Abstract
Chimeric antigen receptor (CAR) T cells represent a potent new approach to treat haematological malignancies. Two CAR T-cell therapies, tisagenlecleucel and axicabtagene ciloleucel, have been approved in Europe and the USA, as well as several other countries, for the treatment of leukaemia and lymphoma. These approvals marked a major milestone in the field of cell and gene therapies. However, the clinical development and regulatory evaluation of these innovative therapies faced several challenges that are considered important lessons learned for future similar products. Here, we examine the products’ non-clinical and clinical data packages to outline the challenges encountered during the regulatory evaluation process in Europe, and to provide an update on their performance after authorisation.
Introduction
On Aug 27, 2018, the European Commission granted marketing authorisation to axicabtagene ciloleucel (Yescarta, Kite Pharma [Gilead]; Santa Monica, USA) and tisagenlecleucel (Kymriah, Novartis; Basel, Switzerland). The products are autologous, genetically modified, chimeric antigen receptor (CAR) T cells that were approved for treating various haematological malignancies. Axicabtagene ciloleucel is approved for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and mediastinal large B-cell lymphoma (appendix p 1). Tisagenlecleucel is approved for the treatment of adult relapsed DLBCL, as well as paediatric and young adult (25 years old or younger) acute lymphoblastic leukaemia. In addition to the EU, both products are approved in the USA, Canada, and Switzerland; tisagenlecleucel is also approved in Japan and Australia.
The novelty in CAR T cells lies in part in the genetically engineered chimeric receptor,1,2 which is a fusion protein with an extracellular antibody-derived domain, known as a single-chain variable fragment (ScFv), and an intracellular signalling component usually comprised of primary and costimulatory signalling domains. The ScFv is responsible for specific antigen recognition on the surface of tumour cells, whereas the intracytoplasmic domains are responsible for T-cell activation, eliciting targeted killing of tumour cells.1,2 The gene encoding the receptor is delivered to T cells via a viral vector or by membrane permeabilisation techniques such as electroporation. Both products use second-generation CAR constructs with CD19 as the target surface antigen, which is expressed on healthy B cells and in B-cell malignancies (appendix p 2). The primary T-cell signalling domain in both products is CD3ζ. The costimulatory signalling domain in axicabtagene ciloleucel is CD28 and the costimulatory signal in tisagenlecleucel is produced by 4–1BB (CD137, TNSFR9).3,4 CD28 and 4–1BB are the most widely used costimulatory domains in clinical studies investigating CAR T-cell therapy.5 CD28 promotes effector T-cell differentiation with an exhausted phenotype (potent, short-lived cells), leading to an initial intense activation and cytokine production that diminishes rapidly,4 whereas 4–1BB induces differentiation predominantly to memory cell subtypes that promote cellular persistence and less cytokine production.4,6 By harnessing the specificity of antibodies and the cytotoxicity of T cells, CAR T cells have shown high potency in treating haematological malignancies, with new generations of CAR T cells being tested for the treatment of many subtypes of haematological malignancies and solid tumours.7,8
In the EU, CAR T cells are subject to the advanced therapy medicinal product (ATMP) legislation and guidelines. The scientific evaluation of marketing authorisation applications for ATMPs is assessed by the European Medicines Agency (EMA) via a mandatory centralised procedure.9 Given the complex nature of developing a living drug, meeting the traditional data requirements for marketing authorisation is challenging. As a result, regulatory guidance and incentives have been continuously evolving to address the unique biomanufacturing characteristics of ATMPs, the lack of suitable animal models, and the restrictive nature of the targeted medical indications. For instance, CAR T-cell products aim to treat life threatening or debilitating conditions and thus qualify for multiple regulatory initiatives to accelerate their development,10 such as the priority medicines scheme (PRIME) and the orphan drug designation programme (appendix p 1). However, some doubts were cast on the completeness and strength of clinical evidence submitted to the EMA to support the marketing authorisation of these products.11–14 Furthermore, the initial negative evaluation of the products by reimbursement bodies supported the argument that authorisation decisions on these drugs were premature.11–13 Nevertheless, the EMA tries to strike a balance between timely market availability, patient safety, and postmarketing knowledge gains, by subjecting such products to more stringent postauthorisation measures.
Since their approval in 2018, tisagenlecleucel and axicabtagene ciloleucel are subject to additional monitoring, and their developers are obligated to supplement the safety and efficacy evidence by conducting postauthorisation studies and close follow-up of treated patients for an extended period (between 5 years and 15 years).15–17 Understanding the added clinical value of these products, and analysing gaps in evidence, could provide essential information and lessons for future ATMP development. Moreover, having two CAR T-cell products approved at the same time for similar indications created an unprecedented opportunity to scrutinise the ability of these different development pathways to inform clinical and regulatory decisions for orphan oncology therapies (figure 1). In this analysis, we examined the preauthorisation data packages submitted to the EMA to obtain marketing authorisation and then identified regulatory objections and concerns raised during the evaluation of both products. Finally, we present the postauthorisation evidence-generation strategies to fulfil the regulatory requirements and summarise the real-world data available on the use of these products.
Figure 1: Development timeline of axicabtagene ciloleucel and tisagenlecleucel.
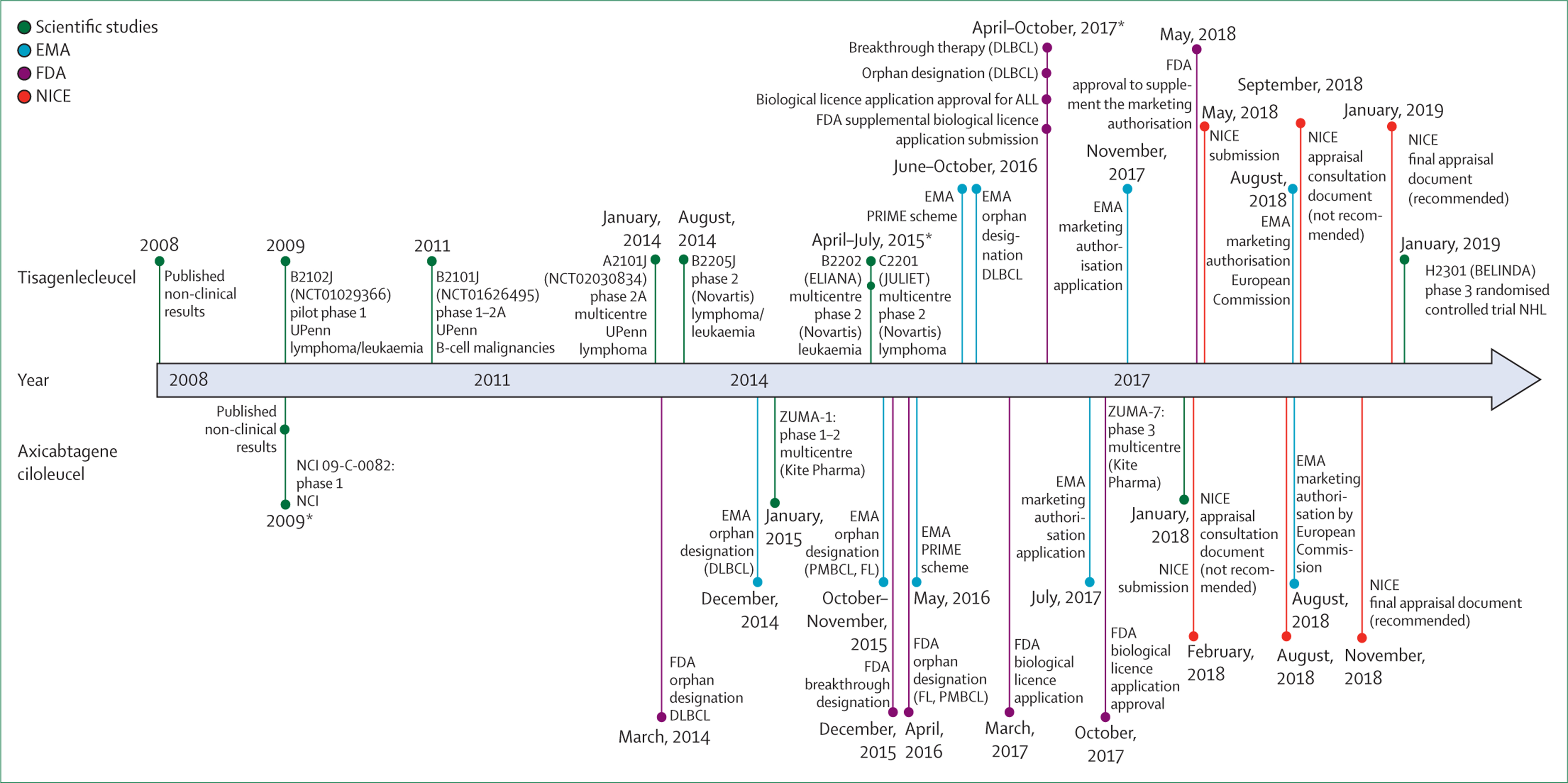
The spaces between the lines are not to scale. EMA=European Medicines Agency. FDA=US Food and Drug Administration. NICE=National Institute for Health and Care Excellence. UPenn=University of Pennsylvania. NCI=National Cancer Institute. DLBCL=diffuse large B-cell lymphoma. PRIME scheme=Priority Medicines scheme. PMBCL=primary mediastinal B-cell lymphoma. FL=follicular lymphoma.
ALL=acute lymphoblastic leukaemia. NHL=non-Hodgkin lymphoma. *Points on the same line represent the events arranged chronologically from top to bottom.
Non-clinical proof-of-concept assessment
Our analysis reveals that the majority of the regulatory concerns raised during the evaluation of axicabtagene ciloleucel and tisagenlecleucel pertained to the clinical data and product quality packages, whereas more regulatory flexibility was shown with the non-clinical data (table 1). Nevertheless, animal models provided valuable information about the pharma cokinetics, pharmacodynamics, pharmacodynamics, and some toxicological aspects of the products. Axicabtagene ciloleucel was tested by use of a CD19-expressing 38c13 mouse lymphoma cell line in an immunocom petent syngeneic lymphoma mouse model,18 whereas tisagenlecleucel treatment studies used an immunodeficient NOD/Shi-scid IL-2Rγnull human leukaemia xenograft mouse model.6
Table 1:
Major objections and concerns raised by the EMA during the evaluation of tisagenlecleucel and axicabtagene ciloleucel
| Tisagenlecleucel | Axicabtagene ciloleucel | |
|---|---|---|
| Quality aspects | ||
| Major objections | Documentation of GMP compliance | Inconsistent viral transduction |
| Other concerns | NA | No initial data on comparability and equivalence of the different processes (CLP 1.0. and CLP 2.2.); lower transduction rate in the last manufacturing process |
| Recommendations | Characterisation and testing of the viral vector, leukapheresis starting material, and the finished product | Enhancing manufacturing process and control of the product |
| Non-clinical aspects | ||
| Major objections | NA | NA |
| Other concerns | Not using both CD28 and 4–1BB as the intracellular domain in the CAR construct | NA |
| Recommendations | NA | NA |
| Clinical pharmacology | ||
| Major objections | NA | NA |
| Other concerns | No dose exposure relationship; less proliferation of the cells in patients with DLBCL than patients with ALL; high variability of cellular kinetics in the study groups | No relation between product characteristics and efficacy outcomes; no correlation between biomarkers and positive treatment outcomes |
| Recommendations | Investigate cellular kinetic parameters | NA |
| Clinical efficacy | ||
| Major objections | Absence of CD19 tumour expression as a requirement for infusion in the summary of product characteristics | NA |
| Other concerns | ALL: delayed assessment of the tumour stage after patient enrolment affects baseline characteristics; not reflecting the study population for the submitted indication; DLBCL: testing the null hypothesis of overall response at 20% against the EMA scientific advice recommendation (overall response of 40%); excluding the effect of bridging therapy in the clinical assessment by use of modified intention-to-treat analysis; long time span (54 days) from enrolment to the infusion of tisagenlecleucel due to longer than expected manufacturing time (4–5 weeks); patients dropping out of the study with poor prognostic factors due to disease progression; introducing bias to the efficacy analysis by use of the infused modified intention-to-treat population; not including stable disease and progressive disease populations in the overall survival analysis; different baseline characteristics between non-infused patients and infused patients | DLBCL: Not doing the baseline-PET scan in the prespecified time before conditioning chemotherapy; not reflecting the study population for the submitted indication; absence of comparison with SCHOLAR-1 for a worst-case scenario by excluding patients with an Eastern Cooperative Oncology Group score of 2–4 or unknown |
| Recommendations | NA | NA |
| Clinical safety | ||
| Major objections | NA | NA |
| Other concerns | Severe and life-threatening adverse effects; missing information in several patient groups | High incidence of adverse drug reactions; missing information in several patient groups |
| Recommendations | NA | NA |
GMP=good manufacturing practice. NA=not applicable. CAR=chimeric antigen receptor. DLBCL=diffuse large B-cell lymphoma. ALL=acute lymphoblastic leukaemia. EMA=European Medicines Agency.
The disadvantage of using immunocompetent mice in the axicabtagene ciloleucel studies is that these mice only support the growth of mouse lymphoma, which hampered the efficacy and safety testing of the human-derived CAR T cells. As a result, murine-derived CAR T cells were developed and tested as a surrogate model for the proposed CAR T-cell therapy. The main limitation of these cells is that their manufacturing and cellular dynamics differ from the final human CAR T-cell product. The EMA highlighted this point and accepted the animal studies as a proof-of-concept, deeming the murine model as the most appropriate for testing.15
Conversely, the immunodeficient mice used in tisagenlecleucel non-clinical studies could be injected with human acute lymphoblastic leukaemia cells, allowing for the testing of the human CAR T-cell product. However, the absence of an intact immune system in this model less accurately simulates the disease in humans than does a model using immunocompetent mice, and the safety testing of on-target–off-tumour activity and cytokine-release syndrome could not be done.19 Moreover, several CAR constructs were tested in the leukaemia model (second-generation CD28, second-generation 4–1BB, and third-generation CD28 and 4–1BB).6 Although CAR T cells with the third-generation CD28 and 4–1BB construct persisted for longer in the tumour-bearing mice, 4–1BB was the construct of choice for clinical testing, a decision that was accepted during the regulatory evaluation process.16
Notably, no lymphoma animal model was developed and tested as a proof of concept for tisagenlecleucel. The EMA flagged this observation; nevertheless, the agency found the absence of this animal model acceptable considering the available clinical experience and approved the product for this indication.16 Overall, the regulatory flexibility in accepting suboptimal non-clinical data packages for both products was evident.
Clinical investigation of CAR T-cell pharmacology
Data on axicabtagene ciloleucel pharmacology were generated by the phase 1–2 ZUMA-1 trial20 and the supportive National Cancer Institute 09-C-00082 study21 (figure 1, table 2), whereas tisagenlecleucel relied on the pivotal phase 2 ELIANA trial22 and supportive studies (Pedi CART1923 [NCT01626495] and ENSIGN22 [NCT02228096]) for the acute lymphoblastic leukaemia indication, and the JULIET study24 for the DLBCL indication (figure 1, table 2). In these trials, proliferation, distribution, and persistence of anti-CD19 CAR T cells were measured in peripheral blood and bone marrow by qPCR and flow cytometry.25,26
Table 2:
Pivotal clinical trials for CAR T-cell products and historical controls submitted in the marketing authorisation application to the EMA
| ELIANA (NCT02435849) | JULIET (NCT02445248) | ZUMA-1 (NCT02348216) | SCHOLAR-1 | |
|---|---|---|---|---|
| Treatment | Tisagenlecleucel | Tisagenlecleucel | Axicabtagene ciloleucel | Salvage chemotherapy |
| Centres in countries | 25 in 11 | 27 in 10 | 24 in 1 | NA |
| Study population | Paediatric and young adult patients with relapsed or refractory B-cell ALL | Relapsed or refractory DLBCL after two lines or more of chemotherapy and not eligible for stem cell transplantation | Relapsed or refractory DLBCL, PMLBCL, or FL after two lines or more of chemotherapy or an autologous stem cell transplantation | Refractory aggressive B-cell non-Hodgkin lymphoma (DLBCL, PMBCL, or TFL) |
| Median age, years (range) | 11 (3–23) | 59 (22–76) | 58 (23–76) | 55 (19–81) |
| Study design | Phase 2, single-arm, open-label, multicentre | Phase 2, single-arm, open-label, multicentre compared with historical data | Phase 2, single-arm, open-label, multicentre compared with historical data | Retrospective meta-analysis |
| Conditioning chemotherapy | Fludarabine (30 mg/m2, intravenous daily for four doses) and cyclophosphamide (500 mg/m2, intravenous daily for two doses); cytarabine (500 mg/m2 daily for 2 days) and etoposide (150 mg/m2 daily for 3 days) | Fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2); intravenous daily for three doses | Fludarabine (30 mg/m2) and cyclophosphamide (500 mg/m2); intravenous daily for three doses; treatment starts 5 days before infusion of the CAR T cells | NA |
| Dose | 0·2–5·0 × 106 cells per kg (for patients < 50 kg) and 0·1–2·5 × 108 cells (for patients >50 kg) | 1·0–5·0 × 108 cells single infusion | 2 × 106 (± 20%) cells per kg (minimum 1 × 106 cells per kg) | Salvage chemotherapy with an anti-CD20 monoclonal antibody such as rituximab |
| Enrolled/infused | 92/75 | 165/111 | 111/101 | 636/523 |
| Primary endpoints | ||||
| Overall response | Best overall disease response as a CR or CRi | Best overall disease response as a CR or PR | Best overall disease response as a CR or PR | .. |
| Response | .. | .. | .. | Best response as a CR or PR |
| Secondary endpoints | Overall response (CR and CRi) from US manufacturing facilities; percentage of patients with a best overall response of CR or CRi, with negative MRD, from all manufacturing facilities; percentage of patients with a best overall response of CR or CRi, with negative MRD, from US manufacturing facilities | Duration of response, overall survival, time to relapse, event-free survival, progression-free survival | Duration of response, progression-free survival, overall survival | CR and overall survival |
| Safety endpoints | Incidence of adverse events | Incidence of adverse events | Incidence of adverse events | NA |
CAR=chimeric antigen receptor. EMA=European Medicines Agency. NA=not applicable. ALL=acute lymphoblastic leukaemia. DLBCL=diffuse large B-cell lymphoma. PMLBCL=primary mediastinal large B-cell lymphoma. FL=follicular lymphoma. TFL= transformed follicular lymphoma. CR=complete response. CRi=complete response with incomplete haematological recovery. PR=partial response. MRD=minimal residual disease.
The non-compartmental analysis of tisagenlecleucel in ELIANA and supportive studies showed an initial rapid expansion of CAR T cells in acute lymphoblastic leukaemia responders, reaching the maximal expansion in peripheral blood (Cmax) after nearly 10 days (Tmax).22,23 Acute lymphoblastic leukaemia responders showed 68% more cellular expansion (Cmax) and 43% higher exposure (area under the curve for 0–28 days; AUC0–28) of tisagenlecleucel than did non-responders. The cells persisted in responders for longer than in non-responders, with the median time until the last measured concentration being 170·0 days in responders versus 28·9 days in non-responders. The pharmacokinetic properties of tisagenlecleucel in the peripheral blood have shown a direct correlation with endpoints in the trial for acute lymphoblastic leukaemia, including event-free survival for more than 3 months and overall response at day 28. Conversely, in the JULIET study,24 a correlation between the cellular kinetics of tisagenlecleucel in the peripheral blood and treatment efficacy could not be shown in patients with lymphoma as no differences in the geometric means of the Cmax or AUC0–28 were observed between responders and non-responders.
Patients with lymphoma who responded to axicabtagene ciloleucel in the ZUMA-1 trial showed a 205% higher median Cmax (43·6 cells per μL vs 21·2 cells per μL) and two times higher median AUC0–28 (7·1 days per cells per μL vs 222·0 days per cells per μL) than did non-responders. The number of cells then declined to near background amounts within 3 months, with a median of 0·4 cells per μL (range of 0–15.8 cells per μL). Unlike with tisagenlecleucel, the Cmax and AUC0–28 of axicabtagene ciloleucel directly correlated with the clinical response in patients with lymphoma (responders tended to have more cells and longer exposure). In axicabtagene ciloleucel, the robust cellular proliferation and cytokine release promoted by the CD28 signalling domain might have influenced the high response observed in lymphoma. However, previous studies reported that CD28 CAR T cells might lack durability and persistence, raising questions about the actual value of treatment, long-term efficacy, and the possible need for subsequent treatment.4,27,28 The UK National Institute for Health and Care Excellence (NICE) also raised this concern during their health technology assessment of axicabtagene ciloleucel.29
Other factors, such as disease burden and location, T-cell phenotype, T-cell subpopulations, conditioning chemotherapy, and the tumour microenvironment have also been reported to affect the cellular kinetics of CAR T cells.30,31 For instance, differences in the cellular kinetics of CAR T-cells between leukaemia and lymphoma might be attributed in part to the fact that leukaemia cells are often present in peripheral blood, whereas lymphoma cells mostly reside in lymphoid tissues. As noted, 4–1BB costimulation promotes cellular differentiation of memory cell phenotypes leading to longer persistence but weaker initial response compared to CD28 costimulation. Such characteristics of 4–1BB, coupled with the difference in microenvironment, can partially explain the observed variation in tisagenlecleucel’s cellular kinetics between lymphoma and leukaemia. These factors prompted the EMA to recommend further characterisation of the cellular kinetics of tisagenlecleucel for both indications as part of the postauthorisation measures. In their efforts to address this point, the developers of tisagenlecleucel established a mixed-effects model describing the effect of tocilizumab and corticosteroids—treatments that are used to manage cytokine-release syndrome—on cellular kinetics.32 The model can be adapted to characterise the expansion and persistence of CAR T cells across different disease indications, within various cell types, and between different costimulatory domains.32
Post-treatment outcomes and analysis of results
We further analysed the European public assessment reports for the submitted clinical data packages of both products.15,16 Tisagenlecleucel showed a clear efficacy profile in patients with acute lymphoblastic leukaemia. The results of the ELIANA study (data cutoff: April 25, 2017) showed that of 92 patients, 61 (66%) achieved an overall response and 45 (49%) a complete response using the intention-to-treat population, and of 75 patients, 61 (81%) achieved an overall response and 45 (60%) a complete response using the infused modified intention-to-treat population, with a median overall survival of 19·4 months after a median follow-up of 10·5 months.16 However, when exploring the results of lymphoma clinical trials for tisagenlecleucel, there were more noticeable differences between the intention-to-treat population versus the infused modified intention-to-treat population in the efficacy analysis of JULIET (data cutoff: Dec 8, 2017; figure 2; appendix pp 3–4). These differences also extended to the median overall survival, which was 8·2 months for the intention-to-treat analyses and 11·7 months for the modified intention-to-treat analysis.16 These differences were not seen for axicabtagene ciloleucel in the ZUMA-1 trial, where overall and complete response proportions were similar between the intention-to-treat and modified intention-to-treat analyses (figure 2), as was median overall survival (17·4 months in the intention-to-treat analysis and not reached in the modified intention-to-treat analysis [data cutoff: Aug 11, 2017]; figure 2; appendix p 4).15 The results of pivotal trials in lymphoma and leukaemia with both CAR T-cell products met the primary endpoint of best overall response in more than 20% of patients—an endpoint that was decided based on data obtained from historical studies.23,24,33
Figure 2: Unadjusted aggregated efficacy results for JULIET (tisagenlecleucel), ZUMA-1 (axicabtagene ciloleucel), SCHOLAR-1, and CORAL extension studies with different analysis populations for the treatment of DLBCL.

Error bars represent the CI. The number of patients in each study analysis group (n=165, 93, 111, 101, 523, or 278) in order of the key from top to bottom. Data cutoff for ZUMA-1: Aug 11, 2017, with a median follow-up of 15·1 months.
Data cutoff for JULIET: Dec 8, 2017, with a median follow-up of 13·9 months. DLBCL=diffuse large B-cell lymphoma.
For the tisagenlecleucel JULIET study, the EMA explored the reasons for the variability seen in the different analyses of clinical outcomes. They found that this variability in results could be attributed to the high dropout (30%), which changed the number of patients included in each analysis. This dropout resulted from a strict inclusion criterion where enrolled patients should not have had any substantial worsening of their disease status before the administration of the cellular product.16 However, the median time from enrolment to infusion was 54 days due to manufacturing delays, which led to patient deterioration and exclusion from the study.16 As such, the EMA concluded that selection bias was introduced in the modified intention-to-treat population. Additionally, 20% of patients who dropped out had a response to the bridging chemotherapy that was administered while waiting for product manufacturing (patients in the axicabtagene ciloleucel ZUMA-1 trial did not receive bridging chemotherapy). These observations have prompted the Inter-Committee Scientific Advisory Group on Oncology to advise the EMA that the evaluation of the intervention should be based on the whole treatment regimen, and not only on the infused cellular product. Taking all these points into consideration, the EMA concluded that the reliability of using the outcomes of the infused modified intention-to-treat population as efficacy estimators was not sufficient to reflect an accurate assessment of clinical benefit (table 1). As such, the EMA used the enrolled intention-to-treat population data to evaluate the differences in outcomes against the historical controls, and to conduct the benefit–risk assessment for both products.16
The role of historical controls in evaluating clinical outcomes
The assessment of treatment benefit for both tisagenlecleucel and axicabtagene ciloleucel was supplemented by comparisons with historical control groups. In the case of single-arm studies with no control arms, regulatory and health technology assessment agencies show more flexibility in allowing comparisons with historical data. Analytical tools, such as matching-adjusted indirect comparisons and network meta-analyses, have been introduced for regulatory submissions and health technology assessments.34,35 However, the choice of a suitable comparator remains challenging, and caution is needed during the interpretation and evaluation of the results.35 Novartis tried to establish a comparison for the leukaemia indication for tisagenlecleucel by pooling data from their leukaemia studies (ELIANA [NCT02435849], ENSIGN [NCT02228096] and Pedi CART19 [NCT01626495]) and matching the data to other studies of marketed therapies, such as blinatumomab; a combination of clofarabine, cyclophosphamide, and etoposide; and clofarabine monotherapy.36–40 Despite the potential bias due to small sample size, confounding patient populations, and matching on few variables, tisagenlecleucel showed consistent superiority across all the comparators, endpoints, and sensitivity analyses.16
In the lymphoma indication, tisagenlecleucel and axicabtagene ciloleucel were compared with SCHOLAR-1,41 which is a retrospective, patient-level, pooled analysis of the outcome of currently available standard of care in patients with refractory, aggressive non-Hodgkin lymphoma. The comparison of response in ZUMA-1 with SCHOLAR-1 is shown in figure 2 for the unmatched and unadjusted data. The reliability of SCHOLAR-1 as a comparator with ZUMA-1 was thoroughly assessed during health technology assessments by NICE,29 and was eventually accepted. This acceptance was attributed to the availability of individual patient data to Kite Pharma (Gilead), which was the sponsor of SCHOLAR-1, enabling the company to match patients in both trials. In the matched analysis, axicabtagene ciloleucel showed superiority over the standardised historical data, even after adjusting the populations to a stricter baseline in a worst-case scenario analysis (figure 3A).
Figure 3: Matched comparisons of results from axicabtagene ciloleucel and tisagenlecleucel pivotal clinical trials with historical comparators for the treatment of DLBCL.
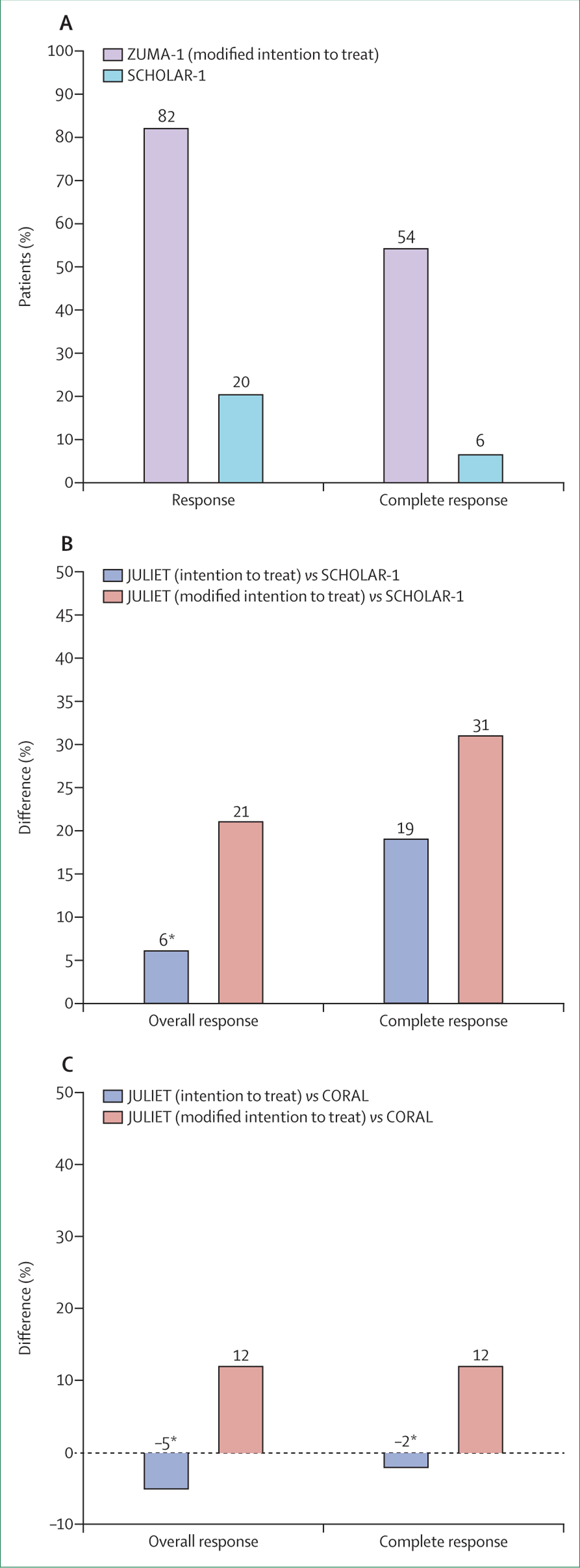
Figures are reproduced from data presented in the European public assessment reports for both products, and a published article.15,16,42 (A) Comparison of responses between ZUMA-1 and SCHOLAR-1 (data cutoff ZUMA-1: Aug 11, 2017, median follow-up 15·1 months). (B) The differences in overall response and complete response between JULIET and SCHOLAR-1 by analysis population (data cutoff: Dec 8, 2017, median follow-up 13·9 months). (C) The differences in overall response and complete response between JULIET and CORAL extension studies (data cutoff: Dec 8, 2017, median follow-up 13·9 months).
*No significant difference in responses (p>0·05).
Since only the published aggregated data of SCHOLAR-1 were available to developers of tisagenlecleucel, other historical comparators were explored. In addition to SCHOLAR-1, the pooled CORAL extension data were used for comparisons (figure 2, appendix p 4).16 The pooled CORAL extensions emerged from the main CORAL study and were considered by the EMA and NICE as a more suitable comparator than SCHOLAR-1 for evaluating tisagenlecleucel due to similarities in the populations enrolled.43 The main CORAL study44 compared salvage chemotherapy regimens followed by stem cell transplantation, whereas the pooled extension studies followed up patients who did not proceed to stem cell transplantation, or had a second relapse after transplantation.45,46 Novartis used matching-adjusted indirect comparisons to match the individual patients from JULIET to both historical controls. When running the matched analysis with the modified intention-to-treat population, tisagenlecleucel showed a significant difference in overall response and complete response compared with that in both the pooled CORAL extensions and SCHOLAR-1 (figure 3B, C). However, when analysing the intention-to-treat population, the product did not show a significant difference in overall response when compared with the pooled CORAL extensions and SCHOLAR-1 studies (figure 3B, C). Nevertheless, tisagenlecleucel showed a significantly longer median overall survival (10·6 months for intention to treat and 16·3 months for modified intention to treat) compared with the pooled CORAL results where the median overall survival was 5·8 months.
Due to the aforementioned inconsistencies in efficacy analysis for the different populations, 12 members of two EMA committees involved in the evaluation process disagreed with granting authorisation for tisagenlecleucel in the lymphoma indication. Eventually, the product was authorised in lymphoma by taking into account the higher response durability in tisagenlecleucel compared with the controls. Nevertheless, Novartis was mandated to do extensive postauthorisation efficacy studies in the form of data collection on treated patients in dedicated registries and an interventional phase 3, randomised, controlled trial of tisagenlecleucel versus platinum-based immunochemotherapy (BELINDA; NCT03570892; table 3). BELINDA began enrolment in May, 2019, with a target enrolment of 318 patients across the USA, Australia, Germany, Japan, and Spain.
TABLE 3.
Postauthorization studies for tisagenlecleucel and axicabtagene ciloleucel based on the submitted risk management plan
| Indication | Primary objective | Obligatory by EMA | Study type | Phase | Control | Randomised | Start date | Number of Patients | Current status | |
|---|---|---|---|---|---|---|---|---|---|---|
| Tisagenlecleucel | ||||||||||
| Stein et al (2019)32 | ALL or DLBCL | Cellular kinetic parameters and the effect of CRS medications | No | Experimental | NA | NA | NA | NA | NA | Published mixed-effects model analysing the effects of CRS medications on cellular kinetics |
| CCTL019B240147 | ALL | Evaluate the efficacy in patients with ALL younger than 3 years | Yes | Observational; registry-based | Phase 4 | NA | NA | Q4, 2018 | NA | Data from EBMT and CIBMTR registries will be used for the observational study; February, 2019: statistical plan for the study submitted to the committee for advanced therapies |
| ELIANA48 (NCT02435849, CCTL019B2202) | ALL | Long-term efficacy and safety of tisagenlecleucel in the ELIANA study | No | Follow-up | Phase 2 multicentre | No | No | April, 2015 | 97 enrolled, 79 infused at last data cutoff | Official 24-month report of ELIANA (expected Q4, 2019); last published results: April, 2018, data cutoff; 24-month median follow-up; median duration of response not reached; median overall survival not reached; 66% overall survival (modified intention to treat; 24 months) |
| CCTL019B240147 | DLBCL | Evaluate efficacy outcome measures, including the manufacturing time | Yes | Observational; registry-based | Phase 4 | No | No | Q4, 2018 | NA | February, 2019: statistical plan for the study submitted to the committee for advanced therapies |
| JULIET49 (NCT02445248, CCTL019C2201) | DLBCL | Long-term efficacy and safety of tisagenlecleucel in the JULIET study | Yes | Follow-up | Phase 2 multicentre | No | No | July, 2015 | 167 enrolled, 115 infused | Official 24-month report of JULIET (expected in September, 2019); last published results: May, 2018, data cutoff; 19-month median follow-up; median duration of response not reached; median overall survival of 11–1 months for infused patients; 43% overall survival at 18 months |
| BELINDA (CCTL019H2301, NCT03570892) | DLBCL | Efficacy of tisagenlecleucel vs standard of care in adult patients with refractory or relapsed NHL | Yes | Interventional | Phase 3 multicentre | Yes (active comparator) | Yes | May, 2019 | 318 (estimated) | Recruiting; primary endpoint: event-free survival |
| CCTL019B240147 | ALL or DLBCL | Long-term safety of tisagenlecleucel in patients with ALL and DLBCL based on disease registry | Yes | Observational; registry-based | Phase 4 | NA | NA | Q4, 2018 | NA | February, 2019: statistical plan for the study submitted to the committee for advanced therapies |
| (NCT02445222, CCTL019A2205B)16 | ALL or DLBCL | Long-term follow-up of patients exposed to lentiviral-based CD19 directed CAR T-cell therapy | Yes | Observational; registry-based | Phase 4 | NA | NA | Nov, 2015 | 620 (estimated) | Follow-up of all the patients who have been infused with tisagenlecleucel for 15 years; annual safety reports and 5-yearly interim reports will be submitted to the EMA; final report of study results in December, 2038 |
| Axicabtagene ciloleucel | ||||||||||
| ZUMA-150 (NCT02348216) | DLBCL, PMLBCL, or FL | Long-term efficacy and safety of axicabtagene ciloleucel in the ZUMA-1 study | No | Follow-up | Phase 2 multicentre | No | No | January, 2015 | 111 enrolled, 101 infused | EMA 24-month result update based on intention-to-treat (n=111); 68% overall response; 50% CR; median duration of response not reached; median overall survival of 17–4 months; 48% 24-month overall survival |
| Non-interventional registry study15 | DLBCL | Long-term safety of axicabtagene ciloleucel in the postmarketing setting | Yes | Observational; registry-based | Phase 4 | NA | NA | NA | NA | Planned |
| ZUMA-2 (NCT02601313) | MCL | Efficacy of axicabtagene ciloleucel in patients with refractory or relapsed MCL | No | Interventional | Phase 2 multicentre | No | No | November, 2015 | 105 | Active; expected primary completion date in July, 2019; primary endpoint: overall response |
| ZUMA-351 (NCT02614066) | ALL | Safety and efficacy of axicabtagene ciloleucel in adult participants with refractory or relapsed ALL | No | Interventional | Phase 1–2 multicentre | No | No | March, 2016 | 100 (estimated) | Recruiting; expected primary completion date in January, 2020; end of phase 1 results: September, 2018, data cutoff; 45 infused patients; 41 evaluable patients; 16-month median follow-up; 68% overall response (CR + CRi); 73% minimal residual disease negative; no DLT |
| ZUMA-452 (NCT02625480) | ALL | Safety and efficacy of axicabtagene ciloleucel in paediatric and adult participants with refractory or relapsed ALL | No | Interventional | Phase 1–2 multicentre | No | No | February, 2016 | 100 | Recruiting; expected primary completion date in July, 2021; end of phase 1 results October, 2018, data cutoff; 24 infused patients; 13-month median follow-up; overall response of 100% (2 × 106), 64% (1 × 106; 68 mL), and 71% (1 × 106; 40 mL) in three dose groups |
| ZUMA-5 (NCT03105336) | NHL | Safety and efficacy of axicabtagene ciloleucel in patients with indolent refractory or relapsed indolent NHL | No | Interventional | Phase 2 multicentre | No | No | June, 2017 | 160 (estimated) | Recruiting; expected primary completion date in March, 2020; primary endpoint: overall response |
| ZUMA-653 (NCT02926833) | DLBCL | Safety and efficacy of axicabtagene ciloleucel in combination with atezolizumab in adults with refractory or relapsed DLBCL | No | Interventional | Phase 1–2 multicentre | No | No | September, 2016 | 37 (estimated) | Active; end of phase 1 results: January, 2018, cutoff; 12 infused patients; 4–4 median follow-up; dose-limiting toxicity in 1 patient; all patients had at least one adverse effect (92%, grade ≥3); overall response in 9 (90%) of 10 evaluable patients |
| ZUMA-7 (NCT03391466) | DLBCL | Efficacy of axicabtagene ciloleucel against the standard of care in relapsed or refractory DLBCL | No | Interventional | Phase 3 multicentre | Yes | Yes | December, 2017 | 350 (estimated) | Recruiting; 71 study locations (Europe, North America, Australia, Israel); primary endpoint: event-free survival; secondary endpoints: overall response, overall survival, progression-free survival, duration of response |
EMA=European Medicines Agency. ALL=acute lymphoblastic leukaemia. DLBCL=diffuse large B-cell lymphoma. CRS=cytokine release syndrome. NA=not applicable. Q4=fourth quarter of the year (October, November, and December). EBMT=European Society for Blood and Marrow Transplantation. CIBMTR=Center for International Blood and Marrow Transplant Research. NHL=non-Hodgkin lymphoma. CAR=chimeric antigen receptor. PMLBCL=primary mediastinal large B-cell lymphoma. FL=follicular lymphoma. CR=complete response. MCL=mantle cell lymphoma. CRi=complete response with incomplete haematological recovery. DLT=dose limiting toxicity.
Associated risks and measures to ensure patient safety
Both tisagenlecleucel and axicabtagene ciloleucel used integrating viral vectors (appendix p 2), which might raise the concern of insertional oncogenesis due to semi-random integration patterns. Lentivectors used in tisagenlecleucel are considered safer than γ-retroviral vectors used in axicabtagene ciloleucel, as their integration patterns do not favour transcriptional start sites.54 However, mature T cells are resistant to malignant transformation after transduction with an integrating viral vector,55 which was Kite Pharma’s (Gilead) justification for using a γ-retroviral vector.15 Notably, axicabtagene ciloleucel received advice from the EMA in the form of early discussions on the risks of insertional mutagenesis under the PRIME scheme. Another concern of the use of viral vectors is the generation of a replication-competent virus.56 The risk of replication-competent virus formation was considered low by the EMA as both vectors are replication incompetent and stringently tested for the absence of replication-competent virus.15,16 Studies have shown that the risk of formation of replication-competent virus either by a lentiviral or retroviral vector is very low.57 As a result, the US Food and Drug Administration is revising the regulations on testing for replication-competent virus, which might result in a reduction of follow-up testing in the case of vectors where there is substantial experience with safety.58 Nevertheless, to ensure patient safety and accumulate more data about the products, the EMA requires postauthorisation safety studies where data from patients treated with these products must be collected for a period of up to 15 years to assess the long-term safety of both vector types as part of the risk minimisation plan (table 3).
During clinical testing, all patients infused with either of the two products had adverse events (appendix p 5). Serious adverse events were mainly attributed to cytokine-release syndrome and neurological complications. Other frequent serious adverse events were infections, tumour lysis syndrome, and febrile neutropenia. Axicabtagene ciloleucel showed a higher incidence of cytokine-release syndrome and neurological events than did tisagenlecleucel (appendix p 5), and these events were associated with higher concentrations of cytokines and a higher maximum number of axicabtagene ciloleucel cells in the blood (Cmax).33 The clinical management plan for adverse events in both studies was seen as sufficient by the EMA. For instance, CAR T-cell therapies were to be provided only in qualified centres that also had available tocilizumab as a treatment for cytokine-release syndrome. Additionally, the clinical trial sponsors had to offer an educational programme for each participating centre that was targeted towards centre personnel and patients. As part of the postauthorisation measures, each applicant had to collect postauthorisation safety data in dedicated registries. For tisagenlecleucel, the data were collected through the European Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research registries.59 As part of the ongoing effort, the EMA released the proposed data elements that should be fulfilled by the registries to capture all the necessary information on the safety and efficacy of CAR T-cell products.60
Complex logistics and regulatory considerations
Although clear clinical benefits were obtained from clinical trials investigating tisagenlecleucel and axicabtagene ciloleucel, issues pertaining to manufacturing and supply chain management should be highlighted. For instance, the locations of the studies might have influenced the outcomes of both treatments and their evaluation by the EMA. ZUMA-1 was done in the USA, except for one patient, who was treated in Israel (table 2). Due to the absence of European patients in ZUMA-1, the developer was advised, under the PRIME scheme, to include European patients in the planned phase 3 trial (ZUMA-7, NCT03391466).15 Conversely, JULIET was done at 27 sites in ten countries across four continents. Even though clinical, collection, and infusion sites were global, tisagenlecleucel for JULIET was mainly manufactured in the USA, with some manufacturing in Germany. This restricted capacity of the manufacturing might have posed a challenge to the product supply chain and manufacturing coordination, and prolonged the time from enrolment to infusion in the JULIET study. As a result, details on tisagenlecleucel manufacturing turnaround time was required by the EMA as part of the postauthorisation efficacy studies.16
Postmarketing performance of CAR T-cell products
The up-to-date clinical follow-up shows that both tisagenlecleucel and axicabtagene ciloleucel elicit a durable response in the approved leukaemia and lymphoma indications (table 3). In patients with leukaemia, the median duration of response and overall survival were not reached at a median follow-up of 24 months in the ELIANA study.48 In patients with lymphoma, the last update from the tisagenlecleucel JULIET trial showed a median overall survival of 11·1 months, and the median duration of response was not reached (table 3).49 The 24-month results of the axicabtagene ciloleucel ZUMA-1 study showed a median overall survival of 17·4 months, and the median duration of response was not reached.15
Two postmarketing real-world studies were published evaluating patients with non-Hodgkin lymphoma that were treated with standard-of-care axicabtagene ciloleucel in the USA.61,62 Nastoupil and colleagues61 reported results of 295 patients treated as of August, 2018, at 17 academic USA centres. 240 of 274 patients had cytokine-release syndrome, of which 18 individuals were grade 3 or worse, and 85 patients had grade 3 or worse neurological complications.63 Overall response was seen in 81% of patients after a median follow-up of 3·9 months.61,64 Jacobson and colleagues62 reported a lower overall response in 67 (71%) of 95 patients infused with axicabtagene ciloleucel, after a median follow-up of 5·6 months.62,65,66 95% of the patients had cytokine-release syndrome, of which 17 (16%) patients were grade 3 or worse, whereas neurological complications were reported in 29 (38%) of the treated patients.65 These real-world experiences extend earlier clinical evidence generated from investigational trials. Further real-world safety and efficacy data on the use of axicabtagene ciloleucel in the USA is expected through the expanded access trial, ZUMA-9 (NCT03153462).
In September, 2019, the European Society for Blood and Marrow Transplantation reported that 155 patients treated with either commercial (80%) or investigational (20%) CAR T cells in 40 centres across nine countries in Europe were registered in their registry.67 Individual clinical reports on patients receiving CAR T cells in different European countries have also been released. In Germany, of 23 patients who underwent leukapheresis, 20 patients with acute lymphoblastic leukaemia were given tisagenlecleucel, while the remaining 3 patients could not be treated as the manufactured products did not meet the prespecified release criteria.68 Of these patients, nine (45%) were in remission at the last follow-up visit. The study reported that at a median follow-up of 11 months, the overall survival was 69% and event-free survival was 65%. 17 (74%) of the 23 enrolled patients received tisagenlecleucel either through the expanded access programme (n=6) or as a commercial product (n=11).68 Grade 4 cytokine-release syndrome was reported in three patients. In Spain, a report released in January, 2019, showed that seven hospitals had treated 84 patients with CAR T cells, out of which only six patients received the product in commercial settings, with the remaining treated in clinical trials.69 In France, 60 patients with DLBCL were treated with either tisagenlecleucel (n=30) or axicabtagene ciloleucel (n=30) across five centres between April, 2018, and February, 2019, under the temporary authorisation for use programme.70 Although the actual numbers of treated patients are yet to be disclosed, the uptake of this treatment in Europe has been steady but smaller compared with the USA.
To investigate the activities of specialised treatment centres in adopting CAR T-cell therapies in Europe, a survey study was done between November, 2018, and January, 2019. 566 European Society for Blood and Marrow Transplantation centres were surveyed, of which 134 centres across 22 countries responded.69 The study showed that 34 centres have already administered CAR T cells to patients, primarily within clinical trials (93% of patients). Furthermore, 57 additional centres located in Europe were planning to administer a CAR T-cell product within the 6 months following the study.69 In the UK, patients of the National Health Service with acute lymphoblastic leukaemia (children, adolescents, and young adults [up to 25 years old]) can receive CAR T-cell therapy in nine centres and adult patients with DLBCL can receive CAR T-cell products in seven centres, with more centres planning to enrol patients in the future.71 Although the data indicate a limited number of centres currently available in Europe for commercial CAR T-cell treatments, they also reflect a strong willingness toward the adoption of the therapy.
Ongoing investigations of authorised products in other oncology indications
Tisagenlecleucel and axicabtagene ciloleucel are being investigated for other indications and treatment strategies. A phase 3 trial (OBERON, NCT03628053) is expected to start in late 2019 to further test the efficacy and safety of tisagenlecleucel for the treatment of acute lymphoblastic leukaemia compared with bispecific (blinatumomab) and monoclonal (inotuzumab ozogamicin) antibody-based therapies. Tisagenlecleucel is also being investigated as a treatment for high-risk paediatric acute lymphoblastic leukaemia (positive minimal residual disease at the end of consolidation) in a phase 2 trial (CASSIOPEIA, NCT03876769). Concurrently, axicabtagene ciloleucel is expanding into chronic lymphocytic leukaemia (ZUMA-8, NCT03624036) and acute lymphoblastic leukaemia indications (ZUMA-3, NCT02614066; ZUMA-4, NCT02625480).51,52 Preliminary results from phase 1 trials were promising,51,52 and axicabtagene ciloleucel has moved on to phase 2 testing for the treatment of these two conditions (table 3).
In lymphoma, tisagenlecleucel is being tested in combination with pembrolizumab, a PD-1 inhibitor, and with ibrutinib, a BTK inhibitor, in patients with DLBCL (NCT03630159, NCT03876028). The product is also being tested for the treatment of paediatric non-Hodgkin lymphoma (NCT03610724) and relapsed or refractory follicular lymphoma (NCT03568461). In large B-cell lymphoma, axicabtagene ciloleucel is being tested in combination with various anticancer drugs: a PD-1 inhibitor, atezolizumab, with promising results (ZUMA-6, NCT02926833), a 4–1BB agonist (utomilumab; ZUMA-11, NCT03704298), and rituximab or lenalidomide (ZUMA-14, NCT04002401). The developer is also testing axicabtagene ciloleucel as a first-line treatment in high-risk large B-cell lymphoma (ZUMA-12, NCT03761056), and as a treatment for mantle cell lymphoma and indolent non-Hodgkin lymphoma. Data generated from these axicabtagene ciloleucel studies will support the pharmacovigilance plan of this product in Europe.
Conclusion
The two approved CAR T-cell products, tisagenlecleucel and axicabtagene ciloleucel, provided a unique opportunity to explore the effect of choices made by developers during product development on the regulatory evaluation processes. Due to the still undetermined long-term benefits and high price tag, the products face tremendous pressure to have proven long-lasting clinical benefits, particularly when compared with other established treatment options in the market that are more cost-effective, such as haemopoietic stem cell transplantation. The clinical efficacy of the products was identified as the most challenging aspect during development because of the nature of the disease under study, the single-arm study designs, the complex treatment regimens, and the absence of suitable comparators. Both developers were able to implement effective measures to partially mitigate serious adverse events during clinical testing. Further measures were mandated by the regulators in the postmarketing setting to ensure patient safety. The products are being tested for various indications, and more data will further inform their benefit–risk profile. Our analysis suggests that regulatory authorities tend to accept more uncertainty in the evidence generated for CAR T-cell therapies at the time of marketing authorisation submissions compared with small molecules and conventional biologics. Of note, the outlined hurdles and challenges faced by these two products should not discourage more developers from pursuing CAR T-cell therapy development, nor are they intended to call for stricter regulatory assessments. This analysis of the development experiences and regulatory approval processes provide a roadmap to improve the generation of evidence and dossiers for future CAR T-cell therapies, and their integration into routine clinical practice.
Supplementary Material
Search strategy and selection criteria.
We obtained the European public assessment reports from the database of the European Medicines Agency website (accessed April 11, 2019). We extracted the manufacturing and product quality, and non-clinical and clinical data into a spreadsheet. When needed, the relevant scientific literature mentioned in the European public assessment reports was also reviewed. Revision of the clinical data packages in the European public assessment reports relied on datasets with the longest possible follow-up time: 12-month update of the ZUMA-1 clinical study of axicabtagene ciloleucel in lymphoma (data cutoff: Aug 11, 2017), the JULIET clinical study of tisagenlecleucel in lymphoma (data cutoff: Dec 8, 2017), and the ELIANA study of tisagenlecleucel in leukaemia (data cutoff: April 25, 2017). To control for investigator bias, we relied on the results reported by the central independent review committee, rather than the results stated by the investigators. Regarding historical comparators, the SCHOLAR-1 study outcomes used as a comparator for both products were extracted from the axicabtagene ciloleucel European public assessment reports. Outcomes of the pooled CORAL extension studies used as a comparator for tisagenlecleucel were not detailed in the European public assessment reports. To reproduce the pooled analysis of the studies, we extracted the data published in scientific literature that were referenced in the European public assessment reports. The population of the pooled studies comprised patients that had relapsed after a second stem cell transplantation (n=75) and patients who did not proceed to stem cell transplantation (n=203). The responses achieved by patients in the clinical studies and historical comparators were then reproduced with the extracted patient numbers. Postauthorisation studies submitted as additional pharmacovigilance activities were extracted from the risk management plan section in the European public assessment reports for each product. To collect the latest published results of these studies, we searched ClinicalTrials.gov, PubMed, Google, the agendas, minutes, and reports of the Committee for Advanced Therapies using the developers and the ClinicalTrials.gov identifiers of the studies (data cutoff: July, 2019).
Acknowledgments
ME received funding from the Arab-German Young Academy of Sciences and Humanities–a project of the Berlin-Brandenburg Academy of Sciences and Humanities–and the Federal Ministry of Education and Research. ASW was supported, in part, by award number P30CA014089 from the National Cancer Institute.
Declaration of interests
BLL reports grants and personal fees from Novartis, during the conduct of the work; personal fees from Novartis, Avectas, Brammer Bio, Incysus, CRC Oncology/Cure Genetics, Novartis, Vycellix, Immuneel, and Ori Biotechand, and equity in Tmunity Therapeutics of which he is a cofounder, outside the submitted work. He has patent methods for the treatment of cancer (US 8906682, US 8916381, US 9101584), patent compositions for the treatment of cancer (US 8911993, US 9102761, US 9102760), a patent method for treating chronic lymphocytic leukaemia (US 9161971), patent compositions and methods for the treatment of cancer (US 9464140, US 9518123, US 9481728, US 9540445), a patent use of CAR-modified T cells to treat cancer (US 9328156, US 9499629), and patent method for assessing the suitability of transduced T cells for administration (US 9572836), with all royalties paid to the University of Pennsylvania. ASW reports advisory board membership and consultation fees from Servier, and grants and consultation fees from Kite Pharma, during the conduct of the work; consultation fees from AbbVie, and grants and consultation fees from Spectrum Pharmaceuticals, outside the submitted work. ME and MA declare no competing interests.
Footnotes
See Online for appendix
References
- 1.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018; 379: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res 2017; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol 2015; 36: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salter AI, Ivey RG, Kennedy JJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal 2018; 11: eaat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 2018; 53: 164–81. [DOI] [PubMed] [Google Scholar]
- 6.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009; 17: 1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359: 1361–65. [DOI] [PubMed] [Google Scholar]
- 8.Grigor EJM, Fergusson D, Kekre N, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev 2019; 33: 98–110. [DOI] [PubMed] [Google Scholar]
- 9.Elsanhoury A, Sanzenbacher R, Reinke P, Abou-El-Enein M. Accelerating patients’ access to advanced therapies in the EU. Mol Ther Methods Clin Dev 2017; 7: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU Market. Cell Stem Cell 2016; 19: 293–97. [DOI] [PubMed] [Google Scholar]
- 11.The Lancet Oncology. CAR T-cell therapy: perceived need versus actual evidence. Lancet Oncol 2018; 19: 1259. [DOI] [PubMed] [Google Scholar]
- 12.The Lancet Oncology. Calling time on the immunotherapy gold rush. Lancet Oncol 2017; 18: 981. [DOI] [PubMed] [Google Scholar]
- 13.Abou-El-Enein M, Hey SP Cell and gene therapy trials: are we facing an ‘evidence crisis’? EClinicalMedicine 2019; 7: 13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulthess D, Gassull D, Makady A, et al. Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants. BMJ Evid Based Med 2019; pii:bmjebm-2019–111226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EMA. European public assessment report, Yescarta (EMA/481168/2018) Committee for Medicinal Products for Human Use. Amsterdam: European Medicines Agency, 2018. [Google Scholar]
- 16.EMA. European public assessment report, Kymriah (EMA/485563/2018) Committee for Medicinal Products for Human Use. Amsterdam: European Medicines Agency, 2018. [Google Scholar]
- 17.EMA. Guideline on follow-up of patients administered with gene therapy medicinal products (EMEA/CHMP/GTWP/60436/2007) Committee for Medicinal Products for Human Use. Amsterdam: European Medicines Agency, 2009. [Google Scholar]
- 18.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 2010; 116: 3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theocharides AP, Rongvaux A, Fritsch K, Flavell RA, Manz MG. Humanized hemato-lymphoid system mice. Haematologica 2016; 101: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33: 540–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller KT, Waldron E, Grupp SA, et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res 2018; 24: 6175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 2017; 130: 2317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert JA. CAR T-cells for relapsed B-cell ALL in adults. Lancet Oncol 2018; 19: e143. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378: 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NICE. Single technology appraisal: axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma after 2 or more systemic therapies [ID 1115]. London: National Institute for Health and Care Excellence, 2018. [Google Scholar]
- 30.Milone MC, Bhoj VG. The pharmacology of T cell therapies. Mol Ther Methods Clin Dev 2018; 8: 210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beatty GL, Moon EK. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology 2014; 3: e970027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein AM, Grupp SA, Levine JE, et al. Tisagenlecleucel model-based cellular kinetic analysis of chimeric antigen receptor-T cells. CPT Pharmacometrics Syst Pharmacol 2019; 8: 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US FDA. Meta-analyses of randomized controlled clinical trials to evaluate the safety of human drugs or biological products (draft guidance document). White Oak, MD: US Food and Drug Administration, 2018. [Google Scholar]
- 35.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making 2018; 38: 200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 2016; 34: 4381–89. [DOI] [PubMed] [Google Scholar]
- 37.von Stackelberg A, Völzke E, Kühl JS, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM study group. Eur J Cancer 2011; 47: 90–97. [DOI] [PubMed] [Google Scholar]
- 38.Locatelli F, Testi AM, Bernardo ME, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol 2009; 147: 371–78. [DOI] [PubMed] [Google Scholar]
- 39.Miano M, Pistorio A, Putti MC, et al. Clofarabine, cyclophosphamide and etoposide for the treatment of relapsed or resistant acute leukemia in pediatric patients. Leuk Lymphoma 2012; 53: 1693–98. [DOI] [PubMed] [Google Scholar]
- 40.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 2011; 118: 6043–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2019; 130: 1800–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neelapu S, Locke F, Bartlett N, et al. SCHOLAR-1 versus ZUMA-1: a standardized comparison of outcomes in patients (pts) with refractory, aggressive non-hodgkin lymphoma (rNHL). Clin Lymphoma Myeloma Leuk 2017; 17: S362–63. [Google Scholar]
- 43.NICE. Single technology appraisal: tisagenlecleucel-T for treating relapsed or refractory diffuse large B-cell lymphoma [ID1166]. London: National Institute for Health and Care Excellence, 2018. [Google Scholar]
- 44.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 2016; 51: 51–57. [DOI] [PubMed] [Google Scholar]
- 46.Van Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant 2017; 52: 216–21. [DOI] [PubMed] [Google Scholar]
- 47.EMA. Committee for advanced therapies: agenda for the meeting on 20–22 February 2019. Amsterdam: European Medicines Agency, 2019. [Google Scholar]
- 48.Grupp SA, Maude SL, Rives S, et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood 2018; 132: 895. [Google Scholar]
- 49.Schuster SJ, Bishop MR, Tam C, et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: an updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel. Blood 2018; 132: 1684. [Google Scholar]
- 50.EMA. Yescarata summary of product characteristics. Amsterdam: European Medicines Agency, 2018. [Google Scholar]
- 51.Shah BD, Bishop MR, Oluwole OO, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). J Clin Oncol 2019; 37: 7006. [Google Scholar]
- 52.Wayne AS, Huynh V, Hijiya N, et al. Phase 1 results of ZUMA-4: KTE-x19, an anti-CD19 chimeric antigen receptor t cell therapy, in pediatric and adolescent patients with relapsed/refractory B cell acute lymphoblastic leukemia. HemaSphere 2019; 3: 433. [Google Scholar]
- 53.Jacobson CA, Locke FL, Miklos DB, et al. End of phase 1 results from Zuma-6: axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Biol Blood Marrow Transplant 2019; 25: S173. [Google Scholar]
- 54.Modlich U, Navarro S, Zychlinski D, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther 2009; 17: 1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugman MH, Fehse B, Baum C, et al. Resistance of mature T cells to oncogene transformation. Blood 2008; 112: 2278–86. [DOI] [PubMed] [Google Scholar]
- 56.EMA. Committee for advanced therapies: guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products. Amsterdam: European Medicines Agency, 2018. [Google Scholar]
- 57.Marcucci KT, Jadlowsky JK, Hwang WT, et al. Retroviral and lentiviral safety analysis of gene-modified T cell products and infused HIV and oncology patients. Mol Ther 2018; 26: 269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.US FDA. Testing of retroviral vector-based 2 human gene therapy products for replication competent retrovirus during product manufacture and patient follow-up (draft guidance document). White Oak, MD: US Food and Drug Administration, 2018. [Google Scholar]
- 59.Abou-El-Enein M, Grainger DW, Kili S. Registry contributions to strengthen cell and gene therapeutic evidence. Mol Ther 2018; 26: 1172–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.EMA. Report on CAR T-cell therapy registries workshop. Amsterdam: European Medicines Agency, 2018. [Google Scholar]
- 61.Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (Axi-cel) CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world experience. Blood 2018; 132: 91. [Google Scholar]
- 62.Jacobson CA, Hunter B, Armand P, et al. Axicabtagene ciloleucel in the real world: outcomes and predictors of response, resistance and toxicity. Blood 2018; 132: 92. [Google Scholar]
- 63.Nastoupil LJ. CAR T-cell therapy for NHL: current and future directions. Southern California Blood Cancer Conference; Anaheim, CA, USA; March 2, 2019. [Google Scholar]
- 64.Bradley S. Real-world efficacy and safety outcomes with axi-cel in patients with relapsed/refractory large B-cell lymphoma comparable to the ZUMA-1 clinical trial. LymphomaHub, 2018. https://lymphomahub.com/medical-information/real-world-efficacy-and-safety-outcomes-with-axicabtagene-ciloleucel-in-patients-with-relapsed-refractory-large-b-cell-lymphoma-comparable-to-the-zuma-1-clinical-trial (accessed Oct 9, 2019). [Google Scholar]
- 65.Oncology Learning Network. Real-world results support axi-cel use in B-cell lymphomas outside of clinical trials. 2018. https://www.oncnet.com/news/real-world-results-support-axi-cel-use-b-cell-lymphomas-outside-clinical-trials (accessed Nov 6, 2019).
- 66.Dangi-Garimella S. Real-world evidence with axicabtagene ciloleucel CAR T treatment similar to ZUMA-1 trial findings. AJMC, 2018. https://www.ajmc.com/conferences/ash-2018/realworld-evidence-with-axicabtagene-ciloleucel-car-t-treatment-similar-to-zuma1-trial-findings (accessed Nov 6, 2019). [Google Scholar]
- 67.European Society for Blood and Marrow Transplantation. EBMT centre members and national registries are requested to document CAR T cell therapies. 2019. https://www.ebmt.org/ebmt/news/ebmt-centre-members-and-national-registries-are-requested-document-car-t-cell-therapies (accessed Oct 9, 2019).
- 68.Bader P. Real life experience in the treatment of pediatric, adolescent and young adult ALL patients using commercially available CAR-T cells. 1st European CAR T cell meeting; Paris, France; Feb 14–16, 2019. [Google Scholar]
- 69.Urbano-Ispizua Á Current status of CAR-T cell development in Europe and elsewhere. 1st European CAR T cell meeting report. 1st European CAR T cell meeting; Paris, France; Feb 14–16, 2019. [Google Scholar]
- 70.Thieblemont C, Le Gouill S, Di Blasi R, et al. Real-world results on CD19 CAR T-cell for 60 french patients with relapsed/refractory diffuse large B-cell lymphoma included in a temporary authorization for use program. Hematol Oncol 2019; 37: 301. [Google Scholar]
- 71.NHS. CAR-T therapy. https://www.england.nhs.uk/cancer/cdf/car-t-therapy/ (accessed Oct 8, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


