Abstract
BACKGROUND:
Estrogen deficiency decreases bone density and increases the risk of osteoporosis and fracture, thereby necessitating reconstruction of bone regeneration. As bone marrow mesenchymal stem cell (BMSCs) lose viability and differentiation potential under osteoporotic conditions, it is impossible to use autologous BMSCs for osteoporosis treatment. As an alternative, adipose-derived stem cells (ADSCs) may serve as the source of therapeutic cells.
METHOD:
We evaluated the effects of osteoporosis on the functional characteristics of ADSCs. Osteoporosis was induced in ovariectomy (OVX) rat model, and the ADSCs from Sham and OVX groups were cultured and analyzed comparatively.
RESULTS:
As a result, the viability was higher for the ADSCs from Sham group than those from OVX group. The analysis of the paracrine potential of ADSCs revealed the elevated levels of inflammatory and cellular senescence factors in the ADSCs from OVX group. The ADSCs from OVX group had much higher differentiation potential into adipocytes than those from the Sham group. Osteoporotic environment had no effect on the osteogenic potential of ADSCs.
CONCLUSION:
Osteoporosis may reduce the activity and influence immune response of ADSCs by modulating paracrine action and adipogenic potential. These characteristics of ADSCs should be given consideration for therapeutic purpose.
Electronic supplementary material
The online version of this article (10.1007/s13770-020-00289-x) contains supplementary material, which is available to authorized users.
Keywords: Adipose-derived stem cell, Osteoporosis, Differentiation, Paracrine action
Introduction
Osteoporosis is an autoimmune disorder accompanied with the failure of hormonal regulation. This metabolic skeletal disease is characterized with the deterioration of the microstructure and a chronic decrease in the bone mass, leading to the risk of fracture. Women are much more likely to suffer from this condition than men. While premenopausal women have more estrogen than men, they may experience a dramatic reduction in estrogen production level owing to menopause [1, 2]. Estrogen deficiency is highly related to the immune system and causes low-grade inflammatory responses through elevated levels of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-17, and tumor necrosis factor (TNF)-α [3–6], accompanied by fat accumulation. This inflammatory condition activates osteoclast to degrade bone tissue, leading to bone loss and furthermore development of osteoporosis.
Autologous bone transplantation is the gold standard regimen, owing to its high osteogenic potential and absence of any immune reaction. However, bone transplantation is associated with the risk of pain and infection at the site of bone harvesting [7–9]. The current state of therapeutic management of osteoporosis is divided into the use of bone resorption inhibitors and anabolic agents. Bone resorption inhibitors, including bisphosphonate, calcitonin, estrogen, selective estrogen receptor modulators, and RANKL inhibitors, may not restore the bone mass but block bone ingestion [10–14]. Anabolic agents, including sclerostin, parathyroid hormone (PTH), and PTH-related protein, induce bone formation through osteoblasts [15–18]. However, the efficacy of these agents is less than that expected. Moreover, conventional drugs for osteoporosis exert serious side-effects depending on dosage [19, 20]. Thus, the development of a novel therapy for bone regeneration is desirable.
Stem cells possess the ability to differentiate in any human tissue; hence, they have great potential to serve as therapeutic agents for tissue regeneration and repair. Various types of stem cells have been used for cell therapy. In particular, bone marrow stem cells (BMSCs) are key therapeutics for diverse diseases [21–23], owing to their ability to secrete paracrine factors and directly differentiate at the site of the damaged tissue [24–26]. Treatment of bone diseases has employed stem cell therapy as a novel strategy, but BMSCs are difficult to isolate under osteoporotic conditions owing to bone fragility and moreover, the BMSCs from patients with osteoporosis are less viable and lack the differentiation potential in vitro, which limit their applications for auto-transplantation.
As an alternative, the adipose-derived stem cells (ADSCs) may be used as the source of cell therapeutics. ADSCs are known to exhibit multi-differentiation abilities as BMSCs as well as repopulation capacity upon late passages [27–29]. In addition, ADSCs are free from ethical controversies and immunogenic barriers, comparing to BMSCs. Notably, the underlying disease condition is known to have little effect on ADSC activity. These characteristics of ADSCs have facilitated their applications as therapeutic agents for critical diseases and a number of studies have been attempted in pre-clinically or clinically.
However, despite these benefits, it would need to contemplate the possibility that critical disease with strong inflammatory situation may affect ADSC functions. Previous investigations proved the therapeutic effect of ADSC from osteoporosis animal. However, impairment of ADSC activity can be dependent on severity of disease and patients who require stem cell therapy have serious statue of disease. Thus, the information for the effect of extreme disease condition on ADSCs is required for further applications of ADSCs. Namely, prior to the application of ADSCs to patients, it is important to evaluate their functions and characteristics under disease condition.
In the present study, we attempted to evaluate the effects of osteoporosis on ADSCs. To obtain ADSCs from osteoporotic conditions, we developed a non-clinical osteoporosis rat model with ovary ligation and removal (ovariectomy; OVX) [30–32]. ADSCs were isolated following a distinct reduction in the bone density with systemic inflammation, and these cells were cultured in vitro for several passages. ADSCs from the ovariectomized rats and surgery-operated control rats were compared in terms of morphology, viability, population doubling time, paracrine potential, and differentiation potential into adipocytes and osteoblasts.
Materials and methods
Materials
Minimum essential medium-alpha (MEM-α) and fetal bovine serum (FBS) were supplied by Gibco (Grand Island, NY). Alizarin red S, and oil red O stains were purchased from Sigma-Aldrich (St. Louis, MO, USA); penicillin/streptomycin, 0.25% trypsin–EDTA solution, and phosphate buffered saline (PBS) from Welgene (Daegu, Korea); Rat Transforming growth factor beta-1 (TGF-β1), Hepatocyte growth factor (HGF), TNF-α and vascular endothelial growth factor (VEGF) ELISA kits were obtained from R&D Systems (Minneapolis, MN, USA).
Animals
We obtained 7-week-old SD female rats from Daehan Bio link (Seoul, Korea). Animals were maintained in an animal holding room and received standard chow and water ad libitum. Animal experiments were approved by the Ethical Committees for Experimental Animals at Kyung Hee University (KHMC-IACUC 2018-40).
OVX rat model
Rats were randomly divided into two groups, namely, the Sham surgery and the OVX groups. OVX was performed in female rats through an incision in the back under general anesthesia with ketamine (100 mg/kg, Yuhan co., Seoul, Korea) and rumpun (1.2 mg/kg, Bayer Healthcare, Seoul, Korea). Approximately 1.5 cm of the skin, abdominal cavity, and muscles were incised, and the ovaries were exposed. The oviduct was ligated with a silk thread, and the rats were subjected to bilateral OVX. Sham operation was performed by exposing bilateral ovaries and returning them to the original position. Bone density and was evaluated with SkyScan 1173 (Resolution: 13.85 μm) for 12 weeks.
ADSC isolation and expansion
Adipose tissue was washed several times to remove contaminating blood and immune cells and incubated for 60 min with collagenase (Sigma) to facilitate digestion. Following centrifugation, the digested samples were separated into a floating population of mature adipocytes and collected stromal vascular fraction (SVF). After washing with PBS (Welgene), the stromal fraction was cultured in growth medium (alpha-MEM supplemented with 10% FBS and 1% penicillin/streptomycin). The expression of the specific marker on ADSCs was examined with fluorescence-activated cell sorting (Table S1).
WST-1 assay
ADSC (passage 2) was seeded on 96 well plate in concentration of 1 × 10 5 cell/ml (200ul) and incubated for 24 h. WST-1 solution (Roche, Indianapolis, IN, USA) was added to each sample at 10% total volume of the medium, and the plate was incubated for 2 h at 37 °C and 5% CO2. The absorbance was measured every 30 min at a wavelength of 450 nm using an microplate reader (Molecular device). The activity of ADSCs from Sham group was set to 100%, while that of the ADSCs from OVX group was expressed as percentage relative to control.
Proliferation assay
ADSCs (2 × 105, passage 2) from Sham and OVX groups were cultured at 75T flask for 5 days. Total cell number was counted before subculture and then, population doubling time (PDT) was obtained, using increased fold of cell number and culture period.
Western blot analysis
Cells were washed with PBS and lysed with 1xlysis buffer (Cell signaling, Danvers, MA, USA). The supernatants were collected by centrifugation (rotor radius: 70 mm) at 12,000 rpm for 10 min at 4 °C. Protein concentrations of lysates were determined with bicinchoninic acid reagents (Thermo Fisher, Rockford, IL, USA). The lysates were denatured and electrophoresed using sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the separated protein bands were transferred onto a nitrocellulose membrane. The membrane was blocked with 5% skim milk for 1 h, and then probed with primary anti-osteopontin (Abcam, Cambridge, UK) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Abcam, Cambridge, UK) antibodies, followed by incubation with an anti-IgG horseradish peroxidase-conjugated secondary antibody (Bio-Rad, Hercules, CA, USA). The blots were visualized using Amersham imager chemiluminescence (GE Healthcare, Chicago, IL, USA).
Cytokine measurement
To obtain serum, blood was collected from OVX rat at 4, 8, and 12 week post induction of OVX and then centrifuged at 12,000 rpm for 5 min to separate serum and red blood cells. After centrifugation, supernatant was collected.
In order to get conditioned medium of ADSC, ADSC was plated in 12 well plate in concentration of 4 × 104 and 24 h later, media was replaced with fresh media. This was sustained for 3 days and then, conditioned medium was collected for ELISA.
TNF-α and IL-17A in serum and transforming growth factor (TGF)-β, TNF-α, hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) in the conditioned medium of ADSCs were quantified with ELISA kits, according to the manufacturer’s instructions (R&D system, Minneapolis, MN, USA). Briefly, samples and standards were added to the wells of 96-well plates coated with anti-TNF-α, anti-IL-17A, anti-TGF-β, anti-HGF, and anti-VEGF antibodies and incubated for appropriate time points at room temperature. The supernatant was discarded and the wells were treated with a horseradish peroxidase-conjugated secondary antibody at room temperature. After washing four times, a substrate solution was added and the reaction was terminated with the addition of a stop solution. The absorbance was measured at 450 and 540 nm using ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA). The readings were obtained by subtracting the absorbance at 540 nm from that at 450 nm.
FACS analysis
For analysis of Th17 cell in spleen, spleen of OVX rats was isolated at 4, 8 and 12 post induction and then single cells were obtained from spleen. Then, these cells were incubated with APC-conjugated CD4 (Biolegend, San Diego, CA, USA) and PE-conjugated IL-17 antibody (Thermofisher, Rockford, IL, USA) for 40 min at 4 °C in the dark. After washing twice with FACS buffer (BD bioscience), the proportion of Th17 cells was analyzed using a FACS Calibur flow cytometer and the CELLQuest software (Becton–Dickinson, San Jose, CA, USA).
Zymography
ADSC was cultured in six well plates and supernatant of ADSCs from Sham and OVX groups were collected to be separated on 8% SDS–polyacrylamide resolving gels containing 0.1% gelatin. The gels were renatured in 2.5% Triton X-100 for 30 min and incubated in a buffer comprising 50 mM Tris (pH 7.4), 0.5 mM calcium chloride (CaCl2), and 1 μM zinc chloride (ZnCl2) for 24 h at 37 °C. The gels were stained with Coomassie Brilliant Blue R-250 staining solution (Bio-Rad Lab) for 1 h and de-stained with a buffer comprising 10% isopropanol and 10% acetic acid until white bands were observed. The intensity of the area with degraded gelatin was quantified using ImageJ software.
Differentiation of ADSCs
ADSCs were induced for differentiation in rat adipocyte differentiation medium (Amsbio, Abingdon, UK). 4 × 105 ADSCs were plated into six well plate and medium from each well was changed every 3 days. The cycle was carried out for 21 days. Neutral lipids were detected with the staining of cells in 0.5% Oil red O solution (Sigma) after fixation with 10% formaldehyde. For quantification, stained Oil red O was extracted by 100% isopropanol.
Osteogenic differentiation was performed using rat osteocyte differentiation medium (Amsbio). 4 × 105 ADSCs were plated into six well plate and differentiation was induced. Half of the medium from each well was changed every 3 days. This step was repeated for 21 days. Mineralization was assessed by staining the cells with a 2% Alizarin red S (pH 4.3) solution after fixation with 10% formaldehyde.
Statistical analysis
All data are presented as the mean standard deviation (SD) of three independent experiments. Statistical analysis of all data was carried out by an unpaired, two-tailed Student t test. Probability values less than 0.05 were interpreted to indicate statistically significant differences (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Osteoporotic environment is accompanied by inflammatory responses
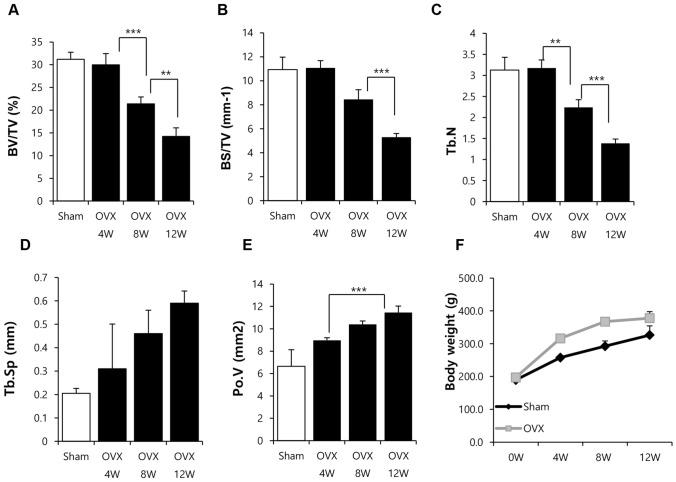
To generate a non-clinical model of osteoporosis, ovaries were excised and bone density was determined with micro-computed tomography for 12 weeks. As shown in Fig. 1, the bone density remained stable for 4 weeks but decreased at 8 weeks post OVX. Severe bone loss was observed at 12 weeks post OVX (Fig. 1A–E). The difference in body weight was observed from 4 weeks post OVX and maintained until 12 weeks post OVX (Fig. 1F). Analysis of bone density suggested that bone loss was initiated from 8 weeks and aggravated at 12 week post OVX.
Fig. 1.
Analysis of bone density (micro-CT) of OVX rats. A–E Ovaries were excised from SD rats (female) and the bone density was monitored for 12 weeks. BV/TV: bone volume/total volume; BS/TV: bone surface density; Tb.N: trabecular number; Tb.Sp: trabecular spacing; Po.V: volume of pores. F Body weight was measured for 12 weeks. Results are shown as the mean ± SD (**p < 0.01, and ***p < 0.001). n = 7/each group
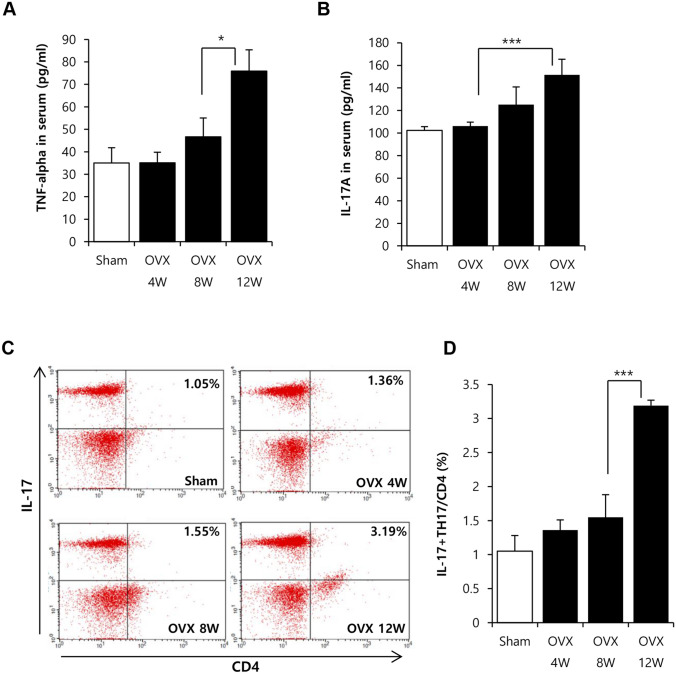
Next, it was examined the relation of systemic inflammation with the reduction of bone density. IL-17 and TNF-α are well known to promote bone loss in osteoporosis by activating osteoclast and immune cells. Thus, we measured the change of concentration of IL-17 and TNF-α as an indicator for inflammation in OVX rat. Serum cytokine profile revealed a considerable increase in the levels of IL-17 and TNF-α at 12 weeks but not at 8 weeks (Fig. 2A and B). Additionally, as source of IL-17 is Th17 cell and generation of Th17 is occurred in 2nd lymphoid organ including spleen, Th17 cell level was determined in spleen by FACS (Fig. 2C and D). FACS analysis showed that CD4+IL-17+ Th17 cells were firmly elevated in spleen at 12 weeks post OVX induction, which pattern was consistent with increase of IL-17 in serum.
Fig. 2.
Analysis of inflammatory indicator under severe bone loss. A–B After OVX induction, serum and spleen were collected at 4, 8 and 12 week and then, analyzed. Serum cytokine was analyzed by ELISA (A) TNF-α. B IL-17A. C–D Spleen was dissociated into single cells and then, portion of Th17 cell of splenocytes was analyzed by FACS Results are shown as the mean ± SD (*p < 0.05, ***p < 0.001). n = 7/each group
This result suggests that 12 weeks post OVX induction is estimated to be status to have both of strong inflammatory responses and severe bone loss. Hence, 12 weeks post OVX induction was considered as the time point suitable for the analysis of ADSCs affected by osteoporotic stress.
Osteoporotic environment influences cell vitality of ADSC
To investigate whether osteoporosis affects ADSC activity, we obtained adipose tissues from 12 weeks OVX rats and cultured them in vitro (Fig. 3A). Cellular morphology was not different between surgery-operated control group (Sham) and OVX group (Fig. 3B). We failed to observe any change in the expression of the specific marker after osteoporosis (Table S1).
Fig. 3.
Effect of osteoporosis on the activity of ADSCs. A ADSCs were isolated from OVX rats at 12 weeks after the removal of ovaries and cultured in vitro. B Light microscopy images of cell morphology. Original magnification 40×; scale bar: 100 μm. C WST-1 assay were performed to compare cellular viability of ADSC from sham or OVX rat D Population doubling time was analyzed. Results are shown as the mean ± SD of at least three replicate wells for each group (***p < 0.001). n = 7/each group
The cellular viability and doubling time of ADSCs from Sham and OVX groups were relatively analyzed. The cellular viability of ADSCs from OVX group was approximately 35% lower than that of ADSCs from Sham group (Fig. 3C). ADSCs from OVX and Sham groups were cultured to compare population doubling time. As a result, there is no difference in these parameters between Sham and OVX groups (Fig. 3D; Sham: 32.14 ± 2.94 h, OVX: 29.8 ± 3.3 h, non-significant).
Collectively, the morphology and specific marker expression on ADSC was not affected by osteoporotic stress. Also, ADSCs maintained their ability to repopulate during in vitro long term cultures regardless of the osteoporotic condition, indicative of their suitability for transplantation. However, their cellular viability was clearly impaired in response to the exposure to osteoporotic conditions.
Osteoporotic environment may affect the paracrine action of ADSCs by modulating the secretion of cytokines
The paracrine action of stem cells is highly associated with their therapeutic effects. Osteoporosis is characterized with persistent low-grade inflammation. This environment may alter the paracrine potential of ADSCs by creating inflammatory environments. We evaluated if the paracrine potential of ADSCs was affected by osteoporosis.
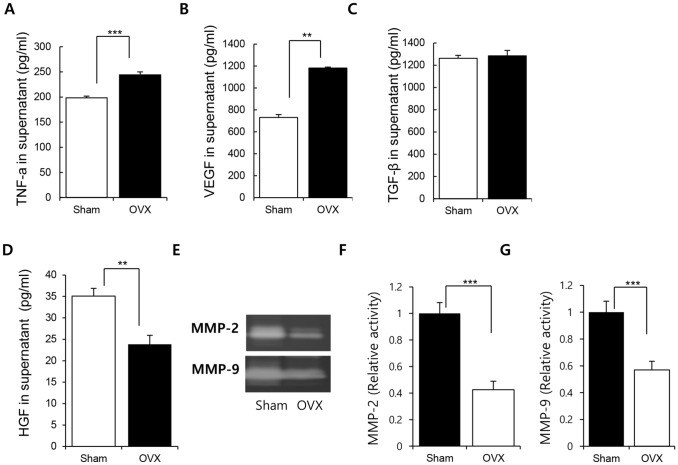
ADSCs from Sham and OVX groups were cultured and the conditioned medium was collected for cytokine analysis with ELISA. In this study, inflammation, angiogenesis and cell senescence-relating factors were employed to check the paracrine potential of ADSCs.
TNF-α production was obviously higher for the ADSCs from OVX group (Fig. 4A; Sham: 198.4354 ± 4.813 pg/mL, OVX: 244.119 ± 8.429 pg/mL, p < 0.005). Furthermore, VEGF secretion was higher in OVX group than in Sham group (Fig. 4B, Sham: 730.0127 ± 27.127 pg/mL, OVX: 1181.158 ± 8.756 pg/mL, p < 0.01). TGF-β is known to be involved in immune suppression and bone formation. Osteoporotic condition did not affect the TGF-β secretion from ADSCs (Fig. 4C; Sham: 1262.233 ± 26.415 pg/mL, OVX: 1284.516 ± 48.07472 pg/mL). HGF/c-met signaling, indicative of the cellular activity of stem cells, decreases with the progression of stem cell senescence owing to the long-term cultivation ex vivo [33]. HGF is also involved in cell motility and morphogenesis. We found that HGF level was clearly lower in the ADSCs from OVX group than in those from Sham group (Fig. 4D, Sham: 35.08 ± 1.8 pg/mL, OVX: 23.84 ± 2.1 pg/mL, p < 0.001).
Fig. 4.
Effect of osteoporosis on the paracrine potential of ADSCs. ADSCs were cultured for 3 days and the culture medium was collected for the quantification of cytokines. Quantification for A TNF-a, B VEGF, C TGF-β, and D HGF was performed with ELISA. E–G Zymography for MMP-2 and MMP-9 activity analysis was performed and the degraded area was quantified with Image J. Results are shown as the mean ± SD of at least three replicate wells for each group (**p < 0.01, and ***p < 0.001). n = 7/each group
Matrix metalloproteases (MMPs) play a role in diverse cellular functions, including proliferation, migration, apoptosis, and differentiation. We compared the secretion of MMPs and found that the levels of MMP-2 and MMP-9 secreted by the ADSCs from OVX group were lower than those secreted by the ADSCs from Sham group (Fig. 4E–G).
These results provide evidence that the osteoporotic environment affects the paracrine action of ADSCs in vivo by modulating the production of soluble factors. This effect may influence the efficacy of stem cell therapy after transplantation.
Osteoporotic environment affects the differentiation potential of ADSCs
ADSCs have the ability to undergo multi-lineage differentiation. To determine the effect of osteoporosis on the differentiation ability of ADSCs, differentiation of ADSCs into osteoblasts and adipocytes was induced in vitro.
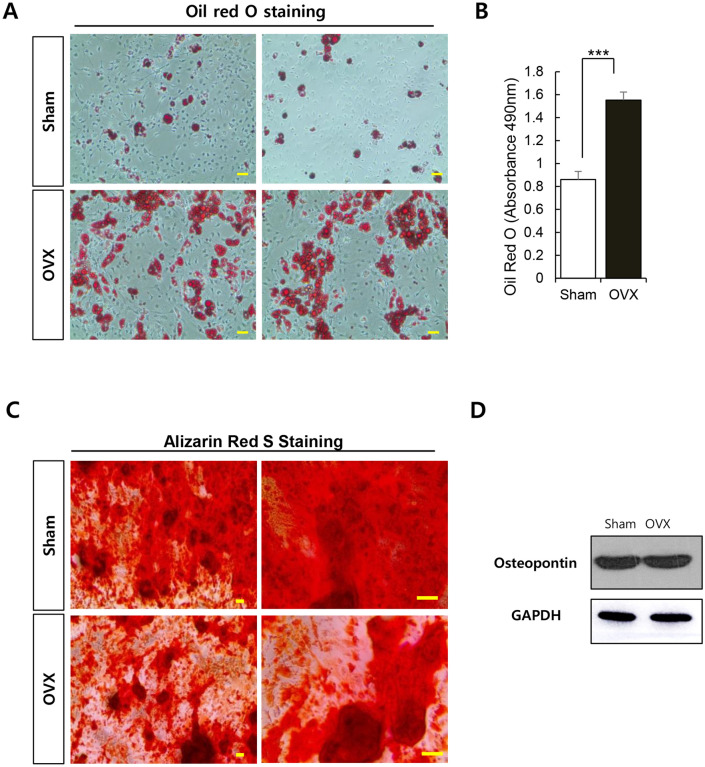
ADSCs were cultured in adipogenic differentiation medium for 2 weeks and adipogenesis was determined with Oil red O staining. The ADSCs from OVX group showed better differentiation into adipocytes than the ADSCs from Sham group during the same period (Fig. 5A–B and Fig. S2).
Fig. 5.
Effect of osteoporosis on the differentiation potential of ADSCs. A Adipogenic induction of ADSCs (passage 2) was performed for 2 weeks. ADSCs were seeded at a density of 4 × 105 cells/well, and the medium was changed every 3 days. After induction, cells were fixed and stained with Oil red O. Original magnification 40×; scale bar: 100 μm. B Quantification of Oil red O in adipocytes. C Osteogenic induction was carried out for 21 days. ADSCs (passage 2) were seeded at a density of 4 × 105 cells/well, and the medium was replaced every 3 days. ADSCs were fixed post osteogenic induction and stained with Alizarin red S. Calcium deposition was observed with the red color of Alizarin red S solution. Scale bar, 100 μm. D Gross view of alizarin red staining. D Western blotting was performed for osteopontin expression analysis in ADSCs. Results are shown as the mean ± SD of at least three replicate wells for each group (***p < 0.001)
We investigated the osteogenic differentiation potential of ADSCs from Sham and OVX groups. With the progression of osteogenesis, calcium deposition on cell layer was observed (Fig. S3). Calcium deposition was determined with Alizarin red S staining (Fig. 5C and Fig. S4). The osteogenic differentiation was not much different between the two groups. The expression of osteopontin, implicated in bone modeling, on ADSCs was also unaffected by osteoporotic conditions.
Thus, the osteogenic potential of ADSCs was not likely to be significantly impaired by osteoporotic condition, which promoted adipogenesis.
Osteoporosis may influence the stem cell activity and functions in the bone marrow. We found that BMSCs under osteoporotic conditions have low level of activity and also, lacked the potential to differentiate into osteoblasts and transformed into adipocytes in response to differentiation induction (Fig. S5, S6). However, as shown in Fig. 5, the osteogenic capacity of ADSCs was not impaired in response to osteoporotic stress, even though cellular activity of ADSC was affected (Fig. 5C–D).
Collectively, this finding suggests that ADSCs may potentially be applied for bone formation even in patients with severe bone loss. However, importantly, ADSC from OVX can be differentiated into adipocyte rapidly, in response to specific signaling compared to ADSC from Sham. Facilitated adipogenesis can enrich pool of adipocyte, which inflammatory environment by providing free fatty acid. Thus, it should be taken differentiation potential of ADSC into consideration when ADSCs was transplanted into specific conditions.
Discussion
Osteoporosis is a medically incurable disease. Estrogen deficiency in postmenopausal women elevates the systemic level of inflammatory cytokines and eventually activates the immune response [34]. The consequence involves reduction in bone density, eventually leading to osteoporosis.
Drugs such as PTH or bisphosphonate used for the clinical treatment of osteoporosis have serious side-effects [35, 36]. Stem cell therapy has been explored as a novel strategy for the treatment of diverse diseases. BMSCs have been tested in various clinical trials. However, the therapeutic potential of BMSCs is highly affected by the disease condition. In particular, patients with osteoporosis have low BMSCs activity, which may limit the therapeutic applications.
ADSCs show characteristics similar to those of BMSCs, including differentiation potential and immune modulation. Therefore, the application of ADSCs to osteoporosis was thought to offer results better than those with BMSC therapy. However, it is important to examine the effects of osteoporosis on the activity of ADSCs.
Bone loss and initiation of strong inflammation were observed at 12 weeks post OVX induction. We obtained ADSCs from osteoporosis rats under this condition and cultured them in vitro. Osteoporosis could decrease the activity of ADSCs and altered the secretion of paracrine factors including TNF-α, VEGF and HGF. However, the level of TGF-β, involved in immune modulation and bone formation, was unaffected by osteoporosis. Change of paracrine factors implies that the transplantation of ADSCs from patients with osteoporosis may cause inflammatory responses and have low survivability in vivo.
The ADSCs from osteoporotic conditions could better differentiate into adipocytes than those from the Sham group. Under osteogenic condition, ADSCs from OVX and Sham groups showed similar osteogenic potentials. That is, the osteogenic potential of ADSCs was unaffected by osteoporosis. On the contrary, the adipogenic capacity of ADSCs was enhanced in response to the exposure to osteoporotic conditions. Previous reports revealed fatty change under osteoporosis [37] and this fatty change can provide detrimental effect on immune system by promoting pro-inflammation through free fatty acid. Thus, OVX-enhanced adipogenesis might be related to inflammation in vivo and thus this feature should be considered for ADSC therapy.
ADSCs have the potential as future therapeutics for osteoporosis; it is imperative to characterize the ADSCs from osteoporotic conditions before their clinical applications. We demonstrated that the ADSCs obtained from osteoporotic conditions show low activity, which is accompanied with elevated levels of inflammatory signals and high adipogenic potential. Additionally, cell surface marker was not good surrogate marker to represent cellular state.
Collectively, our study results provide fundamental information for the development of ADSC therapy. The modulation of ADSC functions by preconditioning or supplementation during ex vivo culture may extend their applications for the treatment of diverse pathologies, including bone diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B0704104813).
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to report.
Ethical statement
Animal experiments were approved by the Ethical Committees for Experimental Animals at Kyung Hee University (KHMC-IACUC 2018-40).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ji MX, Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med. 2015;1:9–13. doi: 10.1016/j.cdtm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-y-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, et al. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/S0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- 6.D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complication of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 8.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9:210–218. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese G, Giuffrida R, Forte S, Fabbi C, Figallo E, Salvatorelli L, et al. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci Rep. 2017;7:7110. doi: 10.1038/s41598-017-07672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 11.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher JC. Role of estrogens in the management of postmenopausal bone loss. Rheum Dis Clin North Am. 2001;27:143–162. doi: 10.1016/S0889-857X(05)70191-5. [DOI] [PubMed] [Google Scholar]
- 13.Hadji P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric. 2012;15:513–523. doi: 10.3109/13697137.2012.688079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129:3214–23. doi: 10.1172/JCI125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen L, Xie X, Su Y, Luo C, Zhang C, Zeng B. Parathyroid hormone versus bisphosphonate treatment on bone mineral density in osteoporosis therapy: a meta-analysis of randomized controlled trials. PLoS One. 2011;6:e26267. doi: 10.1371/journal.pone.0026267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller PD. Optimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapy. Clin Ther. 2005;27:361–376. doi: 10.1016/j.clinthera.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 18.Jódar-Gimeno E. Full length parathyroid hormone in the treatment of osteoporosis in postmenopausal women. Clin Interv Aging. 2007;2:163–174. doi: 10.2147/ciia.2007.2.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponnapakkam T, Katikaneni R, Sakon J, Stratford R, Gensure RC. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today. 2014;19:204–208. doi: 10.1016/j.drudis.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baroncelli GI, Bertelloni S. The use of Bisphosphonates in Pediatrics. Horm Res Paediatr. 2014;82:290–302. doi: 10.1159/000365889. [DOI] [PubMed] [Google Scholar]
- 21.Biehl JK, Russell B. Introduction to stem cell therapy. J Cardiovasc Nurs. 2009;24:98–103. doi: 10.1097/JCN.0b013e318197a6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. Tissue Eng Regen Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci. 2016;17:982. doi: 10.3390/ijms17060982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdon TJ, Paul A, Noiseux N, Prakash S, ShumTim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes Y, Ojeda M, Araya D, Dueñas F, Fernández MS, Peralta OA. Isolation and multilineage differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet Res. 2013;9:133. doi: 10.1186/1746-6148-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 28.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother. 2016;43:268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–430. [PMC free article] [PubMed] [Google Scholar]
- 31.Sophocleous A, Idris AI. Rodent models of osteoporosis. Bonekey Rep. 2014;3:614. doi: 10.1038/bonekey.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khajuria DK, Razdan R, Mahapatra DR. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol. 2012;52:462–470. doi: 10.1590/S0482-50042012000300016. [DOI] [PubMed] [Google Scholar]
- 33.Boichuck M, Zorea J, Elkabets M, Wolfson M, Fraifeld VE. c-Met as a new marker of cellular senescence. Aging Albany NY. 2019;11:2889–97. doi: 10.18632/aging.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao R. Immune regulation of bone loss by Th17 cells in estrogen-deficient osteoporosis. Eur J Clin Invest. 2013;43:1195–1202. doi: 10.1111/eci.12158. [DOI] [PubMed] [Google Scholar]
- 35.Fliefel R, Tröltzsch M, Kühnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44:568–585. doi: 10.1016/j.ijom.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Liu X, Zuo B, Zhang L. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging Dis. 2015;7:514–25. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.