Abstract
Gene fusions resulting in oncogenic activation of various receptor tyrosine kinases, including NTRK1–3, ALK, and RET, have been increasingly recognized in soft tissue tumors (STTs), displaying a wide morphologic spectrum and therefore diagnostically challenging. A subset of STT with NTRK1 rearrangements were recently defined as lipofibromatosis-like neural tumors (LPFNTs), being characterized by mildly atypical spindle cells with a highly infiltrative growth in the subcutis and expression of S100 and CD34 immunostains. Other emerging morphologic phenotypes associated with kinase fusions include infantile/adult fibrosarcoma and malignant peripheral nerve sheath tumor-like patterns. In this study, a large cohort of 73 STT positive for various kinase fusions, including 44 previously published cases, was investigated for the presence of an LPFNT phenotype, to better define the incidence of this distinctive morphologic pattern and its relationship with various gene fusions. Surprisingly, half (36/73) of STT with kinase fusions showed at least a focal LPFNT component defined as >10%. Most of the tumors occurred in the subcutaneous tissues of the extremities (n = 25) and trunk (n = 9) of children or young adults (<30 years old) of both genders. Two-thirds (24/36) of these cases showed hybrid morphologies with alternating LPFNT and solid areas of monomorphic spindle to ovoid tumor cells with fascicular or haphazard arrangement, while one-third (12/36) had pure LPFNT morphology. Other common histologic findings included lymphocytic infiltrates, staghorn-like vessels, and perivascular or stromal hyalinization, especially in hybrid cases. Mitotic activity was generally low (<4/10 high power fields in 81% cases), being increased only in a minority of cases. Immunoreactivity for CD34 (92% in hybrid cases, 89% in pure cases) and S100 (89% in hybrid cases, 64% in pure cases) were commonly present. The gene rearrangements most commonly involved NTRK1 (75%), followed by RET (8%) and less commonly NTRK2, NTRK3, ROS1, ALK, and MET.
Keywords: lipofibromatosis-like neural tumor, MET, NTRK, RET
1 |. INTRODUCTION
With the advent of next generation sequencing (NGS), an increasing number of kinase fusions have been identified across a spectrum of tumor types spanning various lineages, including carcinomas, melanomas, gliomas, and soft tissue tumors (STT).1,2 STT characterized by activating kinase fusions involving NTRK, ALK, and RET genes among others, exhibit a wide morphologic spectrum.3–9 Except for a few well-established pathologic entities, such as inflammatory myofibroblastic tumor (IMT) and infantile fibrosarcoma (IFS), many of these represent novel and emerging tumor types, which were previously unclassified or misclassified under various diagnoses. One of the first morphologic-molecular correlates was that of NTRK1 gene fusions with a group of superficial spindle cell tumors resembling lipofibromatosis, defined by infiltrative growth into surrounding adipose tissues and exhibiting immunoreactivity for S100 and CD34. These tumors typically occur in the extremities of children and young adults and, based on their distinctive features, the term lipofibromatosis-like neural tumor (LPFNT) was proposed for these neoplasms.10 In addition to LPFNT, NTRK1-related gene fusions were also reported in other spindle cell STT reminiscent of infantile or adult fibrosarcoma (FS), malignant peripheral nerve sheath tumor (MPNST), and less commonly with a myopericytoma/hemangiopericytoma phenotype.3,5,9,11 Interestingly, recent studies of STT characterized by RET gene fusions revealed a morphologic spectrum similar to that of tumors with NTRK fusions,6,7 thereby suggesting a degree of phenotypic overlap across kinase fusions. Since STT with kinase fusions are potential candidates for targeted therapies, it is critical to better define their morphologic spectrum and diagnostic criteria in order to facilitate the recognition of these tumors in clinical practice. In this study, we investigated a large cohort of 73 kinase fusion-positive STTs for the incidence of an LPFNT morphology, either as the predominant or focal component, and its correlations with kinase fusion types. The aim was to elucidate whether kinase fusions define a broader morphologic spectrum than initially expected.
2 |. MATERIALS AND METHODS
2.1 |. Case selection
Seventy-three STTs harboring receptor tyrosine kinase fusions, including NTRK1, NTRK2, NTRK3, ALK, ROS1, RET, and MET, were collected from departmental archives and consultation files, including 44 cases previously published by our group and 29 cases diagnosed after the initial publication.3,4,7,9,10,12 Well-established pathologic entities, such as IMT with ALK, ROS1 or NTRK3 fusions, and IFS with ETV6-NTRK3 or alternative gene fusions were not included in the study. The gene fusions/rearrangements were identified either by NGS methods or by fluorescence in situ hybridization (FISH) screening. The screening criteria included spindle cell STT with morphologic features resembling LPFNT, FS, or MPNST. Although most tumors were positive for S100 and CD34, this immunoprofile was not an inclusion criterion in this study.
The available hematoxylin-eosin stained slides were re-reviewed and evaluated for the presence of an LPFNT pattern in at least 10% of the tumor. In tumors displaying LPFNT areas, other coexisting morphologic features were recorded, such as the presence of a solid component (non-LPFNT), stromal and perivascular hyalinization, nuclear pleomorphism, mitotic activity, inflammatory infiltrate, and so forth. Immunostaining results for CD34 (790–2927, Ventana) and S100 (Z0311, 1:8000, Dako) were also reviewed, however, most cases had a much more extensive immunowork-up, typically including SOX10, NTRK1, pan-Trk, and so forth (Supplementary Table S1). Tumors displaying both LPFNT and solid growth were classified as hybrid cases. The presence of a solid component was defined as a compact nodular growth of monomorphic spindle cells, without overt entrapment of nonneoplastic tissues, usually adipose tissues, occupying at least one low power field. Follow-up information was obtained from the electronic medical records or referring pathologists. The study was approved by the Institutional Review Board.
2.2 |. Molecular testing
Kinase gene fusions were identified in eight cases by NGS-based methods, including by whole transcriptome sequencing (n = 4), anchored multiplex RNA sequencing (Archer Dx) (n = 2), targeted RNA sequencing with TruSight RNA Fusion Panel (Illumina, San Diego, California) (n = 1), and FoundationOne CDx (n = 1). RNA was extracted from frozen tissues for whole transcriptome sequencing and from formalin-fixed paraffin-embedded (FFPE) tissues for the other platforms. The sequencing and analysis process were as reported previously.3,4,7,9,10,12
2.3 |. Fluorescence in situ hybridization
FISH was performed on FFPE tissue sections of all cases using break-apart probe designs. Custom FISH probes for NTRK1, NTRK2, NTRK3, ALK, ROS1, RET, and MET were made from bacterial artificial chromosomes after DNA isolation, nick translation, and metaphase validation as previously described.3,4,7,9,10,12 Tumors with histomorphology resembling LPFNT, FS, or MPNST were screened by FISH for various kinase fusion gene rearrangements. For cases with known gene fusions identified by sequencing methods, FISH was performed for the known kinase genes to validate the sequencing results.
3 |. RESULTS
3.1 |. Tumors with either pure or hybrid LPFNT pattern commonly occur in the extremity of children and are associated with a benign clinical course
An LPFNT pattern was identified in 36 of 73 (49%) kinase fusion-positive STTs (Table 1). The 36 patients ranged from 0 to 77 years old, (mean: 15.7, median: 12), with both genders being equally affected (19 males, 17 females). Most of the patients were children (69%) or young adults (22%, ≤30 years old), and 7 (19%) cases presented with congenital lesions or were diagnosed in infants. The tumors occurred most frequently in the extremities, including both lower (n = 13) and upper (n = 12) extremities, followed by trunk (n = 9), and uncommonly in the scalp (n = 2). Most tumors involved the subcutaneous adipose tissue, with or without extension to the adjacent dermis or skeletal muscle.
TABLE 1.
STTs with LPFNT pattern and various kinase gene fusions
| No. | Age (y) | Sex | Location | Depth | LI | SV | PH | Mitosis (/10HPFs) | S100 | CD34 | Gene fusion | Fusion partner | FURef | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure | ||||||||||||||
| 1 | 7 | F | Hand | SC | N | Y | N | 1 | + | + | NTRK1 | TPRW | LR (4.2 y), NED (9.7 y) | 10 |
| 2 | 4 | F | Thigh | SC | Y | N | Y | 2 | + | + | NTRK1 | LMNA | NED (8 m) | 10 |
| 3 | 15 | M | Forearm | SC, FS | Y | N | N | 1 | + | NA | NTRK1 | LMNA | 10 | |
| 4 | 4 m | M | Hip | DM, SC | N | N | N | NA | − | + | NTRK1 | LMNA | ||
| 5 | 6 | F | Hand | SC, IM | N | Y | N | 0–1 | NA | NA | NTRK1 | LMNA | ||
| 6 | 77 | F | Thigh | DM, SC | Y | N | Y | 5 | + | + | NTRK1 | TPR | 3 | |
| 7 | 12 | M | Arm | SC, IM | Y | N | N | 0 | − | NA | NTRK1 | LMNA | ||
| 8 | 13 | F | Abd. wall | Superficial | NA | NA | NA | NA | − | + | RET | 7 | ||
| 9 | 1 | F | Ankle | DM, SC | N | N | N | 0–1 | + | + | RET | CCDC6A | AWD (3 y) | 7 |
| 10 | 0 | F | Foot | Superficial and deep (bone) | NA | NA | NA | NA | + | + | RET | NCOA4FM | AWD (2 y) | 7 |
| 11 | 64 | M | Forearm | SC, IM | Y | N | N | 1 | − | + | ALK | CLIP1W | NED (5 y) | |
| 12 | 14 | F | Buttock | IM | N | Y | N | 0–1 | + | − | ALK | NED (3 y) | 10 | |
| Hybrid | ||||||||||||||
| 13 | 10 | F | Antecubital | SC, IM | Y | Y | Y | 0–1 | + | + | NTRK1 | TPM3W | NED (2.2 y) | 10 |
| 14 | 27 | F | Forearm | IM | Y | Y | N | 0–1 | + | NA | NTRK1 | NED (4.5 y) | 10 | |
| 15 | 28 | F | Flank | SC | Y | Y | Y | 0–1 | + | NA | NTRK1 | LMNA | NED (2 m) | 10 |
| 16 | 25 | M | Foot | SC | N | Y | N | 0–1 | + | + | NTRK1 | LR, NED (5 y) | 10 | |
| 17 | 38 | F | Scalp | DM, SC | Y, focal | Y | Y, focal | 1 | + | + | NTRK1 | 10 | ||
| 18 | 10 | M | Leg | DM, SC | Y | Y | Y | 0–1 | + | + | NTRK1 | LRx2, NED (4 y) | 10 | |
| 19 | 12 | M | Arm | DM, SC | N | Y | N | 0–1 | + | + | NTRK1 | LMNA | NED (1 m) | 10 |
| 20 | 0 | M | Lower back | DM, SC | focal | N | N | NA | + | + | NTRK1 | LMNA | NED (8 m) | 10 |
| 21 | 5 m | M | Forearm | DM, SC | Y | Y | N | 6 | + | NA | NTRK1 | LR (7.5 y) | ||
| 22 | 30 | M | Scalp | DM, SC | Y | N | N | 25 | + | − | NTRK1 | |||
| 23 | 0 | F | Shoulder | DM, SC, IM | N | Y | N | 0–1 | − | NA | NTRK1 | |||
| 24 | 23 | M | Arm | Superficial | Y | N | N | 2 | − | + | NTRK1 | TPM3 | Mets (lung, abd.), AWD (3 y) | 3 |
| 25 | 5 | M | Leg | SC | Y | N | Y | 0 | + | + | NTRK1 | LMNA | NED (3 m) | 3 |
| 26 | 15 | F | Thigh | SC | N | N | N | 2 | + | + | NTRK1 | LMNA | NED (14 m) | 3 |
| 27 | 3 | F | Sacral area | SC, IM | Y | Y | N | 0 | NA | NA | NTRK1 | TPR | ||
| 28 | 17 | M | Thigh | SC | N | Y | N | 13 | NA | NA | NTRK1 | |||
| 29 | 6 | M | Abd. wall | DM, SC | Y | N | N | 0 | NA | NA | NTRK1 | LMNA | ||
| 30 | 10 | M | Abd. wall | SC, IM | Y | N | N | 1 | NA | NA | NTRK1 | TPM3 | ||
| 31 | 20 | F | Trunk | SC, IM | Y | Y | Y | 0 | NA | NA | NTRK1 | LMNA | ||
| 32 | 18 | M | Knee | SC | Y | Y | N | 1 | + | NA | NTRK1 | TPR | ||
| 33 | 21 | F | Paraspinal | IM | Y | Y | Y | 11 | + | + | NTRK3 | TFGAIa | NED (5 m) | 4 |
| 34 | 3 m | M | Back | DM, SC | N | N | N | 1 | NA | NA | MET | TFGW | ||
| 35 | 20 | M | Forearm | NA | NA | NA | Y | NA | + | + | NTRK2 | SPECC1LT | 3 | |
| 36 | 12 | M | Thigh | SC | Y | N | Y, focal | 8 | + | + | ROS1 | LR, NED (9 m) | 10 |
Note: y, years; m, months; F, female; M, male; Abd., abdominal; SC, subcutis; FS, fascia; DM, dermis; IM, intramuscular; NA, not available; LI, lymphocytic infiltrate; N, no; Y, yes; SV, staghorn vessel; PH, perivascular hyalinization; HPF, high power field; W, whole transcriptome sequencing; A, ArcherDx; FM, foundation medicine; I, MSK IMPACT; T, targeted RNA sequencing; FU, follow-up; LR, local recurrence; NED, no evidence of disease; AWD, alive with disease; Mets, metastasis; Ref, citation of cases previously published.
Abbreviations: LPFNT, lipofibromatosis-like neural tumor; SSTs, soft tissue tumors.
MSK IMPACT: p53 mutation, CDKN2A/B deletion.
One third of the cases (12/36) had a pure LPFNT growth pattern with monotonous spindle cells forming vague fascicles intervening between and admixed with the subcutaneous adipose tissue (Figure 1A) and skeletal muscle (Figure 1B). The tumor cells were most commonly ovoid to short spindle cells with vesicular to mildly hyperchromatic nuclei and indistinct cell borders (Figure 1C). Two-thirds of the cases (24/36) displayed a hybrid phenotype, composed of alternating LPFNT pattern (Figure 1D) with solid components of sheets of spindle to ovoid cells organized in a fascicular or haphazard arrangement, devoid of entrapped adipose tissue (Figure 2).
FIGURE 1.
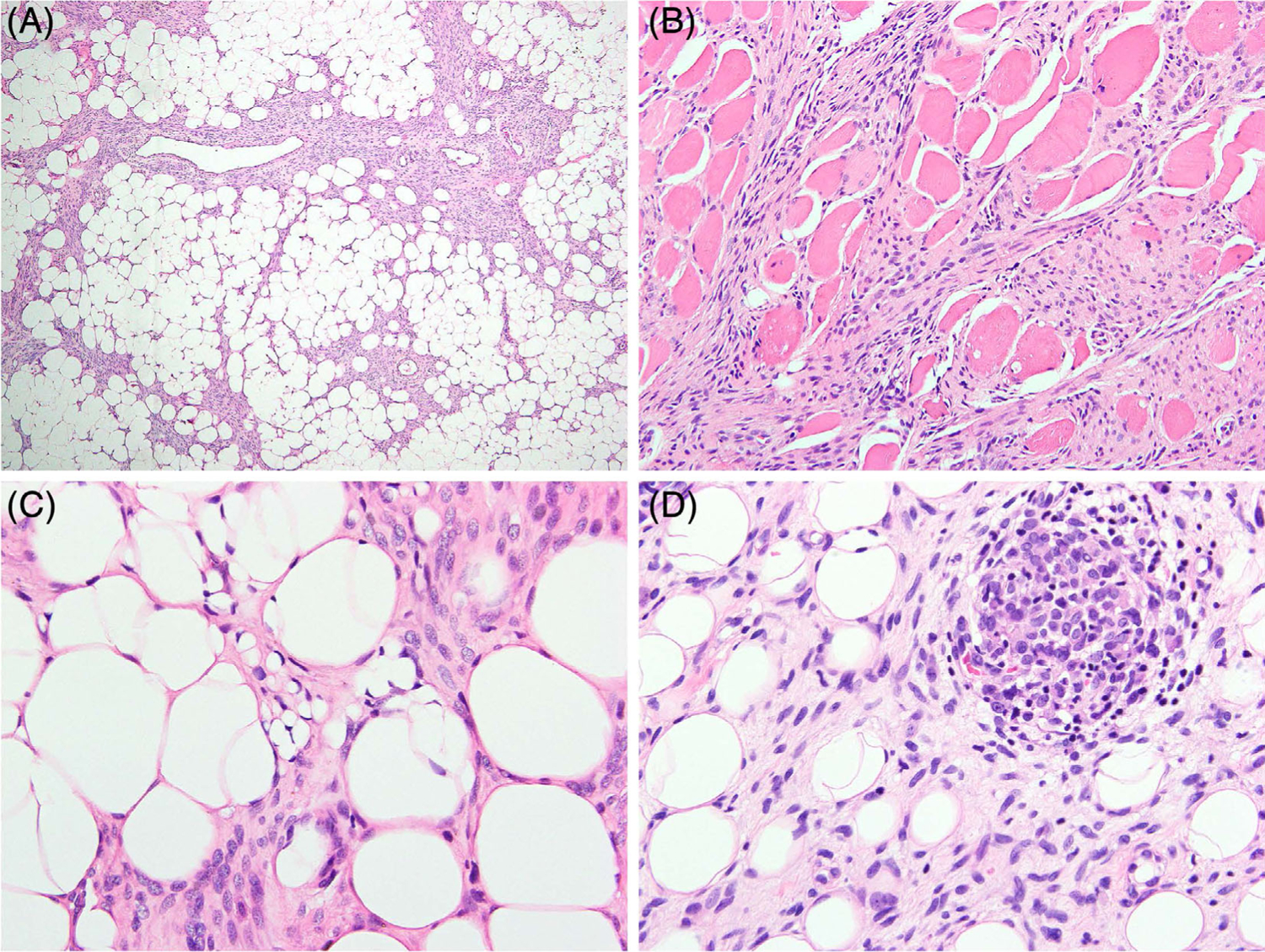
Lipofibromatosis-like neural tumor with kinase gene fusions. Spindle cells infiltrating adipose tissue, A, and skeletal muscle, B. Focal lipoblast-like cells resembling those of lipofibromatosis are seen in some cases, C. The tumor cells are usually relatively monotonous ovoid to short spindle cells with vesicular chromatin and indistinct cell borders, C, and some cases show mild atypia and hyperchromasia, D. Scattered cellular aggregates of round and primitive-appearing cells are also present in rare cases, D. (A–C, case 5; D, case 30) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
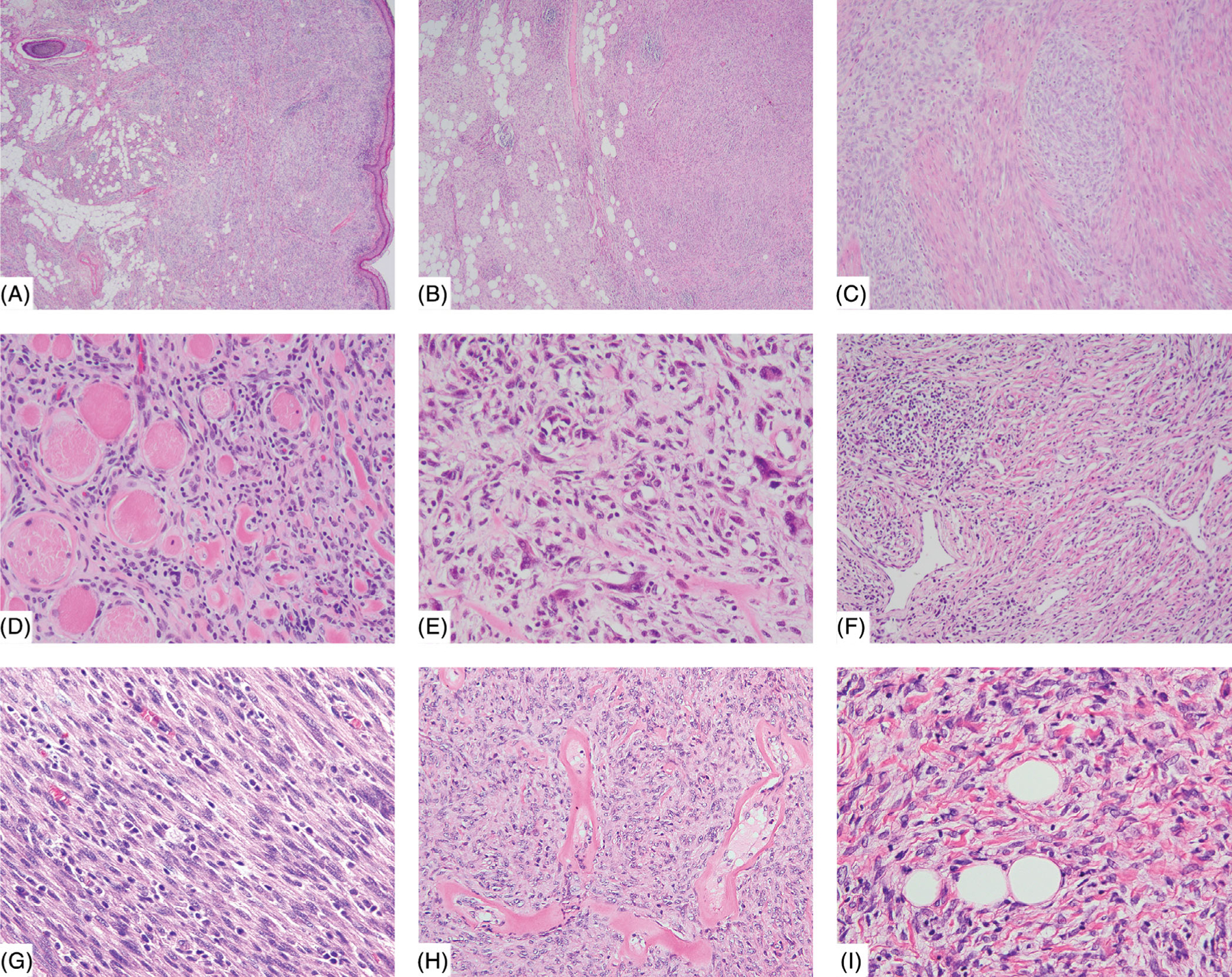
Tumors with hybrid lipofibromatosis-like neural tumor (LPFNT) and solid patterns. Many cases show solid areas of compact spindle cell proliferation as well as lipofibromatosis-like pattern, A,B. Some cases have the solid area in the dermis and LPFNT pattern in the subcutis (A, case 17), and others have both components in the subcutis (B, case 15). The solid areas often show sheets of spindle to ovoid cells in a fascicular or haphazard arrangement (C, case 21). Skeletal muscle involvement is also seen in some cases (D, case 14). A minority of cases show occasional nuclear atypia and hyperchromasia (E, case 15). Other accompanying features include staghorn vessels (F, case 15), lymphocytic infiltrate (F,G, cases 15 and 24), perivascular hyalinization (H, case 13), and ropey collagen fibers admixed with tumor cells (I, case 29) [Color figure can be viewed at wileyonlinelibrary.com]
Patients with pure LPFNT morphology were mostly children (0–15 years), with the exception of a few outlier elderly patients. Those with a hybrid morphology ranged from children to young adults (0–38 years). The extremities were the most common anatomic sites for both groups. Tumors arising in the trunk were more commonly observed to have hybrid morphology (n = 8) than pure LPFNT morphology (n = 1). Deep intramuscular or bone involvement was observed in both morphologic groups (5 of 12 pure LPFNT; 7 of 23 hybrid cases).
In addition to the distinctive LPFNT morphology, some other recurrent features were noted, such as a lymphocytic infiltrate admixed with the tumor cells in the majority of the hybrid cases (17/23, 74%) and in half of the pure LPFNT group (5/10, 50%) (Figure 2F, G). A vascular network composed of thin-walled staghorn vessels was observed in many of the hybrid group cases (14/23, 61%) and some pure LPFNT cases (3/10, 30%) (Figure 2F). Distinct perivascular hyalinization was present in 9/24 hybrid cases (38%) and 2/10 (20%) pure LPFNT cases (Figure 2H). Some cases showed ropey-like collagen bundles (Figure 2I). Most of the tumors had mild to no nuclear atypia. Exceptionally, the only hybrid case with NTRK3 fusion (case 33) showed focally marked nuclear pleomorphism among a variety of different growth patterns and cytologic features, as described previously.4 Mitotic activity was generally low in most cases. Eight of 9 pure LPFNT cases (89%) and 17 of 22 hybrid cases (77%) with available mitotic counts had less than 4 mitotic figures per 10 high power fields (HPFs). However, a subset of hybrid cases (5/22, 23%) showed mitotic activities >5/10 HPFs, prompting classification as low-grade sarcoma in three cases and high-grade sarcoma in two cases. The latter two cases showed in addition to >10 MF/10 HPFs, marked nuclear pleomorphism in one case, and necrosis in the other. Immunohistochemically, CD34 staining was present in most hybrid cases (12/13, 92%), as well as pure LPFNT cases (8/9, 89%). Similarly, S100 immunoreactivity was observed in the majority of hybrid cases (16/18, 89%) and more than half of pure LPFNT cases examined (7/11, 64%). The staining pattern of both CD34 and S100 ranged from focal, multifocal, to diffuse.
Clinical follow-up information was available in 19 patients (6 pure LPFNT and 13 hybrid cases), including one case in each group with extended follow-up period after our previous publication and one new case in each group. In total, local recurrence was observed in 5 of 19 patients (26%), including 1 with pure LPFNT morphology (1/6, 17%) and 4 with hybrid morphology (4/13, 31%), likely related to their incomplete primary surgical resections. Distant metastasis to the lungs and abdomen was only seen in one patient with a superficial arm tumor harboring TPM3-NTRK1 fusion (case 24), showing hybrid morphology with compact monotonous spindle cell proliferation, with increased cellularity and low mitotic activity (2/10HPFs) (Figure 2G), morphologically suggestive of a low-grade malignancy. Most patients had no evidence of disease at last follow-up (15/19, 79%), and none died of the disease.
3.2 |. Various kinase fusions define tumors with LPFNT phenotype, including a novel TFG-MET fusion
NTRK1 gene rearrangement remained the most common genetic alteration (27/36, 75%) in tumors with LPFNT morphology, with LMNA, TPR, and TPM3 being the common fusion partner genes. Less common kinase gene fusions included RET (n = 3, one each with CCDC6-RET, NCOA4-RET and unknown fusion partner), ALK (n = 2, one with CLIP1-ALK and one with unknown fusion partner), NTRK3 (n = 1, TFG-NTRK3), NTRK2 (n = 1, SPECC1L-NTRK2), ROS1 (n = 1, unknown fusion partner), and a recently identified novel MET (n = 1, TFG-MET) fusions.
Histologically, most NTRK1 fusion cases (20/27, 74%), as well as the NTRK3, NTRK2, ROS1, and MET fusion cases, showed hybrid morphology with LPFNT areas and also solid areas devoid of entrapped adipocytes, whereas the 3 RET,2 ALK, a small subset of NTRK1 fusion (7/27, 26%) cases showed pure LPFNT pattern without solid growth (Table 2). No obvious correlation between genotype and age group or tumor location was found. The percentage of tumors showing any LPFNT feature is lowest in the subset with NTRK3 fusions (1/13, 8%), which most commonly present as high-grade sarcomas without LPFNT pattern.
TABLE 2.
Histologic distribution of mesenchymal tumors with kinase gene fusions
| NTRK1 | RET | ALK | NTRK3 | NTRK2 | ROS1 | MET | Total | |
|---|---|---|---|---|---|---|---|---|
| Pure LPF-like | 7 | 3 | 2 | 0 | 0 | 0 | 0 | 12 |
| Hybrid | 20 | 0 | 0 | 1 | 1 | 1 | 1 | 24 |
| Subtotal (pure LPF-like+ hybrid) | 27 | 3 | 2 | 1 | 1 | 1 | 1 | 36 |
| No LPF area (denominator) | 19 | 3 | 0 | 12 | 1 | 0 | 2 | 37 |
| Total | 46 | 6 | 2 | 13 | 2 | 1 | 3 | 73 |
The tumor with a TFG-MET fusion was identified by RNA sequencing in a back mass of a 3-month-old boy. The lesion was diagnosed at birth and was thought to represent a spinal lipoma. The MRI findings showed a well circumscribed, partially exophytic 5.2 cm mass, confined to the subcutis of the lumbar spine areas. The tumor involved the dermis and subcutaneous tissues and showed a peculiar triphasic pattern, with loosely arranged primitive short spindle cells alternating with more interlacing fascicles of dense fibrous tissue, infiltrating into the adipocytic component (Figure 3A–D). The tumor cells had mild nuclear atypia, fine chromatin, small distinct nucleoli, and low mitotic activity (Figure 3D). The tumor cells showed patchy positivity for CD34, while S100 only highlighted the adipocytes. The patient remains free of disease after 18 months follow-up. This case represented the only one of three MET-rearranged tumors in this study showing an LPFNT pattern. The other two MET-positive cases also occurred in infants, but showed IFS-like morphology with hyper-cellular monomorphic spindle cells arranged in fascicles. One of them, as previously reported, was a 4-month-old patient presented with a pelvic soft tissue mass with S100 immunoreactivity and a similar TFG-MET fusion.12 The other case we encountered recently was a 5-week-old girl with a 6.3 cm tumor in the right masseter muscle, abutting the skull base, which showed RBPMS-MET fusion that was identified by anchored multiplex RNA sequencing (Archer Dx).
FIGURE 3.

A congenital superficial back lipofibromatosis-like neural tumor (LPFNT) harboring TFG-MET fusion (case 34). The tumor shows solid areas in the dermis, A, and an infiltrative, lipofibromatosis-like pattern in the subcutis, B. A triphasic pattern with hypocellular collagenous areas alternating with loosely arranged primitive ovoid cells within the adipocytic component, C,D [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
LPFNT is a superficial and locally aggressive, nonmetastasizing STT characterized by an infiltrative growth pattern of spindle cells within adipose tissues, resembling lipofibromatosis, a pediatric acral fibroblastic tumor. In our original report, LPFNT occurred mostly in the extremities, head and neck, or trunk regions of children and young adults and showed coexpression of S100 and CD34 by IHC and frequent NTRK1 fusions.10 A few subsequent reports also showed cases with similar clinicopathologic characteristics.13,14 In addition to LPFNT, NTRK1 gene fusions have been increasingly recognized in STT with an IFS-like or MPNST-like morphology,3,5,9 and less often with a myopericytoma/hemangiopericytoma phenotype.11 Distinctive stromal keloidal collagen and perivascular hyalinization are observed in many of the low-grade MPNST-like cases.3 In addition, STTs with other kinase gene fusions, such as RET, NTRK3, and MET, also appear to show histomorphology that falls within this spectrum.4,7,12 Although some degree of genotype-phenotype correlations may exist, for example, NTRK3 and MET-rearranged spindle cell tumors reported so far show a higher propensity to be high-grade tumors,4,12,15–18 no morphologic parameter is entirely specific for any genetic alteration and vice versa. A proportion of tumors with NTRK1 fusions has been shown to have both low-grade MPNST-like features and focal LPFNT growth.3 However, it is still unclear how often these morphologic features coexist in the same tumor and if there is any association between morphology and other clinicopathological parameters. In this study, we reviewed the histomorphology of 73 STTs positive for various kinase fusions and document the presence of a LPF-like pattern in about half of the cases (36/73, 49%) along with a frequently coexisting solid component (hybrid phenotype) and other common morphologic features, including lymphocytic infiltrate, staghorn vessels, and perivascular hyalinization. We also demonstrate that most NTRK1-rearranged STTs (63%) show at least focal areas of LPFNT morphology, while NTRK3-rearranged STTs rarely show LPFNT area (8%).
Adding to the previous LPFNT study,10 we examined a broader spectrum of tumors with known kinase fusions to determine the incidence of LPFNT features and other previously described histologic characters and their association. Tumors in the pure and hybrid groups show overlapping clinicopathologic features, regarding patient age group, tumor location, immunoprofile, fusion genes, and so forth. The presence of cases with hybrid LPFNT areas and solid components of spindle cell fascicles are in keeping with a morphologic spectrum rather than separate entities within STT with kinase gene fusions. The LPFNT pattern, along with other histologic features (ie, perivascular hyalinization), may serve as a morphologic clue in diagnosing this emerging group of tumors. In this study, we also found that some cases lack S100 immunoreactivity, but had otherwise similar clinicopathologic presentations. In clinical practice, tumors with predominantly solid areas can be diagnostically challenging on limited biopsy specimens, especially when CD34 and S100 stains are very focal or negative. An awareness of this clinicopathologic spectrum and identification of other common morphologic features could help guide further immunostaining and molecular testing.
The clinical outcome of patients with kinase fusion-positive STTs varies greatly. In our original series of LPFNT, local recurrence was noted in 5 of 12 (42%) patients, all of whom had incomplete initial excisions.10 However, no metastatic disease or death of disease was noted. A subsequent report of 10 LPFNTs also revealed local recurrence in 3 (30%) patients and no distant metastasis.14 Our more recent studies of a broader spectrum of kinase-rearranged STTs, included low- and high-grade tumors (defined by increased cellularity and/or mitotic activity), which were associated with different clinical behaviors based on histologic grade.3,4 All five patients with low-grade lesions had no evidence of disease at last follow-up, including one patient who had a local recurrence, while two of three patients with high-grade tumors died of the disease. In a recent study of 30 pediatric cases with IFS morphology and NTRK1/2/3 fusions, the patients had an overall local recurrence rate of 29% and an overall metastatic rate of 22% (mostly to the lungs).5 The current investigation focuses on patients with kinase fusion-positive STTs displaying at least focal LPFNT morphology and found an overall recurrence rate of 26% and only a rare metastatic event (1/36, 3%). Therefore, cases with an LPFNT pattern may represent the lower grade end of this disease spectrum. Further investigation is required to evaluate if any other clinicopathologic parameter can help predict patient outcome and facilitate the identification of patients who can benefit from targeted therapy.
With the increasing application of molecular techniques in clinical practice, tumors traditionally diagnosed as lipofibromatosis appear to encompass a heterogeneous group of tumors. Among 20 cases of lipofibromatosis, Al-Ibraheemi et al found four cases with FN1-EGF fusions, suggesting overlapping with calcifying aponeurotic fibroma, and one case each with EGFR-BRAF, SPARC-PDGFRB, VCL-RET, and TPR-ROS1 fusions.19 Although immunoprofiles of individual cases are not available, the lipofibromatosis cases in that study showed variable expression of SMA and CD34, while S100 was negative. The latter cases could be closely related to LPFNT. The sensitivity of CD34 and S100 stains in tumors with less common (non-NTRK1) fusion variants requires further study.
In this study, we also report two additional STT cases with MET gene fusions. The first case is a 3-month-old boy with a superficial dermal and subcutaneous back tumor showing hybrid LPFNT with solid areas and a peculiar triphasic appearance, carrying a TFG-MET fusion. To our knowledge, this is the first MET fusion STT reported to have an LPFNT pattern. A similar TFG-MET fusion has been identified in an infantile pelvic sarcoma with IFS-like morphology, perivascular myoid proliferation, and S100 expression.12 Another MET fusion tumor in this report was a high-grade IFS-like sarcoma with RBPMS-MET fusion. Albeit with small case numbers, STTs with MET fusions also demonstrate a similar morphologic spectrum to those with NTRK and RET fusions.
The recent advances in the molecular-based classification have resulted in an increasing number of genetically defined entities showing significant overlap in morphologic patterns, often associated with a nonspecific immunophenotype. These findings have triggered inherent challenges on the appropriate terminology applied, reenacting ongoing debates on what defines a pathologic entity, specifically the supremacy of morphology vs molecular gold standard. One such notable example being the wide spectrum of STTs harboring kinase fusions. Although LPFNT was initially described in conjunction with a strict immunoprofile (coexpression of S100 and CD34) and mainly associated with NTRK1 fusions, further studies from our group and others have identified similar morphologic features (either in a pure form or intermixed with other histologic features) associated with a nonspecific immunoprofile and other kinase fusions. Should these lesions be defined based on their microscopic features, that is, “tumors with LPFNT phenotype,”, regardless of the kinase abnormality involved, or should these lesions be defined based on the type of kinase involved, that is, “NTRK-fusion positive STT,” regardless of the morphologic appearance. As most of the kinase fusion-positive mesenchymal tumor studies are very recent and data are still evolving, these very important questions cannot be definitively answered at this time. However, as LPFNT is not only the first, but also one of the most established entity in this molecular setting, with findings validated by various groups, we believe that it represents a good foundation to build further investigations addressing key questions, such as the role of morphology vs molecular gold standard.
In summary, through reviewing a large cohort of STT with kinase gene fusions, we show that an LPFNT pattern is observed in half of these tumors. The tumors may either present as pure LPFNT or hybrid with solid growth areas and can coexist with other common histologic findings, such as lymphocytic infiltrate, staghorn vessels, and perivascular hyalinization. Immunoreactivity for CD34 and S100 is commonly present, but a minority of cases can have a nonspecific immunoprofile. Among various kinase gene fusions, LPFNT pattern is most often associated with tumors with NTRK1 fusions, followed by RET and less commonly NTRK2, NTRK3, ROS1, ALK, and MET fusions.
Supplementary Material
Acknowledgments
Funding information
Center for Scientific Review, Grant/Award Numbers: CA 008748, CA 140146, CA 217694; Memorial Sloan Kettering Cancer Center (Cycle for Survival); St Baldrick Foundation
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147–153. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao SJ, Zehir A, Sireci AN, Aisner DL. Detection of tumor NTRK gene fusions to identify patients who may benefit from tyrosine kinase (TRK) inhibitor therapy. J Mol Diag. 2019;21(4):553–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suurmeijer AJH, Dickson BC, Swanson D, et al. A novel group of spindle cell tumors defined by S100 and CD34 co-expression shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes. Genes Chromosomes Cancer. 2018;57(12):611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suurmeijer AJ, Dickson BC, Swanson D, et al. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes Chromosomes Cancer. 2019;58(11):739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis JL, Lockwood CM, Stohr B, et al. Expanding the spectrum of pediatric NTRK-rearranged mesenchymal tumors. Am J Surg Pathol. 2019;43(4):435–445. [DOI] [PubMed] [Google Scholar]
- 6.Davis JL, Vargas SO, Rudzinski ER, et al. Recurrent RET gene fusions in pediatric spindle mesenchymal neoplasms. Histopathology. 2020;76(7): 1032–1041. [DOI] [PubMed] [Google Scholar]
- 7.Antonescu CR, Dickson BC, Swanson D, et al. Spindle cell tumors with RET gene fusions exhibit a morphologic spectrum akin to tumors with NTRK gene fusions. Am J Surg Pathol. 2019;43(10):1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JC, Zhang L, Drilon AE, et al. Expanding the molecular characterization of thoracic inflammatory myofibroblastic tumors beyond ALK gene rearrangements. J Thorac Oncol. 2019;14(5):825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao YC, Fletcher CDM, Alaggio R, et al. Recurrent BRAF gene fusions in a subset of pediatric spindle cell sarcomas: expanding the genetic spectrum of tumors with overlapping features with infantile fibrosarcoma. Am J Surg Pathol. 2018;42(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agaram NP, Zhang L, Sung YS, et al. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive lipofibromatosis-like neural tumors. Am J Surg Pathol. 2016;40(10):1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haller F, Knopf J, Ackermann A, et al. Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J Pathol. 2016;238(5):700–710. [DOI] [PubMed] [Google Scholar]
- 12.Flucke U, van Noesel MM, Wijnen M, et al. TFG-MET fusion in an infantile spindle cell sarcoma with neural features. Genes Chromosomes Cancer. 2017;56(9):663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartenstein DW, Coe TM, Gordon SC, et al. Lipofibromatosis-like neural tumor: case report of a unique infantile presentation. JAAD Case Rep. 2018;4(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lao IW, Sun M, Zhao M, Yu L, Wang J. Lipofibromatosis-like neural tumour: a clinicopathological study of ten additional cases of an emerging novel entity. Pathology. 2018;50(5):519–523. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki F, Nakatani F, Asano N, et al. Novel NTRK3 fusions in fibrosarcomas of adults. Am J Surg Pathol. 2019;43(4):523–530. [DOI] [PubMed] [Google Scholar]
- 16.Olson N, Rouhi O, Zhang L, et al. A novel case of an aggressive superficial spindle cell sarcoma in an adult resembling fibrosarcomatous dermatofibrosarcoma protuberans and harboring an EML4-NTRK3 fusion. J Cutan Pathol. 2018;45(12):933–939. [DOI] [PubMed] [Google Scholar]
- 17.Chiang S, Cotzia P, Hyman DM, et al. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol. 2018;42(6):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croce S, Hostein I, Longacre TA, et al. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod Pathol. 2019;32(7):1008–1022. [DOI] [PubMed] [Google Scholar]
- 19.Al-Ibraheemi A, Folpe AL, Perez-Atayde AR, et al. Aberrant receptor tyrosine kinase signaling in lipofibromatosis: a clinicopathological and molecular genetic study of 20 cases. Mod Pathol. 2019;32(3): 423–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


