Abstract
With the advent of microvascular surgery, the choice of reconstruction following resection of the primary has an important bearing on the final functional and cosmetic outcome in surgical oncology. The vertical rectus abdominis myocutaneous (VRAM) flap is arguably the most widely used and versatile flap in reconstructive surgery. All patients undergoing a VRAM flap reconstruction following resection of their tumor in the Surgical Oncology Department of a tertiary cancer center from 2012 to 2019 were included in the study. Defects ranged from the breast (40), head and neck (10), groin (3), and perineum (5). The primary outcome measure was incidence of complete and partial flap necrosis, while incidence of hematoma, seroma, incisional hernia, wound dehiscence, and infection were secondary outcomes measured. The patients were followed up for a minimum period of 1 year. The incidence of complete flap necrosis was 5.1% (3) and partial loss 12% (7). Incidence of minor complications such as seroma was 13.7% (8), hematoma 6.8% (4), wound dehiscence 10.3% (6), and wound infection 5.1% (3). Incisional hernia and donor site wound-related complications were not seen in any. On binary regression analysis, the presence of diabetes mellitus, smoking, and the use of adjuvant treatment were associated significantly with increased odds of flap loss. This study demonstrates the versatility and reliability of the VRAM flap in primary reconstruction of defects in surgical oncology. Optimization of risk factors such as diabetes, smoking, and weight gain can reduce flap loss and improve outcomes.
Keywords: Vertical rectus , Abdominis myocutaneous flap, Microvascular reconstruction, Flap loss
Introduction
The rectus abdominis myocutaneous flap was first described by Mathes and Bostwick in 1977 [1]. The pedicled flap has been used for decades, especially in breast reconstruction. With the advent of microvascular anastomosis and free-tissue transfer, the vertical rectus abdominis flap (VRAM) has become arguably, the most versatile flap used to reconstruct a wide range of defects from the head and neck, breast, chest wall to the groin and perineal region. Overtime, along with the latissimus dorsi and radial forearm flap, it has come to be known as the “workhorse of reconstructive plastic surgery.” A reliable blood supply, the ability to harvest a large bulk, and a good cosmetic outcome make it a favored option by most reconstructive surgeons.
In reconstruction of defects of cancer patients, incidence of wound breakdown can be high, given that most patients have compromised blood supply following radiation. The VRAM flap is especially useful in such a scenario in preventing wound-related complications since it has a robust blood supply. It can be harvested as a muscle-only flap or as a myocutaneous flap. The flap can be further contoured to the desired cosmetic requirement by excising the subcutaneous tissue and placing a skin graft over the muscle or debulking the muscle. A pedicle of adequate length can be harvested for anastomosis in the region of interest, be it in the head and neck, chest, groin, or perineum. Blood supply to the skin depends on perforators from the underlying rectus muscle; therefore, a cutaneous free flap without muscle cannot be harvested [2].
We present our experience with the use of the VRAM flap in primary reconstruction of a wide range of defects in surgical oncology department of a tertiary cancer center in India and highlight the versatility of this technique which can be mastered to deliver satisfying cosmetic outcomes even in a resource-limited setting.
Methods
A retrospective analysis of all patients who underwent a VRAM flap reconstruction following resection of the primary tumor in the Surgical Oncology Department of a tertiary cancer center in Bangalore from 2012 to 2019 was carried out. Patient demographics, along with co-morbidities, diagnosis, body mass index (BMI), utilization of preoperative chemotherapy or radiation, and postoperative complications were recorded in a secure database. BMI of 18.5–24.9 was considered normal, 25.0–29.9 as overweight, 30.0–34.9 as obese, and more than 35 as morbidly obese. Patients with any history of smoking or tobacco chewing in the past or present were considered positive. Following the surgery, patients were reviewed in the outpatient at 2 weeks, 4 weeks, and 6 weeks and then 3 monthly as per the institutional protocol with a median follow up period of at least 1 year.
The primary outcome measure was incidence of complete and partial flap loss. A total loss of both skin and muscle was defined as complete flap loss, while any necrosis of skin or muscle short of complete loss was considered as partial flap loss. Incidence of hematoma, seroma, donor site incisional hernia, flap edema, wound dehiscence, and wound infection were secondary outcomes measured.
The collected data was analyzed using IBM SPSS Statistics software (version 25). Categorical variables were analyzed using frequency distribution tests. The relationships between dichotomous variables of interest were assessed using the Χ2 test or Fisher’s exact test for nominal and categorical variables. A binary logistic regression analysis was done with the presence or absence of flap as the dependent variable. Covariates included risk factors such as smoking, diabetes, hypertension, BMI, and adjuvant treatment could lead to flap necrosis (dichotomous-dependent variable). A p value of ≤ 0.05 was considered statistically significant.
Relevant Anatomy
The rectus abdominis is a long broad strap muscle arising from the fifth, sixth, and seventh costal cartilages and inserting in to the crest of the pubis below. The muscle is enclosed in the rectus sheath consisting of an anterior and posterior lamina. The anterior lamina is formed by the splitting of the aponeurosis of the internal oblique muscle and reinforced by the aponeurosis of the external oblique, while the posterior lamina is reinforced by the aponeurosis of the transverse abdominis muscle. The muscle has a dual dominant blood supply from the superior epigastric artery which is a terminal branch of the internal thoracic artery and the inferior epigastric artery which arises from the external iliac artery at the level of the inguinal ligament. Both vessels run posterior to the muscle in a plane between the posterior rectus sheath and the rectus muscle. The skin paddle is supplied by perforators which traverse the rectus muscle. These perforators tend to lie along the medial third of the muscle and above the arcuate line. Both vessels, being dominant, either may be ligated and the flap will survive.
Operative Technique
In this study, the reconstruction was carried out by a single reconstruction team for all cases using the standard technique. The size of the flap was determined by the dimensions of the defect to be reconstructed but can extend from the xiphoid process to the pubis, about 30-cm long in an average adult. The width may vary depending on the skin tension keeping in mind that the defect must be closed primarily.
An ipsilateral vertically superiorly based RAM flap was employed to reconstruct defects of the breast. The axis of rotation of this flap is the point of entry of the superior epigastric artery in to the rectus abdominis muscle at the subcostal margin. The arc of rotation is from 0 to 180 degrees in either the clockwise or counter-clockwise direction. The sites of reach include parasternal, paramanubrial area, and lateral chest wall as far as the axilla. Defects of the groin and perineum were closed using the inferior vertical RAM flap with point of rotation being the origin of the inferior epigastric artery at the level of inguinal ligament. The sites of reach for the inferiorly based flap include low anterior abdominal wall, inguinal region, and the perineum.
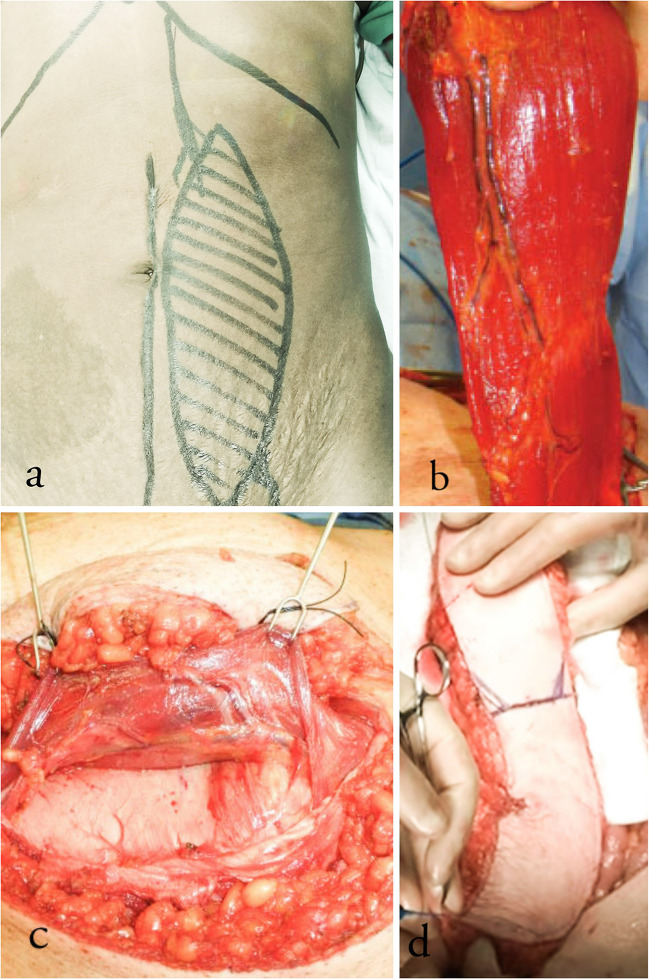
An elliptical skin paddle of appropriate size was marked, while the patient was awake preoperatively (Fig. 1a). Subcutaneous tissue was cut down to the anterior rectus sheath which was incised as an ellipse and sutured to the dermis to prevent shearing strain on the perforators (Fig. 1b). The muscle was dissected from the posterior sheath by blunt and sharp dissection from lateral to medial. The origin of the muscle from the costal cartilage was detached to facilitate rotation of the flap. Depending on whether a superior or inferiorly based flap was being harvested, the inferior or superior epigastric vessels were ligated, respectively. In case of a pedicled flap, the feeding vessels were not dissected. In case of microvascular flap, the pedicle was dissected, and depending on the length required, the pedicle was ligated proximally (Fig. 1c). Oral cavity defects were closed using free-tissue VRAM flap transfer. While harvesting flap for perineum and groin defects, the attachment of the rectus to the symphysis pubis was left intact to prevent torsion of the pedicle (Fig. 1d). In cases of pedicled flap, the muscle was tunneled subcutaneously, making sure the pedicle did not undergo torsion. The abdominal wall defect was closed with prolene mesh reinforcement. Closed suction drains were placed before closing the skin. All reconstructions were carried out primarily in the same sitting (Figs. 2, 3, and 4).
Fig. 1.
a Elliptical skin incision was marked and incised. b Superiorly based VRAM flap harvested with vascular pedicle seen running under the rectus muscle. c Inferior epigastric artery running in a plane between the rectus muscle and the posterior sheath. d Inferiorly based flap harvested retaining the attachment to symphysis pubis intact
Fig. 2.
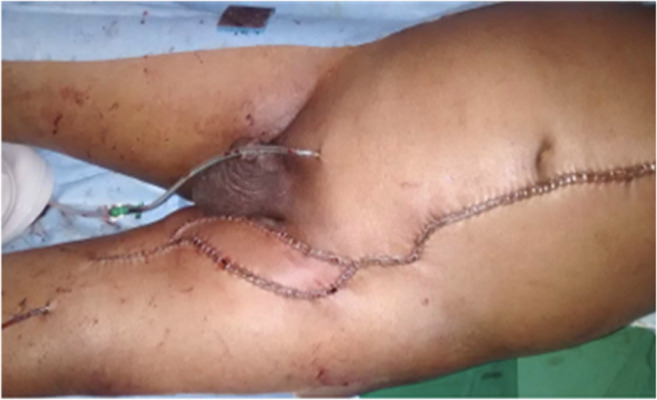
Pedicled VRAM flap used to reconstruct a groin defect following an inguinal block dissection
Fig. 3.
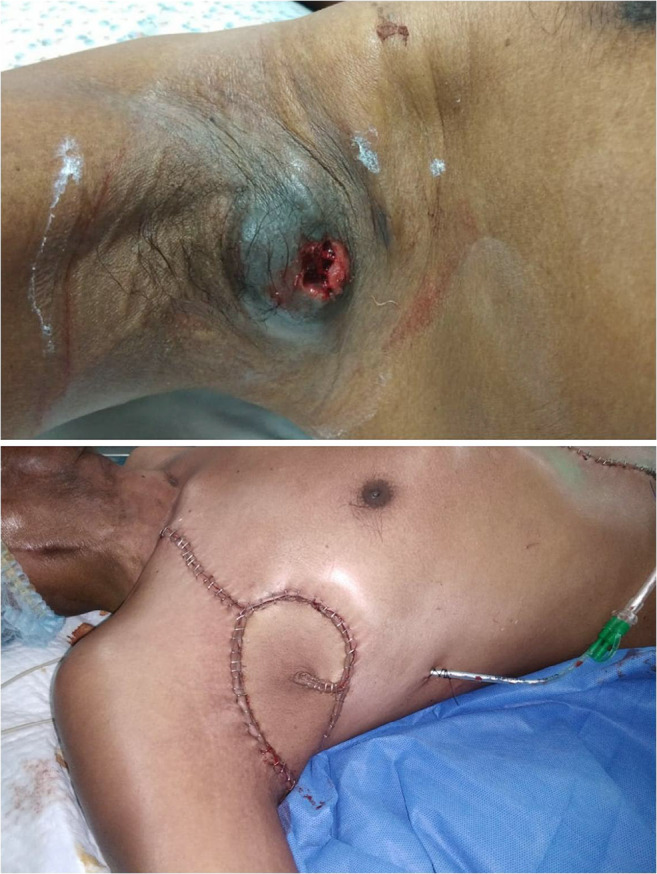
Pedicled VRAM flap used to cover axillary defect in a patient following axillary clearance of nodes with skin involvement
Results
Patient Demographics
The patient demographics are elucidated in Table 1. Fifty-eight patients underwent VRAM flap reconstruction and were included in the study. The median age of the patients was 57 years (range 39–79 years). There were 16 males and 42 females. The site-wise distribution of the cases was as follows: Forty cases of breast reconstruction, 10 oral cavity, 3 cases of carcinoma penis for which the flap was used for reconstructing groin defects following inguinal block dissection and 5 perineal reconstructions following abdominoperineal resection. In oral cavity, the free flap was used to reconstruct large defects such as the following: total glossectomy, maxillectomy, and hard palate. Thirty-eight patients received neoadjuvant chemotherapy, 6 received radiotherapy of which 4 received concurrent chemotherapy, and 14 patients were treated with upfront surgery.
Table 1.
Patient demographics and procedure details
| Frequency | Percent (%) | |
|---|---|---|
| Median age | 57 | 100 |
| Sex | ||
| Male | 16 | 27.6% |
| Female | 42 | 72.4% |
| Adjuvant treatment | ||
| Neoadjuvant CT | 38 | 65.5% |
| Neoadjuvant RT | 2 | 3.4% |
| Concurrent ChemoRT | 4 | 6.9% |
| Primary cases | 14 | 24.1% |
| Breast reconstruction | 40 | 68.9% |
| Oral cavity | 10 | 17.2% |
| Groin | 3 | 5.1% |
| Perineum | 5 | 8.6% |
Table 2 outlines the patients with risk factors that could compromise the vascularity of the flap and wound healing. Eighteen patients (31%) were either current or past smokers. Twenty-three patients (39.7%) were overweight, 12 (20.7%) obese and 2 (3.4%) patients were morbidly obese. Thirty- four (58.6%) patients had type II diabetes while 22 (37.9%) had hypertension.
Table 2.
Risk factors and complications
| Risk factor/complication | Frequency | Percent |
|---|---|---|
| Smoking | 18 | 31% |
| Overweight | 23 | 39.7% |
| Obese | 12 | 20.7% |
| Morbidly obese | 2 | 3.4% |
| Diabetes | 34 | 58.6% |
| Hypertension | 22 | 37.9% |
| Complete flap necrosis | 3 | 5.1% |
| Partial loss | 7 | 12% |
| Seroma | 8 | 13.7% |
| Hematoma | 4 | 6.8% |
| Wound dehiscence | 6 | 10.3% |
| Wound infection | 3 | 5.1% |
| Incisional hernia | 0 | 0 |
Complications
The incidence of complete flap necrosis was seen in 3 patients (5.1%) and partial loss in 7 (12%) which required a debridement and secondary suturing or a salvage flap. Incidence of minor complications such as seroma was 13.7% (8), hematoma 6.8% (4), wound dehiscence 10.3% (6), and wound infection 5.1% (3). Incisional hernia and donor site wound-related complications were not seen in any patient after a follow-up of 1 year. No patients reported functional deficits from removal of the rectus muscle. A summary of the complications is given in Table 2.
The results of the binary regression analysis assessing the odds of flap loss with respect to risk factors such as smoking, diabetes, hypertension, BMI, and neoadjuvant/adjuvant treatment are shown in Table 3. Smoking was associated with 4.5 times increased odds of flap necrosis compared with non-smokers (OR, 4.5; 95% CI, 1.08 to 18.6) which was statistically significant. Patients with diabetes had 8.2 times increased odds of flap necrosis compared with those without diabetes (OR, 8.28; 95% CI, 0.972 to 70.529). Patients who underwent adjuvant treatment had 3.8 times increased odds of flap necrosis (OR, 3.8; 95% CI, 1.438 to 10.414). Increasing BMI was strongly associated with increased odds of flap necrosis with overweight patients having 2.5 times (OR, 2.5; 95% CI, 0.62 to 10.073), obese patients 1.8 times (OR, 1.8; 95% CI, 0.390 to 8.401), and morbidly obese patients having up to 9.4 times increased odds of flap necrosis.
Table 3.
Results of binary regression analysis
| Covariates | B | S.E | Wald | df | Sig. | Exp (B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Smoking | 1.504 | 0.726 | 4.286 | 1 | 0.038* | 4.500 | 1.084 | 18.689 |
| Sex | − 0.693 | 0.726 | 0.910 | 1 | 0.340 | 0.500 | 0.120 | 2.077 |
| Hypertension | − 0.424 | 0.751 | 0.320 | 1 | 0.572 | 0.654 | 0.150 | 2.848 |
| Diabetes mellitus | 2.114 | 1.093 | 3.740 | 1 | 0.053* | 8.280 | 0.972 | 70.529 |
| (Neo) adjuvant treatment | 1.353 | 0.505 | 7.182 | 1 | 0.007* | 3.870 | 1.438 | 10.414 |
| Overweight | 0.916 | 0.711 | 1.661 | 1 | 0.198 | 2.500 | 0.620 | 10.073 |
| Obese | 0.593 | 0.783 | 0.573 | 1 | 0.449 | 1.810 | 0.390 | 8.401 |
| Morbidly obese | 22.974 | 28,420.7 | 0.000 | 1 | 0.999 | 9.491 | 0.000 | |
*p value < 0.05
Discussion
The VRAM flap is a Mathes and Nahai type III flap with dual perfusion from the deep superior and inferior epigastric arteries [3]. The rectus abdominis myocutaneous flap has become an essential tool in the reconstructive armamentarium of the plastic surgeon since it was first described by Mathes and Bostwick in 1977 for reconstruction of abdominal defects. Surgical oncology practice today covers a number of anatomic areas from the head and neck to the perineum and groin as shown in this study. Our study demonstrates the versatility of the rectus flap since it can be used to cover a wide range of defects.
On reviewing existing literature dealing with the VRAM flap, we came across a number of studies demonstrating its utility over a wide range of sites. Autologous reconstruction of the breast has made tremendous progress since the rediscovery of musculocutaneous unit and cutaneous vascular territories along with the advent of microsurgery. Depending on the defect, autologous breast reconstruction options include the pedicled latissimus dorsi flap, abdominal-based flaps (e.g., deep inferior epigastric artery perforator (DEIP), TRAM, and VRAM flaps), or gluteal and thigh-based flaps. Olivari first reintroduced the latissimus dorsi (LD) musculocutaneous flap for breast reconstruction in 1976 [4]. The extended LD was developed by Hokin (1983) and includes the whole muscle with a large area of skin, allowing folding of the flap, thus forming the breast mound and avoiding use of prosthesis. However, its main disadvantage is donor site scar and limited volume [5]. Today the rectus abdominis is one of the preferred regional flaps after latissimus dorsi flap for breast reconstruction. The main advantage of using an abdominal flap in breast reconstruction is that skin type matches the breast and the abdominal fat deposit provides sufficient volume for the breast mound reconstruction without an implant. The VRAM flap is preferred over a latissimus flap in those with a previous thoracotomy transecting the latissimus muscle, those in whom the muscle was used previously as a free flap or those in whom a previous latissimus muscle flap failed, and those who strongly prefer a donor-site scar on the anterior abdominal wall instead of the back. [6]
The pedicled TRAM flap was the most widely used autologous flap for breast reconstruction after it was introduced by Tai and Hasegawa in 1976 using a medially based transverse abdominal skin flap by preserving the perforating vessels from the superior epigastric artery and vein. It has since fallen out of favor due to complications of abdominal wall hernia and increased flap loss. Boyd et al. in 1984 demonstrated that the deep inferior epigastric artery was the main artery of the rectus muscle, explaining the high rate of flap loss [7]. The deep inferior epigastric perforator (DIEP) flap was first introduced in breast reconstruction in 1994 by Allen [8]. This flap can carry the same tissue as the TRAM flap, without the sacrifice of the rectus muscle or fascia, thereby minimizing donor-site morbidity, including bulge, hernia, weakness, and length of recovery time. In general, women who would benefit from an abdominoplasty are ideal candidates for this flap. Even if the DIEP flap is actually considered as the gold standard for autologous breast reconstruction, it is more challenging and requires a longer learning curve in comparison with VRAM and TRAM flaps.
In the head and neck region, the rectus abdominis myocutaneous flap has been shown to provide a good cosmetic and functional outcome as demonstrated by Nakatsuka et al. in their study of 200 cases [9]. Furthermore, Parrett et al. from their retrospective study of 51 patients have shown the reliability of the rectus flap for coverage of irradiated groin and perineal oncological wounds [10].
Flap necrosis is the most dreaded complication following reconstruction with the VRAM flap since it increases patient morbidity, prolongs hospital stay, and leads to a poor cosmetic outcome. In this regard, this flap is extremely reliable, and complication of flap loss in a pedicled flap is invariably technical in nature. We saw a complete flap loss rate of 5.1%, two of which were free flaps and one pedicled flap and partial flap loss rate of 12%. This compares favorably with most reported studies which range from 9 to 19% [1–6].
The patients with complete flap loss required major surgical debridement followed by alternative reconstruction options, while the patients with partial flap loss required either daily dressing or minor debridement and secondary closure. The importance of proper surgical technique and gentle tissue handling while harvesting the flap cannot be emphasized more in preventing this complication. McMenamin et al. have described the increased incidence of flap loss after division of the attachment of the rectus to the symphysis pubis, while reconstructing defects in the perineum, in their retrospective study of 16 patients. This may be due to the increased probability of the pedicle undergoing torsion. The attachment should ideally be preserved but may be divided in cases where extra length is required, taking extra precaution to prevent torsion of the pedicle [11].
We observed increased wound-related complications in patients with co-morbidities such as diabetes, history of smoking, and obesity as demonstrated by the results of the binary logistic modeling shown in Table 3. The presence of diabetes was associated with increased odds of flap loss up to 8.2 times. This underlines the importance of strict perioperative control of blood sugar levels. The use of neoadjuvant or adjuvant chemotherapy/radiation had a significant negative impact with up to 3.8 times increased odds of flap loss. This is due to the vascular damage associated with chemotherapy/radiation which can be minimized by limiting the dose to tumor bed and employing intensity-modulated RT.
The concern of abdominal wall hernias with raising a rectus flap has been largely unfounded. Most studies report rates between 0 and 11%. We routinely used a prolene mesh to reinforce the abdominal wall and found no case of incisional hernia after a mean follow-up of 1 year. McMenamin et al. have reported a 19% incisional hernia rate in their series; however, all of them were seen in patients that were closed using Nylon suture. We recommend using a mesh reconstruction of the donor site especially in cases where a large flap was harvested and a tension closure is expected.
Conclusion
This study demonstrates the versatility and reliability of the VRAM flap in primary reconstruction of a wide range of defects in surgical oncology. The donor site functional deficit is minimal. The flap can be harvested using readily available instruments, which provides a large volume as well as contour that can be customized to the defect. Comorbidities such as diabetes, smoking, and obesity adversely impact wound-related outcomes and should be optimized in the perioperative period.
Compliance with Ethical Standards
Conflict of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mathes S, Bostwick J., 3rd A rectus abdominis myocutaneous flap to reconstruct abdominal wall defects. Br J Plast Surg. 1977;30:282–283. doi: 10.1016/0007-1226(77)90118-7. [DOI] [PubMed] [Google Scholar]
- 2.Shkula HS, Hughes LE. The rectus abdominis flap for perineal wounds. Ann R Coll Surg Engl. 1984;66:337–339. [PMC free article] [PubMed] [Google Scholar]
- 3.Küntscher MV, Mansouri S, Noack N, Hartmann B. Versatility of vertical rectus abdominis musculocutaneous flaps. Microsurgery. 2006;26(5):363–369. doi: 10.1002/micr.20253. [DOI] [PubMed] [Google Scholar]
- 4.Olivari N. The latissimus flap. Br J Plast Surg. 1976;29:126–128. doi: 10.1016/0007-1226(76)90036-9. [DOI] [PubMed] [Google Scholar]
- 5.Hokin JA. Mastectomy reconstruction without a prosthetic implant. Plast Reconstr Surg. 1983;72:810–818. doi: 10.1097/00006534-198312000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Dinner MI, Labandter HP, Dowden RV. Role of the rectus abdominis myocutaneous flap in breast reconstruction. Plast Reconstr Surg. 1982;69:209–213. doi: 10.1097/00006534-198202000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Boyd JB, Taylor GI, Corlett R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast Reconstr Surg. 1984;73:1–16. doi: 10.1097/00006534-198401000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32–38. doi: 10.1097/00000637-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuka T, Harii K, Yamada A, et al. Versatility of a free inferior rectus abdominis flap for head and neck reconstruction: analysis of 200. Cases Plast Reconstr Surg. 1994;93(4):762–769. doi: 10.1097/00006534-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Parrett B, Winograd JM, Garfein ES, et al. The vertical and extended rectus abdominis myocutaneous flap for irradiated thigh and groin defects. Plast Reconstr Surg. 2008;122(1):171–177. doi: 10.1097/PRS.0b013e3181774330. [DOI] [PubMed] [Google Scholar]
- 11.McMenamin DM, Clements D, Edwards TJ, et al. Rectus abdominis myocutaneous flaps for perineal reconstruction: modifications to the technique based on a large single- centre experience. Ann R Coll Surg Engl. 2011;93:375–381. doi: 10.1308/003588411X572268. [DOI] [PMC free article] [PubMed] [Google Scholar]