongue by bilateral removal of the superior cervical ganglia, inhibited tumour growth and invasion in a carcinogen-induced mouse model of squamous cell carcinoma16, and transection of the epigastric nerve to the rat inguinal (groin) mammary gland (anatomically similar to the thoracic mammary gland found in both rodents shown in Figure 1 and humans) as well as intratumoural genetic depletion of sympathetic nerves (achieved by intratumoral injection of a viral vector carrying diphtheria toxin antigen under the sympathetic-specific tyrosine hydroxylase (TH) promoter) inhibited orthotopic breast cancer growth8,26. Furthermore, denervation by surgical ganglionectomy [G] of the celiac and superior mesenteric ganglia was found to improve the efficacy of chemotherapy in a transgenic mouse model of pancreatic cancer; however the effect of ganglionectomy alone on cancer progression was not assessed4.
To disrupt specific neural input to an organ without the permanent effect of surgical transection, several chemical techniques have been developed to temporarily inhibit neural signalling by diverse mechanisms (Table 1). One commonly used denervation technique is chemical poisoning of adrenergic nerve [G] terminals with the dopamine mimetic 6-OH dopamine (6OHDA). Since this drug is polar, it does not cross the blood–brain barrier of adult mice, and thus does not affect the CNS or adrenal glands27. While neonatal 6OHDA administration results in permanent sympathectomy, its effects are temporary in the adult, as the adrenergic nerve terminals regrow after several weeks28,29. In both transgenic and orthotopic mouse models of breast cancer, chronic chemical sympathectomy with 6OHDA decreases intratumoral noradrenaline [G] and inhibits tumour growth30. Similar inhibitory effects on tumour growth were reported after 6OHDA sympathectomy in orthotopic and transgenic mouse models of melanoma and prostate cancer2,5,31. Interestingly gene expression analysis from laser microdissection of prostate stroma revealed similar gene expression patterns from both surgically, as well as chemically denervated prostates, indicating that the two approaches produce equivalent results32.
While prostate cancer derives from glandular epithelium, which is heavily innervated by adrenergic nerves33, pancreatic cancer largely derives from ductal epithelium in the exocrine [G] portion of the pancreas, which receives considerable sensory and adrenergic innervation, with some contribution from cholinergic innervation as well34,35. In contrast to adrenergic innervation that is equally distributed throughout the pancreas, sensory innervation is greatest at the head (within the exocrine portion) of the pancreas35, which derives in equal parts from the vagus nerve and celiac ganglia36. The head of the pancreas is also where the majority of pancreatic cancers occurs37. Using chemical denervation of sensory nerves (Table 1), three groups independently demonstrated that neonatal depletion of sensory nerves reduces initiation of pancreatic intraepithelial neoplasia (PanIN) precursor lesions and inhibits progression from the PanIN stage to pancreatic ductal adenocarcinoma (PDAC) in autochthonous transgenic mouse models of pancreatic cancer that faithfully reproduce human disease10,11,38. Similar to the pro-tumorigenic contributions of sensory nerves to pancreatic cancer progression, depletion of the sensory neuropeptide substance P was also shown to reduce metastasis in an orthotopic mouse model of breast cancer7. Interestingly the majority of breast cancers derive from the ductal epithelium39, thus further implicating sensory nerves in ductal carcinomas.
In addition to its large sensory component in the abdomen, the vagus nerve also provides parasympathetic innervation to the pancreas40. Parasympathetic cholinergic nerves mainly innervate the stroma and glandular epithelium within the pancreas35,41. In contrast to chemical denervation of the sensory component of the vagus nerve (using resiniferatoxin or capsaicin), surgical transection of the vagus nerve (which severs both parasympathetic and sensory axons in this mixed nerve) was independently shown by two groups to accelerate pancreatic cancer progression from the PanIN stage to PDAC42,43. Both groups found that vagotomy increased pancreatic inflammation and recruitment of tumour-associated macrophages (TAMs), two interrelated, well known pro-tumorigenic factors38,44,45, which suggests changes in the composition of the TME that we will discuss in detail below.
By contrast to the cancer inducing effects of vagotomy in pancreatic cancer, transection of the vagus nerve in gastric cancer has been shown to have an anti-tumorigenic effect3. Greater than 95% of gastric cancers are adenocarcinomas, deriving from the gastric glandular epithelium46. The gastric secretory epithelium is highly innervated by cholinergic nerves that derive from ganglia embedded within the stomach wall (the myenteric plexus), which in turn are regulated by the vagus nerve36. Using several different transgenic autochthonous mouse models of gastric cancer, it was found that parasympathetic denervation by vagotomy with pyloroplasty [G] (as denervation inhibits gastric emptying) or by gastric injection of the botulinum neurotoxin [G], inhibited progression from the preneoplastic stage to adenocarcinoma when performed early, as well as prevented disease progression and improved mouse survival when performed in later disease stages3. Furthermore, denervation by chemical destruction of the myenteric plexus was found to inhibit tumour growth in a carcinogen-induced autochthonous model of gastric cancer47.
Modulation of neurotransmitters and cognate receptors in cancer
Just as denervation has largely been found to have an inhibitory effect on cancer initiation and progression, increased nerve signalling has been suggested to promote cancer progression. Early histological studies demonstrated that continuous electrical stimulation of the superior cervical ganglion, which provides adrenergic innervation to the salivary glands, induced glandular hyperplasia48,49. While long-term electrode stimulation is damaging to nerves, other strategies have been used to chronically increase adrenergic activity. A recent study employed genetic engineering to induce expression of long-acting bacterial sodium channels in intratumoral adrenergic nerves. Expression of these long-acting channels increased adrenergic nerve activity and intratumoral levels of the adrenergic neurotransmitter noradrenaline, resulting in accelerated growth of orthotopic and carcinogen-induced breast tumours in mice26.
Chronic stress is associated with an increased incidence of cancer50, portends worse clinical outcomes51, and increases in circulating as well as intratumoral catecholamine [G] levels, especially noradrenaline2,52,53. Pre-clinical models of malignancy have shown that increased adrenergic activity through stress promotes progression of a variety of cancers including ovarian, prostate, breast, and pancreatic18,53–55. In addition, chronic stress models (such as the physical restraint stress model [G]) increases systemic as well as intratumoral levels of catecholamines4,53. The adrenal glands (specifically the adrenal medulla), are considered sympathetic ganglia (Figure 1), and are responsible for endocrine production of catecholamines, mainly adrenaline (epinephrine). While adrenalectomy in unstressed mice with cancer does not influence tumour growth or cancer progression26, removal of the bilateral adrenal glands in chronically stressed mice reduced tumour development in a transgenic autochthonous pancreatic cancer model4. These data suggest that adrenal derived catecholamines play a role in cancer initiation, but further studies are needed to assess their role in cancer progression and metastasis.
Sympathetic adrenergic nerves release noradrenaline that signals through α- and β-adrenergic receptors [G] on target cells. Several studies have shown that administration of β-adrenergic agonists recapitulates the pro-tumorigenic and pro-metastatic effects of stress. In a transgenic model of breast cancer, chronic administration of the non-selective β-adrenergic agonist isoproterenol reproduced the effects of stress causing increased lymph node metastasis and lymphatic vascular density, but interestingly did not affect primary tumour growth56. Conversely, the study also showed that adrenergic inhibition with the non-selective β-blocker propranolol inhibited lymph node metastasis and lymphangiogenesis [G]. Similar results were also seen in orthotopic mouse models of metastatic breast cancer56,57. Increased adrenergic activity was also found to promote pancreatic disease progression from the pre-neoplastic PanIN stage to adenocarcinoma in transgenic mouse models of pancreatic cancer4. Similarly, chronic treatment with isoproterenol accelerated disease progression, and treatment with the antagonist propranolol inhibited disease progression4,18. In transgenic mouse models of prostate cancer, pharmacological inhibition of the β2-adrenergic receptor, embryonic deletion of the gene encoding the β2-adrenergic receptor Adrb2 and Adrb3, or genetic deletion of Adrb2 in adult endothelial cells inhibited cancer progression2,54, further suggesting that β-adrenergic receptors in the TME mediate the pro-tumorigenic effects of adrenergic nerves.
Increased parasympathetic activity has similarly been shown to have pro-tumorigenic effects. Parasympathetic nerves, as well as a small subset of specialized chemosensory cells such as gastrointestinal tuft epithelial cells58, release acetylcholine that signals through nicotinic receptors [G] and muscarinic receptors [G] on target cells. Gastric cancers upregulate expression of the muscarinic acetylcholine receptor 3 (M3R)3. In transgenic and carcinogen-induced models of gastric cancer, genetic deletion of M3R in gastric epithelial cells or pharmacological inhibition slowed tumour growth and progression3,59. In transgenic and orthotopic xenograft models of prostate cancer, stimulation of the M1R with the agonist carbachol stimulated lymph node metastasis, whereas pharmacological inhibition or genetic deletion of M1R prevented metastasis5. In pancreatic cancer, removal of parasympathetic (and sensory) input by transection of the vagus nerve accelerates cancer progression42,43. Furthermore, stimulation of cholinergic signalling with the non-selective muscarinic agonist bethanechol, inhibits pancreatic cancer progression in transgenic and orthotopic xenograft models, whereas global genetic deletion of M1R stimulates progression43. A similar inhibitory role for cholinergic nerves was recently demonstrated in both human xenograft and transgenic mouse models of breast cancer26. Using intratumoral injection of an adeno-associated virus (AAV) vector to express long-acting bacterial sodium channels in tumour cholinergic nerves, the activity of these nerves was constitutively increased and found to slow tumour growth. As the mammary gland is a derivative of the skin, it has been shown to have a similar innervation pattern as skin, receiving sensory and sympathetic fibres, but no parasympathetic input as demonstrated by retrograde tracing studies, and little evidence of cholinergic fibres in immunohistochemical studies60–62. While further retrograde nerve tracing studies are needed in breast tumours, it is possible that cholinergic differentiation of adrenergic nerves occurs, as has been observed in sweat glands of the skin63. Interestingly, it was found that disease recurrence in patients with breast cancer was positively correlated with tumoral adrenergic nerve density and inversely correlated with cholinergic nerve density in the original tumour specimen26.
Taken together, these results suggest that while adrenergic and sensory signals exert pro-tumorigenic effects, cholinergic signals exhibit tissue-dependent effects. However, the molecular mechanisms that underlie the context-dependent effects of parasympathetic signals are not well understood. This lacuna stems in part from the lack of tools to specifically target parasympathetic nerves (Table 1). However, cell-specific deletion of muscarinic receptors, as was carried out in gastric cancer mouse models59, will help tease out the contributions of tumour epithelial cells versus stromal cells in mediating cholinergic signals in the TME. Further understanding of the signalling events downstream of the β-adrenergic receptor (such as β2-adrenergic receptor mediated cAMP activation of cAMP response element-binding protein (CREB)) and muscarinic receptor subtypes (such as M1R activation of the PI3K–MAPK pathway, and M3R activation of downstream WNT signalling)43,59 will likely help explain the tissue specific effects of autonomic signalling in various cancers64.
Nerves in haematological malignancies and CNS tumours
In addition to regulating solid tumours outside of the CNS, which are largely derived from epithelial cells, nerves play a role in other types of malignancy. The haematopoietic stem and progenitor cells (HSPCs) from which blood cancers arise, are regulated by distinct microenvironments known as niches, such as the bone marrow, which are innervated by adrenergic nerves65–67. During normal ageing, there is loss of adrenergic nerve density in the bone marrow, which alters the niche and leads to a decline in HSPC function66,67. In mouse models of acute myeloid leukaemia (AML), the loss of adrenergic nerves promotes malignancy due to reduced β-adrenergic signalling in the niche68. Similarly, neuropathy [G] occurs in myeloproliferative neoplasms, and this loss of adrenergic nerves and Schwann cells [G] promotes malignancy through a reduction of niche cells, and can be partially restored with β3-adrenergic receptor agonists69. Thus, while adrenergic signals in the TME of epithelial cancers promote tumour growth and progression, these same signals in the bone marrow niche are protective against aberrant proliferation and expansion of HSPCs, further underlying the complexity and tissue-specific as well as context-dependent roles of nerves in the microenvironment.
A comparable phenomenon of nerve mediated cancer growth has been observed in primary and metastatic tumours of the CNS. Unlike the periphery, the CNS exhibits an extremely high density of nerves accounting for approximately half of all cells in the brain70,71. While neurons communicate with each other via synaptic transmission, several recent studies have shown that gliomas (brain tumours derived from glial cells) can also form a microtube network of excitatory synapses with glutamatergic nerves in the brain driving tumour growth72,73. Similarly, a recent study found that breast cancer metastases in the brain form excitatory synapses with glutamatergic nerves fuelling their growth through tumour-expressed metabotropic glutamate receptors known as N-methyl-D-aspartate receptors (NMDARs)74. Interestingly, tumour-expressed NMDARs have also been implicated in the aggressiveness of several tumours outside the CNS, including pancreatic and ovarian cancers75. While subtypes of sensory neurons in the periphery secrete glutamate76, it was shown that pancreatic tumours produce their own glutamate thus signalling in an autocrine manner75. Taken together, these data suggest that autonomic (adrenergic, cholinergic, and sensory peptidergic [G]) signalling influences epithelial-derived tumours in the periphery, whereas glutamatergic signalling in the CNS regulates primary and metastatic tumours in the brain.
REACTIVATION OF NERVE-MEDIATED PATHWAYS
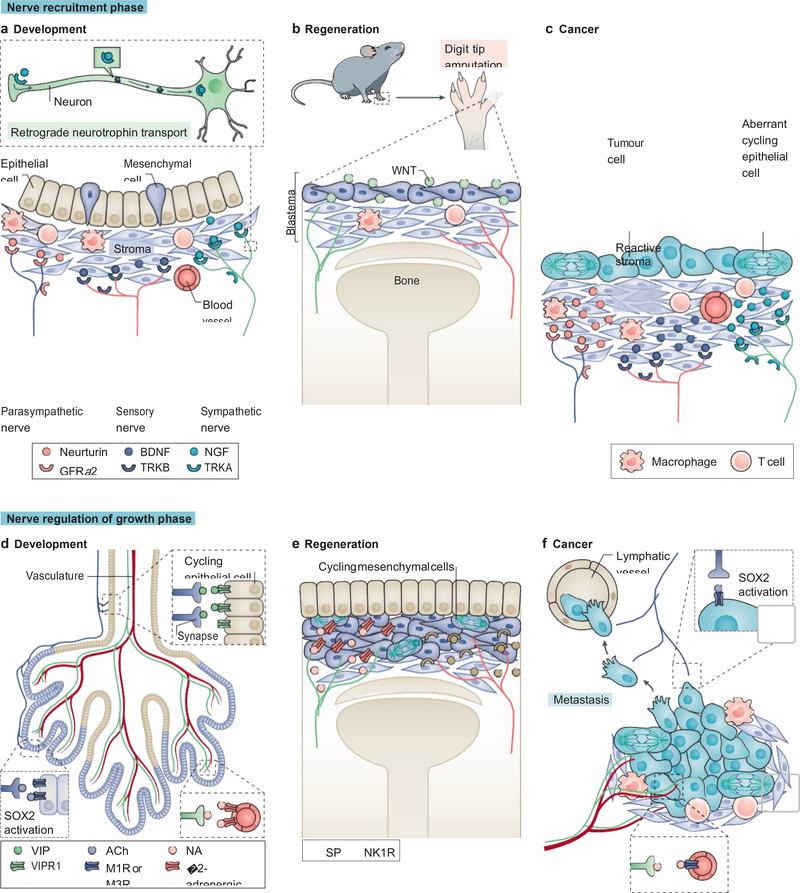
To better understand the mechanisms by which nerves interact with the TME to drive cancer, insights can be gained from the impact of nerves on development and regeneration (Figure 2). During gland development, epithelial organs undergo a spatial and temporal patterning known as lobulation. This process has been shown to be heavily reliant on the recruitment and growth of nerves77–82 (Figure 2A and 2D). As a model system, morphogenesis of the embryonic submandibular gland has been the best studied, owing to its ability to be cultured ex vivo. Like many glands, the submandibular salivary gland maximizes space and surface area through a series of branching ducts and acini to produce the necessary volume of secretory fluid for daily function83. To achieve the morphogenic patterning of epithelial cells within the mature interconnected network of ducts and acini, the embryonic epithelial mesenchyme secretes neurotrophic growth factors (neurotrophins) that recruit nerves. These include nerve growth factor (NGF), its high-affinity receptor (tropomyosin receptor kinase A (TRKA)) and low-affinity p75 neurotrophin receptor (p75NTR), and the glial cell line derived neurotrophic factor (GDNF) neurturin and its receptor GDNF family receptor α2 (GFRα2)77,84. The epithelial end-buds and ducts secrete neurturin, which causes unidirectional axonal outgrowth from the parasympathetic submandibular ganglion by way of neuronally-expressed GFRα277. These parasympathetic nerves in turn release acetylcholine that signals through muscarinic receptors in SRY-box 2 (SOX2)+ epithelial progenitors inducing acinar bud [G] branching and maturation, and release vasoactive intestinal peptide (VIP) that induces tubulogenesis (duct formation in cycling luminal epithelial cells) (Figure 2D)77–79,85.
Figure 2. Reactivation of nerve-mediated developmental and regenerative pathways in cancer.
Nerve recruitment phase (a to c). During development, the epithelial, mesenchymal, and stromal compartments of organs secrete neurotrophins to recruit the three types of peripheral nerves (a). Neurotrophin binding to its cognate receptor on nerves leads to a signal that travels in a retrograde manner to the soma, affecting gene expression and axonal growth (inset). Similarly, in mammalian digit tip regeneration the stromal and mesenchymal compartments secrete neurotrophins (in this case, WNT) to recruit sympathetic and sensory nerves (b). In cancer, aberrantly mitotic malignant epithelial cells and the surrounding reactive stroma, release neurotrophins to recruit nerves necessary for their growth (c). Nerve-mediated regulation of the growth phase (d to f). During organ patterning, parasympathetic nerves initiate ductal epithelial cell (in beige) tubulogenesis (involving mitosis and migration) through vasoactive intestinal peptide (VIP) signalling (upper inset); parasympathetic nerves also regulate glandular or acinar epithelium (in purple) growth and patterning through acetylcholine (ACh) signalling by activating SRY-box 2 (SOX2) through a calcium-dependent pathway (lower left inset);,whereas sympathetic nerves pattern the vasculature (lower right inset) (d). During digit tip regeneration, sympathetic and sensory nerves provide mitotic and differentiation cues to the mesenchymal and overlying epithelial cells (e). Similarly, in cancer, sympathetic nerves pattern the neovasculature supplying the growing tumour, and parasympathetic nerves provide mitotic and migratory cues to tumour cells, which in turn lead to growth expansion and formation of micrometastases, respectively (f). BDNF, brain derived neurotrophic factor; GFRα2, glial cell line derived neurotrophic factor family receptor α2; NA, noradrenaline; NGF, nerve growth factor; NK1R, neurokinin 1 receptor; SP, substance P; TRK, tropomyosin receptor kinase.
Adrenergic nerves similarly play an important role in gland development. During the late prenatal period, adrenergic nerves begin to innervate the salivary glands, and the development of these nerves parallels the maturation of the glandular acini and patterning of the vasculature49,80 (Figure 2D). This innervation is necessary for organogenesis as sympathectomy, or genetic deletion of the major adrenergic neurotrophin NGF, inhibits gland formation86,87. NGF is critical for the initiation and maintenance of gland innervation. However, when organ patterning is complete, NGF levels drop off and axonogenesis correspondingly decreases88. Gland-derived NGF signals in a retrograde manner, binding to its cognate TRKA receptor on the neuronal pre-synaptic membrane, which is then incorporated into endosomes that travel from the distal axon back to the neuronal cell body and dendrites, influencing gene expression and axonogenesis89,90 (Figure 2A). In the developing pancreas, the onset of adrenergic innervation is associated with a rapid phase of glandular growth and maturation, and genetic deletion of NGF or adrenergic nerve-specific deletion of TRKA leads to incomplete adrenergic innervation of the pancreas and disruption of glandular architecture, also phenocopied by sympathectomy81,87,91. In addition to the NGF and GDNF family of neurotrophins, brain-derived neurotrophic factor (BDNF) and its cognate receptor TRKB are involved in glandular innervation (Figure 2A). During mammary gland development, the mammary mesenchyme secretes high levels of BDNF which signals through TRKB receptors on sensory neurons, recruiting these nerves to establish innervation of the ductal tree82. In this study, embryonic deletion of BDNF or TRKB, sensory neuron-specific deletion of TRKB, or genetic TRKB inactivation were all sufficient to inhibit mammary gland innervation.
Akin to the contribution of nerves to organogenesis, nerves are also essential for limb patterning and growth during development. In the developing embryo, some of the highest NGF levels are found in the limb bud at its axillary site, expressed in the undifferentiated mesenchyme adjacent to the apical ectodermal ridge (a thin epithelial layer necessary for proper limb patterning)88. Prior to limb differentiation and patterning, sensory nerves appear in the mesenchyme of the limb bud92, and condensation of the mesenchyme (the initial step in differentiation of the limb structure) is observed in close association (both in time and space) with the arborisation of the innervating nerves93,94.
A similar involvement of nerves is seen in limb regeneration (Figure 2B and 2E). In salamanders, regeneration of limb structures distal to the site of amputation is dependent on the presence of nerves, as denervation of layers deeper than the level of amputation (i.e. proximal to the amputation site) inhibits regeneration95. These nerves signal to the overlying epithelial and mesenchymal cells (the blastema) that pattern cell migration and control cell proliferation96 (Figure 2B and 2E). Nerves are not only important for vascular patterning during organogenesis97,98, but are also involved in vascular patterning during regeneration99. This phenomenon of vascular and epithelial patterning has been demonstrated in Xenopus laevis following amputation of the forelimb and subsequent surgical rerouting of hind limb innervation; the result was hyperinnervation at the amputated site, and accelerated regeneration and patterning100. In this case, the dependence on nerves for regeneration is based on a combination of the effects of nerve-derived neurotransmitters and growth factors, such as the salamander specific secreted glycoprotein newt anterior gradient protein (nAG), which does not have a functionally similar ortholog in mammals101. However, in mammals (including humans) nerve-dependent regeneration of the digit tip occurs102, and was found to be reliant on WNT signalling (Figure 2B). Conditional WNT deletion in epithelial cells at the digit tip reduced expression of neurotrophins and inhibited axonal regrowth and digit tip regeneration in mice103. The dependence upon WNT for axonal patterning in regeneration is a common pathway shared with organogenesis during embryonic development103–105. There are also other conditions under which nerves support regeneration and these include regeneration of the bone marrow following recovery from myeloablative therapy, which is promoted by β-adrenergic signals106, and wound re-epithelialization, which requires epithelial cell proliferation and vascular patterning, both of which can be disrupted by denervation107–109.
During cancer initiation and the early stages of cancer progression, tumours reactivate nerve-dependent pathways similar to those in development and regeneration to enable their continued growth and maintenance (Figure 2C and 2F). As we discussed in the previous section, nerve density more than doubles during the pre-malignant stages of cancer2,10,14, similar to the increased innervation observed during initiation of gland patterning during organogenesis and blastema formation in regeneration. This increase in nerve density is paralleled by an increase in neurotrophin production110 (Figure 2C and 2F). In transgenic mouse models of pancreatic cancer, a doubling in expression of neurotrophic factors including NGF, GDNF, BDNF, and neurturin, as well as expression of their cognate receptors (TRKA, GFRα1, TRKB, and GFRα2, respectively) occurs during the pre-neoplastic PanIN stage111. In this study, neurotrophin levels continued to rise as disease progressed to aggressive adenocarcinoma, reaching levels > 7-fold compared with age-matched controls. In addition, mice with PDAC were found to have a 10-fold elevation in nerve density compared with age-matched controls (one third of these nerves being adrenergic)4. Furthermore, in this study, elevated Ngf levels were found in the epithelial compartment of the pancreatic tumour. When the authors selectively overexpressed NGF in the pancreatic epithelium using an inducible transgenic NGF knock-in model, there was an increase in adrenergic nerve density and induction of PanIN in wild-type mice, with acceleration of the progression from PanIN to PDAC in transgenic cancer models. Conversely, reduction of NGF expression by genetic depletion using small interfering RNA (siRNA), or by blockade with NGF antibodies, inhibits pancreatic cancer progression and metastasis112,113. In contrast to the epithelial expression of NGF in mouse PDAC, neurotrophin levels in human PDAC specimens were elevated in the stromal compartment, whereas their cognate receptors were elevated in the epithelial compartment4,114, suggesting that further research is needed to elucidate the source of neurotrophin production that contributes to cancer.
Increased neurotrophin expression has been linked to poor clinical outcomes in a variety of cancer types. In human prostate cancer specimens, increased expression of pro-NGF, the precursor protein of NGF, was associated with more aggressive pathology and the majority of NGF and BDNF was found in the stromal compartment of these tumours115,116. Similarly, increased NGF was found in human breast cancer tissues, and elevated levels of BDNF were found in human ovarian tumours and were associated with higher nerve density and increased mortality117,118. Mimicking these observations in humans, overexpression of NGF in gastric epithelial cells increased innervation of the gastric mucosa and induced gastric adenocarcinoma by 18 months of age in wild-type mice59. Soluble WNT signalling has also been shown to be both a key neurotrophic factor in the recruitment of nerves, which guides digit tip regeneration as well as the recruitment of nerves in cancer3,103. In clinical gastric cancer specimens, elevated WNT levels correlated with both greater nerve density as well as tumour stage3. Equally, gastric denervation in mouse models of gastric cancer decreased WNT levels and tumour growth, further implicating embryonic and regenerative pathways in cancer initiation and progression.
In organogenesis and regeneration, nerves provide several functions including stimulus for epithelial proliferation, migration, and epithelial and stromal patterning. While parasympathetic nerves regulate acinar cell expansion and patterned migration through M1R signalling to SOX2+ epithelial progenitor cells that exhibit stem cell like characteristics in the developing salivary glands79, certain cancers can co-opt nerves to activate similar pathways (Figure 2D and 2F). Prostate cancer derives from acinar epithelial cells, and recent studies have shown that increased parasympathetic signals promote prostate cancer metastasis through M1R signalling, and furthermore that mouse and human prostate tumours exhibit increased expression of SOX2 in stem cell-like cancer cells, promoting androgen resistance in advanced disease5,119. Additional evidence that parasympathetic nerves regulate putative cancer stem cells (CSCs) in tumours of glandular origin have come from transgenic mouse models of cancer. For example, cholinergic nerves innervate gastric stem cells positive for expression of the transcription factor MIST1 (also known as bHLHa15), and conditional Chrm3 deletion in these cells inhibits gastric tumour growth in vivo59. As parasympathetic nerves have an opposite effect in pancreatic cancer mouse models (i.e. they suppress tumour growth), administration of the muscarinic receptor agonist bethanechol suppressed pancreatic CSC numbers43. However, further studies are needed to assess the innervation of CSCs in various tumours to determine whether adrenergic innervation is directly involved in CSC expansion (especially as during development adrenergic nerves mainly associate with the stroma, and pattern vascular branching and aid in the development of smooth muscle, while cholinergic nerves associate with the glandular epithelium to pattern organ morphogenesis), and to characterize the autonomic nerve receptors expressed by CSCs that mediate these effects.
Innervation in development relies on the combination of neural migration (to establish intra-glandular parasympathetic ganglia) and axonogenesis. A recent study has now found increased numbers of doublecortin-expressing cells (a marker associated with neural progenitors as well as the axonal growth cone of migrating central and peripheral neurons120,121) in transgenic mouse prostate tumours122. This finding suggests neural progenitors may travel through the bloodstream from the brain to seed the prostate. Whether a similar process occurs in other tumour types or in human cancers requires further investigation. However, many additional questions arise from this observation such as how do progenitors cross the blood–brain barrier, what are the signalling cues to direct them from the brain to the prostate tumour, and whether these progenitors differentiate into bona fide functional autonomic nerves. As prostate cancer cells can also express doublecortin123, in-depth studies will be required to determine the origin (through neurogenesis or axonogenesis) of newly formed axons in tumours.
NEURAL REGULATION OF THE TME
Recent advances in genetic engineering and imaging have enabled a greater mechanistic understanding of the molecular basis of neural regulation in cancer. Initial in vitro experiments suggested that neurotransmitters signalled directly to tumour cells, promoting cell proliferation, survival, and migration as reviewed previously124. It is of note that direct innervation of the epithelial compartment (that is, the cells from which solid tumours derive) may indeed play a role in cancer initiation and progression as shown for gastric cancer59. However, in some tissues such as the prostate, epithelial cells are histologically separated from nerves by a barrier of smooth muscle, whereas in others such as the salivary glands, epithelial cells may receive direct innervation. Thus, epithelial specific conditional knockouts of autonomic and sensory receptors (Adrb2, Adrb3, Chrm1, Chrm3 and the gene encoding the substance P receptor, Nk1r (also known as Tacr1)) in autochthonous cancer mouse models will provide insight into the contribution of the epithelial compartment to nerve-mediated regulation of cancer. Nevertheless, histological studies indicate that nerves run through the stromal compartment and directly innervate stromal structures35,125,126. In vivo animal studies suggest that there is a dynamic interaction in the TME among nerves, the stroma, and epithelial compartment. For instance, our group has shown that adrenergic nerves indirectly regulate tumour cell proliferation by modulating angiogenesis and thus nutrient availability to the tumour2. In this section, we will discuss the impact of nerves on individual components of the TME (Figure 3).
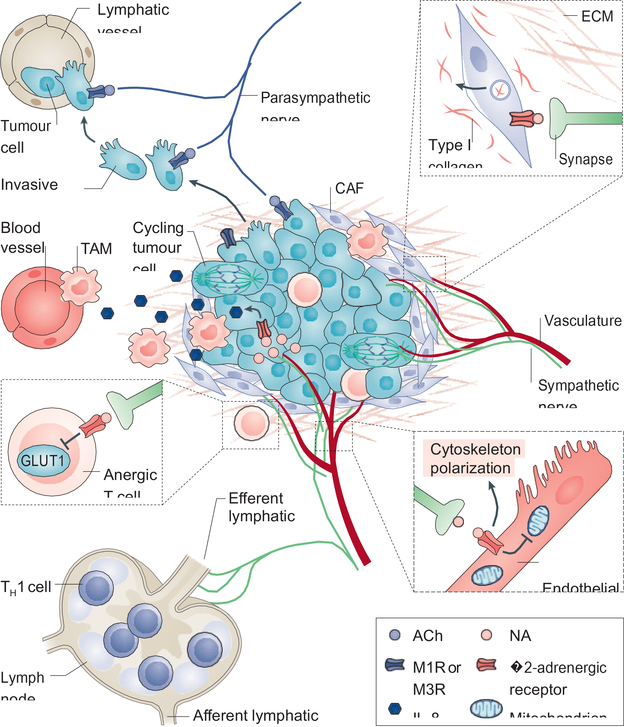
Figure 3. Neural regulation of the tumour microenvironment.
Nerves interact with multiple stromal and malignant epithelial components to promote tumour growth and dissemination. The tumour microenvironment (TME) is largely immunosuppressive. Signalling from adrenergic nerves stimulates secretion of interleukin-8 (IL-8) from tumour cells, which then recruits tumour-associated macrophages (TAMs) that contribute to angiogenesis and further immunosuppression. Adrenergic nerves also signal to T cells through the β2-adrenergic receptor, suppressing metabolic activation by inhibiting expression of the glucose transporter GLUT1, and thus maintaining them in an anergic state (see inset). While immune-responsive T helper 1 (TH1) cells can be recruited to the tumour, they are often hindered from reaching it as increased adrenergic signalling to the lymphatic endothelium constricts the efferent lymphatic channels, thus trapping these cells in nearby lymph nodes.Angiogenesis, a key component of tumour development is intimately regulated by nerves. Signalling through endothelial-expressed β2-adrenergic receptor suppresses oxidative metabolism and promotes endothelial cell migration and vessel formation (see inset). In addition, cancer associated fibroblasts (CAFs) remodel the extracellular matrix through production of type I collagen in response to noradrenaline (NA) signalling (see inset). As mentioned in Figure 2, parasympathetic signalling through tumour cell-expressed cholinergic receptors, promotes tumour cell migration and formation of micrometastases. ACh, acetylcholine; M1R, muscarinic acetylcholine receptor 1.
Angiogenesis and lymphangiogenesis
Angiogenesis, the formation of new blood vessels from the existing vasculature, is necessary for tumour growth127. In the stromal component of tissues, adrenergic nerves closely associate with the vasculature (mainly arterioles [G] and capillaries), sharing patterning cues including the same axon guidance molecules128,129. Our recent study found that adrenergic nerves regulate the initiation and patterning of angiogenesis in the early stages of prostate cancer through a mechanism termed the angiometabolic switch2 (Figure 3). Endothelial cells normally rely on a glycolytic metabolic program for directed cell migration necessary for angiogenesis during development and in cancer130,131. In the TME of a mouse model of prostate cancer, endothelial cells were found to exhibit increased Adrb2 expression, and sympathectomy or conditional endothelial cell-specific deletion of Adrb2 inhibited angiogenesis by shifting endothelial cell metabolism from glycolysis to oxidative phosphorylation through upregulation of cytochrome c oxidase assembly factor 6 (Coa6), a protein involved in the electron transport chain2. Similar to the vasculature, the lymphatic system is highly innervated by adrenergic nerves, as the flow of lymph and egress of lymphocytes are regulated by these nerves132,133. In orthotopic and transgenic models of breast cancer, lymphangiogenesis and lymphatic remodelling were dependent on adrenergic signalling through lymphatic endothelial Adrb2, which promoted metastasis56. Akin to its effects on tumour angiogenesis, sympathetic denervation has been shown to decrease lymphatic vessel formation, which correlated with reduced cancer aggressiveness16.
Immunity and inflammation
Within the TME, autonomic nerve fibres innervate the tissue resident immune network. In the spleen, adrenergic innervation was found to stimulate acetylcholine production from β2-adrenergic receptor-expressing T cells in the white pulp134. T cell-derived acetylcholine in turn inhibits tumour necrosis factor (TNF) production in nicotinic acetylcholine receptor (nAChR)-expressing macrophages135. While this neuro-immune circuit, termed the inflammatory reflex, is responsible for immune suppression under conditions of stress, autonomic innervation also directly influences immune cell recruitment and fate in the TME.
Tumour lymphocyte infiltration and activation are key components of the anti-tumour immune response of the host136. Elevated stress has been linked to increased lymphocyte activation through production of proinflammatory cytokines such as interleukin-6 (IL-6)137. Ovarian tumours resected from stressed patients compared to non-stressed age and stage-matched patients, exhibit increased intratumoral norepinephrine and IL-6 levels138. In vitro studies showed that norepinephrine activated ovarian tumour cell expression of the proinflammatory cytokines IL-6 and IL-8 through β2-adrenergic receptor signalling138,139. However, highly innervated tissues such as the pancreas and prostate exhibit low levels of activated T helper 1 (TH1) cells136,140–142. Interestingly, adrenergic nerves contribute to this immunosuppressive environment in several ways (Figure 3). The lymphatic system, which is responsible for lymphocyte trafficking, is highly innervated by adrenergic nerves and β2-adrenergic receptor signalling in the lymph nodes decreases tissue lymphocyte infiltration by inhibiting T cell egress143. Furthermore, norepinephrine-induced signalling via the β2-adrenergic receptor on CD8+ T cells inhibits their activation by altering lymphocyte glucose metabolism, decreasing glycolytic function144. Similarly, increased adrenergic signalling in the periphery after stroke was found to induce immune phenotype switching (reduced production of pro-inflammatory TH1-type cytokines and increased production of immunosuppressive TH2-type cytokines), this immunosuppression was reversed by chemical sympathectomy or administration of β-blockers145. In breast cancer, adrenergic nerves closely associate with immune checkpoint-expressing (programmed cell death 1 (PD1)), and forkhead box P3 (FOXP3)-expressing tumour-infiltrating lymphocytes (TILs) of the TME26. As these TILs also express β2-adrenergic receptor, sympathetic denervation or pharmacological inhibition of the β-adrenergic receptor was able to reduce the expression of PD1 and FOXP3 in TILs as well as PD1 ligand 1 (PDL1) expression in the tumour tissue itself. Myeloid derived suppressor cells (MDSCs) also express β2-adrenergic receptors, and in an orthotopic mouse model of breast cancer Adrb2 knockout in MDSCs slowed tumour growth, reduced expression of PDL1, and decreased serum levels of immunosuppressive cytokines146. These observations, and the fact that cancers with a robust response to immunotherapy appear to have a high infiltration of TH1 cells136, suggest that denervation or abrogation of adrenergic signals may provide novel approaches to improve immunotherapy responses of highly innervated cancers147.
TNF is a major chemoattractant for cells of the innate immune system such as macrophages. Vagal stimulation activates post-synaptic adrenergic nerves in the celiac ganglion that innervates the spleen, causing inhibition of TNF release from splenic macrophages, and vagotomy removes this immunosuppression thereby elevating systemic TNF levels134,148. Although the spleen lacks cholinergic innervation, adrenergic nerves can signal to splenic T-cells expressing β2-adrenergic receptor to secrete acetylcholine in response to norepinephrine stimulation134. Acetylcholine in turn stimulates nicotinic acetylcholine receptors on splenic macrophages inhibiting TNF release148. In transgenic models of pancreatic cancer, vagotomy substantially increased TNF levels, leading to increased TAM recruitment42,43. In an orthotopic model of breast cancer, increased adrenergic signalling under conditions of stress increased the number of intratumoral TAMs57. These TAMs, which expressed β2-adrenergic receptor, were similarly recruited to the tumour after administration of the β2-adrenergic receptor agonist isoproterenol, and administration of the antagonist propranolol inhibited TAM recruitment and metastasis. Similarly, in prostate and pancreatic cancer a nerve-dependent increase in TAMs was observed as cancer progressed, whereas macrophage depletion inhibited tumour growth18,42,43,45,149. Therefore, the sympathetic and parasympathetic nervous systems appear to have opposite regulatory effects on TAM recruitment. While parasympathetic signals appear to suppress TNF release and TAM recruitment, stress and elevated sympathetic signals increase TAM recruitment and TNF release promoting tumour growth. Taken together, these data further suggest that the neuro-immune connection is an essential regulatory component of the TME where the separate branches of the autonomic nervous system act in opposition to each other, thus providing a delicate balance that is perturbed in cancer.
Fibroblasts and the Extracellular Matrix
Changes in the three-dimensional structure and composition of the TME greatly influence tumour progression and metastasis (Figure 3). For instance, in many tumours the dense extracellular matrix (ECM) deposited as part of the desmoplastic reaction acts as a physical and chemical barrier to immune-cell infiltration, creating an immune-privileged environment150. At the same time, changes in the composition of the ECM, towards a type I collagen-rich environment, acts as an angiogenic superpolymer, aiding in the migration of neo-vasculature and nerves151–154. Additionally, whereas the increased density of the ECM helps prevent immune entry during the early stages of cancer, ECM degradation by matrix metalloproteases (MMPs) enables cancer cell migration and dissemination during the late metastatic stages of disease progression155.
Cancer-associated fibroblasts (CAFs) are a major component of the TME and are largely responsible for ECM production and remodelling150. Under inflammatory conditions such as cirrhosis of the liver, there is elevated adrenergic signalling156. In response to elevated norepinephrine in the liver, there is increased fibroblast proliferation and production of type I collagen157. In more advanced stages of disease, collagen remodelling is essential for cancer dissemination. In an orthotopic mouse model of PDAC, increased adrenergic signalling caused by stress enhanced MMP expression > 100-fold in the stromal compartment, increasing metastasis, while β-adrenergic receptor blockade with propranolol inhibited both phenotypes158. Similarly, in an orthotopic mouse model of breast cancer, adrenergic signalling in the stroma increased collagen remodelling through stromal adrenergic receptors thereby enhancing metastasis, whereas norepinephrine depletion inhibited this process159. In addition, nerves themselves provide a structural conduit for cell migration in a process called perineural invasion that is associated to varying degrees with cancer metastasis (Box1).
Box 1:
Perineural invasion (PNI), the histological presence of malignant cells in neural tissue of large nerves (not isolated individual axons) of the peripheral nervous system is a finding in many solid tumours. Presence of PNI is thought to correlate with advanced aggressive disease and even as a potential route of metastasis203. However, PNI has also been observed in benign diseases of the breast and prostate204,205.
Retrospective studies have shown that PNI has mixed prognostic significance in cancer depending on the tissue type from which the tumour derives. For instance, PNI in head and neck cancers (an area that is anatomically close to many major nerves) portends a worse prognosis206, whereas PNI in prostate cancer (when accounting for confounders, such as age and disease stage) did not 207–209.
Mechanistically, neural regulation of cancer and PNI are distinct processes with separate aetiologies. Whereas neural innervation of cancer involves the recruitment of autonomic and sensory axons into the tumour microenvironment that influence tumour progression, PNI involves tumour cell invasion of large nerve bundles. Emerging evidence suggests that macrophages in the innermost connective tissue layer of nerve bundles, termed the endoneurium, secrete chemokines that recruit tumour cells aiding PNI203. However, our understanding of PNI remains limited by the lack of autochthonous models that accurately model the complex interactions between tumour, nerve bundles, and stroma.
TARGETING TUMOUR INNERVATION
As neural signalling is intimately involved in cancer initiation and progression, therapeutically targeting these pathways has become an area of great clinical interest160. Reports dating from the early 19th century of surgical denervation for cancer control were imprecise, involving transection of large nerve trunks containing mixed motor and autonomic nerve fibres, resulting in disfigurement and major side effects13. With advances in surgical techniques and greater understanding of the autonomic neuroanatomy, more precise denervation procedures were developed. For instance, intraoperative chemical denervation of the pancreatic bed termed splanchnicectomy for uncontrollable pain in unresectable pancreatic cancer, showed survival benefit in randomized placebo-controlled clinical trials161. However, as the authors demonstrated, chemical denervation was not permanent, and innervation and eventual progression occurred with time. While temporary denervation of orthotopic prostate cancer in mice with Botulinum toxin was found to be effective, in so long as the mice received scheduled repeat injections32, human trials were not met with similar success (NCT01520441)162. The frequency, dosage, and duration of temporary denervation treatments still require further study. However, the effect of surgical denervation in the clinical setting has only been studied in a few instances. In the treatment of gastric cancer, those patients who received vagotomy in addition to gastrectomy [G], displayed a reduced recurrence of gastric cancer compared with those who received gastrectomy alone3. This suggests that denervation can serve as an adjunct therapy to increase the success of surgical management of cancer.
Pharmacological inhibition of neural signalling has emerged as a promising therapeutic target in anticancer therapy. As discussed earlier in this Review, pre-clinical data suggests that adrenergic nerves signal to the TME in large part through β-adrenergic receptors. β-adrenergic antagonists or blockers are some of the best studied agents targeting adrenergic signalling. Use of this class of drug, originally developed to treat cardiac disorders, has been found in retrospective studies to reduce the risk of, and mortality from, multiple types of solid tumour including pancreatic, breast, prostate and ovarian cancer as well as melanoma18,163–166. Additionally, β-blockers were found to augment the efficacy of chemotherapeutic agents in pre-clinical cancer models, and their incidental use prior to standard of care therapies in patients with newly diagnosed cancer have similarly been associated with improved outcomes as shown in epidemiological studies4,166–168. Several lines of evidence suggest that β-blockers may even have immediate therapeutic benefit in the perioperative setting. Catecholamines are elevated during the perioperative period, which is thought to be due in part to surgical manipulation of the tumour or tissue as well as the stress of surgery169–171. Several seminal studies have shown that perioperative β-blocker use reduced in-hospital cardiac complications after major noncardiac surgery172,173. Within the patient group who received β-blockers, those who underwent surgery for tumour resection were found to have lower rates of cancer recurrence and greater disease-free survival than control patients who did not receive β-blockers174,175; although in some studies this association did not reach statistical significance176,177. Additionally, the roles of catecholamine-blocking anaesthetics, combined β-adrenergic receptor and prostaglandin inhibition, and stress-related immunosuppression on cancer outcomes in the perioperative period, have been extensively studied and reviewed elsewhere178.
Inhibiting the neurotrophin signalling pathways that contribute to nerve recruitment is another emerging area of clinical interest. Current clinical trials have mainly focused on NGF and its cognate TRKA receptor. While targeting TRKA signalling in cancer in preclinical studies in rodents showed promising results, clinical trials have had mixed results. In theory, targeting TRKA in adults should inhibit nerve infiltration while exerting minimal effects on established nerves as sensory and sympathetic neurons lose NGF trophic dependence in adulthood179. Although small molecule receptor tyrosine kinase inhibitors that target the TRKA receptor have improved survival in malignancies where the cancer itself expresses aberrant TRKA fusion receptors, they have not been shown to impact survival or progression in solid tumours with low rates of TRK chromosomal rearrangements180–183. In addition, as these small molecules have affinity for other receptor tyrosine kinases, they have been shown to have a variety of off-target side-effects184. Targeting NGF itself with NGF antibodies is well tolerated with minimal neuronal or cognitive side effects in humans. While the NGF antibody tanezumab was found to effectively decrease pain owing to bone metastases (NCT00830180)185,186, its effect on cancer progression has yet to be assessed.
CONCLUSIONS AND FUTURE DIRECTIONS
In this Review, we have presented evidence that the reactivation of developmental and regenerative pathways to recruit nerves is an essential component to the establishment and progression of cancer. The contributions of different autonomic and sensory nerve populations vary by tumour type and are dependent on the native tissue type from which the malignancy arises as well as its native innervation pattern. Despite the recent advances in genetic engineering as well as imaging technologies that have enabled advances in the study of neural contributions to the TME, many questions remain unanswered. For instance, it has been established that there is an increase in nerve density during the early stages of cancer paralleled by a rise in neurotrophin levels, but what is yet to be elucidated is which cells in the TME are the source of the neurotrophins and what is the nature of the stimuli that initiates the neurotrophin production. Additionally, studies have demonstrated that developing nerves compete for NGF and only the fittest nerves survive187. Therefore, what is unique about the surviving nerves and how can we specifically target these pathways without affecting established neuronal circuits elsewhere in the body? While inhibition of nerve signalling pathways has dramatic effects on preventing cancer progression in pre-clinical models, translation of the techniques and technologies to manipulate nerves are still at the earliest stages and will require multidisciplinary collaboration to bring viable nerve-based therapies to the clinic. Emerging technologies such as electroceuticals [G] may bridge this gap and provide minimally invasive tools that enable granular control of neural signalling to inhibit malignancy promoting nerves, while stimulating those that inhibit progression. Along the same lines, as autonomic neuronal phenotypes have been shown to be plastic188–191, manipulating molecules that determine neuronal fate such as leukaemia inhibitory factor (LIF) and BDNF to push adrenergic nerves toward a cholinergic phenotype may provide additional minimally invasive therapeutic options. Taken together, these data suggest that the recruitment of nerves is an emerging hallmark of cancer, and that multiple surgical, pharmacological, and genetic approaches to influence their signalling in the TME is a promising novel therapeutic strategy in the treatment of cancer.
ACKNOWLEDGEMENTS
We thank Dr. S. Pinho for helpful discussions and advice on the drawing of figures. We are grateful to the National Institutes of Health (NIH) for support: Training Grant T32 NS007098 and GM007288, and the National Cancer Institute (NCI) Ruth L. Kirschstein National Research Service Award (NRSA) predoctoral M.D./Ph.D. fellowship (F30 CA203446) support (to A.H.Z.), and R01 grant funding HL097700, DK056638, HL069438, and U01 DK116312 (to P.S.F.). Our laboratory and institute have been supported the New York State Department of Health (NYSTEM Program, C029154; Prostate Cancer Hypothesis Development Research C030318GG).
Glossary
- Innervation
receiving neural input or synapse from a nerve or cluster of nerves.
- Sympathetic nervous system (SNS)
a division of the autonomic nervous system that originates in the thoracic and lumbar portions of the spinal cord, travels to a paravertebral or intra-abdominal or intra-pelvic ganglion where it synapses with a postganglionic nerve that commonly uses noradrenaline as its main neurotransmitter.
- Parasympathetic nervous system (PSNS)
a division of the autonomic nervous system that originates in the brain stem and sacral portions of the spinal cord, travels to ganglia located in proximity to the organ that it will innervate, where it synapses with a postganglionic nerve that commonly uses acetylcholine as its main neurotransmitter.
- Ganglion
a cluster of nerve cell bodies found in the autonomic and sensory nervous systems.
- Sympathectomy
the removal of sympathetic innervation to a target organ by mechanical, chemical, genetic, or other means.
- Ganglionectomy
a form of denervation in which a cluster of nerve cell bodies known as a ganglion, is removed.
- Adrenergic nerve
a postganglionic sympathetic nerve that produces the neurotransmitter noradrenaline.
- Noradrenaline
also known as norepinephrine. A neurotransmitter of the catecholamine family often released by sympathetic nerves.
- Exocrine
referring to a family of glands that release their contents onto an epithelial surface (eg. sweat glands, salivary glands and glands of the gastrointestinal and genitourinary tracts).
- Pyloroplasty
a surgery to widen the lower part of the stomach in order to facilitate the emptying of gastric contents into the duodenum.
- Botulinum neurotoxin
a neurotoxic protease that cleaves synaptic proteins preventing acetylcholine release from nerve terminals.
- Catecholamine
a monoamine neurotransmitter derived from tyrosine (includes noradrenaline).
- Physical restraint stress model
a commonly used laboratory model to induce stress, involving immobilizing an animal in a small space such as a plastic tube.
- β-adrenergic receptors
G-protein coupled transmembrane receptors for noradrenaline and adrenaline.
- Lymphangiogenesis
the formation of new lymphatic vessels from pre-existing lymphatic vessels.
- Nicotinic receptors
Ion channel transmembrane receptors that allow cation diffusion upon acetylcholine binding.
- Muscarinic receptors
G-protein coupled transmembrane receptors for acetylcholine.
- Neuropathy
nerve damage or dysfunction usually resulting from an underlying disease process..
- Schwann cells
cells that ensheath axons of peripheral nerves, helping protect axons and forming the myelin sheath in myelinated axons.
- Peptidergic
pertaining to a neuron that expresses short peptide chain neurotransmitters (neuropeptides).
- Acinar bud
an epithelial budding during organogenesis that will later form a functional secretory unit in the mature organ known as an acinus..
- Arterioles
small diameter branches of an artery in tissue microcirculation that further segment to form capillaries.
- Gastrectomy
surgical removal of part or the entirety of the stomach, often performed to treat cancer.
- Electroceuticals
a class of therapeutic agents that target nerve signalling by altering their firing patterns, such as with the use of implantable electrodes.
Footnotes
COMPETING INTERESTS
P.S.F. serves as consultant for Pfizer, has received research funding from Ironwood Pharmaceuticals and is shareholder of Cygnal Therapeutics. A.H.Z declares no competing interests.
PEER REVIEW INFORMATION
Nature Reviews Cancer thanks E. Repasky, S. Ben-Eliyahu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674, doi: 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Zahalka AH et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326, doi: 10.1126/science.aah5072 (2017).This paper shows that adrenergic nerves regulate the vasculature in the TME to promote tumour growth and cancer progression.
- 3.Zhao CM et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 6, 250ra115, doi: 10.1126/scitranslmed.3009569 (2014).This paper shows that surgical transection of the vagus nerve inhibits development of gastric cancer.
- 4.Renz BW et al. beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell, doi: 10.1016/j.ccell.2017.11.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnon C et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361, doi: 10.1126/science.1236361 (2013).This paper showed a role for adrenergic and cholinergic nerves in prostate tumour growth and metastasis.
- 6.Langley J The Autonomic Nervous System, part 1 Cambridge: Heffer W. Lewis T.(1927). The Blood Vessels of the Human Skin and Their Responses (1921). [Google Scholar]
- 7.Erin N, Zhao W, Bylander J, Chase G & Clawson G Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat 99, 351–364, doi: 10.1007/s10549-006-9219-7 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Kappos EA et al. Denervation leads to volume regression in breast cancer. J Plast Reconstr Aesthet Surg 71, 833–839, doi: 10.1016/j.bjps.2018.03.012 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Peterson SC et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412, doi: 10.1016/j.stem.2015.02.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha S et al. PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk. Cancer Research 77, 1868–1879, doi: 10.1158/0008-5472.can-16-0899 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saloman JL et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A 113, 3078–3083, doi: 10.1073/pnas.1512603113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesalius A De Humani Corporis Fabrica (The Fabric of the Human Body). (Johannes Oporinus, 1543). [Google Scholar]
- 13.Jobert M New Treatment of Cancer. The Lancet 34, 112, doi: 10.1016/s0140-6736(02)98476-x (1840). [DOI] [Google Scholar]
- 14.Ayala GE et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14, 7593–7603, doi: 10.1158/1078-0432.CCR-08-1164 (2008).This study provides evidence for an increase in adrenergic nerve density in human cancer.
- 15.Albo D et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 117, 4834–4845, doi: 10.1002/cncr.26117 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Raju B, Haug SR, Ibrahim SO & Heyeraas KJ Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience 149, 715–725, doi: 10.1016/j.neuroscience.2007.07.048 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Huang D et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine (Baltimore) 93, e172, doi: 10.1097/MD.0000000000000172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partecke LI et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 16, 423–433, doi: 10.1016/j.pan.2016.03.005 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Shao JX et al. Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac Cancer 7, 588–598, doi: 10.1111/1759-7714.12374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoucas E, Nilsson C, Alm P & Ihse I Selective microsurgical sympathetic denervation of the rat pancreas. Eur Surg Res 28, 367–373 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Hayashi A et al. Retrograde labeling in peripheral nerve research: it is not all black and white. J Reconstr Microsurg 23, 381–389, doi: 10.1055/s-2007-992344 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Huang ZJ & Zeng H Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci 36, 183–215, doi: 10.1146/annurev-neuro-062012-170307 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Tabatai M, Booth AM & de Groat WC Morphological and electrophysiological properties of pelvic ganglion cells in the rat. Brain Res 382, 61–70 (1986). [DOI] [PubMed] [Google Scholar]
- 24.McVary KT et al. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod 51, 99–107 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Diaz R et al. Histological modifications of the rat prostate following transection of somatic and autonomic nerves. An Acad Bras Cienc 82, 397–404 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Kamiya A et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci 22, 1289–1305, doi: 10.1038/s41593-019-0430-3 (2019).This study developed novel tools for manipulating nerve activity in the TME.
- 27.Thoenen H & Tranzer JP Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-hydroxydopamine. Naunyn-Schmiedebergs Archiv für Pharmakologie und Experimentelle Pathologie 261, 271–288, doi: 10.1007/bf00536990 (1968). [DOI] [PubMed] [Google Scholar]
- 28.Krukoff TL, Fernandez MC & Vincent DH Effects of neonatal sympathectomy with 6-hydroxydopamine or guanethidine on survival of neurons in the intermediolateral cell column of rat spinal cord. J Auton Nerv Syst 31, 119–126 (1990). [DOI] [PubMed] [Google Scholar]
- 29.de Champlain J Degeneration and regrowth of adrenergic nerve fibers in the rat peripheral tissues after 6-hydroxydopamine. Can J Physiol Pharmacol 49, 345–355 (1971). [DOI] [PubMed] [Google Scholar]
- 30.Szpunar MJ, Belcher EK, Dawes RP & Madden KS Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain, Behavior, and Immunity 53, 223–233, doi: 10.1016/j.bbi.2015.12.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvathova L et al. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress 19, 528–534, doi: 10.1080/10253890.2016.1213808 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Coarfa C et al. Influence of the neural microenvironment on prostate cancer. The Prostate 78, 128–139, doi: 10.1002/pros.23454 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson EM Jr., Cantor E & Douglas JR Jr. Biochemical and functional evaluation of the sympathectomy produced by the administration of guanethidine to newborn rats. J Pharmacol Exp Ther 193, 503–512 (1975). [PubMed] [Google Scholar]
- 34.Madden ME & Sarras MP Jr. The pancreatic ductal system of the rat: cell diversity, ultrastructure, and innervation. Pancreas 4, 472–485 (1989). [DOI] [PubMed] [Google Scholar]
- 35.Lindsay TH et al. A quantitative analysis of the sensory and sympathetic innervation of the mouse pancreas. Neuroscience 137, 1417–1426, doi: 10.1016/j.neuroscience.2005.10.055 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Fasanella KE, Christianson JA, Chanthaphavong RS & Davis BM Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol 509, 42–52, doi: 10.1002/cne.21736 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau MK, Davila JA & Shaib YH Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas 39, 458–462, doi: 10.1097/MPA.0b013e3181bd6489 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Bai H et al. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis 32, 1689–1696, doi: 10.1093/carcin/bgr191 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makki J Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin Med Insights Pathol 8, 23–31, doi: 10.4137/CPath.S31563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berthoud HR & Neuhuber WL Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85, 1–17, doi: 10.1016/S1566-0702(00)00215-0 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Alm P, Liedberg G & Owman C Gastric and Pancreatic Sympathetic Denervation in the Rat. Scandinavian Journal of Gastroenterology 6, 307–312, doi: 10.3109/00365527109181125 (1971). [DOI] [PubMed] [Google Scholar]
- 42.Partecke LI et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFalpha in a murine pancreatic cancer model. Oncotarget 8, 22501–22512, doi: 10.18632/oncotarget.15019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renz BW et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov 8, 1458–1473, doi: 10.1158/2159-8290.CD-18-0046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity 47, 323–338 e326, doi: 10.1016/j.immuni.2017.07.014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Partecke LI et al. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer. Pancreatology 13, 508–516, doi: 10.1016/j.pan.2013.06.010 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Dicken BJ et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 241, 27–39, doi: 10.1097/01.sla.0000149300.28588.23 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polli-Lopes AC, Zucoloto S, de Queirós Cunha F, da Silva Figueiredo LA & Garcia SB Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Letters 190, 45–50, doi: 10.1016/s0304-3835(02)00584-0 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Muir TC, Pollock D & Turner CJ The effects of electrical stimulation of the autonomic nerves and of drugs on the size of salivary glands and their rate of cell division. J Pharmacol Exp Ther (1975). [PubMed] [Google Scholar]
- 49.Srinivasan R & Chang WW Effect of neonatal sympathectomy on the postnatal differentiation of the submandibular gland of the rat. Cell Tissue Res 180, 99–109 (1977). [DOI] [PubMed] [Google Scholar]
- 50.Lillberg K et al. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol 157, 415–423, doi: 10.1093/aje/kwg002 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Chida Y, Hamer M, Wardle J & Steptoe A Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 5, 466–475, doi: 10.1038/ncponc1134 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Antoni MH et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6, 240–248, doi: 10.1038/nrc1820 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thaker PH et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12, 939–944, doi: 10.1038/nm1447 (2006).This study identified that elevated norepinephrine levels in the TME accelerate tumour growth.
- 54.Hassan S et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 123, 874–886, doi: 10.1172/JCI63324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schuller HM, Al-Wadei HA, Ullah MF & Plummer HK 3rd. Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis 33, 191–196, doi: 10.1093/carcin/bgr251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le CP et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 7, 10634, doi: 10.1038/ncomms10634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sloan EK et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70, 7042–7052, doi: 10.1158/0008-5472.CAN-10-0522 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westphalen CB et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 124, 1283–1295, doi: 10.1172/JCI73434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayakawa Y et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31, 21–34, doi: 10.1016/j.ccell.2016.11.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hebb C & Linzell JL Innervation of the mammary gland. A histochemical study in the rabbit. The Histochemical Journal 2, 491–505, doi: 10.1007/bf01003127 (1970). [DOI] [PubMed] [Google Scholar]
- 61.Köves K, Györgyi Z, Szabó F & Boldogkői Z Characterization of the autonomic innervation of mammary gland in lactating rats studied by retrograde transynaptic virus labeling and immunohistochemistry. Acta Physiologica Hungarica 99, 148–158, doi: 10.1556/APhysiol.99.2012.2.8 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Gerendai I et al. Transneuronal labelling of nerve cells in the CNS of female rat from the mammary gland by viral tracing technique. Neuroscience 108, 103–118, doi: 10.1016/s0306-4522(01)00399-2 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Stanke M et al. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp130 signaling. Development 133, 383–383, doi: 10.1242/dev.02247 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Cole SW & Sood AK Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18, 1201–1206, doi: 10.1158/1078-0432.CCR-11-0641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinho S et al. Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches. Dev Cell 44, 634–641 e634, doi: 10.1016/j.devcel.2018.01.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maryanovich M et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med, doi: 10.1038/s41591-018-0030-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinho S & Frenette PS Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol 20, 303–320, doi: 10.1038/s41580-019-0103-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanoun M et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15, 365–375, doi: 10.1016/j.stem.2014.06.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arranz L et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81, doi: 10.1038/nature13383 (2014). [DOI] [PubMed] [Google Scholar]
- 70.von Bartheld CS, Bahney J & Herculano-Houzel S The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J Comp Neurol 524, 3865–3895, doi: 10.1002/cne.24040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Azevedo FA et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513, 532–541, doi: 10.1002/cne.21974 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Venkataramani V et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538, doi: 10.1038/s41586-019-1564-x (2019). [DOI] [PubMed] [Google Scholar]
- 73.Venkatesh HS et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545, doi: 10.1038/s41586-019-1563-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng Q et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531, doi: 10.1038/s41586-019-1576-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L & Hanahan D Hijacking the Neuronal NMDAR Signaling Circuit to Promote Tumor Growth and Invasion. Cell 153, 86–100, doi: 10.1016/j.cell.2013.02.051 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Montoya J, Avendano C & Negredo P The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity. Int J Mol Sci 19, doi: 10.3390/ijms19010069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knox SM et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 4, 1494, doi: 10.1038/ncomms2493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nedvetsky PI et al. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell 30, 449–462, doi: 10.1016/j.devcel.2014.06.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emmerson E et al. SOX2 regulates acinar cell development in the salivary gland. Elife 6, doi: 10.7554/eLife.26620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Champlain J, Malmfors T, Olson L & Sachs C Ontogenesis of peripheral adrenergic neurons in the rat: pre- and postnatal observations. Acta Physiol Scand 80, 276–288, doi: 10.1111/j.1748-1716.1970.tb04791.x (1970). [DOI] [PubMed] [Google Scholar]
- 81.Borden P, Houtz J, Leach SD & Kuruvilla R Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep 4, 287–301, doi: 10.1016/j.celrep.2013.06.019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y et al. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science 338, 1357–1360, doi: 10.1126/science.1228258 (2012).This study showed that gland-derived neurotrophins are necessary for nerve recruitment and organogenesis.
- 83.Mattingly A, Finley JK & Knox SM Salivary gland development and disease. Wiley Interdiscip Rev Dev Biol 4, 573–590, doi: 10.1002/wdev.194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levi-Montalcini R & Booker B Excessive Growth of the Sympathetic Ganglia Evoked by a Protein Isolated from Mouse Salivary Glands. Proc Natl Acad Sci U S A 46, 373–384, doi: 10.1073/pnas.46.3.373 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knox SM et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645–1647, doi: 10.1126/science.1192046 (2010).This study showed that parasympathetic nerves pattern gland growth and development.
- 86.Bloom GD, Carlsoo B, Danielsson A, Hellstrom S & Henriksson R Trophic effect of the sympathetic nervous system on the early development of the rat parotid gland: a quantitative ultrastructural study. Anat Rec 201, 645–654, doi: 10.1002/ar.1092010409 (1981). [DOI] [PubMed] [Google Scholar]
- 87.Glebova NO & Ginty DD Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci 24, 743–751, doi: 10.1523/JNEUROSCI.4523-03.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wheeler EF & Bothwell M Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. The Journal of Neuroscience 12, 930–945, doi: 10.1523/jneurosci.12-03-00930.1992 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riccio A, Pierchala BA, Ciarallo CL & Ginty DD An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science 277, 1097–1100, doi: 10.1126/science.277.5329.1097 (1997). [DOI] [PubMed] [Google Scholar]
- 90.Lehigh KM, West KM & Ginty DD Retrogradely Transported TrkA Endosomes Signal Locally within Dendrites to Maintain Sympathetic Neuron Synapses. Cell Rep 19, 86–100, doi: 10.1016/j.celrep.2017.03.028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burris RE & Hebrok M Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience 150, 592–602, doi: 10.1016/j.neuroscience.2007.09.079 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCredie J, Cameron J & Shoobridge R Congenital Malformations and the Neural Crest. The Lancet 312, 761–763, doi: 10.1016/s0140-6736(78)92649-1 (1978). [DOI] [PubMed] [Google Scholar]
- 93.Cameron J & McCredie J Innervation of the undifferentiated limb bud in rabbit embryo. J Anat 134, 795–808 (1982). [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor AC Development of the innervation pattern in the limb bud of the frog. The Anatomical Record 87, 379–413, doi: 10.1002/ar.1090870409 (1943). [DOI] [Google Scholar]
- 95.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA & Brockes JP Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318, 772–777, doi: 10.1126/science.1147710 (2007).This study identified neurotrophins in the regenerating limb mesenchyme that mediate axonogenesis and nerve-regulated limb patterning.
- 96.Boilly B, Faulkner S, Jobling P & Hondermarck H Nerve Dependence: From Regeneration to Cancer. Cancer Cell 31, 342–354, doi: 10.1016/j.ccell.2017.02.005 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Properzi G, Cordeschi G & Francavilla S Postnatal development and distribution of peptide-containing nerves in the genital system of the male rat. An immunohistochemical study. Histochemistry 97, 61–68 (1992). [DOI] [PubMed] [Google Scholar]
- 98.James JM & Mukouyama YS Neuronal action on the developing blood vessel pattern. Semin Cell Dev Biol 22, 1019–1027, doi: 10.1016/j.semcdb.2011.09.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rageh MA et al. Vasculature in pre-blastema and nerve-dependent blastema stages of regenerating forelimbs of the adult newt, Notophthalmus viridescens. J Exp Zool 292, 255–266 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Mitogawa K, Makanae A & Satoh A Hyperinnervation improves Xenopus laevis limb regeneration. Dev Biol 433, 276–286, doi: 10.1016/j.ydbio.2017.10.007 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Grassme KS et al. Mechanism of Action of Secreted Newt Anterior Gradient Protein. PLoS One 11, e0154176, doi: 10.1371/journal.pone.0154176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller TJ, Deptula PL, Buncke GM & Maan ZN Digit Tip Injuries: Current Treatment and Future Regenerative Paradigms. Stem Cells Int 2019, 9619080, doi: 10.1155/2019/9619080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takeo M et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 499, 228–232, doi: 10.1038/nature12214 (2013).This study discovered a potential mechanism for nerve-mediated regeneration in mammals.
- 104.Zhang Y et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135, 2161–2172, doi: 10.1242/dev.017459 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Knosp WM et al. Submandibular parasympathetic gangliogenesis requires sprouty-dependent Wnt signals from epithelial progenitors. Dev Cell 32, 667–677, doi: 10.1016/j.devcel.2015.01.023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucas D et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med 19, 695–703, doi: 10.1038/nm.3155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rinkevich Y et al. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc Natl Acad Sci U S A 111, 9846–9851, doi: 10.1073/pnas.1410097111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ekstrand AJ et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A 100, 6033–6038, doi: 10.1073/pnas.1135965100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin P Wound Healing--Aiming for Perfect Skin Regeneration. Science 276, 75–81, doi: 10.1126/science.276.5309.75 (1997). [DOI] [PubMed] [Google Scholar]
- 110.Griffin N, Faulkner S, Jobling P & Hondermarck H Targeting neurotrophin signaling in cancer: The renaissance. Pharmacol Res 135, 12–17, doi: 10.1016/j.phrs.2018.07.019 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Stopczynski RE et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res 74, 1718–1727, doi: 10.1158/0008-5472.CAN-13-2050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lei Y et al. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun 8, 15130, doi: 10.1038/ncomms15130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saloman JL et al. Systemic Depletion of Nerve Growth Factor Inhibits Disease Progression in a Genetically Engineered Model of Pancreatic Ductal Adenocarcinoma. Pancreas 47, 856–863, doi: 10.1097/MPA.0000000000001090 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miknyoczki SJ et al. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer 81, 417–427, doi: (1999). [DOI] [PubMed] [Google Scholar]
- 115.Pundavela J et al. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am J Pathol 184, 3156–3162, doi: 10.1016/j.ajpath.2014.08.009 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Weeraratna AT, Arnold JT, George DJ, DeMarzo A & Isaacs JT Rational basis for Trk inhibition therapy for prostate cancer. Prostate 45, 140–148 (2000). [DOI] [PubMed] [Google Scholar]
- 117.Pundavela J et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol 9, 1626–1635, doi: 10.1016/j.molonc.2015.05.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Allen JK et al. Sustained Adrenergic Signaling Promotes Intratumoral Innervation through BDNF Induction. Cancer Res 78, 3233–3242, doi: 10.1158/0008-5472.CAN-16-1701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mu P et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88, doi: 10.1126/science.aah4307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Francis F et al. Doublecortin Is a Developmentally Regulated, Microtubule-Associated Protein Expressed in Migrating and Differentiating Neurons. Neuron 23, 247–256, doi: 10.1016/s0896-6273(00)80777-1 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Schaar BT, Kinoshita K & McConnell SK Doublecortin Microtubule Affinity Is Regulated by a Balance of Kinase and Phosphatase Activity at the Leading Edge of Migrating Neurons. Neuron 41, 203–213, doi: 10.1016/s0896-6273(03)00843-2 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Mauffrey P et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature, doi: 10.1038/s41586-019-1219-y (2019). [DOI] [PubMed] [Google Scholar]
- 123.Ayanlaja AA et al. Distinct Features of Doublecortin as a Marker of Neuronal Migration and Its Implications in Cancer Cell Mobility. Front Mol Neurosci 10, 199, doi: 10.3389/fnmol.2017.00199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA & Sood AK Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15, 563–572, doi: 10.1038/nrc3978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujiwara T & Uehara Y The cytoarchitecture of the wall and the innervation pattern of the microvessels in the rat mammary gland: a scanning electron microscopic observation. Am J Anat 170, 39–54, doi: 10.1002/aja.1001700104 (1984). [DOI] [PubMed] [Google Scholar]
- 126.Kepper M & Keast J Immunohistochemical Properties and Spinal Connections of Pelvic Autonomic Neurons That Innervate the Rat Prostate-Gland. Cell and Tissue Research 281, 533–542 (1995). [DOI] [PubMed] [Google Scholar]
- 127.Folkman J, Watson K, Ingber D & Hanahan D Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339, 58–61, doi: 10.1038/339058a0 (1989). [DOI] [PubMed] [Google Scholar]
- 128.Eichmann A & Brunet I Arterial innervation in development and disease. Sci Transl Med 6, 252ps259, doi: 10.1126/scitranslmed.3008910 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Carmeliet P & Tessier-Lavigne M Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200, doi: 10.1038/nature03875 (2005). [DOI] [PubMed] [Google Scholar]
- 130.De Bock K et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663, doi: 10.1016/j.cell.2013.06.037 (2013). [DOI] [PubMed] [Google Scholar]
- 131.Schoors S et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19, 37–48, doi: 10.1016/j.cmet.2013.11.008 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Felten DL & Felten SY Sympathetic noradrenergic innervation of immune organs. Brain Behav Immun 2, 293–300 (1988). [DOI] [PubMed] [Google Scholar]
- 133.McHale NG & Thornbury KD Sympathetic stimulation causes increased output of lymphocytes from the popliteal node in anaesthetized sheep. Exp Physiol 75, 847–850 (1990). [DOI] [PubMed] [Google Scholar]
- 134.Rosas-Ballina M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101, doi: 10.1126/science.1209985 (2011).This study showed that the autonomic nervous system can directly regulate the immune system.
- 135.Wang H et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388, doi: 10.1038/nature01339 (2003). [DOI] [PubMed] [Google Scholar]
- 136.Salmon H, Remark R, Gnjatic S & Merad M Host tissue determinants of tumour immunity. Nat Rev Cancer, doi: 10.1038/s41568-019-0125-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maes M et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10, 313–318, doi: 10.1006/cyto.1997.0290 (1998). [DOI] [PubMed] [Google Scholar]
- 138.Cole SW et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A 107, 5681–5686, doi: 10.1073/pnas.0911515107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shahzad MM et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem 285, 35462–35470, doi: 10.1074/jbc.M110.109579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feig C et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 110, 20212–20217, doi: 10.1073/pnas.1320318110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miller AM et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 177, 7398–7405, doi: 10.4049/jimmunol.177.10.7398 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Bronte V et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med 201, 1257–1268, doi: 10.1084/jem.20042028 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakai A, Hayano Y, Furuta F, Noda M & Suzuki K Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med 211, 2583–2598, doi: 10.1084/jem.20141132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiao G et al. beta-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol Immunother 68, 11–22, doi: 10.1007/s00262-018-2243-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wong CHY, Jenne CN, Lee WY, Leger C & Kubes P Functional Innervation of Hepatic iNKT Cells Is Immunosuppressive Following Stroke. Science 334, 101–105, doi: 10.1126/science.1210301 (2011). [DOI] [PubMed] [Google Scholar]
- 146.Mohammadpour H et al. beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. The Journal of clinical investigation, doi: 10.1172/JCI129502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bucsek MJ et al. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res 77, 5639–5651, doi: 10.1158/0008-5472.CAN-17-0546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Borovikova LV et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462, doi: 10.1038/35013070 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Cheng Y et al. Depression-Induced Neuropeptide Y Secretion Promotes Prostate Cancer Growth by Recruiting Myeloid Cells. Clin Cancer Res 25, 2621–2632, doi: 10.1158/1078-0432.CCR-18-2912 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Joyce JA & Fearon DT T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80, doi: 10.1126/science.aaa6204 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Hisasue S et al. Cavernous nerve reconstruction with a biodegradable conduit graft and collagen sponge in the rat. J Urol 173, 286–291, doi: 10.1097/01.ju.0000141578.84536.80 (2005). [DOI] [PubMed] [Google Scholar]
- 152.Twardowski T, Fertala A, Orgel J & San Antonio J Type I Collagen and Collagen Mimetics as Angiogenesis Promoting Superpolymers. Current Pharmaceutical Design 13, 3608–3621, doi: 10.2174/138161207782794176 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Tuxhorn JA et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 8, 2912–2923 (2002). [PubMed] [Google Scholar]
- 154.Burns-Cox N, Avery NC, Gingell JC & Bailey AJ Changes in Collagen Metabolism in Prostate Cancer: A Host Response That May Alter Progression. The Journal of Urology 166, 1698–1701, doi: 10.1016/s0022-5347(05)65656-x (2001). [DOI] [PubMed] [Google Scholar]
- 155.Egeblad M & Werb Z New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2, 161–174, doi: 10.1038/nrc745 (2002). [DOI] [PubMed] [Google Scholar]
- 156.Henriksen JH, Christensen NJ & Ring-Larsen H Noradrenaline and adrenaline concentrations in various vascular beds in patients with cirrhosis Relation to haemodynamics. Clinical Physiology 1, 293–304, doi: 10.1111/j.1475-097X.1981.tb00898.x (1981). [DOI] [PubMed] [Google Scholar]
- 157.Oben JA, Yang S, Lin H, Ono M & Diehl AM Norepinephrine and neuropeptide Y promote proliferation and collagen gene expression of hepatic myofibroblastic stellate cells. Biochemical and Biophysical Research Communications 302, 685–690, doi: 10.1016/s0006-291x(03)00232-8 (2003). [DOI] [PubMed] [Google Scholar]
- 158.Kim-Fuchs C et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40, 40–47, doi: 10.1016/j.bbi.2014.02.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Szpunar MJ, Burke KA, Dawes RP, Brown EB & Madden KS The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 6, 1262–1272, doi: 10.1158/1940-6207.CAPR-13-0079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen D & Ayala GE Innervating Prostate Cancer. N Engl J Med 378, 675–677, doi: 10.1056/NEJMcibr1714003 (2018). [DOI] [PubMed] [Google Scholar]
- 161.Lillemoe KD et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg 217, 447–455; discussion 456–447 (1993).This randomized placebo-controlled trial showed that denervation improved survival in patients with cancer and elevated sensory nerve activity.
- 162.US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT01520441, 2015). [DOI] [PubMed]
- 163.Al-Wadei HA, Al-Wadei MH & Schuller HM Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs 20, 477–482, doi: 10.1097/CAD.0b013e32832bd1e3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Powe DG et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1, 628–638, doi: 10.18632/oncotarget.101009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Giorgi V et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171, 779–781, doi: 10.1001/archinternmed.2011.131 (2011). [DOI] [PubMed] [Google Scholar]
- 166.Diaz ES, Karlan BY & Li AJ Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 127, 375–378, doi: 10.1016/j.ygyno.2012.07.102 (2012). [DOI] [PubMed] [Google Scholar]
- 167.Grytli HH, Fagerland MW, Fossa SD, Tasken KA & Haheim LL Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73, 250–260, doi: 10.1002/pros.22564 (2013). [DOI] [PubMed] [Google Scholar]
- 168.Wang HM et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 24, 1312–1319, doi: 10.1093/annonc/mds616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Neeman E, Zmora O & Ben-Eliyahu S A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res 18, 4895–4902, doi: 10.1158/1078-0432.CCR-12-1087 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bahnson RR, Andriole GL, Clayman RV & Catalona WJ Catecholamine excess: Probable cause of postoperative tachycardia following retroperitoneal lymph node dissection (RPLND) for testicular carcinoma. Journal of Surgical Oncology 42, 132–135, doi: 10.1002/jso.2930420213 (1989). [DOI] [PubMed] [Google Scholar]
- 171.HALME A, PEKKARINEN A & TURUNEN M ON THE EXCRETION OF NORADRENALINE, ADRENALINE, 17-HYDROXYCORTICOSTEROIDS AND 17-KETOSTEROIDS DURING THE POSTOPERATIVE STAGE. 24, S5, doi: 10.1530/acta.0.024S005 (1957). [DOI] [PubMed] [Google Scholar]
- 172.Lindenauer PK et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 353, 349–361, doi: 10.1056/NEJMoa041895 (2005). [DOI] [PubMed] [Google Scholar]
- 173.Blessberger H et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev 3, CD004476, doi: 10.1002/14651858.CD004476.pub3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Al-Niaimi A et al. The impact of perioperative beta blocker use on patient outcomes after primary cytoreductive surgery in high-grade epithelial ovarian carcinoma. Gynecol Oncol 143, 521–525, doi: 10.1016/j.ygyno.2016.09.019 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Yap A et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br J Anaesth 121, 45–57, doi: 10.1016/j.bja.2018.03.024 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Musselman RP et al. Association between perioperative beta blocker use and cancer survival following surgical resection. Eur J Surg Oncol 44, 1164–1169, doi: 10.1016/j.ejso.2018.05.012 (2018). [DOI] [PubMed] [Google Scholar]
- 177.Cata JP et al. Perioperative beta-blocker use and survival in lung cancer patients. J Clin Anesth 26, 106–117, doi: 10.1016/j.jclinane.2013.10.004 (2014). [DOI] [PubMed] [Google Scholar]
- 178.Horowitz M, Neeman E, Sharon E & Ben-Eliyahu S Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 12, 213–226, doi: 10.1038/nrclinonc.2014.224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Denk F, Bennett DL & McMahon SB Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci 40, 307–325, doi: 10.1146/annurev-neuro-072116-031121 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Smith M et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol 34, 3005–3013, doi: 10.1200/JCO.2015.65.5597 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Collins C et al. Preclinical and clinical studies with the multi-kinase inhibitor CEP-701 as treatment for prostate cancer demonstrate the inadequacy of PSA response as a primary endpoint. Cancer Biol Ther 6, 1360–1367, doi: 10.4161/cbt.6.9.4541 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Drilon A et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378, 731–739, doi: 10.1056/NEJMoa1714448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chan E et al. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest New Drugs 26, 241–247, doi: 10.1007/s10637-008-9118-3 (2008). [DOI] [PubMed] [Google Scholar]
- 184.Shabbir M & Stuart R Lestaurtinib, a multitargeted tyrosine kinase inhibitor: from bench to bedside. Expert Opin Investig Drugs 19, 427–436, doi: 10.1517/13543781003598862 (2010). [DOI] [PubMed] [Google Scholar]
- 185.US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT00830180, 2014). [DOI] [PubMed]
- 186.Sopata M et al. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 156, 1703–1713, doi: 10.1097/j.pain.0000000000000211 (2015). [DOI] [PubMed] [Google Scholar]
- 187.Barford K, Keeler A, Deppmann C & Winckler B TrkA Bumps into Its Future Self. Dev Cell 42, 557–558, doi: 10.1016/j.devcel.2017.09.002 (2017). [DOI] [PubMed] [Google Scholar]
- 188.Spitzer NC Neurotransmitter Switching? No Surprise. Neuron 86, 1131–1144, doi: 10.1016/j.neuron.2015.05.028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Habecker BA & Landis SC Noradrenergic regulation of cholinergic differentiation. Science 264, 1602–1604, doi: 10.1126/science.8202714 (1994).This study showed that the sympathetic neurotransmitter phenotype is plastic and mediated by the tissue-specific microenvironment.
- 190.Yang B, Slonimsky JD & Birren SJ A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci 5, 539–545, doi: 10.1038/nn853 (2002). [DOI] [PubMed] [Google Scholar]
- 191.Yamamori T et al. The Cholinergic Neuronal Differentiation Factor from Heart-Cells Is Identical to Leukemia Inhibitory Factor. Science 246, 1412–1416, doi:DOI 10.1126/science.2512641 (1989). [DOI] [PubMed] [Google Scholar]
- 192.Zurborg S et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain 7, 66, doi: 10.1186/1744-8069-7-66 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lau J et al. Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse. Mol Pain 7, 100, doi: 10.1186/1744-8069-7-100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nassar MA et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A 101, 12706–12711, doi: 10.1073/pnas.0404915101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen X et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron 46, 13–21, doi: 10.1016/j.neuron.2005.03.009 (2005). [DOI] [PubMed] [Google Scholar]
- 196.Karai L et al. Deletion of vanilloid receptor 1_expressing primary afferent neurons for pain control. Journal of Clinical Investigation 113, 1344–1352, doi: 10.1172/jci20449 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Erin N, Boyer PJ, Bonneau RH, Clawson GA & Welch DR Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res 24, 1003–1009 (2004). [PubMed] [Google Scholar]
- 198.Vincenzi FF Effect of botulinum toxin on autonomic nerves in a dually innervated tissue. Nature 213, 394–395 (1967). [DOI] [PubMed] [Google Scholar]
- 199.Ben-Shaanan TL et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat Commun 9, 2723, doi: 10.1038/s41467-018-05283-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K & Delp SL Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Sci Transl Med 8, 337rv335, doi: 10.1126/scitranslmed.aad7577 (2016). [DOI] [PubMed] [Google Scholar]
- 201.Sternson SM & Roth BL Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37, 387–407, doi: 10.1146/annurev-neuro-071013-014048 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Lin CW et al. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron 65, 32–39, doi: 10.1016/j.neuron.2009.12.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Amit M, Na’ara S & Gil Z Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 16, 399–408, doi: 10.1038/nrc.2016.38 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Taylor HB & Norris HJ Epithelial invasion of nerves in benign diseases of the breast. Cancer 20, 2245–2249 (1967). [DOI] [PubMed] [Google Scholar]
- 205.Ali TZ & Epstein JI Perineural involvement by benign prostatic glands on needle biopsy. Am J Surg Pathol 29, 1159–1163 (2005). [DOI] [PubMed] [Google Scholar]
- 206.Cracchiolo JR et al. Patterns of recurrence in oral tongue cancer with perineural invasion. Head Neck. 40, 1287–1295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Al-Hussain T, Carter HB & Epstein JI Significance of prostate adenocarcinoma perineural invasion on biopsy in patients who are otherwise candidates for active surveillance. J Urol 186, 470–473, doi: 10.1016/j.juro.2011.03.119 (2011). [DOI] [PubMed] [Google Scholar]
- 208.Beard CJ et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. International Journal of Radiation Oncology*Biology*Physics 58, 19–24, doi: 10.1016/s0360-3016(03)01433-0 (2004). [DOI] [PubMed] [Google Scholar]
- 209.Kraus RD et al. The Perineural Invasion Paradox: Is Perineural Invasion an Independent Prognostic Indicator of Biochemical Recurrence Risk in Patients With pT2N0R0 Prostate Cancer? A Multi-Institutional Study. Adv Radiat Oncol 4, 96–102, doi: 10.1016/j.adro.2018.09.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]