SUMMARY
The superior colliculus is a conserved sensorimotor structure that integrates visual and other sensory information to drive reflexive behaviors. Although the evidence for this is strong and compelling, a number of experiments reveal a role for the superior colliculus in behaviors usually associated with the cerebral cortex, such as attention and decision-making. Indeed, in addition to collicular outputs targeting brainstem regions controlling movements, the superior colliculus also has ascending projections linking it to forebrain structures including the basal ganglia and amygdala, highlighting the fact that the superior colliculus, with its vast inputs and outputs, can influence processing throughout the neuraxis. Today, modern molecular and genetic methods combined with sophisticated behavioral assessments have the potential to make significant breakthroughs in our understanding of the evolution and conservation of neuronal cell types and circuits in the superior colliculus that give rise to simple and complex behaviors.
Keywords: optic tectum, attention, decision-making, defensive behaviors, escape behaviors, prey capture, orienting, avoidance, lamination, neural cartography, sensory maps, motor maps, evolution, adaptation, conservation, vision, action
IN BRIEF
Basso, Bickford and Cang provide a new look at the superior colliculus. Focusing on classic and recent work, the review highlights how the colliculus is positioned to affect much of the neuraxis and plays key roles in both simple and complex behaviors.
INTRODUCTION
The tectum (roof in Latin) lies on the dorsal surface of the midbrain. Our knowledge of the anatomy, physiology and function of the tectum stems from work performed in all classes of vertebrates: from the primitive jawless fish, the lamprey, to bony and cartilagenous fish, amphibians, reptiles, birds, and mammals, including primates (Figure 1). The tectum is referred to as the superior colliculus (SC) in mammals, and the optic tectum (OT) in non-mammals. In this review, we shall use SC as a general term unless specifically referring to non-mammals. The SC has been studied for over 100 years (Ramón y Cajal, 1909, 1995). During this time, the SC became and remains one of the most well studied structures in the brain. Based on extensive fundamental research, we now understand the SC to be a system integral for encoding spatial locations and transforming them into stimulus-directed orienting and approach behaviors. The SC also plays a role in avoidance behaviors and, more recently revealed, in higher cognitive functions such as visual spatial attention and decision-making (Basso and May, 2017). Much of our knowledge of the SC however, remains at the level of layers and regions rather than neuronal cell types and circuits. With the explosion of molecular and genetic methods, new results are appearing and we are well poised to answer long-standing questions regarding neuronal types and circuits across species that will shed light on critical comparative and evolutionary questions. We also now have a rich opportunity to address how neurons and circuits relate to behaviors such as orienting, escape and freezing, and even more complex behaviors such as attention and decision-making. Moreover, we can begin asking whether similar behaviors across different species are mediated by homologous or different neurons and circuits. In what follows, we introduce what is known about the organization and function of the SC and provide an overview of exciting, new discoveries that are emerging. We intersperse and close with highlights of important future directions made possible by novel neuroscientific methods.
Figure 1.

The relative size of the SC varies across evolution. Cladogram of tree terminations showing schematic images of the brains of different species. The blue coloring highlights the SC indicating the relative size differences. Note that in mammals, the SC lies under the cerebral cortex. Brain images are not to scale.
CONSERVED AND VARIANT FEATURES OF THE SUPERIOR COLLICULUS
Those of us who study the SC often say it is highly conserved. But what exactly do we mean when we say that? The evidence supporting the assertion of conservation stems from at least three observations. First, in all vertebrates examined, the SC consists of alternating layers of neurons and neuronal fibers that are more or less differentiated depending upon the species. A second key observation is that the lamination pattern in the SC forms maps of sensory space and actions that, in all vertebrates examined, are topographically arranged and in register. A third key observation is that some inputs to and outputs from the different layers of the SC are common to all vertebrate species. For example, in addition to receiving topographically organized visual, auditory and somatosensory inputs, the SC contains neurons that project to similar targets in the thalamus and the brainstem and, in all species examined, the neurons in the superficial and deeper layers of the SC often display similar morphologies and response properties, and mediate similar behaviors.
Two major descending pathways arising from the SC and targeting the brainstem include a crossed tectobulbar pathway that targets the contralateral brainstem and spinal cord and mediates approach behaviors, and an uncrossed tectopontine pathway that targets the ipsilateral pons and mediates avoidance behaviors (Comoli et al., 2012; Dean et al., 1989; Isa et al., 2020; Redgrave et al., 1993). The approach and avoidance behaviors mediated by the SC are considered innate or reflexive actions that can be carried out with little involvement of forebrain circuits. The ascending outputs of the SC however, far outnumber the descending ones, but a lag in the initial identification of the ascending outputs led to the long-held belief that the SC subserves reflexive behaviors primarily. In fact, Ramón y Cajal said, “…la nature exclusivement réflexe de ce tubercle c’est à dire sa non-participation à la formation de l’image optique mentale.” (Ramón y Cajal, 1909, 1995), in other words, this tubercle, referring to the tectum, is reflexive in nature and does not participate in image formation. Following this logic, the relative size of the OT compared to the SC suggests a greater reliance on reflexive behaviors in non-mammals. Moreover, the smaller size of the SC relative to the larger forebrain of many mammals suggests that the SC receives commands from the forebrain, but has little influence on non-reflexive functions mediated by cerebral cortical processing (Figure 1). The traditional view of the SC as a reflex organ is inconsistent with a number of observations. First, the size of the SC varies even within vertebrate classes, and depends more on various aspects of visual processing, such as the degree of binocularity or the rod to cone ratio in the retina (Gaillard et al., 2013). Second, recent experimental results reviewed below, provide compelling evidence for the involvement of the SC in cognitive functions including attention and decision-making that are usually attributed to the forebrain. Finally, in all vertebrate species examined, the largest output systems of the SC are the ascending projections (Basso and May, 2017), suggesting that SC and forebrain circuits are much more integrated than previously thought. The challenge moving forward is to understand the function of the interactions between the SC and the forebrain and how the interactions evolved from non-mammalian vertebrates to mammals including primates.
Laminar organization
The alternating layers of neurons and fibers in the SC of all vertebrates can be divided roughly into three; superficial, intermediate and deep, or for the OT in non-mammals, superficial, central and periventricular. Figure 2 panels a-f show coronal sections through the tectum of multiple species illustrating the varied patterns of differentiation within the SC. In non-mammals, the deep layers are referred to as periventricular because a distinguishing feature of the OT (with the exception of the hagfish) is the presence of a tectal ventricle (Figure 2e). In the adult lamprey, one of the most ancient vertebrates still in existence, the OT has seven layers whereas in cartilaginous fish, the OT is comparatively less well-differentiated with only four distinguishable layers (Vanegas, 1984). With the transition to land and flight and correspondingly, a moveable head and reliance on vision, the tectum increased in complexity in some amphibians and reptiles, and especially in birds, where up to 15 alternating neuronal and fiber layers are distinguishable (Figure 2e). In amphibians and reptiles, the layers in the OT are numbered sequentially from the ventricle outward, whereas in birds the layers are numbered sequentially from the dorsal surface inward toward the ventricle.
Figure 2.

The SC shows varying lamination and retinal axon origin. Nissl stained coronal sections through the left SC of six species showing differences in layer differentiation and laminar nomenclature. a. SC of monkey (Macaca mulatta); b. California grey squirrel (Sciurus griseus); c. OT of English sparrow (Passer domesticus); d. SC of mouse (Mus musculus). e. OT of the pigeon (Columba livia domestica) f. SC of the tree shew (Tupaia belangeri). Note that the layering is more pronounced in birds than mammals. a-d scale bars = 1mm. a-e from the PhD thesis of Daniel Major, images kindly provided by Dr. Harvey Karten. See text for abbreviations. g. Schematic diagram of the patterns of retinal afferents entering the OT superficially and terminating in different layers forming separate visual channels. h. Same as in g for the mammalian SC with retinal afferents entering the SC from below. Adapted with permission from (Yamagata et al., 2006).
In mammals, the layers of the SC are labeled from dorsal to ventral: stratum zonale (SZ), stratum griseum superficiale (SGS; sometimes further divided into sublayers 1, 2 and 3), stratum opticum (SO), stratum griseum intermediale (SGI; sometimes divided into sublayers, a, b and c), stratum griseum profundum (SGP) and stratum album profundum (SAP; Figure 2f). The labeling system varies greatly in the literature, but retains the general features first introduced by Ramón y Cajal and Huber and Crosby (Huber and Crosby, 1933; Ramón y Cajal, 1909, 1995). Among mammals, squirrels, tree shrews and some arboreal primates, have the most distinct lamination within the superficial layers, likely related to the importance of visual processing in these species. That many mammals have fewer distinguishable superficial layers than some reptiles and birds, may stem from the nocturnal origins of mammals, when carnivorous reptiles inhabited the earth, as well as the increased reliance on vision for flight in birds (Knudsen, 2020; Northcutt, 2002; Stein and Gaither, 1981). Many of the comparisons made between the layers of the tectum of different vertebrate species used diurnal, highly visual reptiles and birds, and crepuscular or nocturnal mammals, such as the cat and some species of rodent, such as the hamster (Baldwin and Kaas, 2012; Baldwin et al., 2013b; Baldwin et al., 2019; Chalupa and Rhoades, 1977; Harting and Guillery, 1976; Harting et al., 1992; Rhoades et al., 1987; Vanegas, 1984). More detailed comparisons across species with similar visual capabilities (e.g., binocularity and stereopsis, color vision) combined with modern molecular methods for identifying neuronal cell types is a rich area for future investigation to explore the evolution of the SC, its layers, and its neuronal cell types.
In all vertebrates, the SO includes axons originating from the retina. In birds and reptiles, the SO occupies the outermost layer (farthest from the ventricle), whereas in mammals the SO divides the superficial and the intermediate layers. Although the outermost layer, SZ, contains a thin layer of retinal axons in mammals, the difference in the entrance of the majority of retinal fibers translates into substantial differences in the layered organization of the tectum between non-mammals and mammals. In non-mammals the retinal fibers travel from superficial to deep to meet their target dendrites, whereas in mammals the majority of retinal fibers travel in the opposite direction (Figure 2g and h). The retinal axons that exit the SO form orderly arrays of axons and terminals that innervate specific SC laminae or sublaminae despite the differences in the location of the SO. The layered arrangement of retinotectal terminals was initially noted by Ramón y Cajal using Golgi staining techniques. Retrograde labeling of retinal ganglion cells that project to the SC, reconstructions of single retinal axons and their targets, immunocytochemical labeling of subsets of retinotectal axons and their laminar arrangements, as well as the labeling of subsets of retinal ganglion cell terminations in transgenic mice and zebrafish, subsequently revealed that different types of retinal ganglion cells innervate specific laminae and/or sublaminae (Hofbauer and Dräger, 1985; Hong et al., 2011; Kuljis and Karten, 1988; Rivlin-Etzion et al., 2011; Robles et al., 2013; Tamamaki et al., 1995; Yamagata et al., 2006). The precision of the overlap of dendritic endings of different classes of SC neurons with the termination zones of specific ganglion cell classes suggests that the SC maintains parallel channels of visual information. For example, direction-selective neurons in the superficial layers of the mouse SC inherit their selectivity from the retina and the population of direction-selective neurons declines with depth in the SGS, consistent with the projection patterns of direction selective ganglion cells (Barchini et al., 2018; Huberman et al., 2008; Huberman et al., 2009; Inayat et al., 2015; Kay et al., 2011; Kim et al., 2010; Shi et al., 2017). Furthermore, transsynaptic labeling in mice identified a biased sampling of retinal ganglion cell types by different kinds of SC projection neurons, suggesting again that the information carried by different retinal ganglion cell types is maintained in parallel SC channels (Reinhard et al., 2019).
Alignment of sensory maps with action maps
How does the SC transform retinal ganglion cell signals into movements? Sensory information innervating the SC terminates in different layers, forming orderly, topographical maps that are typically in register with one another. For example, input from the cerebral cortex is arranged so that visual areas innervate the superficial layers, and auditory, somatosensory, multimodal and association areas innervate the deeper layers (reviewed in Basso and May, 2017; May, 2006). In the 1940’s, Julia Apter found that the retina projected to the SC of the cat (Felis catus), with the right hemifield mapped onto the left SC and the left hemifield mapped to the right SC (Apter, 1945; Hess et al., 1946). Apter also found that applying strychnine (which causes excitation by blocking glycine receptors) to the surface of the SC produced eye movements directed to the part of the visual field that projected to the manipulated location within the SC (Apter, 1946). Using electrical stimulation in the monkey, David Robinson characterized the map of saccadic eye movement space in the intermediate layers further (Robinson, 1972). The nasal to temporal axis is represented anterior to posterior and the upper to lower axis is represented medial to lateral (Figure 3). This organization is seen in all vertebrates examined including humans (Savjani et al., 2018; Schneider and Kastner, 2005), but with the exception of the iguana (Iguana iguana), in which the maps of space are rotated 90° compared to other vertebrates (Stein and Gaither, 1981). Why the rotation occurs, remains unknown.
Figure 3.

The SC forms topographical maps of space and action. a. Schematic illustration of the right SC map from mouse (Mus musculus) and b. monkey (Macaca mulatta). In the monkey, the central 15° takes up much of the SC visual map whereas in mice, the nasal and temporal visual fields are represented uniformly across the SC. Adapted from (Robinson, 1972; Dräger and Hubel, 1976 and Cang et al., 2018 with permission).
The presence or absence of a fovea often determines the detailed organization of the visual maps across species. For example, in the mouse lacking a fovea, the map approximately uniformly represents the visual field (Cang et al., 2018; Dräger and Hubel, 1976), whereas in the monkey, a highly visual species with a fovea, the central 15° takes up almost half of the SC area (Figure 3a and b). However, in a crepuscular mammal the golden hamster, there also appears a nasal visual field expansion (Finlay et al., 1978). Using electrophysiological and modeling methods, McIlwain examined how retinal afferents create spatial distributions of activity across the surface of the SC and how the SC population activity would be transformed to create spatial distributions of activity in the deeper layers (McIlwain, 1986, 1991). Exactly how receptive field structures are dictated by the dendritic architecture of SC neurons, their receipt of parallel or convergent retinal innervation, as well as their intrinsic connections within the SC are avenues of investigation that are critical for further elucidating how the SC processes and transforms incoming retinal signals for actions. For example, intrinsic GABAergic connections are particularly dense within the superficial layers of the mammalian SC (Whyland et al., 2020) and inhibition of GABAergic neurons in the mouse SC dramatically alters visual receptive field properties (Gale and Murphy, 2016). However, there are an incredible number of interneuron subtypes even in the zebrafish (Förster et al., 2020). Thus, a challenge going forward will be to tease apart the intrinsic circuits of the SC to determine how each neuronal cell type shapes activity patterns and subsequent behavior.
Interestingly, the map of auditory space in owls and monkeys scales to follow the topography of the retinal map in the SC, a scaling that is not predicted by the topography of auditory spatial cues. Indeed, the auditory map is calibrated and aligned in the SC during development in owls and ferrets based on a visual instructive signal, which in owls originates in the OT and reaches the inferior colliculus (Carr, 2002; Hyde and Knudsen, 2002). It is likely that other sensory maps in the SC are aligned following the same principle. The somatosensory representation in the intermediate layers of the mouse SC shows an expansion anteriorly for the whiskers, similar to the foveal representation in monkeys (Dräger and Hubel, 1976). In species with special senses such as infrared detection from pit organs in snakes (Crotalis viridis; Hartline et al., 1978), electroreception in lampreys, electric fish (eg., Gnathonemus petersii; Zeymer et al., 2018), and some acquatic mammals (Czech-Damal et al., 2013), and even magnetoreception in the Zambian mole rat (Crytomys anselli; Nemec et al., 2001), the topography of the special sensory maps is arranged similarly and coincides with the visual maps located above. An interesting example of the common organization and adaptation of the sensory maps in the SC comes from the star-nosed mole (Condylura cristata; Crish et al., 2003). The star-nosed mole is a mammal that lives underground and relies not on vision for navigation, but on tactile receptors in its snout. Within the SC of the star nosed mole, there is a reduction of the visual superficial layers, and the map of tactile inputs arising from the snout receptors follows the nasal to temporal axis representation in the anterior to posterior deeper layers. One of the snout appendages even shows saccade-like rapid movements during exploration and its representation is expanded in the most rostral SC, similar to that seen for infrared receptors located nasally in the snake (Hartline et al., 1978), and the foveal retinal ganglion cells in the monkey (Chen et al., 2019; Robinson, 1972). The motor map in the SC is also likely calibrated and aligned with the retinal map by early visual experience (du Lac and Knudsen, 1991; Wang et al., 2015). Thus, the retinal map of space, which is genetically encoded (Cang and Feldheim, 2013), serves as a template for aligning sensory and motor maps in the SC.
How are the SC maps of sensory space and action coordinated to initiate behavioral responses? Schiller proposed that visual maps in the superficial layers of the monkey SC are linked directly to eye movement maps in the deeper layers. Schiller’s idea, termed the foveation hypothesis, was similar to the idea proposed earlier by Hess termed the visual grasp reflex (Hess et al., 1946). The foveation hypothesis proposed that a saccade generated by activity in the motor layer map, would be directed to the retinotopic location activated by the overlying visual neurons (Schiller, 1972). In other words, the motor layer neurons served to visually-grasp the target located in the periphery. In rodents, Dräger & Hubel (Dräger and Hubel, 1975) proposed that somatosensory maps were organized relative to how body parts would be seen from the eye, implying that information from modalities other than vision is converted to retinotopic coordinates. The work of David Sparks and colleagues in the monkey however, revealed that neurons in the intermediate SC layers were organized according to spatial motor error - the position of the eyes relative to the position of the sensory target of interest (Gandhi and Katnani, 2011; Sparks and Mays, 1990). An important implication of the motor error hypothesis is that the sensory activity within the SC is not stationary but changes as the location of the target of interest relative to current eye position changes. How information arising from the visual, auditory, and somatosensory maps is translated into SC motor error signals for eye, head, or limb movements remains to be determined. With the advent of new molecular and genetic tools, we are well poised to begin dissecting SC circuits to define neurons and the computations they perform leading to the conversion of sensory information into movement commands. As an example, significant progress is being made on these questions using lampreys and zebrafish (Helmbrecht et al., 2018; Suzuki et al., 2019). Translating the molecular and genetic tools to monkeys and other highly visual mammals will be critical for understanding these circuit-based functions of the SC.
Linking superficial and deep layers
In the 1980’s Edwards proposed that the SC should be divided into two: the superficial layers above the SO that link to the rest of the visual system and the intermediate and deep layers below the SO whose neurons appear more like those in the reticular formation (Edwards, 1980). Edwards argued that since the superficial layers had inputs from visual structures, outputs converging on predominantly visual thalamic channels, and neurons with responses to visual stimuli, the layers above the SO were better considered a sensory system. The intermediate layers in contrast, receive input from virtually the entire cerebral cortex, contain neurons with responses to multimodal stimuli, and extend outputs through crossed tectobulbar and uncrossed tectopontine pathways. Edwards concluded therefore, that the layers below the SO were better aligned with the reticular formation than the overlying visuosensory layers.
Edwards’ hypothesis was controversial and hinged on the premise that the superficial and intermediate layers were not linked (Casagrande et al., 1972; Mays and Sparks, 1980; Schiller and Stryker, 1972). Subsequent anatomical experiments however, suggested a connection between neurons of the different layers (Behan and Appell, 1992; Kardamakis et al., 2015; Lee and Hall, 1995; Mooney et al., 1988; Rhoades et al., 1989; Tardif et al., 2005). Experiments using a novel in vitro slice preparation of the rodent and tree shrew SC confirmed a direct link between the superficial and intermediate layers. Superficial layer stimulation evokes excitatory postsynaptic potentials in intermediate layer neurons and application of bicuculline, a GABA-A receptor antagonist, enhances these post-synaptic potentials, resulting in robust bursts of action potentials in intermediate layer neurons (Helms et al., 2004; Isa et al., 1998; Lee et al., 2001; Özen et al., 2004; Vokoun et al., 2010). Pathways linking the superficial and deeper layers are also evident in pigeons (Vega-Zuniga et al., 2014).
Work using the rodent slice preparation also revealed at least two other pathways from the intermediate layers to the superficial layers. Intermediate layer neurons have recurrent collaterals that terminate on local GABAergic interneurons. The GABAergic interneurons in turn, suppress the activity of superficial layer neurons that project to the dorsal lateral geniculate (dLGN) and pulvinar nuclei (Lee et al., 2007; Phongphanphanee et al., 2011). A second pathway also arises from neurons in the intermediate layers that excites superficial layer neurons (Ghitani et al., 2014). The excitatory deep to superficial pathway appears sparser than the inhibitory pathway, but it may underlie the enhancement of visual responses in superficial layer neurons (Li and Basso, 2008) or possibly, our ability to see seamless visual images in spite of saccadic eye movements that move our retinae around so frequently (Dunn et al., 2010; Goldberg and Wurtz, 1972; Wurtz and Mohler, 1976). The inhibitory pathway arising from the intermediate layer neurons may contribute to saccadic suppression (the suppression of motion perception during eye movements).
The anatomical and physiological data together provide solid evidence for the existence of a disynaptic pathway from the retina to the intermediate layer neurons, providing support for the hypothesis that the superficial and intermediate layers of the mammalian SC are parts of one connected structure. However, whether functional connectivity between the layers exists in primates is unknown. The disynaptic circuits linking retinal inputs to motor outputs in the deeper layers of the SC may underlie the generation of ultrafast express saccades (Isa and Hall, 2009) and may be analogous to the monosynaptic circuit that links retinal afferents to the distal dendrites of periventricular neurons seen in lamprey (Kardamakis et al., 2015). As such, intracollicular circuitry remains an area rich for future investigation, particularly now with the advent of novel neuronal cell type markers and transynaptic labeling techniques.
Conserved ascending neuronal pathways of the SC: functional implications
Several conserved ascending pathways from the SC target the thalamus to influence forebrain areas. A prime example is the ascending projection to the reptilian and avian nucleus rotundus, and the mammalian pulvinar (or lateral posterior) nucleus (Baldwin et al., 2013a; Chomsung et al., 2008; Fredes et al., 2012; Luksch et al., 1998; Marín et al., 2003; Robson and Hall, 1977; Zhou et al., 2017). Across species, the tectal-rotundal and SC-pulvinar projections arise from a distinctive neuronal cell type (located in the superficial layers of the mammalian SC and in layer 13 of the avian OT) that displays widespread dendritic arbors that terminate in tufts termed bottlebrush endings (widefield vertical neurons, WFV; Figure 4; Gale and Murphy, 2014; Gale and Murphy, 2018; Luksch et al., 1998; Major et al., 2000; Masterson et al., 2019). The somata of WFV neurons (a.k.a. tectal gangion cells or widefield tectal ganglion cells in non-mammals) are located below the retinorecipient layers (e.g., Figure 4b,d), but their extensive dendritic arbors extend vertically to receive retinal input on the bottlebrush endings. Reflecting their widespread dendritic arbors, recordings from WFV neurons in both birds and mice demonstrate that these neurons have very large receptive fields. They respond robustly to very small objects that move through their large receptive fields, as well as small discs that expand to fill the visual field (Bloomingdale; Gale and Murphy, 2014, 2016; Verhaal and Luksch, 2015, 2016; Wu et al., 2005). Thus, WFV neurons are uniquely configured to detect motion. The apparent importance of this function across species is reflected in the highly conserved morphology of these unique neurons (Figure 4).
Figure 4.

Wide field vertical (WFV) neuron morphology is conserved across species. a. Lizard (Anolis carolinensis), from (Ramón y Cajal, 1995). b. Chicken (Galllus gallus), from (Luksch et al., 1998). c. Mouse (Mus musculus), from (Masterson et al., 2019). d. Ground squirrel (Spermophilius beecheyi), from (Major et al., 2000). e. Grey squirrel (Sciurus carolinensis) from (May, 2006). Images adapted with permission.
In the nucleus rotundus of birds, and the regions of the pulvinar that receive SC input (Zhou et al., 2017), responses to visual motion are particularly well-represented (Bennett et al., 2019; Berman and Wurtz, 2010; Chalupa et al., 1983; Foik et al., 2020; Merabet et al., 1998; Mooney et al., 1984; Sun and Frost, 1998). Reflecting their input from WFV neurons, nucleus rotundus/pulvinar neurons respond best to small moving stimuli presented at any location within a relatively large region of space. In addition, some nucleus rotundus/pulvinar neurons signal the time to collision of a looming stimulus (Bennett et al., 2019; de Vries and Clandinin, 2012; Gabbiani et al., 1999; Liu et al., 2011; Sun and Frost, 1998). The nucleus rotundus of birds projects to the entopallium, and in reptiles such as turtles to the core nucleus of the dorsoventricular ridge (Hall and Ebner, 1970), both considered to be homologous to the extrastriate cortical areas targeted by the SC-recipient zones of the mammalian pulvinar nucleus. Motion-detection in posterior and post-rhinal cortical areas in mice depends on the WFV-pulvinar-cortex pathway and appears independent from area V1 input (Beltramo and Scanziani, 2019). Thus, the SC provides a major source of motion information that bypasses the primary pathway from the retina to the dLGN to V1 (striate cortex). On the other hand, SC neurons with restricted dendritic fields (narrow field vertical, NFV, or stellate neurons in the SGS) also project to subregions of the dLGN which can subsequently influence the activity of neurons in V1 of mammals, or its homologues the visual Wulst in birds, or the dorsal cortex in turtles and pallial thickening in lizards (Albano et al., 1979; Bickford et al., 2015; Cruz-Martín et al., 2014; Diamond et al., 1991; Fernández et al., 2020; Gale and Murphy, 2018; Hellmann and Güntürkün, 2001; Hendry and Yoshioka, 1994; Leventhal, 1979). The SC and OT also form reciprocal connections with the ventral lateral geniculate nucleus and pretectum, and in mammals, other ascending projections arising from the SC target regions surrounding the medial geniculate nucleus and form reciprocal connections with the zona incerta (Abramson and Chalupa, 1988; Baldauf et al., 2003; Edwards et al., 1979; Horie et al., 2013; Katoh and Benedek, 1995; Kim et al., 1992; Linke, 1999; May, 2006; May and Basso, 2018; Vega-Zuniga et al., 2014; Whyland et al., 2020). Finally, in mammals, including rats and monkeys, neurons comprising the crossed tectobulbar pathway send collateral projections to the mediodorsal (MD) nucleus of the thalamus which influence areas of the frontal cortex (Bickford and Hall, 1989; Moschovakis and Karabelas, 1985; Sommer and Wurtz, 2004a; Sommer and Wurtz, 2004b). In primates, at least some of the SC-MD-cortex circuit plays a role in providing the prefrontal cortex with information about impending eye movements (Sommer and Wurtz, 2004a; Sommer and Wurtz, 2004b), but whether this circuit plays a role in other behaviors remains unexplored. Basal ganglia inputs to the SC have been studied extensively, mostly with respect to the role of disinhibition of SC neurons and the generation of orienting movements and saccadic eye movements (Grillner and Robertson, 2016; Hikosaka et al., 2006; Karabelas and Moschovakis, 1985). However, the SC also targets the input nucleus of the basal ganglia, the striatum, via the pulvinar and MD nuclei forming a loop which is only recently beginning to be explored (Day-Brown et al., 2010; McHaffie et al., 2005; Zhou et al., 2018). Indeed, recent work suggests that the role of the basal ganglia even in simple movements may be more complicated than some of the original models propose (Cui et al., 2013; Villalobos and Basso, 2020; Yttri and Dudman, 2016). The extensive inputs and outputs of the SC that appear in most vertebrate species examined, show that the SC can impact virtually the entire neuraxis (Figure 5), and therefore, has the potential to play a role in a variety of behaviors, from simple rapid approach and avoidance behaviors, to complex behaviors such as attention and decision-making.
Figure 5.

The mammalian SC and avian OT influence the entire neuraxis through their extensive inputs and outputs. A selected sample of inputs appear on the left and outputs on the right. Orange shows intermediate and deeper layer inputs and outputs and blue shows superficial layer inputs and outputs. Tectopontine refers to uncrossed pathways. Tectobulbar refers to crossed pathways. Adapted with permission from (Luksch, 2003).
Visually-guided approach and avoidance behaviors
Cajal proposed that the SC participates in neither image forming vision nor volitional behavior (Ramón y Cajal, 1909, 1995). Cajal drew this conclusion based on his observation that the mammalian SC appeared less well-developed compared to the reptilian and avian OT and that he observed only descending and not ascending pathways from the SC. Likely as a result of these initial observations and conclusions about the SC, most experiments aimed at revealing the function of the SC focus on the control of movement, particularly reflexive approach and avoidance movements. For example, the early experiments in the OT of teleost fish and amphibians focused on prey capture and predator avoidance (Vanegas, 1984). Even in primates, beginning in the 1970s and through the 1990s much of the work on the SC stressed the relationship of the intermediate and deeper layer neuronal activity and the descending outputs of these neurons with the control of saccadic eye movements in response to briefly presented visual stimuli (Schiller and Stryker, 1972; Sparks, 1978; Wurtz and Goldberg, 1971). In rodents, early studies focused on the two descending pathways, the crossed tectobulbar pathway and the uncrossed tectopontine pathway. Using traditional anatomical, lesion and electrophysiological techniques, and more recent optogenetic methods, the neurons of the these two pathways were found to be segregated; the crossed pathway arising from neurons located in the lateral intermediate SC and the uncrossed pathway arising from neurons located in the medial intermediate SC (Dean et al., 1989; Isa et al., 2020; Sahibzada et al., 1986). Indeed the size of the crossed pathway appears larger in carnivorous, predatory animals, consistent with its role in orienting and prey capture (Barton and Dean, 1993). Today, studies using mouse and zebrafish models and novel tools that allow specific neurons and circuits to be visualized, mapped, and manipulated continue this trend (Bollmann, 2019; Cang et al., 2018). These new studies reveal a number of novel circuits originating from the SC that underlie reflex-like approach and avoidance behaviors.
Laboratory animals such as hamsters and mice can catch crickets using visual cues (Finlay et al., 1980; Hoy et al., 2019). In hamsters, SC lesions result in prey capture deficits (Finlay et al., 1980). In mice, SC neurons, mostly in the intermediate and deep layers, are activated during prey capture behavior, and global silencing of the SC impairs capture behavior (Shang et al., 2019). Furthermore, excitatory projections from the deep SC to the zona incerta trigger prey capture (Shang et al., 2019). On the other hand, chemogenetic inactivation of superficial layer NFV and WFV neurons affects distinct aspects of prey capture, which appear to be consistent with the visual response properties of the NFV and WFV neuronal cell types (Hoy et al., 2019). Similarly, specific neuronal cell types in the OT of larval zebrafish and lamprey respond selectively to different visual features such as size and speed, and mediate the behavioral choice between orienting and avoidance (Barker and Baier, 2015; Suzuki et al., 2019). In mice, visual information required to detect prey appears to be processed in the WFV and NFV neurons and is then transmitted (via intrinsic or extrinsic SC circuits) to intermediate and deep layer neurons for the decision and generation of behavior, whereas in lampreys and zebrafish, visual information distinguishing between prey and predator appears to be conveyed monosynaptically, directly to the premotor output neurons (Barker and Baier, 2015; Suzuki et al., 2019). Knowing the details of the different neuronal cell types involved in the sensory and motor aspects of these behaviors, as well as how the intrinsic circuits relate across species, will provide important insight into the evolution of the SC.
Defensive responses can be triggered in mice by overhead stimuli mimicking the approaching of an aerial predator (Yilmaz and Meister, 2013). An expanding (“looming”) dark disc often evokes an escape, whereas a sweeping stimulus triggers a freezing response (De Franceschi et al., 2016). These neuronal responses are presumably subject to modulation as a looming stimulus can induce both rapid escape and freezing, depending on environmental context like the presence of a shelter (Vale et al., 2017) or on stimulus parameters such as loom speed and size (Yang et al., 2020). A highly visual diurnal rodent, the common degu (Octodon degus), also shows looming-evoked escape (Deichler et al., 2020). In mice, superficial layer neurons respond to visual looming (Zhao et al., 2014), and optogenetic inhibition of the excitatory SC neurons in the medial region of the intermediate layer reduce looming-induced freezing (Wei et al., 2015). Excitatory parvalbumin (PV+) neurons in the superficial layers could be “loom detectors”, since they appear activated selectively by looming stimuli and their activation induces rapid escape followed by freezing (Shang et al., 2015). However, PV+ neurons in the SC include both excitatory and inhibitory neurons (Villalobos et al., 2018), and respond to more visual stimuli than just looming disks (Hoy et al., 2019), arguing against the entire population of PV+ neurons being restricted to the detection of looming stimuli. In fact, almost all neurons in the superficial layers of the SC of mice respond to other types of visual stimuli in addition to looming stimuli (Zhao et al., 2014). In contrast, neurons in the deep layers of the SC are more selective for looming stimuli such as expanding dark disks, have larger receptive fields, and show habituation to repeated stimulation (Lee et al., 2020), indicating a “sifting” of behaviorally relevant visual information either via intrinsic SC circuits or via extrinsic circuits that subsequently impact the deep layers of the SC.
Several pathways downstream of the SC can mediate looming-evoked behavioral responses (Figure 5), as demonstrated using optogenetic manipulation of neuronal activity and circuit mapping in mice. First, PV+ neurons in the superficial layers project to the amygdala through the parabigeminal nucleus (PBG), and activation of this pathway elicits escape followed by freezing (Shang et al., 2015). Second, another study identified a medial SC - lateral posterior nucleus (LP) - amygdala pathway as mediating the freezing response to looming (Wei et al., 2015). A later study tried to reconcile these two pathways and showed that the PV+ neurons in the superficial layers actually project to both PBG and LP, and activation of the two pathways trigger escape (via PBG) and freezing (via the LP) (Shang et al., 2018). This result suggests that some PV+ neurons would be WFV neurons given the well-established findings that only WFV neurons project to the LP in mammals (Gale and Murphy, 2014; Gale and Murphy, 2018; May, 2006), but this is unlikely to be the case as other studies show that PV+ neurons are located more superficially compared to WFV neurons (Hoy et al., 2019; Villalobos et al., 2018). Additionally, another report showed that the excitatory projections from the deep layers of the medial SC to the dorsal periaqueductal grey (dPAG), but not the SC-LP or SC-PBG pathway, are required for the initiation of looming-evoked escape (Evans et al., 2018). This latter study also demonstrated that SC neurons encode the saliency of the looming stimulus while the PAG neurons compute the escape decision by integrating strong SC inputs to overcome the weak and unreliable SC-dPAG synapses (Evans et al., 2018). Furthermore, yet another brain structure, the ventral tegmental area, also mediates looming-evoked escape, directly downstream of the SC and upstream of the central nucleus of the amygdala (Zhou et al., 2019). In addition, stress accelerates looming-evoked escape in mice, and the locus coeruleus mediates the effect of stress via a projection from tyrosine hydroxylase positive neurons to the intermediate layer of the SC (Li et al., 2018). Finally, the projection of layer 5 neurons in the mouse retrosplenial cortex to the SC appears to encode the direction of shelter, as its inactivation disrupts sound-induced and shelter-directed escape behavior (Vale et al., 2020). Even in humans, the SC - pulvinar - amygdala pathway is activated when exposed to threat (Koller et al., 2019). Humans can mount an escape response to avoid threats but also can orient toward threat if desired, which makes understanding the relationship of this circuit to forebrain pathways an interesting direction for future studies. The SC-pulvinar-amygdala circuit may also underlie the ability of humans with blindsight to recognize fearful emotion in facial expressions (Gerbella et al., 2019). The similarities and differences between different species, different responses to threats and, how threatening stimuli are interpreted presumably by forebrain circuits, is an interesting direction for future work.
Visual spatial attention
Visual spatial attention, a form of selective attention, is one process by which individual visual stimuli or objects are identified and highlighted for further perceptual processing or to serve as goals for actions. Orienting and prey capture can be considered to be the result of attentional selection (Güntürkün and Bugnyar, 2016; Ingle, 1975; Krauzlis et al., 2018). Attention enhances sensory signals through a variety of physiological mechanisms, that facilitate sensory information processing. Attention is also thought to be the purview of the cerebral cortex (e.g., Fiebelkorn and Kastner, 2020; Katsuki and Constantinidis, 2014). How can we reconcile the behavioral similarities across vertebrate species with and without a six-layered cerebral cortex to control attention? The multiple links via the ascending pathways between the SC and the forebrain likely hold the key.
In the 1960’s, Sprague discovered that hemianopia (half visual field blindness) resulted from unilateral removal of occipital cortex in cats and that subsequent removal of the ipsilateral SC exacerbated the hemianopia but could be reversed by removal of the contralateral SC (Sprague, 1966). This surprising finding led Sprague to conclude that “… visual attention and perception are mediated at both forebrain and midbrain levels, which interact in their control of visually guided behavior”. Since Sprague’s original discovery, research on the role of the SC in attention followed two distinct paths. One followed from an appreciation for the connectivity between the SC and other brainstem structures, such as the isthmus nuclei and its cholinergic and GABAergic modulation of SC circuits (Graybiel, 1978; Knudsen, 2018; Sridharan et al., 2014; Wang et al., 2004). The second, referred to as the premotor theory of attention, proposes that the neurons involved in preparing eye movements are also those that control shifts of attention (Deubel and Schneider, 1996; Kowler et al., 1995; Rizzolatti et al., 1987). In both conceptions of attention, the SC plays a critical role.
The isthmus nuclei: a satellite attentional system
In all vertebrate species thus far examined, the isthmus nuclei are reciprocally connected to the OT. The two main isthmic nuclei are the nucleus isthmi pars parvocellularis (Ipc) and the nucleus isthmi pars magnocellularis (Imc). The former contains neurons that express cholinergic markers, whereas the latter contains GABAergic neurons. The mammalian PBG is considered the homologue of the avian Ipc and the lateral tegmental area adjacent to the PBG may be homologous to the Imc. These nuclei in mammals are likewise reciprocally connected to the SC (Appell and Behan, 1990; Graybiel, 1978; Gruberg et al., 2006; Hall et al., 1989; Jiang et al., 1996; Knudsen, 2011; Mysore and Knudsen, 2011; Sereno and Ulinski, 1987; Wang, 2003; Wang et al., 2006). In birds, where the connections between the OT and isthmus nuclei have been studied in the most detail, and in mammals, the isthmus nuclei are described as a satellite attentional system (Graybiel, 1978; Knudsen, 2018). Both the isthmus nuclei and the PBG receive visual input from the OT/SC via neurons that exhibit narrow dendritic fields (Brn3a-immunoreactive Shepherd’s crook neurons in the OT, GABAergic and nonGABAergic/PV+ NFV neurons in the SC; Whyland et al., 2020; Woodson et al., 1991).
Projections from the Ipc to the avian OT, described as “paintbrush axon terminals” (a.k.a. brush-like endings; Figure 6, blue), express cholinergic markers and provide a focal input to primarily the retinorecipient layers (1–7) but also layers 10–13 of the OT (Gruberg et al., 2006; Knudsen, 2011; Mysore and Knudsen, 2011; Ramón y Cajal, 1909, 1995; Sereno and Ulinski, 1987; Wang, 2003; Wang et al., 2006). The input from the Ipc provides a way to enhance the visual responsiveness and possibly spatial selectivity of neurons with visual responses within the OT, potentially through activation of nicotinic receptors on retinal ganglion cell axons and through the activation of both nicotinic and muscarinic receptors on intrinsic GABAergic neurons (Binns and Salt, 1997; Endo et al., 2005; King, 1990; King and Schmidt, 1991; Lee et al., 2001; Prusky et al., 1988). The Ipc terminals also target WFV neurons where they form complex synaptic arrangements with WFV neuron bottlebrush endings, in close proximity to retinal terminals that also innervate WFV neurons (Figure 6, blue terminals and grey discs; (González-Cabrera et al., 2016)). This arrangement presumably underlies the finding that OT responses to visual motion are transmitted to higher visual areas (via the nucleus rotundus) only if boosted by feedback signals from the nucleus isthmi (Marín et al., 2012).
Figure 6.

Schematic of retino-isthmo-tectal circuit. Shepard’s crook neurons (red) in the OT project to the Ipc nucleus and the Ipc neurons (blue) in turn, provide cholinergic (ACh) feedback to the OT. Retinal terminals (black arising from the top layer 1) make synaptic contacts with dendrites of OT tectal ganglion neurons (grey discs and complex dendritic fields). The GABAergic input from the Imc is widespread (green). Note that the circuit depicted in red, blue and green is repeated throughout the OT but only one section is highlighted here for clarity. Adapted with permission from (González-Cabrera et al., 2016).
The Imc provides a GABAergic input to the OT that is broad but omits the region of the OT that provides input to it (Figure 6, green). Simultaneous recordings from Imc and Ipc neurons and experiments inactivating Imc neurons in pigeons support the idea that the GABAergic input from the Imc underlies the suppression of activity in unattended regions of the map in the OT (Goddard et al., 2014). Neurons in the cholinergic Ipc show reduced visual activity in the presence of two visual stimuli, which can be reversed after inactivation of the Imc. Thus, the model of attention in the OT-isthmus nuclei system suggests that neurons in the OT combine forebrain inputs with bottom up sensory information to create a priority map of space (Itti and Koch, 2000; Koch and Ullman, 1985; Mysore and Knudsen, 2011; Mysore and Knudsen, 2014). The region of space is further highlighted by acetylcholine-mediated enhancement of visual responses and GABA-mediated surround suppression. The isthmus-OT circuit also plays a role in generating and enhancing oscillatory activity like that appearing in thalamic and cortical regions during attention (Fiebelkorn and Kastner, 2019; Sridharan and Knudsen, 2015). Some evidence suggests that the relative timing of activity in neurons across the SC may signal information about orienting movements in mammals too (Brecht et al., 2004). However, whether oscillations occur in the mammalian SC during attention remains unknown.
Cholinergic inputs to the deeper layers of the mammalian SC may also contribute to selective attention (Naimoli et al., 2018). Whereas the cholinergic PBG and Ipc inputs provide dense innervation of the retinorecipient layers of the OT and SC (Graybiel, 1978; Tokuoka et al., 2020), the cholinergic pedunculopontine tegmentum (PPT) and laterodorsal tegmental nucleus (LDT) provide cholinergic input to the intermediate layers of the mammalian SC (Fitzpatrick et al., 1988; Hall et al., 1989). The major cholinergic cell groups in the isthmic tegmentum of birds (i.e., PPN and LDT) resemble the mammalian PPT and LDT respectively (Medina and Reiner, 1994), and comparable cholinergic neuronal cell groups have also been described in reptiles (Medina and Smeets, 1992; Medina et al., 1993; Powers and Reiner, 1993). These projections may account for the fact that nicotine enhances the generation of express saccades when injected in the monkey SC (Aizawa et al., 1999; Watanabe et al., 2005). Optogenetic activation of cholinergic inputs from the PPT in vivo significantly increases the activity of a subset of neurons in the deep layers of the SC (Stubblefield et al., 2015) and in vitro studies identified a variety of cholinergic responses in neurons of the deep layers of the SC, including excitatory responses in neurons that contribute to the crossed tectobulbar pathway, via nicotinic receptors, which can enhance their responses to activation of the neurons in the superficial layers (Sooksawate et al., 2008; Sooksawate and Isa, 2006; Sooksawate et al., 2012).
The mammalian midbrain circuits for attention are an area rich for studies capitalizing on novel molecular and imaging methods. For example, are the functions of the PBG-SC circuits similar to those of the nucleus isthmi-OT circuits? How do the PBG-SC circuits differ from the PPT and LDT innervation of the deep SC layers? What cellular compartments of the SC and OT receive inputs from the cholinergic and GABAergic nuclei and how do they relate to terminations from other inputs such as those arising from the cerebral cortex (Wulst and ectopallium in birds) and the basal ganglia and cerebellum? Finally, the differentiation and size of the isthmus system seems to be most elaborated in birds compared to mammals. The structural similarities in mammals and birds suggests a similar function, but the reason for the elaboration in birds is unknown. It may be that the role of this system in visual spatial attention is unique to birds.
The premotor theory of attention
Experiments performed in the 60s and 70s gave rise to the premotor theory of attention. Eye movements facilitate perceptual judgements made at the location of the eye movement (Crovitz and Daves, 1962), and visual responses of neurons in the superficial layers of the monkey SC show enhanced activity when monkeys make saccades to visual targets located in the neurons’ receptive fields, compared to when monkeys remain fixating (Goldberg and Wurtz, 1972; Wurtz and Mohler, 1976). Indeed, visual enhancement appears in overt attentional tasks and even covertly, when a saccade is cued but before it is made (Gattass and Desimone, 1996; Li and Basso, 2008). Consistent with the premotor theory of attention, low-intensity electrical stimulation of the SC without evoking saccades, enhances motion direction discrimination (Muller et al., 2005) and detection (Cavanaugh et al., 2006; Cavanaugh and Wurtz, 2004). These combined results suggest a signal from motor preparatory regions in the deep layers of the SC modulates the sensory responsiveness of neurons in the superficial layers or elsewhere (Ghitani et al., 2014; Wurtz and Mohler, 1976).
Krauzlis and colleagues followed up on the findings of Cavanaugh and Wurtz with crucial experiments (Lovejoy and Krauzlis, 2010). Trained monkeys performed an attention task in which they detected a change in motion direction of a stimulus located in the periphery. Importantly, the monkeys reported the location of the change not by looking at that location but by looking elsewhere. This simple manipulation provided an important control to decouple the location of attention and the location of the prepared eye movement, allowing confidence in interpreting results in terms of attention rather than the ability to make an eye movement. In other experiments monkeys performed the same task but reported the change with a button press or joy stick release, thus completely eliminating the need for an eye movement. Inactivation of a small region of the SC corresponding to the cued location, resulted in monkeys ignoring the cued motion direction change and reporting the motion direction change occurring at the foil or opposite location. Conversely, when the foil signal appeared in the inactivated region of the SC, the monkeys ignored this and reported the motion direction change from the cued location. Interestingly though, inactivation of the SC combined with simultaneous recordings from neurons in cerebral cortical areas MT and MST, where motion direction information is processed and interpreted in primates, revealed that the signatures of attention, such as enhancement and variability changes, all occurred during performance of the attention task and remained after SC inactivation (Zenon and Krauzlis, 2012). Therefore, exactly how inactivation of intermediate layer neurons of the SC causes motion change detection impairments, and through what circuits, remain enigmatic. A recent report using optogenetics in mice suggests that it is the early visual responses in SC neurons that encode the change detection (Wang et al., 2020), suggesting that intrinsic circuits may play a role. A recent fMRI result suggests ascending information to the cerebral cortex could also play a role (Bogadhi et al., 2020).
Visual perceptual decision-making
A popular model of perceptual decision-making proposes that sensory evidence gradually accumulates over time until a bound is reached, at which point a decision is made and reported. In simple, two-choice perceptual decisions, two bounds represent the two alternatives and the relative strength of evidence for the two choices is the accumulated decision variable (Mazurek et al., 2003; Ratcliff et al., 2016). The process of evidence accumulation is generally considered to require processing in sensorimotor regions of the forebrain including parietal and frontal cortices, and the basal ganglia (Brody and Hanks, 2016; de Lafuente et al., 2015; Ding and Gold, 2013; Harvey et al., 2012; Licata et al., 2017). As discussed below, neurons in the intermediate layers of the monkey SC are also implicated in evidence accumulation, a role usually attributed to forebrain structures.
Neurons in the intermediate layers of the SC show activity that correlates with the onset of visual stimuli and the onset of saccadic eye movements. Some SC neurons also show delay-period activity: tonic neuronal discharges that occur during the interval between vison and action, while a monkey waits to make a saccadic eye movement (Gandhi and Katnani, 2011; Glimcher and Sparks, 1992; Munoz and Wurtz, 1995). One of the first reports suggesting that the SC may play a role in decision-making showed that, when monkeys had to choose one target from among multiple possible targets, delay-period signals correlated with the certainty of the impending saccade, rather than being obligately linked to the eye movement (Basso and Wurtz, 1997, 1998). Subsequent reversible inactivation of neurons in the intermediate layers provided direct, causal evidence for the SC’s role in saccade choice (McPeek and Keller, 2004). Muscimol injections in the SC caused monkeys to choose distractors more often (Figure 7a – c). Importantly, making the task more difficult by reducing the color difference or the contrast between the target and distractors, resulted in greater impairment after inactivation, indicating that the SC plays a role in the choice of the saccade target and not simply in the generation of the saccade. A similar result occurs even if monkeys report their decision with an arm movement, indicating that the decision activity in the SC is likely to be a general purpose signal and not only for saccades (Song et al., 2011).
Figure 7.
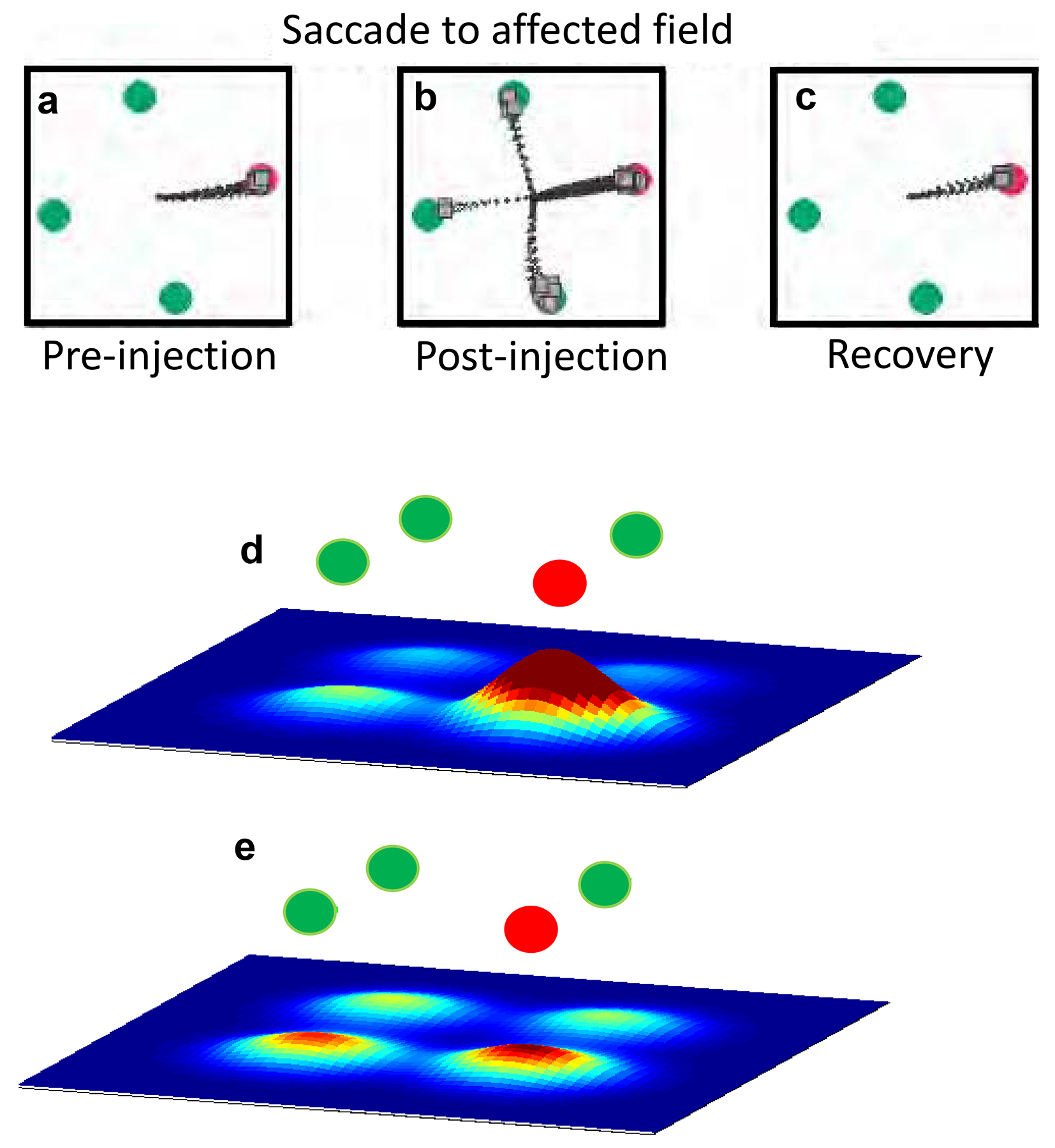
SC neuronal activity encodes saccade choice. a. Schematic of the odd-ball target selection task in which a monkey correctly chose the red target on all trials. Black lines show saccade trajectories. b. After muscimol injection into the SC, monkeys made more frequent errors selecting the distractor stimuli. c. During recovery, 24 hours after muscimol, monkeys performed accurately again. Adapted from (McPeek and Keller, 2004). d. Schematic of the odd-ball target selection task in which a monkey showed good (>75% accuracy) and e. poor (<75% accuracy) target selection performance. Black lines show saccade trajectories. Scale bars indicate 5°. f. Population responses to targets compared to distractors simulated from multiple SC neuron recordings, showed higher discriminability during good performance compared to g. poor performance. Warmer colors indicate higher spike rates. Adapted from (Kim and Basso, 2008).
The inactivation results point toward an hypothesis for understanding how the SC chooses a saccade. If the appearance of each possible target drives a population of neurons encoding that spatial location, then the relative level of activity between the population activities should determine the choice (Figure 7d–g; Kim and Basso, 2008). A read-out mechanism based on probabilistic population codes, provides strong evidence that it is the relative level of activity across the maps of both SCs that determines saccade choice (Kim and Basso, 2010). Mechanistic explanations of population dynamics related to saccade choice at the level of specific neurons and circuits in the SC are as yet unknown. Applying neuroscientific tools designed to identify neuronal cell types and local circuit connectivity to in vivo monkey experiments will be critical to address these issues.
In a series of studies in the early 2000s, Horwitz and Newsome recorded from SC neurons in the intermediate layers while trained monkeys performed a two-choice, motion direction discrimination task, sometimes referred to as the random dot motion task (RDM; Horwitz et al., 2004; Horwitz and Newsome, 1999; Horwitz and Newsome, 2001). Trained monkeys reported their decisions by making a saccade to a choice target located in a position corresponding to the direction of motion; left target for leftward motion and right target for rightward motion. Perceptual decision tasks such as the RDM task are based on the premise that decision-related activity recorded from neurons in sensorimotor areas of the brain depends on the strength of the evidence informing the decision (more dots moving coherently = stronger evidence). Parameterizing the difficulty of the decision by varying the proportion of dots moving in the same direction allows this assessment. Purely motor activity, in contrast to decision activity, would be linked obligately to movement metrics and should therefore be independent of evidence strength. SC neurons show modulation in the RDM task in much the same way as do neurons in cerebral cortical areas LIP and prefrontal cortex area 46. The decision-related activity in the SC occurs in some neurons even when the direction of the discrimination and the position of the target used to report the decision are decoupled, confirming that the decision-related signals in SC are not simply motor signals (Horwitz et al., 2004). Subsequent experiments using a brightness discrimination task in which the sensory evidence and the decision report varied on different dimensions as well as analysis of single spike trains from SC neurons, provide further, compelling evidence that SC neurons with delay-period activity participate in evidence accumulation for perceptual decisions (Ha-Cho et al., 2020; Ratcliff et al., 2003; Ratcliff et al., 2007).
Current models of decision-making propose that sensory information such as motion direction is transmitted from sensory areas of the cerebral cortex such as area MT to sensorimotor regions of the cerebral cortex such as area LIP, where evidence for or against a particular decision is integrated and accumulated (Mazurek et al., 2003). Decision information is then passed to the SC where the decision bound crossing is detected resulting in the saccade choice (Lo and Wang, 2006). Although the electrophysiological signatures of decision-making in SC neurons are remarkably similar to those seen in cerebral cortical areas, the SC activity is often interpreted as simply reflecting the decision-making processing ongoing within the cerebral cortex. This interpretation is in line with classical notions that the role of the mammalian SC in visual processes is minimized and SC is a simple relay of cortical information en route to the brainstem for the generation of movements. However, in light of the known ascending projections from the SC to the forebrain and the results described above, the classical notion warrants revisiting.
Two recent experiments provide compelling evidence that intermediate layer neurons of the monkey SC play a causal role in perceptual decision-making and are not simply passive recipients of cortical information processing used to drive saccades. In one experiment, monkeys assessed whether a visual stimulus contained orientation information. If monkeys perceived orientation, they reported ‘Yes’ by making a saccade to a green target located in either the left or right visual field. If they did not perceive orientation, monkeys reported ‘No’ by making a saccade to a red target located in either the left or right visual field. Importantly, monkeys performed this task under different priming conditions, liberal priming (more ‘Yes” presentations) or conservative priming (more “no” presentations). The priming manipulation caused monkeys to change their decision criterion on the final set of trials, when the “Yes’ and ‘No’ stimuli occurred again with equal frequency. Interestingly, electrical stimulation of the SC in lieu of the stimulus priming produced similar shifts in decision criteria (cf., Figure 8a and b). SC neuronal activity correlated with the changes in decision criterion. After the set of conservative priming trials, the difference in ‘Yes’ and ‘No’ activity decreased compared to the initial baseline differences (cf., Figure 8c and d) whereas after the liberal priming trials the difference in ‘Yes’ and ‘No’ activity increased (cf., Figure 8c and e). Thus, the relative level of SC neuronal activity signals the position of the decision criterion (Crapse et al., 2018). These results are consonant with a series of previous studies in the SC in monkeys and rodents finding decision biases following manipulations of neurons in the SC (Carello and Krauzlis, 2004; Felsen and Mainen, 2008; Lovejoy and Krauzlis, 2010; McPeek and Keller, 2004; Muller et al., 2005; Nummela and Krauzlis, 2010; Thevarajah et al., 2009). Thus, neurons in the intermediate layers of the monkey SC establish the position of the decision criterion and possibly also how aspects of attention are expressed (Basso and May, 2017; Luo and Maunsell, 2015).
Figure 8.
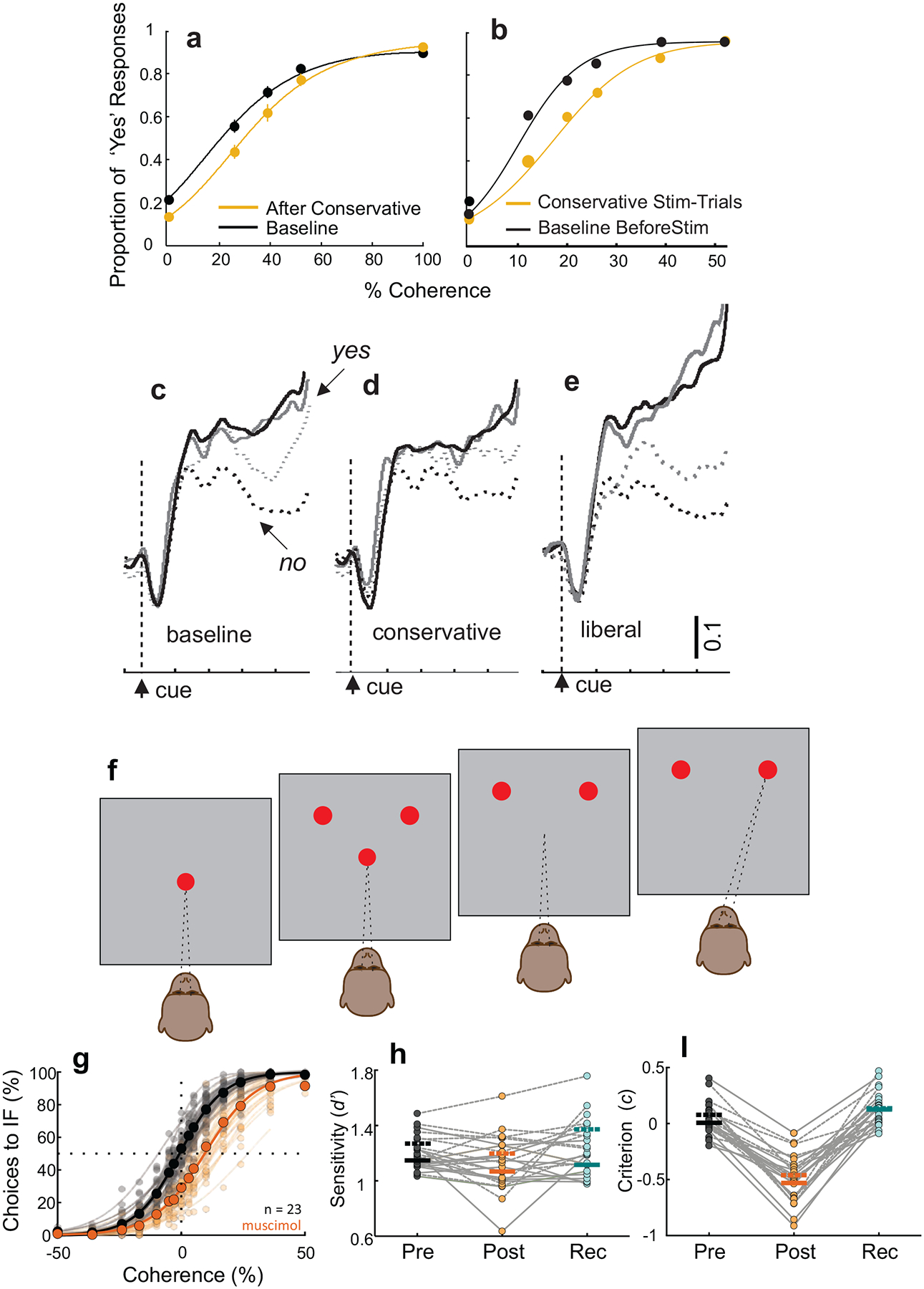
The SC of monkeys plays a causal role in perceptual decision-making. a. Example proportion of ‘Yes’ responses of a monkey in the conservative priming trials (orange more ‘No” trials). Black shows equal “yes’ ‘No” trails (Baseline). b. Same as in a for trials with (orange) and without (black) stimulation of the SC, which mimicked behavioral changes in decision criterion stemming from changes in stimulus frequency. c - e. Neuronal activity from SC neurons during the decision epoch for “Yes’ (solid) and “No” (dashed) trials. After conservative priming the difference in neuronal activity decreased whereas after liberal priming the difference increased. Grey and black lines show different coherences. Adapted with permission from (Crapse et al., 2018). f. Schematic of the spatial arrangement of the two-choice orientation discrimination decision task. Red circles show the fixation point and the two choice targets and the pattern in the center shows the orientation cue. Monkeys reported their orientation decision with an eye movement corresponding to the perceived orientation. g. Choice performance before (black) and after (orange) muscimol inactivation of the intermediate layers of the SC. Decisions became biased away from the inactivated field (IF) after muscimol. h. Sensitivity plotted pre- post- and 24 hours after muscimol injection. d. Criterion plotted pre- post- and 24 hours after muscimol. Adapted from (Jun et al., 2020).
In the second experiment, trained monkeys performed a two choice orientation discrimination task in which they reported seeing leftward or rightward orientation in a visual stimulus and reported their decision on each trial with a saccade to a target located in the right or left visual field. This task is identical to the RDM task, with the exception that the stimulus to be discriminated is orientation rather than motion direction (Figure 8f). Inactivation of neurons in the intermediate layers of the SC in monkeys results in significant decision biases away from the inactivated field (Figure 8g–i). Importantly, in this experiment, monkeys also performed a saccade selection task in which they made saccades to the same two locations as the choice targets, but neither perceptual ambiguity nor orientation discrimination occurred. Here, muscimol did not impair the monkeys’ ability to distribute their saccadic choices equally between the two locations, ruling out interpretations based on motor or attentional biases (Jun et al., 2020).
An extensive modeling effort revealed that muscimol inactivation produced a decrease in the relative evidence for decisions made to the inactivated field (Jun et al., 2020), consistent with previous modeling efforts in the SC (eg., Ratcliff et al., 2003; Ratcliff et al., 2007). Interestingly, inactivation of cortical area LIP showed impairments only in sensory aspects of decisions and not in decision-making per se (Katz et al., 2016; Zhou and Freedman, 2019). One exciting possibility is that the SC plays an active role in evidence accumulation within the SC, or by modulating the accumulation process performed in other areas of the brain. These new results highlight the need to begin exploration of the ascending circuits from the SC to the forebrain, circuits that have been long overlooked.
CONCLUSIONS AND FUTURE DIRECTIONS
The SC and OT have been studied for more than 100 years. There are numerous similarities in the organization of these structures, and many conserved circuits have been identified. Elaborations and adaptations have also occurred over the course of evolution. How the conserved neuronal cell types and conserved, elaborated and adapted circuits give rise to simple and complex behaviors remains to be fully resolved. To address these questions, new technological developments in neuroscience allow for experiments that were previously only dreamed of to be performed. The experiments reviewed here shine a spotlight on the complex organization of visual and other inputs to the SC and OT, their intrinsic circuitry and their vast interconnections with the forebrain. The traditional notion that the SC is simply a relay of retina and cortical information to produce reflex movements is no longer tenable. Applying novel tools that allow specific neuronal cell types and circuits to be visualized, mapped, and manipulated, combined with sophisticated new behavioral tasks in genetically tractable animal models, are exciting future directions that can lead to the unraveling of conserved and divergent circuits underlying simple behaviors from approach and avoidance, to complex behaviors, such as attention and decision-making.
ACKNOLWEDGEMENTS:
We thank Dr. Harvey Karten for extensive discussions and Drs. Tony Reiner, Eric Knudsen and Alice Powers for comments on the manuscript. We thank Nancy Dinh and Brittany Florkiewicz for illustrations. Work in the authors’ labs is supported by EY013692, EY019663, AG063090 and NS107668 (MAB), EY024173 and EY031322 (MEB), EY026286 and EY020950 (JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abramson BP, and Chalupa LM (1988). Multiple pathways from the superior colliculus to the extrageniculate visual thalamus of the cat. J Comp Neurol 271, 397–418. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Kobayashi Y, Yamamoto M, and Isa T (1999). Injection of nicotine Into the superior colliculus facilitates occurrence of express saccades in monkeys. Journal of Neurophysiology 82, 1642–1646. [DOI] [PubMed] [Google Scholar]
- Albano JE, Norton TT, and Hall WC (1979). Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Research 173, 1–11. [DOI] [PubMed] [Google Scholar]
- Appell PP, and Behan M (1990). Sources of subcortical GABAergic projections to the superior colliculus of the cat. The Journal of Comparative Neurology 302, 143–158. [DOI] [PubMed] [Google Scholar]
- Apter JT (1945). Projection of the retina on superior colliculus of cats. J Neurophysiol 8, 123–134. [Google Scholar]
- Apter JT (1946). Eye movements following strychninzation of the superior colliculus of cats. Journal of Neurophysiology 9, 73–86. [DOI] [PubMed] [Google Scholar]
- Baldauf ZB, Wang XP, Wang S, and Bickford ME (2003). Pretectotectal pathway: an ultrastructural quantitative analysis in cats. J Comp Neurol 464, 141–158. [DOI] [PubMed] [Google Scholar]
- Baldwin MK, Balaram P, and Kaas JH (2013a). Projections of the superior colliculus to the pulvinar in prosimian galagos (Otolemur garnettii) and VGLUT2 staining of the visual pulvinar. J Comp Neurol 521, 1664–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MK, and Kaas JH (2012). Cortical projections to the superior colliculus in prosimian galagos (Otolemur garnetti). J Comp Neurol 520, 2002–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Wei H, Reed JL, Bickford ME, Petry HM, and Kaas JH (2013b). Cortical projections to the superior colliculus in tree shrews (Tupaia belangeri). Journal of Comparative Neurology 521, 1614–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Young NA, Matrov D, and Kaas JH (2019). Cortical projections to the superior colliculus in grey squirrels (Sciurus carolinensis). Eur J Neurosci 49, 1008–1023. [DOI] [PubMed] [Google Scholar]
- Barchini J, Shi X, Chen H, and Cang J (2018). Bidirectional encoding of motion contrast in the mouse superior colliculus. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AJ, and Baier H (2015). Sensorimotor decision making in the zebrafish tectum. Current biology : CB 25, 2804–2814. [DOI] [PubMed] [Google Scholar]
- Barton RA, and Dean P (1993). Comparative evidence indicating neural specialization for predatory behaviour in mammals. Proc Biol Sci 254, 63–68. [DOI] [PubMed] [Google Scholar]
- Basso MA, and May PJ (2017). Circuits for action and cognition: A view from the superior colliculus. Annual Review of Vision Science 3, 197–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, and Wurtz RH (1997). Modulation of neuronal activity by target uncertainty. Nature 389, 66–69. [DOI] [PubMed] [Google Scholar]
- Basso MA, and Wurtz RH (1998). Modulation of neuronal activity in superior colliculus by changes in target probability. Journal of Neuroscience 18, 7519–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, and Appell PP (1992). Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol 315, 230–243. [DOI] [PubMed] [Google Scholar]
- Beltramo R, and Scanziani M (2019). A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 363, 64–69. [DOI] [PubMed] [Google Scholar]
- Bennett C, Gale SD, Garrett ME, Newton ML, Callaway EM, Murphy GJ, and Olsen SR (2019). Higher-order thalamic circuits channel parallel streams of visual information in mice. Neuron 102, 477–492.e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, and Wurtz RH (2010). Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci 30, 6342–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford M, and Hall W (1989). Collateral projections of predorsal bundle cells of the superior colliculus in the rat. Journal of Comparative Neurology 283, 86–106. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Zhou N, Krahe TE, Govindaiah G, and Guido W (2015). Retinal and tectal “driver-like” inputs converge in the shell of the mouse dorsal lateral geniculate nucleus. The Journal of Neuroscience 35, 10523–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns KE, and Salt TE (1997). Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. The Journal of Physiology 504, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomingdale LM (1984). In Attention deficit disorder: diagnostic, cognitive and therapeutic understanding (Spectrum).
- Bogadhi AR, Katz LN, Bollimunta A, Leopold DA, and Krauzlis RJ (2020). Midbrain activity shapes high-level visual properties in the primate temporal cortex. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH (2019). The zebrafish visual system: from circuits to behavior. Annu Rev Vis Sci 5, 269–293. [DOI] [PubMed] [Google Scholar]
- Brecht M, Singer W, and Engel A,K (2004). Amplitude and direction of saccadic eye movements depend upon the synchronicity of collicular population activity. Journal of Neurophysiology 92, 424–432. [DOI] [PubMed] [Google Scholar]
- Brody CD, and Hanks TD (2016). Neural underpinnings of the evidence accumulator. Curr Opin Neurobiol 37, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, and Feldheim DA (2013). Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci 36, 51–77. [DOI] [PubMed] [Google Scholar]
- Cang J, Savier E, Barchini J, and Liu X (2018). Visual function, organization, and development of the mouse superior colliculus. Annu Rev Vis Sci 4, 239–262. [DOI] [PubMed] [Google Scholar]
- Carello CD, and Krauzlis RJ (2004). Manipulating intent:evidence for a causal role of the superior colliculus in target selection. Neuron 43, 575–583. [DOI] [PubMed] [Google Scholar]
- Carr C (2002). Sounds, signals and space maps. Nature 415, 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, and Martin GF (1972). Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science 177, 444–447. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Alvarez BD, and Wurtz RH (2006). Enhanced performance with brain stimulation: attentional shift or visual cue? J Neurosci 26, 11347–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, and Wurtz RH (2004). Subcortical modulation of attention counters change blindness. Journal of Neuroscience 24, 11236–11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa L, Williams R, and Hughes M (1983). Visual response properties in the tectorecipient zone of the cat’s lateral posterior-pulvinar complex: a comparison with the superior colliculus. The Journal of Neuroscience 3, 2587–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, and Rhoades RW (1977). Responses of visual, somatosensory, and auditory neurones in the golden hamster’s superior colliculus. J Physiol 270, 595–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Hoffmann KP, Distler C, and Hafed ZM (2019). The foveal visual representation of the primate superior colliculus. Curr Biol 29, 2109–2119.e2107. [DOI] [PubMed] [Google Scholar]
- Chomsung RD, Petry HM, and Bickford ME (2008). Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. The Journal of Comparative Neurology 510, 24–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, and Redgrave P (2012). Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front Neuroanat 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Lau H, and Basso MA (2018). A role for the superior colliculus in decision criteria. Neuron 97, 181–194.e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish S, Comer C, Marasco P, and Catania K (2003). Somatosensation in the superior colliculus of the star-nosed mole. The Journal of comparative neurology 464, 415–425. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, and Daves W (1962). Tendencies to eye movement and perceptual accuracy. Journal of Experimental Psychology 63, 495–498. [DOI] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, and Huberman AD (2014). A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, and Vogel SS (2013). Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech-Damal NU, Dehnhardt G, Manger P, and Hanke W (2013). Passive electroreception in aquatic mammals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199, 555–563. [DOI] [PubMed] [Google Scholar]
- Day-Brown JD, Wei H, Chomsung RD, Petry HM, and Bickford ME (2010). Pulvinar projections to the striatum and amygdala in the tree shrew. Front Neuroanat 4, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi G, Vivattanasarn T, Saleem AB, and Solomon SG (2016). Vision guides selection of freeze or flight defense strategies in mice. Curr Biol 26, 2150–2154. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Jazayeri M, and Shadlen MN (2015). Representation of accumulating evidence for a decision in two parietal areas. The Journal of Neuroscience 35, 4306–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries SE, and Clandinin TR (2012). Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol 22, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Redgrave P, and Westby GW (1989). Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci 12, 137–147. [DOI] [PubMed] [Google Scholar]
- Deichler A, Carrasco D, Lopez-Jury L, Vega-Zuniga T, Márquez N, Mpodozis J, and Marín GJ (2020). A specialized reciprocal connectivity suggests a link between the mechanisms by which the superior colliculus and parabigeminal nucleus produce defensive behaviors in rodents. Scientific Reports 10, 16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, and Schneider WX (1996). Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Research 36, 1827–1837. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Conley M, Fitzpatrick D, and Raczkowski D (1991). Evidence for separate pathways within the tecto-geniculate projection in the tree-shrew. Proceedings of the National Academy of Sciences 88, 1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, and Gold Joshua I. (2013). The basal ganglia’s contributions to perceptual decision making. Neuron 79, 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger UC, and Hubel DH (1975). Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. Journal of Neurophysiology 38, 690–713. [DOI] [PubMed] [Google Scholar]
- Dräger UC, and Hubel DH (1976). Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol 39, 91–101. [DOI] [PubMed] [Google Scholar]
- du Lac S, and Knudsen EI (1991). Early visual deprivation results in a degraded motor map in the optic tectum of barn owls. Proc Natl Acad Sci U S A 88, 3426–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CA, Hall NJ, and Colby CL (2010). Spatial updating in monkey superior colliculus in the absence of the forebrain commissures: dissociation between superficial and intermediate layers. Journal of Neurophysiology 104, 1267–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SB (1980). The deep cell layers of the superior colliculus: their retirular characteristics and structural organization. In The Reticular Formation Revisted, Hobson JA, and Brazier MAB, eds. (New York: Raven Press; ), pp. 193–209. [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, and Stein BE (1979). Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol 184, 309–329. [DOI] [PubMed] [Google Scholar]
- Endo T, Yanagawa Y, Obata K, and Isa T (2005). Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J Neurophysiol 94, 3893–3902. [DOI] [PubMed] [Google Scholar]
- Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, and Branco T (2018). A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsen G, and Mainen ZF (2008). Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M, Morales C, Durán E, Fernández-Colleman S, Sentis E, Mpodozis J, Karten HJ, and Marín GJ (2020). Parallel organization of the avian sensorimotor arcopallium: Tectofugal visual pathway in the pigeon (Columba livia). J Comp Neurol 528, 597–623. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, and Kastner S (2019). A rhythmic theory of attention. Trends Cogn Sci 23, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, and Kastner S (2020). Functional specialization in the attention network. Annual Review of Psychology 71, 221–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Schneps SE, Wilson KG, and Schneider GE (1978). Topography of visual and somatosensory projections to the superior colliculus of the golden hamster. Brain Res 142, 223–235. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Sengelaub DR, Berg AT, and Cairns SJ (1980). A neuroethological approach to hamster vision. Behavioural Brain Research 1, 479–496. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Conley M, Luppino G, Matelli M, and Diamond IT (1988). Cholinergic projections from the midbrain reticular formation and the parabigeminal nucleus to the lateral geniculate nucleus in the tree shrew. J Comp Neurol 272, 43–67. [DOI] [PubMed] [Google Scholar]
- Foik AT, Scholl LR, Lean GA, and Lyon DC (2020). Visual response characteristics in lateral and medial subdivisions of the rat pulvinar. Neuroscience 441, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster D, Helmbrecht TO, Mearns DS, Jordan L, Mokayes N, and Baier H (2020). Retinotectal circuitry of larval zebrafish is adapted to detection and pursuit of prey. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredes F, Vega-Zuniga T, Karten H, and Mpodozis J (2012). Bilateral and ipsilateral ascending tectopulvinar pathways in mammals: a study in the squirrel (Spermophilus beecheyi). Journal of Comparative Neurology 520, 1800–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, and Laurent G (1999). Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci 19, 1122–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard F, Karten HJ, and Sauvé Y (2013). Retinorecipient areas in the diurnal murine rodent Arvicanthis niloticus: A disproportionally large superior colliculus. J Comp Neurol 521, Spc1. [DOI] [PubMed] [Google Scholar]
- Gale SD, and Murphy GJ (2014). Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. The Journal of Neuroscience 34, 13458–13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, and Murphy GJ (2016). Active dendritic properties and local inhibitory input enable selectivity for object motion in mouse superior colliculus neurons. The Journal of Neuroscience 36, 9111–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, and Murphy GJ (2018). Distinct cell types in the superficial superior colliculus project to the dorsal lateral geniculate and lateral posterior thalamic nuclei. J Neurophysiol 120, 1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, and Katnani HA (2011). Motor functions of the superior colliculus. Annual Review of Neuroscience 34, 205–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, and Desimone R (1996). Responses of cells in the superior colliculus during performance of a spatial attention task in the macaque. Reviews of Brasilian Biology 56, 257–279. [PubMed] [Google Scholar]
- Gerbella M, Caruana F, and Rizzolatti G (2019). Pathways for smiling, disgust and fear recognition in blindsight patients. Neuropsychologia 128, 6–13. [DOI] [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, and Basso MA (2014). Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. The Journal of Neuroscience 34, 6822–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, and Sparks DL (1992). Movement selection in advance of action in the superior colliculus. Nature 355, 542–545. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Mysore SP, Bryant AS, Huguenard JR, and Knudsen EI (2014). Spatially reciprocal inhibition of inhibition within a stimulus selection network in the avian midbrain. PLoS ONE 9, e85865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, and Wurtz RH (1972). Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol 35, 560–574. [DOI] [PubMed] [Google Scholar]
- González-Cabrera C, Garrido-Charad F, Mpodozis J, Bolam JP, and Marín GJ (2016). Axon terminals from the nucleus isthmi pars parvocellularis control the ascending retinotectofugal output through direct synaptic contact with tectal ganglion cell dendrites. J Comp Neurol 524, 362–379. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (1978). A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res 145, 365–374. [DOI] [PubMed] [Google Scholar]
- Grillner S, and Robertson B (2016). The basal ganglia over 500 million years. Current Biology 26, R1088–R1100. [DOI] [PubMed] [Google Scholar]
- Gruberg E, Dudkin E, Wang Y, Marín G, Salas C, Sentis E, Letelier J, Mpodozis J, Malpeli J, Cui H, et al. (2006). Influencing and interpreting visual input: the role of a visual feedback system. J Neurosci 26, 10368–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntürkün O, and Bugnyar T (2016). Cognition without cortex. Trends Cogn Sci 20, 291–303. [DOI] [PubMed] [Google Scholar]
- Ha-Cho S, Crapse T, Grimaldi P, Lau H, and Basso MA (2020). Statistical structure of spike trains in monkey superior colliculus neurons varies with task demands. In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, and Ebner FF (1970). Thalamotelencephalic projections in the turtle (Pseudemys scripta). J Comp Neurol 140, 101–122. [DOI] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, and Raczkowski D (1989). Cholinergic innervation of the superior colliculus in the cat. The Journal of Comparative Neurology 287, 495–514. [DOI] [PubMed] [Google Scholar]
- Harting JK, and Guillery RW (1976). Organization of retinocollicular pathways in the cat. Journal of Comparative Neurology 166, 133–144. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, and Van Lieshout DP (1992). Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol 324, 379–414. [DOI] [PubMed] [Google Scholar]
- Hartline P, Kass L, and Loop M (1978). Merging of modalities in the optic tectum: infrared and visual integration in rattlesnakes. Science 199, 1225–1229. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Coen P, and Tank DW (2012). Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann B, and Güntürkün O (2001). Structural organization of parallel information processing within the tectofugal visual system of the pigeon. J Comp Neurol 429, 94–112. [DOI] [PubMed] [Google Scholar]
- Helmbrecht TO, Dal Maschio M, Donovan JC, Koutsouli S, and Baier H (2018). Topography of a Visuomotor Transformation. Neuron 100, 1429–1445.e1424. [DOI] [PubMed] [Google Scholar]
- Helms MC, Ozen G, and Hall WC (2004). Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. Journal of Neurophysiology 91, 1706–1715. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, and Yoshioka T (1994). A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science 264, 575–577. [DOI] [PubMed] [Google Scholar]
- Hess WR, Burgi S, and Bucher V (1946). Motorisch funktion des tektal und temgentalgebietes. Psychiat Neurol 112, 1–52. [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, and Nakahara H (2006). Basal ganglia orient eyes to reward. J Neurophysiol 95, 567–584. [DOI] [PubMed] [Google Scholar]
- Hofbauer A, and Dräger UC (1985). Depth segregation of retinal ganglion cells projecting to mouse superior colliculus. J Comp Neurol 234, 465–474. [DOI] [PubMed] [Google Scholar]
- Hong YK, Kim IJ, and Sanes JR (2011). Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J Comp Neurol 519, 1691–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Tsukano H, Hishida R, Takebayashi H, and Shibuki K (2013). Dual compartments of the ventral division of the medial geniculate body projecting to the core region of the auditory cortex in C57BL/6 mice. Neuroscience Research 76, 207–212. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, and Newsome WT (2004). Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol 91, 2281–2296. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, and Newsome WT (1999). Separate signals for target selection and movement specification in the superior colliculus. Science 284. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, and Newsome WT (2001). Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86, 2543–2558. [DOI] [PubMed] [Google Scholar]
- Hoy JL, Bishop HI, and Niell CM (2019). Defined cell types in superior colliculus make distinct contributions to prey capture behavior in the mouse. Curr Biol 29, 4130–4138.e4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber GC, and Crosby EC (1933). A phylogenetic consideration of the optic tectum. Proc Natl Acad Sci U S A 19, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, and Barres BA (2008). Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, and Barres BA (2009). Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62, 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde PS, and Knudsen EI (2002). The optic tectum controls visually guided adaptive plasticity in the owl’s auditory space map. Nature 415, 73–76. [DOI] [PubMed] [Google Scholar]
- Inayat S, Barchini J, Chen H, Feng L, Liu X, and Cang J (2015). Neurons in the most superficial lamina of the mouse superior colliculus are highly selective for stimulus direction. J Neurosci 35, 7992–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D (1975). Focal attention in the frog: behavioral and physiological correlates. Science 188, 1033–1035. [DOI] [PubMed] [Google Scholar]
- Isa K, Sooksawate T, Kobayashi K, Kobayashi K, Redgrave P, and Isa T (2020). Dissecting the tectal output channels for orienting and defense responses. eNeuro 7, ENEURO.0271–0220.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Endo T, and Saito Y (1998). The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci 18, 8496–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, and Hall WC (2009). Exploring the superior colliculus in vitro. Journal of Neurophysiology 102, 2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, and Koch C (2000). A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research 40, 1489–1506. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, King AJ, and Moore DR (1996). Topographic organization of projection from the parabigeminal nucleus to the superior colliculus in the ferret revealed with fluorescent latex microspheres. Brain Res 743, 217–232. [DOI] [PubMed] [Google Scholar]
- Jun EJ, Bautista A, Nunez MD, Tak T, Alvarez E, and Basso MA (2020). Embodied cognition and decision-making in the primate superior colliculus. bioRxiv, 2020.2008.2014.251777 [Google Scholar]
- Karabelas AB, and Moschovakis AK (1985). Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol 239, 309–329. [DOI] [PubMed] [Google Scholar]
- Kardamakis AA, Saitoh K, and Grillner S (2015). Tectal microcircuit generating visual selection commands on gaze-controlling neurons. Proceedings of the National Academy of Sciences 112, E1956–E1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh YY, and Benedek G (1995). Organization of the colliculo-suprageniculate pathway in the cat: a wheat germ agglutinin-horseradish peroxidase study. J Comp Neurol 352, 381–397. [DOI] [PubMed] [Google Scholar]
- Katsuki F, and Constantinidis C (2014). Bottom-up and top-down attention: different processes and overlapping neural systems. Neuroscientist 20, 509–521. [DOI] [PubMed] [Google Scholar]
- Katz LN, Yates JL, Pillow JW, and Huk AC (2016). Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535, 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, and Sanes JR (2011). Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31, 7753–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, and Basso MA (2008). Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28, 2991–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, and Basso MA (2010). A probabilistic strategy for understanding action selection. J Neurosci 30, 2340–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Meister M, and Sanes JR (2010). Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci 30, 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Gregory E, and Hall WC (1992). Pathway from the zona incerta to the superior colliculus in the rat. J Comp Neurol 321, 555–575. [DOI] [PubMed] [Google Scholar]
- King WM (1990). Nicotinic depolarization of optic nerve terminals augments synaptic transmission. Brain Res 527, 150–154. [DOI] [PubMed] [Google Scholar]
- King WM, and Schmidt JT (1991). The long latency component of retinotectal transmission: enhancement by stimulation of nucleus isthmi or tectobulbar tract and block by nicotinic cholinergic antagonists. Neuroscience 40, 701–712. [DOI] [PubMed] [Google Scholar]
- Knudsen EI (2011). Control from below: the role of a midbrain network in spatial attention. European Journal of Neuroscience 33, 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI (2018). Neural circuits that mediate selective attention: a comparative perspective. Trends in Neurosciences 41, 789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI (2020). Evolution of neural processing for visual perception in vertebrates. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, and Ullman S (1985). Shifts in selective visual attention: towards the underlying neural circuitry. Human Neurobiology 4, 219–227. [PubMed] [Google Scholar]
- Koller K, Rafal RD, Platt A, and Mitchell ND (2019). Orienting toward threat: Contributions of a subcortical pathway transmitting retinal afferents to the amygdala via the superior colliculus and pulvinar. Neuropsychologia 128, 78–86. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, and Blaser E (1995). The role of attention in the programming of saccades. Vision Research 35, 1897–1916. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bogadhi AR, Herman JP, and Bollimunta A (2018). Selective attention without a neocortex. Cortex; a journal devoted to the study of the nervous system and behavior 102, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis RO, and Karten HJ (1988). Neuroactive peptides as markers of retinal ganglion cell populations that differ in anatomical organization and function. Vis Neurosci 1, 73–81. [DOI] [PubMed] [Google Scholar]
- Lee KH, Tran A, Turan Z, and Meister M (2020). The sifting of visual information in the superior colliculus. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, and Hall WC (1995). Interlaminar connections of the superior colliculus in the tree shrew. II: projections from the superficial gray to the optic layer. Vis Neurosci 12, 573–588. [DOI] [PubMed] [Google Scholar]
- Lee PH, Schmidt M, and Hall WC (2001). Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci 21, 8145–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, and Hall WC (2007). Identity of a pathway for saccadic suppression. Proc Natl Acad Sci U S A 104, 6824–6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG (1979). Evidence that the different classes of relay cells of the cat’s lateral geniculate nucleus terminate in different layers of the striate cortex. Exp Brain Res 37, 349–372. [DOI] [PubMed] [Google Scholar]
- Li L, Feng X, Zhou Z, Zhang H, Shi Q, Lei Z, Shen P, Yang Q, Zhao B, Chen S, et al. (2018). Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol 28, 859–871.e855. [DOI] [PubMed] [Google Scholar]
- Li X, and Basso MA (2008). Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci 28, 4561–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata AM, Kaufman MT, Raposo D, Ryan MB, Sheppard JP, and Churchland AK (2017). Posterior parietal cortex guides visual decisions in Rats. J Neurosci 37, 4954–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R (1999). Differential projection patterns of superior and inferior collicular neurons onto posterior paralaminar nuclei of the thalamus surrounding the medial geniculate body in the rat. Eur J Neurosci 11, 187–203. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Wang Q, and Li B (2011). Neuronal responses to looming objects in the superior colliculus of the cat. Brain, Behav Evol 77, 193–205. [DOI] [PubMed] [Google Scholar]
- Lo CC, and Wang XJ (2006). Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9, 956. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, and Krauzlis RJ (2010). Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksch H (2003). Cytoarchitecture of the avian optic tectum: neuronal substrate for cellular computation. Rev Neurosci 14, 85–106. [DOI] [PubMed] [Google Scholar]
- Luksch H, Cox K, and Karten HJ (1998). Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the tectofugal pathway. J Comp Neurol 396, 399–414. [PubMed] [Google Scholar]
- Luo Thomas Z., and Maunsell John H.R. (2015). Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron 86, 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major DE, Luksch H, and Karten HJ (2000). Bottlebrush dendritic endings and large dendritic fields: Motion-detecting neurons in the mammalian tectum. The Journal of Comparative Neurology 423, 243–260. [PubMed] [Google Scholar]
- Marín G, Letelier JC, Henny P, Sentis E, Farfán G, Fredes F, Pohl N, Karten H, and Mpodozis J (2003). Spatial organization of the pigeon tectorotundal pathway: an interdigitating topographic arrangement. J Comp Neurol 458, 361–380. [DOI] [PubMed] [Google Scholar]
- Marín GJ, Durán E, Morales C, González-Cabrera C, Sentis E, Mpodozis J, and Letelier JC (2012). Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. The Journal of Neuroscience 32, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson SP, Zhou N, Akers BK, Dang W, and Bickford ME (2019). Ultrastructural and optogenetic dissection of V1 corticotectal terminal synaptic properties. J Comp Neurol 527, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ (2006). The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151, 321–378. [DOI] [PubMed] [Google Scholar]
- May PJ, and Basso MA (2018). Connections between the zona incerta and superior colliculus in the monkey and squirrel. Brain Struct Funct 223, 371–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE, and Sparks DL (1980). Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol 43, 207–232. [DOI] [PubMed] [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, and Shadlen MN (2003). A role for neural integrators in perceptual decision making. Cereb Cortex 13, 1257–1269. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, and Redgrave P (2005). Subcortical loops through the basal ganglia. Trends Neurosci 28, 401–407. [DOI] [PubMed] [Google Scholar]
- McIlwain JT (1986). Point images in the visual system: New interest in an old idea. Trends Neurosci 9, 354–358. [Google Scholar]
- McIlwain JT (1991). Distributed spatial coding in the superior colliculus: a review. Vis Neurosci 6, 3–13. [DOI] [PubMed] [Google Scholar]
- McPeek RM, and Keller EL (2004). Deficits in saccade target selection after inactivation of superior colliculus. Nature Neuroscience 7, 757–763. [DOI] [PubMed] [Google Scholar]
- Medina L, and Reiner A (1994). Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. The Journal of Comparative Neurology 342, 497–537. [DOI] [PubMed] [Google Scholar]
- Medina L, and Smeets WJ (1992). Cholinergic, monoaminergic and peptidergic innervation of the primary visual centers in the brain of the lizards Gekko gecko and Gallotia galloti. Brain Behav Evol 40, 157–181. [DOI] [PubMed] [Google Scholar]
- Medina L, Smeets WJ, Hoogland PV, and Puelles L (1993). Distribution of choline acetyltransferase immunoreactivity in the brain of the lizard Gallotia galloti. J Comp Neurol 331, 261–285. [DOI] [PubMed] [Google Scholar]
- Merabet L, Desautels A, Minville K, and Casanova C (1998). Motion integration in a thalamic visual nucleus. Nature 396, 265–268. [DOI] [PubMed] [Google Scholar]
- Mooney RD, Fish SE, and Rhoades RW (1984). Anatomical and functional organization of pathway from superior colliculus to lateral posterior nucleus in hamster. Journal of Neurophysiology 51, 407–431. [DOI] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, Hess PR, Allen Z, Lewin AC, and Rhoades RW (1988). The projection from the superficial to the deep layers of the superior colliculus: an intracellular horseradish peroxidase injection study in the hamster. J Neurosci 8, 1384–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, and Karabelas AB (1985). Observations on the somatodendritic morphology and axonal trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol 239, 276–308. [DOI] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG, and Newsome WT (2005). Microstimulation of the superior colliculus focuses attention without moving the eyes. PNAS 102, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, and Wurtz RH (1995). Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73, 2313–2333. [DOI] [PubMed] [Google Scholar]
- Mysore SP, and Knudsen EI (2011). The role of a midbrain network in competitive stimulus selection. Curr Opin Neurobiol 21, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore Shreesh P., and Knudsen Eric I. (2014). Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron 84, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimoli V, Donnelly-Greenberg J, Gabel NM, Libby DJ, Panigrosso ER, Rhindress K, and Powers AS (2018). The role of acetylcholine in attention in turtles (Chrysemys picta). Brain Behav Evol 92, 71–81. [DOI] [PubMed] [Google Scholar]
- Nemec P, Altmann J, Marhold S, Burda H, and Oelschlager HH (2001). Neuroanatomy of magnetoreception: the superior colliculus involved in magnetic orientation in a mammal. Science 294, 366–368. [DOI] [PubMed] [Google Scholar]
- Northcutt RG (2002). Understanding vertebrate brain evolution. Integrative and comparative biology 42, 743–756. [DOI] [PubMed] [Google Scholar]
- Nummela SU, and Krauzlis RJ (2010). Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. Journal of Neurophysiology 104, 1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özen G, Helms MC, and Hall WC (2004). The intracollicular neuronal network. In The superior colliculus: new approaches for studying sensorimotor integration, Hall WC MA, ed. (Boca Raton, FL: CRC Press; ), pp. 147–158. [Google Scholar]
- Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, and Hall WC (2011). A circuit model for saccadic suppression in the superior colliculus. J Neurosci 31, 1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AS, and Reiner A (1993). The distribution of cholinergic neurons in the central nervous system of turtles. Brain Behav Evol 41, 326–345. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Shaw C, and Cynader MS (1988). The distribution and ontogenesis of [3H]nicotine binding sites in cat visual cortex. Brain Res 467, 161–176. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S (1909). Histologie du système nerveux de l’homme & des vertébrés, Vol 1 (Paris: Maloine; ). [Google Scholar]
- Ramón y Cajal S (1995). Histology of the Nervous System of Man and Vertebrates (Oxford University Press; ). [Google Scholar]
- Ratcliff R, Cherian A, and Segraves M (2003). A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol 90, 1392. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, and Segraves MA (2007). Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol 97, 1756–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, and McKoon G (2016). Diffusion decision model: Current issues and history. Trends in Cognitive Sciences 20, 260–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Westby GW, and Dean P (1993). Functional architecture of rodent superior colliculus: relevance of multiple output channels. Prog Brain Res 95, 69–77. [DOI] [PubMed] [Google Scholar]
- Reinhard K, Li C, Do Q, Burke EG, Heynderickx S, and Farrow K (2019). A projection specific logic to sampling visual inputs in mouse superior colliculus. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Belford GR, and Killackey HP (1987). Receptive-field properties of rat ventral posterior medial neurons before and after selective kainic acid lesions of the trigeminal brain stem complex. J Neurophysiol 57, 1577–1600. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Rohrer WH, Nikoletseas MM, and Fish SE (1989). Organization of the projection from the superficial to the deep layers of the hamster’s superior colliculus as demonstrated by the anterograde transport of Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology 283, 54–70. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, and Feller MB (2011). Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci 31, 8760–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, and Umiltá C (1987). Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25, 31–40. [DOI] [PubMed] [Google Scholar]
- Robinson DA (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vis Res 12, 1795–1808. [DOI] [PubMed] [Google Scholar]
- Robles E, Filosa A, and Baier H (2013). Precise lamination of retinal axons generates multiple parallel input pathways in the tectum. J Neurosci 33, 5027–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson JA, and Hall WC (1977). The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). II. Synaptic organization and comparisons with the dorsal lateral geniculate nucleus. J Comp Neurol 173, 389–416. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, and Redgrave P (1986). Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci 6, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani RR, Katyal S, Halfen E, Kim JH, and Ress D (2018). Polar-angle representation of saccadic eye movements in human superior colliculus. NeuroImage 171, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH (1972). The role of the monkey superior colliculus in eye movement and vision. Invest Ophthalmol 11, 451–460. [PubMed] [Google Scholar]
- Schiller PH, and Stryker M (1972). Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35, 915–924. [DOI] [PubMed] [Google Scholar]
- Schneider KA, and Kastner S (2005). Visual responses of the human superior colliculus: a high-resolution functional magnetic resonance imaging study. Journal of Neurophysiology 94, 2491–2503. [DOI] [PubMed] [Google Scholar]
- Sereno MI, and Ulinski PS (1987). Caudal topographic nucleus isthmi and the rostral nontopographic nucleus isthmi in the turtle, pseudemys scripta. The Journal of Comparative Neurology 261, 319–346. [DOI] [PubMed] [Google Scholar]
- Shang C, Chen Z, Liu A, Li Y, Zhang J, Qu B, Yan F, Zhang Y, Liu W, Liu Z, et al. (2018). Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat Commun 9, 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Liu A, Li D, Xie Z, Chen Z, Huang M, Li Y, Wang Y, Shen WL, and Cao P (2019). A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nature Neuroscience 22, 909–920. [DOI] [PubMed] [Google Scholar]
- Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, Li D, and Cao P (2015). A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477. [DOI] [PubMed] [Google Scholar]
- Shi X, Barchini J, Ledesma HA, Koren D, Jin Y, Liu X, Wei W, and Cang J (2017). Retinal origin of direction selectivity in the superior colliculus. Nat Neurosci 20, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, and Wurtz RH (2004a). The dialogue between cerebral cortex and superior colliculus: implications for saccadic target selection and corollary discharge. In The Visual Neurosciences, Chalupa LM, and Werner JS, eds. (Cambridge: MIT Press; ), pp. 1466–1484. [Google Scholar]
- Sommer MA, and Wurtz RH (2004b). What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol 91, 1403–1423. [DOI] [PubMed] [Google Scholar]
- Song J-H, Rafal RD, and McPeek RM (2011). Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proceedings of the National Academy of Sciences 108, E1433–E1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooksawate T, Isa K, and Isa T (2008). Cholinergic responses in crossed tecto-reticular neurons of rat superior colliculus. J Neurophysiol 100, 2702–2711. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, and Isa T (2006). Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. European Journal of Neuroscience 24, 3096–3108. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Yanagawa Y, and Isa T (2012). Cholinergic responses in GABAergic and non-GABAergic neurons in the intermediate gray layer of mouse superior colliculus. European Journal of Neuroscience 36, 2440–2451. [DOI] [PubMed] [Google Scholar]
- Sparks DL (1978). Functional properties of neurons in the monkey superior colliculus: coupling of neuronal activity and saccade onset. Brain Res 156, 1–16. [DOI] [PubMed] [Google Scholar]
- Sparks DL, and Mays LE (1990). Signal transformations required for the generation of saccadic eye movements. Annual Review of Neuroscience 13, 309–336. [DOI] [PubMed] [Google Scholar]
- Sprague JM (1966). Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153, 1544–1547. [DOI] [PubMed] [Google Scholar]
- Sridharan D, and Knudsen EI (2015). Gamma oscillations in the midbrain spatial attention network: linking circuits to function. Curr Opin Neurobiol 31, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Schwarz JS, and Knudsen EI (2014). Selective attention in birds. Curr Biol 24, R510–513. [DOI] [PubMed] [Google Scholar]
- Stein BE, and Gaither NS (1981). Sensory representation in reptilian optic tectum: some comparisons with mammals. J Comp Neurol 202, 69–87. [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Thompson JA, and Felsen G (2015). Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. Journal of Neurophysiology 114, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, and Frost BJ (1998). Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci 1, 296–303. [DOI] [PubMed] [Google Scholar]
- Suzuki DG, Pérez-Fernández J, Wibble T, Kardamakis AA, and Grillner S (2019). The role of the optic tectum for visually evoked orienting and evasive movements. Proc Natl Acad Sci U S A 116, 15272–15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Uhlrich DJ, and Sherman SM (1995). Morphology of physiologically identified retinal X and Y axons in the cat’s thalamus and midbrain as revealed by intraaxonal injection of biocytin. J Comp Neurol 354, 583–607. [DOI] [PubMed] [Google Scholar]
- Tardif E, Delacuisine B, Probst A, and Clarke S (2005). Intrinsic connectivity of human superior colliculus. Experimental Brain Research 166, 316–324. [DOI] [PubMed] [Google Scholar]
- Thevarajah D, Mikulic A, and Dorris MC (2009). Role of the superior colliculus in choosing mixed-strategy saccades. J Neurosci 29, 1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka K, Kasai M, Kobayashi K, and Isa T (2020). Anatomical and electrophysiological analysis of cholinergic inputs from the parabigeminal nucleus to the superficial superior colliculus. J Neurophysiol 124, 1968–1985. [DOI] [PubMed] [Google Scholar]
- Vale R, Campagner D, Iordanidou P, Arocas OP, Tan YL, Stempel AV, Keshavarzi S, Petersen RS, Margrie TW, and Branco T (2020). A cortico-collicular circuit for accurate orientation to shelter during escape. bioRxiv, 2020.2005.2026.117598 [Google Scholar]
- Vale R, Evans DA, and Branco T (2017). Rapid spatial learning controls instinctive defensive behavior in mice. Curr Biol 27, 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, ed. (1984). Comparative Neurology of the Optic Tectum (New York: Plenum Press; ). [Google Scholar]
- Vega-Zuniga T, Mpodozis J, Karten HJ, Marín G, Hain S, and Luksch H (2014). Morphology, projection pattern, and neurochemical identity of Cajal’s “centrifugal neurons”: the cells of origin of the tectoventrogeniculate pathway in pigeon (Columba livia) and chicken (Gallus gallus). J Comp Neurol 522, 2377–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaal J, and Luksch H (2015). Processing of motion stimuli by cells in the optic tectum of chickens. Neuroreport 26, 578–582. [DOI] [PubMed] [Google Scholar]
- Verhaal J, and Luksch H (2016). Neuronal responses to motion and apparent motion in the optic tectum of chickens. Brain Res 1635, 190–200. [DOI] [PubMed] [Google Scholar]
- Villalobos CA, and Basso MA (2020). Optogenetic activation of the inhibitory nigro-collicular circuit evokes orienting movements in mice. bioRxiv, 2020.2005.2021.107680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos CA, Wu Q, Lee PH, May PJ, and Basso MA (2018). Parvalbumin and GABA Microcircuits in the Mouse Superior Colliculus. Frontiers in Neural Circuits 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokoun CR, Jackson MB, and Basso MA (2010). Intralaminar and interlaminar activity within the rodent superior colliculus visualized with voltage imaging. J Neurosci 30, 10667–10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu M, Segraves MA, and Cang J (2015). Visual experience Is required for the development of eye movement maps in the mouse superior colliculus. J Neurosci 35, 12281–12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, McAlonan K, Goldstein S, Gerfen CR, and Krauzlis RJ (2020). A causal role for mouse superior colliculus in visual perceptual decision-making. The Journal of Neuroscience 40, 3768–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-R (2003). The nucleus isthmi and dual modulation of the receptive field of tectal neurons in non-mammals. Brain Research Reviews 41, 13–25. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, and Karten HJ (2006). Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): A possible substrate for synchronizing tectal channels. The Journal of Comparative Neurology 494, 7–35. [DOI] [PubMed] [Google Scholar]
- Wang Y, Major DE, and Karten HJ (2004). Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus). The Journal of Comparative Neurology 469, 275–297. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, and Isa T (2005). Effects of Local Nicotinic Activation of the Superior Colliculus on Saccades in Monkeys. J Neurophysiol 93, 519–534. [DOI] [PubMed] [Google Scholar]
- Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y, Li J, et al. (2015). Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun 6, 6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyland KL, Slusarczyk AS, and Bickford ME (2020). GABAergic cell types in the superficial layers of the mouse superior colliculus. J Comp Neurol 528, 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson W, Reiner A, Anderson K, and Karten HJ (1991). Distribution, laminar location, and morphology of tectal neurons projecting to the isthmo-optic nucleus and the nucleus isthmi, pars parvocellularis in the pigeon (Columba livia) and chick (Gallus domesticus): a retrograde labelling study. J Comp Neurol 305, 470–488. [DOI] [PubMed] [Google Scholar]
- Wu LQ, Niu YQ, Yang J, and Wang SR (2005). Tectal neurons signal impending collision of looming objects in the pigeon. Eur J Neurosci 22, 2325–2331. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, and Goldberg ME (1971). Superior colliculus responses related to eye movement in awake monkeys. Science 171, 82–84. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, and Mohler CW (1976). Organization of monkey superior colliculus: enhanced visual response of superficial layer cells. J Neurophysiol 39, 745–765. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Dulac C, Roth KA, and Sanes JR (2006). Labeled lines in the retinotectal system: markers for retinorecipient sublaminae and the retinal ganglion cell subsets that innervate them. Mol Cell Neurosci 33, 296–310. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu Q, Zhong J, Song R, Zhang L, and Wang L (2020). A simple threat-detection strategy in mice. BMC Biol 18, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, and Meister M (2013). Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol 23, 2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yttri EA, and Dudman JT (2016). Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533, 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenon A, and Krauzlis RJ (2012). Attention deficits without cortical neuronal deficits. Nature 489, 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeymer M, von der Emde G, and Wullimann MF (2018). The mormyrid pptic tectum is a topographic interface for active electrolocation and visual sensing. Front Neuroanat 12, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu M, and Cang J (2014). Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Masterson SP, Damron JK, Guido W, and Bickford ME (2018). The mouse pulvinar nucleus links the lateral extrastriate cortex, striatum, and amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou NA, Maire PS, Masterson SP, and Bickford ME (2017). The mouse pulvinar nucleus: Organization of the tectorecipient zones. Vis Neurosci 34, E011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, and Freedman DJ (2019). Posterior parietal cortex plays a causal role in perceptual and categorical decisions. Science 365, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Liu X, Chen S, Zhang Z, Liu Y, Montardy Q, Tang Y, Wei P, Liu N, Li L, et al. (2019). A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron 103, 473–488.e476. [DOI] [PubMed] [Google Scholar]


