Abstract
Objectives:
To determine the optimal scan delay corresponding to individual hemodynamic status for pancreatic parenchymal phase in dynamic contrast-enhanced CT of the abdomen.
Methods:
One hundred and fourteen patients were included in this retrospective study (69 males and 45 females; mean age, 67.9 ± 12.1 years; range, 39–87 years). These patients underwent abdominal dynamic contrast-enhanced CT between November 2019 and May 2020. We calculated and recorded the time from contrast material injection to the bolus-tracking trigger of 100 Hounsfield unit (HU) at the abdominal aorta (s) (TimeTRIG) and scan delay from the bolus-tracking trigger to the initiation of pancreatic parenchymal phase scanning (s) (TimeSD). The scan delay ratio (SDR) was defined by dividing the TimeSD by TimeTRIG. Non-linear regression analysis was conducted to assess the association between CT number of the pancreas and SDR and to reveal the optimal SDR, which was ≥120 HU in pancreatic parenchyma.
Results:
The non-linear regression analysis showed a significant association between CT number of the pancreas and the SDR (p < 0.001). The mean TimeTRIG and TimeSD were 16.1 s and 16.8 s, respectively. The SDR to peak enhancement of the pancreas (123.5 HU) was 1.00. An SDR between 0.89 and 1.18 shows an appropriate enhancement of the pancreas (≥120 HU).
Conclusion:
The CT number of the pancreas peaked at an SDR of 1.00, which means TimeSD should be approximately the same as TimeTRIG to obtain appropriate pancreatic parenchymal phase images in dynamic contrast-enhanced CT with bolus-tracking method.
Advances in knowledge:
The hemodynamic state is different in each patient; therefore, scan delay from the bolus-tracking trigger should also vary based on the time from contrast material injection to the bolus-tracking trigger. This is necessary to obtain appropriate late hepatic arterial or pancreatic parenchymal phase images in dynamic contrast-enhanced CT of the abdomen.
Introduction
Dynamic contrast-enhanced CT of the abdomen is used for initial evaluation, preoperative staging, and postoperative follow-up of focal hepatic and pancreatic lesions such as hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (PDAC). 1–4 Late hepatic arterial phase (LHAP) imaging is used to detect hypervascular HCC. 5,6 This phase imaging shows the maximal enhancement of hypervascular HCC neovascularity and minimal enhancement of the surrounding liver parenchyma, which maximizes the HCC-to-liver contrast and tumor detection rate. 7,8 Pancreatic parenchymal phase (PPP) imaging allows improved visualization of hypovascular PDAC and also enables assessment of peripancreatic arteries. 9,10 This phase shows the maximal enhancement of pancreatic parenchyma and maximizes the attenuation difference between PDAC and the surrounding pancreatic parenchyma. 11
After the administration of contrast material, LHAP and PPP can be obtained with the same scan delays, around 40 s. 12,13 Previous research revealed that several factors can affect the scan timing of LHAP and PPP, such as cardiovascular status and whether or not bolus-tracking method is used. 14–16 As cardiovascular status is reduced, the circulation of contrast material slows, resulting the delay contrast bolus arrival and delayed peak arterial enhancement. 17 The optimal scan delay after the contrast material reaches the abdominal aorta was determined in previous studies; however, the time from contrast material injection to the bolus-tracking trigger was ill-considered. When applying a fixed scan delay, inappropriate LHAP or PPP images could be obtained in patients with abnormal cardiovascular status. We hypothesized that dynamic contrast-enhanced CT imaging protocol for LHAP and PPP with bolus-tracking method would allow additional investigation of the effect of the time from contrast material injection to the bolus-tracking trigger. Therefore, the purpose of this study was to determine the optimal scan delay corresponding to individual hemodynamic status to obtain the appropriate LHAP and PPP in dynamic contrast-enhanced CT of the abdomen.
Methods and materials
Patients
This retrospective study was approved by our institutional review board, and written informed consent was waived. One hundred and twenty-one consecutive patients who underwent dynamic contrast-enhanced CT of the abdomen to screen hepatic or pancreatic diseases between November 2019 and May 2020 were included. Seven out of 121 patients were excluded for different contrast material injection protocol (n = 5), different scan protocol (n = 1), or huge mass at the hepatic portal region because of concerns about hemodynamic changes (n = 1). The remaining 114 patients (mean age, 67.9 ± 12.1 years; age range, 39–87 years) included 69 males (mean age, 68.9 ± 10.9 years; age range, 39–87 years), and 45 females (mean age, 66.5 ± 13.7 years; age range, 40–87 years). Twenty-five HCCs (mean maximal diameter, 19.1 ± 18.0 mm; maximal diameter range, 6.6–97.3 mm) were identified in 15 patients. One HCC was diagnosed by histopathological examination and the remaining 24 were diagnosed by the imaging findings (arterial enhancement and portal- or equilibrium-phase washout) with increased serological tumor markers.
CT scan protocol and contrast material injection
We used a fast kilovoltage-switching dual-energy CT scanner (Revolution CT; GE Healthcare, Milwaukee, WI, USA) but scanned using single-energy scan mode. The CT imaging parameters were as follows: X-ray tube voltage, 120 kilovolt peak (kVp); noise index, 7.0 at 5 mm slice collimation; tube current, variable; detector configuration, 80 detectors with 0.625-mm section thickness; beam collimation, 80 mm; rotation time, 0.35 s; pitch, 0.508:1; scan field-of-view, large body; and display field-of-view, 40 cm. Raw data were reconstructed using an adaptive statistical iterative reconstruction-Veo (ASiR-V; GE Healthcare) of 40% with 5-mm section thickness and 0% overlap.
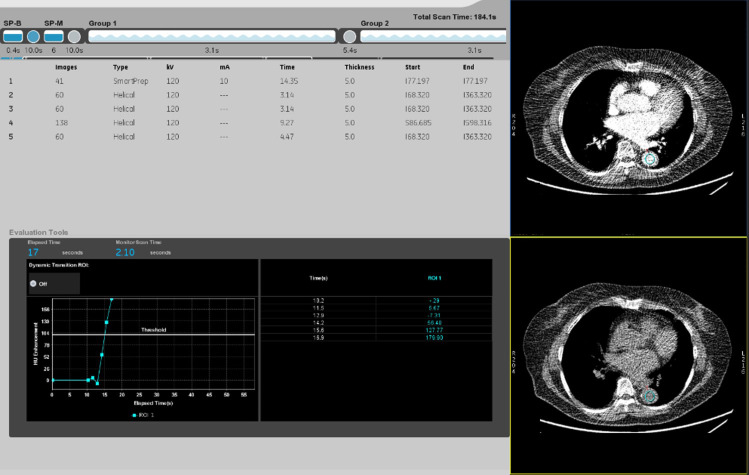
The contrast material, containing 300-mg iohexol per milliliter adjusted to the patient’s body weight (600 mgI/kg), was intravenously injected with a fixed injection duration of 30 s. A circle with a diameter of 15–20 mm was placed as a region-of-interest (ROI) in the abdominal aorta at the level of the first lumbar vertebral body. Real-time fluoroscopic monitoring scans (120 kVp, 10 mA) were initiated 10 s after contrast injection. Diagnostic CT scanning was initiated with an additional delay of 20 s for the LHAP/PPP, 60 s for the portal venous phase, and 170 s for the equilibrium phase after a bolus-tracking program (SmartPrep; GE Healthcare) detected a bolus-tracking trigger of 100 Hounsfield unit (HU) in the abdominal aorta (Figure 1).
Figure 1.
Time enhancement curve and record of SmartPrep scan. The time from contrast material injection to the bolus-tracking trigger of 100 HU at the abdominal aorta (s) (TimeTRIG) and the scan delay from the bolus-tracking trigger of 100 HU to the initiation of LHAP/PPP scanning (s) (TimeSD).
Qualitative image analysis
Two experienced radiologists (N.K. and Y.N., with 8 and 9 years of post-training experience in interpreting body CT images, respectively), who were unaware of the time enhancement curve and record of the SmartPrep scan, reviewed LHAP/PPP images and assessed the scan timing adequacy of LHAP/PPP using three classifications: Early, Appropriate, or Late in consensus. The following imaging findings defined these categories 14 :
Early: maximal aortic and hepatic arterial enhancement, no hepatic parenchymal enhancement, no portal or hepatic venous enhancement, and minimal or no splenic enhancement.
Appropriate: maximal aortic and hepatic arterial enhancement, mild to moderate hepatic parenchymal enhancement, mild to moderate portal venous enhancement, scarce hepatic venous enhancement, and heterogeneous splenic enhancement.
Late: maximal aortic and hepatic arterial enhancement, moderate to high hepatic parenchymal enhancement, moderate to high portal and hepatic venous enhancement, and uniform splenic enhancement.
Calculation of scan delay ratio
We recorded the time immediately before and after reaching the bolus-tracking trigger of 100 HU and CT number in an ROI at the abdominal aorta at that moment in time with reference to the time enhancement curve and record of the SmartPrep scan (Figure 1). According to these data, the time from contrast material injection to the bolus-tracking trigger of 100 HU at the abdominal aorta (s) (TimeTRIG) was calculated. Consecutively, the scan delay from the bolus-tracking trigger of 100 HU to the initiation of LHAP/PPP scanning (s) (TimeSD) was calculated. The scan delay ratio (SDR) was defined by dividing TimeSD by TimeTRIG.
Quantitative image analysis
A radiologist (T.K., with 2 years of post-training experience in interpreting body CT images) measured the CT numbers of the abdominal aorta, portal vein, liver (mean of CT numbers in the anterior and posterior segments of the right hepatic lobe and the medial and lateral segments of the left hepatic lobe), and pancreas on LHAP/PPP images using an ROI. For patients with HCC, the CT numbers of HCCs were also measured. The CT numbers of the abdominal aorta were measured using an ROI of approximately 100 mm2, which encompassed as much of the vascular lumen as possible, devoid of vascular walls, calcification, thrombus, and artifacts. Similarly, the CT numbers of the portal vein were measured using an ROI of approximately 50 mm2, encompassing as much of the vascular lumen as possible. The CT numbers of the liver were measured using an ROI of approximately 25 mm2, carefully avoiding large vessels or bile ducts, focal lesions, and artifacts. The CT numbers of the pancreas were measured using an ROI of approximately 25 mm2, carefully avoiding the main pancreatic duct, visible vessels, and artifacts. For HCCs, the CT numbers were measured using a circular ROI drawn to encompass as much of the lesion as possible.
For each patient, one standard deviation (SD) of the CT number of the homogeneous anterior abdominal wall fat tissue was determined as the background noise. The HCC-to-liver contrast-to-noise ratio (CNR) was calculated for HCCs using the following equation: CNR = (HUHCC–HUliver)/SDnoise, 18 where HUHCC denotes the mean CT number of HCC, HUliver represents that of liver parenchyma, and SDnoise denotes 1 SD of the CT number of the anterior abdominal wall fat tissue.
Statistical analysis
We summarized continuous variables by mean and SD, and categorical variables by counts and percentages. The non-linear regression analysis was performed to assess the association between CT number of the pancreas and SDR. The SDR was modeled with the use of restricted cubic splines to allow for non-linear associations with CT number of the pancreas. We estimated the optimal SDR using predicted values obtained from the non-linear regression model.
We determined the optimal SDR, which was the value predicted the CT number of the pancreas peaked, and that a range of SDRs with predicted value of CT number of the pancreas above 120 HU was optimal range. The CT number of the liver is affected by the severity of fatty liver; therefore, we used the CT number of the pancreas to determine if the scan timing of LHAP/PPP was adequate. Because previous studies demonstrated the optimal CT number of the pancreas for LHAP/PPP ranges from 100 to 120 HU, 11,13,19 we applied 120 HU.
Comparisons of the proportion of ≥120 HU between three classification of LHAP/PPP images (Early, Appropriate, and Late) were performed using the Fisher’s exact test. Median CT numbers of the abdominal aorta, portal vein, liver, and pancreas were compared between three classification of LHAP/PPP images (Early, Appropriate, and Late) by Kraskal–Wallis and Mann–Whitney U-tests. Median CNR was compared between Early and Appropriate +Late of LHAP/PPP images by Mann-Whitney U test. All p-values were two-sided. p values of <0.05 were considered statistically significant. All statistical analyses were performed using R v.4.0.2 (www.r-project.org).
Results
Qualitative image analysis
We used qualitative image analysis to classify the scan timing adequacy of the LHAP/PPP images as Early (n = 16), Appropriate (n = 76), or Late (n = 22). Figure 2 overlays these three classifications on a non-linear regression curve. As shown in Figure 2, no Early cases (0%) showed ≥120 HU in the pancreas. In Late cases, only 6 out of 22 (27.3%) showed ≥120 HU in the pancreas. Appropriate cases showed ≥120 HU in the pancreas in 41 out of 76 (53.9%), and there was a significant difference in frequency of an adequate pancreatic enhancement (p < 0.001).
Figure 2.
Overlayed image of three classifications on the non-linear regression curve. The SDR to peak enhancement of the pancreas (123.5 HU) was 1.00 and that showing ≥120 HU in the pancreas ranged from 0.89 to 1.18.
Scan delay ratio
Non-linear regression analysis showed a significant association between the CT number of the pancreas and the SDR (p < 0.001). The mean TimeTRIG, TimeSD, and SDR were 16.1 ± 2.5 s (range, 11.1–23.4 s), 16.8 ± 3.2 s (range, 5.8–21.1 s), and 1.1 ± 0.3 (range, 0.3–1.6), respectively. All three parameters were significantly different between the Early, Appropriate, and Late groups (p < 0.001) (Table 1) (Figures 3–5). The SDR to peak enhancement of the pancreas (123.5 HU) was 1.00 and that showing ≥120 HU in the pancreas ranged from 0.89 to 1.18 (Figure 2).
Table 1.
TimeTRIG, TimeSD, and Scan Delay Ratio between scan timing adequacy
| All patients (n = 114) | Early (n = 16) | Appropriate (n = 76) | Late (n = 22) | p value | |
|---|---|---|---|---|---|
| TimeTRIG (s) | 16.4 ± 2.5 (11.1–23.4) | 16.9 ± 2.5 (12.7–23.3) | 16.6 ± 2.4 (12.1–23.4) | 14.0 ± 1.5 (11.1–17.2) | <0.001 |
| TimeSD (s) | 16.8 ± 3.2 (5.8–21.1) | 11.3 ± 3.0 (5.8–20.3) | 17.5 ± 2.5 (10.1–21.1) | 18.5 ± 0.8 (16.8–19.9) | <0.001 |
| SDR | 1.1 ± 0.3 (0.3–1.6) | 0.7 ± 0.2 (0.3–1.1) | 1.1 ± 0.2 (0.7–1.5) | 1.3 ± 0.1 (1.0–1.6) | <0.001 |
SDR, scan delay ratio.
Note. TimeTRIG = the time from contrast material injection to the bolus-tracking trigger of 100 HU at the abdominal aorta. TimeSD = the scan delay from the bolus-tracking trigger of 100 HU to the initiation of late hepatic arterial or pancreatic parenchyma phase scan.
Data are means ± 1 standard deviation with ranges in parentheses.
Figure 3.
A 72-year-old male with liver metastases from rectal cancer. Axial images show maximal aortic enhancement, minimal hepatic and pancreatic parenchymal enhancement, and no portal venous enhancement. TimeTRIG, TimeSD, and SDR were 12.7 s, 10.2 s, and 0.80, respectively, and the images were classified into the Early group.
Figure 4.
A 70-year-old male with liver metastases from sigmoid colon cancer. Axial images show maximal aortic and pancreatic parenchymal enhancement, mild hepatic parenchymal enhancement, and moderate portal venous enhancement. TimeTRIG, TimeSD, and SDR were 16.6 s, 17.1 s, and 1.03, respectively, and the images were classified into the Appropriate group.
Figure 5.
A 87-year-old male with intrahepatic cholangiocellular carcinoma. Axial images show maximal aortic enhancement, moderate hepatic parenchymal enhancement, and moderate to high portal venous enhancement. TimeTRIG, TimeSD, and SDR were 14.0 s, 19.4 s, and 1.39, respectively, and the images were classified into the Late group.
Quantitative image analysis
CT numbers
Table 2 summarizes the comparison results of the CT numbers of anatomical structures of the Early, Appropriate, and Late groups. The CT numbers of the portal vein and liver in the Late group were significantly higher than those in the Early and Appropriate groups and were higher in the Appropriate group than in the Early group (p < 0.001). The CT number of the pancreas in the Appropriate group was significantly higher than those in the Early and Late groups and was higher in the Late group than in the Early group (p < 0.001). No significant difference was found in the CT number of the abdominal aorta between the three groups (p = 0.31).
Table 2.
CT numbers of the anatomical structures between scan timing adequacies
| Anatomical structure | Early (n = 16) | Appropriate (n = 76) | Late (n = 22) | p value a |
|---|---|---|---|---|
| Abdominal aorta | 337.6 ± 57.1 (236.5–470.3) | 350.7 ± 54.9 (223.6–464.2) | 329.5 ± 67.1 (168.3–438.1) | 0.31 |
| Early vs Appropriate | 0.26 | |||
| Early vs Late | 1.00 | |||
| Appropriate vs Late | 0.21 | |||
| Portal vein | 74.2 ± 15.0 (46.6–94.9) | 130.9 ± 22.2 (86.0–184.5) | 154.5 ± 19.5 (119.5–198.1) | <0.001 |
| Early vs Appropriate | <0.001 | |||
| Early vs Late | <0.001 | |||
| Appropriate vs Late | <0.001 | |||
| Liver | 69.0 ± 5.7 (54.9–76.4) | 76.1 ± 10.3 (34.5–96.7) | 82.5 ± 12.8 (58.5–120.7) | <0.001 |
| Early vs Appropriate | 0.0014 | |||
| Early vs Late | <0.001 | |||
| Appropriate vs Late | 0.0096 | |||
| Pancreas | 100.6 ± 11.4 (79.9–116.4) | 122.3 ± 13.7 (90.4–150.7) | 114.3 ± 12.3 (91.5–136.9) | <0.001 |
| Early vs Appropriate | <0.001 | |||
| Early vs Late | 0.005 | |||
| Appropriate vs Late | 0.015 | |||
Data are means ± 1 standard deviation with ranges in parentheses.
The Kruskal-Wallis test was used for comparisons between the three groups and the Mann-Whitney U test for comparisons between the two groups.
HCC-to-liver contrast-to-noise ratio
Twenty-five HCCs were identified in the Early (n = 7), Appropriate (n = 17), and Late (n = 1) groups. The CNR was significantly higher in the Appropriate group than in the other groups (p = 0.007). Because the sample size of Late group was small and the non-linear regression curve (Figure 2) demonstrated that the CT number of the pancreas was almost flat after the SDR of 1.18, we also compared the CNR between the Early and Appropriate +Late groups. The CNR was significantly higher in the Appropriate +Late groups (8.1 ± 3.6) than in the Early group (4.4 ± 2.9) (p = 0.009) (Table 3).
Table 3.
HCC-to-liver contrast-to-noise ratios between scan timing adequacy based on visual assessment
| Early (n = 7) | Appropriate (n = 17) | Late (n = 1) | p value | |
|---|---|---|---|---|
| CNR | 4.4 ± 2.9 (2.3–10.9) | 8.4 ± 3.4 (3.9–17.4) | 2.7 (2.7) | 0.007 |
| Early vs Appropriate | 0.0047 | |||
| Early vs Late | N.A. | |||
| Appropriate vs Late | N.A. | |||
| 4.4 ± 2.9 (2.3–10.9) | 8.1 ± 3.6 (2.7–17.4) | 0.009 | ||
CNR, Hepatocellular carcinoma-to-liver contrast-to-noise ratio; N.A, Not applicable.
Data are means ± 1 standard deviation with ranges in parentheses.
Discussion
Our study demonstrated that the mean TimeTRIG (16.4 s) and TimeSD (16.8 s) were almost the same, and the SDR to peak enhancement of the pancreas (123.5 HU) was just 1.00. In addition, the range of SDRs with predicted values of CT number of the pancreas above 120 HU was from 0.89 to 1.18. In this study, we visually assessed the scan timing adequacy of LHAP/PPP images by classifying them into the categories of Early, Appropriate, or Late. The results of the SDRs and a qualitative assessment of the scan timing adequacy showed that LHAP/PPP had links to the SDRs: the SDRs of the Appropriate group (1.1) were in the optimal SDR range of 0.89–1.18, and the Early (0.7) and Late (1.3) groups were below and above the optimal SDR range, respectively.
The optimal scan delay times for LHAP/PPP have been reported to be approximately 10–20 s after the bolus-tracking trigger. 16,20–22 This phase is crucial for detecting hypervascular HCC and hypovascular PDAC. The TimeSD of 16.8 s in our study was an optimal scan delay time, as previously reported. Contrast arrival time in the abdominal aorta, which was TimeTRIG in this study, was 15–18 s in patients with normal circulation. 17 This value was comparable with TimeTRIG of 16.4 s in our study. The range was 11.1–23.4 s in our study, and a previous report gave a variety of patient contrast arrival times, from 10 to 36 s depending on the hemodynamic state of the patient. 17 In the Late group, TimeTRIG was earlier (14.0 s) than in the Early (16.9 s) and Appropriate (16.6 s) groups. Additionally, TimeSD was early in the Early group (11.3 s) and late in the Late group (18.5 s) compared with the Appropriate group (17.5 s). Except for TimeTRIG in the Late group, however, the remaining TimeTRIG and TimeSD were comparable with previous data as noted above. Nevertheless, the cases classified into the Appropriate group were only 66.7% (n = 76/114). This result means a conventional method, which is a previously demonstrated optimal scan delay, still not be enough for obtaining appropriate LHAP/PPP images. We proposed the concept of SDR in this study, and the SDRs between the three groups were significantly different. The SDR is absolutely novel concept which is considering the information of the arrival time of contrast material. We believe that this means adjusting scan delay based on the time of arrival of contrast material in the abdominal aorta is better than using a fixed scan delay time from the bolus-tracking trigger.
There are other options beyond bolus-tracking technique to measure the transit time of contrast material. The test-bolus method is predicated on injecting a small among of contrast material (10–20 ml) prior scanning diagnostic CT with a full volume of contrast material. However, this method requires two separate contrast material injections and needs additional examination time. 23 Another option is the splenic-triggering protocol, the scan delay is determined by assessing the time to peak splenic enhancement. A previous study demonstrated that this method has better image quality, higher signal-to-noise ratio, and higher lesion conspicuity compared with aortic-triggering protocol. 24 Some patients who are planning abdominal dynamic contrast-enhanced CT have pancreatic cancer or already splenectomy. Splenic arterial and/or venous invasion is often seen in patients with pancreatic cancer, and this might lead to change of hemodynamics. The latter cannot be obtained splenic enhancement. Therefore, bolus-tracking technique monitoring at the aorta permits more broad utility.
In the assessment of the scan timing adequacy of LHAP/PPP, the difference between the Early and Appropriate groups was relatively clear, as shown in Figure 2. In contrast, the difference between the Appropriate and Late groups was unclear. Figure 2 demonstrates that the CT number of the pancreas is almost flat after the SDR of 1.18, which indicates the difficulty of dividing the Appropriate and Late groups in a visual matter. Therefore, we compared the CNRs between the Early and Appropriate + Late groups, and it was significantly higher in the Appropriate + Late group than in the Early group. This suggests that the Early group should be absolutely differentiated from the Appropriate and Late groups, that is, the LHAP/PPP scanning initiation should be delayed, depending on the TimeTRIG.
To certainly obtain the appropriate LHAP/PPP images, tailor-made scan delay is needed. However, TimeTRIG cannot be predict before scanning in the present clinical setting. If we could automatically initiate the diagnostic CT scan using CT scanner with automated scanning program designed to be able to automatically set TimeSD depends on TimeTRIG during CT examination and resulting in the SDR of 1.0, the appropriate LHAP/PPP images can be obtained easily regardless of TimeTRIG.
Our study had several limitations. First, the sample size was relatively small, especially the Late group with HCC, which might have caused a selection bias. Second, we determined the optimal SDR to obtain an appropriate LHAP/PPP in dynamic contrast-enhanced CT using bolus-tracking method; however, we only evaluated HCC-to-liver CNR. We could not evaluate PDAC-to-pancreas CNR because we had no PDAC case in this study, and therefore further clinical studies are required to validate our results.
In conclusion, appropriate LHAP/PPP image acquisition was achieved when the SDRs ranged from 0.89 to 1.18 and pancreatic enhancement peaked at an SDR of 1.00. This means TimeSD should be approximately the same as TimeTRIG to obtain appropriate LHAP/PPP images in dynamic contrast-enhanced CT with bolus-tracking method.
Contributor Information
Yoshifumi Noda, Email: noda1031@gifu-u.ac.jp.
Nobuyuki Kawai, Email: noburtcom@yahoo.co.jp.
Takuma Ishihara, Email: tishiha@gifu-u.ac.jp.
Yoshiki Tsuboi, Email: g.yoshiki.t.5125@gmail.com.
Tetsuro Kaga, Email: kagatetsurow@gmail.com.
Toshiharu Miyoshi, Email: t-miyosi@gifu-u.ac.jp.
Fuminori Hyodo, Email: hyodof@gifu-u.ac.jp.
Masayuki Matsuo, Email: matsuo_m@gifu-u.ac.jp.
REFERENCES
- 1. Park S, Joo I, Lee DH, Bae JS, Yoo J, Kim SW, et al. Diagnostic performance of LI-RADS treatment response algorithm for hepatocellular carcinoma: adding ancillary features to MRI compared with enhancement patterns at CT and MRI. Radiology 2020; 296: 554–61. doi: 10.1148/radiol.2020192797 [DOI] [PubMed] [Google Scholar]
- 2. Lee S, Kim Y-Y, Shin J, Hwang SH, Roh YH, Chung YE, et al. CT and MRI liver imaging reporting and data system version 2018 for hepatocellular carcinoma: a systematic review with meta-analysis. J Am Coll Radiol 2020; 17: 1199–206. doi: 10.1016/j.jacr.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 3. Jang JK, Byun JH, Kang JH, Son JH, Kim JH, Lee SS, et al. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following Folfirinox therapy. Eur Radiol 2021; 31: 813–23. doi: 10.1007/s00330-020-07188-8 [DOI] [PubMed] [Google Scholar]
- 4. Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, et al. CT evaluation after neoadjuvant Folfirinox chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 2017; 27: 3104–16. doi: 10.1007/s00330-016-4632-8 [DOI] [PubMed] [Google Scholar]
- 5. Sultana S, Awai K, Nakayama Y, Nakaura T, Liu D, Hatemura M, et al. Hypervascular hepatocellular carcinomas: bolus tracking with a 40-detector CT scanner to time arterial phase imaging. Radiology 2007; 243: 140–7. doi: 10.1148/radiol.2431060069 [DOI] [PubMed] [Google Scholar]
- 6. Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle MP, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology 2001; 218: 763–7. doi: 10.1148/radiology.218.3.r01mr39763 [DOI] [PubMed] [Google Scholar]
- 7. Marin D, Nelson RC, Samei E, Paulson EK, Ho LM, Boll DT, et al. Hypervascular liver tumors: low tube voltage, high tube current multidetector CT during late hepatic arterial phase for detection--initial clinical experience. Radiology 2009; 251: 771–9. doi: 10.1148/radiol.2513081330 [DOI] [PubMed] [Google Scholar]
- 8. Schindera ST, Nelson RC, Mukundan S, Paulson EK, Jaffe TA, Miller CM, et al. Hypervascular liver tumors: low tube voltage, high tube current multi-detector row CT for enhanced detection--phantom study. Radiology 2008; 246: 125–32. doi: 10.1148/radiol.2461070307 [DOI] [PubMed] [Google Scholar]
- 9. Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-Phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology 1996; 199: 697–701. doi: 10.1148/radiology.199.3.8637990 [DOI] [PubMed] [Google Scholar]
- 10. Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997; 168: 1439–43. doi: 10.2214/ajr.168.6.9168704 [DOI] [PubMed] [Google Scholar]
- 11. McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi--detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology 2001; 220: 97–102. doi: 10.1148/radiology.220.1.r01jl1897 [DOI] [PubMed] [Google Scholar]
- 12. Kanematsu M, Goshima S, Kondo H, Nishibori H, Kato H, Yokoyama R, et al. Optimizing scan delays of fixed duration contrast injection in contrast-enhanced biphasic multidetector-row CT for the liver and the detection of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr 2005; 29: 195–201. doi: 10.1097/01.rct.0000155062.50236.59 [DOI] [PubMed] [Google Scholar]
- 13. Goshima S, Kanematsu M, Kondo H, Yokoyama R, Miyoshi T, Kato H, et al. Pancreas: optimal scan delay for contrast-enhanced multi-detector row CT. Radiology 2006; 241: 167–74. doi: 10.1148/radiol.2411051338 [DOI] [PubMed] [Google Scholar]
- 14. Hussain HK, Londy FJ, Francis IR, Nghiem HV, Weadock WJ, Gebremariam A, et al. Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradient-recalled-echo sequence: comparison with test-bolus method. Radiology 2003; 226: 558–66. doi: 10.1148/radiol.2262011593 [DOI] [PubMed] [Google Scholar]
- 15. Osimani M, Rengo M, Paolantonio P, Ferrari R, De Cecco CN, Bellini D, et al. Sixty-four-multidetector-row computed tomography angiography with bolus tracking to time arterial-phase imaging in healthy liver: is there a correlation between quantitative and qualitative scores? J Comput Assist Tomogr 2010; 34: 883–91. doi: 10.1097/RCT.0b013e3181dd80c8 [DOI] [PubMed] [Google Scholar]
- 16. Kondo H, Kanematsu M, Goshima S, Miyoshi T, Shiratori Y, Onozuka M, et al. Mdct of the pancreas: optimizing scanning delay with a bolus-tracking technique for pancreatic, peripancreatic vascular, and hepatic contrast enhancement. AJR Am J Roentgenol 2007; 188: 751–6. doi: 10.2214/AJR.06.0372 [DOI] [PubMed] [Google Scholar]
- 17. Bae KT, Heiken JP. Scan and contrast administration principles of MDCT. Eur Radiol 2005; 15 Suppl 5(Suppl 5): e46–59. doi: 10.1007/s10406-005-0165-y [DOI] [PubMed] [Google Scholar]
- 18. Noda Y, Kanematsu M, Goshima S, Kondo H, Watanabe H, Kawada H, et al. Reducing iodine load in hepatic CT for patients with chronic liver disease with a combination of low-tube-voltage and adaptive statistical iterative reconstruction. Eur J Radiol 2015; 84: 11–18. doi: 10.1016/j.ejrad.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 19. Fletcher JG, Wiersema MJ, Farrell MA, Fidler JL, Burgart LJ, Koyama T, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 2003; 229: 81–90. doi: 10.1148/radiol.2291020582 [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi I, Kidoya E, Suzuki M, Kimura H. Optimizing scan timing of hepatic arterial phase by physiologic pharmacokinetic analysis in bolus-tracking technique by multi-detector row computed tomography. Radiol Phys Technol 2011; 4: 43–52. doi: 10.1007/s12194-010-0105-y [DOI] [PubMed] [Google Scholar]
- 21. Goshima S, Kanematsu M, Kondo H, Yokoyama R, Miyoshi T, Nishibori H, et al. Mdct of the liver and hypervascular hepatocellular carcinomas: optimizing scan delays for bolus-tracking techniques of hepatic arterial and portal venous phases. AJR Am J Roentgenol 2006; 187: W25–32. doi: 10.2214/AJR.04.1878 [DOI] [PubMed] [Google Scholar]
- 22. Kagawa Y, Okada M, Yagyu Y, Kumano S, Kanematsu M, Kudo M, et al. Optimal scan timing of hepatic arterial-phase imaging of hypervascular hepatocellular carcinoma determined by multiphasic fast CT imaging technique. Acta Radiol 2013; 54: 843–50. doi: 10.1177/0284185113485571 [DOI] [PubMed] [Google Scholar]
- 23. Kirchner J, Kickuth R, Laufer U, Noack M, Liermann D. Optimized enhancement in helical CT: experiences with a real-time bolus tracking system in 628 patients. Clin Radiol 2000; 55: 368–73. doi: 10.1053/crad.2000.0376 [DOI] [PubMed] [Google Scholar]
- 24. Mileto A, Husarik DB, Bellini D, Marin D, Reiner CS, Nelson RC. Adoption of splenic enhancement to time and trigger the late hepatic arterial phase during MDCT of the liver: proof of concept and clinical feasibility. AJR Am J Roentgenol 2016; 207: 310–20. doi: 10.2214/AJR.15.15808 [DOI] [PubMed] [Google Scholar]